Abstract
The contributions of gonadal hormones to the development of human behavioral sex differences are subjects of intense scientific and social interest. Isolated gonadotropin-releasing-hormone deficiency (IGD) is a rare endocrine disorder that can reveal a possible role of early gonadal hormones. IGD is characterized by low or absent gonadal hormone production after the first trimester of gestation, but external genitalia and hence gender of rearing are concordant with chromosomal and gonadal sex. We investigated recalled childhood gender nonconformity in men (n = 65) and women (n = 32) with IGD and typically developing men (n = 463) and women (n = 1,207). Men with IGD showed elevated childhood gender nonconformity, particularly if they also reported undescended testes at birth, a marker of low perinatal androgens. Women with IGD did not differ from typically developing women. These results indicate that early androgen exposure after the first trimester contributes to male-typical gender-role behaviors in childhood.
Keywords: childhood gender nonconformity, isolated gonadotropin-releasing-hormone deficiency, sex differences, sex hormones, androgens, open data, open materials
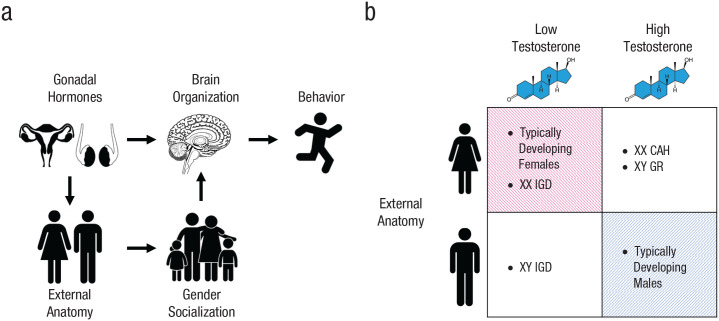
Sex differences in gender-role behaviors emerge early in development (Goldberg & Lewis, 1969; Kung et al., 2018; Todd et al., 2017), persist across adolescence (Kung et al., 2018), and are among the largest observed human sex differences in psychology and behavior (Collaer & Hines, 1995). The developmental factors that shape these phenotypes are the topic of vigorous debate (Eagly & Wood, 2013; Lippa, 2005; Wallen, 1996). Androgens such as testosterone are steroid hormones critical to the development of male characteristics in vertebrates, including behaviors (Swift-Gallant & Monks, 2017; Wallen & Baum, 2002). In nonhuman mammals, androgen administration early in development masculinizes behavior and underlying neural structures by influencing gene expression in the developing brain (X. Xu et al., 2012). By shaping external anatomy, androgens also influence an organism’s interactions with its environment, including its social environment, and socialization as female or male may be particularly germane to human behavioral sexual differentiation (Carter, 2014; Eagly & Wood, 2013; Fig. 1).
Fig. 1.
Some putative causes of sex differences in behavior and possible combinations of external anatomy and perinatal testosterone exposure. Behavioral sex differences may result from direct effects of gonadal hormones on patterns of gene expression in the developing brain (a, top row) and/or effects on external anatomy, which influences interactions with the environment, including gender socialization (a, bottom row). These factors can be partially disentangled by examining individuals whose perinatal testosterone exposure differs from that of typically developing individuals with similar external anatomy (unshaded cells in b). XX IGD = females with isolated gonadotropin-releasing-hormone deficiency; XY IGD = males with isolated gonadotropin-releasing-hormone deficiency; XX CAH = females with congenital adrenal hyperplasia; XY GR = males whose gender was reassigned to female in infancy.
It is difficult to disentangle these two nonmutually exclusive factors to test whether androgens influence sexually differentiated behaviors independently of their effects on external anatomy. An ideal experiment would compare groups randomly assigned to differ in early androgen action but not in external appearance and hence gender of rearing, yet such an experiment would be infeasible and unethical in humans. However, some endocrine conditions or other “experiments of nature” can provide close analogs. Girls who experienced elevated prenatal androgen levels because of congenital adrenal hyperplasia have been found to exhibit more male-typical play preferences and behaviors compared with unaffected girls (Berenbaum et al., 2018; Berenbaum & Hines, 1992; Hines et al., 2004; Meyer-Bahlburg et al., 2004; Pasterski et al., 2005). Similarly, natal boys whose gender was reassigned to female in infancy because of cloacal exstrophy or penile ablation during circumcision have also been found to exhibit behavioral masculinization in comparison with typically developing girls (Diamond & Sigmundson, 1997; Reiner & Gearhart, 2004). If androgens influence behavior by shaping brain development directly, then it should also be the case that, among individuals raised as boys, those exposed to reduced androgens during their early development should exhibit less masculine behavior. However, this complementary evidence is critically missing.
Statement of Relevance.
Childhood gender-role behaviors are among the largest human behavioral sex differences. Experiments in laboratory animals show that hormones produced by the testes contribute to sex differences in behavior, but such experiments in humans would be infeasible and unethical. We measured recalled childhood gender-role behavior in typically developing individuals and in individuals with isolated gonadotropin-releasing-hormone deficiency (IGD). People with IGD are raised as their chromosomal and gonadal sex but do not produce gonadal hormones after the first trimester of gestation. Men with IGD reported higher recalled childhood gender nonconformity, particularly if they possessed a biomarker of low testicular hormone levels during gestation. Women with and without IGD did not reliably differ. These results indicate that early exposure to testicular hormones contributes to sexually differentiated behaviors in humans.
Isolated gonadotropin-releasing-hormone deficiency (IGD) is a rare endocrine disorder affecting approximately 1 in 130,000 live births (Boehm et al., 2015; Laitinen et al., 2011) that can provide this evidence. In typically developing fetuses, gonadal hormone production begins roughly 8 weeks into gestation and remains elevated until just prior to birth, rising again during minipuberty, the period a few months after birth when gonadal hormones reach near-adult levels (Hakim et al., 2017; Lanciotti et al., 2018; Pardue & Wizemann, 2001; Siiteri & Wilson, 1974; Fig. 2). During the first trimester of gestation, human chorionic gonadotropin (hCG) produced by the placenta (Braunstein & Hershman, 1976) binds to luteinizing hormone (LH) receptors in the fetal gonads to stimulate steroid hormone production (Choi & Smitz, 2014; Seminara et al., 1998). As placental hCG levels wane, gonadotropin-releasing hormone from the hypothalamus takes over, inducing the pituitary gland to secrete LH and follicle-stimulating hormone (FSH), which in turn signal the gonads to produce sex steroids (Witchel & Plant, 2013).
Fig. 2.
Approximate human chorionic gonadotropin (hCG) and gonadal sex-steroid production in males (upper graph) and females (lower graph). In typical males, androgen production begins at roughly the 8th week of gestation with the differentiation of the bipotential gonads into testes (Negri-Cesi et al., 2004; Pardue & Wizemann, 2001; Siiteri & Wilson, 1974) and persists until the 24th week (Forest et al., 1973). In typical females, estrogen levels progressively increase across gestation, peaking perinatally, although ovarian activity transiently ceases in the immediate postpartum period between birth and the onset of minipuberty (Lanciotti et al., 2018). In individuals with isolated gonadotropin-releasing-hormone deficiency (IGD), gonadal hormone production declines as circulating hCG levels wane. Figure adapted from Lanciotti et al. (2018). FSH = follicle-stimulating hormone; LH = luteinizing hormone.
By contrast, IGD is characterized by the congenital absence or lack of function of a network of neurons in the hypothalamus that secrete gonadotropin-releasing hormone (Crowley & Pitteloud, 2017; Han & Bouloux, 2012). Gonadal hormone production therefore ceases after the first trimester because of gonadotropin-releasing-hormone deficiency. Individuals with IGD also do not experience minipuberty (Lanciotti et al., 2018), and hormone replacement therapy is required to initiate puberty, although adrenal androgen production is unaffected (Han & Bouloux, 2012). Because gonadal hormones decline in IGD after the first trimester when external genitalia have already sexually differentiated, external genitalia are unambiguous and concordant with chromosomal and gonadal sex (Balasubramanian & Crowley, 2011). An estimated 20% to 40% of men diagnosed later in life with IGD were diagnosed at birth with cryptorchidism (undescended testes) or microphallus (Crowley & Pitteloud, 2017), consistent with low androgen exposure (Foresta et al., 2008; Welsh et al., 2008). Other developmental abnormalities such as anosmia and cleft lip/cleft palate may be present (Balasubramanian & Crowley, 2011; see Section S1 at https://osf.io/m8hn9/). Nevertheless, individuals with IGD are raised as their chromosomal and gonadal sex, almost always without their condition being known until secondary sex characteristics fail to develop spontaneously at puberty (Balasubramanian & Crowley, 2011). IGD thus represents a rare opportunity to help disentangle the direct influence of early gonadal hormones on the central nervous system and behavior from their indirect effects via external anatomy and gender socialization (Fig. 1b). Here, we used IGD to understand the effects of chronically low perinatal gonadal hormone action on sexually differentiated childhood play and gender-role behavior.
Method
Participants
Participants with IGD were recruited in two ways. A first group was referred to the study by the Reproductive Endocrine Unit at Massachusetts General Hospital or the Reproductive Physiology and Pathophysiology Group at the National Institute of Child Health and Human Development (henceforth referred to as the IGD-clinical group; n = 44). Diagnosis for all IGD-clinical participants was confirmed using the following criteria: clinical evidence of absent or incomplete puberty by age 18 years, hypogonadal sex-steroid levels (testosterone < 100 ng/dL in males, estradiol < 20 pg/ml in females), low LH and FSH, and no functional etiology or hypothalamic or pituitary lesions on an MRI. A second group was recruited through posts on Web-based IGD support groups and forums (henceforth, the IGD-Web group; n = 53). Diagnosis was not confirmed with a physician for this group.
Typically developing participants (henceforth, control participants) were recruited in two separate studies on hormones and behavior, combined to take advantage of all available data. A first set of control men (n = 233) and women (n = 395) participated in a study of siblings recruited through emails and letters sent to everyone at Michigan State University who shared the same last name and permanent address of at least one other person at the university (see also Doll et al., 2016; Puts et al., 2010, 2013). A second set of control men (n = 230) and women (n = 812) was recruited via advertisements on social media (e.g., Facebook) and local newspaper and radio in central Pennsylvania, as well as from The Pennsylvania State University Department of Psychology research participant pool (see also Shirazi et al., 2019, 2020).
All participants were 18 years or older, permanent residents of the United States, and fluent in English. All procedures were approved by the institutional review board, and participants provided informed consent.
General procedure
Control participants completed the study at private computer workstations in the laboratory. Participants with IGD participated remotely and were instructed to complete all questionnaires in one sitting. Control participants were compensated with either course credit or money, and participants with IGD received monetary compensation.
Questionnaires
The childhood gender nonconformity questionnaire (CGNQ; Freund & Langevin, 1977) measures recalled childhood behaviors, attitudes, and desires that have been found to differ between boys and girls. The CGNQ includes 24 questions representing several behavioral and psychological categories, including peer preferences, toy preferences, dress-up play, fantasy play, and career aspirations (Zucker et al., 2006). On the basis of categorizations of each response as either male typical or female typical, we coded and scored responses to each question as gender conforming (score of −1), gender nonconforming (score of +1), or gender neutral (score of 0). Responses were then scaled within sexes and averaged to create childhood gender-nonconformity scores; higher scores indicate higher recalled childhood gender nonconformity. Cronbach’s α was .78 in both men and women. For further validation, we tested for sex differences on the subset of items (13 of 24) that were scored symmetrically and worded identically for men and women, and we also created a second, separate composite CGNQ masculinity-femininity score using this subset of items, multiplying women’s scores by −1 to yield a measure of childhood gender masculinity-femininity that could be compared between sexes. Significant sex differences were found in the expected directions for all individual items (see Table S1 at https://osf.io/m8hn9/), as well as for CGNQ masculinity-femininity scores (Cohen’s d = 3.00; see Fig. S1 at https://osf.io/m8hn9/). Comparisons of CGNQ masculinity-femininity scores across all groups are reported in Section S2 and Table S2 at https://osf.io/m8hn9/.
We administered a shortened version of the Klein Sexual Orientation Questionnaire (Klein et al., 1985) to control participants recruited from The Pennsylvania State University and participants with IGD. Responses to questions about sexual feelings and activity over the past year were averaged to create a sexual-orientation score ranging from 0 (exclusively heterosexual) to 6 (exclusively homosexual). We administered a sexual-orientation questionnaire based on the Kinsey scale (Kinsey et al., 1948) to control participants recruited at Michigan State University. Responses to items on sexual attraction and sexual fantasies were averaged to create a sexual-orientation score, again ranging from 0 to 6.
Data on cryptorchidism and microphallus at birth were available from patient files for a subset of IGD-clinical men. For 17 IGD-clinical participants, data were available for cryptorchidism; eight indicated its presence at birth, and nine indicated its absence. Data for microphallus were available for 14 men; four reported microphallus at birth, and 10 reported not presenting with microphallus at birth (Table 1). IGD-clinical men for whom these data were unavailable were not included in analyses relating these phenotypes to childhood gender nonconformity.
Table 1.
Demographic Statistics for Men and Women in the Sample
| Variable | Men | Women | ||||
|---|---|---|---|---|---|---|
| Control (n = 463) |
IGD-clinical (n = 30) |
IGD-Web (n = 35) |
Control (n = 1,207) |
IGD-clinical (n = 14) |
IGD-Web (n = 18) |
|
| Mean age (years) | 20.71 (4.45) | 41.20 (15.85) | 37.46 (13.90) | 20.18 (3.98) | 34.29 (9.41) | 31.39 (6.22) |
| Race/ethnicity | ||||||
| White, non-Hispanic | 80% | 83% | 69% | 77% | 71% | 78% |
| Asian, non-Hispanic | 9.7% | 10% | 8.6% | 10% | 7.1% | 0% |
| Black, non-Hispanic | 4.1% | 0% | 0% | 5% | 0% | 0% |
| Hispanic | 4.5% | 3.3% | 17% | 5.9% | 7.1% | 22% |
| Other | 1.3% | 3.3% | 5.7% | 2.1% | 14% | 0% |
| Mean childhood gender nonconformity | –0.04 (0.33) | 0.24 (0.44) | 0.34 (0.59) | –0.00 (0.37) | 0.02 (0.55) | 0.35 (0.67) |
| Mean sexual orientation | 0.25 (0.94) | 0.31 (0.71) | 0.94 (1.48) | 0.37 (0.79) | 0.36 (0.37) | 1.37 (1.68) |
| Microphallus at birth | 29% | |||||
| Cryptorchidism at birth | 47% | |||||
Note: Standard deviations are given in parentheses. Participants with isolated gonadotropin-releasing-hormone deficiency (IGD) were recruited either from clinical settings or via the Internet.
Data analysis
Analyses for men and women were conducted separately. As sibling pairs were recruited in the Michigan State University sample, we ran linear regressions as multilevel models with individuals nested within sibling units. Current age and sexual orientation were entered as covariates. We included sexual orientation as a covariate because it is reliably associated with childhood gender-role behavior (Drummond et al., 2008; Singh et al., 2021), and we were interested in exploring relationships with childhood gender-role behavior independently of sexual orientation. Regressions simultaneously evaluated the effect of diagnosis (IGD vs. control) and IGD group (IGD-clinical vs. IGD-Web) using orthogonal contrast coding, with one contrast term per comparison. All regression estimates are reported as standardized.
Results
Sample characteristics
Sample demographics are displayed in Table 1.
Men
Men with IGD had higher CGNQ scores than control men (β = 0.24, 95% confidence interval [CI] = [0.10, 0.37], p < .001), and scores did not differ between IGD-clinical and IGD-Web participants (β = −0.001, 95% CI = [–0.11, 0.11], p = .977; Fig. 3a). A post hoc test comparing CGNQ scores in only IGD-clinical participants (for whom we had confirmation of diagnosis) and control men revealed higher CGNQ scores in IGD-clinical men (β = 0.17, 95% CI = [0.06, 0.28], p = .003).
Fig. 3.
Mean childhood gender-nonconformity scores in men. Comparisons are shown separately for (a) the two groups who had isolated gonadotropin-releasing-hormone deficiency (IGD) and the control group, (b) the control group and individuals born with and without cryptorchidism (cryp+ and cryp–, respectively), and (c) the control group and individuals born with and without microphallus (micro+ and micro–, respectively). Larger symbols with white shading and associated error bars show group means and 95% confidence intervals, respectively. Smaller symbols show individual scores, and the width of the shaded areas indicates the density of the data. Vertical and horizontal jitter were added to individual points to aid in data visualization. Significance values are shown in (a) between the control group and the two IGD groups together and between the two IGD groups only and in (b) and (c) for both linear and quadratic effects.
To probe the robustness of our findings, we conducted several additional sets of analyses. First, we conducted analyses in which we initially tested for differences between IGD groups, and if differences were not significant, we combined IGD groups and tested for differences between the combined IGD group and the control group (see Section S3 at https://osf.io/m8hn9/). Second, we restricted our main analyses to participants older than 23 years to eliminate probable university students and thus minimize demographic differences observed between control and IGD participants (see Section S4 at https://osf.io/m8hn9/). Third, we analyzed the structure of the CGNQ by performing a principal components analysis and repeated our main analyses, replacing principal components scores as the dependent variable (see Section S5 at https://osf.io/m8hn9/). Fourth, we repeated the analyses presented above but removing sexual orientation as a covariate (see Section S6 at https://osf.io/m8hn9/). Results of these robustness checks were consistent with results of our main analyses.
We next compared men in the IGD-clinical group with and without cryptorchidism at birth with control participants to further test a connection with low gonadal hormones. We created two contrast terms indexing linear and quadratic terms of prenatal androgen action on the basis of diagnosis and whether cryptorchidism was present at birth; the lowest levels were in participants with both IGD and cryptorchidism, higher levels were in participants with IGD but without cryptorchidism, and the highest levels were in control participants. In cases in which linear or quadratic effects were significant, we performed post hoc tests to further clarify our pattern of results. There was a significant linear effect of level of presumed perinatal androgen action (β = 0.13, 95% CI = [0.02, 0.24], p = .019); the quadratic effect was not significant (β = −0.03, 95% CI = [–0.13, 0.07], p = .534). In follow-up regressions with group analyzed as a categorical variable, IGD-clinical men with cryptorchidism at birth (β = 0.12, 95% CI = [0.02, 0.22], p = .019) exhibited higher CGNQ scores than control men (Fig. 3b); although IGD-clinical men without cryptorchidism at birth also had higher CGQ scores than control men, this difference was not statistically significant (β = 0.03, 95% CI = [–0.06, 0.13], p = .489). Scores did not differ significantly between IGD-clinical men with and without cryptorchidism (β = 0.09, 95% CI = [–0.03, 0.20], p = .130).
We repeated this analysis for microphallus present at birth, finding no significant linear or quadratic effects (Fig. 3c).
Women
Women with IGD had higher CGNQ scores than control women (β = 0.14, 95% CI = [0.06, 0.23], p < .001), but CGNQ scores were lower in IGD-clinical than in IGD-Web women (β = −0.11, 95% CI = [–0.19, –0.02], p = .001; Fig. 4). A post hoc test comparing CGNQ scores in only IGD-clinical and control women revealed no difference (β = −0.01, 95% CI = [–0.07, 0.05], p = .673).
Fig. 4.

Mean childhood gender-nonconformity scores in women, separately for the two groups who had isolated gonadotropin-releasing-hormone deficiency (IGD) and the control group. Larger symbols with white shading and associated error bars show group means and 95% confidence intervals, respectively. Smaller symbols show individual scores, and the width of the shaded areas indicates the density of the data. Vertical and horizontal jitter were added to individual points to aid in data visualization. Significance values are shown between the control group and the two IGD groups together and between the two IGD groups only.
Discussion
We examined the effects of congenitally low gonadal steroids from the second trimester of gestation through minipuberty on recalled childhood gender nonconformity by comparing typically developing men and women with those who have IGD. We found less masculine recalled sex-typed childhood behaviors in men with IGD compared with unaffected men. This effect was robust to any differences in ascertainment bias between clinically recruited and Web-recruited IGD groups, consistent across all robustness analyses, and most pronounced in IGD men with cryptorchidism at birth. These findings suggest that androgen action between the second trimester and neonatal period is important in the development of male-typical gender-role behaviors in childhood.
Our data do not suggest that social learning lacks a role in shaping child gender-role behaviors (Blakemore et al., 2008; Lindsey & Mize, 2001; Lytton & Romney, 1991), but they offer a unique convergence of evidence regarding a role of early androgen exposure. Experimental hormonal manipulations demonstrate that early androgen action masculinizes the brain and behavior in nonhuman mammals (Thornton et al., 2009; X. Xu et al., 2012). Prior studies in humans have shown greater behavioral masculinity in individuals raised as girls who experienced elevated perinatal androgens because of congenital adrenal hyperplasia (Berenbaum et al., 2018; Berenbaum & Hines, 1992; Hines et al., 2004; Meyer-Bahlburg et al., 2004; Pasterski et al., 2005) or having male-typical prenatal endocrine profiles but male-to-female sex reassignment in infancy (Diamond & Sigmundson, 1997; Reiner & Gearhart, 2004). The present findings provide evidence that low or absent early androgen action leads to more female-typical behavioral patterns in humans. However, it is also important to note that men with IGD were more similar in recalled childhood gender-role behaviors to control men than they were to control women (see Table S2 and Fig. S1). This finding may reflect influences of testosterone in the first trimester, sex chromosome complement (Arnold & McCarthy, 2016), and/or gender socialization (Carter, 2014).
Women for whom we had a physician-confirmed IGD diagnosis did not differ from control women in recalled childhood gender nonconformity, suggesting that sex-typical childhood gender-role behavior can develop in human females largely independently of ovarian hormone production after the first trimester. These results also provide further evidence that androgen action at or below female-typical levels—for example, because of complete androgen insensitivity syndrome in XY individuals (Hughes et al., 2012) or IGD in those with XX karyotype—leads to female-typical childhood gender-role behaviors. Some studies have found less female-typical behavior in women with Turner syndrome, in whom gonadal hormone production is also chronically low (Collaer et al., 2002). However, women with Turner syndrome experience a later decline in ovarian hormones and possess a single functioning X chromosome rather than two X chromosomes as women with IGD do. Behavioral differences between women with Turner syndrome and those with IGD may thus reflect these other endocrine and chromosomal differences (Arnold & McCarthy, 2016; Davies & Wilkinson, 2006).
Limitations
IGD is present in fewer than 1 in 130,000 live births (Boehm et al., 2015; Laitinen et al., 2011), and hence, recruiting samples with sufficient power presents a significant challenge. Although our sample sizes provided 80% power to detect small to medium effects (Cohen’s d of 0.4 and 0.5 in men and women, respectively), it is possible that we were unable to detect some small differences between IGD and control individuals.
Our observational data also cannot demonstrate causal links between early sex-hormone exposure and adult phenotypes. For example, we cannot rule out a role of differential treatment by parents, physicians, or other individuals resulting from developmental anomalies associated with IGD, such as cleft lip, cryptorchidism, and microphallus. However, we know of no research suggesting socially mediated effects of such developmental anomalies on childhood gender-role behaviors. Moreover, there is no sexual ambiguity in the clinical presentation of individuals with IGD (Brioude et al., 2010), and IGD is not usually diagnosed until incomplete or absent pubertal development triggers referral to a physician (Brioude et al., 2010; Crowley & Pitteloud, 2017). IGD can be suspected in cases of cryptorchidism or microphallus, but our finding of no differences in childhood gender nonconformity relative to microphallus presence, along with the relative conspicuousness of this trait, also suggests that differential parental treatment does not play a major role (although this finding may also reflect the limited sample for which these data were available).
It is also possible that demographic factors contributed to differences between individuals with IGD and control individuals. However, age and sexual orientation were statistically controlled, findings were robust when college-age participants were excluded from analyses, and there is no compelling evidence suggesting a causal relationship between childhood socioeconomic status and childhood gender nonconformity (Y. Xu et al., 2019). The finding of an apparent dose–response relationship between perinatal androgens, as indicated by IGN diagnosis with or without cryptorchism, and childhood gender nonconformity also provides evidence for a role of androgen action.
Conclusion
We used IGD as a model to elucidate the effects of chronically low perinatal sex-hormone exposure on the sexual differentiation of human behavior. Our results indicate that low gonadal sex-hormone exposure in mid-to-late gestation and early infancy predicts higher recalled childhood gender nonconformity in men but not women. These results suggest that androgen action is critical to the organization of male-typical play and gender-role behaviors and that lower androgen levels associated with IGD in males, or with androgen production at or below sex-typical levels in females, are associated with more female-typical behaviors.
Transparency
Action Editor: Steven W. Gangestad
Editor: Patricia J. Bauer
Author Contributions
K. Dawood, L. L. M. Welling, R. Cárdenas, R. Balasubramanian, A. Delaney, S. M. Breedlove, and D. A. Puts conceived and designed the study. H. Self, K. Dawood, R. Cárdenas, R. Balasubramanian, A. Delaney, and D. A. Puts acquired the data. T. N. Shirazi, K. A. Rosenfield, J. M. Bailey, S. M. Breedlove, and D. A. Puts analyzed and interpreted the data. T. N. Shirazi and D. A. Puts wrote the initial draft of the manuscript. All the authors revised the manuscript and approved the final version for submission.
Declaration of Conflicting Interests: The author(s) declared that there were no conflicts of interest with respect to the authorship or the publication of this article.
Funding: This work was supported by the National Science Foundation, American Institute of Bisexuality, and National Institute of Mental Health.
Open Practices: All data, analysis scripts, questionnaires, and scoring methods have been made publicly available via OSF and can be accessed at https://osf.io/vtf6d/. The design and analysis plan for the study were not preregistered. This article has received the badges for Open Data and Open Materials. More information about the Open Practices badges can be found at http://www.psychologicalscience.org/publications/badges.


References
- Arnold A. P., McCarthy M. M. (2016). Sexual differentiation of the brain and behavior: A primer. In Pfaff D. W. (Ed.), Neuroscience in the 21st century (pp. 2139–2168). Springer. [Google Scholar]
- Balasubramanian R., Crowley W. F. (2011). Isolated GnRH deficiency: A disease model serving as a unique prism into the systems biology of the GnRH neuronal network. Molecular and Cellular Endocrinology, 346(1), 4–12. 10.1016/j.mce.2011.07.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berenbaum S. A., Beltz A. M., Bryk K., McHale S. (2018). Gendered peer involvement in girls with congenital adrenal hyperplasia: Effects of prenatal androgens, gendered activities, and gender cognitions. Archives of Sexual Behavior, 47(4), 915–929. 10.1007/s10508-017-1112-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berenbaum S. A., Hines M. (1992). Early androgens are related to childhood sex-typed toy preferences. Psychological Science, 3(3), 203–206. [Google Scholar]
- Blakemore J., Berenbaum S. A., Liben L. S. (2008). Gender development. Psychology Press. [Google Scholar]
- Boehm U., Bouloux P.-M., Dattani M. T., de Roux N., Dodé C., Dunkel L., Dwyer A. A., Giacobini P., Hardelin J.-P., Juul A., Maghnie M., Pitteloud N., Prevot V., Raivio T., Tena-Sempere M., Quinton R., Young J. (2015). Expert consensus document: European Consensus Statement on congenital hypogonadotropic hypogonadism-pathogenesis, diagnosis and treatment. Nature Reviews Endocrinology, 11(9), 547–564. 10.1038/nrendo.2015.112 [DOI] [PubMed] [Google Scholar]
- Braunstein G. D., Hershman J. M. (1976). Comparison of serum pituitary thyrotropin and chorionic gonadotropin concentrations throughout pregnancy. Journal of Clinical Endocrinology and Metabolism, 42(6), 1123–1126. 10.1210/jcem-42-6-1123 [DOI] [PubMed] [Google Scholar]
- Brioude F., Bouligand J., Trabado S., Francou B., Salenave S., Kamenicky P., Brailly-Tabard S., Chanson P., Guiochon-Mantel A., Young J. (2010). Non-syndromic congenital hypogonadotropic hypogonadism: Clinical presentation and genotype-phenotype relationships. European Journal of Endocrinology, 162(5), 835–851. 10.1530/EJE-10-0083 [DOI] [PubMed] [Google Scholar]
- Carter M. J. (2014). Gender socialization and identity theory. Social Sciences, 3(2), 242–263. 10.3390/socsci3020242 [DOI] [Google Scholar]
- Choi J., Smitz J. (2014). Luteinizing hormone and human chorionic gonadotropin: Distinguishing unique physiologic roles. Gynecological Endocrinology, 30(3), 174–181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collaer M. L., Geffner M. E., Kaufman F. R., Buckingham B., Hines M. (2002). Cognitive and behavioral characteristics of Turner syndrome: Exploring a role for ovarian hormones in female sexual differentiation. Hormones and Behavior, 41(2), 139–155. 10.1006/hbeh.2001.1751 [DOI] [PubMed] [Google Scholar]
- Collaer M. L., Hines M. (1995). Human behavioral sex differences: A role for gonadal hormones during early development? Psychological Bulletin, 118(1), 55–107. 10.1037//0033-2909.118.1.55 [DOI] [PubMed] [Google Scholar]
- Crowley W. F., Pitteloud N. (2017). Congenital hypogonadotropic hypogonadism. In Winters S. J. (Ed.), Male hypogonadism: Basic, clinical, and therapeutic principles (pp. 81–100). Humana Press. [Google Scholar]
- Davies W., Wilkinson L. S. (2006). It is not all hormones: Alternative explanations for sexual differentiation of the brain. Brain Research, 1126, 36–45. [DOI] [PubMed] [Google Scholar]
- Diamond M., Sigmundson H. K. (1997). Sex reassignment at birth: Long-term review and clinical implications. Archives of Pediatrics and Adolescent Medicine, 151, 298–304. [DOI] [PubMed] [Google Scholar]
- Doll L. M., Cárdenas R. A., Burriss R. P., Puts D. A. (2016). Sexual selection and life history: Earlier recalled puberty predicts men’s phenotypic masculinization. Adaptive Human Behavior and Physiology, 2, 134–149. 10.1007/s40750-015-0031-7 [DOI] [Google Scholar]
- Drummond K. D., Bradley S. J., Peterson-Badali M., Zucker K. J. (2008). A follow-up study of girls with gender identity disorder. Developmental Psychology, 44(1), 35–45. [DOI] [PubMed] [Google Scholar]
- Eagly A. H., Wood W. (2013). The nature–nurture debates: 25 years of challenges in understanding the psychology of gender. Perspectives on Psychological Science, 8(3), 340–357. 10.1177/1745691613484767 [DOI] [PubMed] [Google Scholar]
- Forest M. G., Cathiard A. M., Bertrand J. A. (1973). Evidence of testicular activity in early infancy. Journal of Clinical Endocrinology and Metabolism, 148, 148–151. [DOI] [PubMed] [Google Scholar]
- Foresta C., Zuccarello D., Garolla A., Ferlin A. (2008). Role of hormones, genes, and environment in human cryptorchidism. Endocrine Reviews, 29(5), 560–580. 10.1210/er.2007-0042 [DOI] [PubMed] [Google Scholar]
- Freund K., Langevin R. (1977). Extension of the Gender Identity Scale for Males. Archives of Sexual Behavior, 6(6), 507–519. [DOI] [PubMed] [Google Scholar]
- Goldberg S., Lewis M. (1969). Play behavior in the year-old infant: Early sex differences. Child Development, 40(1), 21–31. [PubMed] [Google Scholar]
- Hakim C., Padmanabhan V., Vyas A. K. (2017). Gestational hyperandrogenism in developmental programming. Endocrinology, 158(2), 199–212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han T. S., Bouloux P. M. G. (2012). Kallmann syndrome and other causes of hypothalamic hypogonadism and related development disorders. In Fink G., Pfaff D., Levine J. (Eds.), Handbook of neuroendocrinology (pp. 597–617). Academic Press. 10.1016/B978-0-12-375097-6.10027-7 [DOI] [Google Scholar]
- Hines M., Brook C., Conway G. S. (2004). Androgen and psychosexual development: Core gender identity, sexual orientation, and recalled childhood gender role behavior in women and men with congenital adrenal hyperplasia (CAH). Journal of Sex Research, 41(1), 75–81. 10.1080/00224490409552215 [DOI] [PubMed] [Google Scholar]
- Hughes I. A., Davies J. D., Bunch T. I., Pasterski V., Mastroyannopoulou K., MacDougall J. (2012). Androgen insensitivity syndrome. The Lancet, 380(9851), 1419–1428. 10.1016/S0140-6736(12)60071-3 [DOI] [PubMed] [Google Scholar]
- Kinsey A. C., Pomeroy W. B., Martin C. E. (1948). Sexual behavior in the human male. Saunders. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klein F., Sepekoff B., Wolf T. J. (1985). Sexual orientation: A multi-variable dynamic process. Journal of Homosexuality, 11, 35–49. [DOI] [PubMed] [Google Scholar]
- Kung K. T. F., Li G., Golding J., Hines M. (2018). Preschool gender-typed play behavior at age 3.5 years predicts physical aggression at age 13 years. Archives of Sexual Behavior, 47(4), 905–914. 10.1007/s10508-017-1005-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laitinen E.-M., Vaaralahti K., Tommiska J., Eklund E., Tervaniemi M., Valanne L., Raivio T. (2011). Incidence, phenotypic features and molecular genetics of Kallmann syndrome in Finland. Orphanet Journal of Rare Diseases, 6, Article 41. 10.1186/1750-1172-6-41 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lanciotti L., Cofini M., Leonardi A., Penta L., Esposito S. (2018). Up-to-date review about minipuberty and overview on hypothalamic-pituitary-gonadal axis activation in fetal and neonatal life. Frontiers in Endocrinology, 9, Article 410. 10.3389/fendo.2018.00410 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindsey E. W., Mize J. (2001). Contextual differences in parent-child play: Implications for children’s gender role development. Sex Roles, 44(3–4), 155–176. 10.1023/A:1010950919451 [DOI] [Google Scholar]
- Lippa R. A. (2005). Gender, nature, and nurture. Routledge. [Google Scholar]
- Lytton H., Romney D. M. (1991). Parents’ differential socialization of boys and girls: A meta-analysis. Psychological Bulletin, 109(2), 267–296. 10.1037//0033-2909.109.2.267 [DOI] [Google Scholar]
- Meyer-Bahlburg H. F. L., Dolezal C., Baker S. W., Carlson A. D., Obeid J. S., Newton M. I. (2004). Prenatal androgenization affects gender-related behavior but not gender identity in 5–12-year-old girls with congenital adrenal hyperplasia. Archives of Sexual Behavior, 33(2), 97–104. [DOI] [PubMed] [Google Scholar]
- Negri-Cesi P., Colciago A., Celotti F., Motta M. (2004). Sexual differentiation of the brain: Role of testosterone and its active metabolites. Journal of Endocrinological Investigation, 27, 120–127. [PubMed] [Google Scholar]
- Pardue M. L., Wizemann T. M. (2001). Exploring the biological contributions to human health: Does sex matter? National Academies Press. [PubMed] [Google Scholar]
- Pasterski V. L., Geffner M. E., Hindmarsh P., Brook C., Brain C., Hines M. (2005). Prenatal hormones and postnatal socialization by parents as determinants of male-typical toy play in girls with congenital adrenal hyperplasia. Child Development, 76(1), 264–278. 10.1111/j.1467-8624.2005.00843.x [DOI] [PubMed] [Google Scholar]
- Puts D. A., Bailey D. H., Cárdenas R. A., Burriss R. P., Welling L. L. M., Wheatley J. R., Dawood K. (2013). Women’s attractiveness changes with estradiol and progesterone across the ovulatory cycle. Hormones and Behavior, 63(1), 13–19. 10.1016/j.yhbeh.2012.11.007 [DOI] [PubMed] [Google Scholar]
- Puts D. A., Cárdenas R. A., Bailey D. H., Burriss R. P., Jordan C. L., Breedlove S. M. (2010). Salivary testosterone does not predict mental rotation performance in men or women. Hormones and Behavior, 58(2), 282–289. 10.1016/j.yhbeh.2010.03.005 [DOI] [PubMed] [Google Scholar]
- Reiner W. G., Gearhart J. P. (2004). Discordant sexual identity in some genetic males with cloacal exstrophy assigned to female sex at birth. New England Journal of Medicine, 350(4), 333–341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seminara S. B., Hayes F. J., Crowley W. F. (1998). Gonadotropin-releasing hormone deficiency in the human (idiopathic hypogonadotropic hypogonadism and Kallmann’s syndrome): Pathophysiological and genetic considerations. Endocrine Reviews, 19(5), 521–539. [DOI] [PubMed] [Google Scholar]
- Shirazi T. N., Self H., Dawood K., Cárdenas R., Welling L. L. M., Rosenfield K. A., Ortiz T. L., Carré J. M., Balasubramanian R., Delaney A., Crowley W., Breedlove S. M., Puts D. A. (2020). Pubertal timing predicts adult psychosexuality: Evidence from typically developing adults and adults with isolated GnRH deficiency. Psychoneuroendocrinology, 119, Article 104733. 10.1016/j.psyneuen.2020.104733 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shirazi T. N., Self H., Dawood K., Rosenfield K. A., Penke L., Carré J. M., Ortiz T., Puts D. A. (2019). Hormonal predictors of women’s sexual motivation. Evolution and Human Behavior, 40(3), 336–344. 10.1016/j.evolhumbehav.2019.02.002 [DOI] [Google Scholar]
- Siiteri P. K., Wilson J. D. (1974). Testosterone formation and metabolism during male sexual differentiation in the human embryo. Journal of Clinical Endocrinology and Metabolism, 38(1), 113–125. [DOI] [PubMed] [Google Scholar]
- Singh D., Bradley S. J., Zucker K. J. (2021). A follow-up study of boys with gender identity disorder. Frontiers in Psychiatry, 12, Article 632784. 10.3389/fpsyt.2021.632784 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swift-Gallant A., Monks D. A. (2017). Androgenic mechanisms of sexual differentiation of the nervous system and behavior. Frontiers in Neuroendocrinology, 46, 32–45. 10.1016/j.yfrne.2017.04.003 [DOI] [PubMed] [Google Scholar]
- Thornton J., Zehr J. L., Loose M. D. (2009). Effects of prenatal androgens on rhesus monkeys: A model system to explore the organizational hypothesis in primates. Hormones and Behavior, 55(5), 633–644. 10.1016/j.yhbeh.2009.03.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Todd B. K., Barry J. A., Thommessen S. A. O. (2017). Preferences for ‘gender-typed’ toys in boys and girls aged 9 to 32 months. Infant and Child Development, 26(3), Article e1986. 10.1002/icd.1986 [DOI] [Google Scholar]
- Wallen K. (1996). Nature needs nurture: The interaction of hormonal and social influences on the development of behavioral sex differences in rhesus monkeys. Hormones and Behavior, 30(4), 364–378. 10.1006/hbeh.1996.0042 [DOI] [PubMed] [Google Scholar]
- Wallen K., Baum M. J. (2002). Masculinization and defeminization in altricial and precocial mammals: Comparative aspects of steroid hormone action. Hormones, Brain and Behavior, 4, 385–423. 10.1016/B978-012532104-4/50071-8 [DOI] [Google Scholar]
- Welsh M., Saunders P. T., Fisken M., Scott H. M., Hutchinson G. R., Smith L. B., Sharpe R. M. (2008). Identification in rats of a programming window for reproductive tract masculinization, disruption of which leads to hypospadias and cryptorchidism. Journal of Clinical Investigation, 118(4), 1479–1490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Witchel S. F., Plant T. M. (2013). Puberty: Gonadarche and adrenarche. In Strauss J. F., III, Barbieri R. L. (Eds.), Yen and Jaffe’s reproductive endocrinology (7th ed., pp. 377–421). Elsevier. 10.1016/B978-1-4557-2758-2.00018-4 [DOI] [Google Scholar]
- Xu X., Coats J. K., Yang C. F., Wang A., Ahmed O. M., Alvarado M., Izumi T., Shah N. M. (2012). Modular genetic control of sexually dimorphic behaviors. Cell, 148(3), 596–607. 10.1016/j.cell.2011.12.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu Y., Norton S., Rahman Q. (2019). Early life conditions and adolescent sexual orientation: A prospective birth cohort study. Developmental Psychology, 55(6), 1226–1243. 10.1037/dev0000704 [DOI] [PubMed] [Google Scholar]
- Zucker K. J., Mitchell J. N., Bradley S. J., Tkachuk J., Cantor J. M., Allin S. M. (2006). The recalled childhood gender identity/gender role questionnaire: Psychometric properties. Sex Roles, 54(7–8), 469–483. 10.1007/s11199-006-9019-x [DOI] [Google Scholar]