Abstract
Background
Superior sulcus tumours (SSTs) are relatively uncommon and one of the most intractable lung cancers among non-small cell lung cancer (NSCLC). We planned a multicenter, single-arm confirmatory trial of new multidisciplinary treatment using immune-checkpoint inhibitor. The aim is to evaluate the safety and efficacy of new multidisciplinary treatment with perioperative durvalumab after chemoradiotherapy (CRT).
Methods
The primary endpoint is 3-year overall survival. Patients receive induction CRT with sequential two courses of durvalumab, followed by surgical resection for resectable SST. The regimen for CRT is two courses of cisplatin and S-1, and concurrent radiotherapy (66 Gy/33 Fr). After surgery, 22 courses of post-operative durvalumab therapy are administered. For unresectable SST, an additional 22 courses of durvalumab are administered after induction durvalumab.
Results
In two cases as a safety cohort, the safety of intervention treatment up to 30 days after surgery was examined, and there were no special safety signals. Patient enrollment has now resumed in the main cohort.
Conclusions
The results of this study may contribute to the establishment of a new standard of care for SST, which is an intractable NSCLC.
Keywords: superior sulcus tumour, Pancoast, immune-checkpoint inhibitor, chemoradiotherapy, multidisciplinary
The aim was to evaluate the safety and efficacy in multidisciplinary treatment of perioperative durvalumab after CRT followed by surgery for resectable SST and durvalumab maintenance for unresectable SST.
Introduction
Superior sulcus tumour (SST) of lung cancers, frequently termed Pancoast tumour, which was first reported by Henry Pancoast in 1932, is relatively uncommon and is one of the most intractable lung cancers because it often involves vital structures in the cervicothoracic region, including the first rib, vertebra, sympathetic nerve trunk, brachial plexus, recurrent nerve, subclavian vein and artery (1). Patients with SST often present with characteristic symptoms, such as edema of the upper limb, sensory deficits, motor deficits, pain, and Horner’s syndrome (miosis, ptosis, abnormal sweating). Because of the close proximity of these important organs, it is difficult to secure a surgical margin, and the treatment results are poor (2). SST is a group of diseases with a poor prognosis in non-small cell lung cancer (NSCLC), and treatment development in a framework different from other NSCLCs has been conducted. In the 2014 annual report published by the Japanese Society of Thoracic Surgery, the number of SST surgeries was reported to be 98 (0.26%) of 37 008 annual surgeries for primary lung cancer surgery in Japan (3). SST is a rare fraction among NSCLC, and the development of treatments has been delayed globally.
The standard of care for SST is based on the results of the Japan Clinical Oncology Group (JCOG) study, JCOG9806, conducted in Japan and the SouthWest Oncology Group9416/intergroup trial 0160 (SWOG9416/INT0160) study conducted mainly in North America (4,5). JCOG9806 examined the safety and efficacy of surgery after two courses of MVP therapy (mitomycin + vindesine + cisplatin) and 45 Gy of concurrent radiotherapy to the local + ipsilateral supraclavicular lymph node without prophylactic irradiation for SST patients. In addition to concurrent radiotherapy 45 Gy, 21.6 Gy/12 fractions of irradiation (66.6 Gy totally) and irradiation to the hilum lesion (optional) were additionally performed for unresectable cases.
SWOG9416/INT0160 also examined the safety and efficacy of surgery after 45 Gy of concurrent radiotherapy with two courses of EP therapy (etoposide + cisplatin) for the same subject.
Therefore, at present, 2 cycles of platinum combination chemotherapy and concurrent radiation therapy with a total dose of 45 Gy, and subsequent surgery are applied to the SST patients with cT3-4 N0-1 M0 (ipsilateral supraclavicular lymph node metastasis without mediastinal lymph node metastasis (N3) is included). Chemoradiotherapy (CRT) is used in patients with T3-4N2-3 SST who are not eligible in previous studies even if the tumour lesion is within the range that can be irradiated. If irradiation is not possible due to distant metastases or pleural dissemination, systemic therapies such as cytotoxic chemotherapy, immune-checkpoint inhibitors (ICIs) or molecular targeted drugs are used. The 5-year overall survival (OS) from Stage IIB to Stage IIIC patients diagnosed with SST are not sufficient at 56% and 42% in the JCOG9806 and SWOG9416/INT0160 trials, respectively, and further treatment development is required. The therapeutic outcome has improved with time; however, the incidence of distant recurrence, including brain metastasis, remains high and ~40% of all patients in both trials recurred.
Based on these evidences, CRESSST study was planned and conducted in Japan to evaluate the efficacy of increased radiation dose and change to third-generation chemotherapy regimen (CDDP + S-1 + RT 66 Gy/33 Fr) in preoperative concurrent CRT.
The study enrolled patients between 2014 and 2019, and patient enrollment has been completed. Follow-up for the period up to 2022 is underway and a survival analysis will be conducted (UMIN000014386).
In recent years, for advanced lung cancer, the use of ICIs alone or in combination has become important in lung cancer treatment strategies. Patients with NSCLC with N2 disease have an equally high incidence of metastatic recurrence in distant sites after CRT as patients with SST. Double-blind studies comparing durvalumab and placebo as consolidation therapy with platinum-combined chemotherapy and concurrent definitive thoracic radiotherapy in patients with unresectable Stage III locally advanced lung cancer were reported in 2017 (PACIFIC trial: NCT02125461) (6). The sequential therapy of durvalumab showed manageable toxicities and efficacy in patients with Stage III unresectable NSCLC who had received CRT with a progression-free survival (PFS) hazard ratio (HR) of 0.51 [95% confidence interval (CI), 0.41–0.63] and OS HR of 0.68 (99.73% CI, 0.47–0.997). Thus, durvalumab is expected to enhance the existing standard of care for patients with SST, which is a similar advanced lung cancer.
Additionally, we will conduct a comprehensive analysis of immunostaining, flow cytometry, exome sequencing, ribonucleic acid (RNA) sequencing, immunoprofiling, genetic abnormalities, and expression with immunostaining, flow cytometry, exome sequencing and RNA sequencing, in some enrolled patients with SST as translational research. From these analyses, it may be possible to clarify the changes in the tumour microenvironment due to CRT and durvalumab, which will lead to the elucidation of the mechanism of treatment resistance and drug discovery targeting the elucidated molecules in the future. We also plan to measure circulating tumour deoxyribonucleic acid (DNA) in each treatment phase. No studies have been conducted on SST to examine the association between clinical data on efficacy/safety and immunoprofiling/genetic abnormality data. By clarifying the immunoprofiling and genetic abnormality data of SST based on clinical information, if the predictive markers of therapeutic effect and prognosis predictive markers of CRT and durvalumab are identified, it may lead to personalized medicine for SST.
Protocol digest of JCOG1807C
Objectives
The aim of this study is to evaluate the safety and efficacy of multidisciplinary treatment with perioperative durvalumab after CRT followed by surgery for resectable SST and durvalumab maintenance therapy after CRT for unresectable SST.
Study setting
The study is a multi-institutional single-arm confirmatory trial.
Endpoints
Primary objectives
• Three-year OS.
Secondary objectives
Safety.
Three-year PFS.
Five-year OS.
Five-year PFS.
Recurrence type.
Proportion of local recurrence.
Response rate to preoperative adjunctive therapy.
Proportion of performing surgical treatment.
Proportion of pathological complete resection.
Pathological complete response rate.
Major pathological response rate.
Operation time.
Blood loss.
Relapse/survival and programmed death ligand 1 (PD-L1) expression before and after CRT.
Key eligibility criteria
Key inclusion criteria
Histologically or cytologically confirmed NSCLC.
-
All the following are satisfied (Union for international cancer control tumour-node-metastasis (UICC-TNM) classification 8th edition):
-
(i)
For the primary site, the chest computed tomography (CT) meets any of the following:
-
(i)
Direct invasion of the apical chest wall.
-
Direct invasion of the subclavian artery and/or subclavian vein.
-
(ii)
Regional lymph nodes satisfy any of the following by chest CT and fluorodeoxyglucose-positron emission tomography/CT (FDG-PET/CT):
-
(ii)
cN0
cN1 without metastases to any of #10, #11 and #12 lymph node.
cN3 (same-side supraclavicular lymph node metastasis) and no regional lymph node (N1 or N2) metastasis other than #13 and #14 lymph nodes distant metastasis (including intrapulmonary metastasis within the same lung lobe and ipsilateral lung lobe) was not found upon imaging, including FDG-PET/CT.
It is judged that radical lobectomy is possible.
Upon consultation with the radiation oncologist, it is judged that the following conditions are met:
-
(i)
Radiation therapy is possible according to the protocol.
-
(ii)
The radiation field does not reach the hilar region.
20–79 years old.
Performance status of 0 or 1 according to Eastern Cooperative Oncology Group (ECOG) criteria.
Whether benign or malignant, there is no history of surgery.
No history of chemotherapy, including treatment for other cancer types.
If patients have a history of radiation therapy including other cancer types, lung, hilar, mediastinum and supraclavicular region were not included in the radiation field.
Sufficient oral intake.
Written informed consent obtained from patients regarding study participation.
Key exclusion criteria
Having active double cancer with a disease-free period of 3 years or less.
Receiving continuous systemic administration of steroids or other immunosuppressive drugs.
Having uncontrolled diabetes.
Having uncontrolled hypertension.
Having uncontrolled gastrointestinal disease.
Having unstable angina pectoris or a history of myocardial infarction within 6 months.
Chest CT shows severe pulmonary emphysema.
History of active primary immune deficiency.
Known allergy or hypersensitivity to any of the study drugs or any of the study drug excipients.
Treatment methods
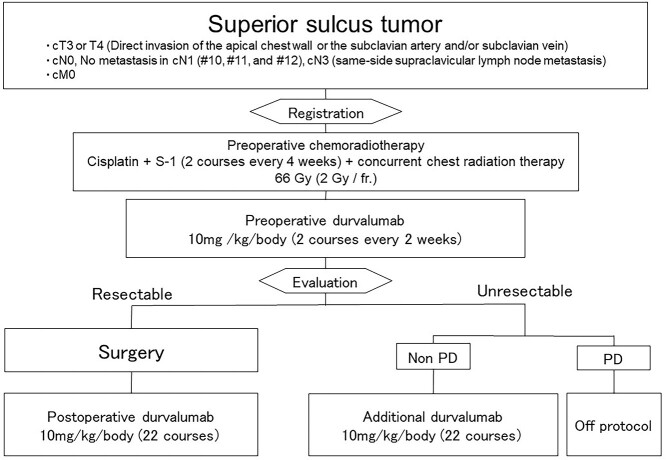
Study schema is shown in Figure 1.
Figure 1.
Study schema.
Induction phase
Patients will receive induction CRT with sequential durvalumab, followed by surgical resection for resectable SST. The specific regimen for CRT is two courses of cisplatin (cisplatin 60 mg/m2 IV drip, day 1) and S-1 (tegafur, gimeracil and oteracil potassium: 80–120 mg/day orally, days 1–14) and thoracic radiotherapy (66 Gy/33 Fr). Day 1 of the first course of thoracic radiation therapy will begin on the same day as chemotherapy. Irradiation will be performed 44 Gy/22 fractions, including for the primary lesion and metastatic lymph nodes, and the prophylactic irradiation area set for each site of the primary lesion will be undertaken. Additional irradiation of 22 Gy/11 fraction as ‘ clinical target volume (CTV) boost’ focused on the lesion is performed after irradiation with 44 Gy/22 fraction as ‘CTV initial’ including ipsilateral supraclavicular lymph node region 5 days a week, 2 Gy once. In cN1 cases, hilar lymph nodes #10, #11 and #12 metastases will be excluded in order to avoid irradiation of the hilar region, and hilar irradiation will also strictly be avoided in intrapulmonary lymph node #13 and #14 metastases. The total dose is 66 Gy, the treatment period is 45–63 days. If it is difficult to comply with spinal cord dose constraints with 3D conformal radiation, intensity-modulated radiotherapy (IMRT) is permitted only at facilities approved by the Radiation Therapy Committee.
Two courses of durvalumab (10 mg/kg/body intravenous injection, day 1) will be received sequentially after CRT.
Surgery phase
If the criteria for performing surgery are met after preoperative treatment, surgery is scheduled.
Lobectomy (including bilobectomy) should be basically performed, but pneumonectomy is also acceptable depending on the intraoperative situation.
Depending on the intraoperative findings, it is permissible to perform segmentectomy, wedge resection in addition to lobectomy and bilobectomy. Segmentectomy or wedge resection alone is not allowed.
Additional phase
Post-operative durvalumab therapy will begin between days 28 and 63 after surgery on day 1. The 22 courses of durvalumab will be administered for ~1 year.
An additional 22 courses of durvalumab will be administered for ~1 year for inoperable SST without progressive disease after induction therapy. Additional durvalumab therapy will commence between days 15 and 28 of the second course of preoperative durvalumab therapy.
In patients with SST who show disease progression during or after induction therapy, protocol treatment will be terminated, and physician choice therapy will be permitted as a post-study treatment.
Statistical setting and considerations
This study is a single-arm confirmatory trial and the rationale of this design is as follows: the first reason is SST is very rare, and the feasibility of a randomized controlled trial was considered to be low. Secondly, the standard of care for SST has been determined based on the results of single-arm studies such as JCOG9806 and SWOG9416/INT0160. Third, the patient selection criteria in this study are similar to those in single-arm trials such as JCOG9806 and CRESSST, thus limiting the selection bias in this study. Therefore, we decided to conduct this study as a single-arm confirmatory study.
In a clinical trial report on surgery after CRT using MVP therapy for SST conducted in Japan, the 3-year OS was 61% (95% CI: 49–71%). In this study, the 3-year OS in patients who received this therapeutic regimen set a threshold value of 66% and an expected value of 80%, expecting an additional effect of 14%.
In this condition, the required number of patients was calculated as 81 with a one-sided significance level of 5%, power of 90%, the threshold 3-year OS of 66% and the expected one of 80%. The final planned number of patients was 84, taking into account for a few ineligible and lost-to-follow-up.
In the primary analysis, OS will be calculated by the Kaplan–Meier method and its 90% CI will be estimated by Greenwood’s formula for all eligible patients. If the lower bound of the 90% CI exceeds 66%, the efficacy of the multidisciplinary treatment of pre-/post-operative durvalumab after CRT or durvalumab maintenance therapy is confirmed. On the other hand, if the lower bound of the 90% CI of the 3-year OS is 66% or less, the standard therapy will be CRT (45 Gy) followed by surgery if resectable and definitive CRT (66 Gy) is unresectable.
Interim analysis and monitoring
All statistical analyses will be conducted at the JCOG Data Center. An interim analysis is not planned for this study because it is a single-arm study, and any safety issues with subjects can be ascertained through periodic monitoring. After registration of two patients as a run-in-cohort, enrollment is suspended and the safety of this new multidisciplinary therapy up to 30 days after surgery will be evaluated through consultation with the Independent Data and Safety Monitoring Committee and Advance Medical Care System of the Japanese Ministry of Health, Labor, and Welfare. The safety of two patients was confirmed, the patient accrual is reopened in April 2021. In-house monitoring will be performed every 6 months by the JCOG Data Center to evaluate the study progress and improve the quality of the data.
Patient protection
All researchers involved in this study conducted this study according to ‘Declaration of Helsinki’ (http://dl.med.or.jp/dl-med/wma/helsinki2013j.pdf), ‘Clinical Research Law in Japan’ (http://www.mhlw.go.jp/stf/seisakunitsuite/bunya/0000163417.html) and related notifications.
Conclusion
This study is a multi-institutional, single-arm, confirmatory trial to confirm the efficacy and safety of a novel multidisciplinary strategy, including an ICI for SST. The results of this study may contribute to the establishment of a new SOC for SST, which is an intractable NSCLC.
Participating institutions (from North to South)
Yamagata Prefectural Central Hospital, National Cancer Center Hospital East, National Cancer Center Hospital, Kyorin University Faculty of Medicine, Tokyo Medical University Hospital, Juntendo University Hospital, Kanagawa Cancer Center, Shizuoka Cancer Center, Nagoya University School of Medicine, Osaka International Cancer Institute, Osaka City General Hospital, Kobe University Hospital, Hyogo Cancer Center, Kurashiki Central Hospital, Hiroshima University Hospital, National Hospital Organization Shikoku Cancer Center, Hospital of the University of Occupational and Environmental Health, Kyushu University Hospital, National Hospital Organization Kyushu Cancer Center, Oita University Hospital.
Funding
AstraZeneca, the National Cancer Center Research and Development Fund (2020-J-3), and the Project Promoting Clinical Trials for Development of New Drugs (20lk0201111h0001) from the Japan Agency for Medical Research and Development (AMED).
Contributor Information
Keiju Aokage, Division of Thoracic Surgery, National Cancer Center Hospital East, Chiba.
Masahiro Tsuboi, Division of Thoracic Surgery, National Cancer Center Hospital East, Chiba.
Yoshitaka Zenke, Division of Thoracic Oncology, National Cancer Center Hospital East, Chiba.
Hidehito Horinouchi, Division of Thoracic Oncology, National Cancer Center Hospital, Tokyo.
Naoki Nakamura, Department of Radiology, St. Marianna University School of Medicine, Kawasaki, Kanagawa.
Satoshi Ishikura, Division of Radiology, Nagoya City University Hospital, Nagoya.
Hiroyoshi Nishikawa, Division of Cancer Immunology, Research Institute/Exploratory Oncology Research & Clinical Trial Center (EPOC), National Cancer Center, Tokyo, Chiba.
Shogo Kumagai, Division of Cancer Immunology, Research Institute/Exploratory Oncology Research & Clinical Trial Center (EPOC), National Cancer Center, Tokyo, Chiba.
Shohei Koyama, Division of Cancer Immunology, Research Institute/Exploratory Oncology Research & Clinical Trial Center (EPOC), National Cancer Center, Tokyo, Chiba.
Keisuke Kanato, JCOG Data Center/Operations Office, National Cancer Center Hospital, Tokyo.
Tomoko Kataoka, JCOG Data Center/Operations Office, National Cancer Center Hospital, Tokyo.
Masashi Wakabayashi, JCOG Data Center/Operations Office, National Cancer Center Hospital, Tokyo.
Miki Fukutani, Clinical Research Support Office, National Cancer Center Hospital East, Kashiwa, 277-8577.
Haruhiko Fukuda, JCOG Data Center/Operations Office, National Cancer Center Hospital, Tokyo.
Yuichiro Ohe, Division of Thoracic Oncology, National Cancer Center Hospital, Tokyo.
Shun-ichi Watanabe, Division of Thoracic Surgery, National Cancer Center Hospital, Tokyo, Japan.
References
- 1. Pancoast HK. Superior pulmonary sulcus tumor: tumor characterized by pain, HORNER'S syndrome, destruction of bone and atrophy of hand muscles CHAIRMAN'S address. JAMA 1932; 99:1394-1396. [Google Scholar]
- 2. Detterbeck FC. Pancoast (superior sulcus) tumors. Ann Thorac Surg 1997;63:1810–8. [DOI] [PubMed] [Google Scholar]
- 3. Committee for Scientific Affairs TJAfTS, Masuda M, Okumura M, et al. Thoracic and cardiovascular surgery in Japan during 2014: annual report by the Japanese Association for Thoracic Surgery. Gen Thorac Cardiovasc Surg 2016;64:665–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Kunitoh H, Kato H, Tsuboi M, et al. Phase II trial of preoperative chemoradiotherapy followed by surgical resection in patients with superior sulcus non-small-cell lung cancers: report of Japan clinical oncology group trial 9806. J Clin Oncol 2008;26:644–9. [DOI] [PubMed] [Google Scholar]
- 5. Rusch VW, Giroux DJ, Kraut MJ, et al. Induction chemoradiation and surgical resection for superior sulcus non-small-cell lung carcinomas: long-term results of Southwest Oncology Group Trial 9416 (Intergroup Trial 0160). J Clin Oncol 2007;25:313–8. [DOI] [PubMed] [Google Scholar]
- 6. Antonia SJ, Villegas A, Daniel D, et al. Durvalumab after Chemoradiotherapy in stage III non-small-cell lung cancer. N Engl J Med 2017;377:1919–29. [DOI] [PubMed] [Google Scholar]