Abstract
Background
Myasthenia gravis is an autoimmune disease mediated by auto‐antibodies most often directed against the nicotinic acetylcholine receptor. Less than five per cent of patients have auto‐antibodies to a muscle tyrosine kinase. Patients would be expected to benefit from plasma exchange.
Objectives
To examine the efficacy of plasma exchange in the short‐ and long‐term treatment of myasthenia gravis.
Search methods
We searched the Cochrane Neuromuscular Disease Group Specialized Register (31 January 2011), the Cochrane Central Register of Controlled Trials (CENTRAL) (31 January 2011, Issue 1 2011 in the Cochrane Library), MEDLINE (January 1966 to January 2011) and EMBASE (January 1980 to January 2011) using the term 'myasthenia gravis'. We checked the bibliographies of trial reports and contacted one author for additional data.
Selection criteria
All randomised controlled trials (RCTs) or quasi‐RCTs including all patients with generalised myasthenia gravis. We considered treatment trials of plasma exchange alone or combined with steroids or immunosuppressive drugs. The primary outcome measures were:(1) for exacerbation: change in a specific muscle score;(2) for chronic myasthenia gravis: change in a functional scale.
Data collection and analysis
One author extracted and a second checked the data.
Main results
We identified four RCTs with 148 participants in total. In the first one, of 14 participants with moderate or severe myasthenia gravis, improvement after one month was not significantly greater for participants treated with plasma exchange and prednisone than for those treated with prednisone alone. A randomised controlled cross‐over trial of 12 participants with moderate to severe myasthenia gravis found no statistically significant difference in the efficacy of plasma exchange or intravenous immunoglobulins after four weeks. A trial including 87 participants with myasthenia gravis exacerbation found no statistically significant difference between plasma exchange and immunoglobulin after two weeks. The fourth RCT, with 35 participants, showed a statistically significant difference in favour of plasma exchange before thymectomy. However these trials, except the third, are at high risk of bias and have a weak statistical power.
Authors' conclusions
No adequate RCTs have been performed to determine whether plasma exchange improves the short‐ or long‐term outcome for chronic myasthenia gravis or myasthenia gravis exacerbation. However, many studies with case series report short‐term benefit from plasma exchange in myasthenia gravis, especially in myasthenic crisis. In severe exacerbations of myasthenia gravis one RCT did not show a significant difference between plasma exchange and intravenous immunoglobulin. Further research is need to compare plasma exchange with alternative short‐term treatments for myasthenic crisis or before thymectomy and to determine the value of long‐term plasma exchange for treating myasthenia gravis.
Plain language summary
Plasma exchange for generalised myasthenia gravis
Myasthenia gravis is caused by antibodies in the blood which attack the junctions between nerves and muscles they stimulate. Plasma exchange removes these circulating auto‐antibodies. Many case series suggest that plasma exchange helps to treat myasthenia gravis. Four randomised controlled trials were identified. In the first one, of 14 participants with moderate or severe myasthenia gravis, the myasthenic muscular score after one month was not significantly different for participants treated with plasma exchange and prednisone than for those treated with prednisone alone but there can be only low statistical confidence in the results of this study because of its small size. A randomised controlled cross‐over trial of only 12 participants reported the same efficacy, after four weeks, of plasma exchange or intravenous immunoglobulins for the treatment of moderate to severe myasthenia gravis, but because of bias and a very weak statistical power the data prevent any conclusion. The third, including 87 participants, showed the same efficacy, after two weeks, of plasma exchange or intravenous immunoglobulins for the treatment of myasthenia gravis exacerbation. The fourth randomised controlled trial involving 35 participants reported a benefit from plasma exchange before thymectomy but this trial was heavily biased. No trial addressed the new subtype with antibodies to a muscle specific kinase. Further research is needed to determine the value of long‐term plasma exchange for treating myasthenia gravis and to compare plasma exchange with alternative short‐term treatments for myasthenic crisis or before thymectomy in both types of autoimmune myasthenia.
Summary of findings
Background
Myasthenia gravis is an autoimmune disease mediated by auto‐antibodies most often directed against the nicotinic acetylcholine receptor. Less than five per cent of patients have auto‐antibodies to a muscle tyrosine kinase. Experimental autoimmune myasthenia gravis can be induced by injecting rabbits with acetylcholine receptors (AChR) from the electric organs of eels (Patrick 1973), which causes AChR antibodies to be demonstrated and the rabbits to become paralysed. In other experiments, clinical and morphological features of myasthenia gravis have been reproduced in animals by passive transfer of human myasthenic serum immunoglobulin G (Toyka 1975), or AchR‐specific monoclonal antibodies (Richman 1980). Myasthenia gravis is characterised by weakness and fatigability of voluntary muscle, which changes over time. Acute exacerbations are life‐threatening because they can cause swallowing difficulties or respiratory failure. Historically, with treatment ‐ including thymectomy, steroids, and immunosuppressive drugs ‐ after one to 21 (mean 12) years, 6% of patients went into remission, 36% improved, 42% were unchanged, and 2% were worse (Grob 1981). In recent years, expert opinion has highlighted the greater efficacy of combined immunosuppressive treatments (Cornelio 1993; Hohlfeld 1993; Hohlfeld 1996; Oosterhuis 1997). A new subtype of myasthenia is associated with autoantibodies to a muscle specific kinase and these antibodies are also pathogenic on passive transfer (Cole 2008).
Therapeutic plasma exchange has been used for many years to remove toxic factors or antibodies. The technique consists of taking blood from one vein, separating plasma from blood cells using membrane filtration or centrifugation and then returning blood with a plasma substitute into another vein. Cells are re‐infused while plasma is removed, with diluted albumin, colloids, or crystalloids used to maintain volume and oncotic equilibrium. Plasma filtration using haemodialysis pumps, and plasma separation using cell centrifuges are both established procedures. An alternative method of removing antibodies is immunoabsorption. In this the plasma is passed down an absorbant column, which removes antibodies, and then returned to the patient. Formal prospective studies to compare the two methods are not available. Plasma exchange has significant constraints and morbidity. It is usual to exchange one plasma volume and takes about three hours. Specific devices and teams trained in the use of extracorporeal circulation are needed.
Plasma exchange was introduced in 1976 as a short‐term therapy for acute exacerbations of myasthenia gravis (Dau 1977; Pinching 1976). It is thought to work because the exchange removes circulating anti‐AChR antibodies. However, improvement has also been reported in so‐called seronegative myasthenia gravis (where no anti‐AChR antibodies can be detected) following plasma exchange (Miller 1981). A symposium held in 1978 (Dau 1979), and numerous papers (Dau 1980; Olarte 1981; Perlo 1981), have recognised the short‐term benefit of plasma exchanges (NIH Consensus 1986). The use of repeated plasma exchange over a long period in refractory myasthenia gravis has also been reported (Kornfeld 1981; Rodnitzky 1984). Plasma exchange is used worldwide for the treatment of myasthenia gravis but despite the published case series and the conferences of experts many questions remains unanswered concerning its efficacy for the treatment of chronic, more or less severe, myasthenia gravis as well as of myasthenic exacerbation or crisis and its efficacy in comparison with other treatments. Few randomised controlled trials have been published (Gajdos 1983; Gajdos 1997; Kamel 2009; Ronager 2001).
This is an update of a review first published in 2002. This review takes account of variables which could affect the result of plasma exchanges, including its use for exacerbations or for the chronic form of myasthenia gravis, and concomitant use of steroids and/or immunosuppressive drugs. The number of randomised studies is limited and we have supplemented the text with a brief review of the non‐randomised studies in the discussion.
Objectives
To examine the efficacy and tolerability of plasma exchange in the short‐ and long‐term treatment of acquired autoimmune myasthenia gravis.
Methods
Criteria for considering studies for this review
Types of studies
We searched for all randomised controlled trials (RCTs) or quasi‐RCTs involving plasma exchange for generalised myasthenia gravis. Because of the lack of controlled trials we also assessed uncontrolled trials (controlled but non‐randomised studies and case series reporting more than 15 patients) and have included a discussion of these under 'Non‐randomised literature' in the Discussion.
Types of participants
We included children and adults with generalised myasthenia gravis.
The diagnosis of myasthenia gravis was based on the following three criteria:
acquired weakness of voluntary muscles including those innervated by cranial nerves;
fluctuation of weakness or fatigability;
concentration of anti‐AChR antibodies greater than 1 nM, decremental electromyographic response (at least a 10% decrease in the amplitude of the muscle action potential when stimulated at three to five Hz), or positive single fibre electromyography (SFEMG, mean jitter more than 20 µs), or an objective improvement with anticholinesterase drugs (e.g. edrophonium test).
We considered two categories of patients as follows.
Patients with major exacerbations of generalised myasthenia gravis, defined as the appearance or reappearance of at least one of the following symptoms: (a) difficulty in swallowing; (b) acute respiratory failure (needing mechanical ventilation); (c) major functional disability responsible for the discontinuation of physical activity.
Patients with chronic generalised myasthenia treated for reasons other than exacerbation (i.e. pre‐operative management, chronic use of plasma exchanges, myasthenia gravis refractory to steroids or immunosuppressive drugs).
We did not include patients with pure ocular myasthenia.
In none of the trials were participants separated by or stratified for having autoantibodies to MuSK instead of AChR.
Types of interventions
We included treatment with plasma exchange alone or plasma exchange associated with steroids and/or immunosuppressive drugs.
Types of outcome measures
Primary outcomes
In patients treated for exacerbation, the primary outcome measure was the change in a specific score between the day before and days seven to fifteen after first plasma exchange (or day of randomisation). The specific score reported in each individual study was used.
In patients treated for chronic myasthenia gravis, the primary outcome measure was an improvement by at least one grade in a functional scale between the day before and six months after the first plasma exchange (or day of randomisation).
Secondary outcomes
In patients treated for exacerbation: (a) an improvement by at least one grade in a functional scale including five to six grades (from complete remission to very severe disease requiring admission to hospital) between the day before and seven to fifteen days after the first plasma exchange (or day of randomisation); (b) percentage weaned from ventilation before day 15 after the first plasma exchange (or day of randomisation); (c) absolute mean reduction after plasma exchange of serum AChR antibodies concentration.
In patients treated for chronic myasthenia gravis: (a) percentage in remission one year after first plasma exchange (or day of randomisation). Remission was defined as the absence of symptoms, or symptoms that were infrequent, or sufficiently mild that they did not interfere with normal activities; (b) delay until the first relapse.
Percentage of adverse events related to the procedure. The following were considered to be adverse events: haemorrhage requiring blood transfusion or a surgical procedure, hypotension requiring vascular expansion, and fever (temperature greater than 38°C).
Percentage of patients in whom plasma exchanges had to be discontinued due to adverse events.
Search methods for identification of studies
We searched the Cochrane Neuromuscular Disease Group Specialized Register (31 January 2011) using 'myasthenia gravis' and 'plasma exchange' or plasmapheresis as the search terms. We adapted this search for The Cochrane Central Register of Controlled Trials (CENTRAL) (Issue 1, 2011 in The Cochrane Library), MEDLINE (January 1966 to January 2011) and EMBASE (January 1980 to January 2011). We checked the bibliographies in reports of the randomised trials and contacted one author to identify additional published or unpublished data.
The search strategies for MEDLINE, EMBASE and CENTRAL can be found in Appendix 1, Appendix 2 and Appendix 3.
Data collection and analysis
Selection of studies
Two authors checked titles and abstracts identified from the register. Both authors independently assessed the full text of all potentially relevant studies. The authors decided which trials fitted the inclusion criteria and graded their methodological quality. The authors resolved disagreements about inclusion criteria by discussion.
Data extraction and management
A single author (PG) performed data extraction and a second author (KVT) checked the data extraction. We obtained missing data from the trial authors whenever possible.
Assessment of risk of bias in included studies
We assessed risk of bias using the following criteria: sequence generation, allocation concealment, binding, incomplete outcome data, selective outcome reporting and other sources of bias, as described in Chapter 8 of the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2008). We judged the risk of bias for each entry where an answer 'Yes indicates a low risk of bias, 'No' a high risk of bias and 'Unclear' an unknown risk of bias. One author (PG) assessed the risk of bas and a second author (KVT) checked the risk of bias.
Data synthesis
Had more than one trial been found for which meta‐analysis was possible, we would have calculated a weighted treatment effect across trials using the Cochrane statistical software, Review Manager 5.0 (RevMan 2008). We analysed all the primary and secondary outcomes under consideration whenever the data allowed.
Subgroup analysis and investigation of heterogeneity
We intended to analyse subgroups of interest. These predefined subgroups were chosen because of their prognostic importance in previous prospective studies and trials. The subgroups were defined as follows:
patients who were being treated because of worsening symptoms during the initiation of steroid treatment;
patients who were being treated prior to thymectomy;
patients who were being treated with plasma exchange alone, or patients who were being treated with plasma exchange and steroids and/or immunosuppressive drugs;
patients who were being treated with immunoabsorption.
Results
Description of studies
The number of papers found by the new, current strategies are: MEDLINE ‐ 200 (15 new papers), EMBASE ‐ 487 (42 new papers), NMD REGISTER ‐ 28 (3 new papers), CENTRAL ‐ 37 papers. Four trials suitable for inclusion were identified. No trials were excluded on methodological grounds. Given the fundamental differences in these trials concerning participants (severe or more or less stable myasthenia gravis of variable duration, myasthenia gravis exacerbation, preparation of patients for thymectomy), design of trial, and outcome, no meta‐analysis was deemed possible.
One trial (Gajdos 1983) compared the long‐term effect of prednisone (Group I) versus prednisone and plasma exchange (Group II) for myasthenia gravis. Two RCTs compared plasma exchange versus IVIg, one for myasthenia exacerbation (Gajdos 1997) and another for moderate to severe myasthenia gravis (Ronager 2001). The fourth (Kamel 2009) compared the short‐term outcome of thymectomy with or without plasma exchange delivered before thymectomy. See Characteristics of included studies.
Plasma exchange for chronic myasthenia gravis
In the first trial (Gajdos 1983) group I, participants initially received prednisone 1 mg/kg/d for one month and then a decreasing dosage. In case of failure at the end of the first month, cyclophosphamide 2 mg/kg/d was added. Prednisone was increased when a relapse occurred. Group II participants received the same treatment with the addition of three plasma exchanges over a 10 day period. Plasma exchanges were continued, if required, at the rate of once a week or resumed when relapse occurred. Seven participants were included in each arm.
In this trial the outcome measures were: (1) evolution of a myasthenic muscle score (MMS) which is the sum of nine independent observations of trunk, limbs, neck and cranial muscles which when added yield an overall numerical rating between 0 for a maximum deficit and 100 for normal strength (Gajdos 1983), (2) number of relapses, (3) daily dosage of prednisone, (4) anti‐AChR antibody titre after one year. The minimum follow‐up was one year. MMS values were available for one, three, six and 12 months after randomisation. Functional scales were not available.
Ronager et al. (Ronager 2001) compared the efficacy of IVIg to plasma exchange using a cross‐over design, in people with moderate to severe myasthenia gravis in a stable phase of their disease. Participants were included if (1) they were in class 3 to 5 of a modified Osserman classification (i.e. class 3: oculofacial, mild limb‐girdle and pharyngeal weakness; class 4: generalized moderate weakness; class 5: generalized severe weakness) and if they were restricted in daily activities or completely dependent on skilled care for support; (2) if they were treated with prednisone or azathioprine; (3) they had anti‐AChR antibodies and a significant (15 %) decrement on electromyography (EMG). Participants were randomly assigned to receive either IVIg 0.4 g/kg on five subsequent days and 16 weeks later five plasma exchanges every other day, or vice versa. The main endpoint was the clinical improvement measured seven days after each treatment using the quantified myasthenia gravis score (QMGS) developed by Besinger and Toyka (Besinger 1983). Secondary endpoints were a decrease in anti‐AChR antibody titre, change in decrement and the clinical effect assessed four, eight and 16 weeks after each treatment. The QMGS was performed by only one observer who was blinded to the treatment given. The trial was powered to identify a difference in QMGS of 0.3 or 20% in response. Twelve participants were included.
Plasma exchange for myasthenic exacerbation
Gajdos et al. (Gajdos 1997), compared the efficacy and tolerability of IVIg and plasma exchange in myasthenia gravis exacerbations, and also compared two doses of IVIg. Participants were eligible if: (1) they fulfilled criteria for the diagnosis of myasthenia gravis (Vincent 2001); and (2) the participant had an exacerbation defined as the appearance of at least one of the following symptoms within the last month: difficulty in swallowing, acute respiratory failure or major functional disability responsible for the discontinuation of physical activity.
In the plasma exchange group, participants received three plasma exchanges of 1.5 plasma volumes performed once every two days. The IVIg group had two arms: in one arm participants received IVIg 0.4 g/kg for three days (total 1.2 g/kg) and in the other arm participants received 0.4 g/kg for five days (total 2 g/kg).
In all groups, immunosuppressive treatment with corticosteroids or other drugs was continued without change.
The main endpoint was the variation of a myasthenic muscle score (MMS) between randomisation and day 15. Other endpoints were: (1) the time to the occurrence of a treatment response within the first two weeks, defined as an increase in MMS of at least 20 points compared with the initial value; (2) the relative variation of anti‐AChR antibody titre between day 0 and day 15; (3) side effects. The trial was adequately powered to detect a 50% difference in the change in the mean MMS between groups. Eighty seven participants were included, 41 in the plasma exchange group and 46 in the IVIg group (23 in the low dose IVIg and 23 in the high dose IVIg regimens).
Plasma exchange before thymectomy
In the trial that aimed to evaluate the effectiveness of plasma exchange on the short‐term outcome of thymectomy (Kamel 2009), consecutive patients referred for thymectomy were included. Participants were randomly assigned to receive either three plasma exchanges in the week before operation or no plasma exchange. End points defined for this trial were the duration of post operative mechanical ventilation, of intensive care unit (ICU) and hospital stay, number of participants with myasthenic crisis (defined as post operative mechanical ventilation of more than 48 hours) and post operative blood loss.
Thirty‐five participants were included, 19 of whom were treated with pre‐operative plasma exchange (group 1) and 16 not treated (group 2).
Risk of bias in included studies
Plasma exchange for chronic myasthenia gravis
In the study of Gadjos et al (Gajdos 1983) the risk of bias was considered to be high (see Risk of bias in included studies and Figure 1). The method of randomisation was not given in the report. There was no placebo (sham exchange) in the control group. Number needed to treat was not calculated and the low number of participants included would have detected only dramatic effects of plasma exchange. Neither participants nor observers were blinded. There were some differences between randomised groups in participant characteristics (more females and more participants under 40 years in the prednisone and plasma exchange group), but severity of myasthenia gravis was identical in both treatment arms. One participant (in the prednisone only group) was lost to follow‐up at 12 months.
1.
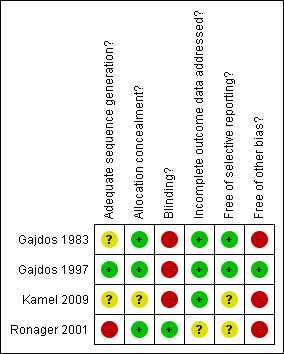
Methodological quality summary: review authors' judgements about each methodological quality item for each included study.
The trial by Ronager et al (Ronager 2001) was also considered to be at high risk of bias. The method of randomisation was done by means of sealed envelopes, but the allocation was skewed with eight participants randomised to IVIg followed by plasma exchange and four to the opposite regimen. The number of participants required was calculated but not obtained. Observers, but not participants, were blinded.
Plasma exchange for myasthenic exacerbation
In the trial by Gajdos et al (Gajdos 1997) the method of randomisation was clearly described, stratified by centre and according to the previous use of corticosteroids or other immunosuppressive drugs. No participants were lost to follow‐up. The study was not blinded but the outcome was assessed by a validated score which is reported to have a good interobserver agreement (Gajdos 2003b). This study was considered to be at low risk of bias.
Plasma exchange before thymectomy
In the trial by Kamel (Kamel 2009) the method of randomisation and of allocation concealment are unknown. Neither participants nor observers were blinded. This study was considered to be at high risk of bias.
Effects of interventions
See: Table 1; Table 2; Table 3; Table 4
Summary of findings for the main comparison. Prednisone versus prednisone and plasma exchange (Gajdos 1983).
| Outcome |
Mean difference (95% CI) |
Number of participants (studies) | Quality of the evidence (Grade) | Comments |
| Score (MMS) day 30 |
+ 17 ( ‐ 6.05 to + 40.05) | 14 (1 study) | Low | No placebo No blinding Few participants (number not calculated) |
| Score (MMS) month 12 |
‐ 9.00 (‐ 23.76 to + 5.76) | 14 (1 study) | Low | No placebo No blinding Few participants (number not calculated) |
Abbreviations: CI confidence interval; MMS myasthenic muscle score.
Summary of findings 2. IVIg versus plasma exchange for chronic myasthenia gravis (Ronager 2001).
| Outcome |
Mean fall in QMGSa PE |
Mean fall in QMGSa IVIg |
Number of participants (studies) |
Quality of the evidence (Grade) |
Comments |
| Week 1 | 0.23 | 0.10 | 12 (1 study) | Very low | Cross‐over study SDb not published Underpowered |
| Week 4 | 0.23 | 0.23 | 12 (1 study) | Very low | Cross‐over study SDb not published Underpowered |
aSee comments. Abbreviations: QMGS quantified myasthenia gravis score; SD standard deviation.
Summary of findings 3. PE versus IVIg for myasthenia gravis exacerbation (Gajdos 1997).
| Outcome |
Mean difference (95% CI) |
Participants (studies) |
Quality of the evidence (Grade) |
Comments |
| Change in score (MMS) day 0 to day 15 |
+ 1.00 (‐ 5.72 to + 7.72) | 87 (1 study) | High | Well powered No loss to follow‐up Evaluation on a validated score |
Abbreviations: IVIg intravenous immunoglobulin G; CI confidence interval; MMS myasthenic muscle score.
Summary of findings 4. PE versus no plasma exchange before thymectomy (Kamel 2009).
| Outcome |
Mean difference (95% CI) |
Participants (studies) | Quality of the evidence(Grade) | Comments |
| Duration of MV (days) |
‐1.10 (‐ 2.12 to ‐ 0.08) |
45 (1 study) | Low | No blinding Randomisation method unknown Underpowered |
| Duration of ICU stay (days) |
‐ 1.20 (‐ 2.30 to ‐ 0.10) |
45 (1 study) | Low | No blinding Randomisation method unknown Underpowered |
Abbreviations: PE plasma exchange; CI confidence interval; MV mechanical ventilation; ICU intensive care unit.
Plasma exchange for chronic myasthenia gravis
In the trial by Gajdos et al (Gajdos 1983), at the time of randomisation, in each group, four participants were in myasthenic crisis requiring mechanical ventilation and three were less severely affected without recent exacerbation. The severity at the time of randomisation measured by the Osserman classification and MMS was identical between the two groups. The participants did not receive steroids or immunosuppressive drugs before randomisation.
By the end of the first month, the mean (SD) MMS was 79 (22) in the plasma exchange and prednisone group and 62 (22) in the prednisone group. The mean difference of 17 points between the two randomised arms was not significant (95% confidence interval (CI): ‐ 6.05 to + 40.05, P = 0.15) (Figure 2, Analysis 1.1).
2.
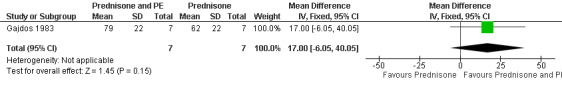
Forest plot of comparison: 1 Prednisone and PE versus prednisone, outcome: 2.1 Score day 30.
1.1. Analysis.
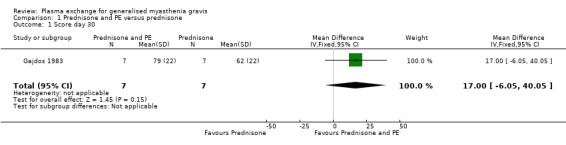
Comparison 1 Prednisone and PE versus prednisone, Outcome 1 Score day 30.
All four participants ventilated in the prednisone and plasma exchange group were weaned before 14 days, and two out of the four participants ventilated in the prednisone alone group were weaned before day 14. The mean (SD) MMS values after one year were 82 (19) in the prednisone and plasma exchange group and 91 (6) in the prednisone alone group. The mean difference of ‐ 9 points between the two randomised arms was not significant (95% CI ‐ 23.76 to + 5.76, P = 0.23) (Figure 3, Analysis 1.2). The evolution of the mean muscle score is summarised in Table 5. Five first relapses were observed in the prednisone and plasma exchange group but only one relapse occurred in the prednisone alone group during the first year following randomisation (P = 0.01 Fisher exact test). The anti‐AChR antibody titre fell to 34% (24) of its baseline level in the prednisone and plasma exchange group and to 29% (13) in the prednisone alone group.
3.

Forest plot of comparison: 1 Prednisone and PE versus prednisone outcome: 1.2 Score month 12.
1.2. Analysis.
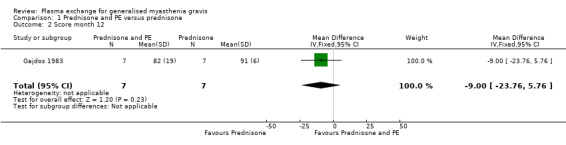
Comparison 1 Prednisone and PE versus prednisone, Outcome 2 Score month 12.
1. Evolution of muscle score: mean (SD) (Gajdos 1983).
| Treatment | Before randomisation | Month 1 | Month 3 | Month 6 | Month 12 |
| Prednisone | 42 (17) | 62 (22) | 78 (19) | 90 (11) | 91 (6) |
| Prednisone and PE | 39 (18) | 79 (22) | 85 (10) | 64 (23) | 82 (19) |
In the trial comparing plasma exchange and IVIg in moderate to severe myasthenia gravis (Ronager 2001), the mean fall in QMGS from baseline to one week after plasma exchange was 0.23 (P < 0.05) and after IVIG was 0.10 (not significant). From baseline to four weeks, the mean fall in QMGS after plasma exchange was still significant and after IVIg was 0.23 (P < 0.05). The change at eight or 16 weeks was also not significant for either plasma exchange or for IVIg. The difference in the changes in QMGS at any date cannot be calculated from the data published.
Plasma exchange for myasthenic exacerbation
In the trial comparing plasma exchange and IVIg for myasthenia gravis exacerbations (Gajdos 1997) participants' characteristics at the time of randomisation were well balanced without any significant difference in age, disease duration, mean MMS, number of thymectomised participants, treatments with prednisone or azathioprine or mechanical ventilation, or presence of anti‐AChR antibodies. At day 15, the mean change in the MMS score was 16.6 (16) in the plasma exchange group and 15.6 (15.9) in the IVIg group. The mean difference of 1 point in change between the two arms was not significant (95% CI: ‐ 5.72 to 7.72, P = 0.77)(Figure 4, Analysis 2.1) and there was no significant difference between the two IVIg groups. Of the 87 participants included, 48 treatment responses were observed, 26 in the plasma exchange group and 22 in the IVIg group (14 in the low dose and 8 in the high dose group).
4.
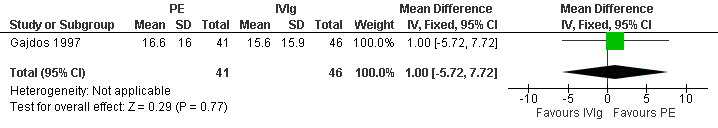
Forest plot of comparison: 2 Plasma exchange versus IVIg for MG exacerbation, outcome: 2.1 Change in MMS after 15 days.
2.1. Analysis.
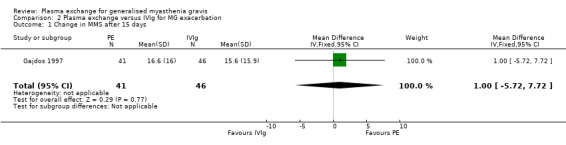
Comparison 2 Plasma exchange versus IVIg for MG exacerbation, Outcome 1 Change in MMS after 15 days.
Among the 63 participants with detectable anti‐AChR antibodies, 39 (62%) exhibited a decrease from baseline and there was no significant difference between treatment groups. The mean change in anti‐AChR antibodies titre was 13.8% (95% CI ‐ 40.8 to + 13.2) decrease in the plasma exchange group and 16.8% (95% CI ‐ 24.9% to + 58.5%) increase in the IVIg group (P = 0.36 Wilcoxon). The mean change in anti‐AChR antibody titre was not significantly different between the low and high dose IVIg groups. The percentage of apparently "seronegative" participants that might have had auto‐antibodies to the muscle specific kinase (MuSK) in this trial was unknown because this test was not known at the time of the trial.
Plasma exchange before thymectomy
In the trial aimed to evaluate the effectiveness of preoperative plasma exchange (Kamel 2009), the duration of postoperative mechanical ventilation was 1.8 ((1.3) hours in group 1 versus 2.9 (1.7) hours in group 2 with a mean difference of 1.10 hours (95% CI ‐ 2.12 to ‐ 0.08, P = 0.03) (Figure 5, Analysis 3.1). The duration of ICU stay was 1.4 (1.3) days in group 1 versus 2.6 (1.9) days in group 2 with a mean difference of 1.20 days (95% CI: ‐ 2.30 to ‐ 0.10, P = 0.03) (Figure 6, Analysis 3.2). The duration of hospital stay was 8.1 (95% CI 6.86 to 9.34) days in group 1 versus 10.8 (95% CI 9.2 to 12.4) in group 2 (P = 0.05). The proportion of participants with antibodies to AChR or to MuSK was not reported and most likely not tested for.
5.

Forest plot of comparison: 3 Prethymecthomy plasma exchange, outcome: 3.1 duration of MV in hours.
3.1. Analysis.
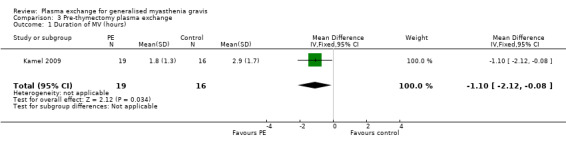
Comparison 3 Pre‐thymectomy plasma exchange, Outcome 1 Duration of MV (hours).
6.
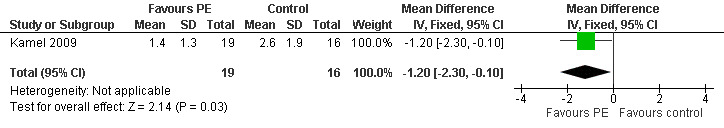
Forest plot of comparison: 3 Prethymectomy plasma exchange, outcome: 3.2 Duration of ICU stay in days.
3.2. Analysis.

Comparison 3 Pre‐thymectomy plasma exchange, Outcome 2 Duration of ICU stay (days).
Adverse events
Adverse events were reported in two studies (Gajdos 1997; Ronager 2001). In the Gajdos study eight participants in the plasma exchange group developed at least one adverse event (haemolysis n = 1; haematoma n = 2; venous thrombosis n = 1; fever n = 2; nausea n = 1; arterial hypotension n = 2; tachycardia n = 1) compared with one participant in the IVIg group (headaches n = 1). Thus 19.5% of participants had adverse events in the plasma exchange group and 2.2% in the IVIg group (P = 0.01 Fisher test). Adverse events led to discontinuation of plasma exchange in two participants.
In the study of Ronager 2001 eight adverse events were reported with plasma exchange (hypotension n = 4; septicaemia n = 1; vomiting n = 1; deep venous thrombosis n = 1 and arterial bleeding n = 1). In the IVIg group 15 adverse effects were reported (fever n = 5; headache n = 7; nausea and vomiting n = 3).
Discussion
Plasma exchange for chronic myasthenia gravis
The RCT by Gajdos et al (Gajdos 1983) failed to demonstrate a short‐ or a long‐term benefit of prednisone and plasma exchange versus prednisone alone. Moreover, it showed a higher incidence of relapses in the plasma exchange group, suggesting that people with myasthenia gravis undergoing plasma exchange may, in fact, do worse in the long term. However, methodological flaws and a substantial risk of bias in this early study leave any strong conclusions from this trial open to question.
The conclusions of the RCT comparing plasma exchange to IVIg for moderate to severe but stable myasthenia gravis (Ronager 2001) are also unreliable because of significant methodological flaws and a high risk of bias.
Plasma exchange for myasthenia gravis exacerbation
The RCT comparing plasma exchange with IVIg for exacerbations of myasthenia gravis (Gajdos 1997) was judged to be at low risk of bias. This trial showed no significant difference in outcomes of treatment with plasma exchange or IVIg for the treatment of myasthenia gravis exacerbations. However, very few patients with myasthenic crisis were included in this trial and the efficacy of IVIg versus plasma exchange for treatment of crisis needs further trial.
Plasma exchange before thymectomy
The trial on the effectiveness of plasma exchange before thymectomy (Kamel 2009) had many methodological flaws and was therefore considered at high risk of bias, providing low quality evidence of any benefit. Because the trial conclusions cannot be relied upon, further trials are needed to judge the efficacy of plasma exchange used as pre‐thymectomy treatment. In any further trial a stratification for a comparable severity and stability of myasthenia before any treatment would be recommended.
Overall, none of the published RCTs demonstrate the efficacy of PE to improve the short‐ or long‐term outcome for chronic stable myasthenia gravis or myasthenia gravis exacerbation. However, PE is used worldwide especially for myasthenia gravis exacerbations, with the justification based on numerous open uncontrolled trials and several consensus statements (for example Bergamini 1983; NIH Consensus 1986). The National Institutes of Health (NIH) consensus conference concluded that "controlled trials comparing plasma exchange with sham exchange or no treatment would be difficult to perform in this life‐threatening disease on the basis of available evidence".
Non‐randomised literature
We have included here a review of the non‐randomised literature. It should be stressed that this does not form part of high quality evidence that can be obtained through performing well designed, fully powered RCTs.
There have been two non‐randomised controlled studies of PE for chronic myasthenia gravis which showed a short‐term but not a long‐term benefit of PE. A non‐randomised but controlled study (Kornfeld 1979) compared six participants who received plasma exchanges over two to two‐and‐a‐half weeks, and six participants who were assigned to a control group. None of the control participants improved, while all treated participants improved strikingly. However, there were no data on clinical characteristics of participants, outcome measurements or associated treatments. A controlled but non‐randomised study (Newsom‐Davis 1979) compared the long‐term effect of plasma exchange plus immunosuppressive drug in seven participants with myasthenia gravis to the effect of immunosuppressive drug alone in seven participants with myasthenia gravis. Plasma exchange was associated with short‐term improvement in all seven participants. At 6 to 12 months clinical outcome as well as AChR antibody titre decline were similar in both groups.
A retrospective multicentre chart review study (Qureshi 1999) compared the efficacy and tolerability of plasma exchange and IVIg in treatment of 54 episodes of myasthenic crisis defined as a forced vital capacity < 1 litre or requirement for mechanical ventilation. Participants were treated with five or six plasma exchanges or with IVIg 0.4 g/kg/d for five days. One week after initiation of treatment, mean (SD) severity score in the IVIg group improved from 7.5 (1.7) to 10.3 (3.2) (P = 0.05) and in the plasma exchange group from 6.9 (1.7) to 11.1 (2.5) (P = 0.009). Ventilatory status at two weeks and outcome at one month were significantly better in the plasma exchange group but total hospital stay was longer in the plasma exchange group. The complication rate was higher with plasma exchange compared to IVIg (13 versus 5 complications). However, this study was retrospective. There was a hospital bias toward selection of a particular treatment. There were few data on concomitant treatments (corticosteroids or immunosuppressive drugs).
Two conferences of experts (NIH Consensus 1986; AAN 1996) recommended the use of plasma exchange before thymectomy, although this recommendation was based only on retrospective studies and expert opinions. D'Empaire et al. (D'Empaire 1985) in a retrospective study, found a significantly decreased time on mechanical ventilation: mean (SD) 1.02 (0.40) versus 3.43 (0.60) days and a shorter stay in the intensive care unit (3.09 (0.99) versus 5.15 (0.66) days) for 11 MG patients with respiratory weakness who were treated with pre‐operative plasma exchange compared with 26 myasthenia gravis patients who did not receive plasma exchange. Patients with respiratory weakness who received pre‐thymectomy plasma exchange required less time on mechanical ventilation (1.02 (0.40) versus 2.73 (0.88) days) and a shorter stay in the intensive care unit (3.09 (0.99) versus 4.46 (1.08) days) than those patients without respiratory weakness who did not receive plasma exchange.
Yeh 2005 reported a retrospective study of 29 myasthenia gravis patients treated pre‐operatively with double filtration plasmapheresis. They found a correlation between a higher removal rate of AchR antibodies and a shorter duration of ICU and postoperative hospital stay (P = 0.001 and 0.019 respectively).
Perez‐Nellar 2001 compared a prospective group of 33 people with myasthenia gravis treated with IVIg with a historical group of 38 people with myasthenia gravis treated with plasma exchange during the peri‐operative period of thymectomy. In the prospective group, participants received 2 g/kg IVIg (two‐thirds of the dose before and one‐third after thymectomy). In the retrospective group, participants were treated with three plasma exchanges on alternate days before and two plasma exchanges on alternate days after thymectomy. The duration of mechanical ventilation was not different between the two treatment groups: 14.1 hours (95% CI 10.71 to 17.31) in the IVIg group and 17.24 hours (95% CI 12.54 to 21.94) in the plasma exchange group, mean difference ‐ 3.23 (95% CI ‐ 8.71 to 2.31). The time in the intensive care unit was shorter in the IVIg group (3.36 days (95% CI 2.9 to 3.82)) compared with 4.34 days (95% CI 3.76 to 4.92) in the plasma exchange group, mean difference ‐ 0.98 (95% CI ‐1.72 to ‐ 0.24). Jensen 2008 compared plasma exchange and IVIg as pre‐operative therapy in a retrospective study. Nine myasthenia gravis patients (Osserman grade 2 or 3 only) treated with IVIg were matched for Osserman grade, gender and age with nine myasthenia gravis patients receiving plasma exchange. Postoperative change in Osserman grade was 1.00 (95% CI 0.53 to 1.47) in the plasma exchange group and 0.78 (95% CI 0.23 to 1.33) for IVIg (P = 0.55).
Seven reports were published of at least 15 patients (Antozzi 1991; Behan 1979; Chiu 2000; Dau 1981; Fornasari 1985; Olarte 1981; Perlo 1981) (Table 6). In these seven open studies, 316 patients were described and an improvement rate of 76.4% was reported. However, one must interpret these case series cautiously. Most series lacked precision about the clinical condition (acute or chronic), outcome measurements, associated treatment such as steroids or immunosuppressive drugs, plasma exchange protocols and side‐effects due to plasma exchange. However, the improvement reported immediately, or a few days following treatment in these case series, suggests that plasma exchange may be effective in the short term. This is corroborated by a rapid fall in antibody titre. The long‐term effectiveness of plasma exchange alone cannot be evaluated in these case series since all patients received steroids and immunosuppressive drugs. Side‐effects, such as hypotension, bradycardia, haematoma, catheter‐related venous thrombosis, vomiting and skin rash, were reported in 15% to 20% of plasma exchanges (Antozzi 1991; Gajdos 1997).
2. Results of seven open studies of at least 15 participants.
| Authors | Year | Participants | Prednisone | Immunosuppressant | PEs/patient | Litres exchanged | Effecta (per cent) |
| Behan | 1979 | 21 | Y | Y | ? | 16‐32 | 100 |
| Dau | 1981 | 60 | 48 | 48 | 9‐33 | 73 | |
| Olarte | 1981 | 21 | 13 | 12 | 2‐10 | 81 | |
| Perlo | 1981 | 17 | ? | ? | 3‐5 | 65 | |
| Fornasari | 1985 | 33 | 11 | 11 | 4‐8 | 61 | |
| Antozzi | 1991 | 70 | ? | ? | 2 | 70 | |
| Chiu | 2000 | 94 | ? | ? | 4‐5 | 85 | |
| Total | 316 | 76.4 |
aEffect is the percentage of patients improved reported in each series whatever the definition of improvement. See text.
Several studies were designed to compare different protocols of PE or to test new techniques associated with PE, namely double filtration or immunoadsorption. Two studies compared the efficacy of different protocols of plasma exchange. Mantegazza (Mantegazza 1987) in a comparative but non‐randomised trial compared three plasma exchanges on alternate days with fresh frozen plasma from multiple donors as replacement fluid and two plasma exchanges on alternate days with fresh frozen plasma from single donors. The two schedules gave similar efficacy: 81.8% improved in the multiple donors group and 93.3% in the single donor group, but there were fewer adverse events in the single donor schedule (P = 0.0003). In a RCT (Yeh 1999), participants were treated with five double‐filtration plasma exchanges daily or every other day. Improvement of the quantified myasthenia gravis score was significantly higher in the daily group (median reduction of the modified QMGS: 4.5 in the daily group and 2 in the alternate daily group. P < 0.05). In 1987, a small case series of patients with myasthenic crisis compared reported treatment using a combination of plasma exchange with a semi‐selective immunoadsorption (tryptophane‐polyvinylalcohol resins) with standard plasma exchange alone in the same patients in two subsequent myasthenic crises, showing similar efficacy in reducing AChR antibodies but a lesser depletion of other proteins (Heininger 1987). Yeh (Yeh 2000) compared the efficacy of double filtration plasma exchange and of immunoadsorption. Five participants were treated with five double filtration plasma exchanges for a first exacerbation of myasthenia gravis and then, four months to two years later, with five immunoadsorptions for a second exacerbation. The mean reduction in a modified quantified myasthenia gravis score was not significantly different between the two groups (2.6 versus 2.2).
Given the disappointing results of RCTs which provide low quality evidence of any benefit of plasma exchange, the numerous questions raised by the non‐randomised literature and the paradoxical extended use of plasma exchange for the treatment of generalised myasthenia gravis, new RCTs are needed. The RCT comparing prednisone alone to plasma exchange plus prednisone and the RCT of pre‐thymectomy plasma exchange both have many methodological flaws. The short‐ and long‐term effectiveness of plasma exchange for chronic but more or less stable myasthenia gravis and of myasthenia gravis crisis remains unsupported by high quality evidence. Further trials are needed to demonstrate the efficacy of plasma exchange for myasthenic crisis, in preparation for thymectomy, and in repeated use for myasthenia gravis resistant to immunosuppressive treatment. The relative efficacy of other techniques such as immunoadsorption or double filtration is not known.
A Cochrane systematic review of therapeutic immunoglobulin in myasthenia gravis treatment has been published (Gajdos 2003a).
Authors' conclusions
Implications for practice.
No adequate randomised controlled trials have been performed to determine whether plasma exchange improves the short‐ or long‐term outcome for chronic myasthenia gravis or myasthenia gravis exacerbation. However, many case series studies convincingly report short‐term benefit from plasma exchange in myasthenia gravis, especially in myasthenic exacerbation or crisis. In severe exacerbations of myasthenia gravis one randomised controlled trial did not show a significant difference between plasma exchange and intravenous immunoglobulin.
Implications for research.
Further research is needed to compare plasma exchange with alternative short‐term treatments for myasthenic crisis or before thymectomy and to determine the value of long‐term plasma exchange for treating myasthenia gravis.
The design of such studies should be adapted to each situation. It would be ethically difficult to compare plasma exchange with placebo or sham plasma exchange for the treatment of exacerbation and not possible in myasthenic crisis. In this situation, plasma exchange could be compared with other techniques such as immunoadsorption or double filtration or alternative treatments such as intravenous immunoglobulin. In other situations such as preparation for thymectomy or treatment of resistant myasthenia gravis, the data in the literature are so inconclusive that a prospective comparison of plasma exchange with no treatment or placebo in a RCT would be ethically justified.
In all studies inclusion criteria should be clearly defined and the severity of myasthenia gravis and concomitant treatment described. Other treatments during the trial should be standardised. Outcome measures should be clinically and statistically significant and validated, and at present there are no standard outcome measures in myasthenia. The primary end point for a RCT looking for a short‐term improvement should be the change of a score of muscle strength 15 or 30 days after initiation of plasma exchange. If plasma exchange is evaluated for preparation for thymectomy, the outcome measure could be the postoperative duration of mechanical ventilation and change in muscle strength. In a RCT looking for a long‐term improvement (treatment resistant myasthenia gravis), the best outcome would be the time to reach a change in functional class and a change in the post‐intervention status one year after initiation of plasma exchange (Jaretzki 2000). The difference in the cumulative dose of steroids could also be considered but any combination of drugs would interfere with the interpretation of long‐term outcome data. In all such trials, adverse events should be considered as secondary endpoints.
Trials should be adequately powered to allow valid conclusions about the main comparison. Power is a major limiting factor in view of the evidence from the open trials where it was shown that immunoadsorption and standard plasma exchange may yield similar results. To show equal efficacy, even higher numbers of participants would be needed. Owing to the number of participants required, the rigorous inclusion criteria needed and the low prevalence of myasthenia gravis, multicentre RCTs are recommended.
What's new
| Date | Event | Description |
|---|---|---|
| 17 May 2010 | New search has been performed | Searches updated to January 2011. Three new trials. Updated 'Risk of bias' methodology and included 'Summary of findings' tables. |
History
Protocol first published: Issue 3, 2000 Review first published: Issue 4, 2002
| Date | Event | Description |
|---|---|---|
| 28 October 2008 | Amended | Converted to new review format. |
| 27 February 2008 | New search has been performed | We updated the search of the Cochrane Neuromuscular Disease Group Trials Register (April 2007), MEDLINE January 2002 to February 2007 and EMBASE (January 1980 to February 2007). No new randomised controlled trials were identified. |
| 10 March 2005 | New search has been performed | We updated the search of the Cochrane Neuromuscular Disease Group specialised register (January 2005), MEDLINE January 2002 to 10 March 2005 and EMBASE (January 1980 to 10 March 2005). No new randomised controlled trials were identified. |
| 10 June 2002 | New citation required and conclusions have changed | Substantive amendment |
Acknowledgements
We thank Prof Djillali Annane for his technical assistance and Prof Richard Hughes for correction of the English version.
Appendices
Appendix 1. MEDLINE (Ovid SP) search strategy
1 randomized controlled trial.pt. 2 controlled clinical trial.pt. 3 randomized.ab. 4 placebo.ab. 5 drug therapy.fs. 6 randomly.ab. 7 trial.ab. 8 groups.ab. 9 or/1‐8 10 exp animals/ not humans.sh. 11 9 not 10 12 exp Myasthenia Gravis/ 13 myastheni$.tw. 14 12 or 13 15 Plasma Exchange/ 16 (plasmapheresis or plasma exchange).tw. 17 15 or 16 18 11 and 14 and 17
Appendix 2. EMBASE (OvidSP) search strategy
‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐ 1 Randomized Controlled Trial/ 2 Clinical Trial/ 3 Multicenter Study/ 4 Controlled Study/ 5 Crossover Procedure/ 6 Double Blind Procedure/ 7 Single Blind Procedure/ 8 exp RANDOMIZATION/ 9 Major Clinical Study/ 10 PLACEBO/ 11 Meta Analysis/ 12 phase 2 clinical trial/ or phase 3 clinical trial/ or phase 4 clinical trial/ 13 (clin$ adj25 trial$).tw. 14 ((singl$ or doubl$ or tripl$ or trebl$) adj25 (blind$ or mask$)).tw. 15 placebo$.tw. 16 random$.tw. 17 control$.tw. 18 (meta?analys$ or systematic review$).tw. 19 (cross?over or factorial or sham? or dummy).tw. 20 ABAB design$.tw. 21 or/1‐20 22 human/ 23 nonhuman/ 24 22 or 23 25 21 not 24 26 21 and 22 27 25 or 26 28 Myasthenia Gravis/ 29 myastheni$.mp. 30 28 or 29 31 Plasmapheresis/ 32 (plasmapheresis or plasma exchange).mp. 33 31 or 32 34 27 and 30 and 33
Appendix 3. Cochrane CENTRAL
#1myastheni* #2plasma exchange #3plasmapheresis #4(#2 OR #3) #5(#1 AND #4)
Data and analyses
Comparison 1. Prednisone and PE versus prednisone.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Score day 30 | 1 | 14 | Mean Difference (IV, Fixed, 95% CI) | 17.0 [‐6.05, 40.05] |
| 2 Score month 12 | 1 | 14 | Mean Difference (IV, Fixed, 95% CI) | ‐9.0 [‐23.76, 5.76] |
Comparison 2. Plasma exchange versus IVIg for MG exacerbation.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Change in MMS after 15 days | 1 | 87 | Mean Difference (IV, Fixed, 95% CI) | 1.00 [‐5.72, 7.72] |
Comparison 3. Pre‐thymectomy plasma exchange.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Duration of MV (hours) | 1 | 35 | Mean Difference (IV, Fixed, 95% CI) | ‐1.10 [‐2.12, ‐0.08] |
| 2 Duration of ICU stay (days) | 1 | 35 | Mean Difference (IV, Fixed, 95% CI) | ‐1.20 [‐2.30, ‐0.10] |
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Gajdos 1983.
| Methods | RCT | |
| Participants | 14 participants with myasthenia gravis | |
| Interventions | Group 1: prednisone Group 2: prednisone and plasma exchange | |
| Outcomes | End points: muscle score, relapses, prednisone dose mean (SD) | |
| Notes | RCT aimed at comparing the long‐term effect of two therapeutic regimens, namely: prednisone (Group I) versus prednisone and plasma exchange (Group II) for chronic stable myasthenia gravis | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Unclear risk | Information is lost but it was probably a computer random number generator (personal communication) |
| Allocation concealment? | Low risk | Sequentially numbered, opaque, sealed envelopes |
| Blinding? All outcomes | High risk | No blinding |
| Incomplete outcome data addressed? All outcomes | Low risk | No missing data at one month. Only one participant missing at one year |
| Free of selective reporting? | Low risk | The published report included all outcomes pre‐specified in the protocol |
| Free of other bias? | High risk | Imbalances between groups (gender and age). See text |
Gajdos 1997.
| Methods | RCT: 2 parallel groups End point: MMS | |
| Participants | 87 participants with myasthenia gravis exacerbation | |
| Interventions | Group 1: 3 PEs Group 2: received either IVIg 2 g/kg or IVIg 1.2 g/kg |
|
| Outcomes | Change in outcome scores at day 15 after randomisation or PE. Outcomes were final MMS, time to reach significant MMS score (>20 point increase from baseline), change in AChR Ab titre and adverse events | |
| Notes | RCT aimed at comparing PE and IVIg for treatment of myasthenia gravis exacerbation | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Low risk | Randomisation through a centralised telephone, stratified by center and previous treatment |
| Allocation concealment? | Low risk | Central allocation and pharmacy controlled |
| Blinding? All outcomes | High risk | Not blinded |
| Incomplete outcome data addressed? All outcomes | Low risk | Missing data were imputed using appropriate methods |
| Free of selective reporting? | Low risk | The published report include all outcomes pre‐specified in the protocol |
| Free of other bias? | Low risk | No other bias suspected |
Kamel 2009.
| Methods | RCT. Two parallel groups | |
| Participants | 35 participants with generalised myasthenia gravis | |
| Interventions | Group 1 (n = 19) 3 PE before thymectomy Group 2 ( n = 16) no PE before thymectomy |
|
| Outcomes | Duration of mechanical ventilation Duration of intensive care unit stay Duration of hospital stay |
|
| Notes | RCT aimed at evaluating the effectiveness of pre‐thymectomy plasma exchange | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Unclear risk | No information |
| Allocation concealment? | Unclear risk | No information |
| Blinding? All outcomes | High risk | Neither participants nor observers were blinded |
| Incomplete outcome data addressed? All outcomes | Low risk | No missing outcome data |
| Free of selective reporting? | Unclear risk | The outcomes are not really specified and therefore impossible to judge |
| Free of other bias? | High risk | There was no calculation of the sample size necessary for a sufficient power |
Ronager 2001.
| Methods | RCT: cross‐over study End point: QMGS | |
| Participants | 12 participants with moderate to severe myasthenia gravis | |
| Interventions | Group 1: IVIg 0.4 g/kg for 5 days and 16 weeks later 5 plasma exchanges Group 2: opposite schedule with plasma exchange followed by IVIg | |
| Outcomes | No difference in the change in QMGS after either treatment | |
| Notes | RCT aimed at comparing the efficacy of IVIg versus plasma exchange in people with moderate to severe myasthenia gravis in a stable phase | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | High risk | Eight participants were randomised to IVIg followed by plasma exchange and four to the opposite regimen |
| Allocation concealment? | Low risk | Sealed envelopes |
| Blinding? All outcomes | Low risk | Only observers were blinded ‐ single blind study |
| Incomplete outcome data addressed? All outcomes | Unclear risk | The publication did not provide the appropriate information. |
| Free of selective reporting? | Unclear risk | The published report include all outcomes pre‐specified in the protocol |
| Free of other bias? | High risk | The number of participants required was calculated but not obtained |
RCT randomised controlled trial; MMS mean muscle score; IVIg intravenous immunoglobulin; PE plasma exchange; AChR acetylcholine receptor; Ab antibody; QMGS quantified myasthenia gravis score.
Differences between protocol and review
'Risk of bias' methodology updated in accordance with the 2008 Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2008) and 'Summary of findings' tables included.
Contributions of authors
Philippe Gajdos extracted the data and wrote the first draft of the review. Sylvie Chevret and Klaus Toyka checked the data and the draft.
Sources of support
Internal sources
None, Not specified.
External sources
None, Not specified.
Declarations of interest
None known
New search for studies and content updated (no change to conclusions)
References
References to studies included in this review
Gajdos 1983 {published data only}
- Gajdos P, Simon N, Rohan‐Chabot P, Raphaël JC, Goulon M. Long term effects of plasma exchange in myasthenia. Results from a randomized study [Effet à long terme des échanges plasmatiques au cours de la myasthénie. Résultat d'une étude randomisée]. Presse Médicale 1983;12(15):939‐42. [PUBMED: 6221247] [PubMed] [Google Scholar]
Gajdos 1997 {published and unpublished data}
- Gajdos P, Chevret S, Clair B, Tranchant C, Chastang C. Clinical trial of plasma exchange and high‐dose immunoglobulin in myasthenia gravis. Myasthenia Gravis Clinical Study Group. Annals of Neurology 1997;41(6):789‐96. [PUBMED: 9189040] [DOI] [PubMed] [Google Scholar]
Kamel 2009 {published data only}
- Kamel A, Essa M. Effectiveness of prethymecthomy plasmapheresis on short‐term outcome of non‐thymomatous generalized myasthenia gravis. Egyptian Journal Neurology, Psychiatry and Neurosurgurgery 2009;46(1):161‐8. [Google Scholar]
Ronager 2001 {published data only}
- Rønager J, Ravnborg M, Hermansen I, Vorstrup S. Immunoglobulin treatment versus plasma exchange in patients with chronic moderate to severe myasthenia gravis. Artificial Organs 2001;25(12):967‐73. [PUBMED: 11843764] [DOI] [PubMed] [Google Scholar]
Additional references
AAN 1996
- Assessment of plasmapheresis. Report of the therapeutics and technology assessment subcommittee of the American Academy of Neurology. Neurology 1996;47(3):840‐3. [PubMed] [Google Scholar]
Antozzi 1991
- Antozzi C, Gemma M, Regi B, Berta E, Confalonieri P, Peluchetti D, et al. A short plasma exchange protocol is effective in severe myasthenia gravis. Journal of Neurology 1991;238(2):103‐7. [DOI] [PubMed] [Google Scholar]
Behan 1979
- Behan PO, Shakir RA, Simpson JA, Burnett AK, Allan TL, Haase G. Plasma‐exchange combined with immunosuppressive therapy in myasthenia gravis. Lancet 1979;2(8140):438‐40. [DOI] [PubMed] [Google Scholar]
Bergamini 1983
- Bergamini L, Cocito D, Durelli L, Quattrocolo G. Opinions about plasma exchange and associated treatments in the therapy of myasthenia gravis. Muscle and Nerve 1983;6(6):457‐8. [DOI] [PubMed] [Google Scholar]
Besinger 1983
- Besinger UA, Toyka KV, Hömberg M, Heininger K, Hohlfeld R, Fateh‐Moghadam A. Myasthenia gravis: long‐term correlation of binding and bungarotoxin blocking antibodies against acetylcholine receptors with changes in disease severity. Neurology 1983;33(10):1316‐21. [DOI] [PubMed] [Google Scholar]
Chiu 2000
- Chiu HC, Chen WH, Yeh JH. The six year experience of plasmapheresis in patients with myasthenia gravis. Therapeutic Apheresis: official journal of the International Society for Apheresis and the Japanese Society for Apheresis 2000;4(4):291‐5. [DOI] [PubMed] [Google Scholar]
Cole 2008
- Cole RN, Reddel SW, Gervasio OL, Phillips WD. Anti‐MuSK patient antibodies disrupt the mouse neuromuscular junction.. Ann Neurol 2008;63(6):782‐9. [DOI] [PubMed] [Google Scholar]
Cornelio 1993
- Cornelio F, Antozzi C, Mantegazza R, Confalonieri P, Berta E, Peluchetti D, et al. Immunosuppressive treatments. Their efficacy on myasthenia gravis patients' outcome and on the natural course of the disease. Annals of the New York Academy of Sciences 1993;681:594‐602. [DOI] [PubMed] [Google Scholar]
D'Empaire 1985
- d'Empaire G, Hoaglin DC, Perlo VP, Pontoppidan H. Effect of prethymecthomy plasma exchange on postoperative respiratory function in myasthenia gravis. Journal of Thoracic and Cardiovascular Surgery 1985;89(4):592‐596. [PubMed] [Google Scholar]
Dau 1977
- Dau PC, Lindstrom JM, Cassel CK, Denys EH, Shev EE, Spitler LE. Plasmapheresis and immunosuppressive drug therapy in myasthenia gravis. New England Journal of Medicine 1977;297(21):1134‐40. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Dau 1979
- Dau PC. Plasmapheresis and the immunology of myasthenia gravis. Boston: Hougton‐Mifflin, 1979. [Google Scholar]
Dau 1980
- Dau PC. Plasmapheresis therapy in myasthenia gravis. Muscle and Nerve 1980;3(6):468‐82. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Dau 1981
- Dau PC. Response to plasmapheresis and immunosuppressive drug therapy in sixty myasthenia gravis patients. Annals of the New York Academy of Sciences 1981;377:700‐8. [DOI] [PubMed] [Google Scholar]
Fornasari 1985
- Fornasari PM, Riva G, Piccolo G, Cosi V, Lombardi M. Short and long‐term clinical effects of plasma exchange in 33 cases of myasthenia gravis. The International Journal of Artificial Organs 1985;8(3):159‐62. [PubMed] [Google Scholar]
Gajdos 2003a
- Gajdos P, Chevret S, Toyka K. Intravenous immunoglobulin for myasthenia gravis. Cochrane Database of Systematic Reviews 2003, Issue 2. [DOI: 10.1002/14651858.CD002277.pub3] [DOI] [PubMed] [Google Scholar]
Gajdos 2003b
- Gajdos P, Shashar T, Chevret S. Standards of measurements in myasthenia gravis. Annals of the New York Academy of Sciences 2003;998:445‐52. [DOI] [PubMed] [Google Scholar]
Grob 1981
- Grob D, Brunner NG, Namba T. The natural course of myasthenia gravis and effect of therapeutic measures. Annals of the New York Academy of Science 1981;377(1):652‐69. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Heininger 1987
- Heininger K, Hartung HP, Toyka KV, Gaczkowski A, Borberg H. Therapeutic plasma exchange in myasthenia gravis: semiselective adsorption of anti‐AChR autoantibodies with tryptophane linked polyvinyalcohol gels. Annals of the New York Academy of Sciences 1987;505:898‐900. [Google Scholar]
Higgins 2008
- Higgins JPT, Altman DG (editors). Chapter 8: Assessing risk of bias in included studies. Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions. Chichester, UK: John Wiley & Sons, 2008. [Google Scholar]
Hohlfeld 1993
- Hohlfeld R, Toyka KV. Therapies. In: Baets MH, Oosterhuis H editor(s). Myasthenia Gravis. CRC Press, 1993:236‐57. [Google Scholar]
Hohlfeld 1996
- Hohlfeld R, Melms A, Toyka KV, Drachman DB. Therapy for myasthenia gravis and myasthenic syndromes. Neurological disorders: course and treatment. Academic Press, 1996. [Google Scholar]
Jaretzki 2000
- Jaretzki A, Barohn RJ, Ernstoff RM, Kaminski HJ, Keesey JC, Penn AS, et al. Myasthenia gravis. Recommendations for clinical research standards. Neurology 2000;55(1):16‐23. [DOI] [PubMed] [Google Scholar]
Jensen 2008
- Jensen P, Bril V. A comparison of the effectiveness of intravenous immunoglobulin and plasma exchange as preoperative therapy of myasthenia gravis. Journal of Clinical Neuromuscular Disease 2008;9(3):352‐5. [DOI] [PubMed] [Google Scholar]
Kornfeld 1979
- Kornfeld P, Ambinder EP, Papatestas AE, Bender AN, Genkins G. Plasmapheresis in myasthenia gravis: controlled study. Lancet 1979;2(8143):629. [DOI] [PubMed] [Google Scholar]
Kornfeld 1981
- Kornfeld P, Ambinder EP, Mittag T, Bender AN, Papatestas AE, Goldberg J, et al. Plasmapheresis in refractory generalized myasthenia gravis. Archives of Neurology 1981;38(8):478‐81. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Mantegazza 1987
- Mantegazza R, Bruzzone E, Regi B, Peluchetti D, Marconi M, Sirchia G, et al. Single donor plasma in therapeutic exchange for myasthenia gravis. International Journal of Artificial Organs 1987;10(5):315‐8. [PubMed] [Google Scholar]
Miller 1981
- Miller RG, Milner‐Brown HS, Dau PC. Antibody‐negative acquired myasthenia gravis. Successful therapy with plasma exchange (letter). Muscle and Nerve 1981;4(3):255. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Newsom‐Davis 1979
- Newsom‐Davis J, Wilson SG, Vincent A, Ward CD. Long‐term effects of repeated plasma exchange in myasthenia gravis. Lancet 1979;1(8114):464‐8. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
NIH Consensus 1986
- NIH Consensus Conference. The utility of therapeutic plasmapheresis for neurological disorders. NIH Consensus Development.. Journal of the American Medical Association 1986;256(10):1333‐7. [MEDLINE: ] [PubMed] [Google Scholar]
Olarte 1981
- Olarte MR, Schoenfeldt RS, Penn AS, Lovelace RE, Rowland LP. Effect of plasmapheresis in myasthenia gravis 1978‐1980. Annals of the New York Academy of Sciences 1981;377:725‐8. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Oosterhuis 1997
- Oosterhuis HJGH. Myasthenia gravis. Groningen: Groningen Neurological Press, 1997. [Google Scholar]
Patrick 1973
- Patrick J, Lindstrom J. Autoimmune response to acetylcholine receptor. Science 1973;180(88):871‐2. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Perez‐Nellar 2001
- Perez‐Nellar J, Dominguez AM, Llorens‐Figueroa JA, Ferra‐Betancourt A, Pardo A, Quiala M, et al. A comparative study of intravenous immunoglobulin and plasmapheresis preoperatively in myasthenia [Estudio comparativo entre immunoglobulina intravenosa y plasmaferesis en el perioperatorio de la miastenia gravis]. Revista de Neurologia 2001;33(5):413‐6. [PubMed] [Google Scholar]
Perlo 1981
- Perlo VP, Shahani BT, Huggins CE, Hunt J, Kosinski K, Potts F. Effect of plasmapheresis in myasthenia gravis. Annals of the New York Academy of Sciences 1981;377:709‐24. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Pinching 1976
- Pinching AS, Peters DK. Remission of myasthenia gravis following plasma exchange. Lancet 1976;2(8000):1373‐6. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Qureshi 1999
- Qureshi AI, Choudhry MA, Akbar MS, Mohammad Y, Chua HC, Yahia AM, et al. Plasma exchange versus intravenous immunoglobulin treatment in myasthenic crisis. Neurology 1999;52(3):629‐32. [DOI] [PubMed] [Google Scholar]
RevMan 2008 [Computer program]
- The Nordic Cochrane Centre, The Cochrane Collaboration. Review Manager (RevMan). Version 5. Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2008.
Richman 1980
- Richman DP, Gomez CM, Berman PW, Burres SA, Fitch FW, Arnason BG. Monoclonal anti acetylcholine receptor antibodies can cause experimental myasthenia. Nature 1980;286(5774):738‐9. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Rodnitzky 1984
- Rodnitzky RL, Bosch EP. Chronic long‐interval plasma exchange in myasthenia gravis. Archives of Neurology 1984;41(7):715‐7. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Toyka 1975
- Toyka KV, Drachman DB, Pestronk A, Kao I. Myasthenia gravis: passive transfer from man to mouse. Science 1975;190(4212):397‐9. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Vincent 2001
- Vincent A, Palace J, Hilton‐Jones D. Myasthenia gravis. Lancet 2001;357(9274):2122‐8. [DOI] [PubMed] [Google Scholar]
Yeh 1999
- Yeh JH, Chiu HC. Plasmapheresis in myasthenia gravis. A comparative study of daily versus alternately daily schedule. Acta Neurologica Scandanavica 1999;99(3):147‐51. [DOI] [PubMed] [Google Scholar]
Yeh 2000
- Yeh JH, Chiu HC. Comparison between double‐filtration plasmapheresis and immunoadsorption plasmapheresis in the treatment of patients with myasthenia gravis. Journal of Neurology 2000;247(7):510‐3. [DOI] [PubMed] [Google Scholar]
Yeh 2005
- Yeh JH, Chen WH, Huang KM, Chiu HC. Prethymecthomy plasmapheresis in myasthenia gravis. Journal of Clinical Apheresis 2005;20(4):217‐221. [DOI] [PubMed] [Google Scholar]


