Abstract
The unprecedented COVID-19 pandemic situation forced the scientific community to explore all the possibilities from various fields, and so far we have seen a lot of surprises, eureka moments and disappointments. One of the approaches from the cellular therapists was exploiting the immunomodulatory and regenerative potential of mesenchymal stromal cells (MSCs), more so of MSC-derived extracellular vesicles (EVs)—particularly exosomes, in order to alleviate the cytokine storm and regenerate the damaged lung tissues. Unlike MSCs, the EVs are easier to store, deliver, and are previously shown to be as effective as MSCs, yet less immunogenic. These features attracted the attention of many and thus led to a tremendous increase in publications, clinical trials and patent applications. This review presents the current landscape of the field and highlights some interesting findings on MSC-derived EVs in the context of COVID-19, including in silico, in vitro, in vivo and case reports. The data strongly suggests the potential of MSC-derived EVs as a therapeutic regime for the management of acute lung injury and associated complications in COVID-19 and beyond.
Graphical abstract
Keywords: COVID19, Viral pneumonia, Acute lung injury, Exosomes, Cell therapy, Regenerative medicine
Introduction
Reporting more than 5.7 million deaths currently, COVID-19 (coronavirus disease 2019) a viral disease characterized by its high contagion caused by the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) has had a major impact on health systems worldwide (WHO Coronavirus (COVID-19) Dashboard [1]). COVID was first reported from a cluster of pneumonia of unknown origin in Wuhan, China in December 2019 [2]. A 13-fold increase in cases over the preceding timeline provoked WHO action and on March 11, 2020, WHO took the historic step of declaring this disease as a global pandemic [3]. Subsequently, the unbiased sequencing of pathogenic samples [4] from pneumonia-affected populations isolated a novel coronavirus signature [5]. The 2019-nCoV demonstrates a distinct difference from MERS CoV, SARS CoV and two other coronaviruses that infect humans. Common strains of Coronaviruses when infective to humans demonstrate common cold symptoms with no severe sequelae [6]. During the last 2003 SARS-CoV outbreak there were no traces of disease transmission before symptom onset [7]. Incisive detective work by several groups of researchers has determined that 40–80% of transmission of novel coronavirus disease happens before symptoms are demonstrated. This window may range from two to four days [8]. This has complicated public health measures aimed at the control of disease transmission necessitating debilitating lockdown measures in most parts of the world. In turn, it has precipitated the unprecedented spreading of the disease [9].
Therapeutic options that are currently available are antivirals, monoclonal antibodies, anti-inflammatory agents, immunomodulators with more on the way. Anti-viral or antibody-based treatments are most effective during the early or replicative state of the disease. With later phases of the disease, it is ideal to seek control over inflammation, provide modulation as required and combine as required to achieve required endpoints [10]. A relatively new field, regenerative medicine has gained much popularity in the exploratory management of COVID-19. Regenerative medicine is accepted to be the culmination of multidisciplinary research and development that aims to be a definitive branch of medicine that will generate approaches to regenerate/repair defective tissues. A keystone of regenerative medicine is the application of mesenchymal stem cells (MSCs) derived from multifarious sources [11]. Adult stem cells are being commoditized for application in immunomodulation, angiogenesis, signaling and tissue remodeling applications [12]. The observation that during stem cell infusions effects are observed that are plausible only via secreted bioactive factors has been accepted [13, 14]. Deployment to address Graft versus Host Disease [15] also reveals that most effects noted are due to paracrine cross-talk [16]. Long-distance communication has been via secretion of vesicles that range from 20 to 120 nm in size and function by transport of genetic material or protein signals [17]. The International Society of Extracellular Vesicles has proposed the generic term “extracellular vesicles (EVs)” for the membrane bound vesicles released from the cell, and typically, three primary classes of EVs are recognized based on size viz. apoptotic bodies ranging between – 500 to 4000 nm, microvesicles ranging between – 100 to 1000 nm, and exosomes ranging between – 40 and 120 nm [18]. The use of EVs, particularly exosomes, has been explored in cardiovascular insults and cancer therapy. The use of these molecules as biomarkers and diagnostic tidemarks has been discussed and is under active investigation [19].
Exploratory studies on MSC-derived EVs, particularly exosomes, in the context of COVID-19 are growing recently. Briefly, MSC-derived EVs are being explored in various aspects of COVID19, including their use as biomarkers for diagnosis purpose, engineered EVs as immune-modulating agents, targeted therapeutic cargo as well as vaccines. The literature review indicated that various preclinical and clinical studies are underway with regard on finding an effective tissue source, effective dose, effective formulation, administrative dose, etc. Subsequent to completion of these studies, a comprehensive meta-analysis could derive an effective MSC-EVs based therapeutic regime for the management of acute lung injury in the context of COVID19. Several notable works have been reported recently on various aspects of stem cells and exosomes in the context of COVID19 [20–23]. However, this review standout as a unique collection of the current landscape in terms of preclinical, clinical and patent data sets reported to date.
COVID-19 and acute lung Injury
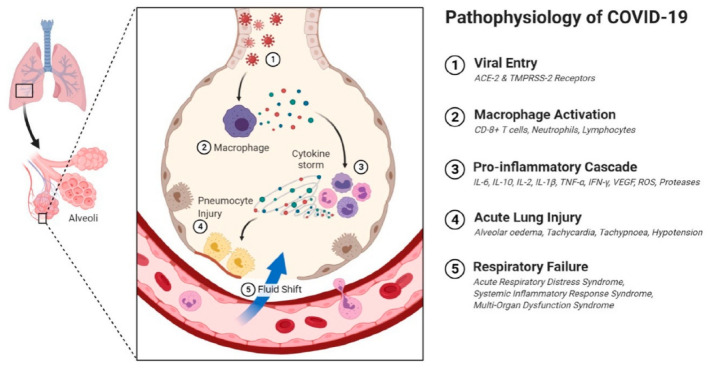
Based on the infection route and tissues affected, the COVID-19 disease progression can be represented as three phases [24]. Inhalation of virus particles causes the binding via Angiotensin-converting enzyme 2 (ACE2) receptor to the respiratory epithelial cells. ACE2 for entry and transmembrane serine protease 2 (TMPRSS2) for S protein priming have been pinpointed as its access and could be inhibited for clinical action [25]. Local propagation to the primary infection site and preliminary immune response initiation may happen in the initial days, with folks being asymptomatic carriers. Viral propagation and transition to lower respiratory tract along conducting airways and a more robust response from the host is generated over the next few days with clinical manifestation of the disease. Increased expression of Interferon-Inducible Protein-10 (CXCL-10) during the first 7 days of SARS was associated with an adverse outcome [26]. Assessing host immune response via monitoring of chemokine agents provides early insight to disease progression with [27] 80% of infected patients will have mild to moderate symptoms and can be managed with conservative therapy. In stage 3 of the disease affected parties may develop pulmonary infiltrates with a final fatality rate of around 2% with an age-related distribution [24]. Stage 3 has disruption of gas exchange units and moves into alveolar type II cells which are typical in SARS medicated infections [28]. Diffuse alveolar damage with a disordered wound healing process is a precursor to scarring with attendant fibrosis [29] with any process that increases the ACE2 receptor population causing overall deterioration. With lessened secretory clearance and poor repair gas exchange units in the elderly are especially prone to non—reversible damage [30].
The acute lung injury that is the most severe aftershock of COVID-19 has no specific definitive treatment. Characteristics include lymphopenia and cytokine storm as part of immune pathological changes [31]. Host response via multiple streams contributes to damage in pulmonary tissue, reduction in total function and reduced lung capacity depending on lack of control over immune response [32]. T—cells on priming with SARS CoV-2 antigens kick off generation of chemokines and cytokines (IL-16,8,21, TNF—alpha) among others that sparks the cytokine storm. Recruitment of immune cellular component to the site of infection proceeds with nuclear factor-kappa B presence and activation being a strong predictor of immune response and inflammation associated respiratory tissue damage [33]. Subsets of severe COVID 19 diagnosed patients may present with elevated markers for inflammation, systemic inflammation and cascading multiple organ failure [33, 34]. Cytokine storm in this disease context would refer to the dysregulation in cytokine release on systems being challenged with infection or other insults [34]. Increased IL-6 in plasma of acute cases of COVID 19 has been documented by several studies [35–38]. Elevated cytokine storm events contribute to pathological intra—alveolar fibrin accumulation and hikes production of growth factors [39] TGF, PDGF with a corresponding drop in surfactants in pulmonary tissue that causes coalescing alveoli leading to a decrease in effective lung volume [40]. This provides a niche conducive to fibroblast activity with rapid collagen deposition and the development of lung fibrosis [41]. A schematic showing pathophysiology of COVID-19 is presented in Fig. 1.
Fig. 1.
A schematic depicting the molecular pathophysiology of lungs in COVID-19-affected patients. Reproduced from [114], ©The Authors. Licensee MDPI, Basel, Switzerland
Stem Cells in COVID-19 and acute lung injury
In chronic and acute lung fibrosis there have been no treatment breakthroughs but with the current pandemic and its reach, there is a large fibrosis population that seeks to be addressed via bioengineering approaches. Damage in elderly or vulnerable populations [42] coupled with progenitor cell aging, accelerated senescence and aberrant regulation throws up multiple challenges in deciding therapeutic approaches [43]. Transient day-to-day insults to lung tissue are addressed by a resident progenitor cell population [44]. With aging, this gets more complicated with a complex interplay of elements with pathological levels of ECM deposition and enhanced circulating fibrocyte accumulation [45] in a bleomycin-induced lung injury model. Lowered reparative capacity hampers lung reserves and is dependent on the age and patency of cytokine response pathways [46]. Acute Respiratory Distress Syndrome (ARDS) based lung insults resulting in fibrosis are of concern under the current pandemic conditions. Incidence of ARDS although underreported was at 10–86 cases per 10,000 with a high fatality rate [47]. Currently, around 70% of mortality in COVID 19 has been directly linked to ARDS [48] with diffuse alveolar damage a hallmark in a majority of the cases [49].
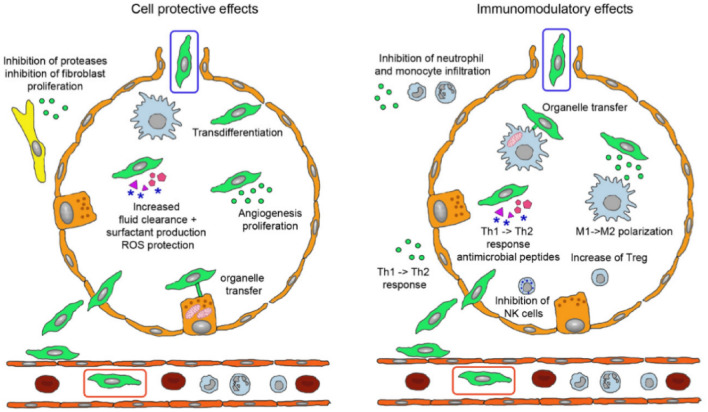
Stem cells have been an accepted tool in the regenerative medicine approach due to their adaptability, ability to derive multiple cell types, compatibility with scaffold systems and also provision of extracellular vesicle-based therapies as the field progresses [50]. These undifferentiated cells survive within specialized niches in a self-renewing, clonogenic and multipotent state with the ability to activate and proliferate in response to stimuli within major organ systems [51]. A schematic representation of MSCs in acute lung injury management is depicted in Fig. 2. Several authors have reported the application of MSC could reduce severity in lung injury in preclinical models with a reduction in E. coli endotoxin-induced lung injury reported in mice [52]. Later reports reinforce MSC therapy in mice and ex vivo lung models challenged with live bacteria [53, 54] with enhanced bacterial clearance and improvement in sepsis outcomes also reported [55, 56]. Further investigation of MSC’s mediation of repair has revealed the role of angiopoietin—1 [57], antagonists of interleukin-1 receptor and keratinocyte growth factor in this process [56]. Mitochondrial transfer mediated by exosomal vesicles is also postulated in this scenario [58, 59]. Delivery of reparative signals from culture-derived MSC extracellular vesicles would address these concerns without cell delivery. This simplifies the process and highlights a novel route to address ARDS and its long-term effects on respiratory tissue.
Fig. 2.
A schematic depicting the possible physiological effects of MSCs administered through intravenous (green cell in red box) and inhalation routes in recovering acute lung injury (green cell in blue box). Reproduced from [115], ©2021 The Authors, Licensee MDPI, Basel, Switzerland
Stem cell-derived extracellular vesicles in COVID-19 and acute lung injury
Lightning advances in understanding the underlying mechanisms of MSC-based cellular therapy have shown their anti-inflammatory effects, regeneration ability, immune escaping characteristics and ability in modulating various other extra/intra -cellular pathways. Many of such therapeutic activities of MSCs are attributed to the paracrine effects, wherein MSCs send signals to the neighboring cells through extracellular vesicles (EVs) [60]. EVs are micron/ sub-micron scale membrane-bound structures with sizes ranging between 30 nm − 1 μm and are secreted by almost all types of cells. EVs are mainly comprised of three types,1. Exosomes, which are small endosomal derived membrane vesicles with size of 30–150 nm size; 2. Microvesicles with the size between 150 and 1000 nm and 3. Apoptotic bodies with greater size than the microvesicles. Initially they were misjudged as cellular waste and later found to be a functional vehicle that carry cargo to communicate neighbouring cells and influence various cellular signalling. The invasion of the endosomal membrane and fusion with membrane of multi-vesicular body leads to exosome formation, whereas microvesicles and apoptotic bodies are formed by outward budding of plasma membrane from healthy and apoptotic cells, respectively. EVs enclose various biologically active molecules, including various growth factors, cytokines, chemokines, miRNA, etc., however, their contents may vary, depending on the type of parental cell from which they are secreted and their environment. Nevertheless, the cell adhesion molecules present on the surface of EVs help them to fuse with the plasma membrane of the recipient cell so as to release the cargo and consequently trigger downstream signaling cascades [61]. In contrast to MSCs alone, the MSC-derived EVs offer many advantages as cell-free therapeutics such as high stability, easy storage, low immunogenicity and yet replicate the therapeutic effect of the MSCs [62, 63]. A detailed account of EV’s biogenesis, composition and functions are beyond the scope of this review, and the readers are referred to the latest review articles for the same [64–67].
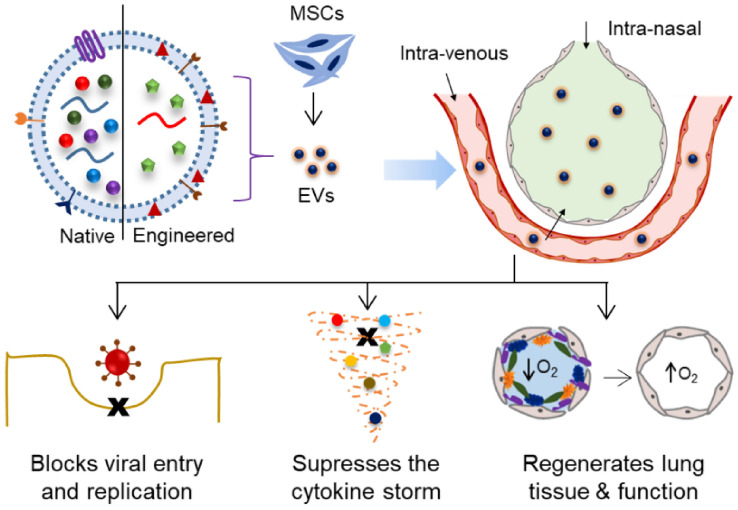
The initial reports indicating the therapeutic potential of EVs in cardioprotection were published in 2009 [68]. Subsequently, several studies published various molecular and biochemical evidence to unravel the EVs mechanism of action in controlling viral replication, reducing the inflammatory reaction and regenerating the damaged tissues (Fig. 3). For instance, Kesimer et al. reported that EVs from airway epithelial cell cultures showed a neutralizing effect on the human influenza virus, therefore suggested that these structures may be involved in diverse physiological processes in airway biology, including innate mucosal defense [69]. Other studies demonstrated that the EVs play a key role in suppressing the cytokine storm and rejuvenating the antiviral defense system of the host [61]. The EVs were reported to reduce the levels of virus-induced lung lesions including apoptosis in lung cells, by the production of growth factors such as keratinocyte growth factor, by reducing the influx of cytokines, inflammatory cells, by increasing the alveolar fluid clearance [70–72]. The MSC-EVs have the potential to repair and regenerate tissue, wherein some reports showed that the EVs induced the repopulation of type II alveolar cells which serve as progenitor cells for lung epithelium [73]. These and several other studies reviewed elsewhere offered possible mechanism of therapeutic action of MSC-derived EVs in the lung damage in general and in the COVID-19 management in particular [74–78]. Lately, The International Society for Cellular and Gene Therapies and the International Society for Extracellular Vesicles released a statement that they recognize, however do not endorse yet, the potential of EVs as treatments for COVID-19, and therefore encouraged research and trials in this regard [79].
Fig. 3.
A schematic representation of MSC-derived EVs, both naïve as well as engineered, in the management of viral infection and the lung damage in the context of COVID-19 [77, 116–118]
Publications landscape
Pre-clinical reports
In silico studies
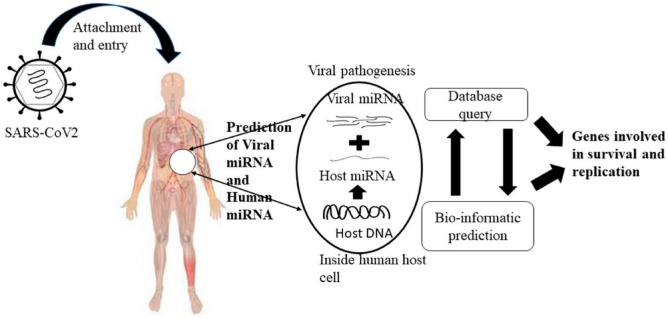
The in silico approaches, such as docking, is well-regarded and validated for predicting new drug-target associations. Several computational studies are reported on the virtual drug repurposing screening and prediction of potential drug-like occurring molecules against SARS-CoV-2; however, many of them explore natural or other molecules known in the literature [80–82]. MSC-derived EVs were known to contain miRNAs that are being investigated for their role in the progression, diagnosis and treatment of various diseases [83]. Several in silico studies are being undertaken to investigate the miRNA that can potentially aid in COVID-19 management (Fig. 4) [84–86]. For instance, in silico analysis done by Schultz et al. reported the capability of MSC-EVs miRNA to modulate the increased cytokines, cell death and coagulation disturbs caused by COVID-19 [87]. Using computational tools, four datasets of miRNA of different stem cell sources (bone marrow, adipose tissue and umbilical cord) and one dataset of mRNA (bone marrow) were analyzed. The result predicted 258 miRNAs for aggravated cytokines and chemokines, 266 miRNAs for cell death genes and 148 miRNAs for coagulation cascades. Amongst these, the study reported some miRNAs with the ability to reduce cytokine storm, protect from cell death, block coagulation cascade, and thereby protect from tissue damage [87]. However, there are not many studies on the interaction of MSC-EVs cargo molecules reported in the literature with several components of SARS-CoV-2 in silico.
Fig. 4.
A schematic depicting computational biology based in silico approach in predicting target molecules against SARS-CoV2. Reproduced with permission from [86], Copyright® Elsevier BV
In vitro reports
In vitro assays are yet another attractive platform for performing proof of concept experiments in a precise, cost-effective, time-saving and high-throughput manner. In the context of EVs in COVID-19 management, a handful of interesting studies were reported using in vitro assays [61, 88],Q. [89, 90]. For instance, Zhang et al. reported the presence of hACE2 on EVs secreted by various cell types, and the expression of the same could be enhanced by treatment with IFNα/β [89, 90]. Further, they demonstrated that EVs bearing hACE2 explicitly block the SARS-CoV-2 entry and thereby inhibit viral replication. The findings indicated a potential antiviral strategy involving EVs with an upregulated viral receptor that could competitively block the virus entry (Fig. 5). Although not related to MSC-EVs, in another interesting study, Wang et al. identified four miRNAs (miR-7-5p, miR-24-3p, miR-145-5p and miR-223-3p) in serum EVs that can directly inhibit S protein expression and SARS-CoV-2 replication [91]. However, these miRNA content has drastically decreased in serum exosomes in the elderly and diabetic patients. This study indicated that the varying levels of EVs-miRNA could be attributed to varying levels of treatment outcomes. Interestingly, there are not many in vitro reports on the MSC-derived EVs in the context of COVID-19,perhaps, this could be due to the requirement of high containment laboratories and the risks involved.
Fig. 5.
Extracellular vesicles (EVs), tagged with receptor binding domain (RBD) of the viral spike, were able to recognize and target ACE2 protein on the surface of lung cells in vitro and suppress the infection of pseudovirus in hACE2 micein vivo. Reproduced with permission from [95], Copyright® Elsevier BV
In vivo reports
It is known that the in vitro models are cost-effective, relatively quick and offer control over test parameters, but unfortunately, they fail to mimic the native tissue physiology. In the context of COVID-19, several developments on in vivo models are underway in order to understand the mechanisms of pathogenesis and to establish the safety and efficacy of potential therapeutic agents and vaccines [92–94]. In the context of EVs in COVID-19 management, Fu and Xiong demonstrated the engineering of EVs tagged with S-protein receptor-binding domain (RBD) of SARS-CoV-2 and their ability to suppress SARS-CoV-2 infection in Transgenic mice that express human ACE2 (hACE2 mouse) (Fig. 5). The RBD domain plays a critical role in the adhesion, fusion and cellular entry of the virus. The study demonstrated the ability of RBD-tagged EVs, encapsulated with siRNAs, to recognize and target ACE2 protein on the surface of lung cells and suppress the infection of pseudovirus in hACE2 mice. They also showed that the RBD-tagged EVs were able to specifically target other organs such as kidneys, heart, etc., and therefore, these engineered EVs can be explored for the delivery of antiviral agents to treat multi-organ failure in SARS-CoV-2 [95].There are no in vivo studies on MSC-EVs in the in vivo models infected with the SARS-CoV-2 virus or its pseudo variants. However, many studies are reported on the use of MSC-EVs in animal models with acute lung injury induced by other agents like LPS [96–100].
Case reports
Owing to the rapid spread of COVID-19 and due to lack of effective therapy, several clinical studies were approved by ethical committees on emergency basis across the world. Some of these explored MSC-derived EVs as therapeutic regime for COVID-19 management. For instance, Sengupta et al. studied the efficacy and safety of allogeneic bone marrow MSC-derived EVs (ExoFlo) as therapeutic for treatment of severe COVID-19. In this study, ExoFlo was administrated to 24 COVID-19 patients with moderate-to-severe ARDS. All the patients were given a single intravenous dose of 15 mL ExoFlo. The status of patients was evaluated from days 1 to 14, wherein, all met safety endpoints with no adverse events observed within 72 h and an overall survival rate of 83% [101]. Few other reports on EVs, not from MSCs but from amniotic fluid-derived, were also reported by Mitrani et al. and team [102, 103]. These studies demonstrated that Zofin (amniotic fluid-derived EVs based formulation) was effective with prominent respiratory improvements in a COVID-19 long hauler. The same team reported another paper on the successful use of Zofin in the management of three severely ill COVID-19 patients. Both studies were completed without any adverse events or safety concerns. Recently, Khanh et al. reported that the Wharton's jelly MSC-derived EVs showed significant reduction in nuclear NF-κB p65 and suppression of the cytokine storm in COVID-19 patients suffering from diabetes mellitus or renal disease [104].
Clinical trials landscape
The first phase I clinical trial using ex vivo expanded bone marrow MSCs as cellular pharmaceutical agents in patients with hematologic malignancies was performed in 1995, followed by their wide use in various applications [105]. The registered clinical trials with MSCs from 2010 to December 2021, based on the data obtained from clinicaltrils.gov, shows a steady increase in the number of trials and therefore the increasing belief in the therapeutic potential of MSCs. Overwhelming in vitro and in vivo studies revealed that the MSCs suppress the over-activated inflammatory response, release factors including cytokines and extracellular vesicles, which promote neighbouring cell survival and growth and possess differentiation potential. In terms of lung regeneration, mainly, the immunomodulatory function of MSCs shown to help in the recovery of lung function and reduce pulmonary fibrosis [106]. According to the International Clinical Trials Registry Platform at World Health Organization, over twelve thousand clinical trials are undertaken on COVID-19 as of December 2021, where 131 studies are using stem cells to attenuate the detrimental effect caused by the SARS-CoV2. Amongst these, 22 trials were completed, 05 were withdrawn (due to ethical issues and patient transfer) and 104 trials others are in progress.
The ethical constraints using cell-based therapy further provoked researchers to use secretome, mainly extracellular vesicles from cultured MSCs for clinical applications. The first clinical trial on EVs was started in 2015 in Kumamoto University, Japan, where they used plasma-derived EVs for cutaneous wound healing (NCT02565264). More than 100 registered clinical studies on EVs are underway for therapeutic applications and beyond. With the previous history on the successful use of MSC-derived EVs in other conditions, clinical trials on the use of MSC-derived EVs in COVID-19 management quickly appeared on the clinical trials registry. A complete list of clinical trials on the use of MSC-derived EVs in COVID-19 treatment is given in Table 1. It is evident that the trials are ongoing (i) with MSC-derived EVs in various forms viz. injectable, inhalable, etc.; (ii) with MSCs derived from various tissue sources viz. bone marrow, umbilical cord, etc.; (iii) to investigate the effective dose of EVs; and (iv) involving various routes of EVs administration viz. intravenous, nasal, etc.
Table 1.
List of clinical trials* on the use of stem cell-derived exosomes in COVID-19 management
| Reference | Official title | Sponsor | Status |
|---|---|---|---|
| NCT04657458 | Bone marrow mesenchymal stem cell-derived extracellular vesicles infusion treatment: expanded access protocol for patients with COVID-19-associated ARDS who do not qualify for Phase II randomized control trial | Direct Biologics, LLC, USA | Completed (results available) |
| NCT04491240 | Evaluation of safety and efficiency of method of exosome inhalation in SARS-CoV-2-associated pneumonia | State Financed Health Facility, Russia | Completed (results available) |
| NCT04276987 | A pilot clinical study on aerosol inhalation of the exosomes derived from allogenic adipose mesenchymal stem cells in the treatment of severe patients with novel coronavirus pneumonia | Ruijin Hospital, China | Completed (results not available) |
| NCT04493242 | Bone marrow mesenchymal stem cell-derived extracellular vesicles infusion treatment for COVID-19 associated acute respiratory distress syndrome (ARDS): a Phase II clinical trial | Direct Biologics, LLC, United States | Completed (results not available) |
| IRCT20130812014333N164 | Investigation of the effects of the exosomes derived from placental mesenchymal stem cells on the oxygen saturation and biological markers of patients with COVID-19 | Kermanshah University of Medical Sciences, Iran | Ongoing |
| ISRCTN33578935 | Rationale and investigational study for the treatment of COVID-19 with severe viral pneumonia with isolated, placental, mesenchymal stem cell exosomes | Kimera Labs, United States | Ongoing |
| NCT04902183 | A Phase II randomized, single-blind dose study to evaluate the safety and efficacy of exosomes overexpressing CD24 in 109 dose versus 1010 dose, for the prevention of clinical deterioration in patients with moderate or severe COVID-19 | Athens Medical Society, Greece | Recruiting |
| IRCT20190101042197N2 | Evaluation of the safety and efficiency of mesenchymal stem cell-derived exosomes in patients with ARDS of COVID-19; An interventional randomized double-blind controlled clinical trial: phase I and II | Tarmim Ava Baran Knowledge Based Company, Iran | Recruiting |
| IRCT20201202049568N3 | Evaluation of the safety and efficiency of human umbilical cord derived mesenchymal stem cell exosomes in patients with ARDS of COVID-19; an interventional randomized double-blind controlled clinical trial: phase I and II | Tarmim Ava Baran Knowledge Based Company, Iran | Recruiting |
| IRCT20200413047063N2 | Exosomes derived from placental mesenchymal stem cells as treatment for severe COVID-19: Phase 1 & 2 clinical trials | Omid Cell and Tissue center, Iran | Recruiting |
| NCT04747574 | A Phase I feasibility study to evaluate the safety of CD24-exosomes in patients with moderate/severe COVID-19 infection | Tel-Aviv Sourasky Medical Center, Israel | Recruiting |
| IRCT20200510047385N1 | Effect of stromal vascular fraction (SVF), blood and condition medium derived exosomes on treatment of covid-19 patients with acute respiratory distress syndrome (ARDS)/clinical Trial | Tabriz University of Medical Sciences, Iran | Recruiting |
| NCT04602442 | The extended protocol of evaluation of safety and efficiency of method of exosome inhalation in COVID-19-associated two-sided pneumonia | Olga Tyumina, Russia | Recruiting |
| NCT05191381 | Immune modulation by exosomes in COVID-19 (IMECOV19) | University of Ulm, Germany | Recruiting |
| NCT04798716 | Mesenchymal stem cell exosomes for the treatment of COVID-19-positive patients with acute respiratory distress syndrome and/or novel coronavirus pneumonia | AVEM HealthCare, United States | Not yet recruiting |
| NCT04389385 | Aerosol inhalation of the exosomes derived from allogenic COVID-19 T cell in the treatment of early stage novel coronavirus pneumonia | TC Erciyes University, Turkey | Not yet recruiting |
| ChiCTR2000030484 | HUMSCs and exosomes treating patients with lung injury following novel coronavirus pneumonia (COVID-19) | Hubei Shiyan Taihe hospital, China | Not yet recruiting |
| ChiCTR2000030261 | A study for the key technology of mesenchymal stem cells exosomes atomization in the treatment of novel coronavirus pneumonia (COVID-19) | Wuxi Fifth People's Hospital, China | Not yet recruiting |
| NCT04969172 | A Phase II randomized, double-blind, placebo-controlled study to evaluate the safety and efficacy of exosomes overexpressing CD24 to prevent clinical deterioration in patients with moderate or severe COVID-19 infection | Eli Sprecher, MD, United States | Not yet recruiting |
*Obtained from trialsearch.who.int, clinicaltrials.gov and clinicaltrialsregister.eu using “extracellular vesicles” or “exosomes” as search strings, with results restricted to COVID-19
From the completed studies, two studies involve the inhalation of EVs-based formulations at the concentration of 0.5–2 × 1010 (twice a day during 10 days—Phase I and now enrolling for Phase II; NCT04491240) and 2 × 108 (5 times at Day 1, Day 2, Day 3, Day 4, Day 5—Phase I; NCT04276987) respectively. Another study involved intravenous administration of bone marrow MSC-derived EVs (800 Billion and 1.2 Trillion—Phase II; NCT04493242). AVEM Healthcare proposed to use intravenous administration of MSC-derived EVs as escalating dose of 2 × 109, 4 × 109, 8 × 109/mL (phase I) and 8 × 109, 4 × 109, 8 × 109/mL (phase II) every other day for a period of 5 days (NCT04798716). Athens medical society (Greece) and Tel-Aviv Sourasky Medical Center (Israel) registered the use of EVs that were engineered to overexpress CD24 from human embryonic kidney T-REx™-293 cells (once daily by inhalation for 5 days—Phase II; NCT04969172 and NCT04747574). TC Erciyes University (Turkey) uses the exosome derived from COVID-19 specific T-cells, where the T-cells are activated in vitro with COVID-19 viral peptide fragments in the presence of cytokines (2 × 108 exosome five-time daily for 5 days—Phase I; NCT04389385).
In addition to MSC-derived EVs alone, Organicell Regenerative Medicine (USA) developed Zofin (an acellular, minimally manipulated product, derived from human amniotic fluid)—shown to have growth factors, cytokines, chemokines and other extracellular vesicles, and is in a clinical trial for COVID-19 (2–5 × 1011 particles/mL of Zofin is administered intravenously on day 0, day 4 and day 8—Phase I and II; NCT04384445). It is therefore evident that the MSCs, as well as the MSC-derived EVs, have once again attracted huge attention as an effective cell therapy owing to the COVID-19 situation. It is anticipated that the outcome of these trials would yield hope in regenerative medicine, both in viral pneumonia conditions and beyond.
Patent landscape
Since the COVID-19 pandemic, several innovative developments happened towards COVID-19 management, both from diagnostic as well as therapeutic perspectives. Innovations in MSC-derived EVs have also come into the limelight and several interesting patent applications were filed. These innovations range from exploring EVs as biomarkers for diagnostics purposes, to the use of engineered EVs to counter SARS-Cov2 replication and to the use of EVs in lung regeneration. For instance, a human bone marrow-derived MSC line stably expressing human ACE2 protein was made and the method of extracting exosome rich in ACE2 protein was developed (patent no. CN112430581A). Similarly, the exosome expressing human ACE2 protein on the surface was made by inducing the HEK293T cells with IFN α/β, which in turn induces the expression of the ACE2 protein. The exosome rich in ACE2 on the surface could specifically interact with the RBD of spike (S) protein. The competitive inhibition-mediated binding of the exosome to the SARS-CoV2 could minimize the viral entry to human cells (patent no. CN112646781A). Another patent suggests that the vesicle expressing a chimeric ACE2 receptor could deliver medicines like Reidesvir to the position where new coronaviruses bind (patent no. CN112522203A). A composition comprising cell-derived particles presenting heterologous CD24, which is well-known oncogene that plays important role in negative regulation of T-cell mediated inflammation. (Patent No: EP3895697A1).
Exosome-mediated vaccine development was proposed by making exosomes that express engineered S protein of SARS-COV2 fused with Vesicular Stomatitis Virus Glycoprotein (VSVG). The injection of this nano-scale exosome is proposed to act like a vaccine and stimulate antibody production (Patent No. CN112439058A). The delivery of EVs or exosome comprising a fragment of a viral peptide or virus interacting protein could induce the host immune response that act against virus (Patent No. WO2021181399A1). In another report, a recombinant plasmid was constructed with Spike-lamp2b and transfected in mouse dendritic cells. The secreted exosomes by the transfected cells were reported to carry S-protein and induce antibody synthesis while injecting into humans (Patent No. CN112111513A). On the other hand, Nano-antibody for S-protein that tightly combined with the spurt protein on the exosome was developed, which can inhibit the interaction between the spike protein and ACE2 receptor (Patent No. CN112626030A). The cargos of EVs from placental MSCs shown to have biomolecules that have inherent anti-inflammatory effect and miRNAs that efficiently disintegrates the RNA genome of coronavirus (WO2021225214A1). The exosome expressing recombinant SARS-CoV2 S-protein fused with VSVG was encapsulated with SARS-CoV2 siRNA, which could specifically inhibit the virus replication (Patent No. CN112226413A). Similarly, the miRNA targeting SARS-COV2 S-protein has enriched in NK cell-derived exosome, which could inhibit the virus replication (Patent No. CN111494416B). The suitable methods for delivering stable EVs to the lungs such as intranasal spray or intranasal nebulizer have been developed (patent no. CN113384597A and WO2021237100A1). Besides traditional vaccines and the new mRNA vaccine, these innovative developments could bring new avenues for the treatment and prevention of SARS-COV2 infection, further, the beneficial effects of MSC-derived EVs could be multitude by incorporating other biomolecules and drugs.
Summary and future prospects
Extracellular vesicles are membrane-bound sub-micron scale structures released into extracellular space by the cells in all living systems. It was recently shown that the production of several factors via EVs orchestrates the main modes of action of MSCs following infusion, and MSC-derived EVs have a number of advantages over live cells, including the ability to reduce undesired side effects such as infusional toxicities. Therefore, MSC-EVs have received significant attention in both academia and industry, as reflected by a flare-up in the number of publications, clinical trials and patent applications. The reports demonstrated that MSC-derived EVs can control the inflammatory response, repair the alveolar damage and avert pulmonary fibrosis. As discussed in this review, MSC-EVs, both pristine as well as engineered, are now being explored for their use as biomarkers, immune-modulating agents, therapeutics as well as vaccines, in the context of COVID-19. The data shows that the studies are ongoing to find out the effective formulation such as injectable, inhalable, etc. Studies are ongoing with EVs from MSCs derived from various tissue sources viz. bone marrow, umbilical cord, etc. to dig deep into tissue-source dependant differences if any. Some other studies are ongoing to investigate the effective dose of EVs. Also, various routes of EVs administration viz. intravenous, nasal, etc. are being investigated.
Though current understanding of therapeutic potential mainly comes from pristine MSCs cultured under routine culture conditions, a variety of other approaches are under investigation by various groups mainly to enhance the potency of MSC-EVs. These futuristic approaches include specialized culture conditions, cellular reprogramming, nanotechnology enabled modification and more [107–109]. For instance, the MSCs may be subjected to various biophysical cues such as electric pulsing, mechanical cues, 2D and 3D scaffolds/scaffold-free culture, etc. and the EVs derived from such specially treated MSCs may be explored for their potency check. Other cases of MSCs cultures in presence of various biochemical factors are also emerging. For instance, bacteria-derived molecules like lipopolysaccharides and the cytokines are included in the culture medium to induce EVs primed with anti-inflammatory ability. Approaches involving overexpression of certain transcription factors, signalling molecules and miRNAs. Bioengineering of EVs to enhance their potency is also being considered in two ways, firstly, by altering their tropism towards target cells, and secondly, by loading the EVs with desired cargo [110]. Nanotechnology based approaches are also under experimentation for engineering of smart EVs for precision medicine [111]. As with many cellular therapies, scale-up and clinical grade manufacturing of EVs is a significant process engineering challenge, however, efforts are on to overcome these barriers towards offering clinically meaningful MSC and EVs based therapies [112, 113].
It is anticipated that, once the ongoing studies are completed, a comprehensive meta-analysis of the results would enable us to derive an effective MSC-EVs based therapeutic regime in the management of COVID-19 and beyond. Further, additional developments in enhancing the potency of EVs and in scaling-up of EVs production would surely make the EVs-based cell-free regenerative therapy a reality in the days to come.
Acknowledgements
NK: Conceptualization, AK, SM, FBF and NK: Data curation, AK and NK: Writing – original draft, SM, FBF and NK: Writing – review & editing.
Authors acknowledge the Science and Engineering Research Board, Department of Science and Technology, Government of India for funding through CRG-COVID-19 special call (No. CVD/2020/000224).
Declarations
Conflict of interest
All Authors declare that they have no conflict of interest.
Ethical statement
There are no animal experiments carried out for this article.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.WHO Coronavirus (COVID-19) Dashboard. (n.d.). Retrieved January 31, 2022, from https://covid19.who.int
- 2.Wiersinga WJ, Rhodes A, Cheng AC, Peacock SJ, Prescott HC. Pathophysiology, transmission, diagnosis, and treatment of coronavirus disease 2019 (COVID-19): a review. JAMA. 2020;324:782–793. doi: 10.1001/jama.2020.12839. [DOI] [PubMed] [Google Scholar]
- 3.Cucinotta D, Vanelli M. WHO declares COVID-19 a pandemic. Acta Bio-Med. 2020;91:157–160. doi: 10.23750/abm.v91i1.9397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Jonsdottir HR, Dijkman R. Coronaviruses and the human airway: a universal system for virus-host interaction studies. Virol J. 2016;13:24. doi: 10.1186/s12985-016-0479-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zhu N, Zhang D, Wang W, Li X, Yang B, Song J, Zhao X, Huang B, Shi W, Lu R, Niu P, Zhan F, Ma X, Wang D, Xu W, Wu G, Gao GF, Tan W, China Novel Coronavirus Investigating and Research Team A novel coronavirus from patients with pneumonia in China, 2019. The New England Journal of Medicine. 2020;382:727–733. doi: 10.1056/NEJMoa2001017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Weiss SR, Leibowitz JL. Coronavirus pathogenesis. Adv Virus Res. 2011;81:85–164. doi: 10.1016/B978-0-12-385885-6.00009-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Peiris JSM, Yuen KY, Osterhaus ADME, Stöhr K. The severe acute respiratory syndrome. N Engl J Med. 2003;349:2431–2441. doi: 10.1056/NEJMra032498. [DOI] [PubMed] [Google Scholar]
- 8.Tindale LC, Stockdale JE, Coombe M, Garlock ES, Lau WYV, Saraswat M, Zhang L, Chen D, Wallinga J, Colijn C. Evidence for transmission of COVID-19 prior to symptom onset. Elife. 2020 doi: 10.7554/eLife.57149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Nishiura H, Linton NM, Akhmetzhanov AR. Serial interval of novel coronavirus (COVID-19) infections. Int J Infect Dis. 2020;93:284–286. doi: 10.1016/j.ijid.2020.02.060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gandhi RT, Lynch JB, Del Rio C. Mild or moderate Covid-19. N Engl J Med. 2020;383:1757–1766. doi: 10.1056/NEJMcp2009249. [DOI] [PubMed] [Google Scholar]
- 11.Sampogna G, Guraya SY, Forgione A. Regenerative medicine: historical roots and potential strategies in modern medicine. J Microsc Ultrastruct. 2015;3:101–107. doi: 10.1016/j.jmau.2015.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jones BJ, Brooke G, Atkinson K, McTaggart SJ. Immunosuppression by placental indoleamine 2,3-dioxygenase: a role for mesenchymal stem cells. Placenta. 2007;28:1174–1181. doi: 10.1016/j.placenta.2007.07.001. [DOI] [PubMed] [Google Scholar]
- 13.Chu D-T, Phuong TNT, Tien NLB, Tran DK, Thanh VV, Quang TL, Truong DT, Pham VH, Ngoc VTN, Chu-Dinh T, Kushekhar K. An update on the progress of isolation, culture, storage, and clinical application of human bone marrow mesenchymal stem/stromal cells. Int J Mol Sci. 2020;21:708. doi: 10.3390/ijms21030708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kim HJ, Kim G, Lee J, Lee Y, Kim J-H. Secretome of stem cells: roles of extracellular vesicles in diseases, stemness, differentiation, and reprogramming. Tissue Eng Regener Med. 2022;19:19–33. doi: 10.1007/s13770-021-00406-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kordelas L, Rebmann V, Ludwig A-K, Radtke S, Ruesing J, Doeppner TR, Epple M, Horn PA, Beelen DW, Giebel B. MSC-derived exosomes: a novel tool to treat therapy-refractory graft-versus-host disease. Leukemia. 2014;28:970–973. doi: 10.1038/leu.2014.41. [DOI] [PubMed] [Google Scholar]
- 16.Ferraris VA. How do cells talk to each other?: Paracrine factors secreted by mesenchymal stromal cells. J Thorac Cardiovasc Surg. 2016;151:849–851. doi: 10.1016/j.jtcvs.2015.11.035. [DOI] [PubMed] [Google Scholar]
- 17.Li X, Corbett AL, Taatizadeh E, Tasnim N, Little JP, Garnis C, Daugaard M, Guns E, Hoorfar M, Li ITS. Challenges and opportunities in exosome research-perspectives from biology, engineering, and cancer therapy. APL Bioeng. 2019;3:011503. doi: 10.1063/1.5087122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Nederveen JP, Warnier G, Di Carlo A, Nilsson MI, Tarnopolsky MA. Extracellular vesicles and exosomes: insights from exercise science. Front Physiol. 2021;11:604274. [DOI] [PMC free article] [PubMed]
- 19.Barani B, Rajasingh S, Rajasingh J. Exosomes: outlook for future cell-free cardiovascular disease therapy. In: Xiao J, Cretoiu S, editors. Exosomes in cardiovascular diseases: biomarkers, pathological and therapeutic effects. Berlin: Springer; 2017. pp. 285–307. [Google Scholar]
- 20.da Silva da Costa FA, Soares MR, Malagutti-Ferreira MJ, da Silva GR, dos Reis Lívero FA, Ribeiro-Paes JT. Three-dimensional cell cultures as a research platform in lung diseases and COVID-19. Tissue Eng Regener Med. 2021;18:735–745. doi: 10.1007/s13770-021-00348-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lee JY, Kim H-S. Extracellular vesicles in regenerative medicine: potentials and challenges. Tissue Eng Regener Med. 2021;18:479–484. doi: 10.1007/s13770-021-00365-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wang X, Wang H, Lu J, Feng Z, Liu Z, Song H, Wang H, Zhou Y, Xu J. Erythropoietin-modified mesenchymal stem cells enhance anti-fibrosis efficacy in mouse liver fibrosis model. Tissue Eng Regener Med. 2020;17:683–693. doi: 10.1007/s13770-020-00276-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zakaria DM, Zahran NM, Arafa SAA, Mehanna RA, Abdel-Moneim RA. Histological and physiological studies of the effect of bone marrow-derived mesenchymal stem cells on bleomycin induced lung fibrosis in adult albino rats. Tissue Eng Regener Med. 2021;18:127–141. doi: 10.1007/s13770-020-00294-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wu Z, McGoogan JM. Characteristics of and important lessons from the coronavirus disease 2019 (COVID-19) outbreak in China: summary of a report of 72 314 cases from the Chinese Center for Disease Control and Prevention. JAMA. 2020;323:1239–1242. doi: 10.1001/jama.2020.2648. [DOI] [PubMed] [Google Scholar]
- 25.Hoffmann M, Kleine-Weber H, Schroeder S, Krüger N, Herrler T, Erichsen S, Schiergens TS, Herrler G, Wu N-H, Nitsche A, Müller MA, Drosten C, Pöhlmann S. SARS-CoV-2 cell entry depends on ACE2 and TMPRSS2 and is blocked by a clinically proven protease inhibitor. Cell. 2020;181:271–280.e8. doi: 10.1016/j.cell.2020.02.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Tang NL-S, Chan PK-S, Wong C-K, To K-F, Wu AK-L, Sung Y-M, Hui DS-C, Sung JJ-Y, Lam CW-K. Early enhanced expression of interferon-inducible protein-10 (CXCL-10) and other chemokines predicts adverse outcome in severe acute respiratory syndrome. Clin Chem. 2005;51:2333–2340. doi: 10.1373/clinchem.2005.054460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rockx B, Baas T, Zornetzer GA, Haagmans B, Sheahan T, Frieman M, Dyer MD, Teal TH, Proll S, van den Brand J, Baric R, Katze MG. Early upregulation of acute respiratory distress syndrome-associated cytokines promotes lethal disease in an aged-mouse model of severe acute respiratory syndrome coronavirus infection. J Virol. 2009;83:7062–7074. doi: 10.1128/JVI.00127-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Weinheimer VK, Becher A, Tönnies M, Holland G, Knepper J, Bauer TT, Schneider P, Neudecker J, Rückert JC, Szymanski K, Temmesfeld-Wollbrueck B, Gruber AD, Bannert N, Suttorp N, Hippenstiel S, Wolff T, Hocke AC. Influenza A viruses target type II pneumocytes in the human lung. J Infect Dis. 2012;206:1685–1694. doi: 10.1093/infdis/jis455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Nikolaidis NM, Noel JG, Pitstick LB, Gardner JC, Uehara Y, Wu H, Saito A, Lewnard KE, Liu H, White MR, Hartshorn KL, McCormack FX. Mitogenic stimulation accelerates influenza-induced mortality by increasing susceptibility of alveolar type II cells to infection. Proc Natl Acad Sci. 2017;114:E6613–E6622. doi: 10.1073/pnas.1621172114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ho JC, Chan KN, Hu WH, Lam WK, Zheng L, Tipoe GL, Sun J, Leung R, Tsang KW. The effect of aging on nasal mucociliary clearance, beat frequency, and ultrastructure of respiratory cilia. Am J Respir Crit Care Med. 2001;163:983–988. doi: 10.1164/ajrccm.163.4.9909121. [DOI] [PubMed] [Google Scholar]
- 31.Cao X. COVID-19: immunopathology and its implications for therapy. Nat Rev Immunol. 2020;20:269–270. doi: 10.1038/s41577-020-0308-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Li G, Fan Y, Lai Y, Han T, Li Z, Zhou P, Pan P, Wang W, Hu D, Liu X, Zhang Q, Wu J. Coronavirus infections and immune responses. J Med Virol. 2020;92:424–432. doi: 10.1002/jmv.25685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Fu Y, Cheng Y, Wu Y. Understanding SARS-CoV-2-mediated inflammatory responses: from mechanisms to potential therapeutic tools. Virol Sin. 2020;35:266–271. doi: 10.1007/s12250-020-00207-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ye Q, Wang B, Mao J. The pathogenesis and treatment of the `Cytokine Storm’ in COVID-19. J Infect. 2020;80:607–613. doi: 10.1016/j.jinf.2020.03.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Chen N, Zhou M, Dong X, Qu J, Gong F, Han Y, Qiu Y, Wang J, Liu Y, Wei Y, Xia J, Yu T, Zhang X, Zhang L. Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan, China: a descriptive study. Lancet (London, England) 2020;395:507–513. doi: 10.1016/S0140-6736(20)30211-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ruan Q, Yang K, Wang W, Jiang L, Song J. Clinical predictors of mortality due to COVID-19 based on an analysis of data of 150 patients from Wuhan, China. Intensive Care Med. 2020;46:846–848. doi: 10.1007/s00134-020-05991-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wu C, Chen X, Cai Y, Xia J, Zhou X, Xu S, Huang H, Zhang L, Zhou X, Du C, Zhang Y, Song J, Wang S, Chao Y, Yang Z, Xu J, Zhou X, Chen D, Xiong W, Song Y, et al. Risk factors associated with acute respiratory distress syndrome and death in patients with coronavirus disease 2019 pneumonia in Wuhan, China. JAMA Internal Med. 2020;180:934–943. doi: 10.1001/jamainternmed.2020.0994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Zhou F, Yu T, Du R, Fan G, Liu Y, Liu Z, Xiang J, Wang Y, Song B, Gu X, Guan L, Wei Y, Li H, Wu X, Xu J, Tu S, Zhang Y, Chen H, Cao B. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet (London, England) 2020;395:1054–1062. doi: 10.1016/S0140-6736(20)30566-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wygrecka M, Jablonska E, Guenther A, Preissner KT, Markart P. Current view on alveolar coagulation and fibrinolysis in acute inflammatory and chronic interstitial lung diseases. Thromb Haemost. 2008;99:494–501. doi: 10.1160/TH07-11-0666. [DOI] [PubMed] [Google Scholar]
- 40.Stinson SF, Ryan DP, Hertweck S, Hardy JD, Hwang-Kow SY, Loosli CG. Epithelial and surfactant changes in influenzal pulmonary lesions. Arch Pathol Lab Med. 1976;100:147–153. [PubMed] [Google Scholar]
- 41.Burkhardt A. Alveolitis and collapse in the pathogenesis of pulmonary fibrosis. Am Rev Respir Dis. 1989;140:513–524. doi: 10.1164/ajrccm/140.2.513. [DOI] [PubMed] [Google Scholar]
- 42.Cho SJ, Stout-Delgado HW. Aging and lung disease. Annu Rev Physiol. 2020;82:433–459. doi: 10.1146/annurev-physiol-021119-034610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Melo-Narváez MC, Stegmayr J, Wagner DE, Lehmann M. Lung regeneration: Implications of the diseased niche and ageing. Eur Respir Rev. 2020 doi: 10.1183/16000617.0222-2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kotton DN, Morrisey EE. Lung regeneration: mechanisms, applications and emerging stem cell populations. Nat Med. 2014;20:822–832. doi: 10.1038/nm.3642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Xu J, Gonzalez ET, Iyer SS, Mac V, Mora AL, Sutliff RL, Reed A, Brigham KL, Kelly P, Rojas M. Use of senescence-accelerated mouse model in bleomycin-induced lung injury suggests that bone marrow-derived cells can alter the outcome of lung injury in aged mice. J Gerontol Ser A. 2009;64:731–739. doi: 10.1093/gerona/glp040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Bustos ML, Huleihel L, Kapetanaki MG, Lino-Cardenas CL, Mroz L, Ellis BM, McVerry BJ, Richards TJ, Kaminski N, Cerdenes N, Mora AL, Rojas M. Aging mesenchymal stem cells fail to protect because of impaired migration and antiinflammatory response. Am J Respir Crit Care Med. 2014;189:787–798. doi: 10.1164/rccm.201306-1043OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Thompson BT, Chambers RC, Liu KD. Acute respiratory distress syndrome. N Engl J Med. 2017;377:562–572. doi: 10.1056/NEJMra1608077. [DOI] [PubMed] [Google Scholar]
- 48.Zhang B, Zhou X, Qiu Y, Song Y, Feng F, Feng J, Song Q, Jia Q, Wang J. Clinical characteristics of 82 cases of death from COVID-19. PLoS ONE. 2020;15:e0235458. doi: 10.1371/journal.pone.0235458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Xu Z, Shi L, Wang Y, Zhang J, Huang L, Zhang C, Liu S, Zhao P, Liu H, Zhu L, Tai Y, Bai C, Gao T, Song J, Xia P, Dong J, Zhao J, Wang F-S. Pathological findings of COVID-19 associated with acute respiratory distress syndrome. Lancet Respir Med. 2020;8:420–422. doi: 10.1016/S2213-2600(20)30076-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Zakrzewski W, Dobrzyński M, Szymonowicz M, Rybak Z. Stem cells: past, present, and future. Stem Cell Res Ther. 2019;10:68. doi: 10.1186/s13287-019-1165-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Gurusamy N, Alsayari A, Rajasingh S, Rajasingh J. Adult stem cells for regenerative therapy. Prog Mol Biol Transl Sci. 2018;160:1–22. doi: 10.1016/bs.pmbts.2018.07.009. [DOI] [PubMed] [Google Scholar]
- 52.Gupta N, Su X, Popov B, Lee JW, Serikov V, Matthay MA. Intrapulmonary delivery of bone marrow-derived mesenchymal stem cells improves survival and attenuates endotoxin-induced acute lung injury in mice. J Immunol. 2007;179:1855–1863. doi: 10.4049/jimmunol.179.3.1855. [DOI] [PubMed] [Google Scholar]
- 53.Gupta N, Krasnodembskaya A, Kapetanaki M, Mouded M, Tan X, Serikov V, Matthay MA. Mesenchymal stem cells enhance survival and bacterial clearance in murine Escherichia coli pneumonia. Thorax. 2012;67:533–539. doi: 10.1136/thoraxjnl-2011-201176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Lee JW, Krasnodembskaya A, McKenna DH, Song Y, Abbott J, Matthay MA. Therapeutic effects of human mesenchymal stem cells in ex vivo human lungs injured with live bacteria. Am J Respir Crit Care Med. 2013;187:751–760. doi: 10.1164/rccm.201206-0990OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Mei SHJ, Haitsma JJ, Dos Santos CC, Deng Y, Lai PFH, Slutsky AS, Liles WC, Stewart DJ. Mesenchymal stem cells reduce inflammation while enhancing bacterial clearance and improving survival in sepsis. Am J Respir Crit Care Med. 2010;182:1047–1057. doi: 10.1164/rccm.201001-0010OC. [DOI] [PubMed] [Google Scholar]
- 56.Walter J, Ware LB, Matthay MA. Mesenchymal stem cells: mechanisms of potential therapeutic benefit in ARDS and sepsis. Lancet Respir Med. 2014;2:1016–1026. doi: 10.1016/S2213-2600(14)70217-6. [DOI] [PubMed] [Google Scholar]
- 57.Fang X, Neyrinck AP, Matthay MA, Lee JW. allogeneic human mesenchymal stem cells restore epithelial protein permeability in cultured human alveolar type II cells by secretion of angiopoietin-1 *♦. J Biol Chem. 2010;285:26211–26222. doi: 10.1074/jbc.M110.119917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Islam MN, Das SR, Emin MT, Wei M, Sun L, Westphalen K, Rowlands DJ, Quadri SK, Bhattacharya S, Bhattacharya J. Mitochondrial transfer from bone-marrow–derived stromal cells to pulmonary alveoli protects against acute lung injury. Nat Med. 2012;18:759–765. doi: 10.1038/nm.2736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Zhu Y, Feng X, Abbott J, Fang X, Hao Q, Monsel A, Qu J, Matthay MA, Lee JW. Human mesenchymal stem cell microvesicles for treatment of Escherichia coli endotoxin-induced acute lung injury in mice. Stem Cells. 2014;32:116–125. doi: 10.1002/stem.1504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Babajani A, Soltani P, Jamshidi E, Farjoo MH, Niknejad H. Recent advances on drug-loaded mesenchymal stem cells with anti-neoplastic agents for targeted treatment of cancer. Front Bioeng Biotechnol. 2020;8:748. doi: 10.3389/fbioe.2020.00748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Yu B, Zhang X, Li X. Exosomes derived from mesenchymal stem cells. Int J Mol Sci. 2014;15:4142–4157. doi: 10.3390/ijms15034142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Gnecchi M, Danieli P, Malpasso G, Ciuffreda MC. Paracrine mechanisms of mesenchymal stem cells in tissue repair. In: Gnecchi M, editor. Mesenchymal stem cells. New York: Springer; 2016. pp. 123–146. [DOI] [PubMed] [Google Scholar]
- 63.Yin K, Wang S, Zhao RC. Exosomes from mesenchymal stem/stromal cells: a new therapeutic paradigm. Biomarker Res. 2019;7:8. doi: 10.1186/s40364-019-0159-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Abels ER, Breakefield XO. Introduction to extracellular vesicles: biogenesis, RNA cargo selection, content, release, and uptake. Cell Mol Neurobiol. 2016;36:301–312. doi: 10.1007/s10571-016-0366-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Colombo M, Raposo G, Théry C. Biogenesis, secretion, and intercellular interactions of exosomes and other extracellular vesicles. Annu Rev Cell Dev Biol. 2014;30:255–289. doi: 10.1146/annurev-cellbio-101512-122326. [DOI] [PubMed] [Google Scholar]
- 66.Keshtkar S, Azarpira N, Ghahremani MH. Mesenchymal stem cell-derived extracellular vesicles: novel frontiers in regenerative medicine. Stem Cell Res Ther. 2018;9:63. doi: 10.1186/s13287-018-0791-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.van Niel G, D’Angelo G, Raposo G. Shedding light on the cell biology of extracellular vesicles. Nat Rev Mol Cell Biol. 2018;19:213–228. doi: 10.1038/nrm.2017.125. [DOI] [PubMed] [Google Scholar]
- 68.Lai RC, Arslan F, Lee MM, Sze NSK, Choo A, Chen TS, Salto-Tellez M, Timmers L, Lee CN, El Oakley RM, Pasterkamp G, de Kleijn DPV, Lim SK. Exosome secreted by MSC reduces myocardial ischemia/reperfusion injury. Stem Cell Res. 2010;4:214–222. doi: 10.1016/j.scr.2009.12.003. [DOI] [PubMed] [Google Scholar]
- 69.Kesimer M, Scull M, Brighton B, DeMaria G, Burns K, O’Neal W, Pickles RJ, Sheehan JK. Characterization of exosome-like vesicles released from human tracheobronchial ciliated epithelium: a possible role in innate defense. FASEB J. 2009;23:1858–1868. doi: 10.1096/fj.08-119131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Gennai S, Monsel A, Hao Q, Park J, Matthay MA, Lee JW. Microvesicles derived from human mesenchymal stem cells restore alveolar fluid clearance in human lungs rejected for transplantation: msc microvesicles rehabilitate marginal lungs. Am J Transplant. 2015;15:2404–2412. doi: 10.1111/ajt.13271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Khatri M, Richardson LA, Meulia T. Mesenchymal stem cell-derived extracellular vesicles attenuate influenza virus-induced acute lung injury in a pig model. Stem Cell Res Ther. 2018;9:17. doi: 10.1186/s13287-018-0774-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Monsel A, Zhu Y, Gennai S, Hao Q, Hu S, Rouby J-J, Rosenzwajg M, Matthay MA, Lee JW. Therapeutic effects of –derived microvesicles in severe pneumonia in mice. Am J Respir Crit Care Med. 2015;192:324–336. doi: 10.1164/rccm.201410-1765OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Laffey JG, Matthay MA. Fifty years of research in ARDS cell-based therapy for acute respiratory distress syndrome biology and potential therapeutic value. Am J Respir Crit Care Med. 2017;196:266–273. doi: 10.1164/rccm.201701-0107CP. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Gurunathan S, Kang MH, Kim J-H. Diverse effects of exosomes on COVID-19: a perspective of progress from transmission to therapeutic developments. Front Immunol. 2021;12:716407. doi: 10.3389/fimmu.2021.716407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Jamshidi E, Babajani A, Soltani P, Niknejad H. Proposed mechanisms of targeting COVID-19 by delivering mesenchymal stem cells and their exosomes to damaged organs. Stem Cell Rev Rep. 2021;17:176–192. doi: 10.1007/s12015-020-10109-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Pillalamarri N, Abdullah RG, Khan L, Ullah A, Jonnakuti S, Ullah M. Exploring the utility of extracellular vesicles in ameliorating viral infection-associated inflammation, cytokine storm and tissue damage. Transl Oncol. 2021;14:101095. doi: 10.1016/j.tranon.2021.101095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Pinky SG, Krishnakumar V, Sharma Y, Dinda AK, Mohanty S. Mesenchymal stem cell derived exosomes: a nano platform for therapeutics and drug delivery in combating COVID-19. Stem Cell Rev Rep. 2021;17(1):33–43. doi: 10.1007/s12015-020-10002-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Popowski KD, Dinh PC, George A, Lutz H, Cheng K. Exosome therapeutics for COVID-19 and respiratory viruses. VIEW. 2021;2:20200186. doi: 10.1002/VIW.20200186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Börger V, Weiss DJ, Anderson JD, Borràs FE, Bussolati B, Carter DRF, Dominici M, Falcón-Pérez JM, Gimona M, Hill AF, Hoffman AM, de Kleijn D, Levine BL, Lim R, Lötvall J, Mitsialis SA, Monguió-Tortajada M, Muraca M, Nieuwland R, Giebel B, et al. International society for extracellular vesicles and international society for cell and gene therapy statement on extracellular vesicles from mesenchymal stromal cells and other cells: considerations for potential therapeutic agents to suppress coronavirus disease-19. Cytotherapy. 2020;22:482–485. doi: 10.1016/j.jcyt.2020.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Bharti R, Shukla SK. Molecules against Covid-19: an in silico approach for drug development. J Electron Sci Technol. 2021;19:100095. doi: 10.1016/j.jnlest.2021.100095. [DOI] [Google Scholar]
- 81.Shah B, Modi P, Sagar SR. In silico studies on therapeutic agents for COVID-19: drug repurposing approach. Life Sci. 2020;252:117652. doi: 10.1016/j.lfs.2020.117652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Teli DM, Shah MB, Chhabria MT. In silico screening of natural compounds as potential inhibitors of SARS-CoV-2 main protease and spike RBD: targets for COVID-19. Front Mol Biosci. 2021;7:599079. doi: 10.3389/fmolb.2020.599079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Asgarpour K, Shojaei Z, Amiri F, Ai J, Mahjoubin-Tehran M, Ghasemi F, ArefNezhad R, Hamblin MR, Mirzaei H. Exosomal microRNAs derived from mesenchymal stem cells: cell-to-cell messages. Cell Commun Signal. 2020;18:149. doi: 10.1186/s12964-020-00650-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Devi A, Chaitanya NSN. Targeting sars cov2 (Indian isolate) genome with mirna: an in silico study. IUBMB Life. 2020;72:2454–2468. doi: 10.1002/iub.2373. [DOI] [PubMed] [Google Scholar]
- 85.El-Nabi SH, Elhiti M, El-Sheekh M. A new approach for COVID-19 treatment by micro-RNA. Med Hypotheses. 2020;143:110203. doi: 10.1016/j.mehy.2020.110203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Sarma A, Phukan H, Halder N, Madanan MG. An in-silico approach to study the possible interactions of miRNA between human and SARS-CoV2. Comput Biol Chem. 2020;88:107352. doi: 10.1016/j.compbiolchem.2020.107352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Schultz IC, Bertoni APS, Wink MR. Mesenchymal stem cell-derived extracellular vesicles carrying miRNA as a potential multi target therapy to COVID-19: an in silico analysis. Stem Cell Rev Rep. 2021;17:341–356. doi: 10.1007/s12015-021-10122-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Troyer Z, Alhusaini N, Tabler CO, Sweet T, Carvalho KIL, Schlatzer DM, Carias L, King CL, Matreyek K, Tilton JC. Extracellular vesicles carry SARS-CoV-2 spike protein and serve as decoys for neutralizing antibodies. J Extracell Vesicles. 2021 doi: 10.1002/jev2.12112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Zhang J, Huang F, Xia B, Yuan Y, Yu F, Wang G, Chen Q, Wang Q, Li Y, Li R, Song Z, Pan T, Chen J, Lu G, Zhang H. The interferon-stimulated exosomal hACE2 potently inhibits SARS-CoV-2 replication through competitively blocking the virus entry. Signal Transduct Target Ther. 2021;6:189. doi: 10.1038/s41392-021-00604-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Zhang Q, Jeppesen DK, Higginbotham JN, Franklin JL, Crowe JE, Coffey RJ. Angiotensin-converting enzyme 2–containing small extracellular vesicles and exomeres bind the severe acute respiratory syndrome coronavirus 2 spike protein. Gastroenterology. 2021;160:958–961.e3. doi: 10.1053/j.gastro.2020.09.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Wang Y, Zhu X, Jiang X-M, Guo J, Fu Z, Zhou Z, Yang P, Guo H, Guo X, Liang G, Zeng P, Xiao G, Ma J, Yin X, Zhang L-K, Yan C, Zhang C-Y. Decreased inhibition of exosomal miRNAs on SARS-CoV-2 replication underlies poor outcomes in elderly people and diabetic patients. Signal Transduct Target Ther. 2021;6:300. doi: 10.1038/s41392-021-00716-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Johansen MD, Irving A, Montagutelli X, Tate MD, Rudloff I, Nold MF, Hansbro NG, Kim RY, Donovan C, Liu G, Faiz A, Short KR, Lyons JG, McCaughan GW, Gorrell MD, Cole A, Moreno C, Couteur D, Hesselson D, Hansbro PM, et al. Animal and translational models of SARS-CoV-2 infection and COVID-19. Mucosal Immunol. 2020;13:877–891. doi: 10.1038/s41385-020-00340-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Muñoz-Fontela C, Dowling WE, Funnell SGP, Gsell P-S, Riveros-Balta AX, Albrecht RA, Andersen H, Baric RS, Carroll MW, Cavaleri M, Qin C, Crozier I, Dallmeier K, de Waal L, de Wit E, Delang L, Dohm E, Duprex WP, Falzarano D, Barouch DH, et al. Animal models for COVID-19. Nature. 2020;586:509–515. doi: 10.1038/s41586-020-2787-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Rosa RB, Dantas WM, do Nascimento JCF, da Silva MV, de Oliveira RN, Pena LJ. In vitro and in vivo models for studying SARS-CoV-2, the etiological agent responsible for COVID-19 pandemic. Viruses. 2021;13:379. doi: 10.3390/v13030379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Fu Y, Xiong S. Tagged extracellular vesicles with the RBD of the viral spike protein for delivery of antiviral agents against SARS-COV-2 infection. J Control Rel. 2021;335:584–595. doi: 10.1016/j.jconrel.2021.05.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Abraham A, Krasnodembskaya A. Mesenchymal stem cell-derived extracellular vesicles for the treatment of acute respiratory distress syndrome. Stem Cells Transl Med. 2020;9:28–38. doi: 10.1002/sctm.19-0205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Kaspi H, Semo J, Abramov N, Dekel C, Lindborg S, Kern R, Lebovits C, Aricha R. MSC-NTF (NurOwn®) exosomes: a novel therapeutic modality in the mouse LPS-induced ARDS model. Stem Cell Res Ther. 2021;12:72. doi: 10.1186/s13287-021-02143-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Silva JD, de Castro LL, Braga CL, Oliveira GP, Trivelin SA, Barbosa-Junior CM, Morales MM, dos Santos CC, Weiss DJ, Lopes-Pacheco M, Cruz FF, Rocco PRM. Mesenchymal stromal cells are more effective than their extracellular vesicles at reducing lung injury regardless of acute respiratory distress syndrome etiology. Stem Cells Int. 2019;2019:1–15. doi: 10.1155/2019/8262849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Su Y, Guo H, Liu Q. Effects of mesenchymal stromal cell-derived extracellular vesicles in acute respiratory distress syndrome (ARDS): current understanding and future perspectives. J Leukoc Biol. 2021;110:27–38. doi: 10.1002/JLB.3MR0321-545RR. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Zhang L, Hei F. Mesenchymal stem cell–derived exosomes: are they another therapeutic method for extracorporeal membrane oxygenation–supported acute respiratory distress syndrome? Am J Respir Crit Care Med. 2020;202:1602–1603. doi: 10.1164/rccm.202007-2895LE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Sengupta V, Sengupta S, Lazo A, Woods P, Nolan A, Bremer N. Exosomes derived from bone marrow mesenchymal stem cells as treatment for severe COVID-19. Stem Cells Dev. 2020;29:747–754. doi: 10.1089/scd.2020.0080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Mitrani MI, Bellio MA, Meglin A, Khan A, Xu X, Haskell G, Arango A, Shapiro GC. Treatment of a COVID-19 long hauler with an amniotic fluid-derived extracellular vesicle biologic. Respir Med Case Rep. 2021;34:101502. doi: 10.1016/j.rmcr.2021.101502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Mitrani MI, Bellio MA, Sagel A, Saylor M, Kapp W, VanOsdol K, Haskell G, Stewart D, Abdullah Z, Santos I, Milberg J, Arango A, Mitrani A, Shapiro GC. Case report: administration of amniotic fluid-derived nanoparticles in three severely Ill COVID-19 patients. Front Med. 2021;8:583842. doi: 10.3389/fmed.2021.583842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Khanh VC, Fukushige M, Chang YH, Hoang NN, Yamashita T, Obata-Yasuoka M, Hamada H, Osaka M, Hiramatsu Y, Ohneda O. Wharton’s jelly mesenchymal stem cell-derived extracellular vesicles reduce SARS-CoV2-induced inflammatory cytokines under high glucose and uremic toxin conditions. Stem Cells Dev. 2021;30:758–772. doi: 10.1089/scd.2021.0065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Lazarus HM, Haynesworth SE, Gerson SL, Rosenthal NS, Caplan AI. Ex vivo expansion and subsequent infusion of human bone marrow-derived stromal progenitor cells (mesenchymal progenitor cells): implications for therapeutic use. Bone Marrow Transplant 1995;16:557–564 [PubMed]
- 106.Chuang HM, Shih TE, Lu KY, Tsai SF, Harn HJ, Ho LI. Mesenchymal stem cell therapy of pulmonary fibrosis: improvement with target combination. Cell Transplant 2018;27:1581–1587. [DOI] [PMC free article] [PubMed]
- 107.Choi H, Choi Y, Yim HY, Mirzaaghasi A, Yoo J-K, Choi C. Biodistribution of exosomes and engineering strategies for targeted delivery of therapeutic exosomes. Tissue Eng Regener Med. 2021;18:499–511. doi: 10.1007/s13770-021-00361-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Park DJ, Seo YJ. Engineering of extracellular vesicles based on payload changes for tissue regeneration. Tissue Eng Regener Med. 2021;18:485–497. doi: 10.1007/s13770-021-00349-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Park K-S, Bandeira E, Shelke GV, Lässer C, Lötvall J. Enhancement of therapeutic potential of mesenchymal stem cell-derived extracellular vesicles. Stem Cell Res Ther. 2019;10:288. doi: 10.1186/s13287-019-1398-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Johnson J, Shojaee M, Mitchell Crow J, Khanabdali R. From mesenchymal stromal cells to engineered extracellular vesicles: a new therapeutic paradigm. Front Cell Dev Biol. 2021;9:705676. doi: 10.3389/fcell.2021.705676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Tran PHL, Xiang D, Tran TTD, Yin W, Zhang Y, Kong L, Chen K, Sun M, Li Y, Hou Y, Zhu Y, Duan W. Exosomes and nanoengineering: a match made for precision therapeutics. Adv Mater. 2020;32:1904040. doi: 10.1002/adma.201904040. [DOI] [PubMed] [Google Scholar]
- 112.Hahm J, Kim J, Park J. Strategies to enhance extracellular vesicle production. Tissue Eng Regener Med. 2021;18:513–524. doi: 10.1007/s13770-021-00364-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Levy O, Kuai R, Siren EMJ, Bhere D, Milton Y, Nissar N, De Biasio M, Heinelt M, Reeve B, Abdi R, Alturki M, Fallatah M, Almalik A, Alhasan AH, Shah K, Karp JM. Shattering barriers toward clinically meaningful MSC therapies. Sci Adv. 2020;6:eaba6884. doi: 10.1126/sciadv.aba6884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Jha N, Jeyaraman M, Rachamalla M, Ojha S, Dua K, Chellappan D, Muthu S, Sharma A, Jha S, Jain R, Jeyaraman N, Gs P, Satyam R, Khan F, Pandey P, Verma N, Singh S, Roychoudhury S, Dholpuria S, Kesari K, et al. Current understanding of novel coronavirus: molecular pathogenesis, diagnosis, and treatment approaches. Immuno. 2021;1:30–66. doi: 10.3390/immuno1010004. [DOI] [Google Scholar]
- 115.Fröhlich E. Therapeutic potential of mesenchymal stem cells and their products in lung diseases—intravenous administration versus inhalation. Pharmaceutics. 2021;13:232. doi: 10.3390/pharmaceutics13020232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Abdelgawad M, Bakry NS, Farghali AA, Abdel-Latif A, Lotfy A. Mesenchymal stem cell-based therapy and exosomes in COVID-19: current trends and prospects. Stem Cell Res Ther. 2021;12:469. doi: 10.1186/s13287-021-02542-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Gardin C, Ferroni L, Chachques JC, Zavan B. Could mesenchymal stem cell-derived exosomes be a therapeutic option for critically Ill COVID-19 patients? J Clin Med. 2020;9:2762. doi: 10.3390/jcm9092762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Lee B-C, Kang I, Yu K-R. Therapeutic features and updated clinical trials of mesenchymal stem cell (MSC)-derived exosomes. J Clin Med. 2021;10:711. doi: 10.3390/jcm10040711. [DOI] [PMC free article] [PubMed] [Google Scholar]