Abstract
In Parkinson’s disease (PD) state, with progressive loss of dopaminergic neurons in the substantia nigra, the striatal dopamine (DA) and glutamate (Glu) levels change, resulting in dysfunction of basal ganglia motor regulation. The PD patient presents motor dysfunction such as resting tremor, bradykinesia, and muscular rigidity. To investigate the mechanism of aerobic exercise to improve PD-related motor dysfunction, in the current study, 6-hydroxydopamine (6-OHDA) was used to induce the PD mice model, and the motor function of PD mice was comprehensively evaluated by open-field test, rotarod test, and gait test. The co-expression of prodynorphin (PDYN) and proenkephalin (PENK) with extracellular signal-regulated kinase (Erk1/2) and phosphorylation Erk1/2 (p-Erk1/2) were detected by double-labeling immunofluorescence. The results showed that a 4-week aerobic exercise intervention could effectively improve the motor dysfunction of PD mice. Moreover, it was found that the expressions of Erk1/2 and p-Erk1/2 in the dorsal striatum (Str) of PD mice were significantly increased, and the number of positive cells co-expressed by Erk1/2, p-Erk1/2, and PENK was significantly higher than PDYN. The above phenomenon was reversed by a 4-week aerobic exercise intervention. Therefore, this study suggests that the mechanism by which aerobic exercise improves PD-related motor dysfunction may be related to that the aerobic exercise intervention alleviates the activity of extracellular signal-regulated kinase/mitogen-activated protein kinases (Erk/MAPK) signaling pathway in striatal medium spiny neurons expressing D2-like receptors (D2-MSNs) of PD mice by regulating the striatal DA and Glu signaling.
Supplementary Information
The online version contains supplementary material available at 10.1007/s00221-022-06360-4.
Keywords: Aerobic exercise, Parkinson’s disease, Motor function, Striatal medium spiny neurons, Erk/MAPK signaling
Introduction
Parkinson’s disease (PD) is a progressive neurodegenerative disease, and its main clinical presentations include resting tremor, bradykinesia, muscular rigidity, abnormal posture and gait, and other behavioral dysfunctions (Olanow et al. 2009). Its pathological features involve degeneration of the nigrostriatal dopamine (DA) pathway and complex physiological changes in the basal ganglia. Decreased striatal DA is accompanied by increased glutamate (Glu), which leads to imbalances in excitatory and inhibitory afferent inputs (Jamwal et al. 2019). Furthermore, the motor symptoms of PD are directly or indirectly related to neurotransmitter imbalances in the brain (Jamwal et al. 2017; Jamwaland and Kumar 2019). Although DA replacement therapy is effective in alleviating the early motor symptoms of PD, it is unable to control the progression of the disease, which eventually results in severe motor dysfunction and cognitive impairments (Coelho et al. 2012; Del Tredici et al. 2016). Surgical treatment, on the other hand, has large side effects and places an immense burden on patients and their families, and a lack of a neuroprotective strategy. Therefore, it remains of crucial significance to find effective measures for the prevention and delay of PD. As early as 1992, Sasco discovered that high-intensity physical exercise can effectively reduce the risk of PD (Sasco et al. 1992). Research has now clearly shown that exercise has a positive effect on improving the behavioral performance and clinical symptoms of PD patients (Muhlack et al. 2007; Slomski 2019). Therefore, a variety of treatment strategies that include exercise and body functional training have been applied to PD management, and exercise therapy has also become an important supplement to PD treatments. Earlier studies have shown that treadmill exercise can effectively improve the motor function of PD rats, while also reducing the overexcitation of the cortico-striatal pathway and Glu levels induced by depletion of striatal DA (Chen et al. 2015, 2017b). This implies that the ability of physical exercise to improve behavioral impairments in PD may be related to its amelioration of striatal neurotransmitter imbalances. However, the exact regulatory mechanisms involved are still unclear. The 95% of striatal neurons were medium spiny neurons (MSNs), which can be divided into two subgroups: one is involved in the direct pathway and expresses dopamine D1-like receptors (D1-MSNs) and the other is involved in the indirect pathway and expresses dopamine D2-like receptors (D2-MSNs). However, the regulatory role and specific molecular mechanism of different subtypes of MSNs in the improvement of PD-related behavioral dysfunction by exercise have not been clarified. It has been confirmed that exercise intervention can improve motor disorders by regulating the imbalance of DA and Glu neurotransmitters in the stream. Extracellular signal-regulated kinase (Erk1/2) is the classic signaling pathway of Mitogen-Activated Protein Kinases (MAPK). It is the key modulation point of Glu from the cerebral cortex and DA from substantia nigra projecting to striatum MSNs to regulate neuronal activity. PD-related motor dysfunction is closely related to abnormal activation of Erk/MAPK signal an imbalance of DA and Glu in the striatum (Mariani et al. 2019). Therefore, we can infer that Erk/MAPK pathway may be involved in the ability of exercise to improve PD behavioral impairments, and its mechanism may be related to the regulation of striatal Glu and DA signaling. Given the above, this study established a unilateral lesion model of PD using 6-hydroxydopamine (6-OHDA), to measure the activity of Erk/MAPK signaling pathways in different striatal MSNs using double-labeling immunofluorescence, which was combined with behavioral tests for the comprehensive evaluation of behavioral performance, thereby further elucidating the regulatory mechanism underlying the improvement of PD behavioral impairments by exercise.
Materials and methods
Animals and group assignment
C57BL6 male mice, weighing 25 ± 2 g, were used in this study, which was provided by Beijing Vital River Laboratory Animal Technology Co., Ltd. All experiments were carried out with the approval of the Life Sciences School Animal Ethics Committee of Hebei Normal University Fig. 1. The animals were housed in separate cages and given free access to food and water under conditions of 12 h alternating light–dark cycles, the temperature of 20–25 ℃, and relative humidity of 45–50%. After acclimatization for a week, the animals were randomly assigned to two groups: saline group (n = 24) and 6-OHDA group (n = 30). After model establishment, the saline group was randomly divided into the control group (C, n = 12) and control plus exercise group (CE, n = 12); while the 6-OHDA group was randomly divided into the Parkinson’s disease group (PD, n = 15) and Parkinson’s disease plus exercise group (PE, n = 15). All animal experiments are approved by the Biomedical Ethics Committee of Hebei normal university.
Fig. 1.
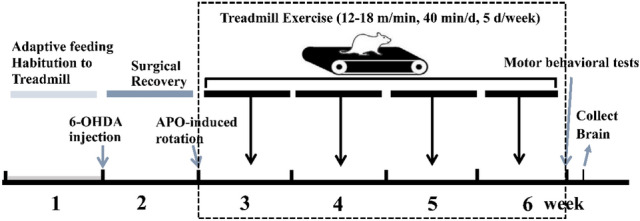
Protocol of the experimental trials
Establishment and evaluation of PD model
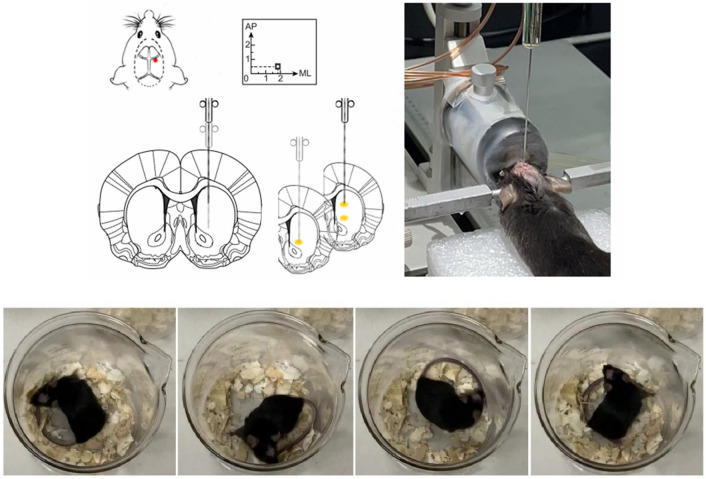
After fasting for 24 h, the mice were induced to deep anesthesia with 3% isoflurane (RWD), fixed on a brain stereotaxic device (RWD) in the flat-skull position, and the animal’s body temperature was maintained using a heating pad. Desipramine (25 mg/kg) was injected intraperitoneally to protect noradrenergic neurons. After 30 min, the skin was disinfected, the epidermis and periosteum were cut, 35% H2O2 was used to remove the surface tissue of the skull, then the anterior 0.5 cm of the frontal suture to the posterior 0.4 cm of the posterior fontanel was exposed. The right medial Str was located based on Franklin’s stereotaxic atlas of the mouse brain. A high-speed stereotaxic drill was used to drill a hole, and 4 μL of 6-OHDA (APExBIO, B 7099) solution was injected into the right Str by 2-point using a microinjector Fig. 2. C and CE mice were injected with the same volume of saline containing 0.02% ascorbic (APExBIO, B 2064) acid using the same protocol. On the seventh day after surgery, the mice were subjected to apomorphine (APO) (APExBIO, B 6936) induced rotation test. APO solution (0.5 µg/g) was injected intraperitoneally, and the number of rotations was recorded after 3 min Fig. 2. A difference of > 100 r between the number of left rotations and the number of right rotations in 30 min was used as the criterion for successful PD model creation.
Fig. 2.
Schematic diagram of PD model establishment and verification
Exercise program
CE and PE mice began treadmill exercise (Anhui Zhenghua) 7 days after being injected with 6-OHDA. The training involved running at 12–18 m/min (12 m/min × 10 min, 14 m/min × 10 min, 16 m/min × 10 min, 18 m/min × 10 min), for 40 min/day, 5 days/week at 16:00–18:00 from Monday to Friday, for 4 weeks consecutive. The exercise intensity was approximately 45%–55% VO2 max (Hoydal et al. 2007). At the same time, mice in the C and PD groups were placed on the stationary treadmill for the same time.
Evaluation of motor behavioral function
Open-field test
The open-field (RWD) test measures the spontaneous locomotor activity of the mice. The mice were placed in a 40 cm × 40 cm × 40 cm opaque box, first allowed to habituate to the laboratory environment for 5 min, after which their free movement was recorded for 10 min. After each experiment uses 95% ethanol solution to clean the bottom and walls for 5 min, data were collected and analyzed using SMART 3.0 software (Panlab, Spain).
Rotarod test
The rotarod (RWD) test evaluates the balance function of mice. The mice were placed in the uniform accelerated rotary rod. The initial speed is 5 rpm/min, and the speed is increased to 40 rpm/min after 300 s. The stay time of the mice on the rotary rod was recorded accurately. The test was performed 3 times a day for 5 consecutive days with an interval of at least 60 min. Clean the rotating stick with 95% alcohol after each experiment.
Gait analysis
The gait analysis (made by myself) evaluates the coordination of mice. The mice were placed in a channel mold of 55 cm × 7 cm. A piece of clean and flat printing paper was laid on the bottom of the mold. In addition, mice were allowed to pass through the channel freely. Different pigments were applied on the front and rear limbs of mice to distinguish the front and rear limbs of the mice. At least three complete steps of each mouse were measured to calculate the average value. Use 95% alcohol to clean passages after each experiment.
Brain slices and tissue extraction were prepared
After fasting for 24 h, the mice were induced to deep anesthesia with 3% isoflurane. Then, the brain tissues were quickly removed through left ventricular perfusion with normal saline (Damao). The striatum was separated on the ice surface, and then stored at -80 ℃. The whole brain of mice was decapitated and placed in 4% paraformaldehyde for 24 h. The whole brain was dehydrated with sucrose solution of different concentrations. After the last dehydration, the whole brain was embedded with a frozen embedding agent and stored at − 80 ℃. The German Leica (CM 1950) frozen slicer with a thickness of 15 μm was used for slicing. All slices were stored at − 80 ℃ after drying.
Molecular experiment
Immunohistochemistry
The expression of tyrosine hydroxylase (TH)-positive fibers in the striatum and the number of TH-positive cells in substantia nigra were detected by immunohistochemistry. 24 h after the last exercise session, the mice were induced to deep anesthesia with 3% isoflurane, followed by perfusion with 37 ℃ physiological saline and 4 ℃ 4% paraformaldehyde via the left ventricle-ascending aorta. The whole brain was removed and fixed in 4% paraformaldehyde for 24 h. Brains were removed and cryoprotected in different concentrations of sucrose in 0.1 M PBS and then frozen in O.C.T. compound. Immunohistochemistry was then performed on 15 μm sections. The slices were first incubated in H2O2 (30%) solution to avoid light for 20 min and then placed in citric acid antigen repair buffer (PH = 6.0) for antigen repair in a microwave oven. Heat the slices for 6 min and bring to a boil. After 2 min, reduce to a low heat for 10 min. After natural cooling, the slides were placed in PBS (PH = 7.4) and washed 3 times, every time 5 min. The sections were blocked with serum and BSA was dropped for 30 min. Add primary antibody (TH 1:1000, Abcam, AB 6211) and incubate overnight at 4 ℃. The second antibody was added to cover the tissues and the cells were incubated at room temperature for 50 min. Wash with PBS (PH = 7.4) for 3 times, 5 min for each time, and then DAB reagent for color rendering. Rinse with tap water for 20 min to terminate the reaction. Then, add alcohol with different concentrations for gradient dehydration, and finally seal the tablet with neutral resin adhesive. Immunohistochemical results were analyzed using Image J software, and positive cell expression of TH in substantia nigra was measured. Integral optical density (IOD) values were measured for TH in the dorsal striatum region.
Western blot
The expression of TH protein in the striatum was detected by Western blot. 24 h after the last exercise session, the mice were induced to deep anesthesia with 3% isoflurane, and the whole brain was quickly removed and stored in liquid nitrogen. The brain tissue was removed from storage during testing, the right striatum was quickly separated on ice, and an appropriate amount of tissue was placed in a pre-cooled homogenizer. After the sample was sufficiently homogenized, an appropriate amount of lysis solution was added for 30 min, then centrifuged at 12,000 r/min for 10 min, and the supernatant was collected for the quantification of protein concentration using the BCA method. The samples then underwent loading, electrophoresis, transfer, blocking, and incubation overnight with diluted monoclonal antibodies (TH: 1:1000, Abcam, AB 6211). Horseradish peroxidase-labeled secondary antibodies were added and incubated for 60 min. ECL working solution was added to the PVDF membrane and incubated at room temperature for 5 min. Then, the PVDF membrane was wrapped in cling film, developed, exposed, washed, and blocked. The β-actin monoclonal antibody was diluted at 1:500 in the primary antibody dilution solution, shaken at room temperature for 60 min, and the internal reference band was obtained according to the method above. The film was analyzed with the Quantity One software (Bio-Rad Laboratories, Inc. California, Berkeley, USA) to obtain the molecular weight and integrated optical density of the target band.
Immunofluorescence
The co-expression of Erk and p-Erk proteins in the striatum with the marker PDYN of D1-MSNs and the marker PENK of D2-MSNs were observed by immunofluorescence double-label technique. PDYN and PENK are precursors of DYN and ENK, respectively. The sections were obtained as described above. Briefly, the slices were placed in citric acid antigen repair buffer (PH = 6.0) for antigen repair in the microwave oven: heat the slices for 6 min and bring to a boil. After 2 min, reduce to a low heat for 10 min. After natural cooling, the slides were washed in PBS (PH = 7.4) 3 times, 5 min each time. Then, the sections were blocked with normal goat serum for 30 min. The primary antibody was then incubated (Erk 1:100, Cell Signaling, 4696; p-Erk 1:200, Cell Signaling 5726; PDYN 1:500, Gene Tex, GTX 113,515; PENK 1:500, Gene Tex, GTX 80,743) at 4 ℃ overnight. After extensive washes in PBS, tissue sections were incubated in Alexa Fluor 488 goat anti-mouse antibody (Immunoway, 1:200, RS 23,210) and Alexa Fluor 549 goat anti-rabbit antibody (Immunoway, 1:200, RS 23,320) for 50 min at room temperature. Then, wash with PBS (PH = 7.4) 3 times, 5 min each time. Then, drop DAPI dye solution, and incubate at room temperature avoid light for 10 min. Quench tissues with spontaneous fluorescence quench agent for 5 min. The tablets were then sealed with an anti-fluorescence quencher. The images were observed and collected under a fluorescence microscope.
Image J software was used to quantitatively analyze the immunofluorescence results, and the number of positive cells was measured. The expression of immunopositive cells in the dorsal striatum was measured. Results: DAPI stained nucleus was blue under UV excitation, and positive expression was the corresponding fluorescein-labeled green light (Erk; p-Erk) or red light (PDYN; PENK).
Data processing
All data from this study were expressed as mean ± standard error of the mean (SEM). Two-way ANOVA was used for inter-group index comparison, multiple comparisons were performed by Tukey post hoc test. P < 0.05 indicated the difference was statistically significant. All the above analyses were performed with the SPSS statistical software (V24.0) (SPSS, IBM, New York) and GraphPad Prism 6.0 (GraphPad Software., CA, USA).
Research results
Identification of PD model mice
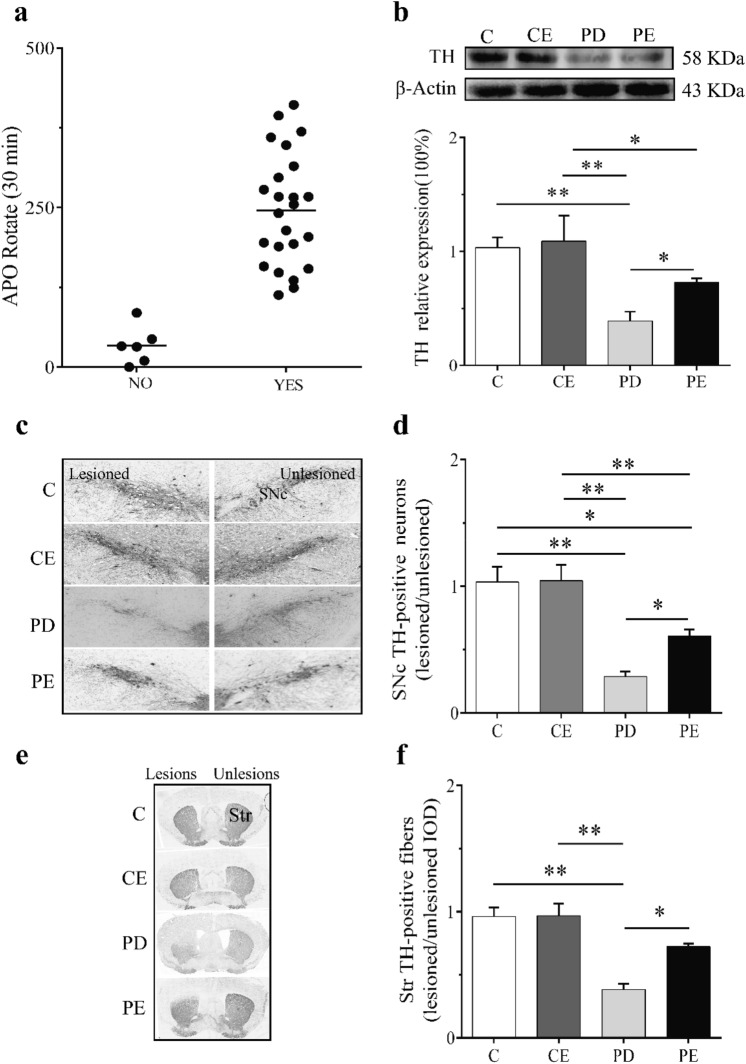
In the evaluation of the PD model, the success of the model was first judged by the number of rotation circles induced by APO. After aerobic exercise intervention, the expression of TH protein in the striatum of some animals was detected by Western blot, and the expression of TH protein in striatum and SNc of some animals were detected by immunohistochemical technique to improve the reliability of the model. On the seventh day after injection of 6-OHDA, the APO-induced rotation test was carried out in the model mice. Among the 30 mice injected with 6-OHDA, there were 6 mice whose rotation circles were less than 100 r, and the PD modeling rate was 80% (see Fig. 3a).
Fig. 3.
The schematic diagram to verify the results of PD mouse models. a Diagram of 6-OHDA-treated mice rotated for 30 min by APO. b The schematic diagram of TH protein expression in the striatum was compared with that in the striatum of each group. c Schematic diagram of expression of TH-positive neurons in substantia nigra (200 ×). d The expression of TH-positive cells in substantia nigra of mice in each group. e Schematic diagram of TH-positive nerve fiber expression in dorsal striatum area (100 ×). f The expression of TH-positive nerve fibers in the striatum of mice in each group. **P < 0.01. *P < 0.05
After 4 weeks of aerobic training, the expression of TH protein in the striatum of mice in each group was detected by Western blot. Compared with C, the protein expression of TH in the striatum of PD mice decreased significantly (P < 0.01); compared with PD, the expression of TH protein increased significantly in PE mice (P < 0.05); and the protein expression of TH in the striatum of PD decreased significantly compared with CE mice (P < 0.01). Compared with CE, the protein expression of TH in the striatum of PE mice decreased significantly (P < 0.05) (see Fig. 3b).
Immunohistochemistry showed that the number of TH-positive neurons in substantia nigra of PD was significantly lower than that of C mice (P < 0.01). Compared with C, the expression of TH neurons in substantia nigra of PE mice decreased significantly (P < 0.05). Compared with PD, the expression of TH neurons in substantia nigra of PE mice increased significantly (P < 0.05). Compared with CE, the number of positive cells of TH neurons in substantia nigra of PD mice decreased significantly (P < 0.01), and that of TH neurons in substantia nigra of PE mice decreased significantly compared with CE (P < 0.01) (see Fig. 3d).
Compared with C, the gray value of TH-positive nerve fibers in the dorsal striatum of PD mice was significantly decreased (P < 0.01), and that of TH-positive nerve fibers in the dorsal striatum of PE mice was significantly higher than that of PD (P < 0.05), and that of TH-positive nerve fibers in the dorsal striatum of PD mice was significantly lower than that of CE (P < 0.01) (see Fig. 3f).
Aerobic exercise improved motor behavior in PD mice
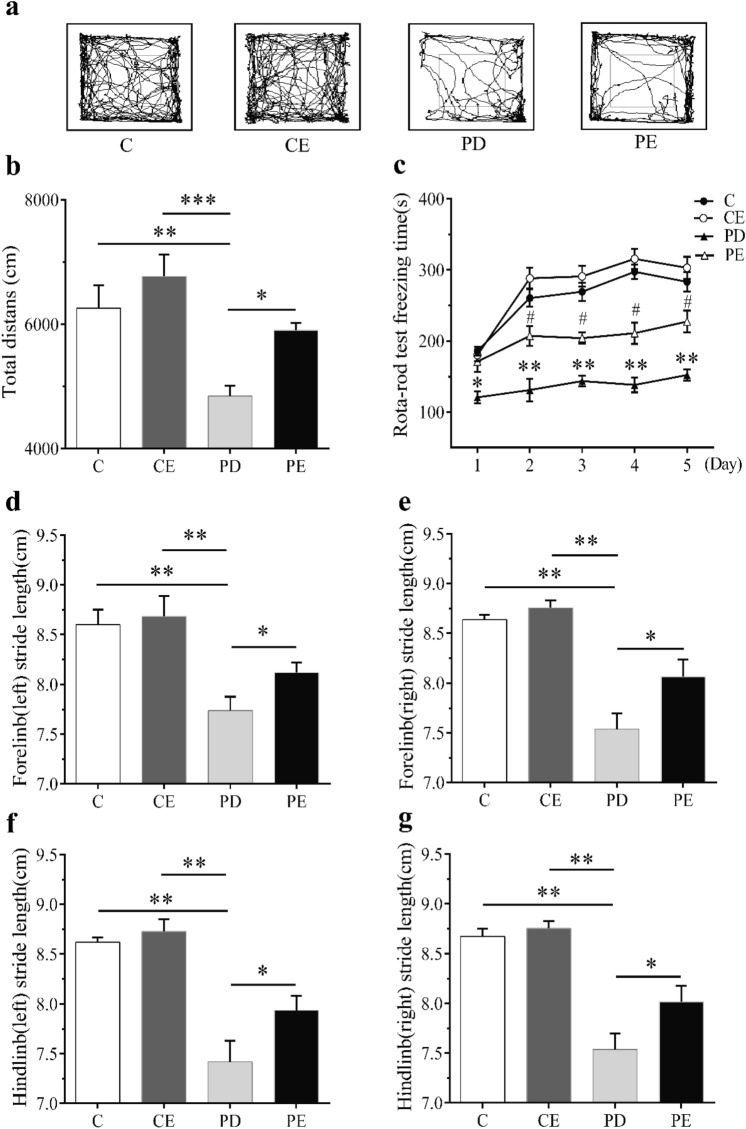
In the open-field experiment, compared with C, the total distance of PD mice decreased significantly (P < 0.01), and compared with PD, the total distance of PE mice increased with significant difference (P < 0.05), compared with CE, the total distance of PD mice decreased significantly (P < 0.001) (see Fig. 4b).
Fig. 4.
The statistical results of open-field total distance, gait test, and rotating rod test. a Track diagram of open-field moving distance. b After 4 weeks of aerobic training, the distance of mice in each group in the open field was compared. c After 4 weeks of aerobic training, the maintenance time of mice in each group on the rotating rod was compared. d Comparison of left forelimb movement steps of mice in each group after 4 weeks of aerobic training. e After 4 weeks of aerobic training, the moving steps of the right forelimb of mice in each group were compared. f After 4 weeks of aerobic training, the moving steps of the left hindlimb of mice in each group were compared. g After 4 weeks of aerobic training, the moving steps of the right hindlimb of mice in each group were compared.*P < 0.05, **P < 0.01, c: comparison between PD and C, *P < 0.01, **P < 0.01; compared with PD and PE, #P < 0.05
In the rotating rod experiment, compared with C, the maintenance time of PD mice on the rod decreased significantly on the first day (P < 0.05) and on the second to fifth days (P < 0.01). Compared with PD, the maintenance time of PE mice on the rod increased significantly from day 2 to 5 (P < 0.05) (see Fig. 4c).
In the gait experiment, there was a significant difference in the movement step of mice in each group in the channel. Compared with C, the limb step of PD mice decreased significantly (P < 0.01). Compared with PD, the limb step length of PE mice increased significantly (P < 0.05). Compared with CE, the limb step of PD mice decreased significantly (P < 0.01) (see Fig. 4d-g).
Aerobic exercise effectively regulated striatal D2-MSNs-Erk1/2 expression in PD mice
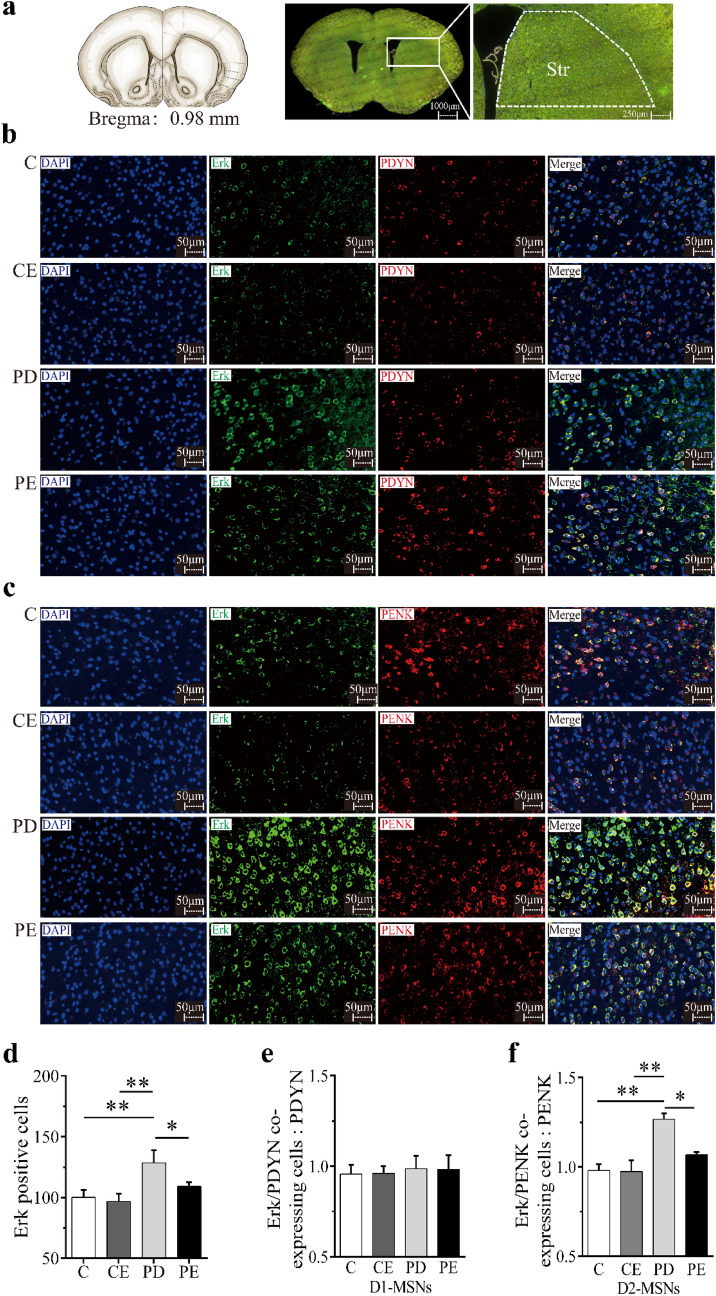
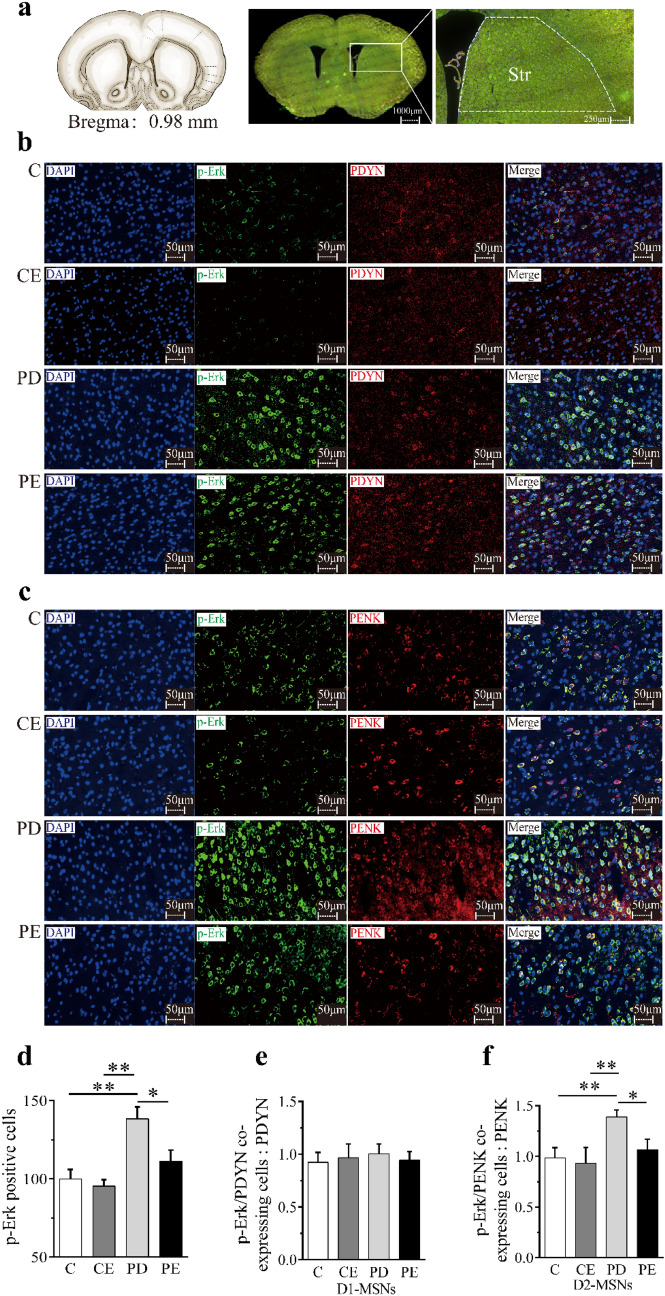
D1-MSNs and D2-MSNs are identified by PDYN and PENK, respectively. After the fourth week, compared with C, the number of Erk-positive cells in the dorsal striatum of PD mice increased significantly (P < 0.01); Compared with PD, the number of Erk-positive cells in PE mice decreased significantly (P < 0.05). Compared with CE, the number of Erk-positive cells in the dorsal striatum of PD mice increased significantly (P < 0.01) (see Fig. 5d).
Fig. 5.
Immunofluorescence expression of Erk in the striatum of mice in each group after 4 week aerobic exercise intervention. a Schematic diagram of the dorsal striatum section. b Schematic diagram of co-expression image of Erk and PDYN immunofluorescence, DAPI immunofluorescence (blue), Erk immunostaining (green), PDYN immunostaining (red), scale bar = 50 μm (400 ×). c Schematic diagram of Erk and PENK immunofluorescence co-labeling image, DAPI immunofluorescence (blue), Erk immunostaining (green), PENK immunostaining (red), bar = 50 μm (400 ×). d The expression of Erk-positive cells in mice of each group was compared. e Comparison of co-expression of Erk and PDYN in mice of each group. f Comparison of co-expression of Erk and PENK in mice of each group. *P < 0.05, **P < 0.01
There was no significant difference in the number of positive cells co-expressed by Erk and PDYN between groups (P > 0.05) (see Fig. 5e). But there was an inter-group difference in the number of positive cells co-expressed by Erk and PENK, compared with C, the number of positive cells co-expressing Erk and PENK in the dorsal striatum of PD mice increased significantly (P < 0.01), compared with PD, the number of positive cells co-expressing Erk and PENK in the dorsal striatum of PE mice decreased significantly (P < 0.05). Compared with CE, the number of positive cells co-expressing Erk and PENK in the dorsal striatum of PD mice increased significantly (P < 0.01) (see Fig. 5f).
Aerobic exercise effectively regulated striatal D2-MSNs-p-Erk1/2 expression in PD mice
D1-MSNs and D2-MSNs are identified by PDYN and PENK, respectively. After the fourth week, compared with C, the number of p-Erk-positive cells in the dorsal striatum of PD mice increased significantly (P < 0.01), compared with PD, the number of p-Erk-positive cells in PE mice decreased significantly (P < 0.05). Compared with CE, the number of p-Erk-positive cells in the dorsal striatum of PD mice increased significantly (P < 0.01) (see Fig. 6d).
Fig. 6.
Immunofluorescence expression of p-Erk in the striatum of mice in each group after 4-week aerobic exercise intervention. a Schematic diagram of the dorsal striatum section. b Schematic diagram of co-expression image of p-Erk and PDYN immunofluorescence, DAPI immunofluorescence (blue), p-Erk immunostaining (green), PDYN immunostaining (red), scale bar = 50 μm (400 ×). c Schematic diagram of p-Erk and PENK immunofluorescence co-labeling image, DAPI immunofluorescence (blue), p-Erk immunostaining (green), PENK immunostaining (red), bar = 50 μm (400 ×). d The expression of p-Erk-positive cells in mice of each group was compared. e Comparison of co-expression of p-Erk and PDYN in mice of each group. f Comparison of co-expression of p-Erk and PENK in mice of each group. *P < 0.05, **P < 0.01
There was no significant difference in the number of positive cells co-expressed by p-Erk and PDYN between groups (P > 0.05) (see Fig. 6e). But there was an inter-group difference in the number of positive cells co-expressed by p-Erk and PENK, compared with C, the number of positive cells co-expressing p-Erk and PENK in the dorsal striatum of PD mice increased significantly (P < 0.01), compared with PD, the number of positive cells co-expressing p-Erk and PENK in the dorsal striatum of PE mice decreased significantly (P < 0.05). Compared with CE, the number of positive cells co-expressing p-Erk and PENK in the dorsal striatum of PD mice increased significantly (P < 0.01) (see Fig. 6f).
Discussion
The motor symptoms of Parkinson’s disease mainly include muscle rigidity, bradykinesia, gait disorder, unstable body posture, and other motor behavior disorders. Epidemiological investigations have shown that physical activity can significantly reduce the risk of PD (Dibble et al. 2009; Xu et al. 2010). A survey with COVID-19 as the social background showed that PD patients’ motor and non-motor symptoms were aggravated due to lack of exercise during the epidemic (Song et al. 2020). Exercise can effectively improve the clinical symptoms of PD patients, and also significantly reduce the motor dysfunction of PD animals. Forouzan et al. (Rafie et al. 2017) showed that exercise can significantly improve the balance ability of PD animals. In this study, a PD mouse model was established by a 2-point injection of 6-OHDA into the striatum. It was found that after 4 weeks of aerobic exercise intervention, the autonomous activity ability, balance, and coordination ability of PD mice were improved, and the expression of TH in the substantia nigra and striatum was up-regulated. In this study, no significant changes in TH were observed in the control exercise group. This effect only occurred in the mice in the PD exercise, and the possible target was caused by inhibiting the activation of the Erk pathway. The mechanism of exercise retarding TH loss of DA energy is still not well studied. There are several sources for the recovery of DA energetic neurons after exercise intervention. Damaged DA energetic neurons may not show up in staining, and these neurons may be detected again after exercise intervention (Sanchez-Ramos et al. 1988). Exercise intervention may activate the germination of DA neurons (Stanic et al. 2003). Exercise increases the compensation response of GABA interneurons (Ibanez-Sandoval et al. 2010). Exercise generates new DA neurons. In this study, more DA was also detected after exercise intervention, and PD motor disorders induced by 6-OHDA neurotoxin were also improved. However, such exercise improvement effect did not appear significantly in mice that did not receive 6-OHDA injection, which may be because behavioral types were selected mainly for PD animal models. In addition, mice in the control exercise group did not lose DA, while the effect of exercise intervention was mainly reflected in PD mice. Previous studies have suggested that MSNs in the striatum may be involved in the improvement of PD symptoms by aerobic exercise.
Basal ganglion is an important nucleus for controlling the body’s voluntary movement, the striatum is the central processing area of basal ganglia, and the number of MSNs accounts for 95% of the total number of neurons (Lang et al. 2004). According to different projection sites and functions, MSNs can be divided into two subgroups. Namely, direct pathway MSNs (D1-MSNs) and indirect pathway MSNs (D2-MSNs). Among them, D1-MSNs emitted axons directly projecting onto the medial part of globus pallidus /substantia nigra reticular complex (GPi/SNr), mainly expressing dopamine I receptor (D1R), substance P (SP), and strong enkephalin (DYN) coupled with Golf protein. D2-MSNs first project to the (globus pallidus external) lateral part of the globus pallidus (GPe) and mainly expresses gi-protein-coupled dopamine II receptors (D2R) and enkephalin (ENK). A small part of MSNs projects to both GPe and GPi/SNr, expressing D1R and D2R together.
MSNs receive both cortical Glu projection (cortical-striatum pathway) and substantia nigra DA projection (substantia nigra striatum pathway) to coordinate voluntary motion. The direct pathway first projects information from the cerebral cortex to the striatum, which is processed by the striatum and then transferred to the ventral anterior nucleus and ventral lateral nucleus of the thalamus through the medial part of the globus pallidus, and finally back to the cortex. The information of indirect pathways is projected from the cerebral cortex to the striatum, and then from the striatum to the lateral part of the globus pallidus and the subthalamic nucleus, and finally back to the cerebral cortex. In addition, parallel branches of motor behavior regulation, reciprocal connections, and parallel connections in the form of feedback loops also exist in the cortex and basal ganglia (Albin et al. 1989). Therefore, DA and Glu signals jointly regulate neuronal activity in MSNs aggregation and MSNs become an important functional target neuron in the regulation of basal ganglia motor function. Previous studies have confirmed that the mechanism of exercise intervention in improving behavioral disorders in PD animals is related to MSNs plasticity.
Lee et al. (2016) applied optogenetics technology combined with functional magnetic resonance imaging (fMRI) to conduct directed activation of neurons in the two pathways and found that MSNs of different subtypes have different responses to motion. Sheng et al. (2019) used MSNs with specific markers of direct and indirect pathways in knockout mice to find that both types of neurons are involved in the execution of motion, with D1-MSNs participating in the initiation of motion and D2-MSNS participating in the inhibition of actions unrelated to target motion. The two types of neurons cooperate to coordinate the execution of motor behaviors.
Extracellular signal-regulated kinase (Erk) is a member of the mitogen-activated protein kinase (MAPK) family, including five subtypes (Erk1–Erk5). The MSNs of striatum mainly distributed Erk1 and Erk2, with highly similar protein sequences and complementary functions. Glu can activate Erk through Ras-/Raf/MEK signaling pathway, while DA can modulate Erk/MAPK signaling activity differently according to its different receptor subtypes. Erk plays an important role as a signal integrator of Glu and DA and reshapes the structure and function of MSNs by regulating gene expression and transcription factor activity. Therefore, Erk/MAPK signaling pathway is also considered as the core cellular and molecular mechanism of DA-mediated behavioral adaptation in the striatum (Cerovic et al. 2013).
In PD animals, MSNs dendritic spines abscission and electrical activity changes are observed, which may be related to the occurrence of motor behavior disorders (Chen et al. 2015a; Liu et al. 2014). For example, Gagnon et al. (2017) showed that 6-OHDA-induced PD mice significantly reduced the total dendritic length and dendrite branches of D1-MSNs and D2-MSNs. Suarez et al. (2014) observed MPTP-induced PD animals by immunoelectron microscopy and found similar results. Other studies have shown that the occurrence of PD involves changes in Erk/MAPK signal pathway transduction. When the Erk signaling pathway is activated, the corresponding transcription factors are activated by phosphorylation, resulting in changes in the expression and activity of specific proteins, and ultimately changes in cell function (Chang et al. 2007). Erk/MAPK signaling pathway plays an important role in regulating striatum related motor behavior in different MSNs. Scott et al. (Hutton et al. 2017) found that Erk/MAPK deletion in D1-MSNs can lead to reduced motor behavior of animals. In contrast, in D2-MSNs, deletion of Erk/MAPK leads to excessive motor behavior, and Erk/MAPK signal deficiency leads to a significant decrease in dendrite spine density and inhibition of neuronal excitability. These results suggest that the Erk/MAPK signaling pathway of MSNs is involved in the regulation of motor behavior and is closely related to the morphological and functional plasticity of neurons. After Erk/MAPK signal is blocked by drugs, motor skill learning, instrumental learning, and habit formation of animals are further consolidated (Bureau et al. 2010; Shiflett et al. 2010; 2011). Louise et al. (Mariani et al. 2019) also showed that cAMP, Ca2+, and Erk/MAPK signal responses in D1-MSNs and D2-MSNs showed significant cell type specificity. These results suggest that the dynamic characteristics of signaling pathways caused by different subtypes of MSNs need to be considered in PD treatment. It has been reported that changes in Erk/MAPK signaling pathway are related to changes in cortical-striatum pathway activity in PD patients and DA neuron injury animal models (Gerfen et al. 2002). Exercise intervention can significantly affect the activity of the cortex-striatum pathway in PD animals (Chen et al. 2015b). In this study, it was found that exercise intervention could have different regulatory effects on Erk/MAPK signaling pathways of different MSNs in the striatum of PD mice. The co-expression levels of Erk and its phosphorylated protein in the striatum of PD mice were significantly increased with enkephalin, while the co-expression levels with strong enkephalin showed no difference between groups, suggesting that the activation of Erk/MAPK signaling pathway in the striatum of PD mice mainly occurred in D2-MSNs. In addition, after 4 weeks of exercise intervention, the Erk/MAPK signaling pathway activity of D2-MSNs in the striatum of PD mice decreased. This may be related to the improvement of motor behavior disorder in PD mice. The loss of DA in PD mice weakened the inhibitory effect on Glu and increased the excitatory toxicity of Glu., as a result of Erk as nigra striatum can DA pathway and glu cortex in the striatum of integration, this makes the Erk anomalies and to some extent, after the exercise intervention reduced PD mice glu excitatory toxic effect, it has proved in the previous studies, in this experiment, exercise intervention improved the excessive activation of Erk PD mice, This was consistent with the inhibitory effect of DA on Glu.
The activation of the Erk/MAPK signaling pathway is different between D1-MSNs and D2-MSNs, and the activation of the Erk/MAPK signaling pathway in D2-MSNs is mainly related to the hyperactivation of the cortex-striatum Glu pathway. Previous studies have found that exercise intervention can reduce presynaptic Glu release and inhibit D2-MSNs excitability by upregulation of D2R expression in the striatum, and exercise intervention can selectively protect dendritic spines in D2-MSNs (Chen et al. 2017, 2015b; Real et al. 2013). This is logically consistent with the results of the present study.
Conclusions
The present study suggests that 4 weeks of aerobic exercise intervention can effectively promote the improvement of motor function in PD mice, and inhibit the transitional activation of the Erk/MAPK signaling pathway in the striatal D2-MSNs, which may be an important regulatory mechanism of exercise-dependent plasticity in the striatal D2-MSNs of PD mice.
Supplementary Information
Below is the link to the electronic supplementary material.
Acknowledgements
We thank the Life Sciences School Animal Ethics Committee of Hebei Normal University for the approval of the animal protocols used for the experiments included in this work. All the animal procedures described in this study were performed in adherence with the Guide for the Care and Use of Laboratory Animals published by the Ministry of Science and Technology of the People’s Republic of China.
Author contributions
WC and YL designed the research. WC and JC searched the scientific literature. XW and YW performed the histological staining and western blot. XW performed the behavior experiment. XW drafted and wrote the manuscript. The results were analyzed by JL Collected data and analyzed the data. All the authors read and approved the final manuscript.
Funding
This work was supported by the National Natural Science Fund of China (32071171) and the Hebei Province Natural Science Fund of China (C2020205011).
Data availability
The data used to support the findings of this study are included in the article.
Declarations
Conflict of interest
The authors declare that there is no conflict of interest regarding the publication of this article.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- Albin RL, Young AB, Penney JB. The functional anatomy of basal ganglia disorders. Trends Neurosci. 1989;12:366–375. doi: 10.1016/0166-2236(89)90074-X. [DOI] [PubMed] [Google Scholar]
- Bureau G, Carrier M, Lebel M, Cyr M. Intrastriatal inhibition of extracellular signal-regulated kinases impaired the consolidation phase of motor skill learning. Neurobiol Learn Mem. 2010;94:107–115. doi: 10.1016/j.nlm.2010.04.008. [DOI] [PubMed] [Google Scholar]
- Cerovic M, D’Isa R, Tonini R, Brambilla R. Molecular and cellular mechanisms of dopamine-mediated behavioral plasticity in the striatum. Neurobiol Learn Mem. 2013;105:63–80. doi: 10.1016/j.nlm.2013.06.013. [DOI] [PubMed] [Google Scholar]
- Chang SJ, Wang TY, Lee YH, Tai CJ. Extracellular ATP activates nuclear translocation of ERK1/2 leading to the induction of matrix metalloproteinases expression in human endometrial stromal cells. J Endocrinol. 2007;193:393–404. doi: 10.1677/JOE-06-0168. [DOI] [PubMed] [Google Scholar]
- Chen W, Shi KX, Liu XL. Exercise intervention improves behavioral function in PD rats through modulation of striatal MSNs structural plasticity. Chin J Sports Med. 2015;34:228–234. doi: 10.16038/j.1000-6710.2015.03.002. [DOI] [Google Scholar]
- Chen W, Wei X, Liu XL, Yan KL. Effect of exercise on cortex -striatum glutamatergic neurotransmission in PD model rats. J Beijing Sport Univ. 2015;38:61–66. doi: 10.19582/j.cnki.11-3785/g8.2015.02.011. [DOI] [Google Scholar]
- Chen W, Qiao DC, Liu XL, Shi KX. Treadmill exercise improves motor dysfunction and hyperactivity of the corticostriatal glutamatergic pathway in rats with 6-OHDA-induced Parkinson's disease. Neural Plast. 2017;2017:2583910. doi: 10.1155/2017/2583910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coelho M, Ferreira JJ. Late-stage Parkinson disease. Nat Rev Neurology. 2012;8:435–442. doi: 10.1038/nrneurol.2012.126. [DOI] [PubMed] [Google Scholar]
- Del Tredici K, Braak H. Review: sporadic Parkinson's disease: development and distribution of alpha-synuclein pathology. Neuropathol Appl Neurobiol. 2016;42:33–50. doi: 10.1111/nan.12298. [DOI] [PubMed] [Google Scholar]
- Dibble LE, Hale TF, Marcus RL, Gerber JP, LaStayo PC. High intensity eccentric resistance training decreases bradykinesia and improves quality of life in persons with Parkinson's disease: a preliminary study. Parkinsonism Relat Disord. 2009;15:752–757. doi: 10.1016/j.parkreldis.2009.04.009. [DOI] [PubMed] [Google Scholar]
- Gagnon D, Petryszyn S, Sanchez MG, Bories C, Beaulieu JM, De Koninck Y, Parent A, Parent M. Striatal neurons expressing D1 and D2 receptors are morphologically distinct and differently affected by dopamine denervation in mice. Sci Rep. 2017;7:41432. doi: 10.1038/srep41432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerfen CR, Miyachi S, Paletzki R, Brown P. D1 dopamine receptor supersensitivity in the dopamine-depleted striatum results from a switch in the regulation of ERK1/2/MAP kinase. J Neurosci Off J Soc Neurosci. 2002;22:5042–5054. doi: 10.1002/neu.10053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoydal MA, Wisloff U, Kemi OJ, Ellingsen O. Running speed and maximal oxygen uptake in rats and mice: practical implications for exercise training. Eur J Cardiovasc Prev Rehabil. 2007;14:753–760. doi: 10.1097/HJR.0b013e3281eacef1. [DOI] [PubMed] [Google Scholar]
- Hutton SR, Otis JM, Kim EM, Lamsal Y, Stuber GD, Snider WD. ERK/MAPK signaling is required for pathway-specific striatal motor functions. J Neurosci Off J Soc Neurosci. 2017;37:8102–8115. doi: 10.1523/JNEUROSCI.0473-17.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ibanez-Sandoval O, Tecuapetla F, Unal B, Shah F, Koos T, Tepper JM. Electrophysiological and morphological characteristics and synaptic connectivity of tyrosine hydroxylase-expressing neurons in adult mouse striatum. J Neurosci. 2010;30:6999–7016. doi: 10.1523/JNEUROSCI.5996-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jamwal S, Kumar P. L-theanine, a component of green tea prevents 3-nitropropionic acid (3-NP)-induced striatal toxicity by modulating nitric oxide pathway. Mol Neurobiol. 2017;54:2327–2337. doi: 10.1007/s12035-016-9822-5. [DOI] [PubMed] [Google Scholar]
- Jamwal S, Kumar P. Insight into the emerging role of striatal neurotransmitters in the pathophysiology of Parkinson's disease and Huntington's disease: a review. Curr Neuropharmacol. 2019;17:165–175. doi: 10.2174/1570159X16666180302115032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lang AE, Obeso JA. Challenges in Parkinson's disease: restoration of the nigrostriatal dopamine system is not enough. Lancet Neurol. 2004;3:309–316. doi: 10.1016/s1474-4422(04)00740-9. [DOI] [PubMed] [Google Scholar]
- Lee HJ, Weitz AJ, Bernal-Casas D, Duffy BA, Choy M, Kravitz AV, Kreitzer AC, Lee JH. Activation of direct and indirect pathway medium spiny neurons drives distinct brain-wide responses. Neuron. 2016;91:412–424. doi: 10.1016/j.neuron.2016.06.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu XL, Shi KX, Qiao DC. Effects of exercise on striatum’s neuronal activity in rats with Parkinson’s disease. J Beijing Sport Univ. 2014;37:62–66. doi: 10.19582/j.cnki.11-3785/g8.2014.05.009. [DOI] [Google Scholar]
- Mariani LL, Longueville S, Girault JA, Herve D, Gervasi N. Differential enhancement of ERK, PKA and Ca(2+) signaling in direct and indirect striatal neurons of Parkinsonian mice. Neurobiol Dis. 2019;130:104506. doi: 10.1016/j.nbd.2019.104506. [DOI] [PubMed] [Google Scholar]
- Muhlack S, Welnic J, Woitalla D, Muller T. Exercise improves efficacy of levodopa in patients with Parkinson's disease. Mov Disord. 2007;22:427–430. doi: 10.1002/mds.21346. [DOI] [PubMed] [Google Scholar]
- Olanow CW, Stern MB, Sethi K. The scientific and clinical basis for the treatment of Parkinson disease (2009) Neurology. 2009;72:S1–136. doi: 10.1212/WNL.0b013e3181a1d44c. [DOI] [PubMed] [Google Scholar]
- Rafie F, Shabazi M, Sheikh M, Naghdi N, Sheibani V. Effects of voluntary exercise on motor function in Parkinson's disease model of rats. Ann Appl Sport Sci. 2017;5:81–86. doi: 10.18869/acadpub.aassjournal.5.2.81. [DOI] [Google Scholar]
- Real CC, Ferreira AFB, Chaves-Kirsten GP, Torro AS, Pires RS, Britto LRG. BDNF receptor blockade hinders the beneficial effects of exercise in a rat model of Parkinson's disease. Neuroscience. 2013;237:118–129. doi: 10.1016/j.neuroscience.2013.01.060. [DOI] [PubMed] [Google Scholar]
- Sanchez-Ramos JR, Michel P, Weiner WJ, Hefti F. Selective destruction of cultured dopaminergic neurons from fetal rat mesencephalon by 1-methyl-4-phenylpyridinium: cytochemical and morphological evidence. J Neurochem. 1988;50:1934–1944. doi: 10.1111/j.1471-4159.1988.tb02500.x. [DOI] [PubMed] [Google Scholar]
- Sasco AJ, Paffenbarger RJ, Gendre I, Wing AL. The role of physical exercise in the occurrence of Parkinson's disease. Arch Neurol. 1992;49:360–365. doi: 10.1001/archneur.1992.00530280040020. [DOI] [PubMed] [Google Scholar]
- Sheng MJ, Lu D, Shen ZM, Poo MM. Emergence of stable striatal D1R and D2R neuronal ensembles with distinct firing sequence during motor learning. Proc Natl Acad Sci. 2019;116:11038–11047. doi: 10.1073/pnas.1901712116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shiflett MW, Balleine BW. Contributions of ERK signaling in the striatum to instrumental learning and performance. Behav Brain Res. 2011;218:240–247. doi: 10.1016/j.bbr.2010.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shiflett MW, Brown RA, Balleine BW. Acquisition and performance of goal-directed instrumental actions depends on ERK signaling in distinct regions of dorsal striatum in rats. J Neurosci Off J SocNeurosci. 2010;30:2951–2959. doi: 10.1523/JNEUROSCI.1778-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slomski A. Intense exercise improved motor function in Parkinson disease. JAMA. 2019;322:1948. doi: 10.1001/jama.2019.18983. [DOI] [PubMed] [Google Scholar]
- Song J, Ahn JH, Choi I, Mun JK, Cho JW, Youn J. The changes of exercise pattern and clinical symptoms in patients with Parkinson's disease in the era of COVID-19 pandemic. Parkinsonism Relat Disord. 2020;80:148–151. doi: 10.1016/j.parkreldis.2020.09.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stanic D, Finkelstein DI, Bourke DW, Drago J, Horne MK. Timecourse of striatal re-innervation following lesions of dopaminergic SNpc neurons of the rat. Eur J Neurosci. 2003;18:1175–1188. doi: 10.1046/j.1460-9568.2003.02800.x. [DOI] [PubMed] [Google Scholar]
- Suarez LM, Solis O, Carames JM, Taravini IR, Solis JM, Murer MG, Moratalla R. L-DOPA treatment selectively restores spine density in dopamine receptor D2-expressing projection neurons in dyskinetic mice. Biol Psychiat. 2014;75:711–722. doi: 10.1016/j.biopsych.2013.05.006. [DOI] [PubMed] [Google Scholar]
- Xu Q, Park Y, Huang X. Physical activities and future risk of Parkinson disease. Neurology. 2010;75:341–348. doi: 10.1212/WNL.0b013e3181ea1597. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data used to support the findings of this study are included in the article.