Abstract
Pancreatic cancer is a disease with high unmet clinical need. Pancreatic cancer is also characterised by an intense fibrotic stroma, which harbours many immune cells. Studies in both human and animal models have demonstrated that the immune system plays a crucial role in modulating tumour onset and progression. In human pancreatic ductal adenocarcinoma, high B-cell infiltration correlates with better patient survival. Hence, B cells have received recent interest in pancreatic cancer as potential therapeutic targets. However, the data on the role of B cells in murine models is unclear as it is dependent on the pancreatic cancer model used to study. Nevertheless, it appears that B cells do organise along with other immune cells such as a network of follicular dendritic cells (DCs), surrounded by T cells and DCs to form tertiary lymphoid structures (TLS). TLS are increasingly recognised as sites for antigen presentation, T-cell activation, B-cell maturation and differentiation in plasma cells. In this review we dissect the role of B cells and provide directions for future studies to harness the role of B cells in treatment of human pancreatic cancer.
Keywords: B cells, Pancreatic cancer, Cancer immunology, Tertiary lymphoid structures, Anti-tumour immunoglobulins, Plasma cells
Core Tip: The role of B cells in pancreatic ductal adenocarcinoma tumorigenesis is controversial. Human studies show clusters of B cells, interacting with other immune cells, forming active sites of the immune response, called tertiary lymphoid structures. In vitro experiments and in vivo studies using B-cell deficient mice suggest the role of an immuno-suppressive B cell phenotype to induce tumour-progression. These discordant findings highlight the need of further studies using better murine models to recapitulate pancreatic cancer and its immune infiltrate.
INTRODUCTION
Pancreatic cancer and its immune infiltration
The majority (about 95%) of pancreatic cancers (adenocarcinomas) arise from the exocrine pancreas, most likely from the epithelial cells lining the pancreatic duct, to form gland-like structures, and hence, are commonly referred to as pancreatic ductal adenocarcinoma (PDAC), though mucinous tumours are the second most common histological type of pancreatic cancer[1]. PDAC is the gastrointestinal tumour with the poorest prognosis, with 80% of the patients presenting with advanced disease. A mere 15%-20% of the patients are suitable for surgical resection, which currently represents the only curative option for pancreatic cancer. For advanced PDAC, the most common systemic treatment is single-agent gemcitabine which is increasingly being replaced with a combination of chemotherapeutics (e.g., FOLFIRINOX or gemcitabine-nab-paclitaxel), at least in patients with good performance status as first-line treatment[2]. Although immunotherapies have gained success in other cancers, there are no approved immunotherapies for PDAC[2].
Many immuno-therapeutic approaches are under investigation for PDAC. Immune-checkpoint inhibition has shown clinical benefit in 2% of PDAC patients harbouring a DNA mismatch repair (MMR) deficiency[3,4]. Vaccination strategies are also being tested including “personalised” dendritic cell (DC)-vaccines loaded with the antigen[5,6]. GVAX [granulocyte-macrophage colony-stimulating factor (GM-CSF)-secreting, allogeneic PDAC vaccine, NCT01417000, NCT00727441, NCT00084383] are being investigated further[7,8]. Furthermore specific use of immune cells is being explored by adoptive transfer of T cells carrying chimeric antigen receptors[9-12], or recover the immuno-suppression and chemo-sensitivity using Ibrutinib, the inhibitor of Bruton’s tyrosine kinase (BTK), a member of the B-cell receptors (BCR) signalling pathway (NCT02436668), targeting regulatory B cells and macrophages.
PDAC is conventionally known as a “cold tumour”, due to low inflammatory cytokine profile and hypoxia, low mutational load and exclusion of infiltrating lymphocytes[13,14]. Recent research has identified an “immunogenic subtype” enriched in genes associated to B-cell signalling, CD4+ and CD8+ T cells, and antigen presentation[13]. Furthermore, the combination of genetic, stromal, and immunological features of PDAC can lead to further definition of novel immune-subtypes which may have prognostic value and the possibility of identifying tumours with immuno-therapeutic potential[15]. Whilst spatial distribution and infiltration of T cells and the formation of clusters with B cells is associated with better outcome in human and murine models of PDAC[16,17], in vivo studies of B cell depletion in murine models of PDAC describe a pro-tumorigenic role of B cells[18-21]. These discordant findings can be ascribed to the different tumour sub-types analysed and to the use of dissimilar murine models. For example, mice that are genetically lacking in B cells might behave differently to those where depletion of B cells is conducted by a depleting antibody[22]. In this review, we critically discuss the evidence for the perceived dichotomous role of B cells in pancreatic cancer.
TUMOUR-SUPPRESSING ROLE OF B CELLS IN SOLID CANCERS
Immuno-histochemical analysis using CD20, and metagene analysis for B-cell signature, showed a positive correlation between B-cell infiltration and patient prognosis in many different cancer types. For example, work in primary cutaneous melanoma (n = 106, immunostaining, multivariate analysis) demonstrated that intra- and peri-tumoral B cells are important, in particular CD20+/OX40+ cell density[23]. In high-grade serous ovarian cancer (n = 70, immunostaining of tissues and FACS of peripheral blood), suggested a role for CD27-memory B cells[24]. In basal-like breast cancer (n = 728, breast cancer, TCGA dataset, B-cell mRNA signature) and non-small cell lung cancer (NSCLC) (n = 74, untreated patients with early-stage NSCLC and 122 patients with treated advanced-stage NSCLC; immunostaining and FACS analysis) demonstrated a prognostic value for follicular B cells[25,26]. In sarcoma (n = 608, soft-tissue sarcomas; gene expression profiles) led to the identification of different immuno-phenotypes, and the B-cell enriched demonstrated improved survival and response to immunotherapy[27]. Some studies included the organisation of tumour-infiltrating B cells into tertiary lymphoid structures (TLS) in addition to the B-cell density[28,29]. B cells are known to act as antigen-presenting cells (APCs) or antibody-producing cells[30]. Thus, presence of B cells or at least their subsets or organisation within cancer tissues seem to confer prognostic benefit suggesting a role for humoral immunity in the anti-tumour response mounted by the host[31].
Tumours can express antigens recognised as non-self by the immune system to induce a specific anti-tumour immune response, collectively referred to as the “cancer immunome”[32]. In this context, B cells with high affinity for a specific tumour-associated antigen (TAA), engulf and process the antigen to display it on their cell surface; thus, acting as APCs. This complex is recognised by activated T helper cells, which induce B-cell proliferation and clonal expansion. Some B cells may serve as memory cells whilst others act as effector cells that differentiate into antibody-producing plasma cells[33]. The antibody–TAA binding also initiates the destruction of the tumour cells expressing the TAA by several mechanisms, such as opsonisation and macrophage recognition and phagocytosis, or blocking of the receptors associated with tumour cell proliferation and survival, or uptake via Fcγ receptors, leading to antigen cross-presentation and vigorous CD4+ and CD8+ T cell responses, complement-dependent cytotoxicity (CDC), or antibody-dependent cellular cytotoxicity (ADCC).
Antibody-production
B cell affinity maturation and differentiation to plasma cells have been described within TLS in several cancers, in addition to the usual places of maturation such as lymph nodes[34]. Tumour-specific B cells may acquire somatic hyper-mutations (SHMs) in TLS and extra-follicular B cells maturation has been described[35-37]. Furthermore expansion of tumour-specific B cells without SHMs may reflect a mechanism of T cell-independent or T cell-dependent but germinal centre-independent B cell activation[38]. For example, in gastric cancer, tumour-infiltrating B cells showed broad variations in the degrees of SHMs, with some producing functional antibodies directed against sulfated glycosaminoglycan with, at least, tumour growth-suppressive properties in vitro[39].
Since cancer is driven by mutations in “self-proteins”, cancer-associated auto-antibodies are detectable[40]. These antibodies may be in response to “self-antigens” which are either over-expressed [e.g., human epidermal growth factor receptor 2 (HER2/neu)] or aberrantly expressed (e.g., cancer-testis antigen) during tumorigenesis. Mechanisms for secretion of cancer-related auto-antibodies include changes in the expression levels, altered protein structures, presentation of dying tumour cells (due to chemo/radiotherapy for example) to the immune system leading an abnormal exposure of autologous intracellular antigens[40]. Antigen load and duration of exposure may induce humoral immune responses since antibodies against several TAA (such as p53, New York esophageal squamous cell carcinoma-1 (NY-ESO-1), surviving, tyrosinase) were more frequently found in advanced tumour stages[41]. Antibodies produced by tumour infiltrating B cells may induce lysis of cancer cells by ADCC or CDC, leading to the direct killing of the cancer cells[42]. Murine models demonstrate binding of tumour B-cell antibodies to mouse tumours in an antigen-specific manner and complement-dependent lysis[43-45]. Binding of C3 components to CD21 (the complement receptor 2) induces B-cell activation to promote anti-cancer responses[46].
Promoting T cell response
B cells may represent the most abundant APC since DCs are scarce in the tissue[47]. Tumour infiltrating B cells can also provide antigen-independent help to cytolytic T cells (CTLs) within the tumour, by interaction between CD27 expressed on helper B cells and CD70 on CTLs, promoting their antigen-independent survival and proliferation of T cells[48].
Activation of bystander B cells
B cells can also be stimulated by transactivation of bystander B cells not in direct contact with the antigen, via transfer of human leukocyte antigen-peptide complexes or BCRs contained in exosomes or cytonemes[49]. These activated bystander B cells can per se produce antibodies and/or serve as APC, but also release T-cell activating cytokines, thus amplifying the cellular and humoral immune response, even with a limited antigen load[49].
Interaction with T follicular helper cells, in intra-tumour TLS
Presence of TLS within the tumour parenchyma correlates with better patient survival[50,51]. Within TLS, B cells in close proximity to T cells and interact with T follicular helper cells and follicular DCs and promote germinal centre (GC) reaction, which results in B-cell differentiation into memory B cells and long-term surviving plasma cells. Within TLS, B cells can act as APCs and produce anti-tumour antibodies, exhibiting tumour-specific humoral responses in situ[26,36,37]. NSCLC-infiltrating B cells were shown to produce in vitro immunoglobulin (Ig) G and IgA directed against tumour antigens (MAGE, LAGE-1, NY-ESO-1, P53)[26]. Micro-dissected TLS-derived B cells from breast cancer showed poly-clonality and high mutation rate, suggestive of an affinity maturation occurring within TLS[36]. Moreover colorectal cancer-infiltrating B cells were shown to produce IgG which bound epitopes on the cell membrane of different tumour cell lines[37,52]. TLS may also be artificially induced by neo-adjuvant treatment such as with anti-programmed cell death protein (PD) 1 in NSCLC, or vaccination against human papilloma virus (HPV) in cervical cancer patients[53,54]. Furthermore, presence of TLS is associated with response to immuno-therapy in NSCLC, melanoma and sarcoma patients[27,50,55,56].
TUMOUR-PROMOTING ROLE OF B CELLS IN SOLID CANCERS
Immuno-histochemical characterisation of the tumoral immune infiltrate has shown a negative correlation between B cell/plasma cell infiltration with patient survival in melanoma, prostate cancer, lung cancer and ovarian cancer[57-60]. Furthermore, the detection of tumour specific (auto)-antibodies in the sera of cancer patients was associated with poor prognosis[61]. Depending on the tumour type studied and murine model investigated, a number of mechanisms for the pro-tumorigenic nature of B cells have been suggested.
Antibody production
Whilst several human studies show a positive correlation between antibodies directed against Her2/neu or mucin 1 (MUC-1) with favourable patient prognosis, high serum anti-p53 antibody levels are associated with poor prognosis[61-64]. It has been speculated that this may be due to high antigen load and exposure rather than a reflection of poor immune activity. Antibodies activate the complement system once they have bound the antigen in the immune-complexes[65]. However, murine studies showed that, counter-intuitively, some antibodies might contribute to the progression of tumours by formation of circulating immune-complexes (CICs). These CICs can bind to myeloid cells within tumours, and activate their Fcγ receptors to induce myeloid suppressor cell activity which promotes tumorigenesis[65,66]. Immune-complexes formation can lead also to the activation of complement cascades resulting in formation of C3 and C5a anaphylatoxins, which can induce the recruitment of inflammatory cells which, in turn, may provide a rich pro-angiogenic and pro-tumoral environment[66]. Deposition of complement components per se does not induce chronic inflammation during tumorigenesis in HPV16/recombination activating gene 2-/- murine model of skin cancers. However, transfer of competent B cells as well as serum from immuno-competent animals could enhance pre-malignant to malignant transformation for skin cancer, raising the speculation that B-cell derived antibodies home into the neoplastic tissue and activate the complement cascade, mediating recruitment of innate immune cells; thus, modulating a tumour-promoting chronic inflammation[66].
Furthermore, different IgG subclasses have distinct biological function[67]. IgG4 is associated with chronic antigen exposure, typical of cancer disease, and in vitro and in vivo studies have demonstrated that this subclass counteracts anti-tumour immunity by antagonising IgG1-mediated immunity[68]. The presence of IgG4 in tumour microenvironment (TME) not only prevents IgG1-FcR-mediated effector functions, contributing to tumour evasion to humoral immunity, but could also impair therapeutic antibody effector function[69].
Cytokine production by B cells
B cells have been shown to directly inhibit cytotoxic T-cell responses in several tumour models via the production of B-cell-derived factor[70,71]. The negative correlation between high tumour-infiltrating B cells and prognosis in prostate cancer was ascribed to the production of lymphotoxin by tumoral B cells recruited by chemokine (C-X-C motif) ligand 13 (CXCL13) signalling, after androgen ablation by castration in a mouse prostate cancer model[58,72]. Lymphotoxin activates non-canonical and canonical nuclear factor kappa-B signalling and signal transducer and activator of transcription 3 in the remaining cancer cells, resulting in androgen-refractory growth and tumour progression[72].
B-regulatory functions
Akin to T-cell subtypes, phenotypically and functionally distinct B-cell subpopulations have been identified. In presence of chronic exposure to the antigen and chronic inflammation, such auto-immune encephalomyelitis or colitis, and cancer, B cells may acquire a regulatory phenotype[73-75]. This subset of B cells have been shown to have immunosuppressive properties, alongside with myeloid-derived suppressor cells or T-regulatory cells (Tregs), thus expanding the team of the suppressive immune players within the TME[76,77]. These B-regulatory cells act as tumour promoters by affecting the function of other immune cells, through immunosuppressive factors, such as transforming-growth factor (TGF)-β, interleukin (IL)-4, and IL-10, which are associated with Th2 skewing of T cells, IL-13 and IL-35, that support tumour-cell growth as well as M2 polarisation of tumour-associated macrophages (TAMs). Moreover, immunosuppression is further induced through PD1 expression, which, by binding to PD-L1 on the surface of tumour cells, can abrogate tumour recognition and killing. In addition to these indirect mechanisms, B regulatory cells can be directly pro-tumourigenic, for example, B-cell derived TGF-β promotes epithelial-mesenchymal transition in colorectal cancer, or through CD40/CD154 signalling pathway drives primary liver cancer[78,79]. These distinct B-cell phenotypes and mechanisms may account for the paradoxical tumour-promoting role of B cells observed in human studies and murine models.
However, the depletion of B cells using a B-cell depleting antibody, for treatment of renal cell carcinoma, melanoma or colorectal cancer, did not show any clinical benefit[80,81]. In particular, in an old early phase clinical trial involving patients with advanced colorectal cancer (n = 14), a reduction of the tumour size was observed after treatment with Rituximab, a humanised monoclonal antibody directed against human CD20, and was associated with a reduction of hyper-positive CD21 B cells in peripheral blood[81]. Surprisingly, this observation has not been further explored in later phase clinical trials. Nevertheless this observation is substantiated by in vivo studies using syngeneic tumour implantation models[82]. The vast majority of these studies, using genetically deficient murine models for B cells, show that B-cell infiltration within the TME produces worse outcomes in mouse models[71]. In contrast, acute B-cell depletion using anti-CD20 antibody did not recapitulate these findings[83]. It is important to note that B-cell deficient mice manifest several secondary immune abnormalities that may contribute to their tumour-suppressive phenotype[83].
B cell exhaustion
Akin to T cell exhaustion, recent reports describe a reversible state of B cell dysfunction, different from anergy and senescence, named B-cell exhaustion. Exhausted B cells, identified in viremic HIV patient blood[84], and described in older and auto-immune patients[85], are phenotypically characterised by low CD21 and CD27 expression, high expression of inhibitory receptors, and deficient effector functions[86]. In NSCLC and breast cancer, exhausted B cells, also named tissue-like memory B cells, were found to correlate with T regulatory cells and exhausted PD1+ CD4+/CD8+ T cells[87,88].
B CELLS IN PDAC
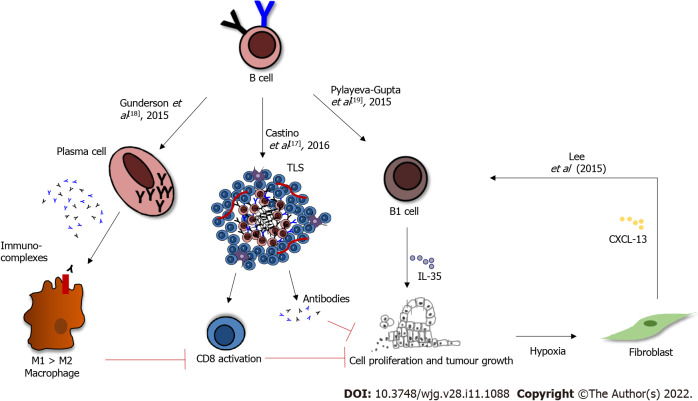
Similar to other cancers, the role of B cells in pancreatic cancer is controversial, perhaps due to model selection in various studies. B cells are generally associated with an improved outcome in PDAC patients[14], and yet often, their presence correlates with tumour growth and shorter survival in murine models of pancreatic cancer, with various mechanisms offered for this pro-tumorigenic role[18-21]. For example, immunoglobulins produced by splenic B cells may form immune complexes, that can bind TAMs and induce M2 polarisation, consequently suppressing the CD8+ T-cell cytotoxic activity; thus, driving tumour progression (Figure 1). Both B cells and macrophages were shown to express activated Bruton’s tyrosine kinase (BTK). In vitro the use of the BTK inhibitor Ibrutinib blocked the M2 polarisation of macrophages that occurred following co-culture with B cells, suggesting that B cells promote the pro-tumorigenic macrophage phenotype, and that BTK signalling is tumour-promoting in both these immune cell types. The use of the BTK inhibitor Ibrutinib in orthotopic pancreatic tumours in vivo reduced tumour growth[18]. A subset of regulatory B cells, called B1 (identified as CD1dhi , CD5+, 10% of all B cells within the murine tumours), express relatively high levels IL-12a and Ebi3 transcripts, which encode for IL-35: An immunosuppressive cytokine (Figure 1)[19]. The injection of IL12a-/- B cells was unable to restore tumour growth, implying that B-cell-derived IL-35 drives tumour cell proliferation[19]. Moreover in the presence of hypoxia, induced by HIF1α deletion, fibroblasts secrete CXCL13, which recruits B cells to the tumour site; in particular the B1 regulatory B cells, which promote tumour growth (Figure 1)[20].
Figure 1.
B-cell role in pancreatic cancer. B cells mature in plasma cells, which can produce immunoglobulin G, and are able to reprogram the M1 macrophage phenotype to M2 via Bruton’s tyrosine kinase activation. B regulatory cells are able to produce immune-suppressive cytokines, which inhibit the anti-tumour immune response, leading to tumour growth. Furthermore, in presence of hypoxia, stromal fibroblasts can secrete chemokine (C-X-C motif) ligand 13, which recruit B regulatory cells (CD1dhiCD5+) and B1 B cells, resulting in faster tumour growth. Clusters of B cells, with follicular dendritic cells and T cells, are sites for T cell priming and B cell maturation and differentiation into antibody-producing cells, with anti-tumoral effect. TLS: Tertiary lymphoid structures; IL-35: Interleukin-35; CXCL-13: Chemokine (C-X-C motif) ligand 13.
However, these immuno-suppressive B cells represent a mere 10% of the entire B-cell population in PDAC. Therefore, their pro-tumorigenic role might be overcome by the presence of a much larger proportion of pro-inflammatory B cells. The genetic analysis of bulk intra-tumoral B-cell population showed a pro-inflammatory and immuno-stimulatory phenotype in both orthotopic and the KPC (KrasG12D-Pdx1-Cre) genetic models of PDAC[22]. Indeed, the phenotype of splenic B cells differs from the intra-tumour B-cell phenotype[22]. Since Gunderson and colleagues used splenic and not intra-tumour B cells, in co-culture experiments with bone-marrow-derived macrophages, the immune-suppressive role of B-cells described by them might be irrelevant within the tumour microenvironment[18]. Furthermore, in independent experiments, it appears that the regulatory phenotype is not acquired in the tumour microenvironment. CD1dhi CD5+ B cells isolated from a healthy spleen and injected into a μMT mice (genetic depletion of B cells from birth) before orthotopic cancer cell injection rapidly restored tumour cell growth[19].
Interestingly, most studies investigating the role of B cells in cancer immunity were conducted in B-cell-deficient mice, where the absence of B cells restricted tumour growth in a variety of tumour models, generally suggesting that B cells inhibit rather than enhance spontaneous anti-tumour immunity[18-20,82,83,89]. On the other hand, the majority of models using an acute B-cell depletion in an established tumour (for example, achieved by treatment with a B-cell depleting antibody, anti-CD20) enhanced tumour growth, suggesting that B cells may have an anti-tumoral role[89-91]. Since, this anti-tumoral aspect is not confirmed if B-cell depletion occurs before initiation of tumour growth, we can speculate that B cells play an initial immunosuppressive/pro-tumoral role; perhaps a role played by circulating or peripheral B cells. However, over the course of tumour development, as B cells infiltrate tumours, they form TLS and acquire a pro-inflammatory phenotype that sustains DC recruitment and activation and antigen presentation, resulting in an anti-tumoral role[14,89,92]. Of note, depletion of B cells earlier in PDAC development in a more relevant pre-clinical model of PDAC, KPC (KrasG12D-Pdx1-Cre) mice, did not impact disease progression[22]. In contrast, B-cell compartment is competent before and during human PDAC tumourigenesis. Lastly, it is now well understood that B-cell-deficient murine models harbour several immune abnormalities, such as defects in myeloid subsets, which may render those mice tumour-resistant[93]. Therefore, acute B-cell depletion in tumour-bearing mice may represent a more reliant model to study the effect of B cells in cancer (Figure 1) [14,22,92].
Based on these considerations, B-cell depletion may prevent TLS formation, suggesting that removing B cells in PDAC patients may be detrimental, as the tumours are deprived of sites of DC localisation and anti-tumour immune response[34,94]. Presence of TLS has been shown to be associated with improved patient survival in PDAC[16,17,95]. The location of TLS (peri-tumoral and intra-tumoral) may be important since those with intra-tumoral TLS had better outcome[16]. PDAC tissues with intra-tumoral TLS showed significantly higher infiltration of T and B cells and lower infiltration of immunosuppressive cells, as well as significantly higher expression of Th1- and Th17-related genes.
It is possible that the dual behaviour of B cells in non-metastatic PDAC patients is dependent on their spatial organisation[17]. Favourable clinical outcome was observed when B cells were organised in TLS, whilst worse patient survival was observed when B cells were scattered at the tumour-stroma edge. The two studies show a different TLS distribution, probably due at the different approaches used for the identification. Hiraoka et al[16] demonstrate a near-universal presence of TLS within human PDAC tissue based on H&E staining, whilst Castino et al[17] identify the aggregate pattern only in a subset of patients. This apparent discrepancy, described also in other cancers, can be resolved thorough TLS functional characterisation, such as activation status and composition, through use of key phenotypic markers; thus, rendering them more useful in predicting patients’ outcome[95-97].
In the KPC transgenic murine model, more closely mimicking human cancer, sporadic presence of TLS was observed, but in the orthotopic model of PDAC, lacking the characteristic desmoplastic stroma, TLS were not observed[17]. Not only TLS developed spontaneously within the tumour parenchyma of the KPC mice, but also their formation could be enhanced by injection with the immunotherapeutic DNA-vaccine encoding the glycolytic enzyme ENO1. The vaccination induced a higher number of TLS, PD1+ GC formation and increased antigen-specific T-cells infiltration[17]. Furthermore injection of chemokine (C-C motif) ligand 21 (CCL21) in a subcutaneous PDAC murine model showed a beneficial effect, by inhibiting tumour growth, decreasing distant metastasis, and recruiting T and DCs within the TME[98]. In keeping with these observations, NSCLC patients are receiving intra-tumoral injections of CCL21-transduced autologous DCs in a phase I clinical trial (NCT00601094, NCT01574222)[99].
Several studies report the development of TLS after anti-tumour vaccination protocols, including pancreatic cancer[6,95,99]. Lutz et al[95] used an irradiated, GVAX given as a single agent or in combination with low-dose cyclophosphamide to deplete regulatory T cells, showing a way to convert a “non-immunogenic” neoplasm such as PDAC, into an “immunogenic” neoplasm, by inducing infiltration of T cells and development of TLS in the TME. The study describes the presence of TLS as defined by a core of B cells and follicular DCs, Ki67 positivity, suggesting the presence of a germinal centre, and CD3+ T cells. Among these, there were CD4+ cells in close vicinity to mature DCs (CD83+ and DC-LAMP+), and monocyte/macrophages, suggesting that these aggregates exhibited adaptive immunity[95]. A better characterisation of the T-cell subsets suggested the presence of negative regulatory signals in the aggregates: most of the aggregates presented FoxP3+ cells and upregulated PD-L1 expression. Thus, the activities of GVAX included both the recruitment of effector T cells into the TME and the upregulation of immunosuppressive regulatory mechanisms, specifically the expression of PD-L1 and T-regs infiltration. But the net impact of the infiltration of both T-effector (T-eff) and Tregs, expressed as ratios of interferon γ-producing Teff/Tregs, were higher in vaccinated patients, suggesting that GVAX can alter the balance of T-eff and T-regs, in favour of an anti-tumour response. The number of TLS resulted increased after combination of GVAX with cyclophosphamide[95].
TLS are also known to be site for the formation of antigen-specific B cells and development of memory response and represent an “antibody factory” within non-lymphoid tissues. Intra-tumoral B cells have been shown to produce high-affinity anti-tumour antibodies, mostly IgG, in several human and murine model studies, providing evidence that tumour-specific humoral responses can be generated in situ, within TLS[26,36,37,52]. Such evidence of humoral response is provided by the presence of germinal centres and follicular DC network in PDAC-associated TLS[16]. Furthermore there is evidence of antibody production by intra-tumour derived B cells in PDAC[100]. IgG production against wild type and mutant KRAS targets (a common occurrence in human PDAC) was assessed to study the antigen specificity of PDAC infiltrating B cells[100]. Incubation of tumour infiltrating B-cell supernatant suggests that B-cell responses targeting mutant and not wild-type KRAS are present in the parenchyma of PDAC, yet not detectable in the serum[100].
The identification of TAA-directed immunoglobulins in PDAC would be of great use in cancer therapy. For example, IgG1 antibody PAM4, identified by vaccination of mice with mucin purified from human pancreatic cancer cells, has been applied in radio-immunotherapy and diagnosis[101]. In PDAC, serum titre of MUC-1 specific immunoglobulins correlates with improved patients survival[62]. Examples of anti-MUC1 antibody-based therapeutics developed against pancreatic cancer and that are in clinical trials are huPAM4, PankoMab-GEX (Gatipotuzumab), AR20.5[102-104]. Many other pancreatic cancer specific antigens could serve as valid clinical targets[11,105-108].
CONCLUSION
B cells play a different role in human and murine cancers. In PDAC, high B-cell infiltrate is associated with better prognosis, especially when those B-cells cluster in TLS (Figure 2). Yet this is discordant with data obtained using orthotopic models of PDAC, where B-cell depletion suggests an early, pro-tumour function of B cells (Figure 2). This apparent paradox can be explained with B cells playing different roles as the tumour progresses and evolves. Firstly, there are differences between intra-tumour and peripheral immune-responses, as demonstrated by in vivo studies in both PDAC[22] and other cancers[109,110]. The more complex cell-cell interactions within the TME may influence B cell phenotype. There are inherent difficulties to recapitulate these features in murine models where the desmoplasia, a characteristic feature of human PDAC may not always be present[111-113]. Furthermore, as with T cells, multiple B-cell subsets have been extensively described in murine models of cancer, but not in human PDAC; and this would be the new frontier of investigation. Despite the current failure of immunotherapy in PDAC, exploring new successful immuno-therapeutic avenues may still be possible. For example, immuno-therapy with immune-checkpoint inhibitors appears effective in the small percentage of PDAC patients harbouring MMR deficiency. Target immunotherapy should be considered for the different PDAC (immune)-subtypes, and should aim to enhance the potential in situ anti-tumour response, which arises within some tumours (TLS+ve patients), with a possibility to revert the immune-suppressive TME. Current immuno-therapeutics under investigation in PDAC in relation to B-cell modulation, include promoting the anti-tumour response [the GVAX vaccine induces the in situ formation of active clusters of T and B cells (TLS)] or inducing the immuno-tolerance (Ibrutinib, BTK inhibitor). Combination with other stromal modulating approaches may yield substantial benefits[111,114,115]. An extensive immuno-genetic and immuno-phenotypic profiling of tumour infiltrating B cells may pave the way towards the understanding of integrated tumoral immune system in PDAC and generate crucial new therapeutic insights.
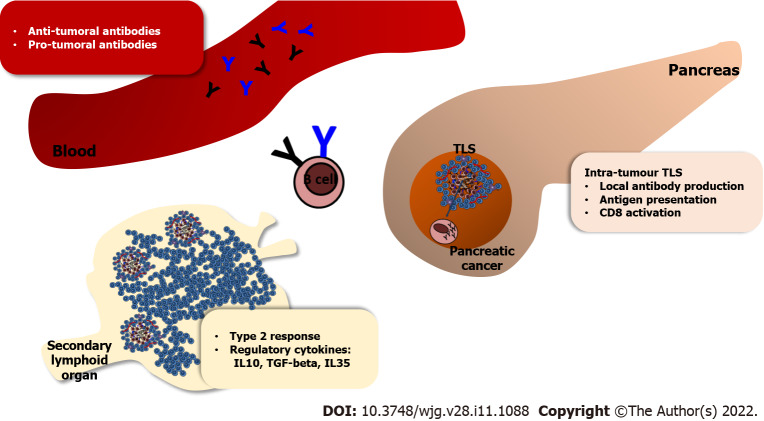
Figure 2.
Mechanisms by which B cells regulate tumour growth. Different conclusions drawn from human and mouse studies can be accommodated within this model which takes into account the different intra-tumour and peripheral immune-responses. In vitro and in vivo studies usually measure the functional immune response in secondary lymphoid organs or blood, rather than studying the infiltration and the spatial organisation of different immune cells within the tumour microenvironment. In the pancreas, B cells can form clusters with T cells, named tertiary lymphoid structures, which are sites of antigen presentation, CD78 activation and antibody production. However, in secondary lymphoid organ the presence of B cells during T cell priming can skew the immune response towards Th2, attenuating Type 1 response. Furthermore, B-regulatory cells can produce immune-suppressive cytokines, which inhibit the anti-tumour immune-response. Finally, a positive correlation is found between serum immunoglobulin G (IgG) 1 and increased survival. However, repeated isotype switching within IgG subclasses generates in human IgG4, an isotype that has been linked to regulatory functions, in mouse models IgG2a, with pro-inflammatory functions. TLS: Tertiary lymphoid structures; IL: Interleukin; TGF-beta: Transforming-growth factor-β.
Footnotes
Conflict-of-interest statement: There are no conflicts of interest to declare.
Provenance and peer review: Invited article; Externally peer reviewed.
Peer-review model: Single blind
Peer-review started: April 9, 2021
Article in press: February 19, 2022
Specialty type: Oncology
Country/Territory of origin: United Kingdom
Peer-review report’s scientific quality classification
Grade A (Excellent): A
Grade B (Very good): 0
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Li C S-Editor: Chang KL L-Editor: A P-Editor: Chang KL
Contributor Information
Francesca Romana Delvecchio, William Harvey Research Institute, Queen Mary University of London, London EC1M 6BQ, United Kingdom; Barts Cancer Institute, Queen Mary University of London, London EC1M 6BQ, United Kingdom.
Michelle R Goulart, Barts Cancer Institute, Queen Mary University of London, London EC1M 6BQ, United Kingdom.
Rachel Elizabeth Ann Fincham, Barts Cancer Institute, Queen Mary University of London, London EC1M 6BQ, United Kingdom.
Michele Bombadieri, William Harvey Research Institute, Queen Mary University of London, London EC1M 6BQ, United Kingdom.
Hemant M Kocher, Barts Cancer Institute, Queen Mary University of London, London EC1M 6BQ, United Kingdom; Barts and the London HPB Centre, Barts Health NHS Trust, London E1 1BB, United Kingdom. h.kocher@qmul.ac.uk.
References
- 1.Kocher HM, Alrawashdeh W. Pancreatic cancer. BMJ Clin Evid. 2010;2010 [PMC free article] [PubMed] [Google Scholar]
- 2.Neuzillet C, Rousseau B, Kocher H, Bourget P, Tournigand C. Unravelling the pharmacologic opportunities and future directions for targeted therapies in gastro-intestinal cancers Part 1: GI carcinomas. Pharmacol Ther. 2017;174:145–172. doi: 10.1016/j.pharmthera.2017.02.028. [DOI] [PubMed] [Google Scholar]
- 3.Royal RE, Levy C, Turner K, Mathur A, Hughes M, Kammula US, Sherry RM, Topalian SL, Yang JC, Lowy I, Rosenberg SA. Phase 2 trial of single agent Ipilimumab (anti-CTLA-4) for locally advanced or metastatic pancreatic adenocarcinoma. J Immunother. 2010;33:828–833. doi: 10.1097/CJI.0b013e3181eec14c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Le DT, Durham JN, Smith KN, Wang H, Bartlett BR, Aulakh LK, Lu S, Kemberling H, Wilt C, Luber BS, Wong F, Azad NS, Rucki AA, Laheru D, Donehower R, Zaheer A, Fisher GA, Crocenzi TS, Lee JJ, Greten TF, Duffy AG, Ciombor KK, Eyring AD, Lam BH, Joe A, Kang SP, Holdhoff M, Danilova L, Cope L, Meyer C, Zhou S, Goldberg RM, Armstrong DK, Bever KM, Fader AN, Taube J, Housseau F, Spetzler D, Xiao N, Pardoll DM, Papadopoulos N, Kinzler KW, Eshleman JR, Vogelstein B, Anders RA, Diaz LA Jr. Mismatch repair deficiency predicts response of solid tumors to PD-1 blockade. Science. 2017;357:409–413. doi: 10.1126/science.aan6733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Middleton G, Silcocks P, Cox T, Valle J, Wadsley J, Propper D, Coxon F, Ross P, Madhusudan S, Roques T, Cunningham D, Falk S, Wadd N, Harrison M, Corrie P, Iveson T, Robinson A, McAdam K, Eatock M, Evans J, Archer C, Hickish T, Garcia-Alonso A, Nicolson M, Steward W, Anthoney A, Greenhalf W, Shaw V, Costello E, Naisbitt D, Rawcliffe C, Nanson G, Neoptolemos J. Gemcitabine and capecitabine with or without telomerase peptide vaccine GV1001 in patients with locally advanced or metastatic pancreatic cancer (TeloVac): an open-label, randomised, phase 3 trial. Lancet Oncol. 2014;15:829–840. doi: 10.1016/S1470-2045(14)70236-0. [DOI] [PubMed] [Google Scholar]
- 6.Le DT, Wang-Gillam A, Picozzi V, Greten TF, Crocenzi T, Springett G, Morse M, Zeh H, Cohen D, Fine RL, Onners B, Uram JN, Laheru DA, Lutz ER, Solt S, Murphy AL, Skoble J, Lemmens E, Grous J, Dubensky T Jr, Brockstedt DG, Jaffee EM. Safety and survival with GVAX pancreas prime and Listeria Monocytogenes-expressing mesothelin (CRS-207) boost vaccines for metastatic pancreatic cancer. J Clin Oncol. 2015;33:1325–1333. doi: 10.1200/JCO.2014.57.4244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Le DT, Picozzi VJ, Ko AH, Wainberg ZA, Kindler H, Wang-Gillam A, Oberstein P, Morse MA, Zeh HJ 3rd, Weekes C, Reid T, Borazanci E, Crocenzi T, LoConte NK, Musher B, Laheru D, Murphy A, Whiting C, Nair N, Enstrom A, Ferber S, Brockstedt DG, Jaffee EM. Results from a Phase IIb, Randomized, Multicenter Study of GVAX Pancreas and CRS-207 Compared with Chemotherapy in Adults with Previously Treated Metastatic Pancreatic Adenocarcinoma (ECLIPSE Study) Clin Cancer Res. 2019;25:5493–5502. doi: 10.1158/1078-0432.CCR-18-2992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lutz E, Yeo CJ, Lillemoe KD, Biedrzycki B, Kobrin B, Herman J, Sugar E, Piantadosi S, Cameron JL, Solt S, Onners B, Tartakovsky I, Choi M, Sharma R, Illei PB, Hruban RH, Abrams RA, Le D, Jaffee E, Laheru D. A lethally irradiated allogeneic granulocyte-macrophage colony stimulating factor-secreting tumor vaccine for pancreatic adenocarcinoma. A Phase II trial of safety, efficacy, and immune activation. Ann Surg. 2011;253:328–335. doi: 10.1097/SLA.0b013e3181fd271c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tran E, Robbins PF, Lu YC, Prickett TD, Gartner JJ, Jia L, Pasetto A, Zheng Z, Ray S, Groh EM, Kriley IR, Rosenberg SA. T-Cell Transfer Therapy Targeting Mutant KRAS in Cancer. N Engl J Med. 2016;375:2255–2262. doi: 10.1056/NEJMoa1609279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Raj D, Yang MH, Rodgers D, Hampton EN, Begum J, Mustafa A, Lorizio D, Garces I, Propper D, Kench JG, Kocher HM, Young TS, Aicher A, Heeschen C. Switchable CAR-T cells mediate remission in metastatic pancreatic ductal adenocarcinoma. Gut. 2019;68:1052–1064. doi: 10.1136/gutjnl-2018-316595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Raj D, Nikolaidi M, Garces I, Lorizio D, Castro NM, Caiafa SG, Moore K, Brown NF, Kocher HM, Duan X, Nelson BH, Lemoine NR, Marshall JF. CEACAM7 Is an Effective Target for CAR T-cell Therapy of Pancreatic Ductal Adenocarcinoma. Clin Cancer Res. 2021;27:1538–1552. doi: 10.1158/1078-0432.CCR-19-2163. [DOI] [PubMed] [Google Scholar]
- 12.Posey AD Jr, Schwab RD, Boesteanu AC, Steentoft C, Mandel U, Engels B, Stone JD, Madsen TD, Schreiber K, Haines KM, Cogdill AP, Chen TJ, Song D, Scholler J, Kranz DM, Feldman MD, Young R, Keith B, Schreiber H, Clausen H, Johnson LA, June CH. Engineered CAR T Cells Targeting the Cancer-Associated Tn-Glycoform of the Membrane Mucin MUC1 Control Adenocarcinoma. Immunity. 2016;44:1444–1454. doi: 10.1016/j.immuni.2016.05.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bailey P, Chang DK, Nones K, Johns AL, Patch AM, Gingras MC, Miller DK, Christ AN, Bruxner TJ, Quinn MC, Nourse C, Murtaugh LC, Harliwong I, Idrisoglu S, Manning S, Nourbakhsh E, Wani S, Fink L, Holmes O, Chin V, Anderson MJ, Kazakoff S, Leonard C, Newell F, Waddell N, Wood S, Xu Q, Wilson PJ, Cloonan N, Kassahn KS, Taylor D, Quek K, Robertson A, Pantano L, Mincarelli L, Sanchez LN, Evers L, Wu J, Pinese M, Cowley MJ, Jones MD, Colvin EK, Nagrial AM, Humphrey ES, Chantrill LA, Mawson A, Humphris J, Chou A, Pajic M, Scarlett CJ, Pinho AV, Giry-Laterriere M, Rooman I, Samra JS, Kench JG, Lovell JA, Merrett ND, Toon CW, Epari K, Nguyen NQ, Barbour A, Zeps N, Moran-Jones K, Jamieson NB, Graham JS, Duthie F, Oien K, Hair J, Grützmann R, Maitra A, Iacobuzio-Donahue CA, Wolfgang CL, Morgan RA, Lawlor RT, Corbo V, Bassi C, Rusev B, Capelli P, Salvia R, Tortora G, Mukhopadhyay D, Petersen GM, Australian Pancreatic Cancer Genome Initiative, Munzy DM, Fisher WE, Karim SA, Eshleman JR, Hruban RH, Pilarsky C, Morton JP, Sansom OJ, Scarpa A, Musgrove EA, Bailey UM, Hofmann O, Sutherland RL, Wheeler DA, Gill AJ, Gibbs RA, Pearson JV, Biankin AV, Grimmond SM. Genomic analyses identify molecular subtypes of pancreatic cancer. Nature. 2016;531:47–52. doi: 10.1038/nature16965. [DOI] [PubMed] [Google Scholar]
- 14.Ene-Obong A, Clear AJ, Watt J, Wang J, Fatah R, Riches JC, Marshall JF, Chin-Aleong J, Chelala C, Gribben JG, Ramsay AG, Kocher HM. Activated pancreatic stellate cells sequester CD8+ T cells to reduce their infiltration of the juxtatumoral compartment of pancreatic ductal adenocarcinoma. Gastroenterology. 2013;145:1121–1132. doi: 10.1053/j.gastro.2013.07.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Knudsen ES, Vail P, Balaji U, Ngo H, Botros IW, Makarov V, Riaz N, Balachandran V, Leach S, Thompson DM, Chan TA, Witkiewicz AK. Stratification of Pancreatic Ductal Adenocarcinoma: Combinatorial Genetic, Stromal, and Immunologic Markers. Clin Cancer Res. 2017;23:4429–4440. doi: 10.1158/1078-0432.CCR-17-0162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hiraoka N, Ino Y, Yamazaki-Itoh R, Kanai Y, Kosuge T, Shimada K. Intratumoral tertiary lymphoid organ is a favourable prognosticator in patients with pancreatic cancer. Br J Cancer. 2015;112:1782–1790. doi: 10.1038/bjc.2015.145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Castino GF, Cortese N, Capretti G, Serio S, Di Caro G, Mineri R, Magrini E, Grizzi F, Cappello P, Novelli F, Spaggiari P, Roncalli M, Ridolfi C, Gavazzi F, Zerbi A, Allavena P, Marchesi F. Spatial distribution of B cells predicts prognosis in human pancreatic adenocarcinoma. Oncoimmunology. 2016;5:e1085147. doi: 10.1080/2162402X.2015.1085147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gunderson AJ, Kaneda MM, Tsujikawa T, Nguyen AV, Affara NI, Ruffell B, Gorjestani S, Liudahl SM, Truitt M, Olson P, Kim G, Hanahan D, Tempero MA, Sheppard B, Irving B, Chang BY, Varner JA, Coussens LM. Bruton Tyrosine Kinase-Dependent Immune Cell Cross-talk Drives Pancreas Cancer. Cancer Discov. 2016;6:270–285. doi: 10.1158/2159-8290.CD-15-0827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Pylayeva-Gupta Y, Das S, Handler JS, Hajdu CH, Coffre M, Koralov SB, Bar-Sagi D. IL35-Producing B Cells Promote the Development of Pancreatic Neoplasia. Cancer Discov. 2016;6:247–255. doi: 10.1158/2159-8290.CD-15-0843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lee KE, Spata M, Bayne LJ, Buza EL, Durham AC, Allman D, Vonderheide RH, Simon MC. Hif1a Deletion Reveals Pro-Neoplastic Function of B Cells in Pancreatic Neoplasia. Cancer Discov. 2016;6:256–269. doi: 10.1158/2159-8290.CD-15-0822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mirlekar B, Michaud D, Lee SJ, Kren NP, Harris C, Greene K, Goldman EC, Gupta GP, Fields RC, Hawkins WG, DeNardo DG, Rashid NU, Yeh JJ, McRee AJ, Vincent BG, Vignali DAA, Pylayeva-Gupta Y. B cell-Derived IL35 Drives STAT3-Dependent CD8+ T-cell Exclusion in Pancreatic Cancer. Cancer Immunol Res. 2020;8:292–308. doi: 10.1158/2326-6066.CIR-19-0349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Spear S, Candido JB, McDermott JR, Ghirelli C, Maniati E, Beers SA, Balkwill FR, Kocher HM, Capasso M. Discrepancies in the Tumor Microenvironment of Spontaneous and Orthotopic Murine Models of Pancreatic Cancer Uncover a New Immunostimulatory Phenotype for B Cells. Front Immunol. 2019;10:542. doi: 10.3389/fimmu.2019.00542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ladányi A, Kiss J, Mohos A, Somlai B, Liszkay G, Gilde K, Fejös Z, Gaudi I, Dobos J, Tímár J. Prognostic impact of B-cell density in cutaneous melanoma. Cancer Immunol Immunother. 2011;60:1729–1738. doi: 10.1007/s00262-011-1071-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Nielsen JS, Sahota RA, Milne K, Kost SE, Nesslinger NJ, Watson PH, Nelson BH. CD20+ tumor-infiltrating lymphocytes have an atypical CD27- memory phenotype and together with CD8+ T cells promote favorable prognosis in ovarian cancer. Clin Cancer Res. 2012;18:3281–3292. doi: 10.1158/1078-0432.CCR-12-0234. [DOI] [PubMed] [Google Scholar]
- 25.Iglesia MD, Vincent BG, Parker JS, Hoadley KA, Carey LA, Perou CM, Serody JS. Prognostic B-cell signatures using mRNA-seq in patients with subtype-specific breast and ovarian cancer. Clin Cancer Res. 2014;20:3818–3829. doi: 10.1158/1078-0432.CCR-13-3368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Germain C, Gnjatic S, Tamzalit F, Knockaert S, Remark R, Goc J, Lepelley A, Becht E, Katsahian S, Bizouard G, Validire P, Damotte D, Alifano M, Magdeleinat P, Cremer I, Teillaud JL, Fridman WH, Sautès-Fridman C, Dieu-Nosjean MC. Presence of B cells in tertiary lymphoid structures is associated with a protective immunity in patients with lung cancer. Am J Respir Crit Care Med. 2014;189:832–844. doi: 10.1164/rccm.201309-1611OC. [DOI] [PubMed] [Google Scholar]
- 27.Petitprez F, de Reyniès A, Keung EZ, Chen TW, Sun CM, Calderaro J, Jeng YM, Hsiao LP, Lacroix L, Bougoüin A, Moreira M, Lacroix G, Natario I, Adam J, Lucchesi C, Laizet YH, Toulmonde M, Burgess MA, Bolejack V, Reinke D, Wani KM, Wang WL, Lazar AJ, Roland CL, Wargo JA, Italiano A, Sautès-Fridman C, Tawbi HA, Fridman WH. B cells are associated with survival and immunotherapy response in sarcoma. Nature. 2020;577:556–560. doi: 10.1038/s41586-019-1906-8. [DOI] [PubMed] [Google Scholar]
- 28.Kroeger DR, Milne K, Nelson BH. Tumor-Infiltrating Plasma Cells Are Associated with Tertiary Lymphoid Structures, Cytolytic T-Cell Responses, and Superior Prognosis in Ovarian Cancer. Clin Cancer Res. 2016;22:3005–3015. doi: 10.1158/1078-0432.CCR-15-2762. [DOI] [PubMed] [Google Scholar]
- 29.Bergomas F, Grizzi F, Doni A, Pesce S, Laghi L, Allavena P, Mantovani A, Marchesi F. Tertiary intratumor lymphoid tissue in colo-rectal cancer. Cancers (Basel) 2011;4:1–10. doi: 10.3390/cancers4010001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Tarlinton D. B cells still front and centre in immunology. Nat Rev Immunol. 2019;19:85–86. doi: 10.1038/s41577-018-0107-2. [DOI] [PubMed] [Google Scholar]
- 31.Delvecchio FR, Fincham REA, Spear S, Clear A, Roy-Luzarraga M, Balkwill FR, Gribben JG, Bombardieri M, Hodivala-Dilke K, Capasso M, Kocher HM. Pancreatic Cancer Chemotherapy Is Potentiated by Induction of Tertiary Lymphoid Structures in Mice. Cell Mol Gastroenterol Hepatol. 2021;12:1543–1565. doi: 10.1016/j.jcmgh.2021.06.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Anderson KS, LaBaer J. The sentinel within: exploiting the immune system for cancer biomarkers. J Proteome Res. 2005;4:1123–1133. doi: 10.1021/pr0500814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Goodnow CC, Fazekas de St Groth B, Barbara BF, Vinuesa CG. Cellular and genetic mechanisms of self tolerance and autoimmunity. Nature. 2005;435:590–597. doi: 10.1038/nature03724. [DOI] [PubMed] [Google Scholar]
- 34.Dieu-Nosjean MC, Giraldo NA, Kaplon H, Germain C, Fridman WH, Sautès-Fridman C. Tertiary lymphoid structures, drivers of the anti-tumor responses in human cancers. Immunol Rev. 2016;271:260–275. doi: 10.1111/imr.12405. [DOI] [PubMed] [Google Scholar]
- 35.Di Niro R, Lee SJ, Vander Heiden JA, Elsner RA, Trivedi N, Bannock JM, Gupta NT, Kleinstein SH, Vigneault F, Gilbert TJ, Meffre E, McSorley SJ, Shlomchik MJ. Salmonella Infection Drives Promiscuous B Cell Activation Followed by Extrafollicular Affinity Maturation. Immunity. 2015;43:120–131. doi: 10.1016/j.immuni.2015.06.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Nzula S, Going JJ, Stott DI. Antigen-driven clonal proliferation, somatic hypermutation, and selection of B lymphocytes infiltrating human ductal breast carcinomas. Cancer Res. 2003;63:3275–3280. [PubMed] [Google Scholar]
- 37.Maletzki C, Jahnke A, Ostwald C, Klar E, Prall F, Linnebacher M. Ex-vivo clonally expanded B lymphocytes infiltrating colorectal carcinoma are of mature immunophenotype and produce functional IgG. PLoS One. 2012;7:e32639. doi: 10.1371/journal.pone.0032639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Takemori T, Kaji T, Takahashi Y, Shimoda M, Rajewsky K. Generation of memory B cells inside and outside germinal centers. Eur J Immunol. 2014;44:1258–1264. doi: 10.1002/eji.201343716. [DOI] [PubMed] [Google Scholar]
- 39.Katoh H, Komura D, Konishi H, Suzuki R, Yamamoto A, Kakiuchi M, Sato R, Ushiku T, Yamamoto S, Tatsuno K, Oshima T, Nomura S, Seto Y, Fukayama M, Aburatani H, Ishikawa S. Immunogenetic Profiling for Gastric Cancers Identifies Sulfated Glycosaminoglycans as Major and Functional B Cell Antigens in Human Malignancies. Cell Rep. 2017;20:1073–1087. doi: 10.1016/j.celrep.2017.07.016. [DOI] [PubMed] [Google Scholar]
- 40.Zaenker P, Gray ES, Ziman MR. Autoantibody Production in Cancer--The Humoral Immune Response toward Autologous Antigens in Cancer Patients. Autoimmun Rev. 2016;15:477–483. doi: 10.1016/j.autrev.2016.01.017. [DOI] [PubMed] [Google Scholar]
- 41.Reuschenbach M, von Knebel Doeberitz M, Wentzensen N. A systematic review of humoral immune responses against tumor antigens. Cancer Immunol Immunother. 2009;58:1535–1544. doi: 10.1007/s00262-009-0733-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Mizukami M, Hanagiri T, Yasuda M, Kuroda K, Shigematsu Y, Baba T, Fukuyama T, Nagata Y, So T, Ichiki Y, Sugaya M, Takenoyama M, Sugio K, Yasumoto K. Antitumor effect of antibody against a SEREX-defined antigen (UOEH-LC-1) on lung cancer xenotransplanted into severe combined immunodeficiency mice. Cancer Res. 2007;67:8351–8357. doi: 10.1158/0008-5472.CAN-06-3889. [DOI] [PubMed] [Google Scholar]
- 43.Li Q, Teitz-Tennenbaum S, Donald EJ, Li M, Chang AE. In vivo sensitized and in vitro activated B cells mediate tumor regression in cancer adoptive immunotherapy. J Immunol. 2009;183:3195–3203. doi: 10.4049/jimmunol.0803773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Patel D, Bassi R, Hooper AT, Sun H, Huber J, Hicklin DJ, Kang X. Enhanced suppression of melanoma tumor growth and metastasis by combined therapy with anti-VEGF receptor and anti-TYRP-1/gp75 monoclonal antibodies. Anticancer Res. 2008;28:2679–2686. [PubMed] [Google Scholar]
- 45.Albanesi M, Mancardi DA, Jönsson F, Iannascoli B, Fiette L, Di Santo JP, Lowell CA, Bruhns P. Neutrophils mediate antibody-induced antitumor effects in mice. Blood. 2013;122:3160–3164. doi: 10.1182/blood-2013-04-497446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Afshar-Kharghan V. The role of the complement system in cancer. J Clin Invest. 2017;127:780–789. doi: 10.1172/JCI90962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Milne K, Köbel M, Kalloger SE, Barnes RO, Gao D, Gilks CB, Watson PH, Nelson BH. Systematic analysis of immune infiltrates in high-grade serous ovarian cancer reveals CD20, FoxP3 and TIA-1 as positive prognostic factors. PLoS One. 2009;4:e6412. doi: 10.1371/journal.pone.0006412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Deola S, Panelli MC, Maric D, Selleri S, Dmitrieva NI, Voss CY, Klein H, Stroncek D, Wang E, Marincola FM. Helper B cells promote cytotoxic T cell survival and proliferation independently of antigen presentation through CD27/CD70 interactions. J Immunol. 2008;180:1362–1372. doi: 10.4049/jimmunol.180.3.1362. [DOI] [PubMed] [Google Scholar]
- 49.Quah BJ, Barlow VP, McPhun V, Matthaei KI, Hulett MD, Parish CR. Bystander B cells rapidly acquire antigen receptors from activated B cells by membrane transfer. Proc Natl Acad Sci U S A. 2008;105:4259–4264. doi: 10.1073/pnas.0800259105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Helmink BA, Reddy SM, Gao J, Zhang S, Basar R, Thakur R, Yizhak K, Sade-Feldman M, Blando J, Han G, Gopalakrishnan V, Xi Y, Zhao H, Amaria RN, Tawbi HA, Cogdill AP, Liu W, LeBleu VS, Kugeratski FG, Patel S, Davies MA, Hwu P, Lee JE, Gershenwald JE, Lucci A, Arora R, Woodman S, Keung EZ, Gaudreau PO, Reuben A, Spencer CN, Burton EM, Haydu LE, Lazar AJ, Zapassodi R, Hudgens CW, Ledesma DA, Ong S, Bailey M, Warren S, Rao D, Krijgsman O, Rozeman EA, Peeper D, Blank CU, Schumacher TN, Butterfield LH, Zelazowska MA, McBride KM, Kalluri R, Allison J, Petitprez F, Fridman WH, Sautès-Fridman C, Hacohen N, Rezvani K, Sharma P, Tetzlaff MT, Wang L, Wargo JA. B cells and tertiary lymphoid structures promote immunotherapy response. Nature. 2020;577:549–555. doi: 10.1038/s41586-019-1922-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Di Caro G, Bergomas F, Grizzi F, Doni A, Bianchi P, Malesci A, Laghi L, Allavena P, Mantovani A, Marchesi F. Occurrence of tertiary lymphoid tissue is associated with T-cell infiltration and predicts better prognosis in early-stage colorectal cancers. Clin Cancer Res. 2014;20:2147–2158. doi: 10.1158/1078-0432.CCR-13-2590. [DOI] [PubMed] [Google Scholar]
- 52.Yasuda M, Mizukami M, Hanagiri T, Shigematsu Y, Fukuyama T, Nagata Y, So T, Ichiki Y, Sugaya M, Takenoyama M, Sugio K, Yasumoto K. Antigens recognized by IgG derived from tumor-infiltrating B lymphocytes in human lung cancer. Anticancer Res. 2006;26:3607–3611. [PubMed] [Google Scholar]
- 53.Cottrell TR, Thompson ED, Forde PM, Stein JE, Duffield AS, Anagnostou V, Rekhtman N, Anders RA, Cuda JD, Illei PB, Gabrielson E, Askin FB, Niknafs N, Smith KN, Velez MJ, Sauter JL, Isbell JM, Jones DR, Battafarano RJ, Yang SC, Danilova L, Wolchok JD, Topalian SL, Velculescu VE, Pardoll DM, Brahmer JR, Hellmann MD, Chaft JE, Cimino-Mathews A, Taube JM. Pathologic features of response to neoadjuvant anti-PD-1 in resected non-small-cell lung carcinoma: a proposal for quantitative immune-related pathologic response criteria (irPRC) Ann Oncol. 2018;29:1853–1860. doi: 10.1093/annonc/mdy218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Maldonado L, Teague JE, Morrow MP, Jotova I, Wu TC, Wang C, Desmarais C, Boyer JD, Tycko B, Robins HS, Clark RA, Trimble CL. Intramuscular therapeutic vaccination targeting HPV16 induces T cell responses that localize in mucosal lesions. Sci Transl Med. 2014;6:221ra13. doi: 10.1126/scitranslmed.3007323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Thommen DS, Koelzer VH, Herzig P, Roller A, Trefny M, Dimeloe S, Kiialainen A, Hanhart J, Schill C, Hess C, Savic Prince S, Wiese M, Lardinois D, Ho PC, Klein C, Karanikas V, Mertz KD, Schumacher TN, Zippelius A. A transcriptionally and functionally distinct PD-1+ CD8+ T cell pool with predictive potential in non-small-cell lung cancer treated with PD-1 blockade. Nat Med. 2018;24:994–1004. doi: 10.1038/s41591-018-0057-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Cabrita R, Lauss M, Sanna A, Donia M, Skaarup Larsen M, Mitra S, Johansson I, Phung B, Harbst K, Vallon-Christersson J, van Schoiack A, Lövgren K, Warren S, Jirström K, Olsson H, Pietras K, Ingvar C, Isaksson K, Schadendorf D, Schmidt H, Bastholt L, Carneiro A, Wargo JA, Svane IM, Jönsson G. Tertiary lymphoid structures improve immunotherapy and survival in melanoma. Nature. 2020;577:561–565. doi: 10.1038/s41586-019-1914-8. [DOI] [PubMed] [Google Scholar]
- 57.Bosisio FM, Wilmott JS, Volders N, Mercier M, Wouters J, Stas M, Blokx WA, Massi D, Thompson JF, Scolyer RA, van Baren N, van den Oord JJ. Plasma cells in primary melanoma. Prognostic significance and possible role of IgA. Mod Pathol. 2016;29:347–358. doi: 10.1038/modpathol.2016.28. [DOI] [PubMed] [Google Scholar]
- 58.Woo JR, Liss MA, Muldong MT, Palazzi K, Strasner A, Ammirante M, Varki N, Shabaik A, Howell S, Kane CJ, Karin M, Jamieson CA. Tumor infiltrating B-cells are increased in prostate cancer tissue. J Transl Med. 2014;12:30. doi: 10.1186/1479-5876-12-30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Kurebayashi Y, Emoto K, Hayashi Y, Kamiyama I, Ohtsuka T, Asamura H, Sakamoto M. Comprehensive Immune Profiling of Lung Adenocarcinomas Reveals Four Immunosubtypes with Plasma Cell Subtype a Negative Indicator. Cancer Immunol Res. 2016;4:234–247. doi: 10.1158/2326-6066.CIR-15-0214. [DOI] [PubMed] [Google Scholar]
- 60.Dong HP, Elstrand MB, Holth A, Silins I, Berner A, Trope CG, Davidson B, Risberg B. NK- and B-cell infiltration correlates with worse outcome in metastatic ovarian carcinoma. Am J Clin Pathol. 2006;125:451–458. [PubMed] [Google Scholar]
- 61.Lai CL, Tsai CM, Tsai TT, Kuo BI, Chang KT, Fu HT, Perng RP, Chen JY. Presence of serum anti-p53 antibodies is associated with pleural effusion and poor prognosis in lung cancer patients. Clin Cancer Res. 1998;4:3025–3030. [PubMed] [Google Scholar]
- 62.Hamanaka Y, Suehiro Y, Fukui M, Shikichi K, Imai K, Hinoda Y. Circulating anti-MUC1 IgG antibodies as a favorable prognostic factor for pancreatic cancer. Int J Cancer. 2003;103:97–100. doi: 10.1002/ijc.10801. [DOI] [PubMed] [Google Scholar]
- 63.Kurtenkov O, Klaamas K, Mensdorff-Pouilly S, Miljukhina L, Shljapnikova L, Chuzmarov V. Humoral immune response to MUC1 and to the Thomsen-Friedenreich (TF) glycotope in patients with gastric cancer: relation to survival. Acta Oncol. 2007;46:316–323. doi: 10.1080/02841860601055441. [DOI] [PubMed] [Google Scholar]
- 64.Montgomery RB, Makary E, Schiffman K, Goodell V, Disis ML. Endogenous anti-HER2 antibodies block HER2 phosphorylation and signaling through extracellular signal-regulated kinase. Cancer Res. 2005;65:650–656. [PubMed] [Google Scholar]
- 65.Andreu P, Johansson M, Affara NI, Pucci F, Tan T, Junankar S, Korets L, Lam J, Tawfik D, DeNardo DG, Naldini L, de Visser KE, De Palma M, Coussens LM. FcRgamma activation regulates inflammation-associated squamous carcinogenesis. Cancer Cell. 2010;17:121–134. doi: 10.1016/j.ccr.2009.12.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.de Visser KE, Korets LV, Coussens LM. De novo carcinogenesis promoted by chronic inflammation is B lymphocyte dependent. Cancer Cell. 2005;7:411–423. doi: 10.1016/j.ccr.2005.04.014. [DOI] [PubMed] [Google Scholar]
- 67.Jefferis R. Isotype and glycoform selection for antibody therapeutics. Arch Biochem Biophys. 2012;526:159–166. doi: 10.1016/j.abb.2012.03.021. [DOI] [PubMed] [Google Scholar]
- 68.Karagiannis P, Gilbert AE, Josephs DH, Ali N, Dodev T, Saul L, Correa I, Roberts L, Beddowes E, Koers A, Hobbs C, Ferreira S, Geh JL, Healy C, Harries M, Acland KM, Blower PJ, Mitchell T, Fear DJ, Spicer JF, Lacy KE, Nestle FO, Karagiannis SN. IgG4 subclass antibodies impair antitumor immunity in melanoma. J Clin Invest. 2013;123:1457–1474. doi: 10.1172/JCI65579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.van Schouwenburg PA, Krieckaert CL, Nurmohamed M, Hart M, Rispens T, Aarden L, Wouters D, Wolbink GJ. IgG4 production against adalimumab during long term treatment of RA patients. J Clin Immunol. 2012;32:1000–1006. doi: 10.1007/s10875-012-9705-0. [DOI] [PubMed] [Google Scholar]
- 70.Shah S, Divekar AA, Hilchey SP, Cho HM, Newman CL, Shin SU, Nechustan H, Challita-Eid PM, Segal BM, Yi KH, Rosenblatt JD. Increased rejection of primary tumors in mice lacking B cells: inhibition of anti-tumor CTL and TH1 cytokine responses by B cells. Int J Cancer. 2005;117:574–586. doi: 10.1002/ijc.21177. [DOI] [PubMed] [Google Scholar]
- 71.Tadmor T, Zhang Y, Cho HM, Podack ER, Rosenblatt JD. The absence of B lymphocytes reduces the number and function of T-regulatory cells and enhances the anti-tumor response in a murine tumor model. Cancer Immunol Immunother. 2011;60:609–619. doi: 10.1007/s00262-011-0972-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Ammirante M, Luo JL, Grivennikov S, Nedospasov S, Karin M. B-cell-derived lymphotoxin promotes castration-resistant prostate cancer. Nature. 2010;464:302–305. doi: 10.1038/nature08782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Mizoguchi A, Mizoguchi E, Takedatsu H, Blumberg RS, Bhan AK. Chronic intestinal inflammatory condition generates IL-10-producing regulatory B cell subset characterized by CD1d upregulation. Immunity. 2002;16:219–230. doi: 10.1016/s1074-7613(02)00274-1. [DOI] [PubMed] [Google Scholar]
- 74.Schioppa T, Moore R, Thompson RG, Rosser EC, Kulbe H, Nedospasov S, Mauri C, Coussens LM, Balkwill FR. B regulatory cells and the tumor-promoting actions of TNF-α during squamous carcinogenesis. Proc Natl Acad Sci U S A. 2011;108:10662–10667. doi: 10.1073/pnas.1100994108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Sumimoto K, Uchida K, Kusuda T, Mitsuyama T, Sakaguchi Y, Fukui T, Matsushita M, Takaoka M, Nishio A, Okazaki K. The role of CD19+ CD24high CD38high and CD19+ CD24high CD27+ regulatory B cells in patients with type 1 autoimmune pancreatitis. Pancreatology. 2014;14:193–200. doi: 10.1016/j.pan.2014.02.004. [DOI] [PubMed] [Google Scholar]
- 76.Watanabe R, Ishiura N, Nakashima H, Kuwano Y, Okochi H, Tamaki K, Sato S, Tedder TF, Fujimoto M. Regulatory B cells (B10 cells) have a suppressive role in murine lupus: CD19 and B10 cell deficiency exacerbates systemic autoimmunity. J Immunol. 2010;184:4801–4809. doi: 10.4049/jimmunol.0902385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Yanaba K, Bouaziz JD, Haas KM, Poe JC, Fujimoto M, Tedder TF. A regulatory B cell subset with a unique CD1dhiCD5+ phenotype controls T cell-dependent inflammatory responses. Immunity. 2008;28:639–650. doi: 10.1016/j.immuni.2008.03.017. [DOI] [PubMed] [Google Scholar]
- 78.Peng X, Luo Z, Kang Q, Deng D, Wang Q, Peng H, Wang S, Wei Z. FOXQ1 mediates the crosstalk between TGF-β and Wnt signaling pathways in the progression of colorectal cancer. Cancer Biol Ther. 2015;16:1099–1109. doi: 10.1080/15384047.2015.1047568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Shao Y, Lo CM, Ling CC, Liu XB, Ng KT, Chu AC, Ma YY, Li CX, Fan ST, Man K. Regulatory B cells accelerate hepatocellular carcinoma progression via CD40/CD154 signaling pathway. Cancer Lett. 2014;355:264–272. doi: 10.1016/j.canlet.2014.09.026. [DOI] [PubMed] [Google Scholar]
- 80.Aklilu M, Stadler WM, Markiewicz M, Vogelzang NJ, Mahowald M, Johnson M, Gajewski TF. Depletion of normal B cells with rituximab as an adjunct to IL-2 therapy for renal cell carcinoma and melanoma. Ann Oncol. 2004;15:1109–1114. doi: 10.1093/annonc/mdh280. [DOI] [PubMed] [Google Scholar]
- 81.Barbera-Guillem E, Nelson MB, Barr B, Nyhus JK, May KF Jr, Feng L, Sampsel JW. B lymphocyte pathology in human colorectal cancer. Experimental and clinical therapeutic effects of partial B cell depletion. Cancer Immunol Immunother. 2000;48:541–549. doi: 10.1007/PL00006672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Qin Z, Richter G, Schüler T, Ibe S, Cao X, Blankenstein T. B cells inhibit induction of T cell-dependent tumor immunity. Nat Med. 1998;4:627–630. doi: 10.1038/nm0598-627. [DOI] [PubMed] [Google Scholar]
- 83.Guy TV, Terry AM, Bolton HA, Hancock DG, Shklovskaya E, Fazekas de St. Groth B. Pro- and anti-tumour effects of B cells and antibodies in cancer: a comparison of clinical studies and preclinical models. Cancer Immunol Immunother. 2016;65:885–896. doi: 10.1007/s00262-016-1848-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Moir S, Ho J, Malaspina A, Wang W, DiPoto AC, O'Shea MA, Roby G, Kottilil S, Arthos J, Proschan MA, Chun TW, Fauci AS. Evidence for HIV-associated B cell exhaustion in a dysfunctional memory B cell compartment in HIV-infected viremic individuals. J Exp Med. 2008;205:1797–1805. doi: 10.1084/jem.20072683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Portugal S, Obeng-Adjei N, Moir S, Crompton PD, Pierce SK. Atypical memory B cells in human chronic infectious diseases: An interim report. Cell Immunol. 2017;321:18–25. doi: 10.1016/j.cellimm.2017.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Li H, Borrego F, Nagata S, Tolnay M. Fc Receptor-like 5 Expression Distinguishes Two Distinct Subsets of Human Circulating Tissue-like Memory B Cells. J Immunol. 2016;196:4064–4074. doi: 10.4049/jimmunol.1501027. [DOI] [PubMed] [Google Scholar]
- 87.Bruno TC, Ebner PJ, Moore BL, Squalls OG, Waugh KA, Eruslanov EB, Singhal S, Mitchell JD, Franklin WA, Merrick DT, McCarter MD, Palmer BE, Kern JA, Slansky JE. Antigen-Presenting Intratumoral B Cells Affect CD4+ TIL Phenotypes in Non-Small Cell Lung Cancer Patients. Cancer Immunol Res. 2017;5:898–907. doi: 10.1158/2326-6066.CIR-17-0075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Garaud S, Buisseret L, Solinas C, Gu-Trantien C, de Wind A, Van den Eynden G, Naveaux C, Lodewyckx JN, Boisson A, Duvillier H, Craciun L, Ameye L, Veys I, Paesmans M, Larsimont D, Piccart-Gebhart M, Willard-Gallo K. Tumor infiltrating B-cells signal functional humoral immune responses in breast cancer. JCI Insight. 2019;5 doi: 10.1172/jci.insight.129641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Affara NI, Ruffell B, Medler TR, Gunderson AJ, Johansson M, Bornstein S, Bergsland E, Steinhoff M, Li Y, Gong Q, Ma Y, Wiesen JF, Wong MH, Kulesz-Martin M, Irving B, Coussens LM. B cells regulate macrophage phenotype and response to chemotherapy in squamous carcinomas. Cancer Cell. 2014;25:809–821. doi: 10.1016/j.ccr.2014.04.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.DiLillo DJ, Yanaba K, Tedder TF. B cells are required for optimal CD4+ and CD8+ T cell tumor immunity: therapeutic B cell depletion enhances B16 melanoma growth in mice. J Immunol. 2010;184:4006–4016. doi: 10.4049/jimmunol.0903009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Forte G, Sorrentino R, Montinaro A, Luciano A, Adcock IM, Maiolino P, Arra C, Cicala C, Pinto A, Morello S. Inhibition of CD73 improves B cell-mediated anti-tumor immunity in a mouse model of melanoma. J Immunol. 2012;189:2226–2233. doi: 10.4049/jimmunol.1200744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Watt J, Kocher HM. The desmoplastic stroma of pancreatic cancer is a barrier to immune cell infiltration. Oncoimmunology. 2013;2:e26788. doi: 10.4161/onci.26788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Crowley MT, Reilly CR, Lo D. Influence of lymphocytes on the presence and organization of dendritic cell subsets in the spleen. J Immunol. 1999;163:4894–4900. [PubMed] [Google Scholar]
- 94.Goc J, Germain C, Vo-Bourgais TK, Lupo A, Klein C, Knockaert S, de Chaisemartin L, Ouakrim H, Becht E, Alifano M, Validire P, Remark R, Hammond SA, Cremer I, Damotte D, Fridman WH, Sautès-Fridman C, Dieu-Nosjean MC. Dendritic cells in tumor-associated tertiary lymphoid structures signal a Th1 cytotoxic immune contexture and license the positive prognostic value of infiltrating CD8+ T cells. Cancer Res. 2014;74:705–715. doi: 10.1158/0008-5472.CAN-13-1342. [DOI] [PubMed] [Google Scholar]
- 95.Lutz ER, Wu AA, Bigelow E, Sharma R, Mo G, Soares K, Solt S, Dorman A, Wamwea A, Yager A, Laheru D, Wolfgang CL, Wang J, Hruban RH, Anders RA, Jaffee EM, Zheng L. Immunotherapy converts nonimmunogenic pancreatic tumors into immunogenic foci of immune regulation. Cancer Immunol Res. 2014;2:616–631. doi: 10.1158/2326-6066.CIR-14-0027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Posch F, Silina K, Leibl S, Mündlein A, Moch H, Siebenhüner A, Samaras P, Riedl J, Stotz M, Szkandera J, Stöger H, Pichler M, Stupp R, van den Broek M, Schraml P, Gerger A, Petrausch U, Winder T. Maturation of tertiary lymphoid structures and recurrence of stage II and III colorectal cancer. Oncoimmunology. 2018;7:e1378844. doi: 10.1080/2162402X.2017.1378844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Yamaguchi K, Ito M, Ohmura H, Hanamura F, Nakano M, Tsuchihashi K, Nagai S, Ariyama H, Kusaba H, Yamamoto H, Oda Y, Nakamura M, Akashi K, Baba E. Helper T cell-dominant tertiary lymphoid structures are associated with disease relapse of advanced colorectal cancer. Oncoimmunology. 2020;9:1724763. doi: 10.1080/2162402X.2020.1724763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Turnquist HR, Lin X, Ashour AE, Hollingsworth MA, Singh RK, Talmadge JE, Solheim JC. CCL21 induces extensive intratumoral immune cell infiltration and specific anti-tumor cellular immunity. Int J Oncol. 2007;30:631–639. [PubMed] [Google Scholar]
- 99.Lee JM, Lee MH, Garon E, Goldman JW, Salehi-Rad R, Baratelli FE, Schaue D, Wang G, Rosen F, Yanagawa J, Walser TC, Lin Y, Park SJ, Adams S, Marincola FM, Tumeh PC, Abtin F, Suh R, Reckamp KL, Lee G, Wallace WD, Lee S, Zeng G, Elashoff DA, Sharma S, Dubinett SM. Phase I Trial of Intratumoral Injection of CCL21 Gene-Modified Dendritic Cells in Lung Cancer Elicits Tumor-Specific Immune Responses and CD8+ T-cell Infiltration. Clin Cancer Res. 2017;23:4556–4568. doi: 10.1158/1078-0432.CCR-16-2821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Meng Q, Valentini D, Rao M, Maeurer M. KRAS RENAISSANCE(S) in Tumor Infiltrating B Cells in Pancreatic Cancer. Front Oncol. 2018;8:384. doi: 10.3389/fonc.2018.00384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Han S, Jin G, Wang L, Li M, He C, Guo X, Zhu Q. The role of PAM4 in the management of pancreatic cancer: diagnosis, radioimmunodetection, and radioimmunotherapy. J Immunol Res. 2014;2014:268479. doi: 10.1155/2014/268479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Gold DV, Karanjawala Z, Modrak DE, Goldenberg DM, Hruban RH. PAM4-reactive MUC1 is a biomarker for early pancreatic adenocarcinoma. Clin Cancer Res. 2007;13:7380–7387. doi: 10.1158/1078-0432.CCR-07-1488. [DOI] [PubMed] [Google Scholar]
- 103.Fiedler W, DeDosso S, Cresta S, Weidmann J, Tessari A, Salzberg M, Dietrich B, Baumeister H, Goletz S, Gianni L, Sessa C. A phase I study of PankoMab-GEX, a humanised glyco-optimised monoclonal antibody to a novel tumour-specific MUC1 glycopeptide epitope in patients with advanced carcinomas. Eur J Cancer. 2016;63:55–63. doi: 10.1016/j.ejca.2016.05.003. [DOI] [PubMed] [Google Scholar]
- 104.Mehla K, Tremayne J, Grunkemeyer JA, O'Connell KA, Steele MM, Caffrey TC, Zhu X, Yu F, Singh PK, Schultes BC, Madiyalakan R, Nicodemus CF, Hollingsworth MA. Combination of mAb-AR20.5, anti-PD-L1 and PolyICLC inhibits tumor progression and prolongs survival of MUC1.Tg mice challenged with pancreatic tumors. Cancer Immunol Immunother. 2018;67:445–457. doi: 10.1007/s00262-017-2095-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Lines KE, Chelala C, Dmitrovic B, Wijesuriya N, Kocher HM, Marshall JF, Crnogorac-Jurcevic T. S100P-binding protein, S100PBP, mediates adhesion through regulation of cathepsin Z in pancreatic cancer cells. Am J Pathol. 2012;180:1485–1494. doi: 10.1016/j.ajpath.2011.12.031. [DOI] [PubMed] [Google Scholar]
- 106.Li NF, Gemenetzidis E, Marshall FJ, Davies D, Yu Y, Frese K, Froeling FE, Woolf AK, Feakins RM, Naito Y, Iacobuzio-Donahue C, Tuveson DA, Hart IR, Kocher HM. RhoC interacts with integrin α5β1 and enhances its trafficking in migrating pancreatic carcinoma cells. PLoS One. 2013;8:e81575. doi: 10.1371/journal.pone.0081575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Haider S, Wang J, Nagano A, Desai A, Arumugam P, Dumartin L, Fitzgibbon J, Hagemann T, Marshall JF, Kocher HM, Crnogorac-Jurcevic T, Scarpa A, Lemoine NR, Chelala C. A multi-gene signature predicts outcome in patients with pancreatic ductal adenocarcinoma. Genome Med. 2014;6:105. doi: 10.1186/s13073-014-0105-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Reader CS, Vallath S, Steele CW, Haider S, Brentnall A, Desai A, Moore KM, Jamieson NB, Chang D, Bailey P, Scarpa A, Lawlor R, Chelala C, Keyse SM, Biankin A, Morton JP, Evans TJ, Barry ST, Sansom OJ, Kocher HM, Marshall JF. The integrin αvβ6 drives pancreatic cancer through diverse mechanisms and represents an effective target for therapy. J Pathol. 2019;249:332–342. doi: 10.1002/path.5320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Zirakzadeh AA, Marits P, Sherif A, Winqvist O. Multiplex B cell characterization in blood, lymph nodes, and tumors from patients with malignancies. J Immunol. 2013;190:5847–5855. doi: 10.4049/jimmunol.1203279. [DOI] [PubMed] [Google Scholar]
- 110.Zhang Z, Ma L, Goswami S, Ma J, Zheng B, Duan M, Liu L, Zhang L, Shi J, Dong L, Sun Y, Tian L, Gao Q, Zhang X. Landscape of infiltrating B cells and their clinical significance in human hepatocellular carcinoma. Oncoimmunology. 2019;8:e1571388. doi: 10.1080/2162402X.2019.1571388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Kocher HM, Basu B, Froeling FEM, Sarker D, Slater S, Carlin D, deSouza NM, De Paepe KN, Goulart MR, Hughes C, Imrali A, Roberts R, Pawula M, Houghton R, Lawrence C, Yogeswaran Y, Mousa K, Coetzee C, Sasieni P, Prendergast A, Propper DJ. Phase I clinical trial repurposing all-trans retinoic acid as a stromal targeting agent for pancreatic cancer. Nat Commun. 2020;11:4841. doi: 10.1038/s41467-020-18636-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Froeling FE, Kocher HM. Homeostatic restoration of desmoplastic stroma rather than its ablation slows pancreatic cancer progression. Gastroenterology. 2015;148:849–850. doi: 10.1053/j.gastro.2015.02.043. [DOI] [PubMed] [Google Scholar]
- 113.Kadaba R, Birke H, Wang J, Hooper S, Andl CD, Di Maggio F, Soylu E, Ghallab M, Bor D, Froeling FE, Bhattacharya S, Rustgi AK, Sahai E, Chelala C, Sasieni P, Kocher HM. Imbalance of desmoplastic stromal cell numbers drives aggressive cancer processes. J Pathol. 2013;230:107–117. doi: 10.1002/path.4172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Munasinghe A, Malik K, Mohamedi F, Moaraf S, Kocher H, Jones L, Hill NJ. Fibronectin acts as a molecular switch to determine SPARC function in pancreatic cancer. Cancer Lett. 2020;477:88–96. doi: 10.1016/j.canlet.2020.02.031. [DOI] [PubMed] [Google Scholar]
- 115.Neuzillet C, Tijeras-Raballand A, Ragulan C, Cros J, Patil Y, Martinet M, Erkan M, Kleeff J, Wilson J, Apte M, Tosolini M, Wilson AS, Delvecchio FR, Bousquet C, Paradis V, Hammel P, Sadanandam A, Kocher HM. Inter- and intra-tumoural heterogeneity in cancer-associated fibroblasts of human pancreatic ductal adenocarcinoma. J Pathol. 2019;248:51–65. doi: 10.1002/path.5224. [DOI] [PMC free article] [PubMed] [Google Scholar]