Abstract
Background
The COVID-19 pandemic has contributed to the widespread disruption of immunization services, including the postponement of mass vaccination campaigns.
Methods
In May 2020, the World Health Organization and partners started monitoring COVID-19-related disruptions to mass vaccination campaigns against cholera, measles, meningitis A, polio, tetanus-diphtheria, typhoid, and yellow fever through the Immunization Repository Campaign Delay Tracker. The authors reviewed the number and target population of reported preventive and outbreak response vaccination campaigns scheduled, postponed, canceled, and reinstated at 4 time points: May 2020, December 2020, May 2021, and December 2021.
Findings
Mass vaccination campaigns across all vaccines were disrupted heavily by COVID-19. In May 2020, 105 of 183 (57%) campaigns were postponed or canceled in 57 countries because of COVID-19, with an estimated 796 million postponed or missed vaccine doses. Campaign resumption was observed beginning in July 2020. In December 2021, 77 of 472 (16%) campaigns in 54 countries, mainly in the African Region, were still postponed or canceled because of COVID-19, with about 382 million postponed or missed vaccine doses.
Interpretation
There is likely a high risk of vaccine-preventable disease outbreaks across all regions because of an increased number of susceptible persons resulting from the large-scale mass vaccination campaign postponement caused by COVID-19.
Keywords: COVID, SARS-CoV-2 pandemic, vaccine-preventable diseases, campaign, vaccination, global
RESEARCH IN CONTEXT.
Evidence before this study
The SARS-CoV-2 pandemic has brought severe disruptions to immunization activities worldwide. Independent reports from regions and countries indicated that the planning and implementation of all vaccine-preventable disease mass vaccination campaigns had been adversely affected by COVID-19. However, the global impact and the progress of campaign resumption after COVID-19 disruption have not been quantified and thoroughly studied.
The added value of this study
The World Health Organization (WHO) Immunization Repository Campaign Delay Tracker has created an opportunity for the planning and execution of global vaccine-preventable diseases control/elimination/eradication programs to be tracked under 1 global campaign tracking mechanism. This was unprecedented and created synergistic opportunities to plan and deliver multi-antigen campaigns. It avoids duplicated efforts for global partners in collecting updates and for countries and regions in reporting. The data collected are used for operational planning, advocacy for urgent actions to avert vaccine-preventable disease outbreaks, resource mobilization, analysis, and modeling to understand the potential impact of campaign cancellations and delays on population immunity and the potential for outbreaks of highly transmissible vaccine-preventable diseases. The data and analysis presented here is the most comprehensive overview of how the COVID-19 pandemic affected mass vaccination campaigns.
Implications of all the available evidence
Both outbreak response and preventive mass vaccination campaigns across all vaccines were disrupted heavily by COVID-19, leaving millions of children at risk of devastating but preventable diseases. The prompt resumption of immunization activities, and an efficient catch-up strategy that aims to catch up on the immunization of children through a combination of enhanced routine immunization services and campaigns, are crucial to prevent the accumulation of susceptible persons and thus reduce the risk of disease outbreaks.
Alt-text: Unlabelled box
Background
The World Health Organization (WHO) declared COVID-19 a global pandemic on March 11, 2020 (WHO, 2020a). By December 26, 2021, a total of 278 million cases of SARS-CoV-2 had resulted in 5.4 million reported deaths since the start of the COVID-19 pandemic (WHO, 2021a). The pandemic has disrupted essential health services, including immunization-related activities such as preventive vaccination campaigns, outbreak response, routine immunization (fixed post and outreach), and vaccine-preventable disease (VPD) surveillance activities (WHO, 2020b, 2020c, 2020d; Local Burden of Disease Vaccine Coverage Collaborators, 2021; Shet et al., 2022; Lassi et al., 2021). Even a brief postponement of routine immunization activities can lead to a critical increase in the number of susceptible individuals and create immunity gaps that predispose them to VPD outbreaks within a short period, particularly for highly infectious diseases such as measles. In the wake of the immunization service disruptions caused by the Ebola epidemic of 2014 to 2015, West African countries experienced a 25% decrease in measles vaccination rate as compared with the previous years (Suk et al., 2016; Masresha et al., 2020). Similarly, COVID-19-associated disruptions to immunization programs have the potential to reverse years of progress in VPD control worldwide.
On March 24, 2020, the Polio Oversight Board of the Global Polio Eradication Initiative (GPEI) recommended pausing all polio campaigns until June 1, 2020, given the uncertainties associated with the pandemic (GPEI, 2020). The same month, the Strategic Advisory Group of Experts on Immunization advising WHO recommended temporarily suspending mass vaccination campaigns because of the risk of SARS-CoV-2 transmission and advised countries to monitor and re-evaluate the risks and benefits of delaying mass vaccination campaigns at regular intervals (WHO, 2020e). As the negative impact of disrupted essential health services, including immunization, on public health became evident, WHO developed guidance to promote safe practices during vaccination activities based on an improved understanding of SARS-CoV-2 transmission (WHO, 2020f, 2020g, 2020h, 2020i). The Strategic Advisory Group of Experts subsequently urged and reinforced countries to preserve mass vaccination campaigns and prioritize the implementation of catch-up vaccination policies and strategies where appropriate (WHO, 2020j, 2020k).
Beginning in May 2020, WHO, in collaboration with partners, tracked and monitored the impact of COVID-19-related disruptions on preventive and outbreak response vaccination campaigns. In this study, the authors describe the systems put in place and the trends in campaign postponement and resumption since the COVID-19 pandemic began to disrupt programmatic activities and the campaign monitoring in May 2020 through December 2021. (Figs. 1 and 2 ; Tables 1a , Tables 1b , Tables 1c , Tables 1d )
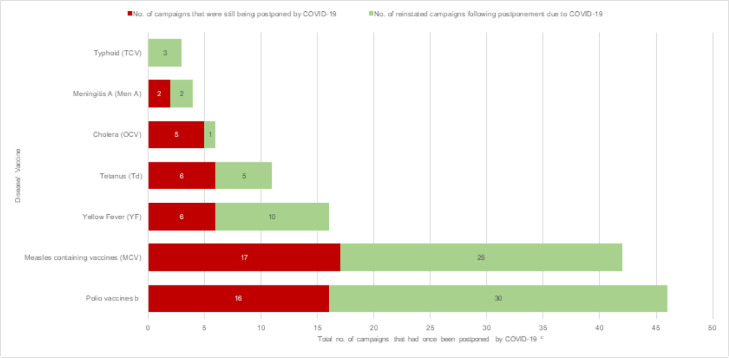
Figure 1b.
Number of campaigns postponed because of COVID-19 and campaigns reinstated a after postponement because of COVID-19, as of May 15, 2021
a The cumulative number of campaigns reinstated after an initial postponement caused by COVID-19.
b 100% (22/22) monovalent oral poliovirus type2 (mOPV2), 43% (3/7) bivalent oral poliovirus types 1&3 (bOPV) and 28% (5/18) inactivated polio vaccine (IPV) campaigns were reinstated after postponement because of COVID-19. No trivalent oral polio vaccine (tOPV) or novel oral polio vaccine type 2 (nOPV2) campaign was recorded as “postponed COVID” or “reinstated COVID”.
c This column includes those campaigns that had ever been postponed at any point between March 2020 and May 2021 and represent the cumulative number of reinstated campaigns plus the number of campaigns that were still being postponed/ projected to be postponed because of COVID-19 as of May 2021.
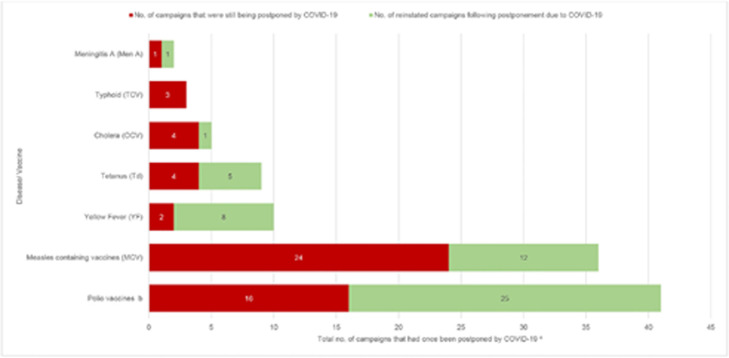
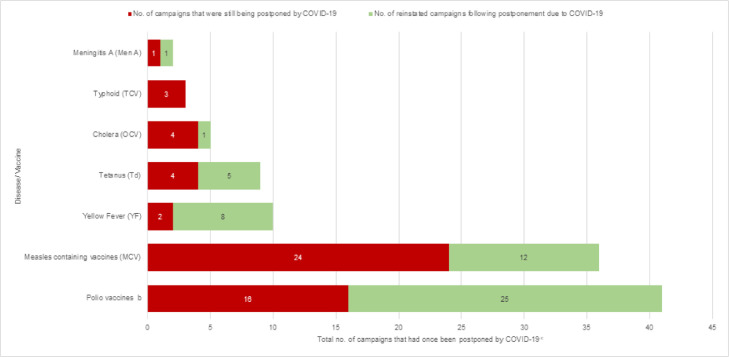
Figure 1c.
Number of campaigns postponed because of COVID-19 and campaigns reinstated a after postponement because of COVID-19, as of December 1, 2021
a The cumulative number of campaigns reinstated after an initial postponement caused by COVID-19.
b 100% (22/22) monovalent oral poliovirus type2 (mOPV2), 50% (3/6) bivalent oral poliovirus types 1&3 (bOPV) and 28% (5/18) inactivated polio vaccine (IPV) campaigns were reinstated after postponement because of COVID-19. No trivalent oral polio vaccine (tOPV) or novel oral polio vaccine type 2 (nOPV2) campaign was recorded as “postponed COVID” or “reinstated COVID”.
c This column includes those campaigns that had ever been postponed at any point between March 2020 and December 2021 and represent the cumulative number of reinstated campaigns plus the number of campaigns that were still being postponed/ projected to be postponed because of COVID-19 as of December 2021.
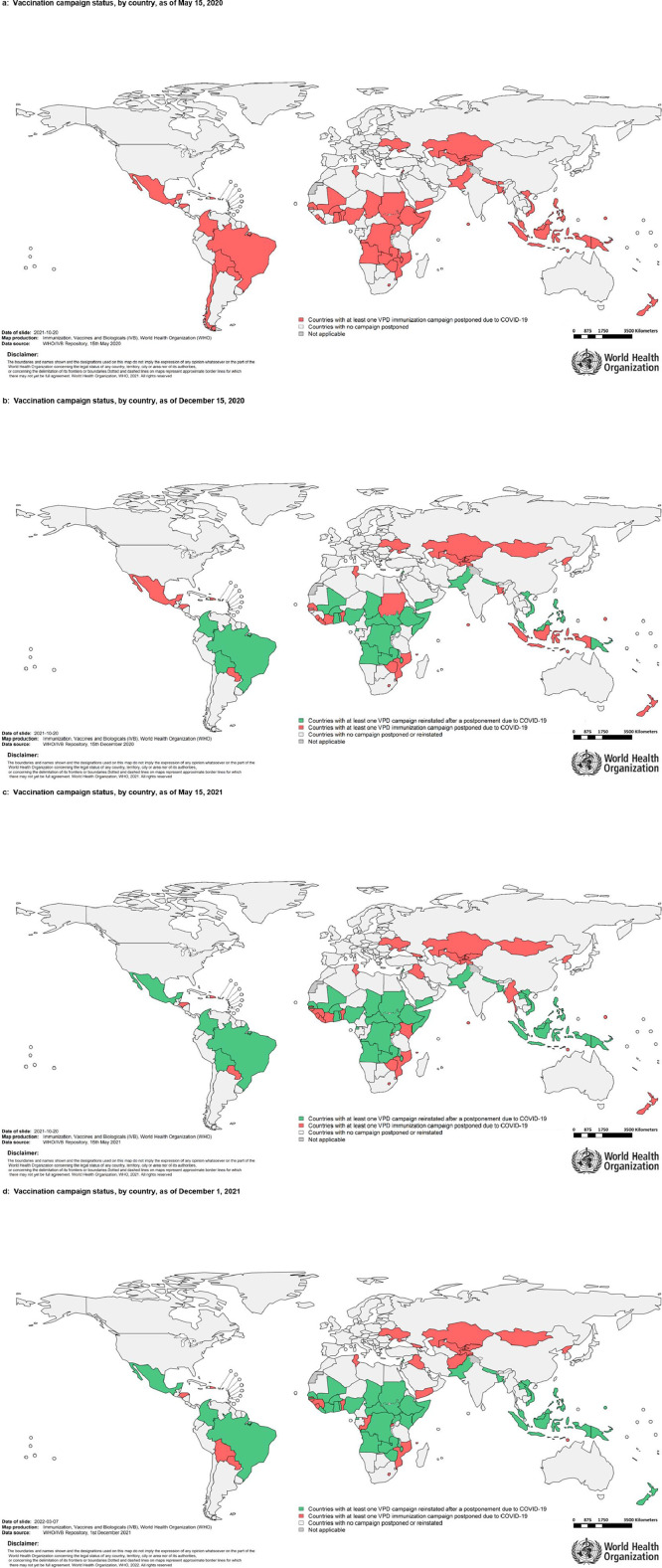
Figure 3.
a: Vaccination campaign status, by country, as of May 15, 2020
b: Vaccination campaign status, by country, as of December 15, 2020
c: Vaccination campaign status, by country, as of May 15, 2021
d: Vaccination campaign status, by country, as of December 1, 2021
Figure 1a.
Number of campaigns postponed because of COVID-19 and campaigns reinstated a after postponement because of COVID-19, as of December 15, 2020
a The cumulative number of campaigns reinstated after an initial postponement caused by COVID-19.
b 90% (19/21) monovalent oral poliovirus type2 (mOPV2), 38% (3/8) bivalent oral poliovirus types 1&3 (bOPV) and 25% (3/12) inactivated polio vaccine (IPV) campaigns were reinstated after postponement because of COVID-19.
c This column includes those campaigns that had ever been postponed at any point between March and December 2020 and represent the cumulative number of reinstated campaigns plus the number of campaigns that were still being postponed/ projected to be postponed because of COVID-19 as of December 2020.
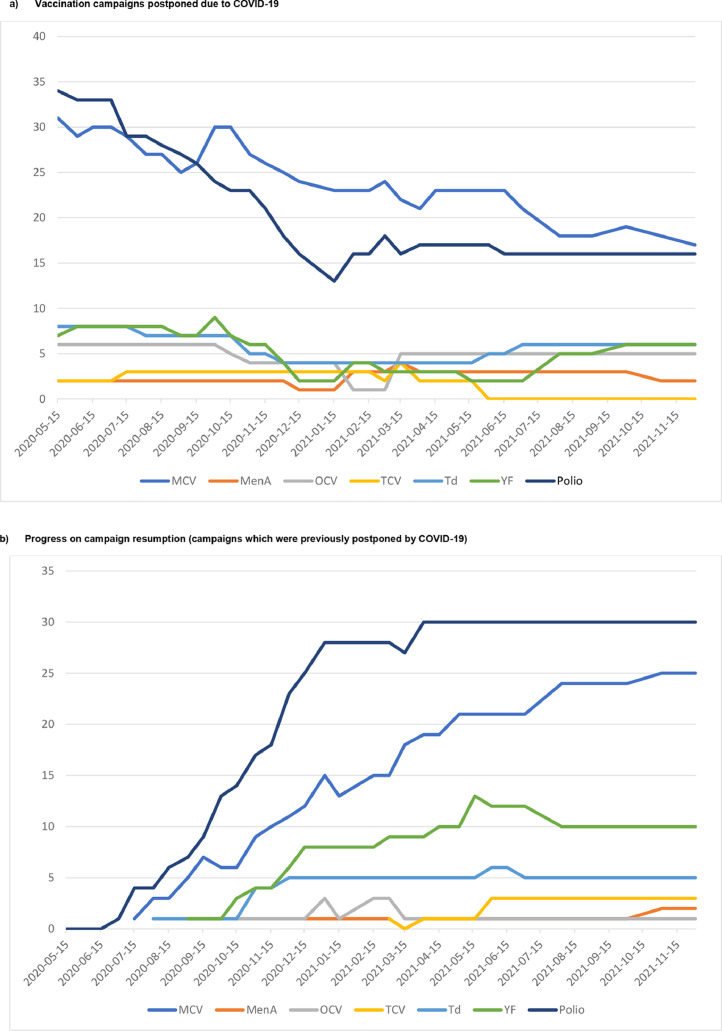
Figure 2.
COVID-19 related campaign postponement and resumption, by vaccine *, May 2020 to December 2021
(a) Vaccination campaigns postponed because of COVID-19.
Note: This figure depicts the total number of campaigns that had previously been planned to take place, but that were projected to be postponed because of COVID-19. Campaigns that had been reinstated, even if implementation was delayed from the originally planned date, were removed from the postponement tally. This graph does not include the number of campaigns that were postponed because of non-COVID related reasons or the number of canceled campaigns. No trivalent oral polio vaccine (tOPV) or novel oral polio vaccine type 2 (nOPV2) campaign was recorded as “postponed COVID”.
* Measles containing vaccines (MCV) including measles, measles rubella (MR), and measles mumps rubella (MMR); polio vaccines including bivalent oral polio vaccine types 1 and 3 (bOPV), monovalent oral polio vaccine type 2 (mOPV2) and inactivated polio vaccine (IPV); meningitis A conjugate vaccine (MenA); tetanus-diphtheria vaccine (Td); yellow fever vaccine (YF); typhoid conjugate vaccine (TCV); and oral cholera vaccine (OCV).
(b) Progress on campaign resumption (campaigns that were previously postponed by COVID-19)
Note: This figure depicts the total number of campaigns that had previously been postponed because of COVID-19 but have restarted. The date refers to when the campaign was conducted. This graph does not include the number of campaigns reinstated after an initial postponement because of non-COVID-related reasons. No trivalent oral polio vaccine (tOPV) or novel oral polio vaccine type 2 (nOPV2) campaign was recorded as “postponed COVID” or “reinstated COVID”.
* Measles containing vaccines (MCV) including measles, measles rubella (MR), and measles mumps rubella (MMR); polio vaccines including bivalent oral polio vaccine types 1 and 3 (bOPV), monovalent oral polio vaccine type 2 (mOPV2) and inactivated polio vaccine (IPV); meningitis A conjugate vaccine (MenA); tetanus-diphtheria vaccine (Td); yellow fever vaccine (YF); typhoid conjugate vaccine (TCV); and oral cholera vaccine (OCV).
Table 1a.
Number of campaigns planned to take place from March 2020 to December 2020 that were postponed or canceled (fully or partially) because of COVID-19, as of May 15, 2020
| Diseases/ Vaccines | No. of countries with postponed or canceled campaigns | No. of postponed or canceled campaign/Total no. of planned campaign |
No. of campaigns postponed or canceled by regions |
|||||
|---|---|---|---|---|---|---|---|---|
| AFR | AMR | EMR | EUR | SEAR | WPR | |||
| Measles containing vaccines (MCV) | 30 | 31/52 (60%) | 6 | 8 | 4 | 5 | 5 | 3 |
| Polio vaccines | 26 * | 49/91 (54%) | 33 | 2 | 5 | 0 | 3 | 6 |
| a) Inactivated polio vaccine (IPV) | 8 | 8/24 (33%) | 7 | 0 | 0 | 0 | 0 | 1 |
| b) Bivalent oral polio vaccine types1&3 (bOPV) | 13 | 14/31 (45%) | 8 | 1 | 1 | 0 | 3 | 1 |
| c) Monovalent oral polio vaccine type2 (mOPV2) | 12 | 27/36 (75%) | 18 | 1 | 4 | 0 | 0 | 4 |
| Meningitis A (Men A) | 2 | 2/5 (40%) | 2 | 0 | 0 | 0 | 0 | 0 |
| Yellow fever (YF) | 6 | 7/10 (70%) | 5 | 2 | 0 | 0 | 0 | 0 |
| Typhoid (TCV) | 2 | 2/3 (67%) | 1 | 0 | 1 | 0 | 0 | 0 |
| Cholera (OCV) | 5 | 6/7 (86%) | 3 | 0 | 1 | 0 | 2 | 0 |
| Tetanus- diphtheria (Td) | 7 | 8/15 (53%) | 3 | 0 | 3 | 0 | 0 | 2 |
| Total | 57* | 105/183 (57%) | 53 | 12 | 14 | 5 | 10 | 11 |
* Total no. of countries with at least 1 VPD immunization campaign postponed (fully or partially)
Table 1b.
Number of campaigns planned to take place from March 2020 to December 2020 that were postponed or canceled (fully or partially) because of COVID-19, as of December 15, 2020
| Diseases/ Vaccines | No. of countries with postponed or canceled campaigns | No. of postponed or canceled campaign/ Total no. of planned campaign |
No. of campaigns postponed or canceled by regions |
|||||
|---|---|---|---|---|---|---|---|---|
| AFR | AMR | EMR | EUR | SEAR | WPR | |||
| Measles containing vaccines (MCV) | 23 | 24/56 (43%) | 3 | 7 | 1 | 5 | 3 | 5 |
| Polio vaccines | 25 * | 38/188 (20%) | 26 | 1 | 5 | 0 | 5 | 1 |
| a) Inactivated polio vaccine (IPV) | 9 | 9/24 (38%) | 7 | 0 | 0 | 0 | 1 | 1 |
| b) Bivalent oral polio vaccine types1&3 (bOPV) | 15 | 19/67 (28%) | 10 | 1 | 4 | 0 | 4 | 0 |
| c) Monovalent oral polio vaccine type2 (mOPV2) | 5 | 10/97 (10%) | 9 | 0 | 1 | 0 | 0 | 0 |
| Meningitis A (Men A) | 1 | 1/7 (14%) | 1 | 0 | 0 | 0 | 0 | 0 |
| Yellow fever (YF) | 1 | 2/11 (18%) | 2 | 0 | 0 | 0 | 0 | 0 |
| Typhoid (TCV) | 3 | 3/3 (100%) | 2 | 0 | 1 | 0 | 0 | 0 |
| Cholera (OCV) | 4 | 5/12 (42%) | 2 | 0 | 1 | 0 | 2 | 0 |
| Tetanus- diphtheria (Td) | 3 | 4/15 (27%) | 1 | 0 | 1 | 0 | 0 | 2 |
| Total | 50* | 77/292 (26%) | 37 | 8 | 9 | 5 | 10 | 8 |
* Total no. of countries with at least 1 VPD immunization campaign postponed (fully or partially)
Table 1c.
Number of campaigns planned to take place from March 2020 to December 2021 that were postponed or canceled (fully or partially) because of COVID-19, as of May 15, 2021
| Diseases/ Vaccines | No. of countries with postponed or canceled campaigns | No. of postponed or canceled campaign/ Total no. of planned campaigns |
No. of campaigns postponed or canceled by regions |
|||||
|---|---|---|---|---|---|---|---|---|
| AFR | AMR | EMR | EUR | SEAR | WPR | |||
| Measles containing vaccines (MCV) | 23 | 23/78 (29%) | 4 | 6 | 2 | 6 | 3 | 2 |
| Polio vaccines | 30* | 40 /246 (16%) | 28 | 1 | 5 | 0 | 5 | 1 |
| a) Inactivated polio vaccine (IPV) | 13 | 13/26 (50%) | 10 | 0 | 0 | 0 | 2 | 1 |
| b) Bivalent oral polio vaccine types1&3 (bOPV)/Trivalent oral polio vaccine (tOPV) | 15 | 18/90 (20%) | 10 | 1 | 4 | 0 | 3 | 0 |
| c) Monovalent oral polio vaccine type2 (mOPV2)/ Novel oral polio vaccine type 2 (nOPV2) | 5 | 9/130 (7%) | 8 | 0 | 1 | 0 | 0 | 0 |
| Meningitis A (Men A) | 3 | 3/7 (43%) | 3 | 0 | 0 | 0 | 0 | 0 |
| Yellow fever (YF) | 2 | 2/21 (10%) | 1 | 0 | 1 | 0 | 0 | 0 |
| Typhoid (TCV) | 2 | 2/3 (67%) | 2 | 0 | 0 | 0 | 0 | 0 |
| Cholera (OCV) | 5 | 5/16 (31%) | 2 | 0 | 1 | 0 | 2 | 0 |
| Tetanus- diphtheria (Td) | 4 | 4/15 (27%) | 1 | 0 | 1 | 0 | 0 | 2 |
| Total | 53* | 79/386 (20%) | 41 | 7 | 10 | 6 | 10 | 5 |
* Total no. of countries with at least 1 VPD immunization campaign postponed (fully or partially)
Table 1d.
Number of campaigns planned to take place from March 2020 to December 2021 that were postponed or canceled (fully or partially) because of COVID-19, as of December 1, 2021
| Diseases/ Vaccines | No. of countries with postponed or canceled campaigns | No. of postponed or canceled campaign/ Total no. of planned campaign |
No. of campaigns postponed or canceled by regions |
|||||
|---|---|---|---|---|---|---|---|---|
| AFR | AMR | EMR | EUR | SEAR | WPR | |||
| Measles containing vaccines (MCV) | 18 | 18/85 (21%) | 3 | 5 | 2 | 6 | 1 | 1 |
| Polio vaccines | 30 * | 40/319 (13%) | 28 | 1 | 5 | 0 | 5 | 1 |
| a) Inactivated polio vaccine (IPV) | 13 | 13/26 (50%) | 10 | 0 | 0 | 0 | 2 | 1 |
| b) Bivalent oral polio vaccine types1&3 (bOPV)/ Trivalent oral polio vaccine (tOPV) | 15 | 18/114 (16%) | 10 | 1 | 4 | 0 | 3 | 0 |
| a) Monovalent oral polio vaccine type2 (mOPV2)/ Novel oral polio vaccine type 2 (nOPV2) | 5 | 9/179 (5%) | 8 | 0 | 1 | 0 | 0 | 0 |
| Meningitis A (Men A) | 2 | 2/7 (29%) | 2 | 0 | 0 | 0 | 0 | 0 |
| Yellow fever (YF) | 6 | 6/21 (29%) | 3 | 2 | 1 | 0 | 0 | 0 |
| Typhoid (TCV) | 0 | 0/6 (0%) | 0 | 0 | 0 | 0 | 0 | 0 |
| Cholera (OCV) | 4 | 5/18 (28%) | 2 | 0 | 1 | 0 | 2 | 0 |
| Tetanus- diphtheria (Td) | 5 | 6/16 (38%) | 2 | 0 | 2 | 0 | 0 | 2 |
| Total | 54* | 77/472 (16%) | 40 | 8 | 11 | 6 | 8 | 4 |
* Total no. of countries with at least 1 VPD immunization campaign postponed (fully or partially)
Methods
WHO and partners established an online repository Campaign Delay Tracker in May 2020, using an existing WHO Immunization Repository Platform. The tracker includes campaigns with the following vaccines: measles-containing vaccines (MCV) including measles, measles-rubella (MR), or measles-mumps-rubella (MMR) vaccines; bivalent oral polio vaccine types 1 and 3 (bOPV); monovalent oral polio vaccine type 2 (mOPV2); inactivated polio vaccine (IPV); meningitis A conjugate vaccine (MenA); tetanus-diphtheria vaccine (Td); yellow fever vaccine (YF); typhoid conjugate vaccine (TCV) and oral cholera vaccine (OCV). Trivalent oral polio vaccines (tOPV) and novel oral polio vaccine type 2 (nOPV2) were added to the analysis starting in 2021, tOPV was reintroduced because of the dual threat of different strains of poliovirus and mOPV2 was replaced by nOPV2 in some vaccination campaigns. Additional variables collected for each campaign included the following: WHO and United Nations Children's Fund (UNICEF) Region, country, the geographic scope of the campaign (national or sub-national), original implementation date, postponed implementation date, target population age range, and size, type of vaccination campaign (preventive or outbreak response), funding source, and other services including co-administered vaccines or other health interventions.
The status for each campaign activity was classified into one of the following categories: “on track”, refers to campaigns that had proceeded or would proceed on time as planned or with slight postponement of less than 1 month; “postponed COVID”, campaigns with confirmed delay fully or partially because of COVID-19 related reasons such as programmatic challenges of campaign implementation in the COVID-19 context or competing priorities with COVID-19 response activities or COVID-19 vaccine rollout; “postponed other”, campaigns with confirmed delay because of non-COVID-19 reasons; “canceled”, campaigns which have been canceled and will not be implemented in the foreseeable future; “reinstated COVID”, campaigns which were previously postponed fully or partially because of COVID-19 but were subsequently implemented; “reinstated others”, campaigns which were previously postponed because of non-COVID-19 reasons but were subsequently implemented; “unknown”, campaigns with no information received on whether they will be implemented or had been implemented on time or postponed.
WHO Headquarters received updates on the campaigns through reports, teleconferences, email exchanges with partners and regional and country colleagues, and direct country reports. The bOPV, mOPV2, tOPV, and nOPV2 data were extracted from the Polio Information System (POLIS) to the tracker. POLIS is an integrated approach to polio real-time data management, whereby data from member states are collected, consolidated, harmonized, and made available for dissemination and analysis for GPEI partners and modelers. Given the complexity and dynamic characteristic of polio vaccination campaigns with bOPV, mOPV2, tOPV, and nOPV2, the data of these 4 vaccines in this manuscript were cleaned and analyzed separately by the POLIS focal point (ALB).
The total number of campaigns reflected in the tracker increased over time as countries made formal plans to conduct campaigns. With 2021 planned campaign data added to the Campaign Delay Tracker in late January 2021, the repository data from February 1, 2021, reflected a sudden increase in the total number of campaigns. The tracker was updated biweekly until June 2021, and then monthly to reflect these updates, and the extracted data were disseminated to a wide range of immunization partners. Datapoints reflecting previous campaign status were updated retrospectively as new data became available; hence, the final database used for this analysis may not completely match the data in the partner reports.
We analyzed and compared the number of campaigns canceled and postponed and the main reasons for cancellation or delay at 4 time points: May 15, 2020, December 15, 2020, May 15, 2021, and December 1, 2021. The estimates as of May 15, 2020, and December 15, 2020, only covered campaigns planned to take place from March 2020 to December 2020, whereas the estimates as of May 15, 2021, and December 1, 2021, covered campaigns planned to take place from March 2020 to December 2021. The figures were disaggregated by vaccines and the 6 WHO regions.
Limitations
The data presented here are subject to several limitations. The Campaign Delay Tracker contains information obtained from various sources, not only data officially reported by WHO member states. Hence some data may be incomplete or erroneous. In addition, the data are evolving dynamically and reflect programmatic decisions taken at the country level to schedule, postpone, implement and/or cancel campaign activities. New data and corrections were entered as new updates came in, resulting in shifting denominators. Furthermore, the data on vaccination doses are approximate and do not represent unique individuals but rather the number of vaccines to be administered.
The design of Campaign Delay Tracker and this study focus on the COVID-19 disruptions brought to other vaccine-preventable diseases. Because COVID-19 vaccines had received WHO's Emergency Use Listing in December 2020 and very few low and middle-income countries had started using COVID-19 vaccines by March 2021, we excluded COVID-19 vaccine data from this study. Furthermore, it is challenging to compare the Campaign Delay Tracker data with COVID-19 data from WHO COVID-19 Dashboard and Our World in Data, which targets the number of cases and vaccinations but not the trend of campaign implementation, postponement, or cancellation. A baseline analysis of campaign postponement and cancellation before COVID-19 is not feasible because of missing historical data on campaigns disruptions at the global level. Nevertheless, the data and analysis presented here are the most comprehensive overview of how the COVID-19 pandemic affected vaccination campaigns, a broadly used strategy to supplement routine immunization and respond to VPD outbreaks, especially in settings where the routine immunization programs fail to reach very high vaccination coverage levels.
Results
May 2020
As of May 15, 2020, 105 of 183 (57%) campaigns with a previously planned implementation date from March 2020 to December 2020 were postponed or canceled because of COVID-19; 13 (4 bOPV, 4 mOPV2, 2 Td, 2 IPV, 1 Men A) were postponed or canceled because of reasons other than COVID-19; 24 (5 bOPV, 5 mOPV2, 4 MCV, 4 Td, 3 IPV, 1 MenA, 1 OCV, 1YF) were on track, and the status of 41 (17 MCV, 11 IPV, 8 bOPV, 2 YF, 1 MenA, 1 TCV, 1 Td) was considered unknown.
The 105 campaigns postponed or canceled because of COVID-19 affected 57 countries and resulted in an estimated 796 million postponed or missed vaccine doses. Forty (38%) campaigns were for outbreak response.
December 2020
As of December 15, 2020, 77 of 292 (26%) campaigns with a previously planned implementation date from March 2020 to December 2020 were postponed or canceled because of COVID-19, 41 (15 bOPV, 14 mOPV2, 4 MCV, 4 MenA, 2 IPV, 2 Td) were postponed or canceled because of non-COVID-19 reasons, 52 (19 mOPV2, 12 MCV, 8 YF, 5 Td, 3 bOPV, 3 IPV, 1 Men A, 1 OCV) were reinstated after a postponement because of COVID-19, 6 (2 mOPV2, 2 MCV, 1 IPV, 1 OCV) were reinstated after non-COVID disruptions. A total of 94 campaigns (48 mOPV2, 25 bOPV, 11 MCV,4 OCV, 3 Td, 2 IPV, 1 YF) were on track, and the status of 22 campaigns (7 IPV, 5 bOPV, 4 mOPV2, 3 MCV, 1 MenA, 1 OCV, 1 Td) was considered unknown.
The 77 campaigns postponed or canceled because of COVID-19 were in 50 countries, resulting in an estimated 365 million postponed or missed vaccine doses. Nineteen (25%) campaigns were outbreak response immunization.
An increasing number of previously postponed campaigns because of COVID-19 started in mid-July 2020. As of December 15, 2020, 52 of 106 (49%) of the VPD campaigns, which had initially been planned to take place between March 2020 and December 2020, had been reinstated after a postponement caused by COVID-19. In total, the delivery of approximately 333 million vaccine doses was resumed in 2020.
May 2021
As of May 15, 2021, 79 of 386 (20%) campaigns with a previously planned implementation date from March 2020 to December 2021 were postponed or canceled because of COVID-19, 53 (21 bOPV, 19 mOPV2, 4 IPV, 3 MCV, 2 MenA, 2 OCV, 2 Td) were postponed or canceled because of non-COVID reasons; 72 (22 mOPV2, 21 MCV, 13 YF, 5 IPV, 5 Td, 3 bOPV, 1 Men A, 1 OCV, 1 TCV) campaigns were reinstated after postponement because of COVID-19; 14 (5 mOPV2, 3 MCV, 3 IPV, 2 bOPV, 1 YF) were reinstated after non-COVID disruptions. Then 136 (67 mOPV2, 29 bOPV, 12 MCV,7 tOPV, 7 OCV, 5 nOPV2, 5 YF, 3 Td, 1 Men A) were on track and the status of 32 campaigns (16 MCV, 10 bOPV, 3 mOPV2, 1 IPV, 1 OCV, 1Td) was considered unknown.
The 79 campaigns that were postponed or canceled because of COVID-19 were in 53 countries, resulting in an estimated 390 million postponed or missed vaccine doses. Twelve (15%) were outbreak responses.
As of May 15, 2021, 72 of 128 (56%) VPD campaigns initially planned to take place between March 2020 and December 2021 had been reinstated after an initial postponement caused by COVID-19. In total, the delivery of accumulated 433 million vaccine doses was resumed.
December 2021
As of December 1, 2021, 77 of 472 (16%) campaigns with a previously planned implementation date from March 2020 to December 2021 were postponed or canceled because of COVID-19, 76 (23 bOPV, 19mOPV2, 12 nOPV2, 10 MCV, 4 IPV, 4 Td, 1 YF, 1 MenA, 1 TCV, 1 OCV) were postponed or canceled because of non-COVID reasons; 76 (25 MCV, 22 mOPV2, 10 YF, 5 IPV, 5 Td, 3 bOPV, 3 TCV, 2 MenA, 1 OCV) campaigns were reinstated after postponement because of COVID-19; 21 (8 mOPV2, 4 bOPV, 4 MCV, 3 IPV, 1 MenA, 1 YF) were reinstated after non-COVID disruption. Then 197 (71 mOPV2, 41 bOPV, 35 nOPV2, 22 MCV, 13 tOPV, 10 OCV, 3 YF, 1 MenA, 1 Td) were on track and the status of 25 (10 bOPV, 6 MCV, 3 mOPV2, 2 tOPV, 2 TCV, 1 IPV, 1 OCV) was considered unknown.
The 77 campaigns that were postponed or canceled because of COVID-19 were in 54 countries, resulting in an estimated 382 million postponed or missed vaccine doses. Twelve (16%) were outbreak responses.
After an initial postponement caused by COVID-19, 76 of 128 (59%) VPD campaigns initially planned to take place between March 2020 and December 2021 had been reinstated. In total, the delivery of accumulated 478 million vaccine doses was resumed.
Discussion
Our results indicate that both outbreak response and preventive campaigns across all vaccines were disrupted heavily by the COVID-19 pandemic. The main reasons reported early in the pandemic for the disruption of services were insufficient personal protective equipment for healthcare workers, unclear public health protocol for safe administration of vaccines in a mass campaign environment, low availability of healthcare workers, extensive lockdown measures, and closure of health facilities and services. In addition, interruptions in the supply of health products because of travel restrictions, decreased demand as population movement was restricted, and fear of infection when going to a health facility occurred (WHO 2020b, 2020c, 2020d, Local Burden of Disease Vaccine Coverage Collaborators, 2021; Shet et al., 2022). Starting from mid-July 2020, the implementation of vaccination campaigns regained momentum despite the challenges brought by the pandemic. The proportion of campaigns postponed or canceled because of COVID-19 decreased from 57% in May 2020 to 16% in December 2021. Among all, mOPV2 campaigns show the fastest rate of resumption. However, at least 77 campaigns were still postponed or canceled because of COVID-19 as of December 2021, leaving millions of children at risk from devastating but preventable diseases. In addition to the disruptions to mass vaccination campaigns presented here, results from the global pulse polls, the official WHO and UNICEF estimates of national immunization coverage (WUENIC) in 2020, and multiple publications have all pointed to the widespread disruption to immunization services in countries of all income levels, unraveling years of progress in immunization (WHO 2020b, 2020c, 2020d; WHO 2021b; Local Burden of Disease Vaccine Coverage Collaborators, 2021; Shet et al., 2022; Causey et al., 2021). Innovative analytics and modeling provided information about the risk of cancellations and delays on population immunity and the potential for outbreaks of highly transmissible VPDs (Gaythorpe et al., 2021; Abbas et al., 2020; Makvandi-Nejad, 2021; Kalkowska et al., 2021).
The accrued learning about transmission risk resulted in guidance measures that were updated during the pandemic and reflected in the resumption of campaign activities. Research shows that with appropriate precautions, there would be a minimal risk for SARS-CoV-2 transmission during VPD campaigns (WHO, 2020l). In October 2020, WHO again emphasized the need to restore campaigns, immunization clinics, and outreach activities rapidly to prevent outbreaks and deaths from VPDs (WHO, 2020m). WHO and UNICEF issued an emergency call to action in November 2020 to avert major measles and polio epidemics as COVID-19 continued to disrupt immunization services worldwide, leaving millions of children at heightened risk of preventable childhood disease (UNICEF and WHO, 2020). Based on country experiences with immunization activities during COVID-19, multi-antigen or integrated mass intervention campaigns should be considered to maximize efficiencies. Digital tools facilitate using real-time data for campaign planning (UNICEF, 2021). The prompt resumption of immunization activities, and an efficient catch-up strategy that aims to catch up on the immunization of children through a combination of enhanced routine immunization services and campaigns, is crucial to prevent the accumulation of susceptible persons, especially with the anticipation of near-term events that may again disrupt immunization systems, including new waves of genetically evolving SARS-CoV-2 variants and the introduction of COVID-19 vaccines.
There are opportunities to learn from multiple success stories of campaigns conducted during the pandemic. For example, Nepal was the first country to conduct a mass measles vaccination campaign during the pandemic (WHO, 2020n). Ethiopia vaccinated 14.5 million children amidst COVID-19 community circulation and conflict. Thanks to a strong coordination mechanism, effective community engagement, and innovative vaccination delivery strategies, the campaign attained 96% coverage (WHO, 2020o). Sudan implemented an innovative and integrated approach to combine catch-up activities for children in host communities with the vaccination for refugees (EYE, 2020). Increasing numbers of countries have re-instated campaigns with multiple antigens, adjusted to the current circumstances and each local context.
However, conducting campaigns and immunization services during COVID-19 is not without challenges. The planning and implementation of these campaigns are still adversely affected by the COVID-19 pandemic. Current data indicate that the pandemic has continued to limit the quality of immunization activities and surveillance sensitivity. Personnel and resources are stretched to cover the increased demand for COVID-19 diagnoses. Some countries have prioritized COVID-19 vaccine rollout over other vaccination campaigns. Although the use of resources has been reprogramed from other VPD campaigns to COVID-19 campaigns, this analysis did not track the implementation of COVID-19 campaigns enabled by this reprogramming. Public health and coordination structures have been shifted to COVID-19 control. Global attention and additional resources are still urgently needed to address dangerous immunity gaps across all VPDs. As stated by the Independent Panel on Pandemic Preparedness and Response, investment in strengthening health system resilience is essential in preparation for future health threats like COVID-19 (WHO, 2021c).
The WHO Campaign Delay Tracker has created an opportunity for all global VPDs control/elimination/eradication programs to have 1 global campaign tracking mechanism. This was unprecedented and created synergistic opportunities to plan and deliver multi-antigen campaigns. The WHO Campaign Delay Tracker serves as a centralized database for monitoring preventive and outbreak response vaccination campaigns, documenting postponements, and providing data for partner coordination and programmatic monitoring. The data were also used to inform analysis and modeling to understand the potential impact of campaign cancellations and delays on population immunity and the potential for outbreaks of highly transmissible VPDs (Gaythorpe, 2021), with modeling studies quantifying the impact of campaign delays (Gaythorpe, 2021; Kalkowska et al., 2021). The lessons learned from establishing the Campaign Delay Tracker illustrate the importance of clearly defining each variable to minimize information bias caused by different categorizations of campaign status or other variables. The methodology used and limitations of the analysis need to be clearly stated and well communicated with immunization partners to avoid misinterpretations. Building synergies with other campaign data systems is crucial to avoid duplicated data collection and management efforts.
Since the start of the pandemic, the implementation of a significant number of vaccination campaigns was adversely affected by the COVID-19 pandemic. Consequently, a large number of persons susceptible to VPDs has been and is likely accumulating, and thus the risk of VPD outbreaks is high. Delaying outbreak response will likely result in a more immediate increase in cases and deaths than delaying preventive campaigns. As mass immunization campaigns have gotten delayed, some children mature out of the target age, thus remaining unexposed to additional vaccination opportunities. At the writing of this study, some vaccinations have resumed and are being implemented across the member states, with infection prevention and control measures in place and with a move toward increased integration. This gradual resumption is a testament to the work of countries to protect their populations against VPDs. It is still crucial to urgently reduce the remaining immunity gaps caused by disrupted immunization services to prevent extensive VPD outbreaks under a health system already strained by the COVID-19 pandemic.
Conflict of interest
The authors declare no competing interests.
Acknowledgments
Funding source
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Ethical approval statement
The authors have read and complied with the policy stated in Ethics in publishing.
Contributors
LLH, SG, HDN, CS, and ALB developed the conceptual framework. HDN, SG, LLH, CS, and ALB developed the data collection instruments and reporting structure from countries. LLH, SG, HDN, CS, and ALB extracted, cleaned, and cataloged data, conducted data analysis and developed graphs. LLH and KK wrote the first draft of the manuscript. KK, MCD-H, SVS, and IM provided critical review, meaningful comments and revisions. All authors have reviewed and approved the final version of the manuscript.
Data sharing
All data used in this study are available either in the manuscript and supplementary materials or in publications referenced in the study. The raw data used for analysis are accessible by registered stakeholders and have been disseminated broadly to immunization partners by email. To obtain permission to access the raw data, please contact co-author HDN at nicolash@who.int.
Disclaimer
The authors alone are responsible for the views expressed in this study, and they do not necessarily represent the views, decisions, or policies of the institutions with which they are affiliated.
Acknowledgments
The authors wish to thank all the persons that make immunization possible, even under challenging circumstances like the current COVID-19 pandemic. Additional thanks go to Malika Bouhenia, Philippe Barboza; Valentina Bigoni, Vachagan Harutyunyan, William Mbabazi, Karim Djibaoui, Thera Sinaly, Alejandro Ramirez Gonzalez, Antoine Durupt, Jenny Walldorf, Carole Tevi Benissan; Nasir Yusuf, Jennifer Horton, Laurence Cibrelus, Diana Chang- Blanc, Alexander Rosewell, William Perea, Desiree Pastor, Regina Duron, Syeda Aslam, Tigran Avagyan for compiling and reviewing the data shared in this study.
References
- Abbas K., Procter S.R., Van Zandvoort K., et al. Routine childhood immunization during the COVID-19 pandemic in AFRICA: A benefit–risk analysis of health benefits Versus excess risk of Sars-cov-2 infection. The Lancet Global Health 2020. 2020 doi: 10.1016/S2214-109X(20)30308-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Causey K, Fullman N, Sorensen RJD, Galles NC, Zheng P, Aravkin A, et al. Estimating global and regional disruptions to routine childhood vaccine coverage during the COVID-19 pandemic in 2020: a modeling study. Lancet 2021. 2021;398:522–534. doi: 10.1016/S0140-6736(21)01337-4. https://www.thelancet.com/pdfs/journals/lancet/PIIS0140-6736(21)01337-4.pdf [DOI] [PMC free article] [PubMed] [Google Scholar]
- EYE newsletter August 2020. https://mailchi.mp/c01237c9ed5c/eye-newsletter-august-2020?e=e98b6f0e1c (accessed March 20, 2022).
- Gaythorpe K, Abbas K, Huber J, Karachaliou A, Thakkar N, Woodruff K, et al. Impact of COVID-19-related disruptions to measles, meningococcal A, and yellow fever vaccination in 10 countries. bioRxiv 2021. 2021 doi: 10.1101/2021.01.25.21250489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Global Polio Eradication Initiative: GPEI (2020). Polio Eradication in the Context of the COVID-19 Pandemic: Summary of urgent country and regional recommendations from the Polio Oversight Board meeting of March 24, 2020. https://polioeradication.org/wp-content/uploads/2020/04/POB-meeting-summary-20200324.pdf (accessed March 20, 2022).
- Kalkowska D.A., Voorman A., Pallansch M.A., Wassilak S.G., Cochi S.L., Badizadegan K., Thompson K.M. The impact of disruptions caused by the COVID-19 pandemic on global polio eradication. Vaccine. 2021 doi: 10.1016/j.vaccine.2021.04.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalkowska DA, Pallansch MA, Wassilak SGF, Cochi SL, Thompson KM. Serotype 2 oral poliovirus vaccine (OPV2) choices and the consequences of delaying outbreak response. Vaccine. 2021 doi: 10.1016/j.vaccine.2021.04.061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lassi ZS, Naseem R, Salam RA, Siddiqui F, Das JK. The Impact of the COVID-19 Pandemic on Immunization Campaigns and Programs: A Systematic Review. Int J Environ Res Public Health. 2021;2021:18. doi: 10.3390/ijerph18030988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Local Burden of Disease Vaccine Coverage Collaborators Mapping routine measles vaccination in low- and middle-income countries. Nature. 2021;589:415–419. doi: 10.1038/s41586-020-03043-4. 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Makvandi-Nejad S, Økland J-M, Haaland ØA, Johansson KA (2021). Reducing Childhood Immunization to Scale Up COVID-19 Immunization in Low- and Lower Middle-Income Countries: Impact on Under-Five Mortality [Preprint]. 2021. doi: 10.2139/ssrn.3927767. [DOI]
- Masresha BG, Luce R, Jr, Weldegebriel G, Katsande R, Gasasira A, Mihigo R. The impact of a prolonged Ebola outbreak on measles elimination activities in Guinea, Liberia and Sierra Leone, 2014-2015. The Pan African medical journal. 2020;35(Suppl 1):8. doi: 10.11604/pamj.supp.2020.35.1.19059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shet A, Carr K, Danovaro-Holliday MC, Sodha SV, Prosperi C, Wunderlich J, Wonodi C, Reynolds HW, Mirza I, Gacic-Dobo M, O'Brien KL, Lindstrand A. Impact of the SARS-CoV-2 pandemic on routine immunization services: evidence of disruption and recovery from 170 countries and territories. Lancet Glob Health. 2022;10(2):e186–e194. doi: 10.1016/S2214-109X(21)00512-X. Epub 2021 Dec 21. https://pubmed.ncbi.nlm.nih.gov/34951973/ [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suk JE, Paez Jimenez A, Kourouma M, Derrough T, Baldé M, Honomou P, et al. Post-Ebola Measles Outbreak in Lola, Guinea, January-June 2015(1) Emerging infectious diseases. 2016;22(6):1106–1108. doi: 10.3201/eid2206.151652. https://doi.org/10.3201/eid2206.151652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- UNICEF and WHO (2020). UNICEF and WHO call for emergency action to avert major measles and polio epidemics. https://www.who.int/news/item/06-11-2020-unicef-and-who-call-for-emergency-action-to-avert-major-measles-and-polio-epidemics#:∼:text=UNICEF%20and%20the%20World%20Health,risk%20of%20preventable%20childhood%20diseases (accessed March 20, 2022).
- UNICEF (2021). Lessons Learned and Good Practices. Country-Specific Case Studies on Immunization Activities During the COVID-19 Pandemic, April 2021. https://www.unicef.org/documents/lessons-learned-and-good-practices-country-specific-case-studies-immunization-activities (accessed March 20, 2022)
- WHO (2020a). WHO Director-General's opening remarks at the media briefing on COVID-19 - March 11, 2020. https://www.who.int/director-general/speeches/detail/who-director-general-s-opening-remarks-at-the-media-briefing-on-covid-19—11-march-2020 (accessed March 20, 2022).
- WHO (2020b). Global Immunization News (GIN). Understanding the disruption to programs through rapid polling, March- April 2020. https://www.who.int/publications/m/item/gin-march-april-2020 (accessed March 20, 2022).
- WHO (2020c). Global Immunization News (GIN). Special feature: immunization and COVID-19, June 2020. https://www.who.int/publications/m/item/gin-june-2020 (accessed March 20, 2022).
- WHO (2020d). Pulse survey on continuity of essential health services during the Covid-19 pandemic: Interim report, August 27, 2020. https://www.who.int/publications/i/item/WHO-2019-nCoV-EHS_continuity-survey-2020.1 (accessed March 20, 2022).
- WHO (2020e). Guiding principles for immunization activities during the COVID-19 pandemic: interim guidance, March 262020. https://apps.who.int/iris/handle/10665/331590 (accessed March 20, 2022).
- WHO (2020f). Immunization in the context of COVID-19 pandemic: frequently asked questions (FAQ), 16 April 2020. https://apps.who.int/iris/handle/10665/331818 (accessed March 20, 2022).
- WHO (2020g). Maintaining essential health services: operational guidance for the COVID-19 context: interim guidance, June 1, 2020. https://apps.who.int/iris/handle/10665/332240 (accessed March 20, 2022).
- WHO (2020h). Framework for decision-making: implementation of mass vaccination campaigns in the context of COVID-19: interim guidance, May 22, 2020. https://apps.who.int/iris/handle/10665/332159 (accessed March 20, 2022).
- WHO (2020i). Leave no one behind: guidance for planning and implementing catch-up vaccination. April 2021. https://www.who.int/publications/i/item/leave-no-one-behind-guidance-for-planning-and-implementing-catch-up-vaccination (accessed March 20, 2022).
- WHO (2020j). Immunization as an essential health service: guiding principles for immunization activities during the COVID-19 pandemic and other times of severe disruption, November 1, 2020. https://www.who.int/publications/i/item/immunization-as-an-essential-health-service-guiding-principles-for-immunization-activities-during-the-covid-19-pandemic-and-other-times-of-severe-disruption (accessed March 20, 2022).
- WHO (2020k). Meeting of the Strategic Advisory Group of Experts on Immunization, October 2020 – conclusions and recommendations. Available at https://apps.who.int/iris/handle/10665/337109 (accessed March 20, 2022).
- WHO (2020l). Department of Immunization, Vaccines and Biologicals (IVB). The risk of SARS-CoV-2 transmission to communities and to health workers in LMICs under different health service delivery conditions. Meeting of the Advisory Committee on Immunization and Vaccines related Implementation Research (IVIR-AC), 63-116. https://terrance.who.int/mediacentre/data/sage/IVIR-AC_Pink_Book_sept_2020.pdf (accessed March 20, 2022).
- WHO (2020m). WHO director-general's opening remarks at the media briefing on covid-19 – October 9, 2020. https://www.who.int/director-general/speeches/detail/who-director-general-s-opening-remarks-at-the-media-briefing-on-covid-19—9-october-2020 (accessed March 20, 2022).
- WHO (2020n). COVID-19 WHO's Action in Countries. July feature countries: A monthly selection of case studies (accessed March 20, 2022).
- WHO (2020o). Ethiopia vaccinates nearly 15 million children against measles despite Covid-19 challenges. Available at https://www.afro.who.int/news/ethiopia-vaccinates-nearly-15-million-children-against-measles-despite-covid-19-challenges (accessed March 20, 2022).
- WHO (2021a). World Health Organization Coronavirus (COVID-19) Dashboard https://covid19.who.int/ (accessed December 26, 2021).
- WHO (2021b). Immunization, Vaccines, Biologicals. Progresses and Challenges with Sustaining and Advancing Immunization Coverage During the COVID-19 Pandemic 2021. https://www.who.int/publications/i/item/progresses-and-challenges-with-sustaining-and-advancing-immunization-coverage-during-the-covid-19-pandemic (accessed March 20, 2022).
- WHO (2021c). Health Systems Resilience during COVID-19: Lessons for building back better. November 2021. https://eurohealthobservatory.who.int/publications/i/health-systems-resilience-during-covid-19-lessons-for-building-back-better (accessed March 20, 2022). [PubMed]