Abstract
BACKGROUND
Gastric injury is the most common digestive system disease worldwide and involves inflammation, which can lead to gastric ulcer or gastric cancer (GC). Matrix metallopeptidase-9 [MMP-9 (gelatinase-B)] plays an important role in inflammation and GC progression. Quercetin and quercetin-rich diets represent potential food supplements and a source of medications for treating gastric injury given their anti-inflammatory activities. However, the effects and mechanisms of action of quercetin on human chronic gastritis and whether quercetin can relieve symptoms remain unclear.
AIM
To assess whether tumor necrosis factor-α (TNF-α)-induced MMP-9 expression mediates the anti-inflammatory effects of quercetin in normal human gastric mucosal epithelial cells.
METHODS
The normal human gastric mucosa epithelial cell line GES-1 was used to establish a normal human gastric epithelial cell model of TNF-α-induced MMP-9 protein overexpression to evaluate the anti-inflammatory effects of quercetin. The cell counting Kit-8 assay was used to evaluate the effects of varying quercetin doses on cell viability in the normal GES-1 cell line. Cell migration was measured using Transwell assay. The expression of proto-oncogene tyrosine-protein kinase Src (c-Src), phospho (p)-c-Src, extracellular-signal-regulated kinase 2 (ERK2), p-ERK1/2, c-Fos, p-c-Fos, nuclear factor kappa B (NF-κB/p65), and p-p65 and the effects of their inhibitors were examined using Western blot analysis and measurement of luciferase activity. p65 expression was detected by immunofluorescence. MMP-9 mRNA and protein levels were measured by quantitative reverse transcription polymerase chain reaction (qRT–PCR) and gelatin zymography, respectively.
RESULTS
qRT-PCR and gelatin zymography showed that TNF-α induced MMP-9 mRNA and protein expression in a dose- and time-dependent manner. These effects were reduced by the pretreatment of GES-1 cells with quercetin or a TNF-α antagonist (TNFR inhibitor) in a dose- and time-dependent manner. Quercetin and TNF-α antagonists decreased the TNF-α-induced phosphorylation of c-Src, ERK1/2, c-Fos, and p65 in a dose- and time-dependent manner. Quercetin, TNF-α antagonist, PP1, U0126, and tanshinone IIA (TSIIA) reduced TNF-α-induced c-Fos phosphorylation and AP-1–Luciferase (Luc) activity in a dose- and time-dependent manner. Pretreatment with quercetin, TNF-α antagonist, PP1, U0126, or Bay 11-7082 reduced TNF-α-induced p65 phosphorylation and translocation and p65–Luc activity in a dose- and time-dependent manner. TNF-α significantly increased GES-1 cell migration, and these results were reduced by pretreatment with quercetin or a TNF-α antagonist.
CONCLUSION
Quercetin significantly downregulates TNF-α-induced MMP-9 expression in GES-1 cells via the TNFR-c-Src–ERK1/2 and c-Fos or NF-κB pathways.
Keywords: Anti-inflammatory, Quercetin, Matrix metallopeptidase-9, Tumor necrosis factor-α, Normal human gastric epithelial cells
Core Tip: Gastric inflammation is a common digestive system disease. Proinflammatory cytokines, such as tumor necrosis factor-α (TNF-α), and degradation induced by matrix metallopeptidases (MMPs) play a crucial role in gastric injury. We investigated whether quercetin prevents TNF-α-induced gastric inflammation in normal human gastric mucosa epithelial cells. Quercetin significantly blocked MMP-9 activity and cell migration and protected against TNF-α-induced gastric injury. Quercetin appeared to block TNF-α-induced gastric injury by downregulating MMP-9 via the TNF-α antagonist-c-Src–extracellular-signal-regulated kinase 1/2 and c-Fos or nuclear factor kappa B pathways. Our data suggest that quercetin, which exhibits high bioavailability, may be effective as an adjuvant therapy for the treatment of gastric inflammation.
INTRODUCTION
Gastric diseases are among the most common aliments of the digestive system worldwide and include chronic gastritis, duodenal and gastric ulceration, gastric mucosa-associated lymphoid tissue lymphoma, and gastric cancer (GC)[1-3]. Gastric diseases have multifaceted causes that are related to the growing use of nonsteroidal anti-inflammatory drugs (NSAIDs), pathogen-mediated factors [such as Helicobacter pylori (H. pylori) infection], environmental factors (such as alcohol consumption, salt intake, and cigarette smoking), and host genetic and other factors (such as genetic polymorphisms, psychological stress, oxidative stress, and acidity)[4,5]. Gastric diseases accompanied by chronic inflammation involve increased neutrophil and mononuclear cell activities and the release of proinflammatory chemokines and cytokines, such as tumor necrosis factor-α (TNF-α)[6], interleukin (IL)-1[7], IL-6, IL-8, and IL-10[8], cell adhesion molecules [such as circular dichroism 44 and intercellular adhesion molecule-1 (ICAM-1)][9], and metalloproteinases (MMPs)[10]. The induction of MMPs is a major pathological feature and plays a critical role in the progression of gastric diseases[2,11,12]. Among the variants of genetic polymorphisms related to proinflammatory cytokines, those involving ILs and TNF-α are the most extensively studied.
TNF-α is a multifunctional cytokine that was first identified as a serum factor causing necrosis of transplanted tumors. This cytokine participates in the regulation of immune–inflammatory reactions involved in host defense against infectious, autoimmune, and endocrine diseases and cancer, and its actions help to determine the survival or death of various cells[13-16]. The upregulation of TNF-α and its related pathways is associated with some gastric diseases. Excessive TNF-α expression is associated with tumor promotion via a strong immune–inflammatory response and angiogenesis, which can modify the risk for gastric, breast, hepatocellular, cervical, or bladder carcinoma[17-20]. Harris et al[21] reported that H. pylori infection upregulates TNF-α expression and that this upregulation decreases gastric acid secretion[21]. Fan et al[22] reported that high expression of TNF-α can stimulate normal gastric epithelial cells to develop into precancerous cells and that these cells may eventually develop into GC. A similar mechanism is also noted in chronic gastritis, intestinal metaplasia, and dysplasia[22].
MMPs are a key group of enzymes that can destroy nearly all elements of the extracellular matrix, including components of the basement membrane[23,24]. MMP-9 expression damages the extracellular matrix, including components of the basement membrane, inducing gastric disease. MMP-9 is often upregulated or dysregulated in severe gastric disease, where it may accelerate severe gastric inflammation and possibly contribute to the development of GC. High MMP-9 expression is also associated with a poor survival rate in patients with GC[25-28]. MMP-9 is induced in a nuclear factor kappa B (NF-κB)-dependent manner[29,30]. Understanding the roles of inflammatory mediators may provide a basis for new therapeutic approaches for treating gastric inflammatory diseases.
Recent research suggests that dietary intake may alter the risk of gastric injury[31,32]. A frequently studied dietary component, quercetin, is a flavonoid polyphenolic compound that is abundant in fruits, red wine, and vegetables. Studies on the bioactivity of quercetin have found that it exerts many favorable effects on human health, such as antioxidant, anti-inflammatory, anticancer, antiallergic, antiviral, free radical-scavenging, and neuroprotective activities[33,34].
Quercetin protects gastric mucosal epithelial cells against injury caused by ischemia–reperfusion[35], ethanol[36], indomethacin[9], hydrogen peroxide[10], or H. pylori[37] in various animal models[38]. Quercetin also exerts gastroprotective activity against TNF-α-induced injury to the gastric mucosal epithelium, but the mechanisms responsible for this effect remain unknown. Here, we explored whether quercetin protects against TNF-α-induced MMP-9 damage to gastric mucosal epithelial cells. We examined the responses of the human gastric mucosal epithelial cell line GES-1 to challenge with immune–inflammatory reactions mediated by TNF-α and explored the underlying molecular mechanisms.
MATERIALS AND METHODS
Materials
Roswell Park Memorial Institute (RPMI) 1640 medium, fetal bovine serum (FBS), and TRIzol were purchased from Invitrogen (Carlsbad, CA, United States). Polyvinylidene fluoride (PVDF) membranes, enhanced chemiluminescence (ECL), and Western blot detection systems were obtained from GE Healthcare Biosciences (Chalfont St Giles, Buckinghamshire, United Kingdom). Anti-phospho-c-Src (Cat#2101), anti-phospho-ERK1/2 (Cat#4377), anti-phospho-c-Fos (Cat#5348), anti-phospho-NF-κB (p65) (Cat#3033), c-Src (Cat#2109), ERK2 (Cat#9108), c-Fos (Cat#2250), NF-κB (Cat#8242), and anti-glyceraldehyde-3-phosphate dehydrogenase (GAPDH) (Cat#2118) antibodies were purchased from Cell Signaling Technology (Danvers, MA, United States). Anti-mouse (Cat# 7056) and anti-rabbit (Cat# 5054) horseradish peroxidase-conjugated secondary antibodies were purchased from Santa Cruz Biotechnology (Santa Cruz, CA, United States). 4’,6-Diamidino-2-phenylindole was purchased from Vector Laboratories (Burlingame, CA, United States). TurboFect Transfection Reagent was purchased from Thermo Fisher Scientific (Waltham, MA, United States). The iScript cDNA Synthesis Kit, SsoFast EvaGreen Supermix, and CFX Connect Real-Time polymerase chain reaction (PCR) detection system were obtained from Bio–Rad Laboratories, Inc. (Hercules, CA, United States). A dual-luciferase reporter system was obtained from Promega (Madison, WI, United States). All inhibitors, including a TNF-α antagonist (SC-356159), PP1 (SC-203212A), U0126 (SC-222395), tanshinone IIA (TSIIA) (SC-200932), and Bay 11-7082 (SC-202490), were purchased from Santa Cruz Biotechnology. Quercetin was obtained from Biotic Chemical Co., Ltd. (New Taipei City, Taiwan). A stock solution of quercetin was resuspended in a 1:9 (v/v) solution of dimethyl sulfoxide (DMSO) and 99% ethanol and diluted to the presented final concentration with culture medium. Human recombinant TNF-α was obtained from R&D Systems (AFL210) (Minneapolis, MN, United States). MMP9 inhibitor (MMP9i) (Cat#15942-500) was purchased from Biomol (Plymouth Meeting, PA, United States). Cell counting Kit-8 (CCK-8) kit reagent was obtained from MedChemExpress Ltd. (Hercules, CA, United States). All other enzymes, reagents, and chemicals were obtained from Sigma–Aldrich (St. Louis, MO, United States).
Cell culture
GES-1 cells were obtained from Xiamen University in China. The cells were cultured in RPMI 1640 medium containing 10% FBS, 100 IU/mL penicillin G, 100 mg/mL streptomycin sulfate, and nonessential amino acids at 37 °C in a humidified atmosphere with 5% CO2 according to a previously described standard protocol[39]. The cells were either untreated or treated with quercetin or inhibitors of the TNF-α antagonist, MMP-9i, c-Src (PP1), ERK1/2 (U0126), c-Fos (TSIIA), or NF-κB (BAY). These substances were added to the cell culture 1 h before the addition of TNF-α in all experiments.
MMP zymography
Growth-arrested cells were incubated with different treatments for the indicated time intervals. The cells were either untreated or treated with quercetin or inhibitors of TNF-α antagonist, c-Src (PP1), ERK1/2 (U0126), c-Fos (TSIIA), or NF-κB (BAY). After treatment, the conditioned media were collected and mixed with 5 × nonreducing sample buffer and analyzed on a 10% gel containing 0.15% gelatin. After electrophoresis, the gel was washed, incubated, stained, and destained. The gelatinolytic activity appeared as parallel white bands on a blue background[40].
CCK-8 assay
GES-1 cells (5000 cells/well) were seeded into 96-well plates with serum-free RPMI 1640 medium and cultured for 24 h at 37 °C in a humidified atmosphere with 5% CO2. The culture medium was replaced by quercetin diluted in series to concentrations of 0, 0.01, 0.1, 1, 10, or 100 mg/mL, and the cells were cultured for 24 or 48 h. After incubation with quercetin, 10 mL of the CCK-8 kit reagent was added to each well (100 mL/well), and the incubation was continued for 1 h. The optical density at 450 nm was measured using a multifunction microplate reader (SpectraMax i3; Kelowna International Scientific Inc., New Taipei, Taiwan) using a previously described standard protocol[41]. Cell viability is expressed as the percentage of living cells relative to the control.
Quantitative reverse transcription–PCR
GES-1 cells were seeded into 9-cm dishes with serum-free RPMI 1640 medium, cultured for 24 h at 37 °C, and then incubated with TNF-α for 0, 16, 20, or 24 h. Total RNA was extracted using TRIzol reagent, and the concentration of RNA was measured on a Nano100 Micro-Spectrophotometer (CLUBIO; Taipei, Taiwan). cDNA was then synthesized using an iScript cDNA Synthesis Kit and amplified on a spectrofluorometric thermal cycler (iCycler; Bio–Rad Laboratories)[42]. To evaluate MMP9 mRNA expression in GES-1 cells, qRT–PCR was performed according to a previously described standard protocol using GAPDH as the internal control[42]. Fluorescence emitted by SsoFast EvaGreen Supermix was measured using the CFX Connect Real-Time PCR detection system. The following primers were used for qRT–PCR: Human MMP9 (sense, 5’–AGTTTGGTGTCGCGGAGCAC–3’ and antisense, 5’–TACAT GAGCGCTTCCGGCAC–3’) and human GAPDH (sense, 5’–ACAGTCAGCCGCATCTTCTT–3’ and antisense, 5’–GACAAGCTTCCCGTTCTCAG–3’). Relative gene expression is expressed using the Delta-Delta-Ct (ddCt) method, where Ct represents the mean threshold cycle.
Western blot analysis
Growth-arrested cells were incubated with different treatments for the indicated time intervals. The cells were either untreated or treated with quercetin or inhibitors of TNF-α antagonist, c-Src (PP1), ERK1/2 (U0126), c-Fos (TSIIA), or NF-κB (BAY). After treatment, the cells were washed with PBS, scraped, lysed in lysis buffer, and collected as a whole-cell extract at 4 °C using a previously described standard protocol[42]. Samples were loaded onto a 10% SDS-PAGE gel, electrophoresis was performed, and the bands were transferred to a PVDF membrane. The PVDF membrane was incubated overnight with specific primary antibodies, including anti-phospho-c-Src, anti-phospho-ERK1/2, anti-phospho-c-Fos, anti-phospho-NF-κB, or anti-GAPDH. The PVDF membranes were washed and then incubated with anti-mouse or anti-rabbit horseradish peroxidase-conjugated secondary antibody (dilution, 1:2000) for 1 h. The relative expression levels of c-Src, ERK1/2, c-Fos, and NF-κB were normalized to that of GAPDH. Finally, the immunoreactive bands were incubated in enhanced chemiluminescence reagent, and images were recorded on a UVP BioSpectrum 500 Imaging System (UVP, Inc.; Upland, CA, United States). Densitometry was quantified using UN-SCAN-IT gel software (Silk Scientific, Inc.; Orem, UT, United States).
Cell migration assay
To evaluate the influence of TNF-α-induced MMP-9 expression on the metastatic activity of GES-1 cells, an in vitro Transwell assay (Becton-Dickinson, Franklin Lakes, NJ, United States) was used. Cells at a concentration of 1 × 105 cells/100 mL in serum-free RPMI 1640 medium were added to the upper chamber, and the Matrigel-uncoated insert was inserted in the lower chamber, which contained 20% FBS + RPMI 1640 medium. The migration assay was run for 24 h following a previously described standard protocol[39,42].
Immunofluorescence
The influence of TNF-α-induced MMP-9 expression in GES-1 cells and the potential involvement of NF-κB/p65 translocation from the cytoplasm into the nucleus in this process were investigated. GES-1 cells were seeded into culture plates with coverslips. The cells were either untreated or treated with quercetin or inhibitors of c-Src (PP1), ERK1/2 (U0126), or NF-κB (BAY). After treatment, the cells were washed, fixed, permeabilized, blocked, and stained with anti-NF-κB. The coverslips were affixed to slides with mounting medium and stained with 4’,6-diamidino-2-phenylindole as previously described[43]. Fluorescence images were photographed using a fluorescence microscope (Leica Microsystems, Wetzlar, Germany).
Luciferase assay
The human AP-1–Luc and NF-κB–Luciferase (Luc) response element reporter plasmids pGL4.44GL4.44 [luc2P/AP1 RE/Hygro] (No. E411A; JQ858516) and pGL4.32 [luc2P/NF-κB-RE/Hygro] (No. E849A; EU581860), and the dual-luciferase reporter system were obtained from Promega (Madison). Two plasmids were transfected into GES-1 cells using TurboFect Transfection Reagent according to a previously reported protocol[44]. The cells were either untreated or treated with quercetin or inhibitors of TNF-α antagonist, c-Src (PP1), ERK1/2 (U0126), or NF-κB (BAY). After treatment, the cells were collected, lysed, and centrifuged, and aliquots of the supernatants were tested for promoter activity using the dual-luciferase assay system. Firefly luciferase activity was standardized to that of Renilla luciferase.
Statistical analysis
All data were obtained from at least three independent experiments and are presented as the mean ± SEM. The data were analyzed using one-way analysis of variance (ANOVA) followed by Tukey’s post-hoc test to identify significant differences between multiple groups. Statistical analyses were performed using GraphPad Prism 6.0 (GraphPad Software, Inc.; La Jolla, CA, United States). A P value < 0.05 was considered significant. The statistical methods of this study were reviewed by Dr. Jian-Hao Chen from Estat Statistics Consulting Company (Taipei, Taiwan).
RESULTS
Omics approach to identify inflammation-related proteins involved in GC
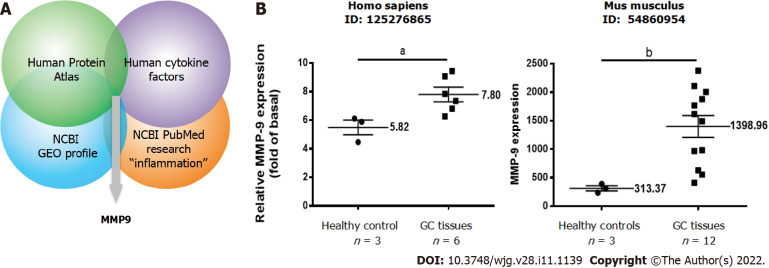
To examine the potential roles of inflammation-related proteins in GC, we used an omics approach based on four datasets from the following sources: (1) The public Human Protein Atla (https://www.proteinatlas.org); (2) The public United States National Center for Biotechnology Information (NCBI) Gene Expression Omnibus (GEO) profiles (https://www.ncbi.nlm.nih.gov/geoprofiles/); (3) Human cytokine factors (https://www.abcam.com/human-cytokine-antibody-array-membrane-174-targets-ab193657.html); and (4) NCBI PubMed research “inflammation” papers. The combination of the four datasets revealed significant upregulation of MMP9 in GC relative to paired normal tissues, as shown in Figure 1A. The NCBI GEO profiles revealed consistent significant MMP9 upregulation (Human, ID: 125276865; Mouse, ID:54860954) in GC tissues relative to paired normal tissues (Figure 1B). Therefore, we selected MMP9 for further study.
Figure 1.
Identification studies of matrix metallopeptidase-9, a potential inflammation-related protein involved in gastric cancer. A: Identification of potential inflammation-related proteins involved in gastric cancer (GC) based on an omics approach including four integrated datasets. The strategy included proteomic profiling; B: Relative expression levels of matrix metallopeptidase-9 in paired GC and adjacent normal tissues in humans and mice. A t test was used for comparisons between the two groups. Data are expressed as the mean ± SEM of three independent experiments. aP < 0.05, bP < 0.01 vs controls. GC: Gastric cancer; GEO: Gene Expression Omnibus; MMP-9: Matrix metallopeptidase-9; NCBI: National Center for Biotechnology Information.
Role of MMP-9 expression in TNF-a-induced inflammation in GES-1 cells
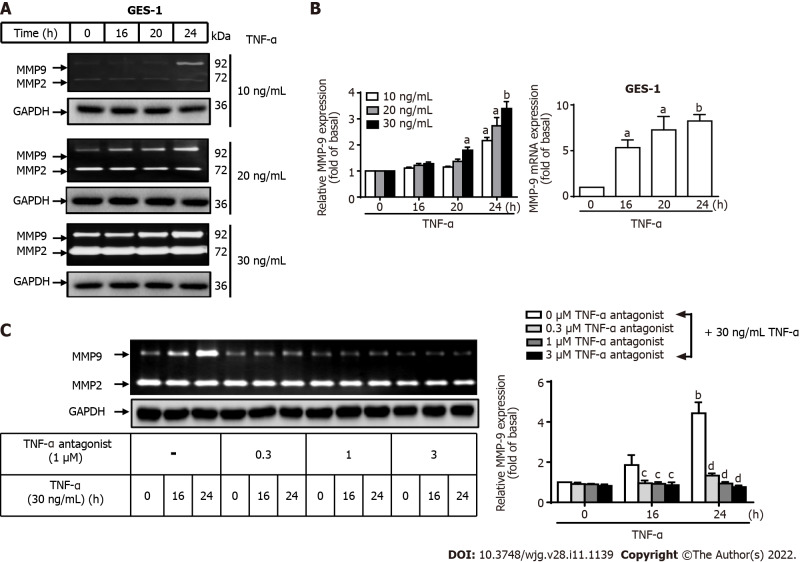
MMP-9 expression is involved in gastric inflammation, may contribute to the development of GC, and is associated with poor survival rates[25-28]. To explore the role of TNF-α-activated MMP-9 in GC, GES-1 cells were treated with different concentrations of TNF-α (10, 20, or 30 ng/mL) for designated times (0, 16, 20, or 24 h), and MMP9 mRNA and protein levels were measured. TNF-α at a dose of 10 mg/mL for 16 or 20 h did not affect MMP-9 protein expression in GES-1 cells, but exposure for 24 h increased MMP-9 expression compared with 0 h (P < 0.05). TNF-α at a dose of 20 mg/mL for 24 h significantly increased MMP-9 expression compared with 0 h (P < 0.05). TNF-α at a dose of 30 mg/mL for 20 or 24 h resulted in the highest MMP-9 expression of GES-1 cells compared with 0 h (P < 0.05 and P <0.01, respectively). TNF-α induced MMP-9 enzymatic activity in a time- and concentration-dependent manner (Figure 2A). qRT–PCR was performed to measure the MMP-9 mRNA level in TNF-a-induced GES-1 cells. After TNF-α stimulation, MMP-9 mRNA was significantly upregulated in a time-dependent manner after 16, 20, and 24 h of incubation compared with 0 h (P < 0.05, P < 0.05, and P < 0.001, respectively) (Figure 2B). These observations suggest that MMP-9 expression was induced by TNF-α at both the transcriptional and translational levels in GES-1 cells. Therefore, we chose to use 30 ng/mL TNF-α in GES-1 cells for further assessments of TNF-α-induced changes in MMP-9 expression. We explored whether a TNF-α antagonist can repress TNF-α-activated MMP-9 expression in GES-1 cells. The cells were either untreated or treated with a TNF-α antagonist (0.3, 1, and 3 mM) for 1 h before the addition of TNF-α. MMP-9 inhibition significantly repressed TNF-α-activated MMP-9 expression in GES-1 cells in a time-dependent manner after 16 and 24 h of incubation compared with 0 h (P < 0.01 and P < 0.001, respectively) (Figure 2C).
Figure 2.
Tumor necrosis factor-α induces matrix metallopeptidase-9 expression via tumor necrosis factor-α receptor in normal human gastric mucosa epithelial cells. A: Tumor necrosis factor-α (TNF-α) was used at concentrations of 10, 20, and 30 ng/mL to stimulate normal human gastric mucosa epithelial cells (GES-1) for 0, 16, 20, or 24 h, and matrix metallopeptidase-9 (MMP-9) enzymatic activity was measured by gelatin zymography as described in the Materials and Methods section; B: MMP-9 transcripts were analyzed by quantitative reverse transcription-polymerase chain reaction; C: Cells were either untreated or treated with a TNF-α antagonist (TNFR inhibitor) (1 mM) 1 h before the addition of TNF-α (30 ng/mL). The TNF-α antagonist repressed TNF-α-activated MMP-9 expression in GES-1 cells. One-way ANOVA was used for comparisons among different treatment time points (aP < 0.05, bP < 0.01 vs control cells in 0 h). One-way ANOVA was used for comparisons among different treatments (cP < 0.05, dP < 0.01 vs TNF-α-stimulated cells). Data are expressed as the mean ± SEM of three independent experiments. GAPDH: Glyceraldehyde-3-phosphate dehydrogenase; GES-1: Normal human gastric mucosa epithelial cell line; MMP: Matrix metallopeptidase; TNF-α: Tumor necrosis factor-α.
Effects of quercetin on TNF-α-induced MMP-9 expression in GES-1 cells
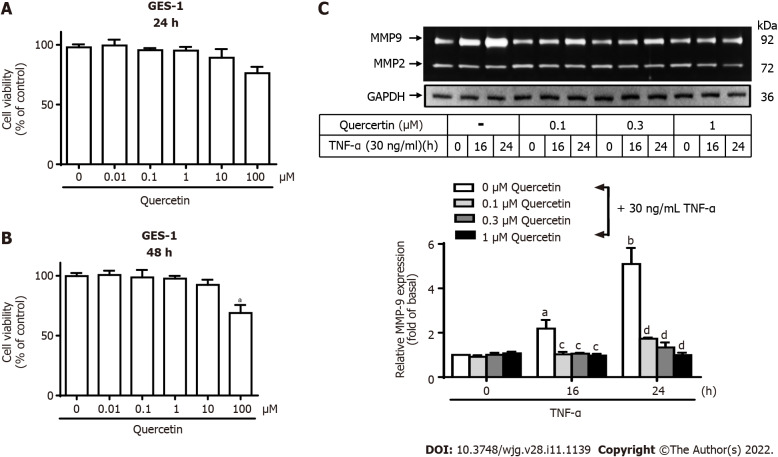
Quercetin has anti-inflammatory effects[33,34]. We first measured the viability of GES-1 cells after quercetin treatment. Quercetin treatment at concentrations of 0, 0.01, 0.1, 1, or 10 mM for 24 to 48 h had almost no effect on GES-1 cell viability (Figure 3A and B). In contrast, 100 mM quercetin significantly reduced GES-1 cell viability after 48 h compared with 0 mM (P < 0.01). Therefore, quercetin was used at concentrations up to 1 mM in the following experiments to explore whether quercetin represses TNF-α-activated MMP-9 expression in GES-1 cells and its mechanism of action. The cells were either untreated or treated with quercetin (0, 0.1, 0.3, or 1 mM) for 1 h. Then, TNF-α was added, and the cells were incubated for 0, 16, or 24 h (Figure 3C). Quercetin significantly repressed TNF-α-activated MMP-9 expression in GES-1 cells at doses of 0.1, 0.3, and 1 mM and in a time-dependent manner after 16 and 24 h of incubation.
Figure 3.
Quercetin suppresses tumor necrosis factor-α-induced matrix metallopeptidase-9 expression in normal human gastric mucosa epithelial cells. A and B: Effects of quercetin on the viability of normal human gastric mucosa epithelial cells (GES-1). GES-1 cells were treated with different concentrations of quercetin (0, 0.01, 0.1, 1, 10, or 100 mM) for 24 h (A) or 48 h (B), and cell viability was measured using the cell counting Kit-8 assay; C: Cells were either untreated or treated with quercetin (0, 0.1, 0.3, or 1 mM) for 1 h before the addition of tumor necrosis factor-α (TNF-α) (30 ng/mL) and then incubated for 0, 16, or 24 h as described in the Materials and Methods section. Matrix metallopeptidase-9 zymogen activity was analyzed by gelatin zymography. One-way ANOVA was used for comparisons among different treatment time points (aP < 0.05, bP < 0.01 vs control cells in 0 h). One-way ANOVA was used for comparisons among different treatments (cP < 0.05, dP < 0.01 vs TNF-α-stimulated cells). Data are expressed as the mean ± SEM of three independent experiments. CCK-8: Cell Counting Kit-8; GAPDH: Glyceraldehyde-3-phosphate dehydrogenase; GES-1: Normal human gastric mucosa epithelial cell line; MMP-9: Matrix metallopeptidase-9; TNF-α: Tumor necrosis factor-α.
Involvement of c-Src in TNF-α-induced MMP-9 expression in GES-1 cells
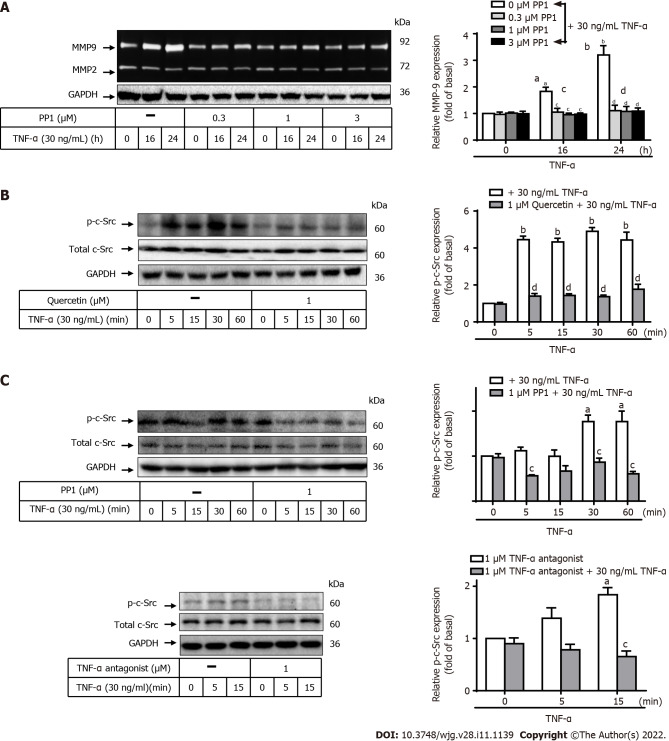
Kim et al[45] reported that TNF-α-induced MMP-9 expression is mediated via mitogen-activated protein kinase (MAPK) in human GC cells[45]. We next explored the pathway through which quercetin reduces TNF-α-induced MMP-9 expression in GES-1 cells. To determine the role of c-Src in TNF-α-induced MMP-9 expression, two inhibitors of TNF-α antagonist or c-Src (PP1) were used. GES-1 cells were pretreated with the TNF-α antagonist (1 mM) or PP1 (0, 0.3, 1, or 3 mM) for 1 h, and the cells were then incubated with TNF-α for 0, 16, or 24 h (Figure 4A). Pretreatment with PP1 significantly attenuated TNF-α-induced MMP-9 expression in cells incubated for 16 or 24 h in a time-dependent manner. This finding suggests that c-Src was involved in these responses. To confirm this effect, Western blot analysis was used to examine the phosphorylation of c-Src in cells exposed to TNF-α for 0, 5, 15, 30, or 60 min (Figure 4B and C). Pretreatment with quercetin, PP1, or TNF-α antagonist blocked TNF-α-activated phosphorylation of c-Src in a time-dependent manner with the exception of incubation with PP1 + TNF-α and TNF-α antagonist + TNF-α for 15 min (Figure 4C). These findings suggest that TNF-α induced MMP-9 expression via TNFR-c-Src in GES-1 cells.
Figure 4.
Quercetin suppresses tumor necrosis factor-α-induced matrix metallopeptidase-9 expression via tumor necrosis factor-α antagonist-proto-oncogene tyrosine-protein kinase Src in normal human gastric mucosa epithelial cells. A: Cells were either untreated or treated with PP1 (0, 0.3, 1, or 3 mM) for 1 h before the addition of tumor necrosis factor-α (TNF-α) (30 ng/mL) and then incubated for 0, 16, or 24 h as described in the Materials and Methods section. Matrix metallopeptidase-9 (MMP-9) zymogen activity was analyzed by gelatin zymography; B and C: Cells were either untreated or treated with quercetin (B; 0 or 1 mM) or PP1 (C; 0 or 1 mM) or TNF-α antagonist (1 mM) for 1 h before the addition of TNF-α (30 ng/mL) and incubated for 0, 16, or 24 h. The phosphorylation of c-Src was measured using Western blot analysis after incubation of cells with TNF-α for 0, 5, 15, 30, or 60 min. One-way ANOVA was used for comparisons among different treatment time points (aP < 0.05, bP < 0.01 vs control cells in 0 h). One-way ANOVA was used for comparisons among different treatments (cP < 0.05, dP < 0.01 vs TNF-α-stimulated cells). Data are expressed as the mean ± SEM of three independent experiments. c-Src: Proto-oncogene tyrosine-protein kinase Src; GAPDH: Glyceraldehyde-3-phosphate dehydrogenase; GES-1: Normal human gastric mucosa epithelial cell line; MMP-9: Matrix metallopeptidase-9; TNF-α: Tumor necrosis factor-α.
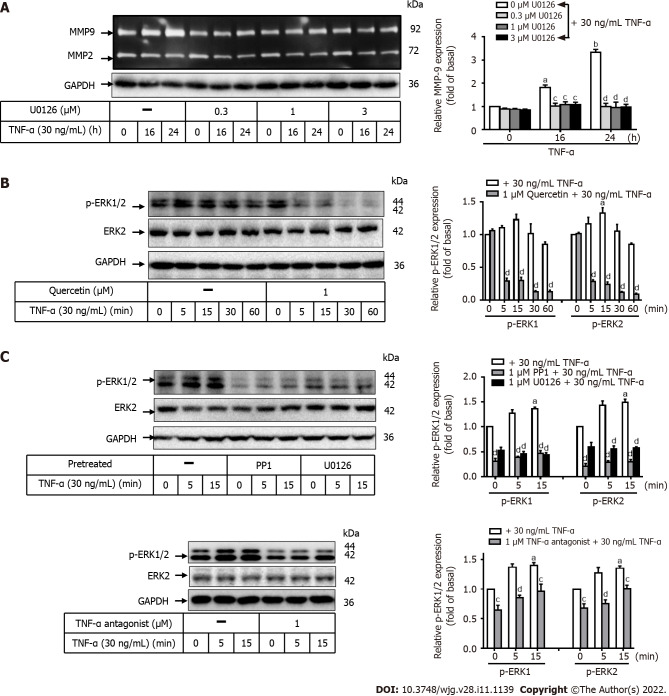
Involvement of ERK1/2 in TNF-a-induced MMP-9 expression in GES-1 cells
To evaluate whether ERK1/2 contributes to TNF-α-induced MMP-9 expression, two inhibitors of TNF-α antagonist or ERK1/2 (U0126) were used. GES-1 cells were pretreated with the TNF-α antagonist (1 mM) or U0126 (0, 0.3, 1, or 3 mM) for 1 h and then incubated with TNF-α for 0, 16, or 24 h (Figure 5A). Pretreatment with U0126 significantly attenuated TNF-α-induced MMP-9 expression after 16 and 24 h of incubation in a time-dependent manner. This finding suggests that ERK1/2 is involved in this response. To further explore the effects of quercetin, U0126, PP1, or TNF-α antagonist on TNF-α-activated ERK1/2 phosphorylation, Western blot analysis was used to measure ERK1/2 phosphorylation after exposure for 0, 5, 15, 30, or 60 min. Pretreatment with quercetin, U0126, PP1, or TNF-α antagonist blocked TNF-α-activated ERK1/2 phosphorylation in a time-dependent manner (Figure 5B and C). These data suggest that TNF-α-induced MMP-9 expression may be mediated through TNFR-c-Src-ERK1/2 in GES-1 cells.
Figure 5.
Quercetin suppresses tumor necrosis factor-α-induced matrix metallopeptidase-9 expression via tumor necrosis factor-α antagonist-c-Src-extracellular-signal-regulated kinase 1/2 in normal human gastric mucosa epithelial cells. A: Normal human gastric mucosa epithelial cells (GES-1) were either untreated or treated with U0126 (0, 0.3, 1, or 3 mM) for 1 h before the addition of tumor necrosis factor-α (TNF-α) (30 ng/mL) and incubated for 0, 16, or 24 h as described in the Materials and Methods section. Matrix metallopeptidase-9 zymogen activity was measured using gelatin zymography; B and C: Cells were either untreated or treated with quercetin (B; 0 or 1 mM), PP1 (C; 0 or 1 mM), U0126 (0 or 1 mM), or TNF-α antagonist (1 mM) for 1 h before the addition of TNF-α (30 ng/mL) and incubated for 0, 16, or 24 h. The phosphorylation of extracellular-signal-regulated kinase 1/2 was examined by Western blot analysis in cells incubated for 0, 5, 15, 30, or 60 min. One-way ANOVA was used for comparisons among different treatment time points (aP < 0.05, bP < 0.01 vs control cells in 0 h). One-way ANOVA was used for comparisons among different treatments (cP < 0.05, dP < 0.01 vs TNF-α-stimulated cells). Data are expressed as the mean ± SEM of three independent experiments. ERK: Extracellular-signal-regulated kinase; GAPDH: Glyceraldehyde-3-phosphate dehydrogenase; GC: Gastric cancer; GES-1: Normal human gastric mucosa epithelial cell line; MMP-9: Matrix metallopeptidase-9; TNF-α: Tumor necrosis factor-α.
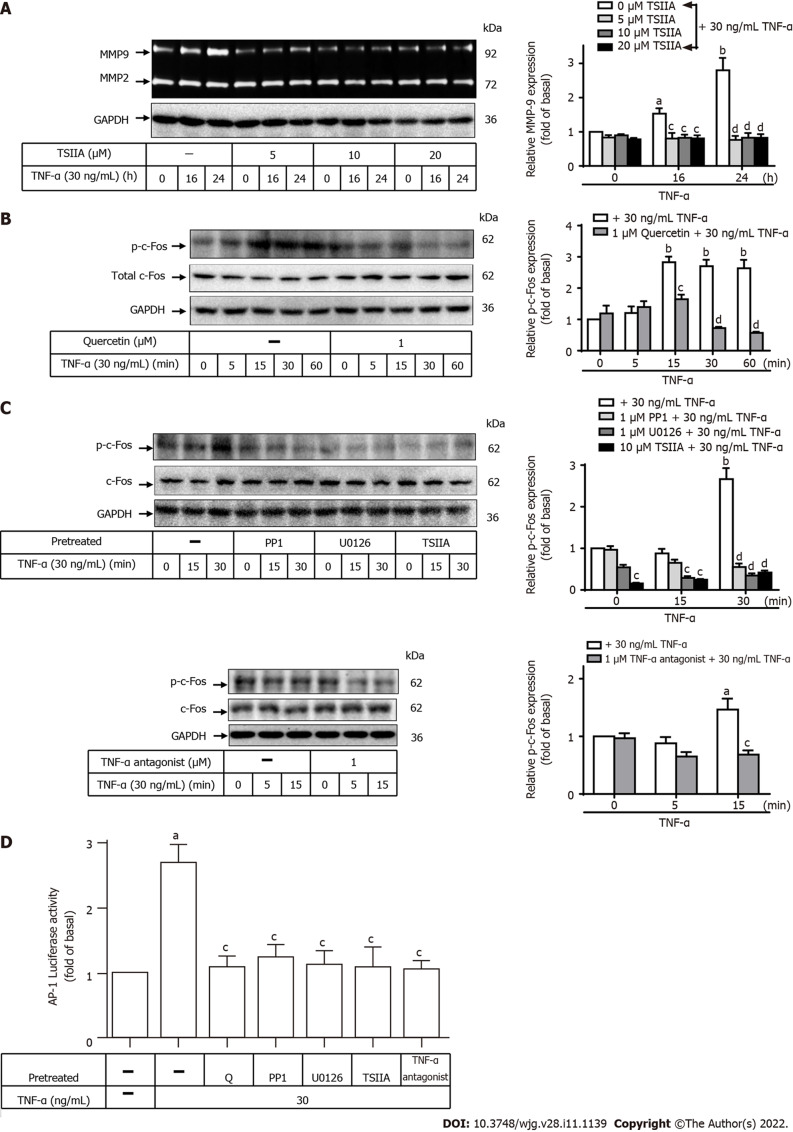
Involvement of c-Fos in TNF-a-induced MMP-9 expression in GES-1 cells
AP-1 and NF-κB play important regulatory roles in the human MMP-9 promoter region, where they react with cytokines or growth factors[46]. To examine the role of c-Fos in TNF-α-induced MMP-9 expression, two inhibitors of TNF-α antagonist or c-Fos (TSIIA) were used. GES-1 cells were pretreated with TNF-α antagonist (1 mM) or TSIIA (0, 5, 10, or 20 mM) for 1 h and incubated with TNF-α for 0, 16, or 24 h (Figure 6A). Pretreatment with TSIIA significantly attenuated TNF-α-induced MMP-9 expression after 16 and 24 h of incubation in a time-dependent manner. This finding suggests that c-Fos was involved in these responses. To further explore the effects of quercetin, TSIIA, U0126, PP1, and TNF-α antagonist on TNF-α-activated c-Fos phosphorylation, Western blot analysis was used to measure c-Fos phosphorylation in cells incubated for various time intervals (Figure 6B and C). Pretreatment with quercetin or TSIIA, U0126, PP1, or TNF-α antagonist blocked TNF-α-activated phosphorylation of c-Fos in a time-dependent manner. An assay to analyze the activity of human AP-1–Luc response element reporter plasmids was used to explore whether the quercetin-induced reduction in AP-1 promoter reporter activity occurs through the signaling pathways mentioned above. Quercetin attenuated AP-1 promoter reporter activity (Figure 6D), and this effect may occur through the TNFR-c-Src-ERK1/2-c-Fos pathway. Taken together, these results suggest that quercetin reduces TNF-α-induced MMP-9 expression in GES-1 cells via the TNFR-c-Src-ERK1/2-c-Fos pathway.
Figure 6.
Quercetin suppresses tumor necrosis factor-α-induced matrix metallopeptidase-9 expression via tumor necrosis factor-α antagonist-c-Src-extracellular-signal-regulated kinase 1/2–c-Fos in normal human gastric mucosa epithelial cells. A: Normal human gastric mucosa epithelial cells (GES-1) were either untreated or treated with Tanshinone IIA (TSIIA) (0, 5, 10, or 20 mM) 1 h before the addition of tumor necrosis factor-α (TNF-α) (30 ng/mL) and incubated for 0, 16, or 24 h as described in the Materials and Methods section. Matrix metallopeptidase-9 zymogen activity was measured using gelatin zymography; B and C: Cells were either untreated or treated with quercetin (B; 0 or 1 mM), PP1 (C; 0 or 1 mM), U0126 (0 or 1 mM), TSIIA (0 or 10 mM), or TNF-α antagonist (1 mM) for 1 h before the addition of TNF-α (30 ng/mL) and incubated for 0, 16, or 24 h. The phosphorylation of c-Fos was measured by Western blot analysis in cells incubated for 0, 5, 15, 30, or 60 min; D: GES-1 cells were transfected with human AP-1–Luc response element reporter plasmids containing the dual-luciferase reporter system. The cells were then pretreated with PP1 (0 or 1 mM), U0126 (0 or 1 mM), TSIIA (0 or 10 mM), TNF-α antagonist (1 mM), or quercetin (1 mM) for 1 h and exposed to TNF-α for 1 h, and luciferase activity was measured. Firefly luciferase activity was standardized to Renilla luciferase activity. One-way ANOVA was used for comparisons among different treatment time points (aP < 0.05, bP < 0.01 vs control cells in 0 h). One-way ANOVA was used for comparisons among different treatments (cP < 0.05, dP < 0.01 vs TNF-α-stimulated cells). Data are expressed as the mean ± SEM of three independent experiments. ERK: Extracellular-signal-regulated kinase; AP-1: Activator protein-1; GAPDH: Glyceraldehyde-3-phosphate dehydrogenase; GES-1: Normal human gastric mucosa epithelial cell line; Luc: Luciferase; GES-1: Normal human gastric mucosa epithelial cell line; MMP-9: Matrix metallopeptidase-9; Q: quercetin; TNF-α: Tumor necrosis factor-α; TSIIA: Tanshinone IIA.
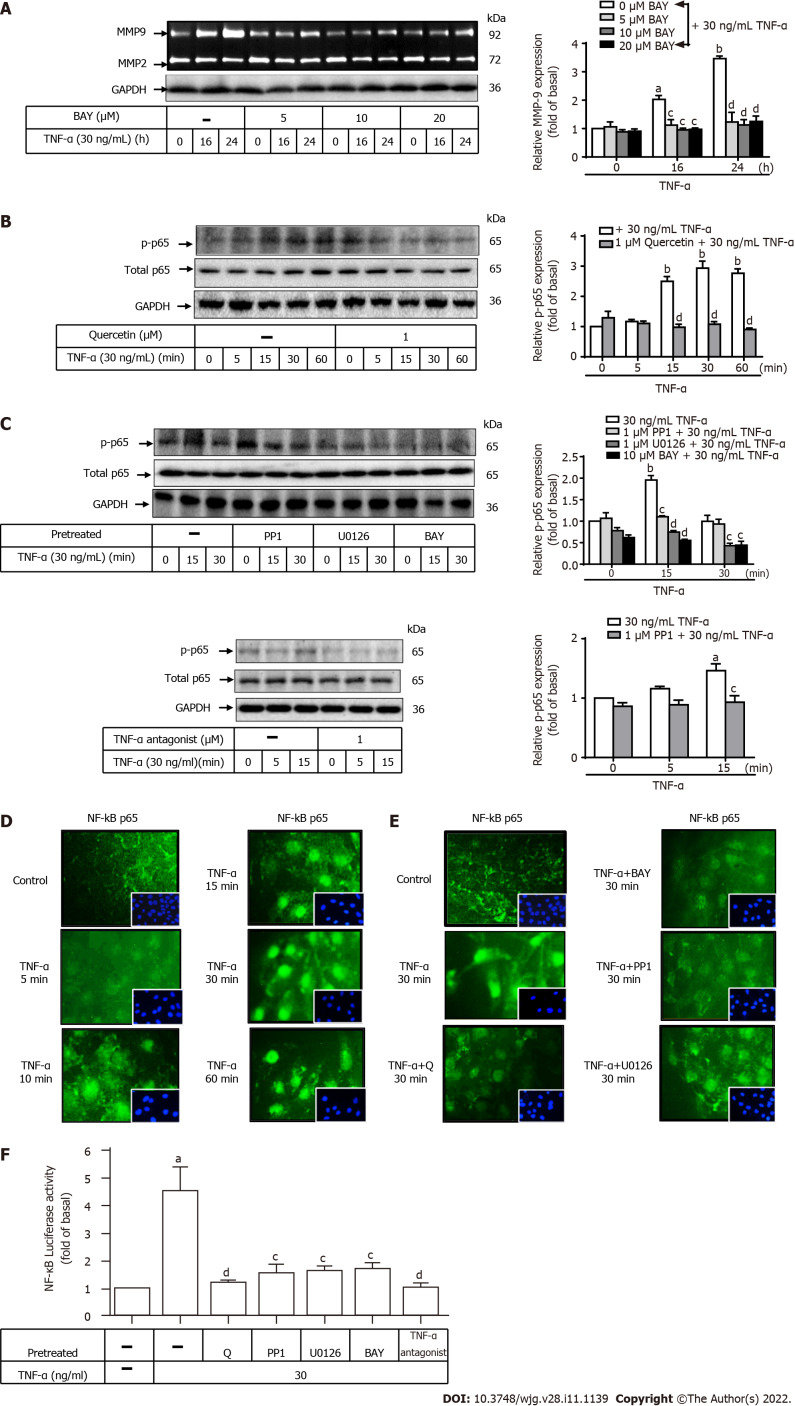
Involvement of NF-κB in TNF-a-induced MMP-9 expression in GES-1 cells
To clarify the role of NF-κB (p65) in TNF-α-induced MMP-9 expression, two inhibitors of TNF-α antagonist or NF-κB (p65) (BAY) were used. GES-1 cells were pretreated with TNF-α antagonist (1 mM) or BAY (0, 5, 10, or 20 mM) for 1 h before the addition of TNF-α and incubated for 0, 16, or 24 h (Figure 7A). Pretreatment with BAY significantly attenuated TNF-α-induced MMP-9 expression after 16 and 24 h of incubation (P < 0.01; P < 0.05) in a time- and dose-dependent manner. This finding suggests that NF-κB (p65) was involved in these responses. To further examine the effects of quercetin, BAY, U0126, PP1, or TNF-α antagonist on TNF-α-activated NF-κB (p65) phosphorylation, Western blot analysis was used to measure NF-κB (p65) phosphorylation in cells incubated for the indicated time intervals. Pretreatment with quercetin, BAY, U0126, PP1, or TNF-α antagonist blocked TNF-α-activated phosphorylation of NF-κB (p65) in a time-dependent manner (Figure 7B and C).
Figure 7.
Quercetin suppresses tumor necrosis factor-α-induced tumor necrosis factor-α antagonist-c-Src–extracellular-signal-regulated kinase 1/2–nuclear factor kappa B (p65) activation in normal human gastric mucosa epithelial cells. A: Normal human gastric mucosa epithelial cells (GES-1) were either untreated or treated with BAY (0, 5, 10, or 20 mM) for 0, 16, or 24 h; compounds were added 1 h before the addition of tumor necrosis factor-α (TNF-α) (30 ng/mL) as described in the Materials and Methods section. Matrix metallopeptidase-9 zymogen activity was measured by gelatin zymography. B and C: Cells were either untreated or treated with quercetin (B; 0 or 1 mM), PP1 (C; 0 or 1 mM), U0126 (0 or 1 mM), BAY (0 or 10 mM), or TNF-α antagonist (1 mM) for 1 h before the addition of TNF-α (30 ng/mL) and incubated for 0, 16, or 24 h. Nuclear factor kappa B (NF-κB) phosphorylation was examined by Western blot analysis; D and E: GES-1 cells were treated with 30 ng/mL TNF-α for 0, 5, 10, 15, 30, or 60 min (D) or quercetin (1 mM), PP1 (1 mM), U0126 (1 mM), or BAY (10 mM) for 1 h (E) before exposure to TNF-α (30 ng/mL) for 30 min; F: GES-1 cells were transfected with the human NF-κB–Luc response element reporter plasmids containing the dual-luciferase reporter system. The cells were then pretreated with PP1 (0 or 1 mM), U0126 (0 or 1 mM), BAY (0 or 10 mM), quercetin (1 mM), or TNF-α antagonist (1 mM) for 1 h and exposed to TNF-α for 1 h. Then, luciferase activity was measured. Firefly luciferase activity was standardized to that of Renilla luciferase. One-way ANOVA was used for comparisons among different treatment time points (aP < 0.05, bP < 0.01 vs control cells in 0 h). One-way ANOVA was used for comparisons among different treatments simultaneously (cP < 0.05, dP < 0.01 vs TNF-α-stimulated cells). Data are expressed as the mean ± SEM of three independent experiments. ERK: Extracellular-signal-regulated kinase; GAPDH: Glyceraldehyde-3-phosphate dehydrogenase; GES-1: Normal human gastric mucosa epithelial cell line; MMP-9: Matrix metallopeptidase-9; NF-κB: Nuclear factor kappa B; Q: Quercetin; TNF-α: Tumor necrosis factor-α.
We used immunofluorescence to assess the effects of quercetin on NF-κB translocation from the cytoplasm into the nucleus. Cells were first stimulated with TNF-α (30 ng/mL) for 0, 5, 10, 15, 30, or 60 min. The data showed that TNF-α induced NF-κB translocation in cells in a time-dependent manner. A maximal response was achieved within 30 min and sustained over 60 min during the period of observation (Figure 7D). Quercetin, PP1, BAY, and U0126 reduced TNF-α-induced NF-κB translocation. PP1 and BAY downregulated MMP9 expression in TNF-α-induced GES-1 cells (Figure 7E). An assay to measure human NF-κB–Luc response element reporter plasmid activity was used to examine whether quercetin could reduce NF-κB promoter reporter activity through the signaling pathways mentioned above. Quercetin attenuated NF-κB promoter reporter activity, and this effect may occur through the TNFR-c-Src-ERK1/2–NF-κB pathway (Figure 7F). Taken together, these results suggest that quercetin reduced TNF-α-induced MMP-9 expression in GES-1 cells via the TNFR-c-Src–ERK1/2–c-Fos and NF-κB pathways.
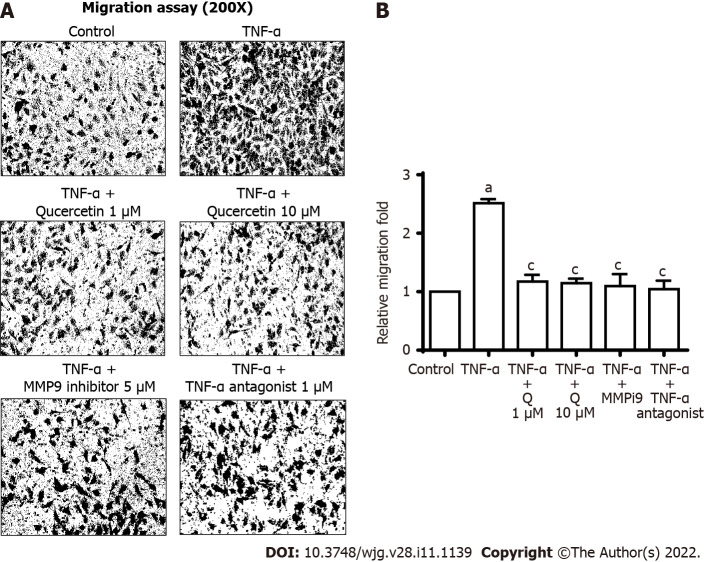
In vitro antimetastatic activities of quercetin
Upregulation of MMP9 is involved in the inflammatory response and cell migration[47-49]. Therefore, we further investigated the effects of quercetin on TNF-α-induced MMP9-mediated functional changes in GES-1 cells. First, images of GES-1 cell migration were observed 24 h after TNF-α treatment. Pretreatment with quercetin (1 mM or 10 mM), a TNF-α antagonist (1 mM), or MMP9i (5 mM) significantly blocked TNF-α-induced cell migration (Figure 8). This finding suggests that quercetin repressed TNF-α-induced cell migration by reducing MMP-9 expression in GES-1 cells.
Figure 8.
Quercetin exhibit antimetastatic activities in vitro. A: Cells were plated in 24-well culture plates, grown to confluence, and starved with serum-free medium for 24 h. Cells were pretreated with a tumor necrosis factor-α (TNF-α) antagonist (1 mM), matrix metallopeptidase-9 inhibitor (5 mM), or quercetin (1 or 10 mM) for 1 h, and monolayer cells were incubated with TNF-α (30 ng/mL) for 24 h as described in the Materials and Methods section; B: One-way ANOVA was used for comparisons between the two groups (aP < 0.05, bP < 0.01 vs control cells). One-way ANOVA was used for comparisons among different treatments (cP < 0.05, dP < 0.01 vs TNF-α-stimulated cells). Data are expressed as the mean ± SEM of three independent experiments. GES-1: Normal human gastric mucosa epithelial cell line; MMP-9: Matrix metallopeptidase-9; MMP9i: MMP-9 inhibitor; TNF-α: Tumor necrosis factor-α.
DISCUSSION
Quercetin belongs to a member of the polyphenolic flavonoid family, which has many favorable effects on human health, such as antioxidant, anti-inflammatory, anticancer, antiallergic, antiviral, free radical-scavenging, and neuroprotective activities[33,34]. Quercetin was used in this study due to its anti-inflammatory and gastroprotective effects.
Gastric inflammation is essential to the development of gastric epithelial injury and gastric diseases. Hu et al[10] reported that quercetin can protect GES-1 cells from hydrogen peroxide-induced production of reactive oxygen species in a mouse model of acute gastric mucosal injury. Quercetin is thought to be protective against oxidative stress, mitochondrial dysfunction, and apoptosis and to be involved in the initiation of an antioxidant defense[10]. Quercetin has also been observed to modulate the inflammatory response of gastric mucosal epithelial cells against injury caused by ischemia–reperfusion[35], ethanol[36,38], indomethacin[9], and H. pylori[37] in animal models. In other cells, quercetin has been reported to inhibit the lipopolysaccharide-induced MMP-9 expression in lung inflammation and the production of proinflammatory cytokines[50]. Ying et al[51] reported that quercetin inhibits IL-1b-induced ICAM-1 expression mediated by the MAPK pathway in lung inflammation[51,52], retinal pigment epithelial cells[53], and human endothelial cells[54] in vivo and in vitro.
The mechanism through which quercetin exerts gastroprotective activity against TNF-α-induced injury to gastric mucosal epithelial cells is unknown. Gastric inflammation and injury induced by proinflammatory cytokines, such as TNF-α produced by activated immune cells, depend on NF-κB and MMP-9 activation through the NF-κB pathway[29,30]. Here, we explored whether quercetin protects against TNF-α-induced MMP-9 damage in GES-1 cells. We challenged these cells by inducing an immune–inflammatory reaction mediated by TNF-α and found that quercetin significantly reduced MMP-9 expression in GES-1 cells. Our findings suggest that quercetin helps to maintain the gastric mucosa and has potential as a treatment for preventing the progression of inflammation.
Investigators have provided evidence that c-Src[55], the MAPK family[56-58], and NF-κB[57,59] participate in pathophysiological processes involved in gastric inflammation and GC. Three major groups of MAPKs have been identified, including ERK1/2, p38, and JNK1/2, which play critical roles in cell proliferation, differentiation, and inflammation[60]. TNF-α-induced MMP-9 expression is regulated through the MAPK pathway in human trophoblastic cells[61], human urinary bladder cancer HT1376 cells[62], lung cancer cells[63], osteoblasts[64], and skeletal muscle cells[65]. This evidence suggests that MMP-9 expression induced by inflammatory mediators, such as TNF-α, is associated with MAPKs in diverse cell types. We observed similar results in our studies. We observed the in vitro antimetastatic and anti-inflammatory activities of quercetin in GES-1 cells. Quercetin protected against TNF-α-induced MMP-9 damage in GES-1 cells challenged by induction of the immune–inflammatory reaction mediated by TNF-α. PP1 (c-Src inhibitor), U0126 (ERK1/2 inhibitor), TSAIIA (c-Fos inhibitor), BAY (NF-κB inhibitor), and TNF-α antagonist (TNFR inhibitor) were used to investigate the interactions of c-Src, ERK1/2, c-Fos, NF-κB, and TNFR in the TNF-α-induced response. Using immunofluorescence, we found that TNF-α induced NF-κB (p65) phosphorylation and translocation and that NF-κB/p65–Luc and AP-1–Luc activities were mediated via the TNFR-c-Src–ERK1/2–c-Fos and NF-κB pathways. We also found that quercetin reduced TNF-α-induced MMP-9 expression in GES-1 cells via the proinflammatory c-Src–ERK1/2–c-Fos and NF-κB pathways. Moreover, quercetin may indeed directly inhibit upstream molecules (such as TNFR or c-Src) and subsequently cause the inhibition of downstream molecules, such as ERK1/2, c-Fos, and NF-κB.
NF-κB plays an important role in the regulation of inflammation. Stimulation of proinflammatory cytokines, such as TNF-α, increases NF-κB activity and involves the phosphorylation and degradation of IκBa, which induces the translocation of active NF-κB to the nucleus and the expression of genes encoding proinflammatory factors, such as cytokines, chemokines, and adhesion molecules[66-69]. In our study, pretreatment with BAY attenuated TNF-α-induced MMP-9 expression in GES-1 cells. MMP-9 is a potential target for new therapies and is being investigated by several research groups[70-72]. A better understanding of the roles of inflammatory mediators will help in the development of new therapeutic approaches for treating gastric inflammatory diseases. Reducing MMP-9 production may help to limit gastric damage in the treatment of gastric diseases. Given the increasing use of NSAIDs worldwide, it is important to identify other agents to control inflammation. Quercetin and a quercetin-rich diet may exhibit potential as food supplements for the prevention of early pathological changes associated with gastric inflammation.
We evaluated the effects of different concentrations of quercetin on the normal GES-1 cell line using the cell viability assay (Figure 3A and B). We found that cells treated with quercetin at concentrations ≤ 10 mM for 24 to 48 h had almost no effect on GES-1 cell viability. Additionally, many studies have confirmed that the safety and efficacy of quercetin remain inconclusive[73]. In 2010, the American Food and Drug Administration considered that the utilization of high-purity quercetin as a food ingredient in different food products is GRAS (“Generally Recognized As Safe”) (https://www.cfsanappsexternal.fda.gov/scripts/fdcc/index.cfm?set=GRASNotices&sort=GRN_No&order=DESC&startrow=1&type=basic&search=341) (GRN No. 341). Based on several published human intervention studies, daily doses of oral quercetin are usually in the range of up to 1000 mg/d (they are the safest at 500 mg/d). Therefore, the daily supplement doses of oral quercetin were noticeably higher than the background daily doses of oral quercetin. Side effects subsequent to supplemental quercetin intake have rarely been described naturally. Moreover, supplemental isolated quercetin at doses clearly exceeding dietary quercetin intake will not aggravate gastric injuries, ulcers, or GC[74-76]. However, based on several published in vivo data, quercetin promotes insulin secretion, and insulin can increase tumor growth[77]. Oral quercetin products have a few potential crucial safety behaviors[78], and potential opposite effects in diabetes. In patients who have developed nephropathy or estrogen-dependent cancer, the use of quercetin should be tracked and researched in the future[79-82].
CONCLUSION
We investigated the in vitro effects of quercetin on antimetastatic and anti-inflammatory activities in GES-1 cells. Quercetin protected against TNF-α-induced damage caused by MMP-9 in human gastric mucosal epithelial cells (GES-1) challenged by TNF-α-mediated immune–inflammatory reactions. PP1 (c-Src inhibitor), U0126 (ERK1/2 inhibitor), TSAIIA (c-Fos inhibitor), BAY (NF-κB inhibitor), and TNF-α antagonist (TNF-α inhibitor) were used to investigate the roles of c-Src, ERK1/2, c-Fos, and NF-κB in the TNF-α-induced response. Quercetin reduces TNF-α-induced MMP-9 expression in GES-1 cells via the proinflammatory TNFR-c-Src–ERK1/2–c-Fos and NF-κB pathways. We propose that quercetin exhibits potential as a novel therapeutic drug for protecting gastric mucosal epithelial cells from damage caused by disease (Figure 9). Quercetin and a quercetin-rich diet may be useful as food supplements for the prevention of early pathological changes associated with gastric inflammation.
Figure 9.
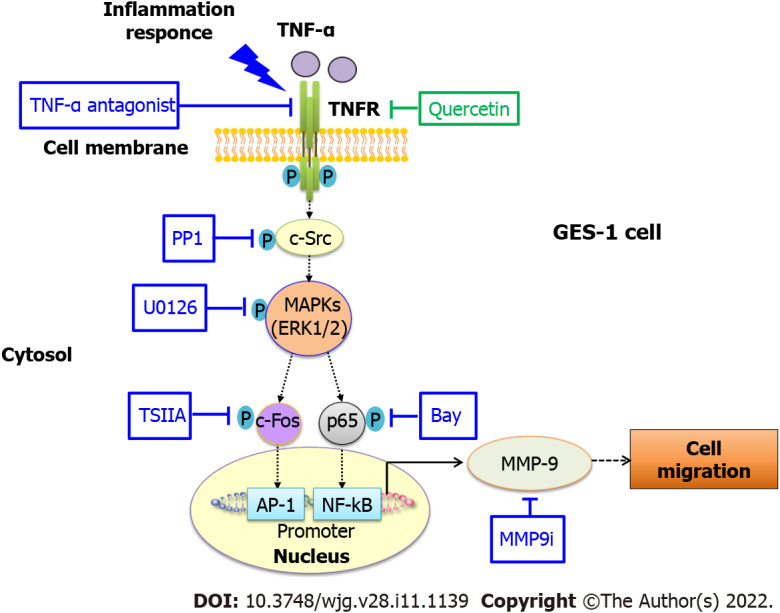
Schematic representation of the effects of quercetin on tumor necrosis factor-α-induced matrix metallopeptidase-9 expression and cell migration in normal human gastric mucosa epithelial cells. Schematic diagram of the signaling pathways for quercetin-mediated attenuation of tumor necrosis factor-α (TNF-α)-induced inflammation via downregulation of matrix metallopeptidase-9 (MMP-9) expression in normal human gastric mucosa epithelial cells. Quercetin attenuates TNF-α-induced MMP-9 expression in normal human gastric mucosa epithelial cells through the proinflammatory TNFR-c-Src–extracellular-signal-regulated kinase 1/2–c-Fos and nuclear factor kappa B pathways. AP-1: Activator protein-1; c-Src: Proto-oncogene tyrosine-protein kinase Src; ERK: Extracellular-signal-regulated kinase; GES-1: Normal human gastric mucosa epithelial cell line; MAPK: Mitogen-activated protein kinase; MMP-9: Matrix metallopeptidase-9; MMP9i: MMP-9 inhibitor; NF-κB: Nuclear factor kappa B; TNF-α: Tumor necrosis factor-α; TSIIA: Tanshinone IIA.
ARTICLE HIGHLIGHTS
Research background
Gastric injury involving inflammation is one of the most common diseases of the digestive system worldwide and is associated with gastric ulcers and gastric cancer (GC). Matrix metallopeptidase-9 (MMP-9) plays an important role in the inflammation and progression of GC. Quercetin exhibits anti-inflammatory activities, but its effects and mechanism of action on human chronic gastritis remain unclear.
Research motivation
To assess whether tumor necrosis factor-α (TNF-α)-induced MMP-9 expression is involved in the anti-inflammatory effects of quercetin in normal human gastric mucosa epithelial cell line GES-1.
Research objectives
The objective of this study was to evaluate the anti- inflammatory effects and mechanisms of quercetin in GES-1 cells.
Research methods
A GES-1 cell model was established to evaluate the anti-inflammatory effects of quercetin on TNF-α-induced overexpression of the proinflammatory MMP-9 protein. The cell counting Kit-8 assay was used to examine the effects of quercetin dose on GES-1 cell viability. Cell migration was measured using the Transwell assay. The expression of c-Src, phospho (p)-c-Src, p- extracellular-signal-regulated kinase (ERK) 1/2, ERK2, p-c-Fos, c-Fos, p-p65, and nuclear factor kappa B (NF-κB)/p65 and the effects of their inhibitors were examined using Western blot analysis and the measurement of luciferase activity. p65 expression was detected by immunofluorescence. MMP-9 mRNA and protein expression levels were determined by quantitative reverse transcription polymerase chain reaction (qRT–PCR) and gelatin zymography, respectively.
Research results
qRT–PCR and gelatin zymography showed that TNF-α induced MMP-9 mRNA and protein expression in a dose- and time-dependent manner. These effects were reduced by pretreatment of GES-1 cells with quercetin or TNF-α antagonist in a dose- and time-dependent manner. Quercetin decreased the TNF-α-induced phosphorylation of c-Src, ERK1/2, c-Fos, and p65 in a dose- and time-dependent manner. Quercetin, PP1, U0126, TSIIA, and a TNF-α antagonist reduced TNF-α-induced c-Fos phosphorylation and AP-1–Luc activity in a dose- and time-dependent manner. Pretreatment with quercetin, PP1, U0126, Bay 11-7082, or TNF-α antagonist reduced TNF-α-induced p65 phosphorylation and translocation and p65–Luc activity in a dose- and time-dependent manner. TNF-α significantly increased GES-1 cell migration, and this effect was reduced by pretreatment with quercetin and a TNF-α antagonist.
Research conclusions
Quercetin significantly downregulates TNF-α-induced MMP-9 expression in GES-1 cells via the TNFR-c-Src–ERK1/2–c-Fos and NF-κB pathways.
Research perspectives
We propose that quercetin potentially represents a new approach for reducing reliance on nonsteroidal anti-inflammatory drugs and an effective therapeutic agent for protecting gastric mucosal epithelial cells. Quercetin and quercetin-rich diets may exhibit potential as food supplements for the prevention of early pathological changes associated with gastric inflammation.
ACKNOWLEDGEMENTS
The corresponding author would like to thank the World Journal of Gastroenterology for the opportunity to present this work and acknowledge the hard work of all supporting authors. The authors wish to thank Dr. Chen QX and Dr. Zhou DW at Xiamen University (China) for providing GES-1 cells.
Footnotes
Institutional review board statement: No human specimens were involved in this study.
Conflict-of-interest statement: All authors have nothing to disclose.
Provenance and peer review: Invited article; Externally peer reviewed.
Peer-review model: Single blind
Peer-review started: September 2, 2021
First decision: November 7, 2021
Article in press: February 12, 2022
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: Taiwan
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Ren JY, Tong GD S-Editor: Fan JR L-Editor: Wang TQ P-Editor: Yuan YY
Contributor Information
Hsi-Lung Hsieh, Department of Nursing, Division of Basic Medical Sciences, Chang-Gung University of Science and Technology, Taoyuan 333, Taiwan; Research Center for Chinese Herbal Medicine, College of Human Ecology, Chang Gung University of Science and Technology, Taoyuan 333, Taiwan; Department of Neurology, Chang Gung Memorial Hospital, Taoyuan 333, Taiwan.
Ming-Chin Yu, Department of General Surgery, New Taipei Municipal TuCheng Hospital, New Taipei 236, Taiwan; College of Medicine, Chang Gung University, Taoyuan 333, Taiwan; Department of General Surgery, Chang Gung Memorial Hospital, Taoyuan 333, Taiwan.
Li-Ching Cheng, Department of Nursing, Division of Basic Medical Sciences, Chang-Gung University of Science and Technology, Taoyuan 333, Taiwan; Department of General Surgery, Chang Gung Memorial Hospital, Taoyuan 333, Taiwan.
Mei-Yi Chu, Research Center for Chinese Herbal Medicine, College of Human Ecology, Chang Gung University of Science and Technology, Taoyuan 333, Taiwan.
Tzu-Hao Huang, Department of Nursing, Division of Basic Medical Sciences, Chang-Gung University of Science and Technology, Taoyuan 333, Taiwan.
Ta-Sen Yeh, College of Medicine, Chang Gung University, Taoyuan 333, Taiwan; Department of General Surgery, Chang Gung Memorial Hospital, Taoyuan 333, Taiwan.
Ming-Ming Tsai, Department of Nursing, Division of Basic Medical Sciences, Chang-Gung University of Science and Technology, Taoyuan 333, Taiwan; Research Center for Chinese Herbal Medicine, College of Human Ecology, Chang Gung University of Science and Technology, Taoyuan 333, Taiwan; Department of General Surgery, New Taipei Municipal TuCheng Hospital, New Taipei 236, Taiwan; Department of General Surgery, Chang Gung Memorial Hospital, Taoyuan 333, Taiwan. mmtsai@mail.cgust.edu.tw.
Data sharing statement
No additional data are available.
References
- 1.Ernst P. Review article: the role of inflammation in the pathogenesis of gastric cancer. Aliment Pharmacol Ther. 1999;13 Suppl 1:13–18. doi: 10.1046/j.1365-2036.1999.00003.x. [DOI] [PubMed] [Google Scholar]
- 2.Zhang XY, Zhang PY, Aboul-Soud MA. From inflammation to gastric cancer: Role of Helicobacter pylori. Oncol Lett. 2017;13:543–548. doi: 10.3892/ol.2016.5506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kim SS, Ruiz VE, Carroll JD, Moss SF. Helicobacter pylori in the pathogenesis of gastric cancer and gastric lymphoma. Cancer Lett. 2011;305:228–238. doi: 10.1016/j.canlet.2010.07.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Choi Y, Kim JW, Nam KH, Han SH, Ahn SH, Park DJ, Lee KW, Lee HS, Kim HH. Systemic inflammation is associated with the density of immune cells in the tumor microenvironment of gastric cancer. Gastric Cancer. 2017;20:602–611. doi: 10.1007/s10120-016-0642-0. [DOI] [PubMed] [Google Scholar]
- 5.Boland CR, Luciani MG, Gasche C, Goel A. Infection, inflammation, and gastrointestinal cancer. Gut. 2005;54:1321–1331. doi: 10.1136/gut.2004.060079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Senthilkumar C, Niranjali S, Jayanthi V, Ramesh T, Devaraj H. Molecular and histological evaluation of tumor necrosis factor-alpha expression in Helicobacter pylori-mediated gastric carcinogenesis. J Cancer Res Clin Oncol. 2011;137:577–583. doi: 10.1007/s00432-010-0921-9. [DOI] [PubMed] [Google Scholar]
- 7.El-Omar EM, Carrington M, Chow WH, McColl KE, Bream JH, Young HA, Herrera J, Lissowska J, Yuan CC, Rothman N, Lanyon G, Martin M, Fraumeni JF Jr, Rabkin CS. Interleukin-1 polymorphisms associated with increased risk of gastric cancer. Nature. 2000;404:398–402. doi: 10.1038/35006081. [DOI] [PubMed] [Google Scholar]
- 8.Xue H, Wang YC, Lin B, An J, Chen L, Chen J, Fang JY. A meta-analysis of interleukin-10 -592 promoter polymorphism associated with gastric cancer risk. PLoS One. 2012;7:e39868. doi: 10.1371/journal.pone.0039868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Yan XM, Joo MJ, Lim JC, Whang WK, Sim SS, Im C, Kim HR, Lee SY, Kim IK, Sohn UD. The effect of quercetin-3-O-β-D-glucuronopyranoside on indomethacin-induced gastric damage in rats via induction of mucus secretion and down-regulation of ICAM-1 expression. Arch Pharm Res. 2011;34:1527–1534. doi: 10.1007/s12272-011-0915-4. [DOI] [PubMed] [Google Scholar]
- 10.Hu XT, Ding C, Zhou N, Xu C. Quercetin protects gastric epithelial cell from oxidative damage in vitro and in vivo. Eur J Pharmacol. 2015;754:115–124. doi: 10.1016/j.ejphar.2015.02.007. [DOI] [PubMed] [Google Scholar]
- 11.Polk DB, Peek RM Jr. Helicobacter pylori: gastric cancer and beyond. Nat Rev Cancer. 2010;10:403–414. doi: 10.1038/nrc2857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Verbeke H, Geboes K, Van Damme J, Struyf S. The role of CXC chemokines in the transition of chronic inflammation to esophageal and gastric cancer. Biochim Biophys Acta. 2012;1825:117–129. doi: 10.1016/j.bbcan.2011.10.008. [DOI] [PubMed] [Google Scholar]
- 13.Thalmaier U, Lehn N, Pfeffer K, Stolte M, Vieth M, Schneider-Brachert W. Role of tumor necrosis factor alpha in Helicobacter pylori gastritis in tumor necrosis factor receptor 1-deficient mice. Infect Immun. 2002;70:3149–3155. doi: 10.1128/IAI.70.6.3149-3155.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Varfolomeev EE, Ashkenazi A. Tumor necrosis factor: an apoptosis JuNKie? Cell. 2004;116:491–497. doi: 10.1016/s0092-8674(04)00166-7. [DOI] [PubMed] [Google Scholar]
- 15.Bazzoni F, Beutler B. The tumor necrosis factor ligand and receptor families. N Engl J Med. 1996;334:1717–1725. doi: 10.1056/NEJM199606273342607. [DOI] [PubMed] [Google Scholar]
- 16.Wang L, Du F, Wang X. TNF-alpha induces two distinct caspase-8 activation pathways. Cell. 2008;133:693–703. doi: 10.1016/j.cell.2008.03.036. [DOI] [PubMed] [Google Scholar]
- 17.Sterne JA, Egger M, Smith GD. Systematic reviews in health care: Investigating and dealing with publication and other biases in meta-analysis. BMJ. 2001;323:101–105. doi: 10.1136/bmj.323.7304.101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Strieter RM, Kunkel SL. The immunopathology of chemotactic cytokines. Adv Exp Med Biol. 1993;351:19–28. doi: 10.1007/978-1-4615-2952-1_3. [DOI] [PubMed] [Google Scholar]
- 19.Canedo P, Durães C, Pereira F, Regalo G, Lunet N, Barros H, Carneiro F, Seruca R, Rocha J, Machado JC. Tumor necrosis factor alpha extended haplotypes and risk of gastric carcinoma. Cancer Epidemiol Biomarkers Prev. 2008;17:2416–2420. doi: 10.1158/1055-9965.EPI-08-0413. [DOI] [PubMed] [Google Scholar]
- 20.Fei BY, Xia B, Deng CS, Xia XQ, Xie M, Crusius JB, Pena AS. Association of tumor necrosis factor genetic polymorphism with chronic atrophic gastritis and gastric adenocarcinoma in Chinese Han population. World J Gastroenterol. 2004;10:1256–1261. doi: 10.3748/wjg.v10.i9.1256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Harris PR, Mobley HL, Perez-Perez GI, Blaser MJ, Smith PD. Helicobacter pylori urease is a potent stimulus of mononuclear phagocyte activation and inflammatory cytokine production. Gastroenterology. 1996;111:419–425. doi: 10.1053/gast.1996.v111.pm8690207. [DOI] [PubMed] [Google Scholar]
- 22.Fan XG, Chua A, Fan XJ, Keeling PW. Increased gastric production of interleukin-8 and tumour necrosis factor in patients with Helicobacter pylori infection. J Clin Pathol. 1995;48:133–136. doi: 10.1136/jcp.48.2.133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Deryugina EI, Quigley JP. Matrix metalloproteinases and tumor metastasis. Cancer Metastasis Rev. 2006;25:9–34. doi: 10.1007/s10555-006-7886-9. [DOI] [PubMed] [Google Scholar]
- 24.Overall CM, Dean RA. Degradomics: systems biology of the protease web. Pleiotropic roles of MMPs in cancer. Cancer Metastasis Rev. 2006;25:69–75. doi: 10.1007/s10555-006-7890-0. [DOI] [PubMed] [Google Scholar]
- 25.Wang M, Busuttil RA, Pattison S, Neeson PJ, Boussioutas A. Immunological battlefield in gastric cancer and role of immunotherapies. World J Gastroenterol. 2016;22:6373–6384. doi: 10.3748/wjg.v22.i28.6373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Umakoshi M, Takahashi S, Itoh G, Kuriyama S, Sasaki Y, Yanagihara K, Yashiro M, Maeda D, Goto A, Tanaka M. Macrophage-mediated transfer of cancer-derived components to stromal cells contributes to establishment of a pro-tumor microenvironment. Oncogene. 2019;38:2162–2176. doi: 10.1038/s41388-018-0564-x. [DOI] [PubMed] [Google Scholar]
- 27.Wang Y, Zhai J, Zhang T, Han S, Zhang Y, Yao X, Shen L. Tumor-Associated Neutrophils Can Predict Lymph Node Metastasis in Early Gastric Cancer. Front Oncol. 2020;10:570113. doi: 10.3389/fonc.2020.570113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wu CY, Wu MS, Chiang EP, Chen YJ, Chen CJ, Chi NH, Shih YT, Chen GH, Lin JT. Plasma matrix metalloproteinase-9 Level is better than serum matrix metalloproteinase-9 Level to predict gastric cancer evolution. Clin Cancer Res. 2007;13:2054–2060. doi: 10.1158/1078-0432.CCR-06-2299. [DOI] [PubMed] [Google Scholar]
- 29.Lin Y, Ukaji T, Koide N, Umezawa K. Inhibition of Late and Early Phases of Cancer Metastasis by the NF-κB Inhibitor DHMEQ Derived from Microbial Bioactive Metabolite Epoxyquinomicin: A Review. Int J Mol Sci. 2018;19 doi: 10.3390/ijms19030729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lambrou GI, Hatziagapiou K, Vlahopoulos S. Inflammation and tissue homeostasis: the NF-κB system in physiology and malignant progression. Mol Biol Rep. 2020;47:4047–4063. doi: 10.1007/s11033-020-05410-w. [DOI] [PubMed] [Google Scholar]
- 31.Mahady GB, Pendland SL, Stoia A, Hamill FA, Fabricant D, Dietz BM, Chadwick LR. In vitro susceptibility of Helicobacter pylori to botanical extracts used traditionally for the treatment of gastrointestinal disorders. Phytother Res. 2005;19:988–991. doi: 10.1002/ptr.1776. [DOI] [PubMed] [Google Scholar]
- 32.Patra KC, Jayaram Kumar K, Ahirwar DK. Gastroprotective effect of standardized extract of Amukkara choornam on experimental gastric ulcer in rats. J Nat Med. 2014;68:284–294. doi: 10.1007/s11418-013-0792-x. [DOI] [PubMed] [Google Scholar]
- 33.Boots AW, Haenen GR, Bast A. Health effects of quercetin: from antioxidant to nutraceutical. Eur J Pharmacol. 2008;585:325–337. doi: 10.1016/j.ejphar.2008.03.008. [DOI] [PubMed] [Google Scholar]
- 34.Manach C, Scalbert A, Morand C, Rémésy C, Jiménez L. Polyphenols: food sources and bioavailability. Am J Clin Nutr. 2004;79:727–747. doi: 10.1093/ajcn/79.5.727. [DOI] [PubMed] [Google Scholar]
- 35.Mojzis J, Hviscová K, Germanova D, Bukovicová D, Mirossay L. Protective effect of quercetin on ischemia/reperfusion-induced gastric mucosal injury in rats. Physiol Res. 2001;50:501–506. [PubMed] [Google Scholar]
- 36.Kahraman A, Erkasap N, Köken T, Serteser M, Aktepe F, Erkasap S. The antioxidative and antihistaminic properties of quercetin in ethanol-induced gastric lesions. Toxicology. 2003;183:133–142. doi: 10.1016/s0300-483x(02)00514-0. [DOI] [PubMed] [Google Scholar]
- 37.González-Segovia R, Quintanar JL, Salinas E, Ceballos-Salazar R, Aviles-Jiménez F, Torres-López J. Effect of the flavonoid quercetin on inflammation and lipid peroxidation induced by Helicobacter pylori in gastric mucosa of guinea pig. J Gastroenterol. 2008;43:441–447. doi: 10.1007/s00535-008-2184-7. [DOI] [PubMed] [Google Scholar]
- 38.Chakraborty S, Stalin S, Das N, Choudhury ST, Ghosh S, Swarnakar S. The use of nano-quercetin to arrest mitochondrial damage and MMP-9 upregulation during prevention of gastric inflammation induced by ethanol in rat. Biomaterials. 2012;33:2991–3001. doi: 10.1016/j.biomaterials.2011.12.037. [DOI] [PubMed] [Google Scholar]
- 39.Tsai MM, Lin HC, Yu MC, Lin WJ, Chu MY, Tsai CC, Cheng CY. Anticancer Effects of Helminthostachys zeylanica Ethyl acetate Extracts on Human Gastric Cancer Cells through Downregulation of the TNF-α-activated COX-2-cPLA2-PGE2 Pathway. J Cancer. 2021;12:7052–7068. doi: 10.7150/jca.64638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lee TH, Liu PS, Tsai MM, Chen JL, Wang SJ, Hsieh HL. The COX-2-derived PGE2 autocrine contributes to bradykinin-induced matrix metalloproteinase-9 expression and astrocytic migration via STAT3 signaling. Cell Commun Signal. 2020;18:185. doi: 10.1186/s12964-020-00680-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Cai L, Qin X, Xu Z, Song Y, Jiang H, Wu Y, Ruan H, Chen J. Comparison of Cytotoxicity Evaluation of Anticancer Drugs between Real-Time Cell Analysis and CCK-8 Method. ACS Omega. 2019;4:12036–12042. doi: 10.1021/acsomega.9b01142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Tsai MM, Wang CS, Tsai CY, Chen CY, Chi HC, Tseng YH, Chung PJ, Lin YH, Chung IH, Lin KH. MicroRNA-196a/-196b promote cell metastasis via negative regulation of radixin in human gastric cancer. Cancer Lett. 2014;351:222–231. doi: 10.1016/j.canlet.2014.06.004. [DOI] [PubMed] [Google Scholar]
- 43.Lee TH, Liu PS, Wang SJ, Tsai MM, Shanmugam V, Hsieh HL. Bradykinin, as a Reprogramming Factor, Induces Transdifferentiation of Brain Astrocytes into Neuron-like Cells. Biomedicines. 2021;9 doi: 10.3390/biomedicines9080923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Lee TH, Chen JL, Liu PS, Tsai MM, Wang SJ, Hsieh HL. Rottlerin, a natural polyphenol compound, inhibits upregulation of matrix metalloproteinase-9 and brain astrocytic migration by reducing PKC-δ-dependent ROS signal. J Neuroinflammation. 2020;17:177. doi: 10.1186/s12974-020-01859-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kim S, Choi MG, Lee HS, Lee SK, Kim SH, Kim WW, Hur SM, Kim JH, Choe JH, Nam SJ, Yang JH, Kim S, Lee JE, Kim JS. Silibinin suppresses TNF-alpha-induced MMP-9 expression in gastric cancer cells through inhibition of the MAPK pathway. Molecules. 2009;14:4300–4311. doi: 10.3390/molecules14114300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Wu HT, Sie SS, Kuan TC, Lin CS. Identifying the regulative role of NF-κB binding sites within promoter region of human matrix metalloproteinase 9 (mmp-9) by TNF-α induction. Appl Biochem Biotechnol. 2013;169:438–449. doi: 10.1007/s12010-012-9958-3. [DOI] [PubMed] [Google Scholar]
- 47.Shan YQ, Ying RC, Zhou CH, Zhu AK, Ye J, Zhu W, Ju TF, Jin HC. MMP-9 is increased in the pathogenesis of gastric cancer by the mediation of HER2. Cancer Gene Ther. 2015;22:101–107. doi: 10.1038/cgt.2014.61. [DOI] [PubMed] [Google Scholar]
- 48.Oku T, Shimada K, Kenmotsu H, Ando Y, Kurisaka C, Sano R, Tsuiji M, Hasegawa S, Fukui T, Tsuji T. Stimulation of Peritoneal Mesothelial Cells to Secrete Matrix Metalloproteinase-9 (MMP-9) by TNF-α: A Role in the Invasion of Gastric Carcinoma Cells. Int J Mol Sci. 2018;19 doi: 10.3390/ijms19123961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Guadagni F, Ferroni P, Palmirotta R, Portarena I, Formica V, Roselli M. Review. TNF/VEGF cross-talk in chronic inflammation-related cancer initiation and progression: an early target in anticancer therapeutic strategy. In Vivo. 2007;21:147–161. [PubMed] [Google Scholar]
- 50.Takashima K, Matsushima M, Hashimoto K, Nose H, Sato M, Hashimoto N, Hasegawa Y, Kawabe T. Protective effects of intratracheally administered quercetin on lipopolysaccharide-induced acute lung injury. Respir Res. 2014;15:150. doi: 10.1186/s12931-014-0150-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Ying B, Yang T, Song X, Hu X, Fan H, Lu X, Chen L, Cheng D, Wang T, Liu D, Xu D, Wei Y, Wen F. Quercetin inhibits IL-1 beta-induced ICAM-1 expression in pulmonary epithelial cell line A549 through the MAPK pathways. Mol Biol Rep. 2009;36:1825–1832. doi: 10.1007/s11033-008-9386-1. [DOI] [PubMed] [Google Scholar]
- 52.Meng L, Lv Z, Yu ZZ, Xu D, Yan X. Protective effect of quercetin on acute lung injury in rats with sepsis and its influence on ICAM-1 and MIP-2 expression. Genet Mol Res. 2016;15 doi: 10.4238/gmr.15037265. [DOI] [PubMed] [Google Scholar]
- 53.Cheng SC, Wu YH, Huang WC, Pang JS, Huang TH, Cheng CY. Anti-inflammatory property of quercetin through downregulation of ICAM-1 and MMP-9 in TNF-α-activated retinal pigment epithelial cells. Cytokine. 2019;116:48–60. doi: 10.1016/j.cyto.2019.01.001. [DOI] [PubMed] [Google Scholar]
- 54.Kobuchi H, Roy S, Sen CK, Nguyen HG, Packer L. Quercetin inhibits inducible ICAM-1 expression in human endothelial cells through the JNK pathway. Am J Physiol. 1999;277:C403–C411. doi: 10.1152/ajpcell.1999.277.3.C403. [DOI] [PubMed] [Google Scholar]
- 55.Liu ST, Pham H, Pandol SJ, Ptasznik A. Src as the link between inflammation and cancer. Front Physiol. 2013;4:416. doi: 10.3389/fphys.2013.00416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Wei Y, Wang R, Ren S, Liu X, Jing M, Li R, Tong Y, Wen J, Yang T, Wang J, Zhao Y. Zuojin Pill ameliorates inflammation in indomethacin-induced gastric injury via inhibition of MAPK pathway. J Ethnopharmacol. 2021;275:114103. doi: 10.1016/j.jep.2021.114103. [DOI] [PubMed] [Google Scholar]
- 57.Akanda MR, Park BY. Involvement of MAPK/NF-κB signal transduction pathways: Camellia japonica mitigates inflammation and gastric ulcer. Biomed Pharmacother. 2017;95:1139–1146. doi: 10.1016/j.biopha.2017.09.031. [DOI] [PubMed] [Google Scholar]
- 58.Magnelli L, Schiavone N, Staderini F, Biagioni A, Papucci L. MAP Kinases Pathways in Gastric Cancer. Int J Mol Sci. 2020;21 doi: 10.3390/ijms21082893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Sokolova O, Naumann M. NF-κB Signaling in Gastric Cancer. Toxins (Basel) 2017;9 doi: 10.3390/toxins9040119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Cobb MH. MAP kinase pathways. Prog Biophys Mol Biol. 1999;71:479–500. doi: 10.1016/s0079-6107(98)00056-x. [DOI] [PubMed] [Google Scholar]
- 61.Cohen M, Meisser A, Haenggeli L, Bischof P. Involvement of MAPK pathway in TNF-alpha-induced MMP-9 expression in human trophoblastic cells. Mol Hum Reprod. 2006;12:225–232. doi: 10.1093/molehr/gal023. [DOI] [PubMed] [Google Scholar]
- 62.Lee SJ, Park SS, Cho YH, Park K, Kim EJ, Jung KH, Kim SK, Kim WJ, Moon SK. Activation of matrix metalloproteinase-9 by TNF-alpha in human urinary bladder cancer HT1376 cells: the role of MAP kinase signaling pathways. Oncol Rep. 2008;19:1007–1013. [PubMed] [Google Scholar]
- 63.Lee IT, Lin CC, Wu YC, Yang CM. TNF-alpha induces matrix metalloproteinase-9 expression in A549 cells: role of TNFR1/TRAF2/PKCalpha-dependent signaling pathways. J Cell Physiol. 2010;224:454–464. doi: 10.1002/jcp.22142. [DOI] [PubMed] [Google Scholar]
- 64.Tsai CL, Chen WC, Hsieh HL, Chi PL, Hsiao LD, Yang CM. TNF-α induces matrix metalloproteinase-9-dependent soluble intercellular adhesion molecule-1 release via TRAF2-mediated MAPKs and NF-κB activation in osteoblast-like MC3T3-E1 cells. J Biomed Sci. 2014;21:12. doi: 10.1186/1423-0127-21-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Srivastava AK, Qin X, Wedhas N, Arnush M, Linkhart TA, Chadwick RB, Kumar A. Tumor necrosis factor-alpha augments matrix metalloproteinase-9 production in skeletal muscle cells through the activation of transforming growth factor-beta-activated kinase 1 (TAK1)-dependent signaling pathway. J Biol Chem. 2007;282:35113–35124. doi: 10.1074/jbc.M705329200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Lawrence T. The nuclear factor NF-kappaB pathway in inflammation. Cold Spring Harb Perspect Biol. 2009;1:a001651. doi: 10.1101/cshperspect.a001651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Zhu S, Xu X, Liu K, Gu Q, Wei F, Jin H. Peptide GC31 inhibits chemokines and ICAM-1 expression in corneal fibroblasts exposed to LPS or poly(I:C) by blocking the NF-κB and MAPK pathways. Exp Eye Res. 2017;164:109–117. doi: 10.1016/j.exer.2017.07.017. [DOI] [PubMed] [Google Scholar]
- 68.Gu R, Lei B, Jiang C, Xu G. Glucocorticoid-Induced Leucine Zipper Suppresses ICAM-1 and MCP-1 Expression by Dephosphorylation of NF-κB p65 in Retinal Endothelial Cells. Invest Ophthalmol Vis Sci. 2017;58:631–641. doi: 10.1167/iovs.16-20933. [DOI] [PubMed] [Google Scholar]
- 69.Wu P, Zhou L, Li YJ, Luo B, Yi LS, Chen SF, Sun HH, Chen Y, Cao ZJ, Xu SC. Protective effects of quercetin against chronic mixed reflux esophagitis in rats by inhibiting the nuclear factor-κB p65 and interleukin-8 signaling pathways. J Dig Dis. 2015;16:319–326. doi: 10.1111/1751-2980.12249. [DOI] [PubMed] [Google Scholar]
- 70.Zhu SH, Liu BQ, Hao MJ, Fan YX, Qian C, Teng P, Zhou XW, Hu L, Liu WT, Yuan ZL, Li QP. Paeoniflorin Suppressed High Glucose-Induced Retinal Microglia MMP-9 Expression and Inflammatory Response via Inhibition of TLR4/NF-κB Pathway Through Upregulation of SOCS3 in Diabetic Retinopathy. Inflammation. 2017;40:1475–1486. doi: 10.1007/s10753-017-0571-z. [DOI] [PubMed] [Google Scholar]
- 71.Mohammad G, Mairaj Siddiquei M, Imtiaz Nawaz M, Abu El-Asrar AM. The ERK1/2 Inhibitor U0126 Attenuates Diabetes-Induced Upregulation of MMP-9 and Biomarkers of Inflammation in the Retina. J Diabetes Res. 2013;2013:658548. doi: 10.1155/2013/658548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Masuzawa K, Goto K, Jesmin S, Maeda S, Miyauchi T, Kaji Y, Oshika T, Hori S. An endothelin type A receptor antagonist reverses upregulated VEGF and ICAM-1 Levels in streptozotocin-induced diabetic rat retina. Curr Eye Res. 2006;31:79–89. doi: 10.1080/02713680500478923. [DOI] [PubMed] [Google Scholar]
- 73.Andres S, Pevny S, Ziegenhagen R, Bakhiya N, Schäfer B, Hirsch-Ernst KI, Lampen A. Safety Aspects of the Use of Quercetin as a Dietary Supplement. Mol Nutr Food Res. 2018;62 doi: 10.1002/mnfr.201700447. [DOI] [PubMed] [Google Scholar]
- 74.Soare A, Weiss EP, Holloszy JO, Fontana L. Multiple dietary supplements do not affect metabolic and cardio-vascular health. Aging (Albany NY) 2014;6:149–157. doi: 10.18632/aging.100597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Javadi F, Ahmadzadeh A, Eghtesadi S, Aryaeian N, Zabihiyeganeh M, Rahimi Foroushani A, Jazayeri S. The Effect of Quercetin on Inflammatory Factors and Clinical Symptoms in Women with Rheumatoid Arthritis: A Double-Blind, Randomized Controlled Trial. J Am Coll Nutr. 2017;36:9–15. doi: 10.1080/07315724.2016.1140093. [DOI] [PubMed] [Google Scholar]
- 76.Shi Y, Williamson G. Quercetin lowers plasma uric acid in pre-hyperuricaemic males: a randomised, double-blinded, placebo-controlled, cross-over trial. Br J Nutr. 2016;115:800–806. doi: 10.1017/S0007114515005310. [DOI] [PubMed] [Google Scholar]
- 77.Anhê GF, Okamoto MM, Kinote A, Sollon C, Lellis-Santos C, Anhê FF, Lima GA, Hirabara SM, Velloso LA, Bordin S, Machado UF. Quercetin decreases inflammatory response and increases insulin action in skeletal muscle of ob/ob mice and in L6 myotubes. Eur J Pharmacol. 2012;689:285–293. doi: 10.1016/j.ejphar.2012.06.007. [DOI] [PubMed] [Google Scholar]
- 78.Cheng Y, Nie J, Li J, Liu H, Yan Z, Kuang L. Synthesis and characterization of core-shell magnetic molecularly imprinted polymers for selective recognition and determination of quercetin in apple samples. Food Chem. 2019;287:100–106. doi: 10.1016/j.foodchem.2019.02.069. [DOI] [PubMed] [Google Scholar]
- 79.Liu KC, Yen CY, Wu RS, Yang JS, Lu HF, Lu KW, Lo C, Chen HY, Tang NY, Wu CC, Chung JG. The roles of endoplasmic reticulum stress and mitochondrial apoptotic signaling pathway in quercetin-mediated cell death of human prostate cancer PC-3 cells. Environ Toxicol. 2014;29:428–439. doi: 10.1002/tox.21769. [DOI] [PubMed] [Google Scholar]
- 80.Vafadar A, Shabaninejad Z, Movahedpour A, Fallahi F, Taghavipour M, Ghasemi Y, Akbari M, Shafiee A, Hajighadimi S, Moradizarmehri S, Razi E, Savardashtaki A, Mirzaei H. Quercetin and cancer: new insights into its therapeutic effects on ovarian cancer cells. Cell Biosci. 2020;10:32. doi: 10.1186/s13578-020-00397-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Hsieh CL, Peng CC, Chen KC, Peng RY. Rutin (quercetin rutinoside) induced protein-energy malnutrition in chronic kidney disease, but quercetin acted beneficially. J Agric Food Chem. 2013;61:7258–7267. doi: 10.1021/jf304595p. [DOI] [PubMed] [Google Scholar]
- 82.Kawai Y. Understanding metabolic conversions and molecular actions of flavonoids in vivo:toward new strategies for effective utilization of natural polyphenols in human health. J Med Invest. 2018;65:162–165. doi: 10.2152/jmi.65.162. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
No additional data are available.