Abstract
Sleep disruptions promote increases of amyloid β (Aβ) and tau in the brain, and increase Alzheimer’s disease (AD) risk, but the precise mechanisms that give rise to sleep disturbances have yet to be defined. The thalamic reticular nucleus (TRN) is essential for sleep maintenance and for the regulation of slow wave sleep (SWS). We examined the TRN in transgenic mice that express mutant human amyloid precursor protein (APP) and found reduced neuronal activity, increased sleep fragmentation, and decreased SWS time as compared to non-transgenic littermates. Selective activation of the TRN using excitatory DREADDs restored sleep maintenance, increased time in SWS, and reduced amyloid plaque load in both hippocampus and cortex. Our findings suggest that the TRN may play a major role in symptoms associated with AD. Enhancing TRN activity might be a promising therapeutic strategy for AD.
One Sentence Summary:
Chemogenetic stimulation of the thalamic reticular nucleus increases slow wave sleep and reduces Aβ accumulation in rodents..
Introduction
Sleep disorders are common in Alzheimer’s disease (AD) and are observed in various stages of disease progression (1-8). Caregivers and patients commonly report sleep disturbances including early awakenings, sleep fragmentation, sleeping either longer or shorter than normal, low sleep efficiency, insomnia, and late sleep onset (9). Studies in humans and mouse models have demonstrated a strong relationship between sleep disturbances and AD (9, 10). Sleep disturbances are evident at very early stages of disease and may be present many years prior to diagnosis (3, 11, 12). Indeed, sleep disturbances measured by self-report or by actigraphy in cognitively normal subjects are associated with increased risk of developing cognitive impairments and Aβ deposition (9, 13-17).
Multiple lines of research indicate that sleep disruptions increase risk of AD by altering the production and/or clearance of disease-relevant proteins such as Aβ or TAU. Aβ and tau concentrationsl in cerebrospinal fluid (CSF) fluctuate with the sleep-wake cycle, and particularly with slow wave sleep (SWS), in both humans and mice (18-21). Disruptions in SWS result in increased accumulation of both Aβ and tau (22-26). Mechanisms by which disrupted sleep may increase the accumulation of such pathological proteins include reduced clearance through the glymphatic system (21, 27), alterations in lymphatic drainage (28), increased activity-dependent production of Aβ and tau, and others (23-25).
Despite the growing evidence linking sleep disturbance and AD risk, little is known about the mechanisms that give rise to such sleep disturbances. Antagonizing or ablating orexin signaling improves sleep and reduces Aβ accumulation in mouse models of AD (14, 29, 30), confirming the relationship between sleep and Aβ and highlighting a role for orexin in the control of sleep/Aβ dynamics. Indeed, patients who have narcolepsy type 1, which results from degeneration of orexinergic neurons, exhibit excessive daytime sleepiness and have been found to have reduced amyloid pathology in their brains (31, 32). An FDA-approved orexin receptor antagonist was recently evaluated in a randomized clinical trial and was found to improve total sleep time in patients with mild-to-moderate probable AD dementia (33), although it was not examined whether the improvement in sleep was accompanied by reductions in Aβ. However, alterations in orexin concentrations become evident late in disease progression (34), suggesting that there may be additional mechanisms that contribute to sleep disturbances early in disease.
Several lines of evidence suggest that the inhibitory thalamic reticular nucleus (TRN) may be a critical brain region that underlies the link between sleep disturbances, as well as other clinical symptoms, and AD. Alterations in gene expression and activity of the TRN have already been associated with some of the symptoms associated with schizophrenia (35, 36), some of which bear similarities to neuropsychiatric features of AD (37, 38). Activity of the TRN is essential for maintaining stable sleep phases (39), by generating the slow wave activity that characterizes SWS (40-44), regulating attention and behavioral state (45-47), and supporting sensory and cognitive processing (45). All of these domains are affected in AD (38, 48, 49). These domains are affected also in schizophrenia (50-53), suggesting that there may be some commonalities underlying neuropsychiatric symptoms observed in diverse neurological disorders. Activity-marker mapping indicates that TRN activity is reduced in young transgenic mice expressing human amyloid precursor protein (APP) (54), a mouse model that is widely used to study the role of APP/Aβ in the pathophysiology of AD (55).
In this study, we investigated the relationship between TRN dysfunction and deficits in sleep maintenance and SWS in APP mice, a mouse model widely used to study AD, determined whether chemogenetic activation of TRN cells improved sleep maintenance and SWS, and tested whether chronic activation of TRN leads to long-lasting improvements in sleep and reduced accumulation of Aβ. Our results indicate that selective activation of the TRN ameliorates sleep disturbances and slow the accumulation of disease-relevant proteins in a rodent model of AD.
Results
Sleep disturbances and Aβ plaque deposition in APP mice
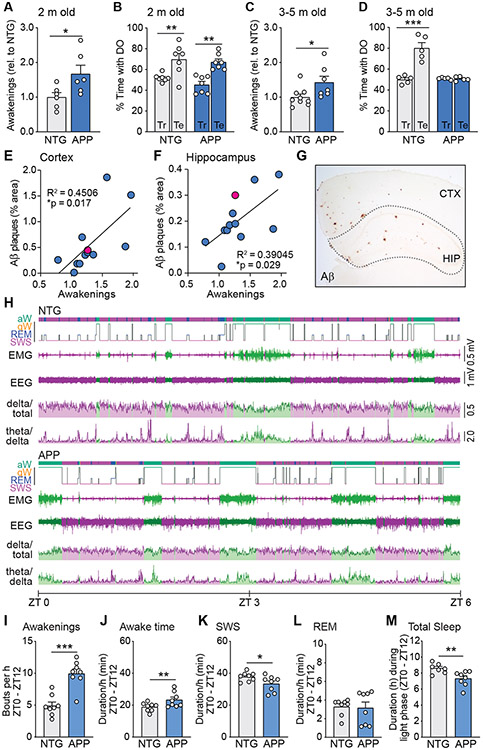
To investigate the role of the TRN in sleep deficits related to AD, we used heterozygous transgenic mice expressing human amyloid precursor protein (APP) carrying Swedish (K670N, M671L) and Indiana (V717F) familial AD mutations (Line J20) (55). We previously demonstrated that these mice exhibit sleep fragmentation at 4-5 months of age, when memory deficits are already present (54). Here we first examined whether sleep fragmentation is present very early in disease progression, prior to the development of memory deficits, since sleep fragmentation in cognitively normal people is associated with increased AD risk (15). We used HomeCageScan, a behavioral software analysis suite to assess whether APP mice exhibit sleep fragmentation at 2 months of age (54). We found an increased number of awakenings during the light phase, which corresponds to the rest period in mice, compared to nontransgenic (NTG) littermate controls (Fig. 1A). At this age, APP mice and NTG mice performed equally well in the object location memory test of spatial memory, as both groups of mice spent more time exploring the displaced object during the test phase than during the training phase (Fig. 1B). However, by 3-5 months of age, APP mice exhibited sleep fragmentation (Fig. 1C), as well as deficits in object location memory (56-58) (Fig. 1D).
Fig. 1. Development of sleep disturbances, memory impairment, and amyloid deposition in APP mice.
Awakenings (A) and spatial memory performance (B) of APP mice at 2 months of age, during the 12-hour light phase. The time that NTG and APP mice spent with the displaced object during testing (Te) and training (Tr) in the object location memory task was used to assess spatial memory. (E to G) Relationship between awakenings from sleep (relative to NTG mice) and amyloid deposition in cortex (E) and hippocampus (F) in APP mice at 6 months of age. Plaque burden shown in (G) indicated by red data points. Example of sleep hypnogram (H) for Zeitgeber Time 0 (ZT0, when lights turn on) to ZT6, generated from wireless EEG-EMG telemetry data (aw: active wake, qw: quiet wake, REM: rapid eye movement sleep, SWS: slow wave sleep). Traces from EMG and EEG, delta ratio (delta/total power in EEG), and theta/delta ratio in 2-3-month-old NTG and APP mice. (I to M) Telemetry data from NTG and APP mice during the 12-hour light phase (ZT0 – ZT12) was analyzed to quantify the number of awakenings (I), time spent awake (J), time in SWS (K), time in REM sleep (L), and total sleep time over the entire 12-hour light phase (M). Bars, mean ± SEM. Circles, individual mice. *p ≤ 0.05, **p < 0.01 using unpaired Student’s t-test (A, C) or Pearson correlation coefficients (E, F). **p < 0.01, ***p < 0.001 using Bonferroni post-hoc test after 2-way repeated measures ANOVA (B, D). For B, there was a significant effect of test/training phase (F(1,12) = 29.90, p = 0.0001) but no effect of genotype (F(1,12) = 1.552, p = 0.2366) or interaction (F(1,12) = 0.2941, p = 0.5976). For D, there was a significant effect of test/training phase (F(1,8) = 27.94, p = 0.0007), of genotype (F(1,8) = 27.12, p = 0.0008), and interaction (F(1,8) = 0.28.85, p = 0.0007).
In this line of APP mice, Aβ reaches reliably measurable concentrations in the brain parenchyma by about 1 month of age and begins to deposit into plaques by about 6 months of age (55). Aβ is produced by active neurons in a diurnal pattern in which Aβ concentrations rise during wakefulness and fall during sleep (21, 27, 59-62). In addition, metabolic waste and proteins like Aβ are cleared through the interstitial fluid by the glymphatic system, which is more active during sleep (21, 27, 62). To assess whether the sleep fragmentation we observed in APP mice might be associated with the accumulation of Aβ at later stages, we examined mice at 6-7 months of age, after plaque deposition has begun. The magnitude of sleep fragmentation was directly related to plaque load in both the hippocampus and cortex in mice of 6 months of age (Fig. 1E-G). Together, these data are consistent with the hypothesis that sleep disturbances begin early in disease and accelerate disease progression and cognitive decline.
SWS is a critical phase of sleep during which neuronal activity is most robustly decreased and clearance through the glymphatic system is enhanced (21, 63). We therefore used EEG/EMG telemetry to assess whether SWS was altered in APP mice (Fig. 1H-M). Wire electrodes were implanted into the frontal lobe for EEG and into the neck muscle for EMG, both connected to a wireless transmitter implanted in the subcutaneous pocket. This system allowed us to evaluate sleep-wake cycles and quantify EEG frequency bands including delta (0.5 – 4 Hz), which is dominant during SWS, and theta (4 – 9 Hz) and gamma (30 – 100 Hz) that predominate during REM sleep, as well as EMG to monitor muscle activity. We also assessed the delta/total EEG power ratio, which is increased during SWS, as well as the theta/delta power ratio which is typically higher during REM sleep (fig. S1). Quantification of sleep architecture from EEG/EMG data confirmed the results from our previous behavioral analysis that APP mice exhibit more frequent awakenings than do NTG mice (Fig. 1I), indicating sleep fragmentation. EEG/EMG data analysis also revealed a small increase in time spent awake (Fig. 1J) and a reduction in time spent in SWS (Fig. 1K). There was no difference in time spent in REM sleep (Fig. 1L). Over the total light phase, APP mice slept less than NTG mice (Fig. 1M). Because APP mice exhibited numerous alterations in sleep parameters during the light cycle when the majority of their sleep occurs, we examined whether APP mice slept more during the dark phase. We found no differences in sleep patterns during the dark phase between APP and NTG mice (fig. S2). Taken together, these results demonstrate that APP mice exhibit disruptions in sleep during the light (rest) phase that could influence the accumulation of pathological proteins. Disruptions in sleep were evident by 5 weeks of age (fig. S2).
DREADD-mediated activation of the TRN
We previously found that the severity of sleep fragmentation in APP mice was directly related to alterations in activity of the thalamocortical network (54). Specifically, activity of neurons in the inhibitory TRN was reduced in APP mice whereas activity in downstream thalamic relay nuclei was increased proportionally to the magnitude of sleep fragmentation (54) (fig. S3). In that study, we mapped expression of FosB/ΔFosB, a marker of chronic neuronal activity that provides an indication of the history of activity (54, 56). Here, to investigate whether other transgenic lines of APP mice also exhibit similar alterations in TRN activity, we examined Tg2576 mice and APP/PS1 mice. We found similar reductions in FosB/ΔFosB expression in Tg2576 mice and in APP/PS1 mice (fig. S3), both of which have been found to exhibit sleep disturbances (64, 65). In contrast, mice from Line I5, which express wild-type human APP and produce only low amounts of Aβ, did not exhibit alterations in FosB/ΔFosB-expressing TRN cells (fig. S3). Even subtle changes in TRN activity have been found to have profound consequences for brain function, including effects on attention, sleep maintenance and SWS, hippocampal function, and cognitive domains affected in AD (9, 30, 38, 45, 46, 66-68). We therefore hypothesized that the reduced activity of the TRN that we identified could be a causal mechanism driving both sleep fragmentation and reduction in SWS in the APP mice used in our studies as well as in other transgenic lines of APP mice with high concentrations of Aβ.
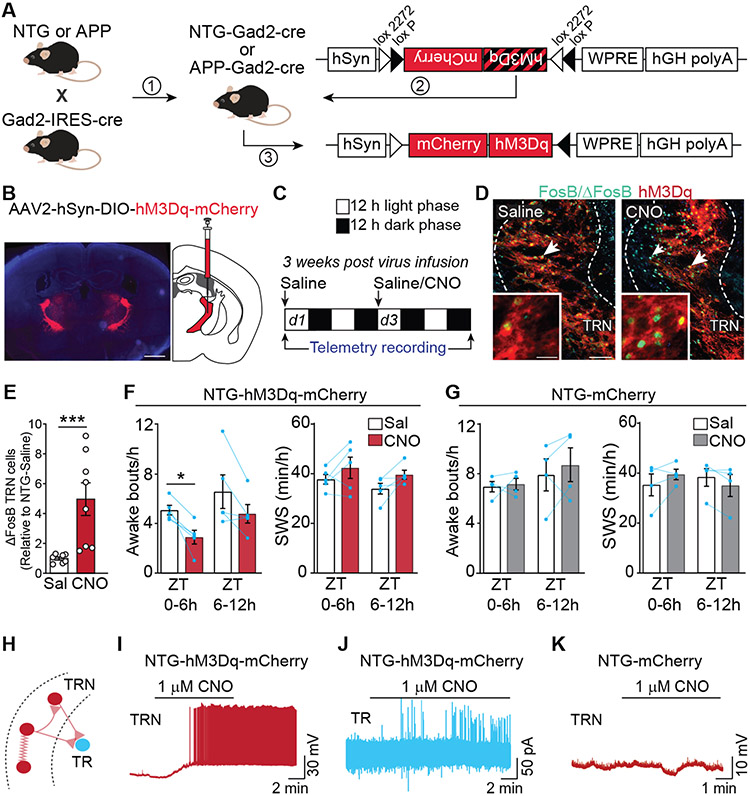
Since TRN neurons exhibit reduced activity but no overt cell loss in APP mice (54), we tested whether activating TRN neurons with the excitatory DREADD (designer receptor exclusively activated by designer drug) hM3Dq (69, 70) could be a viable strategy to increase TRN activity and normalize sleep architecture. To do so, we first crossed APP mice with Gad2-Cre mice to enable selective manipulation of GABAergic populations (Fig. 2A). We then infused AAV-DIO-hM3Dq-mCherry bilaterally into the TRN. We specifically targeted the rostral (Bregma −0.70 to −1.06) part of the TRN (fig. S4) that projects to limbic and sensory thalamic nuclei (71-73), as it displays prominent synchronized activity during SWS (45) and also exhibits the strongest reduction of activity-dependent FosB/ΔFosB expression in APP mice (54). We confirmed that hM3Dq-mCherry was expressed throughout the rostral TRN with little or no spillage into neighboring regions such as zona incerta (Fig. 2B; fig. S4). To verify that stimulation of TRN could be achieved by activating hM3Dq, we first tested whether administration of clozapine N-oxide (CNO) activated TRN neurons and impacted sleep parameters in NTG-Gad2-Cre mice expressing hM3Dq in TRN (NTG-hM3Dq-mCherry mice), following the protocol in Fig. 2C. Saline was first administered intraperitoneally at the start of the light phase (Day 1), followed by administration of either saline again or CNO (5 mg/kg) at the start of the light phase on Day 3. hM3Dq-mediated activation of TRN neurons in vivo was confirmed in post-mortem samples by assessing FosB/ΔFosB expression in the TRN, which was increased in mice that received CNO on Day 3 relative to mice that received saline on Day 3 (Fig. 2D-E). We also analyzed the telemetry data that was collected from these mice. To account for any variability in sleep behaviors between individual mice, the response of mice to saline or CNO on Day 3 was compared with their own response to saline on Day 1 as a baseline control. CNO treatment of NTG-hM3Dq-mCherry mice markedly reduced the number of awakenings during the first 6 hours (ZT0-ZT6) post-CNO administration (Fig. 2F). We did not observe a change in SWS time (Fig. 2F) or in time spent awake (fig. S5), which could relate to the homeostatic processes that regulate the duration of SWS in normal animals (74). To test whether the effects of CNO were specific to hM3Dq and not due to off-target effects, we repeated these experiments in NTG-Gad2-Cre mice in which only mCherry was expressed (NTG-mCherry mice). We found that systemic administration of CNO did not alter sleep-wake behavior, suggesting that neither CNO nor its metabolite clozapine influences these sleep parameters in the absence of hM3Dq expression (Fig. 2G, fig. S5).
Fig. 2. DREADD-induced activation of TRN and modulation of sleep.
(A) AAV expression of double inverted (DIO) cassette carrying hM3Dq in frame with mCherry enables hM3Dq expression in GABAergic cells of Gad2-IRES-cre mice. (B) hM3Dq expression in GABAergic cells of the TRN was achieved by stereotactically infusing AAV2-hSyn-DIO-hM3Dq-mCherry bilaterally into the TRN of 2–3 month old NTG mice; scale bar = 1 mm. (C) 3 weeks after infusion of AAV2-hSyn-DIO-hM3Dq-mCherry or AAV2-hSyn-mCherry into the TRN, mice received saline IP at the start of the light phase (ZT0) on Day 1, followed by saline or CNO at ZT0 on Day 3, with EEG/EMG recording throughout. (D) Example images illustrating FosB/ΔFosB-expression (green) in cells that express hM3Dq (red) denoted by white arrows, in mice treated with saline or with CNO; scale bar = 100 μm. Insets show higher magnification images; scale bar = 25 μm. (E) Quantification of ΔFosB-expressing TRN neurons after CNO treatment. (F) In NTG-hM3Dq-mCherry mice, sleep was quantified after CNO and compared to saline baseline. Graphs show quantification of awakenings and SWS during ZT 0-6h (n = 5; RM ANOVA for Time × Treatment, *p < 0.05 with Bonferroni post-hoc test). Bars, mean ± SEM. (G) In NTG-mCherry mice that do not express hM3Dq, the effects of CNO on awakenings and SWS sleep were quantified (n = 4; RM ANOVA for Time × Treatment with Bonferroni post-hoc test). (H) Schematic of thalamic circuitry probed during in vitro recordings. (I) Current-clamp recordings in TRN neurons in thalamic slices from hM3Dq-expressing mice show effect of bath application of CNO on action potential activity. (J) Voltage clamp recordings in downstream thalamic relay (TR) neurons reveal inhibitory postsynaptic currents following CNO application. (K) Effect of CNO on TRN neuronal firing in slices from animals expressing only mCherry.
The increased expression of FosB/ΔFosB in the TRN after administration of CNO is indicative of increased neuronal activity. To verify that TRN neurons expressing hM3Dq are directly activated by CNO, we performed electrophysiological recordings in acute thalamic slices from NTG-hM3Dq-mCherry mice (Fig. 2H-K). As expected, bath application of 1 μM CNO generated action potentials in TRN cells (Fig. 2I) and inhibitory postsynaptic responses in downstream thalamic relay neurons (TR) in the ventrobasal nucleus (Fig. 2J), whose sole source of inhibitory input arises from the TRN. In contrast, in thalamic slices from NTG-mCherry mice, CNO did not alter TRN activity (Fig. 2K). Together, these results indicate that hM3Dq-mediated activation of TRN neurons is effective and has the potential to impact sleep architecture.
Acute activation of TRN restores sleep in APP mice
We applied the same strategy to activate the TRN in APP mice at 2 months of age (Fig. 3A-B). EEG/EMG telemetry recordings were used to assess sleep architecture after saline treatment (on Day 1) and after CNO treatment (on Day 3). After saline treatment on Day 1, APP-hM3Dq-mCherry mice exhibited fragmented sleep, as expected, characterized by a high number of awakenings. A representative example is shown in the top panel of Fig. 3C, in which interrupted bouts of SWS can be seen on the hypnogram, and short bouts of low frequency activity in the delta range that dominates during SWS can be seen on the spectrogram. After administration of CNO at the start of the light phase on Day 3 (Fig. 3C, bottom), all mice exhibited marked reductions in the numbers of awakenings during the entire 12-hour light phase (Fig. 3D), as well as decreases in the amount of time spent awake during the first 6 hours of the light phase (Fig. 3E). TRN activation also increased the amount of time spent in SWS for the first 6 hours after CNO administration (Fig. 3F), but had no obvious effect on the time spent in REM sleep (Fig. 3G). Overall, APP-hM3Dq-mCherry mice exhibited an increase in the total duration of sleep in the first 6 hours after CNO administration (fig. S6). CNO-induced activation of the TRN did not affect the occurrence of spontaneous seizures or epileptic spikes in APP mice (fig. S7). We noted that during SWS in NTG mice, CNO enhanced the power of low delta frequencies (0.5-3 Hz) whereas it decreased the power of high delta (3-4 Hz), theta (4-8 Hz), alpha (8-12 Hz), beta (12-30 Hz) and gamma (30-80 Hz) frequencies; however, APP mice did not exhibit similar power shifts (fig. S8).
Fig. 3. Modulation of sleep in APP mice by acute activation of TRN neurons using a single dose of CNO.
(A) APP-Gad2-cre mice received stereotactic infusions of AAV2-hSyn-DIO-hM3Dq-mCherry bilaterally into the TRN. (B) 3 weeks after AAV infusion, mice received saline IP at the start of the light phase (ZT0) on Day 1, then saline or CNO at ZT0 on Day 3, with EEG-EMG recording throughout. (C) Example of sleep hypnogram generated from EEG and EMG signal analysis after saline on Day 1 (top) and CNO on Day 3 (bottom), illustrating awakenings and SWS (red lines) after saline or CNO. aW, active wake; qW, quiet wake; REM, REM sleep; SWS, slow wave sleep. Neck muscle EMG, frontal lobe EEG and delta ratio, and spectrograms are shown for ZT 0-6 h. (D to G) Quantification of sleep parameters in APP mice after saline on Day 1 and after CNO on Day 3. The effect of CNO on APP-hM3Dq mice during ZT 0-6h and ZT 6-12h is shown for awakenings (D), time spent awake (E), SWS (F), and REM sleep (G). (H to J) Sleep parameters were compared between APP-hM3Dq mice that received CNO, APP-hM3Dq mice that received saline, and NTG-hM3Dq mice that received saline, on Day 3. Number of awakenings (H), time spent awake (I), and SWS (J), are shown for comparison of the three groups. Bars, mean ± SEM. n = 6 mice (D to G) or 6-8 mice/group (H to J); repeated measures ANOVA for Time x Treatment, *p < 0.05, **p < 0.01, ***p < 0.001 with Bonferroni post-hoc test.
Given that TRN activation reduced the number of awakenings and increased SWS in APP mice, we performed an additional set of experiments to assess whether the improved sleep/wake activity was similar to that observed in NTG mice. We compared three groups of mice (all of which received saline on Day 1): NTG-hM3Dq-mCherry mice that received saline also on Day 3, APP-hM3Dq-mCherry mice that received saline also on Day 3, and APP-hM3Dq-mCherry mice that received CNO (5 mg/kg) on Day 3. We found that relative to NTG-hM3Dq-mCherry mice that received saline on Day 3, APP-hM3Dq-mCherry mice that received saline on Day 3 exhibited the typical increases in awakenings from sleep and increases in time spent awake (Fig. 3H-I), and reductions in SWS (Fig. 3J) as well as in total sleep (fig. S6). However, APP-hM3Dq-mCherry mice that received CNO on Day 3 exhibited a restoration of these parameters to NTG ranges, particularly during the first 6 hours after CNO administration (n = 6; Fig. 3H-J, fig. S6). Together, these results demonstrate that hM3Dq-mediated activation of TRN in APP mice effectively restores SWS and reduces sleep fragmentation.
Chronic activation of the TRN reduces Aβ accumulation
Since sleep disturbances increase the accumulation of Aβ and tau in the brain (22-26, 75), we hypothesized that the selective activation of TRN neurons may reduce Aβ accumulation and plaque load in APP mice. To test this hypothesis, we used APP mice at 6 months of age, just prior to the age at which plaques are reliably observed in cortex and hippocampus. Male and female APP-Gad2-cre mice were stereotactically infused with AAV2-DIO-hM3Dq-mCherry into the TRN bilaterally (Fig. 4A). Two weeks after AAV infusion, animals were implanted with EEG/EMG telemetry transmitters and were allowed to recover for one week before beginning the treatment protocol. All animals were administered one saline injection as baseline. Animals then received daily injections of either saline or CNO (5 mg/kg) for 30 days (Fig. 4B). Telemetry data was collected at baseline and every fifth day for the duration of the treatment. We found that administration of CNO resulted in sustained decreases in the number of awakenings (Fig. 4C; fig. S9), and sustained increases in time spent in SWS (Fig. 4D; fig. S9) throughout the treatment period. There were no obvious effects on time spent in REM sleep (Fig. 4E; fig. S9). CNO administration also increased total sleep over the treatment course (Fig. 4F; fig. S9).
Fig. 4. Effect of chronic activation of the TRN on sleep and amyloid plaque deposition.
(A) 6-month-old APP-Gad2-cre mice received stereotactic infusions of AAV2-hSyn-DIO-hM3Dq-mCherry bilaterally into TRN. (B) 3 weeks after AAV infusion, mice received saline IP at the start of the light phase (ZT0) on Day 1, then on Day 3 began a 30-day treatment of daily injections of either saline or CNO at ZT0 each day, with EEG-EMG recording every fifth day as indicated. (C to F) Effect of chronic activation of TRN on sleep. 2-way repeated measures ANOVA were used to assess effects of CNO on awakenings (C) and SWS (D). Effect of treatment is indicated by the vertical bracket and black asterisks. Dunnett’s post-hoc tests indicate effect of saline or CNO on awakenings and SWS compared to baseline saline injection prior to CNO administration (blue asterisks above or below individual data points). For C, Treatment: F(1, 88) = 84.81, ***p < 0.0001); Days: F(7, 88) = 2.995, **p = 0.0072. For D, Treatment: F(1, 88) = 35.31; ***p < 0.0001; Days: F (7, 88) = 3.490; **p = 0.0024. (E) Effect of CNO on REM sleep (Treatment: F(1, 88) = 0.7066, p = 0.4029; Days: F(7, 88) = 0.3945; p = 0.9032). Effect of CNO on total sleep (F) (Treatment: F(1, 88) = 13.04, ***p = 0.0005; Days: F(7, 88) = 1.992; p = 0.0651). (G and H) Amyloid plaque burden in the hippocampus (HIP) and cortex (CTX) was assessed via Aβ immunostaining (G). Quantification of amyloid plaque burden (H) demonstrates plaque burden in APP-hM3Dq mice treated with CNO (right) compared to saline (left). Red data points indicate animals shown in (G). *p = 0.0265, unpaired Student’s t-test. (I) ΔFosB-expressing cells were quantified in APP-hM3Dq mice treated with saline or CNO to confirm whether CNO treatment increased the number of TRN cells expressing ΔFosB. **p = 0.0011, unpaired Student’s t-test. Graphs show mean ± SEM. n=5/saline and 8/CNO group (C to F), n = 12-13/group (H) and n = 14/group (I). Scale bar, 500 μm (G).
To assess whether sustained improvement in sleep maintenance and SWS time impacted Aβ accumulation, animals were sacrificed after 30 days of treatment (~7.5 months of age) for immunostaining and quantification of Aβ plaque load. We found that animals treated with CNO exhibited approximately 40% reduction in area covered by plaques in cortex and hippocampus compared to animals treated with saline (Fig. 4G-H). To confirm that sustained TRN activation was achieved in these mice, we also immunostained ΔFosB to assess expression in the TRN. We found that the number of TRN cells expressing ΔFosB was increased by more than 2-fold in APP mice treated with CNO relative to APP mice treated with saline (Fig. 4I; fig. S9). These results indicate that chronic stimulation of the TRN in APP mice leads to sustained activation of TRN neurons, sustained improvements in sleep architecture, and reduced accumulation of Aβ.
FosB/ΔFosB expression in the TRN of AD patients correlates with Braak stage
Our results suggest that reduced activity of TRN neurons contribute critically to sleep disturbances in APP mice, and that increasing TRN activity has beneficial effects on sleep and pathology. To understand whether our results in APP mice might be relevant to human disease, we evaluated FosB/ΔFosB expression in the TRN of patients with AD (n = 10), MCI (n = 3), or age-matched controls (n = 15; table S1). Anterior thalamic tissue sections spanning dorsoventral TRN and ventrolateral nucleus of thalamus (VLN) were immunostained for FosB/ΔFosB (Fig. 5A-C), and cells were quantified. We found fewer FosB/ΔFosB-expressing TRN cells in patients with AD compared to age-matched controls, with patients with MCI exhibiting intermediate numbers of FosB/ΔFosB cells (Fig. 5D). We found that the magnitude of reduction in FosB/ΔFosB-expressing cells was associated with increasing Braak stage (Fig. 5E). There was no obvious cell loss in the TRN assessed by hematoxylin-eosin staining (Fig. 5F; fig. S10), or by NeuN staining (Fig. 5G-I), similar to what we previously found in APP mice (54). These results confirm our findings in mice and are consistent with the existence of a relationship between TRN activity and the accumulation of disease-relevant proteins in humans.
Fig. 5. Expression of an activity-dependent marker in TRN of patients with MCI or AD.
(A) Micrographs showing FosB/ΔFosB expression (black arrows) in TRN cells (left) and VLN (right) of age-matched control (CTL), patients with MCI, and patients with AD, with Braak stage indicated in parentheses. (B) Anterior-posterior (A-P) location of area of TRN from which sections were obtained (blue box). (C) Higher magnification of FosB/ΔFosB staining. (D) Quantification demonstrates FosB/ΔFosB-expressing cells in patients with MCI or AD compared to controls. (E) Relationship between Braak stage and the degree of decrease in FosB/ΔFosB-expressing cells. (F) Hematoxylin-eosin staining was used to assess the overall number of cells in TRN or VLN in patients with MCI or AD. Arrowheads indicate examples of hematoxylin-eosin-stained cells. (G) Expression of NeuN-expressing neurons was used to assess the number of neurons in the TRN of controls, patients with MCI, or patients with AD. (H) Relationship between the numbers of NeuN-expressing neurons and FosB/ΔFosB-expressing cells in the TRN of controls, patients with MCI, or patients with AD. (I) Micrographs illustrate the presence of NeuN-expressing neurons in the TRN and VLN of controls, patients with MCI, or patients with AD. VLN: ventrolateral thalamic nucleus. Arrowheads indicate examples of NeuN-expressing cells. n = 15 (controls), 3 (MCI), 10 (AD). **p < 0.01 in ANOVA (D) or Pearson’s correlation (E). Scale bars: 300 μm (A), 100 μm (C, F, and I).
Discussion
Sleep plays a critical role in the equilibrium of both Aβ and tau in the brain, whether by reducing activity-dependent production or by enhancing glymphatic clearance (18, 27, 60, 63, 76). Because sleep disturbances predict risk of AD years before diagnosis (77-80), impairments in sleep early in disease may trigger, or at least exacerbate, disease progression. However, the precise mechanisms that give rise to deficits in sleep in AD have yet to be fully defined.
Our findings demonstrate that decreased activity of the TRN is associated with sleep fragmentation and reduced time spent in SWS in APP mice, and that these alterations occur early in disease progression prior to the development of cognitive deficits and plaque deposition. Moreover, the degree of sleep fragmentation is directly proportional to plaque burden once APP mice reach plaque-bearing age. We found that chemogenetic activation of the TRN in APP mice was sufficient to improve sleep/wake activity and reduce Aβ accumulation, suggesting that the TRN may be a key structure underlying the sleep disturbances that are associated with disease progression in AD. When we examined the TRN of human patients with MCI or AD, we found that the magnitude of reduction in activity marker expression corresponded with increasing Braak stage, supporting the hypothesis that TRN activity modulates pathology in AD. Notably, we found no evidence of overt neuronal loss in the TRN of either human patients or APP mice, supporting the hypothesis that TRN neurons are present and functional, but less active. The precise molecular, cellular, and synaptic mechanisms that underlie such decreased activity of TRN cells remain to be defined, but could relate to alterations in intrinsic excitability or reduced synaptic input from cortex (54) or other structures, among other possibilities. Interestingly, the TRN itself is not a site of robust Aβ accumulation. We found no measurable Aβ plaque deposition in the TRN of APP mice even at 15 months of age, nor in the TRN of patients with AD, consistent with our observations that activity in the TRN is reduced, but it is generally spared from gross pathological alterations.
At least two mechanisms for how sleep modulates the concentration of Aβ or tau in the brain parenchyma have been demonstrated to exist: through the reduction of neuronal activity-dependent production, and through enhanced glymphatic clearance during SWS (18, 21, 22, 60). These mechanisms are not mutually exclusive, and either or both may contribute to the ability of TRN stimulation to reduce Aβ accumulation. We found that the beneficial effects of TRN activation on sleep maintenance and SWS were robust for the first 6 hours post-CNO injection, but less prominent during the second 6-hour period of the rest phase. This timing reflects reported time courses of the actions of CNO in vivo (70) and likely reflects the dynamics of ligand availability and hM3Dq activation. Activation of the TRN daily, at the start of each rest phase, for 30 days produced daily improvements in sleep maintenance and increases in SWS, with no indication of desensitization or loss of effect over the treatment period. Moreover, the reduction of Aβ accumulation observed at the end of the 30 days indicated that daily, transient improvements in sleep are sufficient to produce measurable effects on AD-related pathology.
It should be noted that this study may be limited to conditions in which TRN activity is reduced, such as in AD. Sleep disturbances are associated with a number of disorders and arise from diverse etiologies; targeting the TRN may not be effective if the sleep disturbance is due to unrelated causes such as obstructive sleep apnea or restless leg syndrome. More efforts will be required to assess the extent to which reductions in TRN activity are present in other neurological disorders accompanied by sleep disturbances, such as Down’s syndrome and Parkinson’s disease, which share some similarities in sleep patterns. Although we found that activating the TRN effectively reduced sleep fragmentation and increased SWS in both early and moderate stages of disease progression in APP mice, our study did not address whether continued activation of the TRN is necessary to maintain improved sleep architecture over longer periods of time. Notably, we found that activating the TRN in NTG mice was also effective in enhancing sleep maintenance. This finding suggests that it may also be possible that an intervention targeting the TRN in patients could be initiated once amyloid deposition has begun, even in the absence of clinically symptomatic sleep disturbances.
The activity and function of the TRN has long been appreciated for its roles in sleep and higher order cognitive functions (39, 45). The TRN has been also implicated in some symptoms of schizophrenia; therefore, a comprehensive investigation of the TRN may also provide insight into the biological basis for neuropsychiatric findings in AD. Our studies here provide proof of concept evidence that restoring activity of TRN neurons is sufficient to improve sleep architecture in APP mice. Because a history of sleep disturbances is associated with increased risk of AD, identifying endogenous mechanisms that lead to reduced activity in the TRN may pave the way for the development of therapeutic strategies to improve sleep and modify disease progression in patients, and perhaps also in individuals at risk of developing AD. Ongoing efforts to profile TRN neurons at the transcriptomic, electrophysiological, and circuit levels will lead to a greater understanding of the molecular mechanisms by which TRN neurons regulate their activity, and may reveal effective strategies to do so therapeutically (81-83).
Materials and Methods
Study design:
The aim of our study was to explore whether activation of the TRN could improve sleep architecture and impact the accumulation of Aβ that occurs in AD. We first characterized sleep disturbances in an APP transgenic mouse model often used to study AD, and identified sleep fragmentation and reduced SWS as features of sleep disturbances in these mice (n = 5-12 mice/genotype at each age group studied), in which we previously identified reduced expression of activity-dependent markers in the TRN. We then assessed the ability of chemogenetic strategies to target and acutely activate the TRN in both NTG mice and in APP mice using a combination of slice physiology, activity-dependent gene expression, and efficacy in modulating sleep parameters (n = 5-8 mice/genotype/treatment). To test whether chronic activation of the TRN could reduce the accumulation of Aβ, mice were treated daily with saline or CNO for 30 days, with EEG telemetry recordings occurring every 5th day, prior to assessment of Aβ plaque load (n = 5-8 mice/treatment for telemetry, n = 12-14 mice/treatment for immunohistochemistry). Animals were randomly assigned to treatment groups. To assess relevance to human disease, blocks of anterior thalamic brain samples containing TRN were obtained from the NIH NeuroBioBank (n = 15 controls, 3 MCI, 10 AD). Experimenters were blinded to the genotype of the mice or the diagnosis of the subjects when performing behavioral or biochemical experiments.
Mice:
For this study, we used the J20 mouse line that expresses human amyloid precursor protein (APP). Heterozygous transgenic mice expressing human APP carrying the Swedish (K670N, M671L) and Indiana (V717F) familial AD mutations (hAPP770 numbering) were designated as ‘APP’ mice, and homozygous nontransgenic littermates as ‘NTG’. The J20 mice were crossed for >10 generations onto a C57BL/6 background, and heterozygosity was maintained by breeding male J20 mice with female wild-type C57BL/6 mice from Jackson Laboratory. Targeting of GABAergic neuronal populations was achieved by crossing J20 mice with Gad2-IRES-Cre mice (Gad2tm2(cre)Zjh, #010802) obtained from Jackson Laboratory. Mice were housed in cages with an Enviropak containing nesting material, and maintained on a 12:12 light/dark cycle with ad libitum access to food and water. For tissue collection, mice were anesthetized using SomnaSol Euthanasia-III solution (390 mg pentobarbital sodium/50 mg phenytoin sodium; Henry Schein, Inc.), prior to transcardial perfusion with ice-cold 0.9% NaCl solution. The brains were collected and post-fixed in 4% paraformaldehyde for 48 hours at 4 °C. All procedures were approved by the Institutional Animal Care and Use Committees of Baylor College of Medicine and UT Health.
Stereotactic AAV infusions:
One hour prior to surgical procedures, animals received Buprenorphine SR (1 mg/kg; ZooPharm LLC) subcutaneously. Transdermal administration of 2% lidocaine/0.5% bupivacaine mixture provided local anesthesia, and animals were maintained in a surgical plane of anesthesia using 1% isoflurane for the duration of the infusion.
Expression of hM3Dq in TRN neurons was achieved by infusing 0.5 μl of AAV (5.8 - 6.5 x 1012 GC/ml) carrying the hM3Dq receptor in each hemisphere at stereotactic coordinates: D/V −0.35 mm, A/P −0.7 mm and M/L ±1.3 mm. AAV-2 serotype carrying pAAV-hSyn-DIO-hM3D(Gq)-mCherry (Addgene #44361-AAV2; http://n2t.net/addgene:44361; RRID: Addgene_44361) and pAAV-hSyn-DIO-mCherry (Addgene #50459-AAV2; http://n2t.net/addgene:50459; RRID: Addgene_50459) as control were gifts from Bryan Roth. Virus was allowed to express for 18-22 days before beginning experiments. Virus expression was assessed in all mice. Mice that did not exhibit bilateral expression in the TRN or that exhibited contamination in neighboring regions were excluded from studies (2 mice). To activate hM3Dq receptors, clozapine N-oxide (CNO; Sigma-Aldrich) was administered intraperitoneally at a dose of 5 mg/kg.
Wireless EEG and EMG telemetry recording:
Mice were subcutaneously implanted with an F20-EET (bandwidth of 0.5 – 100 Hz for EEG and 0.5 - 50 Hz for EMG) or HD-X02 (bandwidth of 0.1 – 200 Hz for EEG and EMG) biopotential telemetry transmitter (DSI Harvard Bioscience, Inc., Minneapolis, MN, USA) by performing an elongated incision along the dorsal midline from the posterior margin of the eyes to a point midway between the scapulae. To insert the transmitter into the subcutaneous pocket, animals were positioned in a stereotaxic apparatus in sternal recumbency. The dual channel transmitter was used for frontal lobe epidural EEG and neck muscle EMG recordings. The EEG recording electrode was placed unilaterally into the subdural space in the left frontal cortex (A/P, +1.0; M/L, −1.5), and the reference electrode was placed in the caudal region of the cortex (A/P, −3.0; M/L, +3.0). The EMG lead wire was placed into the lumen of a 21-gauge syringe needle, tunneling through approximately 3 mm of cervical trapezius muscle tissue perpendicular to the long axis of the fiber bundles. The EMG reference wire was placed 2-3 mm posterior to the recording wire. The wires and insulation were secured with sutures. The lead wires on the skull were secured using dental acrylic (Ortho-Jet, LangDental) and the skin was placed back using 5-0 non-absorbable sutures. Post-surgical recovery, EEG-EMG signal recording was performed by placing the mouse’s homecage on a Data Science telemetry receiver (RPC-1) (DSI Harvard Bioscience, Inc.).
Sleep detection and analysis:
Using HomeCageScan:
Sleep-wake monitoring was performed using HomeCageScan software (CleverSys, Inc), which allowed automated detection of homecage behaviors, including sleep-wake, based on movements, body postures, and duration and/or frequency of activities as previously described (39). Each home cage is placed on a platform fitted with an individual visible light- and infrared-sensitive camera (640 x 480 pixels at 30 frames/second), automatic light controller emitting visible or infrared light for 12-hour light-dark cycles, and a video output connected to a video multiplexer for simultaneous multiple video acquisition to a Windows-based computer running HomeCageScan software. For statistical analysis, sleep-wake data were binned in 1-hour intervals and processed through Microsoft Excel.
Using EEG-EMG telemetry:
Telemetry signals including EEG, EMG, activity, and temperature were routed through a matrix (MX2) interface into a Windows 10 OS PC for real-time signal collection. EEG and EMG signals were acquired using a data acquisition software (Ponemah, DSI Harvard Bioscience, Inc.) at sampling rate of 2 kHz. Sleep scoring and data analysis were performed using NeuroScore (DSI Harvard Bioscience, Inc.), Spike2 (version 7.20, Cambridge Electronic Design), MATLAB (MathWorks) with Signal Processing toolboxes, and Python 3.7 (Python Software Foundation) with SciPy and NumPy libraries and MNE package. Epochs of 5 seconds were automatically scored (NeuroScore) and assigned as active wakefulness (aW), quiet wakefulness (qW), REM sleep (REM), and NREM/slow wave sleep (SWS). Automated scoring was further visually inspected by experienced investigators.
Identification of sleep/wake states was based on the following criteria:
Epochs were marked as SWS, if the ratio of delta (δ, 0.5 – 4 Hz) to total power (0.5 – 30 Hz) for frontal EEG was higher than the threshold value (0.4 −0.5), with no muscle activity at EMG.
Epochs were marked as REM, if the ratio of theta (θ, 4 – 8 Hz) to delta (δ, 0.5 – 4 Hz) for frontal EEG was higher than the threshold value (3 - 5), with little or no muscle activity at EMG.
Epochs were scored as aW (active wake) if the EMG signal exceeded threshold, and locomotor movement was reported in the activity channel.
If none of these criteria were met, but a concurrent increase in theta to delta ratio was detected, the epochs were scored as qW (quiet wake).
Quantification of sleep/wake states:
Sleep scoring data was extracted from NeuroScore as the total number of bouts and total time spent in each state in 30-minute bins from the start of the recording.
Spectral analysis:
Data were collected in a single array format. To compute power spectral density, for each mouse we selected five random 30-second-long epochs without any mechanical artifacts or movement noise for each state from ZT 0 to ZT 4 h. Data were down-sampled using FIR filtering to 250 Hz. Data were decomposed into frequency components by using Hann FFT approach. Power spectral density was computed using Welch’s method with no overlapping windows. Band power was calculated by decomposing the range of frequency components for each band using the composite Simpson’s rule. Relative band power was computed by calculating the band power and total power ratio. For analysis of power ratios, the ratio between band powers was calculated.
Spectrogram:
Data were collected and down-sampled to 500 Hz. Spectrograms were computed with non-overlapping windows of 2 seconds and 8192 samples using Hann window/function.
Object location memory testing:
This hippocampus-dependent spatial memory test is comprised of two sessions, a training (Tr) and a testing (Te) session (56-58). For training, two identical 25 mL Erlenmeyer flasks were placed at adjacent corners of the arena (an empty mouse cage) and the mouse was allowed to explore the arena for 3 trials, 3 minutes each with 3 minutes of break in between. The amount of time spent with each flask during each trial was recorded by the experimenter. After a delay of 24 hours, the mouse was returned to the arena for the test session. In this session, one of the flasks was displaced to the adjacent empty corner, so that the two flasks were in opposite, diagonal, positions. The mouse was allowed to explore the arena for 3 minutes, and the percent of time spent with the displaced object (% DO) was computed by calculating the total amount of time spent exploring the displaced object (DO) divided by the total time spent exploring either object. Typically, mice that remember the original position of the DO during the training session spend more time exploring the DO compared with the non-displaced object during the test session. We have previously shown that APP mice are impaired in this task by 3-4 months of age (56-58).
Immunohistochemistry:
Mouse tissue:
Fixed brains were cryoprotected in 30% sucrose in PBS prior to sectioning on a freezing, sliding microtome at a nominal thickness of 30 μm. Serial coronal sections were cut and divided into 8 subseries, each containing every 8th section throughout the rostral-caudal extent of the brain. Each immunostain was performed on an entire subseries of sections, including 3-5 sections containing TRN (bregma −0.58 to −1.46; Paxinos mouse brain atlas) that were used in analyses. For immunofluorescent staining, sections were labeled using rabbit anti-FosB/ΔFosB (1:100, Santa-Cruz Biotechnology Inc., H75) and Alexa Fluor 488–conjugated donkey anti-rabbit (1:500, Life Technologies, A-21206). For diaminobenzidine (DAB) staining, sections were stained with the avidin-biotin method using the following antibodies: rabbit anti-FosB/ΔFosB (1:300, Santa Cruz Biotechnology Inc., H75), rabbit anti-ΔFosB (1:5000, Cell Signaling, D3S8R) and rabbit anti-Aβ (1:1000, Invitrogen/ThermoFisher, #71-5800). Biotinylated goat anti-rabbit (1:200, Vector, BA-1,000) was used as a secondary antibody. DAB was used as the chromogen. For both immunofluorescent and DAB immunostaining, all sections within an experiment were processed, stained, and imaged at the same time using the same parameters and imaging settings. Quantification of FosB/ΔFosB- and ΔFosB-expressing cells was performed manually by an experimenter that was blind to genotype and treatment of the mice. ImageJ (National Institutes of Health) was used for quantification of the percent area covered by Aβ plaques, which was performed by two experts who were blind to the treatment of the mice.
Human tissue:
Anterior thalamus tissue blocks fixed in 4% formalin from individuals with AD, MCI and age-matched controls (table S1) were obtained from the NeuroBioBank operated by the National Institutes of Health. The tissue blocks were sectioned at thickness of 60 μm on a freezing sliding microtome. For DAB staining, two sections per subject were chosen based on anatomical plate locations 28 to 34 (8 mm to 16 mm) as described in www.thehumanbrain.info. Sections underwent antigen retrieval with citrate buffer and formic acid prior to labeling with rabbit anti-FosB/ΔFosB (1:200, Cell Signaling #2251, 5G4), rabbit anti-Aβ (1:1000, Invitrogen/ThermoFisher, #71-5800), or mouse anti-NeuN (1:1000, Millipore #MAB377, clone A60), followed by biotinylated-sp donkey anti-mouse (1:500, Jackson Labs #715-065-150) or biotinylated goat anti-rabbit (1:200, Vector, BA-1000) secondary antibodies. For hematoxylin and eosin staining (Abcam, ab245880), sections were dehydrated with 100% ethanol prior to incubation in hematoxylin for nuclear coloration, and alkaline solution of bluing reagent for permanency of nuclear staining. Cytoplasmic coloration was achieved using an eosin incubation. For quantification and analysis, sections were imaged by the RNA In Situ Hybridization Core facility at Baylor College of Medicine. The images were outlined for the designated area of TRN and VLN of thalamus using Adobe Photoshop CS6 (Adobe Inc.). Counting of cells was performed manually by an experimenter who was unaware of the clinical diagnosis prior to quantification.
In vitro electrophysiology:
Horizontal thalamic brain slices were prepared as described previously (84). Electrophysiological recordings were performed in a chamber perfused with artificial cerebrospinal fluid held at 32-34 °C. For current clamp recordings in TRN, we used a potassium-based internal solution consisting of (in mM): 133 K-Gluconate, 1 KCl, 2 MgCl2, 0.16 CaCl2, 10 HEPES, 0.5 EGTA, 2 Mg-ATP, and 0.4 Na-GTP (adjusted to 290 mOsm and pH 7.3). For voltage clamp recordings of inhibitory postsynaptic currents in neurons of the ventrobasal nucleus of the thalamus (TR – thalamic relay), recording pipettes were filled with a cesium-based internal solution consisting of (in mM): 120 CsMeSO3, 1 MgCl2, 1 CaCl2, 10 CsCl, 10 HEPES, 3 QX-314, 11 EGTA, 2 Mg-ATP, and 0.3 Na-GTP (adjusted to 295 mOsm and pH 7.3). Neurons were held at a holding potential of −40 mV. Recordings were made using a MultiClamp 700B amplifier (Molecular Devices), filtered at 3–10 kHz, and digitized at 20 kHz with a 16-bit analog-to-digital converter (Digidata 1440A; Molecular Devices). Data were acquired using Clampex 10.3 software (Molecular Devices) and analyzed using custom macros written in IGOR Pro (WaveMetrics).
Statistics:
Statistical analyses were performed using Prism 8 (GraphPad). Unless otherwise specified, the results are indicated as sample mean ± SEM. Individual subjects-level data can be found in Data File S1. Differences between experimental groups were assessed by two-tailed Student’s t-test for comparing two groups, paired t-test for comparing treatment groups for before and after effect, one-way ANOVA when comparing multiple groups and two-way ANOVA for multifactorial analyses. Bonferroni and Dunnett’s post-hoc tests were used where appropriate. Correlation analysis was performed using simple regression analyses. No specific randomized method was used in selecting samples or animals, however, mice were semi-randomly assigned to experiments based on age, sex, and genotype.
Supplementary Material
Fig. S1. Sleep scoring in NTG and APP mice using EEG/EMG telemetry.
Fig. S2. Comparison of sleep parameters during the dark (active) phase in 2 month old APP and NTG mice, and during the light phase at 5 weeks of age.
Fig. S3. FosB/ΔFosB-expressing cells in TRN of various APP transgenic mice.
Fig. S4. Stereotactic targeting of TRN with AAV2-hSyn-DIO-hM3Dq-mCherry virus.
Fig. S5. DREADD-induced activation of the TRN and modulation of sleep.
Fig. S6. Acute activation of TRN neurons using a single dose of CNO in APP mice modulates sleep.
Fig. S7. Effect of CNO-mediated activation of TRN cells on the occurrence of spontaneous seizures and epileptic spikes in APP mice; contribution of epileptic spikes to the analysis of power spectral density during SWS or REM sleep.
Fig. S8. Effects on power spectral density by activation of the TRN.
Fig. S9. Changes in sleep architecture after chronic activation of TRN in APP mice.
Fig. S10. Cell counts in the TRN and VLN of patients with MCI or AD.
Fig. S11. Distribution of Aβ plaque deposition APP mice and in patients with AD.
Fig. S12. Comparison of PMIs in controls, patients with MCI, and patients with AD
Table S1. Human anterior thalamic tissue samples.
Data File S1. Individual subjects-level data.
Acknowledgments:
We thank Manuel Silva-Peréz for discussions and critical reading of the manuscript, and Angela Viaene for advice on H&E staining of human tissue sections.
Funding:
This work was supported by the Ruth K Broad Biomedical Research Foundation (to JC) and NIH grants NS085171 (to JC) and AG065290 (to JC and MB). JC and MB are also grateful for the support of the Neurodegeneration Consortium and the Belfer Family Foundation. Research reported in this publication was supported by the Eunice Kennedy Shriver National Institute of Child Health & Human Development of the National Institutes of Health under Award Number P50HD103555 for use of the In situ Hybridization and Imaging Core facilities. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Footnotes
Data and materials availability: All data associated with this study are present in the paper or Supplemental Materials.
References and Notes:
- 1.Mander BA, Local Sleep and Alzheimer's Disease Pathophysiology. Front Neurosci 14, 525970 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Mander BA, Winer JR, Jagust WJ, Walker MP, Sleep: A Novel Mechanistic Pathway, Biomarker, and Treatment Target in the Pathology of Alzheimer's Disease? Trends Neurosci 39, 552–566 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Minakawa EN, Wada K, Nagai Y, Sleep Disturbance as a Potential Modifiable Risk Factor for Alzheimer's Disease. Int J Mol Sci 20, 803 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sprecher KE et al. , High Resolution Topography of Age-Related Changes in Non-Rapid Eye Movement Sleep Electroencephalography. PLoS One 11, e0149770 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Varga AW et al. , Reduced Slow-Wave Sleep Is Associated with High Cerebrospinal Fluid Aβ42 Levels in Cognitively Normal Elderly. Sleep 39, 2041–2048 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.De Gennaro L et al. , The Fall of Sleep K-Complex in Alzheimer Disease. Sci Rep 7, 39688 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gorgoni M et al. , Parietal Fast Sleep Spindle Density Decrease in Alzheimer's Disease and Amnesic Mild Cognitive Impairment. Neural Plast 2016, 8376108 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Westerberg CE et al. , Concurrent impairments in sleep and memory in amnestic mild cognitive impairment. J Int Neuropsychol Soc 18, 490–500 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ju YE, Lucey BP, Holtzman DM, Sleep and Alzheimer disease pathology--a bidirectional relationship. Nat Rev Neurol 10, 115–119 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wang C, Holtzman DM, Bidirectional relationship between sleep and Alzheimer's disease: role of amyloid, tau, and other factors. Neuropsychopharmacology 45, 104–120 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ju YE et al. , Sleep quality and preclinical Alzheimer disease. JAMA Neurol 70, 587–593 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mander BA, Winer JR, Walker MP, Sleep and Human Aging. Neuron 94, 19–36 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bubu OM et al. , Sleep, Cognitive impairment, and Alzheimer's disease: A Systematic Review and Meta-Analysis. Sleep 40, zsw032 (2017). [DOI] [PubMed] [Google Scholar]
- 14.Kang JE et al. , Amyloid-beta dynamics are regulated by orexin and the sleep-wake cycle. Science 326, 1005–1007 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lim AS, Kowgier M, Yu L, Buchman AS, Bennett DA, Sleep Fragmentation and the Risk of Incident Alzheimer's Disease and Cognitive Decline in Older Persons. Sleep 36, 1027–1032 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Spira AP et al. , Self-reported sleep and β-amyloid deposition in community-dwelling older adults. JAMA Neurol 70, 1537–1543 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Yaffe K et al. , Sleep-disordered breathing, hypoxia, and risk of mild cognitive impairment and dementia in older women. Jama 306, 613–619 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Holth JK et al. , The sleep-wake cycle regulates brain interstitial fluid tau in mice and CSF tau in humans. Science 363, 880–884 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mander BA, Disturbed sleep in preclinical cognitive impairment: cause and effect? Sleep 36, 1275–1276 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mander BA et al. , β-amyloid disrupts human NREM slow waves and related hippocampus-dependent memory consolidation. Nat Neurosci 18, 1051–1057 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Xie L et al. , Sleep drives metabolite clearance from the adult brain. Science 342, 373–377 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Barthélemy NR et al. , Sleep Deprivation Affects Tau Phosphorylation in Human Cerebrospinal Fluid. Ann Neurol 87, 700–709 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ju YS et al. , Slow wave sleep disruption increases cerebrospinal fluid amyloid-β levels. Brain 140, 2104–2111 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lucey BP et al. , Effect of sleep on overnight cerebrospinal fluid amyloid β kinetics. Ann Neurol 83, 197–204 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lucey BP et al. , Reduced non-rapid eye movement sleep is associated with tau pathology in early Alzheimer's disease. Sci Transl Med 11, eaau6550 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Winer JR et al. , Sleep as a Potential Biomarker of Tau and β-Amyloid Burden in the Human Brain. J Neurosci 39, 6315–6324 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Nedergaard M, Goldman SA, Glymphatic failure as a final common pathway to dementia. Science 370, 50–56 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Louveau A et al. , Understanding the functions and relationships of the glymphatic system and meningeal lymphatics. J Clin Invest 127, 3210–3219 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Duncan MJ et al. , Effects of the dual orexin receptor antagonist DORA-22 on sleep in 5XFAD mice. Alzheimers Dement (N Y) 5, 70–80 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Roh JH et al. , Potential role of orexin and sleep modulation in the pathogenesis of Alzheimer's disease. J Exp Med 211, 2487–2496 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Dauvilliers YA, Lehmann S, Jaussent I, Gabelle A, Hypocretin and brain β-amyloid peptide interactions in cognitive disorders and narcolepsy. Front Aging Neurosci 6, 119 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gabelle A et al. , Cerebrospinal fluid levels of orexin-A and histamine, and sleep profile within the Alzheimer process. Neurobiol Aging 53, 59–66 (2017). [DOI] [PubMed] [Google Scholar]
- 33.Herring WJ et al. , Polysomnographic assessment of suvorexant in patients with probable Alzheimer's disease dementia and insomnia: a randomized trial. Alzheimers Dement 16, 541–551 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Liguori C et al. , Orexinergic system dysregulation, sleep impairment, and cognitive decline in Alzheimer disease. JAMA Neurol 71, 1498–1505 (2014). [DOI] [PubMed] [Google Scholar]
- 35.Steullet P et al. , The thalamic reticular nucleus in schizophrenia and bipolar disorder: role of parvalbumin-expressing neuron networks and oxidative stress. Mol Psychiatry 23, 2057–2065 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Young A, Wimmer RD, Implications for the thalamic reticular nucleus in impaired attention and sleep in schizophrenia. Schizophr Res 180, 44–47 (2017). [DOI] [PubMed] [Google Scholar]
- 37.Manoach DS, Stickgold R, Abnormal Sleep Spindles, Memory Consolidation, and Schizophrenia. Annu Rev Clin Psychol 15, 451–479 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Jagirdar R, Chin J, Corticothalamic network dysfunction and Alzheimer's disease. Brain Res 1702, 38–45 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lewis LD et al. , Thalamic reticular nucleus induces fast and local modulation of arousal state. Elife 4, e08760 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Timofeev I, Contreras D, Steriade M, Synaptic responsiveness of cortical and thalamic neurones during various phases of slow sleep oscillation in cat. J Physiol 494 (Pt 1), 265–278 (1996). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Fogerson PM, Huguenard JR, Tapping the Brakes: Cellular and Synaptic Mechanisms that Regulate Thalamic Oscillations. Neuron 92, 687–704 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Marks GA, Roffwarg HP, Spontaneous activity in the thalamic reticular nucleus during the sleep/wake cycle of the freely-moving rat. Brain Res 623, 241–248 (1993). [DOI] [PubMed] [Google Scholar]
- 43.Destexhe A, Contreras D, Steriade M, Mechanisms underlying the synchronizing action of corticothalamic feedback through inhibition of thalamic relay cells. J Neurophysiol 79, 999–1016 (1998). [DOI] [PubMed] [Google Scholar]
- 44.Steriade M, Timofeev I, Neuronal plasticity in thalamocortical networks during sleep and waking oscillations. Neuron 37, 563–576 (2003). [DOI] [PubMed] [Google Scholar]
- 45.Halassa MM et al. , State-dependent architecture of thalamic reticular subnetworks. Cell 158, 808–821 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Wimmer RD et al. , Thalamic control of sensory selection in divided attention. Nature 526, 705–709 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.McAlonan K, Cavanaugh J, Wurtz RH, Attentional modulation of thalamic reticular neurons. J Neurosci 26, 4444–4450 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Murphy C, Olfactory and other sensory impairments in Alzheimer disease. Nat Rev Neurol 15, 11–24 (2019). [DOI] [PubMed] [Google Scholar]
- 49.Lucey BP, It's complicated: The relationship between sleep and Alzheimer's disease in humans. Neurobiol Dis 144, 105031 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Shen C et al. , Neurocognitive pattern analysis reveals classificatory hierarchy of attention deficits in schizophrenia. Schizophr Bull 40, 878–885 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Luck SJ, Gold JM, The construct of attention in schizophrenia. Biol Psychiatry 64, 34–39 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Yang C, Winkelman JW, Clinical significance of sleep EEG abnormalities in chronic schizophrenia. Schizophr Res 82, 251–260 (2006). [DOI] [PubMed] [Google Scholar]
- 53.Lauer CJ, Schreiber W, Pollmächer T, Holsboer F, Krieg JC, Sleep in schizophrenia: a polysomnographic study on drug-naive patients. Neuropsychopharmacology 16, 51–60 (1997). [DOI] [PubMed] [Google Scholar]
- 54.Hazra A et al. , Corticothalamic network dysfunction and behavioral deficits in a mouse model of Alzheimer's disease. Neurobiol Aging 44, 96–107 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Mucke L et al. , High-level neuronal expression of abeta 1-42 in wild-type human amyloid protein precursor transgenic mice: synaptotoxicity without plaque formation. J Neurosci 20, 4050–4058 (2000). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Corbett BF et al. , ΔFosB Regulates Gene Expression and Cognitive Dysfunction in a Mouse Model of Alzheimer's Disease. Cell Rep 20, 344–355 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Fu CH et al. , Early Seizure Activity Accelerates Depletion of Hippocampal Neural Stem Cells and Impairs Spatial Discrimination in an Alzheimer's Disease Model. Cell Rep 27, 3741–3751 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.You JC et al. , Epigenetic suppression of hippocampal calbindin-D28k by ΔFosB drives seizure-related cognitive deficits. Nat Med 23, 1377–1383 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Bero AW et al. , Neuronal activity regulates the regional vulnerability to amyloid-β deposition. Nat Neurosci 14, 750–756 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Boespflug EL, Iliff JJ, The Emerging Relationship Between Interstitial Fluid-Cerebrospinal Fluid Exchange, Amyloid-β, and Sleep. Biol Psychiatry 83, 328–336 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Cirrito JR et al. , Synaptic activity regulates interstitial fluid amyloid-beta levels in vivo. Neuron 48, 913–922 (2005). [DOI] [PubMed] [Google Scholar]
- 62.Iliff JJ et al. , A paravascular pathway facilitates CSF flow through the brain parenchyma and the clearance of interstitial solutes, including amyloid β. Sci Transl Med 4, 147ra111 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Hablitz LM et al. , Increased glymphatic influx is correlated with high EEG delta power and low heart rate in mice under anesthesia. Sci Adv 5, eaav5447 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Kam K, Duffy Á M, Moretto J, LaFrancois JJ, Scharfman HE, Interictal spikes during sleep are an early defect in the Tg2576 mouse model of β-amyloid neuropathology. Sci Rep 6, 20119 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Kent BA et al. , Delayed daily activity and reduced NREM slow-wave power in the APPswe/PS1dE9 mouse model of Alzheimer's disease. Neurobiol Aging 78, 74–86 (2019). [DOI] [PubMed] [Google Scholar]
- 66.Beenhakker MP, Huguenard JR, Neurons that fire together also conspire together: is normal sleep circuitry hijacked to generate epilepsy? Neuron 62, 612–632 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Briggs F, Usrey WM, Emerging views of corticothalamic function. Curr Opin Neurobiol 18, 403–407 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Waldemar G et al. , Recommendations for the diagnosis and management of Alzheimer's disease and other disorders associated with dementia: EFNS guideline. Eur J Neurol 14, e1–26 (2007). [DOI] [PubMed] [Google Scholar]
- 69.Alexander GM et al. , Remote control of neuronal activity in transgenic mice expressing evolved G protein-coupled receptors. Neuron 63, 27–39 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Guettier JM et al. , A chemical-genetic approach to study G protein regulation of beta cell function in vivo. Proc Natl Acad Sci U S A 106, 19197–19202 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Fernandez LM et al. , Thalamic reticular control of local sleep in mouse sensory cortex. Elife 7, e39111 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Pinault D, The thalamic reticular nucleus: structure, function and concept. Brain Res Brain Res Rev 46, 1–31 (2004). [DOI] [PubMed] [Google Scholar]
- 73.Vantomme G, Osorio-Forero A, Lüthi A, Fernandez LMJ, Regulation of Local Sleep by the Thalamic Reticular Nucleus. Front Neurosci 13, 576 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Deboer T, Sleep homeostasis and the circadian clock: Do the circadian pacemaker and the sleep homeostat influence each other's functioning? Neurobiol Sleep Circadian Rhythms 5, 68–77 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Winer JR et al. , Sleep Disturbance Forecasts β-Amyloid Accumulation across Subsequent Years. Curr Biol, 4291–4298 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Rasmussen MK, Mestre H, Nedergaard M, The glymphatic pathway in neurological disorders. Lancet Neurol 17, 1016–1024 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Roh JH et al. , Disruption of the sleep-wake cycle and diurnal fluctuation of β-amyloid in mice with Alzheimer's disease pathology. Sci Transl Med 4, 150ra122 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Zhang F et al. , Alteration in sleep architecture and electroencephalogram as an early sign of Alzheimer's disease preceding the disease pathology and cognitive decline. Alzheimers Dement 15, 590–597 (2019). [DOI] [PubMed] [Google Scholar]
- 79.Prinz PN et al. , Changes in the sleep and waking EEGs of nondemented and demented elderly subjects. J Am Geriatr Soc 30, 86–93 (1982). [DOI] [PubMed] [Google Scholar]
- 80.Winer JR, Mander BA, Waking Up to the Importance of Sleep in the Pathogenesis of Alzheimer Disease. JAMA Neurol 75, 654–656 (2018). [DOI] [PubMed] [Google Scholar]
- 81.Martinez-Garcia RI et al. , Two dynamically distinct circuits drive inhibition in the sensory thalamus. Nature 583, 813–818 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Li Y et al. , Distinct subnetworks of the thalamic reticular nucleus. Nature 583, 819–824 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Clemente-Perez A et al. , Distinct Thalamic Reticular Cell Types Differentially Modulate Normal and Pathological Cortical Rhythms. Cell Rep 19, 2130–2142 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Ting JT, Daigle TL, Chen Q, Feng G, Acute brain slice methods for adult and aging animals: application of targeted patch clamp analysis and optogenetics. Methods Mol Biol 1183, 221–242 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Fig. S1. Sleep scoring in NTG and APP mice using EEG/EMG telemetry.
Fig. S2. Comparison of sleep parameters during the dark (active) phase in 2 month old APP and NTG mice, and during the light phase at 5 weeks of age.
Fig. S3. FosB/ΔFosB-expressing cells in TRN of various APP transgenic mice.
Fig. S4. Stereotactic targeting of TRN with AAV2-hSyn-DIO-hM3Dq-mCherry virus.
Fig. S5. DREADD-induced activation of the TRN and modulation of sleep.
Fig. S6. Acute activation of TRN neurons using a single dose of CNO in APP mice modulates sleep.
Fig. S7. Effect of CNO-mediated activation of TRN cells on the occurrence of spontaneous seizures and epileptic spikes in APP mice; contribution of epileptic spikes to the analysis of power spectral density during SWS or REM sleep.
Fig. S8. Effects on power spectral density by activation of the TRN.
Fig. S9. Changes in sleep architecture after chronic activation of TRN in APP mice.
Fig. S10. Cell counts in the TRN and VLN of patients with MCI or AD.
Fig. S11. Distribution of Aβ plaque deposition APP mice and in patients with AD.
Fig. S12. Comparison of PMIs in controls, patients with MCI, and patients with AD
Table S1. Human anterior thalamic tissue samples.
Data File S1. Individual subjects-level data.