Abstract
Genome wide association studies have identified an association between Alzheimer’s disease (AD) and common polymorphisms in the MS4A and TREM loci (each containing a cluster of homologous genes) and should be thoroughly investigated for the presence of potentially functional variations. We conducted a mutation analysis by next generation sequencing of 15 genes within the MS4A and TREM gene clusters; and catalogued rare coding variants detected in a North American data set of 210 cases and 233 controls. Investigation of the 5 homologues genes in the TREM locus revealed potentially damaging rare variants in TREM2, TREML1, TREML2, and TREML4. In agreement with a previous report, we observed a significant enrichment of TREM2-damaging missense substitutions in cases (N 1⁄4 9; 4.2%) compared with controls (N1⁄42; 0.9%; p 1⁄4 0.010; after Yates’ correction p 1⁄4 0.022). Among known AD-associated TREM2 substitutions, we detected p.R47H, p.D87N, and p.H157Y affecting both TREM2 isoforms (NM_018965 and NM_001271821). In addition, we identified 2 cases with novel TREM2 variants (p.L205P and p.G219C), which mapped only to the isoform NM_001271821 at the C-terminus. Investigation of the MS4A gene cluster revealed that potentially damaging missense substitutions and loss-of-function variants were twice as frequent in controls (N 1⁄4 19; 8.2%) than cases (N 1⁄4 9; 4.3%), generating a nominally significant result (p 1⁄4 0.047; after Yates’ correction p 1⁄4 0.07). Validation of our observation in large data sets might address the question whether such variants could contribute to the protective effect of the minor alleles of Genome wide association study- significant single nucleotide polymorphisms at the MS4A locus.
1. Introduction
Genome wide association studies (GWASs) have identified a link between late-onset Alzheimer’s disease (AD) and common single nucleotide polymorphisms (SNPs) in >20 loci (Ghani and Rogaeva, 2014). Apart from the APOE-ε4 allele with an odds ratio (OR) w4, GWAS hits have revealed only a modest effect with ORs of >0.5 for protective or <2 for risk alleles. However, it is possible that rare functional variations could contribute to the GWAS signals and might have bigger effect sizes than the tagging top-significant SNPs. Indeed, recent targeted sequencing of 8 well-confirmed genes detected by GWASs showed that AD cases were significantly enriched (3.1-fold) with nonsynonymous substitutions compared with controls (p 1⁄4 0.002), whereas there was no difference in synonymous variants (Vardarajan et al., 2015).
Some disease-associated SNPs detected by GWAS are located in gene-rich regions with high linkage disequilibrium, which should be thoroughly investigated for the presence of functional mutations, especially within homologous gene clusters. For instance, genome wide sequencing revealed an association between AD and a TREM2 SNP (rs75932628; p.R47H; risk allele frequency 1⁄4 0.0063; p 1⁄4 2 10 12; OR 1⁄4 2.9; Guerreiro et al., 2013; Jonsson et al., 2013), whereas one of the top-significant SNPs (rs9381040) detected by a mega-meta-analysis of GWASs (w74,000 individuals) is located nearby the homologous gene TREML2 (Lambert et al., 2013). Moreover, a recent meta-analysis of w36,000 subjects suggested that the GWAS signals at TREML2 and TREM2 at the chr6p21.1 locus are independent of each other and revealed a common TREML2 substitution (rs3747742; p.S144G) protecting against AD (p 1⁄4 9 10 5) (Benitez et al., 2014). Notably, the ClinVar database does not have any reports of deleterious variants in TREML2, whereas various damaging variations are described for TREM2, including stop mutations (p.E14X, p.Q33X, p.W44X, and p.W78X), frameshift mutations (p.G90Vfs and p.A105Rfs), a splice mutation (NM_018965:c.482þ2T>C), a deletion (NM_018965.3:c.40þ3_40þ5del), and several substitutions (p.R47H, p.V126G, p.D134G, p.H157Y, and p.K186N).
Another example of an AD-associated gene-rich linkage disequilibrium block is at the chr11q12 locus containing GWAS-significant SNPs at the 30UTR (rs610932) and upstream (rs983392) of MS4A6A (NM_152852.2) (Hollingworth et al., 2011; Lambert et al., 2013), as well as downstream of MS4A3 (rs474951; NM_006138) and upstream of MS4A4A (rs1562990; NM_024021Antunez et al., 2011; Lambert et al., 2013; Perez-Palma et al., 2014). These homologous genes encode the membrane-spanning 4A (MS4A) gene family implicated in the immune response (Liang and Tedder, 2001). Increased MS4A6A expression was associated with higher Braak scores in AD brains (Karch et al., 2012; Martiskainen et al., 2015), but no particular mutation in this locus has been functionally evaluated. Recent investigation of MS4A6A revealed a rare potentially functional SNP (rs138650483) predicted to affect splicing of transcript NM_152852 or lead to a missense substitution in the other transcripts (e.g., NM_022349 and NM_001247999) (Vardarajan et al., 2015).
The main purpose of the present study was a comprehensive mutation analysis of the homologous gene clusters at the MS4A and TREM loci by next generation sequencing of a North American case-control AD data set to catalog rare variants with potential risk or protective effects.
2. Materials and methods
2.1. Participants
Written informed consent was obtained from all participants in accordance with the ethics review board. Targeted sequencing of the MS4A and TREM gene clusters was conducted for a Canadian data set that included 210 AD cases (mean age 73 ` 7.3; 50.4% women) and 53 controls (mean age 80.3 ` 3.6; 62.3% women). Of note, the same data set was part of the study investigating 8 AD genes (Vardarajan et al., 2015) that did not include homologous genes within the TREM and MS4A loci. In addition, all rare coding variants across both loci were obtained from whole exome-sequencing data of 180 controls from the National Institutes of Health (NIH; mean age 79.6 ` 9.4; 41.6% women), in total providing us data for 233 controls.
2.2. Sequencing and analyses
At the MS4A locus (Fig. 1A), we sequenced MS4A1 (Entrez Gene: 931), MS4A2 (Entrez Gene: 2206), MS4A3 (Entrez Gene: 932), MS4A4A (Entrez Gene: 51338), MS4A6A (Entrez Gene: 64231) [previously reported (Vardarajan et al., 2015)], MS4A5 (Entrez Gene: 64232), MS4A6E (Entrez Gene: 245802), MS4A7 (Entrez Gene: 58475), MS4A12 (Entrez Gene: 54860), MS4A13 (Entrez Gene: 503497), and MS4A14 (Entrez Gene: 84689). At the TREM locus (Fig. 1B), we sequenced TREM2 (Entrez Gene: 54209), TREML1 (Entrez Gene: 340205), TREML2 (Entrez Gene: 79865), and TREML4 (Entrez Gene: 285852).
Fig. 1.
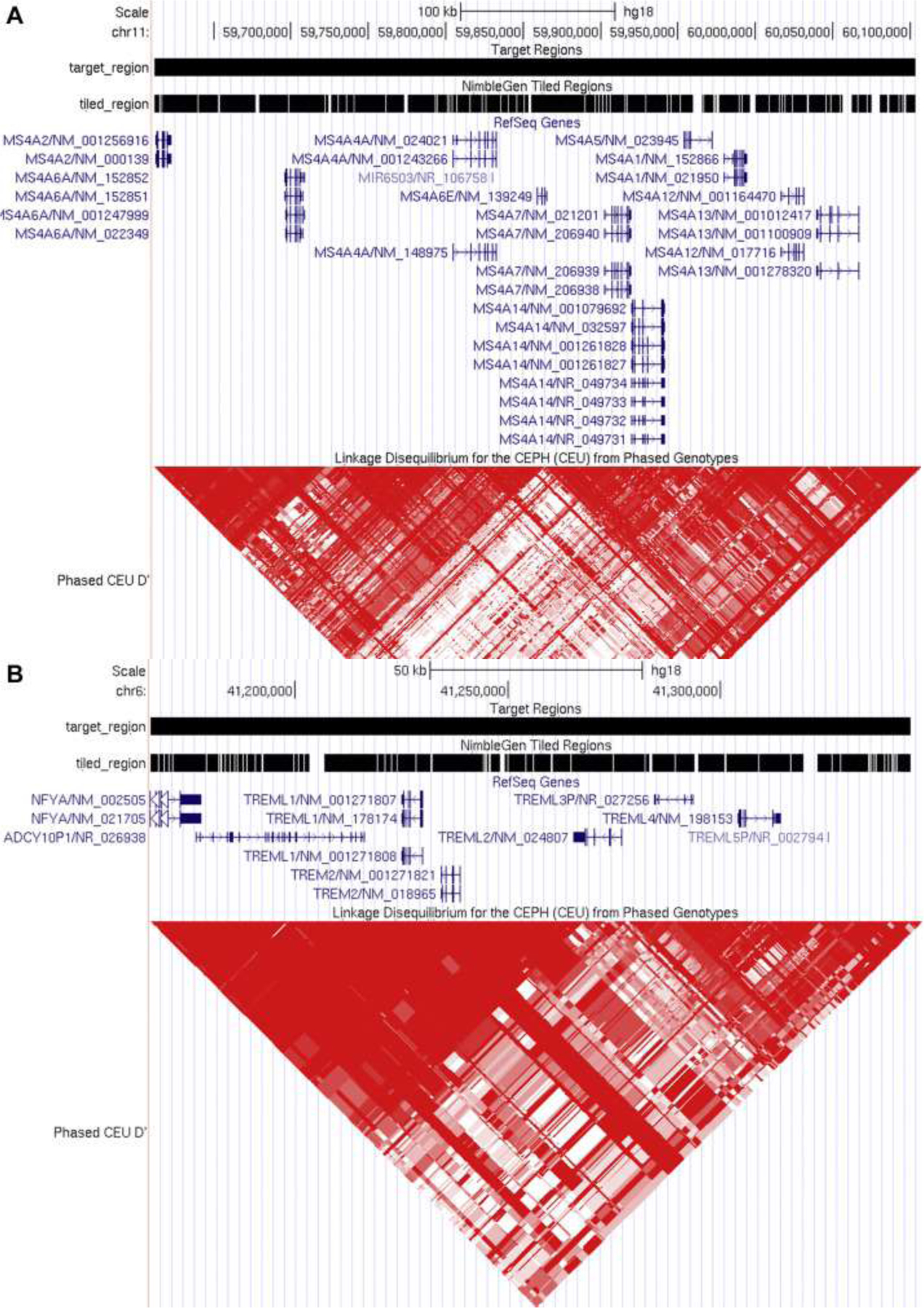
Regions included for targeted sequencing of the MS4A and TREM gene clusters. Roche NimbleGen SeqCap EZ Designs-custom technology was applied to target the MS4A(A) and TREM (B) gene clusters. Genomic regions subjected to deep sequencing are shown by fragmented black bars. The linkage disequilibrium structure (D′ values) for the Caucasian population (CEU) is presented beneath the genomic regions.
Targeted sequencing was performed for the entire regions of interest using the Roche NimbleGen SeqCap EZ Designs-custom as previously described (Vardarajan et al., 2015). On average, we had 16,174,244 reads per sample with 98.5% perfect index reads and 88.7% !Q30 bases. For the whole exome sequencing, the DNA samples were enriched using TruSeq technology (version 1.0) and paired-end sequenced on a HiSeq2000 sequencer according to the manufacturer’s protocol (Illumina, San Diego, CA, USA). Sequence alignment and variant calling were performed against the reference human genome (UCSC hg19) using the Burrows-Wheeler Aligner (Li and Durbin, 2009) and Genome Analysis Toolkit (DePristo et al., 2011; Van der Auwera et al., 2013). Variant calling was done by the Picard software (http://picard.sourceforge.net/index.shtml). Reliably called variants were identified after removing those with DP <30, GQ <20, QD <2.0, MQ <40.0, FS >60, HaplotypeScore >13.0, MQRankSum < 12.5, and ReadPosRankSum < 8.0. Variants were annotated using the ANNOVAR program (Wang et al., 2010) and checked if they have a potentially damaging effect on protein function with either the Sorting Tolerant From Intolerant’ (SIFT) algorithm (http://sift.jcvi.org/) or Polyphen-2 programs. Conservation scores were evaluated with the GERPþþ program (Davydov et al., 2010) implemented in ANNOVAR.
We searched for rare coding variants with minor allele frequencies less than 5% in our controls and not more than 1% in the general population of the 1000 Genomes project, the Exome Aggregation Consortium or NHLBI Exome Sequencing Project Exome Variant Server (Table 1 and Supplementary Table). Where DNA was available, the detected rare variants were validated by Sanger sequencing. All 16 investigated variants in 22 subjects were confirmed, demonstrating the high quality of the obtained genotypes (Fig. S1 Supplementary Material). To assess the distribution of potentially deleterious coding variants among cases and controls, our mutation analysis was supplemented with analyses implemented in the computational tool at Microsoft Research (http://research.microsoft.com/en-us/um/redmond/projects/MSCompBio) (Castagnola et al., 2015; De Moor et al., 2009) that calculates for each variant Fisher’s exact test p-values, pooled p-values, which is based on considering marginal counts of contingency tables as random variables (Carlson et al., 2009), as well as false-positive discovery rates (FDR; Benjamini and Hochberg, 1995) and q-values (the FDR-analog of p-value; Storey, 2003) to address multiple testing for individual variants. In addition, we applied the GraphPad 1-tailed c2 test with Yates’ correction to compare the burden of variants among cases and controls for TREM2 and the MS4A locus (http://www.graphpad.com/quickcalcs/).
Table 1.
Rare potentially deleterious coding variants identified at the TREM and MS4A loci among North American AD patients (N = 210) and controls (N = 233).
| Chr | Location | dbSNP138 | Ref | Alt | Gene | Transcript | Exon | Nucleotide | Amino acid change | Function |
|---|---|---|---|---|---|---|---|---|---|---|
| 6 | 41117594 | rs138237630 | A | T | TREML1 | NM_178174 | 6 | c.T684A | p.D228E | Nonsynonymous |
| 41121999 | rs201565463 | G | A | TREML1 | NM_178174 | 1 | c.C28T | p.L10F | Nonsynonymous | |
| 41126346 | . | C | A | TREM2 | NM_001271821 | 4 | c.G655T | p.G219C | Nonsynonymous | |
| 41126387 | . | A | G | TREM2 | NM_001271821 | 4 | c.T614C | p.L205P | Nonsynonymous | |
| 41127543 | rs2234255 | G | A | TREM2 | NM_018965 | 3 | c.C469T | p.H157Y | Nonsynonymous | |
| 41129133 | rs142232675 | C | T | TREM2 | NM_018965 | 2 | c.G259A | p.D87N | Nonsynonymous | |
| 41129178 | . | G | C | TREM2 | NM_018965 | 2 | c.C214G | p.L72V | Nonsynonymous | |
| 41129252 | rs75932628 | C | T | TREM2 | NM_018965 | 2 | c.G140A | p.R47H | Nonsynonymous | |
| 41162204 | rs115991880 | G | T | TREML2 | NM_024807 | 3 | c.C744A | p.S248R | Nonsynonymous | |
| 41166063 | rs147506354 | C | A | TREML2 | NM_024807 | 2 | c.G160T | p.V54F | Nonsynonymous | |
| 41196683 | rs373300218 | C | G | TREML4 | NM_198153 | 2 | c.C295G | p.L99V | Nonsynonymous | |
| 41204295 | . | T | C | TREML4 | NM_198153 | 5 | c.T578C | p.L193P | Nonsynonymous | |
| 11 | 59829992 | rs140838251 | G | A | MS4A3 | NM_006138 | 3 | c.G208A | p.G70S | Nonsynonymous |
| 59857929 | rs79741566 | T | G | MS4A2 | NM_000139 | 3 | c.T307G | p.W103G | Nonsynonymous | |
| 59861473 | rs138180929 | G | T | MS4A2 | NM_000139 | 6 | c.G574T | p.G192X | Stopgain | |
| 59940500 | rs138650483 | C | T | MS4A6A |
NM_001247999; NM_15285 |
7; 8 | c.G736A; c.651þ1G>A |
p.V246M | Nonsynonymous; splicing |
|
| 59947385 | rs532381110 | T | - | MS4A6A | NM_152852 | 4 | c.201delA | p.A67fs | Frameshift deletion | |
| 60064779 | rs145442198 | C | A | MS4A4A | NM_024021 | 4 | c.C254A | p.T85K | Nonsynonymous | |
| 60105214 | . | G | C | MS4A6E | NM_139249 | 2 | c.G148C | p.V50L | Nonsynonymous | |
| 60160173 | . | A | T | MS4A7 | NM_206939 | 6 | c.A562T | p.M188L | Nonsynonymous | |
| 60160175 | . | G | A | MS4A7 | NM_206939 | 6 | c.G564A | p.M188I | Nonsynonymous | |
| 60160176 | rs143858799 | C | A | MS4A7 | NM_206939 | 6 | c.C565A | p.L189I | Nonsynonymous | |
| 60161278 | . | C | T | MS4A7 | NM_206939 | 7 | c.C667T | p.Q223X | Stopgain | |
| 60183920 | . | A | C | MS4A14 | NM_001261828 | 6 | c.A1578C | p.K526N | Nonsynonymous | |
| 60184404 | rs149015522 | C | T | MS4A14 | NM_001261828 | 6 | c.C2062T | p.Q688X | Stopgain | |
| 60184467 | rs151183005 | C | T | MS4A14 | NM_001261828 | 6 | c.C2125T | p.P709S | Nonsynonymous | |
| 60233409 | rs201245387 | A | C | MS4A1 | NM_152866 | 6 | c.A352C | p.I118L | Nonsynonymous | |
| 60309991 | . | G | T | MS4A13 | NM_001012417 | 7 | c.403–1G>T | . | Splicing |
Frequencies were checked at the 1000 Genomes project (general and European populations), as well as at the Exome Variant Server (data release ESP6500si v2) and Exome Aggregation Consortium (data release exac02). Variant effect on protein function was evaluated with the SIFT and Polyphen2 programs, and conservation scores were determined with GERPþþ. Key: AD, Alzheimer’s disease; FDR, false-positive discovery rates.
3. Results
3.1. MS4A locus
We targeted a 492-Kb region on Chr11q12 (Chr11:59611961–60103563/hg18) containing 11 members of the MS4A gene family that share a CD20 domain (Fig. 1A). Potentially damaging missense and loss-of-function variants in MS4A genes were twice as frequent in controls (N 1⁄4 19; 8.2%) than cases (N 1⁄4 9; 4.3%; Table 1). However, this association was only nominally significant (p 1⁄4 0.047; after Yates’ correction p 1⁄4 0.07) and would require assessment in larger data sets.
In MS4A6A, we detected 1 NIH control with the splice and/or missense variant (rs138650483) that was previously reported in 3 AD cases and 1 control (Vardarajan et al., 2015), as well as a frameshift mutation (rs532381110; NM_152852: c.201delA: p.A67fs) in 2 controls and 1 case (Table 1). Loss-of-function variants were also detected in 4 other MS4A genes, including MS4A7: NM_206939: p.Q223X in 1 case, as well as MS4A2:NM_000139: p.G192X, MS4A14:NM_001261828: p.Q688X, and MS4A13:NM_001012417:exon7:c.403–1G>T, each in 1 control. In addition, 2 MS4A7 substitutions affecting the same codon (MS4A7:NM_206939:c.A562T:p.M188L and c.G564A:p.M188I) were identified in 2 cases, whereas 2 controls showed a potentially damaging substitution at the adjacent residue (MS4A7:NM_206939:p.L189I; Table 1). Both the p.M188 and p.L189 residues in the MS4A7 are highly conserved in evolution (GERPþþ scores 3.63 and 2.72, respectively) and among the CD20 domains of other MS4A proteins (Fig. S2 Supplementary Material).
3.2. TREM locus
We targeted a 178-Kb genomic region on Chr6p21.1 (chr6:41166294–41344491/hg18) with 5 homologous genes encoding the TREM receptor family (Fig. 1B). Potentially damaging rare variants were identified in TREML1, TREML2, TREML4, and TREM2 (Table 1). In TREM2, such variants were observed significantly more frequently in cases (N 1⁄4 9; 4.2%) than controls (N 1⁄4 2; 0.9%; p 1⁄4 0.010; after Yates’ correction p 1⁄4 0.022). This association was in part driven by the known AD-related p.R47H mutation that was marginally more frequent in cases (2.4%) than controls (0.4%; pooled p 1⁄4 0.03; FDR 1⁄4 0.36; q-value 1⁄4 0.26).
Among other previously reported TREM2 variants (Guerreiro et al., 2013), we detected p.H157Y and p.D87N (each in a single AD case; Table 1). Notably, p.D87N, which was also reported in 4 of 5 affected members of a large Italian AD family (Ghani et al., 2015), affects a codon that is even more conserved than p.R47 (GERPþþ score 5.51 and 4.56, respectively). Both the p.D87N and p.R47H variants are located in the extracellular domain of TREM2 generating a soluble form of TREM2 that is known to be increased in patients with multiple sclerosis and CNS inflammation (http://grantome.com/grant/NIH/R21-NS088928–01). Although, the p.D87N was reported in a subject with normal cognitive function and a low level of soluble TREM2 in CSF, this is in contrast with the significantly higher CSF levels of soluble TREM2 in p.R47H carriers versus non-carriers (Piccio et al., 2016).
We also identified 2 novel TREM2 variants (NM_001271821:c.T614C:p.L205P and c.G655T:p.G219C), each in a single AD case, affecting only the alternative TREM2 isoform (NM_001271821) lacking exon 4 of the main isoform (NM_018965) but with a longer coding exon at the C-terminus. The p.L205 codon is conserved (GERPþþ score 2); whereas the p.G219 codon is not (GERPþþ score 3.13) and was predicted to be neutral by PROVEAN prediction (Choi and Chan, 2015) but damaging by SIFT (Table 1).
4. Discussion
We conducted mutation analysis of 15 genes within the MS4A and TREM gene clusters and catalogued rare coding variants in 210 AD cases and 233 controls. Of note, our investigation was not intended as a conventional association study because of the modest size of the data set. A well-powered study for rare variants similar to p.R47H in TREM2 (OR 1⁄4 3; allele frequency 0.006) would require w770 matched case-control pairs to provide the desired 80% power to study a common disorder (e.g., AD), as estimated by Quanto (http://biostats.usc.edu/Quanto.html). Nevertheless, our initial statistical analyses did confirm an association of AD with TREM2 and revealed some new observations for follow-up in large data sets.
In agreement with previous studies, we observed a significant enrichment of TREM2-damaging missense substitutions in cases compared to controls, including the p.R47H variant which is a well-confirmed risk factor for AD (Finelli et al., 2015; Korvatska et al., 2015; Malkki, 2015), especially in the European population (Malkki, 2015; Rosenthal et al., 2015). Recently, the p.R47H mutation was associated with reduced binding of TREM2 to APOE (Atagi et al., 2015; Bailey et al., 2015), an increased level of total-tau in cerebrospinal fluid (Lill et al., 2015), and b-amyloid accumulation due to microglia dysfunction (Wang et al., 2015). It remains to be explored if another allele of this codon (NM_018965.3:c.140G>T: p.R47L) reported in the Latino population at the Exome Aggregation Consortium database (frequency <0.1%) is related to AD.
We also found 2 novel TREM2 variants (p.L205P and p.G219C), which were absent in the European population of the 1000 Genomes project and only affect the alternative TREM2 transcript (NM_001271821). All the missense variants described at the ClinVar database pertain to the common part of the TREM2 transcripts (NM_018965 and NM_001271821), with the exception of the NasuHakola related mutation (p.K186N) affecting only the main TREM2 isoform NM_018965 (Paloneva et al., 2002). Identification of transcript-specific substitutions at the C-terminal part of a particular TREM2 isoform suggests further functional studies to delineate a possible isoform-specific AD mechanism, which is also encouraged by the recently identified proinflammatory role of DAP12 in stabilizing the TREM2 C-terminal fragment (Zhong et al., 2015). In addition, the rare p.S248R substitution in TREML2 could be also prioritized for evaluation in large data sets, because it was observed marginally more frequently in controls (N 1⁄4 5; 2.1%) than cases (N 1⁄4 1; 0.5%), which is in the same direction as a protective GWAS-significant TREML2 p.S144G variation (Benitez et al., 2014). Previously, only the APOE-ε2 allele and the APP p.A673T substitution were reported to protect from AD (Benjamin et al., 1994; Jonsson et al., 2012); the presence of protective variants within other AD-associated genes should be carefully evaluated.
For instance, the potentially damaging missense and loss-of-function variants in MS4A genes were 2 times more frequent in our controls than AD cases, generating nominally significant results. It is tempting to speculate that such variants could contribute or explain the protective effect of minor alleles of GWAS-significant SNPs at the MS4A locus (Hollingworth et al., 2011; Lambert et al., 2013). Importantly, high MS4A6A expression has been associated with increased AD risk (Proitsi et al., 2014), suggesting that a low level of MS4A6A expression (e.g., due to loss-of-function variants) could be protective. Notably, the CD20 domain of MS4A genes was implicated in optimal B-cell immune response (Kuijpers et al., 2010); and MS4A1 CD20 positive inflammatory T-cells were reported in chronic brain lesions of patients with Multiple Sclerosis (Holley et al., 2014) suggesting the value of a similar study for AD.
Supplementary Material
Acknowledgements
This work was supported by grants from the Canadian Institutes of Health Research (Ekaterina Rogaeva, Peter St George-Hyslop), Wellcome Trust, Medical Research Council, Ontario Research Fund Alzheimer Society of Ontario (Peter St George-Hyslop), and the Intramural Research Programs (Z01-AG000949–02) of the National Institutes of Health, National Institute on Aging (Bryan Traynor).
Footnotes
Disclosure statement
The authors have no actual or potential conflicts of interest.
Appendix A. Supplementary data
References
- Antunez C, Boada M, Lopez-Arrieta J, Moreno-Rey C, Hernandez I, Marin J, Gayan J, Alzheimer’s Disease Neuroimaging I, Gonzalez-Perez A, Real LM, Alegret M, Tarraga L, Ramirez-Lorca R, Ruiz A, 2011. Genetic association of complement receptor 1 polymorphism rs3818361 in Alzheimer’s disease. Alzheimer’s Demen. J. Alzheimer’s Assoc 7, e124ee129. [DOI] [PubMed] [Google Scholar]
- Atagi Y, Liu CC, Painter MM, Chen XF, Verbeeck C, Zheng H, Li X, Rademakers R, Kang SS, Xu H, Younkin S, Das P, Fryer JD, Bu G, 2015. Apolipoprotein E is a ligand for triggering receptor expressed on myeloid cells 2 (TREM2). J. Biol. Chem 290, 26043e26050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bailey CC, DeVaux LB, Farzan M, 2015. The triggering receptor expressed on myeloid cells 2 binds apolipoprotein E. J. Biol. Chem 290, 26033e26042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benitez BA, Jin SC, Guerreiro R, Graham R, Lord J, Harold D, Sims R, Lambert JC, Gibbs JR, Bras J, Sassi C, Harari O, Bertelsen S, Lupton MK, Powell J, Bellenguez C, Brown K, Medway C, Haddick PC, van der Brug MP, Bhangale T, Ortmann W, Behrens T, Mayeux R, PericakVance MA, Farrer LA, Schellenberg GD, Haines JL, Turton J, Braae A, Barber I, Fagan AM, Holtzman DM, Morris JC, Group CS consortium, E.Alzheimer’s Disease Genetic, C.Alzheimer’s Disease Neuroimaging, I.Consortium, G., Williams J, Kauwe JS, Amouyel P, Morgan K, Singleton A, Hardy J, Goate AM, Cruchaga C, 2014. Missense variant in TREML2 protects against Alzheimer’s disease. Neurobiol. Aging 35, 1510.e19e1510.e26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benjamin R, Leake A, McArthur FK, Ince PG, Candy JM, Edwardson JA, Morris CM, Bjertness E, 1994. Protective effect of apoE epsilon 2 in Alzheimer’s disease. Lancet 344, 473. [DOI] [PubMed] [Google Scholar]
- Benjamini Y, Hochberg Y, 1995. Controlling the false discovery rate: a practical and powerful approach to multiple testing. J. R. Stat. Soc. Ser. B (methodological) 57, 289e300. [Google Scholar]
- Carlson JM, Heckerman D, Shani G, 2009. Estimating false discovery rates for contingency tables. Microsoft Research Technical Report MSR-TR-2009–53
- Castagnola P, Zoppoli G, Gandolfo S, Monticone M, Malacarne D, Cirmena G, Brown D, Aiello C, Maffei M, Marino R, Giaretti W, Pentenero M, 2015. Genomic DNA copy number aberrations, histological diagnosis, oral subsite and aneuploidy in OPMDs/OSCCs. PLoS One 10, e0142294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi Y, Chan AP, 2015. PROVEAN web server: a tool to predict the functional effect of amino acid substitutions and indels. Bioinformatics 31, 2745e2747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davydov EV, Goode DL, Sirota M, Cooper GM, Sidow A, Batzoglou S, 2010. Identifying a high fraction of the human genome to be under selective constraint using GERPþþ. PLoS Comput. Biol 6, e1001025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Moor MH, Liu YJ, Boomsma DI, Li J, Hamilton JJ, Hottenga JJ, Levy S, Liu XG, Pei YF, Posthuma D, Recker RR, Sullivan PF, Wang L, Willemsen G, Yan H, De Geus EJ, Deng HW, 2009. Genome-wide association study of exercise behavior in Dutch and American adults. Med. Sci. Sports Exerc 41, 1887e1895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DePristo MA, Banks E, Poplin R, Garimella KV, Maguire JR, Hartl C, Philippakis AA, del Angel G, Rivas MA, Hanna M, McKenna A, Fennell TJ, Kernytsky AM, Sivachenko AY, Cibulskis K, Gabriel SB, Altshuler D, Daly MJ, 2011. A framework for variation discovery and genotyping using next-generation DNA sequencing data. Nat. Genet 43, 491e498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finelli D, Rollinson S, Harris J, Jones M, Richardson A, Gerhard A, Snowden J, Mann D, Pickering-Brown S, 2015. TREM2 analysis and increased risk of Alzheimer’s disease. Neurobiol. Aging 36, 546.e9e546.e13. [DOI] [PubMed] [Google Scholar]
- Ghani M, Lang AE, Zinman L, Nacmias B, Sorbi S, Bessi V, Tedde A, Tartaglia MC, Surace EI, Sato C, Moreno D, Xi Z, Hung R, Nalls MA, Singleton A, St George-Hyslop P, Rogaeva E, 2015. Mutation analysis of patients with neurodegenerative disorders using NeuroX array. Neurobiol. Aging 36, 545.e9e545.e14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghani M, Rogaeva E, 2014. Autosomal dominant Alzheimer’s disease: underlying causes. In: Galimberti D, Scarpini E (Eds.), Neurodegenerative Diseases Springer, London, pp. 27e47. [Google Scholar]
- Guerreiro R, Wojtas A, Bras J, Carrasquillo M, Rogaeva E, Majounie E, Cruchaga C, Sassi C, Kauwe JS, Younkin S, Hazrati L, Collinge J, Pocock J, Lashley T, Williams J, Lambert JC, Amouyel P, Goate A, Rademakers R, Morgan K, Powell J, St George-Hyslop P, Singleton A, Hardy J Alzheimer Genetic Analysis, G, 2013. TREM2 variants in Alzheimer’s disease. N. Engl. J. Med 368, 117e127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holley JE, Bremer E, Kendall AC, de Bruyn M, Helfrich W, Tarr JM, Newcombe J, Gutowski NJ, Eggleton P, 2014. CD20þinflammatory T-cells are present in blood and brain of multiple sclerosis patients and can be selectively targeted for apoptotic elimination. Mult. Scler. Relat. Disord 3, 650e658. [DOI] [PubMed] [Google Scholar]
- Hollingworth P, Harold D, Sims R, Gerrish A, Lambert JC, Carrasquillo MM, Abraham R, Hamshere ML, Pahwa JS, Moskvina V, Dowzell K, Jones N, Stretton A, Thomas C, Richards A, Ivanov D, Widdowson C, Chapman J, Lovestone S, Powell J, Proitsi P, Lupton MK, Brayne C, Rubinsztein DC, Gill M, Lawlor B, Lynch A, Brown KS, Passmore PA, Craig D, McGuinness B, Todd S, Holmes C, Mann D, Smith AD, Beaumont H, Warden D, Wilcock G, Love S, Kehoe PG, Hooper NM, Vardy ER, Hardy J, Mead S, Fox NC, Rossor M, Collinge J, Maier W, Jessen F, Ruther E, Schurmann B, Heun R, Kolsch H, van den Bussche H, Heuser I, Kornhuber J, Wiltfang J, Dichgans M, Frolich L, Hampel H, Gallacher J, Hull M, Rujescu D, Giegling I, Goate AM, Kauwe JS, Cruchaga C, Nowotny P, Morris JC, Mayo K, Sleegers K, Bettens K, Engelborghs S, De Deyn PP, Van Broeckhoven C, Livingston G, Bass NJ, Gurling H, McQuillin A, Gwilliam R, Deloukas P, Al-Chalabi A, Shaw CE, Tsolaki M, Singleton AB, Guerreiro R, Muhleisen TW, Nothen MM, Moebus S, Jockel KH, Klopp N, Wichmann HE, Pankratz VS, Sando SB, Aasly JO, Barcikowska M, Wszolek ZK, Dickson DW, Graff-Radford NR, Petersen RC, Alzheimer’s Disease Neuroimaging I, van Duijn CM, Breteler MM, Ikram MA, DeStefano AL, Fitzpatrick AL, Lopez O, Launer LJ, Seshadri S, consortium C, Berr C, Campion D, Epelbaum J, Dartigues JF, Tzourio C, Alperovitch A, Lathrop M, consortium E, Feulner TM, Friedrich P, Riehle C, Krawczak M, Schreiber S, Mayhaus M, Nicolhaus S, Wagenpfeil S, Steinberg S, Stefansson H, Stefansson K, Snaedal J, Bjornsson S, Jonsson PV, Chouraki V, Genier-Boley B, Hiltunen M, Soininen H, Combarros O, Zelenika D, Delepine M, Bullido MJ, Pasquier F, Mateo I, Frank-Garcia A, Porcellini E, Hanon O, Coto E, Alvarez V, Bosco P, Siciliano G, Mancuso M, Panza F, Solfrizzi V, Nacmias B, Sorbi S, Bossu P, Piccardi P, Arosio B, Annoni G, Seripa D, Pilotto A, Scarpini E, Galimberti D, Brice A, Hannequin D, Licastro F, Jones L, Holmans PA, Jonsson T, Riemenschneider M, Morgan K, Younkin SG, Owen MJ, O’Donovan M, Amouyel P, Williams J, 2011. Common variants at ABCA7, MS4A6A/MS4A4E, EPHA1, CD33 and CD2AP are associated with Alzheimer’s disease. Nat. Genet 43, 429e435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jonsson T, Atwal JK, Steinberg S, Snaedal J, Jonsson PV, Bjornsson S, Stefansson H, Sulem P, Gudbjartsson D, Maloney J, Hoyte K, Gustafson A, Liu Y, Lu Y, Bhangale T, Graham RR, Huttenlocher J, Bjornsdottir G, Andreassen OA, Jönsson EG, Palotie A, Behrens TW, Magnusson OT, Kong A, Thorsteinsdottir U, Watts RJ, Stefansson K, 2012. A mutation in APP protects against Alzheimer’s disease and age-related cognitive decline. Nature 488, 96e99. [DOI] [PubMed] [Google Scholar]
- Jonsson T, Stefansson H, Steinberg S, Jonsdottir I, Jonsson PV, Snaedal J, Bjornsson S, Huttenlocher J, Levey AI, Lah JJ, Rujescu D, Hampel H, Giegling I, Andreassen OA, Engedal K, Ulstein I, Djurovic S, Ibrahim-Verbaas C, Hofman A, Ikram MA, van Duijn CM, Thorsteinsdottir U, Kong A, Stefansson K, 2013. Variant of TREM2 associated with the risk of Alzheimer’s disease. N. Engl. J. Med 368, 107e116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karch CM, Jeng AT, Nowotny P, Cady J, Cruchaga C, Goate AM, 2012. Expression of novel Alzheimer’s disease risk genes in control and Alzheimer’s disease brains. PLoS One 7, e50976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korvatska O, Leverenz JB, Jayadev S, McMillan P, Kurtz I, Guo X, Rumbaugh M, Matsushita M, Girirajan S, Dorschner MO, Kiianitsa K, Yu CE, Brkanac Z, Garden GA, Raskind WH, Bird TD, 2015. R47H variant of TREM2 associated with Alzheimer disease in a large late-onset family: clinical, genetic, and neuropathological study. JAMA Neurol 72, 920e927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuijpers TW, Bende RJ, Baars PA, Grummels A, Derks IA, Dolman KM, Beaumont T, Tedder TF, van Noesel CJ, Eldering E, van Lier RA, 2010. CD20 deficiency in humans results in impaired T cell-independent antibody responses. J. Clin. Invest 120, 214e222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lambert JC, Ibrahim-Verbaas CA, Harold D, Naj AC, Sims R, Bellenguez C, DeStafano AL, Bis JC, Beecham GW, Grenier-Boley B, Russo G, Thorton-Wells TA, Jones N, Smith AV, Chouraki V, Thomas C, Ikram MA, Zelenika D, Vardarajan BN, Kamatani Y, Lin CF, Gerrish A, Schmidt H, Kunkle B, Dunstan ML, Ruiz A, Bihoreau MT, Choi SH, Reitz C, Pasquier F, Cruchaga C, Craig D, Amin N, Berr C, Lopez OL, De Jager PL, Deramecourt V, Johnston JA, Evans D, Lovestone S, Letenneur L, Moron FJ, Rubinsztein DC, Eiriksdottir G, Sleegers K, Goate AM, Fievet N, Huentelman MW, Gill M, Brown K, Kamboh MI, Keller L, Barberger-Gateau P, McGuiness B, Larson EB, Green R, Myers AJ, Dufouil C, Todd S, Wallon D, Love S, Rogaeva E, Gallacher J, St George-Hyslop P, Clarimon J, Lleo A, Bayer A, Tsuang DW, Yu L, Tsolaki M, Bossu P, Spalletta G, Proitsi P, Collinge J, Sorbi S, Sanchez-Garcia F, Fox NC, Hardy J, Deniz Naranjo MC, Bosco P, Clarke R, Brayne C, Galimberti D, Mancuso M, Matthews F, European Alzheimer’s Disease I Genetic, Environmental Risk in Alzheimer’s, D.Alzheimer’s Disease Genetic, C.Cohorts for, H.Aging Research in Genomic, E., Moebus S, Mecocci P, Del Zompo M, Maier W, Hampel H, Pilotto A, Bullido M, Panza F, Caffarra P, Nacmias B, Gilbert JR, Mayhaus M, Lannefelt L, Hakonarson H, Pichler S, Carrasquillo MM, Ingelsson M, Beekly D, Alvarez V, Zou F, Valladares O, Younkin SG, Coto E, Hamilton-Nelson KL, Gu W, Razquin C, Pastor P, Mateo I, Owen MJ, Faber KM, Jonsson PV, Combarros O, O’Donovan MC, Cantwell LB, Soininen H, Blacker D, Mead S, Mosley TH Jr., Bennett DA, Harris TB, Fratiglioni L, Holmes C, de Bruijn RF, Passmore P, Montine TJ, Bettens K, Rotter JI, Brice A, Morgan K, Foroud TM, Kukull WA, Hannequin D, Powell JF, Nalls MA, Ritchie K, Lunetta KL, Kauwe JS, Boerwinkle E, Riemenschneider M, Boada M, Hiltuenen M, Martin ER, Schmidt R, Rujescu D, Wang LS, Dartigues JF, Mayeux R, Tzourio C, Hofman A, Nothen MM, Graff C, Psaty BM, Jones L, Haines JL, Holmans PA, Lathrop M, Pericak-Vance MA, Launer LJ, Farrer LA, van Duijn CM, Van Broeckhoven C, Moskvina V, Seshadri S, Williams J, Schellenberg GD, Amouyel P, 2013. Meta-analysis of 74,046 individuals identifies 11 new susceptibility loci for Alzheimer’s disease. Nat. Genet 45, 1452e1458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li H, Durbin R, 2009. Fast and accurate short read alignment with Burrows-Wheeler transform. Bioinformatics 25, 1754e1760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liang Y, Tedder TF, 2001. Identification of a CD20-, FcepsilonRIbeta-, and HTm4-related gene family: sixteen new MS4A family members expressed in human and mouse. Genomics 72, 119e127. [DOI] [PubMed] [Google Scholar]
- Lill CM, Rengmark A, Pihlstrom L, Fogh I, Shatunov A, Sleiman PM, Wang LS, Liu T, Lassen CF, Meissner E, Alexopoulos P, Calvo A, Chio A, Dizdar N, Faltraco F, Forsgren L, Kirchheiner J, Kurz A, Larsen JP, Liebsch M, Linder J, Morrison KE, Nissbrandt H, Otto M, Pahnke J, Partch A, Restagno G, Rujescu D, Schnack C, Shaw CE, Shaw PJ, Tumani H, Tysnes OB, Valladares O, Silani V, van den Berg LH, van Rheenen W, Veldink JH, Lindenberger U, Steinhagen-Thiessen E, Consortium S, Teipel S, Perneczky R, Hakonarson H, Hampel H, von Arnim CA, Olsen JH, Van Deerlin VM, Al-Chalabi A, Toft M, Ritz B, Bertram L, 2015. The role of TREM2 R47H as a risk factor for Alzheimer’s disease, frontotemporal lobar degeneration, amyotrophic lateral sclerosis, and Parkinson’s disease. Alzheimer’s Demen 11, 1407e1416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malkki H, 2015. Alzheimer disease: the involvement of TREM2 R47H variant in Alzheimer disease confirmed, but mechanisms remain elusive. Nat. Rev. Neurol 11, 307. [DOI] [PubMed] [Google Scholar]
- Martiskainen H, Viswanathan J, Nykanen NP, Kurki M, Helisalmi S, Natunen T, Sarajarvi T, Kurkinen KM, Pursiheimo JP, Rauramaa T, Alafuzoff I, Jaaskelainen JE, Leinonen V, Soininen H, Haapasalo A, Huttunen HJ, Hiltunen M, 2015. Transcriptomics and mechanistic elucidation of Alzheimer’s disease risk genes in the brain and in vitro models. Neurobiol. Aging 36, 1221.e15e1221.e28. [DOI] [PubMed] [Google Scholar]
- Paloneva J, Manninen T, Christman G, Hovanes K, Mandelin J, Adolfsson R, Bianchin M, Bird T, Miranda R, Salmaggi A, Tranebjaerg L, Konttinen Y, Peltonen L, 2002. Mutations in two genes encoding different subunits of a receptor signaling complex result in an identical disease phenotype. Am. J. Hum. Genet 71, 656e662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perez-Palma E, Bustos BI, Villaman CF, Alarcon MA, Avila ME, Ugarte GD, Reyes AE, Opazo C, De Ferrari GV Alzheimer’s Disease Neuroimaging, I.Group, N.-L.N.F.S, 2014. Overrepresentation of glutamate signaling in Alzheimer’s disease: network-based pathway enrichment using meta-analysis of genome-wide association studies. PLoS One 9, e95413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piccio L, Deming Y, Del-Aguila JL, Ghezzi L, Holtzman DM, Fagan AM, Fenoglio C, Galimberti D, Borroni B, Cruchaga C, 2016. Cerebrospinal fluid soluble TREM2 is higher in Alzheimer disease and associated with mutation status. Acta Neuropathol. [Epub ahead of print] 10.1007/s00401-016-1533-5. [DOI] [PMC free article] [PubMed]
- Proitsi P, Lee SH, Lunnon K, Keohane A, Powell J, Troakes C, Al-Sarraj S, Furney S, Soininen H, Kloszewska I, Mecocci P, Tsolaki M, Vellas B, Lovestone S, Hodges A, AddNeuroMed C, 2014. Alzheimer’s disease susceptibility variants in the MS4A6A gene are associated with altered levels of MS4A6A expression in blood. Neurobiol. Aging 35, 279e290. [DOI] [PubMed] [Google Scholar]
- Rosenthal SL, Bamne MN, Wang X, Berman S, Snitz BE, Klunk WE, Sweet RA, Demirci FY, Lopez OL, Kamboh MI, 2015. More evidence for association of a rare TREM2 mutation (R47H) with Alzheimer’s disease risk. Neurobiol. Aging 36, 2443.e21e2443.e26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Storey JD, 2003. The positive false discovery rate: a Bayesian interpretation and the q-value 2013e35. 10.1214/aos/1074290335. [DOI]
- Van der Auwera GA, Carneiro MO, Hartl C, Poplin R, Del Angel G, Levy-Moonshine A, Jordan T, Shakir K, Roazen D, Thibault J, Banks E, Garimella KV, Altshuler D, Gabriel S, DePristo MA, 2013. From FastQ data to high confidence variant calls: the Genome Analysis Toolkit best practices pipeline. Curr. Protoc. Bioinformatics 11, 11.10.1e11.10.33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vardarajan BN, Ghani M, Kahn A, Sheikh S, Sato C, Barral S, Lee JH, Cheng R, Reitz C, Lantigua R, Reyes-Dumeyer D, Medrano M, Jimenez-Velazquez IZ, Rogaeva E, St George-Hyslop P, Mayeux R, 2015. Rare coding mutations identified by sequencing of Alzheimer disease genome-wide association studies loci. Ann. Neurol 78, 487e498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang K, Li M, Hakonarson H, 2010. ANNOVAR: functional annotation of genetic variants from high-throughput sequencing data. Nucleic Acids Res 38, e164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y, Cella M, Mallinson K, Ulrich JD, Young KL, Robinette ML, Gilfillan S, Krishnan GM, Sudhakar S, Zinselmeyer BH, Holtzman DM, Cirrito JR, Colonna M, 2015. TREM2 lipid sensing sustains the microglial response in an Alzheimer’s disease model. Cell 160, 1061e1071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhong L, Chen XF, Zhang ZL, Wang Z, Shi XZ, Xu K, Zhang YW, Xu H, Bu G, 2015. DAP12 stabilizes the C-terminal fragment of the triggering receptor expressed on myeloid cells-2 (TREM2) and protects against LPS-induced pro-inflammatory response. J. Biol. Chem 290, 15866e15877. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


