Abstract
Background:
Heat shock proteins (hsp) are intracellular chaperones that possess extracellular immunostimulatory properties when complexed with antigens. A recombinant Hsp110-gp100 chaperone complex vaccine showed antitumor response and prolonged survival in murine melanoma. A Phase Ib dose escalation study of a recombinant human Hsp110-gp100 vaccine in advanced stage melanoma patients was performed to evaluate toxicity, immunostimulatory potential, and clinical response.
Methods:
Patients with pretreated, unresectable Stage IIIB/C/IV melanoma received the chaperone complex vaccine in a dose escalation protocol; 3 vaccinations over a 43-day period. Tumor response, clinical toxicity, and immune response were measured.
Results:
Ten patients (8 female, median age 70) were enrolled and 2 patients had grade 1 adverse events (AE); minor skin rash, hyperhidrosis, and fever (no grade 2 or higher AE). Median progression free survival was longer for lower vaccine doses as compared to the maximum dose of 180 mcg (4.5 vs 2.9 months, p=0.018). The lowest dose patients (30 and 60 mcg) had clinical tumor responses (1 partial response, 1 stable disease). CD8+ T cell IFN-γ responses to gp100 were greater in the clinically responding patients. A pattern of of B cell responses to vaccination was not observed. Regulatory T cell populations and co-stimulatory molecules including CTLA-4 and PD-1 appeared to differ in responders versus non-responders.
Conclusions:
A fully recombinant human Hsp110-gp100 chaperone complex vaccine had minimal toxicity, measurable tumor responses at lower doses, and produced peripheral CD8+ T-cell activation in patients with advanced, pretreated melanoma. Combination with currently available immunotherapies may augment clinical responses.
Keywords: Melanoma, vaccine, heat shock protein, Gp100
INTRODUCTION
Cutaneous melanoma is the most aggressive and a potentially lethal form of skin cancer[1]. It is estimated there will be over 100,000 new cases of melanoma in the United States in 2021 and the overall incidence continues to increase[2]. In the presence of nodal metastases, 5 year overall survival is approximately 65% and decreases to 25% in the presence of distant metastatic disease[3, 4]. Previous treatments for metastatic melanoma offered minimal clinical benefit with significant toxicity. However, with the advent of immunotherapy and targeted molecular treatments for melanoma, significant improvements in prognosis have been achieved over the past decade[1, 2, 5, 6]. As an early attempt at immune stimulation, melanoma antigen targeted vaccines offered initial therapeutic promise, but eventually yielded mixed results[6–9]. With a better understanding of the complex interactions between the human immune system and tumor biology, cancer vaccines may still offer potential as a component of immunotherapy.
Heat shock proteins (hsp) act as essential intracellular chaperones in numerous functions such as translocation of polypeptides, assembly and disassembly of protein complexes, and refolding of misfolded proteins[10–13]. They are constitutively present under normal conditions, but can also be induced by various cellular stresses such as heat, oxidative stress, and toxins[12, 13]. Presumably as a result of their function in polypeptide transport and processing, tumor derived hsp have been shown to carry tumor-specific antigenic peptides and induce tumor-specific immune response[14, 15]. Extensive studies have demonstrated that the immunostimulatory properties of hsp can be attributed to their capability to preferentially interact with receptors on specialized antigen-presenting cells (APCs) leading to efficient antigen uptake, antigen cross-presentation, activation of dendritic cells, and priming of T cells [11, 13, 16–23].
Autologous tumor-derived hsp have shown increased immunological activation and tumor response in multiple clinical studies.[24–26] Indeed, a phase III trial of an autologous hsp vaccine even demonstrated a survival benefit in melanoma patients with Stage IV disease confined to the skin or lungs [27]. Despite these promising results, autologous hsp vaccines carry limitations in clinical use such as requiring a patient specimen, complex procedures of vaccine preparation, and lack of antigenic information for immune monitoring. As such, we instead sought to develop and study a fully recombinant chaperone vaccine by reconstituting Hsp-antigen complexes using the exceptional protein-holding capacity of a large hsp such as Hsp110[11, 28, 29]. While tumor-associated antigen gp100 is highly expressed in over 80% of human melanomas, hsp recombinant vaccines with gp100 had previously never been explored in patients[11, 30, 31]. Preclinical work at our institution using a Hsp110-gp100 recombinant vaccine demonstrated antitumor activity, increased immunostimulation, and prolonged survival in mice bearing B16 melanoma tumors.[11] In light of these promising preclinical results, we performed a Phase Ib dose escalation clinical trial of a recombinant human Hsp110-gp100 vaccine in advanced stage melanoma patients to evaluate toxicity, immunostimulatory potential, and clinical response.
METHODS
Clinical Trial Design
The study was conducted at Roswell Park Comprehensive Cancer Center (RPCCC) after review and approval by the Institutional Review Board. Written, informed consent was obtained from all study participants. Healthy men and non-pregnant women, 18 years of age and above were recruited to enroll in this prospective, single center, Phase Ib clinical vaccine trial (Clinical Trials Registration: NCT01744171). Enrollment of patients took place between March 2013 and January 2018. Patients with Stage IIIB/C/IV melanoma who were either ineligible for or had failed at least one standard of care regional therapy or one non-vaccine based systemic therapy were eligible for enrollment. Patients were required to have measurable disease by RECIST criteria, a good performance status (ECOG 0–2), and a life expectancy greater than 6 months. A full outline of inclusion and exclusion criteria is displayed in Supplemental Tables 1 and 2, respectively.
Vaccine Administration, Monitoring, and Outcomes
Through successive NCI RAID (Rapid Access to Intervention Development) grants (#736285: “Development and Clinical Implementation of a Novel Recombinant Human Hsp110 gp100/ Hsp110-TRP2 Chaperone Complex Polyvalent Melanoma Vaccine” and “Large Scale GMP Production of a Novel Recombinant Human Hsp110 gp100 Chaperone Complex Melanoma Vaccine for Clinical Phase I Assessment.”), the novel human recombinant Hsp110-gp100 chaperone complex melanoma vaccine was successfully GMP mass produced for evaluation in a human Phase I clinical trial. Prior to initiation, the vaccine was approved by the FDA for human clinical trial use (IND 15293). The vaccine was administered on days 1, 15, and 43 following enrollment and was injected intradermally, alternating on the right/left trunk adjacent to nodal basins. The dose for an individual patient was the same for all three vaccinations. The vaccine dose for the first patient was 30 mcg; subsequent patients were enrolled in a dose escalation protocol until the last six patients enrolled each received the planned maximum dose of 180 mcg. Computed topography of the chest, abdomen, and pelvis was performed at days 0, 90, and 180 for target lesion surveillance and response was measured as per RECIST criteria. Clinical visits for toxicity assessments and blood draws were performed at days 0, 13, 29, 41, 57, 90, and 180. The primary outcomes of the study were toxicity and maximum tolerated dosage with secondary outcomes of disease progression, survival, and immunologic response also examined. Classification and reporting of adverse events were based on Common Terminology Criteria for Adverse Events (CTCAE) version 4.0 provided by the NCI.
PBMC Isolation and Cryopreservation
Peripheral blood mononuclear cells (PBMC) were isolated from peripheral blood of melanoma patients by a density gradient centrifugation method using lymphocyte separation media (Corning). PBMC were cryopreserved in CTL-Cryo ABC serum-free freezing media and stored in liquid nitrogen until use.
ELISPOT Assay
Cryopreserved PBMC were thawed in pre-warmed X-VIVO-15 media (Lonza) and the cell number was counted using automated cell counter (Bio-Rad). PBMC (1 × 106 cells) were cultured with 1 μg/ml of gp100 overlapping peptide pool (JPT) in RPMI 1640 media supplemented with 10% human AB serum (Gemini Bio-Products, Inc), L-glutamine, penicillin, streptomycin, and 1% nonessential amino acids (complete media) containing human recombinant IL-2 (10 U/ml; Roche) and IL-7 (10 ng/ml; R&D Systems) into 96-well round-bottom microplates for 13–14 days. The cells were expanded every 3–4 days with complete media containing IL-2 and IL-7. 96-well filter plates (MAHAS4510; Millipore) were coated with IFN-γ mAb (1-D1K; MABTECH) and incubated overnight at 4°C. After the plates were blocked with complete media, cultured PBMC (5 × 104) were resuspended in X-VIVO-15 media and added to each well in the presence or absence of 1 μg/ml of gp100 overlapping peptide pool, and then incubated for 20–22 h in 5% CO2 incubator at 370C. IFN-γ production was developed by substrate (5-bromo-4-chloro-3-indolyl phosphate/nitroblue tetrazolium; Sigma) following incubation with IFN-γ mAb (7-B6–1-biotin; MABTECH) and streptavidin-alkaline phosphatase (MABTECH). Dark-violet spots on the plate membranes were counted by an automated ELISPOT plate reader (CTL). A response was considered positive when spot numbers in triplicate assays stimulated with gp100 peptide were twice higher than the number of spots without peptide and significantly exceeded the cutoff value (cutoff = mean ± 3 SD).
ELISA
Wells were coated in recombinant gp100 in PBS overnight. Each serum was then diluted 1:50 and plated for 1:4 serial dilution in reaction buffer (R&D Systems). The plate was then incubated for 1 hour. After 3 washes with reaction buffer, the plates were blocked with reaction buffer with 1% BSA for 1 hour. After 3 washes, biotinylated goat ant-human IgG+IgM (Jackson ImmunoResearch) was added to each well for 1 hour. After 3 washes, the enzyme reaction was performed by adding Reagent A mixed with Reagent B (R&D systems). The reaction proceeded for 30 minutes at room temperature. Then 0.16M sulfuric acid was added to each well and incubated for 20 minutes. The plates were screened directly using a Hybrid Reader, Synergy H1 (BioTek) at 450 nm with a 570 nm offset. The antibody titer for each serum was defined as the last dilution of serum which gave an absorbance ratio (AR) of 2. The AR was calculated by dividing the OD figure for antibody-positive serum by the OD figure for the antibody-negative serum.
Flow Cytometry
Analytic flow cytometry was performed on FACSCanto II (BD Biosciences) with analysis by FlowJo software (TreeStar). All cells were gated by lymphocytes and live cells by exclusion of cells stained with propidium iodide (PI). Cells were sorted through CD3+CD4+CD8− and CD3+CD4−CD8+ gates initially. For identification of regulatory T cells, gates for CD3, CD4, CD25, CD127, and FoxP3 markers were used. Cells were then put through further gates for PD-1, CTLA-4, Lag 3, and Tim-3 and then quantified.
Statistical Analysis
Progression Free Survival (PFS) was calculated from the time of enrollment on study until the time of disease progression by RECIST criteria or death. Patients alive at last follow up were censored, regardless of disease status. PFS was calculated using Kaplan Meier (KM) methods and curves and the log-rank test was performed. P-values less than 0.05 were considered statistically significant. For ELISPOT, ELISA, and flow cytometry, group comparisons were not performed due to the small number of responders (the study was powered to address toxicity and not responses). All analyses were performed using SAS 9.3 (SAS Institute, Cary, NC).
RESULTS
Patient Characteristics and Dose Escalation
Ten patients were enrolled in the clinical trial. Patient, treatment, and toxicity data are summarized in Table 1. Median age was 70 and the majority of patients were female. Stage of disease at enrollment was 20% Stage IIIC and 80% Stage IV disease. Seven patients had undergone prior surgical treatment with curative intent and the majority of patients (80%) had previously undergone radiation therapy to an involved lymph node basin. Ninety percent of patients received some form of immunotherapy prior to entering the trial. A detailed description of each patient’s clinical history and previous treatments, reflecting the wide range of therapies that ultimately failed prior to entry into this trial, is detailed in Supplemental Table 3. Dose escalation took place in the planned fashion with no dose limiting toxicity; one patient each at 30 mcg, 60 mcg, 90 mcg, and 120 mcg, followed by six patients at the maximum dose of 180 mcg. Toxicity was minimal with only grade 1 events noted in 2 patients (Table 1).
Table 1.
Patient, treatment, and toxicity data for the ten patients who received the recombinant human hsp110-gp100 chaperone complex melanoma vaccine.
| Patient | Age | Gender | Stage | Prior Surgery* | Prior Radiation | PriorImmunotherapy | Dose Received | Toxicity Event | Number of Events | Grade of Event† |
|---|---|---|---|---|---|---|---|---|---|---|
| PT 01 | 66 | F | IV | Yes | No | Yes | 30 mcg | Erythematous Skin Rash | 1 | 1 |
| PT 02 | 79 | M | IV | Yes | Yes | Yes | 60 mcg | None | 0 | 0 |
| PT 03 | 74 | F | IV | Yes | No | No | 90 mcg | None | 0 | 0 |
| PT 04 | 81 | F | IV | No | Yes | Yes | 120 mcg | None | 0 | 0 |
| PT 05 | 72 | F | IV | Yes | Yes | Yes | 180 mcg | None | 0 | 0 |
| PT 06 | 36 | F | IV | Yes | Yes | Yes | 180 mcg | None | 0 | 0 |
| PT 07 | 46 | F | IIIC | Yes | Yes | Yes | 180 mcg | Fever + Hyperhidrosis | 5 + 1 | 1 |
| PT 08 | 85 | F | IV | No | Yes | Yes | 180 mcg | None | 0 | 0 |
| PT 09 | 48 | M | IV | No | Yes | Yes | 180 mcg | None | 0 | 0 |
| PT 10 | 60 | F | IIIC | Yes | Yes | Yes | 180 mcg | None | 0 | 0 |
For therapeutic Intent
No grade 2 or higher adverse events occurred in the cohort
Clinical Outcome
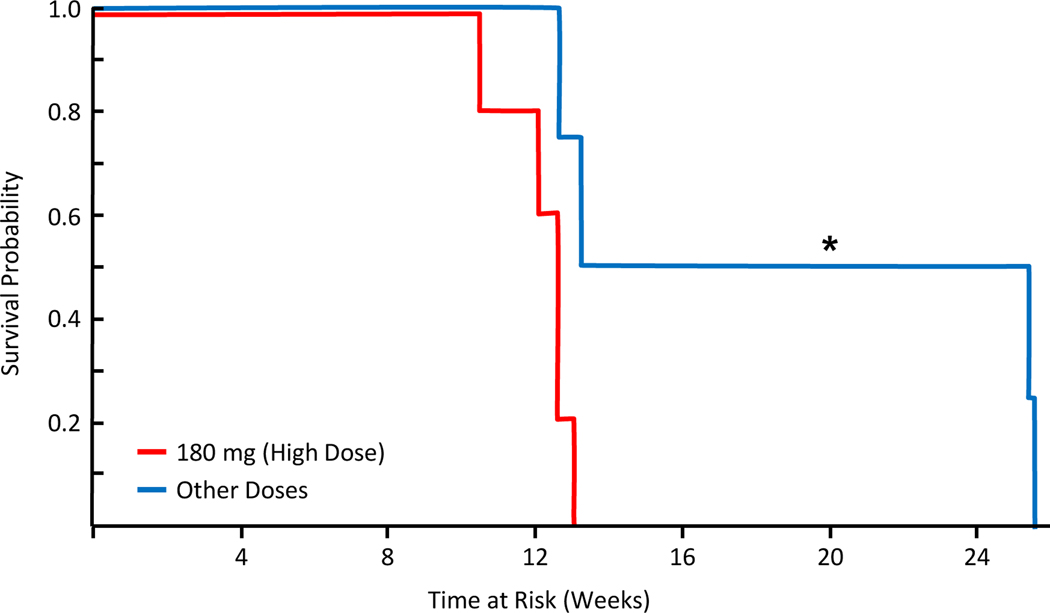
Median progression free survival of the entire cohort was 2.9 months and was found to be longer in patients receiving lower vaccine doses as compared to the maximum dose of 180 mcg (4.5 vs 2.9 months, p=0.018, Figure 1). One patient receiving the 180-mcg dose died at the 10-week time point from disease progression. There were no other patient deaths in the cohort during the time of follow-up. The two patients at the lowest doses (30 and 60 mcg) exhibited clinical tumor responses (1 partial response, 1 stable disease); all other patients exhibited target lesion growth at the first post-vaccination surveillance imaging. A comparison of clinical outcomes from prior immunotherapies versus the vaccine were examined and no associations were noted (Table 2).
Figure 1 –
Lower doses of the Hsp110-gp100 recombinant vaccine were associated with longer progression free survival. Kaplan Meier methods comparing the survival of patients receiving the maximum dose (180mg) of the recombinant vaccine to all others receiving lower doses. Those receiving lower dosage showed increased PFS as measured by RECIST criteria (p=0.018).
Table 2.
A comparison of individual patient clinical outcomes from prior immunotherapies versus the recombinant human hsp110-gp100 chaperone complex melanoma vaccine.
| Patient | Response to Prior Immunotherapy | Vaccine Dose | Number of Vaccine Doses | Outcome of hsp110-gp100 Vaccine |
|---|---|---|---|---|
| PT 01 | Progression | 30 mcg | 3 | Partial Response |
| PT 02 | Partial response and then progression | 60 mcg | 3 | Stable Disease |
| PT 03 | N/A | 90 mcg | 3 | Progression |
| PT 04 | Stable Disease, Progression | 120 mcg | 3 | Progression |
| PT 05 | Progression | 180 mcg | 3 | Progression |
| PT 06 | Progression | 180 mcg | 3 | Progression |
| PT 07 | Mixed Response, Progression | 180 mcg | 3 | Progression |
| PT 08 | Progression | 180 mcg | 2 | Progression |
| PT 09 | Mixed Response, Progression | 180 mcg | 3 | Progression |
| PT 10 | Mixed Response, Progression | 180 mcg | 3 | Progression |
Immunologic Profiles of Peripheral Blood Samples
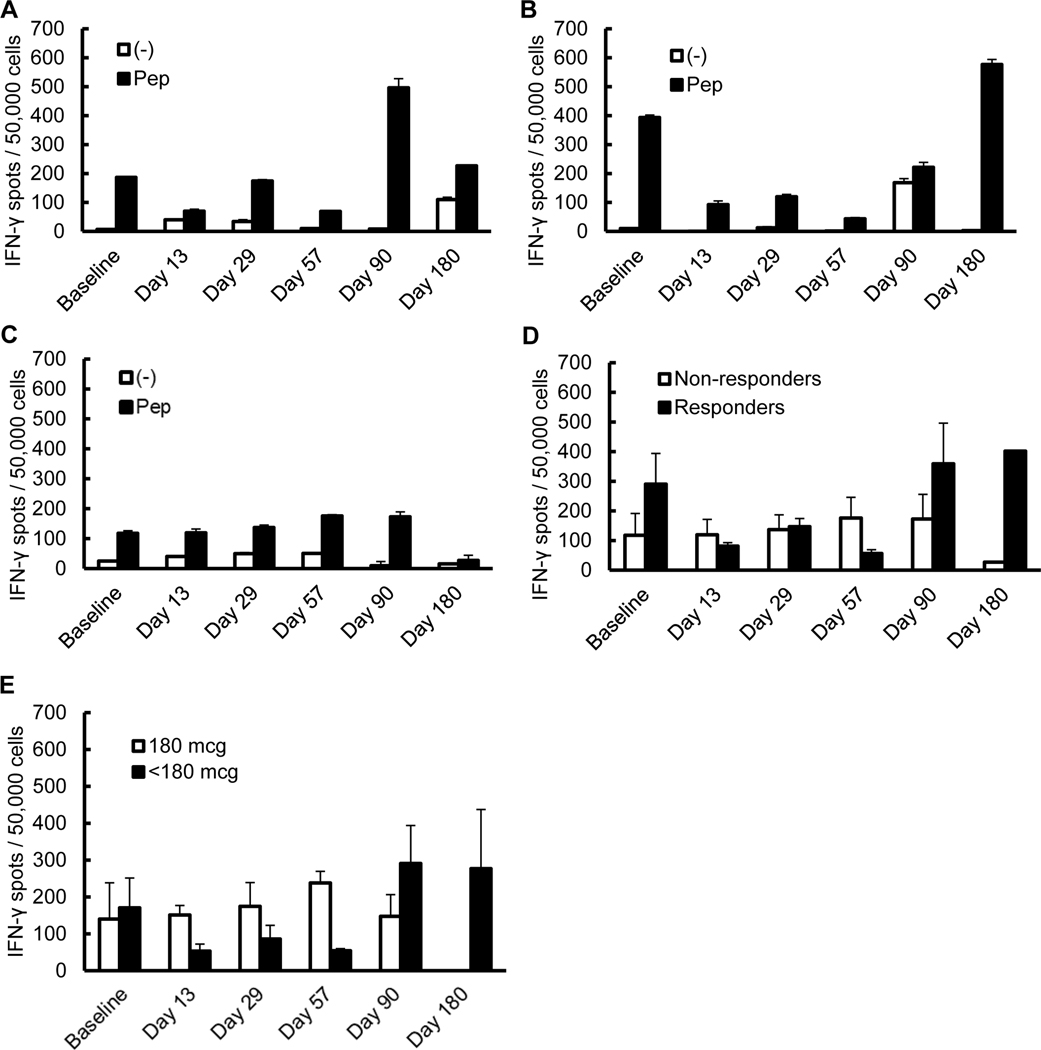
ELISPOT of T-cell samples at designated time points after vaccine inoculation were examined for IFN-γ production with exposure to gp100 versus exposure to a negative control. The highest reactivity was noted in the two patients at the lowest doses who also exhibited clinical tumor response (Figure 2A, B). These responders also had the highest baseline T cell reactivity to gp100 prior to vaccination. Aggregate data across the remaining cohort of non-responders showed increased IFN-γ production with gp100 exposure, but not to the degree of the responders (Figure 2C). Averaged results were compared between responders vs. non-responders and demonstrated a pattern of higher baseline reactivity and higher late time point reactivity in responders (Figure 2D). Patients receiving < 180 mcg doses were also compared to those receiving 180 mcg and were noted to have increased activity, but only at later time points (E).
Figure 2 –
T cell responses to gp100 were noted at higher levels in responders at baseline and following vaccination. ELISPOT assays for IFN-γ production show higher levels in the responders (patient 1 [A] and patient 2 [B]) versus non-responders (C) (patients 3–10, data aggregated in post-hoc analysis). When the groups were compared, patients with clinical responses showed increased reactivity whereas non-responders showed only minor changes and were mostly below the functional readout of the assay (D). Patients receiving < 180 mcg doses versus those receiving 180 mcg were also compared and demonstrated increased activity only at later time points (E).
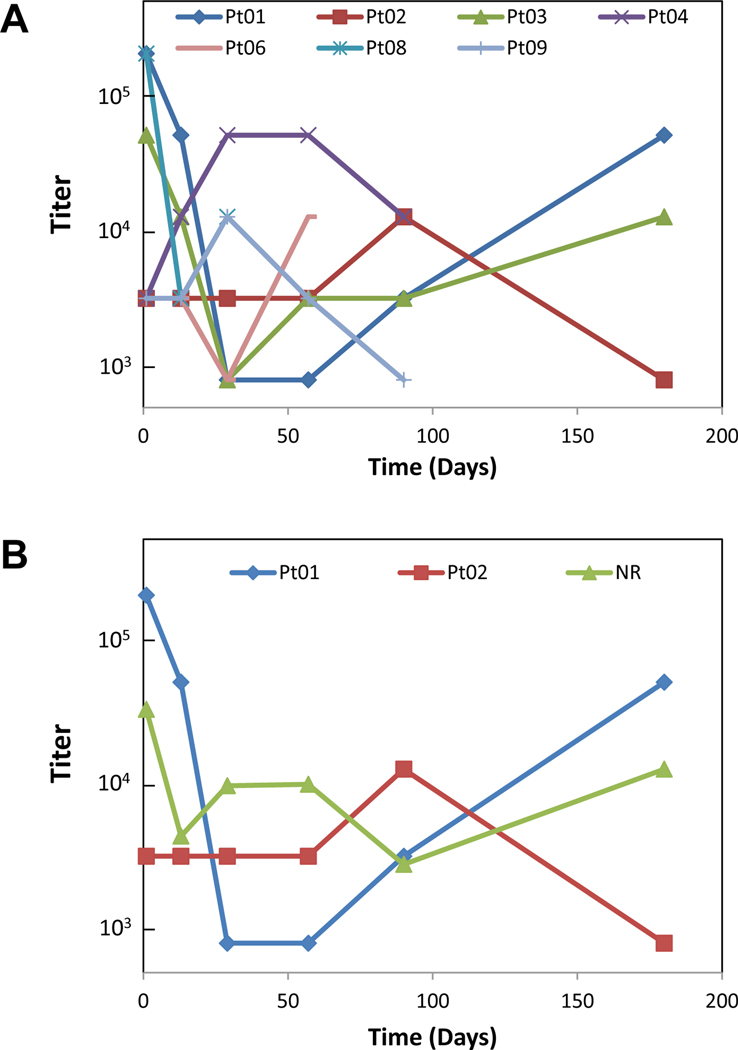
To determine potential B cell responses to the vaccination, gp100-specific IgG antibody was measured by ELISA in the serum of treated patients. Variable responses were noted with no distinct pattern related to the clinical responders (Figure 3A). Similar to the T cell responses, several patients had strong baseline levels of anti-gp100 antibody already present prior to vaccination. Comparing the responders to non-responders, no clear pattern of baseline antibody presence and generation of levels above baseline were noted (Figure 3B).
Figure 3 –
Detection of gp100 specific IgG antibody was variable across patients in the study and had no obvious pattern associated with vaccination. The antibody titers of all available samples were recorded with a variable baseline presence of antibody responses noted (A). Comparison of responders (Pt01 and Pt02) versus non-responders did not exhibit any correlation to clinical outcomes or response to vaccination (B).
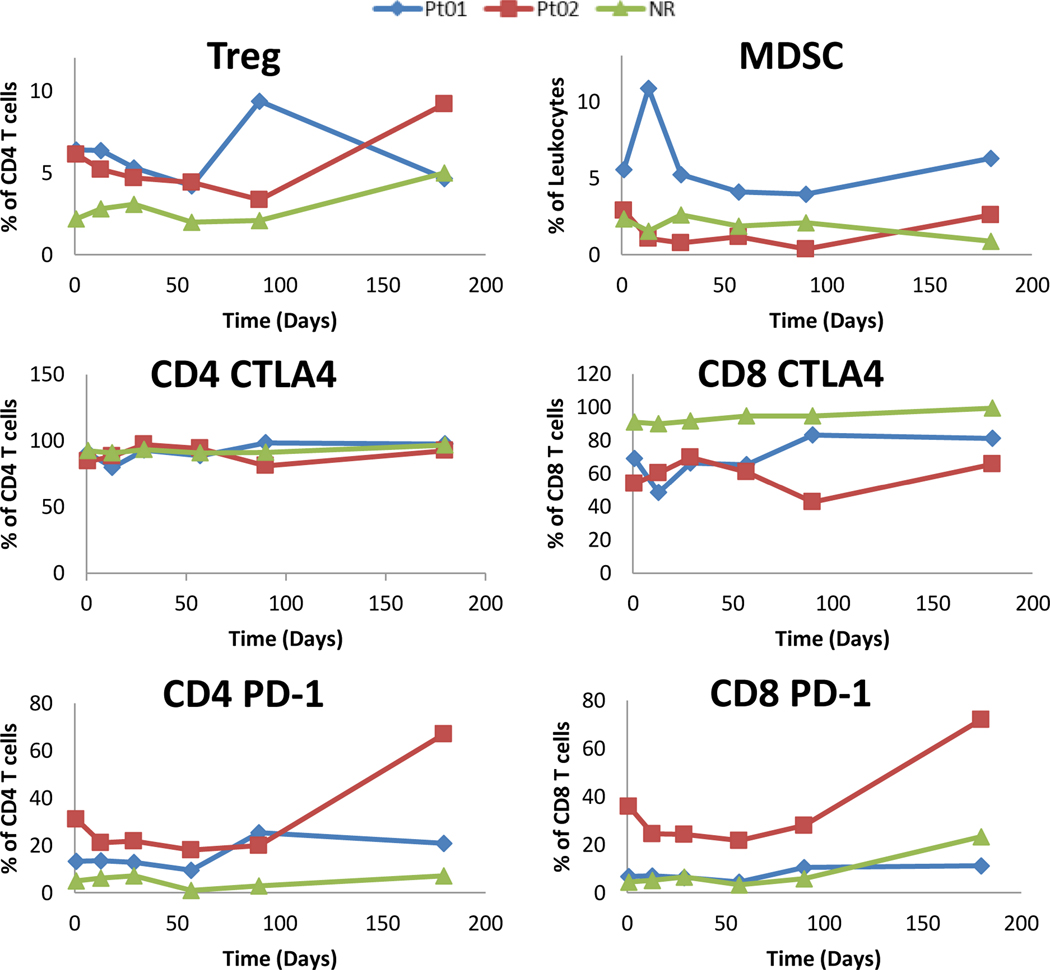
As there appeared to be an association with T cell responses to the vaccine, specific T cell populations were analyzed by flow cytometry and compared between responders and non-responders. There was an increased number of CD4+ T reg already present at higher levels in responders and at the end of the vaccination period (Figure 4). No discernable pattern was noted for MDSC as these tended to be a minor population of the overall number of leukocytes in all patients. The expression of the negative co-stimulatory molecule CTLA4 was uniformly high on all CD4+ T cells measured, but had decreased levels in the CD8+ T cells of responders. Interestingly, the negative co-stimulatory molecule PD-1 appeared upregulated in CD4+ T cells of responders with mixed responses in the CD8+ T cells (Figure 4). No obvious differences or patterns of expression were noted with the co-stimulatory molecules TIM3 or LAG3 in the T cell populations of responders or non-responders (data not shown).
Figure 4 –
Flow cytometry demonstrated higher levels of Treg and PD-1 expressing T cells in responders (Pt01 and Pt02) as compared to non-responders. The majority of patients did not have elevated MDSC populations and CTLA-4 expression was lower in CD8+ T cells of responders as compared to non-responders.
DISCUSSION
Heat shock proteins provide a natural link between the innate and adaptive immune responses by combining properties of antigen carriage (chaperoning) with activation of antigen-presenting cell.[32] Therefore, the relevance of hsp immune activation to cancer may be profound and their potential contributions toward clinical outcomes underappreciated. In colorectal cancer, Hsp110 overexpression has been shown to induce sensitization to anticancer agents such as oxaliplatin and 5-fluorouracil, which are routinely prescribed in the adjuvant setting[33]. Our early preclinical work showed that genetic modification of poorly immunogenic murine cancers for Hsp110 overexpression significantly enhanced tumor immunogenicity[32]. Clinical investigation to leverage Hsp70 in breast cancer and Gp96 in glioma and renal and ovarian cancers is ongoing[34]. Recently, the broad and potent antigen-presenting ability of hsp have also been used for development of prophylactic vaccines against infectious diseases including tuberculosis, HSV, and meningitis[35–37]. The unique properties of hsp have demonstrated clinical efficacy in mouse models of melanoma. The protein antigen carried by Hsp110 contains a large reservoir of potential peptides for stimulating polyepitope-directed T cells, which is also supported by our preclinical observation that the protective antitumor effect elicited by a chaperone-protein antigen complex vaccine was more potent than that achieved by a chaperone-peptide antigen complex vaccine [29].
There were felt to be several ideal features of a fully recombinant Hsp110 gp100 chaperone complex melanoma vaccine approach: low toxicity, simple in vitro production, no need for autologous tumor, no HLA restriction, no co-administration with an “adjuvant”, stimulation of both the cellular and humoral arms of the immune system, and the use of a well-defined tumor antigen which is maximally loaded within the vaccine. The synthetic production of the recombinant chaperone complex also meant that the vaccine could be generated in unlimited quantities with significant uniformity from batch to batch. To date, it has remained stable at minus 70˚C for over 5 years, allowing for the possibility of widespread distribution and standardized multi-institutional use. Finally, a synthetic “building block” approach of combining antigen with hsp could ultimately be used to produce a polyvalent vaccine that could overcome “antigen loss” by a subset of tumor cells secondary to immunologic selection pressure from a single antigen vaccine. While it should be ackowledged that not all tumors and patients share the antigens within a given recombinant vaccine, the numerous clinical advantages listed above still make it an appealing option when compared to autologous derived hsp. In practice, our currently reported Phase Ib study showed that a recombinant human Hsp110-gp100 chaperone complex melanoma vaccine had minimal toxicity across a population of pretreated, advanced stage melanoma patients and produced measurable tumor responses at lower doses, which also correlated with more robust immune responses.
The melanoma patient population accrued to this study was extremely diverse in terms of anatomic location of their primary melanoma, extent of prior therapies, and exposure to contemporaneous systemic therapies. Despite having limited options outside of a clinical trial, these heavily pre-treated patients universally tolerated the vaccine regimen with almost no toxicity. As the vaccination was given as a simple intradermal injection at day 1 then approximately 2 and 6 weeks later, there was broad acceptance by patients. Unlike current intra-tumoral clinical trials, imaging or interventions to access tumors and burdensome injection schedules were not necessary. In this study, 90% of patients also previously received some form of immunotherapy with all of them having progressive disease at the time of enrollment. Although there was no obvious correlation between response to the vaccine and prior immune therapy responses, it was observed that the lowest doses of vaccine appeared to have the best clinical responses and T cell immune activation. While this would seem counterintuitive, there is preclinical data to support this finding. It has been shown that administration of large doses of tumor-derived hsp does not elicit tumor immunity in mice, suggesting that there may be dose restriction of immunogenicity of tumor-derived hsp as a vaccine[38]. Unlike pharmacologic drugs, immune agents such as this vaccine may have an effective biological dose range that is not accurately determined by standard dose escalation/toxicity protocols.
Analysis of the systemic immune changes to the vaccine favored T cell responses as gp100 antibody production did not appear to be induced, consistent with preclinical data[11, 35, 39]. Interestingly, there was some degree of existing immune reactivity towards gp100 as baseline samples had evidence of B and T cell response in most patients. These differences did not appear to correspond to eventual vaccine responses. Specific to the T cell responses, the gp100 antigen-specific production of IFN-γ was notably higher in patients who responded clinically to the vaccine. Interestingly, we noted that the T-cell reactivity of peripheral samples to gp100 in these patients initially seemed to diminish from baseline until 90 days from the first vaccine dose (Figure 2D). By only sampling T-cells peripherally, we are not fully capturing the reaction within the tumor microenvironment (TME). As seen with Tumor Infiltrating Lymphocytes (TIL) in many other melanoma studies, it is likely that the most reactive immune cells become sequestered at the sites of disease and react within the TME rather than peripherally.[5, 40] As such, it is possible that, in isolating the T-cells from the peripheral blood of responders, we were only capturing their circulating populations and not those that were the most responsive to gp100. As the tumors then became more evasive of antigen recognition, these T-cells returned to the circulation and were captured in our later peripheral blood samples. It is also possible that our peripheral blood analysis did not capture all cell types within the TME that could be drivers of the immune response. Future studies would likely benefit from sampling immune cells in resected tumor specimens, if available. Finally, flow cytometric analyses indicated that the clinically responding patients had an on-going immune response with increases in Treg and PD-1 positive T cell populations. Lower levels of CTLA4 in the CD8+ T cells of responders may indicate a pool of T cells that are either not fully activated and/or are less susceptible to tumor-mediated immunosuppression.
There are several potential limitations to our study. The trial was originally designed to primarily assess for adverse events and was performed in a very small cohort of patients, limiting conclusions regarding response. Additionally, the effective dosing range of the vaccine was not yet known, so the escalation schedule may have been suboptimal. Although we did find a statistically significant difference in survival and measurable tumor response between lower versus higher doses, more detailed analyses are statistically limited due to the very small number of patients. Furthermore, while 90% of patients in this trial had previously received some form of immunotherapy, this was done in a non-standardized fashion in terms of timing or selection of therapy. It is possible that delayed immunologic responses or a cumulative reaction from multiple therapies might have been responsible for the observed vaccine responses. As most of the patients had progressive disease from their prior immunotherapy treatments, the potential for a delayed response seems unlikely. Lastly, immunologic data from peripheral blood draws were limited in some patients that were non-responders past 90 days as they chose to leave the trial upon knowledge of disease progression. Observations made from the peripheral blood may also not truly reflect the processes happening in the tumor microenvironment or secondary nodal tissue.
When the concept for this phase I fully recombinant human hsp melanoma vaccine clinical trial was being developed, there were almost no effective therapies for advanced, unresectable melanoma. Overall response rates to interleukin-2 (IL 2) approach 24% with a 7–8% complete response rate[41]. However, treatment related toxicity is very high, limiting its applicability to most patients with metastatic melanoma[42]. The currently available treatments for stage IV melanoma, either anti-PD-1 therapy or BRAF/MEK inhibitors, were just being evaluated in early stage clinical trials. Although these treatments have now dramatically improved survival for advanced melanoma, many patients will still be nonresponders. Only half of melanoma patients will be BRAF mutant, excluding this targeted therapy treatment option. Anti-PD-1 therapies are expensive, require intravenous administration, and can cause prolonged toxicities that might not resolve with discontinuation of treatment as well as a very low, but not inconsequential treatment related mortality. Consequently, there is still a clinical need for low cost, low toxicity immune-based therapies for metastatic melanoma.
Although a Phase 2 study of the Hsp110-gp100 recombinant vaccine alone at a dose of 30–60 mcg in a larger cohort of patients could further define its therapeutic role, a more interesting approach would be to assess its safety and efficacy specifically in combination with other immune therapies. Based on our observation of increasing PD-1 expression over time and its correlation with response, combining the Hsp110-gp100 vaccine with anti-PD-1 immunotherapies may enhance the therapeutic potential. Additional options would include the administration of the vaccine in the adjuvant setting, following surgery in high-risk patients. A next-generation chaperone-based immune modulator with enhanced immunostimulatory capacity could also be considered for vaccine development[43]. Finally, preclinical murine data from our laboratory have shown that coadministration of multiple hsp vaccines complexed with different antigens (ex. a recombinant Hsp110-gp100 vaccine with a second recombinant Hsp110 vaccine utilizing antigen TRP2) inhibited tumor growth more than either vaccine alone[29]. While this strategy has not yet been evaluated in humans, further trials could be performed with a polyvalent Hsp110-melanoma antigen chaperone complex vaccine.
Conclusions
In heavily pretreated patients with advanced melanoma, a recombinant human Hsp110-gp100 chaperone complex vaccine had minimal toxicity and resulted in measurable tumor responses at lower doses (with corresponding immune activation). Further study is warranted in combination with currently available immunotherapies, development of multiantigen melanoma vaccines, and the potential use of Hsp110 vaccine strategies for other cancers.
Supplementary Material
Synopsis.
Recombinant vaccines using heat shock proteins complexed with tumor antigens have shown promising results in murine models. A Phase Ib study of a recombinant Hsp110-gp100 vaccine in human melanoma was undertaken and demonstrated minimal toxicity, measurable tumor response, and T cell activation.
Acknowledgments
Supported by: Grant #736285: “Development and Clinical Implementation of a Novel Recombinant Human Hsp110 gp100/ Hsp110-TRP2 Chaperone Complex Polyvalent Melanoma Vaccine” and “Large Scale GMP Production of a Novel Recombinant Human Hsp110 gp100 Chaperone Complex Melanoma Vaccine for Clinical Phase I Assessment”) and R01 CA099326 “Large heat shock proteins and their role in cancer therapy”
References
- 1).Domingues B, Lopes JM, Soares P, Populo H. Melanoma treatment in review. Immunotargets Ther. 2018;7:35–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2).Siegel RL, Miller KD, FH E, Jemal A Cancer statistics, 2021. CA Cancer J Clin. 2021;71(1):7–33. [DOI] [PubMed] [Google Scholar]
- 3).Howlader N NA, Krapcho M, et al. SEER Cancer Statistics Review, 1975–2017. SEER. 2019. [Google Scholar]
- 4).Cancer Facts & Figures 2021. Atlanta, GA: American Cancer Society; 2021. [Google Scholar]
- 5).Gasser S, Lim LHK, Cheung FSG. The role of the tumour microenvironment in immunotherapy. Endocr Relat Cancer. 2017;24(12):T283–T95. [DOI] [PubMed] [Google Scholar]
- 6).Martins F, Sofiya L, Sykiotis GP, Lamine F, Maillard M, Fraga M, et al. Adverse effects of immune-checkpoint inhibitors: epidemiology, management and surveillance. Nat Rev Clin Oncol. 2019;16(9):563–80. [DOI] [PubMed] [Google Scholar]
- 7).Ott PA, Fritsch EF, Wu CJ, Dranoff G. Vaccines and melanoma. Hematol Oncol Clin North Am. 2014;28(3):559–69. [DOI] [PubMed] [Google Scholar]
- 8).Nakamura K, Okuyama R. Immunotherapy for advanced melanoma: Current knowledge and future directions. J Dermatol Sci. 2016;83(2):87–94. [DOI] [PubMed] [Google Scholar]
- 9).Chi M, Dudek AZ. Vaccine therapy for metastatic melanoma: systematic review and meta-analysis of clinical trials. Melanoma Res. 2011;21(3):165–74. [DOI] [PubMed] [Google Scholar]
- 10).Oh HJ, Easton D, Murawski M, Kaneko Y, Subjeck JR. The chaperoning activity of hsp110. Identification of functional domains by use of targeted deletions. J Biol Chem. 1999;274(22):15712–8. [DOI] [PubMed] [Google Scholar]
- 11).Wang XY, Chen X, Manjili MH, Repasky E, Henderson R, Subjeck JR. Targeted immunotherapy using reconstituted chaperone complexes of heat shock protein 110 and melanoma-associated antigen gp100. Cancer Res. 2003;63(10):2553–60. [PubMed] [Google Scholar]
- 12).Easton DP, Kaneko Y, Subjeck JR. The hsp110 and Grp1 70 stress proteins: newly recognized relatives of the Hsp70s. Cell Stress Chaperones. 2000;5(4):276–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13).Wang XY, Subjeck JR. High molecular weight stress proteins: Identification, cloning and utilisation in cancer immunotherapy. Int J Hyperthermia. 2013;29(5):364–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14).Tamura Y, Peng P, Liu K, Daou M, Srivastava PK. Immunotherapy of tumors with autologous tumor-derived heat shock protein preparations. Science. 1997;278(5335):117–20. [DOI] [PubMed] [Google Scholar]
- 15).Ishii T, Udono H, Yamano T, Ohta H, Uenaka A, Ono T, et al. Isolation of MHC class I-restricted tumor antigen peptide and its precursors associated with heat shock proteins hsp70, hsp90, and gp96. J Immunol. 1999;162(3):1303–9. [PubMed] [Google Scholar]
- 16).Singh-Jasuja H, Toes RE, Spee P, Munz C, Hilf N, Schoenberger SP, et al. Cross-presentation of glycoprotein 96-associated antigens on major histocompatibility complex class I molecules requires receptor-mediated endocytosis. J Exp Med. 2000;191(11):1965–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17).Castellino F, Boucher PE, Eichelberg K, Mayhew M, Rothman JE, Houghton AN, et al. Receptor-mediated uptake of antigen/heat shock protein complexes results in major histocompatibility complex class I antigen presentation via two distinct processing pathways. J Exp Med. 2000;191(11):1957–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18).Asea A, Kraeft SK, Kurt-Jones EA, Stevenson MA, Chen LB, Finberg RW, et al. HSP70 stimulates cytokine production through a CD14-dependant pathway, demonstrating its dual role as a chaperone and cytokine. Nat Med. 2000;6(4):435–42. [DOI] [PubMed] [Google Scholar]
- 19).Basu S, Binder RJ, Suto R, Anderson KM, Srivastava PK. Necrotic but not apoptotic cell death releases heat shock proteins, which deliver a partial maturation signal to dendritic cells and activate the NF-kappa B pathway. Int Immunol. 2000;12(11):1539–46. [DOI] [PubMed] [Google Scholar]
- 20).Singh-Jasuja H, Scherer HU, Hilf N, Arnold-Schild D, Rammensee HG, Toes RE, et al. The heat shock protein gp96 induces maturation of dendritic cells and down-regulation of its receptor. Eur J Immunol. 2000;30(8):2211–5. [DOI] [PubMed] [Google Scholar]
- 21).Facciponte JG, Wang XY, Subjeck JR. Hsp110 and Grp170, members of the Hsp70 superfamily, bind to scavenger receptor-A and scavenger receptor expressed by endothelial cells-I. Eur J Immunol. 2007;37(8):2268–79. [DOI] [PubMed] [Google Scholar]
- 22).Binder RJ HD, Srivastava PK. CD91: a receptor for heat shock protein gp96. Nat Immunol. 2000;1(2):151–5. [DOI] [PubMed] [Google Scholar]
- 23).Basu S BR, Ramalingam T, Srivastava PK. CD91 is a common receptor for heat shock proteins gp96, hsp90, hsp70, and calreticulin. Immunity. 2001;14(3):303–13. [DOI] [PubMed] [Google Scholar]
- 24).Janetzki S PD, Rosenhauer V, et al. Immunization of cancer patients with autologous cancer-derived heat shock protein gp96 preparations: a pilot study. Int J Cancer 2000;88:232–8. [DOI] [PubMed] [Google Scholar]
- 25).Eton O RM, East MJ, et al. Autologous tumor-derived heat-shock protein peptide complex-96 (HSPPC-96) in patients with metastatic melanoma. J Transl Med 2010;8:9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26).Belli F TA, Rivoltini L, et al. Vaccination of metastatic melanoma patients with autologous tumor-derived heat shock protein gp96-peptide complexes: clinical and immunologic findings. J Clin Onc. 2002;20:4169–80. [DOI] [PubMed] [Google Scholar]
- 27).Testori A, Richards J, Whitman E, Mann GB, Lutzky J, Camacho L, et al. Phase III comparison of vitespen, an autologous tumor-derived heat shock protein gp96 peptide complex vaccine, with physician’s choice of treatment for stage IV melanoma: the C-100–21 Study Group. J Clin Oncol. 2008;26(6):955–62. [DOI] [PubMed] [Google Scholar]
- 28).Manjili MH, Wang XY, Chen X, Martin T, Repasky EA, Henderson R, et al. HSP110-HER2/neu chaperone complex vaccine induces protective immunity against spontaneous mammary tumors in HER-2/neu transgenic mice. J Immunol. 2003;171(8):4054–61. [DOI] [PubMed] [Google Scholar]
- 29).Wang XY, Sun X, Chen X, Facciponte J, Repasky EA, Kane J, et al. Superior antitumor response induced by large stress protein chaperoned protein antigen compared with peptide antigen. J Immunol. 2010;184(11):6309–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30).de Vries TJ, Fourkour A, Wobbes T, Verkroost G, Ruiter DJ, van Muijen GN. Heterogeneous expression of immunotherapy candidate proteins gp100, MART-1, and tyrosinase in human melanoma cell lines and in human melanocytic lesions. Cancer Res. 1997;57(15):3223–9. [PubMed] [Google Scholar]
- 31).Rosenberg SA, Yang JC, Schwartzentruber DJ, Hwu P, Marincola FM, Topalian SL, et al. Immunologic and therapeutic evaluation of a synthetic peptide vaccine for the treatment of patients with metastatic melanoma. Nat Med. 1998;4(3):321–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32).Wang XY, Li Y, Manjili MH, Repasky EA, Pardoll DM, Subjeck JR. Hsp110 over-expression increases the immunogenicity of the murine CT26 colon tumor. Cancer Immunol Immunother. 2002;51(6):311–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33).Dorard C, de Thonel A, Collura A, Marisa L, Svrcek M, Lagrange A, et al. Expression of a mutant HSP110 sensitizes colorectal cancer cells to chemotherapy and improves disease prognosis. Nat Med. 2011;17(10):1283–9. [DOI] [PubMed] [Google Scholar]
- 34).McNulty S, Colaco CA, Blandford LE, Bailey CR, Baschieri S, Todryk S. Heat-shock proteins as dendritic cell-targeting vaccines--getting warmer. Immunology. 2013;139(4):407–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35).Zuo D, Subjeck J, Wang XY. Unfolding the Role of Large Heat Shock Proteins: New Insights and Therapeutic Implications. Front Immunol. 2016;7:75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36).Walker KB, Keeble J, Colaco C. Mycobacterial heat shock proteins as vaccines - a model of facilitated antigen presentation. Curr Mol Med. 2007;7(4):339–50. [DOI] [PubMed] [Google Scholar]
- 37).Wald A, Koelle DM, Fife K, Warren T, Leclair K, Chicz RM, et al. Safety and immunogenicity of long HSV-2 peptides complexed with rhHsc70 in HSV-2 seropositive persons. Vaccine. 2011;29(47):8520–9. [DOI] [PubMed] [Google Scholar]
- 38).Chandawarkar RY, Wagh MS, Srivastava PK. The dual nature of specific immunological activity of tumor-derived gp96 preparations. J Exp Med. 1999;189(9):1437–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39).Guo C, Subjeck JR, Wang XY. Creation of Recombinant Chaperone Vaccine Using Large Heat Shock Protein for Antigen-Targeted Cancer Immunotherapy. Methods Mol Biol. 2018;1709:345–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40).Skitzki J CR, Okuyama R, Knibbs R, et al. Donor cell cycling, trafficking, and accumulation during adoptive immunotherapy for murine lung metastases. Cancer Research. 2004;64(6):2183–91. [DOI] [PubMed] [Google Scholar]
- 41).Bastholt L, Svane IM, Bjerregaard JK, Herrstedt J, Hrobjartsson A, Schmidt H. High-dose interleukin-2 and interferon as first-line immunotherapy for metastatic melanoma: long-term follow-up in a large unselected Danish patient cohort. Eur J Cancer. 2019;115:61–7. [DOI] [PubMed] [Google Scholar]
- 42).Atkins MB, Lotze MT, Dutcher JP, Fisher RI, Weiss G, Margolin K, et al. High-dose recombinant interleukin 2 therapy for patients with metastatic melanoma: analysis of 270 patients treated between 1985 and 1993. J Clin Oncol. 1999;17(7):2105–16. [DOI] [PubMed] [Google Scholar]
- 43).Yu X, Guo C, Yi H, Qian J, Fisher PB, Subjeck JR, et al. A multifunctional chimeric chaperone serves as a novel immune modulator inducing therapeutic antitumor immunity. Cancer Res. 2013;73(7):2093–103. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.