Abstract
Background
Internal and external validity are the most relevant components when critically appraising randomized controlled trials (RCTs) for systematic reviews. However, there is no gold standard to assess external validity. This might be related to the heterogeneity of the terminology as well as to unclear evidence of the measurement properties of available tools. The aim of this review was to identify tools to assess the external validity of RCTs. It was further, to evaluate the quality of identified tools and to recommend the use of individual tools to assess the external validity of RCTs in future systematic reviews.
Methods
A two-phase systematic literature search was performed in four databases: PubMed, Scopus, PsycINFO via OVID, and CINAHL via EBSCO. First, tools to assess the external validity of RCTs were identified. Second, studies investigating the measurement properties of these tools were selected. The measurement properties of each included tool were appraised using an adapted version of the COnsensus based Standards for the selection of health Measurement INstruments (COSMIN) guidelines.
Results
38 publications reporting on the development or validation of 28 included tools were included. For 61% (17/28) of the included tools, there was no evidence for measurement properties. For the remaining tools, reliability was the most frequently assessed property. Reliability was judged as “sufficient” for three tools (very low certainty of evidence). Content validity was rated as “sufficient” for one tool (moderate certainty of evidence).
Conclusions
Based on these results, no available tool can be fully recommended to assess the external validity of RCTs in systematic reviews. Several steps are required to overcome the identified difficulties to either adapt and validate available tools or to develop a better suitable tool.
Trial registration
Prospective registration at Open Science Framework (OSF): 10.17605/OSF.IO/PTG4D.
Supplementary Information
The online version contains supplementary material available at 10.1186/s12874-022-01561-5.
Keywords: External validity, Generalizability, Applicability, Measurement properties, Tools, Randomized controlled trial
Background
Systematic reviews are powerful research formats to summarize and synthesize the evidence from primary research in health sciences [1, 2]. In clinical practice, their results are often applied for the development of clinical guidelines and treatment recommendations [3]. Consequently, the methodological quality of systematic reviews is of great importance. In turn, the informative value of systematic reviews depends on the overall quality of the included controlled trials [3, 4]. Accordingly, the evaluation of the internal and external validity is considered a key step in systematic review methodology [4, 5].
Internal validity relates to the systematic error or bias in clinical trials [6] and expresses how methodologically robust the study was conducted. External validity is the inference about the extent to which “a causal relationship holds over variations in persons, settings, treatments and outcomes” [7, 8]. There are plenty of definitions for external validity and a variety of different terms. Hence, external validity, generalizability, applicability, and transferability, among others, are used interchangeably in the literature [9]. Schünemann et al. [10] suggest that: (1) generalizability “may refer to whether or not the evidence can be generalized from the population from which the actual research evidence is obtained to the population for which a healthcare answer is required”; (2) applicability may be interpreted as “whether or not the research evidence answers the healthcare question asked by a clinician or public health practitioner” and (3) transferability is often interpreted as to “whether research evidence can be transferred from one setting to another”. Four essential dimensions are proposed to evaluate the external validity of controlled clinical trials in systematic reviews: patients, treatment (including comparator) variables, settings, and outcome modalities [4, 11]. Its evaluation depends on the specificity of the reviewers´ research question, the review´s inclusion and exclusion criteria compared to the trial´s population, the setting of the study, as well as the quality of reporting these four dimensions.
In health research, however, external validity is often neglected when critically appraising clinical studies [12, 13]. One possible explanation might be the lack of a gold standard for assessing the external validity of clinical trials. Systematic and scoping reviews examined published frameworks and tools for assessing the external validity of clinical trials in health research [9, 12, 14–18]. A substantial heterogeneity of terminology and criteria as well as a lack of guidance on how to assess the external validity of intervention studies was found [9, 12, 15–18]. The results and conclusions of previous reviews were based on descriptive as well as content analysis of frameworks and tools on external validity [9, 14–18]. Although the feasibility of some frameworks and tools was assessed [12], none of the previous reviews evaluated the quality regarding the development and validation processes of the used frameworks and tools.
RCTs are considered the most suitable research design for investigating cause and effect mechanisms of interventions [19]. However, the study design of RCTs is susceptible to a lack of external validity due to the randomization, the use of exclusion criteria and poor willingness of eligible participants to participate [20, 21]. There is evidence that the reliability of external validity evaluations with the same measurement tool differed between randomized and non-randomized trials [22]. In addition, due to differences in requested information from reporting guidelines (e.g. consolidated standards of reporting trials (CONSORT) statement, strengthening the reporting of observational studies in Epidemiology (STROBE) statement), respective items used for assessing the external validity vary between research designs. Acknowledging the importance of RCTs in the medical field, this review focused only on tools developed to assess the external validity of RCTs. The aim was to identify tools to assess the external validity of RCTs in systematic reviews and to evaluate the quality of evidence regarding their measurement properties. Objectives: (1) to identify published measurement tools to assess the external validity of RCTs in systematic reviews; (2) to evaluate the quality of identified tools; (3) to recommend the use of tools to assess the external validity of RCTs in future systematic reviews.
Methods
This systematic review was reported in accordance with the Preferred Reporting Items for Systematic reviews and Meta-Analyses (PRISMA) 2020 Statement [23] and used an adapted version of the PRISMA flow diagram to illustrate the systematic search strategy used to identify clinimetric papers [24]. This study was conducted according to an adapted version of the COnsensus-based Standards for the selection of health Measurement INstruments (COSMIN) methodology for systematic reviews of measurement instruments in health sciences [25–27] and followed recommendations of the JBI manual for systematic reviews of measurement properties [28]. The COSMIN methodology was chosen since this method is comprehensive and validation processes do not differ substantially between patient-reported outcome measures (PROMs) and measurement instruments of other latent constructs. According to the COSMIN authors, it is acceptable to use this methodology for non-PROMs [26]. Furthermore, because of its flexibility, it has already been used in systematic reviews assessing measurement tools which are not health measurement instruments [29–31]. However, adaptations or modifications may be necessary [26]. The type of measurement instrument of interest for the current study were reviewer-reported measurement tools. Pilot tests and adaptation-processes of the COSMIN methodology are described below (see section “Quality assessment and evidence synthesis”). The definition of each measurement property evaluated in the present review is based on COSMIN´s taxonomy, terminology and definition of measurement properties [32]. The review protocol was prospectively registered on March 6, 2020 in the Open Science Framework (OSF) with the registration DOI: 10.17605/OSF.IO/PTG4D [33].
Deviations from the preregistered protocol
One of the aims listed in the review protocol was to evaluate the characteristics and restrictions of measurement tools in terms of terminology and criteria for assessing external validity. This issue has been addressed in two recent reviews with a similar scope [9, 17]. Although our eligibility criteria differed, it was concluded that no novel data was available for the present review to extract, since authors of included tools did not describe the definition or construct of interest or cited the same reports. Therefore, this objective was omitted.
Literature search and screening
A search of the literature was conducted in four databases: PubMed, Scopus, PsycINFO via OVID, and CINAHL via EBSCO. The eligibility criteria and search strategy were predefined in collaboration with a research librarian and is detailed in Table S1 (see Additional file 1). The search strategy was designed according to the COSMIN methodology and consists of the following four key elements: (1) construct (external validity of RCTs from the review authors´perspective), (2) population(s) (RCTs), (3) type of instrument(s) (measurement tools, checklists, surveys etc.), and (4) measurement properties (e.g. validity and reliability) [34]. The four key elements were divided into two main searches (adapted from previous reviews [24, 35, 36]): the phase 1 search contained the first three key elements to identify measurement tools to assess the external validity of RCTs. The phase 2 search aimed to identify studies evaluating the measurement properties of each tool, which was identified and included during phase 1. For this second search, a sensitive PubMed search filter developed by Terwee et al. [37] was applied. Translations of this filter for the remaining databases were taken from the COSMIN website and from other published COSMIN reviews [38, 39] with permission from the authors. Both searches were conducted until March 2021 without restriction regarding the time of publication (databases were searched from inception). In addition, forward citation tracking with Scopus (which is a specialized citation database) was conducted in phase 2 using the ‘cited by’-function. The Scopus search filter was then entered into the ‘search within results’-function. The results from the forward citation tracking with Scopus were added to the database search results into the Rayyan app for screening. Reference lists of the retrieved full-text articles and forward citations with PubMed were scanned manually for any additional studies by one reviewer (AJ) and checked by a second reviewer (KL).
Title and abstract screening for both searches and the full-text screening during phase 2 were performed independently by at least two out of three involved researchers (AJ, KL & TB). For pragmatic reasons, full-text screening and tool/data extraction in phase 1 was performed by one reviewer (AJ) and checked by a second reviewer (TB). This screening method is acceptable for full-text screening as well as data extraction [40]. Data extraction for both searches was performed with a predesigned extraction sheet based on the recommendations of the COSMIN user manual [34]. The Rayyan Qatar Computing Research Institute (QCRI) web app [41] was used to facilitate the screening process (both searches) according to a priori defined eligibility criteria. A pilot test was conducted for both searches in order to reach agreement between the reviewers during the screening process. For this purpose, the first 100 records in phase 1 and the first 50 records in phase 2 (sorted by date) in the Rayyan app were screened by two reviewers independently and subsequently, issues regarding the feasibility of screening methods were discussed in a meeting.
Eligibility criteria
Phase 1 search (identification of tools)
Records were considered for inclusion based on their title and abstract according to the following criteria: (1) records that described the development and or implementation (application), e.g. manual or handbook, of any tool to assess the external validity of RCTs; (2) systematic reviews that applied tools to assess the external validity of RCTs and which explicitly mentioned the tool in the title or abstract; (3) systematic reviews or any other publication potentially using a tool for external validity assessment, but the tool was not explicitly mentioned in the title or abstract; (4) records that gave other references to, or dealt with, tools for the assessment of external validity of RCTs, e.g. method papers, commentaries.
The full-text screening was performed to extract or to find references to potential tools. If a tool was cited, but not presented or available in the full-text version, the internet was searched for websites on which this tool was presented, to extract and review for inclusion. Potential tools were extracted and screened for eligibility as follows: measurement tools aiming to assess the external validity of RCTs and designed for implementation in systematic reviews of intervention studies. Since the terms external validity, applicability, generalizability, relevance and transferability are used interchangeably in the literature [10, 11], tools aiming to assess one of these constructs were eligible. Exclusion criteria: (1) The multidimensional tool included at least one item related to external validity, but it was not possible to assess and interpret external validity separately. (2) The tool was developed exclusively for study designs other than RCTs. (3) The tool contained items assessing information not requested in the CONSORT-Statement [42] (e.g. cost-effectiveness of the intervention, salary of health care provider) and these items could not be separated from items on external validity. (4) The tool was published in a language other than English or German. (5) The tool was explicitly designed for a specific medical profession or field and cannot be used in other medical fields.
Phase 2 search (identification of reports on the measurement properties of included tools)
For the phase 2 search, records evaluating the measurement properties of at least one of the included measurement tools were selected. Reports only using the measurement tool as an outcome measure without the evaluation of at least one measurement property were excluded. If a report did not evaluate the measurement properties of a tool, it was also excluded. Hence, reports providing data on the validity or the reliability of sum-scores of multidimensional tools, only, were excluded if the dimension “external validity” was not evaluated separately.
If there was missing data or information (phase 1 or phase 2), the corresponding authors were contacted.
Quality assessment and evidence synthesis
All included reports were systematically evaluated: (1) for their methodological quality by using the adapted COSMIN Risk of Bias (RoB) checklist [25] and (2) against the updated criteria for good measurement properties [26, 27]. Subsequently, all available evidence for each measurement property for the individual tool were summarized and rated against the updated criteria for good measurement properties and graded for their certainty of evidence, according to COSMIN´s modified GRADE approach [26, 27]. The quality assessment was performed by two independent reviewers (AJ & JB). In case of irreconcilable disagreement, a third reviewer (TB) was consulted to reach consensus.
The COSMIN RoB checklist is a tool [25, 27, 32, 43] designed for the systematic evaluation of the methodological quality of studies assessing the measurement properties of health measurement instruments [25]. Although this checklist was specifically developed for systematic reviews of PROMs, it can also be used for reviews of non-PROMs [26] or measurement tools of other latent constructs [28, 29]. As mentioned in the COSMIN user manual, adaptations for some items in the COSMIN RoB checklist might be necessary, in relation to the construct being measured [34]. Therefore, pilot tests were performed for the assessment of measurement properties of tools assessing the quality of RCTs before data extraction, aiming to ensure feasibility during the planned evaluation of the included tools. The pilot tests were performed with a random sample of publications on measurement instruments of potentially relevant tools. After each pilot test, results and problems regarding the comprehensibility, relevance and feasibility of the instructions, items, and response options in relation to the construct of interest were discussed. Where necessary, adaptations and/or supplements were added to the instructions of the evaluation with the COSMIN RoB checklist. Saturation was reached after two rounds of pilot testing. Substantial adaptations or supplements were required for Box 1 (‘development process’) and Box 10 (‘responsiveness’) of the COSMIN RoB checklist. Minor adaptations were necessary for the remaining boxes. The specification list, including the adaptations, can be seen in Table S2 (see Additional file 2). The methodological quality of included studies was rated via the four-point rating scale of the COSMIN RoB checklist as “inadequate”, “doubtful”, “adequate”, or “very good” [25]. The lowest score of any item in a box is taken to determine the overall rating of the methodological quality of each single study on a measurement property [25].
After the RoB-assessment, the result of each single study on a measurement property was rated against the updated criteria for good measurement properties for content validity [27] and for the remaining measurement properties [26] as “sufficient” (+), “insufficient” (-), or “indeterminate” (?). These ratings were summarized and an overall rating for each measurement property was given as “sufficient” (+), “insufficient” (-), “inconsistent” (±), or “indeterminate” (?). However, the overall rating criteria for good content validity was adapted to the research topic of the present review. This method usually requires an additional subjective judgement from reviewers [44]. Since one of the biggest limitations within this field of research is the lack of consensus on terminology and criteria as well as on how to assess the external validity [9, 12], a reviewers’ subjective judgement was considered inappropriate. After this issue was also discussed with one leading member of the COSMIN steering committee, the reviewers’ rating was omitted. A “sufficient” (+) overall rating was given if there was evidence of face or content validity of the final version of the measurement tool assessed by a user or expert panel. Otherwise, the rating “indeterminate” (?) or “insufficient” (-) was used for the content validity.
The summarized evidence for each measurement property for the individual tool was graded using COSMIN´s modified GRADE approach [26, 27]. The certainty (quality) of evidence was graded as “high”, “moderate”, “low”, or “very low” according to the approach for content validity [27] and for the remaining measurement properties [26]. COSMIN´s modified GRADE approach distinguishes between four factors influencing the certainty of evidence: risk of bias, inconsistency, indirectness, and imprecision. The starting point for all measurement properties is high certainty of evidence and is subsequently downgraded by one to three levels per factor when there is risk of bias, (unexplained) inconsistency, imprecision (not considered for content validity [27]), or indirect results [26, 27]. If there is no study on the content validity of a tool, the starting point for this measurement property is “moderate” and is subsequently downgraded depending on the quality of the development process [27]. The grading process according to COSMIN [26, 27] is described in Table S4. Selective reporting bias or publication bias is not taken into account in COSMIN´s modified GRADE approach, because of a lack of registries for studies on measurement properties [26].
The evidence synthesis was performed qualitatively according to the COSMIN methodology [26]. If several reports revealed homogenous quantitative data (e.g. same statistics, population) on internal consistency, reliability, measurement error or hypotheses testing of a measurement tool, pooling the results was considered using generic inverse variance (random effects) methodology and weighted means as well as 95% confidence intervals for each measurement property [34]. No subgroup analysis was planned. However, statistical pooling was not possible in the present review.
We used three criteria for the recommendation of a measurement tool in accordance with the COSMIN manual: (A) “Evidence for sufficient content validity (any level) and at least low-quality evidence for sufficient internal consistency” for a tool to be recommended; (B) tool “categorized not in A or C” and further research on the quality of this tool is required to be recommended; and (C) tool with “high quality evidence for an insufficient psychometric property” and this tool should not be recommended [26].
Results
Literature search and selection process
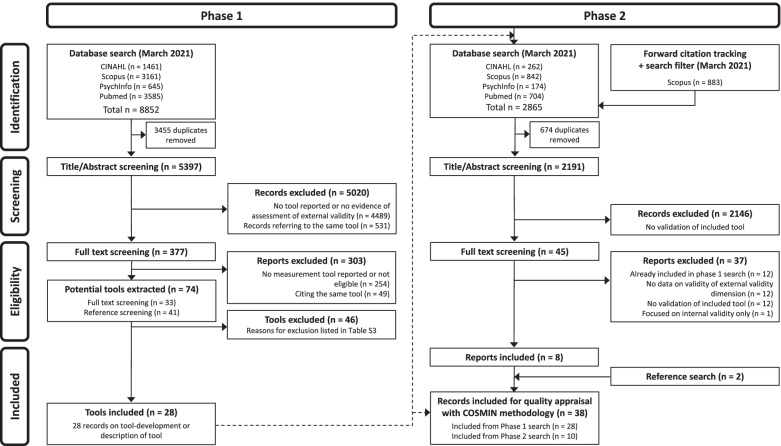
Figure 1 shows the selection process. In the phase 1 search, from 5397 non-duplicate records, 5020 irrelevant records were excluded. 377 reports were screened, and 74 potential tools were extracted. After reaching consensus, 46 tools were excluded (reasons for exclusion are presented in Table S3 (see Additional file 3)) and finally 28 were included. Any disagreements during the screening process were resolved through discussion. There was one case during the full-text screening process in the phase 1 search, in which the whole review team was involved to reach consensus about the inclusion/exclusion of two tools (Agency for Healthcare Research and Quality (AHRQ) criteria for applicability and TRANSFER approach, both listed in Table S3).
Fig. 1.
Flow diagram “of systematic search strategy used to identify clinimetric papers”[24]
In the phase 2 search, 2191 non-duplicate records were screened for title and abstract. 2146 records were excluded as they did not assess any measurement property of the included tools. Of 45 reports, 8 reports were included. The most common reason for exclusion was that reports evaluating the measurement properties of multidimensional tools did not evaluate external validity as a separate dimension. For example, one study assessing the interrater reliability of the GRADE method [45] was identified during full-text screening, but had to be excluded, since it did not provide separate data on the reliability of the indirectness domain (representing external validity). Two additional reports were included during reference screening. Any disagreements during the screening process were resolved through discussion.
Thirty-eight publications on the development or evaluation of the measurement properties of 28 included tools were included for quality appraisal according to the adapted COSMIN guidelines.
We contacted the corresponding authors of three reports [46–48] for additional information. One corresponding author did reply [48].
Methods to assess the external validity of RCTs
During full-text screening in phase 1, several concepts to assess the external validity of RCTs were found (Table 1). Two main concepts were identified: experimental/statistical methods and non-experimental methods. The experimental/statistical methods were summarized and collated into five subcategories giving a descriptive overview of the different approaches used to assess the external validity. However, according to our eligibility criteria, these methods were excluded, since they were not developed for the use in systematic reviews of interventions. In addition, a comparison of these methods as well as appraisal of risk of bias with the COSMIN RoB checklist would not have been feasible. Therefore, the experimental/statistical methods described below were not included for further evaluation.
Table 1.
Experimental/statistical methods to evaluate the EV of RCTs
| 1. Comparing differences of characteristics and/or NNT analysis from not-enrolled eligible patients with enrolled patients [49–52] |
| 2. Conduction of observational studies to assess the “real world” applicability of RCTs [20, 53, 54] |
| 3. Meta-analysis of patient characteristics data from RCTs [55, 56] |
|
4. Comparison of data from RCTs with data from health record database and/or other epidemiological data: |
| 5. Review of exclusion criteria in RCTs which would limit the EV [62] |
Abbreviations: EV external validity, NNT numbers needed to treat, RCT randomized controlled trial
For non-experimental methods, please refer to Table 2
Characteristics of included measurement tools
The included tools and their characteristics are listed in Table 2. Overall, the tools were heterogenous with respect to the number of items or dimensions, response options and development processes. The number of items varied between one and 26 items and the response options varied between 2-point-scales to 5-point-scales. Most tools used a 3-point-scale (n = 20/28, 71%). For 14/28 (50%) of the tools, the development was not described in detail [63–76]. Seven review authors appear to have developed their own tool but did not provide any information on the development process [63–68, 71].
Table 2.
Characteristics of included tools
| Dimension and/or tool | Authors | Construct(s), as described by the authors | Target population | Domains, nr. of items | Response options | Development and validation |
|---|---|---|---|---|---|---|
| “Applicability”-dimension of LEGEND | Clark et al. [77] | Applicability of results to treating patients | P1: RCTs and CCTs P2: reviewers and clinicians | 3 items | 3-point-scale | Deductive and inductive item-generation. Tool was pilot tested among an interprofessional group of clinicians. |
| “Applicability”-dimension of Carr´s evidence-grading scheme | Carr et al. [63] | Generalizability of study population |
P1: clinical trials P:2 authors of SRs |
1 item | 3-point-classification-scale | No specific information on tool development. |
| Bornhöft´s checklist | Bornhöft et al. [78] | External validity (EV) and Model validity (MV) of clinical trials |
P1: clinical trials P2: authors of SRs |
4 domains with 26 items for EV and MV each | 4-point-scale | Development with a comprehensive, deductive item-generation from the literature. Pilot-tests were performed, but not for the whole scales. |
| Cleggs´s external validity assessment | Clegg et al. [64] | Generalizability of clinical trials to England and Wales |
P1: clinical trials P2: authors of SRs and HTAs |
5 items | 3-point-scale | No specific information on tool development |
| Clinical applicability | Haraldsson et al. [66] | Report quality and applicability of intervention, study population and outcomes |
P1: RCTs P2: reviewers |
6 items | 3-point-scale and 4-point-scale | No specific information on tool development |
| Clinical Relevance Instrument | Cho & Bero [79] | Ethics and Generalizability of outcomes, subjects, treatment and side effects |
P1: clinical trials P2: reviewers |
7 items | 3-point-scale |
Tool was pilot tested on 10 drug studies. Content validity was confirmed by 7 reviewers with research experience. - interrater reliability: ICC = 0.56 (n = 127) [80] |
| “Clinical Relevance” according to the CCBRG | Van Tulder et al. [81] | Applicability of patients, interventions and outcomes |
P1: RCTs P2: authors of SRs |
5 items | 3-point-scale (Staal et al., 2008) | Deductive item-generation for Clinical Relevance. Results were discussed in a workshop. After two rounds, a final draft was circulated for comments among editors of the CCBRG. |
| Clinical Relevance Score | Karjalainen et al. [68] | Report quality and applicability of results |
P1: RCTs P2: reviewers |
3 items | 3-point-scale | No specific information on tool development. |
| Estrada´s applicability assessment criteria | Estrada et al. [82] | Applicability of population, intervention, implementation and environmental context to Latin America |
P1: RCTs P2: reviewers |
5 domains with 8 items | 3-point-scale for each domain | Deductive item generation from the review by Munthe-Kaas et al. [17]. Factors and items were adapted, and pilot tested by the review team (n = 4) until consensus was reached. |
| EVAT (External Validity Assessment Tool) | Khorsan & Crawford [83] | External validity of participants, intervention, and setting |
P1: RCTs and non-randomized studies P2: reviewers |
3 items | 3-point-scale | Deductive item-generation. Tool developed based on the GAP-checklist [76] and the Downs and Black-checklist [22]. Feasibility was tested and a rulebook was developed but not published. |
| “External validity”-dimension of the Downs & Black-Checklist | Downs & Black [22] | Representativeness of study participants, treatments and settings to source population or setting |
P1: RCTs and non-randomised studies P2: reviewers |
3 items | 3-point-scale |
Deductive item-generation, pilot test and content validation of pilot version. Final version tested for: - internal consistency: KR-20 = 0.54 (n = 20), - reliability: test-retest: k = -0.05-0.48 and 10–15% disagreement (measurement error) (n = 20), [22] interrater reliability: k = -0.08-0.00 and 5–20% disagreement (measurement error) (n = 20) [22]; ICC = 0.76 (n = 20) [84] |
| “External validity”-dimension of Foy´s quality checklist | Foy et al. [65] | External validity of patients, settings, intervention and outcomes |
P1: intervention studies P2: reviewers |
6 items | not clearly described | Deductive item-generation. No further information on tool development. |
| “External validity”-dimension of Liberati´s quality assessment criterias | Liberati et al. [69] | Report quality and generalizability |
P1: RCTS P2: reviewers |
9 items | dichotomous and 3-point-scale | Tool is a modified version of a previously developed checklist [85] with additional inductive item-generation. No further information on tool development. |
| “External validity”-dimension of Sorg´s checklist | Sorg et al. [71] | External validity of population, interventions, and endpoints |
P1: RCTs P2: reviewers |
4 domains with 11 items | not clearly described | Developed based on Bornhöft et al. [78] No further information on tool development. |
| “external validity”-criteria of the USPSTF | USPSTF Procedure manual [73] | Generalizability of study population, setting and providers for US primary care |
P1: clinical studies P2: USPSTF reviewers |
3 items |
Sum-score- rating: 3-point-scale |
Tool developed for USPSTF reviews. No specific information on tool development. - interrater reliability: ICC = 0.84 (n = 20) [84] |
| FAME (Feasibility, Appropriateness, Meaningfulness and Effectiveness) scale | Averis et al. [70] | Grading of recommendation for applicability and ethics of intervention |
P1: intervention studies P2: reviewers |
4 items | 5-point-scale | The FAME framework was created by a national group of nursing research experts. Deductive and inductive item-generation. No further information on tool development. |
|
GAP (Generalizability, Applicability and Predictability) checklist |
Fernandez-Hermida et al. [76] |
External validity of population, setting, intervention and endpoints |
P1: RCTs P2: Reviewers |
3 items | 3-point-scale | No specific information on tool development. |
| Gartlehner´s tool | Gartlehner et al. [86] | To distinguish between effectiveness and efficacy trials |
P1: RCTs P2: reviewers |
7 items | Dichotomous |
Deductive and inductive item-generation. - criterion validity testing with studies selected by 12 experts as gold standard.: specificity = 0.83, sensitivity = 0.72 (n = 24) - measurement error: 78.3% agreement (n = 24) - interrater reliability: k = 0.42 (n = 24) [86]; k = 0.11–0.81 (n = 151) [87] |
| Green & Glasgow´s external validity quality rating criteria | Green & Glasgow [88] | Report quality for generalizability | P1: trials (not explicitly described) P2: reviewers | 4 Domains with 16 items | Dichotomous |
Deductive item-generation. Mainly based on the Re-Aim framework.[89] - interrater reliability: ICC = 0.86 (n = 14) [90] - discriminative validity: TREND studies report on 77% and non-TREND studies report on 54% of scale items (n = 14) [90] - ratings across included studies (n = 31) [91], no hypothesis was defined |
| “Indirecntess”-dimension of the GRADE handbook | Schünemann et al. [92] | Differences of population, interventions, and outcome measures to research question |
P1: intervention studies P2: authors of SRs, clinical guidelines and HTAs |
4 items |
Overall: 3-point-scale (downgrading options) |
Deductive and inductive item-generation, pilot-testing with 17 reviewers (n = 12) [48]. - interrater reliability: ICC = 0.00–0.13 (n > 100) [93] |
| Loyka´s external validity framework | Loyka et al. [75] |
Report quality for generalizability of research in psychological science |
P1: intervention studies P2: researchers |
4 domains with 15 items |
Dichotomous |
Deductive item generation (including Green & Glasgow [88]) and adaptation for psychological science. No further information on tool development. - measurement error: 60-100% agreement (n = 143) |
| Modified “Indirectness” of the Checklist for GRADE | Meader et al. [94] | Differences of population, interventions, and outcome measures to research question. |
P1: meta-analysis of RCTs P2: authors of SRs, clinical guidelines and HTAs |
5 items |
Item-level: 2-and 3-point-scale Overall: 3-point-scale (grading options) |
Developed based on GRADE method, two phase pilot-tests, - interrater reliability: kappa was poor to almost perfect on item-level [94] and k = 0.69 for overall rating of indirectness (n = 29) [95] |
| external validity checklist of the NHMRC handbook | NHMRC handbook [74] | external validity of an economic study |
P1: clinical studies P2: clinical guideline developers, reviewers |
6 items | 3-point-scale | No specific information on tool development. |
| revised GATE in NICE manual (2012) | NICE manual [72] | Generalizability of population, interventions and outcomes |
P1: intervention studies P2: reviewers |
2 domains with 4 items | 3-point-scale and 5-point-scale | Based on Jackson et al. [96] No specific information on tool development. |
| RITES (Rating of Included Trials on the Efficacy-Effectiveness Spectrum) | Wieland et al. [47] | To characterize RCTs on an efficacy-effectiveness continuum. |
P1: RCTs P2: reviewers |
4 items | 5-point-likert-scale |
Deductive and inductive item-generation, modified Delphi procedure with 69–72 experts, pilot testing in 4 Cochrane reviews, content validation with Delphi procedure and core expert group (n = 14) [47], - interrater reliability: ICC = 0.54-1.0 (n = 22) [97] - convergent validity with PRECIS 2 tool: r = 0.55 correlation (n = 59) [97] |
| Section A (Selection Bias) of EPHPP (Effective Public health Practice Project) tool | Thomas et al. [98] | Representativeness of population and participation rate. |
P1: clinical trials P2: reviewers |
2 items |
Item-level: 4-point-scale and 5-point-scale Overall: 3-point-scale |
Deductive item-generation, pilot-tests, content validation by 6 experts, - convergent validity with Guide to Community Services (GCPS) instrument: 52.5–87.5% agreement (n = 70) [98] - test-retest reliability: k = 0.61–0.74 (n = 70) [98] k = 0.60 (n = 20) [99] |
| Section D of the CASP checklist for RCTs | CASP Programme [100] | Applicability to local population and outcomes |
P1: RCTs P2: participants of workshops, reviewers |
2 items | 3-point-scale | Deductive item-generation, development and pilot-tests with group of experts. |
| Whole Systems research considerations´ checklist | Hawk et al. [67] | Applicability of results to usual practice | P1: RCTs P2: Reviewers (developed for review) | 7 domains with 13 items |
Item-level: dichotomous Overall: 3-point-scale |
Deductive item-generation. No specific information on tool development. |
Abbreviations: CASP Critical Appraisal Skills Programme, CCBRG Cochrane Collaboration Back Review Group, CCT controlled clinical trial, GATE Graphical Appraisal Tool for Epidemiological Studies, GRADE Grading of Recommendations Assessment, Development and Evaluation, HTA Health Technology Assessment, ICC intraclass correlation, LEGEND Let Evidence Guide Every New Decision, NICE National Institute for Health and Care Excellence, PRECIS PRagmatic Explanatory Continuum Indicator Summary, RCT randomized controlled trial, TREND Transparent Reporting of Evaluations with Nonrandomized Designs, USPSTF U.S. Preventive Services Task Force
The constructs aimed to be measured by the tools or dimensions of interest are diverse. Two of the tools focused on the characterization of RCTs on an efficacy-effectiveness continuum [47, 86], three tools focused predominantly on the report quality of factors essential to external validity [69, 75, 88] (rather than the external validity itself), 18 tools aimed to assess the representativeness, generalizability or applicability of population, setting, intervention, and/or outcome measure to usual practice [22, 63–65, 70, 71, 73, 74, 76–78, 81–83, 92, 94, 100], and five tools seemed to measure a mixture of these different constructs related to external validity [66, 68, 72, 79, 98]. However, the construct of interest of most tools was not described adequately (see below).
Measurement properties
The results of the methodological quality assessment according to the adapted COSMIN RoB checklist are detailed in Table 3. If all data on hypotheses testing in an article had the same methodological quality rating, they were combined and summarized in Table 3 in accordance with the COSMIN manual [34]. The results of the ratings against the updated criteria for good measurement properties and the overall certainty of evidence, according to the modified GRADE approach, can be seen in Table 4. The detailed grading is described in Table S4 (see Additional file 4). Disagreements between reviewers during the quality assessment were resolved through discussion.
Table 3.
Methodological quality of included studies based on COSMIN risk of bias (RoB) checklist
| Tool or dimension | Report | Content validity | Internal structure | Remaining measurement properties | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Development |
2.1
CB |
2.2
RE |
2.3
CH |
Structural validity | Internal consistency | Cross-cultural validity | Reliability | Measurement error | Criterion validity | Construct validity | ||
| “Applicability”-dimension of LEGEND | Clark et al. [77] | doubtful | ||||||||||
| “Applicability”-dimension of Carr´s evidence-grading scheme | Carr et al. [63] | inadequate | ||||||||||
| Bornhöft´s checklist | Bornhöft et al. [78] | inadequate | ||||||||||
| Cleggs´s external validity assessment | Clegg et al. [64] | inadequate | ||||||||||
| Clinical Applicability | Haraldsson et al. [66] | inadequate | ||||||||||
| Clinical Relevance Instrument | Cho & Bero [79] | doubtful | doubtful | doubtful | doubtful | |||||||
| Cho & Bero [80] | adequate | |||||||||||
| Clinical Relevance according to the CCBRG | Van Tulder et al. [81] | inadequate | doubtful | doubtful | doubtful | |||||||
| Clinical relevance scores (Karjalainen´s) | Karjalainen et al. [68] | inadequate | ||||||||||
| Estrada´s applicability assessment criteria | Estrada et al. [82] | doubtful | ||||||||||
| EVAT | Khorsan & Crawford [83] | doubtful | ||||||||||
| “External validity”-dimension of the Downs & Black Checklist | Downs & Black [22] | doubtful | doubtful | doubtful | doubtful | doubtful | very gooda | inadequatea | adequate | |||
| very gooda | inadequatea | |||||||||||
| O´Connor et al. [84] | very good | |||||||||||
| “External validity”-dimension of Foy´s quality checklist | Foy et al. [65] | inadequate | ||||||||||
| “External validity”-dimension of Liberati´s quality assessment criteria | Liberati et al. [69] | inadequate | ||||||||||
| “External validity”-dimension of Sorg´s checklist | Sorg et al. [71] | inadequate | ||||||||||
| “External validity”-criteria of the USPSTF | USPSTF manual [73] | inadequate | ||||||||||
| O´Connor et al. [84] | very good | |||||||||||
| FAME scale | Averis et al. [70] | inadequate | ||||||||||
| GAP checklist | Fernandez-Hermida et al. [76] | inadequate | ||||||||||
| Gartlehner´s tool | Gartlehner et al. [86] | inadequate | very good | adequate | adequate | |||||||
| Zettler et al. [87] | very good | |||||||||||
| Green & Glasgow´s external validity quality rating criteria | Green & Glasgow [88] | inadequate | ||||||||||
| Laws et al. [91] | doubtful | |||||||||||
| Mirza et al. [90] | adequate | doubtful | ||||||||||
| “Indirecntess”-dimension from the GRADE Handbook [92] | Atkins et al. [48] | adequate | ||||||||||
| Wu et al. [93] | inadequate | |||||||||||
| Loyka´s external validity framework | Loyka et al.75 | doubtful | adequate | |||||||||
| modified “Indirectness” of the Checklist for GRADE | Meader et al. [94] | adequate | adequateb | |||||||||
| Llewellyn et al. [95] | ||||||||||||
| External validity checklist of the NHMRC Handbook | NHMRMC Handbook [74] | inadequate | ||||||||||
| revised GATE in the NICE manual | NICE Guideline [72] | inadequate | ||||||||||
| RITES tool | Wieland et al. [47] | adequate | adequate | very good | very good | |||||||
| Aves et al. [97, 101] | inadequate | very good | ||||||||||
| “Selection Bias”-dimension (Section A) of the EPHPP tool | Thomas et al. [98] | inadequate | doubtful | doubtful | doubtful | doubtful | doubtful | |||||
| Armijo-Olivo et al. [99] | doubtful | |||||||||||
| Section D of the CASP checklist for RCTs | Critical Appraisal Skills Programme [100] | inadequate | ||||||||||
| Whole Systems research considerations´checklist | Hawk et al. [67] | inadequate | ||||||||||
Fields left blank indicate that those measurement properties were not assessed by the study authors
Abbreviations: CB comprehensibility, RE relevance, CV comprehensiveness, CCBRG Cochrane Collaboration Back Review Group, EPHPP Effective Public Health Practice Project, EVAT External Validity Assessment Tool, FAME Feasibility, Appropriateness, Meaningfulness and Effectiveness, GAP Generalizability, Applicability and Predictability; GATE Graphical Appraisal Tool for Epidemiological Studies, GRADE Grading of Recommendations Assessment, Development and Evaluation; LEGEND Let Evidence Guide Every New Decision, NHMRC National Health & Medical Research Council, NICE National Institute for Health and Care Excellence, RITES Rating of Included Trials on the Efficacy-Effectiveness Spectrum, USPSTF U.S. Preventive Services Task Force
a two studies on reliability (test-retest & inter-rater reliability) in the same article
b results from the same study on reliability reported in two articles [94, 95]
Table 4.
Criteria for good measurement properties & certainty of evidence according to the modified GRADE method
| Tool or dimension | Content validity | Internal consistency | Reliability | Measurement error | Criterion validity | Construct validity |
|---|---|---|---|---|---|---|
| “Applicability”-dimension of LEGEND [77] | ||||||
| CGMP | (?) | |||||
| GRADE | Low | |||||
| “Applicability”-dimension of Carr´s evidence-grading scheme [63] | ||||||
| CGMP | (?) | |||||
| GRADE | Very Low | |||||
| Bornhöft´s checklist [78] | ||||||
| CGMP | (?) | |||||
| GRADE | Very Low | |||||
| Cleggs´s external validity assessment [64] | ||||||
| CGMP | (?) | |||||
| GRADE | Very Low | |||||
| Clinical Applicability [66] | ||||||
| CGMP | (?) | |||||
| GRADE | Very Low | |||||
| Clinical Relevance Instrument [79, 80] | ||||||
| CGMP | (?) | (-) | ||||
| GRADE | Moderate | Moderate | ||||
| Clinical Relevance according to the CCBRG [81] | ||||||
| CGMP | (?) | |||||
| GRADE | Moderate | |||||
| Clinical relevance scores [68] | ||||||
| CGMP | (?) | |||||
| GRADE | Very Low | |||||
| Estrada´s applicability assessment criteria [82] | ||||||
| CGMP | (?) | |||||
| GRADE | Very Low | |||||
| External Validity Assessment Tool (EVAT) [83] | ||||||
| CGMP | (?) | |||||
| GRADE | Low | |||||
| “External validity”-dimension of the Downs & Black Checklist [22, 84] | ||||||
| CGMP | (?) | (?) | (±)a | (?) | (-) | |
| GRADE | Moderate | Very Low | Moderate | Very Low | Very Low | |
| “External validity”-dimension of Foy´s quality checklist [65] | ||||||
| CGMP | (?) | |||||
| GRADE | Very Low | |||||
| “External validity”-dimension of Liberati´s quality assessment criteria [69] | ||||||
| CGMP | (?) | |||||
| GRADE | Very Low | |||||
| “External validity”-dimension of Sorg´s checklist [71] | ||||||
| CGMP | (?) | |||||
| GRADE | Very Low | |||||
| “External validity”-criteria of the USPSTF manual [73, 84] | ||||||
| CGMP | (?) | (+) | ||||
| GRADE | Very Low | Very Low | ||||
| Feasibility, Appropriateness, Meaningfulness and Effectiveness (FAME) scale [70] | ||||||
| CGMP | (?) | |||||
| GRADE | Very Low | |||||
| Generalizability, Applicability and Predictability (GAP) checklist [76] | ||||||
| CGMP | (?) | |||||
| GRADE | Very Low | |||||
| Gartlehner´s tool [86, 87] | ||||||
| CGMP | (?) | (-) | (?) | (+) | ||
| GRADE | Very Low | Moderate | Very Low | Very Low | ||
| Green & Glasgow´s external validity quality rating criteria [88, 90, 91] | ||||||
| CGMP | (?) | (+) | (-) | |||
| GRADE | Very Low | Very Low | Very Low | |||
| “Indirecntess”-dimension from the GRADE Handbook [48, 92, 93] | ||||||
| CGMP | (?) | (-) | ||||
| GRADE | Moderate | Very Low | ||||
| Loyka´s external validity framework [75] | ||||||
| CGMP | (?) | (?) | ||||
| GRADE | Very Low | Low | ||||
| modified “Indirectness” of the Checklist for GRADE [94, 95] | ||||||
| CGMP | (?) | (-) | ||||
| GRADE | Low | Very Low | ||||
| External validity checklist of the National Health & Medical Research Council (NHMRC) Handbook [74] | ||||||
| CGMP | (?) | |||||
| GRADE | Very Low | |||||
| revised Graphical Appraisal Tool for Epidemiological Studies (GATE) [72] | ||||||
| CGMP | (?) | |||||
| GRADE | Very Low | |||||
| Rating of Included Trials on the Efficacy-Effectiveness Spectrum (RITES) [47, 97] | ||||||
| CGMP | (+) | (+) | (+) | |||
| GRADE | Moderate | Very Low | Low | |||
| “Selection Bias”-dimension (Section A) of EPHPP [98, 99] | ||||||
| CGMP | (?) | (-) | (+) | |||
| GRADE | Moderate | Low | Very Low | |||
| Section C of the CASP checklist for RCTs [100] | ||||||
| CGMP | (?) | |||||
| GRADE | Very Low | |||||
| Whole Systems research considerations´checklist [67] | ||||||
| CGMP | (?) | |||||
| GRADE | Very Low | |||||
Abbreviations: CCBRG Cochrane Collaboration Back Review Group, CGMP criteria for good measurement properties, EPHPP Effective Public Health Practice Project, GRADE Grading of Recommendations Assessment, Development and Evaluation, LEGEND Let Evidence Guide Every New Decision, NICE National Institute for Health and Care Excellence, USPSTF U.S. Preventive Services Task Force;
Criteria for good measurement properties: (+) = sufficient; (?) = indeterminate; (-) = insufficient, (±) or inconsistent
Level of evidence according to the modified GRADE approach: high, moderate, low, or very low evidence.Note: the measurement properties “structural validity” and “cross-cultural validity” are not presented in this table, since they were not assessed in any of the included studies
Fields left blank indicate that those measurement properties were not assessed by the study authors
a please refer to Table S4 for more information on reliability of the “external validity”-dimension of the Downs & Black checklist
Content validity
The methodological quality of the development process was “inadequate” for 19/28 (68%) of the included tools [63–66, 68–74, 76, 78, 81, 88, 98, 100]. This was mainly due to insufficient description of the construct to be measured, the target population, or missing pilot tests. Six development studies had a “doubtful” methodological quality [22, 75, 77, 79, 82, 83] and three had an “adequate” methodological quality [47, 48, 94].
There was evidence for content validation of five tools [22, 47, 79, 81, 98]. However, the methodological quality of the content validity studies was “adequate” and “very good” only for the Rating of Included Trials on the Efficacy-Effectiveness Spectrum (RITES) tool [47] and “doubtful” for Cho´s Clinical Relevance Instrument [79], the “external validity”-dimension of the Downs & Black-checklist [22], the “Selection Bias”-dimension of the Effective Public Health Practice Project (EPHPP) tool [98], and the “Clinical Relevance” tool [81]. The overall certainty of evidence for content validity was “very low” for 19 tools (mainly due to very serious risk of bias and serious indirectness) [63–76, 78, 82, 86, 88, 100], “low” for three tools (mainly due to serious risk of bias or serious indirectness) [77, 83, 94] and “moderate” for six tools (mainly due to serious risk of bias or serious indirectness) [22, 47, 79, 81, 92, 98]. All but one tool had an “indeterminate” content validity. The RITES tool [47] had “moderate” certainty of evidence for “sufficient” content validity.
Internal consistency
One study assessed the internal consistency for one tool (“external validity”-dimension of the Downs & Black-checklist) [22]. The methodological quality of this study was “doubtful” due to a lack of evidence on unidimensionality (or structural validity). Thus, this tool had a “very low” certainty of evidence for “indeterminate” internal consistency. Reasons for downgrading were a very serious risk of bias and imprecision.
Reliability
Out of 13 studies assessing the reliability of 9 tools, eleven evaluated the interrater reliability [80, 84, 86, 87, 90, 93–95, 97, 99], one the test-retest reliability [98], and one evaluated both [22]. Two studies had an “inadequate” [93, 101], two had a “doubtful” [98, 99], three had an “adequate” [80, 91, 94, 95], and six had a “very good” methodological quality [22, 84, 86, 87]. The overall certainty of evidence was “very low” for five tools (reasons for downgrading please refer to Table S4) [47, 73, 88, 92, 94]. The certainty of evidence was “low” for the “Selection Bias”-dimension of the EPHPP tool (due to serious risk of bias and imprecision) [98] and “moderate” for Gartlehner´s tool [86], the “external validity”-dimension of the Downs & Black-checklist [22], as well as the clinical relevance instrument [79] (due to serious risk of bias and indirectness).
Out of nine evaluated tools, the Downs & Black-checklist [22] had “inconsistent” results on reliability. The Clinical Relevance Instrument [79], Gartlehner´s tool [86], the “Selection Bias”-dimension of the EPHPP [98], the indirectness-dimension of the GRADE handbook [92] and the modified indirectness-checklist [94] had an “insufficient” rating for reliability. Green & Glasgow´s tool [88], the external validity dimension of the U.S. Preventive Services Task Force (USPSTF) manual [73] and the RITES tool [47] had a “very low” certainty of evidence for “sufficient” reliability.
Measurement error
Measurement error was reported for three tools. Two studies on measurement error of Gartlehner´s tool [86] and Loyka´s external validity framework [75], had an “adequate” methodological quality. Two studies on measurement error of the external validity dimension of the Downs & Black-checklist [22] had an “inadequate” methodological quality. However, all three tools had a “very low” certainty of evidence for “indeterminate” measurement error. Reasons for downgrading were risk of bias, indirectness, and imprecision due to small sample sizes.
Criterion validity
Criterion validity was reported only for Gartlehner´s tool [86]. Although there was no gold standard available to assess the criterion validity of this tool, the authors used expert opinion as the reference standard. The study assessing this measurement property had an “adequate” methodological quality. The overall certainty of evidence was “very low” for “sufficient” criterion validity due to risk of bias, imprecision, and indirectness.
Construct validity (hypotheses testing)
Five studies [22, 90, 91, 97, 98] reported on the construct validity of four tools. Three studies had a “doubtful” [90, 91, 98], one had an “adequate” [22] and one had a “very good” [97] methodological quality. The overall certainty of evidence was “very low” for three tools (mainly due to serious risk of bias, imprecision and serious indirectness) [22, 88, 98] and “low” for one tool (due to imprecision and serious indirectness) [47]. The “Selection-Bias”-dimension of the EPHPP tool [98] had “very low” certainty of evidence for “sufficient” construct validity and the RITES tool [47] had “low” certainty of evidence for “sufficient” construct validity. Both, the Green & Glasgow´s tool [88] and the Downs & Black-checklist [22], had “very low” certainty of evidence for “insufficient” construct validity.
Structural validity and cross-cultural validity were not assessed in any of the included studies.
Discussion
Summary and interpretation of results
To our knowledge this is the first systematic review identifying and evaluating the measurement properties of tools to assess the external validity of RCTs. A total of 28 tools were included. Overall, for more than half (n = 17/28, 61%) of the included tools the measurement properties were not reported. Only five tools had at least one “sufficient” measurement property. Moreover, the development process was not described in 14/28 (50%) of the included tools. Reliability was assessed most frequently (including inter-rater and/or test-retest reliability). Only three of the included tools had “sufficient” reliability (“very low” certainty of evidence) [47, 73, 88]. Hypotheses testing was evaluated in four tools, with half of them having “sufficient” construct validity (“low” and “very low” certainty of evidence) [47, 98]. Measurement error was evaluated in three tools, all with an “indeterminate” quality rating (“low” and “very low” certainty of evidence) [22, 75, 86]. Criterion validity was evaluated for one tool, having “sufficient” with “very low” certainty of evidence [86]. The RITES tool [47] was the measurement tool with the strongest evidence for validity and reliability. Its content validity, based on international expert-consensus, was “sufficient” with “moderate” certainty of evidence, while reliability and construct validity were rated as “sufficient” with “very low” and “low” certainty of evidence, respectively.
Following the three criteria for the recommendation of a measurement tool, all included tools were categorized as ‘B’. Hence, further research will be required for the recommendation for or against any of the included tools [26]. Sufficient internal consistency may not be relevant for the assessment of external validity, as the measurement models might not be fully reflective. However, none of the authors/developers did specify the measurement model of their measurement tool.
Specification of the measurement model is considered a requirement of the appropriateness for the latent construct of interest during scale or tool development [102]. It could be argued that researchers automatically expect their tool to be a reflective measurement model. E.g., Downs and Black [22] assessed internal consistency without prior testing for unidimensionality or structural validity of the tool. Structural validity or unidimensionality is a prerequisite for internal consistency [26] and both measurement properties are only relevant for reflective measurement models [103, 104]. Misspecification as well as lack of specification of the measurement model can lead to potential limitations when developing and validating a scale or tool [102, 105]. Hence, the specification of measurement models should be considered in future research.
Content validity is the most important measurement property of health measurement instruments [27] and a lack of face validity is considered a strong argument for not using or to stop further evaluation of a measurement instrument [106]. Only the RITES tool [47] had evidence of “sufficient” content validity. Nevertheless, this tool does not directly measure the external validity of RCTs. The RITES tool [47] was developed to classify RCTs on an efficacy-effectiveness continuum. An RCT categorized as highly pragmatic or as having a “strong emphasis on effectiveness” [47] implies that the study design provides rather applicable results, but it does not automatically imply high external validity or generalizability of a trial´s characteristics to other specific contexts and settings [107]. Even a highly pragmatic/effectiveness study might have little applicability or generalizability to a specific research question of review authors. An individual assessment of external validity may still be needed by review authors in accordance with the research question and other contextual factors.
Another tool which might have some degree of content or face validity is the indirectness-dimension of the GRADE method [92]. This method is a widely used and accepted method in research synthesis in health science [108]. It has been evolved over the years based on work from the GRADE Working Group and on feedback from users worldwide [108]. Thus, it might be assumed that this method has a high degree of face validity, although it has not been systematically tested for content validity.
If all tools are categorized as ‘B’ in a review, the COSMIN guidelines suggests that the measurement instrument “with the best evidence for content validity could be the one to be provisionally recommended for use, until further evidence is provided” [34]. In accordance with this suggestions, the use of the RITES tool [47] as an provisionally solution might therefore be justified until more research on this topic is available. However, users should be aware of its limitations, as described above.
Implication for future research
This study affirms and supplements what is already known from previous reviews [9, 12, 14–18]. The heterogeneity of characteristics of tools included in those reviews was also observed in the present review. Although Dyrvig et al. [16] did not assess the measurement properties of available tools, they reported a lack of empirical support of items included in measurement tools. The authors of previous reviews could not recommend a measurement tool. Although their conclusions were mainly based on descriptive analysis rather than the assessment of quality of the tools, the conclusion of the present systematic review is consistent with them.
One major challenge on this topic is the serious heterogeneity regarding the terminology, criteria and guidance to assess the external validity of RCTs. Development of new tools and/or further revision (and validation) of available tools may not be appropriate before consensus-based standards are developed. Generally, it may be argued whether these methods to assess the external validity in systematic reviews of interventions are suitable [9, 12]. The experimental/statistical methods presented in Table 1 may offer a more objective approach to evaluate the external validity of RCTs. However, they are not feasible to implement in the conduction of systematic reviews. Furthermore, they focus mainly on the characteristics and generalizability of the study populations, which is insufficient to assess the external validity of clinical trials [109], since they do not consider other relevant dimensions of external validity such as intervention settings or treatment variables etc. [4, 109].
The methodological possibilities in tool/scale development and validation regarding this topic have not been exploited, yet. More than 20 years ago, there was no consensus regarding the definition of quality of RCTs. In 1998, Verhagen et al. [110] performed a Delphi study to achieve consensus regarding the definition of quality of RCTs and to create a quality criteria list. Until now, these criteria list has been a guidance in tool development and their criteria are still being implemented in methodological quality or risk of bias assessment tools (e.g. the Cochrane Collaboration risk of bias tool 1 & 2.0, the Physiotherapy Evidence Database (PEDro) scale etc.). Consequently, it seems necessary to seek consensus in order to overcome the issues regarding the external validity of RCTs in a similar way. After reaching consensus, further development and validation is needed following standard guidelines for scale/tool development (e.g. de Vet et al. [106]; Streiner et al. [111]; DeVellis [112]). Since the assessment of external validity seems highly context-dependent [9, 12], this should be taken into account in future research. A conventional checklist approach seems inappropriate [9, 12, 109] and a more comprehensive but flexible approach might be necessary. The experimental/statistical methods (Table 1) may offer a reference standard for convergent validity testing of the dimension “patient population” in future research.
This review has highlighted the necessity for more research in this area. Published studies and evaluation tools are important sources of information and should inform the development of a new tool or approach.
Strengths and limitations
One strength of the present review is the two-phase search method. With this method we believe that the likelihood of missing relevant studies was addressed adequately. The forward citation tracking using Scopus is another strength of the present review. The quality of the included measurement tools was assessed with an adapted and comprehensive methodology (COSMIN). None of the previous reviews has attempted such an assessment.
There are some limitations of the present review. First, a search for grey literature was not performed. Second, we focused on RCTs only and did not include assessment tools for non-randomized or other observational study design. Third, due to heterogeneity in terminology, we might have missed some tools with our electronic literature search strategy. Furthermore, it was challenging to find studies on measurement properties of some included tools, that did not have a specific name or abbreviation (such as EVAT). We tried to address this potential limitation by performing a comprehensive reference screening and snowballing (including forward citation screening).
Conclusions
Based on the results of this review, no available measurement tool can be fully recommended for the use in systematic reviews to assess the external validity of RCTs. Several steps are required to overcome the identified difficulties before a new tool is developed or available tools are further revised and validated.
Supplementary Information
Acknowledgements
We would like to thank Sven Bossmann and Sarah Tiemann for their assistance with the elaboration of the search strategy.
Abbreviations
- CASP
Critical Appraisal Skills Programme
- CCBRG
Cochrane Collaboration Back Review Group
- CCT
controlled clinical trial
- COSMIN
COnsensus based Standards for the selection of health Measurement Instruments
- EPHPP
Effective Public Health Practice Project
- EVAT
External Validity Assessment Tool
- FAME
Feasibility, Appropriateness, Meaningfulness and Effectiveness
- GATE
Graphical Appraisal Tool for Epidemiological Studies
- GAP
Generalizability, Applicability and Predictability
- GRADE
Grading of Recommendations Assessment, Development and Evaluation
- HTA
Health Technology Assessment
- ICC
intraclass correlation
- LEGEND
Let Evidence Guide Every New Decision
- NICE
National Institute for Health and Care Excellence
- PEDro
Physiotherapy Evidence Database
- PRECIS
PRagmatic Explanatory Continuum Indicator Summary
- RCT
randomized controlled trial
- RITES
Rating of Included Trials on the Efficacy-Effectiveness Spectrum
- TREND
Transparent Reporting of Evaluations with Nonrandomized Designs
- USPSTF
U.S. Preventive Services Task Force
Authors’ contributions
All authors contributed to the design of the study. AJ designed the search strategy and conducted the systematic search. AJ and TB screened titles and abstracts as well as full-text reports in phase (1) AJ and KL screened titles and abstracts as well as full-text reports in phase (2) Data extraction was performed by AJ and checked by TB. Quality appraisal and data analysis was performed by AJ and JB. AJ drafted the manuscript. JB, TB and KL critically revised the manuscript for important intellectual content. All authors read and approved the final manuscript.
Funding
Open Access funding enabled and organized by Projekt DEAL.
Availability of data and materials
All data generated or analyzed during this study are included in this published article (and its supplementary information files).
Declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Andres Jung, Email: a.jung@uni-luebeck.de.
Julia Balzer, Email: j.balzer@eufh-medica.de.
Tobias Braun, Email: to.braun@posteo.de.
Kerstin Luedtke, Email: kerstin.luedtke@uni-luebeck.de.
References
- 1.Bastian H, Glasziou P, Chalmers I. Seventy-five trials and eleven systematic reviews a day: how will we ever keep up? PLoS Med. 2010;7:e1000326. doi: 10.1371/journal.pmed.1000326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Aromataris E, Munn Z (eds). JBI Manual for Evidence Synthesis. JBI Man Evid Synth. 2020. 10.46658/jbimes-20-01
- 3.Knoll T, Omar MI, Maclennan S, et al. Key Steps in Conducting Systematic Reviews for Underpinning Clinical Practice Guidelines: Methodology of the European Association of Urology. Eur Urol. 2018;73:290–300. doi: 10.1016/j.eururo.2017.08.016. [DOI] [PubMed] [Google Scholar]
- 4.Jüni P, Altman DG, Egger M. Systematic reviews in health care: Assessing the quality of controlled clinical trials. BMJ. 2001;323:42–46. doi: 10.1136/bmj.323.7303.42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Büttner F, Winters M, Delahunt E, Elbers R, Lura CB, Khan KM, Weir A, Ardern CL. Identifying the ’incredible’! Part 1: assessing the risk of bias in outcomes included in systematic reviews. Br J Sports Med. 2020;54:798–800. doi: 10.1136/bjsports-2019-100806. [DOI] [PubMed] [Google Scholar]
- 6.Boutron I, Page MJ, Higgins JPT, Altman DG, Lundh A, Hróbjartsson A, Group CBM. Considering bias and conflicts of interest among the included studies. Cochrane Handb. Syst. Rev. Interv. 2021; version 6.2 (updated Febr. 2021)
- 7.Cook TD, Campbell DT, Shadish W. Experimental and quasi-experimental designs for generalized causal inference. Boston: Houghton Mifflin; 2002. [Google Scholar]
- 8.Avellar SA, Thomas J, Kleinman R, Sama-Miller E, Woodruff SE, Coughlin R, Westbrook TR. External Validity: The Next Step for Systematic Reviews? Eval Rev. 2017;41:283–325. doi: 10.1177/0193841X16665199. [DOI] [PubMed] [Google Scholar]
- 9.Weise A, Büchter R, Pieper D, Mathes T. Assessing context suitability (generalizability, external validity, applicability or transferability) of findings in evidence syntheses in healthcare-An integrative review of methodological guidance. Res Synth Methods. 2020;11:760–779. doi: 10.1002/jrsm.1453. [DOI] [PubMed] [Google Scholar]
- 10.Schunemann HJ, Tugwell P, Reeves BC, Akl EA, Santesso N, Spencer FA, Shea B, Wells G, Helfand M. Non-randomized studies as a source of complementary, sequential or replacement evidence for randomized controlled trials in systematic reviews on the effects of interventions. Res Synth Methods. 2013;4:49–62. doi: 10.1002/jrsm.1078. [DOI] [PubMed] [Google Scholar]
- 11.Atkins D, Chang SM, Gartlehner G, Buckley DI, Whitlock EP, Berliner E, Matchar D. Assessing applicability when comparing medical interventions: AHRQ and the Effective Health Care Program. J Clin Epidemiol. 2011;64:1198–1207. doi: 10.1016/j.jclinepi.2010.11.021. [DOI] [PubMed] [Google Scholar]
- 12.Burchett HED, Blanchard L, Kneale D, Thomas J. Assessing the applicability of public health intervention evaluations from one setting to another: a methodological study of the usability and usefulness of assessment tools and frameworks. Heal Res policy Syst. 2018;16:88. doi: 10.1186/s12961-018-0364-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dekkers OM, von Elm E, Algra A, Romijn JA, Vandenbroucke JP. How to assess the external validity of therapeutic trials: a conceptual approach. Int J Epidemiol. 2010;39:89–94. doi: 10.1093/ije/dyp174. [DOI] [PubMed] [Google Scholar]
- 14.Burchett H, Umoquit M, Dobrow M. How do we know when research from one setting can be useful in another? A review of external validity, applicability and transferability frameworks. J Health Serv Res Policy. 2011;16:238–244. doi: 10.1258/jhsrp.2011.010124. [DOI] [PubMed] [Google Scholar]
- 15.Cambon L, Minary L, Ridde V, Alla F. Transferability of interventions in health education: a review. BMC Public Health. 2012;12:497. doi: 10.1186/1471-2458-12-497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Dyrvig A-K, Kidholm K, Gerke O, Vondeling H. Checklists for external validity: a systematic review. J Eval Clin Pract. 2014;20:857–864. doi: 10.1111/jep.12166. [DOI] [PubMed] [Google Scholar]
- 17.Munthe-Kaas H, Nøkleby H, Nguyen L. Systematic mapping of checklists for assessing transferability. Syst Rev. 2019;8:22. doi: 10.1186/s13643-018-0893-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Nasser M, van Weel C, van Binsbergen JJ, van de Laar FA. Generalizability of systematic reviews of the effectiveness of health care interventions to primary health care: concepts, methods and future research. Fam Pract. 2012;29(Suppl 1):i94–i103. doi: 10.1093/fampra/cmr129. [DOI] [PubMed] [Google Scholar]
- 19.Hariton E, Locascio JJ. Randomised controlled trials - the gold standard for effectiveness research: Study design: randomised controlled trials. BJOG. 2018;125:1716. doi: 10.1111/1471-0528.15199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Pressler TR, Kaizar EE. The use of propensity scores and observational data to estimate randomized controlled trial generalizability bias. Stat Med. 2013;32:3552–3568. doi: 10.1002/sim.5802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Rothwell PM. External validity of randomised controlled trials: “to whom do the results of this trial apply?”. Lancet. 2005;365:82–93. doi: 10.1016/S0140-6736(04)17670-8. [DOI] [PubMed] [Google Scholar]
- 22.Downs SH, Black N. The feasibility of creating a checklist for the assessment of the methodological quality both of randomised and non-randomised studies of health care interventions. J Epidemiol Community Health. 1998;52:377–384. doi: 10.1136/jech.52.6.377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Page MJ, Moher D, Bossuyt PM, et al. PRISMA 2020 explanation and elaboration: updated guidance and exemplars for reporting systematic reviews. BMJ. 2021;372:n160. doi: 10.1136/bmj.n160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Clark R, Locke M, Hill B, Wells C, Bialocerkowski A. Clinimetric properties of lower limb neurological impairment tests for children and young people with a neurological condition: A systematic review. PLoS One. 2017;12:e0180031. doi: 10.1371/journal.pone.0180031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mokkink LB, de Vet HCW, Prinsen CAC, Patrick DL, Alonso J, Bouter LM, Terwee CB. COSMIN Risk of Bias checklist for systematic reviews of Patient-Reported Outcome Measures. Qual Life Res. 2018;27:1171–1179. doi: 10.1007/s11136-017-1765-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Prinsen CAC, Mokkink LB, Bouter LM, Alonso J, Patrick DL, de Vet HCW, Terwee CB. COSMIN guideline for systematic reviews of patient-reported outcome measures. Qual Life Res. 2018;27:1147–1157. doi: 10.1007/s11136-018-1798-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Terwee CB, Prinsen CAC, Chiarotto A, Westerman MJ, Patrick DL, Alonso J, Bouter LM, de Vet HCW, Mokkink LB. COSMIN methodology for evaluating the content validity of patient-reported outcome measures: a Delphi study. Qual Life Res. 2018;27:1159–1170. doi: 10.1007/s11136-018-1829-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Stephenson M, Riitano D, Wilson S, Leonardi-Bee J, Mabire C, Cooper K, Monteiro da Cruz D, Moreno-Casbas MT, Lapkin S. Chap. 12: Systematic Reviews of Measurement Properties. JBI Man Evid Synth. 2020 10.46658/JBIMES-20-13
- 29.Glover PD, Gray H, Shanmugam S, McFadyen AK. Evaluating collaborative practice within community-based integrated health and social care teams: a systematic review of outcome measurement instruments. J Interprof Care. 2021;1–15. 10.1080/13561820.2021.1902292. Epub ahead of print. [DOI] [PubMed]
- 30.Maassen SM, Weggelaar Jansen AMJW, Brekelmans G, Vermeulen H, van Oostveen CJ. Psychometric evaluation of instruments measuring the work environment of healthcare professionals in hospitals: a systematic literature review. Int J Qual Heal care J Int Soc Qual Heal Care. 2020;32:545–557. doi: 10.1093/intqhc/mzaa072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Jabri Yaqoob MohammedAl, Kvist F, Azimirad T, Turunen M. A systematic review of healthcare professionals’ core competency instruments. Nurs Health Sci. 2021;23:87–102. doi: 10.1111/nhs.12804. [DOI] [PubMed] [Google Scholar]
- 32.Mokkink LB, Terwee CB, Patrick DL, Alonso J, Stratford PW, Knol DL, Bouter LM, de Vet HCW. The COSMIN study reached international consensus on taxonomy, terminology, and definitions of measurement properties for health-related patient-reported outcomes. J Clin Epidemiol. 2010;63:737–745. doi: 10.1016/j.jclinepi.2010.02.006. [DOI] [PubMed] [Google Scholar]
- 33.Jung A, Balzer J, Braun T, Luedtke K. Psychometric properties of tools to measure the external validity of randomized controlled trials: a systematic review protocol. 2020; 10.17605/OSF.IO/PTG4D
- 34.Mokkink LB, Prinsen CAC, Patrick DL, Alonso J, Bouter LM, de Vet HCW, Terwee CB COSMIN manual for systematic reviews of PROMs, user manual. 2018;1–78. https://www.cosmin.nl/wp-content/uploads/COSMIN-syst-review-for-PROMs-manual_version-1_feb-2018-1.pdf. Accessed 3 Feb 2020.
- 35.Bialocerkowski A, O’shea K, Pin TW. Psychometric properties of outcome measures for children and adolescents with brachial plexus birth palsy: a systematic review. Dev Med Child Neurol. 2013;55:1075–1088. doi: 10.1111/dmcn.12194. [DOI] [PubMed] [Google Scholar]
- 36.Matthews J, Bialocerkowski A, Molineux M. Professional identity measures for student health professionals - a systematic review of psychometric properties. BMC Med Educ. 2019;19:308. doi: 10.1186/s12909-019-1660-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Terwee CB, Jansma EP, Riphagen II, De Vet HCW. Development of a methodological PubMed search filter for finding studies on measurement properties of measurement instruments. Qual Life Res. 2009;18:1115–1123. doi: 10.1007/s11136-009-9528-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sierevelt IN, Zwiers R, Schats W, Haverkamp D, Terwee CB, Nolte PA, Kerkhoffs GMMJ. Measurement properties of the most commonly used Foot- and Ankle-Specific Questionnaires: the FFI, FAOS and FAAM. A systematic review. Knee Surg Sports Traumatol Arthrosc. 2018;26:2059–2073. doi: 10.1007/s00167-017-4748-7. [DOI] [PubMed] [Google Scholar]
- 39.van der Hout A, Neijenhuijs KI, Jansen F, et al. Measuring health-related quality of life in colorectal cancer patients: systematic review of measurement properties of the EORTC QLQ-CR29. Support Care Cancer. 2019;27:2395–2412. doi: 10.1007/s00520-019-04764-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Whiting P, Savović J, Higgins JPT, Caldwell DM, Reeves BC, Shea B, Davies P, Kleijnen J, Churchill R. ROBIS: A new tool to assess risk of bias in systematic reviews was developed. J Clin Epidemiol. 2016;69:225–234. doi: 10.1016/j.jclinepi.2015.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ouzzani M, Hammady H, Fedorowicz Z, Elmagarmid A. Rayyan-a web and mobile app for systematic reviews. Syst Rev. 2016;5:210. doi: 10.1186/s13643-016-0384-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Moher D, Hopewell S, Schulz KF, Montori V, Gøtzsche PC, Devereaux PJ, Elbourne D, Egger M, Altman DG. CONSORT 2010 explanation and elaboration: updated guidelines for reporting parallel group randomised trials. Int J Surg. 2012;10:28–55. doi: 10.1016/j.ijsu.2011.10.001. [DOI] [PubMed] [Google Scholar]
- 43.Mokkink LB, Terwee CB. The COSMIN checklist for assessing the methodological quality of studies on measurement properties of health status measurement instruments: an international Delphi study. 2010;539–549 [DOI] [PMC free article] [PubMed]
- 44.Terwee CB, Prinsen CA, Chiarotto A, De Vet H, Bouter LM, Alonso J, Westerman MJ, Patrick DL, Mokkink LB. COSMIN methodology for assessing the content validity of PROMs–user manual. Amsterdam VU Univ. Med. Cent. 2018; https://cosmin.nl/wp-content/uploads/COSMIN-methodology-for-content-validity-user-manual-v1.pdf. Accessed 3 Feb 2020.
- 45.Mustafa RA, Santesso N, Brozek J, et al. The GRADE approach is reproducible in assessing the quality of evidence of quantitative evidence syntheses. J Clin Epidemiol. 2013;66:735–736. doi: 10.1016/j.jclinepi.2013.02.004. [DOI] [PubMed] [Google Scholar]
- 46.Jennings H, Hennessy K, Hendry GJ. The clinical effectiveness of intra-articular corticosteroids for arthritis of the lower limb in juvenile idiopathic arthritis: A systematic review. Pediatr Rheumatol. 2014 doi: 10.1186/1546-0096-12-23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Wieland LS, Berman BM, Altman DG, et al. Rating of Included Trials on the Efficacy-Effectiveness Spectrum: development of a new tool for systematic reviews. J Clin Epidemiol. 2017;84:95–104. doi: 10.1016/j.jclinepi.2017.01.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Atkins D, Briss PA, Eccles M, et al. Systems for grading the quality of evidence and the strength of recommendations II: pilot study of a new system. BMC Health Serv Res. 2005;5:25. doi: 10.1186/1472-6963-5-25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Abraham NS, Wieczorek P, Huang J, Mayrand S, Fallone CA, Barkun AN. Assessing clinical generalizability in sedation studies of upper GI endoscopy. Gastrointest Endosc. 2004;60:28–33. doi: 10.1016/s0016-5107(04)01307-0. [DOI] [PubMed] [Google Scholar]
- 50.Arabi YM, Cook DJ, Zhou Q, et al. Characteristics and Outcomes of Eligible Nonenrolled Patients in a Mechanical Ventilation Trial of Acute Respiratory Distress Syndrome. Am J Respir Crit Care Med. 2015;192:1306–1313. doi: 10.1164/rccm.201501-0172OC. [DOI] [PubMed] [Google Scholar]
- 51.Williams AC, de Nicholas C, Richardson MK, de Pither PH, FAC Generalizing from a controlled trial: The effects of patient preference versus randomization on the outcome of inpatient versus outpatient chronic pain management. Pain. 1999;83:57–65. doi: 10.1016/s0304-3959(99)00074-3. [DOI] [PubMed] [Google Scholar]
- 52.De Jong Z, Munneke M, Jansen LM, Ronday K, Van Schaardenburg DJ, Brand R, Van Den Ende CHM, Vliet Vlieland TPM, Zuijderduin WM, Hazes JMW. Differences between participants and nonparticipants in an exercise trial for adults with rheumatoid arthritis. Arthritis Care Res. 2004;51:593–600. doi: 10.1002/art.20531. [DOI] [PubMed] [Google Scholar]
- 53.Hordijk-Trion M, Lenzen M, Wijns W, et al. Patients enrolled in coronary intervention trials are not representative of patients in clinical practice: Results from the Euro Heart Survey on Coronary Revascularization. Eur Heart J. 2006;27:671–678. doi: 10.1093/eurheartj/ehi731. [DOI] [PubMed] [Google Scholar]
- 54.Wilson A, Parker H, Wynn A, Spiers N. Performance of hospital-at-home after a randomised controlled trial. J Heal Serv Res Policy. 2003;8:160–164. doi: 10.1258/135581903322029511. [DOI] [PubMed] [Google Scholar]
- 55.Smyth B, Haber A, Trongtrakul K, Hawley C, Perkovic V, Woodward M, Jardine M. Representativeness of Randomized Clinical Trial Cohorts in End-stage Kidney Disease: A Meta-analysis. JAMA Intern Med. 2019;179:1316–1324. doi: 10.1001/jamainternmed.2019.1501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Leinonen A, Koponen M, Hartikainen S. Systematic Review: Representativeness of Participants in RCTs of Acetylcholinesterase Inhibitors. PLoS One. 2015;10:e0124500–e0124500. doi: 10.1371/journal.pone.0124500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Chari A, Romanus D, Palumbo A, Blazer M, Farrelly E, Raju A, Huang H, Richardson P. Randomized Clinical Trial Representativeness and Outcomes in Real-World Patients: Comparison of 6 Hallmark Randomized Clinical Trials of Relapsed/Refractory Multiple Myeloma. Clin Lymphoma Myeloma Leuk. 2020;20:8. doi: 10.1016/j.clml.2019.09.625. [DOI] [PubMed] [Google Scholar]
- 58.Susukida R, Crum RM, Ebnesajjad C, Stuart EA, Mojtabai R. Generalizability of findings from randomized controlled trials: application to the National Institute of Drug Abuse Clinical Trials Network. Addiction. 2017;112:1210–1219. doi: 10.1111/add.13789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Zarin DA, Young JL, West JC. Challenges to evidence-based medicine: a comparison of patients and treatments in randomized controlled trials with patients and treatments in a practice research network. Soc Psychiatry Psychiatr Epidemiol. 2005;40:27–35. doi: 10.1007/s00127-005-0838-9. [DOI] [PubMed] [Google Scholar]
- 60.Gheorghe A, Roberts T, Hemming K, Calvert M. Evaluating the Generalisability of Trial Results: Introducing a Centre- and Trial-Level Generalisability Index. Pharmacoeconomics. 2015;33:1195–1214. doi: 10.1007/s40273-015-0298-3. [DOI] [PubMed] [Google Scholar]
- 61.He Z, Wang S, Borhanian E, Weng C. Assessing the Collective Population Representativeness of Related Type 2 Diabetes Trials by Combining Public Data from ClinicalTrials.gov and NHANES. Stud Health Technol Inform. 2015;216:569–573. [PMC free article] [PubMed] [Google Scholar]
- 62.Schmidt AF, Groenwold RHH, van Delden JJM, van der Does Y, Klungel OH, Roes KCB, Hoes AW, van der Graaf R. Justification of exclusion criteria was underreported in a review of cardiovascular trials. J Clin Epidemiol. 2014;67:635–644. doi: 10.1016/j.jclinepi.2013.12.005. [DOI] [PubMed] [Google Scholar]
- 63.Carr DB, Goudas LC, Balk EM, Bloch R, Ioannidis JP, Lau J. Evidence report on the treatment of pain in cancer patients. J Natl Cancer Inst Monogr. 2004;32:23–31. doi: 10.1093/jncimonographs/lgh012. [DOI] [PubMed] [Google Scholar]
- 64.Clegg A, Bryant J, Nicholson T, et al. Clinical and cost-effectiveness of donepezil, rivastigmine and galantamine for Alzheimer’s disease: a rapid and systematic review. Health Technol Assess (Rockv) 2001;5:1–136. doi: 10.3310/hta5010. [DOI] [PubMed] [Google Scholar]
- 65.Foy R, Hempel S, Rubenstein L, Suttorp M, Seelig M, Shanman R, Shekelle PG. Meta-analysis: effect of interactive communication between collaborating primary care physicians and specialists. Ann Intern Med. 2010;152:247–258. doi: 10.7326/0003-4819-152-4-201002160-00010. [DOI] [PubMed] [Google Scholar]
- 66.Haraldsson BG, Gross AR, Myers CD, Ezzo JM, Morien A, Goldsmith C, Peloso PM, Bronfort G. Massage for mechanical neck disorders. Cochrane database Syst Rev. 2006. 10.1002/14651858.CD004871.pub3. [DOI] [PubMed]
- 67.Hawk C, Khorsan R, AJ L, RJ F. Chiropractic care for nonmusculoskeletal conditions: a systematic review with implications for whole systems research. J Altern Complement Med. 2007;13:491–512. doi: 10.1089/acm.2007.7088. [DOI] [PubMed] [Google Scholar]
- 68.Karjalainen K, Malmivaara A, van Tulder M, et al. Multidisciplinary rehabilitation for fibromyalgia and musculoskeletal pain in working age adults. Cochrane Database Syst Rev. 2000 doi: 10.1002/14651858.CD001984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Liberati A, Himel HN, Chalmers TC. A quality assessment of randomized control trials of primary treatment of breast cancer. J Clin Oncol. 1986;4:942–951. doi: 10.1200/JCO.1986.4.6.942. [DOI] [PubMed] [Google Scholar]
- 70.Averis A, Pearson A. Filling the gaps: identifying nursing research priorities through the analysis of completed systematic reviews. Jbi Reports. 2003;1:49–126. [Google Scholar]
- 71.Sorg C, Schmidt J, Büchler MW, Edler L, Märten A. Examination of external validity in randomized controlled trials for adjuvant treatment of pancreatic adenocarcinoma. Pancreas. 2009;38:542–550. doi: 10.1097/MPA.0b013e31819d7370. [DOI] [PubMed] [Google Scholar]
- 72.National Institute for Health and Care Excellence. Methods for the development of NICE public health guidance, Third edit. National Institute for Health and Care Excellence. 2012; https://www.nice.org.uk/process/pmg4/chapter/introduction. Accessed 15 Apr 2020 [PubMed]
- 73.U.S. Preventive Services Task Force. Criteria for Assessing External Validity (Generalizability) of Individual Studies. US Prev Serv Task Force Appendix VII. 2017; https://uspreventiveservicestaskforce.org/uspstf/about-uspstf/methods-and-processes/procedure-manual/procedure-manual-appendix-vii-criteria-assessing-external-validity-generalizability-individual-studies. Accessed 15 Apr 2020.
- 74.National Health and Medical Research Council NHMRC handbooks. https://www.nhmrc.gov.au/about-us/publications/how-prepare-and-present-evidence-based-information-consumers-health-services#block-views-block-file-attachments-content-block-1. Accessed 15 Apr 2020.
- 75.Loyka CM, Ruscio J, Edelblum AB, Hatch L, Wetreich B, Zabel Caitlin M. Weighing people rather than food: A framework for examining external validity. Perspect Psychol Sci. 2020;15:483–496. doi: 10.1177/1745691619876279. [DOI] [PubMed] [Google Scholar]
- 76.Fernandez-Hermida JR, Calafat A, Becoña E, Tsertsvadze A, Foxcroft DR. Assessment of generalizability, applicability and predictability (GAP) for evaluating external validity in studies of universal family-based prevention of alcohol misuse in young people: systematic methodological review of randomized controlled trials. Addiction. 2012;107:1570–1579. doi: 10.1111/j.1360-0443.2012.03867.x. [DOI] [PubMed] [Google Scholar]
- 77.Clark E, Burkett K, Stanko-Lopp D. Let Evidence Guide Every New Decision (LEGEND): an evidence evaluation system for point-of-care clinicians and guideline development teams. J Eval Clin Pract. 2009;15:1054–1060. doi: 10.1111/j.1365-2753.2009.01314.x. [DOI] [PubMed] [Google Scholar]
- 78.Bornhöft G, Maxion-Bergemann S, Wolf U, Kienle GS, Michalsen A, Vollmar HC, Gilbertson S, Matthiessen PF. Checklist for the qualitative evaluation of clinical studies with particular focus on external validity and model validity. BMC Med Res Methodol. 2006;6:56. doi: 10.1186/1471-2288-6-56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Cho MK, Bero LA. Instruments for assessing the quality of drug studies published in the medical literature. JAMA J Am Med Assoc. 1994;272:101–104. [PubMed] [Google Scholar]
- 80.Cho MK, Bero LA. The quality of drug studies published in symposium proceedings. Ann Intern Med 1996;124:485–489 [DOI] [PubMed]
- 81.van Tulder M, Furlan A, Bombardier C, Bouter L. Updated method guidelines for systematic reviews in the cochrane collaboration back review group. Spine (Phila Pa 1976) 2003;28:1290–1299. doi: 10.1097/01.BRS.0000065484.95996.AF. [DOI] [PubMed] [Google Scholar]
- 82.Estrada F, Atienzo EE, Cruz-Jiménez L, Campero L. A Rapid Review of Interventions to Prevent First Pregnancy among Adolescents and Its Applicability to Latin America. J Pediatr Adolesc Gynecol. 2021;34:491–503. doi: 10.1016/j.jpag.2021.01.022. [DOI] [PubMed] [Google Scholar]
- 83.Khorsan R, Crawford C. How to assess the external validity and model validity of therapeutic trials: a conceptual approach to systematic review methodology. Evid Based Complement Alternat Med. 2014;2014:694804. doi: 10.1155/2014/694804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.O’Connor SR, Tully MA, Ryan B, Bradley JM, Baxter GD, McDonough SM. Failure of a numerical quality assessment scale to identify potential risk of bias in a systematic review: a comparison study. BMC Res Notes. 2015;8:224. doi: 10.1186/s13104-015-1181-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Chalmers TC, Smith H, Blackburn B, Silverman B, Schroeder B, Reitman D, Ambroz A. A method for assessing the quality of a randomized control trial. Control Clin Trials. 1981;2:31–49. doi: 10.1016/0197-2456(81)90056-8. [DOI] [PubMed] [Google Scholar]
- 86.Gartlehner G, Hansen RA, Nissman D, Lohr KN, Carey TS. A simple and valid tool distinguished efficacy from effectiveness studies. J Clin Epidemiol. 2006;59:1040–1048. doi: 10.1016/j.jclinepi.2006.01.011. [DOI] [PubMed] [Google Scholar]
- 87.Zettler LL, Speechley MR, Foley NC, Salter KL, Teasell RW. A scale for distinguishing efficacy from effectiveness was adapted and applied to stroke rehabilitation studies. J Clin Epidemiol. 2010;63:11–18. doi: 10.1016/j.jclinepi.2009.06.007. [DOI] [PubMed] [Google Scholar]
- 88.Green LW, Glasgow RE. Evaluating the relevance, generalization, and applicability of research: issues in external validation and translation methodology. Eval Health Prof. 2006;29:126–153. doi: 10.1177/0163278705284445. [DOI] [PubMed] [Google Scholar]
- 89.Glasgow RE, Vogt TM, Boles SM. Evaluating the public health impact of health promotion interventions: the RE-AIM framework. Am J Public Health. 1999;89:1322–1327. doi: 10.2105/ajph.89.9.1322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Mirza NA, Akhtar-Danesh N, Staples E, Martin L, Noesgaard C. Comparative Analysis of External Validity Reporting in Non-randomized Intervention Studies. Can J Nurs Res. 2014;46:47–64. doi: 10.1177/084456211404600405. [DOI] [PubMed] [Google Scholar]
- 91.Laws RA, St George AB, Rychetnik L, Bauman AE. Diabetes prevention research: a systematic review of external validity in lifestyle interventions. Am J Prev Med. 2012;43:205–214. doi: 10.1016/j.amepre.2012.04.017. [DOI] [PubMed] [Google Scholar]
- 92.Schünemann H, Brożek J, Guyatt G, Oxman A. Handbook for grading the quality of evidence and the strength of recommendations using the GRADE approach (updated October 2013). GRADE Work. Gr. 2013; https://gdt.gradepro.org/app/handbook/handbook.html. Accessed 15 Apr 2020.
- 93.Wu XY, Chung VCH, Wong CHL, Yip BHK, Cheung WKW, Wu JCY. CHIMERAS showed better inter-rater reliability and inter-consensus reliability than GRADE in grading quality of evidence: A randomized controlled trial. Eur J Integr Med. 2018;23:116–122. [Google Scholar]
- 94.Meader N, King K, Llewellyn A, Norman G, Brown J, Rodgers M, Moe-Byrne T, Higgins JPT, Sowden A, Stewart G. A checklist designed to aid consistency and reproducibility of GRADE assessments: Development and pilot validation. Syst Rev. 2014 doi: 10.1186/2046-4053-3-82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Llewellyn A, Whittington C, Stewart G, Higgins JP, Meader N. The Use of Bayesian Networks to Assess the Quality of Evidence from Research Synthesis: 2. Inter-Rater Reliability and Comparison with Standard GRADE Assessment. PLoS One. 2015;10:e0123511. doi: 10.1371/journal.pone.0123511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Jackson R, Ameratunga S, Broad J, Connor J, Lethaby A, Robb G, Wells S, Glasziou P, Heneghan C. The GATE frame: critical appraisal with pictures. Evid Based Med 2006;11:35 LP– 38 [DOI] [PubMed]
- 97.Aves T. The Role of Pragmatism in Explaining Heterogeneity in Meta-Analyses of Randomized Trials: A Methodological Review. 2017; McMaster University. http://hdl.handle.net/11375/22212. Accessed 12 Jan 2021. [DOI] [PMC free article] [PubMed]
- 98.Thomas BH, Ciliska D, Dobbins M, Micucci S. A process for systematically reviewing the literature: providing the research evidence for public health nursing interventions. Worldviews Evidence-Based Nurs. 2004;1:176–184. doi: 10.1111/j.1524-475X.2004.04006.x. [DOI] [PubMed] [Google Scholar]
- 99.Armijo-Olivo S, Stiles CR, Hagen NA, Biondo PD, Cummings GG. Assessment of study quality for systematic reviews: a comparison of the Cochrane Collaboration Risk of Bias Tool and the Effective Public Health Practice Project Quality Assessment Tool: methodological research. J Eval Clin Pract. 2012;18:12–18. doi: 10.1111/j.1365-2753.2010.01516.x. [DOI] [PubMed] [Google Scholar]
- 100.Critical Appraisal Skills Programme. CASP Randomised Controlled Trial Standard Checklist. 2020; https://casp-uk.net/casp-tools-checklists/. Accessed 10 Dec 2020.
- 101.Aves T, Allan KS, Lawson D, Nieuwlaat R, Beyene J, Mbuagbaw L. The role of pragmatism in explaining heterogeneity in meta-analyses of randomised trials: a protocol for a cross-sectional methodological review. BMJ Open. 2017;7:e017887. doi: 10.1136/bmjopen-2017-017887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Diamantopoulos A, Riefler P, Roth KP. Advancing formative measurement models. J Bus Res. 2008;61:1203–1218. [Google Scholar]
- 103.Fayers PM, Hand DJ. Factor analysis, causal indicators and quality of life. Qual Life Res. 1997 doi: 10.1023/A:1026490117121. [DOI] [PubMed] [Google Scholar]
- 104.Streiner DL. Being Inconsistent About Consistency: When Coefficient Alpha Does and Doesn’t Matter. J Pers Assess. 2003;80:217–222. doi: 10.1207/S15327752JPA8003_01. [DOI] [PubMed] [Google Scholar]
- 105.MacKenzie SB, Podsakoff PM, Jarvis CB. The Problem of Measurement Model Misspecification in Behavioral and Organizational Research and Some Recommended Solutions. J Appl Psychol. 2005;90:710–730. doi: 10.1037/0021-9010.90.4.710. [DOI] [PubMed] [Google Scholar]
- 106.De Vet HCW, Terwee CB, Mokkink LB, Knol DL. Measurement in medicine: a practical guide. 2011; 10.1017/CBO9780511996214
- 107.Dekkers OM, Bossuyt PM, Vandenbroucke JP. How trial results are intended to be used: is PRECIS-2 a step forward? J Clin Epidemiol. 2017;84:25–26. doi: 10.1016/j.jclinepi.2016.01.033. [DOI] [PubMed] [Google Scholar]
- 108.Brozek JL, Canelo-Aybar C, Akl EA, et al. GRADE Guidelines 30: the GRADE approach to assessing the certainty of modeled evidence-An overview in the context of health decision-making. J Clin Epidemiol. 2021;129:138–150. doi: 10.1016/j.jclinepi.2020.09.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Burchett HED, Kneale D, Blanchard L, Thomas J. When assessing generalisability, focusing on differences in population or setting alone is insufficient. Trials. 2020;21:286. doi: 10.1186/s13063-020-4178-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Verhagen AP, de Vet HCW, de Bie RA, Kessels AGH, Boers M, Bouter LM, Knipschild PG. The Delphi List: A Criteria List for Quality Assessment of Randomized Clinical Trials for Conducting Systematic Reviews Developed by Delphi Consensus. J Clin Epidemiol. 1998;51:1235–1241. doi: 10.1016/s0895-4356(98)00131-0. [DOI] [PubMed] [Google Scholar]
- 111.Streiner DL, Norman GR, Cairney J. Health measurement scales: a practical guide to their development and use, Fifth edit. Oxford: Oxford University Press; 2015. [Google Scholar]
- 112.DeVellis RF. Scale development: Theory and applications, Fourth edi. Los Angeles: Sage publications; 2017. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All data generated or analyzed during this study are included in this published article (and its supplementary information files).