Abstract
Optimizing cerebral perfusion is key to rescuing salvageable ischemic brain tissue. Despite being an important determinant of cerebral perfusion, there are no effective guidelines for blood pressure (BP) management in acute stroke. The control of cerebral blood flow (CBF) involves a myriad of complex pathways which are largely unaccounted for in stroke management. Due to its unique anatomy and physiology, the cerebrovascular circulation is often treated as a stand-alone system rather than an integral component of the cardiovascular system. In order to optimize the strategies for BP management in acute ischemic stroke, a critical reappraisal of the mechanisms involved in CBF control is needed. In this review, we highlight the important role of collateral circulation and re-examine the pathophysiology of CBF control, namely the determinants of cerebral perfusion pressure gradient and resistance, in the context of stroke. Finally, we summarize the state of our knowledge regarding cardiovascular and cerebrovascular interaction and explore some potential avenues for future research in ischemic stroke.
Keywords: Ischemic stroke, neurovascular physiology, cerebral blood flow
Introduction
An abrupt and profound rise in blood pressure (BP) is a hallmark of ischemic stroke in ∼80% of patients, irrespective of their prior BP or stroke etiology.1,2 This elevation in BP can persist for days following stroke onset, and is accompanied by parallel increases in plasma noradrenaline via sympathetic activation.3,4 In addition, intracranial pressure (ICP) may be elevated, which can be driven directly by accumulation of blood volume as seen in hemorrhagic stroke, 5 or via cerebral oedema following ischemic stroke.6,7 Elevated ICP diminishes the cerebral perfusion pressure (CPP), thus presents a barrier to restoring cerebral blood flow (CBF) to the ischemic brain. 8 A fundamental question is whether intervening to reduce high BP in the acute period after stroke is a help or a hindrance to cerebral perfusion homeostasis. Traditionally, post-stroke hypertension has been viewed as either a pathological response that should be prevented, or a protective physiological response to improve perfusion pressure in the penumbra.9,10 These polarizing views on post-stroke hypertension reflect the current lack of consensus in the literature regarding the effects of elevated BP on long-term outcome after stroke.
Elevated BP during acute stroke has been found to be associated with poor outcomes,6,11,12 improved outcomes, 13 or of minimal prognostic value. 14 Studies have reported a U-shaped relationship between systolic BP on admission and stroke outcome (Figure 1).11,12,15 These findings indicate the ‘least poor’ outcome at a systolic BP of 140–160 mmHg, as judged by the nadir of the U curve. Despite extensive research, findings from large clinical trials have failed to demonstrate clear therapeutic benefits of antihypertensive treatments in acute stroke management. Findings from these clinical studies range from near-negative, 16 to neutral,17–20 to near-positive. 21 One reason for the discrepancies in previous clinical trials may lie in the use of peripheral ‘brachial’ measures of BP as a proxy for cerebral perfusion pressure. The management of BP in acute stroke remains highly controversial due to a lack of reliable and consistent evidence. Accordingly, the 2019 American Heart Association/American Stroke Association guidelines for acute stroke management state “the blood pressure level that should be maintained in patients with acute ischemic stroke to ensure the best outcome is not known”. 22
Figure 1.
Relationship between blood pressure and stroke morality rate at 1 and 12 months. Systolic blood pressure (panel a) and diastolic blood pressure (panel b) on admission. Triangles and squares represent early and late stroke mortality rate, respectively, within groups A and B; Data are presented as mean ± 95% confidence intervals. Reprinted with permission. 15
Although understandable from a logistical and resource constraint perspective, it has been known for over half a century that brachial pressure is a poor surrogate for aortic pressure, and that brachial BP overestimates central BP within the ascending aorta by as much as 40 mmHg.23,24 Since the major arteries supplying the brain are exposed to central pressure, this may partly explain why studies, which focused on brachial BP (i.e., peripheral pressure) as a surrogate of CPP, have failed to demonstrate clear therapeutic benefits of BP management in stroke. In addition, cerebral perfusion is the net balance between CPP and the vascular resistance of the cerebral circulation. CPP can be affected by ICP which acts as a Starling resistor for central venous outflow, 25 while cerebrovascular resistance is under myogenic, physical, metabolic/chemical and neurogenic control pathways. Clearly, the control of CBF is complex and involves a myriad of interwoven mechanisms.
After decades of research, few effective strategies have successfully translated into clinical practice, namely reperfusion/recanalization therapies and acute stroke units. While interventional therapies such as thrombolysis and endovascular thrombectomy can achieve reperfusion rates as high as 85% in eligible patients, and can greatly improve patient outcome, they are ultimately received by fewer than 10% of stroke patients worldwide due to a narrow therapeutic window (typically ∼4.5–6 hours) and other logistical constraints.26,27 In 2011, the Cerebral Autoregulation Network (CARNET, www.car-net.org) was initiated to foster collaboration between researchers and prioritize research into the BP-CBF relationship – termed cerebral autoregulation (CA). The group comprises experienced clinicians and researchers in the different areas such as, neurology, cardiology, cardiovascular physiology, biomedical engineering and neuroscience. In 2016, a new initiative titled ‘Identifying New Targets FOr Management And Therapy in Acute Stroke’ (INFOMATAS) was launched with the objective to set clinical trials to optimize BP management in acute stroke through: 1) a better understanding of CA in acute stroke, 28 and 2) determining the best interventions for improving perfusion to ischemic tissue guided by CA indices. Within the mission statement of INFOMATAS, this review serves to provide a critical reappraisal of the pathophysiological mechanisms involved in CBF regulation following ischemic stroke. We provide an overview of the major determinants of CPP and cerebrovascular resistance in the context of acute stroke, identifying current knowledge gaps and proposing future research directions. We then briefly explore the complex interplay between the BP control (i.e., baroreflex) and CA, and how they may shed light on CBF dysregulation in acute stroke.
The cerebral circulation
The brain has a unique blood vessel anatomy and physiology which serves to protect and maintain CBF relatively constant even in cases of local occlusion. While the large intracranial arteries supply blood to the major regions of the brain, there are some areas bordering the territories supplied by these intracranial arteries – ‘boundary zones’ – which are more vulnerable to decreases in CBF. 29 The large intracranial arteries branch extensively into smaller pial arteries and arterioles that course along the surface of the cerebral cortex before penetrating the brain tissue. The pial arteries form an effective collateral network called leptomeningeal collaterals, such that occlusion of one pial vessel does not appreciably affect the surrounding tissues. As the branching pial arteries course centripetally into the parenchyma, they give rise to the arterioles and then capillaries (intra-parenchymal vessels). In contrast to the extensive network of pial vessels, the penetrating parenchymal arterioles are long and largely unbranched. 30 Due to the lack of collaterals and higher basal myogenic tone, 31 an occlusion of these penetrating parenchymal arterioles will result in significant local hypoperfusion and more rapid infarct expansion to surrounding tissue.32,33
The entire capillary network of the brain, along with arterioles and post-capillary venules lined by cerebral endothelial cells, form an important component of the blood-brain barrier (BBB). The cerebral endothelial cells are connected by tight junctions and surrounded by astrocytic end-feet and pericytes, which makes up the intra-parenchymal vessels. The cerebral endothelial cells, together with pre-capillary vascular smooth muscle cells, astrocytes, pericytes, microglia, neurons, and the extracellular matrix form the neurovascular unit, which regulates local control of microvascular resistance (i.e., terminal arterioles, capillaries and venules) and facilitates the redistribution of blood flow to metabolically active areas of the brain – this is termed neurovascular coupling or functional hyperemia. Due to a lack of functional or anatomical capillary recruitment in the brain, capillary perfusion is increased by vasodilation of upstream arteries and arterioles which increases the microvascular pressure gradient. During cerebral ischemia, a transient constriction of arteriolar smooth muscle cells, but not pericyte-covered capillaries, is responsible for hypoperfusion. 34 However, in vitro evidence suggests that pericyte-mediated capillary constriction occurs in response to ischemia.35–37 In stroke, these in vitro data indicate that pericyte-mediated capillary constriction may prevent microcirculatory reperfusion despite clot removal. It should be acknowledged that the role of pericytes in CBF control is controversial and warrants further studies.
Cerebral venous system
The cerebral venous system holds 70–80% of total cerebral blood volume capacity and yet is often overlooked from a research perspective. This is partly due to the fact that the venous territories are highly variable and less well-characterized in comparison with their arterial counterparts. It is a valveless vascular network comprised of dural sinuses and cerebral veins that is freely communicating and interconnected. Within the grey matter, capillaries drain into venules which converge and join larger venules. These larger venules increase in caliber towards the cortical surface and join with intracortical veins, the latter forming pial vascular-anastomosis with surrounding intracortical arterioles. Due to the absence of valves, the cerebral veins permit retrograde blood flow. Despite the absence of encompasing vascular smooth muscles, the cerebral venous system plays a significant role in CBF control in pathological conditions (see38,39 for reviews). In patients with chronic cerebrospinal venous insufficiency, the degree of parenchymal hypoperfusion is associated with severity of chronic cerebrospinal venous insufficiency, 40 while surgically restoring venous jugular flow ameliorates cerebral hypoperfusion. 41 Likewise, occlusion of cortical ascending venules decreases blood flow, reverses flow direction and elicits upstream capillary dilation. 42
In the infarct core where cytotoxic and vasogenic edema elevates local tissue pressure, the capillaries closest to the venous end will collapse due to higher intramural pressure thereby redirecting blood flow to areas of lower resistance (i.e., the surrounding penumbra). 43 The diverted blood flow manifest as a steal from the infarct core and luxury perfusion to the surrounding penumbral tissue.43,44 Meanwhile, elevated local venous pressure could recruit collapsed vascular network by equilibrating the effective backpressure, thereby correcting perifocal perfusion maldistribution.43,44 This blood flow maldistribution – termed ‘cerebral venous steal’, could explain the larger infarct volumes and poorer patient outcome at three-months in stroke patients exhibiting ipsilateral medullary vein congestion.45,46 Whilst several imaging modalities exist for visualizing the cerebral veins,39,47 it has been largely overlooked in both clinical and research settings. Given the integral role the cerebral veins play in regulating perfusion, routine assessment of the cerebral venous circulation in acute stroke could provide insights into infarct and penumbral progression and serve as prognostic marker for patient outcome.
Collateral circulation
The collateral vessels are an important feature of cerebral circulation as they connect major vessels, either directly or indirectly, and help stabilize regional perfusion when the principal conduit artery fails (i.e., via occlusion). The presence, density and anatomy of cerebral collaterals varies considerably between individuals and has been predictively linked to clinical outcome in cerebrovascular disease. Several studies have found collateral perfusion to be a strong predictor of response to thrombolytic therapies, clinical recovery and infarction volume following stroke, and hemorrhagic transformation in large vessel occlusion stroke.(39,48 for reviews) Likewise, optimal collateral circulatory competence was associated with slower infarct growth rates and prolongation of therapeutic window. 49 In large and small animal models of acute ischemic stroke, nitric oxide (NO) has been shown to selectively dilate collateral arterioles, reducing infarct volume and improving functional outcome. 50 Therefore, a robust collateral circulation appears to be a key determinant of penumbral viability and patient outcome. Conversely, failure to recruit and/or maintain collaterals to ischemic tissue likely explains the rapidly decreasing benefit of reperfusion therapies over time.
Collateral perfusion to ischemic tissues depends on the caliber and patency of primary collateral pathways able to rapidly compensate for decreased blood flow, along with the adequacy of secondary collateral routes. Together with other communicating arteries, the basilar and internal carotid arteries form a complete anastomotic ring at the base of the brain, known as the circle of Willis. In the event of large intracranial artery occlusions, these primary collaterals provide immediate diversion of CBF to ischemic regions through existing anastomoses. Meanwhile, secondary collaterals such as leptomeningeal anastomoses may be anatomically present, although upregulating the capacity of these alternative routes to support CBF may require time. Chronic cerebral ischemia activates angiogenesis pathway during collateral development,51,52 and increased microvascular density appears to be linked to increased tissue survival time following ischemic stroke. 53 Genetic influences have been reported to account for many of the variations in collateral circulation between individuals following acute stroke. 54 Alternatively, Menon et al. 55 have identified older age, hyperuricemia, and metabolic syndrome to be independent predictors of poor leptomenigeal collaterals. Other factors such as dehydration, severe carotid artery stenosis, recent ischemic stroke and anesthesia-induced hypotension during endovascular therapy have also found to be associated with low collateral prevalence and development.56–58 Meanwhile, both exercise and hypoxia-inducible factor 1-α have been shown to promote collateral circulation development.59,60 Promoting more rapid cerebral collateral recruitment could be an effective strategy to improve stroke outcome and should be explored further.
Despite the advent of advanced neuroimaging, the prognostic role of the collateral circulation on stroke outcome remains unclear due to the lack of a standardized grading system. 61 Nevertheless, there are data suggesting that the quality of the collateral circulation is linked to stroke etiology, with lower collateral flow observed in stroke patients with atrial fibrillation compared to those with cervical atherosclerotic steno-occlusive disease. 62 Whilst not an universal finding,63,64 this link between stroke etiology and collateral circulation may explain the poorer patient outcomes seen in atrial-fibrillation-related strokes. 65 Likewise, lower collateral flow could account for the higher mortality rates, greater functional deficits and poorer long-term outcomes observed in atrial fibrillation-related strokes.66,67 While several methods of assessing the anatomical presence and patency of cerebral collaterals exist, 61 they remain underutilized in stroke research. Moreover, the current literature focuses on the anatomical presence or absence of collateral vessels, but the functional responses of the collateral circulation remain largely unstudied. A better understanding of the link between the collateral circulation and stroke outcome could be the key to developing strategies to reduce infarct volume and improve patient outcomes.
Determinants of the cerebral perfusion pressure gradient
Baroreflex
Arterial BP is tightly regulated to ensure adequate perfusion of vital organs through a closed-loop feedback mechanism – termed the baroreflex. Impaired baroreflex sensitivity (BRS) is an important marker of autonomic dysfunction, which plays a major role in long-term development of systemic hypertension and related complications. Numerous studies have reported baroreflex dysfunction following both ischemic and hemorrhagic strokes, which can persist for months following stroke (see Yperzeele et al. 68 for review). Recent data indicate that insula cortex lesions can account for the baroreflex dysfunction observed in stroke patients. 69 Such lesions lower BRS, resulting in sympathetic dominance, which accounts for the occurrence of acute systemic hypertension and elevated BP fluctuations [i.e., BP variability (BPV)] following stroke. Importantly, the degree of BRS has been shown to predict short-term and long-term clinical outcomes in acute ischemic and hemorrhagic stroke, with low BRS associated with the presence of acute systemic hypertension on admission, larger infarct volume, worse clinical outcome and higher mortality rates.70–72 However, extrapolation of previous findings to the general stroke population is difficult due to small sample sizes in those studies. Moreover, these studies only assessed cardiac arm of the baroreflex, thus the effect of stroke on the baroreflex control of peripheral vascular resistance remains unknown. Nevertheless, these findings highlight that autonomic dysfunction and BRS impairment can be important prognostic markers of stroke outcome. It is known that antihypertensive drugs such as angiotensin converting enzyme inhibitors, angiotensin II receptor blockers, calcium (Ca2+) channel and β-blockers improve baroreflex function.73–75 The potential therapeutic benefits of these drugs on BRS in the acute stroke settings warrants further investigation.
Blood pressure variability
BP is a dynamic physiological variable which exhibits both short-term (i.e., beat-to-beat, over 24 hours) and long-term (i.e., day-to-day and over months or years) oscillations. While long-term BP control is predominately under renal modulation, there is a body of evidence showing that short-term BP control may be modulated by autonomic and myogenic function, endothelial-derived NO and the renin-angiotensin system (see Stauss 76 for review). In transient ischemic attack patients, Fan et al. 77 found evidence of impaired myogenic and endothelial NO synthase-derived BPV, which was improved with dietary nitrate supplementation. In the brain, accentuated short- and long-term BPV may result in hypoperfusion and hyperperfusion insults, which could destabilize cerebral tissue oxygenation and lead to BBB breakdown. 78 Irrespective of successful recanalization, 79 increased BPV is associated with adverse events following stroke.80,81 Conversely, low BPV is a predictor of favorable outcome following stroke. 13 These fluctuations in BPV (and therefore cerebral perfusion) exacerbate secondary neuronal damage in the ischemic penumbra, resulting in poorer neurological outcomes following stroke. Both short- and long-term BPV could serve as prognostic markers for patient outcome in acute stroke.
Intracranial pressure
Encased within a rigid cranium, the brain is uniquely vulnerable to changes in ICP. If left unchecked, increases in ICP may result in the compression of cerebral blood vessels and/or neural tissue, and present a barrier to CBF. CPP presents the drive pressure to the brain and is the difference between BP and ICP in conditions where central venous pressure (CVP) is lower than ICP. At rest, ICP is typically low (5–10 mmHg) and largely determined by the balance between cerebrospinal fluid production and drainage. In healthy individuals, the low resting ICP means that CBF is largely determined by arterial pressure. As a result of the enclosed skull, ICP acts as a Starling resistor for cerebral venous outflow, a mechanism that is likely to be of great importance in conditions where ICP and CVP are elevated. The Starling resistor model describes how CPP is quantitatively dependent on the coordinated action of cerebrovascular resistance (i.e., arteriolar and venous resistance). Typically, venous resistance remains stable due to the ability of the arteriolar resistance to rapidly vasoconstrict or vasodilate in response to changes in CPP. However, beyond the limit of this arteriolar network autoregulatory response (i.e., when maximal vasoconstriction or vasodilation is reached), arteriolar resistance becomes constant, and the resistance of the subarachnoid veins change dramatically. 25
In the absence of compensatory increases in arterial pressure, elevations in ICP will reduce CBF; conversely, in the absence of ICP changes, alterations in BP can directly alter CBF. When compensatory rise in BP is prevented, early studies in primates have shown that CBF remains stable with increases of ICP below 50 mmHg, above this point CBF progressively decreases with increasing ICP. 8 During moderate elevations in ICP, compensatory dilation of pial and cortical surface vessels may help prevent cerebral hypoperfusion.82,83 Due to the lack of smooth muscle cells, the cerebral venous system is particularly susceptible to tissue oedema and intracranial hypertension. Dramatic elevations in ICP associated with severe cerebral oedema can easily compress the thin walls of venules and capillaries. The subsequent increases in CVP can lead to an increase in hydrostatic pressure of upstream cerebral veins and reduce blood outflow. Patients with high ICP exhibit narrow venous outlet formation and proximal vascular dilation, resulting in intracranial venous congestion and impaired venous drainage. 84
In large malignant strokes, where moderate increases in ICP are common, 7 the influences of ICP and CVP on CBF may be significant. Since ICP monitoring is invasive and reserved for critically ill patients in intensive care, it is rarely assessed in non-malignant strokes. The role of raised ICP in determining CPP during acute stroke remains poorly understood, in part due to the difficulty of making direct clinical measurements of ICP. Non-invasive strategies to estimate ICP could advance our understanding of how stroke impact ICP (and therefore CPP). The development and validation of non-invasive measures of ICP could advance the clinical management of CPP in stroke.
Determinants of cerebrovascular resistance
In contrast to other organs where large arteries serve as conduit vessels, the entire cerebral circulation is involved in the regulation of cerebrovascular resistance; the large cerebral arteries contribute ∼50% of total resistance in the brain. 85 The cerebral circulation is controlled by several overlapping pathways involving myogenic, physical, metabolic/chemical and neural factors. Under acute physiological conditions, cerebrovascular resistance is determined by the balance between vasodilatory and vasoconstricting inputs (i.e., changes in vascular tone). Over longer timeframes, cerebrovascular resistance can also be influenced by vascular remodeling (i.e., rarefaction, angiogenesis), and changes in vascular stiffness, which can alter the dilation and constriction responses of the vessel during chronic physiological challenges (i.e., prolonged cerebral ischemia/hypoxia). Since CBF is determined as the net balance of CPP and cerebrovascular resistance, efforts to restore perfusion to an ischemic brain by elevating CPP would be less effective if cerebrovascular resistance is concurrently elevated.
Myogenic control
Myogenic control of CBF describes the intrinsic changes in vascular smooth muscle tone in response to variations in perfusion pressure. Typically, increases in intravascular pressure elicit an increase in cerebrovascular smooth muscle tone, which subsequently decreases CBF. Myogenic control of the vasculature is independent of the endothelium as vasoconstriction will occur with increases in transmural pressure in vessels devoid of the endothelial layer. 86 The primary mechanism of myogenic vasoconstriction in the cerebral arterioles is thought to be mediated by increased Ca2+ influx and sensitivity of the vascular smooth muscle via protein kinase C and Rho kinase.87,88
Palomares and Cipolla 33 proposed enhancing myogenic function as a potential therapeutic target for acute stroke. Mechanosensitive ion channels in the vascular smooth muscle response to stretch with a reduction in the smooth muscle membrane potential, an influx of Ca2+ through L-type voltage-gated Ca2+ channels, and subsequent vasoconstriction. 89 As such, the use of pharmacological interventions such as Ca2+ channel blockers have facilitated the exploration of the myogenic control of CBF in humans.90,91 Furthermore, Ca2+ channel blockers are often recommended for stroke prevention primarily due to their antihypertensive effects, but have also been demonstrated to reduce cerebral vasospasm and cerebral ischemia. 92 Tzeng and colleagues found Ca2+ channel blockade with nimodipine reduced BPV and CBF variability.90,91 This BPV- and CBF-variability lowering effect may contribute to the beneficial outcomes observed in stroke following Ca2+ channel blockade.93,94
Shear-stress control
Abrupt increases in BP during acute stroke can directly impact cerebrovascular function via increased shear stress on the cerebral endothelium – the force per unit area created when blood flows over the endothelium. Shear stress is determined as the blood flow velocity and viscosity, inversely proportional to the cube of vessel radius. Under normal conditions, a rise in shear stress elicits a compensatory cerebral vasodilation via endothelial-NO production to restore shear stress.95,96 However, endothelial NO synthase activity appears to be impaired with both chronic hypertension and following transient cerebral ischemia.77,97 Given the prevalence of hypertension in stroke patients, this adaptive response to high shear stress is likely to be greatly impaired in acute stroke patients, contributing to endothelial damage, BBB breakdown and upregulation of atherogenic genes.95,96 Therapeutic strategies to enhance NO production may restore endothelium-mediated cerebral vasodilation in acute stroke, thus improve CBF.
Metabolic/chemical control
The human brain represents ∼2% of total body mass but accounts for ∼20% of the body’s metabolic requirements. 98 Due to the limited capacity for substrate storage and the high metabolic demand, the brain is particularly sensitive to ischemic insults. Adequate supplies of nutrients and oxygen (O2) are crucial in maintaining normal brain functions, and irreversible neuronal damage can occur if CBF is compromised for more than a few minutes. At the regional level, changes in CBF are closely coupled with glucose utilization and O2 consumption through neurovascular coupling. At the whole organ level, global cerebral metabolism appears to be well defended under most physiological challenges such as progressive hypoxia and hypotension.99,100 This remarkable feat is achieved through compensatory increases in cerebral oxygen extraction fraction (OEF) and cerebral perfusion.
Oxygen extraction fraction
In the face of reduced cerebral O2 delivery, the amount of O2 extracted from the blood can be elevated to ensure normal cerebral O2 metabolism. Cerebral OEF is the balance between cerebral O2 delivery and consumption, which can be summarized by the equation
where, CMRO2 is the cerebral metabolic rate for O2, CaO2 is the arterial O2 content, and CBF is cerebral blood flow. Changes in OEF serve as a compensatory mechanism for preserving cerebral delivery of O2 and CMRO2 maintain in the face of fluctuations in CBF (see Lin and Powers 101 for review). At maximal OEF, any further reduction in CBF will result in reduced CMRO2 (Figure 2), which leads to a cascade of cellular events that may result in neuronal cell death. In ischemic stroke patients exhibiting large perfusion-diffusion lesion mismatch, OEF is pathologically elevated in the infarcted hemisphere where perfusion is reduced – termed misery perfusion, which is associated with increased risk of stroke reoccurrence.102,103 In acute stroke, a dramatically increased OEF signals the presence of hypoperfused yet potentially salvageable tissue and could be used as a novel prognostic marker of penumbral development.
Figure 2.
Pathophysiology changes in cerebral perfusion, metabolism and oxygen extraction in cerebral ischemia. The initial decline in regional cerebral blood flow (CBF) is compensated by a mirror rise in regional oxygen extraction fraction (OEF). Since the increase in OEF is unable to sustain the energy requirement of the brain, regional cerebral oxygen metabolism (CMRO2) falls to the level of cerebral oxygen delivery. Over time, CMRO2 falls further despite no further decrease in CBF, resulting in a decrease in OEF. Restoring CBF with reperfusion therapy or recruitment of collateral pathways will increase CBF (i.e., ‘luxury perfusion’) and concurrent decrease in OEF below baseline while CMRO2 remain unchanged. Reprinted with permission. 100
Oxygen reactivity
Under normal physiological conditions, and a constant partial pressure of arterial carbon dioxide (PaCO2), alterations in the partial pressure of arterial O2 (PaO2) within the range of 60–150 mmHg appears to have little influence on CBF. 104 A reduction in PaO2 has been associated with an elevation in CBF, which would help maintain O2 delivery to the brain, 104 but this occurs only below ∼60 mmHg, and also depends on the prevailing PaCO2. 105 The CBF response to hypoxia is heterogeneous within the cerebral circulation, with the posterior and inferior vasculature being more sensitive to hypoxia than anterior regions;104,106 presumably this is an adaptation to preserve the vital homeostatic control centers in those brain regions. The CBF response to pronounced hypoxia could potentially play an important role in helping rescue perfusion to ischemic penumbral tissue following stroke, possibly by inducing local vasodilation. The ischemia-induced vasodilatory response of the cerebral vessels is mediated by adenosine and NO pathways.107,108 Both adenosine and NO pathways have been shown to be neuroprotective in animal models of ischemic stroke. Increasing extracellular adenosine levels with a ketogenic diet improves regional CBF and reduces infarct volume. 109 Similarly, both NO-donors and NO inhalation improved perfusion to the penumbra by selectively dilating collateral arterioles and venules, thereby reducing infarct volume and improving neurological function.50,108
Hyperoxia (i.e., inhalation of 100% O2) results in vasoconstriction of the cerebral blood vessels, which appears to be independent of hypocapnia-induced vasoconstriction. 110 This vasoconstrictive effect of hyperoxia may be related to inactivation of NO in the cerebral endothelium associated with enhanced generation of O2-free radicals. 111 Interestingly, hyperoxia elicits a small reduction in ICP (∼3–4 mmHg), presumably due to its vasoconstrictor effect on the cerebral vessels and/or the small reduction in PaCO2. 112 Recently, acute hyperoxia was shown to elicit a paradoxical increase in CBF in stroke patients, while CBF declined as expected in the healthy controls. 113 The authors attributed this increase in CBF to an impaired vascular response to acute hyperoxia in patients with stroke.
Carbon dioxide reactivity
Changes in PaCO2 and associated hydrogen ion (H+) concentration are potent stimuli for altering vessel caliber and cerebrovascular resistance. A rise in PaCO2 (and subsequent respiratory acidosis) leads to vasodilation in the cerebral vessels, elevating CBF. Conversely, a fall in PaCO2 (and subsequent respiratory alkalosis) leads to cerebral vasoconstriction, lowering CBF. In vivo and in vitro studies have identified NO, C-natriuretic peptide, prostanoids, and endothelin-1 as endogenous mediators of CO2/H+-induced changes in cerebrovascular tone. 114 The response of CBF to CO2, often referred to as ‘cerebrovascular CO2 reactivity’ or ‘cerebral vasomotor reactivity’, represents the dilatory and constrictive capacity of the cerebral vessels to CO2. While this crucial component of CBF control is believed to be mediated by the cerebrovascular endothelium, 114 there is some evidence that cerebral endothelial cells do not play a significant role in the cerebrovascular CO2 reactivity.115,116 In healthy humans, a 1–mmHg change in PaCO2 elicits a 2–8% change in global CBF,104,117 with studies reporting either no regional difference, 104 or higher cerebrovascular CO2 reactivity in the anterior cerebral circulation compared to posterior cerebral regions. 118
In patients with severe carotid artery stenosis or occlusion, the degree of cerebrovascular CO2 reactivity appears to be linked to the patency, type (i.e., ophthalmic or Willisian) and number of collateral pathways,119,120 and is a predictor for ischemic stroke and transient ischemic attack.121–123 Vernieri et al. 120 speculated that the anatomic characteristics of intracranial collateralization may be closely tied to the cerebrovascular reserve. Enhancing NO bioavailability has been shown to elevate cerebrovascular CO2 reactivity, 124 and activate collateral perfusion. 50 Therefore, endogenous NO production may be the underlying mechanism which links cerebrovascular CO2 reactivity with a collateral circulatory response. Therapeutic interventions which increase cerebrovascular CO2 reactivity may concurrently serve to enhance the functional collateral response to ischemia, but these need to be explored further.
A key goal of putative therapies to recruit and/or optimise collateral pathways to the threatened but potentially salvageable tissues of the penumbra should be to slow infarct development and protect the brain until interventional treatment can achieve reperfusion – effectively extending the therapeutic window. Supportive therapies to optimize collateral perfusion would ideally be deliverable safely and flexibly by first-responders, without the need for specialist neuroimaging (to exclude hemorrhagic stroke) prior to treatment. We speculate that one way that this could potentially be achieved is by activating the NO- and/or adenosine-mediated dilatory pathways. Enhancing these dilatory pathways to reduce infarct growth and improve patient outcome following ischemic stroke could be a promising avenue for future studies.
Neurogenic control
Neurogenic control in the context of this review refers to the direct innervation and regulation of the cerebral vasculature, and the subsequent control of vessel caliber and CBF. Studies as early as the 1930s demonstrated that the cerebral vasculature is richly innervated by sympathetic, parasympathetic and sensory nerve fibers, with as much as 90% of arterioles receiving adrenergic innervation.125,126 The pial arteries are innervated by the peripheral nervous system, with fibers originating from the superior cervical ganglion and trigeminal ganglion (extrinsic innervation), while the parenchymal arteries are innervated by nerve afferents from subcortical neurons (intrinsic innervation).127–129 Sympathetic innervation of the cerebral venous system appears to be dependent on vessel caliber, with the large pial veins encased with rich plexuses of adrenergic nerve terminals. 130 In a state of sympathetic activation, as seen in stroke, the heterogeneity in adrenoceptor expression favors dilation in the parenchymal arteries. 131
The neural regulation of CBF in humans has been the focus of debate 132 and numerous reviews.133–135 Much of the disagreement surrounding neural control of the cerebral circulation in humans is associated with the variability in methodological approach, including: 1) the experimental challenge (e.g., exercise, lower body negative pressure, Valsalva maneuver, head-up tilt, cold-pressor test, hypo- and hyper-ventilation); 2) the pharmacological intervention (α- and β-antagonists and agonists); 3) lack of control of confounding factors, such as PaCO2 and arterial pressure; and, 4) difficulty in isolating neural control from other control mechanisms including PaCO2, pH, and vasoactive circulating mediators and neurotransmitters. 134 Furthermore, limitations in the invasive nature of experimental interventions, and the difficulty in making direct measurements of cerebral neural activity, cerebral vessel caliber, CBF, and ICP in conscious humans further contribute to the variation in experimental findings, and subsequent interpretation.
Despite these limitations, accumulating literature from studies in human subjects supports the role of neural control in the regulation of the cerebral vasculature and CBF. In humans, disruption of sympathetic input from the cervical ganglia to the cerebral circulation using either surgical or pharmacological means, has been reported to increase CBF (see ter Laan et al. 134 for review). Cerebral norepinephrine spill-over has only been reported in one human study, due to the invasive and challenging nature of these measures. The authors found that ganglion blockade and central sympathetic inhibition reduced norepinephrine spill-over from the internal jugular vein, suggesting that there is tonic sympathetic activity to the cerebral circulation. 136 In a series of studies assessing the role of sympathetic and cholinergic control of the cerebral vasculature using α-adrenergic and cholinergic blockades, Hamner and colleagues reported that the regulation of CBF in the face of rapid changes in arterial pressure may be dependent on the balance between adrenergic vasoconstriction and cholinergic vasodilation.137,138 Others have found α-adrenergic and ganglionic blockades attenuated the cerebrovascular constrictor response to transient increases in BP, thereby impairing CA.139,140 These findings are consistent with data from animal studies which indicate that tonic cerebral sympathetic nerve activity protects the cerebral circulation against excessive vessel distension and potentially damaging intravascular pressure changes and overperfusion during severe hypoxia, hypercapnia and abrupt increases in BP by increasing cerebrovascular tone.141,142 While known inter-species differences in the neuronal control of CBF mean these findings should be interpreted with caution, 143 the current prevailing hypothesis is that the cerebral sympathetic nerves act to protect the brain against hyperperfusion during transient increases in BP.
Autoregulation
Cerebral autoregulation is a term traditionally used to describe the intrinsic, myogenic cellular phenomena and neurogenic and metabolic mechanisms, responsible for adjusting cerebrovascular resistance to maintain CBF. In 1959, Lassen 144 proposed that CBF remains relatively constant across a range of mean BP (60–150 mmHg), which is often referred to as the ‘autoregulatory plateau’. According to this paradigm, a reduction in arterial pressure (i.e., decreased perfusion pressure) elicits a cerebral vasodilation and a subsequent decrease in vascular resistance in order to maintain a constant CBF. Conversely, an increase in arterial pressure (i.e., increased perfusion pressure) elicits a cerebral vasoconstriction.
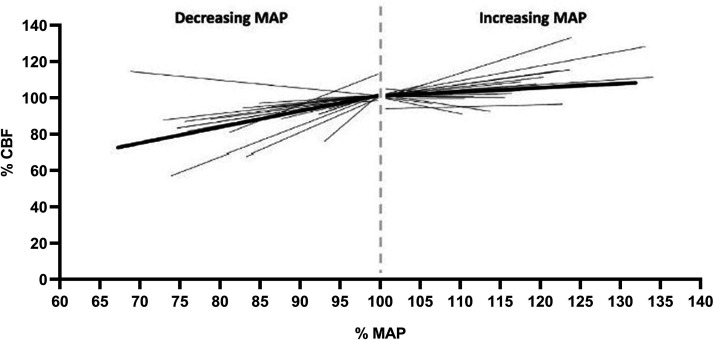
In recent times, this dogma has been challenged for a number of key reasons: 1) the original curve represented a meta-analysis of data from multiple, independent studies, rather than assessing within-subject CBF responses to variations in arterial pressure; 2) hypotensive drugs that may have a direct vasorelaxant effect on the cerebral vessels were used in some of these studies; 3) some of the CBF data was interpreted incorrectly, and the description of some of the subject groups and experimental approaches were not accurate; and, 4) the plateau region of the cerebral autoregulatory curve would require an unusually high feedback gain that is generally not present in a biological system. More recent evidence also clearly indicates that the relationship between CBF and BP is more pressure passive. 145 Moreover, the cerebral vasculature appears to be better adapted to respond to transient increases in BP compared to transient decreases in BP (Figure 3), although this is not a universal finding. 146 Our interpretation is that the brain does have some degree of autoregulatory capacity, but that this is imperfect and likely depends on the magnitude and direction of BP change
Figure 3.
Relationship between mean arterial pressure and cerebral blood flow. All individual lines represent individual studies; the length of each line indicates the range of mean arterial pressure (MAP) change of the study. Average slope (thicker line) was 0.83% MAP/% CBF for decreasing MAP (n = 23), and 0.21% MAP/% CBF for increasing MAP (n = 26). Modified from Numan et al. 166
CA is frequently described as having two representations; static/steady-state CA (i.e., >5 mins to hours; response of CBF to steady-state changes in arterial pressure) and dynamic CA (dCA) (i.e., within seconds; response of CBF to beat-to-beat variations in arterial pressure). The effect of acute stroke on dCA has been extensively reviewed elsewhere,28,147 thus will be only briefly summarized herein. Impairment of dCA has been observed 24–72 h following stroke, which persists for up to two weeks. 147 The degree of initial dCA impairment is predictive of patient outcome, with greater dCA impairment associated with higher risk of hemorrhagic transformation, cerebral oedema, more severe stroke and poor three-month function outcome.148,149 The magnitude of dCA impairment appears to depend on both location and severity of the affected region. 150 Ipsilateral CA impairments have been observed in large artery atherosclerosis strokes, while lacunar strokes and other small artery occlusions results in bilateral CA impairments.151,152 Although the sample sizes are generally small, these studies showcase the diverse nature of CA dysregulation following stroke. Physiological parameters such as BPV, dCA and OEF are potential novel prognostic markers for infarct growth, secondary complications after an initial stroke and long-term patient outcome. However, experimental, preclinical and clinical trials are needed to determine how these parameters might be used to inform stroke management. Establishing normative and pathological thresholds, and identifying therapeutic strategies able to modulate these parameters has the potential to enhance stroke care. But their capacity to improve patient outcome is currently unknown as these parameters are not routinely assessed.
Baroreflex-cerebrovascular crosstalk
It has been hypothesized that BRS and CA share common underlying regulatory mechanisms, and are part of the same continuum for maintaining cerebral perfusion and O2 delivery.153,154 In the presence of unstable perfusion pressure, CA adjusts arteriolar caliber to match CBF to local metabolic demand. Tzeng et al. 153 postulated that depending on the prevailing BRS or BPV, dCA may adjust over time via autonomic modulation, in order to optimize CBF control. Whilst not universal, 155 indices of CA have been shown to inversely relate to BRS and positively relate to BPV in young healthy cohorts.153,156 Similarly, higher or relatively ‘improved’ CA has been observed in patients with spontaneously hypertension. 157 As such, individuals with seemingly poor baroreflex and elevated BPV display relatively efficient CA and vice versa. However, recent studies found no inverse relationship between BRS and dCA in both healthy elderly individuals and patients with Alzheimer’s disease.158–160 While these discrepant findings are difficult to reconcile, they allude to the possibility of disrupted BRS–CA crosstalk with ageing and neurodegeneration. Since both BRS and dCA appear to be impaired following acute stroke, we speculate that BRS–CA crosstalk is likely to be absent in stroke patients. In the face of disrupted BRS following stroke, an impaired BRS–CA crosstalk may partly account for the association between baroreflex dysfunction and stroke-related mortality. 154
The selfish brain hypothesis – cushing’s mechanism
The dichotomic views of post-stroke hypertension as either friend or foe reflects our current knowledge gap surrounding the role of BP in the ischemic brain. One possible explanation can be found in the ‘selfish brain hypothesis’, whereby the energetically expensive brain has evolved ‘selfishly’ to place the utmost priority on maintaining its own blood supply at the expense of systemic hypertension (Figure 4). 161 In a seminal study at the turn of the 20th Century, Cushing 82 first demonstrated that substantial increases in ICP with subdural saline infusion were countered by matching increases in arterial pressure. He proposed that the trigger for the hypertensive response was brainstem ischemia due to ICP induced compression of the cerebral vasculature. If cerebral hypoperfusion and intracranial hypertension cause sympathoexcitation and systemic hypertension, then the brain may directly sense tissue hypoxemia and ICP changes. There are two lines of evidence to support the ‘selfish brain’ hypothesis: 1) experimental data indicate that the increases in cerebrovascular resistance precede the onset of chronic sympathetic hyperactivity and hypertension in humans; 162 and 2) cerebral hypoperfusion associated with either cerebral vasoconstriction or intracranial hypertension elicits sympathetically-medicated compensatory increases HR and BP to restore CBF.8,163,164 In keeping with the selfish brain hypothesis, decreases in BP during acute ischemic stroke are associated with larger infarct volume and worse patient outcome.11,165 Systemic hypertension therefore may play a neuroprotective role to restore perfusion to the penumbra, potentially at the expense of over-perfusing other parts of the brain. If proven, the selfish brain hypothesis would have significant implications on how we manage BP in ischemic stroke patients.
Figure 4.
Schematic representation of the inter-relationship between raised intracranial pressure and decreased cerebral perfusion to sympathetic outflow and arterial pressure, via known signaling pathways in the rostral ventrolateral medulla (RVLM) and nucleus tractus solitarii (NTS). Modified from McBryde et al. 10
Summary
Despite recent advances in treatment therapies, the global burden of stroke is still growing. The pathophysiology of the cerebral circulation following ischemic stroke is complex and in order to ensure optimal O2 and nutrient delivery to the brain, a wide spectrum of overlapping mechanisms are involved in the control of CBF (Figure 5). While our understanding of CBF control in stroke have improved in recent years, several fundamental questions remain unanswered. Does increasing global CBF always translate into better penumbral blood flow, or is a more directed approach necessary? How do we enhance ischemia-mediated cerebral vasodilation and recruit collateral perfusion? Could enhancing myogenic function (i.e., with Ca2+ channel blockers) improve CA in acute stroke? Does elevated cerebral sympathetic nerve activity facilitate or hinder perfusion of ischemic brain tissues? Could collateral perfusion, BPV, OEF and dCA serve as prognostic markers to guide stroke treatment? Should supportive therapies be continued following successful recanalization? In our ongoing endeavour to understand the multifaceted sequelae of ischemic stroke on CBF control, we hope to address these questions. Instead of looking at each physiological system individually, an integrative approach is clearly needed to account for the intricate nature of CBF dysregulation following stroke. A better understanding of CBF control in acute stroke is the first step towards identifying key therapeutic targets for restoring perfusion to ischemic tissues.
Figure 5.
Schematic representation of the effect of ischemic stroke on integrative cerebrovascular physiology. Cerebral perfusion pressure is the net balance of post-stroke hypertension, mediated by increased sympathetic activity, and changes in intracranial and cerebral venous pressure. Increases in intracranial pressure acts to decrease cerebral perfusion pressure and increase cerebral venous pressure. The latter serves to impede outflow of blood from the brain, thereby limit cerebral perfusion. Both baroreflex sensitivity and cerebral autoregulation appears to be impaired following stroke. This increases blood pressure variability and accentuates hyper- and hypoperfusion insults to the brain. Decreases in both cerebral perfusion and metabolic rate for oxygen following stroke are partially compensated by increased oxygen extraction fraction in the brain.
Footnotes
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
Declaration of conflicting interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
ORCID iD: Ricardo C Nogueira https://orcid.org/0000-0003-3309-3760
References
- 1.Wallace JD, Levy LL. Blood pressure after stroke. J Am Med Assoc 1981; 246: 2177–2180. [PubMed] [Google Scholar]
- 2.Britton M, Carlsson A, de Faire U. Blood pressure course in patients with acute stroke and matched controls. Stroke 1986; 17: 861–864. [DOI] [PubMed] [Google Scholar]
- 3.Myers MG, Norris JW, Hachniski VC, et al. Plasma norepinephrine in stroke. Stroke 1981; 12: 200–204. [DOI] [PubMed] [Google Scholar]
- 4.Sander D, Winbeck K, Klingelhofer J, et al. Prognostic relevance of pathological sympathetic activation after acute thromboembolic stroke. Neurology 2001; 57: 833–838. [DOI] [PubMed] [Google Scholar]
- 5.Naredi S, Lambert G, Edén E, et al. Increased sympathetic nervous activity in patients with nontraumatic subarachnoid hemorrhage. Stroke 2000; 31: 901–906. [DOI] [PubMed] [Google Scholar]
- 6.Willmot M, Leonardi-Bee J, Bath PM. High blood pressure in acute stroke and subsequent outcome: a systematic review. Hypertension 2004; 43: 18–24. [DOI] [PubMed] [Google Scholar]
- 7.Hacke W, Schwab S, Horn M, et al. Malignant’ middle cerebral artery territory infarction: clinical course and prognostic signs. Arch Neurol 1996; 53: 309–315. [DOI] [PubMed] [Google Scholar]
- 8.Johnston IH, Rowan JO, Harper AM, et al. Raised intracranial pressure and cerebral blood flow. I. Cisterna magna infusion in primates. J Neurol Neurosurg Psychiatry 1972; 35: 285–296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Appleton JP, Sprigg N, Bath PM. Blood pressure management in acute stroke. Stroke Vasc Neurol 2016; 1: 72–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.McBryde FD, Malpas SC, Paton JF. Intracranial mechanisms for preserving brain blood flow in health and disease. Acta Physiol (Oxf) 2017; 219: 274–287. [DOI] [PubMed] [Google Scholar]
- 11.Castillo J, Leira R, García MM, et al. Blood pressure decrease during the acute phase of ischemic stroke is associated with brain injury and poor stroke outcome. Stroke 2004; 35: 520–526. [DOI] [PubMed] [Google Scholar]
- 12.Leonardi-Bee J, Bath PM, Phillips SJ, et al. Blood pressure and clinical outcomes in the international stroke trial. Stroke 2002; 33: 1315–1320. [DOI] [PubMed] [Google Scholar]
- 13.Yong M, Diener HC, Kaste M, et al. Characteristics of blood pressure profiles as predictors of long-term outcome after acute ischemic stroke. Stroke 2005; 36: 2619–2625. [DOI] [PubMed] [Google Scholar]
- 14.Jensen MB, Yoo B, Clarke WR, et al. Blood pressure as an independent prognostic factor in acute ischemic stroke. Can J Neurol Sci 2006; 33: 34–38. [DOI] [PubMed] [Google Scholar]
- 15.Vemmos KN, Tsivgoulis G, Spengos K, et al. U-shaped relationship between mortality and admission blood pressure in patients with acute stroke. J Intern Med 2004; 255: 257–265. [DOI] [PubMed] [Google Scholar]
- 16.Sandset EC, Bath PM, Boysen G, et al. The angiotensin-receptor blocker candesartan for treatment of acute stroke (SCAST): a randomised, placebo-controlled, double-blind trial. Lancet 2011; 377: 741–750. [DOI] [PubMed] [Google Scholar]
- 17.Robinson TG, Potter JF, Ford GA, et al. Effects of antihypertensive treatment after acute stroke in the continue or stop post-stroke antihypertensives collaborative study (COSSACS): a prospective, randomised, open, blinded-endpoint trial. Lancet Neurol 2010; 9: 767–775. [DOI] [PubMed] [Google Scholar]
- 18.Qureshi AI, Palesch YY, Barsan WG, et al. Intensive blood-pressure lowering in patients with acute cerebral hemorrhage. N Engl J Med 2016; 375: 1033–1043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Investigators ET, Bath PM, Woodhouse L, et al. Efficacy of nitric oxide, with or without continuing antihypertensive treatment, for management of high blood pressure in acute stroke (ENOS): a partial-factorial randomised controlled trial. Lancet 2015; 385: 617–628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Anderson CS, Huang Y, Lindley RI, et al. Intensive blood pressure reduction with intravenous thrombolysis therapy for acute ischaemic stroke (ENCHANTED): an international, randomised, open-label, blinded-endpoint, phase 3 trial. Lancet 2019; 393: 877–888. [DOI] [PubMed] [Google Scholar]
- 21.Anderson CS, Heeley E, Huang Y, et al. Rapid blood-pressure lowering in patients with acute intracerebral hemorrhage. N Engl J Med 2013; 368: 2355–2365. [DOI] [PubMed] [Google Scholar]
- 22.Powers WJ, Rabinstein AA, Ackerson T, Adeoye OM, et al. Guidelines for the early management of patients with acute ischemic stroke: 2019 update to the 2018 guidelines for the early management of acute ischemic stroke: a guideline for healthcare professionals from the American Heart Association/American Stroke Association. Stroke. 2019; 50: e344–e418. [DOI] [PubMed] [Google Scholar]
- 23.Kroeker EJ, Wood EH. Comparison of simultaneously recorded Central and peripheral arterial pressure pulses during rest, exercise and tilted position in man. Circ Res 1955; 3: 623–632. [DOI] [PubMed] [Google Scholar]
- 24.Ohte N, Saeki T, Miyabe H, et al. Relationship between blood pressure obtained from the upper arm with a cuff-type sphygmomanometer and Central blood pressure measured with a catheter-tipped micromanometer. Heart Vessels 2007; 22: 410–415. [DOI] [PubMed] [Google Scholar]
- 25.Gustafsson O, Rossitti S. Intracranial pressure is a fraction of arterial blood pressure. Eur J Neurol 1995; 2: 31–37. [DOI] [PubMed] [Google Scholar]
- 26.Aguiar de Sousa D, von Martial R, Abilleira S, et al. Access to and delivery of acute ischaemic stroke treatments: a survey of national scientific societies and stroke experts in 44 European countries. Eur Stroke J 2019; 4: 13–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Saber H, Khatibi K, Szeder V, et al. Reperfusion therapy frequency and outcomes in mild ischemic stroke in the United States. Stroke 2020; 51: 3241–3249. [DOI] [PubMed] [Google Scholar]
- 28.Beishon L, Minhas JS, Nogueira R, et al. INFOMATAS multi-center systematic review and meta-analysis individual patient data of dynamic cerebral autoregulation in ischemic stroke. Int J Stroke 2020; 15: 807–812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Heistad DD, Kontos HA. Cerebral circulation. In: Shepard JT, Abboud FM, Geiger SR. (eds) Handbook of physiology, section. Bethesda: American Physiologcical Society, 1983, pp. 137–182. [Google Scholar]
- 30.Duvernoy HM, Delon S, Vannson JL. Cortical blood vessels of the human brain. Brain Res Bull 1981; 7: 519–579. [DOI] [PubMed] [Google Scholar]
- 31.Cipolla MJ, Sweet JG, Gokina NI, et al. Mechanisms of enhanced basal tone of brain parenchymal arterioles during early postischemic reperfusion: role of ET-1-induced peroxynitrite generation. J Cereb Blood Flow Metab 2013; 33: 1486–1492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Nishimura N, Schaffer CB, Friedman B, et al. Penetrating arterioles are a bottleneck in the perfusion of neocortex. Proc Natl Acad Sci U S A 2007; 104: 365–370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Palomares SM, Cipolla MJ. Myogenic tone as a therapeutic target for ischemic stroke. Curr Vasc Pharmacol 2014; 12: 788–800. [DOI] [PubMed] [Google Scholar]
- 34.Hill RA, Tong L, Yuan P, et al. Regional blood flow in the normal and ischemic brain is controlled by arteriolar smooth muscle cell contractility and not by capillary pericytes. Neuron 2015; 87: 95–110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hall CN, Reynell C, Gesslein B, et al. Capillary pericytes regulate cerebral blood flow in health and disease. Nature 2014; 508: 55–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Yemisci M, Gursoy-Ozdemir Y, Vural A, et al. Pericyte contraction induced by oxidative-nitrative stress impairs capillary reflow despite successful opening of an occluded cerebral artery. Nat Med 2009; 15: 1031–1037. [DOI] [PubMed] [Google Scholar]
- 37.Fernandez-Klett F, Offenhauser N, Dirnagl U, et al. Pericytes in capillaries are contractile in vivo, but arterioles mediate functional hyperemia in the mouse brain. Proc Natl Acad Sci U S A 2010; 107: 22290–22295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Schaller B. Physiology of cerebral venous blood flow: from experimental data in animals to normal function in humans. Brain Res Brain Res Rev 2004; 46: 243–260. [DOI] [PubMed] [Google Scholar]
- 39.Tong LS, Guo ZN, Ou YB, et al. Cerebral venous collaterals: a new fort for fighting ischemic stroke? Prog Neurobiol 2018; 163–164: 172–193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Zamboni P, Menegatti E, Weinstock-Guttman B, et al. Hypoperfusion of brain parenchyma is associated with the severity of chronic cerebrospinal venous insufficiency in patients with multiple sclerosis: a cross-sectional preliminary report. BMC Med 2011; 9: 22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Zamboni P, Menegatti E, Cittanti C, et al. Fixing the jugular flow reduces ventricle volume and improves brain perfusion. J Vasc Surg Venous Lymphat Disord 2016; 4: 434–445. [DOI] [PubMed] [Google Scholar]
- 42.Nguyen J, Nishimura N, Fetcho RN, et al. Occlusion of cortical ascending venules causes blood flow decreases, reversals in flow direction, and vessel dilation in upstream capillaries. J Cereb Blood Flow Metab 2011; 31: 2243–2254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Pranevicius M, Pranevicius O. Cerebral venous steal: blood flow diversion with increased tissue pressure. Neurosurgery 2002; 51: 1267–1273; discussion 1273-4. [DOI] [PubMed] [Google Scholar]
- 44.Pranevicius O, Pranevicius M, Liebeskind DS. Partial aortic occlusion and cerebral venous steal: venous effects of arterial manipulation in acute stroke. Stroke 2011; 42: 1478–1481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Yu X, Yuan L, Jackson A, et al. Prominence of medullary veins on susceptibility-weighted images provides prognostic information in patients with subacute stroke. AJNR Am J Neuroradiol 2016; 37: 423–429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Payabvash S, Benson JC, Taleb S, et al. Prominent cortical and medullary veins on susceptibility-weighted images of acute ischaemic stroke. Br J Radiol 2016; 89: 20160714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Munuera J, Blasco G, Hernandez-Perez M, et al. Venous imaging-based biomarkers in acute ischaemic stroke. J Neurol Neurosurg Psychiatry 2017; 88: 62–69. [DOI] [PubMed] [Google Scholar]
- 48.Shuaib A, Butcher K, Mohammad AA, et al. Collateral blood vessels in acute ischaemic stroke: a potential therapeutic target. Lancet Neurol 2011; 10: 909–921. [DOI] [PubMed] [Google Scholar]
- 49.Gomez CR. Time is brain: the stroke theory of relativity. J Stroke Cerebrovasc Dis 2018; 27: 2214–2227. [DOI] [PubMed] [Google Scholar]
- 50.Terpolilli NA, Kim SW, Thal SC, et al. Inhalation of nitric oxide prevents ischemic brain damage in experimental stroke by selective dilatation of collateral arterioles. Circ Res 2012; 110: 727–738. [DOI] [PubMed] [Google Scholar]
- 51.Clendenin MA, Conrad MC. Collateral vessel development, following unilateral chronic carotid occlusion in the dog. Am J Vet Res 1979; 40: 84–88. [PubMed] [Google Scholar]
- 52.Clendenin MA, Conrad MC. Collateral vessel development after chronic bilateral common carotid artery occlusion in the dog. Am J Vet Res 1979; 40: 1244–1248. [PubMed] [Google Scholar]
- 53.Krupinski J, Kaluza J, Kumar P, et al. Role of angiogenesis in patients with cerebral ischemic stroke. Stroke 1994; 25: 1794–1798. [DOI] [PubMed] [Google Scholar]
- 54.Kao YJ, Oyarzabal EA, Zhang H, et al. Role of genetic variation in collateral circulation in the evolution of acute stroke: a multimodal magnetic resonance imaging study. Stroke 2017; 48: 754–761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Menon BK, Smith EE, Coutts SB, et al. Leptomeningeal collaterals are associated with modifiable metabolic risk factors. Ann Neurol 2013; 74: 241–248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Badacz R, Przewłocki T, Karch I, et al. Low prevalence of collateral cerebral circulation in the circle of willis in patients with severe carotid artery stenosis and recent ischemic stroke. Postep Kardiol Inter 2015; 11: 312–317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Chang SW, Huang YC, Lin LC, et al. Effect of dehydration on the development of collaterals in acute Middle cerebral artery occlusion. Eur J Neurol 2016; 23: 494–500. [DOI] [PubMed] [Google Scholar]
- 58.Raychev R, Liebeskind DS, Yoo AJ, et al. Physiologic predictors of collateral circulation and infarct growth during anesthesia – detailed analyses of the GOLIATH trial. J Cereb Blood Flow Metab 2020; 40: 1203–1212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Anan M, Abe T, Shimotaka K, et al. Induction of collateral circulation by hypoxia-inducible factor 1alpha decreased cerebral infarction in the rat. Neurol Res 2009; 31: 917–922. [DOI] [PubMed] [Google Scholar]
- 60.Rzechorzek W, Zhang H, Buckley BK, et al. Aerobic exercise prevents rarefaction of pial collaterals and increased stroke severity that occur with aging. J Cereb Blood Flow Metab 2017; 37: 3544–3555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Liu L, Ding J, Leng X, et al. Guidelines for evaluation and management of cerebral collateral circulation in ischaemic stroke 2017. Stroke Vasc Neurol 2018; 3: 117–130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Rebello LC, Bouslama M, Haussen DC, et al. Stroke etiology and collaterals: atheroembolic strokes have greater collateral recruitment than cardioembolic strokes. Eur J Neurol 2017; 24: 762–767. [DOI] [PubMed] [Google Scholar]
- 63.Timsit SG, Sacco RL, Mohr JP, et al. Brain infarction severity differs according to cardiac or arterial embolic source. Neurology 1993; 43: 728–733. [DOI] [PubMed] [Google Scholar]
- 64.Arboix A, Oliveres M, Massons J, et al. Early differentiation of cardioembolic from atherothrombotic cerebral infarction: a multivariate analysis. Eur J Neurol 1999; 6: 677–683. [DOI] [PubMed] [Google Scholar]
- 65.Bang OY, Saver JL, Alger JR, et al. Determinants of the distribution and severity of hypoperfusion in patients with ischemic stroke. Neurology 2008; 71: 1804–1811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Hayden DT, Hannon N, Callaly E, et al. Rebe. Stroke 2015; 46: 3488–3493. [DOI] [PubMed] [Google Scholar]
- 67.Lin HJ, Wolf PA, Kelly-Hayes M, et al. Stroke severity in atrial fibrillation. The Framingham study. Stroke 1996; 27: 1760–1764. [DOI] [PubMed] [Google Scholar]
- 68.Yperzeele L, van Hooff RJ, Nagels G, et al. Heart rate variability and baroreceptor sensitivity in acute stroke: a systematic review. Int J Stroke 2015; 10: 796–800. [DOI] [PubMed] [Google Scholar]
- 69.Sykora M, Diedler J, Rupp A, et al. Impaired baroreceptor reflex sensitivity in acute stroke is associated with insular involvement, but not with carotid atherosclerosis. Stroke 2009; 40: 737–742. [DOI] [PubMed] [Google Scholar]
- 70.Robinson TG, Dawson SL, Eames PJ, et al. Cardiac baroreceptor sensitivity predicts long-term outcome after acute ischemic stroke. Stroke 2003; 34: 705–712. [DOI] [PubMed] [Google Scholar]
- 71.Colivicchi F, Bassi A, Santini M, et al. Prognostic implications of right-sided insular damage, cardiac autonomic derangement, and arrhythmias after acute ischemic stroke. Stroke 2005; 36: 1710–1715. [DOI] [PubMed] [Google Scholar]
- 72.Sykora M, Diedler J, Poli S, et al. Blood pressure course in acute stroke relates to baroreflex dysfunction. Cerebrovasc Dis 2010; 30: 172–179. [DOI] [PubMed] [Google Scholar]
- 73.Chen X, Hassan MO, Jones JV, et al. Baroreflex sensitivity and the blood pressure response to beta-blockade. J Hum Hypertens 1999; 13: 185–190. [DOI] [PubMed] [Google Scholar]
- 74.Munakata M, Aihara A, Nunokawa T, et al. The influence of one-year treatment by angiotensin converting enzyme inhibitor on baroreflex sensitivity and flow-mediated vasodilation of the brachial artery in essential hypertension–comparison with calcium channel blockers. Clin Exp Hypertens 2003; 25: 169–181. [DOI] [PubMed] [Google Scholar]
- 75.Gonsorcik J, Farkaš A, Rajnič A. Candesartan improves baroreflex sensitivity in hypertensive patients with mild chronic renal failure. In: Timio M, Wizerman S, Venanzi S. (eds) Cardionephrology 7. Assisi: Editoriale Bios, 2002, pp. 247–248. [Google Scholar]
- 76.Stauss HM. Identification of blood pressure control mechanisms by power spectral analysis. Clin Exp Pharmacol Physiol 2007; 34: 362–368. [DOI] [PubMed] [Google Scholar]
- 77.Fan J-L, O’Donnell T, Lanford J, et al. Dietary nitrate reduces blood pressure and cerebral artery velocity fluctuations and improves cerebral autoregulation in transient ischemic attack patients. J Appl Physiol (1985) 2020; 129: 547–557. [DOI] [PubMed] [Google Scholar]
- 78.Iadecola C, Davisson RL. Hypertension and cerebrovascular dysfunction. Cell Metab 2008; 7: 476–484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Delgado-Mederos R, Ribo M, Rovira A, et al. Prognostic significance of blood pressure variability after thrombolysis in acute stroke. Neurology 2008; 71: 552–558. [DOI] [PubMed] [Google Scholar]
- 80.Rothwell PM, Howard SC, Dolan E, et al. Prognostic significance of visit-to-visit variability, maximum systolic blood pressure, and episodic hypertension. Lancet 2010; 375: 895–905. [DOI] [PubMed] [Google Scholar]
- 81.Fukuda K, Kai H, Kamouchi M, et al. Day-by-Day blood pressure variability and functional outcome after acute ischemic stroke: Fukuoka stroke registry. Stroke 2015; 46: 1832–1839. [DOI] [PubMed] [Google Scholar]
- 82.Cushing H. Concerning a definitive regulatory mechanism of the vaso-motor centre which controls blood pressure during cerebral compression. Bull Johns Hopkins Hosp 1901; 12: 290–292. [Google Scholar]
- 83.Wolff HG, Forbes HS. The cerebral circulation. V. Observations of the pial circulation during changes in intracranial pressure. Arch Neurol Psychiatry 1928; 20: 1035–1047. [Google Scholar]
- 84.Si Z, Luan L, Kong D, et al. MRI-based investigation on outflow segment of cerebral venous system under increased ICP condition. Eur J Med Res 2008; 13: 121–126. [PubMed] [Google Scholar]
- 85.Heistad DD, Marcus ML, Abboud FM. Role of large arteries in regulation of cerebral blood flow in dogs. J Clin Invest 1978; 62: 761–768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Gebremedhin D, Gopalakrishnan S, Harder DR. Endogenous events modulating myogenic regulation of cerebrovascular function. Curr Vasc Pharmacol 2014; 12: 810–817. [DOI] [PubMed] [Google Scholar]
- 87.Li Y, Brayden JE. Rho kinase activity governs arteriolar myogenic depolarization. J Cereb Blood Flow Metab 2017; 37: 140–152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.De Silva TM, Kinzenbaw DA, Modrick ML, et al. Heterogeneous impact of ROCK2 on carotid and cerebrovascular function. Hypertension 2016; 68: 809–817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Hill MA, Davis MJ, Meininger GA, et al. Arteriolar myogenic signalling mechanisms: implications for local vascular function. Clin Hemorheol Microcirc 2006; 34: 67–79. [PubMed] [Google Scholar]
- 90.Tzeng YC, Chan GS, Willie CK, et al. Determinants of human cerebral pressure-flow velocity relationships: new insights from vascular modelling and Ca2+ channel blockade. J Physiol (Lond) 2011; 589: 3263–3274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Tzeng YC, MacRae BA. Interindividual relationships between blood pressure and cerebral blood flow variability with intact and blunted cerebrovascular control. J Appl Physiol 2013; 114: 888–895. [DOI] [PubMed] [Google Scholar]
- 92.Inzitari D, Poggesi A. Calcium channel blockers and stroke. Aging Clin Exp Res 2005; 17: 16–30. [PubMed] [Google Scholar]
- 93.Gelmers HJ, Gorter K, de Weerdt CJ, et al. A controlled trial of nimodipine in acute ischemic stroke. N Engl J Med 1988; 318: 203–207. [DOI] [PubMed] [Google Scholar]
- 94.Paci A, Ottaviano P, Trenta A, et al. Nimodipine in acute ischemic stroke: a double-blind controlled study. Acta Neurol Scand 1989; 80: 282–286. [DOI] [PubMed] [Google Scholar]
- 95.Humphrey JD. Mechanisms of arterial remodeling in hypertension: coupled roles of wall shear and intramural stress. Hypertension 2008; 52: 195–200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Davies PF. Hemodynamic shear stress and the endothelium in cardiovascular pathophysiology. Nat Clin Pract Cardiovasc Med 2009; 6: 16–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Li Q, Youn JY, Cai H. Mechanisms and consequences of endothelial nitric oxide synthase dysfunction in hypertension. J Hypertens 2015; 33: 1128–1136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Kety SS, Schmidt CF. The nitrous oxide method for the quantitative determination of cerebral blood flow in man: theory, procedure and normal values. J Clin Invest 1948; 27: 476–483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Ainslie PN, Shaw AD, Smith KJ, et al. Stability of cerebral metabolism and substrate availability in humans during hypoxia and hyperoxia. Clin Sci (Lond) 2014; 126: 661–670. [DOI] [PubMed] [Google Scholar]
- 100.Powers WJ, et al. Cerebral blood flow and metabolism: regulation and pathophysiology in cerebrovascular disease. In: Grotta JC, Albers GW, Broderick JP, et al. (eds). Stroke: pathophysiology, diagnosis and management. 6th ed. Amsterdam: Elsevier, 2016, pp. 28–46. [Google Scholar]
- 101.Lin W, Powers WJ. Oxygen metabolism in acute ischemic stroke. J Cereb Blood Flow Metab 2018; 38: 1481–1499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Yamauchi H, Higashi T, Kagawa S, et al. Is misery perfusion still a predictor of stroke in symptomatic major cerebral artery disease? Brain 2012; 135: 2515–2526. [DOI] [PubMed] [Google Scholar]
- 103.Fan AP, Khalil AA, Fiebach JB, et al. Elevated brain oxygen extraction fraction measured by MRI susceptibility relates to perfusion status in acute ischemic stroke. J Cereb Blood Flow Metab 2020; 40: 539–551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Willie CK, Macleod DB, Shaw AD, et al. Regional brain blood flow in man during acute changes in arterial blood gases. J Physiol 2012; 590: 3261–3275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Mardimae A, Balaban DY, Machina MA, et al. The interaction of carbon dioxide and hypoxia in the control of cerebral blood flow. Pflugers Arch 2012; 464: 345–351. [DOI] [PubMed] [Google Scholar]
- 106.Subudhi AW, Fan JL, Evero O, et al. AltitudeOmics: effect of ascent and acclimatization to 5260 m on regional cerebral oxygen delivery. Exp Physiol 2014; 99: 772–781. [DOI] [PubMed] [Google Scholar]
- 107.Meno JR, Ngai AC, Ibayashi S, et al. Adenosine release and changes in pial arteriolar diameter during transient cerebral ischemia and reperfusion. J Cereb Blood Flow Metab 1991; 11: 986–993. [DOI] [PubMed] [Google Scholar]
- 108.Zhang F, White JG, Iadecola C. Nitric oxide donors increase blood flow and reduce brain damage in focal ischemia: evidence that nitric oxide is beneficial in the early stages of cerebral ischemia. J Cereb Blood Flow Metab 1994; 14: 217–226. [DOI] [PubMed] [Google Scholar]
- 109.Yang Q, Guo M, Wang X, et al. Ischemic preconditioning with a ketogenic diet improves brain ischemic tolerance through increased extracellular adenosine levels and hypoxia-inducible factors. Brain Res 2017; 1667: 11–18. [DOI] [PubMed] [Google Scholar]
- 110.Floyd TF, Clark JM, Gelfand R, et al. Independent cerebral vasoconstrictive effects of hyperoxia and accompanying arterial hypocapnia at 1 ATA. J Appl Physiol (1985) 2003; 95: 2453–2461. [DOI] [PubMed] [Google Scholar]
- 111.Pohl U. Endothelial cells as part of a vascular oxygen-sensing system: hypoxia-induced release of autacoids. Experientia 1990; 46: 1175–1179. [DOI] [PubMed] [Google Scholar]
- 112.Hlatky R, Valadka AB, Gopinath SP, et al. Brain tissue oxygen tension response to induced hyperoxia reduced in hypoperfused brain. J Neurosurg 2008; 108: 53–58. [DOI] [PubMed] [Google Scholar]
- 113.Hegeduš I, Milić J, Ćosić A, et al. Cerebrovascular reactivity in acute hyperoxia in patients with acute ischaemic stroke. Brain Inj 2017; 31: 560–566. [DOI] [PubMed] [Google Scholar]
- 114.Edvinsson L, Krause DN. Cerebral blood flow and metabolism. 2nd ed. Philadelphia: Lippincott Williams & Wilkins, 2002. [Google Scholar]
- 115.Wang Q, Pelligrino DA, Koenig HM, et al. The role of endothelium and nitric oxide in rat pial arteriolar dilatory responses to CO2 in vivo. J Cereb Blood Flow Metab 1994; 14: 944–951. [DOI] [PubMed] [Google Scholar]
- 116.Faraci FM, Taugher RJ, Lynch C, et al. Acid-sensing ion channels: novel mediators of cerebral vascular responses. Circ Res 2019; 125: 907–920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Peebles KC, Richards AM, Celi L, et al. Human cerebral arteriovenous vasoactive exchange during alterations in arterial blood gases. J Appl Physiol 2008; 105: 1060–1068. [DOI] [PubMed] [Google Scholar]
- 118.Skow RJ, MacKay CM, Tymko MM, et al. Differential cerebrovascular CO(2) reactivity in anterior and posterior cerebral circulations. Respir Physiol Neurobiol 2013; 189: 76–86. [DOI] [PubMed] [Google Scholar]
- 119.Norrving B, Nilsson B, Risberg J. rCBF in patients with carotid occlusion. Resting and hypercapnic flow related to collateral pattern. Stroke 1982; 13: 155–162. [DOI] [PubMed] [Google Scholar]
- 120.Vernieri F, Pasqualetti P, Matteis M, et al. Effect of collateral blood flow and cerebral vasomotor reactivity on the outcome of carotid artery occlusion. Stroke 2001; 32: 1552–1558. [DOI] [PubMed] [Google Scholar]
- 121.Silvestrini M, Troisi E, Matteis M, et al. Transcranial doppler assessment of cerebrovascular reactivity in symptomatic and asymptomatic severe carotid stenosis. Stroke 1996; 27: 1970–1973. [DOI] [PubMed] [Google Scholar]
- 122.Reinhard M, Schwarzer G, Briel M, et al. Cerebrovascular reactivity predicts stroke in high-grade carotid artery disease. Neurology 2014; 83: 1424–1431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Markus H, Cullinane M. Severely impaired cerebrovascular reactivity predicts stroke and TIA risk in patients with carotid artery stenosis and occlusion. Brain 2001; 124: 457–467. [DOI] [PubMed] [Google Scholar]
- 124.Fan J-L, O’Donnell T, Gray CL, et al. Dietary nitrate supplementation enhances cerebrovascular CO2 reactivity in a sex-specific manner. J Appl Physiol (1985) 2019; 127: 760–769. [DOI] [PubMed] [Google Scholar]
- 125.Busija DW, Heistad DD. Effects of activation of sympathetic nerves on cerebral blood flow during hypercapnia in cats and rabbits. J Physiol 1984; 347: 35–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Edvinsson L, MacKenzie ET, McCulloch J. Perivascular nerve fibers in brain vessels. In: Edvinsson L, MacKenzie ET, McCulloch J. (eds). Cerebra blood flow and metabolism. New York: Raven Press, 1993, pp. 57–91. [Google Scholar]
- 127.Hamel E. Perivascular nerves and the regulation of cerebrovascular tone. J Appl Physiol (1985) 2006; 100: 1059–1064. [DOI] [PubMed] [Google Scholar]
- 128.Iadecola C. Neurovascular regulation in the normal brain and in Alzheimer’s disease. Nat Rev Neurosci 2004; 5: 347–360. [DOI] [PubMed] [Google Scholar]
- 129.Moskowitz MA. The neurobiology of vascular head pain. Ann Neurol 1984; 16: 157–168. [DOI] [PubMed] [Google Scholar]
- 130.Edvinsson L, Hogestatt ED, Uddman R, et al. Cerebral veins: fluorescence histochemistry, electron microscopy, and in vitro reactivity. J Cereb Blood Flow Metab 1983; 3: 226–230. [DOI] [PubMed] [Google Scholar]
- 131.Lincoln J. Innervation of cerebral arteries by nerves containing 5-hydroxytryptamine and noradrenaline. Pharmacol Ther 1995; 68: 473–501. [DOI] [PubMed] [Google Scholar]
- 132.van Lieshout JJ, Secher NH. Point: counterpoint: sympathetic activity does/does not influence cerebral blood flow. J Appl Physiol (1985) 2008; 105: 1364–1366. [DOI] [PubMed] [Google Scholar]
- 133.Brassard P, Tymko MM, Ainslie PN. Sympathetic control of the brain circulation: appreciating the complexities to better understand the controversy. Auton Neurosci 2017; 207: 37–47. [DOI] [PubMed]
- 134.ter Laan M, van Dijk JM, Elting JW, et al. Sympathetic regulation of cerebral blood flow in humans: a review. Br J Anaesth 2013; 111: 361–367. [DOI] [PubMed] [Google Scholar]
- 135.Goadsby PJ. Autonomic nervous system control of the cerebral circulation. Handb Clin Neurol 2013; 117: 193–201. [DOI] [PubMed] [Google Scholar]
- 136.Mitchell DA, Lambert G, Secher NH, et al. Jugular venous overflow of noradrenaline from the brain: a neurochemical indicator of cerebrovascular sympathetic nerve activity in humans. J Physiol 2009; 587: 2589–2597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Hamner JW, Tan CO, Lee K, et al. Sympathetic control of the cerebral vasculature in humans. Stroke 2010; 41: 102–109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Hamner JW, Tan CO, Tzeng YC, et al. Cholinergic control of the cerebral vasculature in humans. J Physiol 2012; 590: 6343–6352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Zhang R, Zuckerman J, Iwasaki K, et al. Autonomic neural control of dynamic cerebral autoregulation in humans. Circulation 2002; 106: 1814–1820. [DOI] [PubMed] [Google Scholar]
- 140.Ogoh S, Brothers RM, Eubank WL, et al. Autonomic neural control of the cerebral vasculature: acute hypotension. Stroke 2008; 39: 1979–1987. [DOI] [PubMed] [Google Scholar]
- 141.Cassaglia PA, Griffiths RI, Walker AM. Sympathetic nerve activity in the superior cervical ganglia increases in response to imposed increases in arterial pressure. Am J Physiol Regul Integr Comp Physiol 2008; 294: R1255–R1261. [DOI] [PubMed] [Google Scholar]
- 142.Cassaglia PA, Griffiths RI, Walker AM. Cerebral sympathetic nerve activity has a major regulatory role in the cerebral circulation in REM sleep. J Appl Physiol 2009; 106: 1050–1056. [DOI] [PubMed] [Google Scholar]
- 143.Heistad DD, Marcus ML, Gross PM. Effects of sympathetic nerves on cerebral vessels in dog, cat, and monkey. Am J Physiol 1978; 235: H544–52. [DOI] [PubMed] [Google Scholar]
- 144.Lassen NA. Cerebral blood flow and oxygen consumption in man. Physiol Rev 1959; 39: 183–238. [DOI] [PubMed] [Google Scholar]
- 145.Tzeng YC, Willie CK, Atkinson G, et al. Cerebrovascular regulation during transient hypotension and hypertension in humans. Hypertension 2010; 56: 268–273. [DOI] [PubMed] [Google Scholar]
- 146.Barnes SC, Ball N, Haunton VJ, et al. The cerebro-cardiovascular response to periodic squat-stand maneuvers in healthy subjects: a time-domain analysis. Am J Physiol Heart Circ Physiol 2017; 313: H1240–H1248. [DOI] [PubMed] [Google Scholar]
- 147.Intharakham K, Beishon L, Panerai RB, et al. Assessment of cerebral autoregulation in stroke: a systematic review and meta-analysis of studies at rest. J Cereb Blood Flow Metab 2019; 39: 2105–2116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Salinet AS, Silva NC, Caldas J, et al. Impaired cerebral autoregulation and neurovascular coupling in middle cerebral artery stroke: influence of severity? J Cereb Blood Flow Metab 2019; 39: 2277–2285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Castro P, Azevedo E, Serrador J, et al. Hemorrhagic transformation and cerebral edema in acute ischemic stroke: link to cerebral autoregulation. J Neurol Sci 2017; 372: 256–261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Reinhard M, Roth M, Guschlbauer B, et al. Dynamic cerebral autoregulation in acute ischemic stroke assessed from spontaneous blood pressure fluctuations. Stroke 2005; 36: 1684–1689. [DOI] [PubMed] [Google Scholar]
- 151.Immink RV, van Montfrans GA, Stam J, et al. Dynamic cerebral autoregulation in acute lacunar and middle cerebral artery territory ischemic stroke. Stroke 2005; 36: 2595–2600. [DOI] [PubMed] [Google Scholar]
- 152.Guo ZN, Liu J, Xing Y, et al. Dynamic cerebral autoregulation is heterogeneous in different subtypes of acute ischemic stroke. PLoS One 2014; 9: e93213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Tzeng YC, Lucas SJ, Atkinson G, et al. Fundamental relationships between arterial baroreflex sensitivity and dynamic cerebral autoregulation in humans. J Appl Physiol (1985) 2010; 108: 1162–1168. [DOI] [PubMed] [Google Scholar]
- 154.Sykora M, Diedler J, Turcani P, et al. Baroreflex: a new therapeutic target in human stroke? Stroke 2009; 40: e678–e682. [DOI] [PubMed] [Google Scholar]
- 155.Xing CY, Tarumi T, Meijers RL, et al. Arterial pressure, heart rate, and cerebral hemodynamics across the adult life span. Hypertension 2017; 69: 712–720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Nasr N, Czosnyka M, Pavy-Le Traon A, et al. Baroreflex and cerebral autoregulation are inversely correlated. Circ J 2014; 78: 2460–2467. [DOI] [PubMed] [Google Scholar]
- 157.Serrador JM, Sorond FA, Vyas M, et al. Cerebral pressure-flow relations in hypertensive elderly humans: transfer gain in different frequency domains. J Appl Physiol (1985) 2005; 98: 151–159. [DOI] [PubMed] [Google Scholar]
- 158.de Heus RAA, de Jong DLK, Sanders ML, et al. Dynamic regulation of cerebral blood flow in patients with alzheimer disease. Hypertension 2018; 72: 139–150. [DOI] [PubMed] [Google Scholar]
- 159.Aengevaeren VL, Claassen JA, Levine BD, et al. Cardiac baroreflex function and dynamic cerebral autoregulation in elderly masters athletes. J Appl Physiol (1985) 2013; 114: 195–202. [DOI] [PubMed] [Google Scholar]
- 160.Maasakkers CM, Melis RJF, Kessels RPC, et al. The short-term effects of sedentary behaviour on cerebral hemodynamics and cognitive performance in older adults: a cross-over design on the potential impact of mental and/or physical activity. Alzheimers Res Ther 2020; 12: 76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Paton JF, Dickinson CJ, Mitchell G. Harvey cushing and the regulation of blood pressure in giraffe, rat and man: introducing ‘cushing’s mechanism’. Exp Physiol 2009; 94: 11–17. [DOI] [PubMed] [Google Scholar]
- 162.Warnert EA, Rodrigues JC, Burchell AE, et al. Is high blood pressure self-protection for the brain? Circ Res 2016; 119: e140–e151. [DOI] [PubMed] [Google Scholar]
- 163.Marina N, Christie IN, Korsak A, et al. Astrocytes monitor cerebral perfusion and control systemic circulation to maintain brain blood flow. Nat Commun 2020; 11: 131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Qureshi AI, Wilson DA, Traystman RJ. Treatment of transtentorial herniation unresponsive to hyperventilation using hypertonic saline in dogs: effect on cerebral blood flow and metabolism. J Neurosurg Anesthesiol 2002; 14: 22–30. [DOI] [PubMed] [Google Scholar]
- 165.Petersen NH, Ortega-Gutierrez S, Wang A, et al. Decreases in blood pressure during thrombectomy are associated with larger infarct volumes and worse functional outcome. Stroke 2019; 50: 1797–1804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Numan T, Bain AR, Hoiland RL, et al. Static autoregulation in humans: a review and reanalysis. Med Eng Phys 2014; 36: 1487–1495. [DOI] [PubMed] [Google Scholar]