Abstract
Functional magnetic resonance imaging (fMRI) has been mainly utilized for the preoperative localization of eloquent cortical areas. However, lesion-induced impairment of neurovascular coupling (NVC) in the lesion border zone may lead to false-negative fMRI results. The purpose of this study was to determine physiological factors impacting the NVC. Twenty patients suffering from brain lesions were preoperatively examined using multimodal neuroimaging including fMRI, magnetoencephalography (MEG) during language or sensorimotor tasks (depending on lesion location), and a novel physiologic MRI approach for the combined quantification of oxygen metabolism, perfusion state, and microvascular architecture. Congruence of brain activity patterns between fMRI and MEG were found in 13 patients. In contrast, we observed missing fMRI activity in perilesional cortex that demonstrated MEG activity in seven patients, which was interpreted as lesion-induced impairment of NVC. In these brain regions with impaired NVC, physiologic MRI revealed significant brain tissue hypoxia, as well as significantly decreased macro- and microvascular perfusion and microvascular architecture. We demonstrated that perilesional hypoxia with reduced vascular perfusion and architecture is associated with lesion-induced impairment of NVC. Our physiologic MRI approach is a clinically applicable method for preoperative risk assessment for the presence of false-negative fMRI results and may prevent severe postoperative functional deficits.
Keywords: Blood-oxygen-level-dependent contrast, functional magnetic resonance imaging, hypoxia, magnetoencephalography, neurovascular coupling
Introduction
Functional magnetic resonance imaging (fMRI) using the blood-oxygen-level-dependent (BOLD) contrast mechanism is one of the dominant in vivo imaging techniques to noninvasively visualize neural activity. 1 , 2 This is mainly due to the fact that BOLD fMRI is characterized by easy implementation and widespread availability combined with relatively high sensitivity and spatial resolution. Cerebral blood flow (CBF) is locally controlled by the neurovascular unit in response to dynamic changes in tissue oxygen tension evoked by stimulus- or task-associated cortex activity: Increased extraction of oxygen from the local capillaries leads to a drop in oxygenated hemoglobin (oxyHb) and an increase in local carbon dioxide (CO2) and deoxygenated hemoglobin (deoxyHb). The hemodynamic response of the neurovascular unit eventually overcompensates for this initial oxygen demand, resulting in a net decrease in local deoxyHb concentration and a net increase in local oxyHb. 3 This large rebound in local blood and tissue oxygenation is detectable with BOLD fMRI because of the differences in magnetic susceptibility between oxyHb (diamagnetic) and deoxyHb (paramagnetic). 4 , 5 Saying so, BOLD fMRI assesses neuronal activity via the hemodynamic response of the neurovascular unit, which is also known as neurovascular coupling (NVC). However, the BOLD effect may not be appropriately correlated with neuronal activity and local pathologic alterations in cerebral blood perfusion, tissue oxygen metabolism, and/or microvascular architecture, which may have a considerable influence on BOLD fMRI findings.
Neurosurgical procedures adjacent to functional important brain circuits are associated with a meaningful risk for the occurrence of postoperative neurological impairment and, therefore, still represent an unmet issue for preoperative decision-finding. BOLD fMRI is the method of choice for the presurgical localization of eloquent cortex areas in the vicinity of brain lesions and is of high relevance for an effective surgical management. 6 Of note, most brain lesions can affect BOLD fMRI due to lesion-induced impairment of NVC, and thus attenuation of the BOLD response. 7 This can lead to false-negative results for localization of functional areas and, in further consequence, to a seriously deteriorated neurological postsurgical state. 8
Magnetoencephalography (MEG) directly measures magnetic fields generated by the electrical currents produced by synchronous activity of thousands of neurons, well known as the local field potentials. 9 Most importantly in our context, these electromagnetic recordings are unaffected by lesion-induced impairment of the NVC. While both methods have a spatial resolution in the mm range, MEG provides high-scale temporal resolution in the range of milliseconds (i.e. at the level of electrophysiological dynamics), contrary to fMRI with a time resolution in the range of seconds. However, due to its limited availability and time-consuming implementation of the presurgical tasks, MEG is not commonly applied in neurosurgical practice. Given these facts, only few studies10–12 combined BOLD fMRI with MEG experiments for the detection of an impaired NVC so far.
Alterations in local hemodynamics 8 , 13 , 14 or oxygen availability 15 have been previously described as the physiological correlate of impaired NVC. The interplay between blood perfusion (i.e. hemodynamics), microvascular architecture, and oxygen metabolism (including tissue hypoxia) is of crucial importance for elucidating the physiological status of cortical regions adjacent to brain lesions. Most of the available techniques, however, are not well suited for in vivo characterization in humans due to their invasiveness (electrodes), limited availability and high costs (15O2 positron emission tomography), or low spatial resolution (near-infrared spectroscopy). In our previously published study, we could demonstrate that a novel multiparametric MRI approach reliably allowed for the combined assessment of local perfusion, microvascular architecture, and oxygen metabolism in noninvasive manner. 16 Hence, this method unifies microvascular architecture mapping (VAM) 17 with multiparametric quantitative BOLD (qBOLD) MRI. 18 VAM is based on the different sensitivity of gradient-echo (GE) and spin-echo (SE) MR imaging to magnetic susceptibility that provides additional characteristics relevant to the tissue microvascularity.19–21 Furthermore, a multiparametric qBOLD approach was proposed to gather quantitative information related to the oxygen metabolism and tissue oxygenation. 18 In our previously performed studies, this approach was used to assess the tumor microenvironment of brain tumors and its changes during therapy and recurrence.22–26
However, no previous study investigated alterations in both local hemodynamics and oxygen metabolism or included investigations of the microvascular architecture. In this study, we used a multimodal approach combining preoperative BOLD fMRI and MEG experiments for detection of lesion-induced impairment of NVC with physiological MRI measurements of perfusion, microvascular architecture, and oxygen metabolism in patients with brain lesions in order to investigate the physiological reasons of impaired NVC.
Materials and methods
Patients and histopathology
The study protocol was approved by the ethics committee of the University of Erlangen-Nürnberg and was in line with the Helsinki Declaration of Human Rights. Informed consent was obtained from all subjects. Twenty adult patients (13 male, 7 female; mean age ± standard deviation: 49.6 ± 16.2 years; age range: 24–76 years) with brain lesions were enrolled. The study cohort comprised 16 patients with histopathological confirmed glioma: 7 glioblastoma (GB, World Health Organization (WHO) grade IV), 3 anaplastic astrocytoma (AA, WHO grade III), 3 anaplastic oligodendroglioma (AOG, WHO grade III), 2 low-grade astrocytoma (WHO grade II), and 1 ganglioglioma (WHO grade I), respectively. Furthermore, two patients suffered from an arteriovenous malformation (AVM), one patient suffered from a cavernoma (Cav), and one patient from a meningioma, respectively. Clinical details of the patients are summarized in Table 1.
Table 1.
Clinical details of the patients.
| ID | Age (y) | Sex | Histo | Location of the lesion | LVol (cm3) | Neurol. deficits |
ShDi (mm) | Impaired NVC | funct. task | r thresh. | |
|---|---|---|---|---|---|---|---|---|---|---|---|
| preop | postop | ||||||||||
| 1 | 55 | m | Men | l temporal | 48.9 | no | no | 0 | no | lang | 0.3 |
| 2 | 76 | m | GB | r frontal | 77.9 | no | no | 2 | yes | sm | 0.13 |
| 3 | 67 | m | AOG | l frontal | 102 | no | no | 22 | yes | lang | 0.12 |
| 4 | 54 | m | GB | r trigonal | 58.1 | yes | no | 9 | yes | sm | 0.21 |
| 5 | 41 | m | AA | l parietal | 71.7 | no | no | 4 | no | sm | 0.3 |
| 6 | 59 | m | GB | r temporal | 232 | yes | no | 13 | yes | sm | 0.17 |
| 7 | 71 | m | GB | l temporal | 24.6 | no | no | 8 | no | lang | 0.3 |
| 8 | 43 | m | AOG | r central | 40.2 | yes | no | 0 | no | sm | 0.3 |
| 9 | 31 | m | LGA | l frontal | 29.9 | no | no | 4 | no | lang | 0.3 |
| 10 | 36 | f | AVM | r temporal | 0.3 | no | no | 2 | no | sm | 0.3 |
| 11 | 73 | m | Cav | l temporal | 5.9 | no | no | 0 | yes | lang | 0.16 |
| 12 | 44 | m | AVM | l postcentral | 7.9 | no | yes | 0 | no | sm | 0.3 |
| 13 | 41 | f | AOG | l frontal | 67.0 | yes | yes | 5 | no | sm | 0.3 |
| 14 | 51 | f | GB | r frontal | 15.7 | yes | no | 2 | yes | sm | 0.18 |
| 15 | 27 | m | AA | l fronto-insular | 35.8 | no | no | 0 | no | lang | 0.3 |
| 16 | 58 | f | GB | l temporal | 36.7 | no | yes* | 9 | no | lang | 0.3 |
| 17 | 45 | m | AA | l fronto-temp. | 58.6 | no | no | 8 | yes | lang | 0.26 |
| 18 | 27 | f | LGA | l frontal | 3.0 | no | yes* | 0 | no | lang | 0.3 |
| 19 | 24 | f | GG | l temporal | 2.7 | no | yes* | 5 | no | lang | 0.3 |
| 20 | 68 | f | GB | l frontal | 53.2 | yes | yes | 0 | no | lang | 0.3 |
Histo: histology of the lesion; LVol: lesion volume; Neurol. deficits preop/postop: preoperative/postoperative neurologic deficits; ShDi: shortest distance between cortical area with important brain function and lesion border; NVC: neurovascular coupling; funct. task: functional task; lang: language task; sm: sensorimotor task; r thresh.: correlation threshold used for BOLD fMRI data evaluation. Note: numbers lesser than 0.3 are the reduced threshold values at that an fMRI activity was visible. Men: meningioma; GB: glioblastoma WHO grade IV; AOG: anaplastic oligodendroglioma WHO grade III; AA: anaplastic astrocytoma WHO grade III; GG: ganglioglioma WHO grade I; LGA: low-grade astrocytoma WHO grade II; AVM: arteriovenous malformation; Cav: cavernoma.
Physiologic MRI data acquisition
Anatomic and physiologic MRI examinations were performed on a 3 tesla clinical scanner (Tim Trio, Siemens, Erlangen, Germany) equipped with a 32-channel head coil. The state-of-the-art MRI protocol included T2-weighted, diffusion-weighted (DWI), fluid-attenuated inversion-recovery, and pre- and postcontrast enhanced T1-weighted MRI, respectively. In addition, the following sequences were performed for physiologic MRI data acquisition: (i) a multiecho GE sequence for T2*-mapping (8 echoes; echo time (TE) = 5–40 ms; repetition time (TR) = 658 ms); (ii) a multiecho SE sequence for T2-mapping (8 echoes; TE = 12–96 ms; TR = 1610 ms); and (iii) a dynamic susceptibility contrast (DSC) bolus-tracking perfusion MRI sequence combined with a hybrid single-shot gradient-echo spin-echo (GESE) echo planar imaging (EPI) readout (TR = 1380 ms; TE[GE] = 16 ms; TE[SE] = 89 ms) were performed. 19 Geometric parameters were chosen identically for the three experimental sequences: axial slice orientation; field-of-view (FoV) = 230 × 230 mm2; in-plane resolution = 1.8 × 1.8 mm2; slice thickness = 4 mm; 8 slices. DSC GESE perfusion examinations were performed with 80 dynamic measurements during administration of a double dose (0.2 mmol/kg bodyweight) of gadoterate meglumine (Dotarem, Guerbet, France) at a rate of 4 ml/s followed by a 20-ml bolus of saline using an MR-compatible injector (Spectris, Medrad Bayer, Leverkusen, Germany). This resulted in 80 dynamic volumes of both GE-EPI and SE-EPI for tracking the first-pass peak contrast media bolus dynamics. The acquisition time (TA) for the three experimental sequences was less than 5 min: TA for T2*-mapping = 50 s; TA for T2-mapping = 2.0 min; and TA for DSC GESE perfusion = 1.8 min. The total MRI TA was about 30 min.
Physiologic MRI data processing
Processing of physiologic MRI data and calculation of MRI biomarker maps for oxygen metabolism, perfusion, and microvascular architecture were performed with custom-made MATLAB (MathWorks, Natick, MA, USA) software. Details about the whole data processing pipeline from MRI data acquisition over preprocessing to biomarker calculation were described previously. 16 , 17 , 25 Briefly, qBOLD data processing included three steps: (i) corrections for background fields of the T2*-mapping data 16 and for stimulated echoes of the R2-mapping data; 27 (ii) calculation of R2*- and R2 maps from the multiecho MR relaxometry data; and (iii) calculation of cerebral blood volume (CBV) and CBF maps from the GE-EPI DSC perfusion MRI data via automatic identification of arterial input functions (AIFs) 28 and correction for remaining contrast agent extravasation. 29 These data were used for the calculation of MRI biomarker maps of oxygen metabolism, including oxygen extraction fraction (OEF), cerebral metabolic rate of oxygen (CMRO2), and the mitochondrial oxygen tension (mitoPO2) using the following equations: 18 , 30
with R2* = 1/T2*; R2 = 1/T2; γ = 2.67502·108 rad/s/T is the nuclear gyromagnetic ratio; Δχ = 0.264·10–6 is the difference between the magnetic susceptibilities of fully oxygenated and fully deoxygenated hemoglobin; Hct = 0.42·0.85 is the microvascular hematocrit fraction, whereby the factor 0.85 stands for a correction factor of systemic Hct for small vessels
where Ca = 8.68 mmol/ml is the arterial blood oxygen content; 31 and
where P50 is the hemoglobin half-saturation tension of oxygen (27 mmHg) and h is the Hill coefficient of oxygen binding to hemoglobin (2.7), and L (4.4 mmol/hg/min) is the tissue oxygen conductivity as defined by Vafaee and Gjedde. 32
In a next step, the calculation of microvascular CBV (µCBV) and microvascular CBF (µCBF) maps from the SE-EPI DSC perfusion MRI data via a separate automatic identification of AIFs was performed. 28
The data processing for the microvascular architecture was performed according to the following steps: (i) correction for remaining contrast agent extravasation; 29 (ii) fitting of the first bolus curves for each voxel of the GE- and SE-DSC perfusion data with gamma-variate function; 33 and (iii) calculation of the ΔR2,GE versus (ΔR2,SE)3/2 diagram 19 —the so-called vascular hysteresis loop (VHL). These data were used for calculation of MRI biomarker maps of the microvessel density (MVD) and vessel size index (VSI, i.e. the microvessel radius) 34 using the following equations:
with Qmax = max[ΔR2,GE]/max[(ΔR2,GE)3/2] obtained from the VHL; ≈ 3.0 µm is the mean vessel lumen radius; ADC is the apparent diffusion constant calculated from the DWI data; and b is a numerical constant (b = 1.6781). 34
Preoperative BOLD fMRI
BOLD fMRI data acquisition was performed on a 1.5 tesla whole-body MR scanner (Magnetom Sonata, Siemens, Erlangen, Germany), which is installed in an operating theater, using a conventional 2 D EPI sequence with the following parameters: FoV = 192 × 192 mm2; acquisition matrix = 64 × 64; slice thickness = 3 mm; 16 slices, TE = 60 ms, TR = 2470 ms, flip angle = 90°. A block paradigm with 180 measurements in 6 blocks (3 resting state and 3 active intervals) with 30 volumes per block was used. The task that was performed by the patients depended on the lesion location, i.e. patients with lesions located in vicinity to the sensory-motor cortex performed a sensory-motor task, whereas patients with lesions in the vicinity of the cortical language areas performed a language task. The total examination time (including instruction and preparation of the patients) for the BOLD fMRI experiments was 1.5 to 2 h.
For localization of cortical motor areas, the patients were instructed to repeatedly flex and extend all digits (exercise 1) or foot and toes (exercise 2) of a designated side and to refrain from any other motor actions. Start and stop commands for the movements were given acoustically, and the patients’ movements were monitored from the control room. In somatosensory measurements, the patients were asked to avoid any motor activity, and the stimulation of the index finger was started and stopped automatically according to the intervals. The sensory-motor task was described in more detail previously. 35
For localization of cortical language areas, the following two tasks were used: (i) Conjugation of a given verb in first person, past tense: 150 short verbs, typically with one syllable, were used. Of this, 80% were irregular verbs, 20% were regular verbs. (ii) Building a simple sentence with a given noun: 150 short nouns, typically 4 to 5 letters long, were used. The language task was described in more detail previously. 10
BOLD fMRI data processing was performed in three steps using a dedicated software package (BrainVoyager, Brain Innovation, Maastricht, the Netherlands): (i) Motion correction was performed using an image-based prospective acquisition correction applying interpolation in the k-space. 36 (ii) For determination of activated areas during each task, a gamma-variate function was convolved with the task reference function (time course of square waveform) and cross-correlated with the BOLD signal on a voxel-wise basis. Cortical activation was determined using a threshold of 0.30 corresponding to a Bonferroni corrected p < 0.01 (significance level of p < 0.000045). 37 Clusters of at least four contiguous voxels were assembled in order to eliminate isolated voxels and fused automatically to an anatomical data set (3-D T1w MRI). 10 , 35 (iii) For detection of lesion-induced impairment of NVC in cortical areas with missing BOLD response which, however, were expected from MEG results (see below), these areas were analyzed by lowering the threshold from 0.3 progressively in 0.01 steps to 0.05 or until occurrence of a BOLD activation.
Additionally, in patients with existing BOLD response, the threshold was progressively increased from 0.1 to 0.7 in steps of 0.05 until disappearance of BOLD activations in the vicinity to the lesions.
Preoperative MEG
MEG experiments were performed prior to the MRI examinations; details were described previously. 10 , 35 Briefly, cortical motor-evoked fields, somatosensory-evoked fields, or language-evoked fields were continually recorded using a 248-magnetometer whole-head MEG system (MAGNES 3600 WH, 4-D Neuroimaging, San Diego, CA, USA) installed in a magnetically shielded room (Vacuumschmelze, Hanau, Germany). MEG signals were acquired at a 678-Hz sampling rate using an online high-pass filter of 0.1 Hz, low-pass filter of 200 Hz, and noise cancellation. For co-registration of MEG data and anatomical MRI data, we used five small coils placed on the surface of the patients’ head at defined positions, and digitization of the patients’ head surface was performed using a 3-D tracking system (Polhemus, Colchester, VT, USA). The total examination time (including instruction and preparation of the patients) for the MEG experiments was about 2 h.
Motor and somatosensory MEG recordings were performed in the same session following the comprehensive sensorimotor protocol where external mechanic (sensory) stimulation serves as a cue for patients’ movements as described previously. 38 Visual language stimulation was achieved using dedicated software (E-Prime, Psychology Software Tools, Sharpsburg, PA, USA). Language tasks were similar to those used in BOLD fMRI experiments: (i) conjugation of verbs and (ii) building of short sentences. The interstimulus intervals were 3000 ms. Patients were instructed to avoid any other motor actions like blinking, swallowing, or moving other parts of the body as effectively as possible. Patients were supervised using a camera installed in the shielded room.
Typically, 300 trials were recorded during each MEG experiment. Trials exhibiting artefacts originating from the patient or external noise were excluded by visual inspection from an experienced MEG investigator. All remaining trials with sufficient data quality (typically around 280) were averaged using a trigger signal for start of stimulation and filtered by band pass (0.3–95 Hz) and notch filter (50 Hz). The noise covariance data were calculated from baseline data in the 500 ms pretrigger interval. Individual brain MRI data (3-D T1w MRI) were used to create individual brain anatomy data set BrainSuite (version 15c) 39 in order to consider for lesion-induced changes in brain anatomy. Localization of MEG-based cortical activation was performed with dynamical Statistical Parametric Mapping (dSPM) 40 as part of the brainstorm software package (version 3.4). 41 dSPM is based on minimal modeling to estimate distributed sources and are applicable on complex data sets or at high noise levels 42 , 43 and uses deep weighting and noise normalization for compensation. Deep weighting was performed by normalizing all sources in the model using a measure of the overall amplitude. A minimization of the localization errors was achieved by normalization of rows of the lead field matrix. 40 Details for MEG data analysis were described previously. 10 , 35
Multimodal data and statistical analysis
Both fMRI- and MEG-based cortical localizations were displayed on an anatomical 3-D T1w MRI data set. The following cortical areas in the motor network were evaluated: M1, premotor area, and supplementary motor area. The evaluated cortical areas in the cortical somatosensory network were the primary and secondary somatosensory cortex. Cortical language areas included in total 13 subareas of Broca’s area, Wernicke’s area, the angular gyrus, and the supramarginal gyrus, respectively. Details about the language subareas were published previously. 10 Areas of motor, sensory, and language function were classified into three subgroups via comparison of the fMRI- and MEG-based cortical activity patterns: (i) cortical activity in both BOLD fMRI and MEG data on the ipsilateral side of the brain lesion; (ii) cortical activity in both BOLD fMRI and MEG data on the contralateral side of the brain lesion; and (iii) cortical activity in MEG data but not in BOLD fMRI data using the conventional threshold for correlation on the ipsilateral side of the brain lesion. The latter cortical areas were interpreted as impaired NVC in functional important brain areas.
Regions of interest (ROIs) were manually defined for the first and second subgroup of cortical localizations (BOLD and MEG ipsilateral, BOLD and MEG contralateral) on axial slices of the BOLD fMRI results bordering the whole cortical activation (see black contour line in Figure 1). For the third subgroup, the same procedure was performed on the MEG results (see black contour lines in Figure 2). The ROIs were subsequently transferred to the co-registered imaging biomarker maps for oxygen metabolism (OEF, CMRO2, and mitoPO2), perfusion (CBV, CBF, µCBV, and µCBF), and microvascular architecture (MVD and VSI), respectively. Mean values of all imaging biomarkers as well as of the z score and the t score for the BOLD fMRI data were calculated for the ROIs and statistically analyzed using dedicated software (SPSS, IBM, Chicago, IL, USA). Differences in MRI biomarkers between subgroups of cortical areas were determined using the analysis of variance method. Dunnett’s T3 test was used as post hoc procedure to be consistent with the assumption that homogeneity of variance was not met and for correction for multiple comparisons. Homogeneity of variance was tested using the Levene’s test. Intraindividual differences in MRI biomarker values between ipsi- and contralateral cortical areas were compared using a Wilcoxon signed-rank test. Significance of differences in lesion volume and shortest distance of cortical function to lesion border between patient subgroups with or without impaired NVC was calculated using a Mann–Whitney U test. The Pearson’s correlation coefficient (R) was calculated as a measure of the strength of the linear relationship between imaging biomarkers and z scores and the t scores, respectively. p values less than 0.05 were considered to indicate significance.
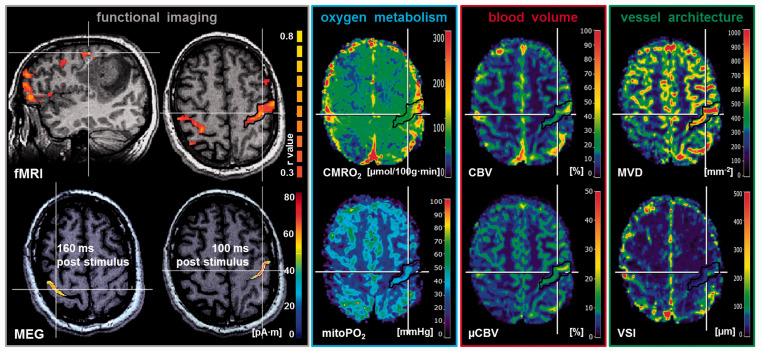
Figure 1.
Multimodal imaging in a 41-year-old patient suffering from an anaplastic astrocytoma (ID 5 in Table 1) with intact NVC in the cortical areas for sensorimotor function. From left to right: BOLD fMRI-based cortical localizations superimposed onto T1w MRI data (in sagittal and axial orientation) showed good spatial congruence with the MEG-based findings measured 160 ms and 100 ms poststimulus, respectively. MR imaging biomarker maps for oxygen metabolism (CMRO2 and mitoPO2), cerebral blood volume (CBV and µCBV), and microvascular architecture (MVD and VSI) revealed no alterations or restrictions and no significant differences between ipsi- and contralateral side to the lesion. Note: White lines indicate the slice intersections; black contour lines are the ROIs for data analysis; color coding bars are positioned on right-hand side of corresponding image. For the fMRI images, the color coding bar represents the correlation coefficient (r value).
fMRI: functional magnetic resonance imaging; MEG: magnetoencephalography; CMRO2: cerebral metabolic rate of oxygen; mitoPO2: mitochondrial oxygen tension; CBV: cerebral blood volume; µCBV: microvascular cerebral blood volume; MVD: microvessel density; VSI: vessel size index.
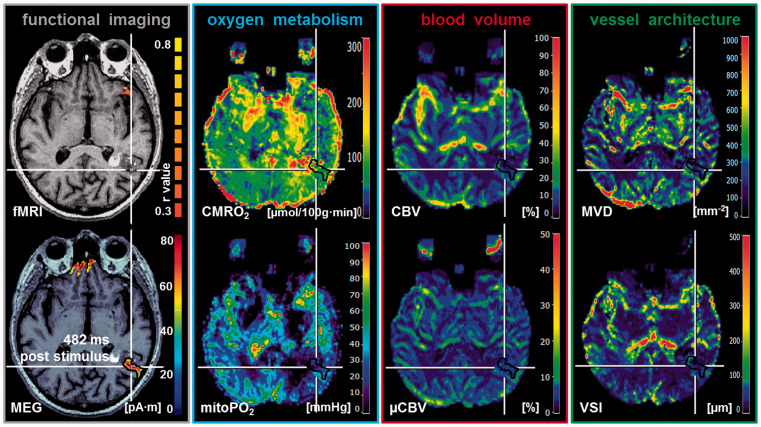
Figure 2.
Multimodal imaging in a 73-year-old patient suffering from a Cav (ID 11 in Table 1) with impaired NVC in a cortical area for language function (posterior superior temporal sulcus (STS) extending into Brodmann’s area 39). From left to right: BOLD fMRI-based cortical localizations superimposed onto axial T1w MRI data showed missing BOLD response when compared with MEG-based cortical localizations of language functions (482 ms poststimulus). MR imaging biomarker maps revealed alterations in oxygen metabolism (high CMRO2 and low mitoPO2) due to restrictions in cerebral blood volume (decreased CBV and µCBV) and microvascular architecture (decreased MVD and VSI) compared to the contralateral side to the lesion. Note: White lines indicate the slice intersections; black contour lines are the ROIs for data analysis; color coding bars are positioned on right-hand side of corresponding image. For the fMRI image, the color coding bar represents the correlation coefficient (r value).
fMRI: functional magnetic resonance imaging; MEG: magnetoencephalography; CMRO2: cerebral metabolic rate of oxygen; mitoPO2: mitochondrial oxygen tension; CBV: cerebral blood volume; µCBV: microvascular cerebral blood volume; MVD: microvessel density; VSI: vessel size index.
Results
Differences in patient and lesion characteristics
Physiological MRI examinations as well as preoperative functional imaging with BOLD fMRI and MEG were successfully performed in all 20 patients. In accordance with the location of the brain lesion, 11 patients obtained multimodal functional imaging in combination with language tasks and 9 patients in combination with sensorimotor tasks, respectively. From the 20 patients, 13 patients showed good congruence between BOLD fMRI and MEG (Figure 1); however, 7 patients (35%) showed indications for impaired NVC (Figure 2), i.e. cortical activity in MEG data but not in BOLD fMRI data using the common threshold for correlation (> 0.3). In all seven patients, however, lowering of the threshold was associated with the appearance of BOLD activation, indicating a reduced BOLD effect due to an impairment of the NVC. The lowered threshold values are summarized in Table 1.
From these seven patients with impaired NVC, three patients performed the language tasks and four patients the sensorimotor tasks, with four patients suffering from a GB (WHO grade IV) and one patient each from an AOG (WHO grade III), an AA (WHO grade III), and a Cav, respectively. Patients with low-grade glioma or AVMs showed no impaired NVC (Table 1).
Although we observed a difference in lesion volume between the subgroups of patients with intact NVC (32.5 ± 24.1 cm3; 0.3–71.7 cm3) and with impaired NVC (78.5 ± 75.4 cm3; 5.9–231.9 cm3), this was statistically not significant (p = 0.081). The shortest distance of cortical function to the brain lesion border was also not significant different (p = 0.115) between these subgroups: intact NVC: 2.8 ± 3.2 mm, 0 to 9 mm; impaired NVC: 8.0 ± 7.7 mm, 0 to 22 mm (Table 1).
Differences in physiologic MRI biomarkers
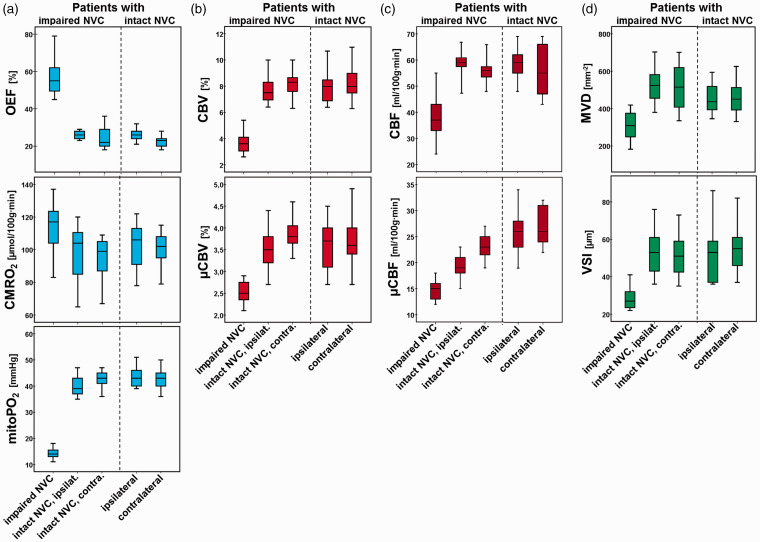
In the 13 patients with intact NVC, i.e. with reliable accordance between all fMRI- and MEG-based cortical localizations, we found no significant differences in physiologic MRI biomarkers comparing ipsi- and contralateral functional brain areas (p = 0.331–1.0). An illustrative case for this patient subgroup is presented in Figure 1. However, in the seven patients with impaired NVC (i.e. missing BOLD response) in one functional brain region, all physiologic MRI biomarkers were significantly different (p = 0.017–0.028) in these functional brain areas compared to the values of the functional brain regions contralateral to the side of the lesion. Furthermore, the physiologic MRI biomarkers in the functional brain areas with impaired NVC were significantly different (p = 0.018–0.028) compared to those in the functional brain areas on the ipsilateral side with intact NVC, except for CMRO2 (p = 0.108). Figure 2 illustrates an example out of this particular subgroup of patients. In general, OEF and CMRO2 were increased, while all other biomarkers were decreased in functional brain areas with impaired NVC (Figure 3). In summary, functional brain areas with lesion-induced impairment of NVC showed increased oxygen metabolism but decreased blood perfusion as well as alterations in microvascular architecture, which resulted in a distinctly reduced tissue oxygen tension, i.e. hypoxia. No significant differences (p = 0.552–1.0) were found between the subgroups of patients for the physiologic imaging biomarkers in the functional brain areas with intact NVC (ipsi- and contralateral). Table 2 displays the physiologic MRI biomarker values for the two subgroups of patients.
Figure 3.
Box-whisker plots demonstrating the minimum, the maximum, the sample median, and the first and third quartiles of the MR imaging biomarker values for (a) oxygen metabolism (OEF, CMRO2, and mitoPO2), (b) cerebral blood volume (CBV and µCBV), (c) cerebral blood flow (CBF and µCBF), and (d) microvascular architecture (MVD and VSI), respectively. The values for the patients with an impaired NVC in a cortical region with important brain function are presented on the left-hand side of the individual panels. The values for the patients with intact NVC are presented on right-hand side separated by a dashed line from the former.
NVC: neurovascular coupling; OEF: oxygen extraction fraction; CMRO2: cerebral metabolic rate of oxygen; mitoPO2: mitochondrial oxygen tension; CBV: cerebral blood volume; µCBV: microvascular cerebral blood volume; CBF: cerebral blood flow; µCBF: microvascular cerebral blood flow; MVD: microvessel density; VSI: vessel size index.
Table 2.
MR imaging biomarker values for oxygen metabolism, perfusion, and microvascular architecture in the patient subgroups with impaired and intact NVC.
| Patients with impaired NVC |
Patients with intact NVC |
||||
|---|---|---|---|---|---|
| Impaired NVC | Intact NVC, ipsilateral | Intact NVC, contralateral | Ipsilateral | Contralateral | |
| OEF | 57 ± 12 | 26 ± 2 | 25 ± 7 | 26 ± 3 | 23 ± 3 |
| (%) | (45–79) | (23–29) | (18–36) | (21–32) | (18–28) |
| CMRO2 | 113 ± 18 | 97 ± 21 | 94 ± 16 | 103 ± 14 | 100 ± 11 |
| (µmol/100g·min) | (83–137) | (65–120) | (67–109) | (78–122) | (79–115) |
| mitoPO2 | 14 ± 2 | 40 ± 5 | 43 ± 4 | 44 ± 4 | 43 ± 4 |
| (mmHg) | (11–18) | (35–47) | (36–47) | (39–51) | (36–50) |
| CBV | 3.7 ± 1.0 | 7.8 ± 1.2 | 8.2 ± 1.2 | 7.9 ± 1.3 | 8.4 ± 1.4 |
| (%) | (2.6–5.4) | (6.4–10.0) | (6.3–10.0) | (6.4–10.7) | (6.3–11.0) |
| µCBV | 2.5 ± 0.3 | 3.5 ± 0.6 | 3.9 ± 0.4 | 3.6 ± 0.5 | 3.7 ± 0.6 |
| (%) | (2.1–2.9) | (2.7–4.4) | (3.3–4.6) | (2.7–4.5) | (2.7–5.0) |
| CBF | 38 ± 11 | 59 ± 6 | 56 ± 6 | 59 ± 7 | 55 ± 10 |
| (ml/100g·min) | (24–55) | (47–67) | (48–66) | (48–69) | (43–69) |
| µCBF | 15 ± 2 | 22 ± 3 | 23 ± 3 | 26 ± 5 | 27 ± 4 |
| (ml/100g·min) | (12–18) | (18–28) | (19–27) | (19–34) | (22–32) |
| MVD | 309 ± 91 | 526 ± 111 | 515 ± 147 | 453 ± 76 | 466 ± 94 |
| (mm–2) | (183–419) | (380–703) | (335–701) | (347–596) | (332–627) |
| VSI | 29 ± 7 | 53 ± 14 | 52 ± 14 | 53 ± 17 | 55 ± 12 |
| (µm) | (22–41) | (36–76) | (35–73) | (36–86) | (37–82) |
NVC: neurovascular coupling; OEF: oxygen extraction fraction; CMRO2: cerebral metabolic rate of oxygen; mitoPO2: mitochondrial oxygen tension; CBV: cerebral blood volume; µCBV: cerebral blood volume in microvasculature; CBF: cerebral blood flow; µCBF: cerebral blood flow in microvasculature; MVD: microvessel density; VSI: vessel size index.
Investigation of attenuated BOLD responses
For the patients with impaired NVC, both the z score and the t score from BOLD fMRI data were calculated for the cortical areas with an attenuated BOLD response. For the patients with intact NVC, z score and the t score values were calculated for the cortical areas in closest vicinity to the lesion. Both z score and the t score values were correlated with the corresponding physiologic MRI biomarker values in the same ROI. For all 20 patients together, the z score showed a significant correlation (R = –0.770–0.802; p < 0.001 to 0.03) with all physiologic MRI biomarkers, except for CMRO2, which revealed no significant correlation (R = –0.136; p = 0.569). The t score, however, additionally showed no significant correlation with CBF (R = 0.421; p = 0.065), MVD (R = 0.335; p = 0.149), and VSI (R = 0.401; p = 0.080), respectively. For the patient subgroups with impaired and intact NVC, respectively, we found no significant correlations between BOLD fMRI metrics (z and t score) and the physiologic MRI biomarker values. The correlation coefficients between the BOLD fMRI metrics and the physiologic MRI biomarkers are summarized in Table 3.
Table 3.
Correlation coefficients between MR imaging biomarker values and BOLD fMRI metrics for all patients as well as for the patient subgroups with impaired and intact NVC.
| All patients |
Patients with impaired NVC |
Patients with intact NVC |
||||
|---|---|---|---|---|---|---|
| z score | t score | z score | t score | z score | t score | |
| OEF | –0.770 | –0.632 | –0.581 | –0.620 | 0.354 | 0.131 |
| (%) | (p < 0.001) | (p = 0.003) | (p = 0.171) | (p = 0.138) | (p = 0.235) | (p = 0.670) |
| CMRO2 | –0.136 | 0.068 | 0.094 | 0.166 | 0.280 | 0.474 |
| (µmol/100g·min) | (p = 0.569) | (p = 0.777) | (p = 0.840) | (p = 0.722) | (p = 0.354) | (p = 0.102) |
| mitoPO2 | 0.802 | 0.619 | –0.144 | –0.185 | 0.038 | –0.029 |
| (mmHg) | (p < 0.001) | (p = 0.004) | (p = 0.759) | (p = 0.692) | (p = 0.902) | (p = 0.924) |
| CBV | 0.683 | 0.538 | –0.115 | 0.036 | –0.144 | –0.094 |
| (%) | (p = 0.001) | (p = 0.014) | (p = 0.806) | (p = 0.938) | (p = 0.638) | (p = 0.761) |
| µCBV | 0.564 | 0.616 | –0.076 | –0.125 | –0.126 | 0.318 |
| (%) | (p = 0.010) | (p = 0.004) | (p = 0.871) | (p = 0.789) | (p = 0.682) | (p = 0.290) |
| CBF | 0.543 | 0.421 | –0.199 | –0.044 | –0.397 | –0.274 |
| (ml/100g·min) | (p = 0.013) | (p = 0.065) | (p = 0.669) | (p = 0.925) | (p = 0.179) | (p = 0.365) |
| µCBF | 0.696 | 0.578 | –0.145 | 0.162 | 0.130 | 0.125 |
| (ml/100g·min) | (p = 0.001) | (p = 0.008) | (p = 0.757) | (p = 0.729) | (p = 0.671) | (p = 0.683) |
| MVD | 0.562 | 0.335 | –0.124 | 0.036 | 0.078 | –0.245 |
| (mm–2) | (p = 0.010) | (p = 0.149) | (p = 0.791) | (p = 0.939) | (p = 0.801) | (p = 0.420) |
| VSI | 0.485 | 0.401 | –0.062 | 0.148 | –0.132 | –0.054 |
| (µm) | (p = 0.030) | (p = 0.080) | (p = 0.894) | (p = 0.751) | (p = 0.668) | (p = 0.861) |
OEF: oxygen extraction fraction; CMRO2: cerebral metabolic rate of oxygen; mitoPO2: mitochondrial oxygen tension; CBV: cerebral blood volume; µCBV: cerebral blood volume in microvasculature; CBF: cerebral blood flow; µCBF: cerebral blood flow in microvasculature; MVD: microvessel density; VSI: vessel size index.
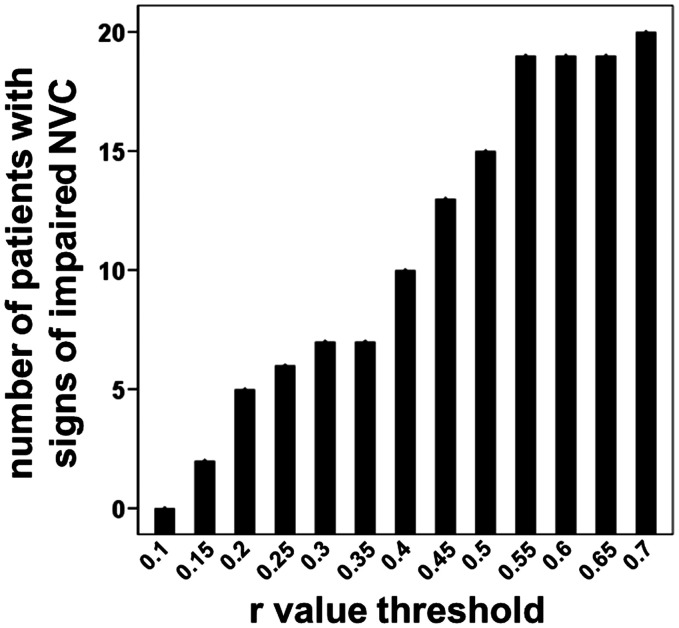
Figure 4 depicts the relationship between the number of patients with signs of impaired NVC and the r value threshold used for BOLD fMRI data processing. A value of 0.3 is commonly used as threshold for correlation. As expected, an increase in the r value threshold was associated with an increase in the assigned number of patients with signs of impaired NVC. Interestingly, an increase of the threshold from 0.3 to 0.35 showed no increase in the number of patients with signs of impaired NVC. This might be interpreted that a threshold of 0.3 is a good compromise for a common threshold for preclinical functional mapping in clinical routine
Figure 4.
Bar plot demonstrating the relationship between the number of patients with signs of impaired NVC and the r value threshold used for BOLD fMRI data processing. A value of 0.3 is commonly used as threshold for correlation.
NVC: neurovascular coupling.
Discussion
In this study, we attempt to investigate the physiological basis for an impaired NVC using a multimodal neuroimaging approach that combined preoperative BOLD fMRI and MEG experiments for detection of lesion-induced attenuation of the BOLD effect with physiological MRI measurements of hemodynamics, microvascular architecture, and oxygen metabolism in patients with brain lesions. In one third of patients, we observed impairment of NVC in perilesional brain regions that, in turn, showed significant brain tissue hypoxia, as well as significantly decreased macro- and microvascular hemodynamics, and microvascular architecture. These seven patients predominantly suffered from high-grade glioma (six patients), and one patient had a Cav.
Neurosurgeons rely on reliable presurgical mapping of eloquent cortical regions in order to achieve maximum safe resection of brain lesions, i.e. maximize the extent of resection while preserving neurological status of the patient. BOLD fMRI, which is considered as a main method for this purpose, however, is not reliable when the eloquent cortex is in close vicinity to the lesion, i.e. in the most crucial situation. Impairment of NVC, also known as neurovascular uncoupling or decoupling, is widely accepted as reason for impaired BOLD response when mapping these crucial cortical areas and therefore may confound interpretation of BOLD fMRI data. 8 , 44 , 45
Holodny et al. 8 , 46 were one of the first who showed that in brain tumor patients, the activation volumes on ipsilateral side of the tumor were significantly smaller compared to the contralateral, unaffected side. They assumed that a loss of autoregulation in the tumor vasculature and compressive effects to venous structures were the cause for this difference. In order to develop an MRI-based method for detection of neurovascular uncoupling, Pillai and Zacà 47 used BOLD cerebrovascular reactivity (CVR) mapping during hypercapnia induction via breath-holding in conjunction with task-based fMRI and found an overall concordance of 95% between areas of abnormally decreased regional CVR with areas of absent BOLD task-based activation in expected eloquent cortical regions infiltrated by or adjacent to the tumors. Hence, they concluded that breath-holding CVR mapping may be an important surrogate marker of NVU potential. However, using this method does not allow for spatial assessment of the functional level of cortical tissue with impaired CVR, i.e. impaired NVC.
Alternatively, in our study, we performed MEG experiments for detection of impaired NVC. Task-evoked fields in cortical areas with attenuated BOLD response have been interpreted as lesion-induced impairment of NVC. This MEG-based approach has been previously used in few studies for this purpose. Rossini et al. 11 compared the neuronal activation in 10 patients with cerebrovascular diseases during somatosensory stimulation measured with both MEG and BOLD fMRI and observed neuronal activity in the sensory cortex areas with MEG that was not observed on fMRI in five patients. Furthermore, they demonstrated that this uncoupling of neuronal activity from fMRI activation was strongly associated to the altered vasomotor reactivity as measured by transcranial Doppler during CO2 inhalation. Further studies that combined BOLD fMRI and MEG during language tasks in patients with focal brain lesions also found MEG activities but missing BOLD response in the fMRI data in 53% 12 and 17% 10 of the patients, respectively. These latter studies, similar to our study, did not use MR-based CVR mapping. Nevertheless, a combination of BOLD fMRI, MEG, and CVR mapping would be of high scientific interest in order to better understand the mechanism of lesion-induced attenuation of the BOLD effect and impairment of the NVC.
In the present study, we were able to uncover physiological reasons for attenuated BOLD response and impaired NVC using an advanced multiparametric MRI method. Our VAM approach revealed alterations in microvascular architecture, i.e. significant decreased MVD and size, in the cortical areas showing attenuated BOLD response. This was associated with significantly decreased hemodynamics in both macro- and microvasculature. Additionally, these cortical regions were affected by altered oxygen metabolism: a significantly increased CMRO2 and OEF, which was associated with strongly decreased tissue oxygen tension, i.e. severe local hypoxia.
The widely accepted view is that tumor infiltration of the brain parenchyma occurs by cooption of preexisting vessels. 48 , 49 With tumor growth, cancer cells migrate along existing blood vessels and invade the perivascular space. 49 , 50 Healthy brain vessels, however, are surrounded by astrocytes, and glioma cell invasion lead to the displacement of the astrocytic end-feet from the vasculature. 51 In addition, glioma cells took over the areas surrounding blood vessels, particularly favoring small capillaries. This leads to compression and destabilization of the vessels and in further consequence to vessel regression and reduced perfusion. 52 This is in accordance with our current study findings of reduced CBF and volume as well as decreased MVD and diameter. Additional studies such the work of Watkins et al. 51 suggested that this prevents vasoactive molecules released by astrocytes from reaching the endothelial cells, and glioma cells can increase their invasion area in the perivascular space by inducing vasoconstriction. 51 Ultimately, both effects may lead to an impairment of the NVC (and an attenuated BOLD response). Furthermore, reduced perfusion combined with increasing energy demands of progressing tumors (increased CMRO2 in our study) results in higher extraction of oxygen (increased OEF in our study) and a paucity of oxygen, i.e. hypoxia (decreased mitoPO2 in our study). 49 A study by Sumiyoshi et al. 15 investigated the effects of oxygen availability on NVC using simultaneous electroencephalography (EEG) and BOLD fMRI in anesthetized rats and found that even mild hypoxic conditions induced significant reductions in fMRI responses to electrical stimulation in the forepaw, but EEG responses remained unchanged. It is also well acknowledged that hypoxia plays an important role in the pathophysiology of the neurovascular unit 53 in cerebrovascular diseases such as Cav, where thrombosis with associated hypoxia and reactive gliosis of adjacent cerebral parenchyma are frequent signs. 54
The main limitation of this study is the relatively small sample size of the patient cohort with different tumor pathologies. We presented results of 20 patients with gliomas (low or high-grade), cerebrovascular diseases (AVM or Cav), or a meningioma, respectively. Furthermore, we did not include CVR mapping during breath-holding or CO2 inhalation, which might be of high scientific and possibly clinical relevance. An additional important limitation is related with the fact that BOLD fMRI was performed at 1.5 tesla. Previous studies investigated the influence of field strength on sensitivity and specificity in BOLD fMRI and demonstrated the advantages of high-field (3 tesla) and ultra-high-field (7 tesla) MR systems. 55 , 56 Further studies demonstrated the usefulness and reliability of 7 tesla BOLD fMRI for presurgical planning. 57 , 58 Therefore, future studies are highly warranted to replicate our findings on a larger scale including CVR mapping and high-field or ultra-high-field BOLD fMRI. The model for calculation of OEF has also several limitations. It assumes that the system is in the static dephasing regime 59 which is associated with the fact that OEF is predominantly weighted to the medium-sized and larger vessels of the venous vascular network. Furthermore, the multiparametric qBOLD approach 18 provides an average blood oxygenation within the entire vasculature, which is different from the venous blood oxygenation (deoxygenated CBV) derived from the original qBOLD approach, 60 and ignores the intravascular component. Additionally, accumulation of hemosiderin and/or proteins in perilesional tissue could bias the OEF estimation. Therefore, it is important to point out that the multiparametric qBOLD approach provides only an estimation of the oxygen metabolism with model-inherent limitations.
Another confounding factor of this study is related to the use of the combined GE-SE perfusion sequence 19 , 21 that does not fully meet the requirements for clinical routine diagnosis due to insufficient spatial coverage as only eight slices are measured. The coverage of the whole brain, however, is mandatory for routine MR perfusion. The efforts to upgrade the combined GE-SE perfusion sequence with the promising Simultaneous MultiSlice technique for full coverage may help to overcome these restrictions. 61 In previous studies, we used two separate MR perfusion sequences, i.e. one for GE-EPI DSC perfusion and one for SE-EPI DSC perfusion measurements instead of the hybrid GESE EPI perfusion sequence used in this study. The approach with two separate MR perfusion sequences, however, provides full compatibility with the efforts of clinical routine MRI. 23 , 24 , 26
Conclusion
Conclusively, our multimodal preoperative mapping approach combining fMRI and MEG provided highly consistent evidence for the presence of lesion-induced impairment of NVC in functional important cortex areas adjacent to brain lesions. This is of high clinical relevance for preoperative planning and postoperative outcome. However, due to the limited availability of MEG devices and the time-consuming implementation of MEG experiments, it is not used routinely in clinical practice. Our physiological MRI approach, which required only a few minutes of extra scanning time, may allow the detection of cortical areas at risk for lesion-induced impairment of NVC. However, CVR mapping should be included in future studies.
Acknowledgements
We thank Martin Kaltenhäuser and Peter Grummich, from the Department of Neurosurgery of the University Clinic Erlangen, for technical assistance.
Funding
The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work was supported by the German Research Foundation (Deutsche Forschungsgemeinschaft; Grant Numbers STA 1331/3-1 and DO 721/9-1) and by the Erlanger Leistungsbezogene Anschubfinanzierung und Nachwuchsförderung program (Grant Number 14-05-21-1-Stadlbauer).
Declaration of conflicting interests
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Authors’ contributions
AS, TMK, AD, MB, MZi, MZa, NB, SB, and IE conceived the study. AS, SB, IE, MZi, MZa, NB, and TMK were involved in the analysis of the data. AS, TMK, AD, MZi, NB, IE, and SB were involved in the interpretation of the data. AS, MB, MZi, NB, and AD contributed to the acquisition of the data. All authors reviewed the manuscript critically for intellectual content, and read and approved the final manuscript.
ORCID iDs
Andreas Stadlbauer https://orcid.org/0000-0001-8348-2620
Thomas M Kinfe https://orcid.org/0000-0002-4888-543X
References
- 1.Ogawa S, Menon RS, Tank DW, et al. Functional brain mapping by blood oxygenation level-dependent contrast magnetic resonance imaging. A comparison of signal characteristics with a biophysical model. Biophys J 1993; 64: 803–812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ogawa S, Lee TM, Kay AR, et al. Brain magnetic resonance imaging with contrast dependent on blood oxygenation. Proc Natl Acad Sci U S A 1990; 87: 9868–9872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hu X, Yacoub E. The story of the initial dip in fMRI. Neuroimage 2012; 62: 1103–1108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gabriel M, Brennan NP, Peck KK, et al. Blood oxygen level dependent functional magnetic resonance imaging for presurgical planning. Neuroimaging Clin N Am 2014; 24: 557–571. [DOI] [PubMed] [Google Scholar]
- 5.Filippi M, Agosta F. Diffusion tensor imaging and functional MRI. Handb Clin Neurol 2016; 136: 1065–1087. [DOI] [PubMed] [Google Scholar]
- 6.Chaudhry AA, Naim S, Gul M, et al. Utility of preoperative blood-oxygen-level-dependent functional MR imaging in patients with a central nervous system neoplasm. Radiol Clin North Am 2019; 57: 1189–1198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Pak RW, Hadjiabadi DH, Senarathna J, et al. Implications of neurovascular uncoupling in functional magnetic resonance imaging (fMRI) of brain tumors. J Cereb Blood Flow Metab 2017; 37: 3475–3487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Holodny AI, Schulder M, Liu WC, et al. The effect of brain tumors on BOLD functional MR imaging activation in the adjacent motor cortex: implications for image-guided neurosurgery. Am J Neuroradiol 2000; 21: 1415–1422. [PMC free article] [PubMed] [Google Scholar]
- 9.Hari R, Parkkonen L, Nangini C. The brain in time: insights from neuromagnetic recordings. Ann N Y Acad Sci 2010; 1191: 89–109. [DOI] [PubMed] [Google Scholar]
- 10.Zimmermann M, Rössler K, Kaltenhäuser M, et al. Refined functional magnetic resonance imaging and magnetoencephalography mapping reveals reorganization in language-relevant areas of lesioned brains. World Neurosurg 2020; 136: e41–e59. [DOI] [PubMed] [Google Scholar]
- 11.Rossini PM, Altamura C, Ferretti A, et al. Does cerebrovascular disease affect the coupling between neuronal activity and local haemodynamics? Brain 2004; 127: 99–110. [DOI] [PubMed] [Google Scholar]
- 12.Grummich P, Nimsky C, Pauli E, et al. Combining fMRI and MEG increases the reliability of presurgical language localization: a clinical study on the difference between and congruence of both modalities. Neuroimage 2006; 32: 1793–1803. [DOI] [PubMed] [Google Scholar]
- 13.Para AE, Sam K, Poublanc J, et al. Invalidation of fMRI experiments secondary to neurovascular uncoupling in patients with cerebrovascular disease. J Magn Reson Imaging 2017; 46: 1148–1455. [DOI] [PubMed] [Google Scholar]
- 14.Orukari IE, Siegel JS, Warrington NM, et al. Altered hemodynamics contribute to local but not remote functional connectivity disruption due to glioma growth. J Cereb Blood Flow Metab 2020; 40: 100–115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sumiyoshi A, Suzuki H, Shimokawa H, et al. Neurovascular uncoupling under mild hypoxic hypoxia: an EEG-fMRI study in rats. J Cereb Blood Flow Metab 2012; 32: 1853–1858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Stadlbauer A, Zimmermann M, Kitzwögerer M, et al. MR imaging-derived oxygen metabolism and neovascularization characterization for grading and IDH gene mutation detection of gliomas. Radiology 2017; 283: 799–809. [DOI] [PubMed] [Google Scholar]
- 17.Stadlbauer A, Zimmermann M, Heinz G, et al. Magnetic resonance imaging biomarkers for clinical routine assessment of microvascular architecture in glioma. J Cereb Blood Flow Metab 2017; 37: 632–643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Christen T, Schmiedeskamp H, Straka M, et al. Measuring brain oxygenation in humans using a multiparametric quantitative blood oxygenation level dependent MRI approach. Magn Reson Med 2012; 68: 905–911. [DOI] [PubMed] [Google Scholar]
- 19.Xu C, Kiselev VG, Möller HE, et al. Dynamic hysteresis between gradient echo and spin echo attenuations in dynamic susceptibility contrast imaging. Magn Reson Med 2013; 69: 981–991. [DOI] [PubMed] [Google Scholar]
- 20.Kiselev VG, Strecker R, Ziyeh S, et al. Vessel size imaging in humans. Magn Reson Med 2005; 53: 553–563. [DOI] [PubMed] [Google Scholar]
- 21.Emblem KE, Mouridsen K, Bjornerud A, et al. Vessel architectural imaging identifies cancer patient responders to anti-angiogenic therapy. Nat Med 2013; 19: 1178–1183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Stadlbauer A, Zimmermann M, Oberndorfer S, et al. Vascular hysteresis loops and vascular architecture mapping in patients with glioblastoma treated with antiangiogenic therapy. Sci Rep 2017; 7: 8508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Stadlbauer A, Zimmermann M, Doerfler A, et al. Intratumoral heterogeneity of oxygen metabolism and neovascularization uncovers 2 survival-relevant subgroups of IDH1 wild-type glioblastoma. Neuro Oncol 2018; 20: 1536–1546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Stadlbauer A, Roessler K, Zimmermann M, et al. Predicting glioblastoma response to bevacizumab through MRI biomarkers of the tumor microenvironment. Mol Imaging Biol 2019; 21: 747–757. [DOI] [PubMed] [Google Scholar]
- 25.Stadlbauer A, Mouridsen K, Doerfler A, et al. Recurrence of glioblastoma is associated with elevated microvascular transit time heterogeneity and increased hypoxia. J Cereb Blood Flow Metab 2018; 38: 422–432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Stadlbauer A, Oberndorfer S, Zimmermann M, et al. Physiologic MR imaging of the tumor microenvironment revealed switching of metabolic phenotype upon recurrence of glioblastoma in humans. J Cereb Blood Flow Metab 2020; 40: 528–538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Prasloski T, Mädler B, Xiang QS, et al. Applications of stimulated echo correction to multicomponent T2 analysis. Magn Reson Med 2012; 67: 1803–1814. [DOI] [PubMed] [Google Scholar]
- 28.Bjornerud A, Emblem KE. A fully automated method for quantitative cerebral hemodynamic analysis using DSC-MRI. J Cereb Blood Flow Metab 2010; 30: 1066–1078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Boxerman JL, Prah DE, Paulson ES, et al. The role of preload and leakage correction in gadolinium-based cerebral blood volume estimation determined by comparison with MION as a criterion standard. Am J Neuroradiol 2012; 33: 1081–1087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Vafaee MS, Vang K, Bergersen LH, et al. Oxygen consumption and blood flow coupling in human motor cortex during intense finger tapping: implication for a role of lactate. J Cereb Blood Flow Metab 2012; 32: 1859–1868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kennan RP, Zhong J, Gore JC. Intravascular susceptibility contrast mechanisms in tissues. Magn Reson Med 1994; 31: 9–21. [DOI] [PubMed] [Google Scholar]
- 32.Vafaee MS, Gjedde A. Model of blood-brain transfer of oxygen explains nonlinear flow-metabolism coupling during stimulation of visual cortex. J Cereb blood flow Metab 2000; 20: 747–754. [DOI] [PubMed] [Google Scholar]
- 33.Ducreux D, Buvat I, Meder JF, et al. Perfusion-weighted MR imaging studies in brain hypervascular diseases: comparison of arterial input function extractions for perfusion measurement. AJNR Am J Neuroradiol 2006; 27: 1059–1069. [PMC free article] [PubMed] [Google Scholar]
- 34.Jensen JH, Lu H, Inglese M. Microvessel density estimation in the human brain by means of dynamic contrast-enhanced echo-planar imaging. Magn Reson Med 2006; 56: 1145–1150. [DOI] [PubMed] [Google Scholar]
- 35.Zimmermann M, Rössler K, Kaltenhäuser M, et al. Comparative fMRI and MEG localization of cortical sensorimotor function: bimodal mapping supports motor area reorganization in glioma patients. PLoS One 2019; 14: e0213371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Thesen S, Heid O, Mueller E, et al. Prospective acquisition correction for head motion with image-based tracking for real-time fMRI. Magn Reson Med 2000; 44: 457–465. [DOI] [PubMed] [Google Scholar]
- 37.Bandettini PA, Jesmanowicz A, Wong EC, et al. Processing strategies for time‐course data sets in functional MRI of the human brain. Magn Reson Med 1993; 30: 161–173. [DOI] [PubMed] [Google Scholar]
- 38.Castillo EM, Simos PG, Wheless JW, et al. Integrating sensory and motor mapping in a comprehensive MEG protocol: clinical validity and replicability. Neuroimage 2004; 21: 973–983. [DOI] [PubMed] [Google Scholar]
- 39.Shattuck DW, Leahy RM. Brainsuite: an automated cortical surface identification tool. Med Image Anal 2002; 6: 129–142. [DOI] [PubMed] [Google Scholar]
- 40.Dale AM, Liu AK, Fischl BR, et al. Dynamic statistical parametric mapping: combining fMRI and MEG for high-resolution imaging of cortical activity. Neuron 2000; 26: 55–67. [DOI] [PubMed] [Google Scholar]
- 41.Tadel F, Baillet S, Mosher JC, et al. Brainstorm: a user-friendly application for MEG/EEG analysis. Comput Intell Neurosci 2011; 2011: 879716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Dale AM, Sereno MI. Improved localization of cortical activity by combining EEG and MEG with MRI cortical surface reconstruction: a linear approach. J Cogn Neurosci 1993; 5: 162–176. [DOI] [PubMed] [Google Scholar]
- 43.Greenblatt RE, Ossadtchi A, Pflieger ME. Local linear estimators for the bioelectromagnetic inverse problem. IEEE Trans Signal Process 2005; 53: 3403–3412. [Google Scholar]
- 44.Zacà D, Jovicich J, Nadar SR, et al. Cerebrovascular reactivity mapping in patients with low grade gliomas undergoing presurgical sensorimotor mapping with BOLD fMRI. J Magn Reson Imaging 2014; 40: 383–390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Agarwal S, Sair HI, Yahyavi-Firouz-Abadi N, et al. Neurovascular uncoupling in resting state fMRI demonstrated in patients with primary brain gliomas. J Magn Reson Imaging 2016; 43: 620-626. [DOI] [PubMed] [Google Scholar]
- 46.Holodny AI, Schulder M, Liu WC, et al. Decreased BOLD functional MR activation of the motor and sensory cortices adjacent to a glioblastoma multiforme: implications for image-guided neurosurgery. Am J Neuroradiol 1999; 20: 609–612. [PMC free article] [PubMed] [Google Scholar]
- 47.Pillai JJ, Zacà D. Comparison of BOLD cerebrovascular reactivity mapping and DSC MR perfusion imaging for prediction of neurovascular uncoupling potential in brain tumors. Technol Cancer Res Treat 2012; 11: 361–374. [DOI] [PubMed] [Google Scholar]
- 48.Leenders WPJ, Küsters B, De Waal RMW. Vessel co-option: how tumors obtain blood supply in the absence of sprouting angiogenesis. Endothel J Endothel Cell Res 2002; 9: 83–87. [DOI] [PubMed] [Google Scholar]
- 49.Holash J, Maisonpierre PC, Compton D, et al. Vessel cooption, regression, and growth in tumors mediated by angiopoietins and VEGF. Science (80-) 1999; 284: 1994–1998. [DOI] [PubMed] [Google Scholar]
- 50.Hardee ME, Zagzag D. Mechanisms of glioma-associated neovascularization. Am J Pathol 2012; 181: 1126–1141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Watkins S, Robel S, Kimbrough IF, et al. Disruption of astrocyte-vascular coupling and the blood-brain barrier by invading glioma cells. Nat Commun 2014; 5: 1–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Padera TP, Stoll BR, Tooredman JB, et al. Cancer cells compress intratumour vessels. Nature 2004; 427: 695. [DOI] [PubMed] [Google Scholar]
- 53.Stanimirovic DB, Friedman A. Pathophysiology of the neurovascular unit: disease cause or consequence. J Cereb Blood Flow Metab 2012; 32: 1207–1221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Cortés Vela JJ, Concepción Aramendía L, Ballenilla Marco F, et al. Cerebral cavernous malformations: spectrum of neuroradiological findings. Radiol (English Ed) 2012; 54: 401–409. [DOI] [PubMed] [Google Scholar]
- 55.Boubela RN, Kalcher K, Nasel C, et al. Scanning fast and slow: current limitations of 3 Tesla functional MRI and future potential. Front Phys 2014; 2: 00001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Beisteiner R, Robinson S, Wurnig M, et al. Clinical fMRI: evidence for a 7T benefit over 3T. Neuroimage 2011; 57: 1015–1021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Cardoso PL, Fischmeister FPS, Dymerska B, et al. Improving the clinical potential of ultra-high field fMRI using a model-free analysis method based on response consistency. Magn Reson Mater Physics, Biol Med 2016; 29: 435–449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Lima Cardoso P, Fischmeister FPS, Dymerska B, et al. Robust presurgical functional MRI at 7 T using response consistency. Hum Brain Mapp 2017; 38: 3163–3174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Yablonskiy DA, Haacke EM. Theory of NMR signal behavior in magnetically inhomogeneous tissues: the static dephasing regime. Magn Reson Med 1994; 32: 749–763. [DOI] [PubMed] [Google Scholar]
- 60.He X, Yablonskiy DA. Quantitative BOLD: mapping of human cerebral deoxygenated blood volume and oxygen extraction fraction: default state. Magn Reson Med 2007; 57: 115–126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Eichner C, Jafari-Khouzani K, Cauley S, et al. Slice accelerated gradient-echo spin-echo dynamic susceptibility contrast imaging with blipped CAIPI for increased slice coverage. Magn Reson Med 2013; 72: 770–778. [DOI] [PMC free article] [PubMed] [Google Scholar]