Abstract
Background
Pre‐eclampsia, a multisystem disorder of pregnancy characterised by high blood pressure and protein in the urine, is associated with endothelial dysfunction. Nitric oxide mediates many functions of the endothelium, including vasodilatation and inhibition of platelet aggregation. Pre‐eclampsia may be associated with nitric oxide deficiency, but the evidence to support this suggestion is contradictory. Nevertheless, it has been hypothesised that agents which increase nitric oxide may prevent pre‐eclampsia.
Objectives
To assess the effectiveness and safety of nitric oxide donors and precursors for preventing pre‐eclampsia and its complications.
Search methods
We searched the Cochrane Pregnancy and Childbirth Group's Trials Register (November 2006), CENTRAL (The Cochrane Library 2006, Issue 3), and EMBASE (2002 to December 2004). We updated the search of the Pregnancy and Childbirth Group's Trials Register on 18 January 2010 and added the results to the awaiting classification section.
Selection criteria
Studies were included if they were randomised trials evaluating nitric oxide donors or precursors for preventing pre‐eclampsia and its complications.
Data collection and analysis
Both review authors independently assessed studies for inclusion. Data were extracted and double checked for accuracy.
Main results
Six trials (310 women) were included. Four were of good quality and two were of uncertain quality. Four trials (170 women) compared nitric oxide donors (glyceryl trinitrate) or precursors (L‐arginine) with either placebo or no intervention. There are insufficient data for reliable conclusions about the effects on pre‐eclampsia (four trials, 170 women; relative risk (RR) 0.83, 95% confidence interval (CI) 0.49 to 1.41) or its complications. One trial (36 women) compared a nitric oxide donor with nifedipine, and another (76 women) compared it with antiplatelet agents. Both were too small for reliable conclusions about possible differential effects.
Glyceryl trinitrate was associated with an increased risk of headache (two trials, 56 women; RR 6.85, 95% CI 1.42 to 33.04), and of stopping treatment (two trials, 56 women; RR 4.02, 95% CI 1.15 to 14.09) compared to placebo. However, the increase for both outcomes was due to an extreme result in one small trial (7/7 versus 0/9 for both outcomes).
Authors' conclusions
There is insufficient evidence to draw reliable conclusions about whether nitric oxide donors and precursors prevent pre‐eclampsia or its complications.
[Note: The 13 citations in the awaiting classification section of the review may alter the conclusions of the review once assessed.]
Plain language summary
Nitric oxide for preventing pre‐eclampsia and its complications
Not enough evidence to say if nitric oxide donors or their precursors are helpful in preventing pre‐eclampsia and its complications.
Pre‐eclampsia is a serious complication of pregnancy occurring in about 10% of women. It is identified by increased blood pressure and protein in the urine, but women often suffer no symptoms initially. It can, through constriction of the blood vessels in the placenta, interfere with food and oxygen passing to the baby, thus inhibiting the baby's growth and causing the baby to be born too soon. Women can be affected by huge swelling and sometimes they go on to have fits. Nitric oxide drugs, like glycerol trinitrate, or their precursors, like L‐arginine, may play a role in helping to prevent pre‐eclampsia through their action in relaxing blood vessel walls. The review of trials found too few women had been studied, so it was not possible to say if nitric oxide drugs help prevent pre‐eclampsia. However, these drugs did cause headaches, often sufficiently severe for women to stop taking the drugs. Future studies need to address these adverse effects and seek women's views.
Background
Hypertension (high blood pressure) is common during pregnancy. Around 10% of women will have raised blood pressure at some point before delivery. The hypertensive disorders of pregnancy comprise a spectrum of conditions that is usually classified into four categories: (i) gestational hypertension, a rise in blood pressure during the second half of pregnancy; (ii) pre‐eclampsia, usually hypertension with proteinuria (protein in urine) during the second half of pregnancy; (iii) chronic hypertension, a rise in blood pressure prior to pregnancy or before 20 weeks' gestation, and (iv) pre‐eclampsia superimposed on chronic hypertension (NHBPEP2000). For women with uncomplicated mild to moderate hypertension, pregnancy outcome is similar to that for women with normal blood pressure. Outcome deteriorates once pre‐eclampsia develops or if the blood pressure is very high. Pre‐eclampsia is a multisystem disorder, involving the liver, kidneys, brain, and placenta. It affects 2% to 8% of pregnancies (WHO 1988), and is associated with a substantive increase in morbidity and mortality for both the woman and her baby (DH 2002). Complications for the mother may include eclampsia (seizures), stroke, liver or kidney failure, and abnormal blood clotting, and problems for the baby may include poor growth and preterm birth.
The cause of pre‐eclampsia is uncertain, but current belief is that reduced blood supply to the placenta leads to abnormal function of endothelial cells, possibly as a result of oxidative stress. Endothelial dysfunction results in generalised vasoconstriction, platelet activation and thrombosis, and decreased plasma volume, with subsequently reduced blood supply to multiple organs. Pre‐eclampsia is discussed in more detail in the generic protocol for this review (Generic Protocol 2005).
In 1987, nitric oxide was first identified as an endothelium derived factor associated with vascular relaxation (Ignarro 1987; Palmer 1987). Although it is now known to have a wide range of biological functions, interest in its possible role in pre‐eclampsia has been generated largely because nitric oxide mediates many functions of the endothelium, including vasodilatation and inhibition of platelet aggregation. Nitric oxide is believed to contribute, at least in part, to the physiological vascular adaptations of normal pregnancy (Sladek 1997). Therefore, reduced availability of nitric oxide may have a role in the pathophysiology of pre‐eclampsia. Although accumulating evidence over the last decade has yielded contradictory results, this hypothesis has raised the possibility that therapeutic agents that increase nitric oxide, or nitric oxide donors and precursors, may prevent or treat pre‐eclampsia.
Technical background
Nitric oxide is produced in the endothelial cells lining blood vessels, from the amino acid L‐arginine, by the action of a group of enzymes called nitric oxide synthases (NOS). It then diffuses into adjacent vascular smooth muscle cells and increases the second messenger cyclic guanosine monophosphate (cGMP), resulting in relaxation of vascular smooth muscle. Nitric oxide promotes vasodilatation (Moncada 1991) and reduces the effect of vasoconstrictors (Myatt 1992). It also inhibits platelet aggregation and adhesion to vascular endothelial surfaces, limits thrombus formation (Radomski 1987), modifies the expression of inflammatory cytokines, and inhibits interactions between immune and endothelial cells (De Caterina 1995; Kubes 1991). The balance between pro‐oxidant and antioxidant forces in endothelial cells may be influenced by nitric oxide, and reduced availability may contribute to development of oxidative stress.
During normal pregnancy, nitric oxide appears to play a role in physiological vasodilatation, decreased responsiveness to vasopressors, and increased uteroplacental blood flow (Sladek 1997). However, data from human studies are conflicting, and it is unclear whether these changes result from an overall increase in nitric oxide production, only a local increase in certain tissues, or increased responsiveness of vascular smooth muscle to normal levels of nitric oxide (Sladek 1997). Similarly, in pre‐eclampsia, although a deficiency of nitric oxide may explain some of its characteristic features, evidence for the role of nitric oxide is contradictory. If the availability of nitric oxide is indeed reduced, it is also unclear whether this is due to increased degradation of nitric oxide or reduced production. Increased degradation of nitric oxide may result from the oxidative stress which occurs in pre‐eclampsia (Hubel 1997; Lowe 2000). Nitric oxide production may be reduced if there is a deficiency of L‐arginine or an increase in NOS enzyme inhibitors. Some investigators have found evidence of decreased nitric oxide production in the serum and urine of women with pre‐eclampsia (Davidge 1996; Seligman 1994), but others have failed to demonstrate such reductions (Conrad 1999; Norris 1999; Ranta 1999; Silver 1996). Reduced serum levels of L‐arginine have been demonstrated but were mirrored by increased nitric oxide production, so are likely to represent increased consumption of L‐arginine (Benedetto 2000). There is evidence that NOS activity within platelets is reduced (Delacretaz 1995; Neri 2000a), and that the normal inhibitor of NOS enzyme, asymmetric dimethylarginine, is increased in women with pre‐eclampsia (Fickling 1993).
In animal studies, chronic inhibition of nitric oxide synthesis produces a clinical picture similar to pre‐eclampsia, with the development of hypertension, proteinuria, thrombocytopenia, reduced plasma volume and intrauterine growth restriction in pregnant rats (Molnar 1994). In humans, in vitro inhibition of nitric oxide synthesis in blood vessels causes vasoconstriction, but less so in blood vessels from women with pre‐eclampsia (Cockell 1997), suggesting that there may already be a basal deficiency of nitric oxide in pre‐eclampsia.
Decreased L‐arginine concentrations (Noris 2004) and reduced NOS activity (Morris 1995) have been found in the placenta of women with pre‐eclampsia, and in vitro stimulation of NOS in umbilical vessels from women with pre‐eclampsia results in less production of nitric oxide compared to normal pregnancy (Pinto 1991). However, others have found increased placental expression of the NOS enzyme (Di Iorio 1998; Shaamash 2001) and increased levels of nitric oxide metabolites in umbilical vessels (Lyall 1995; Norris 1999) and amniotic fluid of women with pre‐eclampsia (Di Iorio 1998). A possible explanation for this apparent paradox is that the increase in nitric oxide production in specific tissues may be a compensatory response to pre‐eclampsia, rather than contributing to its aetiology (Shaamash 2001).
Uterine artery resistance may be increased in pre‐eclampsia, compromising blood flow to the placenta. Some studies have demonstrated that administration of nitric oxide donors is associated with a reduction in uterine artery resistance in women with pre‐eclampsia (Lees 1996; Thaler 1999). Although this observation has not been confirmed in all reports (Grunewald 1995; Makino 1997), it has led some to suggest that nitric oxide may have a role in prevention and treatment of pre‐eclampsia. Others have highlighted concerns that such dilatation of the uterine vessels may not be appropriate in all cases of pre‐eclampsia, and may theoretically result in a relative underfill of the uterine circulation, further compromising blood supply to the placenta (Poston 1998).
Nitric oxide agents
Drugs that can be converted by the body into nitric oxide (known as nitric oxide donors) are widely available, and have been used for years as therapeutic agents in cardiovascular diseases such as angina and hypertension. Commonly used nitric oxide donors include glyceryl trinitrate, isosorbide mononitrate, isosorbide dinitrate, S‐nitroglutathione, and sodium nitroprusside. They may be taken in a range of ways such as oral or sublingual tablets, aerosol spray under the tongue, skin patches, or by intravenous injection. Tolerance can develop quickly, with reduced therapeutic effect, but is minimised by carefully timed, intermittent administration. Common side‐effects include headache, flushing, postural hypotension, and local irritation with patches. There are no known risks associated with the use of nitric oxide donors in pregnancy. A potential risk, associated with all vasodilators, is hypotension with subsequent reduction in the blood supply to the fetus and placenta.
L‐arginine is an amino acid essential for the body's production of nitric oxide (known as a nitric oxide precursor). It is also commercially available, and may be given orally as tablets, or solution, or by intravenous injections. Known side‐effects include diarrhoea. Although L‐arginine has not been associated with any adverse effects on the fetus in the few studies which have evaluated it, there are limited data on safety during pregnancy.
Another group of compounds known as phosphodiesterase inhibitors (PDE5‐selective inhibitors) also enhance the effects of nitric oxide. These drugs act by blocking enzymes which break down the molecule that mediates the effects of nitric oxide in the body, or cGMP. High concentrations of cGMP in smooth muscle cells lead to vascular relaxation. These drugs include sildenafil, tadalafil, and vardenafil, and have been used to treat male sexual impotence and pulmonary hypertension. Although no teratogenic effects have been observed in animal studies, there are currently few data on safety in human pregnancy.
The use of nitric oxide for prevention of pre‐eclampsia appears attractive. However, before nitric oxide agents can be recommended for use in clinical practice, reliable evidence is required on whether they are both effective and safe.
Objectives
To determine the effectiveness and safety of nitric oxide for preventing pre‐eclampsia and its complications.
Methods
Criteria for considering studies for this review
Types of studies
Randomised controlled trials were included. Trials with a quasi‐random design were excluded.
Types of participants
Pregnant women were included, regardless of gestation at trial entry. Whenever possible and relevant, women were grouped on the basis of their risk at trial entry as follows.
(1) Women with normal blood pressure
(a) High risk: defined as having one or more of the following: diabetes, renal disease, thrombophilia, autoimmune disease, previous severe or early onset pre‐eclampsia, or multiple pregnancy. (b) Moderate risk: defined as none of the above, but having either previous pre‐eclampsia that was not severe or early onset (or severity unspecified), or a first pregnancy and at least one of the following: teenager or over 35 years age, family history of pre‐eclampsia, obesity (body mass index of 30 or more), increased sensitivity to Angiotensin II, positive roll‐over test, abnormal uterine artery doppler scan. (c) Low risk: defined as pregnancy that does not qualify as either high or moderate risk. (d) Undefined risk: when the risk is unclear or not specified.
(2) Women with high blood pressure, without proteinuria
These women are all at high risk of developing pre‐eclampsia. They fall into two groups. (a) Gestational hypertension: hypertension detected for the first time after 20 weeks' gestation, in the absence of proteinuria. (b) Chronic hypertension: essential or secondary hypertension detected prior to pregnancy or before 20 weeks' gestation. Some women with chronic hypertension may have longstanding proteinuria due to their underlying disease. We planned to include these women as their proteinuria is not due to pre‐eclampsia.
If a trial included women with pre‐eclampsia as well as those with non‐proteinuric hypertension (gestational or chronic), where possible, we included only the women with non‐proteinuric hypertension in the review. For trials that did not report results separately for the two categories, we had planned to include them but present them as a separate subgroup. We found one such trial (Neri 1999), but because the number of women with pre‐eclampsia was small, we have included it in the main analysis and not presented it as subgroup.
Women were excluded if they had established pre‐eclampsia.
Types of interventions
Studies were included if they were comparisons of any nitric oxide agent with any of the following: (i) placebo or no intervention; (ii) another nitric oxide donor or precursor; (iii) any other intervention for prevention of pre‐eclampsia.
All types of agents enhancing the effects of nitric oxide were included, as were different dosage regimens and routes of administration.
Trials were excluded if the intended duration of therapy at trial entry was less than seven days. This cut off was taken so as to exclude studies evaluating only the short‐term physiological effects of nitric oxide, and because it is unlikely that such a brief intervention could influence substantive pregnancy outcomes.
Types of outcome measures
A range of outcomes for the woman and child were included. These are listed below, with their definitions. Trials that used acceptable variations of these definitions, or that did not define their outcomes were also included. The definitions used in each study, where available, are described in the table of 'Characteristics of included studies'.
For the woman
Main outcome
(1) Pre‐eclampsia: defined as hypertension (blood pressure at least 140 mmHg systolic or 90 mmHg diastolic) with proteinuria (at least 300 mg protein in a 24 hour urine collection or 30 mg/dL in a single sample or 1+ on dipstick or at least 30 mg/mmol urine protein/creatinine ratio). For a woman with chronic hypertension at trial entry, pre‐eclampsia is defined as new onset proteinuria or sudden worsening of proteinuria or hypertension, or both, or other signs and symptoms of pre‐eclampsia after 20 weeks' gestation.
Other outcomes
(2) Death: during pregnancy or up to 42 days after end of pregnancy. (3) Severe morbidity: including eclampsia, liver or renal failure, HELLP (haemolysis, elevated liver enzymes and low platelets) syndrome, disseminated intravascular coagulation, stroke and pulmonary oedema. These outcomes will be reported individually, and as a composite measure where the information is available. (4) Severe pre‐eclampsia: pre‐eclampsia with two or more signs or symptoms of severe disease, such as severe hypertension, severe proteinuria (usually 3 g/24 hour, or 3+ on dipstick), visual disturbances, exaggerated tendon reflexes, upper abdominal pain, pulmonary oedema, impaired liver function tests, high serum creatinine, low platelets, fetal growth restriction, or reduced liquor volume. (5) Early onset of pre‐eclampsia: pre‐eclampsia at or before 33 completed weeks. (6) Severe hypertension: blood pressure at least 160 mmHg systolic or 110 mmHg diastolic. (7) Gestational hypertension: hypertension after 20 weeks' gestation. (8) Use of antihypertensive drugs or need for additional antihypertensive drugs. (9) Abruption of the placenta or antepartum haemorrhage. (10) Elective delivery: induction of labour or caesarean section. (11) Caesarean section: emergency and elective. (12) Postpartum haemorrhage: blood loss of 500 ml or more. (13) Side‐effects: any side‐effects including headache, rashes, flushing, postural hypotension, and tachycardia, nitric oxide donors/precursors stopped due to side‐effects. (14) Use of health service resources: antenatal clinic visit, visit to day care unit, antenatal hospital admission, length of hospital stay, intensive care (admission to intensive care unit, ventilation, dialysis). (15) Women's experiences and views on nitric oxide donors or precursors in relation to pregnancy and childbirth.
For the child
Main outcomes
(1) Death: including all deaths before birth and up to discharge from hospital. (2) Preterm birth: defined as birth at or before 37 completed weeks' gestation. (3) Small‐for‐gestational age: defined as growth below the third centile, or lowest centile reported.
Other outcomes
(4) Death, classified by timing of death: miscarriage (fetal loss at or before 19 completed weeks' gestation or however defined in the study), stillbirth (death in utero at or after 20 weeks' gestation), perinatal death (stillbirth or death in the first seven days of life), neonatal death (death in the first 28 days after birth), infant death (death in the first year of life). (5) Severity of preterm birth: very preterm birth (at or before 33 completed weeks) and extremely preterm birth (at or before 27 completed weeks). (6) Apgar score at five minutes: low (less than seven) and very low (less than four) or lowest reported. (7) Endotracheal intubation or use of mechanical ventilation. (8) Neonatal morbidity: respiratory distress syndrome, chronic lung disease, sepsis, necrotizing enterocolitis, retinopathy of prematurity, and intraventricular haemorrhage. (9) Long‐term growth and development: blindness, deafness, seizures, poor growth, neurodevelopmental delay and cerebral palsy. (10) Side‐effects associated with nitric oxide donors or precursors. (11) Use of hospital resources: admission to neonatal intensive care unit, duration of hospital stay after birth.
Economic outcomes
Costs to health service resources: short term and long term for both mother and baby.
Costs to the woman, her family, and society associated with the intervention.
Search methods for identification of studies
Electronic searches
We searched the Cochrane Pregnancy and Childbirth Group's Trials Register (November 2006). We updated this on 18 January 2010 and added the results to Studies awaiting classification.
The Cochrane Pregnancy and Childbirth Group’s Trials Register is maintained by the Trials Search Co‐ordinator and contains trials identified from:
quarterly searches of the Cochrane Central Register of Controlled Trials (CENTRAL);
weekly searches of MEDLINE;
handsearches of 30 journals and the proceedings of major conferences;
weekly current awareness alerts for a further 44 journals plus monthly BioMed Central email alerts.
Details of the search strategies for CENTRAL and MEDLINE, the list of handsearched journals and conference proceedings, and the list of journals reviewed via the current awareness service can be found in the ‘Specialized Register’ section within the editorial information about the Cochrane Pregnancy and Childbirth Group.
Trials identified through the searching activities described above are each assigned to a review topic (or topics). The Trials Search Co‐ordinator searches the register for each review using the topic list rather than keywords.
In addition, we searched CENTRAL (The Cochrane Library 2006, Issue 3) and EMBASE (2002 to December 2004) by combining the list of terms in Appendix 1 with the CENTRAL and EMBASE search strategy listed in the generic protocol (Generic Protocol 2005):
We did not apply any language restrictions.
Data collection and analysis
Selection of studies
Both review authors independently assessed potentially eligible studies for inclusion. We resolved any differences in opinion by discussion.
Seeking unpublished data
We contacted authors for additional information about the method of allocation to treatment group, and to seek information about important outcomes that had not been reported, whenever relevant and possible.
Assessment of study quality
Both review authors independently assessed the quality of each trial using the criteria outlined in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2005). Methods used for generation of the randomisation sequence are described for each trial, where possible. Each study was assessed for quality of concealment of allocation, completeness of follow up, and blinding.
(1) Allocation concealment
A quality score for concealment of allocation was assigned to each trial, using the following criteria: (A) adequate concealment of allocation, such as telephone randomisation and consecutively‐numbered, sealed, opaque envelopes; (B) unclear whether concealment of allocation was adequate; (C) inadequate concealment of allocation such as random‐number tables, sealed envelopes that are not numbered or opaque.
(2) Completeness of follow up
Completeness of follow up was assessed using the following criteria: (A) less than 5% of participants excluded from analysis; (B) 5% to 10% of participants excluded from analysis; (C) more than 10% and up to and including 20% of participants excluded from analysis.
Studies were excluded if:
more than 20% of participants were excluded from analysis;
more than 10% of participants were not analysed in their randomised groups and it was not possible to restore participants to the correct group;
there was more than 10% difference in loss of participants between groups.
Data were analysed based on the group to which the participants were randomised, regardless of whether they received the allocated intervention or not. Where data were missing, we sought clarification from the authors.
(3) Blinding
Blinding was assessed using the following criteria:
blinding of participants (yes/no/unclear or unspecified);
blinding of caregiver (yes/no/unclear or unspecified);
blinding of outcome assessment (yes/no/unclear or unspecified).
Data extraction and data entry
Both review authors independently extracted data. Data were entered onto the Review Manager software (RevMan 2003), and double checked for accuracy.
Statistical analyses
Statistical analyses was carried out using Review Manager (RevMan 2003). Results are presented as summary relative risk with 95% confidence intervals. The I2 statistic was used to assess heterogeneity between trials. In the absence of significant heterogeneity, results were pooled using a fixed‐effect model. If substantial heterogeneity is detected in future updates (I2 more than 50%), possible causes will be explored and subgroup analyses for the main outcomes will be performed. Heterogeneity that is not explained by subgroup analyses may be modelled using random‐effects analysis, where appropriate.
Sensitivity analyses
Our prespecified sensitivity analysis was to explore the effects of trial quality based on concealment of allocation, by excluding studies with clearly inadequate allocation of concealment (rated C). This analysis will be performed in future updates when sufficient data become available.
Subgroup analyses
The prespecified subgroups were based on whether:
gestation at randomisation was 20 weeks or less, rather than over 20 weeks;
maternal risk of pre‐eclampsia at trial entry was high risk, rather than moderate risk, low risk, or undefined risk;
the intervention was a nitric oxide donor or a nitric oxide precursor or a compound enhancing the effects of nitric oxide.
Only the main outcomes listed above will be included in the subgroup analyses. These subgroup analyses were not conducted as there are insufficient data. Although data are presented separately for nitric oxide donors and precursors, this was done for clarity, and not as part of a subgroup analyses. Subgroup analyses will be included in future updates, once more data are available.
Results
Description of studies
Details of each study can be found in the tables of Characteristics of included studies and Characteristics of excluded studies.
Six small trials, involving a total of 310 women, were included in the review. (Thirteen reports from an updated search in January 2010 have been added to Studies awaiting classification.)
Setting
Three trials were conducted in a single centre and three had two centres. One study was from Australia, the remainder were from Europe.
Participants
All women were at moderate to high risk of developing pre‐eclampsia. Most women had hypertension at trial entry. In one trial, all the women had gestational hypertension and in another, they had chronic hypertension. Two trials included women with either gestational hypertension or pre‐eclampsia. Another trial included women with chronic hypertension as well as those with normal blood pressure but a history of previous early onset pre‐eclampsia or fetal growth restriction. The sixth study included only normotensive women with abnormal doppler scans.
Of the two trials that included women with pre‐eclampsia at trial entry, for one, the authors provided unpublished data for women with non‐proteinuric hypertension for all outcomes except caesarean section (Facchinetti 2002). For the other, data were not available separately for women with non‐proteinuric hypertension and so data for all women were included, as discussed above (Neri 1999).
Gestation at randomisation was less than 20 weeks in two trials, more than 24 weeks in three trials, and not reported in one trial.
Interventions
Five trials compared glyceryl trinitrate transdermal patches (5 to 20 mg/day), either with placebo (two trials), no intervention (one trial), antiplatelet agents (one trial) or nifedipine (one trial). In four of these studies the glyceryl trinitrate patches were applied for between 12 and 16 hours each day. The fifth study had three arms. Glyceryl trinitrate patches were applied for 16 hours in one group, and for 24 hours in another. Women in the control group received nifedipine. Data for the two glyceryl trinitrate arms are combined in the analyses presented here.
One trial compared the nitric oxide precursor L‐Arginine with placebo (Facchinetti 2002).
Outcomes
Definitions used in trials were similar to those specified for this review.
Excluded studies
Seventeen studies were excluded. Two were single‐dose studies (Makino 1997; Thaler 1999). Six had an intended duration of treatment between one to five days (among these, three included women with pre‐eclampsia at trial entry and four did not report relevant clinical outcomes) (Amit 1998; Decano 2000; Neri 2004a; Neri 2004b; Reyna‐Villasmil 2001; Staff 2004). Five other studies included women with established pre‐eclampsia (Hladunewich 2006; Neri 2000b; Ruggenenti 2006; Rytewski 2005; Rytlewski 2006). For one study, it was unclear whether it was randomised (Madhubala 2006) and three did not report any relevant clinical outcomes (Ledingham 1999; Scarpellini 2001; Valensise 2005).
Risk of bias in included studies
Overall, four studies were of good quality, and two were of uncertain quality.
Allocation concealment
Methods used for allocation concealment were adequate in four studies, and not reported in the other two studies.
Follow up
Follow up was complete in two studies, and not reported in two. The remaining two studies excluded six women each from the analysis. In one study, 8% of the women were excluded from analysis for various reasons but it is not clear how many were lost in each arm. In the other study, 17% of women were excluded (four from the glyceryl trinitrate arm and two from the nifedipine arm). Two women from each arm dropped out of the study due to severe pre‐eclampsia and growth restriction requiring delivery, and two from the glyceryl trinitrate arm dropped out because of severe headaches.
Blinding
Participants and caregivers were blinded to the intervention in two studies. In another study only the caregiver was blinded and in a fourth, only the outcome assessor for blood pressure was blinded. Blinding was not discussed for two studies.
Effects of interventions
Comparison one: nitric oxide donors or precursors versus placebo or no intervention
Four trials (170 women) compared nitric oxide donors (glycerol trinitrate) or precursors (L‐Arginine) with control (either placebo or no nitric oxide donor or precursor). Overall, there are insufficient data for reliable conclusions about the effects on pre‐eclampsia (four trials, 170 women; relative risk (RR) 0.83, 95% confidence interval (CI) 0.49 to 1.41), perinatal death (three trials 114 women; RR 0.25, 95% CI 0.03 to 2.34), preterm birth (three trials 154 women; RR 0.48, 95% CI 0.21 to 1.07) or having a small‐for‐gestational‐age baby (two trials 108 women; RR 0.78, 95% CI 0.36 to 1.70).
Glyceryl trinitrate was associated with an increased relative risk of headache compared to placebo (two trials, 56 women; RR 6.85, 95% CI 1.42 to 33.04). Both glyceryl trinitrate and placebo were given as skin patches, and there was no clear difference between the groups in the risk of skin rashes (two trials 56 women; RR 0.68, 95% CI 0.22 to 2.07). Women allocated glyceryl trinitrate were more likely to stop treatment than those allocated placebo (two trials, 56 women; RR 4.02, 95% CI 1.15 to 14.09). This was due to headaches for women allocated glyceryl trinitrate in one small trial (Davis 2001). In the other trial (Lees 1998) equal numbers of women in the two groups stopped treatment due to skin rashes.
The increase in the relative risk of headaches, and stopping treatment, was due to an extreme result in one small study (Davis 2001) (7/7 versus 0/9 for both outcomes). There were no clear differences between the two groups for any other outcome. There was no statistically significant heterogeneity between the trials.
Comparison two: nitric oxide donors versus nifedipine
One trial (36 women) compared nitric oxide donors with nifedipine. The confidence intervals for all outcomes reported were wide, and crossed the line of no effect. There are insufficient data for any reliable conclusions about the differential effects of these agents.
Comparison three: nitric oxide donors versus antiplatelets
One trial (76 women) compared nitric oxide donors with antiplatelets. The confidence intervals for all outcomes reported were wide, and crossed the line of no effect. There are insufficient data for any reliable conclusions about the differential effects of these agents.
Discussion
The aim of this review was to assess the effectiveness and safety of nitric oxide for preventing pre‐eclampsia and its complications. Four small trials have evaluated the effects of using either a nitric oxide donor or a precursor compared with placebo or no intervention in women at increased risk of pre‐eclampsia. Three of these trials were good quality and one was poorly described. There are insufficient data for any reliable conclusions about the effectiveness of these agents. However, the use of glyceryl trinitrate does appear to be associated with an increased risk of headache, and this was sufficiently severe to lead to stopping of treatment in some cases. The use of nitric oxide precursor L‐arginine in one small trial was associated with a reduction in the risk of pre‐eclampsia that was borderline for statistical significance. Although promising, this result should be interpreted with caution as the numbers are so small, and the confidence intervals suggest that the true effect may be anything from a 91% reduction in the risk of pre‐eclampsia to a 2% increase associated with L‐arginine.
Two small trials have compared glyceryl trinitrate with other interventions. In one study the comparison was with nifedipine, in the other it was with antiplatelets. The nifedipine trial was of good quality whereas the antiplatelet trial was of uncertain quality. In both these studies, the confidence intervals for all outcomes reported were wide and crossed the line of no effect. There is therefore insufficient evidence for any reliable conclusions.
Glyceryl trinitrate during pregnancy may be associated with headaches severe enough to stop treatment. Although this trend towards increased risk of headache is consistent across the comparisons, it only achieves statistical significance in the comparison with placebo where it is heavily dependant on the results from one small study (Davis 2001). In this study all seven women allocated glyceryl trinitrate had headaches, and none of those were allocated placebo (Davis 2001). This small study is also unusual in that the risk of headache in the treatment group is considerably higher than in the other studies. Hence, the true impact on the incidence and severity of headaches remains unclear. In addition, although skin patches are theoretically attractive for administration of glyceryl trinitrate during pregnancy, the risk of skin rashes for both the active and placebo patches appears to be around 10%. Strategies to minimise potential side‐effects, such as using a lower dose or different route of administration, or alternative agents that have similar potential benefits but with fewer side‐effects, should be explored. The nitric oxide precursor L‐arginine appears to be a promising alternative, although its use was associated with diarrhoea in some women.
Authors' conclusions
Implications for practice.
Until there is clear evidence that nitric oxide donors and precursors are both effective and safe for prevention of pre‐eclampsia and its complications, they should not be used in clinical practice.
Implications for research.
Further large randomised trials are needed to assess whether nitric oxide reduces the risk of pre‐eclampsia and its complications. In view of the potential association between glyceryl trinitrate and headaches, the side‐effect profile during pregnancy should be further evaluated. This might include using a lower dose or different modes of administration. Alternative agents, such as L‐arginine, also merit further assessment of their role in preventing pre‐eclampsia.
[Note: The 13 citations in the awaiting classification section of the review may alter the conclusions of the review once assessed.]
What's new
| Date | Event | Description |
|---|---|---|
| 18 January 2010 | Amended | Search updated. Thirteen reports added to Studies awaiting classification. |
History
Protocol first published: Issue 2, 2005 Review first published: Issue 2, 2007
| Date | Event | Description |
|---|---|---|
| 20 September 2008 | Amended | Converted to new review format and edited contact details. |
Acknowledgements
Our thanks to Milli Anim‐Somuah for translating a Russian published report, and to Professor Fabio Fachinnetti for providing unpublished data for the three Italian trials, and for his helpful comments on the background.
As part of the pre‐publication editorial process, this review has been commented on by three peers (an editor and two referees who are external to the editorial team), one or more members of the Pregnancy and Childbirth Group's international panel of consumers and the Group's Statistical Adviser.
Appendices
Appendix 1. Search strategy
NITRIC OXIDE (MeSH)
nitric next oxide
NITRIC OXIDE DONORS (MeSH)
nitric next oxide next donors
NITROGLYCERIN (MeSH)
nitroglycerin
glyceryl next trinitrate
GTN
isosorbide dinitrate (MeSH)
isorbide next dinitrate
sodium next nitroprusside
nitrosoglutathione
ARGININE (MeSH)
l‐arginine
#1 or # 2 or #3 or #4 or #5 or #6 or #7 or #8
#9 or #10 or #11 or #12 or #13 or #14
#15 or #16
Data and analyses
Comparison 1. Nitric oxide versus placebo/no intervention.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Pre‐eclampsia | 4 | 170 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.83 [0.49, 1.41] |
| 1.1 Nitric oxide donors | 3 | 124 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.12 [0.61, 2.08] |
| 1.2 Nitric oxide precursors | 1 | 46 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.30 [0.09, 1.02] |
| 2 Severe pre‐eclampsia | 1 | 46 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.10 [0.01, 1.87] |
| 2.1 Nitric oxide donors | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 2.2 Nitric oxide precursors | 1 | 46 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.10 [0.01, 1.87] |
| 3 HELLP syndrome | 1 | 40 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.30 [0.01, 7.02] |
| 3.1 Nitric oxide donors | 1 | 40 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.30 [0.01, 7.02] |
| 3.2 Nitric oxide precursors | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 4 Placental abruption | 2 | 108 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.85 [0.12, 6.04] |
| 4.1 Nitric oxide donors | 2 | 108 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.85 [0.12, 6.04] |
| 4.2 Nitric oxide precursors | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 5 Caesarean section | 1 | 74 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.21 [0.79, 1.87] |
| 5.1 Nitric oxide donors | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 5.2 Nitric oxide precursors | 1 | 74 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.21 [0.79, 1.87] |
| 6 Maternal side‐effects (subgroup by type of side‐effect) | 3 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 6.1 Headache | 2 | 56 | Risk Ratio (M‐H, Fixed, 95% CI) | 6.85 [1.42, 33.04] |
| 6.2 Skin rash | 2 | 56 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.68 [0.22, 2.07] |
| 6.3 Diarrhoea | 1 | 46 | Risk Ratio (M‐H, Fixed, 95% CI) | 5.0 [0.27, 91.52] |
| 7 Stopped treatment due to side‐effects | 2 | 56 | Risk Ratio (M‐H, Fixed, 95% CI) | 4.02 [1.15, 14.09] |
| 7.1 Nitric oxide donors | 2 | 56 | Risk Ratio (M‐H, Fixed, 95% CI) | 4.02 [1.15, 14.09] |
| 7.2 Nitric oxide precursors | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 8 Perinatal or neonatal death | 2 | 114 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.25 [0.03, 2.34] |
| 8.1 Nitric oxide donors | 1 | 68 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.26 [0.01, 6.28] |
| 8.2 Nitric oxide precursors | 1 | 46 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.24 [0.01, 5.55] |
| 9 Preterm birth | 3 | 154 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.48 [0.21, 1.07] |
| 9.1 Nitric oxide donors | 2 | 108 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.38 [0.11, 1.34] |
| 9.2 Nitric oxide precursors | 1 | 46 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.59 [0.21, 1.65] |
| 10 Small‐for‐gestational age | 2 | 108 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.78 [0.36, 1.70] |
| 10.1 Nitric oxide donors | 2 | 108 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.78 [0.36, 1.70] |
| 10.2 Nitric oxide precursors | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 11 Low Apgar score | 1 | 68 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.79 [0.05, 12.11] |
| 11.1 Nitric oxide donors | 1 | 68 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.79 [0.05, 12.11] |
| 11.2 Nitric oxide precursors | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 12 Admission to neonatal intensive care unit | 1 | 68 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.05 [0.25, 4.35] |
| 12.1 Nitric oxide donors | 1 | 68 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.05 [0.25, 4.35] |
| 12.2 Nitric oxide precursors | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 13 Respiratory distress syndrome | 1 | 68 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.79 [0.12, 5.28] |
| 13.1 Nitric oxide donors | 1 | 68 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.79 [0.12, 5.28] |
| 13.2 Nitric oxide precursors | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 14 Intraventricular haemorrhage | 1 | 68 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.26 [0.01, 6.28] |
| 14.1 Nitric oxide donors | 1 | 68 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.26 [0.01, 6.28] |
| 14.2 Nitric oxide precursors | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
1.1. Analysis.

Comparison 1 Nitric oxide versus placebo/no intervention, Outcome 1 Pre‐eclampsia.
1.2. Analysis.
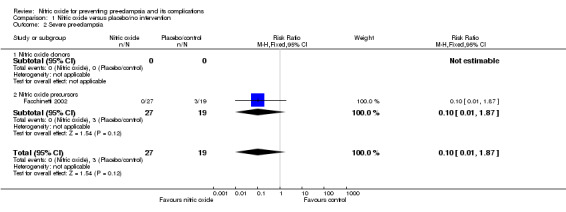
Comparison 1 Nitric oxide versus placebo/no intervention, Outcome 2 Severe pre‐eclampsia.
1.3. Analysis.

Comparison 1 Nitric oxide versus placebo/no intervention, Outcome 3 HELLP syndrome.
1.4. Analysis.

Comparison 1 Nitric oxide versus placebo/no intervention, Outcome 4 Placental abruption.
1.5. Analysis.

Comparison 1 Nitric oxide versus placebo/no intervention, Outcome 5 Caesarean section.
1.6. Analysis.

Comparison 1 Nitric oxide versus placebo/no intervention, Outcome 6 Maternal side‐effects (subgroup by type of side‐effect).
1.7. Analysis.

Comparison 1 Nitric oxide versus placebo/no intervention, Outcome 7 Stopped treatment due to side‐effects.
1.8. Analysis.

Comparison 1 Nitric oxide versus placebo/no intervention, Outcome 8 Perinatal or neonatal death.
1.9. Analysis.

Comparison 1 Nitric oxide versus placebo/no intervention, Outcome 9 Preterm birth.
1.10. Analysis.

Comparison 1 Nitric oxide versus placebo/no intervention, Outcome 10 Small‐for‐gestational age.
1.11. Analysis.

Comparison 1 Nitric oxide versus placebo/no intervention, Outcome 11 Low Apgar score.
1.12. Analysis.

Comparison 1 Nitric oxide versus placebo/no intervention, Outcome 12 Admission to neonatal intensive care unit.
1.13. Analysis.

Comparison 1 Nitric oxide versus placebo/no intervention, Outcome 13 Respiratory distress syndrome.
1.14. Analysis.

Comparison 1 Nitric oxide versus placebo/no intervention, Outcome 14 Intraventricular haemorrhage.
Comparison 2. Nitric oxide versus nifedipine.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Pre‐eclampsia | 1 | 36 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.0 [0.10, 9.96] |
| 2 Severe hypertension | 1 | 36 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.56 [0.07, 35.67] |
| 3 Caesarean section | 1 | 36 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.67 [0.40, 1.12] |
| 4 Maternal side‐effects | 1 | 36 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.6 [0.13, 50.25] |
| 4.1 Headache | 1 | 36 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.6 [0.13, 50.25] |
| 5 Perinatal death | 1 | 36 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 6 Preterm birth | 1 | 36 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.63 [0.20, 1.91] |
| 7 Small‐for‐gestational age | 1 | 36 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.0 [0.10, 9.96] |
2.1. Analysis.

Comparison 2 Nitric oxide versus nifedipine, Outcome 1 Pre‐eclampsia.
2.2. Analysis.

Comparison 2 Nitric oxide versus nifedipine, Outcome 2 Severe hypertension.
2.3. Analysis.

Comparison 2 Nitric oxide versus nifedipine, Outcome 3 Caesarean section.
2.4. Analysis.

Comparison 2 Nitric oxide versus nifedipine, Outcome 4 Maternal side‐effects.
2.5. Analysis.

Comparison 2 Nitric oxide versus nifedipine, Outcome 5 Perinatal death.
2.6. Analysis.

Comparison 2 Nitric oxide versus nifedipine, Outcome 6 Preterm birth.
2.7. Analysis.

Comparison 2 Nitric oxide versus nifedipine, Outcome 7 Small‐for‐gestational age.
Comparison 3. Nitric oxide versus antiplatelet agents.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Progression of gestational hypertension | 1 | 76 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.12 [0.58, 2.18] |
| 2 Placental abruption | 1 | 76 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.85 [0.12, 67.83] |
| 3 Development or progression of renal failure | 1 | 28 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.0 [0.31, 3.23] |
| 4 Death of baby | 1 | 76 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.71 [0.17, 2.97] |
| 5 Preterm birth | 1 | 76 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.78 [0.36, 1.66] |
| 6 Small‐for‐gestational age | 1 | 76 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.08 [0.44, 2.69] |
3.1. Analysis.

Comparison 3 Nitric oxide versus antiplatelet agents, Outcome 1 Progression of gestational hypertension.
3.2. Analysis.

Comparison 3 Nitric oxide versus antiplatelet agents, Outcome 2 Placental abruption.
3.3. Analysis.

Comparison 3 Nitric oxide versus antiplatelet agents, Outcome 3 Development or progression of renal failure.
3.4. Analysis.

Comparison 3 Nitric oxide versus antiplatelet agents, Outcome 4 Death of baby.
3.5. Analysis.

Comparison 3 Nitric oxide versus antiplatelet agents, Outcome 5 Preterm birth.
3.6. Analysis.

Comparison 3 Nitric oxide versus antiplatelet agents, Outcome 6 Small‐for‐gestational age.
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Davis 2001.
| Methods | Randomisation: computer‐generated random numbers. Allocation concealment: by telephone. Follow up: complete (A). Blinding: caregiver blinded only. | |
| Participants | 16 women with gestational hypertension. | |
| Interventions | Exp: glyceryl trinitrate skin patch (10 mg) for 12 hr/day until delivery. Control: no patch. | |
| Outcomes | Women: pre‐eclampsia; side‐effects. | |
| Notes | Study aimed to recruit 200 women. Discontinued early after 16 women recruited, because all women with active patches developed severe headaches, which led them to remove the patch and withdraw from the study. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Low risk | A ‐ Adequate |
Facchinetti 2002.
| Methods | Randomisation: sequence provided by pharmaceutical company. Allocation concealment: investigators provided with a list of consecutive numbers 1‐80, each allocated A or B. Code not known to trialists. Follow up: 6/80 (7.5%) women excluded from analysis but not reported if proteinuric or non‐proteinuric and from which arm: 3 lost to follow up, 1 changed her mind, 2 did not fit inclusion criteria (B). Blinding: participants and caregivers blinded but not outcome assessment. | |
| Participants | 74 women between 24‐36 weeks' gestation with gestational hypertension (46 women (BP > 140/90 X2 after 20 weeks)) or pre‐eclampsia (28 women (as above + proteinuria > 300 g/24 hrs)). Excluded if: BP raised < 20 weeks' gestation, severe hypertension, severe pre‐eclampsia, antiphospholipid syndrome, heart kidney or liver disease, < 2 antenatal visits before enrolment. | |
| Interventions | Exp: L‐arginine 20 g/500 ml IV daily for 5 days, then 4 g/d oral for 2 weeks. Control: placebo. | |
| Outcomes | Women: changes in BP; platelet aggregation; time from trial entry to delivery; pre‐eclampsia; severe pre‐eclampsia (one of: BP > 170/110, proteinuria > 5 g/24 hr, HELLP, coagulation disorders, arrested fetal growth). Babies: perinatal death; preterm birth; birthweight (mean); gestation at birth (mean). | |
| Notes | Authors provided unpublished data for women without proteinuria at trial entry. For most outcomes, data are presented for this subset only. For caesarean section, data are presented for all women randomised as this is all that is available. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Low risk | A ‐ Adequate |
Lees 1998.
| Methods | Randomisation: by study pharmacist in 1 centre. Allocation concealment: randomisation by pharmacist, code not available to clinicians until after the last woman delivered. Follow up: complete (A). Blinding: participants and caregivers blinded, but not outcome assessment. | |
| Participants | 40 women with singleton pregnancy, normal BP and abnormal uterine Doppler scans at 24‐26 weeks (bilateral diastolic notches and resistivity index > 0.58). Excluded if: pre‐existing hypertension, diabetes, chronic renal disease, proteinuria, or on antihypertensive or cardiovascular medication or fetal growth restriction or abnormalities. | |
| Interventions | Exp: glyceryl trinitrate skin patch 5 mg from 7 am‐10 pm (15 hrs) till delivery. Patches discontinued if any of the following: BP > 140/90 +/‐ proteinuria, use of antihypertensives, fetal growth restriction, or any clinical indication to stop the patch (including side‐effects). Control: placebo patches with the same instructions. | |
| Outcomes | Women: pre‐eclampsia; gestation of onset of pre‐eclampsia; maternal BP changes; HELLP; side‐effects (skin rash and headaches); abruption; Doppler indices. Babies: gestation at delivery (mean); preterm birth; small‐for‐gestational age (either abdominal circumference < 5th centile or birthweight < 10th centile); birthweight (mean). | |
| Notes | 2 recruiting centres, UK and Italy. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Low risk | A ‐ Adequate |
Neri 1999.
| Methods | Randomisation: computer‐generated list of random numbers. Allocation concealment: sealed envelopes. The allocation was given by telephone to 1 centre. Follow up: 6 participants (17%) excluded from analysis (2 nifedipine‐1 pre‐eclampsia, 1 severe IUGR; 3 continuous GTN‐2 severe headaches, 1 pre‐eclampsia; 1 intermittent GTN‐severe IUGR) (C). Blinding: participants and investigators not blinded, person assessing BP blinded to allocation. | |
| Participants | 36 women with a singleton pregnancy at > 24 weeks' gestation with either gestational hypertension (> 140 mmHg sBP or > 90 mmHg dBP) (32 women) or pre‐eclampsia (gestational hypertension + proteinuria > 300 mg/24 h) (4 women), and normal blood pressure before 20 weeks' gestation, no antihypertensive treatment, and diastolic mesor > 73 mmHg. Excluded if: fetal abnormality or chromosomal disorders, renal or hepatic disease, or diastolic mesor < 73 mmHg. | |
| Interventions | Exp 1: glyceryl trinitrate skin patch 10 mg continuously. Exp 2: glyceryl trinitrate skin patch 10 mg for 16 hrs/day. Control: oral nifedipine 40 mg/d. | |
| Outcomes | Women: BP changes (recorded with ambulatory BP monitoring device at 2 week interval); caesarean section; stopped treatment due to side‐effects. Babies: birthweight (mean); gestation at delivery (mean). | |
| Notes | 2 recruiting centres. 3‐arm trial. For the analysis, data from the 2 experimental arms combined. Data for excluded women has been re‐instated. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Low risk | A ‐ Adequate |
Picciolo 2000.
| Methods | Randomisation: random‐number list. Allocation concealment: no information. Follow up: not reported. Blinding: not discussed. | |
| Participants | 68 women at < 16 weeks' gestation, with either chronic hypertension or previous history of pre‐eclampsia before 34 weeks or IUGR, or both. | |
| Interventions | Exp: glyceryl trinitrate skin patch 5 mg for 14‐16 hrs/day, from 16‐38 weeks' gestation. Controls: observation only. | |
| Outcomes | Women: pre‐eclampsia; abruption; Doppler notch at 24 weeks. Babies: neonatal death; preterm birth; IUGR; Apgar score; admission to NICU; RDS; IVH. | |
| Notes | 2 centres in Italy. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
Zozulia 1997.
| Methods | Randomisation: 'blinded randomisation' stratified by whether essential hypertension or chronic glomerulonephritis. No further information. Allocation concealment: no information. Follow up: not reported. Blinding: participants not blinded, and blinding of caregiver and outcome assessor not reported. | |
| Participants | 76 women with a singleton pregnancy between 16‐20 weeks' gestation, and mild‐moderate hypertension, with either essential hypertension or CGN. Excluded if: severe hypertension. | |
| Interventions | Exp: glyceryl trinitrate skin patch 5 mg, increasing to 20 mg if tolerated, for 12 hrs/day, from 16‐20 weeks' gestation until delivery. Controls: acetyl salicylic acid 125 mg/day and curantil (dipyridamole) 150‐225 mg/day. | |
| Outcomes | Women: progressive gestational hypertension (not defined); increase in proteinuria (CGN women only); development or progression of renal failure (CGN women only); abruption; total number of pregnancy complications; side‐effects. Baby: miscarriage or stillbirth; preterm birth; IUGR. | |
| Notes | Data on side‐effects not used as not reported for both groups. Headache reported only for nitric oxide group (almost all women on nitric oxide developed headaches which improved with time: 5 had severe headache, 3 requested change of treatment). 2 women had nausea and 1 had skin irritation with patch, but unclear which group. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
BP: blood presssure CGN: chronic glomerulonephritis dBP: diastolic blood pressure Exp: experiment GTN: glyceryl trinitrate HELLP: haemolysis, elevated liver enzymes, low platelets syndrome hr: hour IUGR: intrauterine growth restriction IV: intravenous IVH: intraventricular haemorrhage NICU: neonatal intensive care unit RDS: respiratory distress syndrome sBP: systolic blood pressure
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Amit 1998 | Intended duration of intervention 2 days. No pregnancy outcomes reported as participants were randomised prior to elective termination of pregnancy. Methods: cross‐over randomised trial. Participants: 11 pregnant women between 8‐12 weeks' gestation, prior to elective termination of pregnancy. Intervention: isosorbide dinitrate (5 mg) on 1 day and placebo on the other day (total 2 days duration of intervention). Outcomes: maternal blood pressure and heart rate; Doppler indices. |
| Decano 2000 | Available as abstract only. Intended duration of intervention was 3 days. Participants were women with established pre‐eclampsia. No clinical outcomes reported. Methods: 'randomly assigned'. No further information available. Participants: 16 pregnant women with pre‐eclampsia. Intervention: nitroglycerin (5 mg) transdermal patch for 16 hours on 3 consecutive days versus placebo. Outcomes: Doppler indices. |
| Hladunewich 2006 | Women with established pre‐eclampsia. Most women had therapy for less than 1 day before delivery, and some treated only after delivery. Methods: randomised trial with computer‐generated numbers. Participants: 45 women with pre‐eclampsia. Intervention: L‐arginine 3.5 g 6 hourly orally or 10 g 8 hourly infusion versus placebo, until 10 days postpartum. Outcomes: changes in maternal blood pressure and renal function 3 and 10 days postpartum; biochemical outcomes. |
| Ledingham 1999 | No relevant outcomes reported: nitric oxide used for cervical ripening prior to termination of pregnancy. Methods: women 'randomised' into 2 groups. Participants: 20 women 7‐12 weeks' pregnant undergoing termination of pregnancy. Intervention: isosorbide mononitrate versus no treatment. Outcomes: immunohistochemical effects on cervix. |
| Madhubala 2006 | Unclear if randomised trial. Data cannot be used as presented. Methods: unclear. 2 groups, 1 with intervention, and other without intervention. Participants: 82 women with oligohydramnios at 26‐28 weeks. Interventions: L‐arginine powder 3 g sachet versus no L‐arginine. Outcomes: birthweight; preterm birth; perinatal morbidity and mortality. |
| Makino 1997 | Single‐dose study: intended duration of intervention was 24 hours only. Methods: 'randomly allocated'. No further information available. Participants: 18 pregnant women at midgestation (mean 31 weeks) with raised blood pressure with or without proteinuria. Interventions: single dose of isosorbide dinitrate (40 mg) patch with bed rest versus bed rest alone, for 24 hours. Outcomes: Doppler indices; blood pressure; mode of delivery; gestational age at delivery; birthweight; Apgar score. |
| Neri 2000b | Women with pre‐eclampsia also included. No relevant outcomes reported. Methods: women 'randomised' to 2 groups. Participants: 60 women with pregnancy‐induced hypertension or pre‐eclampsia. Intervention: transdermal GTN 10 mg for 16 h/day versus oral nifedipine 40 mg/day over a 2 week study period. Outcomes: changes in systolic and diastolic blood pressure. |
| Neri 2004a | Physiological study over 1 day. Cross‐over trial. No relevant outcomes. Methods: randomised cross‐over design. Participants: 15 women with gestational hypertension. Interventions: L‐arginine infusion versus saline infusion. Outcomes: changes in maternal blood pressure and fetal heart rate patterns; fetal movements. |
| Neri 2004b | Physiological study over 5 days. 42% women with pre‐eclampsia. No relevant outcomes. Methods: multicentre randomised trial. Participants: 148 women at 24‐34 weeks with gestational hypertension or pre‐eclampsia. Interventions: daily L‐arginine intravenous infusion versus placebo infusion. Bed rest 4 hours for both groups. Outcomes: changes in systolic and diastolic blood pressure. |
| Reyna‐Villasmil 2001 | Available as abstract only. Intended duration of intervention was 2 days. Participants were women with established pre‐eclampsia. No clinical outcomes reported. Methods: 'randomly assigned'. No further information available. Participants: 30 pregnant women with pre‐eclampsia. Interventions: nitroglycerin (7 mg for 12 hours for 2 days) transdermal patch versus placebo. Outcomes: Doppler indices. |
| Ruggenenti 2006 | Women with established pre‐eclampsia. Ongoing trial. Methods: randomised controlled trial. Participants: 20 women with pre‐eclampsia. Interventions: L‐arginine versus placebo. Outcomes: biochemical; time to delivery; birthweight; Apgar score; gestational age at delivery; histopathology of placenta. |
| Rytewski 2005 | Women with established pre‐eclampsia. No relevant outcomes. Methods: 'randomised trial'. Participants: 60 women with pre‐eclampsia. Intervention: 3 g oral L‐arginine versus placebo for 3 weeks. Outcomes: changes in blood pressure; plasma and urine levels of nitric oxide metabolites. |
| Rytlewski 2006 | Women with established pre‐eclampsia. Methods: randomised trial using random‐number tables. Participants: 83 women with pre‐eclampsia. Interventions: L‐arginine 3 g/day versus placebo until delivery. Outcomes: maternal blood pressure; duration of pregnancy; Doppler indices for baby; biophysical profile; birthweight; IUGR; Apgar score; neonatal death. |
| Scarpellini 2001 | Available as abstract only. No clinical outcomes reported. Methods: 2 groups (intervention and control). No further information available. Participants: 24 pregnant women with PIH. Interventions: isosorbide dinitrate sublingual tablets 6 hourly versus nifedipine 20 mg daily. Outcomes: apoptosis in placental blood vessels. |
| Staff 2004 | Intended duration of study was 5 days. Participants were women with established pre‐eclampsia. Methods: 'randomised study'. No further information available. Participants: 30 pregnant women between 28‐36 weeks' gestation with pre‐eclampsia. Interventions: L‐arginine (4 g tds) versus placebo. Outcomes: diastolic blood pressure; interval between start of study and delivery; mode of delivery; birthweight; perinatal mortality; NICU admission; hypoglycaemia; Apgar score. |
| Thaler 1999 | Single‐dose study: intended duration of intervention was < 24 hours. Methods: randomisation by consecutively‐numbered, opaque sealed envelopes provided by a pharmaceutical company. Participants: 23 women with singleton pregnancy and raised blood pressure after 20 weeks' gestation with proteinuria < 300 mg/24 h. Interventions: single dose of isosorbide dinitrate (5 mg) sublingual tablet versus single dose of placebo tablet. Outcomes: maternal blood pressure; pulse; Doppler indices; biochemical criteria; gestation at delivery; birthweight; fetal growth restriction. |
| Valensise 2005 | No relevant outcomes. Methods: women 'randomised to 2 treatment groups'. Participants: 50 women with moderate to severe gestational hypertension between 27‐30 weeks with fetal abdominal circumference < 10th percentile. Interventions: calcium antagonists and bed rest versus calcium antagonists, bed rest, transdermal GTN and intravenous fluid infusion 2 litres in 24 hrs. Outcomes: physiological. |
GTN: glyceryl trinitrate IUGR: intrauterine growth restriction NICU: neotanal intensive care unit PIH: pregnancy‐induced hypertension tds: to be taken three times a day
Contributions of authors
Shireen Meher and Lelia Duley drafted the protocol. Both review authors independently assessed trials for inclusion. Shireen Meher extracted and entered data into Review Manager software and Lelia Duley double checked them for accuracy. The review was drafted by Shireen Meher and Lelia Duley.
Sources of support
Internal sources
The University of Liverpool, UK.
University of Oxford, UK.
External sources
Health Technology Assessment, UK.
Medical Research Council, UK.
Declarations of interest
None known.
Edited (no change to conclusions)
References
References to studies included in this review
Davis 2001 {published and unpublished data}
- Davis G, Brown M. Glyceryl trinitrate patches are unsuitable in hypertensive pregnancy. Australian and New Zealand Journal of Obstetrics and Gynaecology 2001;41(4):474. [DOI] [PubMed] [Google Scholar]
Facchinetti 2002 {published and unpublished data}
- Facchinetti F, Piccinini F, Pizzi C, Bukowski R, Volpe A, Saade G. Effect of arginine supplementation in patients with gestational hypertension. American Journal of Obstetrics and Gynecology 2002;187(6 Pt 2):S213. [Google Scholar]
Lees 1998 {published data only}
- Lees C, Valensise H, Black R, Harrington K, Byiers S, Romanini C, et al. The efficacy and fetal‐maternal cardiovascular effects of transdermal glyceryl trinitrate in the prophylaxis of pre‐eclampsia and its complications: a randomized double‐blind placebo‐controlled trial. Ultrasound in Obstetrics & Gynecology 1998;12(5):334‐8. [CN‐00157005] [DOI] [PubMed] [Google Scholar]
Neri 1999 {published and unpublished data}
- Neri I, Valensise H, Facchinetti F, Menghini S, Romanini C, Volpe A. 24‐hour ambulatory blood pressure monitoring: a comparison between transdermal glyceryl‐trinitrate and oral nifedipine. Hypertension in Pregnancy 1999;18(1):107‐13. [CN‐00233632] [DOI] [PubMed] [Google Scholar]
Picciolo 2000 {published data only}
- Picciolo C, Roncaglia N, Neri I, Pasta F, Arreghini A, Facchinetti F. Nitric oxide in the prevention of pre‐eclampsia. Prenatal and Neonatal Medicine 2000;5(4):212‐5. [CN‐00399568] [Google Scholar]
Zozulia 1997 {published data only}
- Zozulia O, Rogov VA, Piatakova NV, Tareeva IE. Nitric oxide: its role in the development of pregnancy complications and in their prevention in women with hypertension and chronic glomerulonephritis. Terapevticheskii Arkhiv 1997;69(6):17‐20. [PubMed] [Google Scholar]
References to studies excluded from this review
Amit 1998 {published data only}
- Amit A, Thaler I, Paz Y, Itskovitz‐Eldor J. The effect of a nitric oxide donor on Doppler flow velocity waveforms in the uterine artery during the first trimester of pregnancy. Ultrasound in Obstetrics & Gynecology 1998;11(2):94‐8. [CN‐00304196] [DOI] [PubMed] [Google Scholar]
Decano 2000 {published data only}
- Decano MB, Cabrera LT. The effects of transdermal nitroglycerin (nitrol patch) on the uterine and umbilical artery blood flow in preeclampsia: a randomized double blind placebo controlled study [abstract]. XVI FIGO World Congress of Obstetrics & Gynecology (Book 1); 2000 Sept 3‐8; Washington DC, USA. 2000:26. [CN‐00355035]
Hladunewich 2006 {published data only}
- Hladunewich MA, Derby GC, Lafayette RA, Blouch KL, Druzin ML, Myers BD. Effect of L‐arginine therapy on the glomerular injury of preeclampsia. Obstetrics & Gynecology 2006;107(4):886‐95. [DOI] [PubMed] [Google Scholar]
Ledingham 1999 {published data only}
- Ledingham MA, Denison FC, Kelly RW, Young A, Normal JE. Nitric oxide donors stimulate prostaglandin F2alpha and inhibit thromboxane B2 production in the human cervix during first trimester of pregnancy. Molecular Human Reproduction 1999;5(10):973‐82. [DOI] [PubMed] [Google Scholar]
Madhubala 2006 {published data only}
- Madhubala M. Use of L‐arginine in oligohydramnios. 49th All India Congress of Obstetrics and Gynaecology; 2006 January 6‐9; Cochin, Kerala State, India. 2006:42.
Makino 1997 {published data only}
- Makino Y, Izumi H, Makino I, Shirakawa K. The effect of nitric oxide on uterine and umbilical artery flow velocity waveform in pre‐eclampsia. European Journal of Obstetrics & Gynecology and Reproductive Biology 1997;73(2):139‐43. [CN‐00141718] [DOI] [PubMed] [Google Scholar]
Neri 2000b {published data only}
- Neri I, Ternelli G, Facchinetti F, Volpe A. Effectiveness of oral nifedipine and transdermal glyceryltrinitrate on 24‐hour blood pressure values in pregnancy‐induced hypertension. Hypertension in Pregnancy 2000;19(Suppl 1):O65. [Google Scholar]
Neri 2004a {published data only}
- Neri I, Blasi I, Facchinetti F. Effects of acute L‐arginine infusion on non‐stress test in hypertensive pregnant women. Journal of Maternal‐Fetal & Neonatal Medicine 2004;16:23‐6. [DOI] [PubMed] [Google Scholar]
Neri 2004b {published data only}
- Neri I, Jasonni VM, Gori G, Blasi I, Facchinetti F. Intravenous l‐arginine is a new anti‐hypertensive agent for pregnant women. American Journal of Obstetrics and Gynecology 2004;191(6 Suppl 1):S42. [Google Scholar]
Reyna‐Villasmil 2001 {published data only}
- Reyna‐Villasmil E, Prieto‐Franchi M, Guerra‐Velazquez M, Torres‐Montilla M. Effect of transdermal nitroglycerin on umbilical artery blood flow in preeclampsia. Journal of Perinatal Medicine 2001;29 Suppl 1(Pt 2):486. [CN‐00363873] [Google Scholar]
Ruggenenti 2006 {published data only}
- Ruggenenti P. L‐Arginine in pre‐eclampsia. www.clinicaltrials.gov (accessed 21 March 2006).
Rytewski 2005 {published data only}
- Rytlewski K, Olszanecki R, Korbut R, Zdebski Z. Effects of prolonged oral supplementation with L‐arginine on blood pressure and nitric oxide synthesis in pre‐eclampsia. European Journal of Clinical Investigation 2005;35:32‐7. [DOI] [PubMed] [Google Scholar]
Rytlewski 2006 {published data only}
- Rytlewski K, Olszanecki R, Lauterbach R, Grzyb A, Basta A. Effects of oral l‐arginine on the foetal condition and neonatal outcome in preeclampsia: a preliminary report. Basic & Clinical Pharmacology & Toxicology 2006;99:146‐52. [DOI] [PubMed] [Google Scholar]
Scarpellini 2001 {published data only}
- Scarpellini F, Sbracia M, Lecchini S, Bolis P. Nitric oxide donor treatment reduces apoptosis in placental vessels of pregnancy induced hypertension. American Journal of Obstetrics and Gynecology 2001;185(6 Suppl):S177. [CN‐00387652] [Google Scholar]
Staff 2004 {published data only}
- Staff AC, Berge L, Haugen G, Lorentzen B, Mikkelsen B, Henriksen T. Dietary supplementation with L‐arginine or placebo in women with pre‐eclampsia. Acta Obstetricia et Gynecologica Scandinavica 2004;83(1):103‐7. [CN‐00459625] [PubMed] [Google Scholar]
Thaler 1999 {published data only}
- Thaler I, Amit A, Kamil D, Itskovitz‐Eldor J. The effect of isosorbide dinitrate on placental blood flow and maternal blood pressure in women with pregnancy induced hypertension. American Journal of Hypertension 1999;12(4 Pt 1):341‐7. [CN‐00162808] [DOI] [PubMed] [Google Scholar]
Valensise 2005 {published data only}
- Valensise H, Vasapollo B, Novelli GP, Altomare F, Arduini D. Nitric oxide donors and fluid therapy increase fetal growth in gestational hypertension. Ultrasound in Obstetrics & Gynecology 2005;26:440. [Google Scholar]
References to studies awaiting assessment
Di Iorio 2002 {published data only}
- Iorio R, Marinoni E, Gazzolo D, Letizia C, Netta T, Cosmi EV. Maternal nitric oxide supplementation increases adrenomedullin concentrations in growth retarded fetuses. Gynecological Endocrinology 2002;16(3):187‐92. [PubMed] [Google Scholar]
El‐Hamedi 2001 {published data only}
- El‐Hamedi A, Shillito TJA, Simpson NAB, Walker JJ. A prospective randomised controlled trial of nitric oxide donors in at risk pregnancy [abstract]. Journal of Obstetrics and Gynaecology 2001;21 Suppl 1:S22. [Google Scholar]
Facchinetti 2007 {published data only}
- Facchinetti F, Saade GR, Neri I, Pizzi C, Longo M, Volpe A. L‐arginine supplementation in patients with gestational hypertension: a pilot study. Hypertension in Pregnancy 2007;26(1):121‐30. [DOI] [PubMed] [Google Scholar]
Facchinetti 2008 {published data only}
- Facchinetti F. Effects of oral L‐arginine on chronic hypertension in pregnancy. ClinicalTrials.gov (http://clinicaltrials.gov/) (accessed 20 February 2008).
Hubel 2009 {published data only}
- Hubel CA. Oral L‐arginine and ADMA in pregnancy. ClinicalTrials.gov (http://clinicaltrials.gov/) (accessed 4 January 2009) 2009.
Lopez‐Molina 2008 {published data only}
- Lopez‐Molina K, Gonzalez‐Altamirano JC, Valdivia‐Silva JE. Early L‐arginine therapy improves notably the fetal growth in preeclamptic women. A randomized controlled trial. 55th Annual Meeting of the Society of Gynecologic Investigation; 2008 March 26‐29; San Diego, USA 2008:Abstract no: 801.
Neri 2006 {published data only}
- Neri I, Jasonni VM, Gori GF, Blasi I, Facchinetti F. Effect of L‐arginine on blood pressure in pregnancy‐induced hypertension: a randomized placebo‐controlled trial. Journal of Maternal‐Fetal and Neonatal Medicine 2006;19(5):277‐81. [DOI] [PubMed] [Google Scholar]
Noris 2008 {published data only}
- Noris M, Fratelli N, Rampello S, Gherardi G, Platto C, Todeschini M, et al. A double‐blind, randomized, pilot study on L‐arginine oral supplementation in pre‐eclamptic pregnancies. Hypertension in Pregnancy 2008;27(4):505. [Google Scholar]
Samangaya 2009 {published data only}
- Samangaya RA, Mires G, Shennan A, Skillern L, Howe D, McLeod A, et al. A randomised, double‐blinded, placebo‐controlled trial of the phosphodiesterase type 5 inhibitor sildenafil in the treatment of preeclampsia. Hypertension in Pregnancy 2009;28:369‐82. [DOI] [PubMed] [Google Scholar]
Smith 2008 {published data only}
- Smith G. Use of a nitric oxide (ISMN) for the prevention and management of pre‐eclampsia (pilot study). Current Controlled Trials (www.controlled‐trials.com/) (accessed 20 February 2008) 2008.
Winer 2007 {published data only}
- Winer N, Branger B, Azria E, ROze JC, Descamps P, Philippe HJ, et al. Treatment of severe vascular fetal intrauterine growth restriction with L‐arginine: a multicenter prospective double‐blind randomized placebo‐controlled study [abstract]. Ultrasound in Obstetrics and Gynecology 2007;30:381. [Google Scholar]
Xiao 2005 {published data only}
- Xiao XM, Li LP. L‐arginine treatment for asymmetric fetal growth restriction. International Journal of Gynecology & Obstetrics 2005;88(1):15‐8. [DOI] [PubMed] [Google Scholar]
Zhang 2007 {published data only}
- Zhang N, Xiong AH, Xiao X, Li LP. Effect and mechanism of L‐arginine therapy for fetal growth retardation due to pregnancy‐induced hypertension. Nan Fang Yi Ke Da Xue Xue Bao//Journal of Southern Medical University 2007;27(2):198‐200. [PubMed] [Google Scholar]
Additional references
Benedetto 2000
- Benedetto C, Marozio L, Neri I, Giarola M, Volpe A, Facchinetti F. Increased L‐citrulline/L‐arginine plasma ratio in severe preeclampsia. Obstetrics & Gynecology 2000;96(3):395‐9. [DOI] [PubMed] [Google Scholar]
Cockell 1997
- Cockell AP, Poston L. Flow‐mediated vasodilatation is enhanced in normal pregnancy but reduced in preeclampsia. Hypertension 1997;30(2):247‐51. [DOI] [PubMed] [Google Scholar]
Conrad 1999
- Conrad KP, Kerchner LJ, Mosher MD. Plasma and 24‐h NOx and cGMP during normal pregnancy and preeclampsia in women on a reduced NOx diet. American Journal of Physiology 1999;2777(1):F48‐F57. [DOI] [PubMed] [Google Scholar]
Davidge 1996
- Davidge ST, Stranko CP, Roberts JM. Urine but not plasma nitric oxide metabolites are decreased in women with preeclampsia. American Journal of Obstetrics and Gynecology 1996;174(3):1008‐13. [DOI] [PubMed] [Google Scholar]
De Caterina 1995
- Caterina R, Libby P, Peng HB, Thannickal VJ, Rajavashisth TB, Gimbrone MA Jr, et al. Nitric oxide decreases cytokine‐induced endothelial activation. Nitric oxide selectively reduces expression of adhesion molecules and proinflammatory cytokines. Journal of Clinical Investigation 1995;96(1):60‐8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Delacretaz 1995
- Delacretaz E, DeQuay N, Waeber B, Vial Y, Schulz PE, Burnier M, et al. Differential nitric oxide synthase activity in human platelets during normal pregnancy and pre‐eclampsia. Clinical Sciences 1995;88:607‐10. [DOI] [PubMed] [Google Scholar]
DH 2002
- Department of Health, Scottish Executive Health Department and Department of Health, Social Services, Public Safety. Northern Ireland. Why mothers die. The sixth report on confidential enquiries into maternal deaths in the United Kingdom 2000‐2002. London: RCOG Press, 2002. [Google Scholar]
Di Iorio 1998
- Ioro R, Marinoni E, Emiliani S, Villaccio B, Ermelando VC. Nitric oxide in preeclampsia: lack of evidence for decreased production. European Journal of Obstetrics & Gynecology and Reproductive Biology 1998;76(1):65‐70. [DOI] [PubMed] [Google Scholar]
Fickling 1993
- Fickling SA, Williams D, Vallance P, Nussey SS, Whitley GStJ. Plasma concentrations of endogenous inhibitor of nitric oxide synthesis in normal pregnancy and pre‐eclampsia. Lancet 1993;342:242‐3. [DOI] [PubMed] [Google Scholar]
Generic Protocol 2005
- Meher S, Duley L, Prevention of Pre‐eclampsia Cochrane Review Authors. Interventions for preventing pre‐eclampsia and its consequences: generic protocol. Cochrane Database of Systematic Reviews 2005, Issue 2. [DOI: 10.1002/14651858.CD005301] [DOI] [Google Scholar]
Grunewald 1995
- Grunewald C, Kublickas M, Carlstrom K, Lunell N, Nisell H. Effects of nitroglycerin on the uterine and umbilical circulation in severe preeclampsia. Obstetrics & Gynecology 1995;86:600‐4. [DOI] [PubMed] [Google Scholar]
Higgins 2005
- Higgins JPT, Green S, editors. Cochrane Handbook for Systematic Reviews of Interventions 4.2.4 [updated March 2005. In: The Cochrane Library, Issue 2, 2005. Chichester, UK: John Wiley & Sons, Ltd.
Hubel 1997
- Hubel CA, Kagan VE, Kisin ER, McLaughlin MK, Roberts JM. Increased ascorbate radical formation and ascorbate depletion in plasma from women with preeclampsia: implications for oxidative stress. Free Radical Biology and Medicine 1997;23:597‐609. [DOI] [PubMed] [Google Scholar]
Ignarro 1987
- Ignarro LJ, Buga GM, Wood KS, Byrne RE, Chaudhuri G. Endothelium‐derived relaxing factor produced and released from artery and vein is nitric oxide. Proceedings of the National Academy of Sciences of the United States of America 1987;84:9265‐69. [DOI] [PMC free article] [PubMed] [Google Scholar]
Kubes 1991
- Kubes P, Suzuki M, Granger DN. Nitric oxide: an endogenous modulator of leukocyte adhesion. Proceedings of the National Academy of Sciences of the United States of America 1991;88:4651‐5. [DOI] [PMC free article] [PubMed] [Google Scholar]
Lees 1996
- Lees C, Langford E, Brown AS, Belder A, Pickles A, Martin JF. The effects of S‐nitrosoglutathione on platelet activation, hypertension, and uterine and fetal Doppler in severe preeclampsia. Obstetrics & Gynecology 1996;88:14‐9. [DOI] [PubMed] [Google Scholar]
Lowe 2000
- Lowe DT. Nitric oxide dysfunction in the pathophysiology of preeclampsia. Nitric Oxide 2000;4(4):441‐58. [DOI] [PubMed] [Google Scholar]
Lyall 1995
- Lyall F, Young A, Greer IA. Nitric oxide concentrations are increased in the fetoplacental circulation in preeclampsia. American Journal of Obstetrics and Gynecology 1995;173(1):714‐8. [DOI] [PubMed] [Google Scholar]
Molnar 1994
- Molnar M, Suto T, Toth T, Hertelendy F. Prolonged blockade of nitric oxide synthesis in gravid rats produces sustained hypertension, proteinuria, thrombocytopenia, and intrauterine growth retardation. American Journal of Obstetrics and Gynecology 1994;170(1):1458‐66. [DOI] [PubMed] [Google Scholar]
Moncada 1991
- Moncada S, Palmer RMJ, Higgs EA. Nitric oxide. Pharmacological Reviews 1991;43(2):109‐42. [PubMed] [Google Scholar]
Morris 1995
- Morris NH, Sooranna SR, Learmont JG, Poston L, Ramsey B, Peason JD, et al. Nitric oxide synthase activities in placental tissue from normotensive, preeclamptic, and growth retarded fetuses. British Journal of Obstetrics and Gynaecology 1995;102:711‐4. [DOI] [PubMed] [Google Scholar]
Myatt 1992
- Myatt L, Brewer AS, Langdon G, Brockman DE. Attenuation of the vasoconstrictor effects of thromboxane and endothelin by nitric oxide in the human fetal‐placental circulation. American Journal of Obstetrics and Gynecology 1992;166:224‐30. [DOI] [PubMed] [Google Scholar]
Neri 2000a
- Neri I, Piccinini F, Marietta M, Facchinetti F, Volpe A. Platelet responsiveness to L‐arginine in hypertensive disorders of pregnancy. Hypertension in Pregnancy 2000;19(3):323‐30. [DOI] [PubMed] [Google Scholar]
NHBPEP2000
- Gifford RW Jr, August PA, Cunningham G, Green LA, Lindhemier MD, McNellis D, et al. Report of the national high blood pressure education program working group on high blood pressure in pregnancy. American Journal of Obstetrics and Gynecology 2000;183(1):S1‐S22. [PubMed] [Google Scholar]
Noris 2004
- Noris M, Todeschini M, Cassis P, Pasta F, Cappellini A, Bonazzola S, et al. L‐arginine depletion in preeclampsia orients nitric oxide synthase toward oxidant species. Hypertension 2004;43(3):614‐22. [DOI] [PubMed] [Google Scholar]
Norris 1999
- Norris L, Higgins JR, Darling MR, Walshe JJ, Bonnar J. Nitric oxide in the uteroplacental, fetoplacental, and peripheral circulation in preeclampsia. Obstetrics & Gynecology 1999;93(6):958‐63. [DOI] [PubMed] [Google Scholar]
Palmer 1987
- Palmer RMJ, Ferrige AG, Moncada S. Nitric oxide release accounts for the biological activity of endothelium‐derived relaxing factor. Nature 1987;327:524‐6. [DOI] [PubMed] [Google Scholar]
Pinto 1991
- Pinto A, Sorrentino R, Sorrentino P, Guerritore T, Miranda L, Biondi A, et al. Endothelial‐derived relaxing factor released by endothelial cells of human umbilical vessels and its impairment in pregnancy‐induced hypertension. American Journal of Obstetrics and Gynecology 1991;164:507‐13. [DOI] [PubMed] [Google Scholar]
Poston 1998
- Poston L. Nitrovasodilators ‐ will they be useful in lowering uterine artery resistance in pre‐eclampsia and intrauterine growth restriction?. Ultrasound in Obstetrics & Gynecology 1998;11:92‐3. [DOI] [PubMed] [Google Scholar]
Radomski 1987
- Radomski MW, Palmer RM, Moncada S. Endogenous nitric oxide inhibits human platelet adhesion to vascular endothelium. Lancet 1987;2(8567):1057‐8. [DOI] [PubMed] [Google Scholar]
Ranta 1999
- Ranta V, Viinikka L, Halmesmaki E, Ylikorkala O. Nitric oxide production with preeclampsia. Obstetrics & Gynecology 1999;93(3):442‐5. [DOI] [PubMed] [Google Scholar]
RevMan 2003 [Computer program]
- The Cochrane Collaboration. Review Manager (RevMan). Version 4.2 for Windows. Oxford, England: The Cochrane Collaboration, 2003.
Seligman 1994
- Seligman SP, Buyon JP, Clancy RM, Young BK, Abramson SB. The role of nitric oxide in the pathogenesis of preeclampsia. American Journal of Obstetrics and Gynecology 1994;171:944‐8. [DOI] [PubMed] [Google Scholar]
Shaamash 2001
- Shaamash AH, Elsonosy ED, Zakhari MM, Radwan SH, El‐Dien HM. Placental nitric oxide synthase (NOS) activity and nitric oxide (NO) production in normal pregnancy, pre‐eclampsia, and eclampsia. International Journal of Gynecology & Obstetrics 2001;72(2):127‐33. [DOI] [PubMed] [Google Scholar]
Silver 1996
- Silver RK, Kupferminc MJ, Russell TL, Adler L, Mullen TA, Caplan MS. Evaluation of nitric oxide as a mediator of severe preeclampsia. American Journal of Obstetrics and Gynecology 1996;175(4):1013‐7. [DOI] [PubMed] [Google Scholar]
Sladek 1997
- Sladek SM, Magness RR, Conrad KP. Nitric oxide and pregnancy. American Journal of Physiology 1997;272:R441‐63. [DOI] [PubMed] [Google Scholar]
WHO 1988
- World Health Organization International Collaborative Study of Hypertensive Disorders in Pregnancy. Geographic variation in the incidence of hypertension in pregnancy. American Journal of Obstetrics and Gynecology 1988;158:80‐3. [PubMed] [Google Scholar]


