Abstract
Pneumocystis carinii pneumonia remains one of the most serious complications of immunosuppressed patients. In this study, the in vitro pharmacodynamic parameters of four sordarin derivatives (GM 191519, GM 237354, GM 193663, and GM 219771) have been evaluated by a new quantitative approach and compared with the commercially available drugs pentamidine, atovaquone, and trimethoprim-sulfamethoxazole (TMP-SMX). In vitro activities and in vivo therapeutic efficacies of sordarin derivatives against P. carinii were also evaluated. In vitro activity was determined by the broth microdilution technique, comparing the total number of microorganisms in treated and drug-free cultures by using Giemsa staining. The in vitro maximum effect (Emax), the drug concentrations to reach 50% of Emax (EC50), and the slope of the dose-response curve were then estimated by the Hill equation (Emax sigmoid model). Sordarin derivatives were the most potent agents against P. carinii, with EC50s of 0.00025, 0.0007, 0.0043, and 0.025 μg/ml for GM 191519, GM 237354, GM 193663, and GM 219771, respectively. The EC50s of pentamidine, atovaquone, and TMP-SMX were 0.025, 0.16, and 26.7/133.5 μg/ml, respectively. The results obtained with this approach showed GM 237354 and GM 191519 to be approximately 35- and 100-fold more active in vitro than pentamidine, the most active marketed compound. All sordarin derivatives tested were at least 5,000-fold more active in vitro than TMP-SMX. The three sordarin derivatives tested in vivo—GM 191519, GM 237354, and GM 219771—showed a marked therapeutic efficacy, defined as reduction of cyst forms per gram of lung. GM 191519 was the most potent (daily dose reducing 50% of the P. carinii burden in the lungs [ED50], 0.05 mg/kg/day) followed by GM 237354 and GM 219771 (ED50s, 0.30 and 0.49 mg/kg/day, respectively). Good agreement between in vitro parameters and in vivo outcome was obtained when P. carinii pneumonia in rats was treated with sordarin derivatives.
Pneumocystis carinii is an important opportunistic pathogen that remains a significant cause of lethal pneumonia in immunocompromised individuals such as patients with AIDS and patients receiving chemotherapy or immunosuppressive drugs for organ transplantation or other pathological conditions. Patients suffering from P. carinii pneumonia (PCP) are usually treated with trimethoprim-sulfamethoxazole (TMP-SMX) or pentamidine (24). However, the relatively high frequency of adverse reactions to these drugs reflects the need for new therapeutic approaches. For this reason, the pharmaceutical industry is investigating more effective and less toxic agents.
Sordarin derivatives are a new class of antifungal agents that target protein synthesis (11), with marked in vitro activity against P. carinii (13) and excellent in vivo activity in experimental PCP (E. Dei-Cas, E. M. Aliouat, C. Mullet, E. Mazars, and D. Gargallo, Abstr. 37th Intersci. Conf. Antimicrob. Agents Chemother., abstr. F-65, 1997).
Well-defined mouse, rat, or rabbit experimental models (2, 4, 22) can be used to describe the in vivo anti-PCP activity of new compounds. Several in vitro tests for evaluating compound activity against P. carinii have been described using axenic cultures or coculture with feeder cells (8, 10, 14). However, no universally accepted standard method is presently available for the in vitro evaluation of anti-P. carinii molecules (10). The anti-Pneumocystis activity of any given antimicrobial could be evaluated in terms of its intrinsic activity (in vitro) and serum time profile (in vivo) (12). However, the results obtained with different in vitro assays (8, 10) yield limited information on the intrinsic activity of anti-Pneumocystis compounds. Furthermore, comparisons between product activities and extrapolation to in vivo activity remain unreliable.
The Hill equation, which describes sigmoid concentration-effect relationships, has proven its utility by revealing in vitro pharmacodynamic properties of several antibiotics (17, 30). This approach offers at least three parameters which can be used to describe the in vitro activity of antimicrobial compounds (17): the maximum effect (Emax) as a measure of efficacy, the 50% effective concentration (EC50) as a parameter of intrinsic activity, and the slope (γ) of the concentration-effect relationship.
The aim of the present work was to establish the experimental conditions allowing definition of the in vitro pharmacodynamic parameters of tested drugs for defining the intrinsic activity of anti-Pneumocystis molecules, as well as the relationships between their in vitro activities and in vivo effects on microorganisms.
(This work was presented in part at the 38th Interscience Conference on Antimicrobial Agents and Chemotherapy, San Diego, Calif., 24 to 27 September, 1998 [E. M. Aliouat, P. Aviles, E. Dei-Cas, E. Herreros, L. Dujardin, and D. Gargallo-Viola, abstr. J-15].)
MATERIALS AND METHODS
Drugs.
GM 191519, GM 193663, GM 219771, and GM 237354 are new sordarin derivatives synthesized by GlaxoWellcome, S.A. (Madrid, Spain). TMP-SMX (Sigma Chemical Co., St. Louis, Mo.), pentamidine isothionate (Sigma Chemical Co.), and atovaquone (GlaxoWellcome, Greenford, United Kingdom) were also tested. Sordarin derivatives were dissolved in sterile distilled water at a starting concentration of 10 mg/ml. TMP, pentamidine, and atovaquone were dissolved in 100% dimethyl sulfoxide (DMSO) (Sigma Chemical Co.) to produce a 10-mg/ml stock solution. SMX was also dissolved in DMSO but to a concentration of 30 mg/ml. TMP and SMX solutions were mixed appropriately to obtain a final combination of 1:5. Finally, the drug stock solutions were diluted in Dulbecco's modified Eagle's medium (DMEM) (Bio-Whittaker, Boehringer Ingelheim, Brussels, Belgium) supplemented with 10% heat-inactivated fetal calf serum (FCS) (GIBCO BRL, Life Technologies Inc.) to produce the required drug concentrations. Compound solutions were prepared immediately before use.
Source of P. carinii.
Corticosteroid-treated rats were used as the source of P. carinii organisms. Seven-week-old female Wistar rats (Iffa-Credo, Lyon, France) were immunosuppressed with dexamethasone (Fortecortin; Merck, Darmstadt, Germany) administered in drinking water (2 mg/liter) for approximately 10 weeks (24). Animals had access to sterile standard food (gamma-irradiated rodent maintenance diet) and water ad libitum. At the end of the immunosuppression period the rats were sacrificed and P. carinii was recovered from their lungs. The research complied with national legislation, with company policy on the care and use of animals, and with related guidelines (1).
Isolation and quantitation of P. carinii organisms.
P. carinii organisms were isolated as previously described (3), with some modifications. After the immunosuppression period, rat lungs were removed aseptically and cut into small pieces in sterile DMEM. P. carinii organisms were extracted by agitation of lung pieces with a magnetic stirrer for 1 h at 4°C. To remove tissue debris, the resulting homogenate was poured through sterile gauze and centrifuged at 2,900 × g for 10 min at 4°C. After centrifugation, the pellet was resuspended in a buffered hemolytic solution (9:1 solution of 0.15 M NH4Cl in 20 mM Tris-HCl, pH 7.4), incubated for 10 min at 4°C, and centrifuged. Then, the pellet was resuspended in DMEM and filtered successively through 250- to 63-μm-pore-size stainless steel filters. Finally, a polysucrose gradient (Histopaque-1077; Sigma Chemical Co.), to obtain purified Pneumocystis organisms with a minimum of host contamination, was performed as follows. Polysucrose solution and inoculum suspended in DMEM were prepared 1:1 (vol/vol) in a 15-ml tube (Costar Corporation, Cambridge, Mass.) and centrifuged at 1,000 × g for 15 min at 4°C. The band accumulated at the interface between DMEM and polysucrose solution was collected and washed twice with DMEM (2,900 × g for 10 min at 4°C). P. carinii was quantitated on air-dried smears stained with RAL-555 (Réactifs RAL), a rapid panoptic methanol-Giemsa stain, which stains every Pneumocystis life cycle stage. In addition, samples were used to search for putative contaminant organisms. Moreover, final suspension was plated on blood (Difco, Detroit, Mich.) and Sabouraud dextrose agar (Difco) for the detection of bacterial or fungal contamination, respectively. The total numbers of P. carinii forms (trophozoites, precysts, and cysts) were calculated as previously described (3): (n · Sa · R)/Fa, where n is the average number of microorganisms per oil immersion field (10 fields were counted for each smear), Sa is the 2-μl smear area, R is the ratio between total volume of the microorganism suspension and calibrate smear volume (2 μl), and Fa is the oil immersion field area.
Axenic in vitro culture of P. carinii.
In order to determine the in vitro drug susceptibility of P. carinii, axenic cultures of the organism were produced as follows. All the experiments were carried out in 24-well plates (Nalge Nunc International, Roskilde, Denmark) with a final volume of 2 ml of DMEM supplemented with 10% FCS containing a final inoculum of 1.5 × 106 organisms per ml. Plates with organisms were incubated for 5 days in an atmosphere of 5% CO2 at 37°C, and the kinetic patterns of in vitro P. carinii development were determined. Daily for 5 consecutive days, the total volume of each well was removed and centrifuged for 10 min at 2,900 × g, and the pellet was resuspended with 200 μl of phosphate buffer solution Dulbecco (Sigma Chemical Co.). Two-microliter smears were obtained from each suspension. P. carinii organisms were stained with RAL-555 and were quantified as described above. All experiments were performed in triplicate.
In vitro susceptibility studies.
The above method was validated by performing three independent experiments involving GM 237354 as reference compound. Concentration-response curves were calculated, and the corresponding Emax, EC50, and slope were obtained.
In vitro susceptibility studies were performed using the twofold broth microdilution technique. Final drug concentrations ranged from 5 × 101 to 5 × 10−7 μg/ml for GM 191519, GM 193663, GM 219771, GM 237354, pentamidine, and atovaquone. The TMP-SMX combination was tested from 150/750 to 1 × 10−5/5 × 10−5 μg/ml. Plates were incubated for 4 days in an atmosphere of 5% CO2 at 37°C. One drug-free control was included in each assay. Microorganisms were quantified on homogenate smears as described above. All susceptibility assays were performed in triplicate.
Analysis of results.
The anti-Pneumocystis activity of a single concentration of compound may be expressed in terms of percent inhibition, defined as the decrease (expressed as percentage) in P. carinii forms in antifungal-treated cultures with respect to the total microorganism count in compound-free culture. Once all the differences between drug-treated and untreated wells were calculated, the concentration-effect relationship was established by means of the Hill equation (17, 30): ER = ER,max · CS/[(EC50)S + CS], where ER is the effect of each drug concentration (C) upon percent inhibition estimated from experimental results, S is a parameter reflecting the steepness of the concentration-effect relationship curve, and EC50 is the concentration of the compound at which 50% of the maximum effect (ER,max) is obtained.
Once the ER values were fitted, the parameters of the pharmacodynamic model were calculated by nonlinear least-squares regression techniques using commercial software (WinNonlin; Scientific Consulting, Inc.).
In vivo study.
An in vivo pilot study which involved PCP in rats was performed to explore whether in vitro results reflect in vivo efficacy. PCP was established by using a previously described method. Briefly, animals were immunosuppressed with dexamethasone (Fortecortin; Merck) at a concentration of 2 mg/liter in the drinking water for 9 weeks. Tetracycline (Terramicine; Pfizer Laboratories) at 1 g/liter was added as antibacterial prophylactic agent. All animals remained on immunosuppressive therapy with dexamethasone throughout the study. Before treatment, PCP was microscopically verified as previously reported (28). Animals were divided into groups of six, and then, sordarin derivatives (GM 191519, GM 237354, and GM 219771) were dosed at 0.1, 1.0 and 5.0 mg/kg by subcutaneous route. The drugs were given twice a day for 10 consecutive days. Control animals were dosed with sterile water. Twenty-four hours after the last dose all animals were sacrificed by overdosing of sodium pentobarbital (Euthalender; Normon). Lungs were aseptically removed and weighted. P. carinii extractions were performed by using a previously described method (2). P. carinii cystic forms were quantitated by Toluidine Blue-0 staining (Sigma-Aldrich, S.A.). The number of cysts were determined by visual assessment by light microscopy (20 microscopic fields). All results were expressed as log10 of the number of cysts per gram of lung (logQ/g). Daily dose (milligrams per kilogram per day) and logQ/g were plotted and adjusted to an Emax model by nonlinear least-squares regression techniques using commercial software (WinNonlin). Then, ED50 (defined as the daily dose which reduces 50% the P. carinii burden in lungs) was calculated.
RESULTS
In vitro P. carinii growth.
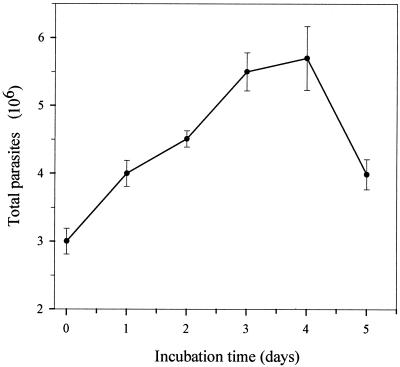
Figure 1 shows the P. carinii growth curve obtained after 5 days of incubation. The curve displays two well-defined portions: a first segment (from day 0 to 3 or 4), where P. carinii gradually increases in number, with doubling times of 85.3 h (up to day 3) and 102.7 h (up to day 4), and a second segment, reflecting P. carinii population decrements after the fourth day of incubation. Moreover, the formation of large, typical trophozoite clusters was observed throughout the incubation period, while the number of cystic forms gradually decreased in proportion, to less than 2% of total microorganisms after 3 days of incubation.
FIG. 1.
Typical growth curve of rat-derived P. carinii cultivated in DMEM supplemented with 10% FCS.
Optimal assay setup conditions.
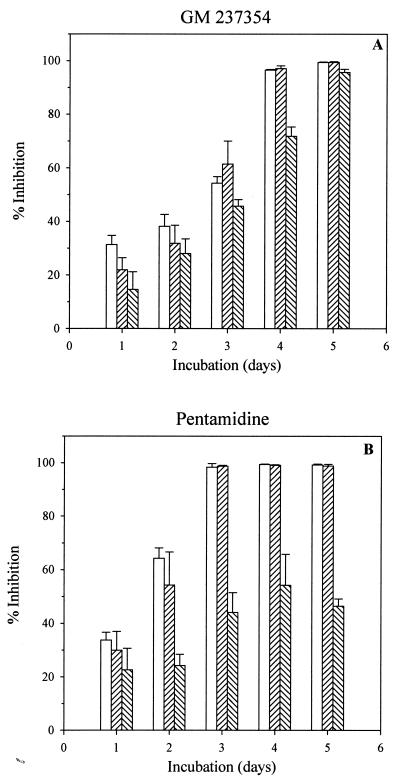
Preliminary experiments were performed using pentamidine and GM 237354. High (10-μg/ml), medium (1-μg/ml), and low (0.01-μg/ml) concentrations of compound were tested against P. carinii for 5 days of culture. Percent inhibition compared to drug-free control was determined (Fig. 2). When P. carinii was incubated for 1 to 3 days with three concentrations of GM 237354, relatively low growth inhibition was observed, even when the highest concentration (10 μg/ml) was tested. Maximum inhibition rates of 38 and 60% were observed after 2 or 3 days of culture, respectively (Fig. 2A). However, after 3 days of culture, pentamidine (10 and 1 μg/ml) reached 100% inhibition (Fig. 2B). Both concentrations, i.e., 10 and 1 μg of pentamidine and GM 237354 per ml, had the maximum effect by the fourth day of incubation. The inhibitory effect of pentamidine at 0.01 μg/ml showed similar levels (44 to 54% inhibition) after 3 to 5 days of culture, whereas the GM 237354 inhibitory effect reached 70 and 96% inhibition at 4 and 5 days postinoculation, respectively (Fig. 2A).
FIG. 2.
Influence of incubation time on the in vitro activities of GM 237354 and pentamidine for 5 consecutive days of culture. Both compounds were tested at three different concentrations. □, 10 μg/ml; ▨, 1 μg/ml; ▧, 0.01 μg/ml. The effect of each compound concentration on P. carinii was expressed as percent inhibition versus the drug-free control.
Considering both these results and the behavior of the P. carinii population, 4 days of culture was selected as the best assay duration for in vitro anti-Pneumocystis drug susceptibility studies under our experimental conditions. In further studies a wider range of drug concentrations was used (5 × 101 to 5 × 10−7 μg/ml).
In vitro susceptibility studies.
The robustness of the method was assayed by performing three different experiments with GM 237354. All the three resulting concentration-response curves obtained essentially overlapped (data not shown). Mean calculated values for Emax (99.06%), EC50 (0.00076 μg/ml), and the slope (0.53) showed variation coefficients of 2, 20, and 11.5%, respectively.
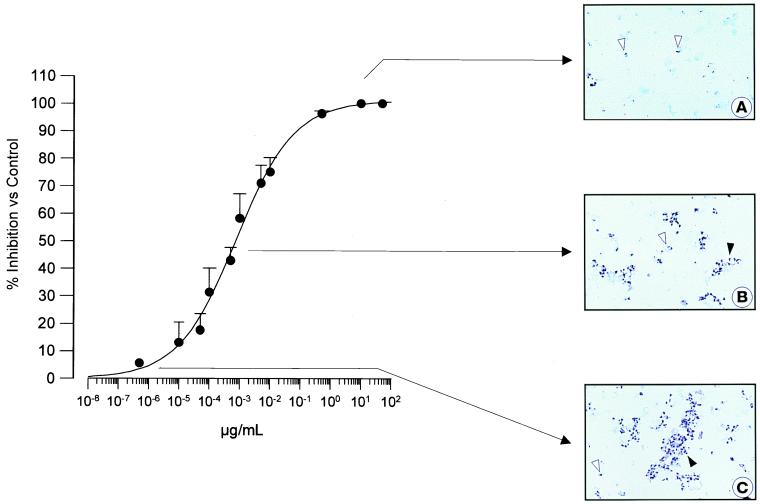
Figure 3 shows a concentration-response curve obtained after 4 days of incubation of P. carinii with GM 237354 (concentration range, 5 × 101 to 5 × 10−7 μg/ml). The reduction in the number of microorganisms detected per field was gradual and concentration dependent. Figures 3A and B show an evident decrease in P. carinii forms when the culture was incubated with 0.005 and 0.5 μg/ml, respectively. These concentrations correspond to the upper and middle portion of the concentration-response curve. Figure 3C shows the characteristic appearance of a drug-free culture.
FIG. 3.
In vitro activity of GM 237354 against P. carinii. (A) Culture medium with 0.5 μg of GM 237354 per ml. A dramatic reduction in the number of microorganisms was observed. (B) Culture medium with 5 × 10−4 μg of GM 237354 per ml. A marked decrease in the number of microorganisms was observed. (C) Drug-free control culture. Trophozoites (open arrowhead) and trophozoite clusters (solid arrowhead) were observed. RAL-555 staining was used. Magnification, ×1,100.
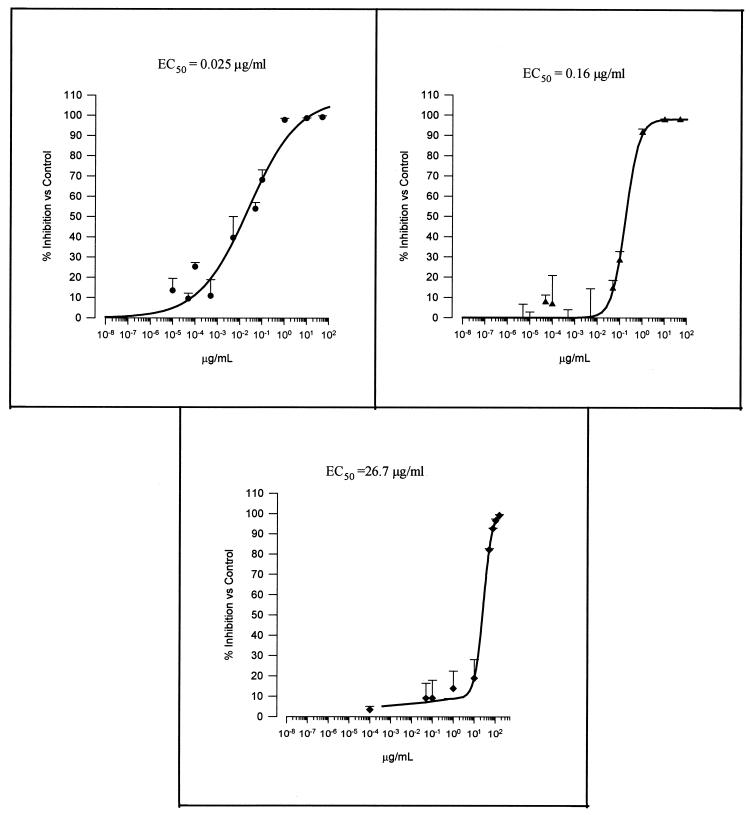
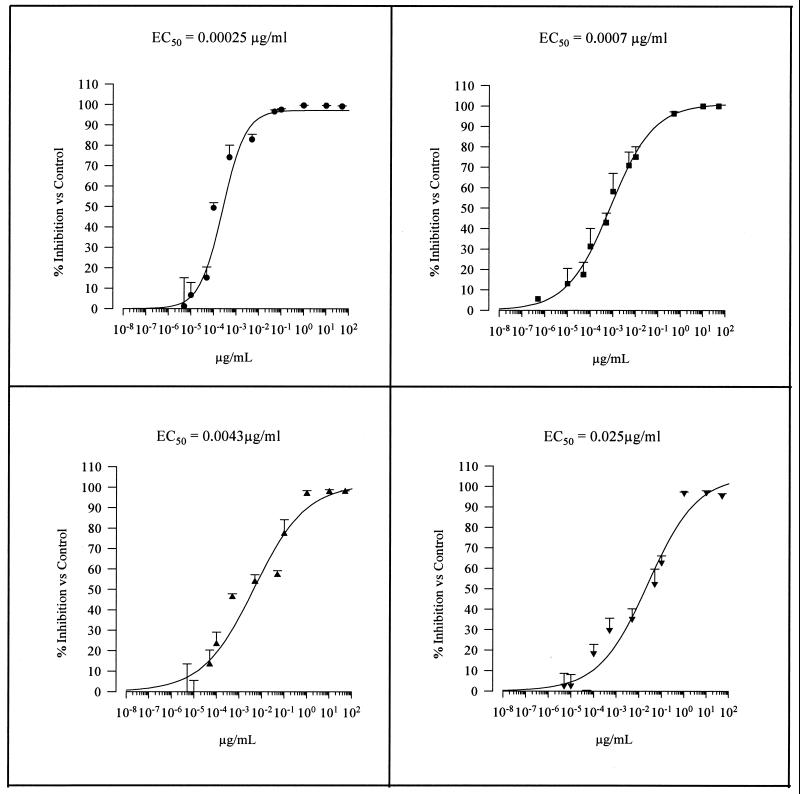
Figures 4 and 5 show the concentration-response curves obtained for sordarin derivatives and marketed compounds, respectively. All tested compounds inhibited P. carinii growth compared with drug-free control cultures. TMP-SMX demonstrated the lowest intrinsic activity, followed by atovaquone and pentamidine (EC50s, 26.7/133.5, 0.16, and 0.025, respectively). In terms of efficacy, both pentamidine and atovaquone reached about 100% inhibition at a concentration of 1 μg/ml. However, TMP-SMX reached the same inhibition level (100%) at high concentrations (75/375 μg/ml).
FIG. 4.
Concentration-in vitro activity relationships of the three commercial compounds against P. carinii. ●, pentamidine; ▴, atovaquone; ⧫, TMP-SMX. Results were calculated after 4 days of incubation.
FIG. 5.
Concentration-in vitro activity relationships of the four sordarin derivatives against P. carinii. ●, GM 191519; ■, GM 237354; ▴, GM 193663; ▾, GM 219771. Results were calculated after 4 days of incubation.
Sordarin derivatives exhibit a differential potency (EC50) ranging from 0.00025 μg/ml (GM 191519) to 0.025 μg/ml (GM 219771) (Fig. 5). In fact, the less potent sordarin derivative (GM 219771), which was 100 times less active than the most efficient derivative, exhibits a potency equal to that of pentamidine, which in turn was the most potent of the marketed compounds. This differential in vitro activity was also reflected when sordarin derivatives were used for the treatment of experimental PCP in rats.
In vivo study.
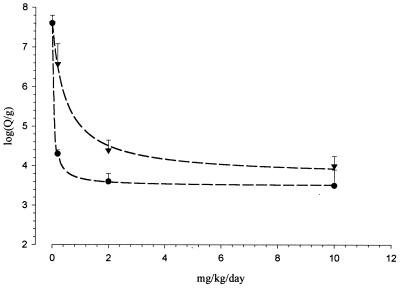
The number of P. carinii cysts per gram of lung in untreated animals which develop PCP reaches 107.6 at the end of the treatment period (73 days after the start of dexamethasone immunosuppression). All the three sordarin derivatives caused a marked reduction in the number of P. carinii cyst forms per gram of lung. Dose-response curves obtained after sordarin derivative treatment of experimental PCP in rats are displayed in Fig. 6. Good agreement between experimentally observed and calculated values was obtained (r2, 0.999, 0.980, and 0.998 for GM 191519, GM 237354, and GM 219771, respectively) for each therapeutic regimen used. Calculated ED50s for sordarin derivatives demonstrated that GM 191519 was the most potent of the three compounds (ED50, 0.05 mg/kg/day) followed by GM 237354 (ED50, 0.30 mg/kg/day) and GM 219771 (ED50, 0.49 mg/kg/day). These in vivo potencies are in the same order as those observed for in vitro results.
FIG. 6.
In vivo efficacies of GM 191519 (●) and GM 219771 (▾) on the P. carinii burden in lung. The data are means with standard deviations for six animals. The dose-effect relationships were calculated according a simple Emax model.
DISCUSSION
A quantitative and reproducible in vitro susceptibility axenic model for evaluating pharmacodynamic parameters of anti-Pneumocystis compounds is described in the present study. The results obtained indicate that in vitro EC50 could be an index of the expected anti-PCP in vivo effect, at least for sordarin derivatives. Pharmacodynamic parameters of anti-Pneumocystis drugs were calculated using the Hill equation, which has been previously used to describe in vitro concentration-effect relationships of antibacterial compounds (17, 30). Antimicrobial agents were evaluated by comparing P. carinii growth in drug-treated versus drug-free control cultures after a 4-day incubation period. Microorganism growth was assessed on dry smears stained by methanol-Giemsa-like staining (RAL-555), which allows total microorganism quantitation. Most P. carinii microorganisms developing in this system are vegetative forms. In agreement with other reports (5, 16), we found that in vitro Pneumocystis proliferation leads to the formation of clusters in which more than 98% are trophozoite forms.
In vitro susceptibility tests are a basic step in any pharmacological screening for new anti-infective drugs. Several factors influence the outcome and reproducibility of susceptibility tests (6), including medium composition, inoculum size, incubation time, and the nature of the microorganism. In the present study, P. carinii organisms were cultivated by means of an axenic culture rather than the usual coculture methods. The main reasons for adopting this approach can be summarized as follows. (i) Mammalian cell growth produces extracellular catabolic products and depletes broth nutrients—processes capable of affecting P. carinii growth and influencing the susceptibility patterns. (ii) Developing cocultured cells could influence the stability of tested compounds. (iii) New compounds can display toxicity against monolayer cells. This fact could affect P. carinii growth and hence the susceptibility results obtained. (iv) Microorganisms attached to cell monolayers could acquire susceptibility patterns different from those of unattached microorganisms in suspension due to several factors, such as different growth rates, metabolic processes, or drug accessibilities. (v) Interactions between unattached microorganisms and compounds are probably easier than those found when microorganisms are attached to target cells. All the above considerations could also negatively affect interlaboratory reproducibility. Furthermore, in order to minimize the presence of a biological matrix in the culture medium, FCS should be replaced by a synthetic substitute, since the presence of serum could have multiple effects in susceptibility testing. Some authors have reported compound inactivation as a result of drug binding to serum components (9), where only the free fraction shows antimicrobial activity. Moreover, considering the behavior of P. carinii in in vitro cultures, some authors have attempted to improve culture performance by using cocultured cells to evaluate susceptibility. However, the presence of mammalian cells in the system could add a poorly controlled variable and potentially result in a lack of assay reproducibility.
Another variable with specific weight in drug susceptibility test outcomes is incubation time. Our results show that this parameter must be considered to establish an effective compromise between microorganism growth in drug-free controls and microorganism inhibition in drug-treated microculture wells.
In the antibacterial field, the MIC is the most widely used parameter for determining susceptibility. For this reason, internationally accepted guidelines have been published (18) for performing the tests so as to avoid or minimize interlaboratory variability. Similar initiatives have been proposed by the international scientific community for other tests which offer complementary information on the in vitro activities of antibacterial drugs (20, 21). More recently a standard method was proposed for pathogenic yeast (19). P. carinii has recently been included in the fungal kingdom, though it may be regarded as an atypical fungus (29): it lacks ergosterol (a target for most antifungals) and refuses to grow well in vitro (25). Recent efforts have been made to improve this situation (16), however.
Antigenic and genomic host species-related differences have been reported among P. carinii isolates, yielding a strong host species specificity (4). However, it has not been proven whether Pneumocystis strains from different mammal hosts can exhibit different drug susceptibility patterns. The development of an in vitro pharmacodynamic model affords an improved tool for the evaluation of susceptibility patterns of Pneumocystis strains from different host species.
Mouton et al. have offered a pharmacological explanation for the parameters in the Hill equation (17). They also suggested that the Hill model could be very useful for understanding changes in susceptibility, relating EC50 values to classical MICs. The findings described herein provide sufficient information for considering EC50 to be an accurate indicator of in vitro activities of anti-Pneumocystis compounds. Such an approach will provide a new tool for selecting new compounds and establishing therapeutic protocols. By using this pharmacodynamic approach, the results obtained revealed a high in vitro anti-Pneumocystis activity on the part of sordarin derivatives which belong to a new family of antifungals targeting fungal protein synthesis (11). Moreover, EC50 seems to reflect in vivo anti-Pneumocystis activity, as sordarin derivative in vivo ED50s suggest. Future experimental in vivo work is warranted to further establish the performance of this new in vitro approach with other anti-Pneumocystis compounds.
TABLE 1.
In vitro susceptibilities of P. carinii to marketed compounds and sordarin derivatives
| Antifungal agent | EC50 (μg/ml) |
|---|---|
| Pentamidine | 0.025 |
| Atovaquone | 0.16 |
| TMP-SMX | 26.7/133.5 |
| Sordarins | |
| GM 191519 | 0.00025 |
| GM 237354 | 0.0007 |
| GM 193663 | 0.0043 |
| GM 219771 | 0.025 |
ACKNOWLEDGMENTS
The Pasteur Institute team was supported by the French Ministry of Research (PRFMMIP program).
We thank María José Guillén and Elena Jiménez for excellent technical assistance.
REFERENCES
- 1.Acred P, Hennessey T D, MacArthur-Clark J A, Merrikin D J, Ryan D M, Smulders H C, Troke P F, Wilson R G, Straughan D W. Guidelines for the welfare of animals in rodent protection tests. A report from the rodent test working party. Lab Anim. 1994;28:13–18. doi: 10.1258/002367794781065870. [DOI] [PubMed] [Google Scholar]
- 2.Aliouat E M, Martinez A, Jimenez E, Dei-Cas E, Mullet C, Delcourt P, Gargallo-Viola D. Development of Pneumocystis animal models: corticosteroid-treated Wistar rat; SCID mouse and Nude rat. J Eukaryot Microbiol. 1997;44:41S–42S. doi: 10.1111/j.1550-7408.1997.tb05765.x. [DOI] [PubMed] [Google Scholar]
- 3.Aliouat E M, Dei-Cas E, Ouassi M A, Palluault F, Soulez B, Camus D. In vitro attachment of Pneumocystis carinii from mouse and rat origin. Biol Cell. 1993;77:209–217. doi: 10.1016/s0248-4900(05)80190-x. [DOI] [PubMed] [Google Scholar]
- 4.Aliouat E M, Mazars E, Dei-Cas E, Cesbron J Y, Camus D. Intranasal inoculation of mouse, rat or rabbit-derived Pneumocystis in SCID mouse. J Protozool Res. 1993;3:94–98. [Google Scholar]
- 5.Aliouat E M, Dujardin L, Martinez A, Duriez T, Dei-Cas E. Pneumocystis carinii growth kinetics in culture systems and in hosts: involvement of each life cycle parasite stage. J Eukaryot Microbiol. 1999;46:116S–117S. [PubMed] [Google Scholar]
- 6.Amsterdam D. Susceptibility testing of antimicrobials in liquid media. In: Lorian V, editor. Antibiotics in laboratory medicine. 3rd ed. Baltimore, Md: Williams & Wilkins, Ltd.; 1991. pp. 53–105. [Google Scholar]
- 7.Barlett M S. Models for evaluating compounds for activity against Pneumocystis carinii. Eur J Clin Microbiol Infect Dis. 1991;10:199–201. doi: 10.1007/BF01964463. [DOI] [PubMed] [Google Scholar]
- 8.Cirioni O, Giacometti A, Scalise G. In vitro activity of atovaquone, sulfamethoxazole and dapsone alone and combined with inhibitors of dihydrofolate reductase and macrolides against Pneumocystis carinii. J Antimicrob Chemother. 1997;39:45–51. doi: 10.1093/jac/39.1.45. [DOI] [PubMed] [Google Scholar]
- 9.Craig W A, Suh B. Protein binding and the antimicrobial effects: methods for the determination of protein binding. In: Lorian V, editor. Antibiotics in laboratory medicine. 3rd ed. Baltimore, Md: Williams & Wilkins, Ltd.; 1991. pp. 367–402. [Google Scholar]
- 10.Cushion M T. In vitro studies of Pneumocystis carinii. J Protozool. 1989;36:45–52. doi: 10.1111/j.1550-7408.1989.tb02691.x. [DOI] [PubMed] [Google Scholar]
- 11.Dominguez J M, Kelly V A, Kinsman O S, Marriott M S, Gomez de las Heras F, Martin J J. Sordarins: a new class of antifungals with selective inhibition of the protein synthesis elongation cycle in yeasts. Antimicrob Agents Chemother. 1998;42:2274–2278. doi: 10.1128/aac.42.9.2274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Drusano G L. Role of pharmacokinetics in the outcome of infections. Antimicrob Agents Chemother. 1988;32:289–297. doi: 10.1128/aac.32.3.289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Herreros E, Martinez C M, Almela M J, Marriott M S, Gomez de las Heras F, Gargallo-Viola D. Sordarins: in vitro activities of new antifungal derivatives against pathogenic yeasts, Pneumocystis carinii, and filamentous fungi. Antimicrob Agents Chemother. 1998;42:2863–2869. doi: 10.1128/aac.42.11.2863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Herreros E, Almela M J, Martinez C M, Lozano S, Jackson H, Aliouat E M, Gargallo-Viola D. Microplate assays for In vitro evaluation of anti-pneumocystis drugs. J Eukaryot Microbiol. 1997;44:43S–44S. doi: 10.1111/j.1550-7408.1997.tb05766.x. [DOI] [PubMed] [Google Scholar]
- 15.Merali S, Meshnick S R. Susceptibility of Pneumocystis carinii to artemisinin in vitro. Antimicrob Agents Chemother. 1991;35:1225–1227. doi: 10.1128/aac.35.6.1225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Merali S, Frevert U, Williams J H, Chin K, Bryan R, Clarkson A B., Jr Continuous axenic cultivation of Pneumocystis carinii. Proc Natl Acad Sci USA. 1999;96:2402–2407. doi: 10.1073/pnas.96.5.2402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mouton J W, Vinks A A T M M, Punt N C. Pharmacokinetic-pharmacodynamic modeling of activity of ceftazidime during continuous and intermittent infusion. Antimicrob Agents Chemother. 1997;41:733–738. doi: 10.1128/aac.41.4.733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.National Committee for Clinical Laboratory Standards. Methods for dilution antimicrobial susceptibility tests for bacteria that grow aerobically. 2nd ed. 1990. Approved standard M7-A2 10. National Committee for Clinical Laboratory Standards, Villanova, Pa. [Google Scholar]
- 19.National Committee for Clinical Laboratory Standards. Reference method for broth dilution antifungal susceptibility testing of yeasts. Approved standard M27-A. Villanova, Pa: National Committee for Clinical Laboratory Standards; 1997. [Google Scholar]
- 20.National Committee for Clinical Laboratory Standards. Methodology for the serum bactericidal test. Approved guideline M21-A. Villanova, Pa: National Committee for Clinical Laboratory Standards; 1999. [Google Scholar]
- 21.National Committee for Clinical Laboratory Standards. Methods for determining bactericidal activity of antimicrobial agents. Approved guideline M26-A. Villanova, Pa: National Committee for Clinical Laboratory Standards; 1999. [Google Scholar]
- 22.Oz H S, Hughes W T. Effect of sex and dexamethasone dose on the experimental host for Pneumocystis carinii. Lab Anim Sci. 1996;46:109–110. [PubMed] [Google Scholar]
- 23.Pifer L L, Pifer D D, Woods D R. Biological profile and response to anti-Pneumocystis agents of Pneumocystis carinii in cell culture. Antimicrob Agents Chemother. 1983;24:673–678. doi: 10.1128/aac.24.5.674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Schmatz D M, Powles M, McFadden D C, Pittarelli L A, Liberator P A, Anderson J W. Treatment and prevention of Pneumocystis carinii pneumonia and further elucidation of the P. carinii life cycle with 1,3-beta-glucan synthesis inhibitor L-671,329. J Protozool. 1991;38:151S–153S. [PubMed] [Google Scholar]
- 25.Sloand E, Laughon B, Armstrong M, Bartlett M S, Blumenfeld W, Cushion M T, Kalica A, Kovacs J A, Martin W, Pitt E, Pesanti E L, Richards F, Rose R, Walzer P. The challenge of Pneumocystis carinii culture. J Eukaryot Microbiol. 1993;40:188–195. doi: 10.1111/j.1550-7408.1993.tb04902.x. [DOI] [PubMed] [Google Scholar]
- 26.Smith J W, Bartlett M S, Queener S F. Development of models and their use to discover new drugs for therapy and prophylaxis of Pneumocystis carinii pneumonia. In: Walzer P D, editor. Pneumocystis carinii pneumonia. New York, N.Y: Marcell Decker, Inc.; 1994. [Google Scholar]
- 27.Soulez B, Dei-Cas E, Camus D. Le lapin, hôte experimental de Pneumocystis carinii. Ann Parasitol Hum Comp. 1988;63:5–15. doi: 10.1051/parasite/19886315. [DOI] [PubMed] [Google Scholar]
- 28.Soulez B, Dei-Cas E, Palluault F, Camus D. Morphological evaluation of Pneumocystis carinii after extraction from infected lung. J Parasitol. 1991;77:449–453. [PubMed] [Google Scholar]
- 29.Stringer J R. Pneumocystis carinii: what is it, exactly? Clin Microbiol Rev. 1996;9:489–498. doi: 10.1128/cmr.9.4.489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Whiting B, Gilman A G. The modeling of drug response. Clin Sci. 1980;59:311–315. doi: 10.1042/cs0590311. [DOI] [PubMed] [Google Scholar]