Abstract
Introduction
The efficacy of dexmedetomidine supplementation for thoracoscopic surgery remains controversial. We conduct a systematic review and meta-analysis to explore the impact of dexmedetomidine for thoracoscopic surgery.
Methods
We have searched PubMed, EMbase, Web of science, EBSCO, and Cochrane library databases through September 2020 for randomized controlled trials (RCTs) assessing the effect of dexmedetomidine supplementation on thoracoscopic surgery. This meta-analysis is performed using the random-effect model.
Results
Six RCTs involving 510 patients are included in the meta-analysis. Overall, compared with control group for thoracoscopic surgery, dexmedetomidine supplementation results in significantly reduced pain scores (SMD = − 1.50; 95% CI = − 2.63–− 0.37; P = 0.009), anesthetic consumption (SMD = − 3.91; 95% CI = − 6.76–− 1.05; P = 0.007), mean heart rate (SMD = − 0.41; 95% CI = − 0.65–− 0.18; P = 0.0007), and the risk ratio (RR) of ICU stay (RR = 0.39; 95% CI = 0.19–0.80; P = 0.01), but showed no obvious effect on mean blood pressure (SMD = − 0.07; 95% CI = − 0.45–0.31; P = 0.72) or hospital stay (SMD = − 0.61; 95% CI = − 1.30–0.08; P = 0.08).
Conclusions
Dexmedetomidine supplementation can substantially improve the analgesic efficacy for thoracoscopic surgery.
Supplementary Information
The online version contains supplementary material available at 10.1186/s13019-022-01803-z.
Keywords: Dexmedetomidine, Thoracoscopic surgery, Analgesic efficacy, Randomized controlled trials
Introduction
Thoracoscopic surgery is widely used to treat various diseases such as esophageal cancer and lung cancer. It results the smaller incision, less pain and inflammatory response, reduced recovery times compared to traditional surgery [1–3]. The pain commonly occurs after the surgery, and negatively affects the postoperative recovery. Various analgesic regimens have developed for the pain management after thoracoscopic surgery, and they mainly include pharmacologic and regional interventions (e.g. nerve block) [4–7].
Dexmedetomidine, a short-acting α2 -adrenoceptor agonist, is reported to provide the sedation and analgesia for various surgeries [8, 9]. Studies demonstrated that dexmedetomidine attenuated surgical stress responses in patients undergoing surgery, and is effective and safe to improve the analgesic efficacy when serving as an adjunctive analgesic [10, 11]. Previous trials demonstrated that dexmedetomidine had opioid-sparing properties, maximized pain relief and minimized analgesic-related side effects [12–14].
However, the efficacy of dexmedetomidine supplementation for thoracoscopic surgery has not been well established. Recently, several studies on the topic have been published, and the results were conflicting [7, 15–17]. For instance, two studies reported that dexmedetomidine supplementation could significantly reduce postoperative pain scores for thoracoscopic surgery [16, 18], but another study found no benefits to pain control after using dexmedetomidine supplementation for thoracoscopic surgery [7]. With accumulating evidence, we therefore perform a systematic review and meta-analysis of RCTs to investigate the analgesic efficacy of dexmedetomidine supplementation for thoracoscopic surgery.
Materials and methods
Ethical approval and patient consent are not required because this is a systematic review and meta-analysis of previously published studies. The systematic review and meta-analysis are conducted and reported in adherence to PRISMA (Preferred Reporting Items for Systematic Reviews and Meta-Analyses) [19].
Search strategy and study selection
Two investigators have independently searched the following databases (inception to September 2020): PubMed, EMbase, Web of science, EBSCO, and Cochrane library databases. The electronic search strategy was conducted using the following keywords: “dexmedetomidine”, and “thoracoscopic” or “thoracoscopy”. We also check the reference lists of the screened full-text studies to identify other potentially eligible trials.
The inclusive selection criteria are as follows: (i) patients underwent thoracoscopic surgery; (ii) intervention treatments were intravenous dexmedetomidine supplementation versus no dexmedetomidine; (iii) study design was RCT.
Data extraction and outcome measures
We have extracted the following information: author, number of patients, age, sex, body mass index, American Society of Anesthesiologists (ASA) and detail methods in each group. The ASA Physical Status Classification System is the most widely used system globally to describe a patient’s preoperative medical condition. The four categories (P1–P4) in the classification have changed little since they were first proposed in 1941 [20]. Data were extracted independently by two investigators, and discrepancies are resolved by consensus. We also contacted the corresponding author to obtain the data when necessary.
The primary outcome was pain scores. Secondary outcomes included analgesic consumption, mean heart rate and blood pressure, ICU stay, and hospital stay.
Quality assessment in individual studies
Methodological quality of the included studies is independently evaluated using the Jadad scale [21]. There are 3 items for Jadad scale: randomization (0–2 points), blinding (0–2 points), dropouts and withdrawals (0–1 points). The score of Jadad Scale varies from 0 to 5 points. An article with Jadad score ≤ 2 is considered to be of low quality. If the Jadad score ≥ 3, the study is thought to be of high quality [22].
Statistical analysis
We estimate the standard mean difference (SMD) with 95% confidence interval (CI) for continuous outcomes (pain scores, analgesic consumption, mean heart rate and blood pressure, and hospital stay) and relative risk (RR) with 95% CI for dichotomous outcomes (ICU stay). The random-effects model was used regardless of heterogeneity. Heterogeneity was reported using the I2 statistic, and I2 > 50% indicated significant heterogeneity [23]. Whenever significant heterogeneity was present, we searched for potential sources of heterogeneity via omitting one study in turn for the meta-analysis or performing subgroup analysis. Publication bias was not evaluated because of the limited number (< 10) of included studies. All statistical analyses were performed using Review Manager Version 5.3 (The Cochrane Collaboration, Software Update, Oxford, UK).
Results
Literature search, study characteristics and quality assessment
A detailed flowchart of the search and selection results was shown in Additional file 1: Fig. S1. 239 potentially relevant articles are identified initially. Finally, six RCTs that meet our inclusion criteria are included in the meta-analysis [7, 15–18, 24].
The baseline characteristics of the six eligible RCTs in the meta-analysis were summarized in Table 1. The six studies were published between 2016 and 2020, and the total sample size was 510. Dexmedetomidine was used before the anesthesia [7, 15, 18, 24], or during surgery [16, 17].
Table 1.
Characteristics of included studies
| No | Author | Dexmedetomidine group | Control group | ||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Number | Age (years) | Sex(male/female) | Body mass index (kg/m2) | ASA (I/II/III) | Methods | Number | Age (years) | Sex(male/female) | Body mass index (kg/m2) | ASA (I/II/III) | Methods | Surgery type | Combined anaesthetics | Outcomes | Jada scores | ||
| 1 | Wang 2020 | 46 | 56.78 ± 12.81 | 17/29 | 22.09 ± 3.22 | 7/39/0 | dexmedetomidine 0.8 μ g/kg administered for 10 min before anesthesia | 44 | 60.48 ± 12.58 | 22/22 | 22.89 ± 2.85 | 10/34/0 | placebo | video-assisted thoracoscopic lung lobectomy | fentanyl, propofol and isoflurane | pain score (numeric rating scale), heart rate, blood pressure | 3 (minus 2, undescribed blindness) |
| 2 | Kim 2019 | 60 | 63 [58–68], median [interquartile range] | 28/32 | 24 ± 3 | 18/42/0 | dexmedetomidine started after inducing anesthesia and continued until the end of surgery at a fixed dose (0.5 ug/kg/h) | 60 | 59 [56–65] | 30/30 | 23 ± 5 | 20/40/0 | placebo | thoracoscopic lung resection surgery | sevoflurane | pain score (numeric rating scale), analgesic consumption (opioids), ICU stay | 5 |
| 3 | Wu 2018 | 30 | 59.0 ± 8.8 | 15/15 | - | 2/14/14 | 0.5 ug/kg/h dexmedetomidine through the surgery | 30 | 58.7 ± 10.1 | 16/14 | - | 1/18/11 | placebo | thoracoscopic surgery | fentanyl, propofol and sevoflurane | heart rate, blood pressure, ICU stay and hospital stay | 4 (minus 1, unclear blindness) |
| 4 | Wang 2016 | 40 | 54.25 ± 9.98 | 20/20 | 21.93 ± 2.12 | - | 0.5 μ g/kg, dexmedetomidine diluted to 20 mL with physiologic saline and infused for 10 min intravenously before the surgery | 40 | 55.63 ± 11.20 | 20/20 | 22.10 ± 2.13 | - | no dexmedetomidine | video-assisted thoracoscopic lobectomy | oxycodone, propofol, fentanyl and sevoflurane | analgesic consumption (oxycodone dose), heart rate, blood pressure | 5 |
| 5 | Lee 2016 | 50 | 62.0 ± 10.5 | 26/24 | 23.6 ± 0.4 | 0/37/13 | dexmedetomidine 1.0 ug/kg for 20 min before the termination of surgery | 50 | 62.0 ± 11.5 | 23/27 | 23.6 ± 0.4 | 0/42/8 | placebo | video-assisted thoracoscopic surgery for lung cancer | propofol, remifentanil, desflurane and fentanyl | pain score (numeric rating scale), analgesic consumption (opioids), ICU stay | 5 |
| 6 | Lee 2016 (2) | 25 | 68.4 ± 6.4 | 12/13 | 22.3 ± 2.7 | 0/11/14 | dexmedetomidine at an initial loading dose of 1.0 ug/kg over 10 min followed by a maintenance dose of 0.5 ug/kg/h during the surgery | 25 | 69.4 ± 8.7 | 11/14 | 22.7 ± 2.1 | 0/12/13 | placebo | thoracoscopy for lung resection | propofol, remifentanil, and sevoflurane | heart rate, blood pressure, ICU stay and hospital stay | 5 |
ASA American Society of Anesthesiologists
Among the six studies included here, three studies reported pain scores [15, 16, 18], three studies reported analgesic consumption [7, 16, 18], four studies reported mean heart rate and blood pressure [7, 15, 17, 18], three studies reported ICU stay [15, 17, 24], and three studies reported hospital stay [17, 18, 24]. Jadad scores of the six included studies vary from 3 to 5, and all six studies are considered to be high-quality ones according to quality assessment.
Primary outcome: pain scores
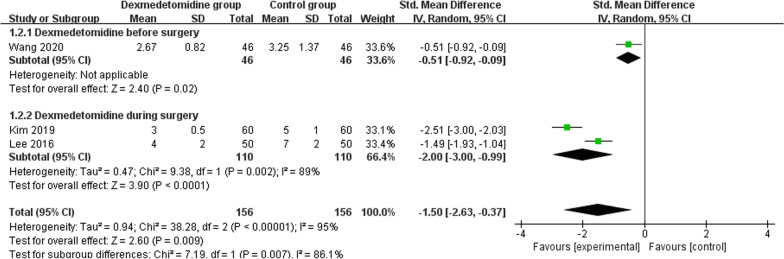
This outcome data was analyzed with the random-effects model, and the pooled estimate of the three included RCTs suggested that compared to control group for thoracoscopic surgery, dexmedetomidine was associated with significantly reduced pain scores (SMD = -1.50; 95% CI = -2.63 to -0.37; P = 0.009), with significant heterogeneity among the studies (I2 = 95%, heterogeneity P < 0.00001) (Fig. 1).
Fig. 1.
Forest plot for the meta-analysis of pain scores
Sensitivity analysis
Significant heterogeneity is observed among the included studies for the primary outcomes, but there is still significant heterogeneity after when performing sensitivity analysis via omitting one study in turn to detect the heterogeneity (I2 ranging from 89 to 97%). In addition, we perform the subgroup analysis based on dexmedetomidine supplementation before vs during surgery, but there is still significant heterogeneity (I2 = 89%). The results find that dexmedetomidine supplementation results in substantially reduced pain scores when administered before surgery (P = 0.02) and during surgery (P < 0.0001, Fig. 2).
Fig. 2.
Subgroup analysis of pain scores based on dexmedetomidine supplementation before surgery versus during surgery
Secondary outcomes
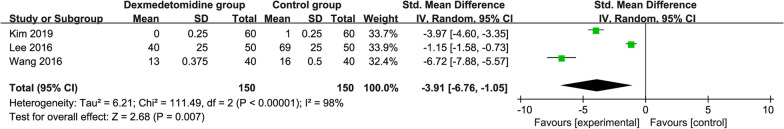
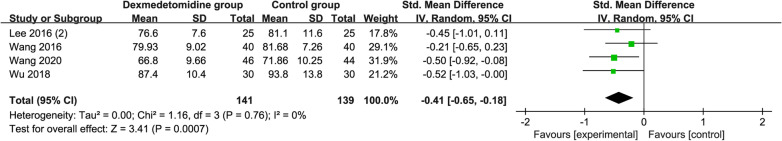
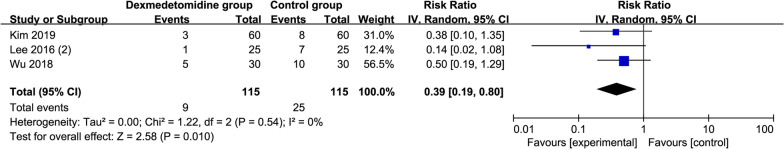
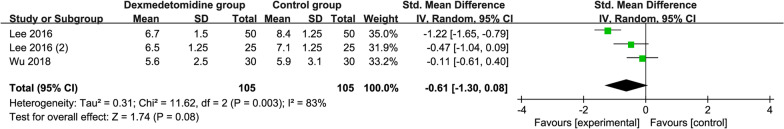
Compared to control group for thoracoscopic surgery, dexmedetomidine can significantly reduce anesthetic consumption (SMD = − 3.91; 95% CI = -6.76–− 1.05; P = 0.007; Fig. 3) and mean heart rate (SMD = − 0.41; 95% CI = − 0.65–− 0.18; P = 0.0007; Fig. 4), but has no important impact on mean blood pressure (SMD = − 0.07; 95% CI = − 0.45–0.31; P = 0.72; Fig. 5). In addition, dexmedetomidine was associated with the decrease in the RR of ICU stay (RR = 0.39; 95% CI = 0.19–0.80; P = 0.01; Fig. 6), but revealed no effect on hospital stay (SMD = − 0.61; 95% CI = − 1.30 to 0.08; P = 0.08; Fig. 7).
Fig. 3.
Forest plot for the meta-analysis of analgesic consumption
Fig. 4.
Forest plot for the meta-analysis of mean heart rate
Fig. 5.
Forest plot for the meta-analysis of mean blood pressure
Fig. 6.
Forest plot for the meta-analysis of ICU stay
Fig. 7.
Forest plot for the meta-analysis of hospital stay
Discussion
Thoracoscopic surgery has been widely used to treat lung cancer because of its minimally invasion, less postoperative pain and shortened hospital stay compared with open thoracotomy [25]. Postoperative pain management, particularly early postoperative pain, still remains a matter of concern for many anesthesiologists and these patients [26, 27]. Opioids are essential during surgery, and many methods are developed to reduce opioid consumption due to the side effects such as delayed recovery from general anesthesia, opioid-induced nausea, and respiratory depression [28, 29].
Intraoperative dexmedetomidine was reported to improve the effects of postoperative analgesia [30–32]. It showed analgesic, sedative and anxiolytic effects, and avoided respiratory depression and the inhibitory effect of sympathetic stimulation as an adjunct to general anesthesia [8]. Our meta-analysis included six RCTs and 510 patients. The results revealed that intravenous dexmedetomidine was associated with substantially reduced pain scores, anesthetic consumption, the RR of ICU stay and mean heart rate after thoracoscopic surgery, but showed no obvious influence on mean blood pressure or hospital stay.
In addition, dexmedetomidine benefited to maintain the stability of the cardiovascular system and decrease the stress response [10]. Intraoperative infusion of dexmedetomidine decreased both norepinephrine and epinephrine. Dexmedetomidine can decrease the release of catecholamines and has analgesic, anxiolytic, and hypnotic effects [33]. Regarding the sensitivity analysis, there is significant heterogeneity. Several reasons may account for the heterogeneity. Firstly, different doses and methods of dexmedetomidine supplementation may produce some bias. For instance, Dexmedetomidine was used before the anesthesia [7, 15, 18, 24] or during surgery [16, 17]. Secondly, dexmedetomidine was applied as the adjunct to different drugs such as oxycodone and sevoflurane, which may result in various analgesic effect. Thirdly, different operation procedures produces various pain intensity, which may affect the pooling results.
This meta-analysis has several potential limitations. Firstly, our analysis is based on only six RCTs, and three of them have a relatively small sample size (n < 100). Overestimation of the treatment effect was more likely in smaller trials compared with larger samples. Next, the doses, methods and combination of anesthetic drugs in included RCTs are different, which may have an influence on the pooling results. Finally, thoracoscopic surgeries are performed for various diseases and operation procedures.
Conclusions
Dexmedetomidine benefits to improve the analgesic efficacy for thoracoscopic surgery.
Supplementary Information
Additional file 1: Figure S1. Flow diagram of study searching and selection process.
Acknowledgements
None.
Abbreviations
- RCTs
Randomized controlled trials
- MDs
Mean differences
- CIs
Confidence intervals
- RRs
Risk ratios
Authors' contributions
CS conducted the design, study planning, data analysis and data interpretation. QL wrote and revised the article. Both authors read and approved the final manuscript.
Funding
Not applicable.
Availability of data
Not applicable.
Declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare no conflict of interest.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Chengjun Song, Email: nbschj@163.com.
Quan Lu, Email: 191973251@qq.com.
References
- 1.Murakawa T, Sato H, Okumura S, Nakajima J, Horio H, Ozeki Y, Asamura H, Ikeda N, Otsuka H, Matsuguma H, Yoshino I, Chida M, Nakayama M, Iizasa T, Okumura M, Shiono S, Kato R, Iida T, Matsutani N, Kawamura M, Sakao Y, Funai K, Furuyashiki G, Akiyama H, Sugiyama S, Kanauchi N, Shiraishi Y, Metastatic Lung Tumor Study Group of Japan Thoracoscopic surgery versus open surgery for lung metastases of colorectal cancer: a multi-institutional retrospective analysis using propensity score adjustmentdagger. Eur J Cardiothorac Surg. 2017;51(6):1157–1163. doi: 10.1093/ejcts/ezx020. [DOI] [PubMed] [Google Scholar]
- 2.Kinjo Y, Kurita N, Nakamura F, Okabe H, Tanaka E, Kataoka Y, Itami A, Sakai Y, Fukuhara S. Effectiveness of combined thoracoscopic-laparoscopic esophagectomy: comparison of postoperative complications and midterm oncological outcomes in patients with esophageal cancer. Surg Endosc. 2012;26(2):381–390. doi: 10.1007/s00464-011-1883-y. [DOI] [PubMed] [Google Scholar]
- 3.Pham TH, Perry KA, Dolan JP, Schipper P, Sukumar M, Sheppard BC, Hunter JG. Comparison of perioperative outcomes after combined thoracoscopic-laparoscopic esophagectomy and open Ivor-Lewis esophagectomy. Am J Surg. 2010;199(5):594–598. doi: 10.1016/j.amjsurg.2010.01.005. [DOI] [PubMed] [Google Scholar]
- 4.Davies RG, Myles PS, Graham JM. A comparison of the analgesic efficacy and side-effects of paravertebral vs epidural blockade for thoracotomy–a systematic review and meta-analysis of randomized trials. Br J Anaesth. 2006;96(4):418–426. doi: 10.1093/bja/ael020. [DOI] [PubMed] [Google Scholar]
- 5.Sztain JF, Gabriel RA, Said ET. Thoracic epidurals are associated with decreased opioid consumption compared to surgical infiltration of liposomal bupivacaine following video-assisted thoracoscopic surgery for lobectomy: a retrospective cohort analysis. J Cardiothorac Vasc Anesth. 2018 doi: 10.1053/j.jvca.2018.06.013. [DOI] [PubMed] [Google Scholar]
- 6.Adhikary SD, Pruett A, Forero M, Thiruvenkatarajan V. Erector spinae plane block as an alternative to epidural analgesia for post-operative analgesia following video-assisted thoracoscopic surgery: a case study and a literature review on the spread of local anaesthetic in the erector spinae plane. Indian J Anaesth. 2018;62(1):75–78. doi: 10.4103/ija.IJA_693_17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wang X, Wang K, Wang B, Jiang T, Xu Z, Wang F, Yu J. Effect of oxycodone combined with dexmedetomidine for intravenous patient-controlled analgesia after video-assisted thoracoscopic lobectomy. J Cardiothorac Vasc Anesth. 2016;30(4):1015–1021. doi: 10.1053/j.jvca.2016.03.127. [DOI] [PubMed] [Google Scholar]
- 8.Carollo DS, Nossaman BD, Ramadhyani U. Dexmedetomidine: a review of clinical applications. Curr Opin Anaesthesiol. 2008;21(4):457–461. doi: 10.1097/ACO.0b013e328305e3ef. [DOI] [PubMed] [Google Scholar]
- 9.Venn RM, Bradshaw CJ, Spencer R, Brealey D, Caudwell E, Naughton C, Vedio A, Singer M, Feneck R, Treacher D, Willatts SM, Grounds RM. Preliminary UK experience of dexmedetomidine, a novel agent for postoperative sedation in the intensive care unit. Anaesthesia. 1999;54(12):1136–1142. doi: 10.1046/j.1365-2044.1999.01114.x. [DOI] [PubMed] [Google Scholar]
- 10.Kim MH, Lee KY, Bae SJ, Jo M, Cho JS. Intraoperative dexmedetomidine attenuates stress responses in patients undergoing major spine surgery. Minerva Anestesiol. 2019;85(5):468–477. doi: 10.23736/S0375-9393.18.12992-0. [DOI] [PubMed] [Google Scholar]
- 11.Shamim R, Srivastava S, Rastogi A, Kishore K, Srivastava A. Effect of two different doses of dexmedetomidine on stress response in laparoscopic pyeloplasty: a randomized prospective controlled study. Anesth Essays Res. 2017;11(4):1030–1034. doi: 10.4103/aer.AER_54_17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kim HC, Lee YH, Jeon YT, Hwang JW, Lim YJ, Park JE, Park HP. The effect of intraoperative dexmedetomidine on postoperative catheter-related bladder discomfort in patients undergoing transurethral bladder tumour resection: a double-blind randomised study. Eur J Anaesthesiol. 2015;32(9):596–601. doi: 10.1097/EJA.0000000000000196. [DOI] [PubMed] [Google Scholar]
- 13.Shorrock P, Heaton T, Cochrane N, Jackson M, Lund K, Plummer N. The effects of dexmedetomidine on postoperative pain. Anaesthesia. 2015;70(3):372. doi: 10.1111/anae.13033. [DOI] [PubMed] [Google Scholar]
- 14.Peng K, Liu HY, Wu SR, Cheng H, Ji FH. Effects of combining dexmedetomidine and opioids for postoperative intravenous patient-controlled analgesia: a systematic review and meta-analysis. Clin J Pain. 2015;31(12):1097–1104. doi: 10.1097/AJP.0000000000000219. [DOI] [PubMed] [Google Scholar]
- 15.Wang YL, Kong XQ, Ji FH. Effect of dexmedetomidine on intraoperative Surgical Pleth Index in patients undergoing video-assisted thoracoscopic lung lobectomy. J Cardiothorac Surg. 2020;15(1):296. doi: 10.1186/s13019-020-01346-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kim JA, Ahn HJ, Yang M, Lee SH, Jeong H, Seong BG. Intraoperative use of dexmedetomidine for the prevention of emergence agitation and postoperative delirium in thoracic surgery: a randomized-controlled trial. Can J Anaesth. 2019;66(4):371–379. doi: 10.1007/s12630-019-01299-7. [DOI] [PubMed] [Google Scholar]
- 17.Wu CY, Lu YF, Wang ML, Chen JS, Hsu YC, Yang FS, Cheng YJ. Effects of dexmedetomidine infusion on inflammatory responses and injury of lung tidal volume changes during one-lung ventilation in thoracoscopic surgery: a randomized controlled trial. Mediators Inflamm. 2018;2018:2575910. doi: 10.1155/2018/2575910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lee SH, Lee CY, Lee JG, Kim N, Lee HM, Oh YJ. Intraoperative dexmedetomidine improves the quality of recovery and postoperative pulmonary function in patients undergoing video-assisted thoracoscopic surgery: a CONSORT-prospective, randomized, controlled trial. Medicine. 2016;95(7):e2854. doi: 10.1097/MD.0000000000002854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Moher D, Liberati A, Tetzlaff J, Altman DG, PRISMA Group Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. J Clin Epidemiol. 2009;62(10):1006–1012. doi: 10.1016/j.jclinepi.2009.06.005. [DOI] [PubMed] [Google Scholar]
- 20.Thackray NM, Gibbs NM. American Society of Anesthesiologists P5: "with or without" definition? Anesthesiology. 2011;114(2):467–468. doi: 10.1097/ALN.0b013e3182065c88. [DOI] [PubMed] [Google Scholar]
- 21.Jadad AR, Moore RA, Carroll D, Jenkinson C, Reynolds DJM, Gavaghan DJ, McQuay HJ. Assessing the quality of reports of randomized clinical trials: Is blinding necessary? Control Clin Trials. 1996;17(1):1–12. doi: 10.1016/0197-2456(95)00134-4. [DOI] [PubMed] [Google Scholar]
- 22.Kjaergard LL, Villumsen J, Gluud C. Reported Methodologic Quality and Discrepancies between Large and Small Randomized Trials in Meta-Analyses. Ann Intern Med. 2001;135(11):982–989. doi: 10.7326/0003-4819-135-11-200112040-00010. [DOI] [PubMed] [Google Scholar]
- 23.Higgins JP, Thompson SG. Quantifying heterogeneity in a meta-analysis. Stat Med. 2002;21(11):1539–1558. doi: 10.1002/sim.1186. [DOI] [PubMed] [Google Scholar]
- 24.Lee SH, Kim N, Lee CY, Ban MG, Oh YJ. Effects of dexmedetomidine on oxygenation and lung mechanics in patients with moderate chronic obstructive pulmonary disease undergoing lung cancer surgery: a randomised double-blinded trial. Eur J Anaesthesiol. 2016;33(4):275–282. doi: 10.1097/EJA.0000000000000405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Steinthorsdottir KJ, Wildgaard L, Hansen HJ, Petersen RH, Wildgaard K. Regional analgesia for video-assisted thoracic surgery: a systematic review. Eur J Cardiothorac Surg . 2014;45(6):959–966. doi: 10.1093/ejcts/ezt525. [DOI] [PubMed] [Google Scholar]
- 26.Falcoz PE, Puyraveau M, Thomas PA, Decaluwe H, Hürtgen M, Petersen RH, Hansen H, Brunelli A. Video-assisted thoracoscopic surgery versus open lobectomy for primary non-small-cell lung cancer: a propensity-matched analysis of outcome from the European Society of Thoracic Surgeon database. Eur J Cardiothorac Surg. 2016;49(2):602–609. doi: 10.1093/ejcts/ezv154. [DOI] [PubMed] [Google Scholar]
- 27.Bendixen M, Jørgensen OD, Kronborg C, Andersen C, Licht PB. Postoperative pain and quality of life after lobectomy via video-assisted thoracoscopic surgery or anterolateral thoracotomy for early stage lung cancer: a randomised controlled trial. Lancet Oncol. 2016;17(6):836–844. doi: 10.1016/S1470-2045(16)00173-X. [DOI] [PubMed] [Google Scholar]
- 28.Clarke H, Soneji N, Ko DT, Yun L, Wijeysundera DN. Rates and risk factors for prolonged opioid use after major surgery: population based cohort study. BMJ (Clin Res ed.) 2014;348:g1251. doi: 10.1136/bmj.g1251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Fletcher D, Martinez V. Opioid-induced hyperalgesia in patients after surgery: a systematic review and a meta-analysis. Br J Anaesth. 2014;112(6):991–1004. doi: 10.1093/bja/aeu137. [DOI] [PubMed] [Google Scholar]
- 30.Grape S, Kirkham KR, Frauenknecht J, Albrecht E. Intra-operative analgesia with remifentanil vs. dexmedetomidine: a systematic review and meta-analysis with trial sequential analysis. Anaesthesia. 2019;74(6):793–800. doi: 10.1111/anae.14657. [DOI] [PubMed] [Google Scholar]
- 31.Ranganathan P, Ritchie MK, Ellison MB, Petrone A, Heiraty P, Tabone LE. A randomized control trial using intraoperative dexmedetomidine during Roux-en-Y gastric bypass surgery to reduce postoperative pain and narcotic use. Surg Obes Relat Dis. 2019;15(4):588–594. doi: 10.1016/j.soard.2019.01.021. [DOI] [PubMed] [Google Scholar]
- 32.Beder El Baz MM, Farahat TEM. Intraperitoneal levobupivacaine alone or with dexmedetomidine for postoperative analgesia after laparoscopic cholecystectomy. Anesth Essays Res. 2018;12(2):355–358. doi: 10.4103/aer.AER_205_17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Gertler R, Brown HC, Mitchell DH, Silvius EN. Dexmedetomidine: a novel sedative-analgesic agent. Proc (Bayl Univ Med Cent) 2001;14(1):13–21. doi: 10.1080/08998280.2001.11927725. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Additional file 1: Figure S1. Flow diagram of study searching and selection process.
Data Availability Statement
Not applicable.