Abstract
Background
Gallbladder carcinoma (GBC) is relatively rare but highly aggressive and it has poor prognosis, especially for metastatic GBC. We aimed to determine the prognostic significance of primary tumor resection on patients with metastatic GBC.
Material/Methods
The records of patients with GBC with distant metastasis from 2010 to 2015 were extracted from the Surveillance, Epidemiology, and End Results (SEER) database. Kaplan-Meier methods were used to compare overall survival (OS) and carcinoma-specific survival (CSS) between patients receiving primary tumor resection and those without surgery. Cox regression analysis was conducted to identity independent factors significantly associated with survival. In addition, a propensity score-matched analysis was performed to adjust for the heterogeneity between the groups.
Results
Of the 1337 patients included, 496 patients underwent primary tumor resection and 841 patients did not. Multivariate Cox regression analysis showed that OS (hazard ratio [HR]: 0.56, 95% confidence interval [CI]: 0.48–0.66, P<0.001) and CSS (HR: 0.57, 95% CI: 0.48–0.66, P<0.001) were significantly improved in patients receiving surgical resection of the primary tumor lesion in the unmatched cohort. Additionally, in the matched cohort, univariate Cox regression analysis similarly indicated that performing surgery at the primary site was associated with better OS (HR: 0.62, 95% CI: 0.50–0.77, P<0.001) and CSS (HR: 0.61, 95% CI: 0.50–0.762, P<0.001).
Conclusions
This study indicated that primary tumor resection might prolong survival in patients with metastatic GBC.
Keywords: Gallbladder Neoplasms, Prognosis, SEER Program
Background
Gallbladder carcinoma (GBC) is the most common malignancy of the biliary system, accounting for approximately two-thirds of biliary system carcinomas and ranking sixth in incidence of digestive system tumors [1,2]. Although GBC is relatively rare among all types of human malignancies, it is highly aggressive and a great threat to health worldwide. Owing to an insidious onset, rapid progression, asymptomatic development, and high propensity to metastatic dissemination, most patients with GBC are diagnosed with an advanced stage or even distant metastasis at initial diagnosis due to the symptoms of biliary obstruction and pain caused by tumor invasion into surrounding structures [3–5]. At the advanced or metastatic stage, GBC progresses rapidly and spreads frequently to adjacent organs, such as the liver, pancreas, colon, and peritoneum as well as to regional and distant lymph nodes. The liver, an adjacent organ of the gallbladder, is the leading metastatic site, accounting for more than half of the occurrences of metastatic GBC [6].
The long-term prognosis of patients with metastatic GBC is dismal, with the 5-year overall survival (OS) rate reported at far less than 10% [7,8]. The clinical management of metastatic GBC generally includes chemotherapy, radiotherapy, clinical trials, and best supportive care [9,10]. However, palliative therapy is not potentially curative, and the primary tumor lesion is rarely eradicated by systemic therapy; therefore, it is feasible to selectively perform local consolidation. Although metastatic GBC has been traditionally considered far beyond the scope of surgical resection [11], there are numerous case reports of successful surgical management among patients with metastatic GBC, mostly based on multidisciplinary therapy [12–14]. Moreover, based on the biological rationale of decreasing tumor dissemination by reducing overall tumor burden [15,16], it is feasible to carry out surgical resection of a primary tumor lesion in carefully selected patients with metastatic GBC. However, the high rates of perioperative complications and mortality cannot be neglected, especially in patients with poor performance status.
To the best of our knowledge, there has not been a large population-based evaluation of the efficacy of primary tumor resection for metastatic GBC [17]. Herein, we aimed to assess the prognostic effect of primary tumor resection among patients with metastatic GBC in a large population cohort.
Material and Methods
Data Source and Patient Selection
A population-based, retrospective study was performed to investigate the influence of primary tumor resection on the survival of patients with metastatic GBC. The Surveillance, Epidemiology, and End Results (SEER) database, which is sponsored by the National Carcinoma Institute, authoritatively collects and reports updated information on patient demographics, tumor incidence and characteristics, therapeutic modalities, and patient survival by covering 18 regional registries and comprising approximately 30% of the US population. Therefore, the SEER database is a valuable source for clinical carcinoma research, especially for rare malignancies, such as GBC. SEER*Stat software (version 8.3.6; NCI, Bethesda, MD, USA) was used to download detailed information from the SEER database. Because the acquired data were de-identified and publicly available, institutional review board approval was waived.
A total of 4471 patients with pathologically confirmed GBC were initially extracted from the database from 2010 to 2015. The inclusion criteria were as follows: (1) patients 18 years or older; (2) GBC was the only or first primary malignancy; (3) patients had distant metastasis at diagnosis; (4) the survival time was no less than 1 month; and (5) details of the therapy of the primary tumor were accessible. According to the above inclusion criteria, 1337 eligible patients were finally included after several rounds of screening. Patients not meeting these inclusion criteria were excluded from the study. The detailed process of cohort selection is displayed in Figure 1
Figure 1.
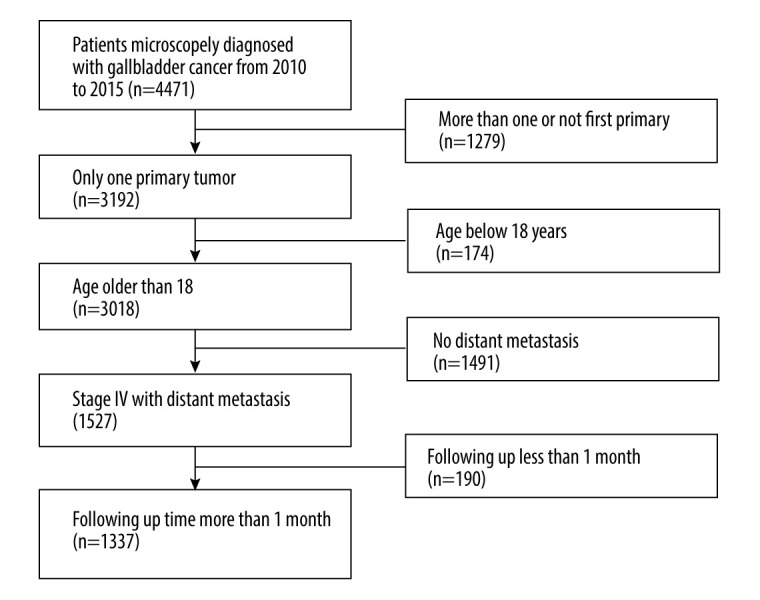
Flowchart of patient selection.
Covariates and Outcomes
Patient demographics, tumor-specific characteristics, treatment modalities, and survival data were extracted from the SEER database. The patient demographics included age at diagnosis, race, sex, and marital status. Age at diagnosis was simply divided into 2 groups by using 65 years of age as the cutoff value. In terms of tumor characteristics, tumor grade, tumor size, histology, primary tumor (T) stage (eighth version of the TNM staging system), regional lymph node involvement (N) stage (eighth version of TNM staging system), and distant metastasis were downloaded and analyzed. Continuous tumor size was divided into ≤1 cm, 1.1–3 cm, 3.1–5 cm, >5 cm, or unknown according to our clinical experience. Treatment modalities included primary tumor resection, radiation, and chemotherapy. The variable “RX Summ – Surg Prim Site” was retrieved from the database to define surgical removal of the primary lesion according to SEER site-specific codes, which categorized eligible patients into a surgery group and non-surgery group.
The main endpoints in this study were OS and CSS. OS referred to death by any cause, including individuals who died of other non-tumor related causes, while CSS was defined as time from the date of diagnosis until death attributed to GBC.
Propensity Score Matching
As this was a retrospective study, the potential covariates were not well balanced between the surgery and non-surgery groups, which may have distorted the real relationship of surgery with OS and DFS. To reduce this influence, propensity score matching (PSM) was used to perform a matched case-control analysis. First, each patient was allocated a propensity score calculated using logistic regression modeling with surgery as a dependent variable. The remaining confounding variables, including sex, race, age, marital status, grade, T and N stage, radiotherapy, chemotherapy, tumor size, and histology, were taken as covariates. Second, patients in the surgery and non-surgery groups were matched at a 1: 1 fixed ratio if they had similar propensity scores. The method we used in this process is called nearest available matching, with a caliper of 0.05. Third, the standard difference acted as an indicator of the matching effect and was calculated for all clinical variables. The value <0.15 indicated that the covariates were well distributed between the 2 groups.
Statistical Analysis
The t test and chi-square test (or Fisher’s exact test) were used to compare continuous variables and categorical variables, respectively, aiming to reveal any differences of clinicopathological characteristics between the 2 groups. The Kaplan-Meier method was used to plot the survival curve before and after PSM, and the log-rank test was used to determine the survival difference between the 2 groups. Cox proportional hazards regression was used to identify prognostic factors for OS and CSS. Variables with a P value less than 0.05 in the univariate model were further incorporated into the multivariate analysis. Results were presented with hazard ratios (HRs) and 95% confidence intervals (CIs). All statistical analyses and figures were performed using R software version 2.15.3 (R Foundation for Statistical Computing, Vienna, Austria), and a 2-sided P<0.05 was suggestive of statistical significance.
Results
Clinicopathological Characteristics of Patients
According to the inclusion and exclusion criteria, 1337 eligible patients were enrolled, including 496 patients who underwent primary tumor resection and 841 patients without surgery. Approximately 37.1% of patients received primary tumor resection. Women presented a higher proportion than men in the overall population. Most patients were White (n=988, 73.9%) and married (n=730, 54.6%). Adenocarcinoma was the most common histological type, accounting for 80.1% of cases. The liver was the most common metastatic organ, followed by the lung, bone, and brain. The baseline characteristics of the study cohort are summarized in Table 1.
Table 1.
Clinicopathological characteristics of the selected patients.
| Non-surgery N=841 |
Surgery N=496 |
P value | |
|---|---|---|---|
| Sex | <0.001 | ||
| Female | 561 (66.7%) | 378 (76.2%) | |
| Male | 280 (33.3%) | 118 (23.8%) | |
| Race | 0.015 | ||
| Black | 136 (16.2%) | 61 (12.3%) | |
| White | 598 (71.1%) | 390 (78.6%) | |
| Other | 107 (12.74%) | 45 (9.07%) | |
| Age | 0.303 | ||
| ≤65 | 371 (44.1%) | 234 (47.2%) | |
| >65 | 470 (55.9%) | 262 (52.8%) | |
| Marital status | 0.112 | ||
| Married | 441 (52.4%) | 289 (58.3%) | |
| Unmarried | 360 (42.8%) | 188 (37.9%) | |
| Unknown | 40 (4.76%) | 19 (3.83%) | |
| Grade | <0.001 | ||
| I | 7 (0.83%) | 38 (7.66%) | |
| II | 58 (6.90%) | 180 (36.3%) | |
| III | 128 (15.2%) | 213 (42.9%) | |
| IV | 7 (0.83%) | 21 (4.23%) | |
| Unknown | 641 (76.2%) | 44 (8.87%) | |
| T | <0.001 | ||
| T1 | 113 (13.4%) | 25 (5.04%) | |
| T2 | 6 (0.71%) | 151 (30.4%) | |
| T3 | 345 (41.0%) | 285 (57.5%) | |
| T4 | 69 (8.20%) | 21 (4.23%) | |
| Unknown | 308 (36.6%) | 14 (2.82%) | |
| N | <0.001 | ||
| N0 | 348 (41.4%) | 266 (53.6%) | |
| N1 | 219 (26.0%) | 151 (30.4%) | |
| N2 | 126 (15.0%) | 44 (8.87%) | |
| Unknown | 148 (17.6%) | 35 (7.06%) | |
| Radiation | 0.016 | ||
| None/Unknown | 784 (93.2%) | 443 (89.3%) | |
| Yes | 57 (6.78%) | 53 (10.7%) | |
| Chemotherapy | 0.036 | ||
| No/Unknown | 326 (38.8%) | 222 (44.8%) | |
| Yes | 515 (61.2%) | 274 (55.2%) | |
| Regional lymph nodes | <0.001 | ||
| Negative | 6 (0.71%) | 48 (9.68%) | |
| Positive | 40 (4.76%) | 133 (26.8%) | |
| Unknown | 795 (94.5%) | 315 (63.5%) | |
| Bone | 0.21 | ||
| No | 779 (92.6%) | 469 (94.6%) | |
| Yes | 62 (7.37%) | 27 (5.44%) | |
| Brain | 0.684 | ||
| No | 829 (98.6%) | 491 (99.0%) | |
| Yes | 12 (1.43%) | 5 (1.01%) | |
| Liver | 0.001 | ||
| No | 248 (29.5%) | 193 (38.9%) | |
| Yes | 593 (70.5%) | 303 (61.1%) | |
| Lung | <0.001 | ||
| No | 707 (84.1%) | 466 (94.0%) | |
| Yes | 134 (15.9%) | 30 (6.05%) | |
| DLN | 0.001 | ||
| No | 684 (81.3%) | 438 (88.3%) | |
| Yes | 157 (18.7%) | 58 (11.7%) | |
| Tumor size | <0.001 | ||
| ≤1 | 23 (2.73%) | 14 (2.82%) | |
| ≤3 | 64 (7.61%) | 121 (24.4%) | |
| ≤5 | 91 (10.8%) | 92 (18.5%) | |
| >5 | 153 (18.2%) | 87 (17.5%) | |
| Unknown | 510 (60.6%) | 182 (36.7%) | |
| Histology | <0.001 | ||
| Adenocarcinomas | 655 (77.9%) | 416 (83.9%) | |
| Epithelial | 103 (12.2%) | 32 (6.45%) | |
| Mucinous | 29 (3.45%) | 34 (6.85%) | |
| Others | 54 (6.42%) | 14 (2.82%) |
Summary of Distant Metastasis
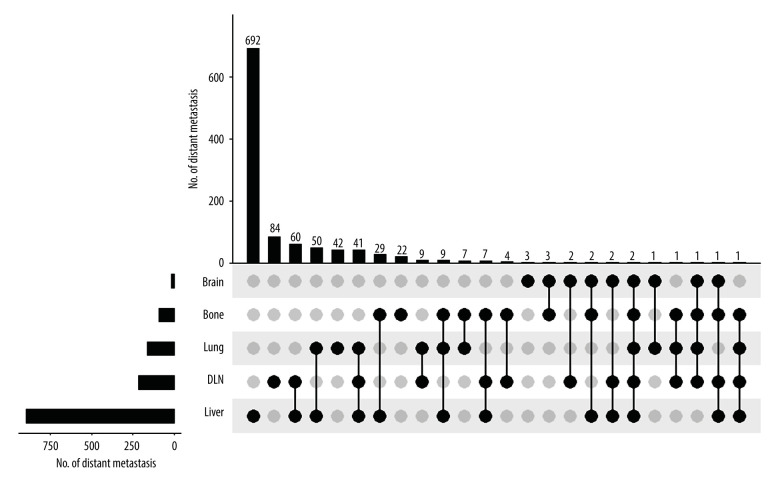
We further analyzed the distribution of distant metastasis sites. As shown in Figure 2, a total of 692 patients had only liver metastasis and 204 patients had more than 1 metastatic organ, including the liver. Moreover, 215 patients had distant lymph node involvement, accounting for 16.1% of the total population. Also, 164 patients had lung metastasis. Bone metastasis and brain metastasis were relatively uncommon, accounting for 89 and 17 patients, respectively.
Figure 2.
Summary of distant metastases.
Survival Analysis and Prognostic Factors Before Matching
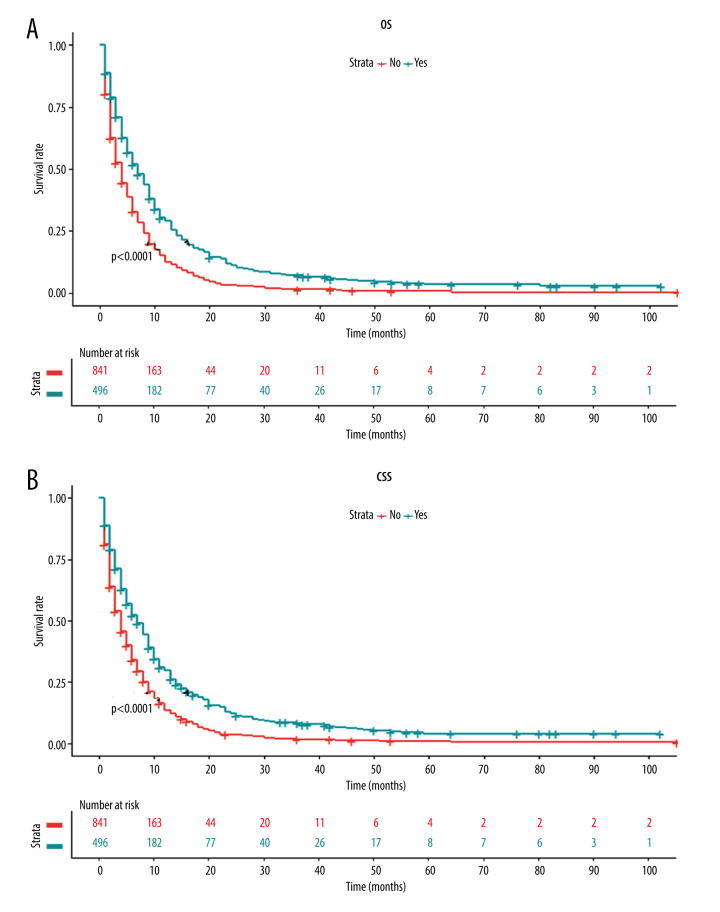
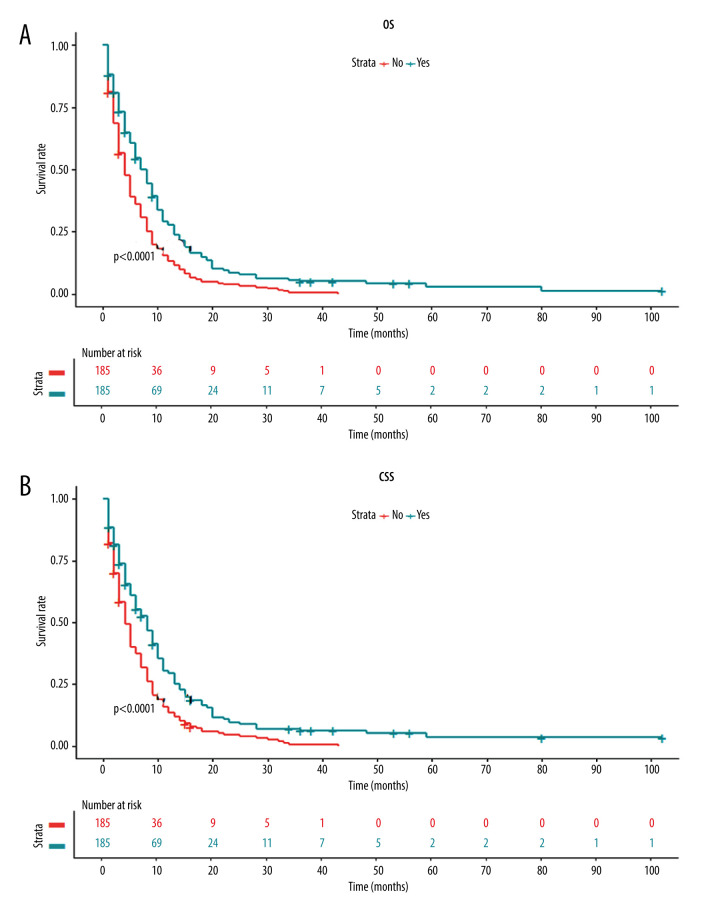
Next, we investigated whether there was any survival difference between patients with and without primary tumor resection. The Kaplan-Meier curve showed that patients receiving primary tumor resection had a significantly higher OS rate than those without surgery (Figure 3A). Similarly, the Kaplan-Meier curve of CSS also revealed significantly better survival in patients following surgery of the primary tumor site (Figure 3B).
Figure 3.
Kaplan-Meier curves for (A) overall survival and (B) carcinoma-specific survival stratified by primary tumor resection in the unmatched cohort.
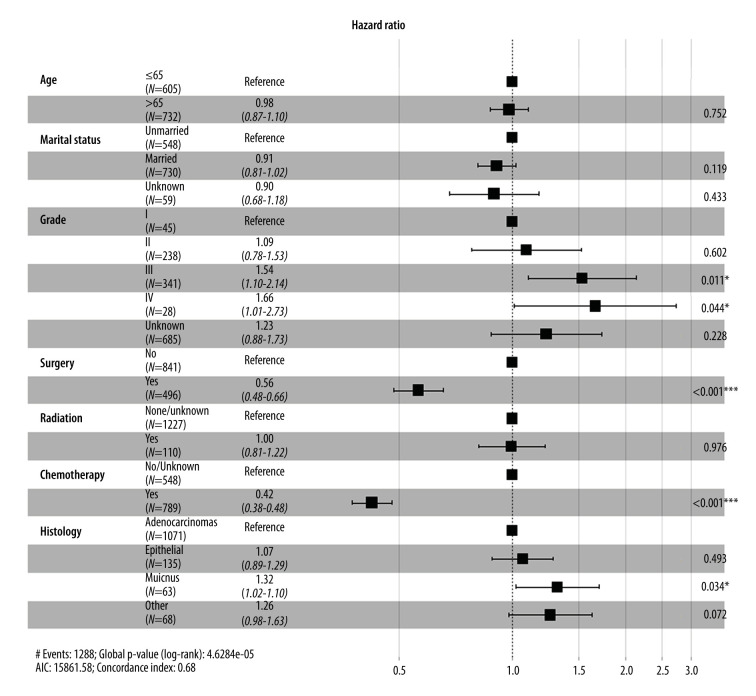
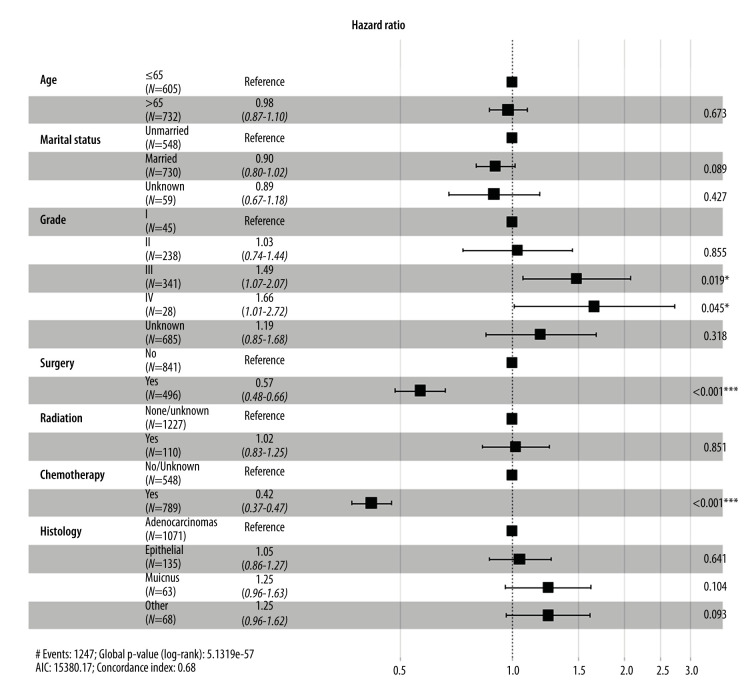
To further confirm the prognostic significance of primary tumor resection in patients with metastatic GBC, Cox analysis was performed. Variables with a P value <0.05 in the univariate analysis were incorporated into the multivariate analysis. Multivariate Cox regression analysis demonstrated that tumor grade, chemotherapy, and surgery of the primary lesion were independent prognostic factors for OS (Figure 4) and CSS (Figure 5). Specifically, primary tumor resection was associated with a significantly better OS rate (HR=0.56, 95% CI: 0.48–0.66, P<0.001). A better CSS hazard ratio was also consistently detected in patients undergoing surgery of the primary tumor site (HR=0.57, 95% CI: 0.48–0.66, P<0.001).
Figure 4.
Forest plot showing results of multivariate Cox regression analysis to explore independent prognostic factors for overall survival.
Figure 5.
Forest plot showing results of multivariate Cox regression analysis to explore independent prognostic factors for carcinoma-specific survival.
In consideration of the possible effects of different metastatic sites on patient survival, we conducted subgroup analysis stratified by metastatic organs. Multivariate Cox regression analysis revealed that primary tumor resection was significantly associated with better OS and CSS rates in patients with GBC with liver metastasis or distant lymph node metastasis (Table 2). Such an association was not detected in patients with GBC with lung or brain metastasis, possibly due to the small sample size.
Table 2.
Univariate and multivariate Cox regression analysis of the prognostic significance of metastatic location in gallbladder cancer.
| Univariate analysis | Multivariate analysis | |||
|---|---|---|---|---|
| HR | p | HR | p | |
| Overall survival | ||||
| Liver | 0.598 (0.517–0.691) | <0.001 | 0.556 (0.458–0.674) | <0.001 |
| DLN | 0627 (0.459–0.857) | 0.003 | 0.390 (0.257–0.593) | <0.001 |
| Lung | 0.744 (0.488–1.133) | 0.168 | – | – |
| Bone | 1.171 (0.732–1.875) | 0.509 | – | – |
| Cancer-specific survival | ||||
| Liver | 0.603 (0.520–0.698) | <0.001 | 0.560 (0.461–0.682) | <0.001 |
| DLN | 0.634 (0.461–0.870) | 0.005 | 0.411 (0.269–0.627) | <0.001 |
| Lung | 0.789 (0.517–1.204) | 0.271 | – | – |
| Bone | 1.210 (0.755–1.941) | 0.428 | – | – |
Survival Analysis and Prognostic Factors After Matching
To balance the covariates between the surgery group and non-surgery group, we performed PSM analysis at a 1: 1 fixed ratio, which identified 370 patients in the matched cohort (185 patients in each group). As shown in Table 3, the standard difference value of various clinicopathological variables was decreased after matching. A standard difference of <0.15 was used as the cutoff value to suggest that covariates were well-balanced between the matched groups.
Table 3.
The distribution of clinicopathological variables with standardized difference before and after propensity score matching.
| Variables | Before matching | After matching | ||||
|---|---|---|---|---|---|---|
| Non-surgery N=841 |
Surgery N=496 |
SD | Non-surgery N=185 |
Surgery N=185 |
SD | |
| Sex | 0.212 | 0.035 | ||||
| Female | 561 (66.7%) | 378 (76.2%) | 131 (70.8%) | 128 (69.2%) | ||
| Male | 280 (33.3%) | 118 (23.8%) | 54 (29.2%) | 57 (30.8%) | ||
| Race | 0.175 | 0.130 | ||||
| Black | 136 (16.2%) | 61 (12.3%) | 20 (10.8%) | 28 (15.1%) | ||
| White | 598 (71.1%) | 390 (78.6%) | 145 (78.4%) | 139 (75.1%) | ||
| Other | 107 (12.74%) | 45 (9.07%) | 20 (10.8%) | 18 (9.73%) | ||
| Age | 0.062 | 0.119 | ||||
| ≤65 | 371 (44.1%) | 234 (47.2%) | 83 (44.9%) | 94 (50.8%) | ||
| >65 | 470 (55.9%) | 262 (52.8%) | 102 (55.1%) | 91 (49.2%) | ||
| Marital status | 0.119 | 0.023 | ||||
| Married | 441 (52.4%) | 289 (58.3%) | 105 (56.8%) | 103 (55.7%) | ||
| Unmarried | 360 (42.8%) | 188 (37.9%) | 68 (36.8%) | 70 (37.8%) | ||
| Unknown | 40 (4.76%) | 19 (3.83%) | 12 (6.49%) | 12 (6.49%) | ||
| Grade | 1.889 | 0.124 | ||||
| I | 7 (0.83%) | 38 (7.66%) | 6 (3.24%) | 6 (3.24%) | ||
| II | 58 (6.90%) | 180 (36.3%) | 44 (23.8%) | 44 (23.8%) | ||
| III | 128 (15.2%) | 213 (42.9%) | 82 (44.3%) | 83 (44.9%) | ||
| IV | 7 (0.83%) | 21 (4.23%) | 5 (2.70%) | 9 (4.86%) | ||
| Unknown | 641 (76.2%) | 44 (8.87%) | 48 (25.9%) | 43 (23.2%) | ||
| T | 1.39 | 0.120 | ||||
| T1 | 113 (13.4%) | 25 (5.04%) | 24 (13.0%) | 22 (11.9%) | ||
| T2 | 6 (0.71%) | 151 (30.4%) | 5 (2.70%) | 6 (3.24%) | ||
| T3 | 345 (41.0%) | 285 (57.5%) | 133 (71.9%) | 130 (70.3%) | ||
| T4 | 69 (8.20%) | 21 (4.23%) | 9 (4.86%) | 14 (7.57%) | ||
| Unknown | 308 (36.6%) | 14 (2.82%) | 14 (7.57%) | 13 (7.03%) | ||
| N | 0.408 | 0.028 | ||||
| N0 | 348 (41.4%) | 266 (53.6%) | 86 (46.5%) | 84 (45.4%) | ||
| N1 | 219 (26.0%) | 151 (30.4%) | 55 (29.7%) | 55 (29.7%) | ||
| N2 | 126 (15.0%) | 44 (8.87%) | 27 (14.6%) | 28 (15.1%) | ||
| Unknown | 148 (17.6%) | 35 (7.06%) | 17 (9.19%) | 18 (9.73%) | ||
| Radiation | 0.139 | 0.020 | ||||
| None/Unknown | 784 (93.2%) | 443 (89.3%) | 169 (91.4%) | 170 (91.9%) | ||
| Yes | 57 (6.78%) | 53 (10.7%) | 16 (8.65%) | 15 (8.11%) | ||
| Chemotherapy | 0.122 | 0.034 | ||||
| No/Unknown | 326 (38.8%) | 222 (44.8%) | 65 (35.1%) | 68 (36.8%) | ||
| Yes | 515 (61.2%) | 274 (55.2%) | 120 (64.9%) | 117 (63.2%) | ||
| Tumor size | 0.605 | 0.118 | ||||
| ≤1 | 23 (2.73%) | 14 (2.82%) | 5 (2.70%) | 6 (3.24%) | ||
| ≤3 | 64 (7.61%) | 121 (24.4%) | 21 (11.4%) | 22 (11.9%) | ||
| ≤5 | 91 (10.8%) | 92 (18.5%) | 28 (15.1%) | 29 (15.7%) | ||
| >5 | 153 (18.2%) | 87 (17.5%) | 39 (21.1%) | 46 (24.9%) | ||
| Unknown | 510 (60.6%) | 182 (36.7%) | 92 (49.7%) | 82 (44.3%) | ||
| Histology | 0.305 | 0.140 | ||||
| Adenocarcinomas | 655 (77.9%) | 416 (83.9%) | 153 (82.7%) | 145 (78.4%) | ||
| Epithelial | 103 (12.2%) | 32 (6.45%) | 16 (8.65%) | 20 (10.8%) | ||
| Mucinous | 29 (3.45%) | 34 (6.85%) | 11 (5.95%) | 11 (5.95%) | ||
| Others | 54 (6.42%) | 14 (2.82%) | 5 (2.70%) | 9 (4.86%) | ||
Univariate Cox regression analysis for OS in the matched cohort revealed that primary tumor resection was a robust prognostic factor for patients with metastatic GBC (HR=0.62, 95% CI: 0.50–0.77, P<0.001, Table 4). Moreover, marital status (HR=0.75, 95% CI: 0.61–0.94, P=0.012), radiation (HR=0.59, 95% CI: 0.41–0.86, P=0.006), and chemotherapy (HR=0.49, 95%CI: 0.40–0.62, P<0.001) were revealed as prognostic factors for OS. Similarly, patients undergoing primary tumor resection had better CSS in the matched cohort (HR=0.61, 95% CI: 0.50–0.76, P<0.001, Table 4).
Table 4.
Univariate Cox regression analysis for assessing the effect of different clinical variables on gallbladder cancer patients’ overall survival and disease-free survival in the matched population.
| Variables | OS | CSS | ||
|---|---|---|---|---|
| HR | P value | HR | P value | |
| Sex | ||||
| Female | Reference | Reference | ||
| Male | 1.048 (0.834–1.316) | 0.688 | 1.036 (0.820–1.307) | 0.769 |
| Race | ||||
| Black | Reference | Reference | ||
| White | 0.976 (0.718–1.328) | 0.878 | 1.013 (0.738–1.391) | 0.935 |
| Other | 0.802 (0.522–1.233) | 0.314 | 0.787 (0.504–1.230) | 0.294 |
| Age | ||||
| ≤65 | Reference | Reference | ||
| >65 | 1.096 (0.889–1.351) | 0.393 | 1.074 (0.868–1.330) | 0.510 |
| Marital status | ||||
| Unmarried | Reference | Reference | ||
| Married | 0.754 (0.605–0.939) | 0.012 | 0.744 (0.595–0.931) | 0.010 |
| Unknown | 1.097 (0.706–1.703) | 0.681 | 1.064 (0.675–1.676) | 0.790 |
| Grade | ||||
| I | Reference | Reference | ||
| II | 0.699 (0.381–1.285) | 0.250 | 0.668 (0.363–1.230) | 0.195 |
| III | 1.034 (0.574–1.861) | 0.912 | 0.995 (0.552–1.793) | 0.986 |
| IV | 2.023 (0.932–4.393) | 0.075 | 2.024 (0.932–4.396) | 0.075 |
| Unknown | 0.939 (0.513–1.718) | 0.838 | 0.895 (0.489–1.641) | 0.721 |
| T | ||||
| T1 | Reference | Reference | ||
| T2 | 1.122 (0.564–2.233) | 0.744 | 1.162 (0.582–2.319) | 0.670 |
| T3 | 0.863 (0.628–1.187) | 0.365 | 0.852 (0.615–1.180) | 0.335 |
| T4 | 0.616 (0.366–1.035) | 0.068 | 0.640 (0.379–1.080) | 0.094 |
| Unknown | 0.979 (0.606–1.580) | 0.930 | 0.976 (0.599–1.591) | 0.923 |
| N | ||||
| N0 | Reference | Reference | ||
| N1 | 0.910 (0.712–1.163) | 0.450 | 0.903 (0.703–1.161) | 0.427 |
| N2 | 0.898 (0.656–1.231) | 0.504 | 0.920 (0.669–1.266) | 0.608 |
| Unknown | 1.499 (1.039–2.164) | 0.031 | 1.521 (1.048–2.209) | 0.027 |
| Radiation | ||||
| None/Unknown | Reference | Reference | ||
| Yes | 0.594 (0.410–0.861) | 0.006 | 0.624 (0.431–0.905) | 0.013 |
| Chemotherapy | ||||
| No/Unknown | Reference | Reference | ||
| Yes | 0.494 (0.397–0.616) | <0.001 | 0.484 (0.388–0.606) | <0.001 |
| Surgery | ||||
| No | Reference | Reference | ||
| Yes | 0.620 (0.502–0.766) | <0.001 | 0.614 (0.495–0.762) | <0.001 |
| Tumor size | ||||
| ≤1 | Reference | Reference | ||
| ≤3 | 0.904 (0.465–1.757) | 0.766 | 0.839 (0.430–1.640) | 0.609 |
| ≤5 | 1.064 (0.556–2.037) | 0.852 | 1.025 (0.534–1.967) | 0.940 |
| >5 | 0.867 (0.460–1.632) | 0.658 | 0.836 (0.444–1.577) | 0.581 |
| Unknown | 1.110 (0.603–2.044) | 0.738 | 1.070 (0.580–1.971) | 0.829 |
| Histology | ||||
| Adenocarcinomas | Reference | Reference | ||
| Epithelial | 1.129 (0.794–1.606) | 0.498 | 1.145 (0.801–1.637) | 0.456 |
| Mucinous | 1.052 (0.681–1.625) | 0.821 | 1.000 (0.634–1.577) | 0.999 |
| Others | 1.282 (0.748–2.196) | 0.367 | 1.336 (0.779–2.291) | 0.292 |
In addition, Kaplan-Meier curves were plotted in the matched cohort. As expected, significantly prolonged OS and CSS rates were observed in patients receiving primary tumor resection compared with those without surgery (Figure 6).
Figure 6.
Kaplan-Meier curves for (A) overall survival and (B) carcinoma-specific survival stratified by primary tumor resection in the matched cohort.
Discussion
Although GBC is relatively less common than other digestive tract malignancies, it is rather aggressive and lethal unless diagnosed and treated at the earliest stage [5,19]. Early-stage GBC is generally detected incidentally after simple cholecystectomy for assumed benign gallbladder disease, such as cholecystitis and gallbladder stones [20]. Curative surgical resection (simple cholecystectomy or extended cholecystectomy) can result in a comparatively favorable long-term survival in patients with early-stage GBC [20]. For patients with advanced or metastatic GBC, the overall prognosis is poor [21,22]. Since the prognostic benefit of surgical resection in advanced or metastatic GBC has been reported in case reports and small population-based studies [11,23–25], the application of surgical resection in advanced or metastatic GBC has gained support [11,26,27]. However, the prognostic impact of surgical resection of the primary tumor site among patients with metastatic GBC has been not assessed in a large population.
In this study, we examined the influence of primary tumor resection on patient survival in metastatic GBC. This population-based analysis revealed that resection of a primary tumor lesion could prolong the survival of patients with metastatic GBC. This finding was further supported by PSM analysis to avoid possible confounding factors, consistently showing that surgical resection of the primary tumor was significantly associated with better OS and CSS rates. Our findings agree with those of Yang et al, whose study was also based on the SEER database. However, our study differs from theirs because we included all histological types of gallbladder carcinoma, including gallbladder adenocarcinoma, and ours was a retrospective study, and the imbalanced covariate variables may have led to bias in the conclusion. Therefore, we chose to use PSM to reduce the effects of these potential factors.
One of the noteworthy characteristics of our study is that we accounted for chemotherapy in the PSM analysis, which is known to affect patient prognosis. Systemic therapy is the mainstay of the standard management of metastatic GBC [28,29]. Unsurprisingly, patients receiving chemotherapy showed significantly improved survival compared with those without chemotherapy in the unmatched and PSM-matched cohorts in our analysis. In addition, we also found that marital status was a protective prognostic factor for OS and CSS in the matched cohort (Table 4). These findings are consistent with those of other types of malignancies, including breast carcinoma, bladder carcinoma, gastrointestinal stromal tumors, and colon carcinoma [30–33]. It is generally understood that married individuals have access to more physiological and financial support than do unmarried individuals [34,35], which can, in turn, bring about survival benefit.
Our findings are consistent with several previous studies on malignancies of the gastrointestinal tract. For instance, Zhang et al reported the positive correlation between local surgery to the primary tumor and survival outcomes among patients with metastatic rectal carcinoma [36]. Similarly, to examine the impact of palliative resection of the primary tumor on survival among patients with metastatic colorectal carcinoma, Gillian et al also revealed that palliative resection of the primary lesion was significantly associated with more favorable prognosis [37]. Despite the seemingly consistent survival improvement following local therapy, the precise reasons for the association between potential prognostic benefit and primary tumor location is unclear. The etiologic explanations might be associated with several theories.
Metastasis of solid tumors occurs in a sequential manner, including local invasion, intravasation, survival in the circulation, extravasation, and colonization [38]. Primary tumors can secrete cytokines to prime a pre-metastatic niche that facilitates the formation of distant metastasis [39], which has generated the hypothesis of the beneficial effects of primary tumor treatment on retarding metastasis, even possibly prolonging survival [40]. In 2 clinical trials to elucidate the value of nephrectomy in metastatic renal cell carcinoma, patients were randomly assigned to undergo the standard systemic therapy alone or in combination with radical nephrectomy [41,42]. The median OS was significantly prolonged from 7 months to 17 months by nephrectomy [42] and from 8 months to 11 months with standard systemic therapy alone [41]. The survival benefit in these 2 clinical trials indicates that resection of the primary tumor could abolish the pro-metastatic role of primary lesions. In addition, removal of primary tumor lesions has been shown to potentially rescue the immunosuppressive status and restore immunocompetence in different animal models [43,44].
However, the opponents of primary tumor resection in patients with distant metastasis argue that the procedure of local tumor resection can delay the initiation of systemic therapy and expose patients to possible postoperative complications, both of which can affect patient survival [45]. Therefore, multidisciplinary evaluation of patients with metastatic GBC should be routinely conducted before decision-making occurs [46,47], aiming to carefully establish personalized therapeutic strategies. Additionally, owing to perioperative complications, postoperative quality of life, and medical costs, patient preference should also be taken into consideration before undertaking this procedure [36].
There are some limitations to this study. First, due to the nature of the SEER database, it was a retrospective study and selection bias was unavoidable. Although PSM was used to balance covariates between the 2 different groups, the outcomes might have been affected by other factors, such as the number of metastasis sites and the general status of patients. Specifically, we do not know if patients undergoing primary tumor resection tended to have better performance status, which could affect the evaluation of the influence of local tumor resection on patient survival. Second, detailed data on chemoradiotherapy regimens and biological targeted therapies are unavailable in the SEER database, which might also affect patient survival.
Conclusions
Collectively, this population-based, propensity matched study showed that surgical resection of the primary tumor could yield an association with favorable survival for patients with metastatic GBC, despite several inevitable limitations. Prospective randomized controlled trials should be performed to confirm our initial conclusions. Moreover, we suggest that a multi-disciplinary team should be generalized for comprehensive clinical management of patients with metastatic GBC.
Footnotes
Conflict of interest: None declared
Availability of Data and Materials
The datasets generated and/or analyzed during the current study are available in the SEER database.
Declaration of Figures’ Authenticity
All figures submitted have been created by the authors, who confirm that the images are original with no duplication and have not been previously published in whole or in part.
Financial support: None declared
References
- 1.Hundal R, Shaffer EA. Gallbladder carcinoma: Epidemiology and outcome. Clin Epidemiol. 2014;6:99–109. doi: 10.2147/CLEP.S37357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Siegel RL, Miller KD, Jemal A. Carcinoma statistics, 2019. Carcinoma J Clin. 2019;69(1):7–34. doi: 10.3322/caac.21551. [DOI] [PubMed] [Google Scholar]
- 3.Yang Y, Tu Z, Ye C, et al. Site-specific metastases of gallbladder adenocarcinoma and their prognostic value for survival: A SEER-based study. BMC Surg. 2021;21(1):59. doi: 10.1186/s12893-021-01068-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Schmidt MA, Marcano-Bonilla L, Roberts LR. Gallbladder carcinoma: Epidemiology and genetic risk associations. Chin Clin Oncol. 2019;8(4):31. doi: 10.21037/cco.2019.08.13. [DOI] [PubMed] [Google Scholar]
- 5.Downing SR, Cadogan KA, Ortega G, et al. Early-stage gallbladder carcinoma in the surveillance, epidemiology, and end results database: Effect of extended surgical resection. Arch Surg. 2011;146(6):734–38. doi: 10.1001/archsurg.2011.128. [DOI] [PubMed] [Google Scholar]
- 6.Wang X, Yu GY, Chen M, et al. Pattern of distant metastases in primary extrahepatic bile-duct carcinoma: A SEER-based study. Carcinoma Med. 2018;7(10):5006–14. doi: 10.1002/cam4.1772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zhu X, Zhang X, Hu X, et al. Survival analysis of patients with primary gallbladder carcinoma from 2010 to 2015: A retrospective study based on SEER data. Medicine (Baltimore) 2020;99(40):e22292. doi: 10.1097/MD.0000000000022292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kiran RP, Pokala N, Dudrick SJ. Incidence pattern and survival for gallbladder carcinoma over three decades – an analysis of 10301 patients. Ann Surg Oncol. 2007;14(2):827–32. doi: 10.1245/s10434-006-9224-4. [DOI] [PubMed] [Google Scholar]
- 9.Wang J, Narang AK, Sugar EA, et al. Evaluation of adjuvant radiation therapy for resected gallbladder carcinoma: A multi-institutional experience. Ann Surg Oncol. 2015;(Suppl 3):S1100–6. doi: 10.1245/s10434-015-4685-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wistuba II, Gazdar AF. Gallbladder carcinoma: Lessons from a rare tumour. Nat Rev Carcinoma. 2004;4(9):695–706. doi: 10.1038/nrc1429. [DOI] [PubMed] [Google Scholar]
- 11.Kang MJ, Song Y, Jang JY, et al. Role of radical surgery in patients with stage iv gallbladder carcinoma. HPB (Oxford) 2012;14(12):805–11. doi: 10.1111/j.1477-2574.2012.00544.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kuga D, Ebata T, Yokoyama Y, et al. Long-term survival after multidisciplinary therapy for residual gallbladder carcinoma with peritoneal dissemination: A case report. Surg Case Rep. 2017;3(1):76. doi: 10.1186/s40792-017-0351-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Somasekar R, Naganathbabu O, Prabhakaran R, et al. Salvage surgery for metastatic gall bladder carcinoma with vanishing liver metastasis following palliative 5-fluorouracil metronomic chemotherapy. J Clin Diagn Res. 2017;11(5):XD03–5. doi: 10.7860/JCDR/2017/26460.9938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tomita K, Takano K, Shimazu M, et al. Long-term survival of a recurrent gallbladder carcinoma patient with lymph node and peritoneal metastases after multidisciplinary treatments. A case report. Surg Case Rep. 2016;2(1):12. doi: 10.1186/s40792-016-0135-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Khan SA, Stewart AK, Morrow M. Does aggressive local therapy improve survival in metastatic breast carcinoma? Surgery. 2002;132(4):620–26. doi: 10.1067/msy.2002.127544. discussion 626–27. [DOI] [PubMed] [Google Scholar]
- 16.Rashid OM, Nagahashi M, Ramachandran S, et al. Resection of the primary tumor improves survival in metastatic breast carcinoma by reducing overall tumor burden. Surgery. 2013;153(6):771–78. doi: 10.1016/j.surg.2013.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kasumova GG, Tabatabaie O, Najarian RM, et al. Surgical management of gallbladder carcinoma: Simple versus extended cholecystectomy and the role of adjuvant therapy. Ann Surg. 2017;266(4):625–31. doi: 10.1097/SLA.0000000000002385. [DOI] [PubMed] [Google Scholar]
- 18.Little RJ, Rubin DB. Causal effects in clinical and epidemiological studies via potential outcomes: Concepts and analytical approaches. Annu Rev Public Health. 2000;21:121–45. doi: 10.1146/annurev.publhealth.21.1.121. [DOI] [PubMed] [Google Scholar]
- 19.Donohue JH, Stewart AK, Menck HR. The national carcinoma data base report on carcinoma of the gallbladder, 1989–1995. Carcinoma. 1998;83(12):2618–28. doi: 10.1002/(sici)1097-0142(19981215)83:12<2618::aid-cncr29>3.0.co;2-h. [DOI] [PubMed] [Google Scholar]
- 20.Yuza K, Sakata J, Prasoon P, et al. Long-term outcomes of surgical resection for t1b gallbladder carcinoma: An institutional evaluation. BMC Carcinoma. 2020;20(1):20. doi: 10.1186/s12885-019-6507-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chijiiwa K, Kai M, Nagano M, et al. Outcome of radical surgery for stage iv gallbladder carcinoma. J Hepatobiliary Pancreat Surg. 2007;14(4):345–50. doi: 10.1007/s00534-006-1186-1. [DOI] [PubMed] [Google Scholar]
- 22.Chen C, Geng Z, Shen H, et al. Long-term outcomes and prognostic factors in advanced gallbladder carcinoma: Focus on the advanced t stage. PLoS One. 2016;11(11):e0166361. doi: 10.1371/journal.pone.0166361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Miyazaki M, Itoh H, Ambiru S, et al. Radical surgery for advanced gallbladder carcinoma. Br J Surg. 1996;83(4):478–81. doi: 10.1002/bjs.1800830413. [DOI] [PubMed] [Google Scholar]
- 24.Dasari BVM, Ionescu MI, Pawlik TM, et al. Outcomes of surgical resection of gallbladder carcinoma in patients presenting with jaundice: A systematic review and meta-analysis. J Surg Oncol. 2018;118(3):477–85. doi: 10.1002/jso.25186. [DOI] [PubMed] [Google Scholar]
- 25.Rao J, Xia J, Yang W, et al. Complete response to immunotherapy combined with an antiangiogenic agent in multiple hepatic metastases after radical surgery for advanced gallbladder carcinoma: A case report. Ann Transl Med. 2020;8(23):1609. doi: 10.21037/atm-20-4420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Shimizu H, Kimura F, Yoshidome H, et al. Aggressive surgical approach for stage IV gallbladder carcinoma based on japanese society of biliary surgery classification. J Hepatobiliary Pancreat Surg. 2007;14(4):358–65. doi: 10.1007/s00534-006-1188-z. [DOI] [PubMed] [Google Scholar]
- 27.Wakabayashi H, Ishimura K, Hashimoto N, et al. Analysis of prognostic factors after surgery for stage III and IV gallbladder carcinoma. Eur J Surg Oncol. 2004;30(8):842–46. doi: 10.1016/j.ejso.2004.06.001. [DOI] [PubMed] [Google Scholar]
- 28.Zhu AX, Hong TS, Hezel AF, Kooby DA. Current management of gallbladder carcinoma. Oncologist. 2010;15(2):168–81. doi: 10.1634/theoncologist.2009-0302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Reni M, Zanon S, Balzano G, et al. A randomised phase 2 trial of nab-paclitaxel plus gemcitabine with or without capecitabine and cisplatin in locally advanced or borderline resectable pancreatic adenocarcinoma. Eur J Carcinoma. 2018;102:95–102. doi: 10.1016/j.ejca.2018.07.007. [DOI] [PubMed] [Google Scholar]
- 30.Martinez ME, Unkart JT, Tao L, et al. Prognostic significance of marital status in breast carcinoma survival: A population-based study. PLoS One. 2017;12(5):e0175515. doi: 10.1371/journal.pone.0175515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Tao L, Pan X, Zhang L, et al. Marital status and prognostic nomogram for bladder carcinoma with distant metastasis: A seer-based study. Front Oncol. 2020;10:586458. doi: 10.3389/fonc.2020.586458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Song W, Tian C. The effect of marital status on survival of patients with gastrointestinal stromal tumors: A seer database analysis. Gastroenterol Res Pract. 2018;2018:5740823. doi: 10.1155/2018/5740823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Yang CC, Cheng LC, Lin YW, et al. The impact of marital status on survival in patients with surgically treated colon carcinoma. Medicine (Baltimore) 2019;98(11):e14856. doi: 10.1097/MD.0000000000014856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Du KL, Bae K, Movsas B, et al. Impact of marital status and race on outcomes of patients enrolled in radiation therapy oncology group prostate carcinoma trials. Support Care Carcinoma. 2012;20(6):1317–25. doi: 10.1007/s00520-011-1219-4. [DOI] [PubMed] [Google Scholar]
- 35.Aizer AA, Chen MH, McCarthy EP, et al. Marital status and survival in patients with carcinoma. J Clin Oncol. 2013;31(31):3869–76. doi: 10.1200/JCO.2013.49.6489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Zhang CH, Pan YB, Zhang QW, et al. The influence of local therapy on the survival of patients with metastatic rectal carcinoma: A population-based, propensity-matched study. J Carcinoma Res Clin Oncol. 2017;143(9):1891–903. doi: 10.1007/s00432-017-2442-2. [DOI] [PubMed] [Google Scholar]
- 37.Gresham G, Renouf DJ, Chan M, et al. Association between palliative resection of the primary tumor and overall survival in a population-based cohort of metastatic colorectal carcinoma patients. Ann Surg Oncol. 2014;21(12):3917–23. doi: 10.1245/s10434-014-3797-0. [DOI] [PubMed] [Google Scholar]
- 38.Nguyen DX, Bos PD, Massague J. Metastasis: From dissemination to organ-specific colonization. Nat Rev Carcinoma. 2019;9(4):274–84. doi: 10.1038/nrc2622. [DOI] [PubMed] [Google Scholar]
- 39.Psaila B, Lyden D. The metastatic niche: Adapting the foreign soil. Nat Rev Carcinoma. 2009;9(4):285–93. doi: 10.1038/nrc2621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Morgan SC, Parker CC. Local treatment of metastatic carcinoma – killing the seed or disturbing the soil? Nat Rev Clin Oncol. 2011;8(8):504–6. doi: 10.1038/nrclinonc.2011.88. [DOI] [PubMed] [Google Scholar]
- 41.Flanigan RC, Salmon SE, Blumenstein BA, et al. Nephrectomy followed by interferon alfa-2b compared with interferon alfa-2b alone for metastatic renal-cell carcinoma. N Engl J Med. 2001;345(23):1655–59. doi: 10.1056/NEJMoa003013. [DOI] [PubMed] [Google Scholar]
- 42.Mickisch GH, Garin A, van Poppel H, et al. European Organisation for Research and Treatment of Cancer (EORTC) Genitourinary Group. Radical nephrectomy plus interferon-alfa-based immunotherapy compared with interferon alfa alone in metastatic renal-cell carcinoma: A randomised trial. Lancet. 2001;358(9286):966–70. doi: 10.1016/s0140-6736(01)06103-7. [DOI] [PubMed] [Google Scholar]
- 43.Danna EA, Sinha P, Gilbert M, et al. Surgical removal of primary tumor reverses tumor-induced immunosuppression despite the presence of metastatic disease. Carcinoma Res. 2004;64(6):2205–11. doi: 10.1158/0008-5472.can-03-2646. [DOI] [PubMed] [Google Scholar]
- 44.Piranlioglu R, Lee E, Ouzounova M, et al. Primary tumor-induced immunity eradicates disseminated tumor cells in syngeneic mouse model. Nat Commun. 2019;10(1):1430. doi: 10.1038/s41467-019-09015-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Chen JN, Shoucair S, Wang Z, et al. Primary tumor resection for rectal carcinoma with unresectable liver metastases: A chance to cut is a chance for improved survival. Front Oncol. 2021;11:628715. doi: 10.3389/fonc.2021.628715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Valle JW, Borbath I, Khan SA, et al. Biliary carcinoma: Esmo clinical practice guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2016;27(Suppl 5):v28–v37. doi: 10.1093/annonc/mdw324. [DOI] [PubMed] [Google Scholar]
- 47.Chakedis J, Schmidt CR. Surgical treatment of metastatic colorectal carcinoma. Surg Oncol Clin N Am. 2018;27(2):377–99. doi: 10.1016/j.soc.2017.11.010. [DOI] [PubMed] [Google Scholar]