Abstract
Background
Glanders is a transmissible zoonotic disease caused by Burkholderia mallei that infects equids and humans. No glanders cases in equids were reported so far in Nepal.
Case presentation
Following suspected glanders in animals with clinical signs in different regions in Nepal, serum samples were tested by CFT, ELISA and Luminex® tests. Two horses and a mule tested positive for glanders by all tests, while two other equids only tested positive by ELISA and Luminex®. Analysis of swabs and pus samples by a PCR system targeting B. mallei confirmed the presence of the bacterium in the samples collected from the 3 equids that yielded positive results in all serological tests. Genotyping of the three PCR positive samples with a SNP-based method identified a genotype closely related to the B. mallei strains circulating in India.
Conclusion
Confirmation of glanders cases underscores the need of implementing a surveillance program in Nepal and a strict control of the animal movement across the borders.
Keywords: Burkholderia mallei, Equids, India
Background
Glanders is an infectious disease caused by Burkholderia mallei. This zoonotic bacterium primarily infects equids [1]. Several outbreaks of glanders in equids have been recently reported in South Asia, the Middle East, and South America (Brazil) [2]. Clinical and laboratory diagnosis of glanders is difficult since limited clinical signs are expressed in the early stage of infection. Symptoms of B. mallei infection include nasal discharge, pneumonia, and ulcerating nodular lesions on the skin. Discharges from the respiratory tract and skin are infectious. Transmission between animals is facilitated by close contact, inhalation, ingestion of contaminated materials (e.g., from infected feed and water troughs), or by inoculation (e.g., via a harness). Diagnostic methods of glanders include immunological tests such as complement fixation test (CFT) or ELISA and/or allergic reaction (malleinization), as well as direct tests such as bacteriological isolation and molecular tests. For B. mallei typing, the high-resolution melting PCR (HRM-PCR) technique targeting single nucleotide polymorphisms (SNPs) allows categorization of strains into three lineages (L1 to L3), as well as branches, sub-branches, and clusters with geographic specificities [3–5].
Case presentation
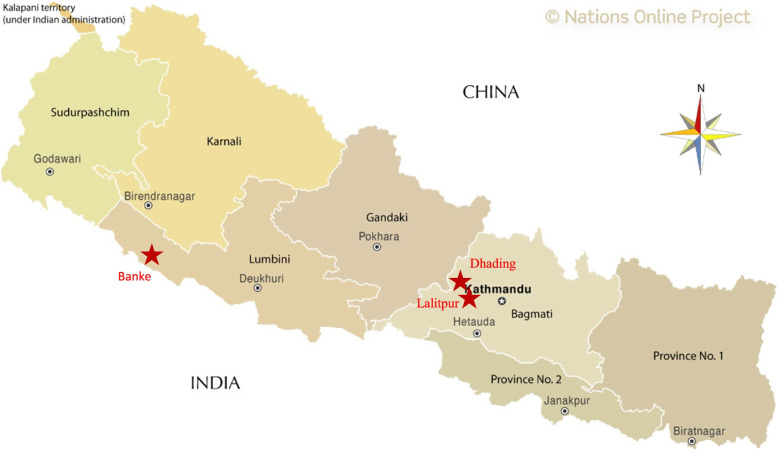
In November 2020, equids in Banke district of mid-western Nepal developed clinical signs and symptoms like high grade fever (up to 40-410C), labored breathing, dry cough, loss of appetite, lameness, thick mucopurulent yellowish nasal discharge, pus filled nodules on different parts of body, especially on thigh area. Later, in December 2020, similar clinical symptoms and signs were observed in mules in Dhading and Lalitpur districts of the Bagmati Province. The death of several equids was also reported in Nepalgunj, Lumbini province (Fig. 1). Available clinical surveillance data are reported in Table 1. Sticky yellowish pus discharge from ulcerated nodules and scabs was noticed on some of the infected equids (Fig. 2). Mules showed more severe symptoms than horses. All the infected animals were isolated and given symptomatic treatment. In most cases, symptoms relapsed after some time, became more severe and animals died of the disease. There is no policy to euthanize glanders infected animals in Nepal.
Fig. 1.
Map showing the geographical locations of the glanders-affected districts in Nepal, between November and December 2020 (map source:
© Nations Online Project, https://www.nationsonline.org/oneworld/map/nepal-administrative-map.htm)
Table 1.
Total risks population reported morbidities and mortalities
| Districts | Administrative Region | Species | Total populationa | Susceptible cases | Cases | Deaths |
|---|---|---|---|---|---|---|
| Lalitpur | Bagmati Province | Equine | 250 | 10 | 2 | 0 |
| Dhading | Bagmati Province | Equine | 293 | 3 | 1 | 0 |
| Banke | Lumbini Province | Equine | 2021 | 77 | 24 | 16 |
aSource: Statistical Information of Nepalese Agriculture 2019-20, Page no. 91, https://www.moald.gov.np/publication/Agriculture%20Statistics
Separate data for horses, mules and donkeys aren’t available. Susceptible cases were asymptomatic equines belonging to the same farms in which cases were found. Cases were equids that developed signs and symptoms of glanders
Fig. 2.
Lesions observed in mules (pus filled nodules and thick yellowish pus discharge from nodules)
For diagnosis and further investigations, sera and tissue samples were collected from three horses and two mules, each from different owners. One horse (L/157) came from the Lalitpur district, while the four other equids came from the Banke district (B/113, B/115, B/117 and B/120).
Sera from these five equids were analyzed with five different serological tests: (i) CFT [6], (ii) ID Screen® Glanders indirect ELISA (IdVet, France) [7], (iii) GLANDA ELISA (IdVet, France) based on two recombinant proteins [8], (iv) Luminex® bead-based assay targeting Hcp1 and GroEL proteins [9], and (v) ELISA based on a glycoengineered protein of Burkholderia recently validated for glanders diagnosis (Glyco ELISA) [10]. Two horses (L/157, B/120) and one mule (B/113) were positive by all tests and positive or undetermined results were obtained for all animals with ID Screen® Glanders indirect ELISA, GLANDA ELISA and Luminex® tests. The CFT and the ELISA test based on a glycoengineered protein identified fewer positive samples (Table 2).
Table 2.
Serological results from the five equids tested with the complement fixation test, ELISA and Luminex® methods
| ID Screen ® Glanders indirect ELISAa | GLANDA ELISAb | Luminex – Hcp1 | Luminex - GroEL | Glyco Elisac | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| CFT | Cut-off > 50 (40<S/P >= 50 : undet.) | cut-off > 50 | cut-off > 43 | cut-off > 45 | cut-off > 40 | ||||||||
| Animal ID | species | Titer | Result | %S/P | Result | %S/P | Result | %S/P | Result | %S/P | Result | %S/P | Result |
| L/157 | Horse | 4441 | P | 119 | P | 281 | P | 121 | P | 123 | P | 150 | P |
| B/113 | Mule | 444443 | P | 117 | P | 301 | P | 100 | P | 110 | P | 164 | P |
| B/115 | Mule | 0 | n | 80 | P | 285 | P | 62 | P | 90 | P | 9 | n |
| B/117 | Horse | 0 | n | 47 | U | 288 | P | 146 | P | 45 | U | 2 | n |
| B/120 | Horse | 444444 | P | 129 | P | 288 | P | 145 | P | 147 | P | 193 | P |
aELISA test based on a semi-purified fraction of B. mallei, bELISA test based on a recombinant protein of B. mallei, cELISA test based on a glycoengineered antigen of Burkholderia
P Positive, U Undetermined, n Negative
For CFT, result were expressed as the intensity of hemolysis inhibition (0=0%, 1=25%, 2=50%, 3=75%, and 4=100%) for reach serum dilution (1/5, 1/10, 1/20, 1/40 and 1/80)
For ELISA and Luminex methods, results were expressed as S/P =((Sample – Negative control)/(Positive control - Negative control))* 100. Cut-off values for each test are mentioned
In parallel, nasal or pus swabs were collected from these five animals. Samples were submitted to DNA extraction and PCR amplifications as previously described [4]. Briefly, after DNA extraction with the High Pure PCR Template Preparation Kit (Roche, Meylan, France), DNA was amplified by real-time PCR using four different PCR systems: fliP (specific for B. mallei), orf11 (specific for B. pseudomallei), and aroA (specific for the B. pseudomallei complex). All samples with a quantification cycle (Cq) over 39 were considered as negatives. Both aroA and fliP PCR detected a positive signel for the three equids (L/157, B/113, B/120) that were positive by all serological tests (Table 3).
Table 3.
Summary of the molecular analyses conducted on samples collected from L/157, B/113, and B/120
| real-time PCR | PCR-HRM clustering | |||||
|---|---|---|---|---|---|---|
| B. pseudomallei complex | B.mallei fliP | B. pseudomallei | ||||
| Horse Id | Sample | IPC | aroA | fliP | orf11 | |
| (Cq value) | (Cq value) | (Cq value) | (Cq value) | |||
| L/157 | swab | 30.3 | 31.6 | 29.4 | - | L2B2s B2 India – Group_2 (large) |
| B/113 | swab | 30 | 37.6 | 34.2 | - | L2B2s B2 India – Group_2 (large) |
| B/115 | swab | 30 | 40a | - | - | / |
| B/117 | swab | 30.2 | - | - | - | / |
| B/1 | pus | 32 | 35.5 | 33.3 | - | L2B2s B2 India – Group_2 (large) |
IPC Internal control with exogen DNA, - (negative), / (not done), PCR-HRM High-resolution melting PCR analysis for the genotyping of Burkholderia mallei
avalue beyond the cut-off (sample considered as negative)
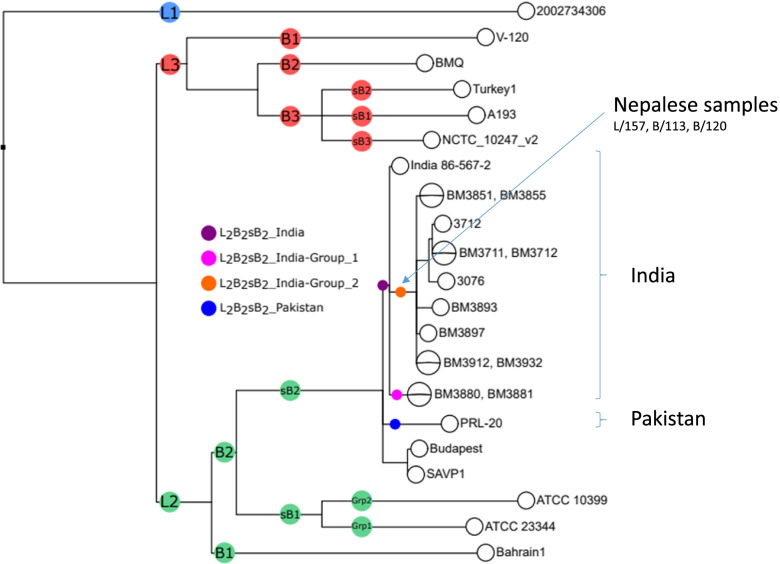
We further genotyped the B. mallei strain from these three PCR positive samples. After a pre-amplification step to increase the amount of template (using the Perfecta® pre-amplification kit (Quantabio) and the corresponding set of primers), DNA samples were analyzed by PCR-HRM [3]. The panel of 15 markers was used to classify the B. mallei strains into one of the three lineages (L1 to L3) and the branches, sub-branches, and groups to which they relate. All samples corresponded to the L2B2sB2branch, which includes B. mallei strains circulating in India and Pakistan. A recent study, using four new SNP markers, classified B. mallei strains from India and Pakistan into two small and large subgroups [5]. This new set of markers were investigated in the three positive Nepalese samples from Lalitpur (L/157) and Banke (B/113 and B/120). The results indicated that all samples clustered in the India_group 2 (large), which includes most of the Indian strains typed so far with this new set [5], all originating from the states of Uttar Pradesh and Haryana, in Northern India (Fig. 3). The origin of the India_group 1 (small) strains is not clearly defined at this time.
Fig. 3.
A SNP tree of Burkholderia mallei incorporating strains from India and Pakistan within the L2B2sB2 branch (5). PCR HRM clustering results for samples L/157, B/113, B/120 in L2B2sB2_India _Group 2 are shown
Discussion and conclusions
Recently Adhikari and colleagues [11] warned about the potential risks of glanders outbreaks in Nepal due to the re-emergence of the disease in neighboring Indian states [12–14], and the unrestricted movement of equids between the two countries. Most equids from India passed through the Nepalgunj quarantine office, the closest to India’s Uttar Pradesh region [11], where glanders cases are regularly reported [12–14]. Our epidemiological investigation indicates the equids were imported from Uttar Pradesh through uncontrolled routes, as there is about 1,770 kilometers of open border between India and Nepal. Further, there is seasonal migration of horses and mules from far western part of Nepal to western and central part and back to the India’s Uttar Pradesh region. Most of the equids in Nepal (an estimated number of 59,762; Ministry of Agriculture and Livestock Development (MOALD), 2019/2020) are used in the brick industry for transportation of bricks, goods and pulling carts. Equids are also popular in the tourism industry for transport of goods by trekkers. Horses are only vehicles for transport of goods and humans in high hill areas where mechanical vehicles cannot be used [15].
In May 2021, Nepal notified to OIE its first outbreak of glanders. Until now, no policy for prevention and control of this disease was implemented. The confirmation of glanders cases, detected in central and mid-western parts of Nepal and reported in this study, should prompt a national surveillance programme and enhanced border control measures. In general, the glanders situation in Asia is very poorly documented and strict measures are necessary to control this re-emergent disease.
Acknowledgments
Not applicable
Abbreviations
- CFT
Complement fixation test
- DNA
Desoxyribose Nucleic Acid
- iELISA
Indirect ELISA
- ELISA
Enzyme-Linked Immuno Assay
- HRM-PCR
High Resolution Melting-PCR
- PCR
Polymerase Chain Reaction
- SNP
Single Nucleotide Polymorphism
Authors’ contributions
K.P., M.M., M.S., and P.K.R. contributed to the collection of samples and performed the clinical and preliminary diagnosis of these first cases of glanders in Nepal. D.T., W.G., V.M.A and K.L performed the complementary diagnosis investigations and molecular typing analysis. All authors contributed to the revising of the manuscript. The authors read and approved the final manuscript.
Funding
This project was supported by the Government of Nepal and by the European Commission’s Directorate-General for Health and Consumers.
Availability of data and materials
All data generated or analyzed during this study are included in this published article. The datasets generated during and/or analysed during the current study are available from the corresponding author.
Declarations
Ethics approval and consent to participate
Samples from naturally infected animal were collected as part of routine veterinary investigation carried out by qualified veterinarians from the Central Veterinary Laboratory of the Government of Nepal.
Consent for publication
Not applicable
Competing interests
The authors have no financial or personal relationships with any individuals or organizations that could inappropriately influence or bias this paper.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Koirala P, Email: paggya2000@gmail.com.
Laroucau K, Email: karine.laroucau@anses.fr.
References
- 1.Khan I, Wieler LH, Melzer F, Elschner MC, Muhammad G, Ali S, Sprague LD, Neubauer H, Saqib M. Glanders in animals: a review on epidemiology, clinical presentation, diagnosis and countermeasures. Transbound Emerg Dis. 2013;60:204–221. doi: 10.1111/j.1865-1682.2012.01342.x. [DOI] [PubMed] [Google Scholar]
- 2.World Organization for Animal Health. 2018. Terrestrial animal health code. Available at: https://www.oie.int/fileadmin/Home/eng/Health_standards/tahm/3.05.11_GLANDERS.
- 3.Girault G, Wattiau P, Saqib M, Martin B, Vorimore F, Singha H, Engelsma M, Roest HJ, Spicic S, Grunow R, Vicari N, De Keersmaecker SCJ, Roosens NHC, Fabbi M, Tripathi BN, Zientara S, Madani N, Laroucau K. High-resolution melting PCR analysis for rapid genotyping of Burkholderia mallei. Infect Genet Evol. 2018;63:1–4. doi: 10.1016/j.meegid.2018.05.004. [DOI] [PubMed] [Google Scholar]
- 4.LaroucauK AR, Vorimore F, Varghese K, Deshayes T, Bertin C, Delannoy S, SamiAM AB, M, El ShorbagyM, AlmutawaaKAW, SaadAlanezi SJ, Alazemi YSN, Guernier- CambertV, Wernery U. A genetic variant of Burkholderia mallei detected in Kuwait: Consequences for the PCR diagnosis of glanders. Transbound Emerg Dis. 2021;68(2):960–963. doi: 10.1111/tbed.13777. [DOI] [PubMed] [Google Scholar]
- 5.Singha H, Vorimore F, Saini Sheetal, Deshayes T, Saqib M, Tripathi BN, Laroucau K. Molecular epidemiology of Burkholderia mallei isolates from India (2015–2016): New SNP markers for strain tracing. Infect Genet Evol. 2021;95:105059. doi: 10.1016/j.meegid.2021.105059. [DOI] [PubMed] [Google Scholar]
- 6.Laroucau K, Colaneri C, Jaÿ M, Corde Y, Drapeau A, Durand B, Zientara S, Beck C, Union E, laboratories involved in glandersserodiagnosis. Interlaboratory ring trial to evaluate CFT proficiency of European laboratories for diagnosis of glanders in equids. Vet Rec. 2016;178(25):632. doi: 10.1136/vr.103617. [DOI] [PubMed] [Google Scholar]
- 7.Elschner MC, Melzer F, Singha H, Muhammad S, Gardner I, Neubauer H. Validation of a commercial glanders ELISA as an alternative to the CFT in international trade of equidae. Front Vet Sci. 2021;8:628389. doi: 10.3389/fvets.2021.628389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Elschner M, Laroucau K, Singha H, El-Adawy H, Khan I, Saqib M, Melzer F, Gardner I, Neubauer H. Evaluation of the prescribed complement fixation test in comparison with western blot and five enzyme linked immune assays for serological diagnosis of glanders. PLoS One. 2019;14(4):e0214963. doi: 10.1371/journal.pone.0214963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Laroucau K, Saqib M, Martin B, Deshayes T, Bertin C, Wernery U, Joseph S, Singha H, Tripathi BN, Beck C. Development of a microsphere-based immunoassay for the serological detection of glanders in equids. Acta Topica. 2020;207:105463. doi: 10.1016/j.actatropica.2020.105463. [DOI] [PubMed] [Google Scholar]
- 10.Wang G, Glaser L, Scott NE, Fathy Mohamed Y, Ingram R, Laroucau K, Valvano MA. A glycoengineered antigen exploiting a conserved protein O-glycosylation pathway in the Burkholderia genus for detection of glanders infections. Virulence. 2021;12(1):493–506. doi: 10.1080/21505594.2021.1876440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Adhikari N, Acharya KP, Wilson RT. The potential for an outbreak of glanders in Nepal. Tropical Medicine and Health. 2019;47:57. doi: 10.1186/s41182-019-0185-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Malik P, Singha H, Khurana SK, Kumar R, Kumar S, Raut AA, Riyesh T, Vaid RK, Virmani N, Singh BK, Pathak SV, Parkale DD, Singh B, Pandey SB, Sharma TR, Chauhan BC, Awasthi V, Jain S, Singh RK. Emergence and re-emergence of glanders in India: a description of outbreaks from 2006 to 2011. Vet Ital. 2012;48(2):167–178. [PubMed] [Google Scholar]
- 13.Malik P, Singha H, Goyal SK, Khurana SK, Tripathi BN, Dutt A, Singh D, Sharma N, Jain S. Incidence of Burkholderia mallei infection among indigenous equines in India. Vet Rec Open. 2015;2(2):e000129. doi: 10.1136/vetreco-2015-000129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Singha H, Shanmugasundaram K, Tripathi BN, Saini S, Khurana SK, Kanani A, Shah N, Mital A, Kanwar P, Bhatt L, Limaye V, Khasa V, Arora R, Gupta S, Sangha S, Sharma H, Agarwal SK, Tapase J, Parnam S, Dubey P, Baalasundaram SK, Mandal BN, Virmani N, Raj Gulati B, Malik P. Serological surveillance and clinical investigation of glanders among indigenous equines in India from 2015 to 2018. Transbound Emerg Dis. 2020 doi: 10.1111/tbed.13475. [DOI] [PubMed] [Google Scholar]
- 15.Bhatt BR, Kafle A, Shrestha S, and Kaphle K.2020. Horse Breeds of Nepal. International Journal of Zoology and Animal Biology. 10.23880/izab-16000216. https://www.moald.gov.np/publication/Agriculture%20Statistics
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All data generated or analyzed during this study are included in this published article. The datasets generated during and/or analysed during the current study are available from the corresponding author.