Abstract
Objective:
To identify clinical factors, including esophageal dilation on chest high-resolution computed tomography (HRCT), that are associated with pulmonary function decline in patients with systemic sclerosis (SSc).
Methods:
Patients fulfilled 2013 SSc criteria and had ≥1 HRCT and ≥2 pulmonary function tests (PFTs). According to published methods, widest esophageal diameter (WED) and radiographic interstitial lung disease (ILD) were assessed, and WED was dichotomized as dilated (≥19mm) vs. not dilated (<19mm). Clinically meaningful PFT decline was defined as %-predicted change in forced vital capacity (FVC) ≥5 and/or diffusion capacity for carbon monoxide (DLCO) ≥15. Linear mixed effect models were used to model PFT change over time.
Results:
138 SSc patients met study criteria: 100 (72%) had radiographic ILD; 49 (35%) demonstrated FVC decline (median follow-up 2.9y). Patients with Scl-70 autoantibodies had 5-year %-predicted FVC decline (−6.3; 95% CI −9.9, −2.8), while patients without Scl-70 autoantibodies demonstrated 5-year FVC stability (+1.78; 95% CI −0.6, 4.15). Esophageal diameter did not distinguish between those with vs. without FVC decline. Patients with esophageal dilation had statistically significant 5-year %-predicted DLCO decline (−5.6; 95% CI −10.0, −1.2), but this decline was unlikely clinically significant. Similar results were observed in sub-analysis of patients with radiographic ILD.
Conclusion:
In patients with SSc, Scl-70 positivity is a risk factor for %-predicted FVC decline at five years. Esophageal dilation on HRCT was associated with a minimal, non-clinically significant decline in DLCO and no change in FVC during 5-year follow-up. These results have prognostic implications for SSc-ILD patients with esophageal dilation.
Keywords: Systemic sclerosis, interstitial lung disease, gastrointestinal disease, biomarkers
Introduction:
Interstitial lung disease (ILD) is a leading cause of death in patients with systemic sclerosis (SSc) (1, 2). Known risk factors for prevalent SSc-ILD include positive anti-topoisomerase I (Scl-70) serum autoantibody status, diagnosis of diffuse cutaneous (dc)-SSc, black race, male sex, and genetic polymorphisms including certain major histocompatibility complex class II human leukocyte alleles (MHC-II HLA-DRB1*11 and HLA-DPB*1301), and non-MHC genes including those for interleukin (IL)-1α, and IL-1β, (2). However, less is known regarding risk factors for SSc-ILD progression. A systematic review of 20 studies between 1994 and 2012 (1,524 SSc patients) found that greater chest HRCT fibrosis severity and shorter SSc disease duration were predictors of ILD progression (3). Subsequently, Liu et al. showed that baseline elevation in C-reactive protein (CRP) predicted SSc-ILD progression, measured by change in %-predicted forced vital capacity (FVC), over a mean time-in-study of 4.4 years (4). Assassi et al. demonstrated that positive Scl-70 autoantibody status was associated with short term (3-year) %-predicted FVC decline (regression coefficient = −2.49, 95% CI −4.62, −0.36; p=0.022) in an SSc cohort where 58% had baseline FVC %-predicted >80. However, beyond three years, autoantibody status was not associated with progression, as assessed by %-predicted FVC change (5).
Symptomatic esophageal disease is present in >50% of patients with SSc, and abnormal esophageal motility of uncertain significance on manometric testing is present in up to 90% of SSc patients (6). Esophageal dilation (defined as >10mm diameter on coronal HRCT images) in patients with SSc is associated with esophageal dysmotility, as assessed by esophageal transit scintigraphy (7); and esophageal dysmotility may be associated with SSc-ILD progression (1, 8). Specifically, Marie et al. reported that in 43 patients with SSc-ILD, those with severe vs. mild-moderate esophageal dysmotility on manometry demonstrated greater two-year decline in %-predicted diffusion capacity for carbon monoxide (DLCO) (−16.04% vs. +1.47%, p=0.022), but not %-predicted FVC (−3.65% vs. +0.09%, p=0.386) (8). We previously showed in a cross-sectional study that esophageal diameter on axial HRCT images correlated positively with the presence of radiographic ILD and negatively with baseline %-predicted FVC and DLCO in patients with SSc (9). Moreover, an esophageal diameter ≥19mm had the best combined sensitivity and specificity for associated radiographic SSc-ILD (10).
Mechanistically, a dilated esophagus may act as a gastric content reservoir allowing for micro-aspiration that induces lung parenchymal damage (1, 8, 11, 12). Gastroesophageal reflux disease (GERD) is associated with idiopathic pulmonary fibrosis, and its treatment has been shown to stabilize lung function, supporting the hypothesis that esophageal dysfunction may also play an important role in SSc-ILD pathogenesis and progression (13, 14). The present study was undertaken to determine if radiographic esophageal dilation is an independent risk factor for pulmonary function decline 1, 2, and 5 years in SSc. We also sought to identify other important patient factors associated with SSc-ILD progression using our large cohort of clinically well-characterized SSc patients.
Methods:
This retrospective study was approved by the Northwestern University Institutional Review Board (STU00066807). Patient consent was obtained through the Northwestern Scleroderma Patient Registry (STU00002669). Patients with sine, limited cutaneous (lc-), or dc-SSc who fulfilled American College of Rheumatology 2013 SSc Classification Criteria and had at least one HRCT and two PFTs were included (15). SSc disease duration was defined as the duration between first non-Raynaud SSc symptom and the baseline HRCT date. Follow-up time was defined as time from baseline PFT to last PFT date between April 2008 and August 2016. Patients with prior pulmonary or gastrointestinal procedures that would independently affect esophageal diameter or PFT measurements, including lung transplant, lobectomy, or esophageal dilatation procedures, were excluded. Important baseline clinical data were obtained by manual review of rheumatology clinic notes within one year of HRCT date. Collected data include proton pump inhibitor (PPI) use, prednisone use (any), tobacco use (current or former), digital ulcer (DU) history, pulmonary and gastrointestinal symptoms, and erythrocyte sedimentation rate (ESR). Pulmonary hypertension was defined as mean pulmonary arterial pressure ≥25 mmHg on right heart catheterization (16).
An experienced thoracic radiologist (RA), blinded to clinical data, manually reviewed HRCT exams to determine widest esophageal diameter (WED) and the presence or absence of ILD. The widest esophageal diameter (WED) was defined as the largest of three esophageal diameters (mucosa to mucosa) at the level of the mid-arch of the aorta, the carina, and the diaphragmatic hiatus on axial HRCT images (9). Patients were dichotomized by WED≥19mm or <19mm, because our previous results showed that a WED cut-point of 19mm had the highest combined sensitivity and specificity for prevalent radiographic SSc-ILD (10). The presence or absence of radiographic ILD was determined based upon methods described by Kazerooni et al. (9, 17). PFT change was analyzed in the full cohort (regardless of ILD status), and sub-analysis was performed in patients with baseline radiographic ILD. We evaluated inter-rater reliability (between two radiologists) and intra-rater reliability (two ratings of one radiologist) for WED using intraclass correlation coefficient (ICC) for two-way random effects model and for ILD presence using Cohen’s kappa coefficient and percent agreement for a subset of 60 HRCT scans. The sample size of 60 was calculated expecting the estimated ICC to be 0.90 or higher compared to the null ICC of 0.80 with at least 80% power and alpha of 0.05.
Baseline PFT was defined as the test closest to, and within 12 months of, the first available HRCT. Subsequent longitudinal PFT data were recorded in months from baseline HRCT. Using National Health and Nutrition Examination Survey (NHANES) III reference populations, predicted FVC was determined by age, sex and race, and predicted DLCO was determined by age and sex and adjusted for hemoglobin (18). The DLCO value was excluded from analysis in patients without an available hemoglobin result within six months of PFT or where inspiratory vital capacity (IVC):FVC ratio was <0.85 (indicating poor test quality) (19).
We performed parallel analyses for FVC and DLCO change. For FVC analyses, we included 138 patients with FVC results and measured change in %-predicted FVC to determine patient factors associated with longitudinal FVC decline. For DLCO analyses, we included 99 patients with DLCO results, and measured change in %-predicted DLCO to determine patient factors associated with longitudinal DLCO decline. Both change in %-predicted FVC and DLCO were used as surrogates for worsening ILD (20, 21). Clinically meaningful PFT worsening was defined as a ≥5-point decrease in %-predicted FVC and/or a ≥15-point decrease in %-predicted DLCO (8, 21-26).
Spaghetti plots were used to visualize %-predicted FVC and DLCO change over time between Scl-70 positive vs. negative groups and WED≥19mm vs. WED<19mm groups. We tested for baseline differences between those with vs. without ILD progression using chi-square tests for categorical variables, and two sample t-tests with unequal variance for continuous variables. We used linear mixed effect models with random intercepts and an unstructured covariance structure to examine between group differences in %-predicted FVC and DLCO change over time. Time since baseline PFT was modeled with a linear term. We tested for group differences by including group*time interaction terms. We analyzed possible differences in %-predicted FVC and DLCO change by WED groups within Scl-70 autoantibody positive patients by including a three-way interaction. We present means adjusted estimates from models adjusted for sex, SSc disease subtype, Scl-70 autoantibody positivity, SSc disease duration, PPI use, prednisone use, and smoking history. All analyses were conducted using SAS version 9.4 (SAS Institute, Cary, NC) and R version 3.5.3 (cran.r-project.org).
Results:
The cohort included 138 patients who fulfilled study criteria with a median (range) follow-up of 2.9 (0.3-7.2) years (Figure 1). Fifteen patients (11%) with baseline HRCT died. The median (range) time between last PFT and death was 1.4 years (0.2-3.9 years). Baseline characteristics for full cohort and for patients with baseline radiographic ILD are described in Table 1. Most patients were female (84%), white (75%), and non-smokers (62%). The mean (SD) age was 50 (11.1) years, and modified Rodnan skin score was 11 (9). Scl-70 autoantibody was positive in 50 of 138 (36%) patients, and 64 (46%) patients had dcSSc. Radiographic ILD was present in 100 out of 138 (72%) patients, of whom 48% had positive Scl-70 autoantibodies and 54% had dcSSc. The mean (SD) baseline %-predicted FVC was 78 (16.7) and DLCO was 60 (20.4) in the full cohort. Among patients with baseline ILD, mean %-predicted FVC was 75 (17.3) and DLCO was 57 (19.9). The mean (SD) WED was 17.6 (8.2) mm in the full cohort and 18.6 (7.8) mm among patients with radiographic ILD. The median (IQR) number of PFT per patient was 3 (2-4).
Figure 1. Derivation of analysis sample.
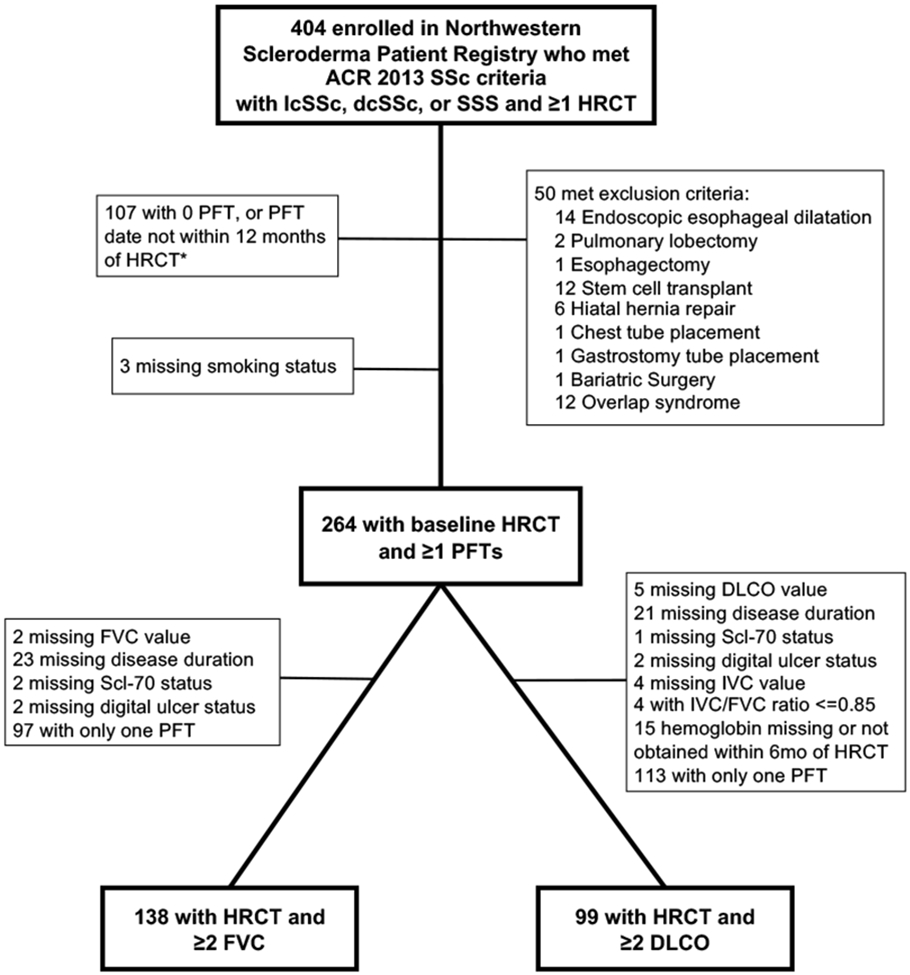
*Patients excluded for missing PFTs may have met multiple exclusion criteria. (HRCT= High Resolution Computed Tomography; lcSSc= Limited Cutaneous Systemic Sclerosis; dcSSc= diffuse cutaneous systemic sclerosis; SSS= scleroderma sine scleroderma; PFT= pulmonary function test; FVC = forced vital capacity; DLCO= diffusing capacity of the lung for carbon monoxide; IVC = inspiratory vital capacity.)
Table 1.
Baseline characteristics of full study cohort (N=138) and patients with baseline radiographic systemic sclerosis (SSc) associated interstitial lung disease (ILD) (N=100)
| Mean (SD) or N (%) | Total Cohort (N=138) |
Patients with radiographic SSc-ILD (N = 100) |
|---|---|---|
| Age at time of HRCT, years | 50.0 (11.1) | 49.5 (11.6) |
| Sex, women | 116 (84.1) | 82 (82.0) |
| Race, white | 104 (75.4) | 70 (70.0) |
| Smoker, current or former | 52 (37.7) | 37 (37.0) |
| Gastrointestinal symptoms, present | 102 (73.9) | 74 (74.0) |
| Pulmonary symptoms, present | 83 (60.1) | 66 (66.0) |
| Proton pump inhibition, current | 81 (58.7) | 60 (60.0) |
| SSc disease subtype, diffuse | 64 (46.4) | 54 (54.0) |
| SSc disease duration, years* | 5.7 (7.7) | 4.7 (5.9) |
| SSc-specific autoantibodies, positive (N=136) | 107 (78.7) | 77 (77.8) |
| Anti-topoisomerase I (Scl-70) | 50 (36.2) | 48 (48.0) |
| Anti-centromere | 24 (17.4) | 6 (6.0) |
| Anti-RNA polymerase III (N=135) | 34 (25.2) | 24 (24.2) |
| Erythrocyte sedimentation rate, mm/h | 24 (22.7) | 22 (18.6) |
| Modified Rodnan skin score | 11 (9.0) | 13 (9.4) |
| Medications, any use | 64 (46.4) | 52 (52.0) |
| Cyclophosphamide | 6 (4.3) | 6 (6.0) |
| Mycophenolate mofetil | 31 (22.5) | 27 (27.0) |
| Prednisone | 35 (25.4) | 27 (27.0) |
| Pulmonary hypertension present among those with RHC (N = 56) | 19 (33.9) | 17 (38.6) |
| Radiographic ILD, present | 100 (72.5) | 100 (100.0) |
| FVC %-predicted, baseline | 78 (16.7) | 75 (17.3) |
| DLCO %-predicted, baseline | 60 (20.4) | 57 (19.9) |
| Digital ulcers, present | 52 (37.7) | 40 (40.0) |
| Widest esophageal diameter, mm | 17.6 (8.2) | 18.6 (7.8) |
SSc disease duration defined as the interval between first non-Raynaud SSc symptom and baseline HRCT date. WED=widest esophageal diameter; HRCT=high-resolution computed tomography of the chest; SSc=systemic sclerosis; ILD=interstitial lung disease; PAH=pulmonary arterial hypertension; RHC=right heart catheterization; FVC=forced vital capacity; DLCO=diffusing capacity for carbon monoxide (adjusted for hemoglobin). Pulmonary and gastrointestinal symptoms defined as positive pulmonary and/or gastrointestinal review of systems in an outpatient rheumatology clinic note within one year of HRCT date.
Reliability.
Because the presence of ILD is based upon clinical judgement, two independent assessors reviewed a subset of chest HRCT exams. The inter-rater reliability, kappa was 0.96 (95% CI: 0.88, 1.00) for ILD presence, and ICC was 0.97 (95% CI: 0.94, 0.98) for WED. The intra-rater reliability, kappa was 0.83 (95% CI: 0.67, 0.99) for ILD, and ICC was 0.97 (95% CI: 0.95, 0.98) for WED.
Baseline characteristics and clinically meaningful pulmonary function change.
We compared baseline characteristics between patients with and without meaningful change in FVC (≥5-point change) and DLCO (≥15-point change) (Tables 2 and 3). In both the full study cohort and among patients with baseline radiographic SSc-ILD, positive Scl-70 autoantibody status was more common in patients with vs. without FVC worsening (full cohort: 51% vs. 28%, p=0.01; radiographic ILD only: 62% vs. 39%, p=0.05). Among patients with baseline radiographic ILD, those with meaningful FVC change less commonly had digital ulcers present (26% vs. 49%, p=0.03). Baseline %-predicted FVC was lower in those who demonstrated significant DLCO decline (full cohort: 67 vs. 78, p=0.03; radiographic ILD only: 64 vs. 76, p=0.04). There was no difference in the presence of pulmonary hypertension, in those with vs. without longitudinal DLCO decline.
Table 2.
Baseline characteristics of patients with systemic sclerosis with vs. without clinically meaningful %-predicted forced vital capacity (FVC) worsening
| Full Analytic Cohort (N=138) |
Radiographic ILD present (N=100) |
|||||
|---|---|---|---|---|---|---|
| Mean (SD) or N (%) | No FVC decline (N =89) |
FVC decline ≥5 (N=49) |
p-value | No FVC decline (N=61) |
FVC decline ≥5 (N=39) |
p-value |
| Age at time of HRCT, years | 49.2 (11.9) | 51.7 (9.4) | 0.179 | 48.6 (12.4) | 51.0 (10.1) | 0.281 |
| Sex, women | 78 (87.6) | 38 (77.6) | 0.191 | 52 (85.2) | 30 (76.9) | 0.430 |
| Race, white | 67 (75.3) | 37 (75.5) | 1.000 | 42 (68.9) | 28 (71.8) | 0.929 |
| Smoker, current or former | 32 (36.0) | 20 (40.8) | 0.704 | 20 (32.8) | 17 (43.6) | 0.379 |
| Gastrointestinal symptoms, present | 66 (74.2) | 36 (73.5) | 1.000 | 44 (72.1) | 30 (76.9) | 0.765 |
| Pulmonary symptoms, present | 53 (59.6) | 30 (61.2) | 0.992 | 38 (62.3) | 28 (71.8) | 0.446 |
| Proton pump inhibition, current | 53 (59.6) | 28 (57.1) | 0.925 | 36 (59.0) | 24 (61.5) | 0.967 |
| SSc disease subtype, diffuse | 42 (47.2) | 22 (44.9) | 0.936 | 36 (59.0) | 18 (46.2) | 0.292 |
| SSc disease duration, years* | 5.6 (7.5) | 5.8 (8.1) | 0.887 | 4.1 (5.1) | 5.5 (6.9) | 0.268 |
| Anti-Scl-70, positive | 25 (28.1) | 25 (51.0) | 0.013 | 24 (39.3) | 24 (61.5) | 0.05 |
| Anti-centromere, positive | 17 (19.1) | 7 (14.3) | 0.632 | 4 (6.6) | 2 (5.1) | 1.000 |
| Anti-RNA polymerase III, positive | 26 (29.9) | 8 (16.7) | 0.137 | 19 (31.7) | 5 (12.8) | 0.058 |
| Erythrocyte sedimentation rate | 24 (25.1) | 23 (17.8) | 0.820 | 20 (18.1) | 25 (19.2) | 0.240 |
| Modified Rodnan skin score | 11.9 (9.7) | 10.5 (7.5) | 0.344 | 13.8 (10.1) | 10.9 (7.8) | 0.116 |
| Medications, any use | 41 (46.1) | 23 (46.9) | 1.000 | 32 (52.5) | 20 (51.3) | 1.000 |
| Cyclophosphamide | 4 (4.5) | 2 (4.1) | 1.000 | 4 (6.6) | 2 (5.1) | 1.000 |
| Mycophenolate mofetil | 18 (20.2) | 13 (26.5) | 0.525 | 15 (24.6) | 12 (30.8) | 0.654 |
| Prednisone | 24 (27.0) | 11 (22.4) | 0.705 | 18 (29.5) | 9 (23.1) | 0.634 |
| Pulmonary hypertension present among those with RHC (N = 56) | 8 (26.7) | 11 (42.3) | 0.342 | 6 (28.6) | 11 (47.8) | 0.317 |
| Radiographic ILD, present | 61 (68.5) | 39 (79.6) | 0.233 | 61 (100.0) | 39 (100.0) | NA |
| FVC %-predicted, baseline | 77 (16.8) | 79 (16.8) | 0.714 | 74 (17.9) | 75 (16.6) | 0.793 |
| DLCO %-predicted, baseline | 60 (19.6) | 59 (21.8) | 0.741 | 59 (20.2) | 54 (19.3) | 0.202 |
| Digital ulcers, present | 38 (42.7) | 14 (28.6) | 0.146 | 30 (49.2) | 10 (25.6) | 0.033 |
| Widest esophageal diameter | 17.2 (7.9) | 18.3 (8.6) | 0.425 | 18.6 (7.6) | 18.6 (8.3) | 0.987 |
SSc disease duration defined as the interval between first non-Raynaud SSc symptom and baseline HRCT date. Widest esophageal diameter measured in mm; HRCT=high-resolution computed tomography of the chest; SSc=systemic sclerosis; ILD=interstitial lung disease; PAH=pulmonary arterial hypertension; RHC=right heart catheterization; FVC=forced vital capacity; DLCO=diffusing capacity of the lungs for carbon monoxide (adjusted for hemoglobin). Pulmonary and gastrointestinal symptoms defined as positive pulmonary and/or gastrointestinal review of systems in an outpatient rheumatology clinic note within one year of HRCT date.
Table 3.
Baseline characteristics of patients with systemic sclerosis with vs. without clinically meaningful %-predicted diffusing capacity of carbon monoxide (DLCO) worsening
| Full Analytic Cohort (N=99) |
Radiographic ILD present (N=73) |
|||||
|---|---|---|---|---|---|---|
| Mean (SD) or N (%) | No significant decline in DLCO (N=88) |
Decline in % predicted DLCO ≥15 (N=11) |
p-value | No significant decline in DLCO (N=64) |
Decline in % predicted DLCO ≥15 (N=9) |
p-value |
| Age at time of HRCT, years | 49.9 (10.6) | 47.3 (12.6) | 0.530 | 49.0 (10.5) | 46.1 (13.7) | 0.564 |
| Sex, women | 72 (81.8) | 9 (81.8) | 1.000 | 52 (81.2) | 7 (77.8) | 1.000 |
| Race, white | 66 (75.0) | 9 (81.8) | 0.901 | 45 (70.3) | 7 (77.8) | 0.944 |
| Smoker, current or former | 30 (34.1) | 4 (36.4) | 1.000 | 22 (34.4) | 3 (33.3) | 1.000 |
| Gastrointestinal symptoms, present | 65 (73.9) | 8 (72.7) | 1.000 | 47 (73.4) | 7 (77.8) | 1.000 |
| Pulmonary symptoms, present | 56 (63.6) | 8 (72.7) | 0.795 | 44 (68.8) | 8 (88.9) | 0.392 |
| Proton pump inhibition, current | 49 (55.7) | 6 (54.5) | 1.000 | 38 (59.4) | 5 (55.6) | 1.000 |
| SSc disease subtype, diffuse | 38 (43.2) | 8 (72.7) | 0.126 | 31 (48.4) | 7 (77.8) | 0.196 |
| SSc disease duration, years* | 5.9 (8.4) | 3.4 (4.6) | 0.142 | 4.6 (5.8) | 3.9 (5.0) | 0.706 |
| Anti-Scl-70, positive | 32 (36.4) | 3 (27.3) | 0.795 | 30 (46.9) | 3 (33.3) | 0.684 |
| Anti-centromere, positive | 16 (18.2) | 0 (0.0) | 0.267 | 3 (4.7) | 0 (0.0) | 1.000 |
| Anti-RNA polymerase III, positive | 24 (27.3) | 4 (40.0) | 0.635 | 17 (26.6) | 3 (33.3) | 0.978 |
| Erythrocyte sedimentation rate | 24 (22.0) | 23 (20.2) | 0.908 | 23 (18.4) | 26 (19.9) | 0.718 |
| Modified Rodnan skin score | 11.0 (9.4) | 16.2 (8.6) | 0.083 | 11.8 (10.1) | 15.4 (9.2) | 0.294 |
| Medications, any use | 42 (47.7) | 7 (63.6) | 0.500 | 33 (51.6) | 6 (66.7) | 0.622 |
| Cyclophosphamide | 5 (5.7) | 0 (0.0) | 0.935 | 5 (7.8) | 0 (0.0) | 0.87 |
| Mycophenolate mofetil | 21 (23.9) | 2 (18.2) | 0.966 | 19 (29.7) | 2 (22.2) | 0.944 |
| Prednisone | 24 (27.3) | 3 (27.3) | 1.000 | 18 (28.1) | 2 (22.2) | 1.000 |
| Pulmonary hypertension present among those with RHC (N = 56) | 9 (25.0) | 4 (44.4) | 0.459 | 8 (30.8) | 4 (50.0) | 0.567 |
| Radiographic ILD, present | 64 (72.7) | 9 (81.8) | 0.777 | 64 (100.0) | 9 (100.0) | NA |
| FVC %-predicted, baseline | 78 (16.6) | 67 (14.1) | 0.034 | 76 (17.5) | 64 (13.6) | 0.038 |
| DLCO %-predicted, baseline | 58 (18.3) | 69 (19.3) | 0.103 | 56 (18.4) | 62 (12.9) | 0.184 |
| Digital ulcers, present | 33 (37.5) | 6 (54.5) | 0.445 | 26 (40.6) | 4 (44.4) | 1.000 |
| Widest esophageal diameter | 17.2 (8.6) | 19.5 (8.9) | 0.438 | 18.1 (8.1) | 20.0 (9.6) | 0.577 |
SSc disease duration defined as the interval between first non-Raynaud SSc symptom and baseline HRCT date. Widest esophageal diameter measured in mm; HRCT=high-resolution computed tomography of the chest; SSc=systemic sclerosis; ILD=interstitial lung disease; PAH=pulmonary arterial hypertension; RHC=right heart catheterization; FVC=forced vital capacity; DLCO=diffusing capacity of the lungs for carbon monoxide (adjusted for hemoglobin). Pulmonary and gastrointestinal symptoms defined as positive pulmonary and/or gastrointestinal review of systems in an outpatient rheumatology clinic note within one year of HRCT date.
Longitudinal pulmonary function change by Scl-70 autoantibody status.
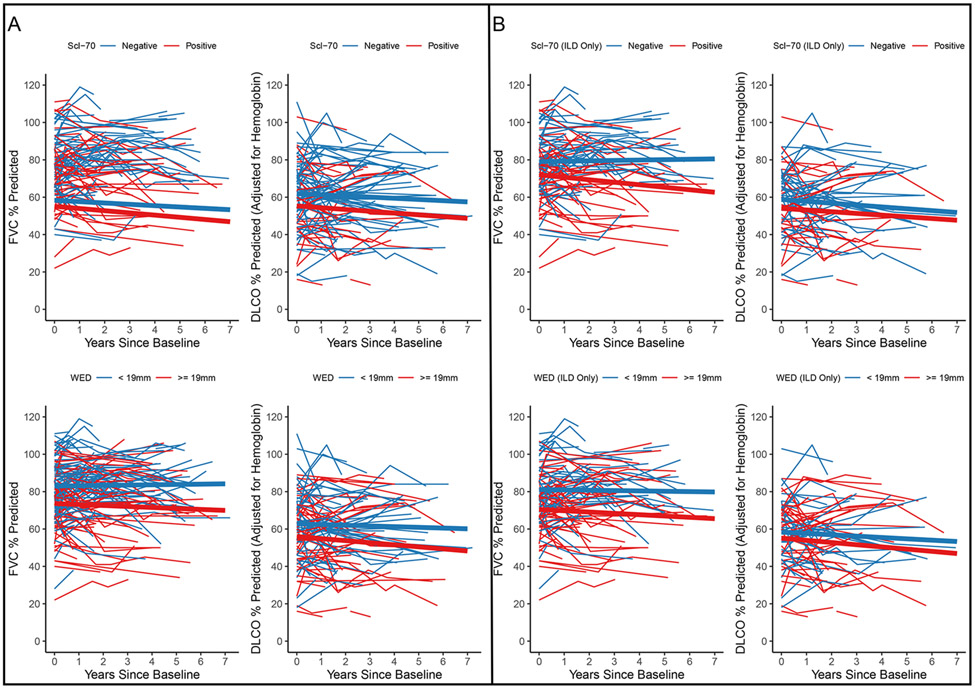
In the full cohort, baseline %-predicted FVC was lower in those with positive vs. negative Scl-70 autoantibody status (72 (95% CI 68, 77) vs. 82 (95% CI 79, 86), respectively; p<0.01). There was a statistically significant decline in %-predicted FVC at 1-, 2-, 3- and 5-years in patients with positive Scl-70 autoantibody status (5-year change: −6.3 (95% CI −9.9, −2.8), p<0.01), adjusted for sex, PPI use, prednisone use, SSc disease subtype, SSc disease duration, smoking history (current or former), and widest esophageal diameter (Table 4, Figure 2). In those lacking Scl-70 autoantibodies, there was no significant change in %-predicted FVC in adjusted model (1.8 (95% CI −0.6, 4.2), p=0.14). The reverse was observed for %-predicted DLCO change where patients lacking Scl-70 autoantibody demonstrated a statistically significant modeled change in %-predicted DLCO from baseline to 1-, 2-, 3- and 5-years (5-year change: −3.3 (95% CI −6.6, −0.05) that did not meet the pre-specified clinical threshold for significance. Patients with positive Scl-70 autoantibodies lacked significant change in %-predicted DLCO over time (5-year change: −4.7 (95% CI −11.0, 1.7) (Table 4, Figure 2). Similar findings were observed when restricting the analysis to only those with radiographic ILD at baseline (Figure 3, Supplemental Table 2).
Table 4.
Model-based estimates for change over time in %-predicted forced vital capacity (n=138) and carbon monoxide diffusing capacity (n=99) in patients with systemic sclerosis by Scl-70 and WED (95% CI)*
| Change from baseline |
FVC | DLCO | ||||||
|---|---|---|---|---|---|---|---|---|
| Scl-70 Negative | Scl-70 Positive | Scl-70 Negative | Scl-70 Positive | |||||
| 1 year | 0.36 | (−0.1, 0.83) | −1.27 | (−2.0, −0.56) | −0.66 | (−1.3, −0.01) | −0.93 | (−2.2, 0.33) |
| 2 years | 0.71 | (−0.2, 1.66) | −2.53 | (−3.9, −1.12) | −1.32 | (−2.6, −0.02) | −1.86 | (−4.4, 0.66) |
| 3 years | 1.07 | (−0.4, 2.49) | −3.80 | (−5.9, −1.67) | −1.99 | (−3.9, -0.03) | −2.79 | (−6.6, 0.99) |
| 5 years | 1.78 | (−0.6, 4.15) | −6.33 | (−9.9, −2.79) | −3.31 | (−6.6, −0.05) | −4.65 | (−11.0, 1.65) |
| WED < 19mm | WED ≥ 19mm | WED < 19mm | WED ≥ 19mm | |||||
| 1 year | 0.14 | (−0.4, 0.68) | −0.49 | (−1.1, 0.11) | −0.43 | (−1.2, 0.34) | −1.12 | (−2.0, −0.23) |
| 2 years | 0.27 | (−0.8, 1.35) | −0.97 | (−2.2, 0.22) | −0.85 | (−2.4, 0.67) | −2.23 | (−4.0, −0.46) |
| 3 years | 0.41 | (−1.2, 2.03) | −1.46 | (−3.2, 0.33) | −1.28 | (−3.6, 1.01) | −3.35 | (−6.0, −0.69) |
| 5 years | 0.68 | (−2.0, 3.38) | −2.43 | (−5.4, 0.55) | −2.13 | (−5.9, 1.68) | −5.58 | (−10.0, −1.15) |
Scl-70 = anti-topoisomerase 1; FVC = forced vital capacity; DLCO = diffusing capacity for carbon monoxide (adjusted for hemoglobin); PFT = pulmonary function test. PFT results shown as %-predicted.
PFT means for Scl-70 analysis adjusted for sex, proton pump inhibitor use, prednisone use, SSc disease subtype, duration since first non-Raynaud (years), smoking history (current or former), and widest esophageal diameter. PFT means for WED analysis adjusted for sex, proton pump inhibitor use, prednisone use, SSc disease subtype, duration since first non-Raynaud (years), smoking history (current or former), and Scl-70 autoantibody status.
Figure 2. Change in pulmonary function by Scl-70 autoantibody status and widest esophageal diameter.
A. Analysis of the full cohort (N=138). B. Analysis of the subset of patients with interstitial lung disease on baseline high-resolution computed tomography scan (N=100). Spaghetti plot depicting change in %-predicted forced vital capacity (FVC) (N=138) and carbon monoxide diffusing capacity (DLCO) (N=99) over time in patients with systemic sclerosis. Thick lines represent estimated % predicted FVC and DLCO from statistical model using data from all patients.
Longitudinal pulmonary function change by widest esophageal diameter.
Baseline characteristics for patients with a WED≥19 vs. <19mm are shown in Supplemental Table 1. At baseline, radiographic ILD was more common in patients with WED≥19 vs. <19mm (52 of 64 (81%) vs. 48 of 74 (65%), p=0.03). The baseline mean %-predicted FVC was lower in WED≥19 compared to those with WED<19mm (73 vs. 82, p<0.01). Similarly, the baseline mean %-predicted DLCO was lower in the wider (vs. narrower) WED group (57 vs. 64, p=0.05). The mean follow-up time was similar for individuals with WED≥19 vs. <19 (3.1 and 3.0 years, respectively).
Longitudinally, patients with WED≥19mm demonstrated a small, statistically significant %-predicted DLCO change from baseline to 5 years (−5.6 (95% CI −10.0, −1.2)) (Table 4, Figure 2), adjusted for sex, PPI use, prednisone use, SSc disease subtype, SSc disease duration, smoking history (current or former), and Scl-70 autoantibody status. There was no statistically significant difference in %-predicted FVC change in patients dichotomized by esophageal diameter (Table 4, Figure 2). Similar findings were observed when restricting the analysis to only patients with baseline radiographic ILD (Figure 2, Supplemental Table 3). Testing the three-way interaction of Scl-70 autoantibody status, WED, and time showed there was no significant difference in FVC decline among patients with positive Scl-70 autoantibody status comparing WED≥19 vs. <19mm (difference in change from baseline to 5 years = −3.2 (95% CI −11.1, 4.8).
Discussion:
To determine the impact of esophageal dilation on SSc-ILD progression, we examined a large cohort of 138 well-characterized SSc patients who had undergone HRCT and serial PFT. We show that esophageal dilation on axial chest HRCT images was associated with a minimal, non-clinically significant decline in DLCO and no change in FVC during 5-year follow-up, and Scl-70 is associated with SSc-ILD worsening as assessed by FVC and/or DLCO decline. In SSc patients, Savarino et al. reported an association between greater number of proximal reflux episodes on pH-impedance testing and pulmonary fibrosis on HRCT, supporting the hypothesis that esophageal dysfunction is related to SSc-ILD (27). Similarly, in a previous study, we showed that a wider esophageal diameter was associated with prevalent radiographic SSc-ILD and lower %-predicted FVC and DLCO (9, 10). However, in the present study, a dilated esophagus on HRCT did not predict longitudinal FVC worsening. Winstone et al., who studied 145 SSc patients (median follow-up 4 years), reported that for every one centimeter increase in esophageal diameter on chest HRCT at baseline, there was a 1.8% higher lung fibrosis score and 5.5% lower %-predicted FVC (p<0.001) after adjustment for age, gender, weight, and body mass index. However, there was no association between esophageal diameter and change in %-predicted FVC at one year of follow-up when adjusting for baseline fibrosis score (28). Our study included %-predicted DLCO that some consider to better estimate ILD extent, as discussed below, and tested a proposed WED threshold of 19mm as an esophageal dilation cut-point that could be important to include in chest HRCT reports in patients with SSc.
We demonstrate that the presence of Scl-70 autoantibodies was associated with FVC but not DLCO worsening over a 2.9-year median follow-up period. Assassi et al. reported that positive Scl-70 autoantibody status was associated with short term (3-year) decline in %-predicted FVC in 244 SSc patients (5). We found a statistically significant (though not likely clinically meaningful) decline in %-predicted DLCO in patients without Scl-70 autoantibodies. This finding may be because DLCO decline is less specific for ILD and can be observed in SSc associated pulmonary arterial hypertension (PAH) (29).
We report a 73% ILD prevalence in our cohort that is similar to other tertiary care center rates (36-84%) (30-32). Our prevalence on the upper end of this reported range is likely due to selection bias due to inclusion criteria requiring patients to have an available chest HRCT. Though many patients undergo HRCT at the baseline visit, it is not standard of care for all patients at our center. Thus, HRCT may be obtained more frequently in patients with pulmonary symptoms or abnormal physical exam findings in whom ILD is present. Further, only 51 of 138 (37%) patients we studied demonstrated clinically meaningful FVC worsening. Reasons for FVC stability may be related to high baseline %-predicted FVC reflecting more mild disease or the use of medications including mycophenolate mofetil and cyclophosphamide for ILD in approximately 30% of patients at the time of baseline HRCT scan (20, 33). In this retrospective study, we were unable to assess the longitudinal use of ILD medications which may have stabilized pulmonary function and confound the relationship with esophageal diameter and ILD. Thus, patients may have received ILD therapies after the baseline HRCT exam. Another possible reason for stability is the interdisciplinary care provided at our scleroderma program that includes aggressive use of PPIs, lifestyle management counseling including head of bed elevation, avoidance of meals before recumbency and importance of attaining/maintaining ideal body weight (the mean (SD) body mass index (BMI) of our group was 26 (5.73)). The control of gastrointestinal reflux with acid suppressive therapy and counseling may be associated with a slower rate of FVC decline (34, 35). We also did not classify patients by ILD pattern (usual interstitial pneumonia (UIP) vs. fibrotic nonspecific interstitial pneumonia (NSIP)) that could have impacted PFT trajectory.
In this study, we defined clinically meaningful PFT worsening a priori as ≥5-point decrease in %-predicted FVC or ≥15-point decrease in %-predicted DLCO (8, 21-26). We chose %-predicted FVC change ≥5 as an intermediate threshold based upon studies using a range of FVC change between 2 and 10 to define meaningful change. Specifically, in idiopathic pulmonary fibrosis (IPF), a 2-6-point decline in %-predicted FVC is defined as clinically meaningful (24). Based upon the Scleroderma Lung Study (SLS)-I and -II, clinically meaningful change in %-predicted FVC at 12-months could be considered as low as a FVC decline of 3-3.3% (25), while the OMERACT Connective Tissue Disease-ILD Working Group suggest FVC decline ≥10% to define progression (26). Compared to SLS-I and -II and the nintedanib trial (36) that each followed patients for only one year, our median follow-up was 2.9 years. Regarding DLCO, some studies suggest that DLCO best estimates SSc-ILD extent although the potential lack of specificity and reproducibility limit its use as an outcome (37, 38). Future studies may help determine the minimally clinically important differences in FVC and DLCO specifically in patients with SSc.
Our study has limitations. As an observational study, no conclusions regarding the causality of esophageal dilation and ILD progression can be inferred, and patient follow-up and PFT timing is not uniform. Also, mean SSc disease duration at time of HRCT is 5.7 years in full cohort (4.7 years among those with baseline radiographic ILD) which limits assessment of early pulmonary function decline and may enrich our cohort for patients with more stable disease. The relatively high mean baseline %-predicted FVC of 75 for patients in our cohort limits our ability to identify patient factors that are associated with longitudinal FVC decline in patients with more severe pulmonary disease at baseline. Also, patients did not routinely undergo esophageal manometry, so we are unable to comment on the relationship between esophageal diameter and dysfunction. Additionally, we defined esophageal dilation as ≥19 mm, because we previously found that this cut-point has the best combined sensitivity and specificity for SSc-ILD. Previous studies investigating esophageal dilation effect on pulmonary function defined esophageal dilation as ≥10 mm or greater, based upon radiographic definitions of ‘normal’, and found no association with esophageal dilation and ILD (7). Our definition, while more precisely related to SSc-ILD, may misclassify some patients with a more mildly dilated esophagus (10-18mm) and bias our results toward the null hypothesis (no difference in FVC decline between groups). Only one expert radiologist determined if radiographic ILD was present vs. absent which is the standard for clinical care.
Study strengths include examination of FVC and DLCO %-predicted as surrogates for worsening pulmonary disease in a sample of well characterized SSc patients. Study coordinators routinely contact outside hospitals to obtain serum autoantibody serologies and PFT records to reduce ascertainment bias. The DLCO measurement accuracy was assured by adjusting for hemoglobin and excluding patients for whom a CBC was not available in the preceding six months and where inspiratory vital capacity (IVC):FVC ratio was <0.85, because these features improve DLCO accuracy. Excluding patients with poor quality DLCO data or lacking hemoglobin values within 6 months may introduce selection bias; however, inclusion of these patients would introduce error into the DLCO measurement. Other strengths include large study size and the evaluation of esophageal diameter as a potential novel predictor for SSc-ILD progression. Also, an expert thoracic radiologist performed all esophageal diameter measurements on axial images and identified the largest diameter among three locations: mid-arch of the aorta, the carina, and the diaphragmatic hiatus. This allowed us to more accurately classify patients by esophageal diameter.
Conclusion:
Scl-70 autoantibody positivity is a risk factor for %-predicted FVC decline in patients with SSc. Esophageal dilation on HRCT was associated with a minimal, non-clinically significant 5-year decline in %-predicted DLCO and no change in %-predicted FVC during follow-up. These results have prognostic implications for SSc-ILD patients with esophageal dilation. Prospective studies that enroll patients with early SSc disease such as the Very Early Diagnosis of Systemic Sclerosis (VEDOSS) and Prospective Registry of Early Systemic Sclerosis (PRESS) registries will enable identification of the relationship between esophageal diameter and early PFT decline and may identify other patient factors and/or biomarkers associated with ILD progression (39, 40).
Supplementary Material
Financial support:
Research was supported by the National Institute of Arthritis and Musculoskeletal and Skin Diseases of the National Institutes of Health under Award Numbers K23 AR059763 (MH), R01 AR073270 (MH), P60 AR064464 (RWC, KK, JL), and P30 AR072579 (RWC, JL) and National Center for Advancing Translational Sciences-Clinical and Translational Science Award Number UL1 TR000150 (JL). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. The Rheumatology Research Foundation (KS), Scleroderma Foundation (KS), and the Scleroderma Research Foundation (MH) also supported this work.
Footnotes
Conflicts of interest: No relevant conflicts of interest
References:
- 1.Christmann RB, Wells AU, Capelozzi VL, Silver RM. Gastroesophageal reflux incites interstitial lung disease in systemic sclerosis: Clinical, radiologic, histopathologic, and treatment evidence. Seminars in arthritis and rheumatism 2010;40:241–9. [DOI] [PubMed] [Google Scholar]
- 2.Wells AU. Interstitial lung disease in systemic sclerosis. Presse Med 2014;43:e329–43. [DOI] [PubMed] [Google Scholar]
- 3.Winstone TA, Assayag D, Wilcox PG, Dunne JV, Hague CJ, Leipsic J, et al. Predictors of mortality and progression in scleroderma-associated interstitial lung disease: A systematic review. Chest 2014;146:422–36. [DOI] [PubMed] [Google Scholar]
- 4.Liu X, Mayes MD, Pedroza C, Draeger HT, Gonzalez EB, Harper BE, et al. Does c-reactive protein predict the long-term progression of interstitial lung disease and survival in patients with early systemic sclerosis? Arthritis care & research 2013;65:1375–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Assassi S, Sharif R, Lasky RE, McNearney TA, Estrada YMRM, Draeger H, et al. Predictors of interstitial lung disease in early systemic sclerosis: A prospective longitudinal study of the genisos cohort. Arthritis research & therapy 2010;12:R166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sjogren RW. Gastrointestinal motility disorders in scleroderma. Arthritis Rheum 1994;37:1265–82. [DOI] [PubMed] [Google Scholar]
- 7.Pitrez EH, Bredemeier M, Xavier RM, Capobianco KG, Restelli VG, Vieira MV, et al. Oesophageal dysmotility in systemic sclerosis: Comparison of hrct and scintigraphy. Br J Radiol 2006;79:719–24. [DOI] [PubMed] [Google Scholar]
- 8.Marie I, Dominique S, Levesque H, Ducrotte P, Denis P, Hellot MF, et al. Esophageal involvement and pulmonary manifestations in systemic sclerosis. Arthritis Rheum 2001;45:346–54. [DOI] [PubMed] [Google Scholar]
- 9.Richardson C, Agrawal R, Lee J, Almagor O, Nelson R, Varga J, et al. Esophageal dilatation and interstitial lung disease in systemic sclerosis: A cross-sectional study. Seminars in arthritis and rheumatism 2016;46:109–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hoffmann A, Lee J, Ma M, Agrawal R, Chang RW, Richardson C, et al. Comment on "esophageal dilatation and interstitial lung disease in systemic sclerosis: A cross-sectional study". Seminars in arthritis and rheumatism 2016;46:e11–2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Johnson DA, Drane WE, Curran J, Cattau EL Jr., Ciarleglio C, Khan A, et al. Pulmonary disease in progressive systemic sclerosis. A complication of gastroesophageal reflux and occult aspiration? Arch Intern Med 1989;149:589–93. [PubMed] [Google Scholar]
- 12.Lock G, Pfeifer M, Straub RH, Zeuner M, Lang B, Scholmerich J, et al. Association of esophageal dysfunction and pulmonary function impairment in systemic sclerosis. Am J Gastroenterol 1998;93:341–5. [DOI] [PubMed] [Google Scholar]
- 13.Lee JS, Collard HR, Raghu G, Sweet MP, Hays SR, Campos GM, et al. Does chronic microaspiration cause idiopathic pulmonary fibrosis? Am J Med 2010;123:304–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Raghu G, Yang ST, Spada C, Hayes J, Pellegrini CA. Sole treatment of acid gastroesophageal reflux in idiopathic pulmonary fibrosis: A case series. Chest 2006;129:794–800. [DOI] [PubMed] [Google Scholar]
- 15.van den Hoogen F, Khanna D, Fransen J, Johnson SR, Baron M, Tyndall A, et al. 2013 classification criteria for systemic sclerosis: An american college of rheumatology/european league against rheumatism collaborative initiative. Arthritis Rheum 2013;65:2737–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Galie N, Hoeper MM, Humbert M, Torbicki A, Vachiery JL, Barbera JA, et al. Guidelines for the diagnosis and treatment of pulmonary hypertension: The task force for the diagnosis and treatment of pulmonary hypertension of the european society of cardiology (esc) and the european respiratory society (ers), endorsed by the international society of heart and lung transplantation (ishlt). Eur Heart J 2009;30:2493–537. [DOI] [PubMed] [Google Scholar]
- 17.Kazerooni EA, Martinez FJ, Flint A, Jamadar DA, Gross BH, Spizarny DL, et al. Thin-section ct obtained at 10-mm increments versus limited three-level thin-section ct for idiopathic pulmonary fibrosis: Correlation with pathologic scoring. AJR Am J Roentgenol 1997;169:977–83. [DOI] [PubMed] [Google Scholar]
- 18.Hankinson JL, Odencrantz JR, Fedan KB. Spirometric reference values from a sample of the general u.S. Population. American journal of respiratory and critical care medicine 1999;159:179–87. [DOI] [PubMed] [Google Scholar]
- 19.Macintyre N, Crapo RO, Viegi G, Johnson DC, van der Grinten CP, Brusasco V, et al. Standardisation of the single-breath determination of carbon monoxide uptake in the lung. Eur Respir J 2005;26:720–35. [DOI] [PubMed] [Google Scholar]
- 20.Tashkin DP, Roth MD, Clements PJ, Furst DE, Khanna D, Kleerup EC, et al. Mycophenolate mofetil versus oral cyclophosphamide in scleroderma-related interstitial lung disease (sls ii): A randomised controlled, double-blind, parallel group trial. Lancet Respir Med 2016;4:708–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Goh NS, Hoyles RK, Denton CP, Hansell DM, Renzoni EA, Maher TM, et al. Short-term pulmonary function trends are predictive of mortality in interstitial lung disease associated with systemic sclerosis. Arthritis Rheumatol 2017;69:1670–8. [DOI] [PubMed] [Google Scholar]
- 22.Goh NS, Desai SR, Anagnostopoulos C, Hansell DM, Hoyles RK, Sato H, et al. Increased epithelial permeability in pulmonary fibrosis in relation to disease progression. Eur Respir J 2011;38:184–90. [DOI] [PubMed] [Google Scholar]
- 23.Bouros D, Wells AU, Nicholson AG, Colby TV, Polychronopoulos V, Pantelidis P, et al. Histopathologic subsets of fibrosing alveolitis in patients with systemic sclerosis and their relationship to outcome. Am J Respir Crit Care Med 2002;165:1581–6. [DOI] [PubMed] [Google Scholar]
- 24.du Bois RM, Weycker D, Albera C, Bradford WZ, Costabel U, Kartashov A, et al. Forced vital capacity in patients with idiopathic pulmonary fibrosis: Test properties and minimal clinically important difference. American journal of respiratory and critical care medicine 2011;184:1382–9. [DOI] [PubMed] [Google Scholar]
- 25.Kafaja S, Clements PJ, Wilhalme H, Tseng CH, Furst DE, Kim GH, et al. Reliability and minimal clinically important differences of forced vital capacity: Results from the scleroderma lung studies (sls-i and sls-ii). Am J Respir Crit Care Med 2018;197:644–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Khanna D, Mittoo S, Aggarwal R, Proudman SM, Dalbeth N, Matteson EL, et al. Connective tissue disease-associated interstitial lung diseases (ctd-ild) - report from omeract ctd-ild working group. J Rheumatol 2015;42:2168–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Savarino E, Bazzica M, Zentilin P, Pohl D, Parodi A, Cittadini G, et al. Gastroesophageal reflux and pulmonary fibrosis in scleroderma: A study using ph-impedance monitoring. American journal of respiratory and critical care medicine 2009;179:408–13. [DOI] [PubMed] [Google Scholar]
- 28.Winstone TA, Hague CJ, Soon J, Sulaiman N, Murphy D, Leipsic J, et al. Oesophageal diameter is associated with severity but not progression of systemic sclerosis-associated interstitial lung disease. Respirology 2018;23:921–6. [DOI] [PubMed] [Google Scholar]
- 29.Steen V. Predictors of end stage lung disease in systemic sclerosis. Ann Rheum Dis 2003;62:97–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Steele R, Hudson M, Lo E, Baron M, Canadian Scleroderma Research G. Clinical decision rule to predict the presence of interstitial lung disease in systemic sclerosis. Arthritis care & research 2012;64:519–24. [DOI] [PubMed] [Google Scholar]
- 31.Suliman YA, Dobrota R, Huscher D, Nguyen-Kim TD, Maurer B, Jordan S, et al. Brief report: Pulmonary function tests: High rate of false-negative results in the early detection and screening of scleroderma-related interstitial lung disease. Arthritis Rheumatol 2015;67:3256–61. [DOI] [PubMed] [Google Scholar]
- 32.Vonk MC, van Die CE, Snoeren MM, Bhansing KJ, van Riel PL, Fransen J, et al. Oesophageal dilatation on high-resolution computed tomography scan of the lungs as a sign of scleroderma. Ann Rheum Dis 2008;67:1317–21. [DOI] [PubMed] [Google Scholar]
- 33.Tashkin DP, Elashoff R, Clements PJ, Goldin J, Roth MD, Furst DE, et al. Cyclophosphamide versus placebo in scleroderma lung disease. N Engl J Med 2006;354:2655–66. [DOI] [PubMed] [Google Scholar]
- 34.Lee JS, Collard HR, Anstrom KJ, Martinez FJ, Noth I, Roberts RS, et al. Anti-acid treatment and disease progression in idiopathic pulmonary fibrosis: An analysis of data from three randomised controlled trials. Lancet Respir Med 2013;1:369–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lee JS, Ryu JH, Elicker BM, Lydell CP, Jones KD, Wolters PJ, et al. Gastroesophageal reflux therapy is associated with longer survival in patients with idiopathic pulmonary fibrosis. Am J Respir Crit Care Med 2011;184:1390–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Distler O, Highland KB, Gahlemann M, Azuma A, Fischer A, Mayes MD, et al. Nintedanib for systemic sclerosis-associated interstitial lung disease. N Engl J Med 2019;380:2518–28. [DOI] [PubMed] [Google Scholar]
- 37.Tashkin DP, Volkmann ER, Tseng CH, Kim HJ, Goldin J, Clements P, et al. Relationship between quantitative radiographic assessments of interstitial lung disease and physiological and clinical features of systemic sclerosis. Ann Rheum Dis 2016;75:374–81. [DOI] [PubMed] [Google Scholar]
- 38.Caron M, Hoa S, Hudson M, Schwartzman K, Steele R. Pulmonary function tests as outcomes for systemic sclerosis interstitial lung disease. Eur Respir Rev 2018;27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Frech TM, Shanmugam VK, Shah AA, Assassi S, Gordon JK, Hant FN, et al. Treatment of early diffuse systemic sclerosis skin disease. Clin Exp Rheumatol 2013;31:166–71. [PMC free article] [PubMed] [Google Scholar]
- 40.Minier T, Guiducci S, Bellando-Randone S, Bruni C, Lepri G, Czirjak L, et al. Preliminary analysis of the very early diagnosis of systemic sclerosis (vedoss) eustar multicentre study: Evidence for puffy fingers as a pivotal sign for suspicion of systemic sclerosis. Ann Rheum Dis 2014;73:2087–93. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.