Abstract
The embolic middle cerebral artery occlusion (eMCAO) model mimics ischemic stroke due to large vessel occlusion in humans and is amenable to thrombolytic therapy with rtPA. However, two major obstacles, the difficulty of the eMCAO surgery and unpredictable occurrence of clot autolysis, had impeded its application in mice. In this study, we modified catheters to produce suitable fibrin-rich embolus and optimized the eMCAO model using cerebral blood flow (CBF) monitored by both laser Doppler flowmetry (LDF) and 2D laser speckle contrast imaging (LSCI) to confirm occlusion of MCA. The results showed that longer embolus resulted in higher mortality. There was a compensatory increase in MCA territory perfusion after eMCAO associated with decreased infarct volume; however, this was only partly dependent on recanalization as clot autolysis was only observed in ∼30% of mice. Cortical CBF monitoring with LSCI showed that the size of peri-core area at 3 h displayed the best correlation with infarct volume that is attributed to compensatory collateral blood flow. The peri-core area best predicted functional outcome after eMCAO. In summary, we developed a reliable eMCAO mouse model that better mimics embolic ischemic stroke in humans, which will increase the potential for successful translation of stroke neuroprotective therapies.
Keywords: Animal model, cerebral blood flow, embolic MCAO, laser speckle, thrombolytic therapy
Introduction
Stroke is the second leading cause of death worldwide and disabilities due to stroke continues to increase.1–3 Ischemic stroke accounts for approximately 87% of all strokes. 3 Intravenous injection of recombinant tissue plasminogen activator (rtPA) within 4.5 h or endovascular thrombectomy within 24 h after stroke onset are two effective therapeutic strategies recommended by the American Heart Association (AHA) for clinical treatment of ischemic strokes. 4 rtPA is still the only Food and Drug Administration (FDA)-approved drug for ischemic stroke; however, a narrow therapeutic window and a high risk of hemorrhagic transformation limit its application in intra-arterial thrombolysis following ischemia.5,6 To this end, suitable ischemic animal models, which more closely simulate events occurring in stroke patients are needed to assess the effect of therapeutic strategies in pre-clinical studies.7,8
To date, a variety of stroke models have been developed in animals, including the transient mechanical occlusion model with intraluminal filament, 9 the embolic occlusion model with preformed fibrin-rich clot,10,11 the thromboembolic occlusion model with thrombin injection,12,13 the permanent focal photothrombotic occlusion model, 14 and atherothrombotic model induced by intra-arterial injection of collagen or thrombin.13,15 Among them, occlusion of the MCA (MCAO) using an intraluminal filament is the most widely used technique in rodents due to its stability and reproducibility. 9 However, recanalization of the cerebral artery after 1 h of ischemia does not simulate the pathological process in most human strokes, as up to 80% of human strokes are triggered by embolism or thrombosis, and have low early reperfusion after ischemia onset. Therefore, thromboembolic MCAO is increasingly being used to investigate thrombolytic and neuroprotective agents, as it more closely resembles the pathophysiology of ischemic stroke with varying degrees of tissue reperfusion, thereby providing a better model in which to test thrombolytic therapies.16,17 The embolic MCAO (eMCAO) model was first reported in rats in 1982, 18 and was further optimized by Overgaard et al. in 1992, 19 and Zhang et al. in 1997. 20 Recently, Zhang et al. 10 developed a standard procedure for focal eMCAO in rats. This autologous clot animal model strongly mimics the thromboembolic stroke in humans, but unpredictable recanalization and variation in infarct volume limit its application in studies examining neuroprotective therapies.10,11,17,21
With the widespread use of transgenic mice in stroke research, it is imperative that a reliable ischemic stroke model be developed in mice. Herein, we optimized eMCAO using standardized catheters to simplify the preparation of emboli to produce more dependable and reproducible infarct volumes. To verify the occlusion of the MCA and the existence of clots, LSCI was used to monitor CBF at various time points along with LDF. Correlational analysis and a receiver operating characteristic (ROC) curve were used to determine whether the indices recorded from LSCI could be used to predict the outcome of eMCAO. Given the controversial outcomes between different treatment time points of rtPA in rodents,22–24 we also examined the effect of intra-arterial rtPA administration at different time points in this eMCAO mouse model to determine if it can be reliably used to assess the effect of therapeutic strategies.
Material and methods
Methodological details beyond the descriptions below are provided in supplemental file.
Animals
Male adult C57BL/6 mice (25–30 g, 10–12 weeks old) were obtained from the Laboratory Animal Center, Chinese Academy of Sciences (Shanghai, China). Animals were housed in a temperature- and humidity-controlled animal facility with a 12-h light/dark cycle. A rodent diet and water were available ad libitum. All animal procedures were approved by the Animal Care and Use Committee of Fudan University and performed by following the National Institutes of Health Guide for the Care and Use of Laboratory Animals. All animal experiments were in compliance with the ARRIVE guidelines. 25
Murine model of eMCAO
Animal surgery was performed under an operating microscope. Mice were anesthetized with 1.5% isoflurane in a 30% O2/70% N2 gas mixture. The left common carotid artery (CCA) and the internal carotid artery (ICA) were exposed through a midline cervical skin incision and temporarily ligated with suture. The superior thyroid artery (STA) was permanently coagulated. The external carotid artery (ECA) was ligated and a small puncture was made approximately 1.5–2.0 mm from the bifurcation. An embolus in the modified catheter (Figure S1(c)) was introduced into the lumen of the ECA through the small puncture. A suture was placed around the origin of the ECA to fasten the catheter into place and to prevent bleeding. After releasing the ICA ligature, the catheter was advanced beyond the pterygopalatine artery (PPA) branch and the clot was injected into the ICA with a short burst gently (Figure S1(e)). Then the catheter was removed, and the ECA was ligated permanently. After release of the CCA ligature, the wound was sutured and disinfected with iodine. Rectal temperature was maintained at 37 ± 0.5°C with a temperature-controlled heating pad. All efforts were made to minimize animal suffering during the surgical procedure. Animals with a decrease in CBF less than 70% of pre-ischemia baseline levels immediately after embolus injection were excluded from further experimentation. The clot was found to be located right at the origin of the MCA in mice with a sharp decrease in CBF to less than 30% of baseline after surgery (Figure S1(d)). Mice in the sham group were similarly anesthetized and underwent the same surgery without clot injection.
Cortical CBF measurements
CBF was measured by LDF (PeriFlux System 5000; Perimed AB, Sweden) and LSCI (PeriCam PSI System; Perimed, Järfälla, Sweden). The laser Doppler probe was affixed to the surface of the left side of the skull around the mid-point between the left ear and left eye with the origin of the MCA cortical bifurcation localized underneath. The initial drop in CBF after MCAO was assessed by LDF. Blood perfusion was also monitored by LSCI at the indicated time points (Figure S1(a), before ischemia, 5 min, 1, 3, and 24 h post ischemia). CBF could not be simultaneously recorded by both LDF and LSCI because of the impact of light derived from LDF on the laser speckle images. The images taken by LSCI were analyzed using PIMsoft (Perimed AB, Stockholm, Sweden). The perfusion unit of the MCA cortical branch was calculated at the origin of this artery in images captured by LSCI and was expressed as the percentage of the perfusion unit which indicates the time point to the perfusion baseline. The core area was reduced blood flow area defined as CBF ≤ 30% of baseline and the peri-core area was reduced blood flow area defined as 30% < CBF ≤ 50% of baseline. We listed the representative image of CBF and the related core and peri-core area calculated based on CBF change at pre, 5 min, 3 h and 24 h in Figure S3(a). These three indices were filtered and measured by investigators blinded to the experimental group assignment.
Statistical analysis
All data are reported as mean ± SD. Pearson product linear regression analysis was used to correlate the 2D laser speckle images with infarct volume and behavioral performance, respectively. ROC analysis was performed to determine which index was the best predictor of ischemic outcomes. The difference in means between two groups was assessed by the two-tailed Student’s t test (normal distribution) or Mann–Whitney test (non-normal distribution). One- or two-way ANOVA followed by the Bonferroni/Dunn post hoc test was used to determine the statistical differences in cerebral blood flow and neurological outcomes among means of multiple groups. p ≤ 0.05 was considered statistically significant. All statistics are summarized in Table S1.
Results
Embolic MCAO induced by autologous and allogeneic blood clots results in comparable outcomes
Autologous blood clots as observed in humans could be mimicked in the mouse eMCAO model by collecting blood from the femoral artery of each mouse one day before MCAO surgery. However, preparation of the clot takes time and restricted blood supply after blood collection surgery could result in sensorimotor deficits in the hindlimb. Thus, considering C57BL/6 mice are an inbred strain, the use of allogeneic blood clots is potentially an alternative to avert the aforementioned disadvantages. Therefore, the differences between autologous and allogeneic emboli were initially compared. We calculated the reduced blood flow area by the CBF reduction monitored by LSCI. The core area was defined as CBF ≤ 30% of baseline and the peri-core area was 30% < CBF ≤ 50% of baseline (Figure S3(a)). Although cortical CBF core area was transiently increased in mice induced by autologous blood clot 5 min after surgery (Figure S2(b)), no significant difference was detected in infarct volume (Figure S2(c) and (d)) between the two groups, suggesting that both autologous and allogeneic emboli could be used for embolic stroke in C57BL/6 mice. To compare the clinical effect of eMCAO induced by the two types of emboli, mice were divided into four outcome categories: dead (died within 24 h after surgery), infarct volume (IV) ≥ 25 mm3, IV < 25 mm3, and failed (no sudden drop in rCBF monitored by LDF). The percentage of death was comparable in the two groups. And the percentage of failure was higher in the mice that received the allogeneic clot, about 43.18% of these mice developed an infarct volume larger than 25 mm3, compared to mice that received the autologous clot (36.84%) (Figure S2(e)). Therefore, allogeneic clots were used in subsequent experiments.
Optimization of clot length to produce a reliable eMCAO model in mice
The length of the MCA thrombus is correlated with clinical outcomes after ischemic stroke. Longer thrombus is associated with worse clinical consequences in patients,26–28 as well as in experimental animals.29,30 Thus, we compared clots with three different lengths (5, 10 and 15 mm) to investigate the impact of clot length on CBF, infarct volume, and neurological function. The area of the ischemic core and peri-core was compared between the clots of different lengths at 5 min, 3 h, and 24 h after ischemic insult. There was a substantial increase in the ischemic core area in the 10 mm- and 15 mm-clot mice compared to the 5 mm-clot mice 5 min and 24 h after ischemia, whereas no significant difference was detected 3 h following ischemia (Figure 1(a) and (b) top panel). In contrast to the ischemic core, no significant difference was observed in the peri-core area between the three groups within 3 h of the ischemic insult (Figure 1(a) and (b) bottom panel). The peri-core area was augmented in mice with the 15 mm clot compared to the 5 mm clot group 24 h after ischemia (Figure 1(b) bottom panel). The data support that eMCAO causes immediate reduction in CBF which is proportional to the clot length (5–15 mm) and sustained for at least 24 h (Figure 1(a) and (b)).
Figure 1.
Longer clots are prone to result in more severe injury. Mice were subjected to eMCAO induced by clots with different lengths (5 mm, 10 mm, or 15 mm). (a) Representative 2D laser speckle images of cortical CBF before ischemia (Pre) and at 5 min, 3 h and 24 h after clot injection. Scale bar = 3 mm. (b) Quantification of the core (CBF ≤ 30% of baseline) and peri-core (30% < CBF ≤ 50% of baseline) area at indicated time points after surgery. n = 20 per group. *p ≤ 0.05, **p ≤ 0.01, ***p ≤ 0.001 using Kruskal–Wallis followed by Dunn post hoc test or two-way ANOVA followed by Bonferroni post hoc test. (c) Representative brain images of TTC staining 24 h after eMCAO induced by clots with different lengths. The black dashed line encompasses the infarct area in each slice. (d) Quantification of infarct volume by TTC staining 24 h after stroke. n = 20 per group. ns: no significant difference using one-way ANOVA followed by Bonferroni post hoc test. (e) The outcomes at 24 h were classified into four categories: (1) dead: mouse dead within 24 h after eMCAO; (2) infarct volume ≥ 25 mm3 and (3) infarct volume < 25 mm3: measured by TTC staining 24 h post eMCAO; (4) failed: no sudden drop in regional cerebral blood flow (rCBF) detected by LDF at the time the clot was injected. n = 31–35 per group. (f) Kaplan–Meyer curves illustrating the survival of mice after ischemia induced by clots with different length. n = 34–51 per group. **p ≤ 0.01 vs. 5 mm group using Long-Rank test for Kaplan–Meyer curves. (g) Sensorimotor dysfunctions after MCAO were assessed by corner and rotarod tests for a week, n = 8 per group. *p ≤ 0.05, **p ≤ 0.01, ***p ≤ 0.001 vs. sham using two-way RM ANOVA followed by Bonferroni post hoc test. (h) Correlation between core or peri-core area in laser speckle at 24 h post eMCAO and latency to fall in the rotarod test at seven days after ischemia. n = 8 per group of different clot length. Dot line represents 95% confidence interval of the best-fit line. All data were presented as mean ± SD.
Infarct volume was calculated by TTC staining 24 h post injury. The mean infarct volume induced by 15 mm-clots was numerically larger than that induced by 5 mm-clots at 24 h after ischemia (46.72 ± 38.02 vs. 27.09 ± 20.99); however, the increase was not statistically significant (p = 0.0761) (Figure 1(c) and (d)). The percentage of the different surgery outcomes induced by clots of different lengths (Figure 1(e)) suggests that the mice received the 10 mm- or 15 mm-clot suffered more severe brain injury, as well as higher acute mortality (Figure 1(f)). Infarct volume was less than 25 mm3 in 39% of the 5 mm-clot mice, which may be driven by more frequent occurrence of clot autolysis or MCA sub-occlusion in this group. The failure rate of ischemia was also slightly higher in the 5 mm-clot group than the other two groups (12% for 5 mm-clot mice, 9% for 10 mm-clot and 15 mm-clot mice).
Pearson correlation analysis was performed to investigate the relationship between the infarct volume and the ischemic core area or peri-core area at the indicated time points following injury. The results indicate that the ischemic core area significantly correlated with infarct volume at the three different time points (Figure S3(b)), while the peri-core area only positively correlated with infarct volume at 3 and 24 h after the ischemic insult (Figure S3(c)). Two indices, core area change between 3 h and 24 h and peri-core area change between 5 min and 3 h were significantly correlated with acute infarct volume (Figure S3(d) and (e)).
Sensorimotor deficits were determined by the corner test and rotarod test for a week following eMCAO. All three groups had obvious neurological deficits compared with the sham group within seven days post-ischemia (Figure 1(g)), suggesting that the eMCAO led to functional deficits regardless of clot length. Pearson correlation analysis was further performed between the area of CBF core or peri-core at 24 h and rotarod performance at seven days after eMCAO. Performance on the rotarod was significantly correlated with the peri-core area (Figure 1(h) bottom panel). However, no significant correlation was detected between the core area and behavioral performance seven days after surgery (Figure 1(h) top panel), suggesting that the acute peri-core area may better predict the functional outcomes in the eMCAO model.
Embolic MCAO results in larger hypoperfusion area and higher mortality than sMCAO
Intraluminal suture MCAO (sMCAO) is an indispensable animal model that resembles human stroke, but transient mechanical occlusion of the MCA does not fully mimic the ischemia and thrombolysis that occurs in humans. To compare the outcome between sMCAO and eMCAO, eMCAO induced by 10 mm length clots was used. Using LSCI to monitor CBF, both filament and embolus induced a dramatic drop in CBF when the arteries were mechanically occluded (Figure 2(a) and (b)). With the reperfusion of MCA 1 h after ischemia, sMCAO showed a significant elevation in the hypoperfusion area referred to as the reperfusion onset due to the withdrawal of filament. Nevertheless, the eMCAO model displayed persistent low perfusion of cortical arteries, although the hypoperfusion area significantly decreased compared to the ischemic onset. As expected, 24 h later, there was no visible difference between the left and right hemispheres with regard to CBF for sMCAO, whereas eMCAO still maintained a distinct CBF decrease in the ischemic hemisphere (Figure 2(a) and (b) top panel). Consistently, significant differences were still observed in the peri-core area 1 and 24 h after MCA occlusion between the two groups (Figure 2(b) bottom panel). Compared with sMCAO, the hypoperfusion condition was sustained longer after eMCAO.
Figure 2.
Larger hypoperfusion area and higher mortality in eMCAO. Mice were subjected to eMCAO induced by a 10 mm blood clot or transient MCAO induced by intraluminal suture (sMCAO). (a) Representative 2D laser speckle images of sMCAO and eMCAO pre-ischemia and at indicated time points after surgery. (b) Quantification of core (CBF ≤ 30% of baseline) and peri-core (30% < CBF ≤ 50% of baseline) area in sMCAO and eMCAO at indicated time points after ischemia. n = 13 per group. **p ≤ 0.01, ***p ≤ 0.001 vs. sMCAO at the same time points using two-tailed Student’s t test or Mann–Whitney test. (c) Quantification of the total infarct volume, and infarct volume in each region, including cortex (CTX), striatum (STR), hippocampus (HIP), thalamus (THA), and deutocerebrum (DEU) 24 h after MCAO by TTC staining. n = 13 per group. *p ≤ 0.05, ***p ≤ 0.001 vs. sMCAO using the two-tailed Student’s t test. (d) Sensorimotor dysfunctions were assessed by corner test and rotarod test within seven days after surgery, n = 7–8 per group. ns: no significant difference, **p ≤ 0.01, ***p ≤ 0.001 vs. sham using two-way RM ANOVA followed by Bonferroni post hoc test. (e) Atrophy/infarct volume was measured by NeuN staining seven days after MCAO, n = 7–8 per group. ns: no significant difference analyzed by a two-tailed Student’s t test. (f) NeuN staining of sMCAO (60 min) and eMCAO seven days after surgery. Scale bar = 2 mm. The white dashed line circles the infarct area, while the red dashed line outlines the relative area of the contralateral hemisphere to illustrate atrophy area in the ipsilateral hemisphere. (g) Kaplan–Meyer curves illustrating the survival of mice after sMCAO and eMCAO. n = 30–34 per group. **p ≤ 0.01 using Long-Rank test for Kaplan–Meyer curves. All data are presented as mean ± SD.
The infarct volume was determined by TTC staining 24 h after surgery. Although the average infarct volume of eMCAO was larger than sMCAO, no significant difference was detected on the total, cortical, or striatal infarct volume. Surprisingly, the infarct volumes of hippocampus, thalamus and deutocerebrum were significantly larger in eMCAO than sMCAO (Figure 2(c)). Neurological functions were then assessed with the rotarod and corner tests for seven days after MCAO. Compared with sham mice, both sMCAO and eMCAO resulted in marked sensorimotor deficits, but no significant difference was detected between the two MCAO groups (Figure 2(d)). Infarct/atrophy volume was further calculated using NeuN staining seven days after ischemic injury, which indicated no significant difference between the two MCAO groups (Figure 2(e) and (f)). However, the eMCAO group showed significantly higher mortality versus sMCAO (Figure 2(g)).
Acute regional CBF reduction after eMCAO predicts infarct volume and mortality
LDF is the most commonly used method to assess acute changes in regional CBF (rCBF) during experimental large artery occlusion due to its reliability and sensitivity. 31 Eighteen mice, which were subjected to eMCAO with 5 mm-length clots, were used to record rCBF for 2 h after surgery. Mice were divided into three groups based on their CBF and outcome after ischemia: type A: rCBF returned to more than 30% of baseline within 1 h; B: mice that survived with rCBF lower than 30% of baseline up to 1 h post-surgery; C: stable decrease in CBF at least 1 h but died within 24 h following ischemia (Figure 3(a)). Five out of 18 mice (27.8%, red) died from a dramatic drop in rCBF to less than 15% of baseline that remained persistently low (less than 20% of baseline) for at least 1 h (Figure 3(a) and (b)). Regional CBF above 30% of baseline indicating perfusion was occurring which resulted in small infarcts in 33.3% of the mice. rCBF decreased to 15–30% of baseline for at least an hour may ensure stable infarct volumes and reduce the mortality of the animals (the grey area in Figure 3(a) and (c)).
Figure 3.
Magnitude of rCBF is negatively correlated to the infarct volume 24 h after eMCAO. (a) The rCBF was monitored continuously at the cortical branch of the MCA by Laser-Doppler flowmetry (LDF). Each curve represents a single mouse. Type A (black): mice with rCBF above 30% of baseline. Type B (blue): mice with persistent rCBF below 30% of baseline that survived 24 h after eMCAO. Type C (red): mice dead within 24 h after eMCAO. (b) The proportion of each class. n = 18 for all mice. (c) The correlation of infarct volume with rCBF 1 h after eMCAO. n = 18 mice (Five mice dead within 24 h, shown with red dots, were NOT included in the analysis of the infarct volume). (d) Three typical representative rCBF profiles measured by LDF throughout the surgery. In mouse a, a sudden increase in CBF occurred within 30 min after ischemia, and blood perfusion returned to baseline after an hour of dramatic fluctuation. CBF started to increase 30 min after embolism, and remained around 50% of baseline for two hours in mouse b, while profound hypoperfusion was detected in mouse c up to 150 min after ischemia. (e) Representative images of TTC-stained brain coronal sections of the mice correspondingly listed in (d). (f) The correlation of infarct volume with the time of rCBF continuously reduced to less than 30% of baseline. Different color represents a different class of rCBF.
Representative images of TTC-stained brain sections from mice with different CBF profiles (Figure 3(d)) during ischemia are shown in Figure 3(e). These results demonstrate that varying degrees of fluctuation in MCA territory perfusion lead to notable differences in infarct volume. Pearson correlation analysis subsequently confirmed that acute MCA blood flow reduction 1 h after ischemia onset is negatively correlated to the infarct volume 24 h after eMCAO (r = -0.7312, p = 0.0045, Figure 3(c)). When the duration of rCBF below 30% of baseline was prolonged, the infarct volume increased accordingly. The upward trend line was based more on a logarithmic rather than a linear function. An hour after occlusion, the curve steadily rose then leveled off indicating that sustained rCBF less than 30% of baseline beyond a certain point did not result in greater damage (Figure 3(f)).
MCA territory perfusion increase is not determined by clot autolysis
Fluctuations in MCA territory perfusion were recorded using LDF in eMCAO mice under anesthetic conditions. Whether the observed increase in subsequent tissue perfusion was due to autolysis of the blood clot was difficult to discern as the level of anesthesia has previously been shown to affect arterial blood pressure after stroke, 32 which can subsequently alter CBF. 33 Therefore, to determine the possible existence and localization of emboli when CBF changes were detected, LSCI was used as an alternative method to monitor CBF, which would preclude the impact of long-lasting anesthesia on CBF. LSCI was captured every 30 min after the onset of ischemia. Once the CBF of the MCA increased more than 20% of baseline, mice were sacrificed to examine the ICA and MCA for emboli. The results showed that a significant increase in CBF correlated with three types of clot conditions – I: no clot in either the MCA or ICA; II: more than a half-length of the clot stuck in the ICA; and III: more than a half-length of the clot localized to the MCA (Figure 4(a)). No significant difference was detected between the clot length of conditions type II and III (Figure 4(b)). Among the 40 mice used in this experiment, no clot was observed in 30% of the mice. Most of the change in CBF took place within 3 h of the eMCAO. Clot analysis was further performed in detail at three different time points when CBF increased above 20% of the baseline. Within the first hour after eMCAO, the clot had dissolved in only 5% of mice, whereas clot autolysis occurred in 20% of mice between 1 and 3 h after ischemia. In mice in which CBF remained depressed beyond 3 h, the clot was still present in 12.5% of these mice when CBF increased (Figure 4(c)). The clot type in each time period was analyzed (Figure 4(d)). MCA perfusion significantly increased in all three types; however, no significant difference was detected among the three types either at the time of ischemia or sacrifice (Figure 4(e)). Due to the increase in MCA perfusion, the core area significantly decreased prior to sacrifice, regardless of clot condition, and there was no significant difference in core area among the three types either at both time points (Figure 4(f)). However, a large decrease in the peri-core area was only detected in type III mice. Although there was no significant difference in clot length between type II and III mice, the peri-core area in type III mice was significantly smaller than type II mice prior to the mice being sacrificed, suggesting that the peri-core area was dependent on MCA perfusion only when the clot was predominantly stuck in the MCA (p = 0.0350, Figure 4(g)). Thus, MCA perfusion was increased regardless of whether a clot existed or had dissolved.
Figure 4.
MCA territory perfusion increase is not determined by clot autolysis in eMCAO mice without rtPA thrombolysis. (a) Mice were sacrificed when MCA CBF increased more than 20% compared to the blood perfusion at the onset of ischemia. Representative images of clot existence/location in brain vessels after being sacrificed, and corresponding 2D laser speckle images at indicated time points of each mouse. The “Y” shape white line marked the area for MCA perfusion analysis. Type I: no clot exists in the artery; Type II: clot in ICA; Type III: clot in MCA. (b) The length of clots for the three types of clot location conditions. n = 40 for all mice. n = 12 for type I, n = 14 for type II, n = 14 for type III. ***p ≤ 0.001 vs. type I using Kruskal–Wallis followed by Dunn post hoc test. (c) The existence of clot at indicated time points when MCA perfusion increased by 20% of baseline. (d) The percentage of three types of clot location conditions at indicated time points when MCA perfusion increased by 20% of baseline. (e) MCA perfusion unit (PU) changes from the onset of ischemia to the time of animal sacrifice. No significant difference was detected among the three types either at the time of ischemia or sacrifice. ***p ≤ 0.001 using two-tailed paired t test or Wilcoxon matched-pairs signed rank test. Isch: ischemia; Sac: sacrifice. (f) Core area changes before animals were sacrificed when the MCA PU increased 20% of the baseline. No significant difference was detected in core area among the three types at both time points. ***p ≤ 0.001 using Wilcoxon matched-pairs signed rank test. (g) Peri-core area changes before animals were sacrificed when the MCA PU increased 20% of the baseline. ns: no significant difference, ***p ≤ 0.001 analyzed by two-tailed paired t test or Wilcoxon matched-pairs signed rank test, *p ≤ 0.05 vs. type II analyzed by Kruskal–Wallis followed by Dunn post hoc test. All data were presented as mean ± SD.
Peri-core area 3 h after ischemia predicts the infarct volume in eMCAO
Although alterations in MCA territory perfusion was not dependent on clot autolysis, the core and peri-core area recorded by LSCI was positively associated with infarct volume. Pearson correlation analysis was therefore performed to investigate the correlation between MCA perfusion and core/peri-core area. MCA perfusion was negatively correlated with core area at the three time points examined after ischemia (Figure 5(a)), while a negative correlation was only detected with the peri-core area at 3 and 24 h, but not at 5 min (Figure 5(b)). In contrast to the scattered distribution in CBF of the IV ≥ 25 mm3 and IV < 25 mm3 groups at 5 min, CBF in most of the mice in the IV ≥ 25 mm3 group was concentrated within the region, where MCA perfusion was less than 50% of baseline 3 and 24 h after eMCAO (Figure 5(a) and (b)). These data suggest that increased CBF could minimize damage incurred after eMCAO. The correlation between MCA perfusion and infarct volume is further summarized in Figure 5(c). For a given infarct volume, mice were classified into eight groups in a Venn diagram using MCA perfusion percentage at 5 min and 3 h after ischemia. In the IV < 25 mm3 group, MCA perfusion was higher than 50% of baseline in more than 70% (17/23) of mice 3 h after ischemia. Infarct volume was larger than 25 mm3 in 70.27% of mice whose MCA perfusion was below 50% of baseline 3 h after ischemia. Surprisingly, there were four mice that had infarct volume less than 25 mm3 whose MCA perfusion was limited to 30% at 5 min and 50% up to 3 h, indicating that CBF of the MCA was not the only factor to influence the size of the infarct (Figure 5(c)). The core and peri-core areas were compared at three time points after ischemia. The results showed that no significant difference existed between the two groups whose MCA perfusion was higher than 50% of baseline 3 h after eMCAO. However, for mice with MCA perfusion lower than 50% of baseline, the peri-core area was markedly bigger in mice with larger infarct volumes 3 h after injury (Figure 5(d)). The existence of collateral flow was detected in the mice with smaller infarct volumes, which may potentially compensate for blood flow in the ischemic area (Figure 5(e)). To determine the best predictor of infarct volume, ROC analysis was performed. Within each CBF index, CBF at 3 h was better than the other two time points. The peri-core area was found to be the best predictor of infarct volume in eMCAO (Figure 5(f), AUC (3 h) = 0.8202).
Figure 5.
CBF monitored by LSCI could predict infarct volume after cerebral ischemia induced by an embolus. (a) The correlation of core area (CBF ≤ 30% of baseline) with CBF of MCA at indicated time points post-ischemia. n = 23 for IV < 25 mm3 group, n = 37 for IV ≥ 25 mm3 group. (b) The correlation of peri-core area (30% < CBF ≤ 50% of baseline) with CBF of MCA at indicated time points post-ischemia. (c) Venn diagram of mice grouped by infarct volume and MCA CBF perfusion at both 5 min and 3 h after surgery. MCA CBF ≥ 50% may indicate the perfusion of the MCA is attributed at least partially to clot dissolution within 3 h after surgery. (d) The peri-core area 3 h post ischemia had significant impact on the infarct volume in the MCA low perfusion (≤50% at 3 h) group. n = 17 for IV < 25 mm3, n = 11 for IV ≥ 25 mm3 in MCA > 50% groups, n = 6 for IV < 25 mm3, n = 26 for IV ≥ 25 mm3 in MCA ≤ 50% groups. *p ≤ 0.05, **p ≤ 0.001 vs. IV < 25 mm3 using two-way RM ANOVA followed by Bonferroni post hoc test. All data are presented as mean ± SD. (e) Representative images of collateral flow indicated by the white arrow. (f) Receiver operating characteristic curve of MCA perfusion (left, AUC (5 min) = 0.5006, AUC (3 h) = 0.7967 and AUC (24 h) = 0.745), core area (CBF ≤30% of baseline, middle, AUC (5 min) = 0.5441, AUC (3 h ) = 0.7991 and AUC (24 h) = 0.7573) and peri-core area (30% < CBF ≤50% of baseline, right, AUC (5 min) = 0.5805, AUC (3 h) = 0.8202 and AUC (24 h) = 0.725).
Thrombolysis of eMCAO by rtPA
To examine whether the eMCAO model could be used to test the efficacy of thrombolytic therapy and if rtPA treatment would lead to hemorrhagic transformation, mice subjected to eMCAO received saline or rtPA intravenous injection 1 or 3 h after clot introduction. Hemorrhagic transformation was assessed with hemoglobin concentrations in the cerebral parenchyma after transcardial perfusion with cold PBS. Treatment with rtPA 1 h after eMCAO decreased infarct volume and did not exacerbate hemorrhagic transformation compared to eMCAO animals that received saline (Figure 6(b) and (c)). However, thrombolysis 3 h post-ischemia provided no therapeutic benefit compared to non-treated eMCAO animals assessed by infarct volume (Figure 6(b)). In addition, delayed rtPA administration led to more severe hemorrhage versus the other two groups (Figure 6(c)), consistent with previous reports in which delayed rtPA-intervention 3 h after ischemic insults exacerbated cerebral injury and resulted in hemorrhagic transformation in mice. 24 No significant difference was detected on mortality and CBF between saline and rtPA groups after thrombolysis (Figure 6(d) and (e)).
Figure 6.
Thrombolysis by rtPA 1 or 3 h after the onset of eMCAO. Mice were treated with the same volume of saline or rtPA (10 mg/kg body weight, Actilyse®, Boehringer Ingelheim Pharma GmbH & Co KG, Ingelheim, Germany) intravenously (I.V.) via the right femoral vein at 1 or 3 h after eMCAO. (a) Representative images of the dorsal (first row) and ventral (second row) surfaces of the brain, coronal section (third row, bregma 0.74 mm), and TTC stained coronal section (bottom row, bregma 0.74 mm). The location of hemorrhage and infarct 24 h post eMCAO (10 mm clot). Mice were treated with saline, or rtPA 1 or 3 h after eMCAO. (b) Quantification of infarct volume in different groups with saline, or rtPA treatment at indicated time points after clot injection, n = 14 per group. ns: no significant difference, ***p ≤ 0.001 using Kruskal–Wallis followed by Dunn post hoc test. (c) Quantification of hemorrhage after rtPA treatment by spectrophotometric hemoglobin assay 24 h post eMCAO, n = 14 per group. ns: no significant difference, ***p ≤ 0.001 using Kruskal–Wallis followed by Dunn post hoc test. (d) Kaplan–Meyer curves illustrating the survival of mice with or without thrombolysis. (e) The area of CBF ≤ 50% of baseline changed throughout the operation in saline, 1 and 3 h thrombolysis mice. n = 7 per saline group, n = 14 per rtPA group, ns: no significant difference, *p ≤ 0.05, **p ≤ 0.01, ***p ≤ 0.001 using one-way RM ANOVA followed by Bonferroni post hoc test. All data are presented as mean ± SD.
Discussion
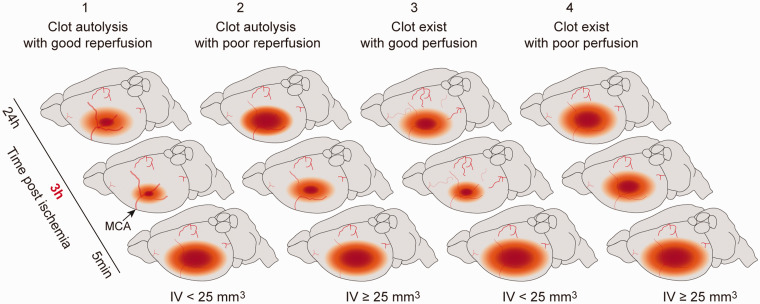
The development of preclinical pharmacotherapies for ischemic stroke with high clinical translational potential requires the use of animal models that closely approximate the human disease. Although eMCAO recapitulates many features of ischemic stroke, issues regarding clot location, clot fragment migration, and varying degrees of spontaneous reperfusion result in variability in CBF and infarct size across animals, which makes using this model to assess neuroprotective agents challenging. In this study, we developed a customized catheter to generate more uniform fibrin-rich clots to address these issues. We aimed to refine the eMCAO mouse model using these catheters and systematically assessed the interdependence of CBF, infarct volume, and neurological deficits. Infarct evolution in the eMCAO mouse model could be influenced by several factors. In this study, we focused on vessel patency and MCA territory perfusion and divided our observations into four categories (Figure 7). Type 1: early reperfusion due to clot dissolution within 3 h after ischemia led to smaller infarct volume. Type 2: poor reperfusion resulted in larger infarct volume. Type 3: vessel occlusion up to 3 h in the setting of sufficient collateral flow led to smaller infarct volume. Type 4: Persistent vessel occlusion led to a stable larger infarct. Furthermore, we examined the validity of using this model to test thrombolytic drugs and/or other neuroprotective agents. Optimizing the eMCAO model in mice would allow researchers to capitalize on the availability of transgenic and knockout mice to examine the underlying mechanism of this predominant form of stroke in humans and to assess thrombolytic and/or the other neuroprotective agents efficacy.
Figure 7.
Clot condition and perfusion of cerebral arteries determine the outcome of eMCAO. Cerebral ischemia induced by thrombus is divided into four categories. Early reperfusion due to clot dissolution within 3 h after ischemia contributes to smaller infarct volume (type 1). However, bad reperfusion, owing to the redistribution of the clot in smaller vessels or vasospasm induced by artery occlusion, may result in larger infarct volume in clot dissolution mice (type 2). The occurrence of collateral flow could not be excluded in type I mice. For the mice with clot occlusion up to 3 h, sufficient collateral flow may contribute to alleviation of the infarction due to reduction of the low perfusion area (CBF ≤ 50% of baseline) (type 3). Persistent occlusion of MCA leads to stable infarct volume, which could be used to mimic conditions in stroke patients and investigate thrombolysis (type 4).
An important impediment to obtaining uniform infarct volumes across animals in the eMCAO model is inherent to the clot itself. Chen et al. 13 developed a reproducible thromboembolic mouse model by introducing a blood clot and thrombin near the bifurcation of the MCA/anterior cerebral artery, resulting in the in situ formation of the blood clot. This model produced reliable infarct volume and the clot responded well to rtPA. However, the red blood cells-dominant embolus could migrate into vessels and was enriched with fibrin and platelets, which is more resistant to thrombolysis.34,35 Thus, the embolus formed under these conditions is different from that formed in situ in stroke patients, while the microemboli prepared in vitro, as in the current study, mimic arterial thrombi, which is fibrin-rich and closer to blood clots observed with embolic stroke in clinical setting.10,35
Lack of uniformity in clot size translates to variations in CBF and the location of clots, as smaller clots are more likely to migrate to smaller vessels to induce unpredictable infarct. 29 Although total infarct volume did not change as a function of clot length, mice with longer clots suffered more severe neurological deficits and higher mortality, as observed in rats and patients as well.19,26,36 Compared to the high failure rate of 5 mm-clot and severe mortality of 15 mm-clot, 10 mm was the optimal length. The infarct volume was more than 25 mm3 in 43% of the 10 mm-clot mice, and the failure rate of ischemia is only 9%, suggesting that 10 mm-clot is prone to induce more stable eMCAO injury. Moreover, seven-day survival of 10 mm-clot mice is 45.2% making it possible for long-term investigation. Comparison of the eMCAO model with the commonly used MCAO intraluminal suture mouse model found no significant difference between the two models with regard to total infarct volume and neurological deficits. However, eMCAO resulted in a larger core area and peri-core area, larger infarct volume in the hippocampus, thalamus, and deutocerebrum, as well as a higher incidence of death after ischemia. The larger hypoperfusion area obtained with eMCAO is likely analogous to the ischemic penumbra in the human stroke due to large vessel occlusion. Compared with the irreversible damage that occurs in the ischemic core, many cells within the penumbra can be saved from becoming part of the growing infarct.37,38 The study of the penumbra is a central focus of stroke research, thus making this eMCAO model a more suitable model for therapeutic development than the widely used sMCAO model. As for the infarct observed in the hippocampus, thalamus, and deutocerebrum, it may result from fragments of the blood clot migrating distally, as observed previously with eMCAO. 29 This phenomenon may also occur in humans and is not mimicked by sMCAO.
Rapid recanalization is critical to reducing stroke-related morbidity. Intravenous thrombolysis using rtPA within 3 h after onset of stroke symptoms has been approved by the FDA since 1996. 39 Although the therapeutic time window may extend up to 4.5 h and beyond,40,41 only 3–5% of stroke patients are currently rtPA-eligible due to the currently recommended therapeutic time window and the higher incidence of hemorrhagic transformation after 4.5 h.6,38,42 The underlying merit of any animal model lies in its ability to closely mimic its targeted clinical condition and to assess therapeutic agents that may be applied in the clinic. The larger penumbral volume observed in our eMCAO model versus the sMCAO model provides a viable target in which to assess neuroprotective agents to act as bridge therapies to reperfusion. Moreover, interventions leading to faster and more effective recanalization could be first tested in the eMCAO model to minimize infarction and neurological deficits that could be more readily translatable to the clinical setting. Thus, we assessed the efficacy of rtPA in our eMCAO mouse model. Intravenous administration of rtPA within 1 h after stroke onset slightly reduced brain infarction without hemorrhage. However, prolonged treatment with rtPA up to 3 h led to an elevated risk of hemorrhagic conversion and failed to reduce infarct volume 24 h after ischemia, as previously observed. 24 We did not investigate the delayed application of rtPA on hemorrhagic transformation, or the long-term neurological deficits after rtPA injection.
In the current study, CBF was monitored by both traditional LDF and the newly developed LSCI method. LDF, first reported by Stern in 1975, 43 is a noninvasive technique that measures microcirculatory blood flow in various tissues, including central and peripheral nervous systems.44,45 LDF commonly used in ischemic stroke to monitor CBF.46,47 Peri-infarct flow assessed by LDF can predict the outcome in experimental ischemic stroke in rats and humans, when an acute MRI facility is not readily available.48,49 However, LDF has limited spatial resolution (roughly 1 mm3) and may contain artifacts, which can be produced by movement, unstable fixation, or probe placement over large vessels. More recently, LSCI has been widely used to image and calculate the perfusion of blood flow in near-surface tissues, as well as the cortex of the brain.50–52 LSCI has the advantage of full view field imaging of surface blood flow and has been used to identify ischemic tissue and to predict infarct volume in rodent cerebral ischemia models and in ischemic stroke victims.53–55 For the first time, we monitored cortical CBF by LSCI in the eMCAO mouse model. Spontaneous recanalization characterized with an increase in MCA territory perfusion occurred in approximately 37.5% of mice within the first hour after eMCAO. However, clot autolysis was detected in only 13.3% of these mice, suggesting that spontaneous recanalization was not completely attributable to clot autolysis. Although the increase in MCA perfusion had no correlation with clot existence and length after eMCAO, clot localization affected the peri-core area rather than the core-area, which was more dependent on MCA perfusion upon ischemia.
MCA perfusion was negatively correlated with the core and peri-core areas after ischemia. MCA perfusion was lower than 50% of baseline in most of the mice whose infarct volume was larger than 25 mm3. There were two mice whose CBF perfusion almost returned to baseline 3 h after surgery that developed large infarcts, suggesting that recovery of microcirculation was a poor predictor of infarct volume, underscoring the complex relation between infarct volume and recanalization of the MCA. Previous researches have reported that unilateral impairment of dynamic cerebral autoregulation was persistent in a patient with MCA occlusion throughout the first week, and back to normal in the second week.56,57 Thus, in the present study, ischemia-induced vascular damage and dysfunction of cerebral autoregulation may have resulted in persistent tissue hypoperfusion even though MCA flow had been restored (Figure 7, type 2). 58
In contrast to restored MCA perfusion with larger infarct volumes, the infarct volume of four mice was smaller than 25 mm3, even with hypoperfusion of the MCA up to 3 h after ischemia. MCA territory hypoperfusion may have been compensated for by sufficient collateral flow after MCAO, which is supported by the smaller peri-core area in the current study (Figure 7, type 3). Collateral circulation upon blockage of “normal” blood flow functions to support the supply of oxygen and nutrition to the tissue. 59 In the clinical setting, collateral circulation is one of the most important indices that determine the loss of an MRI-defined penumbral area in ischemic stroke patients. The presence of good collaterals delays the loss of the penumbra to infarction, and thus could potentially extend the therapeutic window. 60 The existence of collateral flow was also detected in these mice with LSCI, which allowed us to see the CBF of the whole parietal cortex.
Considering the complex factors regulating CBF, it is crucial to determine whether CBF can be used to detect the occurrence of spontaneous recanalization and to predict ischemic outcomes. In this study, our results illustrate that CBF indices (MCA perfusion, core and peri-core area) were significantly correlated with infarct volume and neurological deficits after eMCAO. Within each CBF indices, CBF at 3 h was a stronger predictor of outcomes than the other two time points. More importantly, we for the first time reported that the area of the peri-core 3 h after clot injection was the most efficient index to predict infarct volume 24 h after eMCAO. With LDF monitoring, all experimental mice included in our study had similar CBF levels after surgery since those with rCBF reduction less than 70% of baseline were excluded from further experiment. The cortical CBF changes afterward could be determined by MCA recanalization or the compensatory collateral blood flow after ischemia. Nevertheless, peri-core area at 3 h post eMCAO could be used as a criterion to exclude animals that are not suitable for thrombolysis or other therapeutic treatment, and predict the outcomes after eMCAO in future.
In summary, our refinement of the eMCAO mouse model achieves reproducible ischemic brain damage that better imitates human ischemic stroke. With the aid of genetically altered mice, this model can be used to further study the pathophysiology of cerebral ischemia and hemorrhagic transformation induced by prolonged treatment with rtPA in preclinical research. The existence of a larger penumbra, as compared to the sMCAO model, makes the eMCAO model an ideal model to assess novel therapeutic strategies for stroke, and the similarity of the model to clinical ischemic stroke may expedite clinical translation.
Supplemental Material
Supplemental material, sj-pdf-1-jcb-10.1177_0271678X20917625 for Optimized mouse model of embolic MCAO: From cerebral blood flow to neurological outcomes by Rongrong Wang, Hailian Wang, Yaan Liu, Di Chen, Yangfan Wang, Marcelo Rocha, Ashutosh P Jadhav, Amanda Smith, Qing Ye, Yanqin Gao and Wenting Zhang in Journal of Cerebral Blood Flow & Metabolism
Funding
The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This project was supported by the Chinese Key R&D Plan of the State Ministry of Science and Technology 2017YFC1308403 (to Y.G.). Project Supported by Shanghai Municipal Science and Technology Major Project (No. 2018SHZDZX01) and ZJLab.
Declaration of conflicting interests
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Authors’ contributions
WZ and YG designed the study. RW and YL performed the experiments. RW, DC, YW, QY and WZ analyzed the data. RW, WZ, and AS wrote the manuscript. HW, YG, MR and APJ critically edited the manuscript.
ORCID iDs
Di Chen https://orcid.org/0000-0003-1513-8873
Yanqin Gao https://orcid.org/0000-0002-4915-9819
Supplemental material
Supplemental material for this article is available online.
References
- 1.DALYs GBD, Collaborators H, Murray CJ, et al. Global, regional, and national disability-adjusted life years (DALYs) for 306 diseases and injuries and healthy life expectancy (HALE) for 188 countries, 1990–2013: quantifying the epidemiological transition. Lancet 2015; 386: 2145–2191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gorelick PB. The global burden of stroke: persistent and disabling. Lancet Neurol 2019; 18: 417–418. [DOI] [PubMed] [Google Scholar]
- 3.Benjamin EJ, Muntner P, Alonso A, et al. Heart disease and stroke statistics-2019 update: a report from the American Heart Association. Circulation 2019; 139: e56–e528. [DOI] [PubMed] [Google Scholar]
- 4.Wardlaw JM, Bath PM. Stroke research in 2018: extended time windows, refined benefit, and lifestyle prevention targets. Lancet Neurol 2019; 18: 2–3. [DOI] [PubMed] [Google Scholar]
- 5.Kanazawa M, Takahashi T, Nishizawa M, et al. Therapeutic strategies to attenuate hemorrhagic transformation after tissue plasminogen activator treatment for acute ischemic stroke. J Atheroscler Thromb 2017; 24: 240–253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Yaghi S, Willey JZ, Cucchiara B, et al. Treatment and outcome of hemorrhagic transformation after intravenous alteplase in acute ischemic stroke: a scientific statement for healthcare professionals from the American Heart Association/American Stroke Association. Stroke 2017; 48: e343–e361. [DOI] [PubMed] [Google Scholar]
- 7.Shi L, Rocha M, Leak RK, et al. A new era for stroke therapy: integrating neurovascular protection with optimal reperfusion. J Cereb Blood Flow Metab 2018; 38: 2073–2091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sommer CJ. Ischemic stroke: experimental models and reality. Acta Neuropathol 2017; 133: 245–261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Longa EZ, Weinstein PR, Carlson S, et al. Reversible middle cerebral artery occlusion without craniectomy in rats. Stroke 1989; 20: 84–91. [DOI] [PubMed] [Google Scholar]
- 10.Zhang L, Zhang RL, Jiang Q, et al. Focal embolic cerebral ischemia in the rat. Nat Protoc 2015; 10: 539–547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Jin R, Zhu X, Li G. Embolic middle cerebral artery occlusion (MCAO) for ischemic stroke with homologous blood clots in rats. J Vis Exp 2014; 91: 51956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Alawieh A, Wang W, Narang A, et al. Thromboembolic Model of Cerebral Ischemia and Reperfusion in Mice. Methods Mol Biol 2016; 1462: 357–372. [DOI] [PubMed] [Google Scholar]
- 13.Chen Y, Zhu W, Zhang W, et al. A novel mouse model of thromboembolic stroke. J Neurosci Methods 2015; 256: 203–211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Labat-gest V, Tomasi S. Photothrombotic ischemia: a minimally invasive and reproducible photochemical cortical lesion model for mouse stroke studies. J Vis Exp 2013; 76: 50370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Schunke KJ, Toung TK, Zhang J, et al. A novel atherothrombotic model of ischemic stroke induced by injection of collagen into the cerebral vasculature. J Neurosci Methods 2015; 239: 65–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Venkat P, Yan T, Chopp M, et al. Angiopoietin-1 mimetic peptide promotes neuroprotection after stroke in type 1 diabetic rats. Cell Transplant 2018; 12: 1744–1752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ishrat T, Fouda AY, Pillai B, et al. Dose-response, therapeutic time-window and tPA-combinatorial efficacy of compound 21: a randomized, blinded preclinical trial in a rat model of thromboembolic stroke. J Cereb Blood Flow Metab 2019; 39: 1635–1647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kudo M, Aoyama A, Ichimori S, et al. An animal model of cerebral infarction. Homologous blood clot emboli in rats. Stroke 1982; 13: 505–508. [DOI] [PubMed] [Google Scholar]
- 19.Overgaard K, Sereghy T, Boysen G, et al. A rat model of reproducible cerebral infarction using thrombotic blood clot emboli. J Cereb Blood Flow Metab 1992; 12: 484–490. [DOI] [PubMed] [Google Scholar]
- 20.Zhang Z, Zhang RL, Jiang Q, et al. A new rat model of thrombotic focal cerebral ischemia. J Cereb Blood Flow Metab 1997; 17: 123–135. [DOI] [PubMed] [Google Scholar]
- 21.Asahi M, Asahi K, Wang X, et al. Reduction of tissue plasminogen activator-induced hemorrhage and brain injury by free radical spin trapping after embolic focal cerebral ischemia in rats. J Cereb Blood Flow Metab 2000; 20: 452–457. [DOI] [PubMed] [Google Scholar]
- 22.Orset C, Haelewyn B, Allan SM, et al. Efficacy of alteplase in a mouse model of acute ischemic stroke: a retrospective pooled analysis. Stroke 2016; 47: 1312–1318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mao L, Li P, Zhu W, et al. Regulatory T cells ameliorate tissue plasminogen activator-induced brain haemorrhage after stroke. Brain 2017; 140: 1914–1931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Garcia-Yebenes I, Sobrado M, Zarruk JG, et al. A mouse model of hemorrhagic transformation by delayed tissue plasminogen activator administration after in situ thromboembolic stroke. Stroke 2011; 42: 196–203. [DOI] [PubMed] [Google Scholar]
- 25.Kilkenny C, Browne W, Cuthill IC, et al. Animal research: reporting in vivo experiments–the ARRIVE guidelines. J Cereb Blood Flow Metab 2011; 31: 991–993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Barreto AD, Albright KC, Hallevi H, et al. Thrombus burden is associated with clinical outcome after intra-arterial therapy for acute ischemic stroke. Stroke 2008; 39: 3231–3235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Puetz V, Dzialowski I, Hill MD, et al. Intracranial thrombus extent predicts clinical outcome, final infarct size and hemorrhagic transformation in ischemic stroke: the clot burden score. Int J Stroke 2008; 3: 230–236. [DOI] [PubMed] [Google Scholar]
- 28.Mokin M, Levy EI, Siddiqui AH, et al. Association of clot burden score with radiographic and clinical outcomes following Solitaire stent retriever thrombectomy: analysis of the SWIFT PRIME trial. J Neurointerv Surg 2017; 9: 929–932. [DOI] [PubMed] [Google Scholar]
- 29.Kim DE, Kim JY, Nahrendorf M, et al. Direct thrombus imaging as a means to control the variability of mouse embolic infarct models: the role of optical molecular imaging. Stroke 2011; 42: 3566–3573. [DOI] [PubMed] [Google Scholar]
- 30.Kilic E, Hermann DM, Hossmann KA. A reproducible model of thromboembolic stroke in mice. Neuroreport 1998; 9: 2967–2970. [DOI] [PubMed] [Google Scholar]
- 31.Schmid-Elsaesser R, Zausinger S, Hungerhuber E, et al. A critical reevaluation of the intraluminal thread model of focal cerebral ischemia: evidence of inadvertent premature reperfusion and subarachnoid hemorrhage in rats by laser-Doppler flowmetry. Stroke 1998; 29: 2162–2170. [DOI] [PubMed] [Google Scholar]
- 32.Chuang BTC, Liu X, Lundberg AJ, et al. Refinement of embolic stroke model in rats: effect of post-embolization anesthesia duration on arterial blood pressure, cerebral edema and mortality. J Neurosci Methods 2018; 307: 8–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Slupe AM, Kirsch JR. Effects of anesthesia on cerebral blood flow, metabolism, and neuroprotection. J Cereb Blood Flow Metab 2018; 38: 2192–2208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Undas A, Ariens RA. Fibrin clot structure and function: a role in the pathophysiology of arterial and venous thromboembolic diseases. Arterioscler Thromb Vasc Biol 2011; 31: e88–99. [DOI] [PubMed] [Google Scholar]
- 35.Bacigaluppi M, Semerano A, Gullotta GS, et al. Insights from thrombi retrieved in stroke due to large vessel occlusion. J Cereb Blood Flow Metab 2019; 39: 1433–1451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Gralla J, Burkhardt M, Schroth G, et al. Occlusion length is a crucial determinant of efficiency and complication rate in thrombectomy for acute ischemic stroke. AJNR Am J Neuroradiol 2008; 29: 247–252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Albers GW, Marks MP, Kemp S, et al. Thrombectomy for stroke at 6 to 16 hours with selection by perfusion imaging. N Engl J Med 2018; 378: 708–718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.group ISTc, Sandercock P, Wardlaw JM, et al. The benefits and harms of intravenous thrombolysis with recombinant tissue plasminogen activator within 6 h of acute ischaemic stroke (the third international stroke trial [IST-3]): a randomised controlled trial. Lancet 2012; 379: 2352–2363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.National Institute of Neurological D and Stroke rt PASSG . Tissue plasminogen activator for acute ischemic stroke. N Engl J Med 1995; 333: 1581–1587. [DOI] [PubMed] [Google Scholar]
- 40.Ma H, Campbell BCV, Parsons MW, et al. Thrombolysis guided by perfusion imaging up to 9 hours after onset of stroke. N Engl J Med 2019; 380: 1795–1803. [DOI] [PubMed] [Google Scholar]
- 41.Del Zoppo GJ, Saver JL, Jauch EC, et al. Expansion of the time window for treatment of acute ischemic stroke with intravenous tissue plasminogen activator: a science advisory from the American Heart Association/American Stroke Association. Stroke 2009; 40: 2945–2948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Prabhakaran S, O’Neill K, Stein-Spencer L, et al. Prehospital triage to primary stroke centers and rate of stroke thrombolysis. JAMA Neurol 2013; 70: 1126–1132. [DOI] [PubMed] [Google Scholar]
- 43.Stern MD. In vivo evaluation of microcirculation by coherent light scattering. Nature 1975; 254: 56–58. [DOI] [PubMed] [Google Scholar]
- 44.Frerichs KU, Feuerstein GZ. Laser-Doppler flowmetry. A review of its application for measuring cerebral and spinal cord blood flow. Mol Chem Neuropathol 1990; 12: 55–70. [DOI] [PubMed] [Google Scholar]
- 45.Yalcin N, Chen SP, Yu ES, et al. Caffeine does not affect susceptibility to cortical spreading depolarization in mice. J Cereb Blood Flow Metab 2019; 39: 740–750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Dirnagl U, Kaplan B, Jacewicz M, et al. Continuous measurement of cerebral cortical blood flow by laser-Doppler flowmetry in a rat stroke model. J Cereb Blood Flow Metab 1989; 9: 589–596. [DOI] [PubMed] [Google Scholar]
- 47.Cipolla MJ, Linfante I, Abuchowski A, et al. Pharmacologically increasing collateral perfusion during acute stroke using a carboxyhemoglobin gas transfer agent (Sanguinate) in spontaneously hypertensive rats. J Cereb Blood Flow Metab 2018; 38: 755–766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Luckl J, Dreier JP, Szabados T, et al. Peri-infarct flow transients predict outcome in rat focal brain ischemia. Neuroscience 2012; 226: 197–207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Cuccione E, Versace A, Cho TH, et al. Multi-site laser Doppler flowmetry for assessing collateral flow in experimental ischemic stroke: validation of outcome prediction with acute MRI. J Cereb Blood Flow Metab 2017; 37: 2159–2170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Dunn AK, Bolay H, Moskowitz MA, et al. Dynamic imaging of cerebral blood flow using laser speckle. J Cereb Blood Flow Metab 2001; 21: 195–201. [DOI] [PubMed] [Google Scholar]
- 51.Zhang W, Wang H, Zhang H, et al. Dietary supplementation with omega-3 polyunsaturated fatty acids robustly promotes neurovascular restorative dynamics and improves neurological functions after stroke. Exp Neurol 2015; 272: 170–180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.He J, Lu H, Young L, et al. Real-time quantitative monitoring of cerebral blood flow by laser speckle contrast imaging after cardiac arrest with targeted temperature management. J Cereb Blood Flow Metab 2019; 39: 1161–1171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Hecht N, Muller MM, Sandow N, et al. Infarct prediction by intraoperative laser speckle imaging in patients with malignant hemispheric stroke. J Cereb Blood Flow Metab 2016; 36: 1022–1032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Li Y, Zhu S, Yuan L, et al. Predicting the ischemic infarct volume at the first minute after occlusion in rodent stroke model by laser speckle imaging of cerebral blood flow. J Biomed Opt 2013; 18: 76024. [DOI] [PubMed] [Google Scholar]
- 55.Dunn AK. Laser speckle contrast imaging of cerebral blood flow. Ann Biomed Eng 2012; 40: 367–377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Petersen NH, Ortega-Gutierrez S, Reccius A, et al. Dynamic cerebral autoregulation is transiently impaired for one week after large-vessel acute ischemic stroke. Cerebrovasc Dis 2015; 39: 144–150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Immink RV, van Montfrans GA, Stam J, et al. Dynamic cerebral autoregulation in acute lacunar and middle cerebral artery territory ischemic stroke. Stroke 2005; 36: 2595–2600. [DOI] [PubMed] [Google Scholar]
- 58.Castro P, Azevedo E, Sorond F. Cerebral autoregulation in stroke. Curr Atheroscler Rep 2018; 20: 37. [DOI] [PubMed] [Google Scholar]
- 59.Liebeskind DS. . Collateral circulation. Stroke 2003; 34: 2279–2284. [DOI] [PubMed] [Google Scholar]
- 60.Jung S, Gilgen M, Slotboom J, et al. Factors that determine penumbral tissue loss in acute ischaemic stroke. Brain 2013; 136: 3554–3560. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, sj-pdf-1-jcb-10.1177_0271678X20917625 for Optimized mouse model of embolic MCAO: From cerebral blood flow to neurological outcomes by Rongrong Wang, Hailian Wang, Yaan Liu, Di Chen, Yangfan Wang, Marcelo Rocha, Ashutosh P Jadhav, Amanda Smith, Qing Ye, Yanqin Gao and Wenting Zhang in Journal of Cerebral Blood Flow & Metabolism