Abstract
Growing evidence suggests physical activity and cardiorespiratory fitness are associated with better cognition across the lifespan. However, the neurobiological underpinnings relating fitness and cognition remain unclear, particularly in healthy younger adults. Using a well-established and popular multi-compartment diffusion modeling approach, called Neurite Orientation and Dispersion and Density Imaging (NODDI), we investigated the relationship between physical fitness (measured via a 2-minute walk test), cognition (fluid and crystallized), and gray and white matter microstructure, in a large sample (n=816) of healthy younger adults (ages 22-35 years) from the human connectome project (HCP). Concurrent with previous literature, we found that fitness was positively associated with both fluid and crystallized cognition. Furthermore, we found that physical fitness was negatively associated with white matter orientation dispersion index (ODIWM) around the cerebellar peduncle and was negatively associated with widespread cortical and subcortical gray matter neurite density index (NDIGM). Lower ODIWM of the cerebral peduncle was associated with better fluid cognitive performance, while lower NDIGM was associated with better crystallized cognition. Finally, we found that while ODIWM partially mediated the relationship between fitness and fluid cognition, NDIGM partially mediated the relationship between fitness and crystallized cognition. This study is the first to explore the relationship between physical fitness and white and gray matter microstructure measures using NODDI. Our findings suggest that in addition to improved cognitive performance, higher physical fitness may be associated with lower white matter tract dispersion and lower neurite density in the cortical and subcortical gray matter of healthy younger adults.
Keywords: Neural Efficiency, Exercise, Diffusion-Weighted Imaging, MRI, Human Connectome Project, Cognitive Performance
1. Introduction
Mounting evidence suggests a strong link between physical activity, cardiorespiratory fitness, and cognitive function across the lifespan [1]. Specifically, regular physical activity and higher aerobic fitness are associated with enhanced cognitive function across multiple domains, such as attention, memory, and executive function [1], [2]. However, evidence for associations between fitness and cognition are predominantly focused on young adolescents and older adults, leaving noticeable gaps in our understanding of the relationship between fitness and brain health in young and middle-aged adults [1]. Given that physical activity is a leading modifiable risk factor for preventing and delaying age-related cognitive decline and that early lifestyle interventions may bolster cognitive reserve and make the brain more resilient across the lifespan, a better understanding of the relationship between cardiorespiratory fitness and brain health in younger adults is needed [2]-[5].
In addition to improved cognitive function, higher cardiorespiratory fitness is also linked to structural brain health. Exercise training and improving cardiorespiratory fitness show promise as a means of increasing and preserving both gray and white matter brain tissue volume across the lifespan, particularly in adolescents and older adults [2], [6]-[8]. Volumetric analysis accounts for large macrostructural changes in cortical and subcortical gray and white matter tissue and is a well-established and commonly used measure of brain health. However, while changes in gray and white matter volume are sensitive and used extensively to track cognitive performance across the lifespan, they are highly nonspecific measure of underlying tissue health and microarchitecture [9]-[12]. Furthermore, significant changes in gray and white matter volume tend to occur during later stages of life and pathological processes. Meanwhile, small changes in underlying neurophysiology and tissue microstructure may precede larger volumetric and functional changes in brain health and may be better predictors of age, cognition, and disease pathology [13]-[19]. This highlights a critical opportunity to apply more specific measures of tissue microstructure that are more sensitive to small presymptomatic neurophysiological changes and could provide a more detailed mechanistic understanding of the benefits of physical fitness for brain health in younger adults.
Advancements in diffusion-weighted imaging now allow researchers to probe questions relating to the composition and microarchitecture of underlying brain tissue by measuring the diffusion of water molecules [11], [20]. More specifically, Diffusion Tensor Imaging (DTI) is the most widely used method in humans for quantifying the microstructural integrity of white matter, with measures such as fractional anisotropy (FA) and mean diffusivity (MD) serving as in vivo measures of white matter tract integrity that can be related to aging, behavioral performance, and lifestyle factors [9], [21]-[25]. Additionally, there has been a recent focus on diffusion within gray matter as previous DTI based measures may be more sensitive and provide more functionally relevant insight into cortical and subcortical gray matter tissue health than standard volumetric measures [14], [17], [26]-[29].
However, while DTI can be sensitive to numerous neurophysiological changes such as myelination, axon diameter, and membrane permeability, it is an inherently nonspecific signal representation method that provides summary statistics of the observed signal without incorporating assumptions based on the underlying tissue [30]-[33]. Notably, DTI is generally performed using single-shell diffusion imaging, which also often employs weaker diffusion weighting strength (b-values ~1000 s/mm2) and thus, often suffers from poor spatial resolution and the inability to resolve complex sub-voxel microstructural details [30], [33]. This limitation is particularly critical in white matter tracts with crossing or fanning fibers near the ventricles and in cortical gray matter, where tissue structure and composition is less coherent and homogeneous and where cerebral spinal fluid (CSF) and partial volume effects can greatly influence the quality of tensor measures [12], [29], [30], [34], [35].
Neurite Orientation Dispersion Density Imaging (NODDI, [30]) addresses several limitations of the single-shell DTI technique through multi-shell acquisitions and multi-compartment diffusion modeling [33]. NODDI is arguably the most popular and widely used multi-compartment modeling technique and attempts to parameterize the diffusion signal into three microstructural compartments: intracellular diffusion (restricted diffusion within axons and dendrites), extracellular diffusion (hindered diffusion outside of axons and dendrites, such as within cell bodies, glial cells, extracellular matrices, and vascular structures)), and isotropic diffusion (i.e., free water). More specifically, the NODDI model provides three primary scalar values: the neurite density index (NDI; the proportion of intraneurite diffusion relative to extraneurite diffusion), the orientation dispersion index (ODI; 0 for perfectly parallel and aligned neurites and 1 for completely isotropic neurites), and the volume fraction of isotropic diffusion (ISO; proportion of free water such as CSF). Importantly, NODDI has good test-retest reliability, unlike DTI, accounts for partial volume effects and has undergone histological validation in animals and humans [33], [36]-[39]. Furthermore, gray and white matter NODDI measures are sensitive to cognition and have recently been used to study pathological and normal brain development with great success [16], [19], [31], [33], [35], [40]-[44]. For instance, in healthy older adults and those with mild cognitive impairment or Alzheimer’s disease, lower NDI in gray matter and white matter (NDIGM and NDIWM, respectively), lower ODI in gray matter (ODIGM), and higher ODI in white matter (ODIWM) are generally all indicative of poorer cognitive performance and are thought to represent neural or axonal degeneration [31], [33], [40], [43], [44]. However, this relationship may be somewhat different in younger healthy adults with some research suggesting that lower NDIGM and ODIWM and higher ODIGM is associated with better cognitive performance and that this could be related to synaptic or dendritic pruning to enhance network efficiency [16], [19].
Yet, research focusing on the relationship between lifestyle factors like physical activity or fitness and structural brain health is limited to single-shell DTI methods [45]. Cross-sectional research indicates a positive relationship between higher cardiorespiratory fitness and physical activity levels with higher white matter FA in younger [46]-[48] and older adults [24], [49], [50]. Furthermore, Opel and colleagues [51] recently reported higher white matter FA mediated the relationship between fitness and global cognition in a similar subsample of healthy young adults from the HCP dataset. Meanwhile, previous aerobic exercise training studies focused on white matter integrity have only been conducted in older adults, and such studies have been inconsistent with respect to finding a positive relationship between exercise training and white matter FA [52].
Additionally, far less work has determined the relationship between fitness and cortical and subcortical gray matter diffusion. The few studies that have specifically focused on hippocampal diffusion in older adults have reported a negative association between hippocampal MD and fitness [53], [54] and that improvements in fitness from a 6-month exercise intervention were related to reductions in hippocampal MD [55]. In contrast, we recently reported that a 12-week exercise intervention in healthy older adults and those diagnosed with mild cognitive impairment resulted in increased cortical gray matter MD, which was associated with improvements in verbal fluency and memory recall [56]. Nevertheless, no research has employed multi-shell and multi-compartment diffusion imaging techniques such as NODDI to determine the relationship between fitness, cognition, and white or gray matter microstructure in healthy younger adults. Given the more specific nature of the NODDI modeling method and its ability to overcome some of the primary limitations of DTI, the multi-compartment model provides an opportunity to better understand the relationship between fitness and gray and white matter microstructure in healthy younger adults.
Therefore, the primary purpose of this study was to examine a large representative sample of young healthy adults from the HCP to determine the relationship between physical fitness (2-minute walk test) with whole brain gray and white matter NODDI metrics (ODI and NDI). We hypothesized that higher fitness would be: 1) positively associated with neurite density (NDIWM) and negatively associated neurite orientation dispersion of white matter tracts (ODIWM); and 2) negatively associated with neurite density and dispersion of cortical gray matter (NDIGM) and subcortical gray matter (ODIGM), based on previous work suggesting lower NDIGM and ODIGM are associated with better cognitive performance in healthy younger adults [16], [19], [31], [35]. The secondary purpose of this study was to determine whether fitness was related to two composite behavioral measures (crystallized and fluid cognition) that encompass a range of cognitive domains and whether fitness-related differences in white and gray matter NODDI metrics are associated with these cognitive measures. Finally, given previous evidence for mediating effects of microstructure on the relationship between fitness and cognition [24], [51] we aimed to determine whether the associaitons between fitness and cognition are mediated by NODDI based measures of white and gray matter microstructure.
2. Methods
2.1. Participants
We analyzed behavioral and neuroimaging data from the WU-Minn HCP Young Adults 1200 Subject Data Release [57]. Additional information about the dataset, acquisition protocols, and processing steps for the sample can be found here (https://www.humanconnectome.org/; [57], [58]). Participants were age 22-35 years old and were predominantly recruited from the surrounding Missouri area. Each participant visited Washington University twice, undergoing MRI and behavioral assessments outside the scanner. In short, exclusion criteria included documentation of a neurodevelopmental, neuropsychiatric, or neurological disorders, a diagnosis of diabetes or high blood pressure, or acute alcohol or drug intoxication [57]. All subjects provided written informed consent at the beginning of their first visit. We focused our analysis on subjects from the S1200 Subject data release who had a complete diffusion scan, demographic data, and both fitness and cognitive measures from the NIH-Toolbox, leaving us with a final sample of 818 participants. Data were analyzed at the University of Maryland and use of the HCP data in this study was approved by the institutional review board.
2.2. Fitness Measure
Physical Fitness:
Cardiovascular fitness was measured with a walking endurance test in which participants walked for 2-minutes (2MWT) as a part of the Motor Domain of the NIH-Toolbox. Subjects were asked to walk as fast as they could for 2-minutes on a 50-foot (out and back) course. Raw score was measured as the total distance traveled in 2-minutes in feet and inches. Raw scores were normalized to a scale score with a mean=100 and SD=15. The 2MWT underwent extensive reliability and validity testing before its implementation into the NIH-toolbox [59], showing good test-retest reliability (ICC>.8) and excellent external validity with the 6-minute walk test (r>.96).
Gait Speed:
Participants walked 4 meters at their usual pace and performed one practice and then two timed trials. Gait speed scores were determined as the time in seconds it took to walk 4 meters in each of the timed trials. The better trial was used for scoring and computed scores were then converted to meters per second. Gait speed was positively associated with 2MWT performance (r=.24, p<.001) and was used as a covariate in later analysis to account for variance in cardiovascular fitness that could be attributed to differences in gait speed [60].
2.3. Cognitive Performance
Participants completed extensive behavioral assessment outside of the scanner. The primary set of behavioral measures on the first day was the NIH Toolbox [61], which took approximately 2 hours to complete. The NIH Toolbox consisted of an extensive cognitive battery (Flanker, Dimensional Change Card Sort, Picture Sequence Memory, List Sorting and Pattern Comparison, Picture Vocabulary and Reading Recognitions) that is reflective of general cognitive performance. Two composite scores were then produced to capture cognitive function within broader cognitive domains. A Fluid Cognition Composite Score was composed of average performance on the Dimensional Change Card Sort Test, Flanker, Picture Sequence Memory, List Sorting, and Pattern Comparison test and a Crystallized Cognition Composite Score, consisted of performance on a Picture Vocabulary and Reading Recognition test. Fluid cognitive abilities are based on the capacity to process and integrate information, act quickly, and solve novel problems and are thought to be less dependent on learning, experience, and education [62]. Meanwhile, crystallized cognitive abilities represent the accumulation of learned procedures and knowledge and is thought to be more dependent on cultural experiences and education [63]. Importantly, crystallized and fluid composite scores from the NIH toolbox have been shown to have excellent test-retest reliability and high discriminant and convergent validity with similar gold-standard composites in healthy younger adults [63].
2.4. Diffusion Acquisition
Diffusion data for the HCP was acquired on a customized Siemens 3 T Connectome Scanner using a standard 32-channel Siemens receiver head coil and a “body” transmission coil designed by Siemens. The customized hardware includes gradient coils and gradient power amplifiers that increase the maximum gradient strength from 40 mT/m to 100 mT/m, which specifically provides benefits for the quality of the diffusion imaging [58]. The diffusion sequence consisted of a Spin-echo EPI (TR 5520 ms, TE 89,5 ms, flip angle 78 deg, refocusing flip angle 160 deg, FOV 210 × 180 (RO × PE), matrix 168 × 144 (RO × PE), slice thickness 1.255 mm, 111 slices, 1.25 mm isotropic voxels, multiband factor 3, echo spacing 0.78 ms, BW 1488 Hz/Px, phase partial fourier 6/8), with the full diffusion session including 6 runs (approximately 9 minutes and 50 seconds each) of three different gradient tables acquired in both right-left and left-right phase encoding directions. Each diffusion gradient table included 90 diffusion weighted directions with 6 interspersed b=0 acquisitions. The three diffusion gradients consisted of b=1000, 2000, and 3000s/mm2 shells with approximately equal acquisitions for each run [57], [58].
2.5. Preprocessing Pipeline:
Diffusion data was preprocessed through HCP’s diffusion preprocessing pipeline [64] and downloaded from the S1200 Young Adult Data Release. In short, the diffusion preprocessing pipeline included Intensity normalization, removal of EPI distortions with FSL’s ‘TOPUP’ algorithm, eddy-current-induced and motion correction, correction for gradient-nonlinearities, and subject motion. The tensor model was also fit to the corrected diffusion data to produce FA maps for each participant for use in later analysis steps [65]. Next, we fit the NODDI model [30] to the diffusion data using the Accelerated Microstructure Imaging via Convex Optimization toolbox [66]. This provided us with a neurite density index (NDI), orientation dispersion index (ODI), and percent isotropic cerebrospinal fluid diffusion (ISO) maps for each participant, which were then used in the following voxel-wise analyses.
2.6. Voxelwise White Matter Tract Processing:
FSL’s (Version 6.01) well established Tract-based spatial statistics (TBSS; (Smith et al., 2006)) analysis pipeline was performed to analyze the effects of fitness on white matter microstructure. First, participant FA images were normalized to the FMRIB58 FA template using an affine and non-linear transformation. Normalized images were then averaged to create a mean FA image and then an average skeleton was produced, representing major tracts common across participants, which was thresholded at an FA value of 0.2. Each normalized FA image was then projected onto the mean FA skeleton. Finally, TBSS’s tbss_non_FA algorithm was used on native space ODI and NDI images to project these values onto the mean FA skeleton using the previously established transformation.
2.7. Voxelwise Cortical and Subcortical Gray Matter Processing:
Each participants native space ODI and NDI images were transformed into standard MNI space in a two-step process using Advanced Normalization Tools (ANTS; [68]). For each registration, a linear and then diffeomorphic transformation was performed using ANTS Symmetric Normalization (SyN) algorithm. The first step consisted of registering each participant’s b0 image to its respective T1 anatomical image and then registering the T1 image to the 1x1x1 mm MNI152 standard space template. These two estimated registrations were then combined and applied to both the native space ODI, NDI, and ISO scan to align them with MNI space. All transformed NODDI images were visually inspected for proper processing and registration to standard space, leading to the removal of two subjects from further analysis due to poor registration. Transformed ODI, NDI, and ISO images were then concatenated into a single 4D image and spatial smoothing with a 8-mm FWHM Gaussian kernel was applied. To restrict the analysis to gray matter voxels and reduce likelihood of partial volume effects a global gray matter mask was created, as has been previously detailed [56]. Using the fslmath tool, we first created a brain mask including voxels in which at least 90% of the transformed concatenated images were present. Large white matter tracts were excluded from this mask by overlaying the mean FA image and removing voxels with an average FA greater or equal to 0.2. Finally, to control for CSF contamination, a free water mask was created by isolating voxels in which more than 10% of participants had valid ISO values and then including voxels in which average ISO values were at or above 50%. Voxels that overlapped with the free water mask were removed from our final cortical and subcortical gray matter mask.
3. Statistical Analysis
Of the 818 participants, two were excluded from analysis due to poor registration of the diffusion images into standard space. In the following multiple linear regression analysis, age, sex, body mass index (BMI), systolic blood pressure (SBP), education, and gait speed were included as covariates. We chose to include BMI and SBP because they were found to be related to 2MWT performance independent of age, sex, education, and gait speed (BMI, r=−.292, p<.001; SBP, r=. 107, p=.002) and because they are commonly used health and fitness measures that could be related to brain health and function [69]. First, multiple linear regression analyses were used to determine the independent effect of cardiorespiratory fitness on fluid and crystallized cognition. Of the 816 remaining subjects, only 806 subjects had a composite fluid cognition score and 809 had a composite crystallized cognition score. The number of subjects included in the analysis was further reduced to 794 for composite fluid cognition and 797 for crystallized cognition after excluding participants without the above covariate measures. Second, whole brain voxelwise multiple regression analysis were performed using FSL’s randomize tool (5000 permutations) to determine whether cardiorespiratory fitness was related to ODI and NDI across both the white matter skeleton and cortical and subcortical gray matter regions while controlling for age, sex, and gait speed [70]. Gray matter and white matter skeleton clusters were defined based on voxels in which a significant relationship between fitness and diffusivity, existed after threshold-free cluster enhancement (TFCE) and family-wise error (FWE) correction (p<.05, 5000 permutations, k>20 voxel cluster) was applied. Cluster locations were defined using fslatlasquery function with the JHU DTI-based white-matter atlases for significant white matter clusters and the Harvard-Oxford cortical and subcortical structural atlases for gray matter clusters. Then significant gray and white matter clusters of the same NODDI analysis were combined into a mask, and average NODDI metrics were extracted. We then ran an additional regression analysis further including age, sex, gait speed, education, BMI, and SBP, to determine the magnitude of the independent relationship between these averaged NODDI metrics and fitness. Third, fluid and crystallized cognition were independently regressed on these significant averaged NODDI metrics while including age, sex, gait speed, education, BMI, and SBP as covariates. All multiple linear regression models were tested for data points with abnormal leverage (hat value > 3 times average), influence (Cook’s D > 0.5), and discrepancy (studentized residuals greater > 3), and predictors. Furthermore, collinearity between covariates and predictors in all models were checked for a high variance inflation factor (VIF > 2.5). Based on an exclusionary criterion of violating more than one of these three heuristics, no additional data had to be removed from further analyses. Finally, for cases in which significant associations between fitness, NODDI measures, and cognitive performance existed (n=794 for fluid cognition; 797 for crystallized cognition) and thus, the requirements for mediation analysis were met, we tested whether ODI and NDI values mediated the relationship between fitness and cognition. In these mediation models, fitness was the independent variable (X), gray and white matter NODDI measures were mediators (M), and fluid and crystallized cognitive performance were dependent variables (Y), while age, sex, gait speed, education, BMI, and SBP were included as covariates. For the mediation analysis, a bootstrapping approach (10,000 permutations) was implemented using JASP (JASP Team (2020), Version 0.13.1, https://jasp.stats.org/). Direct and indirect effects were estimated to obtain standardized regression coefficients and significance of an indirect effect was assumed if the 95% confidence interval did not include zero. All statistical analyses were performed using JASP.
4. Results
4.1. Participants
A total of 816 (453 Females) participants were included in the analysis with a mean age of 28.8 (3.6) years, and an average of 14.9 (1.8) years of education. A complete description of participants demographic, fitness, and cognitive scores can be found in Table 1.
Table 1.
Demographic information from the Human Connectome Project (HCP) Younger Adult sample
| Total sample (n=816) |
||
|---|---|---|
| Mean (SD) | ||
| Demographics | ||
| Age (years) | 28.85 (3.57) | |
| Female (n, %) | 453 (56%) | |
| Education (years) | 14.95 (1.81) | |
| Gait Speed (m/s) | 1.33 (.20) | |
| Systolic Blood Pressure (mmHg) | 124.04 (13.84) | |
| Body Mass Index | 26.56 (5.22) | |
| Fitness | ||
| Two Minute Walk (distance) | 109.63 (12.1) | |
| Cognition | ||
| Fluid Cognitive Composite (n=806) | 115.35 (11.5) | |
| Crystallized Cognitive Composite (n=809) | 118.02 (9.93) |
Note. Two Minute Walk distance was normalized across all participants in the study. Gait Speed calculated as meters per second (m/s).
Relations Between Physical Fitness and Cognitive Composite Scores
The overall model incorporating our covariates and fitness explained a significant amount of variance in fluid (F(7,794)=16.06, R2=0.13, p<.001) and crystallized cognition (F(7,797)=49.99, R2=0.30, p<.001)], see Table 2. Specifically, there was a significant independent standardized effect (β) of fitness on crystallized (t(797) = 3.62, p <.001, β = 0.13) and fluid (t(794) = 5.37, p <.001, β = 0.22) cognition scores, with greater fitness associated with better cognitive performance.
Table 2.
Results from linear regression of fitness on fluid and crystallized cognition, controlling for age, sex, education, gait speed, body mass index, and systolic blood pressure.
| Dependent Variable |
Predictor variables |
b (SE) | β | p-value | Adj. R2 | F (df) |
|---|---|---|---|---|---|---|
| Fluid Cognition | 0.13*** | 16.06 (7, 794) | ||||
| Predictor | ||||||
| Fitness | 0.21 (.04) | .22*** | <.001 | |||
| Crystallized Cognition | 0.30*** | 49.99 (7, 797) | ||||
| Predictor | ||||||
| Fitness | 0.11 (.03) | .13*** | <.001 |
Note. Non-Standardized (b) and Standardized beta (β) coefficients, along with Standard errors (SE) are reported.
p < .05
p < .01
p < .001.
4.2. Whole Brain Voxel-wise Relations Between Fitness and White Matter Microstructure
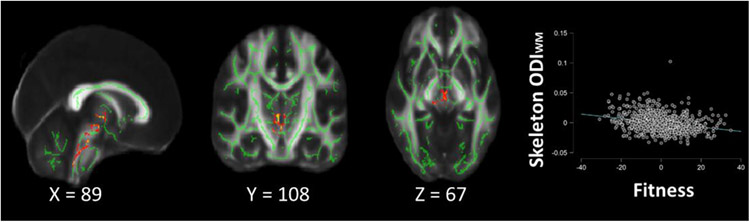
The results of the voxelwise regression analyses for fitness as a predictor of for ODIWM and NDIWM produced three and 15 statistically significant clusters, respectively (see Table 3). Regressing ODIWM, on fitness while controlling for age, sex, and gait speed, we found that higher fitness was associated with lower ODIWM in several small clusters in the cerebellar peduncle (t(811) = −8.09, p <.001, β = −.27), see Figure 1 & Table 3. Furthermore, we found that higher fitness was associated with lower NDIWM in widespread clusters including the cerebellar peduncle, corpus callosum, internal capsules, corona radiata, thalamic radiation, longitudinal fasciculus, uncinate fasciculus, fronto-occipital fasciculus, cingulum, and fornix (t(811) = −3.32, p <.001, β = −.12), see Table 3.
Table 3.
Voxelwise NODDI analysis: Major cluster locations and volumes for the relationship between fitness and white and gray matter NODDI metrics controlling for age, sex, and education, using the Harvard-Oxford atlases and JHU-DTI-81 white matter atlas.
| Analysis (effect direction) |
Primary Cluster Regions (Regions distributed across all clusters) |
Clusters | PFWE | Peak Location | Volume | ||
|---|---|---|---|---|---|---|---|
| x | y | z | |||||
| ODIWM (negative) |
JHU ICBM-DTI-81 White-Matter Labels Cerebellar peduncle, cerebral peduncle, medial lemniscus |
1 | .045 | 85 | 101 | 61 | 548 |
| 2 | .047 | 87 | 111 | 70 | 113 | ||
| 3 | .049 | 90 | 107 | 64 | 24 | ||
| NDIWM * (negative) |
JHU ICBM-DTI-81 White-Matter Labels Cerebellar peduncle, corpus callosum, internal capsules, corona radiata, thalamic radiation, longitudinal fasciculus, uncinate fasciculus, fronto-occipital fasciculus, cingulum, and fornix |
1 | .011 | 64 | 77 | 34 | 28486 |
| 2 | .048 | 121 | 81 | 83 | 310 | ||
| 3 | .050 | 126 | 86 | 71 | 32 | ||
| NDIGM (negative) |
Harvard-Oxford Cortical Structural Atlas Frontal Pole, Fronto Orbital, Insular Cortex, Frontal Gyrus, Precentral Gyrus, Temporal Pole, Temporal Gyrus, Postcentral Gyrus, Partietal Lobule, Supramarginal Gyrus, Occipital Cortex, Occipital Pole, Cingulate Gyrus, Precuneous, Cuneal cortex, Parahippocampal Gyrus, Lingual Gyrus, Fusiform Gyrus |
1 | .016 | 142 | 56 | 63 | 48001 |
| 2 | .010 | 30 | 63 | 59 | 38988 | ||
| 3 | .019 | 44 | 176 | 68 | 6884 | ||
| 4 | .024 | 91 | 55 | 27 | 4546 | ||
| 5 | .037 | 41 | 50 | 38 | 1147 | ||
| 6 | .031 | 131 | 173 | 54 | 718 | ||
| 7 | .046 | 128 | 119 | 79 | 603 | ||
|
Harvard-Oxford Subcortical Structural Atlas Hippocampus, Amygdala, Caudate, Putamen, Pallidium, Accumbens,Thalamus, |
8 | .047 | 124 | 103 | 91 | 210 | |
| 9 | .049 | 67 | 42 | 51 | 132 | ||
| 10 | .049 | 129 | 44 | 112 | 96 | ||
| 11 | .048 | 114 | 146 | 47 | 62 | ||
| 12 | .050 | 83 | 53 | 63 | 45 | ||
Peak location in voxel coordinates. ODIWM; Average white matter orientation dispersion index negatively associated with fitness. NDIWM; Average white matter neurite density index negatively associated with fitness. NDIGM; Average gray matter neurite density index negatively associated with fitness. JHU ICBM-DTI-81; 48 white matter tract labels created by hand segmentation of a standard-space average of diffusion MRI tensor maps from 81 subjects.
Note that the relationship between fitness and NDIWM did not survive with the addition of Body Mass Index, education, and systolic blood pressure as covariates.
Figure 1.
Results of Voxel-wise analysis for the relationship between residualized fitness (2-minute walk time) and residualized white matter dispersion (ODWM; Red and Yellow) and neurite density (NDIWM; Blue) in healthy younger adults after controlling for age, sex, and gait speed. A) Regions in which a significant relationship between fitness and dispersion along the white matter skeleton was found overlayed on the FMRIB58_FA standard image and mean skeleton image (green). On the right are the average extracted ODIWM values from the significant voxels depicted in the voxelwise analysis plotted against residualized fitness scores, when controlling for age, sex, education, BMI, systolic blood pressure, and gait speed.
However, when BMI, education, and SBP were included as additional covariates 2MWT performance remained a significant predictor (t(804) = −5.10, p <.001, β = −.18) of ODIWM, but was no longer a significant predictor of NDIWM (t(804) = −0.82, p = .416, β = −.03).
4.3. Whole Brain Voxel-wise Relations Between Fitness and Gray Matter Microstructure
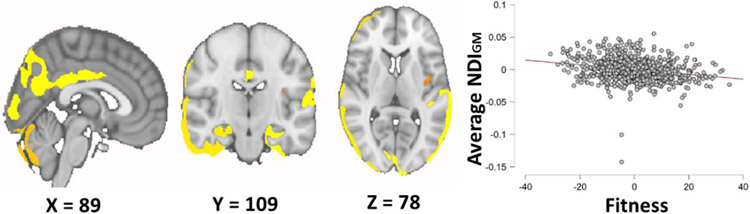
We found that higher fitness was associated with lower NDIGM in widespread clusters throughout the frontal, temporal, parietal, and occipital cortices and the bilateral hippocampus, cerebellum, amygdala, caudate, putamen, and thalamus (t(811) = −6.96, p <.001, β = −.25), see Figure 2 & Table 3. Additionally, 2MWT performance remained a significant predictor of NDIGM (t(804) = −3.10, p = .002, β = −.12) when additionally controlling for BMI, education, and SBP.
Figure 2.
Results of Voxel-wise analysis of the relationship between Fitness (2-minute walk time) and gray matter neurite density (NDIGM) in healthy younger adults. On the left is a depiction of regions in which there was a significant relationship between fitness and NDIGM in cortical and subcortical gray matter overlayed on the MNI152 standard image. On the right are the residualized average extracted NDIGM values plotted against residualized fitness scores, when controlling for age, sex, education, BMI, systolic blood pressure, and gait speed.
4.4. White and Gray Matter Microstructure and Cognition
After controlling for age, sex, education, gait speed, BMI, and SBP, ODIWM of the cerebellar peduncle was negatively associated with fluid (t(789) = −3.17, p =.002, β = −.13), but not crystallized (t(789) = −3.23, p = .175, β = −.05) cognition. Furthermore, NDIGM was negatively associated with crystallized (t(789) = −2.73, p= .006, β = −.09), but not fluid cognition (t(789) = −0.31, p = .754, β = −.01), see Table 4.
Table 4.
Results from linear regression of white matter ODI and gray matter NDI on fluid and crystallized cognition, controlling for age, sex, education, gait speed, body mass index, and systolic blood pressure.
| Dependent Variable |
Independent variables |
b (SE) | β | p-value | Adj. R2 | F (df) |
|---|---|---|---|---|---|---|
| Fluid Cognition | 0.10*** | 11.60 (8, 786) | ||||
| Predictor | ||||||
| ODIWM | −92.11 (29.08) | −.13*** | 0.002 | |||
| NDIGM | −7.97 (25.40) | −.01 | 0.754 | |||
| Crystallized Cognition | 0.31*** | 43.42 (8, 789) | ||||
| Predictor | ||||||
| ODIWM | −29.75 (21.92) | −.05 | 0.175 | |||
| NDIGM | −52.06 (19.07) | −.09** | 0.006 |
Note. Non-Standardized (b) and Standardized beta (β) coefficients, along with Standard errors (SE) are reported.
p < .05
p < .01
p < .001. ODIWM; Average white matter orientation dispersion index from voxelwise analysis. NDIGM; Average gray matter neurite density index from voxelwise analysis
4.5. NODDI as Mediators of Associations Between Fitness and Cognition
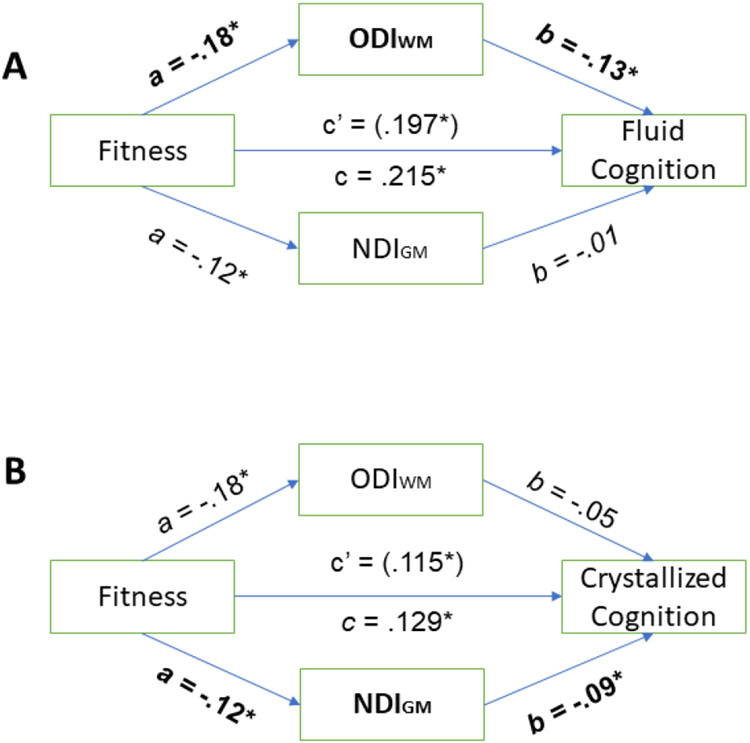
Mediation analyses conducted while controlling for sex, age, education, BMI, SBP, and gait speed suggest that cerebellar ODIWM was a significant partial mediator of the relationship between fitness and fluid cognition (Indirect effect 95% CI [.005 .037]), but not crystallized cognition (Indirect effect 95% CI [−.004 .022]). Furthermore, NDIGM was a significant partial mediator of the relationship between fitness and crystallized cognition (Indirect effect 95% CI [.001 .021])., but not fluid cognition (Indirect effect 95% CI [−.011 .008]), see Figure 3.
Figure 3.
Standardized regression coefficients for the relationship between fitness and fluid cognition (Panel A) and crystallized cognition (Panel B) were partially mediated by white matter orientation dispersion (ODIWM) and gray matter neurite density (NDIGM) while controlling for age, sex, education, gait speed, body mass index, systolic blood pressure, and the additional diffusion metric. Panel A shows the relationship between fitness, ODIWM, NDIGM, and fluid cognition, in which ODIWM partially mediates the relationship between fitness and fluid cognition. Panel B shows the relationship between fitness, ODIWM, NDIGM, and crystallized cognition, in which NDIGM partially mediates the relationship between fitness and crystallized cognition. a and b = standardized regression coefficients. c = total effect. c’ = direct effect. * p<. 05.
5. Discussion
Using a large cross-sectional sample from the Young Adult HCP, we provide evidence for a relationship between 2MWT performance, NODDI based measures of white and gray matter integrity, and fluid and crystallized cognition in healthy younger adults. In addition to a strong relationship between cardiorespiratory fitness and crystallized and fluid cognitive composite scores, we found relations between fitness and ODIWM and NDIGM in healthy younger adults. Specifically, fitness-related associations with ODIWM and NDIGM were related to better fluid and crystallized cognitive performance, respectively. Finally, we found that cerebellar ODIWM partially mediated the relationship between fitness and fluid cognitive performance and that cortical and subcortical NDIGM partially mediated the relationship between fitness and crystallized cognition. Thus, this study provides compelling initial evidence for a relationship between cardiorespiratory fitness, fluid and crystallized cognition, and NODDI based microstructural properties of both gray and white matter tissue in healthy younger adults.
5.1. Fitness and Cognition
Our finding of a significant relationship between fitness and both crystallized and fluid cognition corroborates previous research and meta-analytic findings, which indicate that higher cardiorespiratory fitness is associated with enhanced cognitive performance across the lifespan [1], [71]-[74]. While most research has been conducted in adolescents and older adults, there is still evidence to suggest a positive relationship between fitness and cognition in healthy younger adults. In particular, higher aerobic fitness and physical activity levels are associated with better memory [75] and executive function [76] in healthy younger adults. Furthermore, Opel et al. 2019 [51], recently reported a positive association between 2MWT performance and multiple cognitive domains (executive function, cognitive flexibility, picture vocabulary, processing speed, working memory, spatial orientation, and verbal episodic memory) in the HCP S1200 younger adult sample, while controlling for similar cardiometabolic factors. In the current study, we analyzed a slightly different subsample of the HCP S1200 dataset (n~800) and focused our analysis on two specific composite cognitive scores (fluid and crystallized cognition). We also chose to control for gait speed in our study to account for variance in the 2MWT due to baseline differences in stride length or gait speed to better delineate cardiorespiratory fitness from 2MWT performance [60]. Unsurprisingly, our results support the findings of Opel et al., 2019 [51] in showing widespread associations between 2MWT performance and multiple cognitive domains in healthy younger adults. Our results further suggest that the relationship between cardiorespiratory fitness and fluid and crystallized cognition may develop or be present in early adulthood even after accounting for gait speed. However, these results are cross-sectional and not prospective or longitudinal.
Few randomized controlled studies have tested the effects of exercise training on cognition in younger adults [77]. These studies primarily have focused on a small subset of cognitive domains such as pattern separation [78]. Additionally, most of these studies have employed relatively small sample sizes (n ~ 30-50), have reported smaller effects of exercise training on cognition for younger compared to older adults [77], or have failed to establish significant effects altogether [79]. Nevertheless, research conducted on healthy younger adults constitutes a substantial gap in our current understanding. Several recent reviews indicate insufficient evidence to make strong conclusions about the relationship between exercise and cognition in young adults [1], [2]. Therefore, our finding of a positive relationship between 2MWT performance and cognitive performance, even after controlling for age, sex, education, BMI, SBP, and gait speed, supports the hypothesis that maintaining higher cardiorespiratory fitness is associated with better cognition, even in younger adults considered to be both cognitively intact and cognitively stable. However, to better understand this gap, future studies will need to incorporate larger sample sizes, longitudinal approaches and randomized clinical trials, more precise measures of cardiorespiratory fitness, and explore the relationship by testing multiple cognitive domains.
5.2. Fitness and White Matter Microstructure
Our findings provide several important indicators of GM and WM network integrity that in part explain the associations between better cardiorespiratory fitness and cognition in healthy younger adults. First, we found that cardiorespiratory fitness was negatively associated with cerebellar peduncle ODIWM and NDIWM of widespread white matter tracts. A few papers have previously reported positive associations between physical activity behavior [48], [49], cardiorespiratory fitness [46], [80] and 2MWT performance [51] with DTI based white matter integrity in younger and middle-aged adults. Fitness was positively related to white matter fractional anisotropy (FA) in almost all previous studies. This finding has led to the common interpretation that greater fitness is associated with greater myelination, axon integrity, and axon density [12], [46], [48]. However, FA is a simple summary metric of the underlying direction and magnitude of water diffusion and is inherently nonspecific to underlying tissue properties [33]. Our findings provide novel insight into the relationship between fitness and white matter integrity using multi-shell and multi-compartment modeling approaches such as NODDI, which are more biophysically specific to differences in underlying tissue structure [33], [37], [39].
Our finding of lower white matter orientation dispersion (ODIWM) of the cerebellar peduncle suggests more anisotropic diffusion along the white matter tracts and is generally an indication of greater coherence and integrity of the underlying white matter. This finding is intriguing because Opel et al., 2019 found a positive relationship between FA (higher FA is also generally associated with better white matter integrity) and fitness. However, these effects were more widespread than just the cerebellar peduncle [51]. Yet, their analysis did not include gait speed as a covariate when running their voxel-wise analysis or in any follow-up analysis, which may explain differences between Opel et al. and the current study in the spatial extent of the effects. To test this hypothesis, we analyzed the relationship between fitness with FA and ODIWM without including gait speed as a covariate (Supplementary Figure 1 A&C) and found that the relationship between 2MWT and FA was very similar to that found by Opel and colleagues. Interestingly, whether we controlled for gait speed or not, the extent of the relationship between fitness and ODIWM was less widespread than for FA (Supplementary Figure 1 A-D). This post-hoc analysis suggests that controlling for gait speed does account for some of the association observed. However, it may also indicate that using a more biologically specific measure like ODIWM, may be more selective to differences in fiber dispersion because it better accounts for partial volume effects that influence FA. Furthermore, the lack of a significant effect of 2MWT on NDIWM suggests the relationship between 2MWT and white matter integrity is more likely being driven by differences in white matter tract dispersion as opposed to the density of these white matter tracts.
Fitness-related lower ODIWM of the cerebellar peduncle was found to partially mediate the relationship between fitness and fluid cognition. The cerebellar peduncle affords communication between the cerebellum and the rest of the central nervous system. In addition to being critical to motor control and performance, growing evidence suggests the cerebellum plays an essential role in motor and cognitive performance through communication with cortical and subcortical networks [81]-[84]. Exercise training-induced changes in cerebellar peduncle white matter have been linked to balance and postural control improvements following an 8-week training program in healthy individuals and those who sustained brain injuries [85]. It may therefore seem reasonable to conclude that the integrity of cerebellar white matter may be associated with more efficient movement, which may have led to better performance on the 2MWT. However, given that our analysis controlled for gait speed, a more likely alternative interpretation is that cerebellar ODIWM is independently related to higher cardiorespiratory fitness above and beyond superior motor coordination. Thus, we postulate that lower ODIWM may be indicative of better tract coherence resulting from enhanced communication between the cerebellum and cortical and subcortical structures, which may have partially facilitated our finding of better fluid cognition [81]-[84], [86]-[88].
5.3. Fitness and Gray Matter Microstructure
In gray matter, we found a significant negative relationship between fitness and NDIGM in clusters across the frontal, temporal, parietal, and occipital cortices and the bilateral hippocampus, cerebellum, amygdala, caudate, putamen, and thalamus. Furthermore, lower NDIGM partially mediated the relationship between fitness and crystallized cognition, suggesting that the relationship between fitness and crystallized cognition in healthy younger adults could in part be due to microstructural remodeling of cortical and subcortical gray matter tissue. This finding may appear counterintuitive at first glance due to a standard working hypothesis of “bigger is better” in cognitive neuroscience, as larger cortical and subcortical tissue volume is generally associated with better cognitive performance and is inversely related to age and neurodegenerative disease progression [84], [89]-[93]. The primary argument for this common assumption is that higher cortical and subcortical volume and thickness indicate more neurons and thus, greater computational power. However, volumetric measures are highly nonspecific and generally explain only a small amount of variance in the number of neurons and structure of underlying neural tissue [94], [95]. Furthermore, age-related cortical and volumetric loss in healthy older adults is not associated with a loss of neurons in underlying tissue [94]. The advantage of cortical and subcortical gray matter NODDI measures over gross volumetric indices is that they provide more distinct and independent information about the microarchitecture of underlying dendrites, axons, and glia in development, healthy aging, and neurodegenerative diseases [10], [42], [44], [96]-[98].
Within gray matter, intraneurite diffusion is generally associated with axonal and dendritic density. In contrast, extra neurite diffusion is predominantly a measure of density within neural cell bodies, glial cells, vascular structures, and the extracellular matrix [10], [30], [96]. While lower gray matter neurite density has been associated with age and poorer cognitive performance in older adults [19], [40], [44], Gene et al., 2018 [16] found that lower cortical neurite density was related to higher intelligence in healthy younger adults while accounting for age, sex, and cortical volume. Furthermore, Venkatesh et al., 2020 [19], not only showed that hippocampal gray matter NDI explained more variance in age and cognitive performance than DTI metrics, but they also found that the relationship between extracellular diffusion and pattern separation performance, a measure of episodic memory skill, was age-dependent. Specifically, they found that lower intracellular diffusion (lower NDIGM) was significantly associated with better pattern separation performance in younger adults while the opposite directional relationship (i.e., higher NDIGM was associated with better performance) existed in older adults [19]. This suggests that earlier stages of neural development in adulthood, which can extend into the second and third decade of life, may experience differential changes in tissue microstructure compared to those occurring later in life [99]. Neural development is associated with synaptic growth, followed by synaptic pruning in early adulthood [100]. In contrast, failure to perform proper pruning is associated with poor cognition and neurodevelopmental diseases like autism and Down’s syndrome [100]-[102]. An overabundance of synapse and dendritic connections can impede the differentiation of neural signals from noise leading to poorer network communication and learning[103], [104]. Thus, our finding of lower gray matter neurite density in healthy younger adults, which partially mediated the relationship between fitness and crystallized cognition, suggests that maintaining higher fitness levels may afford beneficial effects for cognition through better differentiation of neural signals, which might promote information storage, integration, and retrieval associated with the higher crystallized cognition observed.
5.4. Mechanisms for the relationship between Cognition, Fitness, and Microstructure
Higher fitness is associated with cortical thinning in healthy younger adults and some cortical regions in middle-aged adults [47], [105]. However, few studies have explored the relationship between fitness and exercise training on gray matter microstructure, and none have determined this relationship in the cortical gray matter of younger adults. Fitness has previously been shown to be positively associated with hippocampal MD in very old adults [53], [54]. Improvements in cardiorespiratory fitness have also been associated with a reduction in hippocampal MD [55], while a single session of aerobic exercise appears to increase hippocampal diffusivity in healthy older adults [106]. Finally, we recently found that a 12-week exercise training program was associated with an increase in insular and cerebellar MD, which was associated with improvements in verbal fluency and memory performance in healthy older adults and those diagnosed with mild cognitive impairment [56]. This increase in MD following the exercise training program was associated with improved cognitive performance and was hypothesized to result from reduced overactivation of neural activity, synaptic pruning, and enhanced network efficiency [56], [83], [88], [107]-[109].
Interestingly, MD is negatively associated with NDI [30] and thus, our finding of fitness being associated with lower NDIGM in widespread cortical and subcortical gray matter tissue, including the cerebellum, is similar to our previous findings of exercise training leading to increased MD [56] and improved cerebellar connectivity [83]. Yet, it can also be said that our finding of lower NDIGM in the hippocampus appears to be opposite of what we might expect based on previous studies showing that higher fitness [53] is associated with lower hippocampal MD and that increased in fitness was associated with decreased hippocampal MD in older adults [55]. However, unlike in our previous exercise training study [56], most other studies have failed to control for partial volume effects when computing gray matter MD measures. Controlling for partial volume effects and CSF contamination within relatively large voxels is critical when analyzing gray matter diffusivity because differences in MD can often be biased by the presence of partial volume effects and the intermixing of surrounding CSF and white matter [110]. This highlights the benefits of the high-resolution multi-shell and multi-compartment NODDI approach we employed here as the free water and CSF signal is modeled separately for each voxel, providing greater confidence in our other measures of intra- and extra-neurite diffusion. Our study not only provides new insight into the relationship between fitness and NODDI metrics, but also extends the literature by reporting the relationships between fitness and gray matter microstructure in healthy younger adults. As previously indicated, the relationship between cognition and gray matter microstructure appears to somewhat dependent on age, as previous studies in older adults suggest higher diffusion is negatively associated with cognition [19], [40], [44], while in younger adults lower cortical diffusion is associated with better cognition [16], [19]. Given previous studies exploring the relationship between fitness and gray matter microstructure have been conducted in older adults, this association between fitness and gray matter microstructure in healthy younger adults should be further explored and could be due to different underlying neurophysiological mechanisms.
The mechanisms by which exercise and fitness may afford benefits for both white and gray matter microstructure in healthy younger adults are likely numerous and remain to be conclusively determined. Since we found higher fitness was associated with lower ODIWM and NDIWM, higher fitness may be related to higher rates of myelination and axon coherence (contributing to lower ODIWM), but could also be coupled with higher capillary and oligodendrocyte density (contributing to lower NDIWM). In support of this view, Brocket et al., 2015 [111] found that wheel running was associated with enhanced cortical and subcortical synaptic and astrocytic markers that were in turn associated with improvements in cognitive flexibility. Similarly, Luo et al., 2019 [112] recently found that wheel running preserved cortical oligodendrocytes against chronic unpredictable stress in young rats. These findings suggest that exercise and greater cardiorespiratory fitness may provide cognitive benefits for healthy younger adults by promoting enhanced network efficiency and maintaining healthy levels of gray matter synaptic, dendritic, and glial morphology and connectivity [113].
5.5. Limitations
Our study is the first to explore the relationship between fitness, cognition, and gray and white matter microstructure using advanced high resolution multi-shell and multi-compartment diffusion imaging approaches. Furthermore, we analyzed a relatively large and representative sample of the US population. However, the study has several limitations, the greatest of which is its cross-sectional design; therefore, it is impossible to imply causation with any of our analyses. Consequently, it is impossible to determine whether differences in fitness drive the observed differences in gray and white matter microstructure or whether they might lead some individuals to perform better on a 2MWT. Furthermore, the 2MWT is not an exact measure of cardiorespiratory fitness. Although the 2MWT has excellent external validity with the 6-minute walk test (r>.96) and we controlled for numerous covariates in all of our analyses, future studies should use more objective and sensitive measures of cardiorespiratory fitness such as a VO2max test to better delineate the relationship between cardiorespiratory fitness and gray and white matter microstructure in healthy younger adults. Another consideration is that this sample does include some siblings, however, we found that accounting for family ID as a random effect in a linear mixed effects model did not change the significant effects, suggesting heritability did not influence the associations. Finally, recent work suggests that the parallel diffusivity parameter used by the NODDI algorithm to differentiate between intraneurite and extraneurite diffusion are less optimal in gray matter compared to white matter (for more discussion, see [114]); although other parameters of the NODDI model are optimized for modeling diffusion in gray matter [30], [115], [116]. Given that our entire sample was acquired using the same high-resolution acquisition protocol, processing steps, and modeling parameters, it is unlikely that this significantly impacts the relationships between fitness and gray and white matter NODDI metrics we found.
6. Conclusions
This study is the first to explore the relationship between fitness and both gray and white matter microstructure using multi-shell imaging and NODDI modeling techniques. We found that fitness was associated with lower white matter neurite dispersion and lower cortical and subcortical gray matter neurite density. Furthermore, we found that these fitness-related differences in cerebellar white matter dispersion and cortical and subcortical gray matter neurite density partially mediated the positive relationship between physical fitness and fluid and crystallized cognition, respectively. These findings indicate the presence of a strong relationship between fitness and cognition and an association with healthier and more efficient white and gray matter microarchitecture in early adulthood. Future studies should extend this work by exploring these relationships in older adults and implementing exercise intervention study designs to determine the relationship between exercise and gray and white matter microstructure across the lifespan.
Supplementary Material
Supplementary Figure 1: Voxel-wise analysis comparing the effects of fitness on Fractional Anisotropy (FA) and Neurite Orientation Dispersion Index (ODIWM) while controlling for age and sex vs age, sex, and gait speed. A) Positive relationship between FA and fitness without controlling for gait speed. B) Positive relationship between FA and fitness while controlling for gait speed. C) Negative relationship between ODIWM and fitness without controlling for gait speed. D) Negative relationship between ODIWM and fitness while controlling for gait speed. Our results are consistent with Opel and colleagues 2019, showing that fitness was associated with better white matter integrity through both higher FA and lower ODIWM. However, ODIWM and the inclusion of gait speed as a covariate led to more concentrated effects.
Highlights.
Two minute walk distance is associated with better fluid and crystallized cognition in healthy younger adults
Two minute walk distance is associated with lower white matter neurite dispersion as well as lower gray matter neurite density.
Lower white matter neurite dispersion and gray matter neurite density were associated with better fluid and crystallized cognition, respectively.
White matter neurite dispersion partially mediates the relationship between two minute walk distance and fluid cognition while gray matter neurite density partially mediates the relationship between two minute walk distance and crystallized cognition
Acknowledgments:
Data of this study were provided by the Human Connectome Project, WU-Minn Consortium (Principal Investigators: David Van Essen and Kamil Ugurbil; 1U54MH091657) and was funded by the 16 NIH Institutes and Centers that support the NIH Blueprint for Neuroscience Research; and by the McDonnell Center for Systems Neuroscience at Washington University.
Funding:
DC contributions to this research were supported in part by NSF award DGE-1632976 and NIH award F31 AG074670-01.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflicts of Interest: None of the authors have conflict of interests to report.
Code availability: The scripts used to process and analyze the data are publicly available in the following repository https://github.com/CallowBrainProject/Connectome_analysis.
Data availability:
The data used for this analysis are part of the “S1200 Subjects Data Release” provided by the Human Connectome Project and can be accessed via its ConnectomeDB platform (https://db.humanconnectome.org/). While all neuroimaging data is open access, some demographic data falls under the restricted access and data use terms put in place by the HCP and researchers must apply and agree to these terms to access the data https://www.humanconnectome.org/study/hcp-young-adult/document/quick-reference-open-access-vs-restricted-data.
References
- [1].Erickson KI et al. , “Physical Activity, Cognition, and Brain Outcomes: A Review of the 2018 Physical Activity Guidelines,” Medicine and Science in Sports and Exercise, vol. 51, no. 6. Lippincott Williams and Wilkins, pp. 1242–1251, Jun. 01, 2019, doi: 10.1249/MSS.0000000000001936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Stillman CM, Esteban-Cornejo I, Brown B, Bender CM, and Erickson KI, “Effects of Exercise on Brain and Cognition Across Age Groups and Health States Challenges of Identifying Mechanisms of Exercise,” 2020, doi: 10.1016/j.tins.2020.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Kuehn BM, “Nearly Half of Dementia Cases Could Be Prevented or Delayed,” JAMA, vol. 324, no. 11, p. 1025, Sep. 2020, doi: 10.1001/jama.2020.16210. [DOI] [PubMed] [Google Scholar]
- [4].Reuter-Lorenz PA and Park DC, “How does it STAC up? Revisiting the scaffolding theory of aging and cognition.,” Neuropsychol. Rev, vol. 24, no. 3, pp. 355–70, Sep. 2014, doi: 10.1007/s11065-014-9270-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Livingston G et al. , “Dementia prevention, intervention, and care: 2020 report of the Lancet Commission,” Lancet, vol. 396, no. 10248, pp. 413–446, Aug. 2020, doi: 10.1016/S0140-6736(20)30367-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Colcombe SJ et al. , “Aerobic Exercise Training Increases Brain Volume in Aging Humans,” Journals Gerontol. Ser. A Biol. Sci. Med. Sci, vol. 61, no. 11, pp. 1166–1170, 2006, doi: 10.1093/gerona/61.11.1166. [DOI] [PubMed] [Google Scholar]
- [7].Sexton CE, Betts JF, Demnitz N, Dawes H, Ebmeier KP, and Johansen-Berg H, “A systematic review of MRI studies examining the relationship between physical fitness and activity and the white matter of the ageing brain.,” Neuroimage, vol. 131, pp. 81–90, 2016, doi: 10.1016/j.neuroimage.2015.09.071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Firth J et al. , “Effect of aerobic exercise on hippocampal volume in humans: A systematic review and meta-analysis,” Neuroimage, 2018, doi: 10.1016/j.neuroimage.2017.11.007. [DOI] [PubMed] [Google Scholar]
- [9].Soares JM, Marques P, Alves V, and Sousa N, “A hitchhiker’s guide to diffusion tensor imaging.,” Front. Neurosci, vol. 7, p. 31, 2013, doi: 10.3389/fnins.2013.00031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Fukutomi H et al. , “Neurite imaging reveals microstructural variations in human cerebral cortical gray matter,” Neuroimage, vol. 182, pp. 488–499, Nov. 2018, doi: 10.1016/j.neuroimage.2018.02.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Le Bihan D, “Looking into the functional architecture of the brain with diffusion MRI,” Nat. Rev. Neurosci, vol. 4, no. 6, pp. 469–480, 2003, doi: 10.1038/nrn1119. [DOI] [PubMed] [Google Scholar]
- [12].Basser PJ and Jones DK, “Diffusion-tensor MRI: theory, experimental design and data analysis - a technical review,” NMR Biomed., vol. 15, no. 7–8, pp. 456–467, Nov. 2002, doi: 10.1002/nbm.783. [DOI] [PubMed] [Google Scholar]
- [13].Fellgiebel A and Yakushev I, “Diffusion Tensor Imaging of the Hippocampus in MCI and Early Alzheimer’s Disease,” J. Alzheimer’s Dis, vol. 26, no. s3, pp. 257–262, Oct. 2011, doi: 10.3233/JAD-2011-0001. [DOI] [PubMed] [Google Scholar]
- [14].Callow DD, Canada KL, and Riggins T, “Microstructural integrity of the hippocampus during childhood: Relations with age and source memory,” Front. Psychol, vol. 11, p. 2352, Sep. 2020, doi: 10.3389/FPSYG.2020.568953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Hayek D, Thams F, Flöel A, and Antonenko D, “Dentate Gyrus Volume Mediates the Effect of Fornix Microstructure on Memory Formation in Older Adults,” Front. Aging Neurosci, vol. 12, p. 79, Mar. 2020, doi: 10.3389/fnagi.2020.00079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Genç E et al. , “Diffusion markers of dendritic density and arborization in gray matter predict differences in intelligence,” Nat. Commun, vol. 9, no. 1, Dec. 2018, doi: 10.1038/s41467-018-04268-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Aribisala BS et al. , “Quantitative multi-modal MRI of the Hippocampus and cognitive ability in community-dwelling older subjects.,” Cortex., vol. 53, no. 100, pp. 34–44, Apr. 2014, doi: 10.1016/j.cortex.2013.12.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Kantarci K et al. , “DWI predicts future progression to Alzheimer disease in amnestic mild cognitive impairment,” Neurology, vol. 64, no. 5, pp. 902–904, Mar. 2005, doi: 10.1212/01.WNL.0000153076.46126.E9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Venkatesh A, Stark SM, Stark CEL, and Bennett IJ, “Age- and memory- related differences in hippocampal gray matter integrity are better captured by NODDI compared to single-tensor diffusion imaging,” Neurobiol. Aging, vol. 96, pp. 12–21, Dec. 2020, doi: 10.1016/j.neurobiolaging.2020.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Le Bihan D, “Diffusion MRI: What water tells us about the brain,” EMBO Mol. Med, vol. 6, no. 5, pp. 569–573, 2014, doi: 10.1002/emmm.201404055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Walhovd KB, Johansen-Berg H, and Káradóttir RT, “Unraveling the secrets of white matter--bridging the gap between cellular, animal and human imaging studies.,” Neuroscience, vol. 276, no. 100, pp. 2–13, Sep. 2014, doi: 10.1016/j.neuroscience.2014.06.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Bennett IJ, Madden DJ, Vaidya CJ, Howard DV, and Howard JH, “Age-related differences in multiple measures of white matter integrity: A diffusion tensor imaging study of healthy aging,” Hum. Brain Mapp, vol. 31, no. 3, pp. 378–390, Mar. 2010, doi: 10.1002/hbm.20872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Madden DJ, Bennett IJ, and Song AW, “Cerebral white matter integrity and cognitive aging: contributions from diffusion tensor imaging.,” Neuropsychol. Rev, vol. 19, no. 4, pp. 415–35, Dec. 2009, doi: 10.1007/s11065-009-9113-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Oberlin LE et al. , “White matter microstructure mediates the relationship between cardiorespiratory fitness and spatial working memory in older adults HHS Public Access,” Neuroimage, vol. 131, pp. 91–101, 2016, doi: 10.1016/j.neuroimage.2015.09.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Bennett and Madden, “Disconnected aging: cerebral white matter integrity and age-related differences in cognition.,” Neuroscience, vol. 276, pp. 187–205, Sep. 2014, doi: 10.1016/j.neuroscience.2013.11.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Carlesimo GA, Cherubini A, Caltagirone C, and Spalletta G, “Hippocampal mean diffusivity and memory in healthy elderly individuals: A cross-sectional study,” Neurology, vol. 74, no. 3, pp. 194–200, Jan. 2010, doi: 10.1212/WNL.0b013e3181cb3e39. [DOI] [PubMed] [Google Scholar]
- [27].Lancaster MA et al. , “Diffusion Tensor Imaging Predictors of Episodic Memory Decline in Healthy Elders at Genetic Risk for Alzheimer’s Disease,” J. Int. Neuropsychol. Soc, vol. 22, pp. 1005–1015, 2016, doi: 10.1017/S1355617716000904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Fellgiebel A, Dellani PR, Greverus D, Scheurich A, Stoeter P, and Müller MJ, “Predicting conversion to dementia in mild cognitive impairment by volumetric and diffusivity measurements of the hippocampus,” Psychiatry Res. - Neuroimaging, vol. 146, no. 3, pp. 283–287, 2006, doi: 10.1016/j.pscychresns.2006.01.006. [DOI] [PubMed] [Google Scholar]
- [29].Assaf Y, “Imaging laminar structures in the gray matter with diffusion MRI,” NeuroImage, vol. 197. Academic Press Inc., pp. 677–688, Aug. 15, 2019, doi: 10.1016/j.neuroimage.2017.12.096. [DOI] [PubMed] [Google Scholar]
- [30].Zhang H, Schneider T, Wheeler-Kingshott CA, and Alexander DC, “NODDI: Practical in vivo neurite orientation dispersion and density imaging of the human brain,” 2012, doi: 10.1016/j.neuroimage.2012.03.072. [DOI] [PubMed] [Google Scholar]
- [31].Nazeri A et al. , “Functional consequences of neurite orientation dispersion and density in humans across the adult lifespan,” J. Neurosci, vol. 35, no. 4, pp. 1753–1762, Jan. 2015, doi: 10.1523/JNEUROSCI.3979-14.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Sampaio-Baptista C and Johansen-Berg H, “White Matter Plasticity in the Adult Brain,” Neuron, vol. 96, no. 6, pp. 1239–1251, 2017, doi: 10.1016/j.neuron.2017.11.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Kamiya K, Hori M, and Aoki S, “NODDI in clinical research,” Journal of Neuroscience Methods, vol. 346. Elsevier B.V., p. 108908, Dec. 01, 2020, doi: 10.1016/j.jneumeth.2020.108908. [DOI] [PubMed] [Google Scholar]
- [34].Jeurissen B, Tournier JD, Dhollander T, Connelly A, and Sijbers J, “Multi-tissue constrained spherical deconvolution for improved analysis of multi-shell diffusion MRI data,” Neuroimage, vol. 103, pp. 411–426, Dec. 2014, doi: 10.1016/j.neuroimage.2014.07.061. [DOI] [PubMed] [Google Scholar]
- [35].Radhakrishnan H, Stark SM, and Stark CEL, “Microstructural Alterations in Hippocampal Subfields Mediate Age-Related Memory Decline in Humans,” Front. Aging Neurosci, vol. 12, Apr. 2020, doi: 10.3389/fnagi.2020.00094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Schneider T, Brownlee W, Zhang H, Ciccarelli O, Miller DH, and Wheeler-Kingshott CG, “Sensitivity of multi-shell NODDI to multiple sclerosis white matter changes: a pilot study,” Funct. Neurol, vol. 32, no. 2, p. 97, 2017, doi: 10.11138/FNEUR/2017.32.2.097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Grussu F et al. , “Neurite dispersion: a new marker of multiple sclerosis spinal cord pathology?,” Ann. Clin. Transl. Neurol, vol. 4, no. 9, pp. 663–679, Sep. 2017, doi: 10.1002/acn3.445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].McCunn P et al. , “Reproducibility of Neurite Orientation Dispersion and Density Imaging (NODDI) in rats at 9.4 Tesla,” PLoS One, vol. 14, no. 4, p. e0215974, Apr. 2019, doi: 10.1371/journal.pone.0215974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Sato K et al. , “Understanding microstructure of the brain by comparison of neurite orientation dispersion and density imaging (NODDI) with transparent mouse brain,” Acta Radiol. Open, vol. 6, no. 4, p. 205846011770381, Apr. 2017, doi: 10.1177/2058460117703816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Parker TD et al. , “Cortical microstructure in young onset Alzheimer’s disease using neurite orientation dispersion and density imaging,” Hum. Brain Mapp, vol. 39, no. 7, pp. 3005–3017, Jul. 2018, doi: 10.1002/hbm.24056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Evans SL, Dowell NG, Prowse F, Tabet N, King SL, and Rusted JM, “Mid age APOE ε4 carriers show memory-related functional differences and disrupted structure-function relationships in hippocampal regions,” Sci. Rep, vol. 10, no. 1, pp. 1–11, Dec. 2020, doi: 10.1038/s41598-020-59272-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Mah A, Geeraert B, and Lebel C, “Detailing neuroanatomical development in late childhood and early adolescence using NODDI,” PLoS One, vol. 12, no. 8, p. e0182340, Aug. 2017, doi: 10.1371/journal.pone.0182340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Beck D et al. , “White matter microstructure across the adult lifespan: A mixed longitudinal and cross-sectional study using advanced diffusion models and brain-age prediction,” Neuroimage, vol. 224, p. 117441, Jan. 2021, doi: 10.1016/j.neuroimage.2020.117441. [DOI] [PubMed] [Google Scholar]
- [44].Vogt NM et al. , “Cortical Microstructural Alterations in Mild Cognitive Impairment and Alzheimer’s Disease Dementia,” Cereb. Cortex, vol. 30, no. 5, pp. 2948–2960, May 2020, doi: 10.1093/cercor/bhz286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Won J et al. , “Evidence for Exercise-Related Plasticity in Functional and Structural Neural Network Connectivity,” Neurosci. Biobehav. Rev, vol. 131, no. October, pp. 923–940, 2021, doi: 10.1016/j.neubiorev.2021.10.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Hayes SM, Salat DH, Forman DE, Sperling RA, and Verfaellie M, “Cardiorespiratory fitness is associated with white matter integrity in aging.,” Ann. Clin. Transl. Neurol, vol. 2, no. 6, pp. 688–98, Jun. 2015, doi: 10.1002/acn3.204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Tarumi T et al. , “Midlife aerobic exercise and brain structural integrity: Associations with age and cardiorespiratory fitness,” Neuroimage, vol. 225, Jan. 2021, doi: 10.1016/j.neuroimage.2020.117512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].Strömmer JM, Davis SW, Henson RN, Tyler LK, and Campbell KL, “Physical activity predicts population-level age-related differences in frontal white matter,” Journals Gerontol. - Ser. A Biol. Sci. Med. Sci, vol. 75, no. 2, pp. 236–243, Feb. 2020, doi: 10.1093/gerona/gly220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [49].Marks BL et al. , “Role of aerobic fitness and aging on cerebral white matter integrity,” Ann. N. Y. Acad. Sci, vol. 1097, pp. 171–174, 2007, doi: 10.1196/annals.1379.022. [DOI] [PubMed] [Google Scholar]
- [50].Smith JC et al. , “Interactive effects of physical activity and APOE-ε4 on white matter tract diffusivity in healthy elders.,” Neuroimage, vol. 131, pp. 102–12, 2016, doi: 10.1016/j.neuroimage.2015.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [51].Opel N et al. , “White matter microstructure mediates the association between physical fitness and cognition in healthy, young adults,” Sci. Rep, vol. 9, no. 1, pp. 1–9, Dec. 2019, doi: 10.1038/s41598-019-49301-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [52].Won J et al. , “Evidence for exercise-related plasticity in functional and structural neural network connectivity,” Neurosci. Biobehav. Rev, vol. 131, pp. 923–940, Dec. 2021, doi: 10.1016/J.NEUBIOREV.2021.10.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [53].Tian Q et al. , “Cardiorespiratory fitness and brain diffusion tensor imaging in adults over 80 years of age.,” Brain Res., vol. 1588, pp. 63–72, Nov. 2014, doi: 10.1016/j.brainres.2014.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [54].Tian Q et al. , “Physical activity predicts microstructural integrity in memory-related networks in very old adults,” Journals Gerontol. - Ser. A Biol. Sci. Med. Sci, vol. 69, no. 10, pp. 1284–1290, Oct. 2014, doi: 10.1093/gerona/glt287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [55].Kleemeyer MM et al. , “Changes in fitness are associated with changes in hippocampal microstructure and hippocampal volume among older adults,” Neuroimage, vol. 131, pp. 155–161, 2016, doi: 10.1016/j.neuroimage.2015.11.026. [DOI] [PubMed] [Google Scholar]
- [56].Callow DD et al. , “Exercise Training-Related Changes in Cortical Gray Matter Diffusivity and Cognitive Function in Mild Cognitive Impairment and Healthy Older Adults,” Front. Aging Neurosci, vol. 13, p. 164, Apr. 2021, doi: 10.3389/fnagi.2021.645258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [57].Van Essen DC, Smith SM, Barch DM, Behrens TEJ, Yacoub E, and Ugurbil K, “The WU-Minn Human Connectome Project: An overview,” Neuroimage, vol. 80, pp. 62–79, Oct. 2013, doi: 10.1016/j.neuroimage.2013.05.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [58].Uǧurbil K et al. , “Pushing spatial and temporal resolution for functional and diffusion MRI in the Human Connectome Project,” Neuroimage, vol. 80, pp. 80–104, Oct. 2013, doi: 10.1016/j.neuroimage.2013.05.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [59].Reuben DB et al. , “Motor assessment using the NIH Toolbox.,” Neurology, vol. 80, no. 11 Suppl 3, p. S65, 2013, doi: 10.1212/wnl.0b013e3182872e01. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [60].Dalgas U, Severinsen K, and Overgaard K, “Relations Between 6 Minute Walking Distance and 10 Meter Walking Speed in Patients With Multiple Sclerosis and Stroke,” 2012, doi: 10.1016/j.apmr.2012.02.026. [DOI] [PubMed] [Google Scholar]
- [61].Gershon RC, Wagster MV, Hendrie HC, Fox NA, Cook KF, and Nowinski CJ, “NIH toolbox for assessment of neurological and behavioral function.,” Neurology, vol. 80, no. 11 Suppl 3, pp. S2–S6, Mar. 2013, doi: 10.1212/wnl.0b013e3182872e5f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [62].Stawski RS, Almeida DM, Lachman ME, Tun PA, and Rosnick CB, “Fluid cognitive ability is associated with greater exposure and smaller reactions to daily stressors,” Psychol. Aging, vol. 25, no. 2, pp. 330–342, 2010, doi: 10.1037/a0018246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [63].Heaton RK et al. , “Reliability and validity of composite scores from the NIH toolbox cognition battery in adults,” J. Int. Neuropsychol. Soc, vol. 20, no. 6, pp. 588–598, 2014, doi: 10.1017/S1355617714000241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [64].Glasser MF et al. , “The minimal preprocessing pipelines for the Human Connectome Project,” Neuroimage, vol. 80, pp. 105–124, Oct. 2013, doi: 10.1016/j.neuroimage.2013.04.127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [65].Pierpaoli C, Jezzard P, Basser P, BArnett A, and Chiro D, “Diffusion Tensor MR Imaging ofthe Human Brain,” Magn. Reson. Med, pp. 637–648, 1996, Accessed: Oct. 21, 2018. [Online]. Available: https://pdfs.semanticscholar.org/f052/fe997aed03a303a21247a84f975f5649b04a.pdf.8892219 [Google Scholar]
- [66].Daducci A, Canales-Rodríguez EJ, Zhang H, Dyrby TB, Alexander DC, and Thiran JP, “Accelerated Microstructure Imaging via Convex Optimization (AMICO) from diffusion MRI data,” Neuroimage, vol. 105, pp. 32–44, Jan. 2015, doi: 10.1016/j.neuroimage.2014.10.026. [DOI] [PubMed] [Google Scholar]
- [67].Smith SM et al. , “Tract-based spatial statistics: Voxelwise analysis of multi-subject diffusion data,” Neuroimage, vol. 31, no. 4, pp. 1487–1505, Jul. 2006, doi: 10.1016/j.neuroimage.2006.02.024. [DOI] [PubMed] [Google Scholar]
- [68].Avants BB, Epstein CL, Grossman M, and Gee JC, “Symmetric diffeomorphic image registration with cross-correlation: Evaluating automated labeling of elderly and neurodegenerative brain,” Med. Image Anal, vol. 12, no. 1, pp. 26–41, Feb. 2008, doi: 10.1016/j.media.2007.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [69].Gorelick PB et al. , “Defining Optimal Brain Health in Adults: A Presidential Advisory From the American Heart Association/American Stroke Association,” Stroke, vol. 48, no. 10, p. e284, Oct. 2017, doi: 10.1161/STR.0000000000000148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [70].Winkler AM, Ridgway GR, Webster MA, Smith SM, and Nichols TE, “Permutation inference for the general linear model,” Neuroimage, vol. 92, no. 100, pp. 381–397, May 2014, doi: 10.1016/j.neuroimage.2014.01.060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [71].Kramer AF and Colcombe S, “Fitness Effects on the Cognitive Function of Older Adults: A Meta-Analytic Study—Revisited,” Perspect. Psychol. Sci, vol. 13, no. 2, 2018, doi: 10.1177/1745691617707316. [DOI] [PubMed] [Google Scholar]
- [72].Colcombe and Kramer AF, “Fitness Effects on the Cognitive Function of Older Adults,” Psychol. Sci, vol. 14, no. 2, pp. 125–130, 2003, doi: 10.1111/1467-9280.t01-1-01430 T4 - A meta-analytic study M4 - Citavi. [DOI] [PubMed] [Google Scholar]
- [73].Hillman CH, Castelli DM, and Buck SM, “Aerobic Fitness and Neurocognitive Function in Healthy Preadolescent Children,” Med. Sci. Sport. Exerc, vol. 37, no. 11, pp. 0–000, 2005, doi: 10.1249/01.mss.0000176680.79702.ce. [DOI] [PubMed] [Google Scholar]
- [74].Gomez-Pinilla F and Hillman C, “The influence of exercise on cognitive abilities.,” Compr. Physiol, vol. 3, no. 1, pp. 403–28, Jan. 2013, doi: 10.1002/cphy.c110063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [75].Suwabe K, Hyodo K, Byun K, Ochi G, Yassa MA, and Soya H, “Acute moderate exercise improves mnemonic discrimination in young adults,” Hippocampus, vol. 27, no. 3, pp. 229–234, Mar. 2017, doi: 10.1002/hipo.22695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [76].Salas-Gomez D et al. , “Physical Activity Is Associated With Better Executive Function in University Students,” Front. Hum. Neurosci, vol. 14, Feb. 2020, doi: 10.3389/fnhum.2020.00011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [77].Stern Y et al. , “Effect of aerobic exercise on cognition in younger adults: A randomized clinical trial,” Neurology, vol. 92, no. 9, pp. E905–E916, Feb. 2019, doi: 10.1212/WNL.0000000000007003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [78].Nauer RK, Dunne MF, Stern CE, Storer TW, and Schon K, “Improving fitness increases dentate gyrus/CA3 volume in the hippocampal head and enhances memory in young adults,” Hippocampus, p. hipo.23166, Oct. 2019, doi: 10.1002/hipo.23166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [79].Verburgh L, Königs M, Scherder EJA, and Oosterlaan J, “Physical exercise and executive functions in preadolescent children, adolescents and young adults: A meta-analysis,” British Journal of Sports Medicine, vol. 48, no. 12. BMJ Publishing Group, pp. 973–979, Jun. 01, 2014, doi: 10.1136/bjsports-2012-091441. [DOI] [PubMed] [Google Scholar]
- [80].Zhu N et al. , “Cardiorespiratory fitness and brain volume and white matter integrity: The CARDIA Study.,” Neurology, vol. 84, no. 23, pp. 2347–53, Jun. 2015, doi: 10.1212/WNL.0000000000001658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [81].Kansal K et al. , “Structural cerebellar correlates of cognitive and motor dysfunctions in cerebellar degeneration,” Brain, vol. 140, no. 3, pp. 707–720, Mar. 2017, doi: 10.1093/brain/aww327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [82].Lin CY, Chen CH, Tom SE, and Kuo SH, “Cerebellar Volume Is Associated with Cognitive Decline in Mild Cognitive Impairment: Results from ADNI,” Cerebellum, vol. 19, no. 2, pp. 217–225, Apr. 2020, doi: 10.1007/s12311-019-01099-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [83].Won J et al. , “Association Between Greater Cerebellar Network Connectivity and Improved Phonemic Fluency Performance After Exercise Training in Older Adults,” The Cerebellum, pp. 1–14, Jan. 2021, doi: 10.1007/s12311-020-01218-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [84].Ramanoël S et al. , “Gray matter volume and cognitive performance during normal aging. A voxel-based morphometry study,” Front. Aging Neurosci, vol. 10, no. AUG, p. 235, Aug. 2018, doi: 10.3389/fnagi.2018.00235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [85].Drijkoningen D et al. , “Training-induced improvements in postural control are accompanied by alterations in cerebellar white matter in brain injured patients,” NeuroImage Clin., vol. 7, pp. 240–251, Jan. 2015, doi: 10.1016/j.nicl.2014.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [86].Stoodley CJ and Schmahmann JD, “Functional topography in the human cerebellum: A meta-analysis of neuroimaging studies,” Neuroimage, vol. 44, no. 2, pp. 489–501, Jan. 2009, doi: 10.1016/j.neuroimage.2008.08.039. [DOI] [PubMed] [Google Scholar]
- [87].Schmahmann JD, “The cerebellum and cognition,” Neuroscience Letters, vol. 688. Elsevier Ireland Ltd, pp. 62–75, Jan. 01, 2019, doi: 10.1016/j.neulet.2018.07.005. [DOI] [PubMed] [Google Scholar]
- [88].Won J et al. , “Hippocampal Functional Connectivity and Memory Performance After Exercise Intervention in Older Adults with Mild Cognitive Impairment,” J. Alzheimer’s Dis, pp. 1–17, Jun. 2021, doi: 10.3233/jad-210051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [89].Raz N et al. , “Regional brain changes in aging healthy adults: General trends, individual differences and modifiers,” Cereb. Cortex, vol. 15, no. 11, pp. 1676–1689, 2005, doi: 10.1093/cercor/bhi044. [DOI] [PubMed] [Google Scholar]
- [90].Pietschnig J, Penke L, Wicherts JM, Zeiler M, and Voracek M, “Meta-analysis of associations between human brain volume and intelligence differences: How strong are they and what do they mean?,” Neuroscience and Biobehavioral Reviews, vol. 57. Elsevier Ltd, pp. 411–432, Oct. 01, 2015, doi: 10.1016/j.neubiorev.2015.09.017. [DOI] [PubMed] [Google Scholar]
- [91].Frangou S et al. , “Cortical thickness across the lifespan: Data from 17,075 healthy individuals aged 3–90 years,” Hum. Brain Mapp, 2021, doi: 10.1002/hbm.25364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [92].Rao SM et al. , “Genetic risk for Alzheimer’s disease alters the five-year trajectory of semantic memory activation in cognitively intact elders,” Neuroimage, vol. 111, pp. 136–146, May 2015, doi: 10.1016/j.neuroimage.2015.02.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [93].Reiter K et al. , “Five-year longitudinal brain volume change in healthy elders at genetic risk for Alzheimer’s disease HHS Public Access,” J Alzheimers Dis, vol. 55, no. 4, pp. 1363–1377, 2017, doi: 10.3233/JAD-160504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [94].Freeman SH et al. , “Preservation of neuronal number despite age-related cortical brain atrophy in elderly subjects without alzheimer disease,” J. Neuropathol. Exp. Neurol, vol. 67, no. 12, pp. 1205–1212, Dec. 2008, doi: 10.1097/NEN.0b013e31818fc72f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [95].Asan L et al. , “Cellular correlates of gray matter volume changes in magnetic resonance morphometry identified by two-photon microscopy,” Sci. Rep, vol. 11, no. 1, p. 4234, Dec. 2021, doi: 10.1038/s41598-021-83491-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [96].Yi SY et al. , “Detecting Microglial Density With Quantitative Multi-Compartment Diffusion MRI,” Front. Neurosci, vol. 13, no. FEB, p. 81, Feb. 2019, doi: 10.3389/fnins.2019.00081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [97].Crombe A et al. , “Deciphering the microstructure of hippocampal subfields with in vivo DTI and NODDI: Applications to experimental multiple sclerosis,” Neuroimage, vol. 172, pp. 357–368, May 2018, doi: 10.1016/j.neuroimage.2018.01.061. [DOI] [PubMed] [Google Scholar]
- [98].Nazeri A, Schifani C, Anderson JAE, Ameis SH, and Voineskos AN, “In Vivo Imaging of Gray Matter Microstructure in Major Psychiatric Disorders: Opportunities for Clinical Translation,” Biological Psychiatry: Cognitive Neuroscience and Neuroimaging, vol. 5, no. 9. Elsevier Inc, pp. 855–864, Sep. 01, 2020, doi: 10.1016/j.bpsc.2020.03.003. [DOI] [PubMed] [Google Scholar]
- [99].Qian W, Khattar N, Cortina LE, Spencer RG, and Bouhrara M, “Nonlinear associations of neurite density and myelin content with age revealed using multicomponent diffusion and relaxometry magnetic resonance imaging,” Neuroimage, vol. 223, p. 117369, Dec. 2020, doi: 10.1016/j.neuroimage.2020.117369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [100].Marsman A et al. , “Glutamate changes in healthy young adulthood,” Eur. Neuropsychopharmacol, vol. 23, no. 11, pp. 1484–1490, Nov. 2013, doi: 10.1016/j.euroneuro.2012.11.003. [DOI] [PubMed] [Google Scholar]
- [101].Craik FIM and Bialystok E, “Cognition through the lifespan: Mechanisms of change,” Trends in Cognitive Sciences, vol. 10, no. 3. Elsevier Current Trends, pp. 131–138, Mar. 01, 2006, doi: 10.1016/j.tics.2006.01.007. [DOI] [PubMed] [Google Scholar]
- [102].Paolicelli RC et al. , “Synaptic pruning by microglia is necessary for normal brain development,” Science (80-.)., vol. 333, no. 6048, pp. 1456–1458, Sep. 2011, doi: 10.1126/science.1202529. [DOI] [PubMed] [Google Scholar]
- [103].Neubauer AC and Fink A, “Intelligence and neural efficiency,” Neuroscience and Biobehavioral Reviews, vol. 33, no. 7. Pergamon, pp. 1004–1023, Jul. 01, 2009, doi: 10.1016/j.neubiorev.2009.04.001. [DOI] [PubMed] [Google Scholar]
- [104].Sporns O, Tononi G, and Edelman GM, “Connectivity and complexity: The relationship between neuroanatomy and brain dynamics,” Neural Networks, vol. 13, no. 8–9. Elsevier Science Ltd, pp. 909–922, Nov. 01, 2000, doi: 10.1016/S0893-6080(00)00053-8. [DOI] [PubMed] [Google Scholar]
- [105].Williams VJ et al. , “Cardiorespiratory fitness is differentially associated with cortical thickness in young and older adults,” Neuroimage, vol. 146, pp. 1084–1092, Feb. 2017, doi: 10.1016/j.neuroimage.2016.10.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [106].Callow DD et al. , “Microstructural Plasticity in the Hippocampus of Healthy Older Adults after Acute Exercise,” Med. Sci. Sports Exerc, vol. 53, no. 9, pp. 1928–1936, Sep. 2021, doi: 10.1249/MSS.0000000000002666. [DOI] [PubMed] [Google Scholar]
- [107].Alfini AJ, Weiss LR, Nielson KA, Verber MD, and Smith JC, “Resting cerebral blood flow after exercise training in mild cognitive impairment,” J. Alzheimer’s Dis, vol. 67, no. 2, pp. 671–684, 2019, doi: 10.3233/JAD-180728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [108].Chirles TJ, Reiter K, Weiss LR, Alfini AJ, Nielson KA, and Smith JC, “Exercise Training and Functional Connectivity Changes in Mild Cognitive Impairment and Healthy Elders,” J. Alzheimer’s Dis, vol. 57, no. 3, pp. 845–856, Apr. 2017, doi: 10.3233/JAD-161151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [109].Smith JC et al. , “Semantic memory functional MRI and cognitive function after exercise intervention in mild cognitive impairment.,” J. Alzheimers. Dis, vol. 37, no. 1, pp. 197–215, 2013, doi: 10.3233/JAD-130467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [110].Henf J, Grothe MJ, Brueggen K, Teipel S, and Dyrba M, “Mean diffusivity in cortical gray matter in Alzheimer’s disease: The importance of partial volume correction.,” NeuroImage. Clin, vol. 17, pp. 579–586, 2018, doi: 10.1016/j.nicl.2017.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [111].Brockett AT, LaMarca EA, and Gould E, “Physical exercise enhances cognitive flexibility as well as astrocytic and synaptic markers in the medial prefrontal cortex,” PLoS One, vol. 10, no. 5, May 2015, doi: 10.1371/journal.pone.0124859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [112].Luo Y et al. , “Running exercise protects oligodendrocytes in the medial prefrontal cortex in chronic unpredictable stress rat model,” Transl. Psychiatry, vol. 9, no. 1, pp. 1–11, Dec. 2019, doi: 10.1038/s41398-019-0662-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [113].Talukdar T et al. , “Aerobic fitness explains individual differences in the functional brain connectome of healthy young adults,” Cereb. Cortex, vol. 28, no. 10, pp. 3600–3609, Oct. 2018, doi: 10.1093/cercor/bhx232. [DOI] [PubMed] [Google Scholar]
- [114].Guerrero JM et al. , “Optimizing the intrinsic parallel diffusivity in NODDI: An extensive empirical evaluation,” PLoS One, vol. 14, no. 9, p. e0217118, Sep. 2019, doi: 10.1371/journal.pone.0217118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [115].Jespersen SN, Leigland LA, Cornea A, and Kroenke CD, “Determination of axonal and dendritic orientation distributions within the developing cerebral cortex by diffusion tensor imaging.,” IEEE Trans. Med. Imaging, vol. 31, no. 1, pp. 16–32, Jan. 2012, doi: 10.1109/TMI.2011.2162099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [116].Hansen MB, Jespersen SN, Leigland LA, and Kroenke CD, “Using diffusion anisotropy to characterize neuronal morphology in gray matter: The orientation distribution of axons and dendrites in the NeuroMorpho.org database,” Front. Integr. Neurosci, no. APR 2013, pp. 1–31, 2013, doi: 10.3389/fnint.2013.00031. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Figure 1: Voxel-wise analysis comparing the effects of fitness on Fractional Anisotropy (FA) and Neurite Orientation Dispersion Index (ODIWM) while controlling for age and sex vs age, sex, and gait speed. A) Positive relationship between FA and fitness without controlling for gait speed. B) Positive relationship between FA and fitness while controlling for gait speed. C) Negative relationship between ODIWM and fitness without controlling for gait speed. D) Negative relationship between ODIWM and fitness while controlling for gait speed. Our results are consistent with Opel and colleagues 2019, showing that fitness was associated with better white matter integrity through both higher FA and lower ODIWM. However, ODIWM and the inclusion of gait speed as a covariate led to more concentrated effects.
Data Availability Statement
The data used for this analysis are part of the “S1200 Subjects Data Release” provided by the Human Connectome Project and can be accessed via its ConnectomeDB platform (https://db.humanconnectome.org/). While all neuroimaging data is open access, some demographic data falls under the restricted access and data use terms put in place by the HCP and researchers must apply and agree to these terms to access the data https://www.humanconnectome.org/study/hcp-young-adult/document/quick-reference-open-access-vs-restricted-data.