Abstract
The demonstrated utility of the nucleoside analog ribavirin in the treatment of certain viral diseases can be ascribed to its multiple distinct properties. These properties may vary in relative importance in differing viral disease conditions and include the direct inhibition of viral replication, the promotion of T-cell-mediated immune responses via an enhanced type 1 cytokine response, and a reduction of circulating alanine aminotransferase (ALT) levels associated with hepatic injury. Ribavirin also has certain known toxicities, including the induction of anemia upon chronic administration. To determine if all these properties are linked, we compared the d-nucleoside ribavirin to its l-enantiomer (ICN 17261) with regard to these properties. Strong similarities were seen for these two compounds with respect to induction of type 1 cytokine bias in vitro, enhancement of type 1 cytokine responses in vivo, and the reduction of serum ALT levels in a murine hepatitis model. In contrast, ICN 17261 had no in vitro antiviral activity against a panel of RNA and DNA viruses, while ribavirin exhibited its characteristic activity profile. Importantly, the preliminary in vivo toxicology profile of ICN 17261 is significantly more favorable than that of ribavirin. Administration of 180 mg of ICN 17261 per kg of body weight to rats by oral gavage for 4 weeks generated substantial serum levels of drug but no observable clinical pathology, whereas equivalent doses of ribavirin induced a significant anemia and leukopenia. Thus, structural modification of ribavirin can dissociate its immunomodulatory properties from its antiviral and toxicologic properties, resulting in a compound (ICN 17261) with interesting therapeutic potential.
Ribavirin (1-β-d-ribofuranosyl-1,2,4-triazole-3-carboxamide) is a nucleoside analog that has demonstrated efficacy in treating viral disease as monotherapy (respiratory syncytial virus [RSV] [15]) and in combination with alpha interferon (IFN-α) (hepatitis C virus [HCV] [27, 36]). Ribavirin has multiple biologic properties that are favorable for treating viral diseases. It can directly inhibit the replication of many DNA and RNA viruses (38). More recently, studies have shown that it can also act as an immunomodulator and thus promote T-cell-mediated immunity against viral infection (18, 25, 30, 39, 40). The central focus of this effect of ribavirin is the augmentation of antiviral type 1 cytokine expression (interleukin-2 [IL-2], gamma interferon [IFN-γ], and tumor necrosis factor alpha [TNF-α]) and concomitant suppression of type 2 cytokine levels [IL-4, IL-5, and IL-10] by activated T cells in both human and murine systems. Finally, ribavirin, alone or in combination with IFN-α, can lower serum alanine aminotransferase (ALT) levels during the course of treatment of HCV infection (11). Elevated serum ALT levels are a marker for liver damage and progressive hepatitis, and hence the ribavirin-mediated lowering of ALT levels is a distinct liver-specific effect of this nucleoside analog.
The therapeutic use of ribavirin is restricted by its toxicology profile. Prolonged administration of ribavirin is frequently associated with anemia, whose severity correlates with dose level and which is reversible upon dose reduction or cessation of treatment. We sought to identify compounds which would retain those properties deemed critical for utility in the treatment of chronic HCV infection, but which would not have the toxicity profile of ribavirin.
We have recently shown (34) that the l-enantiomer of ribavirin, ICN 17261, has similar type-1-cytokine-enhancing activity as ribavirin in vitro in activated human T cells. The objective of this study is to expand on these initial findings by performing a comparative analysis of ICN 17261 and ribavirin relative to the aforementioned properties of ribavirin (direct antiviral activity and cytotoxicity, immunomodulatory effects in vitro and in vivo, effect on serum ALT, and preliminary toxicology profile). The results from this study suggest that the bioactive l-nucleoside ICN 17261 may offer a therapeutic advantage over ribavirin for the treatment of some viral diseases.
MATERIALS AND METHODS
Compounds.
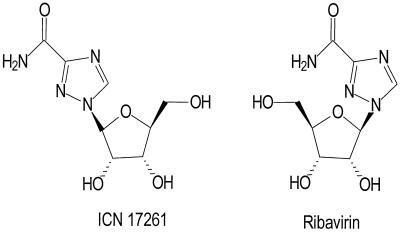
ICN 17261 (1-β-l-ribofuranosyl-1,2,4-triazole-3-carboxamide) is a new chemical entity and is the l-enantiomer of ribavirin. It is synthesized from 1,2,3,5-tetra-O-acetyl-β-l-ribofuranose and methyl 1,2,4-triazole-3-carboxylate (34). ICN 17261 has a molecular weight of 244.21 and is freely soluble in water. The structures of both ICN 17261 and ribavirin are shown in Fig. 1.
FIG. 1.
Structures of ICN 17261 and ribavirin.
Animals.
Six- to eight-week-old female BALB/c mice were purchased from Bantin and Kingman Universal (Fremont, Calif.).
In vitro studies (human).
Peripheral blood mononuclear cells were isolated from healthy donors by density gradient centrifugation followed by T-cell enrichment with Lymphokwik (One Lambda, Canoga Park, Calif.). Contaminating monocytes were removed by adherence to plastic. Purified T cells comprised >99% CD2+, <1% HLA-DR+, and <5% CD25+ and were maintained in RPMI-AP5 (RPMI 1640 medium containing 5% autologous plasma, 1% l-glutamine, 1% penicillin-streptomycin, and 0.05% 2-mercaptoethanol). For determination of cytokine protein levels, T cells (106 cells in a volume of 1 ml) were activated by the addition of either 400 ng of Staphylococcal enterotoxin B (SEB; Sigma, St. Louis, Mo.) or 10 ng of phorbol myristate acetate (PMA) plus 0.5 μg of ionomycin (ION) (Calbiochem, La Jolla, Calif.) and were incubated in 24-well plates in the presence of 0 to 10 μM ICN 17261 or ribavirin for 48 h at 37°C and 5% CO2 in a humidified incubator. Cell-free supernatants were taken and analyzed for human cytokine levels, following appropriate dilution, by using enzyme-linked immunosorbent assay (ELISA) kits specific for IL-2, IFN-γ, TNF-α, IL-4, and IL-5 (Biosource, Camarillo, Calif.). All ELISA results were expressed in picograms per milliliter.
In vitro studies (mouse).
In vitro experiments used lymph node cells from contact allergen-primed BALB/c mice or spleen cells from unsensitized BALB/c mice. Priming with contact allergen was accomplished by application of 20 μl of 0.3% dinitrofluorobenzene (DNFB) (Sigma) in acetone-olive oil, in the ratio 4:1, onto shaved abdomens 5 days prior to sacrifice. Primed mice were sacrificed by cervical dislocation and axillary/lateral axillary lymph nodes were removed. Lymph node cell (LNC) suspensions were then prepared for individual mice. Twenty-four-well plates were coated with 150 μl of a 25-μg/ml preparation of anti-mouse α/β-T cell receptor (TCR) antibody (clone H57-597; Pharmingen, La Jolla, Calif.) for 90 min at 37°C and then plates were washed twice with cold phosphate-buffered saline (PBS). LNC (2 × 106/well) were seeded in 1 ml of complete Dulbecco modified Eagle medium (containing 4.5 g of dextrose per liter [ICN Biomedicals, Costa Mesa, Calif.] and supplemented with 10% fetal bovine serum [Hyclone, Logan, Utah], 1% l-glutamine, 1% penicillin-streptomycin, 10 mM HEPES, 1× nonessential amino acids, and 50 μM 2-mercaptoethanol) in the presence of 0 or 2 μM ICN 17261 or ribavirin and cultured for 48 h at 37°C and 10% CO2 in a humidified incubator.
In spleen cell assays, BALB/c mice were sacrificed by cervical dislocation, and splenocyte suspensions were prepared from individual spleens following removal of contaminating erythrocytes with ACK lysing buffer (0.15 M NH4Cl, 1 mM KHCO3, and 0.1 mM Na2EDTA adjusted to pH 7.2 to 7.4 and filtered). Splenocytes were then seeded in 24-well plates at 2 × 106/well in 1 ml of complete RPMI media (RPMI 1640 medium containing 10% fetal bovine serum, 1% l-glutamine, and 1% penicillin-streptomycin). Splenocytes were activated with 400 ng of SEB and cultured for 48 h at 37°C and 10% CO2 in the presence of 0 to 10 μM ICN 17261 or ribavirin.
In both LNC and spleen cell assays, cell-free supernatants were taken for extracellular cytokine analyses. Murine cytokine levels were determined in cell supernatants, following appropriate dilution, by using ELISA kits specific for IL-2, IL-10, and IFN-γ ELISAs from R & D Systems (Minneapolis, Minn.) or from Biosource. All ELISA results were expressed in picograms per milliliter.
In spleen cell assays, T-cell proliferation was assessed by using a colorimetric assay (MTT cell proliferation kit I; Boehringer Mannheim, Indianapolis, Ind.) based on the conversion of the tetrazolium salt MTT by mitochondrial dehydrogenases to a formazan dye which is readily detectable by measuring the absorbance at 540 nm.
In vivo assay (contact hypersensitivity).
Reactivity to DNFB was determined in BALB/c mice as previously described (41). Briefly, mice were sensitized by the application of 20 μl of 0.3% DNFB in 4:1 acetone-olive oil onto the shaved abdomens of naive mice. For optimal elicitation of contact hypersensitivity (CHS), the mice were challenged on both sides of each ear with 20 μl of 0.12% DNFB, 5 days after sensitization. Unsensitized mice were also challenged and used as controls in each experiment. After 24 h, ear thickness measurements were taken, and response to DNFB was assessed by subtracting postchallenge from prechallenge values. Where indicated, ICN 17261 or ribavirin, at a dose of 10 μg in 50 μl PBS (0.5 mg/kg), was administered by intraperitoneal (i.p.) injection at the time of challenge with DNFB. This dose of ribavirin gave maximal effect in preliminary optimization studies.
In vivo assay (SEB treatment in vivo).
SEB was injected i.p. at a dose of 50 μg per mouse at day 0 into three groups of four mice. One group was injected with ICN 17261 and one group was injected with ribavirin, both at 10 μg in 50 μl PBS (0.5 mg/kg) i.p., 1 h prior to SEB injection. Three more groups of four mice were not treated with SEB but were injected with PBS, ICN 17261, and ribavirin, respectively. This dose of ribavirin gave maximal effect in preliminary optimization studies. All mice were anesthetized 24 h later with an appropriate dose of the inhalation anesthetic Penthrane (Abbott Labs, North Chicago, Ill.) and were exsanguinated by cardiac puncture to obtain whole blood. Serum was obtained from clotted blood and was used for determinations of nitric oxide production. Nitric oxide production was evaluated by measuring its stable end products, nitrite and nitrate. Total nitrite and nitrate levels were determined following the reduction of nitrate to nitrite through a nitrate reductase enzyme reaction followed by a colorimetric assay (Sigma) based on the reduction of total nitrite by Griess reagent to a purple azo compound.
In vivo assay (ConA-induced hepatitis).
BALB/c mice (six per group) were injected intraperitoneally with a single dose of 20 μg (1 mg/kg) of ribavirin or ICN 17261 or with 200 μl of PBS 1 h prior to intravenous tail vein injection with 0.3 mg of ConA (Calbiochem). This dose of ribavirin gave maximal effect in preliminary optimization studies. After 24 h, mice were anesthetized with Penthrane and were exsanguinated by cardiac puncture to obtain whole blood. Serum was obtained from clotted blood and was used for determinations of serum ALT. Serum ALT levels were determined by using an enzyme activity assay (Sigma). ALT catalyses the transamination of α-ketoglutaric acid to produce glutamic acid by using an amino group donated by the substrate alanine. This assay is based on the colorimetric measurement of the products (pyruvic acid and glutamic acid).
Antiviral assays.
In vitro testing for antiviral activity of ICN 17261 and ribavirin against influenza A and B viruses, parainfluenza viruses 1 and 3, and RSV (Institute of Antiviral Research, Utah State University, Logan, Utah) were performed as previously described (3, 4, 17). Anti-human immunodeficiency virus (HIV) activity was assessed by the National Cancer Institute (Bethesda, Md.) by using a procedure designed to detect agents acting at any stage of the virus reproductive cycle (43). Anti-hepatitis B virus (HBV) activity was monitored by measuring intracellular viral DNA levels following a 7-day culture of the 2.2.15 cell line (an HepG2 hepatoblastome cell line stably transfected by HBV DNA [19]) with and without various concentrations of ribavirin or ICN 17261. Viral DNA was evaluated by slot blot DNA hybridization similar to that described in Marion et al. (24) (Hepadnavirus Testing, Inc., Mountain View, Calif.). Anti-HIV activity and cytotoxicity for ribavirin were determined from previous data (2, 26).
Four-week oral gavage toxicity study.
A 4-week oral gavage toxicity study was performed by Covance Laboratories Inc. (Madison, Wis.). Male Crl:CD (SD) IGS BR rats were assigned to four groups (12 males/group in groups 1 to 3 and 15 males in group 4) and were given the 29-day treatment regimen outlined in Table 1. The doses of ICN 17261, ribavirin (used as positive control), and water control were given daily by oral gavage at a volume of 10 ml/kg. Food and water were provided ad libitum. Animals were observed twice daily for mortality and moribundity. Body weight and food consumption data were collected weekly. Blood samples were collected from all animals for drug level determinations on days 30 and 60; plasma samples were analyzed by a liquid chromatography-mass spectrometry methodology for ICN 17261 or ribavirin (M. Larson, O. Oluyedun, and S. V. Ravavendran, Internal Report 6937-101, Covance Laboratories, Inc., 1999). Further blood samples were taken on days 11, 30, and 60 for hematology and clinical chemistry. On day 30, 8 to 10 animals/group, and on day 60, four animals/group, were fasted overnight, anesthetized, weighed, exsanguinated, and necropsied. At necropsy, macroscopic observations were recorded, and selected organs were weighed, collected, and frozen.
TABLE 1.
Group designations and treatment regimen of 4-week gavage toxicity study with ICN 17261 and ribavirin in rats with 4-week recovery
| Group | Test material | No. of malesa | Dose level (mg/kg/day)b | Dose (mg/ml)b |
|---|---|---|---|---|
| 1 | Control | 12 | 0 | 0 |
| 2 | ICN 17261 | 12 | 60–300c | 6.0–30.0c |
| 3 | ICN 17261 | 12 | 180 | 18.0 |
| 4 | Ribavirin | 15 | 180 | 18.0 |
Four animals in each group designated as recovery animals were dosed daily for 29 days, after which dosing was discontinued, and the animals were observed for reversibility, persistence, or delayed occurrence of toxic effects for 31 days posttreatment.
The dose volume was 10 ml/kg.
On day 17, the dose level was increased to 300 mg/kg/day, and the dose concentration was increased to 30 mg/ml.
Statistical analysis.
Trend analysis was assessed by using analysis of variance where the main effects evaluated included donor (random effect), concentration, and nucleoside. Statistical significance, where relevant, was assessed by using the Student-Newman-Keuls multiple comparison method.
RESULTS
In vitro type 1 and type 2 cytokine synthesis in activated human T cells.
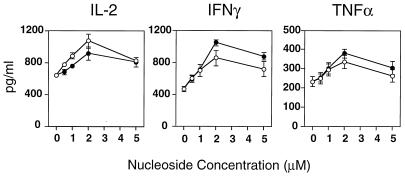
Recently, we have shown that ribavirin can enhance antiviral type 1 cytokines and suppress type 2 cytokine expression in human T cells (40). Here we compared the influence of ICN 17261 and ribavirin on the cytokine pattern induced by the bacterial superantigen SEB in human T cells from five normal donors. After 48 h of stimulation, secreted levels of type 1 cytokines IL-2, TNF-α, and IFN-γ were determined in the cell-free supernatants. Our data, generated from ELISA analyses, show that ICN 17261, like ribavirin, in the dose range 0.5 to 5 μM, augmented IL-2, IFN-γ, and TNF-α (Fig. 2). A significant concentration-dependent effect was observed for both compounds on IFN-γ and TNF-α levels (P < 0.0001) but not on IL-2 levels. Both compounds showed a significantly elevated cytokine response at 2 and 5 μM for IFN-γ and TNF-α (P < 0.05), with a peak effect at about 2 μM for all cytokines. No significant differences were seen between nucleoside effects on all cytokines. ICN 17261 induced a mean peak increase in IL-2, IFN-γ, and TNF-α of 42, 125, and 72% over activated control levels, respectively. For ribavirin, the mean peak increase was 66, 84, and 51% over activated control levels, respectively. We were unable to determine by ELISA whether levels of type 2 cytokines in SEB-stimulated T cells were suppressed by ICN 17261 or ribavirin, as levels of SEB-induced type 2 cytokines were below the level of immunoassay sensitivity.
FIG. 2.
Modulation of SEB-induced type 1 cytokine responses following ICN 17261 and ribavirin treatment in human T cells. Comparison of the dose-dependent (0.5 to 5 μM) augmentation by ICN 17261 (open circles) and ribavirin (filled circles) of IL-2, IFN-γ, and TNF-α levels in SEB-stimulated T cells from five individual human donors. Cytokine levels were determined in cell-free supernatants by ELISA. The absolute level (picograms per milliliter ± SD) of type 1 cytokine secretion was substantially elevated in SEB-stimulated T cells (IL-2, 640 ± 36; IFN-γ, 462 ± 37; TNF-α, 223 ± 27) compared to resting levels (<30 pg/ml for all cytokines).
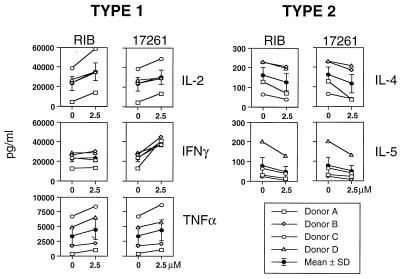
Next, we investigated the influence of ribavirin on the cytokine pattern in human T cells from four donors following activation with the pharmacologic agents, PMA, and ionomycin (ION). After 48 h, levels of type 1 cytokines IL-2, TNF-α, and IFN-γ and the type 2 cytokines IL-4 and IL-5 were determined in the cell-free supernatants. In Fig. 3, both ICN 17261 and ribavirin at 2.5 μM similarly augmented IL-2 (mean peak increase of 28 and 49%, respectively) and TNF-α (mean peak increase of 28 and 33%, respectively) expression in PMA-ION-activated human T cells. A significant positive concentration-dependent effect was observed for both compounds on IL-2 and TNF-α levels (P < 0.0002 and P < 0.003, respectively) but not IFN-γ. Both compounds showed a significantly elevated cytokine response at 2 μM for IL-2 and TNF-α (P < 0.05). The effect on IL-2 and TNF-α levels was not significantly different between both nucleosides. ICN 17261 (mean peak increase of 73%) showed significant enhancement of IFN-γ (P < 0.05), whereas ribavirin did not (2%). This lack of enhancement of IFN-γ by ribavirin in PMA-ION-activated human T cells has been observed previously (40). A significant negative concentration-dependent effect was observed for both compounds on IL-4 and IL-5 levels (P < 0.002 and P < 0.007, respectively). Both ICN 17261 and ribavirin at 2.5 μM significantly (P < 0.05) suppressed type 2 cytokines IL-4 (mean peak decreases of 38 and 26%, respectively) and IL-5 (mean peak decreases of 60 and 66%, respectively) (Fig. 3). These data show that following both physiologic (SEB) or pharmacologic (PMA-ION) T-cell activation, ribavirin and ICN 17261 have the property of inducing a type 1 cytokine bias in human T cells.
FIG. 3.
Modulation of PMA-ION-stimulated type 1 and type 2 cytokine responses following treatment with ICN 17261 and ribavirin. The effect of ICN 17261 (17261) or ribavirin (RIB) (0 and 2.5 μM) on PMA-ION-stimulated T-cell expression of the type 1 (IL-2, IFN-γ, and TNF-α) and type 2 (IL-4 and IL-5) cytokines is shown for four individual human donors, A to D. The mean cytokine responses for all four donors (±SD) at 0 and 2.5 μM of each nucleoside are also shown in addition to the individual data for each donor. Cytokine levels were determined in cell-free supernatants by ELISA. The absolute level (picogram per milliliter ± SD) of PMA-ION-induced type 1 cytokine secretion was 23,568 ± 6,983 for IL-2, 22,954 ± 3,391 for IFN-γ, and 3,414 ± 1,451 for TNF-α. The absolute level (picogram per milliliter ± SD) of PMA-ION-induced type 2 cytokine secretion in PMA-ION-stimulated T cells was 162 ± 40 for IL-4, and 80 ± 41 for IL-5. Resting levels were <30 pg/ml for all cytokines tested.
In vitro and in vivo effects of ICN 17261 on type-1-cytokine-mediated immune responses in mice.
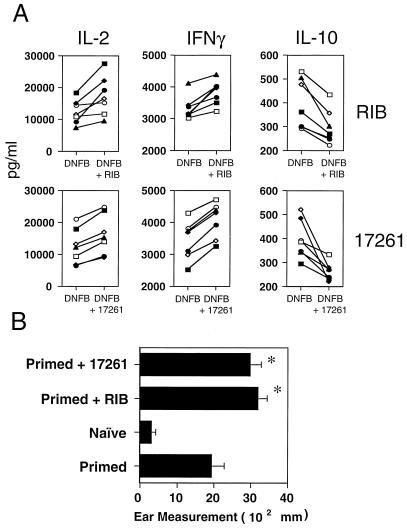
We investigated the comparative effects of ICN 17261 and ribavirin on two type-1-cytokine-mediated immune responses (1, 45) in vitro and in vivo in mice. Firstly, we assessed the effect of ICN 17261 and ribavirin on the in vitro cytokine and in vivo contact hypersensitivity responses to the contact allergen DNFB. In vitro cytokine secretion in individual LNC preparations from seven DNFB-primed mice was induced with plate-bound anti-α/β-TCR antibody in vitro in the presence or absence of 2 μM of ICN 17261 or ribavirin. The peak effect on modulation of type 1 and 2 cytokine levels by ribavirin was previously shown in mice to be at a concentration of 2 μM (39). It is noteworthy that in this previous study (39) the enhancement of contact hypersensitivity by ribavirin in BALB/c mice was dependent on the suppression of IL-10. After 48 h, levels of type 1 cytokines IL-2 and IFN-γ and the type 2 cytokine IL-10 were determined in the cell-free supernatants. In Fig. 4A, in LNC from DNFB-primed mice, both ICN 17261 and ribavirin at 2 μM augmented IL-2 (mean peak increases of 69 and 84%, respectively) and significantly (P < 0.05) augmented IFN-γ (mean peak increases of 27 and 24%, respectively) while concomitantly and significantly (P < 0.05) suppressing the type 2 cytokine IL-10 (mean peak decreases of 37 and 55%, respectively).
FIG. 4.
The effect of ICN 17261 and ribavirin on the in vitro cytokine (A) and in vivo contact hypersensitivity (B) responses to the contact allergen DNFB. (A) The effect of ICN 17261 and ribavirin on secreted type 1 and type 2 cytokine levels in mouse LNC from individual BALB/c mice sensitized with DNFB (see Materials and Methods). Extracellular levels of type 1 (IL-2 and IFN-γ) and the type 2 cytokine IL-10 were determined in anti-mouse α/β-TCR-activated LNC from seven primed mice following a 48-h incubation in the presence of 0 and 2 μM ribavirin (RIB, top panels) or ICN 17261 (17261, bottom panels). The effect of ribavirin or ICN 17261 on cytokine levels was assessed in cell-free supernatants in duplicate by ELISA analyses and are represented as mean concentration. The data shown are representative of three separate experiments each using data from six mice. (B) CHS responses were induced in three groups of five mice by sensitization and challenge with DNFB as described in Materials and Methods. At the time of challenge with 0.12% DNFB, one group was administered 5 μg of ribavirin (RIB) and a second group was administered 5 μg of ICN 17261 (17261), both given in 50 μl of PBS by i.p. injection. The third DNFB-primed group (Primed) was untreated. The ear swelling in all groups (including an unsensitized control group [Naïve]) was measured after 24 h and was compared to prechallenge values. The data shown as mean ear swelling (Ear Measurement) are representative of four separate experiments with three to five animals in each test group. Error bars indicate SDs. Asterisks indicate P < 0.001 when compared to the primed group.
In separate experiments, the functional effect of ICN 17261 and ribavirin on contact hypersensitivity responses in vivo in BALB/c mice were examined. Animals were primed with DNFB (0.3%) epicutaneously on the abdomen and were challenged 6 days later on each ear with 0.12% DNFB. ICN 17261 or ribavirin (0.5 mg/kg) was given i.p. at the time of challenge. Modulation of CHS, as determined from measurements of ear swelling following challenge, was calculated following subtraction of responses in nonsensitized challenged (naïve) mice. CHS responses following priming and challenge in BALB/c mice were significantly greater (P < 0.0001) than those seen in the naïve group (Fig. 4B). Intraperitoneal administration of either ICN 17261 or ribavirin at the time of challenge significantly enhanced CHS responses in DNFB-primed BALB/c mice (P < 0.0001, Fig. 4B). The mean percentage increases in ear thickness (± standard deviation [SD]) following ICN 17261 or ribavirin treatment was 55% ± 13% and 60% ± 14%, respectively, 24 h postchallenge.
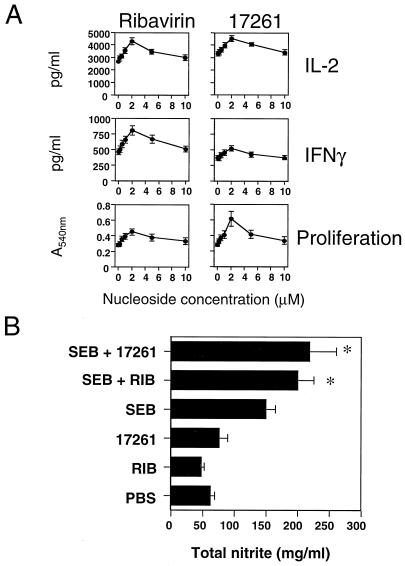
Secondly, we assessed the effect of ICN 17261 and ribavirin on the in vitro cytokine and in vivo inflammatory responses to the bacterial superantigen SEB. In vitro proliferative responses and cytokine secretion in splenocytes from BALB/c mice were induced with 400 ng of SEB in vitro in the presence or absence of 2 μM ICN 17261 or ribavirin. It was important to assess the effect of both nucleosides on IFN-γ, IL-2, and splenocyte proliferation, as IFN-γ is one of the critical mediators of this type of inflammatory response (1) and T-cell proliferative responses are a functional effect of nucleoside-mediated increase in IL-2 production (40). After 48 h, splenocyte proliferation and levels of type 1 cytokines IL-2 and IFN-γ in the cell-free supernatants were determined. Figure 5A shows the effect of ICN 17261 and ribavirin in the dose range 0.125 to 10 μM on type 1 cytokine production in BALB/c splenocytes. A significant positive concentration-dependent effect was observed for both compounds on IL-2 and IFN-γ levels and on splenocyte proliferation (P < 0.0001, P < 0.0003, and P < 0.0001, respectively). The mean peak increase (2 μM, P < 0.05 for both nucleosides) of proliferation and IL-2 and IFN-γ levels over activated control for ICN 17261 was 89, 71, and 52%, respectively, whereas ribavirin caused a mean peak increase of 61, 62, and 75%, respectively. Next, the functional effects of ICN 17261 and ribavirin on inflammatory responses to SEB in vivo in BALB/c mice were examined. Animals were treated with a single challenge i.p. with 50 μg of SEB 24 h prior to sacrifice. ICN 17261 or ribavirin (0.5 mg/kg) was given i.p. at the time of challenge. Modulation of SEB-induced inflammatory responses in SEB-treated mice was determined from serum levels of the inflammatory mediator, nitric oxide (total nitrite) and was calculated following subtraction of responses in mice treated with PBS. Total serum nitrite levels in SEB-treated BALB/c mice were significantly greater (P < 0.0001) than those treated with PBS, ribavirin alone, or ICN 17261 alone in the absence of SEB (Fig. 5B). Administration (i.p.) of both ICN 17261 and ribavirin enhanced serum nitrite levels in SEB-treated BALB/c mice (P < 0.0001) (Fig. 5B), giving mean percentage increases (±SD) following ICN 17261 or ribavirin treatment of 63% ± 30% and 58% ± 20%, respectively, 24 h post-SEB treatment in vivo.
FIG. 5.
The effect of ICN 17261 and ribavirin on the in vitro cytokine (A) and in vivo inflammatory (B) responses to the bacterial superantigen SEB. (A) The effect of ICN 17261 and ribavirin on secreted type 1 and type 2 cytokine levels from SEB-stimulated BALB/c mouse splenocytes (see Materials and Methods). Extracellular levels of type 1 cytokines IL-2 and IFN-γ were determined in individual splenocyte preparations from six BALB/c mice following stimulation for 48 h with 400 ng of SEB in the presence of 0 to 10 μM ribavirin (left panels) or ICN 17261 (17261, right panels). The effects of ribavirin or ICN 17261 on proliferation were assessed by using an MTT cell proliferation kit and are represented as mean absorbance units (at 540 nm) ± SD. Cytokine levels were assessed in cell-free supernatants in quadruplicate by ELISA analyses and are represented as mean concentrations. The data shown are representative of three separate experiments each using data from six mice. (B) In vivo responses to ICN 17261 and ribavirin administration in SEB-treated BALB/c mice. Two groups of four mice were injected i.p. with either 5 μg of ICN 17261 (SEB + 17261) or 5 μg of ribavirin (SEB + RIB) (both in 50 μl of PBS) 1 h prior to i.p. administration with 50 μg of SEB. An additional four groups of four mice were injected with 50 μg of SEB (SEB), 5 μg of ICN 17261 (17261), 5 μg of ribavirin (RIB), or 50 μl of PBS. After 24 h, all mice were anesthetized with inhalation anesthetic, were exsanguinated by cardiac puncture, and were sacrificed. Nitric oxide production was monitored by determining total nitrite levels in the serum obtained from clotted blood by using a colormetric assay (see Materials and Methods). The data shown as mean total nitrite levels are representative of three separate experiments with four animals in each test group. Error bars indicate SDs. Asterisks indicate P < 0.001 when compared to SEB-treated group.
Collectively, these data show that in both models of type 1 cytokine-mediated immune responses, both ICN 17261 and ribavirin induced a type 1 cytokine bias and enhanced the antigen-induced immune response in vivo.
Anti-inflammatory activities of ICN 17261 in ConA-induced hepatitis.
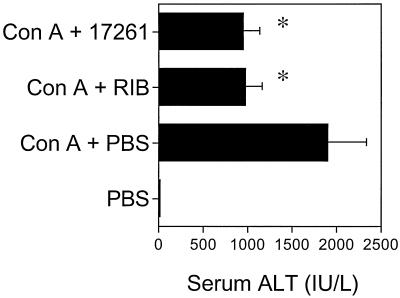
Treatment of chronically HCV-infected patients with ribavirin dramatically reduces serum ALT levels. Recently, a new murine hepatitis model was developed in which liver-specific inflammatory lesions are induced by injection of ConA (29). Significantly, the hepatic injury appears to be a consequence of T-cell activation (29). Here, using the ConA-induced hepatitis model, we compared the influence of ICN 17261 and ribavirin on hepatic-injury-induced serum ALT levels. BALB/c mice were injected i.p. with either ICN 17261 (1 mg/kg), ribavirin (1 mg/kg), or PBS 1 h prior to intravenous injection of 0.3 mg of ConA. Mice were exsanguinated 24 h later, and serum ALT levels were determined. Figure 6 shows that both ICN 17261 and ribavirin were able to substantially reduce ConA-induced serum ALT levels from 1,896 U/ml to 969 ± 192 U/ml (49% inhibition) and 954 ± 179 U/ml (50% inhibition), respectively. Administration of ribavirin or ICN 17261 (1 mg/kg) to normal mice did not affect serum ALT levels when compared to untreated controls. Neither ribavirin nor ICN 17261 interfere with the ALT assay, as serum samples from normal or ConA-treated mice spiked with ribavirin or ICN 17261 showed indistinguishable ALT concentrations from unspiked serum samples from the same mice.
FIG. 6.
ICN 17261 and ribavirin both suppress hepatic injury in a murine hepatitis model measured using serum ALT as a surrogate marker. Three groups of six mice were injected i.p. with 1-mg/kg ICN 17261 (Con A + 17261) 1-mg/kg ribavirin (Con A + RIB), or PBS (Con A + PBS) 1 h prior to intravenous injection of 0.3 mg of ConA. Twenty-four hours later, all mice, including an untreated group, were exsanguinated, and serum ALT levels were determined by using a colorimetric assay. Data were obtained as absorbance values (A505) ± SD and are representative of three separate experiments. Serum ALT concentrations were calculated from a calibration standard curve (Sigma) and were converted to international units per milliliter. Asterisks indicate P < 0.0001 when compared to the ConA-injected group. Error bars indicate SDs.
Antiviral and cytotoxicity activities.
The antiviral activities of ICN 17261 and ribavirin against a variety of viruses were compared in vitro and are shown in Table 2. ICN 17261 showed no cytotoxicity and little or no activity in any of the antiviral tests, whereas ribavirin had the expected profile of antiviral activities and cytotoxicity.
TABLE 2.
Antiviral activities and cytotoxicity in vitro of ICN 17261 and ribavirin against various viruses
| Test material | Activity testedb | Activity against virusesa (no. determinations):
|
||||||
|---|---|---|---|---|---|---|---|---|
| HBV (4) | HIV (6) | INFL. A (6) | INFL. B (6) | PARA. 1 (3) | PARA. 3 (3) | RSV (3) | ||
| 17261 | Antiviral | >100 | >600 | >200 | >200 | >1000 | >1000 | >1000 |
| Cytotoxic | >100 | >600 | >200 | >200 | >1000 | >1000 | >1000 | |
| Ribavirin | Antiviral | >100 | 40c | 5.0 ± 0.9 | 2.6 ± 0.7 | 4.0 ± 5.0 | 50 ± 20 | 40 ± 3.0 |
| Cytotoxic | 53 | >40d | 56 | >100 | >1000 | >4000 | >4000 | |
Viruses tested were HBV, HIV, influenza (INFL.) A and B, parainfluenza (PARA.) 1 and 3, and RSV. Data are presented as means ± SDs (where applicable and available).
Antiviral activity (50% effective concentration) and cytotoxicity (CC50) are given as micromolarity.
From McCormick et al. (26); no SD available.
From Balzarini et al. (2); no SD available.
Preliminary toxicology data.
A 4-week oral gavage toxicity study was performed to assess the toxicity of ICN 17261 in rats. The 29-day treatment regimen (Table 1) was followed by a 31-day recovery period in which no test compound was administered. Animals given 180 mg of ribavirin/kg/day had lower food consumption and lower body weights and body weight gains throughout treatment when compared with those of controls. During recovery, there was a trend of higher weight gains and similar food consumption compared to controls. In contrast, there were no apparent differences in body weight, body weight gain, or food consumption noted in animals given ICN 17261.
Administration of ICN 17261 had no effects on clinical pathology results. Administration of 180 mg of ribavirin/kg/day was associated with decreases in erythrocyte count, hemoglobin, hematocrit, leukocyte count (due to lower absolute neutrophil, lymphocyte, and eosinophil counts) (Table 3), and higher mean corpuscular hemoglobin concentration and platelet count. All of the effects of ribavirin were reversed following the 31-day recovery. The anemia and leukopenia caused by ribavirin were moderately severe. For example, the mean hematocrit at day 30 for the ribavirin-treated animals was 26% lower than that for the control animals (32 versus 43%). The mean leukocyte count for the ribavirin-treated animals was 55% lower than that of control animals (3,800 versus 8,400 cells per μl). The effect on leukocyte count was primarily due to lower absolute lymphocyte count (2,800 versus 7,100 cells per μl).
TABLE 3.
Four-week daily oral administration of ICN 17261 does not induce anemia or leukopenia in rats
| Groupa | Test material | Dose (mg/ml/day)b | Level of:
|
|||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Hemoglobinc
|
Hematocritd
|
Leukocytee
|
Lymphocytef
|
|||||||||||
| Day 11 | Day 30 | Day 60 | Day 11 | Day 30 | Day 60 | Day 11 | Day 30 | Day 60 | Day 11 | Day 30 | Day 60 | |||
| 1 | Control | 0 | 14.1 ± 0.66 | 14.4 ± 0.61 | 15.1 ± 0.64 | 41.4 ± 2.08 | 42.6 ± 1.76 | 44.2 ± 1.78 | 7.5 ± 1.86 | 8.4 ± 2.65 | 11.8 ± 2.52 | 6.5 ± 1.73 | 7.1 ± 2.51 | 10.3 ± 2.64 |
| 2 | ICN 17261 | 60–300 | 13.9 ± 0.63 | 14.4 ± 0.56 | 15.4 ± 0.37 | 40.7 ± 2.13 | 42.4 ± 1.26 | 45.1 ± 1.1 | 7.2 ± 0.83 | 9.0 ± 1.55 | 10.9 ± 1.26 | 6.1 ± 0.79 | 7.4 ± 1.26 | 9.0 ± 1.18 |
| 3 | ICN 17261 | 180 | 14.0 ± 0.61 | 14.6 ± 0.54 | 15.5 ± 0.75 | 40.9 ± 2.01 | 42.9 ± 1.52 | 45.5 ± 2.97 | 6.8 ± 1.57 | 8.4 ± 1.88 | 9.6 ± 2.98 | 5.8 ± 1.31 | 6.8 ± 1.89 | 7.8 ± 2.81 |
| 4 | Ribavirin | 180 | 12.1 ± 1.01g | 11.7 ± 1.54g | 15.9 ± 0.45 | 32.9 ± 4.05g | 31.7 ± 5.96g | 45.3 ± 0.62 | 5.4 ± 1.41g | 3.8 ± 1.10g | 8.2 ± 2.79 | 4.8 ± 1.37g | 2.8 ± 0.94g | 6.8 ± 2.52 |
Measurements at days 11 and 30 were from 12 male rats from each group except for group 4 (15 rats). Day 60 measurements were from four animals from each group designated as recovery animals which were dosed daily for 29 days, after which dosing was discontinued, and the animals were observed for reversibility, persistence, or delayed occurrence of toxic effects for 31 days posttreatment.
Dose regimen as in Table 1.
Hemoglobin values are shown as grams per deciliter.
Hematocrit values are shown as percentage of blood volume.
Leukocyte count shown as cells × 10−3/μl.
Lymphocyte count shown as cells × 10−3/μl.
Group mean is significantly (P ≤ 0.05) different from mean of control group 1.
There was no gross organ weight changes or macroscopic or microscopic findings suggestive of any toxic effect following 29 days of treatment with 180 mg of ICN 17261/kg/day by oral gavage. Animals given 180 mg of ribavirin/kg/day exhibited reduced terminal body weights and increased extramedullary hematopoiesis in the spleen. The increased extramedullary hematopoiesis was secondary to anemia. Terminal body weights and spleen histopathology were normal in ribavirin-treated animals following the 31-day recovery.
Mean plasma levels of ribavirin and ICN 17261 were comparable at terminal sacrifice for animals given 180 mg of ribavirin/kg/day and 60 to 300 mg of ICN 17261/kg/day, respectively, and increased in approximate proportion to dose for animals treated with ICN 17261.
DISCUSSION
In the present study, we compared ribavirin and its l-enantiomer, ICN 17261, with respect to key properties associated with the clinical utility of ribavirin. We found that these compounds have very similar activity in a variety of assays that evaluate enhancement of the type 1 cytokine response by activated T cells. These assays include both human and murine systems and both pharmacologic and antigen-dependent activation in vitro. It is of note that peak activity of both ICN 17261 and ribavirin in vitro was at 2 to 5 μM (0.6 to 1.3 μg/ml), a dose range which encompasses the reported steady-state ribavirin concentration range in patients administered ribavirin at 600 to 1,200 mg/day in combination with IFN-α (13). Also, both ICN 17261 and ribavirin have similar activity in two in vivo assays of type 1 cytokine activation, contact hypersensitivity responses to DNFB and in vivo responses to SEB. Furthermore, both compounds reduced the levels of circulating liver enzymes in a murine inflammatory hepatitis model.
In contrast to these similarities, the two compounds also exhibited marked differences. ICN 17261 was less cytotoxic than ribavirin and was inactive against a range of viruses whose replication is normally inhibited by ribavirin in vitro. Importantly, ICN 17261 did not show apparent toxicity in rats following a 4-week multidose toxicology study. In contrast, ribavirin exhibited multiple toxicities, including the anemia commonly observed in clinical studies. Thus, the studies reported here demonstrate that a compound structurally related to ribavirin can also have multiple biologic properties, some remarkably similar (e.g., type 1 cytokine bias and lowering serum ALT) and others strikingly different (e.g., lack of direct antiviral activity and lack of apparent toxicity) to ribavirin.
The in vivo comparisons of these two enantiomers provide a basis for future work to establish their relative utilities. Drug levels were not assessed as part of the murine efficacy studies, which were short term and used i.p. injection to administer drug; the comparable efficacy of the two compounds in these immunological assays suggests adequate exposure to both compounds, but does not assure similar in vivo potency. Because poor bioavailability of ICN 17261 could explain the absence of toxicity observed in rats treated by gavage with this compound, we assessed the serum levels of ICN 17261 and ribavirin by a liquid chromatography-mass spectrometry methodology (M. Larson, O. Oluyedun, and S. V. Ravavendrun, Internal Report 6937-101, Covance Laboratories, Inc., 1999). The serum levels of ICN 17261 in these rats were proportional to dose and, at the highest dose, not significantly different from the serum levels of ribavirin that showed toxicity. Notwithstanding these encouraging results, the pharmacokinetics of ICN 17261 are of obvious interest and are the subject of ongoing investigations.
The common paradigm for the mechanism of action of antiviral nucleoside analogs involves transport into cells followed by enzymatic phosphorylation, generating nucleoside mono-, di-, and triphosphates. These phosphorylated products act to inhibit various functions critical to viral replication. Differences in enzymatic phosphorylation have been reported for enantiomeric guanosine nucleoside analogs (28). The mechanism(s) for immunomodulation by purine nucleoside analogs may not require phosphorylation (5, 14), and thus differences in phosphorylation may account for at least some of the distinguishing properties we report here for ribavirin and ICN 17261. However, additional work is needed to confirm this hypothesis.
The multiple properties ascribed to ribavirin provide potential utility for the treatment of a range of viral diseases and make determination of the optimal use of the compound more complex. Its demonstrable inhibition of RSV replication in vitro, and the satisfactory clinical performance of aerosol ribavirin in the treatment of RSV pneumonia in pediatric patients, led to its adoption for this use. In contrast, the absence of a clear inhibitory effect on the levels of circulating HCV reduced interest in the use of ribavirin as monotherapy for this disease, even though reproducible improvement in ALT levels was seen (10, 23). The occurrence of anemia in some patients further complicated its clinical use as monotherapy. However, clinical studies using ribavirin in combination with IFN-α 2b demonstrated major improvement in the proportion of patients who clear chronic infection with HCV compared to IFN-α 2b alone (9, 27, 32), leading to widespread adoption of this combination therapy. Thus, it is likely that the relative importance of each of ribavirin's multiple activities varies in the treatment of different viral diseases.
The absence of a substantial effect of ribavirin monotherapy on circulating levels of HCV is consistent with a range of in vitro data. Although direct assessment of inhibition of replication of HCV is difficult, evaluation in primary hepatocytes showed ribavirin to be inactive, whereas IFN-α inhibited HCV replication in this system (6). This result also is consistent with what is known regarding the effects of ribavirin against HCV molecular targets. For example, HCV utilizes an internal ribosome entry site element rather than a 5′-cap structure to initiate translation, so any inhibition by ribavirin of 5′-cap synthesis is unlikely to affect HCV replication. Evaluation of the ability of ribavirin nucleotides to inhibit the HCV helicase has not been reported; however, this enzyme is not selective with regard to ribonucleoside triphosphates (33), and the high intracellular concentrations of other ribonucleotides should compete effectively to minimize inhibition by ribavirin triphosphate. A more likely target is the HCV-dependent RNA polymerase, but the available data are not clear on this point. One group has determined that ribavirin triphosphate did not inhibit this enzyme in vitro (R. Bartenschlager, personal communication), while another has suggested that high concentrations of ribavirin triphosphate in hepatocytes may result in some inhibition of the HCV polymerase (16). It is possible that modest inhibition of the HCV NS5b polymerase results in an antiviral effect that is only clinically detectable when ribavirin is combined with IFN-α. Evaluation of HCV dynamics in combination therapy showed no significant change in the viral clearance rate over a 4-week time frame compared to IFN-α monotherapy (23); a second study did observe differences later in therapy (31). It is not clear that this late effect on viral titer results from direct antiviral activity rather than immunologic mechanisms.
Also of interest is the fact that the clinical response to ribavirin monotherapy in patients chronically infected with hepatitis B virus (12) closely resembles the response to ribavirin monotherapy in patients chronically infected with HCV. In both diseases, ribavirin treatment suppresses liver damage (as measured by a reduction in levels of circulating ALT) with only minor reductions in the levels of circulating virus. As we report here, ribavirin has no in vitro activity against HBV, an observation that reinforces the conclusion that direct inhibition of viral replication by ribavirin does not play a significant role in its utility in treating viral hepatitis.
In contrast to all these data that indicate a minimal direct antiviral effect of ribavirin in viral hepatitis, the role of immunomodulation by ribavirin in chronic HCV infection may be significant. A robust, multispecific T-cell response is seen in the majority of patients who clear their HCV infections, either spontaneously or in response to IFN-α treatment (7, 20–22, 35, 37, 42, 44). Ribavirin and IFN-α combination treatment-induced control of viremia is also associated with the development of HCV-specific T-cell responses with enhanced IFN-γ and low IL-10 production (8). Such an immune response is much less frequent in patients who remain chronically infected following treatment. The favorable clinical interaction of ribavirin and IFN-α is thus consistent with ribavirin enhancement of the type 1 cytokine response in stimulated T cells as reported here and elsewhere (18). The recognition that ribavirin can enhance the type 1 cytokine response provides a rationale for combination use with IFN-α, even in the absence of a direct effect of ribavirin on HCV replication.
Thus, the known properties of ribavirin that appear to be of prime importance in the treatment of chronic viral hepatitis are the suppression of circulating ALT consequent to liver damage and the enhancement of a type 1 cytokine T-cell response. These properties are separable from the direct antiviral activity of ribavirin, and a compound possessing these properties (ICN 17261) has significantly reduced toxicity both in vitro and in vivo. ICN 17261 merits additional evaluation as a clinical candidate.
ACKNOWLEDGMENTS
We thank Jace Collins for excellent administrative assistance.
REFERENCES
- 1.Assenmacher M, Lohning M, Sheffold A, Manz R A, Schmitz J, Radbruch A. Sequential production of IL-2, IFN-gamma, and IL-10 by individual staphylococcal enterotoxin B-activated T helper lymphocytes. Eur J Immunol. 1998;28:1534–1543. doi: 10.1002/(SICI)1521-4141(199805)28:05<1534::AID-IMMU1534>3.0.CO;2-R. [DOI] [PubMed] [Google Scholar]
- 2.Balzarini J, Mitsuya H, De Clerq E, Broder S. Comparative inhibitory effects of suramin and other selected compounds on the infectivity and replication of human T-cell lymphotropic virus (HTLV-III)/lymphadenopathy-associated virus (LAV) Int J Cancer. 1986;37:451–457. doi: 10.1002/ijc.2910370318. [DOI] [PubMed] [Google Scholar]
- 3.Barnard D L, Bischofberger N, Kim C U, Huffman J H, Sidwell R W, Dougherty J P, Lew W, Williams M A, Yang W. Acyclic phosphonomethylether nucleoside inhibitors of respiratory viruses. Antivir Chem Chemother. 1997;8:223–233. [Google Scholar]
- 4.Barnard D L, Hill C L, Gage T, Matheson J E, Huffman J H, Sidwell R W, Otto M J, Schinazi R F. Potent inhibition of respiratory syncytial virus by polyoxometalates of several structural classes. Antiviral Res. 1997;34:27–37. doi: 10.1016/s0166-3542(96)01019-4. [DOI] [PubMed] [Google Scholar]
- 5.Bonnet P A, Robins R K. Modulation of leukocyte genetic expression by novel purine nucleoside analogues. A new approach to antitumor and antiviral agents. J Med Chem. 1993;36:635–653. doi: 10.1021/jm00058a001. [DOI] [PubMed] [Google Scholar]
- 6.Clarysse C, Nevens F, Pirenne J, Carucci P, Aerts R, Soumillion A, Roberts N, Yap S H. Differential effect of IFN-alpha 2b and ribavirin on the HCV-RNA level in primary cultures of human hepatocytes derived from chronic HCV carriers and of human hepatocytes infected in vitro. Hepatology. 1997;26:215A. [Google Scholar]
- 7.Cramp M E, Carucci P, Rossol S, Chokshi S, Maertens G, Williams R, Naoumov N V. Hepatitis C virus (HCV) specific immune responses in anti-HCV positive patients without hepatitis C viraemia. Gut. 1999;44:424–429. doi: 10.1136/gut.44.3.424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cramp M E, Rossol S, Chokshi S, Carucci P, Williams R, Naoumov N V. Hepatitis C virus-specific T cell reactivity during interferon and ribavirin treatment in chronic hepatitis C. Gastroenterology. 2000;118:346–355. doi: 10.1016/s0016-5085(00)70217-4. [DOI] [PubMed] [Google Scholar]
- 9.Davis G L, Esteban-Mur R, Rustgi V, Hoefs J, Gordon S C, Trepo C, Schiffman M L, Zeuzem S, Craxi A, Ling M H, Albrecht J. Interferon alpha-2b alone or in combination with ribavirin for the treatment of relapse of chronic hepatitis C virus. International Hepatitis Interventional Therapy Group. N Engl J Med. 1998;339:1493–1499. doi: 10.1056/NEJM199811193392102. [DOI] [PubMed] [Google Scholar]
- 10.Di Bisceglie A M, Conjeevaram H S, Fried M W, Sallie R, Park Y, Yurdaydin C, Swain M, Kleiner D E, Mahaney K, Hoofnagle J. Ribavirin as therapy for chronic hepatitis. A randomized, double-blind, placebo-controlled trial. Ann Intern Med. 1995;123:897–903. doi: 10.7326/0003-4819-123-12-199512150-00001. [DOI] [PubMed] [Google Scholar]
- 11.Dusheiko G, Main J, Thomas H, Reichard O, Lee C, Dhillon A, Rassam S, Fryden A, Reesink H, Bassendine M, Norkrans G, Cuypers T, Lelie N, Telfer P, Watson J, Weegink C, Sillikens P, Weiland O. Ribavirin treatment for patients with chronic hepatitis C: results of a placebo-controlled study. J Hepatol. 1996;25:591–598. doi: 10.1016/s0168-8278(96)80225-x. [DOI] [PubMed] [Google Scholar]
- 12.Fried M W, Fong T L, Swain M G, Park Y, Beames M P, Banks S M, Hoofnagle J H, Di Bisceglie A M. Therapy of chronic hepatitis B with a 6-month course of ribavirin. J Hepatol. 1994;21:145–150. doi: 10.1016/s0168-8278(05)80387-3. [DOI] [PubMed] [Google Scholar]
- 13.Glue P, Jen F, Gupta S K, Lau J Y N. Interferon-a2b/ribavirin (Rebetron™) treatment for chronic hepatitis C—population pharmacokinetics and pharmacodynamics of ribavirin. Hepatology. 1999;30:190A. [Google Scholar]
- 14.Goodman M G, Reitz A B, Chen R, Bobardt M D, Goodman J H, Pope B L. Selective modulation of elements of the immune system by low molecular weight nucleosides. J Pharmacol Exp Ther. 1995;274:1552–1557. [PubMed] [Google Scholar]
- 15.Hall C B, McBride J T, Walsh E E, Bell D M, Gala C L, Hildreth S, Ten Eyck L G, Hall W J. Aerosolized ribavirin treatment of infants with respiratory syncytial viral infection. N Engl J Med. 1983;308:1443–1447. doi: 10.1056/NEJM198306163082403. [DOI] [PubMed] [Google Scholar]
- 16.Hong Z, Ferrari E, Wright-Minotogue J, Skelton A, Glue P, Zhong W, Lau J Y N. Direct antiviral activity of ribavirin: hepatitis C virus NS5B polymerase incorporates ribavirin triphosphate into nascent RNA products. Hepatology. 1999;30:354A. [Google Scholar]
- 17.Huffman J H, Sidwell R W, Barnard D L, Morrison A, Otto M J, Hill C L, Schinazi R F. Influenza virus-inhibitory effects of a series of germanium- and silicon-centered polyoxometalates. Antivir Chem Chemother. 1997;8:75–83. [Google Scholar]
- 18.Hultgren C, Milich D R, Weiland O, Sällberg M. The antiviral compound ribavirin modulates the T helper (Th)1/Th2 subset balance in hepatitis B and C virus-specific immune responses. J Gen Virol. 1998;79:2381–2391. doi: 10.1099/0022-1317-79-10-2381. [DOI] [PubMed] [Google Scholar]
- 19.Jansen R W, Johnson L C, Averett D R. High-capacity in vitro assessment of anti-hepatitis B virus compound selectivity by a virion-specific polymerase chain reaction assay. Antimicrob Agents Chemother. 1993;37:441–447. doi: 10.1128/aac.37.3.441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Koziel M J. The role of immune responses in the pathogenesis of hepatitis C. J Vir Hepatitis. 1997;4(Suppl.2):31–41. doi: 10.1111/j.1365-2893.1997.tb00178.x. [DOI] [PubMed] [Google Scholar]
- 21.Lasarte J J, Garcia-Granero M, Lopez A, Casares N, Garcia N, Civeira M P, Borras-Cuesta F, Prieto J. Cellular immunity to hepatitis C virus core protein and the response to interferon in patients with chronic hepatitis C. Hepatology. 1998;28:815–822. doi: 10.1002/hep.510280332. [DOI] [PubMed] [Google Scholar]
- 22.Lechmann M, Ihlenfeldt H G, Braunschweiger I, Giers G, Jung G, Matz B, Kaiser R, Sauerbruch T, Spengler U. The role of hepatitis C virus specific CD4+ T lymphocytes in acute and chronic hepatitis C. Hepatology. 1996;24:790–795. doi: 10.1002/hep.510240406. [DOI] [PubMed] [Google Scholar]
- 23.Lee J H, von Wagner M, Roth W K, Teuber G, Sarrazin C, Zeuzem S. Effect of ribavirin on virus load and quasispecies distribution in patients infected with hepatitis C virus. J Hepatol. 1998;29:29–35. doi: 10.1016/s0168-8278(98)80175-x. [DOI] [PubMed] [Google Scholar]
- 24.Marion P L, Cullen J M, Azcarraga R R, Van Davelaar M J, Robinson W S. Experimental transmission of duck hepatitis B virus to Pekin ducks and to domestic geese. Hepatology. 1987;7:724–731. doi: 10.1002/hep.1840070418. [DOI] [PubMed] [Google Scholar]
- 25.Martin M J, Navas S, Quiroga J A, Pardo M, Carreno V. Effects of the ribavirin-interferon alpha combination on cultured peripheral blood mononuclear cells from chronic hepatitis C patients. Cytokine. 1998;79:2381–2391. doi: 10.1006/cyto.1997.0333. [DOI] [PubMed] [Google Scholar]
- 26.McCormick J B, Getchell J P, Mitchell S W, Hicks D R. Ribavirin suppresses replication of lymphadenopathy-associated virus in cultures of human adult T lymphocytes. Lancet. 1984;ii:1367–1369. doi: 10.1016/s0140-6736(84)92060-9. [DOI] [PubMed] [Google Scholar]
- 27.McHutchinson J G, Gordon S C, Schiff E R, Mitchell M D, Shiffmann M L, Lee W M, Rustgi V K, Goodman Z D, Ling M H, Cort S, Albrecht J K. Interferon alpha 2B alone or in combination with ribavirin as initial treatment for chronic hepatitis C. N Engl J Med. 1998;339:1485–1492. doi: 10.1056/NEJM199811193392101. [DOI] [PubMed] [Google Scholar]
- 28.Miller W H, Daluge S M, Garvey E P, Hopkins S, Reardon J E, Boyd F L, Miller R L. Phosphorylation of carbovir enantiomers by cellular enzymes determines the stereoselectivity of antiviral activity. J Biol Chem. 1992;267:21220–21224. [PubMed] [Google Scholar]
- 29.Mizuhara H, Uno M, Seki N, Yamashita M, Yamakoka M, Ogawa T, Kaneda K, Fujii T, Senoh H, Fujiwara H. Critical involvement of interferon-gamma in the pathogenesis of T cell activation-associated hepatitis and regulatory mechanisms of interleukin-6 for the manifestations of hepatitis. Hepatology. 1996;23:1608–1615. doi: 10.1053/jhep.1996.v23.pm0008675184. [DOI] [PubMed] [Google Scholar]
- 30.Ning Q, Brown D, Parodo J, Cattral M, Fung L, Gorczynski R, Cole E, Fung L, Ding J W, Liu M F, Rotstein O, Phillips M J, Levy G. Ribavirin inhibits viral-induced macrophage production of tumor necrosis factor, interleukin-1, procoagulant activity fgl2 prothrombinase and preserves Th1 cytokine production but inhibits Th2 cytokine response. J Immunol. 1998;160:3487–3493. [PubMed] [Google Scholar]
- 31.Nyberg L, Albrecht J, Glue P, Gianelli G, Zambras D, Elliot M, Conrad A, McHutchinson J. Changes in serum hepatitis C virus RNA in interferon nonresponders retreated with interferon plus ribavirin: a preliminary report. J Clin Gastroenterol. 1999;28:313–316. doi: 10.1097/00004836-199906000-00006. [DOI] [PubMed] [Google Scholar]
- 32.Poynard T, Marcellin P, Lee S S, Niederau C, Minuk G S, Ideo G, Bain V, Heathcote J, Zeuzem S, Trepo C, Albrecht J. Randomized trial of interferon alpha2b plus ribavirin plus placebo for 48 weeks for treatment of chronic infection with hepatitis C virus. International Hepatitis Interventional Therapy Group. Lancet. 1998;352:1426–1432. doi: 10.1016/s0140-6736(98)07124-4. [DOI] [PubMed] [Google Scholar]
- 33.Preugschat F, Averett D R, Clarke B E, Porter D J T. A steady-state and pre-steady-state kinetic analysis of the NTPase activity associated with the hepatitis C virus NS3 helicase domain. J Biol Chem. 1996;271:24449–24457. doi: 10.1074/jbc.271.40.24449. [DOI] [PubMed] [Google Scholar]
- 34.Ramasamy, K., R. C. Tam, J. Bard, and D. R. Averett. Monocyclic L-nucleosides with Type 1 cytokine-inducing activity. J. Med. Chem. 43:1019–1028. [DOI] [PubMed]
- 35.Rehermann B, Chang K M, McHutchinson J, Kokka R, Houghton M, Rice C M, Chisari F V. Differential cytotoxic T-lymphocyte responsiveness to the hepatitis B and C viruses in chronically infected patients. J Virol. 1996;70:7092–7102. doi: 10.1128/jvi.70.10.7092-7102.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Reichard O, Norkrans G, Fryden A, Braconier J-H, Sonnerborg A, Weiland O. Randomized, double blind, placebo controlled trial of interferon alpha 2B with and without ribavirin for chronic hepatitis C. Lancet. 1998;351:83–87. doi: 10.1016/s0140-6736(97)06088-1. [DOI] [PubMed] [Google Scholar]
- 37.Sarobe P, Jauregui J I, Lasarte J J, Garcia N, Civeira M P, Borras-Cuesta F, Prieto J. Production of interleukin-2 in response to synthetic peptides from hepatitis C virus E1 protein in patients with chronic hepatitis C: relationship with the response to interferon treatment. J Hepatol. 1996;25:1–9. doi: 10.1016/s0168-8278(96)80320-5. [DOI] [PubMed] [Google Scholar]
- 38.Sidwell R W, Huffman J H, Khare G P, Allen L B, Witkowski J T, Robins R K. Broad-spectrum antiviral activity of Virazole: 1-β-d-ribofuranosyl-1,2,4-triazole-3-carboxamide. Science. 1972;177:705–706. doi: 10.1126/science.177.4050.705. [DOI] [PubMed] [Google Scholar]
- 39.Tam R C, Lim C, Bard J, Pai B. Contact hypersensitivity responses following ribavirin treatment in vivo are influenced by Type 1 cytokine polarization, regulation of IL-10 expression and costimulatory signaling. J Immunol. 1999;163:3709–3717. [PubMed] [Google Scholar]
- 40.Tam R C, Pai B, Bard J, Lim C, Averett D R, Phan U T, Milovanovic T. Ribavirin polarizes human T cell responses towards a Type 1 cytokine profile. J Hepatol. 1999;30:376–382. doi: 10.1016/s0168-8278(99)80093-2. [DOI] [PubMed] [Google Scholar]
- 41.Tam R C, Phan U T, Milovanovic T, Pai B, Lim C, Bard J, He L. Oligonucleotide-mediated inhibition of CD28 expression induces human T cell hyporesponsiveness and manifests impaired contact hypersensitivity in mice. J Immunol. 1997;158:200–208. [PubMed] [Google Scholar]
- 42.Tsai S L, Liaw Y F, Chen M H, Huang C Y, Kuo G C. Detection of type 2-like T helper cells in hepatitis C virus infection: implications for hepatitis C virus chronicity. Hepatology. 1997;25:449–458. doi: 10.1002/hep.510250233. [DOI] [PubMed] [Google Scholar]
- 43.Weislow O W, Kiser R, Fine D, Bader J, Shoemaker R H, Boyd M R. New soluble-formazan assay for HIV-1 cytopathic effects: application to high-flux screening of synthetic and natural products for AIDS-antiviral activity. J Natl Cancer Inst. 1989;81:577–586. doi: 10.1093/jnci/81.8.577. [DOI] [PubMed] [Google Scholar]
- 44.Woitas R P, Lechmann M, Jung G, Kaiser R, Sauerbach T, Spengler U. CD30 induction and cytokine profiles in hepatitis C virus core-specific peripheral blood T lymphocytes. J Immunol. 1997;159:1012–1018. [PubMed] [Google Scholar]
- 45.Xu H, Dilulio N A, Fairchild R L. T cell populations primed to hapten sensitization in contact hypersensitivity are distinguished by polarized patterns of cytokine production: interferon-gamma (Tc1) effector CD8+ T cells and interleukin 4/interleukin 10-producing (Th2) negative regulatory CD4+ T cells. J Exp Med. 1996;183:1001–1012. doi: 10.1084/jem.183.3.1001. [DOI] [PMC free article] [PubMed] [Google Scholar]