Abstract
The oral microbiota are closely related to human health. Nonetheless, to the best of our knowledge, their relationship with membranous nephropathy (MN) remains unstudied. The saliva microbiota collected from 22 patients with MN and 15 healthy controls were analyzed by next-generation sequencing, and bioinformatics analysis of the 16S ribosomal RNA gene was subsequently carried out. The Chao1 and Shannon indices in patients with MN were higher than those in healthy controls. Analysis of similarities revealed that the oral microbiota in the patient group were significantly different from those in the healthy controls. At the genus level, the abundance of Alloprevotella, Granulicatella, Prevotella, Streptococcus and Prevotella_7 was markedly higher in patients with MN than in healthy controls. Six operational taxonomic units (OTUs; OTU5, OTU28, OTU9, OTU15, OTU33 and OTU38) were found to be markedly correlated with the clinical factors creatinine, proteinuria in 24 h, estimated glomerular filtration rate and systolic blood pressure. A total of 28 Kyoto Encyclopedia of Genes and Genomes pathways were obtained from the significant OTUs. The oral microbiota of patients with MN were investigated and it was found that OTU5, OTU28, OTU9, OTU15, OTU33 and OTU38 may be used as biomarkers. The present findings may assist in the diagnosis of patients with MN.
Keywords: 16S ribosomal RNA gene, membranous nephropathy, oral microbiota, biomarkers
Introduction
Membranous nephropathy (MN) is a common glomerular disease characterized by increased thickness of the diffuse glomerular basement membrane and subepithelial immune deposits (1). A total of one-third of MN cases present primary glomerulonephritis and two-thirds present secondary MN (2). In previous years, the number of patients with MN has increased (3) and ~15% of them develop end-stage renal disease (4). The prognosis of patients with MN is poor, with 15–30% of patients relapsing after remission (5).
The microflora, especially that in the intestine, maintains human health and affects physiological functions (6). A number of studies have reported that patients with cancer, diabetes and chronic kidney disease present an imbalance in the intestinal microbiota, and that dysbiosis further stimulates disease development (7,8). Furthermore, the composition of the intestinal microflora shows different characteristics in different diseases. Yu et al (9) found that the composition of the gut microbiome in diabetic kidney disease and MN was markedly different. Furthermore, the oral microbiota is a reflection of health, and the dynamic changes in its diversity affect the balance between disease and health (10). It has also been reported that the oral microbiota is closely related to chronic kidney disease. Duan et al (11) found that the diversity of the saliva microbiota was increased, and its abundance and richness were decreased in patients with end-stage renal disease. Our previous study reported an association between the oral microbiota and immunoglobulin A nephropathy (12). Nonetheless, studies on the relationship between the oral microflora and kidney diseases are rare, especially studies investigating the relationship between the diversity and abundance of oral microbiota and MN. In the present study, saliva samples from 22 patients with MN and 15 healthy controls were collected for oral microbiota characterization and the possibility of using the microbiota to diagnose patients with MN was explored.
Materials and methods
Sample collection
Saliva was collected from 22 patients with MN (mean age, 41.9 years) and 15 healthy controls (mean age, 42.1 years) between March 1, 2019 and June 31, 2020, at Shenzhen Longhua District Central Hospital (Shenzhen, China). The diagnosis of MN was based on routine light and immunofluorescence microscopy examination. The criteria proposed by Ehrenreich and Churg (13) were used to determine histological staging of MN. Subjects underwent comprehensive dental and periodontal examinations by clinicians who performed clinical status assessments. The subjects brushed their teeth in the morning and 2 h after they were required to spit 4–5 ml of saliva directly into sterile collection containers over 30 min. The collected saliva was naturally produced, without any stimulation. After collection, the samples were immediately mixed with RNA later (cat. no. R0901; MilliporeSigma) and stored at −80°C until use. The patients with MN did not receive hormones, antibiotics, immunosuppressive therapy or alternate therapies when the saliva samples were collected. The serum creatinine (CR), estimated glomerular filtration rate (eGFR), systolic blood pressure (SBP), diastolic blood pressure (DBP) and proteinuria in 24 h (Pro-24) were measured before medication therapy and obtained from the records of the patients, and these clinical characteristics are listed in Table I. The present study was approved by the ethics committee of Shenzhen Longhua District Central Hospital (Shenzhen, China). All participants enrolled voluntarily in the study and signed a written consent form.
Table I.
Clinical information of patients with membranous nephropathy and healthy controls.
| A, Patients with membranous nephropathy | ||||||||
|---|---|---|---|---|---|---|---|---|
|
| ||||||||
| ID | Sex | Age, years | CR, µmol/l | eGFR, ml/min/1.73 m2 | SBP, mmHg | DBP, mmHg | Pro-24, g/day | Pathological results |
| 160801 | M | 51 | 304.4 | 20.1 | 137 | 69 | 5.55 | MN, stage III |
| 160901 | M | 30 | 68 | 126.2 | 122 | 83 | 4.36 | MN, stage II |
| 161004 | M | 46 | 90 | 83.8 | 112 | 67 | 7.26 | MN, stage II |
| 161005 | F | 43 | 48 | 130.2 | 126 | 72 | 1.92 | MN, stage II |
| 161007 | F | 44 | 61 | 98.2 | 123 | 83 | 3.66 | MN, stage II |
| 170102 | F | 39 | 67 | 90.3 | 122 | 75 | 4.36 | MN, stage II |
| 170103 | M | 53 | 82 | 90.6 | 139 | 82 | 8.90 | MN, stage II |
| 170201 | M | 42 | 93 | 82.2 | 117 | 77 | 2.20 | MN, stage II |
| 170302 | F | 45 | 83 | 68.5 | 139 | 79 | 1.87 | MN, stage I |
| 170402 | M | 50 | 90 | 82.4 | 161 | 100 | 3.63 | MN, stage II |
| 170403 | M | 30 | 95 | 85.8 | 149 | 73 | 7.50 | MN, stage II |
| 170407 | M | 34 | 64 | 132.0 | 113 | 81 | 5.45 | MN, stage II |
| 170502 | M | 44 | 80 | 96.8 | 162 | 106 | 9.33 | MN, stage II |
| 170903 | M | 50 | 220 | 29.4 | 130 | 74 | 6.33 | MN, stage III |
| 180102 | M | 37 | 79 | 101.8 | 119 | 61 | 0.78 | MN, stage II |
| 180304 | F | 43 | 71 | 82.8 | 139 | 81 | 1.80 | MN, stage III |
| 180305 | F | 24 | 64 | 105.1 | 105 | 70 | 2.94 | MN, stage II |
| 180602 | M | 46 | 77 | 100.3 | 137 | 92 | 5.96 | MN, stage II |
| 180702 | M | 44 | 78 | 99.7 | 105 | 56 | 3.26 | MN, stage II |
| 180801 | M | 35 | 88 | 90.9 | 121 | 81 | 2.93 | MN, stage II |
| 180902 | F | 47 | 54 | 111.6 | 135 | 85 | 3.93 | MN, stage I |
| 181003 | M | 44 | 124 | 58.4 | 108 | 73 | 10.36 | MN, stage II |
|
| ||||||||
| B, Healthy controls | ||||||||
|
| ||||||||
| ID | Sex | Age, years | CR, µmol/l | eGFR, ml/min/1.73 m2 | SBP, mmHg | DBP, mmHg | Pro-24, g/day | Pathological results |
|
| ||||||||
| HE1 | M | 40 | 69 | 117.1 | 121 | 56 | 0.04 | Healthy |
| HE2 | M | 55 | 80 | 92.5 | 98 | 73 | 0.03 | Healthy |
| HE3 | M | 34 | 68 | 123.0 | 105 | 79 | 0.10 | Healthy |
| HE4 | M | 33 | 78 | 105.7 | 123 | 55 | 0.034 | Healthy |
| HE5 | M | 42 | 70 | 114.0 | 130 | 85 | 0.06 | Healthy |
| HE6 | M | 34 | 73 | 113.4 | 116 | 79 | 0.05 | Healthy |
| HE7 | M | 40 | 75 | 106.4 | 108 | 88 | 0.08 | Healthy |
| HE8 | M | 45 | 67 | 118.3 | 123 | 72 | 0.12 | Healthy |
| HE9 | M | 50 | 82 | 91.7 | 113 | 59 | 0.09 | Healthy |
| HE10 | M | 43 | 54 | 119.9 | 125 | 86 | 0.08 | Healthy |
| HE11 | F | 58 | 58 | 98.5 | 108 | 84 | 0.10 | Healthy |
| HE12 | F | 43 | 54 | 113.6 | 107 | 76 | 0.08 | Healthy |
| HE13 | F | 38 | 67 | 90.8 | 98 | 82 | 0.12 | Healthy |
| HE14 | F | 34 | 50 | 130.2 | 106 | 75 | 0.03 | Healthy |
| HE15 | F | 42 | 57 | 107.3 | 113 | 83 | 0.04 | Healthy |
Clinical indicators of each patient and healthy controls are listed. Pathological results of patients with membranous nephropathy are also provided. The normal range of CR is 57–97 µmol/l. SBP and DBP were measured before medication therapy. CR, creatinine; eGFR, estimated glomerular filtration rate; SBP, systolic blood pressure; DBP, diastolic blood pressure; Pro-24, proteinuria at 24 h; M, male; F, female; MN, membranous nephropathy.
Exclusion criteria
Subjects who had diarrhea or other intestinal diseases and had taken antibiotics, probiotics or laxatives in the previous 4 weeks were excluded from the present study.
DNA extraction
A bacterial DNA kit (DP328; Tiangen Biotech Co., Ltd.) was used to extract the microbial DNA from saliva according to the manufacturer's protocols. Agarose gel (1%) electrophoresis was used to check the integrity of the extracted DNA, and the DNA was stored at −20°C for future use.
Library construction for next-generation sequencing based on 16S ribosomal RNA (16S rRNA)
Illumina Bridge PCR-compatible primers, barcode primers and a pair of primers were used to amplify the V4 region of the 16S rRNA gene by DNA Taq polymerase (cat. no. P102-01, Vazyme Biotechnology Co. Ltd.). The PCR conditions were as follows: 95°C for 2 min; 30 cycles at 95°C for 15 sec, 56°C for 30 sec and 72°C for 30 sec; and 72°C for 10 min. The amplicons were purified using DNA binding beads (cat. no. DP705; Tiangen Biotech Co., Ltd.). The sequences of the degenerate primers for amplification of the V4 region were designed by Primer 3 (version 2.4.0; http://primer3.ut.ee/) and they were: V4 forward, 5′-GTGCCAGCMGCCGCGGTAA-3′ and reverse, 5′-GGACTACHVGGGTWTCTAAT-3′. An Agilent 2100 Bioanalyzer (Agilent Technologies, Inc.) was used to assess the size of the amplicons, and Qubit 3.0 (Thermo Fisher Scientific, Inc.) was used to measure the concentration. VAHTS Universal DNA Library Prep Kit for Illumina V3 (cat. no. ND607, Vazyme Biotechnology Co. Ltd.) was used to prepare sequencing library. VAHTS Library Quantification kit for Illumina (cat. no. NQ101, Vazyme Biotechnology Co. Ltd.) was used to quantify the concentration and a loading concentration of 4 pM for sequencing. The Illumina Hiseq X (Illumina, Inc., San Diego, CA, USA) was used to sequence the libraries using read length of 250 bp from each end (paired-end 250).
Quality control for raw data and data assembly
The Trimmomatic (V0.33) software (14) was applied to pair-end raw data for quality control, and all the parameters were set as default to obtain clean reads. The mothur (V1.35.1) software (15) was used to categorize the clean reads for each sample according to their barcodes. The FLASH (V1.2.11) software (16) was used to assemble the paired-end reads to obtain raw tags (original splicing sequence). Clean tags (effective splicing fragments) were obtained after quality control and filtering.
Bioinformatics analysis
All clean tags were clustered by USEARCH (v9.0.2132) (17) and aligned to the operational taxonomic units (OTUs) by UPARSE (V10.0.240) (18) to identify the taxonomy. The parameters for identity were set as the default at 97%. Each sequence was sorted out into OTUs by Quantitative Insights Into Microbial Ecology (QIIME) (V1.9.1) (19) after singleton OTUs were removed by USEARCH and chimeric sequences were removed by UCHIME (V4.1) (20). QIIME was used to select the best representative sequence from optimized QIIME-selected sequences for the final OTU cluster to align with the Human Oral Microbiome Database (21). The species annotation information of OTUs was obtained and the OTUs that were annotated as chloroplast or mitochondrion and those which could not be annotated to any species were deleted. The relative abundance of each OTU was each OTU read normalized to total OTUs reads of each sample (in-house scripts). QIIME was also used to perform α-diversity analysis of all OTU cluster data. The nonparametric Mann-Whitney test (two-tailed; 95% CI) in GraphPad Prism (version 6.0; GraphPad Software, Inc.) was performed to analyze the abundance difference between patients with MN and healthy controls at the genus level. P<0.05 was considered to indicate a statistically significant difference. Analysis of similarities (ANOSIM) was performed between the patients with MN and healthy controls using the Vegan package (V2.4-3) of R language (22). Chao1 and Shannon indices between the patients with MN and healthy controls were analyzed using QIIME. The Kyoto Encyclopedia of Genes and Genomes (KEGG) pathway and Lefse analyses were predicted based on the abundance of OTUs using the Phylogenetic Investigation of Communities by Reconstruction of Unobserved States (PICRUST; V1.1.4) software (23) and Lefse (V1.1.2) (24).
Statistical analysis
The differences of clinical indicators between patients with MN and healthy controls were analysed by one way ANOVA using Tukey's post hoc. Metastats analysis allows a comparison of metagenomic samples (represented as counts of individual features such as organisms, genes and functional groups) from two treatment populations (for example, healthy vs. disease) and identifies those features that statistically distinguish the two populations. Spearman was used to analyse the correlation between OTUs and clinical indicators using Stats package of R language (22).
Results
Characterization of the sequencing results
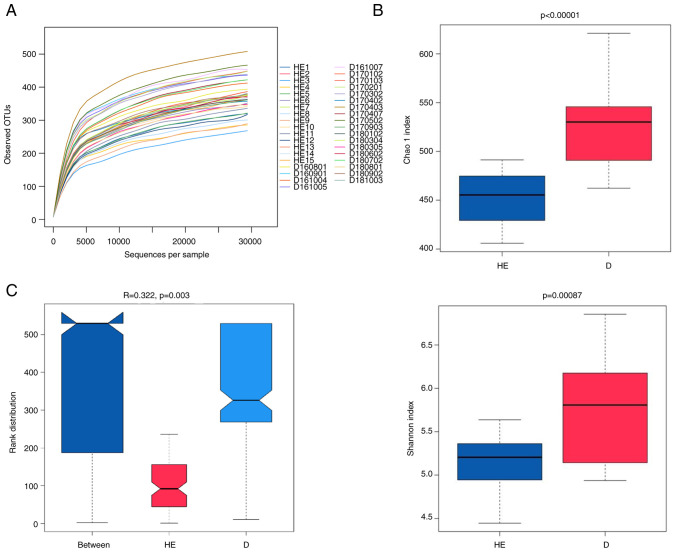
The salivary microbiota of patients with MN and healthy controls were analyzed by sequencing of the 16S rRNA gene using Illumina platform. After quality control, the total number of salivary microbial sequences was 1,534,154 reads at an average of 69,734 reads per sample for the 22 patients with MN, and 1,414,400 at an average of 88,400 reads per sample for the 15 healthy controls. The rarefaction curve plateaued, indicating that the sequencing depth could be used to analyze the composition of the salivary microbiota (Fig. 1A).
Figure 1.
(A) Rarefaction curves of the samples collected from patients with membranous nephropathy and healthy controls. (B) Chao and Shannon index value distribution of samples. (C) ANOSIM results. The y-axis represents the Bray-Curtis rank. OTU, operational taxonomic unit; ANOSIM, analysis of similarities; PC, principal component; HE, healthy control samples; D, membranous nephropathy samples.
Taxonomic analysis of the microbiota
Through the OTU clustering of sequencing reads, a total of 1,362 OTUs were found in the 22 samples of the MN group. The number of OTUs in each sample ranged between 391 and 559, with an average value of 462. The healthy group had fewer OTUs than the group of patients with MN. The number of OTUs in each sample was 344–453, with an average of 401. Bacterial species richness (Chao1 index) and diversity (Shannon index) of the patients with MN were significantly higher than those of the healthy controls (Fig. 1B). The Chao1 index (a value of 521) of patients with MN was higher than that of the healthy controls (which presented a Chao1 index of 452). The P-values for the Chao1 index and Shannon index were <0.00001 and 0.00087, respectively. ANOSIM is a non-parametric statistical test widely used to assess the similarities between groups. According to ANOSIM analysis, the microbial composition in the MN group was statistically different from that in the healthy group (P=0.003; Fig. 1C).
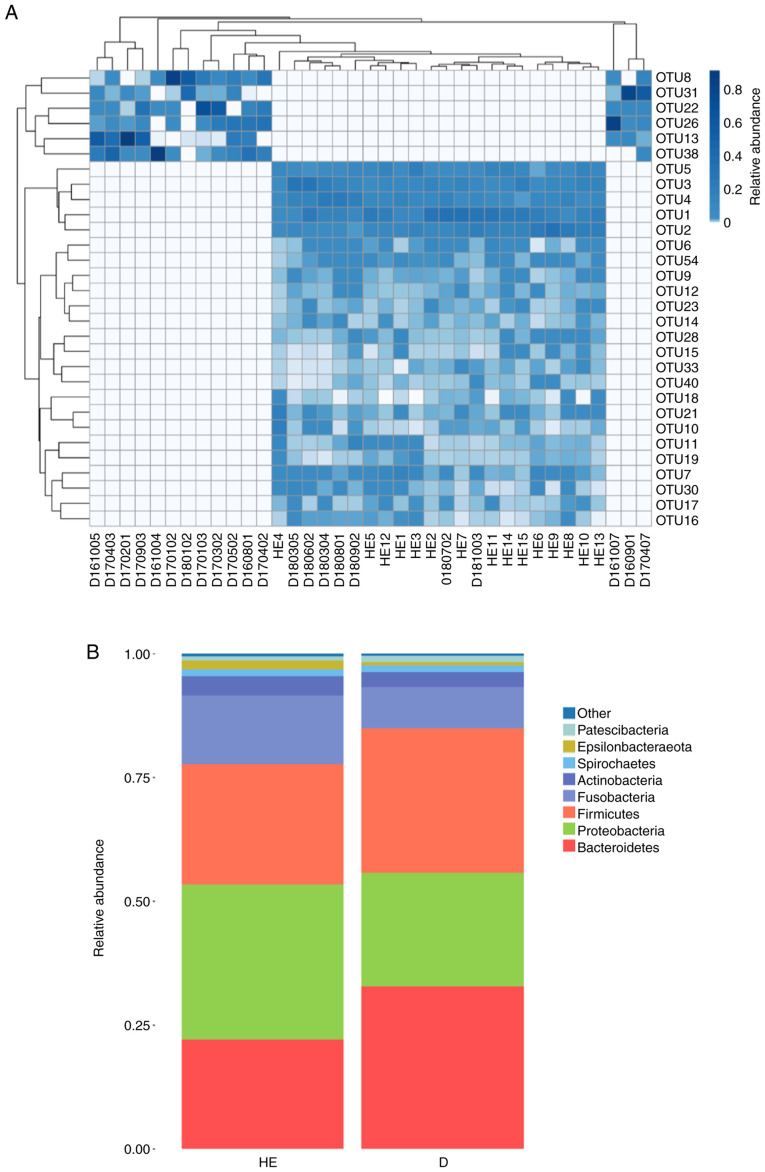
Heatmap analysis
The OTU heatmap showed that the MN group and healthy control group were mostly clustered separately (Fig. 2A; Table SI). In the microbial classification, eight phyla (Bacteroidetes, Firmicutes, Proteobacteria, Fusobacteria, Actinobacteria, Spirochaetes, Patescibacteria and Epsilonbacteraeota) accounted for >99% of the microbiota in the MN group and >99% in the healthy control group (Fig. 2B). Metastat analysis was conducted at the genus level and revealed that there were 47 different genera between the MN group and the healthy control group (P<0.05; Table SII).
Figure 2.
(A) Heatmap analysis based on OTU abundance. Different colors indicate relative abundance. The relative abundance increases from white to blue. In the heatmap, the x-axis indicates the patient ID number and the y-axis indicates the OTU number. (B) Comparison of the relative abundance at the phyla level between patients with membranous nephropathy and healthy controls. OTU, operational taxonomic unit; HE, healthy control samples; D, membranous nephropathy samples.
Correlation analysis of biochemical indicators and OTUs
OTUs of each sample were used to analyze the correlation with the biochemical indicators CR, Pro-24, eGFR, SBP and DBP, which are used clinically (Table SIII). Pro-24, SBP and eGFR were found to be significantly different between the patients with MN and healthy controls (P<0.05; Table SIV). The abundance of OTUs was considered to be related to clinical indicators. Serum CR was negatively correlated with 12 OTUs, of which OTU12, OTU17, OTU23, OTU6, OTU10, OTU33, OTU21 and OTU11 had a P-value <0.05, and OTU5, OTU9, OTU15 and OTU28 had a P-value <0.001, and it was positively correlated with OTU13 (P<0.05) and OTU38 (P<0.001). Pro-24 was negatively correlated with 24 OTUs (OTU4 with P<0.05; OTU1, OTU2, OTU5, OTU6, OTU7, OTU9, OTU11, OTU12, OTU15, OTU18, OTU19, OTU21, OTU28, OTU33, OTU40 and OTU54 with P<0.01; OTU3, OTU10, OTU14, OTU16, OTU17, OTU23 and OTU30 with P<0.001) and positively correlated with 6 OTUs (OTU8, OTU13, OTU22, OTU26 and OTU38 with P<0.05; and OTU31 with P<0.001). eGFR was negatively correlated with 4 OTUs (OTU8, OTU22 and OTU26 with P<0.05; OTU38 with P<0.001) and positively correlated with 11 OTUs (OTU5, OTU7, OTU9, OTU15, OTU16, OTU21, OTU30 and OTU33 with P<0.05; OTU11, OTU19 and OTU28 with P<0.001). The SBP was negatively correlated with 19 OTUs and positively correlated with 6 OTUs. No OTUs were associated with DBP, age or sex (Fig. 3). Among all OTUs, OTU5, OTU9, OTU15, OTU28 and OTU33 were significantly correlated with SBP, Pro-24, eGFR and CR.
Figure 3.
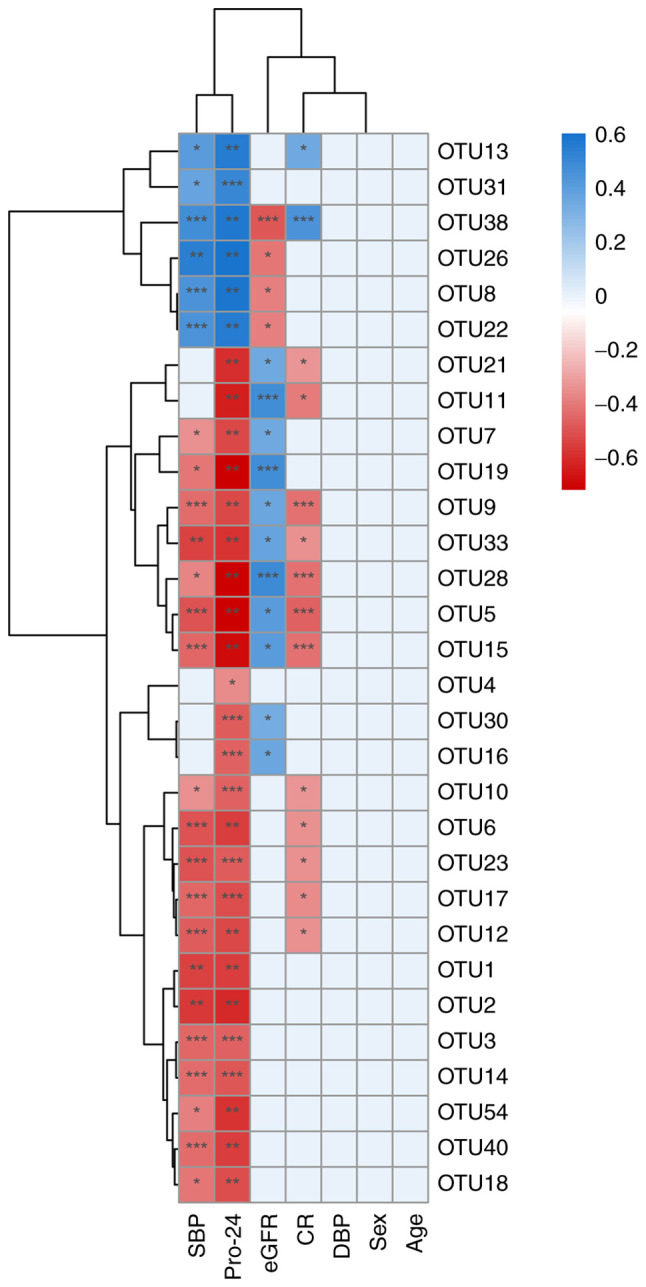
Correlation between OTUs and the clinical indicators. *P<0.05, **P<0.01 and ***P<0.001. Blue indicates a positive correlation and red indicates a negative correlation. OTU, operational taxonomic unit; eGFR, estimated glomerular filtration rate; SBP, systolic blood pressure; Pro-24, proteinuria at 24 h; CR, creatinine; DBP, diastolic blood pressure.
Lefse and PICRUST analyses
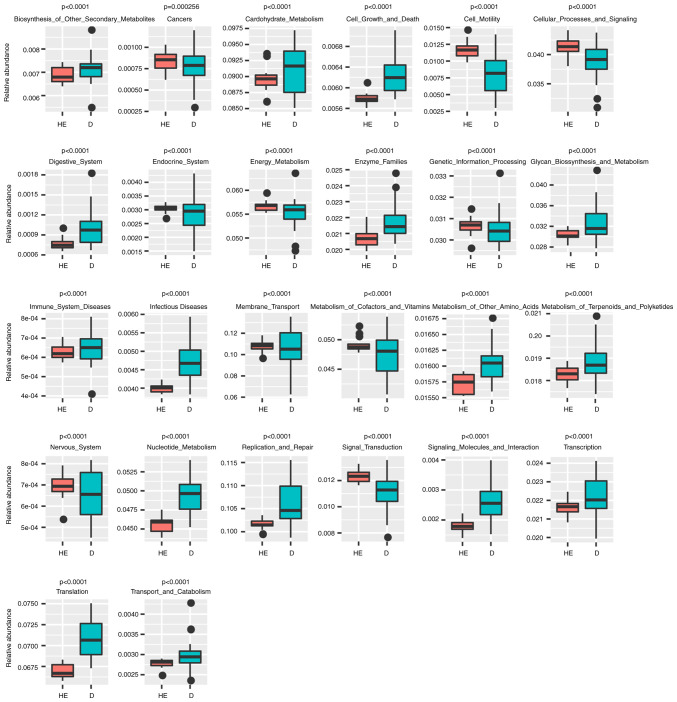
Lefse analysis was conducted to explore possible biomarkers and PICRUST analysis was conducted to explore pathways. Lefse analysis showed that 10 bacteria could be possibly used as biomarkers for patients with MN (Fig. 4), including Bacteroidales (order, P=6.59×10−5), Prevotellaceae (family, P=2.62×10−4), Lactobacillales (order, P=0.0412), Alloprevotella (genus, P=7.81×10−3), Prevotella (family, P=0.0145), Carnobacteriaceae (family, P=0.0172) and Granulicatella (genus, P=0.0328). A total of 26 KEGG pathways were obtained from significant OTUs using PICRUST (Fig. 5; Table SV), including ‘Immune_System_Diseases’ (P=1.81×10−6), ‘Glycan_Biosynthesis_and_Metabolism’ (P=1.91×10−6), ‘Energy_Metabolism’ (P=7.76×10−7), ‘Endocrine_System’ (P=4.05×10−7) and ‘Digestive_System’ (P=1.31×10−4).
Figure 4.

Lefse analysis. Green indicates biomarkers for patients with membranous nephropathy; red indicates biomarkers for healthy controls. HE, healthy control samples; D, membranous nephropathy samples; LDA, linear discriminant analysis.
Figure 5.
Kyoto Encyclopedia of Genes and Genomes pathways predicted by Phylogenetic Investigation of Communities by Reconstruction of Unobserved States software. The number on the Y axis is the relative abundance. HE, healthy control samples; D, membranous nephropathy samples.
Discussion
MN is a common glomerular disease; however, there are few studies on the oral microbiota of patients with MN. Through high-throughput sequencing of the 16S rRNA gene of oral microbiota and the subsequent bioinformatics analysis, the composition and characterization of oral microbiota in patients with MN were thoroughly investigated and the associations between clinically used biochemical indicators and OTUs were evaluated. ANOSIM analysis revealed that there was a significant difference in the composition of microbial species between the patients with MN and healthy controls (P=0.003). α-diversity analysis showed that The Chao1 index of patients with MN was higher than that of the healthy controls, indicating that the total number of oral microbes in the patients with MN was higher than that in the healthy controls. Although α-diversity is normally lower in patients than in controls, some reports have indicated that α-diversity is higher in patients than in the control group and might be associated with clinical benefits (25–27). The exact meaning of the observation that α-diversity in patients with MN was higher than in the controls needs further exploration. Additionally, the Shannon index of the patients with MN was higher than that of the healthy controls (5.72 vs. 5.13), indicating that the microbial diversity of the patients with MN was also higher than that of the healthy controls.
According to Lefse analysis, there were 10 significantly different bacteria in patients with MN. At the phylum and class level, Bacteroidetes, Bacteroidia and Bacilli may be candidate biomarkers. Lactobacillales and Bacteroidales at the order level, Prevotellaceae, Carnobacteriaceae and Prevotella at the family level, and Alloprevotella and Granulicatella at the genus level may serve as potential biomarkers for patients with MN. Streptococcus and Granulicatella produce antimicrobial compounds that inhibit bacterial growth, and thus, are beneficial to the oral cavity (28). The abundance of Streptococcus and Granulicatella was significantly higher in patients with MN than in the control group, which was also observed in the case of immunoglobulin A nephropathy (12). However, the present findings were different from those reported by Piccolo et al (29), and this may be due to the difference in ethnicities. Meanwhile, the phylum Bacteroidetes normally implies poor health in the host (9).
The potential KEGG pathway function of microorganisms was predicted using PICRUST (30). The abundance of the pathway ‘Immune_System_Diseases’ in the MN group was significantly higher than that in the healthy individuals (Fig. 5), indicating that the microbial environment of patients with MN is likely to change according to the immune system condition.
Regarding clinical indicators, CR is a product of muscle metabolism in the human body and is mainly excreted from the body by glomerular filtration. When acute or chronic glomerulonephritis causes the glomerular filtration function to decrease, serum CR levels can increase. eGFR is an indicator of kidney function. It mainly refers to the excretion capacity of the kidneys per unit of time and is used to assess kidney function. Pro-24 is the 24 h urine protein content. Increased urine protein is often present in various glomerular diseases, such as acute nephritis, chronic nephritis, nephrotic syndrome and lupus nephritis (31). Patients with nephritis also often present high blood pressure (32,33). The correlations between the OTU abundance of the 22 patients with MN and these clinical indicators were analyzed to explore the relationship between the microbes and the clinical indicators and the possibility of using them to diagnose and assess the status of the patients with MN. Six OTUs (OTU5, OTU28, OTU9, OTU15, OTU33 and OTU38) were found to be significantly correlated with CR, Pro-24, eGFR and SBP, implying that they could be used as biomarkers for the diagnosis of patients with MN.
The present study has certain limitations. First, the sample size was relatively small. This was a preliminary proof-of-concept study that indicated that the salivary microbiota was associated with MN. The sample size will be increased in subsequent research. Second, it was a retrospective study. For the biomarkers to be used in clinical settings, a prospective study is necessary. Third, the present study enrolled patients from southern China. Their diet is different from patients from northern China (34). Whether and how the different diets affect our conclusions requires further investigation.
In conclusion, to the best of our knowledge, there are no previous reports on the relationship between oral microbiota and MN. The present study was the first to investigate the oral microbiota in patients with MN. The present results revealed that there were significant differences between the microbiota of the patients with MN and that of healthy controls and that certain microbial strains and OTUs (OTU5, OTU28, OTU9, OTU15, OTU33 and OTU38) can be used as biomarkers to facilitate the diagnosis of patients with MN in clinical settings.
Supplementary Material
Acknowledgements
Not applicable.
Funding Statement
The work was supported by grants from the National Natural Science Foundation of China (grant no. 82001336), Guangdong Basic and Applied Basic Research Foundation (grant nos. 2019A1515011009, 2021A1515010683, 2020A1515010225 and 2021A1515010955), Shenzhen Foundation of Science and Technology (grant nos. JCYJ20180306172449376, JCYJ20180306172459580 and JCYJ20180306172502097), Nanjing Municipal Health Science and Technology Development Special Fund Project (grant no. YKK19161) and Shenzhen Longhua District Foundation of Science and Technology (grant no. SZLHQJCYJ202002).
Availability of data and materials
The datasets generated and/or analyzed during the current study are available in the National Center for Biotechnology Information repository (no. PRJNA698736; http://www.ncbi.nlm.nih.gov/bioproject/PRJNA698736).
Authors' contributions
BH and HG conceived and supervised the study. SL, SZ, LP and WH wrote the draft of the manuscript and designed the experiments. HC and XWe analyzed the data. RL, CL, PZ, XWa and WL collected the samples and clinical information and analyzed the data. SL, ZX and YZ designed the experiments, revised the manuscript and provided helpful advice. SL and HG confirm the authenticity of all the raw data. All authors read and approved the final manuscript.
Ethics approval and consent to participate
The study protocol was approved by The Ethics Committee of Shenzhen Longhua District Central Hospital (Shenzhen, China). Informed written consent was obtained from each patient and healthy subject enrolled in the present study.
Patient consent for publication
Consent for publication was obtained from all patients.
Competing interests
The authors declare that they have no competing interests.
References
- 1.Francis JM, Beck LH, Jr, Salant DJ. Membranous nephropathy: A journey from bench to bedside. Am J Kidney Dis. 2016;68:138–147. doi: 10.1053/j.ajkd.2016.01.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cai Q, Hendricks AR. Membranous nephropathy: A ten-year journey of discoveries. Semin Diagn Pathol. 2020;37:116–120. doi: 10.1053/j.semdp.2020.01.001. [DOI] [PubMed] [Google Scholar]
- 3.Tang L, Yao J, Kong X, Sun Q, Wang Z, Zhang Y, Wang P, Liu Y, Li W, Cui M, et al. Increasing prevalence of membranous nephropathy in patients with primary glomerular diseases: A cross-sectional study in China. Nephrology (Carlton) 2017;22:168–173. doi: 10.1111/nep.12739. [DOI] [PubMed] [Google Scholar]
- 4.Cattran DC. Idiopathic membranous glomerulonephritis. Kidney Int. 2001;59:1983–1994. doi: 10.1046/j.1523-1755.2001.0590051983.x. [DOI] [PubMed] [Google Scholar]
- 5.Cattran D. Management of membranous nephropathy: When and what for treatment. J Am Soc Nephrol. 2005;16:1188–1194. doi: 10.1681/ASN.2005010028. [DOI] [PubMed] [Google Scholar]
- 6.Sekirov I, Russell SL, Antunes LC, Finlay BB. Gut microbiota in health and disease. Physiol Rev. 2010;90:859–904. doi: 10.1152/physrev.00045.2009. [DOI] [PubMed] [Google Scholar]
- 7.Sharma S, Tripathi P. Gut microbiome and type 2 diabetes: Where we are and where to go? J Nutr Biochem. 2019;63:101–108. doi: 10.1016/j.jnutbio.2018.10.003. [DOI] [PubMed] [Google Scholar]
- 8.Lin C, Cai X, Zhang J, Wang W, Sheng Q, Hua H, Zhou X. Role of gut microbiota in the development and treatment of colorectal cancer. Digestion. 2019;100:72–78. doi: 10.1159/000494052. [DOI] [PubMed] [Google Scholar]
- 9.Yu W, Shang J, Guo R, Zhang F, Zhang W, Zhang Y, Wu F, Ren H, Liu C, Xiao J, Zhao Z. The gut microbiome in differential diagnosis of diabetic kidney disease and membranous nephropathy. Ren Fail. 2020;42:1100–1110. doi: 10.1080/0886022X.2020.1837869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Pflughoeft KJ, Versalovic J. Human microbiome in health and disease. Annu Rev Pathol. 2012;7:99–122. doi: 10.1146/annurev-pathol-011811-132421. [DOI] [PubMed] [Google Scholar]
- 11.Duan X, Chen X, Gupta M, Seriwatanachai D, Xue H, Xiong Q, Xu T, Li D, Mo A, Tang X, et al. Salivary microbiome in patients undergoing hemodialysis and its associations with the duration of the dialysis. BMC Nephrol. 2020;21:414. doi: 10.1186/s12882-020-02009-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Luan S, Zhang S, Zhong H, Zhang Y, Wei X, Lin R, Li C, Zeng P, Wang X, Li W, Gao H. Salivary microbial analysis of Chinese patients with immunoglobulin A nephropathy. Mol Med Rep. 2019;20:2219–2226. doi: 10.3892/mmr.2019.10480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ehrenreich T, Churg J. Pathology of membranous nephropathy. Pathol Ann. 1968:145–186. [Google Scholar]
- 14.Bolger AM, Lohse M, Usadel B. Trimmomatic: A flexible trimmer for Illumina sequence data. Bioinformatics. 2014;30:2114–2120. doi: 10.1093/bioinformatics/btu170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Schloss PD, Westcott SL, Ryabin T, Hall JR, Hartmann M, Hollister EB, Lesniewski RA, Oakley BB, Parks DH, Robinson CJ, et al. Introducing mothur: Open-source, platform-independent, community-supported software for describing and comparing microbial communities. Appl Environ Microbiol. 2009;75:7537–7541. doi: 10.1128/AEM.01541-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Magoc T, Salzberg SL. FLASH: Fast length adjustment of short reads to improve genome assemblies. Bioinformatics. 2011;27:2957–2963. doi: 10.1093/bioinformatics/btr507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Edgar R. Taxonomy annotation and guide tree errors in 16S rRNA databases. PeerJ. 2018;6:e5030. doi: 10.7717/peerj.5030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Edgar RC. UPARSE: Highly accurate OTU sequences from microbial amplicon reads. Nat Methods. 2013;10:996–998. doi: 10.1038/nmeth.2604. [DOI] [PubMed] [Google Scholar]
- 19.Caporaso JG, Kuczynski J, Stombaugh J, Bittinger K, Bushman FD, Costello EK, Fierer N, Peña AG, Goodrich JK, Gordon JI, et al. QIIME allows analysis of high-throughput community sequencing data. Nat Methods. 2010;7:335–336. doi: 10.1038/nmeth.f.303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Edgar RC, Haas BJ, Clemente JC, Quince C, Knight R. UCHIME improves sensitivity and speed of chimera detection. Bioinformatics. 2011;27:2194–2200. doi: 10.1093/bioinformatics/btr381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Escapa IF, Chen T, Huang Y, Gajare P, Dewhirst FE, Lemon KP. New insights into human nostril microbiome from the expanded human oral microbiome database (eHOMD): A resource for the microbiome of the human aerodigestive tract. mSystems. 2018;3:e00187–18. doi: 10.1128/mSystems.00187-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.R Core Team: R, corp-author. R Foundation for Statistical Computing; Vienna: 2021. A language and environment for statistical computing (V 4.1.1) [Google Scholar]
- 23.Langille MG, Zaneveld J, Caporaso JG, McDonald D, Knights D, Reyes JA, Clemente JC, Burkepile DE, Vega Thurber RL, Knight R, et al. Predictive functional profiling of microbial communities using 16S rRNA marker gene sequences. Nat Biotechnol. 2013;31:814–821. doi: 10.1038/nbt.2676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Segata N, Izard J, Waldron L, Gevers D, Miropolsky L, Garrett WS, Huttenhower C. Metagenomic biomarker discovery and explanation. Genome Biol. 2011;12:R60. doi: 10.1186/gb-2011-12-6-r60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wang J, Gu X, Yang J, Wei Y, Zhao Y. Gut microbiota dysbiosis and increased plasma LPS and TMAO levels in patients with preeclampsia. Front Cell Infect Microbiol. 2019;9:409. doi: 10.3389/fcimb.2019.00409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Fu M, Zhang X, Liang Y, Lin S, Qian W, Fan S. Alterations in vaginal microbiota and associated metabolome in women with recurrent implantation failure. mBio. 2020;11:e03242–19. doi: 10.1128/mBio.03242-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Riquelme E, Zhang Y, Zhang L, Montiel M, Zoltan M, Dong W, Quesada P, Sahin I, Chandra V, San Lucas A, et al. Tumor microbiome diversity and composition influence pancreatic cancer outcomes. Cell. 2019;178:795–806.e12. doi: 10.1016/j.cell.2019.07.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kumar PS, Griffen AL, Moeschberger ML, Leys EJ. Identification of candidate periodontal pathogens and beneficial species by quantitative 16S clonal analysis. J Clin Microbiol. 2005;43:3944–3955. doi: 10.1128/JCM.43.8.3944-3955.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Piccolo M, De Angelis M, Lauriero G, Montemurno E, Di Cagno R, Gesualdo L, Gobbetti M. Salivary microbiota associated with immunoglobulin a nephropathy. Microb Ecol. 2015;70:557–565. doi: 10.1007/s00248-015-0592-9. [DOI] [PubMed] [Google Scholar]
- 30.Kanehisa M, Furumichi M, Sato Y, Ishiguro-Watanabe M, Tanabe M. KEGG: Integrating viruses and cellular organisms. Nucleic Acids Res. 2021;49:D545–D551. doi: 10.1093/nar/gkaa970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Levey AS, Becker C, Inker LA. Glomerular filtration rate and albuminuria for detection and staging of acute and chronic kidney disease in adults: A systematic review. JAMA. 2015;313:837–846. doi: 10.1001/jama.2015.0602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.de Azevedo FVA, Maia DG, de Carvalho JF, Rodrigues CEM. Renal involvement in antiphospholipid syndrome. Rheumatol Int. 2018;38:1777–1789. doi: 10.1007/s00296-018-4040-2. [DOI] [PubMed] [Google Scholar]
- 33.Turrent-Carriles A, Herrera-Felix JP, Amigo MC. Renal involvement in antiphospholipid syndrome. Front Immunol. 2018;9:1008. doi: 10.3389/fimmu.2018.01008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wang L, Xing Y, Yu X, Ming J, Liu X, Li X, Fu J, Zhou J, Gao B, Hu D, et al. Greater macrovascular and microvascular morbidity from type 2 diabetes in northern compared with southern China: A cross-sectional study. J Diabetes Investig. 2020;11:1285–1294. doi: 10.1111/jdi.13262. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets generated and/or analyzed during the current study are available in the National Center for Biotechnology Information repository (no. PRJNA698736; http://www.ncbi.nlm.nih.gov/bioproject/PRJNA698736).