Abstract
MicroRNAs (miRNAs) are small non-coding RNAs that control patterns of gene expression by inducing the degradation of mRNAs. In addition, miRNAs are known to serve an important role in the pathogenesis of atrial fibrillation (AF). In general, AF is diagnosed using electrocardiography. However, the present study investigated whether specific miRNAs derived from microarray analysis of human urine could regulate AF through the inhibition of calcium handling protein phosphorylation in an AF model. Microarray analysis of the transcriptome in the human urine of patients with paroxysmal supraventricular tachycardia and AF revealed that 7 differentially expressed miRNAs were significantly downregulated (miR-3613, 6763, 423, 3162, 1180, 6511, 3197) in patients with AF. In addition, quantitative PCR results demonstrated that collagen I, collagen III, fibronectin and TGF-β, which are fibrosis-related genes, were upregulated in patients with AF. Furthermore, fibrosis-related genes were upregulated in angiotensin II-induced atrial myocytes, which demonstrated that these genes may be targets of miR-423. In the AF cell model transfected with miR-423, the expression of calcium handling proteins, including phosphorylated calmodulin-dependent protein kinase II, was reduced. The transfection of miR-423 attenuated damage to cardiac cells caused by calcium handling proteins. The findings highlight the importance of calcium handling protein phosphorylation changes in fibrosis-induced AF and support miR-423 detection in human urine as a potential novel approach of AF diagnosis.
Keywords: AF, arrhythmia, microRNA-423, CaMKII, urine
Introduction
Atrial fibrillation (AF) is a well-known supraventricular arrhythmia in the general population (1). In animal studies, atrial fibrosis plays an important role in the induction and perpetuation of AF (2–4). Atrial fibrosis causes interatrial inhomogeneity in electrical conduction, creating a substrate for local re-entry and contributing to the progression of AF (4). The rennin-angiotensin system, specifically angiotensin II (Ang II), has been suggested to play a significant role in the development of cardiac remodeling during AF. However, the precise downstream molecules important in the genesis of AF-induced atrial fibrosis are currently unclear (5). The major proteins and mechanisms involved in the remodeling process of AF include the altered expression of ion channels (causing a reduction in action potential duration), Ca2+ handling proteins (impairing intracellular Ca2+ removal and release), or connexins (impairing intra-atrial conduction). In addition, spontaneous sarcoplasmic reticulum Ca2+ release through altered RyR2 can act as a trigger for AF and can induce AF by increasing fibrosis, which acts as a substrate that promotes re-entry and maintenance of AF.
miRNAs are small non-coding, single-stranded RNAs that are18-25 nucleotides in length (6). The expression of various miRNAs has been linked to cardiovascular disorders, including heart failure and left ventricular hypertrophy (7,8). miRNAs represent one of the most actively investigated areas in the cardiovascular field; they are being investigated as potential diagnostic and prognostic biomarkers in a number of cardiovascular pathologies and associated metabolic diseases (9). Urine is a sterile biological fluid containing the final product produced by the metabolism of proteins secreted by the kidneys and can be collected in a non-invasive and simple manner (10). Most urine miRNAs are derived from renal and urethral cells, and the analysis of these cells can determine the health status of patients. Therefore, changes in the miRNAs in AF patients could be confirmed through urine samples (11,12). In this study, we report a newly discovered miRNA that is related to fibrosis and Ca2+ handling proteins in the development of AF and attempt to establish its significance in the diagnosis of AF by examining the changes in the miRNA through urine samples.
Materials and methods
Urine sample collection
Urine samples were collected from patients diagnosed with paroxysmal supraventricular tachycardia (PSVT) and AF using a non-invasive method. Patient samples were recruited from patients with written informed consent at Ewha Womans University Hospital from August 2019 to February 2020. The clinical profiles of patients are shown in Table I. The study protocol was ethically approved by the local ethics committee (Institutional Review Board of Ewha Womans University Hospital (Seoul, Korea); approval no. IRB 2019-10-019). In brief, after the tube was inserted into the patient's urethra, approximately 50 ml of initial urine was collected. Then, the old tube was replaced with a new tube, and experimental urine samples were collected. Aliquoted urine samples were stored at −70°C until miRNA isolation.
Table I.
Clinical profile of patients whose urine was used for mRNA isolation.
| Case | Sex | Age, years | Diagnosis |
|---|---|---|---|
| 1 | Male | 47 | PSVT |
| 2 | Female | 46 | PSVT |
| 3 | Female | 69 | PSVT |
| 4 | Male | 63 | PeAF |
| 5 | Female | 58 | PeAF |
| 6 | Female | 63 | PeAF |
| 7 | Male | 56 | PeAF |
| 8 | Male | 58 | PeAF |
PSVT, Paroxysmal supraventricular tachycardia; PeAF, Persistent atrial fibrillation.
Quantitative gene expression analysis of urine samples from the AF and PSVT groups
Fibrosis-related gene expression (collagen I/III, fibronectin 1, and TGF-β) was analyzed using RNA samples isolated from AF patients' urine and HL-1 cells. To further investigate the effects of the fibrosis markers, fibrosis-related gene expression was examined in Ang II-treated cells.
Cell culture
HL-1 is cardiac muscle cell line purchased from Merck Millipore (Merck Millipore, Germany). Cells were maintained in Complete Claycomb Medium (Sigma-Aldrich, MO, USA) supplemented with 10% FBS, 1% penicillin-streptomycin, 100 µM norepinephrine (Sigma-Aldrich, MO, USA), and 4 mM L-glutamine (Gibco, MA, USA) in plates coated with fibronectin to 0.02% gelatin solution with a final concentration of 12.5 µg/ml. (Sigma-Aldrich, MO, USA). Cells were grown in 5% CO2 incubator at 37°C.
miRNA transfection into HL-1 cells
The negative control miRNA (SMC-2001), miRNA inhibitor negative control (SMC-2101), miR-423 mimic (5-AUAAAGGAAGUUAGGCUGAGGGGCAGAGAGCGAGACUUUUCUAUUUUCCAAAAGCUCGGUCUGAGGCCCCUCAGUCUUGCUUCCUAACCCGCGC-3), and miR-423 inhibitor (5-UGAGGGGCAGAGAGCGAGACUUU-3) were purchased from Bioneer Corporation (Daejeon, Korea). HL-1 cells were plated on a 35 mm dish in 1 ml of Claycomb Medium. The transfection of miRNAs into HL-1 cells was performed with RNAiMax Transfection Reagent. The cells were transfected with Lipofectamine according to the manufacturer's protocol (Invitrogen, Grand Island, NY, USA). Transfection efficacy was evaluated by quantitative PCR. Also, we showed that transfection of miR-423 was successful using miR-423 mimic, inhibitor and negative controls using RT-qPCR (Fig. S1).
Western blotting
HL-1 cells were homogenized and lysed with RIPA buffer (Sigma-Aldrich, MO, USA) containing a protease inhibitor cocktail (Sigma-Aldrich, MO, USA). BCA protein assay kit (Millipore, Billerica, MA, USA) was used to measure the protein concentration according to the manufacturer's instructions. Protein samples (20 µg) of were resolved by SDS-PAGE and then transferred to polyvinylidene fluoride membrane (Millipore, Billerica, MA, USA). The membranes were incubated overnight at 4°C with anti-total CaMKIIδ (1:1,000; Santa Cruz Biotechnology), Thr287 and Thr306/Thr307 phosphorylated CaMKIIδ (1:1,000; Abcam Reagents), total phospholamban (PLB) (1:1,000; Santa Cruz Biotechnology), Thr17 phosphorylated PLB (1:1,000; Santa Cruz Biotechnology), SERCA2A (1:1,000; Santa Cruz Biotechnology), total RyR2 (1:1,000; Abcam Reagents), Ser2808 and Ser2814 phosphorylated RyR2 (1:500 and 1:1,000, Badrilla, Leeds, UK) as indicated. The membranes were incubated with HRP-conjugated mouse or rabbit anti-mouse secondary antibodies (Santa Cruz), and the protein bands were visualized by chemiluminescence (Millipore, Billerica, MA, USA).
Quantitative polymerase chain reaction
Total RNA was extracted from cells using the QIAGEN miRNeasy Mini kit (QIAGEN, CA, USA) according to the manufacturer's instructions. Cell-derived total RNA was reverse transcribed using a miRNA-specific stem-loop real-time reaction with transcription primers. The concentration of total RNA was quantified using an UV–Vis spectrophotometer (Nabi, MicroDigital Co., Ltd., Korea) via the A260/280 ratio; only pure total RNA samples within a ratio were selected. cDNA was synthesized using the RevertAid™ First Strand cDNA Synthesis Kit (Thermofisher, NZ, USA). Quantitative PCR was performed using the iQ5 system (Bio-Rad, ON, USA) with SYBR® Premix Ex Taq™ II (Agilent Technologies Inc. CA, USA) for quantification. Triplicates were tested for each sample. Gene and miRNA expression levels were normalized to GAPDH and U6 snRNA expression levels, respectively. Primer sequences are listed in Table II.
Table II.
Primer sequence.
| Target gene | Forward primer (3′-5′) | Reverse primer (5′-3′) |
|---|---|---|
| TGF-β | TTGCTTCAGCTCCACAGAGA | TGGTTGTAGAGGGCAAGGAC |
| Collagen I | GAGCGGAGAGTACTGGATCG | GCTTCTTTTCCTTGGGGTTC |
| Collagen III | TGATGGAAAACCAGGACCTC | CAGTCTCCCCATTCTTTCCA |
| Fibronectin | GATGCACCGATTGTCAACAG | TGATCAGCATGGACCACTTC |
| GAPDH | ACCAGGTATCTGCTGGTTG | TAACCATGATGTCAGCGTGGT |
| hsa-miR-3613-5p | UGUUGUACUUUUUUUUUUGUUC | |
| hsa-miR-6763-5p | CUGGGGAGUGGCUGGGGAG | |
| hsa-miR-423-5p | UGAGGGGCAGAGAGCGAGACUUU | |
| hsa-miR-1180-5p | GGACCCACCCGGCCGGGAAUA | |
| hsa-miR-6511b-3p | CCUCACCACCCCUUCUGCCUGCA | |
| hsa-miR-3197 | GGAGGCGCAGGCUCGGAAAGGCG | |
| hsa-miR-3162-3p | UCCCUACCCCUCCACUCCCCA | |
| has-u6 | CGCAAGGATGACACGCAAATTC | |
| Universal reverse | GTGCAGGGTCCGAGGT |
miR, microRNA.
Immunofluorescence staining
For immunofluorescence staining, cells from the control and experimental groups were grown on 4-chamber slides and cells were fixed with freshly prepared 4% ice-cold formaldehyde (pH 7.2-7.3) at room temperature. The cells were washed and blocked with 5% bovine serum albumin (BSA) and 0.3% Triton X-100 in PBS for 30 min. After serial washing and blocking, cells were incubated with primary antibody against CaMKII and phosphorylated CaMKII primary antibodies overnight at 4°C to stain cardiomyocyte, followed by Alexa Fluor 488 and Alexa 555 secondary antibodies incubation for an additional 30 min at room temperature. Finally, the cells were counterstained with 4′,6-diamidino-2-phenylindole (DAPI, 1:1,000) during the final wash. Fluorescence images were captured using a confocal microscope (LSM800, Zeiss, Germany).
Dynamic Ca2+ imaging
Images were obtained using a confocal microscope (LSM700; Carl Zeiss, Germany). HL-1 cells were washed three times with a live cell imaging solution (140 mM NaCl, 2.5 mM KCl, 1.8 mM CaCl2, 1 mM MgCl2, 20 mM HEPES-Na, 5.6 mM glucose, pH 7.4) and loaded with 5 µM fluo-4 AM for 20 min at 37°C. Coverslips were mounted in 1 ml capacity chambers and placed in the microscope for fluorescence measurements after excitation with a 488 nm wavelength argon laser beam or filter system. Fluo-4 was excited by a 488 nm line of an Argon laser, and emission signals over 505 nm were collected. Line-scan images are acquired along the longitudinal axis of the cells. Each line was composed of 512 pixels spaced at 0.14 µm intervals. After sequential scanning, a two-dimensional image of 512×10,000 lines or 512×20,000 lines was generated and stored for offline analysis. Image sequences were analyzed using the NIH open-access software ImageJ. Intracellular Ca2+ levels are expressed as the percentage of fluorescence intensity relative to the basal fluorescence intensity.
miRNA microarray screening
Microarray analysis was performed with a GeneChip miRNA 4.0 array containing a set of approximately 2500 human mature miRNA probes annotated in the miRBase20 database. In the case of miRNA expression experiment, 100 ng of total RNA sample was used and biotin-labeling was performed using the poly tail of total RNA. After loading the biotin-labeled sample onto the GeneChip miRNA 4.0 array, hybridization was performed using the GeneChip hybridization system. Image processing was performed using an Affymetrix Gene Array 3000 scanner, and data analysis was performed using GeneSpring GX 14.9.1 software. Quality control and microarray analysis of small RNA experiments were performed at Biocore (Seoul, Korea). A fold change cutoff of 1.5 and a statistical cutoff of P<0.05 were applied to find differentially expressed miRNAs. After that, validation experiments were analyzed using RT-qPCR. Additionally, bioinformatics analysis was performed to predict the target of miRNA-423. We used Targetscan (http://www.targetscan.org/) and miRDB (http://www.mirdb.org) websites.
Statistical analysis
All quantitative data are expressed as the mean ± SEM. The normally distributed values are compared using an unpaired Student's t-test between the two groups. Also, a one-way analysis of variance (ANOVA) with a post hoc Tukey's test or Bonferroni test was used to compare the differences among groups when appropriate. Statistical analyses were performed using SPSS version 23.0 (SPSS, Inc., IL, USA) and GraphPad Prism 5 program (GraphPad Software, Inc., CA, USA). A P value of <0·05 was considered statistically significant.
Results
Quantitative gene expression analysis of urine samples from the AF and PSVT groups
We analyzed fibrosis-related gene expression using RNA samples isolated from AF patients' urine and HL-1 cells. In the urine, the expression of fibrosis-related genes (collagen I/III, fibronectin 1, and TGF-β) tended to be higher in the AF group than in the PSVT group (Fig. 1A). Furthermore, fibrosis-related gene expression was examined in Ang II (1 µM)-treated cells. the expression of collagen I (Col I), collagen III (Col III), fibronectin 1 (Fn 1), and Transforming growth factor-β (TGF-β) was significantly increased in the Ang II group compared with the control group (Fig. 1B).
Figure 1.
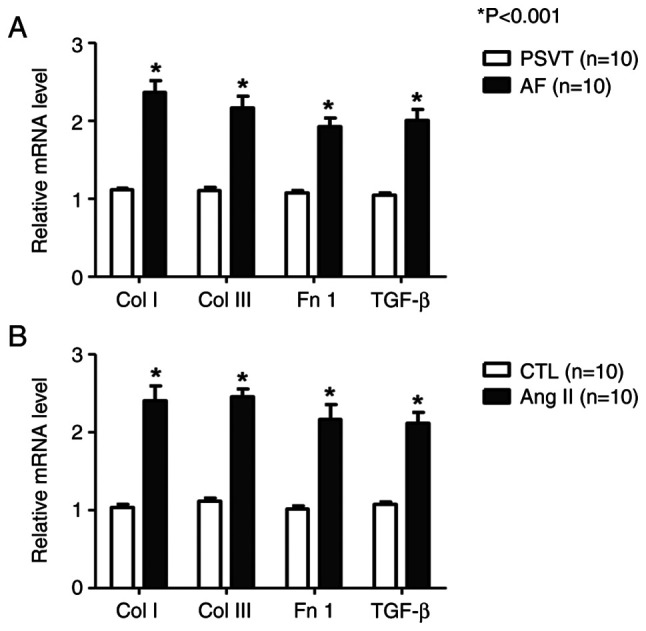
Examination of the expression levels of fibrotic markers by quantitative PCR. (A and B) Quantitative PCR showing the expression levels of Col I, Col III, Fn 1 and TGF-β mRNA compared with GAPDH mRNA. (A) Human urine. (B) HL-1 cells. *P<0.001, vs. PSVT and CTL. n=10. AF, atrial fibrillation; Ang II, angiotensin II; Col I, collagen type 1; Col III, collagen type III; CTL, Control; Fn 1, fibronectin 1; PSVT, paroxysmal supraventricular tachycardia; TGF-β, transforming growth factor-β1.
miRNA microarray screening
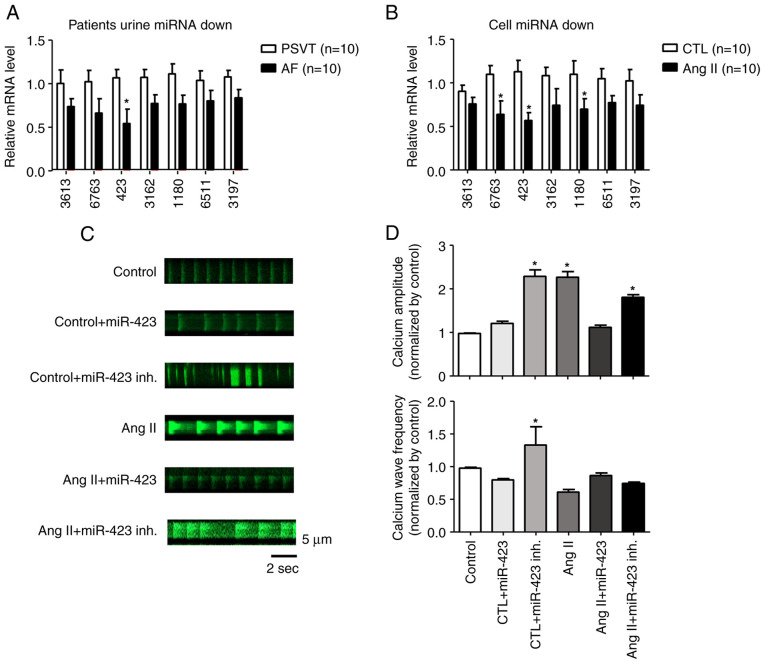
We performed miRNA microarray on two populations of cells using the Sanger miRBase Version 20 miRNA expression microarray (LC Sciences, Houston, TX, USA). We analyzed unique mature miRNAs across biological duplicates of each cell type and found that 7 miRNAs were significantly downregulated in the AF group compared with the PSVT group. In particular, miR-423 was downregulated by approximately 2-fold in live cells (Fig. S2A, B and C). To confirm the results from miRNA microarray, we selected miR-3613, miR-6763, miR-423, miR-3162, miR-1180, miR-6511, and miR-3197 for quantitative PCR analysis. These miRNAs were significantly downregulated in Ang II-treated HL-1 cells (Fig. 2A and B).
Figure 2.
miRNA microarray screening. Quantitative PCR of 7 miRNAs in (A) patients and (B) cells. The expression levels of miR-423 in patients with AF and the AF cell model were significantly lower than those in the PSVT and control cell groups, respectively. (C) Representative line-scan of time-lapse calcium imaging of cells loaded with the intracellular calcium indicator (Fluo-4 AM). Representative records of spontaneous Ca2+ transients according to the group. (D) Summary of the Ca2+ transient amplitude and frequency. The amplitude was significantly increased in control + miR-423 inhibitor, Ang II and Ang II + miR-423 inhibitor groups. *P<0.001 vs. control group n=5. AF, atrial fibrillation; Ang II, angiotensin II; CTL, Control; inh., inhibitor; miRNA/miR, microRNA; PSVT, paroxysmal supraventricular tachycardia; Fluo-4 AM, 4-(6-Acetoxymethoxy-2,7-difluoro-3-oxo-9-xanthenyl)-4-methyl-2,2-(ethylenedioxy)dianiline-N,N,N,N-tetraacetic acid tetrakis(acetoxymethyl) ester.
Fig. 2C shows representative line-scan images of spontaneous Ca2+ transients obtained from HL-1 cells of the control, control + miR-423, control + miR 423 inhibitor, Ang II, Ang II + miR423, and Ang II + miR423 inhibitor groups. Ca2+ amplitude and wave frequency were increased in the control + miR-423 inhibitor (P<0.001, 1.0±0.0 F/F0 to 2.2±0.2 F/F0), Ang II (P<0.001, 1.0±0.0 F/F0 to 2.1±0.1 F/F0), and Ang II + miR-423 inhibitor (P<0.001, 1.0±0.0 F/F0 to 1.8±0.1 F/F0) groups compared with the control group. In contrast, Ca2+ wave frequency and amplitude were restored in cells simultaneously treated with Ang II + miR-423 (1.0±0.0 F/F0 to 1.1±0.1 F/F0) (Fig. 2D). In this study, we found that miR-423 reduced Ca2+ handling proteins in AF with the suppression of excessive CaMKII activity and subsequent restoration of Ca2+ homeostasis-related proteins.
Inhibition of CaMKII activation and reduction in dysregulated Ca2+ handling proteins in Ang II-induced cells following miR-423 administration
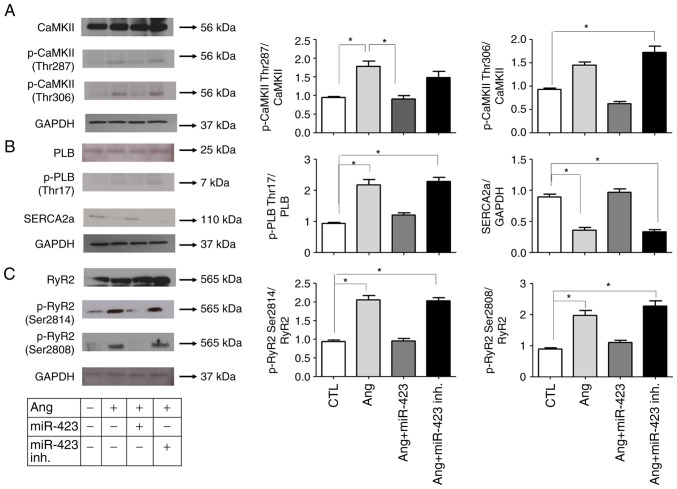
To determine whether CaMKII was activated in Ang II-induced cells, we analyzed the protein levels of phosphorylated-CaMKII (p-CaMKII, at T287 and T306). As shown in Fig. 3A, both p-CaMKII and total CaMKII were significantly increased in Ang II-induced cells but normalized in the Ang II + miR-423 group, suggesting that CaMKII was activated under AF conditions. We also evaluated the CaMKII target phospholamban (PLB). The phosphorylation of PLB by CaMKII at Thr17 will relieve its inhibition of SERCA2 and consequently increase SERCA2 Ca2+ uptake activity (Fig. 3B). Ang II-induced cells exhibited increased protein levels of phosphorylated PLB (p-PLB, at Thr17). However, the dysregulated Ca2+ handling proteins were markedly reduced by miR-423 treatment.
Figure 3.
miR-423 inhibition of CaMKII, PLB and RyR2 activities in Ang II-induced HL-1 cells. (A-C) (left) Western blot analysis of (A) CaMKII (Thr 287, Thr 306), (B) PLB (Thr17) and (C) RyR2 (Ser 2814, Ser 2808). (right) Protein semi-quantification showing an increase in p-CaMKII, p-PLB and p-RyR2 in Ang II-treated HL-1 cells and the miR-423 inhibitor-treated group. However, the phosphorylation of these proteins was decreased in the group treated with miR-423. n=5-6 per group. *P<0.001. Data are presented as the mean ± SEM. Ang II, angiotensin II; CaMKII, calmodulin-dependent protein kinase II; CTL, Control; inh., inhibitor; miR, microRNA; p-, phosphorylated; PLB, phospholamban; RyR2, ryanodine receptor 2; SERCA2a, sarcoplasmic reticulum calcium ATPase.
The alteration of CaMKII expression and activation status could mediate CaMKII-dependent phosphorylation in the ryanodine receptor 2 gene (RyR2), which encodes a cardiac sarcoplasmic reticulum Ca2+ release channel, and phosphorylated RyR2 can increase the probability of opening. Therefore, we examined the activation state of RyR2. RyR2 contains several phosphorylation sites, including Ser2814 (phosphorylated by CaMKII) and Ser2808 (which could be phosphorylated by both CaMKII and cAMP-dependent protein kinase A). We performed western blotting with two phospho-specific antibodies (for RyR2-S2814 and RyR2-S2808). In cells stimulated by Ang II, the phosphorylation of RyR2 was increased at both Ser2814 and Ser2808, suggesting the Ca2+ release properties of RyR2. However, these changes were significantly attenuated by miR-423 treatment (Fig. 3C).
Effects of CaMKII activity in Ang II-induced HL-1 cells
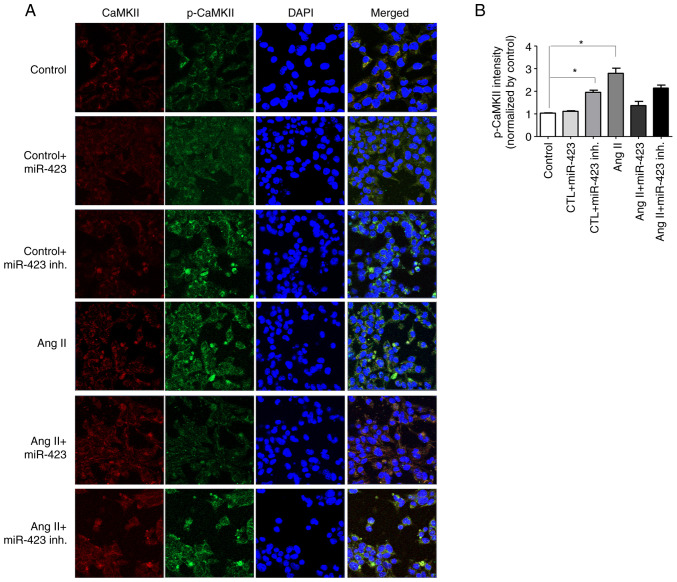
It is well known that the upregulation of the pCaMKII-T286/CaMKII ratio is closely associated with AF. Fig. 4A shows the confocal microscope images of HL-1 cells using immunofluorescence stain method for CaMKII and p-CaMKII. Under Ang II treatment, both CaMKII and p-CaMKII were increased (3.2-fold); however, Ang II + miR-423 effectively prevented the increase in p-CaMKII (1.3-fold, P<0.001 vs. Ang II group). No effect was observed on CaMKII and p-CaMKII in the control group (Fig. 4B).
Figure 4.
Representative immunofluorescence confocal microscopy images. (A) miR-423 activates CaMKII phosphorylation in the control + miR-423 inhibitor group, Ang II group and Ang II + miR-423 inhibitor group but not the Ang II + miR-423 group. Total CaMKII (red fluorescence), p-CaMKII (green fluorescence) and DAPI (blue fluorescence). Magnification, ×400. (B) Quantitative analysis of p-CaMKII intensity. N=5 per group. *P<0.001. Data are presented as the mean ± SEM. Ang II, angiotensin II; CaMKII, calmodulin-dependent protein kinase II; CTL, control; inh., inhibitor; miR, microRNA; p-CaMKII, phosphorylated CaMKII.
Discussion
Importantly, human studies have shown that soluble miRNAs can be isolated from a minimal amount of urine supernatant and still be present at detectable levels. Therefore, the use of miRNAs as biomarkers for heart disease is a feasible and attractive option. Unlike proteins or mRNAs, miRNAs are highly stable and resistant to degradation in clinical urine samples. Studies have demonstrated the stability of circulating miRNAs. Most commonly, plasma-derived miRNAs can be used as indicators of cardiovascular disease (13). However, in the case of paroxysmal AF, there is a limit to diagnosis using only electrocardiogram. For this reason, it is useful clinically to diagnose heart disease using excreted urinary miRNA. Excreted urinary miRNAs already fulfill some of the prerequisites for a good biomarker. Nevertheless, further investigation is needed before preclinical and clinical applications.
Atrial conduction disorder is characterized by a decrease in action potential duration and impulse propagation rate. These changes are often induced in the early stages of atrial remodeling (14). Atrial dilatation and fibrosis observed in AF are characterized by structural remodeling, which leads to conduction disturbances. Until recently, these electrophysiological changes provided the basis for a method to treat AF. However, AF promotes the remodeling process of the atria, contributing to the treatment resistance observed in patients with long-term arrhythmias (15). The molecular basis for the electrical remodeling of the atrium is changes in ion channels and exchangers, including NCX1, NKA and L-type Ca2+ channels and RyR receptors (16). For this reason, we treated HL-1 cells with Ang II to create an environment similar to AF. We performed microarray analysis using the urine from 5 patients with AF and 3 patients with PSVT. The goal of this study was to identify a miRNA predictor of AF in the patients' urine. We found miRNAs with different levels of expression in the AF and PSVT groups. Several miRNAs have been proposed as biomarkers for AF; however, none of them are currently available for diagnostic purposes (17). There are limited data on the association between miRNAs and AF. In comparison with the control group, AF patients had lower levels of miR-423. Similarly, in HL-1 cells treated with Ang II, the expression level of miR-423 was found to be significantly lower than the level in the control group.
CaMKII mutations are associated with AF in humans. Previous studies have shown that mice heterozygous for CaMKII deficiency exhibit atrial electrical dysfunction and increased AF induction. n this study, the expression of miR-423 was decreased in the urine of patients with AF compared to patients with PSVT. In addition, the level of Ca2+ handling protein increased in HL-1 cells treated with Ang II, resulting in electrophysiological changes observed in AF. Also, cells with decreased miR-423 expression exhibited abnormal Ca2+ signaling. Therefore, it was hypothesized that miR-423 downregulation may cause AF. However, the mechanism underlying the aberrant expression of miR-423 in this experiment were not clear.
In the present study, various miRNAs (e.g., miR-423) associated with electrophysiological processes and the expression of Ca2+ handling proteins were performed. The results proved that the levels of CaMKII, PLB, and RyR2 were increased in Ang II-induced HL-1 cells compared with control, and miR-423 treatment reversed this effect. miR-423 was demonstrated in previous studies was to induce coronary artery disease, it was associated with cardiac fibrosis (18). This study showed that atrial fibrillation can be controlled by regulation of miR-423 expression. In addition, alterations in intracellular Ca2+ signaling associated with the regulation of miR-423 expression could be regulated by CaMKII. In Ang II treated cells, overexpression of miR-423 decreased Ca2+ signaling, whereas inhibition of miR-423 increased Ca2+ signaling by Ang II. The impairment of Ca2+ signaling observed in this study after transfection with miR-423 was similar to the early electrophysiological changes in AF. Therefore, miR-423 may be involved in the pathogenesis of AF by modulating CaMKII, which disrupted Ca2+ signaling.
Limitations of this study is limited to the ability to determine the clinical value of a diagnostic test capabilities and AF patient's miRNA signatures because of the small number of patient's urine samples for analysis. Second, according to the results of the current study, it is uncertain whether changes in Ca2+ levels mediate the atrial profibrotic effect of Ang II or whether this is a related but not causally related event. In addition, this study was not include the functional analysis on calcium homeostasis or electrophysiological evaluation. Further research on an atrial tachypacing-induced AF model will be useful for defining the causal relationship in atrial structural remodeling.
In summary, changing miR-423 levels in AF to the normal range may be a novel strategy for converting AF to sinus rhythm in the clinical. Considering that this technique is reported to have good cellular safety and miRNA specificity in in vitro, the miRNA approach appears to be reliable. Although miR-423 acted as an important factor in regulating experimental AF in our study, we do not exclude other mechanisms determining AF and our findings in experimental methods may not directly apply to human AF. Nevertheless, our study shows new insights into AF and other aspects of the mechanism of miR-423 in AF.
Supplementary Material
Acknowledgements
Not applicable.
Glossary
Abbreviations
- AF
atrial fibrillation
- Ang II
angiotensin II
- PSVT
paroxysmal supraventricular tachycardia
- Col I
collagen type 1
- Col III
collagen type III
- Fn1
fibronectin 1
- TGF-β
transforming growth factor-β1
- CaMKII
calmodulin-dependent protein kinase II
- PLB
phospholamban
- RyR2
ryanodine receptor 2
Funding Statement
The present study was supported by research grants from the Basic Science Research Program through the National Research Foundation of Korea (NRF-2017R1E1A1A01078382, 2019R1C1C1003389). This research was supported by the Korea Medical Device Development Fund grant funded by the Korean government (Ministry of Science and ICT, Ministry of Trade, Industry and Energy, Ministry of Health & Welfare, Ministry of Food and Drug Safety) (Project Number: 9991006899). This research was also supported by the Young Medical Scientist Research Grant through the Daewoong Foundation (DY20102P). HP was supported by the RP-Grant 2020 of Ewha Womans University.
Availability of data and materials
The datasets analyzed during the current study are available in the GSE190898 repository (https://www.ncbi.nlm.nih.gov/geo/query/acc.cgi?acc=GSE190898). The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Authors' contributions
JP and HyewP confirm the authenticity of all the raw data. JP and HyewP conceived and designed the study. HyewP and HyelP participated in the experimental design. JP, HyewP and HyelP analyzed the data. All authors have read and approved the final manuscript.
Ethics approval and consent to participate
This study was approved by the IRB of Ewha Womans University Mokdong Hospital (approval no. EUMC 2019-10-019). Written informed consent was obtained from all patients before they were enrolled.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
References
- 1.Benjamin EJ, Wolf PA, D'Agostino RB, Silbershatz H, Kannel WB, Levy D. Impact of atrial fibrillation on the risk of death: The Framingham Heart Study. Circulation. 1998;98:946–952. doi: 10.1161/01.CIR.98.10.946. [DOI] [PubMed] [Google Scholar]
- 2.Everett TH, IV, Olgin JE. Atrial fibrosis and the mechanisms of atrial fibrillation. Heart Rhythm. 2007;4((Suppl 3)):S24–S27. doi: 10.1016/j.hrthm.2006.12.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Burstein B, Nattel S. Atrial fibrosis: Mechanisms and clinical relevance in atrial fibrillation. J Am Coll Cardiol. 2008;51:802–809. doi: 10.1016/j.jacc.2007.09.064. [DOI] [PubMed] [Google Scholar]
- 4.Li D, Fareh S, Leung TK, Nattel S. Promotion of atrial fibrillation by heart failure in dogs: Atrial remodeling of a different sort. Circulation. 1999;100:87–95. doi: 10.1161/01.CIR.100.1.87. [DOI] [PubMed] [Google Scholar]
- 5.Aldhoon B, Melenovsky V, Peichl P, Kautzner J. New insights into mechanisms of atrial fibrillation. Physiol Res. 2010;59:1–12. doi: 10.33549/physiolres.931651. [DOI] [PubMed] [Google Scholar]
- 6.Wang HB, Jiang ZB, Li M. Research on the typical miRNA and target genes in squamous cell carcinoma and adenocarcinoma of esophagus cancer with DNA microarray. Pathol Oncol Res. 2014;20:245–252. doi: 10.1007/s12253-013-9688-z. [DOI] [PubMed] [Google Scholar]
- 7.Elia L, Kunderfranco P, Carullo P, Vacchiano M, Farina FM, Hall IF, Mantero S, Panico C, Papait R, Condorelli G, Quintavalle M. UHRF1 epigenetically orchestrates smooth muscle cell plasticity in arterial disease. J Clin Invest. 2018;128:2473–2486. doi: 10.1172/JCI96121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wong LL, Wang J, Liew OW, Richards AM, Chen YT. MicroRNA and heart failure. Int J Mol Sci. 2016;17:502. doi: 10.3390/ijms17040502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.D'Alessandra Y, Devanna P, Limana F, Straino S, Di Carlo A, Brambilla PG, Rubino M, Carena MC, Spazzafumo L, De Simone M, et al. Circulating microRNAs are new and sensitive biomarkers of myocardial infarction. Eur Heart J. 2010;31:2765–2773. doi: 10.1093/eurheartj/ehq167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Weber JA, Baxter DH, Zhang S, Huang DY, Huang KH, Lee MJ, Galas DJ, Wang K. The microRNA spectrum in 12 body fluids. Clin Chem. 2010;56:1733–1741. doi: 10.1373/clinchem.2010.147405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wang G, Kwan BC, Lai FM, Chow KM, Li PK, Szeto CC. Urinary miR-21, miR-29, and miR-93: Novel biomarkers of fibrosis. Am J Nephrol. 2012;36:412–418. doi: 10.1159/000343452. [DOI] [PubMed] [Google Scholar]
- 12.Cheng L, Quek CY, Sun X, Bellingham SA, Hill AF. The detection of microRNA associated with Alzheimer's disease in biological fluids using next-generation sequencing technologies. Front Genet. 2013;4:150. doi: 10.3389/fgene.2013.00150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Creemers EE, Tijsen AJ, Pinto YM. Circulating microRNAs: Novel biomarkers and extracellular communicators in cardiovascular disease? Circ Res. 2012;110:483–495. doi: 10.1161/CIRCRESAHA.111.247452. [DOI] [PubMed] [Google Scholar]
- 14.Pang H, Ronderos R, Perez-Riera AR, Femenia F, Baranchuk A. Reverse atrial electrical remodeling: A systematic review. Cardiol J. 2011;18:625–631. doi: 10.5603/CJ.2011.0025. [DOI] [PubMed] [Google Scholar]
- 15.Verheule S, Tuyls E, Gharaviri A, Hulsmans S, van Hunnik A, Kuiper M, Serroyen J, Zeemering S, Kuijpers NH, Schotten U. Loss of continuity in the thin epicardial layer because of endomysial fibrosis increases the complexity of atrial fibrillatory conduction. Circ Arrhythm Electrophysiol. 2013;6:202–211. doi: 10.1161/CIRCEP.112.975144. [DOI] [PubMed] [Google Scholar]
- 16.Iwasaki YK, Nishida K, Kato T, Nattel S. Atrial fibrillation pathophysiology: Implications for management. Circulation. 2011;124:2264–2274. doi: 10.1161/CIRCULATIONAHA.111.019893. [DOI] [PubMed] [Google Scholar]
- 17.Komal S, Yin JJ, Wang SH, Huang CZ, Tao HL, Dong JZ, Han SN, Zhang LR. MicroRNAs: Emerging biomarkers for atrial fibrillation. J Cardiol. 2019;74:475–482. doi: 10.1016/j.jjcc.2019.05.018. [DOI] [PubMed] [Google Scholar]
- 18.Nabialek E, Wanha W, Kula D, Jadczyk T, Krajewska M, Kowalowka A, Dworowy S, Hrycek E, Włudarczyk W, Parma Z, et al. Circulating microRNAs (miR-423-5p, miR-208a and miR-1) in acute myocardial infarction and stable coronary heart disease. Minerva Cardioangiol. 2013;61:627–637. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets analyzed during the current study are available in the GSE190898 repository (https://www.ncbi.nlm.nih.gov/geo/query/acc.cgi?acc=GSE190898). The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.