Abstract
Young adolescents in Sub Saharan Africa (SSA) are at high risk of involvement in sexual risk behaviors; and curable sexually transmitted infections (STI), herpes simplex virus type 2 (HSV-2), human immunodeficiency virus (HIV) and unintended pregnancies remain persistently high in this population. Evidence based strategies are urgently needed to improve these outcomes. The aim of this systematic review was to synthesize the evidence from randomized controlled trials (RCT) to determine whether school-based interventions promote safe sex behaviors, reduce sexual risk behaviors and risk of curable STIs, HSV-2, HIV and unintended pregnancies among young adolescents aged 9 to 19 years in SSA. Electronic databases were searched for published studies and manual searches were conducted through reviewing of references of cited literature in the English language up to December 2019. Two independent reviewers screened and abstracted the data. We identified 428 articles and data from nine RCTs (N=14,426 secondary school students) that fulfilled the selection criteria were analysed. Two studies measured pregnancy as an outcome and showed significant declines in unintended pregnancies. Of the five studies that measured HIV/AIDS related-knowledge, condom-use outcomes (normative beliefs, knowledge, and self-efficacy) and attitudes to HIV testing, four showed significant improvements. Of the six studies that measured sexual debut, four reported moderate but non-significant declines and in two studies sexual debut information was either incomplete or unreliable. One study measured curable STIs and found no significant declines; whilst the second study that measured HSV-2 and HIV, no significant declines were observed. This review highlights the need to undertake well-designed research studies to provide evidence on the impact of interventions on curable STIs, HSV-2 and HIV, critical to improving the health of young adolescents.
Keywords: Systematic review, sub-Saharan Africa, schools, interventions, young adolescents, sexual risk behaviors, sexually transmitted infections, HIV, unintended pregnancies
Introduction
Sub-Saharan Africa (SSA) bears a disproportionate burden of curable sexually transmitted infections (STIs) including herpes simplex virus type 2 (HSV-2) and human immunodeficiency virus (HIV) [1–4]. Despite the scale up and widespread coverage of prevention and treatment programs for STIs and HIV, the overall prevalence and incidence of these infections remains unacceptably high [3, 5].
The HIV epidemic in SSA is characterized as heterosexually driven and many countries in the region experience epidemics that are generalized and hyper-endemic with HIV prevalence exceeding 15% in the adult population. The largest number of new infections occur in young women 15–24 years [1, 6, 7] and contribute to about 25% of new HIV infections [1] and about 80% of all HIV positives [1, 8]. Whilst young women acquire HIV at least 5–7 years earlier [1, 9, 10] and HIV prevalence is at least seven to eight times higher than in similar aged young men [6, 8, 9, 11], men tend to acquire infection later in life and prevalence increase rapidly [9, 11–13].
Data from school-based surveys have shown the persistently high HIV prevalence among high school students and these surveys have provided an opportunity to better understand the HIV epidemic in younger populations, time to HIV acquisition and risk factors contributing to HIV acquisition [14–16]. Furthermore, the surveys showed that one in four students were already sexually active, pregnancy prevalence was 3.3%, HIV prevalence was 6.8% in girls and 2.7% in boys and girls with older sex partners were three to four times more likely to be HIV positive [15]. Clues on HIV transmission among high school students, showed that there was limited spread among students within and across schools and that young girls were more likely to have acquired their infection from community members [16]. Similarly, studies from the region have shown that young adolescents have high rates of unintended pregnancies and are particularly vulnerable to curable STIs including HSV-2, which are significant risk factors for HIV acquisition and transmission, and poor health, and birth outcomes [17–20]. These findings suggest that schools should be considered as important venues for intensifying prevention efforts to improve adolescent health and reduce unintended pregnancies, STIs and STI related HIV acquisition and transmission.
Adolescence is a crucial stage for psychosocial, social, cognitive, and emotional development [21–23] and contributes to determining adolescents’ ability to perceive and judge risk effectively. Furthermore, multiple complex and diverse structural, social, behavioral, and biological cofactors contribute to influencing risk (Supplementary Figure 1). However, appropriate information, education, guidance and support could help adolescents as they transition through this developmental period [23]. STI and HIV risk reduction programs in schools are crucial to develop young people’s identities and characters to enhance self-esteem and rational decision-making to reduce or delay early sexual debut towards safer adulthood [21, 24]. Nevertheless, a high proportion of high school students are already sexually active [16], suggesting that early sexual debut persists [11, 25]. In addition to the high prevalence of HIV, unintended pregnancies and STIs, and poor school completion rates also lead to poor health and economic outcomes among adolescents [11, 25–27]. As majority of adolescents are likely to be in schools, the school environment provides an opportunity for the delivery of programs to initiate and promote healthy behaviors and decision-making skills to minimize sexual risk behaviors and reduce the risk of curable STIs, HSV-2, HIV and unintended pregnancies that potentially lead to adverse health, educational and economic outcomes [24, 27–29].
School-based programs should therefore be practical as they are time, place, and population-specific, offer a suitable platform to promote health and minimize health risks [21], have the ability to reach large numbers of young adolescents simultaneously [30] and has the potential to transform country-level HIV epidemics [31]. Numerous school and community-based adolescent focused life-skills programs have been initiated to empower young people in communities to lead positive and healthy lives, pursue successful futures and stay HIV free [11, 32–35]. Several systematic reviews have also assessed and evaluated the effectiveness of interventions for adolescents to improve adolescent health, reduce the risk of curable STIs, HSV-2, HIV and unintended pregnancies [30, 36–42]. However, these reviews included studies of different study designs, were of varying quality and implemented both within schools and the broader community environments which could potentially bias the results [43]. To address the evidence gap, the current systematic review is based on RCTs, as the RCT study design offers the highest level of evidence and implementation within a school environment was expected to ensure maximum exposure, fidelity and reach of the interventions to the target population.
The aim of this systematic review was to synthesize the evidence from RCTs to determine whether school-based interventions promote safe sex behaviors, reduce sexual risk behaviors and risk of curable STIs, HSV-2, HIV and unintended pregnancies among young adolescents aged 9 to 19 years in SSA.
Methods
Search Strategy and selection criteria
To synthesize the research evidence, the systematic review followed the Preferred Reporting Items for Systematic Reviews (PRISMA) guidelines (Supplementary Table 1) [44–46]. Two independent reviewers (NS and NN) systematically searched electronic databases for published studies (PubMed, PubMed Central, Cochrane and ClinicalTrials.gov registry), and conducted manual searches through reviewing of references of cited literature in the English language up to December 2019. Abstracts and studies that assessed programs and interventions for adolescents for reducing sexual risk behaviors and reducing the risk of curable STIs, HSV-2, HIV and unintended adolescent pregnancies were reviewed. The search terms used individually and in combination were “interventions”/ “programs” for adolescents”, “sexually transmitted infections”, “STIs”, “HIV”, “HSV-2”, “acquired immunodeficiency syndrome”. “AIDS”, “HIV prevention strategies/ interventions /programs”, “randomized control trials”, “school-based interventions”, “adolescent pregnancy prevention”, “teenage pregnancies”, “unplanned pregnancies”, “unintended adolescent pregnancies”, “risk reduction”, “sexual debut”, “adolescent risk behaviors”, “sub-Saharan Africa”, SSA and “Africa” for all completed or ongoing studies. Both reviewers reviewed all abstracts independently.
Eligibility criteria
Articles were eligible if studies were individual or cluster RCTs, interventions that promoted positive sexual reproductive health to reduce sexual risk behaviors and risk of curable STIs, HSV-2, HIV and unintended pregnancies among young adolescents aged 9 to 19 years, attending primary school through to high school during the time of study participation; implemented within a school environment; located in SSA and peer-reviewed published articles. Studies were excluded if the interventions were not implemented as RCTs [47], were community-based [48–50], computer-based [51], university-based [52], not based in the SSA [53] or were conference presentations [54].
Study Selection
The two-independent reviewer (NS and NN) searches generated a total of 442 articles (Figure 1). All the articles were combined in Zotero reference manager software (version 5.0.60, Roy Rosenzweig Center for History and New Media, Fairfax, Virginia) and checked for duplicates. A total of 14 duplicates were excluded and the remaining articles were exported to Covidence systematic review software (Melbourne, Australia Veritas Health Innovation) [55] where each reviewer screened the titles and abstracts of articles related to eligibility criteria. Screening results of the two reviewers were merged for comparison and discrepancies were discussed with discrepancy resolution and to reach consensus. Of the 428 articles screened, 394 articles were excluded as the study design, study location and topic/focus did not meet the eligibility criteria. Following a full-text review of the remaining 34 articles, 12 articles were excluded as three were determined to be in the incorrect setting, three were not deemed to be RCTs, four were based on process and cost evaluation and one study was still in progress [56]. Of the remaining 22 articles, nine articles [57–65] contributed to unique independent RCTs that met the eligibility criteria and were included in the final review analysis (Figure 1).
Figure 1.
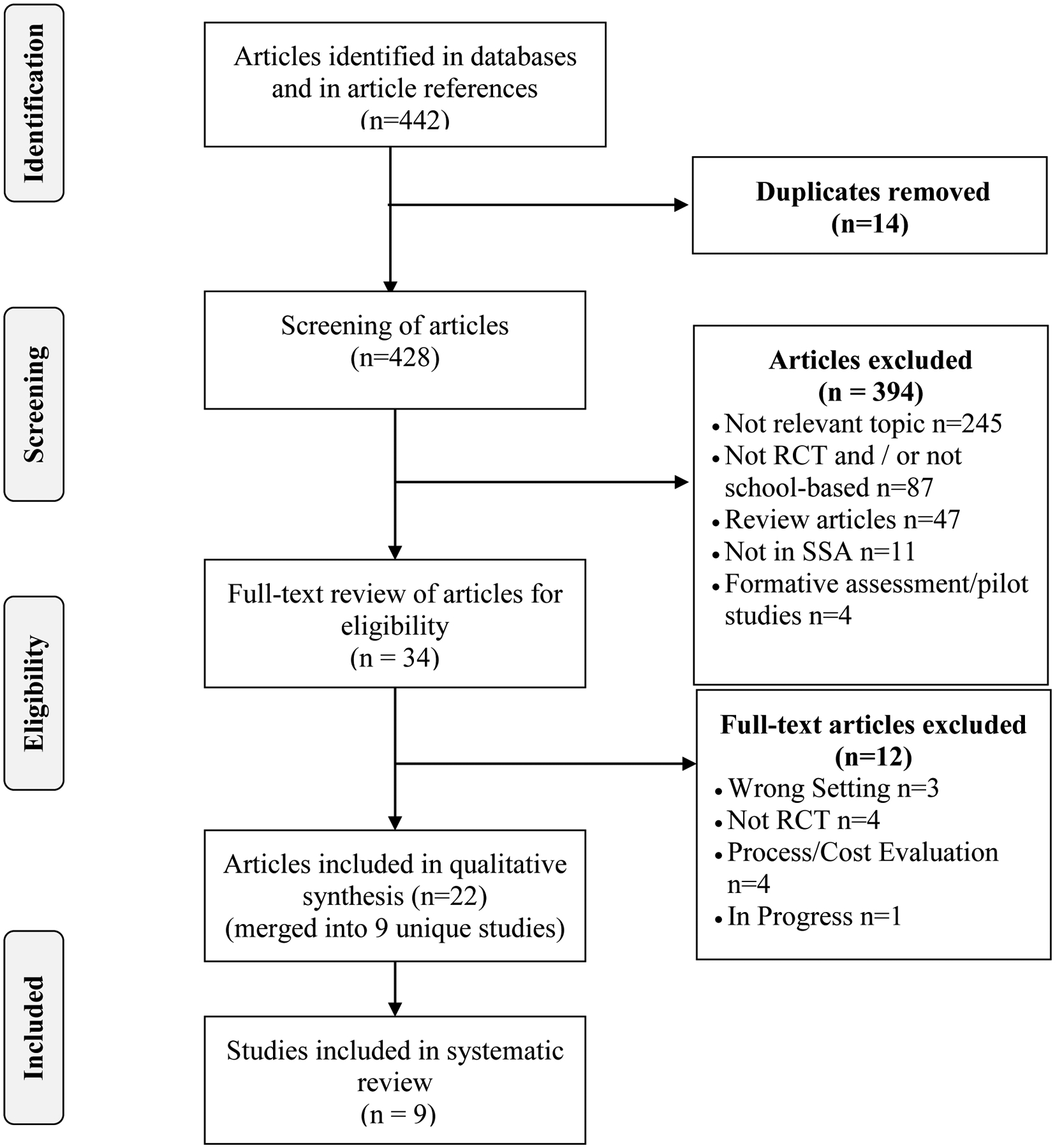
Search strategy and selection of articles for analysis and reported according to PRISMA guidelines
Data extraction and analysis
Data were extracted into Covidence software (Melbourne, Australia Veritas Health Innovation) [55] using the Cochrane guidelines [66]. Data were collected on; context (country and setting), participants (location, grade, gender, and age), study design (intervention details, theoretical framework, implementation, and analysis), and the analyses on study endpoints of sexual risk behaviors of age at sexual debut, condom use, unprotected sex, abstinence, HIV knowledge, school dropout, curable STIs (Chlamydia trachomatis, Neisseria gonorrhoeae, and Trichomonas vaginalis), HSV-2, HIV and pregnancy. Studies with multiple publications on the study design, conceptual framework, baseline, and primary outcome findings were merged to represent the primary study. For the quality assessment, the Cochrane Collaboration’s tool [67] assessed risk of bias by evaluating selection bias-random sequence generation, allocation and concealment; performance bias-blinding of participants and personnel; detection bias-blinding of outcome assessment; attrition bias-complete/incomplete outcome data and reporting bias-selective reporting and completeness of reporting of assessed outcomes. To assess the quality of each study, the risk bias analysis was undertaken and each study was scored and assessed as being of “low risk”, “high risk” or “unclear” [67]. No studies were excluded based on quality.
Results
Quality assessment of eligible studies
Table 1 shows the assessment of the nine studies included in the systematic review. Eight studies reported on sequence generation for randomization and allocation concealment and were at low risk of bias, however, in one study this was unclear [60]. To reduce performance bias eight studies reported that schools were blinded to the intervention by study group [57, 58, 60–65], whilst one study [59] was considered to be at high-risk of bias as the unit of randomization were individuals and not schools, which could potentially result in contamination of the two groups and reduce or exaggerate the effect estimates. To safeguard against detection bias five studies reported that the outcome measurements were blinded [59, 62–65]; compared to four studies where the intervention assignment were known to the assessors [57, 58, 60, 61]. All studies were at low risk of attrition as minimal loss to follow-up was expected unless the learner left school prematurely. There was no evidence of selective reporting of outcomes. However, in some studies, small sample sizes [59, 61] or short follow-up periods [57, 58, 60, 63–65] may have reduced exposure time and require results to be interpreted with caution.
Table 1.
Assessment of risk bias analysis of eligible studies
| Domain | Rusakaniko et al., 1997 [57] | Agha 2002 [58] | Burnett et al., 2010 [59] | Atwood et al., 2012 [60] | Hallfors et al., 2015 [61] | Jemmott et al., 2015 [62] | Mathews et al., 2016 [63] | Mmbaga et al., 2017 [64] | Kemigisha et al., 2019 [65] | |
|---|---|---|---|---|---|---|---|---|---|---|
| Selection Bias | ||||||||||
| Allocation Concealment | + | + | + | ? | + | + | + | + | + | |
| Performance bias | Blinding of participants and personnel | + | + | − | + | + | + | + | + | + |
| Detection Bias | Blinding of outcome assessment | ? | ? | + | ? | ? | + | + | + | + |
| Attrition bias | Incomplete outcome data | + | + | + | + | + | + | + | + | + |
| Reporting Bias | Selective reporting | + | + | + | − | + | + | + | + | + |
+=low risk; −=high risk; ?=unclear [67]
Characteristics of eligible studies
The nine eligible studies were based in seven countries in SSA; two each were based in Zimbabwe [57, 61] and South Africa [62, 63] and one each in Tanzania [64], Zambia [58], Liberia [60], Swaziland [59] and Uganda [65]. The sample sizes across the studies ranged from 135 [59] to 5,091 [64], with aggregate data available for 14,426 participants. Participants were from primary and secondary/high school (grades 6 to 12) and their ages ranged from 9 to 19 years. One study focused on female orphans [61], whilst the remaining eight included both males and females. Table 2 provides an overview of articles included in the systematic review.
Table 2.
Overview of articles included in the systematic review
| Study | Country (province) | Sample Characteristics | Intervention Aim and Framework | Measurement, follow-up and Analysis | Comparison Programs | Primary Outcomes |
|---|---|---|---|---|---|---|
| *Rusakaniko et al. 1997 [57] | Zimbabwe (Mashonaland Central) | Learners from 11 rural and urban secondary schools. N=1,689; Mean age (SD) 13.5 (1.3) years. |
Aim to determine impact of a knowledge-based intervention on sexual and reproductive health Framework not specified |
Questionnaire Follow-up 5 and 9 months. Analysis chi-square test |
Health-promotion program | Knowledge on STDs/HIV, family planning and pregnancy |
| *Agha, 2002 [58] | Zambia (Central, Copperbelt and Lusaka) | Male and female learners from grades 10, 11, and 12, from 5 secondary boarding schools. N=759; Males=56.8%. Mean age 17.9 years, 42.3% were 14–17 years. 33% were in grade 12 |
Aim to evaluate effectiveness of a peer sexual health intervention Framework not specified |
Self-administered questionnaire Follow-up 2 weeks. Analysis Logistic regression analyses |
1 hr. peer water purification intervention | Knowledge, normative beliefs about abstinence and about condoms, perception of personal risk |
| *Burnett et al. 2010 [59] | Swaziland (Manzini) | Male and female learners from grades 9 to 11. N=135, Males=56%, Mean age 17.3 years |
Aim to increase HIV knowledge and change attitudes and behavior among learners Framework Self-Efficacy Theory |
Self-administered questionnaire; Follow-up post-intervention. Analysis Student’s t-test, Chi test, linear regression and logistic regression |
No intervention until after study completion | HIV knowledge, self-efficacy for abstinence, condom use and HIV testing |
|
*Atwood et al. 2012 [60], Atwood et al., 2012 [69], Kennedy et al. 2012 [101] |
Liberia (Monrovia) | Grade 6 learners from 8 public schools. N=820; Mean age (range, SD) 16.4 (13–19, 1.75) years; Males=56% |
Aim to address health needs of school adolescents Framework Social Cognitive Theory and Theory of Reasoned Action |
Self-administered questionnaire, Follow-up 3 and 9 months; Analysis Student’s t-test and Pearson chi-square |
Modified General Health Program (TB, worms, general HIV/STD knowledge | HIV/AIDS knowledge, Condom attitudes, perceived HIV risk, sexual attitudes, self-efficacy, peer norms |
|
*Hallfors et al. 2015 [61], Iritani et al. 2016 [102], Hallfors et al. 2011 [74] |
Zimbabwe (Manicaland) | Grade 6 female orphans from 25 rural primary schools (boarding & non-boarding). N=328; Mean age (range) 15.3 (13–19) years |
Aim to assess the effectiveness of a school structural HIV support intervention Framework Social Development Model and Social Cognitive Theory |
Self-administered survey (audio computer-assisted self-interviewing in English and Shona). Follow-up annually Analysis Chi-squared, ANOVA, and logistic regression |
Delayed partial treatment (school fees from in 2011 for 1.5yr) | Sexual debut, Ever pregnant, School dropout and years of schooling. HIV and HSV-2 as biomarkers (1.5yrs after introduction of partial intervention) |
|
*Jemmott et al. 2015 [62], Jemmott et al. 2010 [68], Jemmott et al. 2013 [103], O’Leary et al. 2012 [104], O’Leary et al. 2015 [105] |
South Africa (Eastern Cape) | 17 matched pairs of primary schools consisting of 6th grade learners who speak isiXhosa and English (urban & rural). N=1,057; Mean age (range) 12.4 (9 to 18) years |
Aim to promote HIV/STD risk-reduction knowledge and to address cultural HIV myths Framework Social Cognitive and Reasoned Action and Planned Behavior |
Self-reported assessment, curable STIs, Follow-up 3, 6, 12, 42 & 54 months. Analysis intent-to-analysis, chi-square and t-test |
Health-promotion program | Unprotected vaginal intercourse within a 12-month follow-up period |
| *Mathews et al. 2016 [63], Mathews et al. 2015 [106],&Aarø et al. 2014 [107],&Namisi et al. 2013 [108] | South Africa (Western Cape) | 41 public high schools consisting of 8th grade learners who spoke the province’s three main languages. N=3,451; Mean age 13.7 years; Males=37.9% |
Aim HIV, IPV prevention and to improve sexual reproductive health Framework Social Cognition Models, Reasoned Action Framework, I-Change theoretical model |
Self-administered questionnaire; Follow-up 6 and 12 months; Analysis Regression | Usual school lessons | Sexual debut, number of sexual partners, condom use and contraception use |
| *Mmbaga et al. 2017 [64],&Aarø et al. 2014 [107],&Namisi et al. 2013 [108] | Tanzania (Dar es Salaam) | 38 public primary schools of standard 5 and 6 learners. N=5,091; Mean age (range) 12.4 (12–14) years. |
Aim to reduce sexual initiation and promote condom use among adolescents Framework Social Cognitive Theory |
Self-administered questionnaire; Follow-up 6 months and then 12 months. Analysis Generalized Estimating Equation modelling |
Not specified | Sexual debut and condom use |
| Kemigisha et al., 2019 [65] Kemigisha et al., 2018 [109] |
Uganda (Mbarara district) | Learners from 33 primary schools. N=864; Mean age (SD) 12.1 (1.13) years. |
Aim to evaluate the effectiveness of a Comprehensive Sexuality Education intervention among adolescents in primary school Framework: Planned Behavior and Social Ecological Model |
Questionnaire & qualitative interview Follow-up 9 months. Analysis Logistic regression analyses |
Not specified | Sexual debut and health knowledge |
Key
Referent article;
articles describing studies undertaken in South Africa (Western Cape) and Tanzania (Dar es Salaam) as part of multicenter studies
Characteristics of study design, intervention implementation, data collection and measurements
Seven studies were guided by a theoretical framework that incorporated the Social Cognitive Theory [60–64], Reasoned Action framework [60, 62, 63], Planned Behavior [62, 65], Social ecological model [65] and Self-efficacy [59]. In some studies, the instruments were adapted from a western-based program [59, 60]. All studies on behavioral interventions provided information to promote positive healthy behaviors and a single study additionally included financial support towards school fees, uniforms, and school supplies [61].
Community members [68], study staff [63], teachers [57, 59, 61, 64, 65, 69] and peer educators [58, 64, 65] facilitated the delivery of interventions. Facilitator training ranged from eight days [68] to two weeks [63], whilst some studies did not specify the duration of facilitator training [58, 61, 64, 65] and others did not specify if training was provided [57, 59, 60]. Intervention form and delivery varied, either delivered thematically as educational-programs and through role-playing in five studies [58, 60, 62–64], incorporated discussions or lessons in five [57–59, 62, 63, 65] or provided with structural support [61]. The intervention programs were delivered weekly for the duration of the study and ranged from 40 minutes to 1 hour 45 minutes per session. Majority of the programs were delivered in schools during school hours [57–62, 64, 65], whilst one was delivered after school hours [63, 64]. Overall, the exposure to the interventions varied with follow-up assessments occurring as short as after two weeks [58] or approximately five years later [61]. Tables 2 and 3 show a summary of studies undertaken, interventions and implementation details.
Table 3.
Summary of study interventions and implementation details
| Study | Intervention | Implementation | Additional components | ||||
|---|---|---|---|---|---|---|---|
| Duration | Main activities/component | Setting | Exposure delivery /duration | Instructors | Training/Duration | ||
| *Rusakaniko et al. 1997 [57] | Lectures, videos, leaflets posters | School | Teacher discretion/NS | Teachers | NS /NS | Topics: Female and male reproductive function, anatomy, sexuality, pregnancy, STDs, HIV/AIDs | |
| *Agha 2002 [58] | 1hr 45min | Interactive Discussion Drama skits |
School | Peer educators/ weekly meetings | Peer educators | Yes / NS | Topics: Abstinence, condoms and STIs; |
| *Burnett et al. 2010 [59] | 1hr/week and half-day Saturday | 4 curricula (life skills for HIV, computer technology, job readiness & community outreach) Discussions | School | 13 weeks | Teachers (facilitators) | NS / NS | Topics: Understanding My Body; Romantic Relationships/Assertive Behavior; HIV and Sexually Transmitted Infections. Used a Western-based program. |
| *Atwood et al. 2012 [69], Atwood et al. 2012 [60], Kennedy et al. 2012 [101] | NS | 8 Modules (role play, HIV-related prevention skills) | School | NS/(9 months | Educators | NS /NS | Topics: Positive condom attitudes, skills and self-efficacy to refuse sex, condom negotiation. Used a Western-based program (HIV/STDs and pregnancy risk reduction). Participants received ~US $2 for each questionnaire |
|
*Hallfors et al. 2015 [61], Iritani et al. 2016 [102], Hallfors et al. 2011 [74] |
N/A | School fees School uniform School supplies |
School | 5 years | Female teachers (helpers) | Yes / NS |
Topics: Monitoring of school attendance, daily feeding scheme. Results for after 5 years |
|
*Jemmott et al. 2015 [62], Jemmott et al. 2010 [68], Jemmott et al. 2013 [103], O’Leary et al. 2012 [104], O’Leary et al. 2015 [105] |
1hr | 12 Modules (activities, games, role-playing, comic workbook, discussions etc.) | School | 54 months | Community members (facilitators) | Yes / 8 days | Topics: HIV/AIDS, stigma, pregnancy, risky behaviors, abstinence, condom use etc. Compensation was given at each follow-up |
| *Mathews et al. 2016 [63], Mathews et al. 2015 [106],&Aarø et al. 2014 [107],&Namisi et al. 2013 [108] | 1hr 30 min | 21 sessions (discussions, readings, role-plays, worksheets etc); school health services; sexual violence prevention program | After school | 3 years | Staff (facilitators) | Yes/ 2 weeks thereafter weekly | Topics: Assertive communication, gender power inequalities, Intimate Partner Violence, HIV etc. |
| *Mmbaga et al. 2017 [64],&Aarø et al. 2014 [107],&Namisi et al. 2013 [108] | 40–80 min, 60–90 min | 16 interactive sessions Role-play/drama |
School After school |
9 weeks | Teachers, peer educators, health providers | Yes / NS | Topics: Lessons were integrated in the primary school science curriculum |
|
*Kemigisha et al., 2019 [65] Kemigisha et al., 2019 [109] |
1 – 2 hr | 11 lessons | School | 9 months | Teachers, university student volunteers | Yes/NS | Topics: Puberty, sexual violence, STIs, sexuality and media influence, prevention of pregnancy, relationships, and emotions etc. |
Key NS=not specified,
Referent article;
articles describing studies undertaken in South Africa (Western Cape) and Tanzania (Dar es Salaam) as part of multicenter studies
All studies measured outcomes through self-administered questionnaires as aided, recall closed-ended questions. However, aided recall questions tend to result in higher effect estimates compared to unaided recall questions and could potentially lead to overreporting, though they are suitable when underreporting is likely to occur especially with sensitive sexual behavior data [70]. Measurement error could have occurred due to misunderstanding of questions using self-administered questionnaires. For instance, results were non-significant when asked “is abstinence effective in preventing HIV” [Adjusted odds ratio (AOR)=1.38; 95% Confidence Interval (CI) 0.62–3.10], but when asked “a person can avoid HIV by abstaining” the intervention group had greater odds of responding correctly (AOR=3.89; 95% CI 2.21–6.85) [58]. Therefore, in the assessment of outcomes, the wording, formatting and ordering of questions was important in preventing bias and variance [70]. In addition, six studies assessed knowledge about HIV/AIDS, however, the ascertainment of this outcome varied by study [57–60, 62, 63]. Burnett et al. [59] measured HIV/AIDS knowledge using a 15-item scale and focused on three HIV prevention methods, risk factors and misconceptions and myths about HIV/AIDS. Conversely, Jemmott et al. [62] focused on transmission and consequences of HIV using a 48-item scale. Moreover, one study used the audio computer-assisted self-interviews [61], considered to be highly suitable for collection of sensitive data to minimize response bias [70]. The measurements assessed knowledge, self-efficacy, attitudes, and behaviors on sexual and reproductive health. Biological measurements included curable STIs [62], HSV-2 and HIV [61] and pregnancy [57, 61].
Programs implemented for the control schools or individuals included a water purification program [58], a modified general health program [60], delayed partial treatment [61] and health promotion programs [57, 62], whilst some studies did not include any programs for the control schools [59, 63]. All studies were RCT in design, however, in one study the analysis of the outcome measurement was analysed as pre and post outcomes compared to the analysis of differences between the control and intervention schools [59], whilst one study placed students who chose not to participate into the control group [60], potentially compromising the robustness of the RCT. Therefore studies without programs for the control schools or who assigned refusals to the control group would be susceptible to bias since blinding would less likely be maintained [71], or eliminated if participants and/or investigators were blinded to the intervention and control groups [71]. More importantly if cash transfer programs as interventions are implemented, blinding those assigned to the intervention is often more difficult. Furthermore, contamination from the intervention and control is possible in instances where both groups were located in the same school or community [38]. Three studies used cluster random assignment of schools to the intervention and controls to limit potential contamination [62–64]. However, cross-contamination is likely to occur if both study groups are located in the same school [38]. A single study ensured that participants including participating schools were blinded to study groups [62]. Agha [58] selected participants in a boarding school to reduce the possibility of contamination from external sources.
Measured Outcomes
The primary outcome measures assessed for each of the studies are shown in table 4, whilst table 5 shows the effect estimates of behavioral and biological outcome measures.
Table 4.
Primary outcomes assessed in the studies
| Study | Primary Outcomes |
|---|---|
| Rusakaniko et al. 1997 [57] | Knowledge on STDs/HIV, family planning and pregnancy |
| Agha 2002 [58] | Knowledge, normative beliefs about abstinence and about condoms, perception of personal risk |
| Burnett et al. 2010 [59] | HIV knowledge, self-efficacy for abstinence, condom use and HIV testing |
| Atwood et al. 2012 [60] | AIDS/HIV knowledge, Condom attitudes, perceived HIV risk, sexual attitudes, self-efficacy, peer norms |
| Hallfors et al. 2015 [61] | Sexual debut, Meals per day, Ever pregnant, School dropout and years of schooling. HSV-2 and HIV as biomarkers (1.5yrs after introduction of partial intervention) |
| Jemmott et al. 2015 [62] | Unprotected vaginal intercourse within a 12-month follow-up period; curable STIs |
| Mathews et al. 2016 [63] | Sexual debut, number of sexual partners, condom use and contraception use, intimate partner violence |
| Mmbaga et al. 2017 [64] | Sex initiation and condom use |
| Kemigisha et al., 2019 [65] | Sexual and reproductive health knowledge, sexual wellbeing and attitudes, sexual debut |
Table 5:
Effect estimates of the measured outcomes in the studies
| Outcome | Rusakaniko et al., 1997 [57] | Agha 2002 [58] | Burnett et al., 2010 [59] | Atwood et al., 2012[60] | †Hallfors et al., 2015 [61] | Jemmott et al., 2015 [62] | Mathews et al., 2016 [63] | Mmbaga et al., 2017 [64] | Kemigisha et al., 2019 [65] |
|---|---|---|---|---|---|---|---|---|---|
| Sexual initiation/Sexual debut |
*15% vs 15%, p=1.00; ⁑22% vs 25%, p=1.00 |
ǂaOR=0.91 | 14.3% vs 23.8%, p=0.04 |
‡aOR=0.83 (95% CI 0.48– 1.45) |
aOR=1.07 (95% CI 0.83–1.40) |
§M: aRR=1.9, p=0.027 §F: aRR=1.6, p=0.019 |
aOR=0.76 (95% CI 0.32–1.80) |
||
| Condom use |
‡aOR=1.47 (95% CI 0.79– 2.71) |
aOR=0.64 (95% CI 0.33–1.25) |
M: β=0.217, (p=0.004 F: β=0.016, p=0.463 |
||||||
| Knowledge about condom use | aOR=1.61 (95% CI 0.91–2.83) |
Overall effect=:≠mdiff=1.02 (95% CI 0.84–1.21); Short-term effect= ≠mdiff=1.29 (95% CI 1.08–1.51); Long-term effect= ≠mdiff= 0.49 (95% CI 0.27–0.71) |
β=0.07 (95% CI 0.04–0.10) p<0.001 |
||||||
| Self-efficacy for Condom use | β=0.16, SE=0.05, p=0.001 |
β=0.07, (p<0.1) |
≠mdiff=0.50 (95% CI 0.31–0.69) |
β=0.19 (95% CI −0.09–0.11) |
|||||
| Knowledge on sexual reproductive health |
&Family planning and contraception 94.3% vs 88.6%, χ2=5.67, p=0.017 &family planning methods 51.2% vs 32.8%, χ2 =1.25, p=0.263 |
aOR= 2.18 (95% CI 1.66–2.86) | |||||||
| Abstinence in preventing STI, HIV and pregnancy | STIs = OR=1.72 (95% CI 0.79–3.72), HIV = OR=1.38 (95% CI 0.62–3.10) Pregnancy= OR=1.73 (95% CI 0.82–3.66) |
β=0.14, SE=0.04, p=0.001 |
|||||||
| Heard of abstinence | aOR=3.42, 95% CI: 1.01–11.54) | ||||||||
| A person can avoid HIV by abstaining from sex | aOR= 3.89, 95% CI: 2.21–6.85) | ||||||||
| Belief that it is normal to propose abstinence | Women= aOR=2.28, 95% CI: 1.11–4.70. Men= aOR=1.96, 95% CI: 1.06–3.62 |
||||||||
| Knowledge about HIV/AIDS |
&73.6% vs 68.5%, χ2=0.26, p=0.607 |
β=0.08, SE=0.02, p=0.001 |
β=−0.05 |
≠mdiff=2.78 (95% CI 2.22–3.35) |
β=0.05 (95% CI 0.02–0.08), p<0.01 |
||||
| Personal risk perception (HIV) | Baseline aOR=1.14 (95% CI 0.88–1.47) Follow-up aOR=0.68 (95% CI 0.56–0.83) |
β=−0.07, p<0.1 |
β=−0.01, (95% CI −0.17–0.14) |
||||||
| Ever tested for HIV | aOR=10.96 (95% CI 4.59–26.15) |
||||||||
| HIV infection | aOR=1.15 (95% CI 0.47–2.79) |
||||||||
| HSV-2 infection | aOR=1.46 (95% CI 0.50– 4.26) |
||||||||
| Pregnancy | & 37.5% vs 24.9%, χ2=10.42, p=0.001 | &11.8% vs 22.2%, χ2=5.60, p=0.02 | |||||||
| Intimate partner violence |
§35.1% vs 40.9%, OR= 0.77, (95% CI 0.61–0.99) |
||||||||
| Curable STIs (C. trachomatis, N. gonorrhoeae, T. vaginalis) |
‡OR = 0.91, (95% CI 0.73–1.12) ǂOR = 0.71, (95% CI 0.54, 0.95), ±OR = 1.15, (95% CI 0.84, 1.57) |
Control vs. intervention at 12 months;
Pre vs. Post intervention at 9 month;
Pre vs. Post Control at 9 months;
Intervention vs control at 9 months;
Intervention vs delayed partial intervention;
Long-term intervention effect, only includes 42 and 54 months follow-up;
Overall intervention effect, includes 3, 6, 12, 42 and 54 months follow-up;
Intervention effect at 42 months;
Intervention effect at 54 months;
aOR=adjusted odds ratio; cRR=crude risk ratio; aRR=adjusted relative risk; mdiff=mean difference, se=standard error;; M=Males; F=Females
Behavioral Outcomes
Of the nine studies, seven [59–65] measured sexual debut or sexual initiation as an outcome measure defined as ‘ever had’ sexual intercourse. Of these, five studies [60–63, 65] showed no significant association on reported sexual debut between the study groups, whilst in one study 33% (44/135) of participants had incomplete information on sexual behavior and those that responded to ever having had sex was too small to be considered for any reliable analysis [59]. However, one study reported the rate of sexual initiation declining from the month 6 to month 12 follow-up in the intervention schools but was almost double among males [Adjusted relative risk (aRR)=1.9, p=0.027] and females (aRR=1.6, p=0.019) in the control schools [64].
Condom related outcomes assessed on knowledge [58, 62, 63], usage [62–64] and self-efficacy [59, 60, 62, 63]. Self-reported knowledge on condom-use improved and showed β of 0.07 (95% CI 0.04–0.10, p<0.001) [63]. The long term intervention effect on self-reported knowledge on condom-use showed significant improvements with an observed mean difference of 0.49 (95% CI 0.27–0.71; p<0.001) [62] and declines in unprotected sexual intercourse averaged over the follow-up period [Odds ratio (OR)=0.42, 95% CI 0.22–0.84], yet these effects were not significantly reduced at 42 and 54 month follow-up compared with 3, 6, and 12-month follow-ups [62]. Similarly, improvements in condom use had been shown among males (β=0.217, p=0.004) but not among females (β=0.016, p=0.463) [64]. Although significant improvements were observed in condom use self-efficacy, negotiation, one’s ability to convince partners to use condoms, one’s ability/technical skills of wearing a condom [62] and/or condom negotiation [60], translating condom use knowledge and self-efficacy to ensuring protected sexual intercourse remains a major challenge, highlighting the potential for high rates of inconsistent condom use.
Knowledge about HIV and AIDS, including questions on prevention, risk factors and/or misconceptions were assessed in five studies [57, 59, 60, 62, 63]. Three of the five studies reported a significant increase on HIV/AIDS related knowledge [59, 62, 63]. Jemmott et al. [62] reported a mean difference of 2.78 (95% CI 2.22–3.35; p<0.001), whilst Burnett et al [59] reported significant differences between the intervention and control groups regarding overall HIV knowledge (β=0.08, SE=0.02, p=0.001) and Rusakaniko showed an upward but non-significant trend in HIV/AIDS related knowledge [57].
Of the three studies that measured HIV risk perception [58, 60, 63], Agha et al, showed that those in the intervention group were 0.68 times as likely as the control group to report that there was no chance of contracting HIV (aOR=0.68 95% CI 0.56–0.83) [58]. For knowledge and normative beliefs about abstinence, Agha showed that students receiving the peer sexual health intervention compared to the control groups were over three times more likely (aOR=3.42, 95% CI: 1.01–11.54) to report that they had ever heard of abstinence; almost four times more likely to report that a person can avoid HIV by abstaining from sex (aOR= 3.89, 95% CI: 2.21–6.85); over two times more likely to believe that it was normal for a women to propose abstinence (aOR=2.28, 95% CI: 1.11–4.70) and approximately two times more likely to believe that it is normal for a man to propose abstinence (aOR=1.96, 95% CI: 1.06–3.62). However, knowledge of students to express that some people find it difficult to abstain from sex (aOR=1.25, 95% CI: 0.73–2.15) and that abstinence was effective in preventing STIs (OR=1.72, 95% CI: 0.79–3.72), HIV (OR=1.38, 95% CI: 0.62–3.10) and pregnancy (OR=1.73, 95% CI: 0.82–3.66) were not significantly different to the control group [58]. Similarly, Burnett et al reported the intervention effect for self-efficacy scales for abstinence was significantly increased (β=0.14, SE=0.04, p=0.001) [59]. Overall, all studies found significant intervention effects on improving HIV related knowledge, normative beliefs in condom use, and positive attitudes toward HIV testing.
Whilst studies examined changes in attitudes and perception in sexual risk behaviors, a single study examined outcomes five years post intervention and found declines in sexual debut (14.3% vs 23.8%, χ2=4.26, p=0.04), ever married (11.2% vs 23.6%, χ2=7.23, p=0.01) and school dropouts (10.6% vs 29.4%, χ2=16.4, p<0.01), whilst improvements in years of schooling (mean 9.51 years vs 8.74 years, χ2=−4.9, p<0.01), meals per day (mean 2.66 vs 2.30, χ2=3.02, p<0.01) and health-related quality of life (mean 0.82 vs 0.79, p=0.03) were observed [61]. Using pre and post analysis Burnett et al reported successful increase in HIV testing in the intervention group and protective behaviors of getting an HIV test [59], improved knowledge in area of sexual and reproductive health [57, 65]. However, one study found no effect of the interventions on self-esteem, body image, or gender equitable norms [65]. Whilst a single study found no differences between the intervention and control groups in sexual risk behaviors, the study found that participants in the intervention group were less likely to report intimate partner violence victimisation (35.1% vs 40.9%, OR= 0.77, 95% CI:0.61–0.99) suggestive of safer intimate partnerships which could potentially reduce the risk for HIV [63].
Biological Outcomes
Of the nine studies, one study reported on curable STIs and HSV-2 [62], whilst the second study reported on HSV-2 and HIV [61]. Of the study that reported on curable STIs (C. trachomatis, N. gonorrhoeae, and T. vaginalis) and HSV-2 [62], among the sexually experienced students, prevalence of curable STIs was 21.1% (n=123) at 42 months follow-up. Despite students provided with treatment for curable STIs and counselled on safer sex practices; at the 54-month follow-up, 19.6% (n=119) tested positive for curable STIs. Whilst the intervention effect on curable STIs averaged over the 42 and 54 month follow-up showed no significant effect (OR=0.91, 95% CI 0.73–1.12) [62], the intervention reduced curable STIs at 42 months follow-up (OR=0.71, 95% CI 0.54–0.95) but not at the 54 month follow-up (OR=1.15, 95% CI 0.84–1.57) [62]. Furthermore, HSV-2 serostatus showed no significant intervention or interaction effect (p>0.66) [62]. Similarly, adjusting for the interaction of the intervention and time, the intervention’s effects on curable STIs and HSV-2 was non-significant (p>0.18). The second study that measured and reported on HSV-2 and HIV [61], found no significant differences between the intervention and control groups for HSV-2 (aOR=1.46, 95% CI 0.50–4.26) or for HIV (aOR=1.15, 95% CI 0.47–2.79). Whilst the study was designed to measure HIV, due to the low HIV prevalence among students the study did not have sufficient power to detect the intervention effect on HIV infection prevention [61]. Two studies that measured pregnancy as an outcome measure [57, 61], showed significant improvements in knowledge on family planning and contraception (χ2=5.67, p=0.017) with a corresponding significant decreasing linear trend on pregnancy risk (χ2=10.42, p=0.001) [57] and significant reduction in being ever pregnant five years post intervention (11.8% vs 22.2%, χ2=5.60, p=0.02) among orphans [61].
Discussion
The systematic review yielded nine studies from SSA countries that assessed school-based interventions designed to reduce sexual risk behaviors, STIs, HSV-2, HIV and unintended pregnancies [57–65]. These studies are important especially in settings with an unprecedented burden of STIs, HSV-2, HIV including and adolescent pregnancies [72]. Furthermore, schools provide an opportunity to maximize exposure to interventions at a much younger age within a structured yet stimulating setting that could be accessible, safe, and comfortable for a positive impact. Whilst the region has made considerable progress in enrolling young adolescents into schools, multiple complex and diverse structural, social, behavioral and biological cofactors play a major role in accessing high quality education [1] and more importantly with over 50% of girls not progressing beyond primary school education [73, 74] or completing high school [16, 27], which potentially contributes to future risk-taking behaviors. Despite the potential to deliver interventions within schools, the intervention effects may be diminished by the quality of schools often characterized by poor infrastructure, overcrowded classrooms and relatively poor educational outcomes which further perpetuates the challenges faced by young people.
Several important insights and themes have emerged from this review. The intervention effects showed that there was positive impact on selected behavioral outcomes, specifically delaying sexual debut [64], improved knowledge on condoms [62, 63], self-efficacy for condom use [59, 60, 62, 63], condom use [64], abstinence self-efficacy [59], knowledge on HIV/AIDS [59, 62] and HIV risk perception [58]. Earlier systematic reviews found that whilst knowledge and attitudes may improve [30, 38], behaviors that modify risk in the longer term are challenging to sustain. Therefore, it is important that these interventions are sustained beyond the study period and benefit young adolescents transitioning to adulthood in the longer term. It is important to note that in this review only three RCTs were conducted for a relatively long duration (3–5 years) [61–63]. These findings underscore the need for comprehensive packages of interventions designed to be desirable for young adolescents to enable reducing risk taking behaviors and risk of acquiring STIs including HIV.
Studies with a positive effect on outcome(s) provided intervention packages grounded on a theoretical framework, offered training to program facilitator, and/or included an HIV educational component. However, the lack of consistency in the design, intervention packages and measurement of outcomes in the studies from SSA highlight the importance of robustly designed RCTs to inform and guide the design of interventions in settings that have the highest HIV burden [12]. Several studies on the implementation of comprehensive behavioral risk reduction programs for adolescents showed reduced risky sexual behavior, reduced HIV and STI acquisition and unintended adolescent pregnancies, though this evidence emanates from non-clinical trials and have been reviewed extensively [30, 36–38, 40–42, 75, 76]. Interventions have either had no effect in changing sexual behaviors [38, 77], or have influenced changes in knowledge and attitudes [30]. Thus, the consideration for interventions is the inclusion of economic and educational risk factors [78, 79] that potentially influence risk taking behaviors are clearly important. In the absence of biological measurements, several large studies have demonstrated increasing risk-taking behaviors predispose to STIs and HIV [39, 80] suggesting that interventions including comprehensive sexual and reproductive health programs are urgently needed, to reach younger adolescents and to be sustained to protect young adolescents in preparation for safe adulthood [1, 16, 81].
Biologically, females are at higher susceptibility to acquiring HIV through vaginal sexual intercourse [10, 82–85], heightening their vulnerability for STI and HIV acquisition. There are also marked differences between young boys and girls in terms of sexual debut, age-disparate sex, transactional sex, high levels of longer term multiple concurrent sexual partnerships, low condom use, inconsistent condom use with casual rather than with longer term partners and STIs, contributing to enhancing risk and potential spread of STIs and HIV [86–90]. Young women are also susceptible to gender inequalities [27, 91], gender-based violence [92] and might have restricted access to healthcare [93], in particular in terms of access to sexual and reproductive services [94–96]. This systematic review found no long-term impact of the interventions on curable STIs, HSV-2 and HIV [61], although there was evidence of improvements in reducing adolescent pregnancies and, in several risk-taking behaviors. Thus, comprehensive interventions incentivized with conditionalities may have an added advantage to overall improvements in reducing STIs, HSV-2, HIV and adolescent pregnancies including reducing sexual risk behaviors, [14, 49, 82, 97, 98].
Strengths and limitations
The strength of this systematic review was the inclusion of RCTs delivered within a school setting and therefore expected to achieve maximum coverage and exposure of the interventions within confined settings such as schools. However, some RCTs may still suffer from biases, limiting the strength of evidence observed, they nevertheless are in the hierarchy of study designs that provide the highest level of evidence as they are designed to be unbiased and have low risk of systematic errors. We have shown that the heterogeneity in the methodology, ascertainment of outcomes, implementation of interventions and mixed effect outcomes presented challenges in comparison of findings. Nevertheless, the RCTs included in this systematic review allowed us to review the evidence, identify gaps for the design and measurement of future studies.
A key limitation was the evaluation of studies with incompatible questionnaires to ensure reasonable and fair comparison of outcome measures. Furthermore, there was heterogeneity in the comparison group. One study conducted the comparison analysis between pre and post intervention outcomes and did not analyze differences between the control and intervention groups, even though the study was designed as an RCT [59]. Moreover, selection of control participants may also present challenges. For instance, one study placed students who chose not to participate into the control group [60]. Thus, RCTs that placed refusals into the control group are susceptible to bias since blinding might have been less likely to be maintained [71]. Moreover, there was considerable variation in the duration of the interventions with the shortest study duration being nine weeks [64] and the longest being five years [61], which leads to question both the short and long-terms effects of the interventions.
Conclusions
This systematic review provides evidence on the successes and the limitations on the effectiveness of school-based interventions. Whilst HIV/AIDS related knowledge improved with declines in sexual risk behaviors and unintended pregnancies, the interventions had no effect on reducing curable STIs, HSV-2 and HIV. With about a third of young people in schools already sexually active; there is an urgent need for interventions for young adolescents who are yet sexually inexperienced will be critical to guide their decision-making process as they transition to sexual debut and towards being sexually experienced.
Recommendations
Future research studies testing the effectiveness of interventions among young adolescents in school settings should be carefully designed and guided by theoretical framework, potentially include comparable questionnaires for behavioral and biological measurements [99, 100] and be of reasonably long duration for more realistic communication of sustainable behavior change.
Supplementary Material
Acknowledgments
This work was supported by the joint South Africa-US Program for Collaborative Biomedical Research from the National Institutes of Health (R01HD083343). Nosipho Shangase was supported by the joint South Africa-US Program for Collaborative Biomedical Research from the National Institutes of Health (R01HD083343) and the South African Department of Science and Innovation and the National Research Foundation’s Centre of Excellence in HIV Prevention (Grant 96354). N. Ntombela and ABM. Kharsany received support from the South African Department of Science and Innovation and the National Research Foundation’s Centre of Excellence in HIV Prevention (Grant 96354). We sincerely thank Professor Charles Poole from the Department of Epidemiology, Gillings School of Global Public Health, University of North Carolina, Chapel Hill, USA for support with the analysis.
Footnotes
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical Approval
This article does not contain any studies with human participants or animals performed by any of the authors.
Publisher's Disclaimer: Disclaimer
Publisher's Disclaimer: The contents of this publication are those of the authors and do not necessarily represent the official position of the funding agencies.
References
- 1.Joint United Nations Programme on HIV/AIDS (UNAIDS). The Gap Report ISBN 978-92-9253-062-4 2014, Available at: http://www.unaids.org/en/media/unaids/contentassets/documents/unaidspublication/2014/UNAIDS_Gap_report_en.pdf Date accessed 05 April 2019.
- 2.South African National Department of Health.: The 2015 National Antenatal Sentinel HIV and Syphilis Survey Report, South Africa. 2017, Available at www.health.gov.za/…/2015…/2015…/2015-04-30-08-21-56?…2015…hiv…survey… Date accessed 15 May 2019.
- 3.Joint United Nations Programme on HIV/AIDS (UNAIDS). Global AIDS Update, 2016 2016, Available at: http://www.unaids.org/sites/default/files/media_asset/global-AIDS-update-2016_en.pdf: Date accessed 05 April 2019.
- 4.United Nations.: The Millennium Development Goals Report 2015. 2015, Available at https://www.un.org/millenniumgoals/2015_MDG_Report/pdf/MDG%202015%20rev%20(July%201).pdf: Date accessed 04 April 2019.
- 5.World Health Organization.: Global health sector strategy on Sexually Transmitted Infections 2016–2021: Towards ending STIs. 2016, Available at: https://www.who.int/reproductivehealth/publications/rtis/ghss-stis/en/: Accessed 4 May 2019.
- 6.Kharsany AB, Abdool Karim Q: HIV Infection and AIDS in Sub-Saharan Africa: Current Status, Challenges and Opportunities. Open AIDS J 2016, 10:34–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.World Health Organization.: Progress report 2016: prevent HIV, test and treat all: WHO support for country impact. 2016, Available at https://apps.who.int/iris/handle/10665/251713(WHO/HIV/2016.24): Date accessed 4 April 2019 [Google Scholar]
- 8.Gouws E, Stanecki KA, Lyerla R, Ghys PD: The epidemiology of HIV infection among young people aged 15–24 years in southern Africa. AIDS 2008, 22 Suppl 4:S5–16. [DOI] [PubMed] [Google Scholar]
- 9.Shisana O, Rehle T, Simbayi LC et al. : South African National HIV Prevalence, Incidence and Behaviour Survey, 2012. Cape Town, HSRC Press. 2014, Available at: http://www.hsrc.ac.za/uploads/pageContent/4565/SABSSM%20IV%20LEO%20final.pdf: Date accessed 20 August 2019. [Google Scholar]
- 10.Abdool Karim Q, Sibeko S, Baxter C: Preventing HIV Infection in Women: A global Health Imperative. Clin Infect Dis 2010, 50 (Supplement_3):S122–S129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Pettifor AE, Rees HV, Kleinschmidt I et al. : Young people’s sexual health in South Africa: HIV prevalence and sexual behaviors from a nationally representative household survey. AIDS 2005, 19(14):1525–1534. [DOI] [PubMed] [Google Scholar]
- 12.Human Sciences Research Council.: The Fifth South African National HIV Prevalence, Incidence, Behaviour And Communication Survey, 2017 (SABSSM V) 2018, Available at: http://www.hsrc.ac.za/uploads/pageContent/9234/SABSSMV_Impact_Assessment_Summary_ZA_ADS_cleared_PDFA4.pdf. : Date accessed 15 March 2019.
- 13.Kharsany ABM, Cawood C, Khanyile D et al. : Community-based HIV prevalence in KwaZulu-Natal, South Africa: results of a cross-sectional household survey. Lancet HIV 2018, 5(8):e427–e437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Abdool Karim Q, Kharsany AB, Leask K et al. : Prevalence of HIV, HSV-2 and pregnancy among high school students in rural KwaZulu-Natal, South Africa: a bio-behavioural cross-sectional survey. Sex Transm Infect 2014, 90(8):620–626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kharsany AB, Mlotshwa M, Frohlich JA et al. : HIV prevalence among high school learners - opportunities for schools-based HIV testing programmes and sexual reproductive health services. BMC Public Health 2012, 12:231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kharsany AB, Buthelezi TJ, Frohlich JA et al. : HIV infection in high school students in rural South Africa: role of transmissions among students. AIDS Res Hum Retroviruses 2014, 30(10):956–965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Francis SC, Holm Hansen C, Irani J et al. : Results from a cross-sectional sexual and reproductive health study among school girls in Tanzania: high prevalence of bacterial vaginosis. Sex Transm Infect 2019, 95(3):219–227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Francis SC, Mthiyane TN, Baisley K et al. : Prevalence of sexually transmitted infections among young people in South Africa: A nested survey in a health and demographic surveillance site. PLoS Med 2018, 15(2):e1002512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Torrone EA, Morrison CS, Chen PL et al. : Prevalence of sexually transmitted infections and bacterial vaginosis among women in sub-Saharan Africa: An individual participant data meta-analysis of 18 HIV prevention studies. PLoS Med 2018, 15(2):e1002511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kharsany AB, McKinnon LR, Lewis L et al. : Population Prevalence of sexually transmitted infections in a high HIV burden district in KwaZulu-Natal, South Africa: Implications for HIV epidemic control. Int J Infect Dis 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Patton GC, Sawyer SM, Santelli JS et al. : Our future: a Lancet commission on adolescent health and wellbeing. Lancet 2016, 387(10036):2423–2478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Blakemore SJ, Mills KL: Is adolescence a sensitive period for sociocultural processing? Annu Rev Psychol 2014, 65:187–207. [DOI] [PubMed] [Google Scholar]
- 23.Sanders RA: Adolescent Psychosocial, Social, and Cognitive Development. Pediatrics in Review 2013, 34(8):354–359. [DOI] [PubMed] [Google Scholar]
- 24.United Nations Childrens Fund.: Opportunity in Crisis: Preventing HIV from early adolescence to young adulthood. 2011, Available at https://www.unicef.org/media/files/Opportunity_in_Crisis_LoRes_EN_05182011.pdf: Date accessed 05 April 2019
- 25.Pettifor AE, van der Straten A, Dunbar MS, Shiboski SC, Padian NS: Early age of first sex: a risk factor for HIV infection among women in Zimbabwe. AIDS 2004, 18(10):1435–1442. [DOI] [PubMed] [Google Scholar]
- 26.Coates TJ, Richter L, Caceres C: Behavioural strategies to reduce HIV transmission: how to make them work better. Lancet 2008, 372(9639):669–684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Pettifor AE, Levandowski BA, MacPhail C, Padian NS, Cohen MS, Rees HV: Keep them in school: the importance of education as a protective factor against HIV infection among young South African women. Int J Epidemiol 2008, 37(6):1266–1273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Eaton L, Flisher AJ, Aaro LE: Unsafe sexual behaviour in South African youth. Soc Sci Med 2003, 56(1):149–165. [DOI] [PubMed] [Google Scholar]
- 29.Macleod CI, Tracey T: A Decade Later: Follow-Up Review of South African Research on the Consequences of and Contributory Factors in Teen-Aged Pregnancy. S Afr J Psychol 2010, 40(1):18–31. [Google Scholar]
- 30.Paul-Ebhohimhen VA, Poobalan A, van Teijlingen ER: A systematic review of school-based sexual health interventions to prevent STI/HIV in sub-Saharan Africa. BMC Public Health 2008, 8:4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Jones A, Cremin I, Abdullah F et al. : Transformation of HIV from pandemic to low-endemic levels: a public health approach to combination prevention. Lancet 2014, 384(9939):272–279. [DOI] [PubMed] [Google Scholar]
- 32.Taylor M, Dlamini SB, Meyer-Weitz A, Sathiparsad R, Jinabhai CC, Esterhuizen T: Changing sexual behaviour to reduce HIV transmission - a multi-faceted approach to HIV prevention and treatment in a rural South African setting. AIDS Care 2010, 22(11):1395–1402. [DOI] [PubMed] [Google Scholar]
- 33.Birdthistle I, Schaffnit SB, Kwaro D et al. : Evaluating the impact of the DREAMS partnership to reduce HIV incidence among adolescent girls and young women in four settings: a study protocol. BMC Public Health 2018, 18(1):912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.George G, Cawood C, Puren A et al. : Evaluating DREAMS HIV prevention interventions targeting adolescent girls and young women in high HIV prevalence districts in South Africa: protocol for a cross-sectional study. BMC Womens Health 2020, 20(1):7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Subedar H, Barnett S, Chaka T et al. : Tackling HIV by empowering adolescent girls and young women: a multisectoral, government led campaign in South Africa. BMJ 2018, 363:k4585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Chen L, Jha P, Stirling B et al. : Sexual risk factors for HIV infection in early and advanced HIV epidemics in sub-Saharan Africa: systematic overview of 68 epidemiological studies. PLoS One 2007, 2(10):e1001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Cowan F, Pettifor A: HIV in adolescents in sub-Saharan Africa. Curr Opin HIV AIDS 2009, 4(4):288–293. [DOI] [PubMed] [Google Scholar]
- 38.Gallant M, Maticka-Tyndale E: School-based HIV prevention programmes for African youth. Soc Sci Med 2004, 58(7):1337–1351. [DOI] [PubMed] [Google Scholar]
- 39.Harrison A, Newell ML, Imrie J, Hoddinott G: HIV prevention for South African youth: which interventions work? A systematic review of current evidence. BMC Public Health 2010, 10:102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Mason-Jones AJ, Sinclair D, Mathews C, Kagee A, Hillman A, Lombard C: School-based interventions for preventing HIV, sexually transmitted infections, and pregnancy in adolescents. Cochrane Database Syst Rev 2016, 11:CD006417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Michielsen K, Temmerman M, Van Rossem R: Limited effectiveness of HIV prevention for young people in sub-Saharan Africa: studying the role of intervention and evaluation. Facts Views Vis Obgyn 2013, 5(3):196–208. [PMC free article] [PubMed] [Google Scholar]
- 42.Sani AS, Abraham C, Denford S, Ball S: School-based sexual health education interventions to prevent STI/HIV in sub-Saharan Africa: a systematic review and meta-analysis. BMC Public Health 2016, 16(1):1069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Peinemann F, Tushabe DA, Kleijnen J: Using multiple types of studies in systematic reviews of health care interventions--a systematic review. PloS one 2013, 8(12):e85035–e85035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Liberati A, Altman DG, Tetzlaff J et al. : The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: explanation and elaboration. PLoS Med 2009, 6(7):e1000100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Higgins JTP, Green S: Cochrane Handbook for Systematic Reviews of Interventions: Cochrane Collaboration; 2008.
- 46.Moher D, Liberati A, Tetzlaff J, Altman DG, Group P: Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med 2009, 6(7):e1000097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Michielsen K, Beauclair R, Delva W, Roelens K, Van Rossem R, Temmerman M: Effectiveness of a peer-led HIV prevention intervention in secondary schools in Rwanda: results from a non-randomized controlled trial. BMC Public Health 2012, 12:729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Dunbar MS, Kang Dufour MS, Lambdin B, Mudekunye-Mahaka I, Nhamo D, Padian NS: The SHAZ! project: results from a pilot randomized trial of a structural intervention to prevent HIV among adolescent women in Zimbabwe. PLoS One 2014, 9(11):e113621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Pettifor A, MacPhail C, Hughes JP et al. : The effect of a conditional cash transfer on HIV incidence in young women in rural South Africa (HPTN 068): a phase 3, randomised controlled trial. Lancet Glob Health 2016, 4(12):e978–e988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Duflo E, Dupas P, Ginn T et al. : HIV prevention among youth: A randomized controlled trial of voluntary counseling and testing for HIV and male condom distribution in rural Kenya. PLoS One 2019, 14(7):e0219535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Musiimenta A: A Controlled Pre-Post Evaluation of a Computer-based HIV/AIDS Education on Students’ Sexual Behaviors, Knowledge and Attitudes. Online J Public Health Inform 2012, 4(1). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Heeren GA, Jemmott JB 3rd, Ngwane Z, Mandeya A, Tyler JC: A randomized controlled pilot study of an HIV risk-reduction intervention for sub-Saharan African university students. AIDS Behav 2013, 17(3):1105–1115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Bell CC, Bhana A, Petersen I et al. : Building protective factors to offset sexually risky behaviors among black youths: a randomized control trial. J Natl Med Assoc 2008, 100(8):936–944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Abdool Karim Q, Leask K, Kharsany A et al. : Impact of conditional cash incentives on HSV-2 and HIV prevention in rural South African high school students: results of the CAPRISA 007 cluster randomized controlled trial. In: 8th International AIDS Society Conference on HIV Pathogenesis Treatment and Prevention Vancouver, Canada 18–22 July 2015 2015, Abstract number: TUAC0101LB (Available at: http://www.abstract-archive.org/): Date accessed 20 Dec 2019. [Google Scholar]
- 55.Veritas Health Innovation Melbourne Australia.: Covidence systematic review software. Available at www.covidence.org.
- 56.Sandoy IF, Mudenda M, Zulu J et al. : Effectiveness of a girls’ empowerment programme on early childbearing, marriage and school dropout among adolescent girls in rural Zambia: study protocol for a cluster randomized trial. Trials 2016, 17(1):588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Rusakaniko S, Mbizvo MT, Kasule J et al. : Trends in reproductive health knowledge following a health education intervention among adolescents in Zimbabwe. Cent Afr J Med 1997, 43(1):1–6. [PubMed] [Google Scholar]
- 58.Agha S: An evaluation of the effectiveness of a peer sexual health intervention among secondary-school students in Zambia. AIDS Educ Prev 2002, 14(4):269–281. [DOI] [PubMed] [Google Scholar]
- 59.Burnett SM, Weaver MR, Mody-Pan PN, Thomas LA, Mar CM: Evaluation of an intervention to increase human immunodeficiency virus testing among youth in Manzini, Swaziland: a randomized control trial. J Adolesc Health 2011, 48(5):507–513. [DOI] [PubMed] [Google Scholar]
- 60.Atwood KA, Kennedy SB, Shamblen S et al. : Impact of school-based HIV prevention program in post-conflict Liberia. AIDS Educ Prev 2012, 24(1):68–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Hallfors DD, Cho H, Rusakaniko S et al. : The impact of school subsidies on HIV-related outcomes among adolescent female orphans. J Adolesc Health 2015, 56(1):79–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Jemmott JB 3rd, Jemmott LS, O’Leary A et al. : HIV/STI risk-reduction intervention efficacy with South African adolescents over 54 months. Health Psychol 2015, 34(6):610–621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Mathews C, Eggers SM, Townsend L et al. : Effects of PREPARE, a Multi-component, School-Based HIV and Intimate Partner Violence (IPV) Prevention Programme on Adolescent Sexual Risk Behaviour and IPV: Cluster Randomised Controlled Trial. AIDS Behav 2016, 20(9):1821–1840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Mmbaga EJ, Kajula L, Aaro LE et al. : Effect of the PREPARE intervention on sexual initiation and condom use among adolescents aged 12–14: a cluster randomised controlled trial in Dar es Salaam, Tanzania. BMC Public Health 2017, 17(1):322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Kemigisha E, Bruce K, Ivanova O et al. : Evaluation of a school based comprehensive sexuality education program among very young adolescents in rural Uganda. BMC Public Health 2019, 19(1):1393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Flemming K, Booth A, Hannes K, Cargo M, Noyes J: Cochrane Qualitative and Implementation Methods Group guidance series-paper 6: reporting guidelines for qualitative, implementation, and process evaluation evidence syntheses. J Clin Epidemiol 2018, 97:79–85. [DOI] [PubMed] [Google Scholar]
- 67.Higgins JP, Altman DG, Gotzsche PC et al. : The Cochrane Collaboration’s tool for assessing risk of bias in randomised trials. BMJ 2011, 343:d5928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Jemmott JB 3rd, Jemmott LS, O’Leary A et al. : School-based randomized controlled trial of an HIV/STD risk-reduction intervention for South African adolescents. Arch Pediatr Adolesc Med 2010, 164(10):923–929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Atwood KA, Kennedy SB, Shamblen S et al. : Reducing sexual risk taking behaviors among adolescents who engage in transactional sex in post-conflict Liberia. Vulnerable Child Youth Stud 2012, 7(1):55–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Lavrakas PJ: Encyclopedia of Survey Research Methods, vol. Available at https://books.google.co.za/books?id=_jxQswEACAAJ: SAGE Publications; 2008. [Google Scholar]
- 71.Kendall JM: Designing a research project: randomised controlled trials and their principles. Emerg Med J 2003, 20(2):164–168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Joint United Nations Programme on HIV/AIDS (UNAIDS). Implementation of the HIV Prevention 2020 road map: First progress report, March 2018. 2018, Available at https://www.unaids.org/sites/default/files/media_asset/jc2927_hiv-prevention-2020-road-map-first-progress-report_en.pdf Date accessed 03 April 2019.
- 73.Sperling G, Winthrop R: What Works in Girls’ Education: Evidence for the World’s Best Investments Council on Foreign Relations 2016, Available at https://books.google.co.za/books?id=1yfECgAAQBAJ&printsec=frontcover&dq=hat+works+in+girls%27+education:+Evidence+and+policies+from+the+developing+world&hl=en&sa=X&ved=0ahUKEwiRr7zN2aPoAhWSxIUKHUz1CjQQ6AEIMzAB: Date accessed 16 Sept 2019.
- 74.Hallfors D, Cho H, Rusakaniko S, Iritani B, Mapfumo J, Halpern C: Supporting adolescent orphan girls to stay in school as HIV risk prevention: evidence from a randomized controlled trial in Zimbabwe. Am J Public Health 2011, 101(6):1082–1088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Harrison A: HIV prevention and research considerations for women in sub-Saharan Africa: moving toward biobehavioral prevention strategies. Afr J Reprod Health 2014, 18(3 Spec No):17–24. [PMC free article] [PubMed] [Google Scholar]
- 76.Operario D, Underhill K, Chuong C, Cluver L: HIV infection and sexual risk behaviour among youth who have experienced orphanhood: systematic review and meta-analysis. J Int AIDS Soc 2011, 14:25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Menna T, Ali A, Worku A: Effects of peer education intervention on HIV/AIDS related sexual behaviors of secondary school students in Addis Ababa, Ethiopia: a quasi-experimental study. Reprod Health 2015, 12:84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Thurman TR, Kidman R, Carton TW, Chiroro P: Psychological and behavioral interventions to reduce HIV risk: evidence from a randomized control trial among orphaned and vulnerable adolescents in South Africa. AIDS Care 2016, 28 Suppl 1:8–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Ivanova O, Rai M, Kemigisha E: A Systematic Review of Sexual and Reproductive Health Knowledge, Experiences and Access to Services among Refugee, Migrant and Displaced Girls and Young Women in Africa. Int J Environ Res Public Health 2018, 15(8). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Johnson BT, Scott-Sheldon LA, Huedo-Medina TB, Carey MP: Interventions to reduce sexual risk for human immunodeficiency virus in adolescents: a meta-analysis of trials, 1985–2008. Arch Pediatr Adolesc Med 2011, 165(1):77–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Kharsany AB, Frohlich JA, Yende-Zuma N et al. : Trends in HIV Prevalence in Pregnant Women in Rural South Africa. J Acquir Immune Defic Syndr 2015, 70(3):289–295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Dellar RC, Dlamini S, Karim QA: Adolescent girls and young women: key populations for HIV epidemic control. J Int AIDS Soc 2015, 18(2 Suppl 1):19408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Pomerantz RJ, de la Monte SM, Donegan SP et al. : Human immunodeficiency virus (HIV) infection of the uterine cervix. Ann Intern Med 1988, 108(3):321–327. [DOI] [PubMed] [Google Scholar]
- 84.Abdool Karim SS, Baxter C, Passmore JS, McKinnon LR, Williams BL: The genital tract and rectal microbiomes: their role in HIV susceptibility and prevention in women. J Int AIDS Soc 2019, 22(5):e25300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Mwatelah R, McKinnon LR, Baxter C, Abdool Karim Q, Abdool Karim SS: Mechanisms of sexually transmitted infection-induced inflammation in women: implications for HIV risk. J Int AIDS Soc 2019, 22(S6):e25346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.de Oliveira T, Kharsany AB, Graf T et al. : Transmission networks and risk of HIV infection in KwaZulu-Natal, South Africa: a community-wide phylogenetic study. Lancet HIV 2017, 4(1):e41–e50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Maughan-Brown B, George G, Beckett S et al. : HIV Risk Among Adolescent Girls and Young Women in Age-Disparate Partnerships: Evidence From KwaZulu-Natal, South Africa. J Acquir Immune Defic Syndr 2018, 78(2):155–162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Maughan-Brown B, Venkataramani A, Kharsany ABM et al. : Recently formed age-disparate partnerships are associated with elevated HIV-incidence among young women in South Africa. AIDS 2020, 34(1):149–154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Schaefer R, Gregson S, Eaton JW et al. : Age-disparate relationships and HIV incidence in adolescent girls and young women: evidence from Zimbabwe. AIDS 2017, 31(10):1461–1470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Stoner MCD, Edwards JK, Miller WC et al. : Effect of Schooling on Age-Disparate Relationships and Number of Sexual Partners Among Young Women in Rural South Africa Enrolled in HPTN 068. J Acquir Immune Defic Syndr 2017, 76(5):e107–e114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Leclerc-Madlala S: Age-disparate and intergenerational sex in southern Africa: the dynamics of hypervulnerability. AIDS 2008, 22 Suppl 4:S17–25. [DOI] [PubMed] [Google Scholar]
- 92.Abdool Karim Q, Baxter C: The dual burden of gender-based violence and HIV in adolescent girls and young women in South Africa. South African medical journal = Suid-Afrikaanse tydskrif vir geneeskunde 2016, 106(12):1151–1153. [DOI] [PubMed] [Google Scholar]
- 93.Joint United Nations Programme on HIV/AIDS (UNAIDS). UNAIDS 2016 | GUIDANCE HIV prevention among adolescent girls and young women. Putting HIV prevention among adolescent girls and young women on the Fast-Track and engaging men and boys. 2016, Available at https://www.unaids.org/sites/default/files/media_asset/UNAIDS_HIV_prevention_among_adolescent_girls_and_young_women.pdf: Date accessed 16 Sept 2019. [Google Scholar]
- 94.Lince-Deroche N, Berry KM, Hendrickson C, Sineke T, Kgowedi S, Mulongo M: Women’s costs for accessing comprehensive sexual and reproductive health services: findings from an observational study in Johannesburg, South Africa. Reprod Health 2019, 16(1):179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Lince-Deroche N, Hargey A, Holt K, Shochet T: Accessing Sexual and Reproductive Health Information and Services: A Mixed Methods Study of Young Women’s Needs and Experiences in Soweto, South Africa. Afr J Reprod Health 2015, 19(1):73–81. [PubMed] [Google Scholar]
- 96.Maharaj P, Cleland J: Integration of sexual and reproductive health services in KwaZulu-Natal, South Africa. Health Policy Plan 2005, 20(5):310–318. [DOI] [PubMed] [Google Scholar]
- 97.Baird SJ, Garfein RS, McIntosh CT, Ozler B: Effect of a cash transfer programme for schooling on prevalence of HIV and herpes simplex type 2 in Malawi: a cluster randomised trial. Lancet 2012, 379(9823):1320–1329. [DOI] [PubMed] [Google Scholar]
- 98.Abdool Karim Q, Kharsany AB, Frohlich JA et al. : HIV incidence in young girls in KwaZulu-Natal, South Africa--public health imperative for their inclusion in HIV biomedical intervention trials. AIDS Behav 2012, 16(7):1870–1876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Hargreaves JR, Delany-Moretlwe S, Hallett TB et al. : The HIV prevention cascade: integrating theories of epidemiological, behavioural, and social science into programme design and monitoring. Lancet HIV 2016, 3(7):e318–322. [DOI] [PubMed] [Google Scholar]
- 100.Shackleton N, Jamal F, Viner RM, Dickson K, Patton G, Bonell C: School-Based Interventions Going Beyond Health Education to Promote Adolescent Health: Systematic Review of Reviews. J Adolesc Health 2016, 58(4):382–396. [DOI] [PubMed] [Google Scholar]
- 101.Kennedy SB, Atwood KA, Harris AO, Taylor CH, Gobeh ME, Quaqua M, Woods DV, Bee EM, Warlonfa M. HIV/STD risk behaviors among in-school adolescents in post-conflict Liberia. J Assoc Nurses AIDS Care. 2012;23(4):350–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Iritani BJ, Cho H, Rusakaniko S, Mapfumo J, Hartman S, Hallfors DD. Educational outcomes for orphan girls in rural Zimbabwe: effects of a school support intervention. Health Care Women Int. 2016;37(3):301–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Jemmott LS, Jemmott JB 3rd, Ngwane Z, Icard L, O’Leary A, Gueits L, Brawner B. ‘Let Us Protect Our Future’ a culturally congruent evidenced-based HIV/STD risk-reduction intervention for young South African adolescents. Health Educ Res. 2014;29(1):166–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.O’Leary A, Jemmott JB 3rd, Jemmott LS, Bellamy S, Ngwane Z, Icard L, Gueits L. Moderation and mediation of an effective HIV risk-reduction intervention for South African adolescents. Ann Behav Med. 2012;44(2):181–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.O’Leary A, Jemmott JB 3rd, Jemmott LS, Teitelman A, Heeren GA, Ngwane Z, Icard LD, Lewis DA. Associations between psychosocial factors and incidence of sexually transmitted disease among South African adolescents. Sex Transm Dis. 2015;42(3):135–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Mathews C, Eggers SM, de Vries PJ, Mason-Jones AJ, Townsend L, Aaro LE, De Vries H. Reaching the hard to reach: longitudinal investigation of adolescents’ attendance at an after-school sexual and reproductive health programme in Western Cape, South Africa. BMC Public Health. 2015;15:608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Aaro LE, Mathews C, Kaaya S, Katahoire AR, Onya H, Abraham C, Klepp KI, Wubs A, Eggers SM, de Vries H. Promoting sexual and reproductive health among adolescents in Southern and Eastern Africa (PREPARE): project design and conceptual framework. BMC Public Health. 2014;14:54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Namisi FS, Aaro LE, Kaaya S, Onya HE, Wubs A, Mathews C. Condom use and sexuality communication with adults: a study among high school students in South Africa and Tanzania. BMC Public Health. 2013;13:874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Kemigisha E, Bruce K, Nyakato VN, Ruzaaza GN, Ninsiima AB, Mlahagwa W, Leye E, Coene G, Michielsen K. Sexual health of very young adolescents in South Western Uganda: a crosssectional assessment of sexual knowledge and behavior. Reprod Health. 2018;15(1):148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Kemigisha E, Ivanova O, Ruzaaza GN, Ninsiima AB, Kaziga R, Bruce K, Leye E, Coene G, Nyakato VN, Michielsen K. Process evaluation of a comprehensive sexuality education intervention in primary schools in South Western Uganda. Sex Reprod Health. 2019;21:51–9. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


