Abstract
The social-signal-transduction theory of depression asserts that people who experience ongoing interpersonal stressors and mount a greater inflammatory response to social stress are at higher risk for depression. The current study tested this theory in two adult samples. In Study 1, physically healthy adults (N = 76) who reported more frequent interpersonal tension had heightened depressive symptoms at Visit 2, but only if they had greater inflammatory reactivity to a marital conflict at Visit 1. Similarly, in Study 2, depressive symptoms increased among lonelier and less socially supported breast-cancer survivors (N = 79). This effect was most pronounced among participants with higher inflammatory reactivity to a social-evaluative stressor at Visit 1. In both studies, noninterpersonal stress did not interact with inflammatory reactivity to predict later depressive symptoms.
Keywords: inflammation, depression, social, interpersonal, stress, interpersonal relationships, loneliness, social-signal-transduction theory of depression
Although inflammation is an adaptive response to injury and is meant to facilitate healing, chronic inflammation can erode mental and physical health. Many depressed individuals have chronic inflammation (Kiecolt-Glaser, Derry, & Fagundes, 2015), and depressed individuals have greater inflammatory reactivity to laboratory social stressors than their nondepressed peers (Fagundes et al., 2013; Miller et al., 2005; Pace et al., 2006). The social-signal-transduction theory of depression posits that elevated inflammatory reactivity is not only a correlate but also a risk factor for depression—especially in the context of chronic or repetitive interpersonal stress (Slavich & Irwin, 2014).
Interpersonal stress, including objective threats such as social isolation or perceived threats such as loneliness, robustly predicts depression (Slavich, 2020). For instance, individuals who experienced chronic interpersonal stress or a recent major interpersonal stressful life event had an enhanced risk of depression onset compared with their less-stressed peers (Vrshek-Schallhorn et al., 2015). In addition, among people who were already experiencing high depressive symptoms, interpersonal stressors preceded a symptom spike, which did not occur following noninterpersonal stressors (Gunthert et al., 2007).
Inflammation may mechanistically link interpersonal stress and depression. Inflammatory cytokines are the primary transducers of social signals, as they can mediate context-appropriate physiological, cognitive, and behavioral shifts (Slavich, 2020). For example, healthy young women who reported higher levels of interpersonal stress had greater expression of pro- and anti-inflammatory signaling molecules 6 months later (Miller et al., 2009). Acute social stress also provokes a strong but transient inflammatory spike (Marsland et al., 2017). An influx of inflammation can increase threat-related neural sensitivity to negative social interactions and boost reward-related neural sensitivity to positive social interactions, leading people to withdraw from distant or negative relationships and affiliate with close, supportive others—behaviors characteristic of sick people (Eisenberger et al., 2017). This marked change in social goals is an appropriate and adaptive response, conserving energy and facilitating recovery. Even so, the social-signal-transduction theory of depression suggests that heightened inflammatory reactivity to social stress is problematic and depressogenic in the context of frequent interpersonal conflict and tension (Slavich & Irwin, 2014). Chronic and repeated conflict can prevent a return to the initial homeostatic set point and drive up systemic inflammation, ultimately motivating more sustained disengagement, such as that observed in depression.
The cross-sectional relationship between depression and heightened inflammatory reactivity has been replicated multiple times (Fagundes et al., 2013; Miller et al., 2005; Pace et al., 2006), but more longitudinal data are needed to determine whether greater inflammatory reactivity precedes and increases risk for depression. One study found that individuals with greater inflammatory reactivity to a laboratory social stressor had elevated depressive symptoms 1 year later (Aschbacher et al., 2012). According to the social-signal-transduction theory of depression, individuals with heightened inflammatory reactivity to social stress may be especially at risk for depression when they have chronic or repetitive interpersonal stress (Slavich & Irwin, 2014).
The Current Study
The current study tested the social-signal-transduction theory of depression using inflammatory reactivity to a laboratory social stressor, self-reported interpersonal stress, and repeated measures of depressive symptoms in two different adult samples—physically healthy married couples (Study 1) and breast-cancer survivors (Study 2). In both studies, participants completed a laboratory social stressor at the baseline visit and reported their depressive symptoms. At follow-up visits, participants reported their depressive symptoms and stress levels. Study 1 assessed the frequency of social stressors, whereas Study 2 assessed perceived social support and loneliness. We hypothesized that individuals with both greater chronic interpersonal stress (as reported at the follow-up visits) and heightened inflammatory reactivity to the laboratory social stressor at baseline would have elevated depressive symptoms at follow-up. We expected that this relationship would be unique to interpersonal stress and would not exist for noninterpersonal stress. The Ohio State University Institutional Review Board approved both studies, and all participants provided written informed consent.
Statement of Relevance.
Psychological stress triggers depression in some people but not others. Characteristics of the stressor and of the individual can help to determine depression onset. For instance, depression is more likely if the stressor is social in nature, long lasting, or frequent. The social-signal-transduction theory of depression suggests that people who have more frequent social stress and higher levels of inflammation in response to this stress are more likely to develop depression over time. The current study tested this theory in two distinct samples—physically healthy adults and breast-cancer survivors—and findings support the theory. In line with prior research, our results suggest that social stress is more relevant than nonsocial stress to depression. These findings point to social stress and inflammation as prime targets for depression treatment and prevention.
Study 1
Method
Participants
We used print- and Web-based announcements to recruit 43 physically healthy couples (86 individuals) for a parent study on inflammatory and metabolic responses to high-fat meals (Kiecolt-Glaser, Jaremka, et al., 2015). Participants were young (age: M = 38.88 years, SD = 8.26, range = 24–61) and mostly White (82%). Couples had been married for at least 3 years (M = 12.17, SD = 6.66; Table 1). The exclusionary criteria related to chronic health conditions and medications are detailed elsewhere (Kiecolt-Glaser, Jaremka, et al., 2015).
Table 1.
Characteristics of Physically Healthy Individuals Included in Study 1 Analyses (N = 76)
| Variable | M (SD) | n (%) |
|---|---|---|
| Age (years) | 38.88 (8.26) | |
| Trunk fat (grams) | 19,326.76 (7,300.78) | |
| Race | ||
| White | 62 (82%) | |
| Black | 14 (18%) | |
| Years married | 12.17 (6.66) | |
| Sex (female) | 38 (50%) | |
| Serum IL-6 before stress (pg/mL) | 2.12 (5.68) | |
| Serum TNF-α before stress (pg/mL) | 4.74 (1.18) | |
| Inflammatory reactivity | 0.01 (0.72) | |
| CES-D | 6.86 (6.33) | |
| TENSE | ||
| Anger | 8.45 (7.64) | |
| Insensitivity | 19.29 (20.43) | |
| Interference | 9.74 (10.69) | |
| TICS-S | ||
| Social Overload | 4.09 (2.63) | |
| Social Performance Pressure | 5.00 (2.38) | |
| Social Isolation | 3.47 (2.34) | |
| Social Tension | 3.29 (2.01) | |
| Lack of Social Recognition | 4.13 (2.17) | |
| Work Overload | 6.00 (2.57) | |
| Work Performance Pressure | 6.08 (2.70) | |
| Work Discontent | 2.84 (1.88) | |
| Overextended at Work | 1.92 (1.66) | |
| PSS-4 | 4.43 (2.89) |
Note: The Test of Negative Social Exchange (TENSE), Trier Inventory of Chronic Stress–short form (TICS-S), and Perceived Stress Scale–four-item short form (PSS-4) were administered only at Visit 2. The other measures were assessed at Visit 1. IL-6 = interleukin-6; TNF-α = tumor necrosis factor-α; CES-D = Center for Epidemiological Studies Depression Scale.
Procedure
Participants completed two full-day study visits spaced 30.07 (SD = 29.57) days apart at The Ohio State University Clinical Research Center. At both admissions, couples arrived at 7:30 a.m. after fasting for 12 hr, a catheter was inserted into each person’s arm, and a baseline blood draw was taken. Participants relaxed for a brief period and then ate a standardized high-fat meal made with either saturated fat or oleic sunflower oil for the parent study’s aim. Blood was also drawn once after the meal, but this measure was not included in our models because of our desire to have only one prestress, true baseline value.
Couples then engaged in a 20-min problem-solving discussion. Prior to the discussion, experimenters conducted a brief interview to identify mutually contentious topics (e.g., finances, sex, in-laws). Blood samples were collected approximately 90 and 300 min after the conflict. At both visits, participants reported their depressive symptoms approximately 3 hr before the problem discussion, and at Visit 2, they also reported their interpersonal and overall stress levels. Trunk fat was measured by dual X-ray absorptiometry at Visit 1.
Self-report measures
The 20-item Center for Epidemiological Studies Depression Scale (CES-D) indexed the frequency of depressive symptoms over the past week (Radloff, 1977; Visit 1: α = .86; Visit 2: α = .88). The revised Test of Negative Social Exchange (TENSE) scale assessed frequency of interpersonal conflict with important others over the past month (Ruehlman & Karoly, 1991; Visit 2: α = .97). The subscales include Anger, Insensitivity, and Interference. Participants responded on a 10-point Likert scale ranging from not at all (0) to frequently (9). The 30-item short-form Trier Inventory of Chronic Stress (TICS-S; Schulz & Schlotz, 2002) assessed frequency of work-related and interpersonal chronic stressors over the past 3 months using a 5-point Likert scale ranging from never (0) to very often (4; Visit 2: α = .92). The social-stress subscales indexed interpersonal stress, and the work-stress subscales measured noninterpersonal stress. Participants also completed the four-item short form of the Perceived Stress Scale (PSS-4; Cohen, 1988), a general measure of perceived stress that does not specifically address interpersonal stress (Visit 2: α = .78).
Inflammatory markers
Serum interleukin-6 (IL-6) and tumor necrosis factor-α (TNF-α) were assayed as previously described (Kiecolt-Glaser, Jaremka, et al., 2015). Each participant’s samples were assayed for all cytokine markers in one run, thus using the same controls for all time points. The sensitivity for these serum cytokines was 0.3 pg/mL. For IL-6 and TNF-α, the intra-assay coefficients of variation were 3.42% and 2.59%, respectively, and the interassay coefficients of variation were 8.43% and 8.14%, respectively.
Analytic strategy
Eight participants were missing at least one inflammatory-marker measurement, which precluded the calculation of their inflammatory reactivity; thus, they were excluded from analyses. Additionally, two individuals did not report their depressive symptoms at Visit 2 and therefore were also excluded. Excluded individuals were younger (M = 33.20 years, SD = 5.57) than those included in the models (M = 38.88 years old, SD = 8.26), t(14.8) = 2.84, p = .01, but did not differ from individuals included in the models on any other variable of interest at Visit 1 (ps > .48).
We first modeled the trajectories of inflammatory markers surrounding the marital discussion using linear mixed-effects models with (categorical) time as the sole predictor. These models had an unstructured participant-level residual-covariance matrix to account for within-participant measurement correlations and a random couple-level intercept to account for clustering within spousal pairs. IL-6 and TNF-α values were log-transformed to better approximate normality of residuals. We then modeled the trajectory of depressive symptoms over time with a similar model, substituting visit (Visit 1, Visit 2) as the predictor.
Next, we created an inflammatory-reactivity variable by (a) calculating the IL-6 and TNF-α slopes from baseline to 90 min after the discussion and from baseline to 300 min after the discussion and (b) averaging the two slopes for each inflammatory marker. We then z-scored the individual inflammatory-marker slope variables and averaged the two z-scores for a composite measure of inflammatory reactivity. We also created a baseline inflammatory-burden covariate to account for regression to the mean; to do so, we z-scored and averaged prestress values of log-transformed IL-6 and TNF-α.
For the primary analyses, we used linear mixed-effects models with Visit 2 depressive symptoms as the outcome, the main effects of Visit 2 chronic interpersonal stress and Visit 1 inflammatory reactivity, and their interaction term. We included Visit 1 depressive symptoms as a covariate so these models would capture the change in depression from Visit 1 to Visit 2. Separate models were constructed for various measures of interpersonal stress (TICS-S and TENSE subscales). To test the specificity of interpersonal stress, we reran these models with measures of noninterpersonal stress (TICS-S work-related subscales and PSS-4). The primary and alternative models controlled for age and trunk fat, which are both linked to depression (Kessler et al., 1992; Speed et al., 2019), as well as meal type, baseline inflammatory burden, and sex. Our previous analyses (Kiecolt-Glaser, Habash, et al., 2015; Kiecolt-Glaser, Jaremka, et al., 2015) showed a postmeal increase in IL-6, but not TNF-α, and no differences between meal types. The random effect for each couple captured the within-couple correlation. When there was a significant interaction, we probed it at the 25th and 75th percentiles of inflammatory reactivity. In follow-up analyses, we tested these interactions with individual inflammatory slopes (e.g., TNF-α from baseline to 90 min after stress) rather than the inflammatory-reactivity composite score (see Table S1 in the Supplemental Material available online). Thus, in total, we ran 13 primary models and 52 follow-up models. Because of the exploratory nature of this theory’s first empirical test, we report p values that are not multiple-test corrected. However, we also note which results remained significant following false-discovery-rate correction using the Benjamini-Hochberg procedure (adjusting as a group all p values reported in each table). In all models, the Kenward-Roger degrees-of-freedom adjustment was used. All analyses were run in SAS (Version 9.4). Two-tailed tests were conducted, and the α level was set to .05.
Results
Preliminary analyses
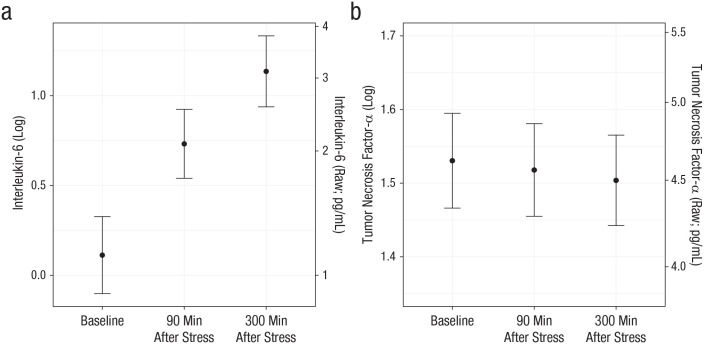
On average, TNF-α did not change after the stressor, p = .16, but IL-6 increased following the stressor, F(74) = 69.04, p < .0001. Specifically, IL-6 increased from before to 90 min after stress, b = 0.62, SE = 0.08, t(75) = 8.03, ps < .0001, and continued to increase from 90 min to 300 min after stress, b = 0.40, SE = 0.07, t(75) = 5.84, p < .0001 (Fig. 1). On average, depressive symptoms did not change from Visit 1 to Visit 2 (p = .33).
Fig. 1.
Estimated marginal mean for (a) interleukin-6 and (b) tumor necrosis factor-α as a function of measurement time in Study 1. For each inflammatory marker, both raw and log-transformed values are graphed. Error bars represent standard errors.
Interpersonal stress, inflammatory reactivity, and depression
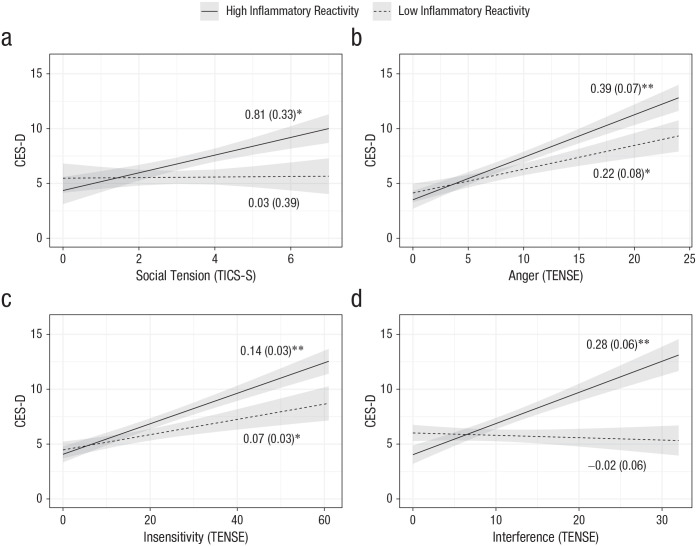
Participants with higher levels of social tension on the TICS-S had greater depressive symptoms at Visit 2, but this relationship was true only for those with high inflammatory reactivity to the social stressor at Visit 1, b = 0.93, SE = 0.36, t(66) = 2.57, p = .01. Similarly, participants who reported more angry, insensitive, or interfering interactions on the TENSE scale had more depressive symptoms at Visit 2, but only if they had high levels of inflammatory reactivity at Visit 1—angry: b = 0.20, SE = 0.09, t(66) = 2.29, p = .03; insensitive: b = 0.08, SE = 0.03, t(66) = 2.5, p = .01; interfering: b = 0.36, SE = 0.08, t(66) = 4.49, p < .001 (Fig. 2). After multiple-test correction, the main effects of the TENSE subscales and the interaction of TENSE Interference and inflammatory reactivity remained significant. Inflammatory reactivity to social stress at Visit 1 marginally interacted with lack of social recognition to predict later depressive symptoms, b = 0.70, SE = 0.37, t(66) = 1.89, p = .06, but did not interact with any other TICS-S social-stress subscale (ps > .63; Table 2). Follow-up analyses revealed that IL-6 reactivity from baseline to 90 min after stress primarily drove significant results (see Table S1 in the Supplemental Material).
Fig. 2.
Relation of scores on the Center for Epidemiological Studies Depression Scale (CES-D) and (a) social tension, (b) anger, (c) insensitivity, and (d) interference in Study 1, separately for participants with high and low inflammatory reactivity. Social tension was measured on the Trier Inventory of Chronic Stress (TICS-S), whereas anger, insensitivity, and interference were measured on the Test of Negative Social Exchange (TENSE). Shaded areas represent standard errors. Models adjusted for age, trunk fat, meal type, baseline inflammatory burden, and sex. The numerical values shown are unstandardized coefficients (with standard errors in parentheses). Asterisks indicate significant slopes (*p < .05, **p < .001).
Table 2.
Results From Mixed-Effects Models Predicting Depressive Symptoms in Study 1
| Predictor | Estimate | SE | t | p |
|---|---|---|---|---|
| Inflammatory reactivity | −0.74 | 1.01 | t(66) = −0.73 | .47 |
| TENSE Anger | 0.31 | 0.07 | t(66) = 4.56 | < .0001 a |
| Inflammatory Reactivity × TENSE Anger | 0.20 | 0.09 | t(66) = 2.29 | .03 |
| Inflammatory reactivity | −0.47 | 0.87 | t(66) = −0.55 | .59 |
| TENSE Insensitivity | 0.11 | 0.03 | t(66) = 4.11 | .0001 a |
| Inflammatory Reactivity × TENSE Insensitivity | 0.08 | 0.03 | t(66) = 2.5 | .01 |
| Inflammatory reactivity | −2.37 | 1.07 | t(66) = −2.21 | .03 |
| TENSE Interference | 0.14 | 0.05 | t(66) = 2.91 | .005 a |
| Inflammatory Reactivity × TENSE Interference | 0.36 | 0.08 | t(66) = 4.49 | < .0001 a |
| Inflammatory reactivity | 1.50 | 1.54 | t(66) = 0.97 | .33 |
| TICS-S Social Overload | 0.23 | 0.24 | t(66) = 0.97 | .33 |
| Inflammatory Reactivity × TICS-S Social Overload | 0.03 | 0.30 | t(66) = 0.09 | .93 |
| Inflammatory reactivity | 0.61 | 2.03 | t(66) = 0.3 | .76 |
| TICS-S Social Performance Pressure | 0.10 | 0.25 | t(66) = 0.4 | .69 |
| Inflammatory Reactivity × TICS-S Social Performance Pressure | 0.17 | 0.35 | t(66) = 0.48 | .63 |
| Inflammatory reactivity | 1.44 | 1.49 | t(66) = 0.97 | .34 |
| TICS-S Social Isolation | 0.12 | 0.28 | t(66) = 0.42 | .67 |
| Inflammatory Reactivity × TICS-S Social Isolation | 0.03 | 0.46 | t(66) = 0.07 | .95 |
| Inflammatory reactivity | −1.34 | 1.29 | t(66) = −1.04 | .30 |
| TICS-S Social Tension | 0.44 | 0.32 | t(66) = 1.38 | .17 |
| Inflammatory Reactivity × TICS-S Social Tension | 0.93 | 0.36 | t(66) = 2.57 | .01 |
| Inflammatory reactivity | −1.88 | 1.86 | t(66) = −1.01 | .32 |
| TICS-S Lack of Social Recognition | 0.45 | 0.26 | t(66) = 1.73 | .09 |
| Inflammatory Reactivity × TICS-S Lack of Social Recognition | 0.70 | 0.37 | t(66) = 1.89 | .06 |
| Inflammatory reactivity | 0.34 | 2.28 | t(66) = 0.15 | .88 |
| TICS-S Work Overload | 0.10 | 0.25 | t(66) = 0.42 | .68 |
| Inflammatory Reactivity × TICS-S Work Overload | 0.22 | 0.37 | t(66) = 0.6 | .55 |
| Inflammatory reactivity | 0.80 | 2.22 | t(66) = 0.36 | .72 |
| TICS-S Work Performance Pressure | 0.16 | 0.21 | t(66) = 0.77 | .45 |
| Inflammatory Reactivity × TICS-S Work-Performance Pressure | 0.10 | 0.30 | t(66) = 0.33 | .74 |
| Inflammatory reactivity | 0.30 | 1.54 | t(66) = 0.19 | .85 |
| TICS-S Work Discontent | 0.45 | 0.31 | t(66) = 1.43 | .16 |
| Inflammatory Reactivity × TICS-S Work Discontent | 0.40 | 0.43 | t(66) = 0.92 | .36 |
| Inflammatory reactivity | 1.98 | 1.29 | t(66) = 1.54 | .13 |
| TICS-S Overextended at Work | 0.46 | 0.39 | t(66) = 1.18 | .24 |
| Inflammatory Reactivity × TICS-S Overextended at Work | −0.18 | 0.50 | t(66) = −0.36 | .72 |
| Inflammatory reactivity | 0.57 | 1.27 | t(65) = 0.45 | .65 |
| PSS-4 | 0.96 | 0.22 | t(64.8) = 4.31 | < .0001 a |
| Inflammatory Reactivity × PSS-4 | 0.23 | 0.24 | t(65) = 0.96 | .34 |
Note: The estimates shown are unstandardized. Models adjusted for age, trunk fat, meal type, baseline inflammatory burden, and sex. TENSE = Test of Negative Social Exchange; TICS-S = Trier Inventory of Chronic Stress–short form; PSS-4 = Perceived Stress Scale–four-item short form.
This value remained significant after false-discovery-rate correction.
Noninterpersonal stress, inflammatory reactivity, and depression
Although participants with higher PSS-4 scores had higher depressive symptoms, b = 0.96, SE = 0.22, t(64.8) = 4.31, p < .0001, this effect did not depend on inflammatory reactivity to social stress (interaction: p = .34). The main effect of PSS-4 on depression remained significant even after multiple-test correction. Work-related-stress subscales on the TICS-S did not predict depressive symptoms (ps > .16), nor did they interact with inflammatory reactivity to predict depressive symptoms (interaction ps > .36; Table 2).
Study 2
Method
Participants
Participants (N = 100) were female breast-cancer survivors (Stage 0–IIIA) from the waitlist control condition of a parent randomized controlled trial involving hatha yoga (clinical-trials identifier: NCT00486525). All were middle aged (age: M = 51.11 years, SD = 8.90), 85% were White, 51% had been diagnosed with early stage (0–I) breast cancer, and a majority had received chemotherapy (63%) or radiation (61%; see Table 3). They had completed cancer treatment (except for hormonal therapy) between 2 months and 3 years (M = 12.04 months, SD = 8.25) prior to the study and were recruited through oncologists’ referrals, community print and Web-based announcements, and breast-cancer groups and events. The exclusionary criteria and randomization procedure are described elsewhere (Kiecolt-Glaser et al., 2014).
Table 3.
Characteristics of Breast-Cancer Survivors Included in Study 2 Analyses (N = 79)
| Variable | M (SD) | n (%) |
|---|---|---|
| Age (years) | 51.11 (8.90) | |
| Sagittal abdominal diameter | 20.88 (3.68) | |
| Months since treatment | 12.04 (8.25) | |
| Race | ||
| White | 67 (85%) | |
| Black | 10 (13%) | |
| Asian American | 2 (3%) | |
| Cancer stage | ||
| 0–I | 40 (51%) | |
| I–II | 31 (39%) | |
| III+ | 8 (10%) | |
| Chemotherapy treatment | 50 (63%) | |
| Radiation treatment | 48 (61%) | |
| No longer menstruating | 64 (81%) | |
| Serum IL-6 before stress (pg/mL) | 2.45 (2.50) | |
| Serum TNF-α before stress (pg/mL) | 7.36 (3.46) | |
| Inflammatory reactivity | −.06 (.81) | |
| CES-D | 10.43 (7.55) | |
| UCLA Loneliness | 37.35 (9.23) | |
| ISEL | 94.61 (15.36) | |
| PSS | 21.76 (8.58) |
Note: For the University of California, Los Angeles (UCLA) Loneliness Scale; Interpersonal Support Evaluation List (ISEL); and Perceived Stress Scale–full version (PPS), values obtained at the first follow-up are shown. Values shown for the other measures were assessed at baseline. IL-6 = interleukin-6; TNF-α = tumor necrosis factor-α; CES-D = Center for Epidemiological Studies Depression Scale.
Procedure
The waitlist control group was told to continue their normal activities and refrain from starting a yoga practice. They completed three visits. Visits 2 and 3 were 4.47 (SD = 1.08) and 7.68 (SD = 1.11) months after Visit 1. After their final assessment, participants were offered yoga classes. At Visit 1, women had a fasting baseline blood draw between 7:00 a.m. and 9:00 a.m. to control for diurnal variability. After the blood draw, they ate a standardized breakfast.
Around 9:00 a.m. at Visit 1 only, they underwent the Trier Social Stress Test, a well-validated psychosocial stressor consisting of a speech and a mental-arithmetic task (Kirschbaum et al., 1993). They had their blood drawn 45 and 120 min after stress to assess their inflammatory reactivity. At all visits, women reported their depressive symptoms, loneliness, social support, and perceived stress. They reported their depressive symptoms shortly before the stressor and their loneliness, social support, and perceived stress 1 hr after the stressor. We gathered cancer-related information (time since treatment, cancer stage at diagnosis, and treatment type) from participants’ medical charts and assessed sagittal abdominal diameter, a measure of belly fat.
Self-report measures
The CES-D, as described in Study 1, indexed depressive symptoms at each visit (.85 < αs < .91 at all visits). The University of California, Los Angeles (UCLA) Loneliness Scale assessed perceptions of social isolation (Russell, 1996; .92 < αs < .94 at all visits). Social support was measured with the 40-item Interpersonal Support Evaluation List (ISEL; Cohen & Hoberman, 1983; αs = .94 at all visits). To measure stress that was not specifically interpersonal, we asked participants to complete the full-length Perceived Stress Scale (PSS; Cohen et al., 1983; .88 < αs < .91 at all visits).
Inflammatory markers
Serum cytokine levels, assessed undiluted in duplicate, were determined using ProInflammatory II 4-Plex Ultra-Sensitive Kit (Meso Scale Discovery, Gaithersburg, MD) per kit instructions. The lower limits of detection for IL-6 and TNF-α were 0.26 pg/mL and 0.37 pg/mL, respectively. The intra-assay coefficients of variation for IL-6 and TNF-α were 1.43% and 4.32%, respectively, and the interassay coefficients of variation were 4.42% and 5.30%, respectively.
Analytic strategy
After randomization for the parent yoga trial, 100 breast-cancer survivors were allocated to the waitlist control group. Overall, 90 women returned for Visit 1 and 87 returned for Visit 2; 11 of these women did not have complete inflammatory-reactivity data at the baseline visit, largely because of blood-draw issues. Therefore, 79 were included in the primary models; the mixed models described below include all participants for which we had at least one observation of the outcome variable. Participants excluded from the models did not differ on key variables of interest at baseline (ps > .11).
We used the same analytic strategy as in Study 1. However, to model depressive symptoms at the two follow-up visits, we used linear mixed-effects models with an unstructured participant-level covariance matrix for the moderation models to account for the high correlation between each participant’s repeated measurements. These models adjusted for age, sagittal abdominal diameter, inflammatory burden, baseline CES-D scores, time since treatment, cancer stage (0–I, I–II, IIIA), chemotherapy treatment (yes or no), and radiation treatment (yes or no). Unlike in Study 1, there was no clustering of participants in spousal pairs, so no models contained a random effect for couple. Significant interactions were probed at the 25th and 75th percentiles of inflammatory reactivity. In follow-up analyses, we tested these interactions with individual inflammatory slopes rather than the inflammatory-reactivity composite score (see Table S2 in the Supplemental Material). As an alternative model, to test the time bound of the theory, we reran the primary Study 2 models with Visit 1 values of chronic stress. The Study 1 chronic-stress measures were not administered at Visit 1, so we were unable to run these alternative models using that sample. Thus, in total, we ran three primary models, three alternative models, and 12 follow-up models. As in Study 1, we noted which results remained significant after false-discovery-rate correction. In all models, the Kenward-Roger degrees-of-freedom adjustment was used. All analyses were run in SAS (Version 9.4). Two-tailed tests were conducted, and the α level was set to .05.
Results
Preliminary analyses
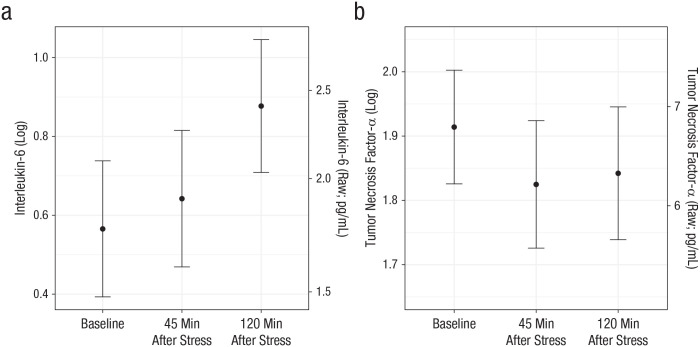
Across participants, TNF-α declined from before stress to 45 min after stress, b = −0.089, SE = 0.03, t(78) = −2.78, p = .007, but not from 45 to 120 min after stress, p = .49. IL-6 marginally increased from before stress to 45 min after stress, b = 0.077, SE = 0.04, t(78) = 1.83, p = .07, and continued to increase from 45 to 120 min after stress, b = 0.24, SE = 0.05, t(78) = 4.37, ps < .0001 (Fig. 3). Depressive symptoms did not change throughout the study (p = .16).
Fig. 3.
Estimated marginal mean for (a) interleukin-6 and (b) tumor necrosis factor-α as a function of measurement time in Study 2. For each inflammatory marker, both raw and log-transformed values are graphed. Error bars represent standard errors.
Interpersonal stress, inflammatory reactivity, and depression
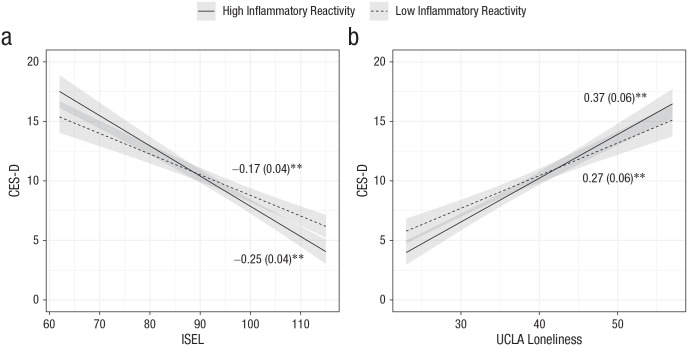
Women who had lower social support had heightened depressive symptoms at the follow-up visits, and this effect was intensified if they had greater inflammatory reactivity to the laboratory social stressor at the baseline visit, b = −0.11, SE = 0.05, t(86.2) = −2.2, p = .03. Similarly, women who were lonelier had greater depressive symptoms at the follow-up visits, and this effect was marginally amplified among those with greater inflammatory reactivity to the social stressor at the baseline visit, b = 0.13, SE = 0.07, t(98.8) = 1.9, p = .06 (Fig. 4). Only the main effects of loneliness and social support remained significant after multiple-test correction. Follow-up analyses revealed that TNF-α stress reactivity primarily drove these results (see Table S2 in the Supplemental Material).
Fig. 4.
Relation of scores on the Center for Epidemiological Studies Depression Scale (CES-D) and (a) scores on the Interpersonal Support Evaluation List (ISEL) and (b) University of California, Los Angeles (UCLA) Loneliness Scale in Study 2, separately for participants with high and low inflammatory reactivity. Shaded areas represent standard errors. Models adjusted for age, sagittal abdominal diameter, baseline inflammatory burden, baseline CES-D score, time since treatment, cancer stage, and cancer-treatment type. The numerical values shown are unstandardized coefficients (with standard errors in parentheses). Asterisks indicate significant slopes (p < .001).
Noninterpersonal stress, inflammatory reactivity, and depression
Although women who reported more stress on the PSS had higher depressive symptoms, b = 0.41, SE = 0.07, t(133) = 5.58, p < .0001, the effect of inflammatory reactivity on later depressive symptoms did not depend on PSS scores, p = .41 (Table 4). The main effect of PSS remained significant after multiple-test correction.
Table 4.
Results From Mixed-Effects Models Predicting Depressive Symptoms in Study 2
| Predictor | Estimate | SE | t | p |
|---|---|---|---|---|
| Inflammatory reactivity | −5.53 | 2.78 | t(96.2) = −1.99 | .049 |
| UCLA Loneliness | 0.33 | 0.06 | t(90.5) = 5.9 | < .0001 a |
| Inflammatory Reactivity × UCLA Loneliness | 0.13 | 0.07 | t(98.8) = 1.9 | .06 |
| Inflammatory reactivity | 9.99 | 4.76 | t(85.8) = 2.1 | .04 |
| ISEL | −0.22 | 0.03 | t(87) = −6.38 | < .0001 a |
| Inflammatory Reactivity × ISEL | −0.11 | 0.05 | t(86.2) = −2.2 | .03 |
| Inflammatory reactivity | 1.98 | 2.13 | t(97.5) = 0.93 | .36 |
| PSS | 0.41 | 0.07 | t(133) = 5.58 | < .0001 a |
| Inflammatory Reactivity × PSS | −0.08 | 0.09 | t(103) = −0.82 | .41 |
Note: The estimates shown are unstandardized. Models adjusted for age, sagittal abdominal diameter, baseline inflammatory burden, baseline Center for Epidemiological Studies Depression Scale (CES-D) scores, time since treatment, cancer stage, and cancer-treatment type. UCLA = University of California, Los Angeles; ISEL = Interpersonal Support Evaluation List; PSS = Perceived Stress Scale–full version.
This value remained significant after false-discovery-rate correction.
Alternative longitudinal model
When using chronic-stress measures at baseline rather than the follow-up visits, we found that women who were lonelier at baseline had depressive-symptom increases at follow-up, and this effect was stronger among those with high inflammatory reactivity, b = 0.22, SE = 0.09, t(67.2) = 2.35, p = .02. Similarly, women who had less social support at baseline had elevated depressive symptoms at follow-up, and lower social support marginally interacted with inflammatory reactivity to predict later depressive symptoms, b = −0.10, SE = 0.05, t(67.1) = −1.93, p = .06. Noninterpersonal stress, as measured by the PSS, did not predict later depressive symptoms (p = .39), nor did it interact with inflammatory reactivity to predict depressive symptoms (p = .17).
Discussion
Across two samples, we found evidence supporting the social-signal-transduction theory of depression (Slavich & Irwin, 2014). Participants who reported more chronic interpersonal stress—both objective (Study 1) and perceived (Study 2)—had elevated depressive symptoms, but this effect depended on the level of inflammatory reactivity to a laboratory social stressor. Among Study 1’s physically healthy couples, frequent interpersonal stress (particularly conflict-related social stress) predicted increased depressive symptoms only among participants with exaggerated inflammatory reactivity to the social stressor; there was no association between interpersonal stress and later depressive symptoms among those with lower inflammatory reactivity. Among Study 2’s breast-cancer survivors, lonelier and less socially supported women had greater depressive symptoms no matter the magnitude of their inflammatory reactivity, but those with high inflammatory reactivity combined with elevated interpersonal stress experienced even greater increases in depressive symptoms over time. Importantly, these findings were specific to interpersonal stress, pointing to the unique role of social relationships in depression etiology. These results generalized across physically healthy individuals and cancer survivors, two laboratory social stressors, multiple measures of chronic interpersonal stress administered at baseline and follow-up, different follow-up periods, and varying time frames of poststress inflammation, demonstrating the theory’s robustness.
These results show that interindividual variability in stress exposure and inflammatory reactivity to social stress may play a role in depression risk. In turn, depression and related cognitive biases (e.g., catastrophizing) may augment stress exposure and inflammatory reactivity, propagating a vicious cycle. According to the stress-generation hypothesis, depressed individuals may unintentionally generate additional stressors because of the nature of depressive symptoms (Hammen, 2006). For instance, someone who is depressed and does not have the motivation to do household chores may have more conflict with their spouse. Notably, our sample differences, such as sex, physical health status, and marriage status, can influence stress exposure and perception. Other factors associated with depression, such as lower subjective social status (Derry et al., 2013), and heightened emotional reactivity (Carroll et al., 2011) are risk factors for elevated inflammatory reactivity to social stress. Given the role that stress exposure and exaggerated inflammatory reactivity may play in depression etiology, further investigation into factors that modulate exposure and reactivity is warranted.
These results extend the developing inflammation–depression narrative. Current evidence suggests that inflammation and depression fuel one another—a vicious cycle (Kiecolt-Glaser, Derry, & Fagundes, 2015; Mac Giollabhui et al., 2021). In addition to higher basal inflammation, depressed individuals have greater inflammatory reactivity to laboratory stressors (Fagundes et al., 2013; Miller et al., 2005; Pace et al., 2006). Our findings suggest that exaggerated inflammatory reactivity is not only a correlate of but also a risk factor for heightened depressive symptoms, especially in the context of chronic or frequent interpersonal stress.
Our preliminary analyses showed that unlike TNF-α, IL-6 consistently increased following laboratory social stressors, in line with meta-analytic evidence that found greater elevations in IL-6 following acute stressors (Marsland et al., 2017). Even so, in Study 2, TNF-α reactivity primarily drove the results; although it did not increase on average across the sample, there was significant variability in TNF-α responses; specifically, some individuals had a steep rise following the stressor. Results from Study 1 also indicated that IL-6 may continue to increase even 5 hr after stress. Few studies have measured inflammatory markers for longer than 2 hr after a laboratory stressor (Marsland et al., 2017); thus, these data add to the literature by showing sustained poststress IL-6 reactivity. Notably, IL-6 has a diurnal rhythm and typically rises in the afternoon, but the elevations we observed 5 hr after stress were greater than what would be expected at that time of day (Nilsonne et al., 2016), pointing to the stressor’s sustained effect. Follow-up analyses showed that inflammatory reactivity 1 hr to 2 hr after stress may be especially important (see the Supplemental Material).
One other notable difference between these two studies is that IL-6 reactivity primarily drove the effects among physically healthy men and women in Study 1, whereas TNF-α reactivity did so among female cancer patients in Study 2. Sample differences—including sex and cancer status—may help to explain this difference. For instance, although a comprehensive meta-analysis did not find evidence that sex moderates cytokine reactivity to stress (Marsland et al., 2017), there are prior reports of stronger IL-6 reactivity among women and stronger TNF-α reactivity among men (e.g., Steptoe et al., 2002). Also, TNF-α not only provokes inflammation but also mediates cell death and is particularly relevant to cancer, which may help to explain why it was more central to Study 2’s sample.
Findings from our alternative model in Study 2 push the time bounds of the social-signal-transduction theory of depression, suggesting that chronic interpersonal stress—even from several months prior—can provoke depression. Although our primary models tested the effect of interpersonal stress reported at the follow-up visits, the alternative model showed that interpersonal stress that occurred before the baseline visit also predicted depressive-symptom increases at follow-up. These alternative models utilized data only from our breast-cancer sample, a demographic that may disproportionately benefit from social support and suffer the consequences of social stress. These results warrant further investigation of interpersonal-stress timing and its relationship with later depression among other samples.
We found that social stress, rather than general stress, predicted depressive-symptom increases over time. Consistent with these findings, a plethora of prior evidence indicates that humans are highly motivated to form and maintain social bonds and, therefore, that threats to social safety most profoundly impact physical and mental health (Slavich, 2020). Social threat is more closely tied to inflammation than are other stressors, perhaps because the body is readying itself for possible wounding and infection, which is more likely when one is separated from the group (Slavich & Irwin, 2014). Our results extend this prior work by demonstrating the aptness of the social-signal-transduction theory of depression’s focus on social stress rather than all life stress.
Our longitudinal findings have prevention and treatment implications. They suggest a role for anti-inflammatory treatments, at least in certain cases of depression (e.g., Kappelmann et al., 2018). Moreover, they corroborate longstanding central tenets of the interpersonal theory of depression, namely that interpersonal stress drives depression and that resolving interpersonal stress may help to quell depression. This idea led to the development of interpersonal therapy for depression, which has strong empirical support (Cuijpers et al., 2011). Our results suggest that inflammation and interpersonal stress may be vital targets not only for depression treatment but also for prevention.
The strengths of these studies include repeated measurement of inflammatory markers before and after well-controlled laboratory psychosocial stressors, as well as measurement of longitudinal depressive symptoms. Additionally, results generalized across multiple methodological variations, revealing the theory’s robustness. Although results were similar among physically healthy couples and female breast-cancer survivors, both samples were mostly White and living in the midwestern United States, so it is unclear whether these results generalize to other populations. Moreover, the inflammatory-composite scores were conceptualized a priori to capture both initial and sustained stress reactivity as a unitary predictor variable, but this is a novel way of conceptualizing reactivity, and it deserves further exploration and replication. Also, the combination of inflammatory reactivity and social stress may not be unique risk factors for depression; in fact, they may predispose people to other forms of psychopathology—an area for further research. Last, because of the exploratory nature of our hypotheses as an initial test of the social-signal-transduction theory, we ran many statistical tests, and most of our significant findings did not survive correction for multiple tests. Even so, these findings form a foundation for future work and need to be replicated in more diverse samples and with other social-stress paradigms.
Conclusion
In two different samples, we found support for the social-signal-transduction theory of depression. Breast-cancer survivors and physically healthy married couples who had greater interpersonal stress had increased depressive symptoms over time, and this relationship was especially pronounced among participants with high inflammatory reactivity to the laboratory social stressor at baseline. These results demonstrate the clinical significance and predictive validity of both chronic interpersonal stress and inflammatory reactivity as they relate to depression. Accordingly, this research lends support to the idea that inflammation and social stress are prime targets for depression prevention and treatment.
Supplemental Material
Supplemental material, sj-docx-1-pss-10.1177_09567976211031225 for Frequent Interpersonal Stress and Inflammatory Reactivity Predict Depressive-Symptom Increases: Two Tests of the Social-Signal-Transduction Theory of Depression by Annelise A. Madison, Rebecca Andridge, M. Rosie Shrout, Megan E. Renna, Jeanette M. Bennett, Lisa M. Jaremka, Christopher P. Fagundes, Martha A. Belury, William B. Malarkey and Janice K. Kiecolt-Glaser in Psychological Science
Footnotes
ORCID iDs: M. Rosie Shrout  https://orcid.org/0000-0001-5751-1782
https://orcid.org/0000-0001-5751-1782
Christopher P. Fagundes  https://orcid.org/0000-0003-3173-2215
https://orcid.org/0000-0003-3173-2215
Janice K. Kiecolt-Glaser  https://orcid.org/0000-0003-4900-9578
https://orcid.org/0000-0003-4900-9578
Supplemental Material: Additional supporting information can be found at http://journals.sagepub.com/doi/suppl/10.1177/09567976211031225
Transparency
Action Editor: Eddie Harmon-Jones
Editor: Patricia J. Bauer
Author Contributions
A. A. Madison developed the study concept. J. K. Kiecolt-Glaser and M. A. Belury designed Study 1’s parent study. J. K. Kiecolt-Glaser designed Study 2’s parent study. J. K. Kiecolt-Glaser, L. M. Jaremka, and C. P. Fagundes collected Study 1’s data. J. K. Kiecolt-Glaser and J. M. Bennett collected Study 2’s data. A. A. Madison and R. Andridge analyzed the data and interpreted the results. A. A. Madison drafted the manuscript. J. K. Kiecolt-Glaser, W. B. Malarkey, M. E. Renna, and M. R. Shrout provided critical revisions. All authors approved the final version of the manuscript for submission.
Declaration of Conflicting Interests: The author(s) declared that there were no conflicts of interest with respect to the authorship or the publication of this article.
Funding: This work was supported in part by National Institutes of Health Grant Nos. TL1TR002735, CA158868, CA172296, CA016058, CA126857, CA131029, and UL1RR025755. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Open Practices: Data and materials for these studies have not been made publicly available, and the design and analysis plans were not preregistered.
References
- Aschbacher K., Epel E., Wolkowitz O., Prather A., Puterman E., Dhabhar F. (2012). Maintenance of a positive outlook during acute stress protects against pro-inflammatory reactivity and future depressive symptoms. Brain, Behavior, and Immunity, 26(2), 346–352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carroll J. E., Low C. A., Prather A. A., Cohen S., Fury J. M., Ross D. C., Marsland A. L. (2011). Negative affective responses to a speech task predict changes in interleukin (IL)-6. Brain, Behavior, and Immunity, 25(2), 232–238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen S. (1988). Perceived stress in a probability sample of the United States. In Spacapan S., Oskamp S. (Eds.), The social psychology of health (pp. 31–67). SAGE. [Google Scholar]
- Cohen S., Hoberman H. M. (1983). Positive events and social supports as buffers of life change stress. Journal of Applied Social Psychology, 13(2), 99–125. [Google Scholar]
- Cohen S., Kamarck T., Mermelstein R. (1983). A global measure of perceived stress. Journal of Health and Social Behavior, 24(4), 385–396. 10.2307/2136404 [DOI] [PubMed] [Google Scholar]
- Cuijpers P., Geraedts A. S., van Oppen P., Andersson G., Markowitz J. C., van Straten A. (2011). Interpersonal psychotherapy for depression: A meta-analysis. American Journal of Psychiatry, 168(6), 581–592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Derry H. M., Fagundes C. P., Andridge R., Glaser R., Malarkey W. B., Kiecolt-Glaser J. K. (2013). Lower subjective social status exaggerates interleukin-6 responses to a laboratory stressor. Psychoneuroendocrinology, 38(11), 2676–2685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eisenberger N. I., Moieni M., Inagaki T. K., Muscatell K. A., Irwin M. R. (2017). In sickness and in health: The co-regulation of inflammation and social behavior. Neuropsychopharmacology, 42(1), 242–253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fagundes C. P., Glaser R., Hwang B. S., Malarkey W. B., Kiecolt-Glaser J. K. (2013). Depressive symptoms enhance stress-induced inflammatory responses. Brain, Behavior, and Immunity, 31, 172–176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gunthert K. C., Cohen L. H., Butler A. C., Beck J. S. (2007). Depression and next-day spillover of negative mood and depressive cognitions following interpersonal stress. Cognitive Therapy and Research, 31(4), 521–532. [Google Scholar]
- Hammen C. (2006). Stress generation in depression: Reflections on origins, research, and future directions. Journal of Clinical Psychology, 62(9), 1065–1082. [DOI] [PubMed] [Google Scholar]
- Kappelmann N., Lewis G., Dantzer R., Jones P., Khandaker G. (2018). Antidepressant activity of anti-cytokine treatment: A systematic review and meta-analysis of clinical trials of chronic inflammatory conditions. Molecular Psychiatry, 23(2), 335–343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kessler R. C., Foster C., Webster P. S., House J. S. (1992). The relationship between age and depressive symptoms in two national surveys. Psychology and Aging, 7(1), 119–126. 10.1037/0882-7974.7.1.119 [DOI] [PubMed] [Google Scholar]
- Kiecolt-Glaser J. K., Bennett J. M., Andridge R., Peng J., Shapiro C. L., Malarkey W. B., Emery C. F., Layman R., Mrozek E. E., Glaser R. (2014). Yoga’s impact on inflammation, mood, and fatigue in breast cancer survivors: A randomized controlled trial. Journal of Clinical Oncology, 32(10), 1040–1049. 10.1200/JCO.2013.51.8860 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiecolt-Glaser J. K., Derry H. M., Fagundes C. P. (2015). Inflammation: Depression fans the flames and feasts on the heat. American Journal of Psychiatry, 172(11), 1075–1091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiecolt-Glaser J. K., Habash D. L., Fagundes C. P., Andridge R., Peng J., Malarkey W. B., Belury M. A. (2015). Daily stressors, past depression, and metabolic responses to high-fat meals: A novel path to obesity. Biological Psychiatry, 77(7), 653–660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiecolt-Glaser J. K., Jaremka L., Andridge R., Peng J., Habash D., Fagundes C. P., Glaser R., Malarkey W. B., Belury M. A. (2015). Marital discord, past depression, and metabolic responses to high-fat meals: Interpersonal pathways to obesity. Psychoneuroendocrinology, 52, 239–250. 10.1016/j.psyneuen.2014.11.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirschbaum C., Pirke K.-M., Hellhammer D. H. (1993). The ‘Trier Social Stress Test’—a tool for investigating psychobiological stress responses in a laboratory setting. Neuropsychobiology, 28(1–2), 76–81. [DOI] [PubMed] [Google Scholar]
- Mac Giollabhui N., Ng T. H., Ellman L. M., Alloy L. B. (2021). The longitudinal associations of inflammatory biomarkers and depression revisited: Systematic review, meta-analysis, and meta-regression. Molecular Psychiatry, 26, 3302–3314. 10.1038/s41380-020-00867-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marsland A. L., Walsh C., Lockwood K., John-Henderson N. A. (2017). The effects of acute psychological stress on circulating and stimulated inflammatory markers: A systematic review and meta-analysis. Brain, Behavior, and Immunity, 64, 208–219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller G. E., Rohleder N., Cole S. W. (2009). Chronic interpersonal stress predicts activation of pro- and anti-inflammatory signaling pathways 6 months later. Psychosomatic Medicine, 71(1), 57–62. 10.1097/PSY.0b013e318190d7de [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller G. E., Rohleder N., Stetler C., Kirschbaum C. (2005). Clinical depression and regulation of the inflammatory response during acute stress. Psychosomatic Medicine, 67(5), 679–687. [DOI] [PubMed] [Google Scholar]
- Nilsonne G., Lekander M., Åkerstedt T., Axelsson J., Ingre M. (2016). Diurnal variation of circulating interleukin-6 in humans: A meta-analysis. PLOS ONE, 11(11), Article e0165799. 10.1371/journal.pone.0165799 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pace T. W., Mletzko T. C., Alagbe O., Musselman D. L., Nemeroff C. B., Miller A. H., Heim C. M. (2006). Increased stress-induced inflammatory responses in male patients with major depression and increased early life stress. American Journal of Psychiatry, 163(9), 1630–1633. [DOI] [PubMed] [Google Scholar]
- Radloff L. S. (1977). The CES-D scale: A self-report depression scale for research in the general population. Applied Psychological Measurement, 1(3), 385–401. [Google Scholar]
- Ruehlman L. S., Karoly P. (1991). With a little flak from my friends: Development and preliminary validation of the Test of Negative Social Exchange (TENSE). Psychological Assessment, 3(1), 97–104. 10.1037/1040-3590.3.1.97 [DOI] [Google Scholar]
- Russell D. W. (1996). UCLA Loneliness Scale (Version 3): Reliability, validity, and factor structure. Journal of Personality Assessment, 66(1), 20–40. [DOI] [PubMed] [Google Scholar]
- Schulz P., Schlotz W. (2002). The Trier Inventory for the Assessment of Chronic Stress—Version 2 (TICS2): Manual. University of Trier. [Google Scholar]
- Slavich G. M. (2020). Social safety theory: A biologically based evolutionary perspective on life stress, health, and behavior. Annual Review of Clinical Psychology, 16, 265–295. 10.1146/annurev-clinpsy-032816-045159 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slavich G. M., Irwin M. R. (2014). From stress to inflammation and major depressive disorder: A social signal transduction theory of depression. Psychological Bulletin, 140(3), 774–815. 10.1037/a0035302 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Speed M. S., Jefsen O. H., Børglum A. D., Speed D., Østergaard S. D. (2019). Investigating the association between body fat and depression via Mendelian randomization. Translational Psychiatry, 9, Article 184. 10.1038/s41398-019-0516-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steptoe A., Owen N., Kunz-Ebrecht S., Mohamed-Ali V. (2002). Inflammatory cytokines, socioeconomic status, and acute stress responsivity. Brain, Behavior, and Immunity, 16(6), 774–784. [DOI] [PubMed] [Google Scholar]
- Vrshek-Schallhorn S., Stroud C. B., Mineka S., Hammen C., Zinbarg R. E., Wolitzky-Taylor K., Craske M. G. (2015). Chronic and episodic interpersonal stress as statistically unique predictors of depression in two samples of emerging adults. Journal of Abnormal Psychology, 124(4), 918–932. 10.1037/abn0000088 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, sj-docx-1-pss-10.1177_09567976211031225 for Frequent Interpersonal Stress and Inflammatory Reactivity Predict Depressive-Symptom Increases: Two Tests of the Social-Signal-Transduction Theory of Depression by Annelise A. Madison, Rebecca Andridge, M. Rosie Shrout, Megan E. Renna, Jeanette M. Bennett, Lisa M. Jaremka, Christopher P. Fagundes, Martha A. Belury, William B. Malarkey and Janice K. Kiecolt-Glaser in Psychological Science