Abstract
Since the beginning of laparoscopic liver surgery, resection of the posterosuperior segments has been considered one of the most challenging procedure due to its difficult access. The main drawbacks of the laparoscopic approach to dome lesions are poor visualization, the difficulty of instrumentation and the greater complexity in the control of bleeding. In the evolution of minimally invasive techniques from hybrid techniques to the current purely laparoscopic approaches, the different authors have established gradually the currents indications and surgical techniques to operate these segments with a similar feasibility and safety than open approach. The standardization in the patient position, the use of intercostal trocars, the learning curve in laparoscopic liver surgery, the management of the hepatic blood flow and the refinement of the technique in the extrahepatic and intrahepatic Glissonean pedicle approaches, has allowed to leave behind the initial contraindications about the laparoscopic approach in these segments. In the present review of the literature, the accumulated experience of the different groups in minimally invasive liver surgery together with the technological advances in the different laparoscopic devices have facilitated the resection of tumors in segments 7 and 8 with similar and even better results than open surgery.
Keywords: Laparoscopy, Hepatectomy, Segment 7, Segment 8
INTRODUCTION
Since the completion of the first laparoscopic liver resection,1 its indications have been increased until today, where it can be carried out in all segments with similar and even better results compared to open surgery. In the evolution of this approach,2-7 the lesions located in the superior part of the right anterior sector and in the posterior sectors, segments 7 and 8, were initially considered as extremely difficult for laparoscopy due to the limited visualization in relation to the diaphragm and ribs, the greater risk of bleeding and difficult control related to a higher transfusion requirement, the higher conversion rate in the initial series, the longer operative time, the greater difficulty in obtaining surgical margins and the greater technical complexity in liver mobilization. This location together with the difficulties for its access make the technical aspects have greater relevance. One of the reasons why the resection of segment 7 and 8 is so complex is because of the difficulty in securing the Glissonean pedicles.8-11 Although the hybrid approaches and hand assisted were described initially to facilitate some surgical maneuvers,12-14 the purely laparoscopic abdominal approach with the possibility of combined intercostal trocars with the growing experience15 in this field and the development of surgical devices have demonstrated their feasibility and safety.
The objective of this review was to analyze the outcomes published in the literature on laparoscopic liver resections in lesions located in segments 7 and 8 and detail the surgical technical aspects described by the different authors.
METHODS
Search strategy
A medical librarian-developed systematic search strategy was utilized to browse through Medline/PubMed, EMBASE, Scopus, ClinicalTrials.gov, the Cochrane Database of Systematic Reviews and the Cochrane Central Register of Controlled Trials using a combination of standardized index terms and plain language to cover the following terms: (laparoscopic liver surgery) AND (posterosuperior segments) AND (segment 7) AND (segment 8). Searches were limited to studies published in English, using standard limitations provided by the respective databases. Screening of titles, abstracts, and bibliographies of relevant review articles and publications in the field was performed by two independent researchers. Quality assessment was based on the Cochrane risk of bias tool. Data were extracted systematically under the following headings: study design (e.g., randomized controlled trial, registry review, cohort study, etc.); study population (dates of recruitment, number of patients,); tumor locations; indications for liver metastases, hepatocellular carcinoma or cholangiocellular carcinoma; tumor sized; surgical technique (surgeon, patient and trocar position, pringle maneuver, transection technique); intraoperative outcomes; morbidity and mortality. Due to the retrospective review of this study, approval was waived by the IRB.
RESULTS
A total of 13 hospital series10,16-27 and 6 clinical cases28-33 that exclusively analyzed segments 7 and/or 8 were included in this review (Table 1, 2).
Table 1.
Preoperative, intraoperative and postoperative characteristics of the studies included in the review
| Author and date hospital series | No. patients and segments | Indication | Surgical technique | Tumor size (mm) | Operative time (min) | Pringle n (%) | Blood losses (ml) | Conversion n (%) | Morbidity n (%) | R0 (%) | Hospital stay (days) |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Huan et al. 2003 | VII (3) | Hemangioma (1); HCC (2) | Partial hepatectomy (3) | 2; 2.5; 2 | 180; 160; 90 | - | 250; 200; 300 | No | - | 100 | 4; 5; 4 |
| Ishizawa et al. 2012 | VII (6) | - | Segmentectomy (10) | 63~267 gr | 180~240 | - | 100~1200 | 1 | 2 (33.3) | - | |
| VIII (4) | 85~189 gr | 132~240 | 100~1100 | 0 | 0 | ||||||
| Coles et al. 2014 | VII (7) | - | Segmentectomy (7) | 13.0 (±6.9) | 252 (±69) | 6 (85.7) | 400±493 | 0 | I-II: 1 (14.2) | 100 | 4.6±2.5 |
| Lee et al. 2014 | VII (3) | CRLM (3); Breast cancer (1); HCC (1) | - | 22±11 | 197±68 | - | 161±138 | - | No | 100 | 7±3.5 |
| VIII (2) | |||||||||||
| Okuda et al. 2014 | VII (6) | Metastases (6) | - | 15 (7~25) | 244 (167~347) | Yes | 70 (20~150) | No | No | 100 | 6 (5~7) |
| Chiow et al. 2015 | CLA: VII or VIII (8) | CLA: CRLM (5); others (3) | - | CLA: 20 (6~34) | CLA: 105 (50~150) | - | CLA: 220 (50~300) | CLA: 0 | CLA: 0 | - | CLA: 2 (1~4) |
| AA: VII or VIII (11) | AA: CRLM (11) | AA: 26 (10~50) | AA: 115 (45 ~255) | AA: 200 (10~1200) | AA:2 (18.2) | AA: 2 (18.2) | AA: 6 (2~24) | ||||
| Ogiso et al. 2015 | CLA: VII (15), VIII (15), VII+VIII (1) | CRLM (36); others metastases (6); HCC (1); Adenoma (1) | - | CLA: 24.5 (8~49) | CLA: 217.5 (90~390) | CLA: 18 (58) | CLA: 200 (20~2900) | CLA: 0 | CLA: I-II: 1 (5), III-IV: 4 (16) | CLA: 93.5 | CLA: 7 (4~22) |
| AA: VII (11), VIII (8), VII+VIII (1) | AA: 15 (8~40) | AA: 165 (75~570) | AA: 7 (35) | AAl: 100 (0~1800) | AA: 1 (5) | AA: I-II: 1 (5), III-IV 4 (21) | AA: 95 | AA: 6 (3~49) | |||
| Ichida et al. 2016 | VII (4) | Metastases (11); HCC (2); Hemangioma (1) | - | 16 (6~25) | 224.5 (109~477) | 11 (78.5)v | 60 (20~310) | No | No | 100 | 7.5 (6~19) |
| VIII (10) | |||||||||||
| Guro et al. 2017 | VII and/or VIII (46) | HCC (46) | Tumorectomy (19) | 2.8 (1.3~6.9) | 330 (195~790) | - | 550 (200~5900) | - | I-II: 2 (4.3) | 97.8 | 8 (5~47) |
| Segmentectomy (10) | III: 5 (10.9) | ||||||||||
| Bisegementectomy (1) | |||||||||||
| RAS (1) | |||||||||||
| RPS (6) | |||||||||||
| RH (8) | |||||||||||
| CB (1) | |||||||||||
| Inoue et al. 2017 | VIII IP (11) | HCC/ICC (13); Metastases/others (16) | - | - | CLA: 183 (130~427) | - | CLA: 50 (0~250) | CLA: 0 | No≥IIIA | CLA: 100 | CLA: 11 (6~20) |
| VIII non-IP (18) | AA: 150 (95~285) | AA: 50 (0~450) | AA: 7 (38.9) | AA: 90.9 | AA: 9 (5~16) | ||||||
| Inoue et al. 2017 | VII IP (15) | HCC/ICC (11); Metastases/others (18) | - | - | CLA: 218 (136~292) | - | CLA: 75 (0~480) | CLA: 1 (6.7) | CLA: 0 | CLA: 92.9 | CLA: 11 (6~16) |
| VII non-IP (14) | AA: 223 (105~415) | AA: 75 (0~250) | AA: 6 (42.9) | AA: 1 (12.5) | AA: 100 | AA: 7 (5~18) | |||||
| Okuda et al. 2017 | VII (6) | Metastases (4); HCC (2) | - | 39 (30~70) | 420 (285~629) | Yes | 200 (40~555) | No | No | 100 | 7.5 (6~12) |
| Martinez-Cecilia et al. 2018 | VIII (13) | CRLM (7); NETM (4); Melanoma (1); Ovarian (1) | Isolated atypical resections | 22±3.7 | 200 (90~240) | 6 (46.1) | 191 (20~400) | 0 | I-II: 3 (23) | 92 | 4 (3~7) |
| Morikawa et al. 2018 | VII and/or VIII (20) | Metastases (13); HCC (5); Bening tumors (1); Cholangiocellular carcinoma (1) | - | 23 (10~75) | 414±126 | - | 318 (10~2205) | - | I-II: 4 (20) | 90 | 9.5 (6~19) |
| Cheng et al. 2011 | VII | HCC | Segmentectomy | 26 | 510 | - | 800 | 0 | - | 100 | 6 |
| Aikawa et al. 2014 | VIII | CRLM | Segmentectomy | 15 | 310 | - | 10 | 0 | - | 100 | 4 |
| Krüger et al. 2014 | VIII | HCC | Segmentectomy | 20 | 75 | - | 20 | 0 | - | 100 | 2 |
| Li et al. 2016 | VIII | HCC | Segmentectomy | 12 | 260 | Yes | 30 | 0 | - | 100 | 5 |
| Kim et al. 2019 | VII | HCC | Segmentectomy | 55 | 330 | - | 300 | 0 | - | 100 | 5 |
| Berardi et al. 2019 | VIII | HCC | Segmentectomy | 30 | 420 | - | 261 | 0 | No | 100 | 8 |
CRLM = colorectal cancer liver metastasis; HCC = hepatocellular carcinoma; ICC = intrahepatic cholangiocarcinoma; CLA = combined laparoscopic approach (additional intercostal ports); AA = abdominal approach; RAS = right anterior sectionectomy; RPS = right posterior sectionectomy; RH = right hepatectomy; CB = central bisectionectomy; GR = grames; IP = intercostal ports; Clavien-Dindo = I-II (minor) and III-IV (major).
Diagnoses and surgical outcomes
In the hospital series analyzed the most frequent indication was metastases (55.4%), followed by hepatocellular carcinoma (28.7%). The average size of the lesions ranged between 13 and 39 mm. Mean surgical times were between 105 and 420 minutes. Six groups performed the pringle maneuver in 8~84% of the patients. Blood losses varied between 50~550 ml with a conversion rate between 0~42.9%. Minor complications were between 0~33.3% and major complications between 0~10.86% with average hospital stays between 2 and 12 days. The resection margin was negative in 90~100% of the resections. All authors used the purely laparoscopic technique for liver resection, except one group that used the hand-assisted technique. In relation to the laparoscopic approach, 4 authors exclusively performed an abdominal approach while the rest combined the placement of abdominal trocars with the insertion of intercostal trocars.
Placement of the patient and surgeons’ position
Regarding the placement of the patient, the most used positions was supine position with the right side approximately 30º elevated; in left, semi-lateral side in a reverse Trendelenburg position; or in the low lithotomy position (“French” position). Other authors recommend left lateral decubitus position with the right arm suspended as a modification that could improve the advantages previously described. While some groups recommend the separation of the legs for the placement of the main surgeon that may later vary depending on the needs of the surgery, others prefer to closed legs with the main surgeon on the right side. Still, none has shown to be superior and the most important issue is the standardization of the position by each group. The two main positions of the surgeon are between the patient’s legs or on the patient’s right side. In cases of placing between the legs, the surgeon will subsequently vary their position (mostly on the patient’s right side), but also on the left side depending on the needs of the surgery. The scopist usually maintains a fixed position on the patient’s left side unless they need to be changed because the main surgeon needs to be placed in this position.
Approach, bleeding control, and trocar position
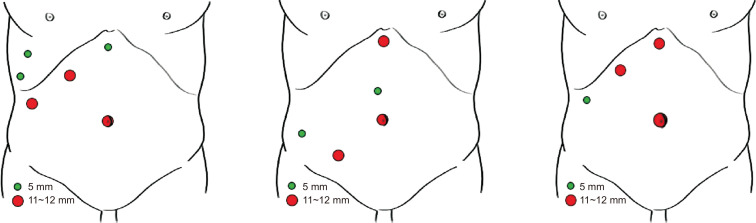
The minimally invasive technique used by most authors is purely laparoscopic approach using a 30 or 45 grades flexible tip scope. The pneumoperitoneum pressure ranges from 10~14 mmHg. The pringle maneuver was performed in most of cases. Several groups have developed the combined approach by the classic abdominal route with the use of intercostal trocars to facilitate the visualization of these difficult to access segments and a better dissection of the hepatic parenchyma. The placement of abdominal trocars, although it depends on each group, usually consists of four to five trocars placed in the upper right quadrant of the abdomen, where three of them are usually adjusted to the right subcostal margin (Fig. 1). In addition to the 11~12 mm umbilical trocar, a trocar is inserted at the level of the midclavicular line (11~12 mm), another in the epigastric area (5 or 11~12 mm), and another in the anterior axillary line (5 or 11~12 mm) in such a way that the trocars of the subcostal arch are placed in a range of 6~7 cm between them from the epigastric trocar. Additional trocars are inserted between the ribs and through the diaphragm or between the ribs in the thoracic cavity and then through the diaphragm. Normally, the placement of two trocars is recommended, although one is sufficient for some authors. They can be 5 mm or 12 mm and placed in the same intercostal space or in two different ones. One is usually cranial and the other caudal and the intercostal space is oscillated from the sixth to the tenth.
Fig. 1.
Different trocar positions to laparoscopic surgical approach for segments 7 and 8.
The type of approach and placement of the trocars is also a key factor. Okuda et al recommend that the devices managed by the main surgeon should be placed in parallel since in these segments the tumor is much farther from the trocars than in other locations of the liver. The Southampton group describes that the “reversed-L” configuration around the medial and inferior part of the tumor allows transection in four planes in line. Against the exclusively abdominal approach, the advantages of intercostal trocar placement have been described. This approach allows a direct and perpendicular view of the right hepatic vein and the vena cava which facilitates access to the operative field. For segment 8, vertical transection planes to the liver surface can be maintained with minimal mobilization of the right liver and less risk of bleeding, ascites and adhesions.34 In addition, on many occasions by the abdominal route the instrumentation and the laparoscope are unable to reach all these segments. This approach presents possible complications such as lung lesions, pneumothorax, hemothorax, biliothorax or diaphragmatic hernias. Therefore, to reduce its appearance, the use of balloon trocars, its introduction during forced expiration or apnea and the closure of the holes is recommended.
Mobilization of the right liver
For better access to segment 7, mobilization of the right side of the liver is fundamental, and it requires division of the falciform ligament to the right and middle hepatic veins along with the complete division of the right triangular and coronary ligament, the division of the round ligament together with the separation of the inferior vena cava, the diaphragm and the retroperitoneal reflection (frequently, the right adrenal gland is also exposed). The root of the right hepatic vein is fully exposed, not only from the anterocranial side but also from the posterior and lateral aspects by dividing the right retrocaval ligament. For the segmentectomy 7 and 8, the round ligament should be divided for better access to the lesions, however, It should be preserved in sever cirrhotic patients with collateral portal veins. in such cases, segmentectomy is out of indication, and only partial resection is available in terms of the liver function. On the other hand, in the resections in segment 8, the complete mobilization of the right hepatic lobe is not necessary. In many occasions with a slight mobilization, the reverse-Trendelemburg position and the retraction of the falciform ligament towards hypogastrium is sufficient for a correct exposure of the lesions in this segment (Table 2).
Table 2.
Summary of the different approaches used for laparoscopic resection of segments VII and VIII in the studies included in the review
| Author and date | Minimally invasive technique | Approach | Laparoscope (grades) | Patient Position | Surgeon position | Pringle maneuver | Pressure (mmHg) | Trocar position (nº of trocars) | Transection technique | Retrieval |
|---|---|---|---|---|---|---|---|---|---|---|
| Huang et al. 2003 | Handport | AA | - | LSD | Left side | - | 12 | 10-mm (1) umbilical; 10-mm (3) RSM; HandPort access 6~8 cm right upper quadrant | Ultrashear ultrasonic; disector; endoclip ligation; inserted hand to directly compress the lesion | HandPort access |
| Cheng et al. 2011 | Pure | - | - | - | - | - | - | - | - | - |
| Ishizawa et al. 2012 | Pure | CLA | - | French position and LLD | - | Taped extra-corporeal | 10~12 | RSM (3), intercostal space (2) | Bipolar forceps and ultrasonic shears; absorbable sutures or clips | - |
| Aikawa et al. 2014 | Pure | CLA | - | LLD | - | - | - | 3 intercostal trocars. | Harmonic scalpel; monopolar coagulator with a ball-shaped tip; Bipolar sealing device. | Enlarged port incision |
| Coles et al. 2014 | Pure | AA | 30 | LSD | Left side/Right side | Taped extra-corporeal | 13~14 | 12-mm umbilical, epigastric, and RIF; 5-mm middle line and RH | Harmonic scalpel; CUSA; hem-o-Lock clips or vascular staplers | Pfannenstiel |
| Krüger et al. 2014 | Pure | CLA | 30 | French position | - | - | 12 | 10-mm right subcostal margin (1) | Bipolar sealing device | Enlarged port incision |
| 12-mm and 10-mm intercostal trocars | ||||||||||
| Lee et al. 2014 | Pure | CLA | 45 | French position and LSD | Between the legs+right side | Taped extra-corporeal | 13 | 11 mm umbilical (1); 11-mm (2) at epigastric and RSM; 5-mm subcostal lateral intercostal ports (2) at the 7th and 9th ICS | Ultrasonic shears; CUSA; clips; sealing device | Enlarged port incision |
| Chiow et al. 2015 | Pure | CLA | 30 | LLD | Right side | Vascular clamp | - | Umbilical (1): 5-mm ports in epigastric and RSM; Additional 5-mm trocars between the ribs below and/or through the diaphragm | LigaSure ¡®V¡¯; hem-o-Lok clips | - |
| Ogiso et al. 2015 | Pure | CLA/AA | - | CLA: LSD | CLA: Right side | Taped extra-corporeal | 10~12 | Umbilical (1): RSM (4) | Bipolar forceps; ultrasonic shears | Enlarged port incision/suprapubic |
| AA: French position | AA: Between the legs/left side | Incision | ||||||||
| Ichida et al. 2016 | Pure | CLA | 30 | LLD and French position | Right side | - | 10~12 | 12 mm abdominal trocars (3 or 4) and 5 mm intercostal ports (2) | Bipolar forceps; vessel-sealing system | - |
| Li et al. 2016 | Pure | CLA | SD+LLD | Right side | Taped extra-corporeal. | 13 | 12-mm abdominal trocars (2; umbilical and right upper quadrant of the abdomen) | Bipolar forceps; Ultrasonic shears. | Enlarged port incision | |
| 12-mm intercostal trocars (3) | ||||||||||
| Inoue et al. 2017 | Pure | CLA | 45 | LSD | Between the legs+right side | Taped extra-corporeal | 12 | 12- mm umbilical; 5- to 12-mm (4) in RSM; 5-mm (2) intercostal | Soft coagulation system; clips or stapling devices | Enlarged port incision |
| Guro et al. 2017 | Pure | CLA | - | French position and LSD | Between the legs+right side | Taped extra-corporeal | <13 | 12-mm umbilical; 12-mm and 5 mm at epigastric and RSM (3); Intercostal trocars (2) | Ultrasonic shears; CUSA; clips; sealing device | Enlarged port incision/suprapubic |
| Incision | ||||||||||
| Okuda et al. 2014 and 2017 | Pure | CLA | 30 | LSD | Right side | Taped extra-corporeal | 10 | 12 mm umbilical; 12-mm and 5 mm at epigastric and RSM (3); Intercostal trocar (1) | CUSA; ultrasonically activated scalpel | Enlarged port incision |
| Martinez–Cecilia et al. 2018 | Pure | AA | 30 | French position | Left side/Right side | Taped extra-corporeal | 13~14 | “Reversed-L” configuration (4 or 5) | Ultrasonic Scalpel; harmonic Ultrasonic; CUSA; Hem-o-Lock clips; vascular staplers | Pfannenstiel |
| Morikawa et al. 2018 | Pure | AA | - | French position and LSD | - | Taped extra-corporeal | ≤12 | 12-mm umbilical; Others 12-mm RSM | Coagulating shears; ultrasonic device; clips; monopolar soft-mode coagulation; hemostatic forceps | Enlarged port incision |
| Berardi et al. 2019 | Pure | AA | - | LSD | Righ side | Taped extra-corporeal | - | 12-mm umbilical; Others 12-mm RSM | Ultrasonic shears; clips; sealing device | - |
AA = abdominal approach; LLD = left lateral decubitus; LSD = left semilateral decubitus; RIF = right iliac fossa; RH = right hypochondrium; RSM = right subcostal margin; SD = supine decubitus; CLA = combined laparoscopic approach (additional intercostal ports); CUSA = cavitron ultrasonic surgical aspirator; ICS = intercostal space.
Approach to the Glissonean Pedicle
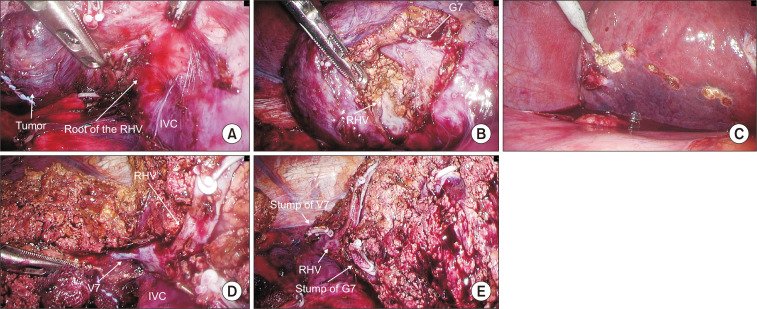
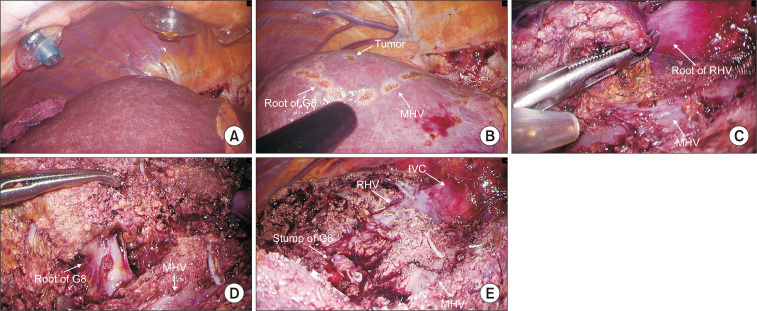
In the case of non-anatomic resections, an ultrasound is performed for the location of the lesion and its relationship with the nearby vasculature. A margin delimitation is performed to ensure a wide resection before starting the hepatic transection For anatomic resection, the intrahepatic Glissonian approach of segment 7 consists in the intrahepatic control of G7 located by ultrasound and the identification of the right hepatic vein to guide parenchymal transection along the intersegmental plane. Once G7 ligated, resection begins from the demarcation that occurs between S6 and S7 to expose the entire length of the RHV. At this point it is recommended to continue dissection from the ventral side in the plane between the RHV and the demarcation line on the liver surface (Fig. 2). In the intrahepatic Glissonian approach of segment 8, the approach begins by taking as a reference the middle hepatic vein that must be exposed from the cranial side to the periphery in the medial part of it. The dissection should continue in this direction more deeply until reaching the root of G8 that will allow an anatomical demarcation of the segment (Fig. 3). segment 7 and 8 are usually supplied by one to three tertiary portal branch.
Fig. 2.
Intrahepatic Glissonean pedicle approach of segment 7. (A) Exposing the root of RHV. (B) Root of G7. (C) Demarcation line. (D) Exposing RHV. (E) Resected surface.
Fig. 3.
Intrahepatic Glissonean pedicle approach of segment 8. (A) Intercostal trocar position. (B) S8 demarcation line. (C) Exposure of MHV. (D) S8 root of G8. (E) Resected surface.
Glissonean branches of segment 7 or 8 can be isolated extrahepatically from hepatic hilum. it is possible through an avascular plane to dissect the right anterior and posterior pedicles in the hepatic hilum until the branches corresponding G7 and G8 are isolated close to their origins. Segmental pedicles to segment 7 can be addressed after dissecting the liver in the Rouviere groove while segments 8 first need to locate the G5 and G8 branches that branch from the right anterior pedicle to subsequently isolate G8 exclusively. Once the correct delimitation of these segments has been verified by ultrasound and fluorescence imaging with preoperative intravenous injection of indocyanine green, its resection is performed.35 However, Ome et al.36 reported this technique was not safe because it has often caused biliary complications due to injury to the bile duct around the hilum.
Parenchymal transection
For hepatic transection the use of ultrasonic shears or bipolar forceps is recommended. Due to the relationship of these segments with the middle and right hepatic veins, a carefully dissection should be performed by Cavitron Ultrasonic Surgical Aspirator or clamp crushing method for a better visualization of the vascular and biliary branches.
DISCUSSION
Segments 7 and 8 present a greater challenge due to its location in the deepest region of the abdominal cavity along with its relationship with the hepatic veins and the large number of interconnected vascular branches from the Glissonean pedicles.37-39 Therefore, although at the beginning of laparoscopic liver surgery the approach of these segments was considered even as a contraindication, nowadays its performance is increasingly recommended in groups with experience in laparoscopic liver surgery.40 To facilitate the surgical approach to these locations different technical modifications have been proposed.
The use of hybrid approaches and the hand-assisted technique were initially used in surgery on the S7 or S7 and 8 as less invasive approaches than the traditional ones.12,16 These techniques facilitated surgical maneuvers with a safer control of possible bleeding and in a shorter surgical time. At the same time, the tactile perception of the liver allowed better control of the surgical margins and the use of a larger incision for the extraction of the surgical specimen. Despite this, these techniques require a larger incision, reducing the intrinsic advantages of minimally invasive surgery. With the development of purely laparoscopic liver surgery, these types of approaches are considered especially useful in these segments with difficult access for control of bleeding from the hepatic veins, mobilization of large tumors and during the beginning of the learning curve.
Although are usually more technically difficult than a major hepatectomy, parenchymal sparing resections in these segments, should be the technique of choice, due to the lower risk of liver failure, especially in cirrhotic patients.35 This complexity is higher with laparoscopic due to the poor operative field in the liver dome and need for a curved transection plane. D’Hondt et al.41 recommend a caudocranial transection of the hepatic parenchyma, as it seems to allow a better identification of intraparenchymal vascular structures compared to the open anterior approach. It is important to emphasize again that for most authors in segment 8 resections, it would not be necessary to carry out a complete mobilization of the right liver unlike those that occur with segment 7.
How to approach G7 and G8 has three methods. Hilar approach with extrahepatic dissection of the Glissonean pedicle, intrahepatic approach after the transection along the major hepatic vein and dyeing to the portal vein. Dyeing method is difficult in laparoscopic surgery, so the other two methods are usually applied. In the Glissonean approach, for the precise performance of an anatomical resection for segment 7 and 8 lesions, the boundaries must be identified by occluding the corresponding portal pedicle to visualize the ischemic regions on the liver surface. This type of approach allows a complete resection of the liver segments where the tumor is located. The use of the ultrasound is crucial for the correct identification of both the relationship of the lesion with the vascular structures and for the proper demarcation before the transection that allows us to obtain a correct surgical margin. For resections in segment 8, the middle hepatic vein and the bifurcation of the segment 8 portal pedicle with the branches of the right and middle hepatic vein are essential.
Only 2 studies included in the review compare open and laparoscopic approach. Guro et al. describes less blood loss, shorter hospital stay, higher number of non-anatomical resection and lower tumor size in laparoscopic group. Morikawa et al describes that the laparoscopic technique required a longer operation time with less intraoperative blood loss, hospital stay and major complications. In the literature, most studies that compare the laparoscopic approach and open in the posterosuperior segments also include segments 4a, 1 or 6. In colorectal liver metastases, the sub-analysis of the OsloCOMET study and Okuno et al. reported shorter hospital stays with similar perioperative results.42,43 With regard to hepatocellular carcinoma, Xiao et al.44 included 41 patients who underwent laparoscopic liver resection and 86 who underwent open liver resection with the same oncological outcomes as conventional procedures, with lower blood loss, fewer postoperative complications, and shorter hospital stay. On the other hand, while Scuderi et al.45 in a multicenter propensity score matched-study reported reduced complication rates in the laparoscopic group, Hond’t et al. found no statistically significant differences in relation to hospital stay and postoperative morbidity.46 Finally, in a recent meta-analysis of the posterosuperior segments, Zeng et al. reported that the operative time was longer and overall complications greater, while the hospital stay was shorter. In addition, there were no differences in blood loss, transfusions, resection margins, major complications, disease-free survival for HCC and CRLM and overall survival in HCC.47
The size, number and location of the lesions has also been considered an aspect of great relevance with respect to the feasibility of these resections. Different authors have recommended that lesions larger than 5 cm are considered less suitable for the laparoscopic approach. In fact, the average size of the lesions collected in the series from this review ranged between 13 and 39 mm. Another factor has been the depth of the lesion in the hepatic parenchyma. Okuno et al.42 reported that surgeons tended to open approach for tumors located at a depth of ≥3 cm in the posterosuperior segments, while Morikawa et al.27 suggested that a tumor depth of ≤3 cm from the liver’s surface could be a good indication for laparoscopic partial liver resection.
CONCLUSIONS
The accumulated experience of the different groups in minimally invasive liver surgery, the standardization of the technique and the technological advances in the different laparoscopic devices have facilitated the resection of tumors in segments 7 and 8 with similar and even better results than open surgery. Even so, the degree of difficulty in laparoscopic approach for this area depends mainly on liver cirrhosis, the location of the tumor, and the size of the tumor.
ACKNOWLEDGMENTS
None.
Footnotes
AUTHORS’ CONTRIBUTIONS
Conceptualization: GH, YO, VLL. Formal analysis: VLL, YK, AGR, RRC. Methodology: GH, YO, VLL, YK, AGR, RRC. Writing-original draft: GH, YO, VLL. Writing-review and editing: GH, YO, YK, VLL.
CONFLICT OF INTEREST
None.
FUNDING
None.
REFERENCES
- Gagner M. Laparoscopic partial hepatectomy for liver tumor [Abstract] Surg Endosc. 1992;6:97–98. [Google Scholar]
- Cherqui D, Husson E, Hammoud R, et al. Laparoscopic liver resections: a feasibility study in 30 patients. Ann Surg. 2000;232:753–762. doi: 10.1097/00000658-200012000-00004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buell JF, Cherqui D, Geller DA, et al. The international position on laparoscopic liver surgery: The Louisville Statement, 2008. Ann Surg. 2009;250:825–830. doi: 10.1097/SLA.0b013e3181b3b2d8. [DOI] [PubMed] [Google Scholar]
- Wakabayashi G, Cherqui D, Geller DA, et al. Recommendations for laparoscopic liver resection: a report from the second international consensus conference held in Morioka. Ann Surg. 2015;261:619–629. doi: 10.1097/SLA.0000000000001184. [DOI] [PubMed] [Google Scholar]
- Nguyen KT, Gamblin TC, Geller DA. World review of laparoscopic liver resection-2,804 patients. Ann Surg. 2009;250:831–841. doi: 10.1097/SLA.0b013e3181b0c4df. [DOI] [PubMed] [Google Scholar]
- Abu Hilal M, Aldrighetti L, Dagher I, et al. The Southampton Consensus Guidelines for Laparoscopic Liver Surgery: From Indication to Implementation. Ann Surg. 2018;268:11–18. doi: 10.1097/SLA.0000000000002524. [DOI] [PubMed] [Google Scholar]
- Ciria R, Cherqui D, Geller DA, Briceno J, Wakabayashi G. Comparative Short-term Benefits of Laparoscopic Liver Resection: 9000 Cases and Climbing. Ann Surg. 2016;263:761–777. doi: 10.1097/SLA.0000000000001413. [DOI] [PubMed] [Google Scholar]
- Yamamoto M, Ariizumi S-I. Glissonean pedicle approach in liver surgery. Ann Gastroenterol Surg. 2018;2:124–128. doi: 10.1002/ags3.12062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Machado MAC, Surjan RC, Basseres T, Schadde E, Costa FP, Makdissi FF. The laparoscopic Glissonian approach is safe and efficient when compared with standard laparoscopic liver resection: Results of an observational study over 7 years. Surgery. 2016;160:643–651. doi: 10.1016/j.surg.2016.01.017. [DOI] [PubMed] [Google Scholar]
- Okuda Y, Honda G, Kobayashi S, et al. Intrahepatic Glissonean Pedicle Approach to Segment 7 from the Dorsal Side During Laparoscopic Anatomic Hepatectomy of the Cranial Part of the Right Liver. J Am Coll Surg. 2018;226:e1–e6. doi: 10.1016/j.jamcollsurg.2017.10.018. [DOI] [PubMed] [Google Scholar]
- Choi Y, Han HS, Sultan AM, Yoon YS, Cho JY. Glissonean pedicle approach in laparoscopic anatomical liver resection. Hepatogastroenterology. 2014;61:2317–2320. [PubMed] [Google Scholar]
- Hasegawa Y, Koffron AJ, Buell JF, Wakabayashi G. Approaches to laparoscopic liver resection: a meta-analysis of the role of hand-assisted laparoscopic surgery and the hybrid technique. J Hepatobiliary Pancreat Sci. 2015;22:335–341. doi: 10.1002/jhbp.214. [DOI] [PubMed] [Google Scholar]
- Fong Y, Jarnagin W, Conlon KC, DeMatteo R, Dougherty E, Blumgart LH. Hand-assisted laparoscopic liver resection: lessons from an initial experience. Arch Surg. 2000;135:854–859. doi: 10.1001/archsurg.135.7.854. [DOI] [PubMed] [Google Scholar]
- Soyama A, Takatsuki M, Adachi T, et al. A hybrid method of laparoscopic-assisted open liver resection through a short upper midline laparotomy can be applied for all types of hepatectomies. Surg Endosc. 2014;28:203–211. doi: 10.1007/s00464-013-3159-1. [DOI] [PubMed] [Google Scholar]
- Periyasamy M, Han HS, Cho JY, et al. Current Status of Laparoscopic Liver Resection: Experiences from Tertiary Center. J Minim Invasive Surg. 2017;20:125–128. doi: 10.7602/jmis.2017.20.4.125. [DOI] [Google Scholar]
- Huang MT, Lee WJ, Wang W, Wei PL, Chen RJ. Hand-assisted laparoscopic hepatectomy for solid tumor in the posterior portion of the right lobe: initial experience. Ann Surg. 2003;238:674–679. doi: 10.1097/01.sla.0000094301.21038.8d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishizawa T, Gumbs AA, Kokudo N, Gayet B. Laparoscopic segmentectomy of the liver: from segment I to VIII. Ann Surg. 2012;256:959–964. doi: 10.1097/SLA.0b013e31825ffed3. [DOI] [PubMed] [Google Scholar]
- Coles SR, Besselink MG, Serin KR, et al. Total laparoscopic management of lesions involving liver segment 7. Surg Endosc. 2015;29:3190–3195. doi: 10.1007/s00464-014-4052-2. [DOI] [PubMed] [Google Scholar]
- Lee W, Han HS, Yoon YS, Cho JY, Choi Y, Shin HK. Role of intercostal trocars on laparoscopic liver resection for tumors in segments 7 and 8. J Hepatobiliary Pancreat Sci. 2014;21:E65–E68. doi: 10.1002/jhbp.123. [DOI] [PubMed] [Google Scholar]
- Okuda Y, Honda G, Kurata M, Kobayashi S, Sakamoto K, Takahashi K. A safe and valid procedure for pure laparoscopic partial hepatectomy of the most posterosuperior area: the top of segment 7. J Am Coll Surg. 2015;220:e17–e21. doi: 10.1016/j.jamcollsurg.2014.11.021. [DOI] [PubMed] [Google Scholar]
- Chiow AKH, Lewin J, Manoharan B, Cavallucci D, Bryant R, O'Rourke N. Intercostal and transthoracic trocars enable easier laparoscopic resection of dome liver lesions. HPB (Oxford) 2015;17:299–303. doi: 10.1111/hpb.12336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ogiso S, Conrad C, Araki K, Nomi T, Anil Z, Gayet B. Laparoscopic Transabdominal With Transdiaphragmatic Access Improves Resection of Difficult Posterosuperior Liver Lesions. Ann Surg. 2015;262:358–365. doi: 10.1097/SLA.0000000000001015. [DOI] [PubMed] [Google Scholar]
- Ichida H, Ishizawa T, Tanaka M, et al. Use of intercostal trocars for laparoscopic resection of subphrenic hepatic tumors. Surg Endosc. 2017;31:1280–1286. doi: 10.1007/s00464-016-5107-3. [DOI] [PubMed] [Google Scholar]
- Guro H, Cho JY, Han HS, et al. Laparoscopic liver resection of hepatocellular carcinoma located in segments 7 or 8. Surg Endosc. 2018;32:872–878. doi: 10.1007/s00464-017-5756-x. [DOI] [PubMed] [Google Scholar]
- Inoue Y, Suzuki Y, Fujii K, et al. Laparoscopic Liver Resection Using the Lateral Approach from Intercostal Ports in Segments VI, VII, and VIII. J Gastrointest Surg. 2017;21:2135–2143. doi: 10.1007/s11605-017-3516-9. [DOI] [PubMed] [Google Scholar]
- Martínez-Cecilia D, Fontana M, Siddiqi NN, Halls M, Barbaro S, Abu-Hilal M. Laparoscopic parenchymal sparing resections in segment 8: techniques for a demanding and infrequent procedure. Surg Endosc. 2018;32:2012–2019. doi: 10.1007/s00464-017-5897-y. [DOI] [PubMed] [Google Scholar]
- Morikawa T, Ishida M, Takadate T, et al. Laparoscopic partial liver resection improves the short-term outcomes compared to open surgery for liver tumors in the posterosuperior segments. Surg Today. 2019;49:214–223. doi: 10.1007/s00595-018-1719-7. [DOI] [PubMed] [Google Scholar]
- Cheng KC, Yeung YP, Hui J, Ho KM, Yip AWC. Multimedia manuscript: laparoscopic resection of hepatocellular carcinoma at segment 7: the posterior approach to anatomic resection. Surg Endosc. 2011;25:3437–3437. doi: 10.1007/s00464-011-1685-2. [DOI] [PubMed] [Google Scholar]
- Aikawa M, Miyazawa M, Okamoto K, et al. Thoracoscopic hepatectomy for malignant liver tumor. Surg Endosc. 2014;28:314–314. doi: 10.1007/s00464-013-3128-8. [DOI] [PubMed] [Google Scholar]
- Krüger JAP, Coelho FF, Perini MV, Herman P. Laparoscopic transthoracic liver resection. Arq Bras Cir Dig. 2014;27:288–290. doi: 10.1590/S0102-67202014000400014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li H, Wei Y, Li B, Peng B. Modified Thoracoscopic Hepatectomy For Segment VIII: A Case Report. Medicine (Baltimore) 2016;95:e3801. doi: 10.1097/MD.0000000000003801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim S, Han HS, Sham JG, Yoon YS, Cho JY. Laparoscopic anatomical S7 segmentectomy by the intrahepatic glissonian approach. Surg Oncol. 2019;28:158. doi: 10.1016/j.suronc.2019.01.004. [DOI] [PubMed] [Google Scholar]
- Berardi G, Wakabayashi G, Igarashi K, et al. Full Laparoscopic Anatomical Segment 8 Resection for Hepatocellular Carcinoma Using the Glissonian Approach with Indocyanine Green Dye Fluorescence. Ann Surg Oncol. 2019;26:2577–2578. doi: 10.1245/s10434-019-07422-8. [DOI] [PubMed] [Google Scholar]
- Ishizawa T, Ichida H, Saiura A. Application of intercostal transthoracic trocars to laparoscopic hepatectomy. Ann Laparosc Endosc Surg. 2017;2:48. doi: 10.21037/ales.2017.02.21. [DOI] [Google Scholar]
- Berardi G, Igarashi K, Li CJ, et al. Parenchymal Sparing Anatomical Liver Resections With Full Laparoscopic Approach: Description of Technique and Short-term Results. Ann Surg. 2019 Aug 23; doi: 10.1097/SLA.0000000000003575. [Epub]. DOI: 10.1097/SLA.0000000000003575. [DOI] [PubMed] [Google Scholar]
- Ome Y, Honda G, Doi M, Muto J, Seyama Y. Laparoscopic Anatomic Liver Resection of Segment 8 Using Intrahepatic Glissonean Approach. J Am Coll Surg. 2020;230:e13–e20. doi: 10.1016/j.jamcollsurg.2019.11.008. [DOI] [PubMed] [Google Scholar]
- Launois B, Tay K, Meunier B. The posterior intrahepatic approach to liver resection. HPB (Oxford) 1999;1:209–214. doi: 10.1016/S1365-182X(17)30672-X. [DOI] [Google Scholar]
- Jia C, Wang H, Chen Y, Fu Y, Liu H. Anatomic liver resection of segments 6, 7, and 8 by the method of selective occlusion of hepatic inflow. Indian J Surg. 2014;76:159–161. doi: 10.1007/s12262-012-0777-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fiorentini G, Ratti F, Cipriani F, et al. Theory of Relativity for Posterosuperior Segments of the Liver. Ann Surg Oncol. 2019;26:1149–1157. doi: 10.1245/s10434-019-07165-6. [DOI] [PubMed] [Google Scholar]
- Jia C, Li H, Wen N, Chen J, Wei Y, Li B. Laparoscopic liver resection: a review of current indications and surgical techniques. Hepatobiliary Surg Nutr. 2018;7:277–288. doi: 10.21037/hbsn.2018.03.01. [DOI] [PMC free article] [PubMed] [Google Scholar]
- D'Hondt M, Yoshihara E, Vansteenkiste F, et al. Laparoscopic parenchymal preserving hepatic resections in semiprone position for tumors located in the posterosuperior segments. Langenbecks Arch Surg. 2016;401:255–262. doi: 10.1007/s00423-016-1375-6. [DOI] [PubMed] [Google Scholar]
- Okuno M, Goumard C, Mizuno T, et al. Operative and short-term oncologic outcomes of laparoscopic versus open liver resection for colorectal liver metastases located in the posterosuperior liver: a propensity score matching analysis. Surg Endosc. 2018;32:1776–1786. doi: 10.1007/s00464-017-5861-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aghayan DL, Fretland ÅA, Kazaryan AM, et al. Laparoscopic versus open liver resection in the posterosuperior segments: a sub-group analysis from the OSLO-COMET randomized controlled trial. HPB (Oxford) 2019;21:1485–1490. doi: 10.1016/j.hpb.2019.03.358. [DOI] [PubMed] [Google Scholar]
- Xiao L, Xiang Lj, Li Jw, Chen J, Fan Yd, Zheng Sg. Laparoscopic versus open liver resection for hepatocellular carcinoma in posterosuperior segments. Surg Endosc. 2015;29:2994–3001. doi: 10.1007/s00464-015-4214-x. [DOI] [PubMed] [Google Scholar]
- Scuderi V, Barkhatov L, Montalti R, et al. Outcome after laparoscopic and open resections of posterosuperior segments of the liver. Br J Surg. 2017;104:751–759. doi: 10.1002/bjs.10489. [DOI] [PubMed] [Google Scholar]
- D'Hondt M, Tamby E, Boscart I, et al. Laparoscopic versus open parenchymal preserving liver resections in the posterosuperior segments: a case-matched study. Surg Endosc. 2018;32:1478–1485. doi: 10.1007/s00464-017-5835-z. [DOI] [PubMed] [Google Scholar]
- Zheng H, Huang SG, Qin SM, Xiang F. Comparison of laparoscopic versus open liver resection for lesions located in posterosuperior segments: a meta-analysis of short-term and oncological outcomes. Surg Endosc. 2019;33:3910–3918. doi: 10.1007/s00464-019-07071-8. [DOI] [PubMed] [Google Scholar]