Abstract
Different surgical approaches have been reported in the management of lateral pelvic lymph node dissection (LPND) including open, laparoscopic and robotic. Since the introduction of Da Vinci robotic system in the early 2000s. It has been useful for more meticulous dissection of deep and narrow spaces and easier to gain access. In this article we describe our approach using the Da Vinci Xi robotic system in LPND and with a supplementary video.
Keywords: Lymph nodes, Robotic surgical procedures, Rectal neoplasms
INTRODUCTION
The current gold standard for patient with T3 or T4 with or without lymph node involvement is neoadjuvant chemoradiotherapy followed by total mesorectal excision (TME).1 There is a debate in the management of lateral pelvic lymph node (LPN) between western and eastern society.2-4 Western society consider LPN involvement as a distant metastasis and they treat it with chemoradiotherapy.2,3 On the other hand, eastern society consider LPN involvement as a local disease and they treat it with LPND.2-4 Different surgical approaches have been reported in the management of lateral pelvic lymph node dissection (LPND) including open, laparoscopic and robotic.5 In this article we will explain our method for LPND. This study was reviewed by the Institutional Review Board of Seoul St. Mary’s Hospital and approved from exemption (IRB number: 2020-1040-0001).
PROCEDURE
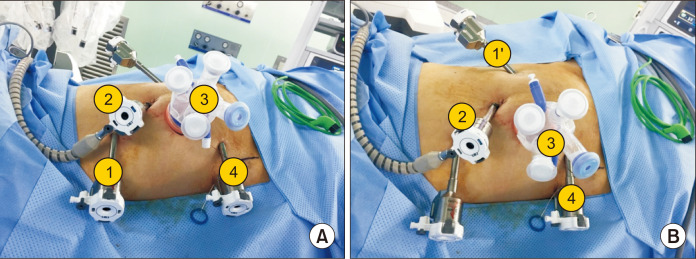
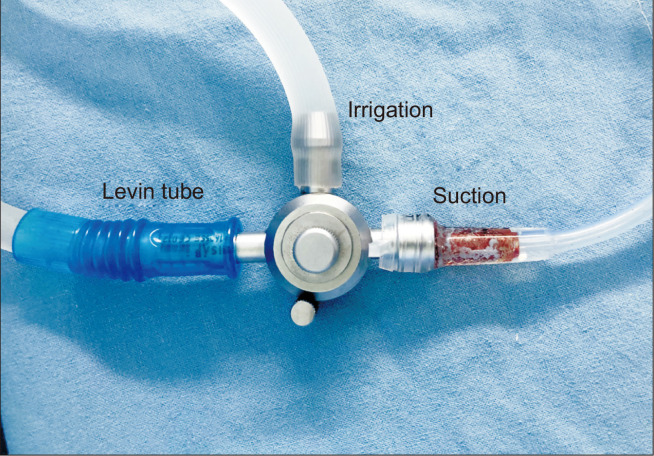
Our technique for robotic low anterior or abdominal perineal resection with or without LPND is totally robotic and it have the same port placement. We use ‘double docking surgery’ Fig. 1. First phase is for inferior mesenteric vessel ligation and splenic mobilization (splenic phase), targeting the scope toward the descending colon with sigmoid junction. After the colonic mobilization, we begin TME phase and LPND (second phase) targeting the scope toward the rectum. We use four 8 mm port and one single port (glove port®, Nelis). Distance among ports were at least 6 cm from each other to avoid collusion. The camera port (Arm 2) inserted at supra-umbilical on midline. After creating pneumoperitoneum, robotic camera is placed and checked the direction of spleen and the right anterior superior iliac spine (ASIS). A line drawn on the skin from the umbilical port toward the Right ASIS. Then at least 6 cm away from the umbilical port toward the right ASIS a transverse 4~4.5 cm incision is made for the glove port (Arm 3) and a 12 mm trocar with adjustable 8 mm cover is inserted (this port used also for specimen retrieval). This multi channeled port made suction (Levin tube Fig. 2) and rectal traction easier.
Fig. 1.
Position of the trocars in splenic phase (A) and TME and LPLD phase (B).
Fig. 2.
Instrument for suction & irrigation made by Levin tube.
On the same line we measure at least 6 cm from port number 3 then go 2 cm toward the pubic tubercle and insert a8 mm port (Arm 4). Next port (Arm 1 used for colon and splenic mobilization only) will be inserted in triangular fashion and at least 6 cm away from the umbilical and glove port. Last port (Arm 1’ in TME and LPND) size 8 mm is inserted at the same umbilical port level toward the left side of the patient (this port used for TME and LPND).
After port insertion patient position in Trendelenburg position. A Cardiere grasper is used in Arm 1 and camera was fixed on arm 2. Stapling and monopolar or energy device were used through arm 3. Fenestrated grasper applied on arm 4. LPND usually start after the colon is completely resected with anvil insertion but before doing the anastomoses.
OPERATIVE STEPS
It can be simply divided to five steps:
First step is to dissect between Ureter and pelvic plexus. Secondly we identify the external iliac artery & vein, and dissect towards the psoas muscle. Then we identify the common iliac artery bifurcation and dissect in between. After that we identify the obturator nerve and artery and dissect along them and ligate the artery. The last step is to dissect the along the internal iliac artery distally towards the Alcock’s canal (pudendal canal). After that the specimen is taken out as en bloc through the glove port in an endobag to avoid spillage of content.
DISCUSSION
Surgery is all about knowing the anatomy and exact planes. Now with the era of laparoscopic and robotic technology, surgeon can gain access to deep structure more easily with an excellent 3D view.
At the beginning of my practice for LPND I focused only for enlarged lymph node. After attending many cadaveric workshops and video presentations I started to know more and more about the anatomy of the lateral pelvic wall and better visualization of my dissection plan. Finally, I reached my goal for meticulous dissection and en bloc resection of the lymph nodes. Gentle traction and counter traction with meticulous dissection will yield a bloodless plan and decrease the incidence of devastating complication. During dissection knowing the major structure and dissection plan is an important step for complete and safe lymph node dissection.
Using the robotic system can give the surgeon the benefit of more steady dissection with an excellent articulation to gain access to the deep pelvic structure. Surgeon should be patient, attending courses and workshops to step up in their learning curve. There is no gold standard until now for the LPND and we are doing our best to standardize this technique for better patient safety and outcome.
Supplemental Materials
ACKNOWLEDGMENTS
None.
Footnotes
AUTHORS’ CONTRIBUTIONS
Conceptualization: In Kyu Lee. Formal analysis: In Kyu Lee. Methodology: Suhail Avdullah Alturkistani and In Kyu Lee. Writing–original draft: Suhail Avdullah Alturkistani and In Kyu Lee. Writing–review and editing: Suhail Avdullah Alturkistani, Alanoud Mohammed Alghanem, and In Kyu Lee.
CONFLICT OF INTEREST
None.
FUNDING
None.
Supplementary video file:
This article contains supplementary material (https://doi.org/10.7602/jmis.2020.23.2.103).
REFERENCES
- 1).Feeney G, Sehgal R, Sheehan M, et al. Neoadjuvant radiotherapy for rectal cancer management. World J Gastroenterol. 2019;25:4850–4869. doi: 10.3748/wjg.v25.i33.4850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2).Moriya Y. Treatment of lateral pelvic nodes metastases from rectal cancer: the future prospective. G Chir. 2013;34:245–248. [PMC free article] [PubMed] [Google Scholar]
- 3).Yano H, Moran BJ. The incidence of lateral pelvic side-wall nodal involvement in low rectal cancer may be similar in Japan and the West. Br J Surg. 2008;95:33–49. doi: 10.1002/bjs.6061. [DOI] [PubMed] [Google Scholar]
- 4).Kobayashi H, Mochizuki H, Kato T, et al. Outcomes of surgery alone for lower rectal cancer with and without pelvic sidewall dissection. Dis Colon Rectum. 2009;52:567–576. doi: 10.1007/DCR.0b013e3181a1d994. [DOI] [PubMed] [Google Scholar]
- 5).Bae SU, Saklani AP, Hur H, et al. Robotic and laparoscopic pelvic lymph node dissection for rectal cancer: short-term outcomes of 21 consecutive series. Ann Surg Treat Res. 2014;86:76–82. doi: 10.4174/astr.2014.86.2.76. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.