Abstract
Prior studies on red and processed meat consumption with breast cancer risk have generated inconsistent results. We performed a systematic review and meta-analysis of prospective studies to summarize the evidence regarding the relation of red meat and processed meat consumption with breast cancer incidence. We searched in MEDLINE and EMBASE databases through January 2018 for prospective studies that reported the association between red meat and processed meat consumption with incident breast cancer. The multivariable-adjusted relative risk (RR) was combined comparing the highest with the lowest category of red meat (unprocessed) and processed meat consumption using a random-effect meta-analysis. We identified 13 cohort, 3 nested case–control and two clinical trial studies. Comparing the highest to the lowest category, red meat (unprocessed) consumption was associated with a 6% higher breast cancer risk (pooled RR,1.06; 95% confidence intervals (95%CI):0.99–1.14; I2 = 56.3%), and processed meat consumption was associated with a 9% higher breast cancer risk (pooled RR, 1.09; 95%CI, 1.03–1.16; I2 = 44.4%). In addition, we identified two nested case–control studies evaluating the association between red meat and breast cancer stratified by N-acetyltransferase 2 acetylator genotype. We did not observe any association among those with either fast (per 25 g/day pooled odds ratio (OR), 1.18; 95%CI, 0.93–1.50) or slow N-acetyltransferase 2 acetylators (per 25 g/day pooled OR, 0.99; 95%CI, 0.91–1.08). In the prospective observational studies, high processed meat consumption was associated with increased breast cancer risk.
Keywords: red meat, processed meat, breast cancer, N-acetyltransferase 2 acetylators, meta-analysis
Introduction
Globally, breast cancer is the most common cancer among women and the second leading cause of cancer death.1 Given the international variations in breast cancer rates and trends,2 the importance of identifying modifiable lifestyle risk factors is widely acknowledged as a means to reduce breast cancer. Red meat is hypothesized to be an important dietary risk factor for several cancer sites, and provides a source of animal fat, heme iron and chemical carcinogens that may accumulate during cooking and/or processing. The International Agency for Research on Cancer (IARC) concluded that consumption of red meat (unprocessed) was a probable human carcinogen, whereas processed meat was classified as “carcinogenic to humans.”3 This classification was largely based on the evidence for colorectal, pancreas and prostate cancers for red meat and colorectal cancer for processed meat.3 Using pooled data from eight cohort studies, Missmer et al.4 observed a null association of red meat and processed meat consumption with breast cancer risk. However, in a recent meta-analysis of 14 studies, Guo et al. provided evidence that red meat and processed meat consumption was associated with higher risk of breast cancer.5 In contrast, Anderson et al. reported that only processed meat might increase risk of breast cancer.6 However, the meta-analysis by Guo et al. had several limitations such as including some studies twice in the analysis4,7–10 as well as including a case–control study.11
Epidemiological studies assessing red meat and processed meat intake with risk of breast cancer based on menopausal status are limited and inconsistent, with most of the studies including largely postmenopausal women.9,10,12–18 In previously meta-analysis, higher processed meat was associated with higher risk of postmenopausal breast cancer, but not breast cancer before menopause.6 Moreover, breast cancer is a heterogeneous disease, and estrogen receptor positive breast tumors (ER+) are more strongly associated with hormone-related factors than estrogen receptor negative tumors (ER−),19 therefore, hormone residues of the exogenous hormones used in beef cattle may increase risk of ER+ tumors.20 However, the association of red meat consumption in relation to tumor hormone receptor status is not well-established8,10,18,21,22 and, to our knowledge, no prior meta-analyses have reviewed this association.
Heterocyclic amines (HCAs) are carcinogenic compounds that form in meat when cooking for a long duration at high temperature.23,24 N-acetyltransferase 2 (NAT2), an important enzyme in the biotransformation of aromatics and HCAs,25,26 is a polymorphic enzyme that segregates individuals into biochemical phenotypes, ranging from slow to fast acetylators.27 It is hypothesized that higher levels of DNA adducts are formed by O-acetylation of HCAs among NAT2 fast acetylators than among slow acetylators.28 Therefore, these differences in enzyme activity may modify the carcinogenic effect of red meat. However, regarding the interaction between meat consumption and NAT2 polymorphisms on risk of breast cancer, evidence in epidemiological studies is sparse and conflicting,29–31 and it has not been evaluated in published meta-analyses.
In our study, we conducted a meta-analysis on the current evidence for effects of red meat and processed meat intake with risk of breast cancer overall, and according to menopausal, and estrogen and progesterone receptor status. We also examined whether NAT2 genotype may modify the association between red meat intake and breast cancer risk.
Subjects and Methods
Study strategy
We followed the checklist of the Meta-analysis of Observational Studies in Epidemiology (MOOSE) for background, design, analysis and interpretation.32 A systematic literature review was conducted in two databases, MEDLINE and EMBASE, related articles and hand-searching of references for all prospective studies describing the association of intake of red meat and processed meat with breast cancer risk until January, 2018. Two authors (M.S.F, E.C.) screened all publications. Only English publications were considered. The search strategy identified 466 unique citations. The definition of red meat and processed meat was based on the IARC Working Group classification.3 Red meat refers to unprocessed mammalian muscle meat including beef, veal, pork, lamb, mutton, horse, or goat meat. Processed meat refers to meat that has been transformed through salting, curing, fermentation, smoking, or other processes to enhance flavor or improve preservation (e.g., bacon, sausage, salami, hot dog).3 To minimize the influence of recall and selection bias that occur in case–control studies, we did not include these studies. Only prospective studies were included with multivariable-adjusted risk estimates (relative risk [RR] or hazard ratio [HR]) of red meat, processed meat, or total red meat (red meat + processed meat) consumption as an exposure and breast cancer as an endpoint. Retrospective (historical), case–control, cross-sectional, or ecological studies were excluded; nonoriginal research (reviews, editorials and letters), meeting abstracts and duplicated publications were also excluded. When multiple manuscripts were published from the same study population, the most up-to-date analyses with the largest number of breast cancer cases were considered for this meta-analysis (Fig. 1).
Figure 1.
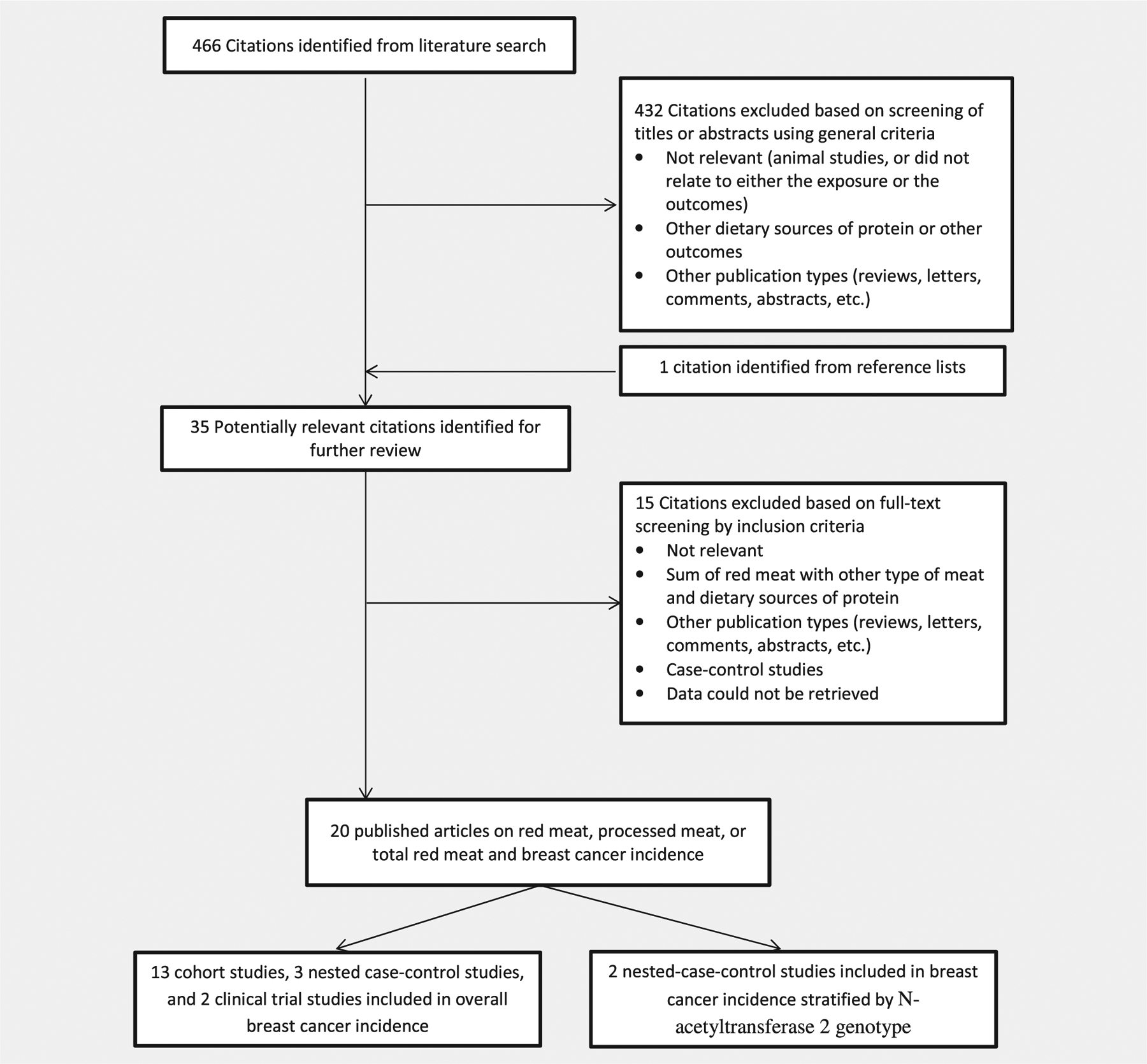
Search, screening and selection process of prospective cohort studies of red meat and processed meat intake and risk of breast cancer.
In a previous paper, using data from the Nurses’ Health Study II (NHSII),8 we reported the association between total red meat intake including unprocessed and processed red meat and risk of breast cancer. For the current meta-analysis, we have updated the previous paper and included the results of red meat and processed meat intake separately.
Data extraction
Information was extracted on study characteristics (first author, publication year, study name, country), duration of follow up (mean, median, or maximum number of follow up), number of participants, number of cases of overall breast cancer, breast cancer before and after menopause, age at baseline (mean, median, or range), dietary assessment method, meat variable definition and covariates in the statistical models. When more than one multivariable model was reported, we extracted the RRs with the greatest number of adjusted variables.
Data synthesis
We conducted separate analyses considering three different variables: “red meat” which included only unprocessed red meat items, “processed meat” which included only processed meats, and “total red meat” which included red meat and processed meats. Because studies reported risk estimates differently (e.g., tertiles, quartiles, or quintiles of intake), we combined the RRs for the highest vs. the lowest category of intake. Forest plots were used to visualize the RRs and corresponding 95% confidence intervals (CIs) of the pertinent studies included in the meta-analysis. Potential heterogeneity among the studies was assessed using the I2 statistic. Random-effects models (DerSimonian and Laird method) were used to calculate the overall RR estimates and 95% CIs. We assessed the possibility of publication bias by visual inspection of a funnel plot and the Begg’s test. Potential heterogeneity among studies was assessed using the I2 statistic, and the heterogeneity was further explored using stratified analysis and the meta-regression method. Sources of heterogeneity included region (North America/other countries), duration of follow-up (<8 years/≥8 years of follow-up), adjustment for energy intake, smoking, benign breast disease, family history of breast cancer, and alcohol intake. A two-tailed p < 0.05 was considered statistically significant. All statistical analyses were conducted using STATA, version 12, software (STATA Corp, College Station, TX).
Results
Study characteristics
After screening the titles and abstracts, 13 prospective cohort studies met inclusion criteria for red meat and processed meat meta-analyses6,8–10,12,14,15,17,18,21,22,33,34 (Table 1). In addition, three nested case–control studies13,35,36 and two clinical trial studies (not testing either red meat or processed meat)16,37 met inclusion criteria for red/processed meat. Furthermore, two studies met inclusion criteria regarding the interaction between red meat consumption and NAT2 polymorphisms on risk of breast cancer29,30 (Table 2).
Table 1.
Characteristics of cohort studies that evaluated red meat and processed meat intake and incidence of breast cancer
| Author and year | Study name and country | Design of the study | Follow-up (year) | Total No. of participants | Numbe of breast cancer events | Menopausal status | ER/PR | Baseline age | Red meat variables (method of assessment) | Adjustment | |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Pre | Post | ||||||||||
| Mills et al.,12 | CSA/US | Cohort | 6 | 20,341 | 215 | – | – | – | 55.4 | Red meat (hamburger, steak and other beef or veal) (FFQ) | Age, age at first live birth, age at menarche, menopausal status, history of BBD, maternal history of breast cancer, educational attainment, BMI |
| Toniolo et al.,35 | NYS/US | nested case-control | 22.2 ± 17.9 months | Control: 829 | 180 | – | – | – | 52.1 ± 8.4 for controls 52.2 ± 8.4 for cases |
Total red meat (beef, veal, lamb, pork, processed luncheon meats: ham, cold cuts and turkey rolls) (FFQ) | Energy |
| Key et al.,33 | RERFLSS/Japan | Cohort | 14 | 34,759 | 427 | – | – | – | Processed meat (ham, sausages) (FFQ) | Age, city, age at time of bombing, radiation dose | |
| Voorrips et al.,13 | NLCS/the Netherlands | Case-Cohort design | 6.3 | Subcohort: 1,598 | 941 | – | 941 | – | 55–69 | Red meat Processed meat (FFQ) | Age, history of BBD, maternal breast cancer, breast cancer in one or more sisters, age at menarche, age at menopause, OC use, parity, age at first childbirth, Quetelet index, education, alcohol use, current cigarette smoking, energy intake |
| Holmes et al.,9 | NHS/US | Cohort | 18 | 88,647 | 4,107 | 854 | 2,936 | – | 46.7 ± 7.2 | Processed meat (hot dogs, bacon and other processed meat) Total red meat (hamburger, beef, pork or lamb as a main dish, beef, pork or lamb as a sandwich and all processed meat) (FFQ) |
Age, total energy intake, alcohol intake, parity and age at first birth, BMI at age 18, weight change since age 18, height, family history of breast cancer, history of BBD, age at menarche, menopausal status, age at menopause HRT, duration of menopause |
| Van der Hel et al.,36 | MPCDRF/the Netherland | nested case-control | 10 | Control: 264 | 229 | – | – | – | 47.0 ± 9.1 for controls 47.5 ± 8.0 for cases |
Red meat (beef and pork) Processed meat (all processed meat items) (FFQ) | Age, menopausal status, town, energy intake, smoking, alcohol, age at menarche, BMI |
| Kabat et al.,14 | CNBSS/Canada | Cohort | 16.4 | 49,654 | 2,491 | 1,171 | 993 | – | 40–59 | Red meat (FFQ) | Age, BMI, menopausal status, parity, age at menarche, family history of breast cancer in a first degree relative, history of BBD, OC use, HRT, energy intake, alcohol intake, education, study center, randomization group, |
| Taylor et al.,15 | UKWCS/UK | Cohort | 8 | 33,725 | 678 | 283 | 395 | – | 52 (35–69) | Red meat (beef, pork, lamb and other red meat included in mixed dishes) Processed meat (bacon, ham, corned beef, spam, luncheon meats, sausages, pies, pasties, sausage rolls, liver pate, salami and meat pizza) (FFQ) |
Age, energy intake, menopausal status, BMI, physical activity, smoking status, HRT, OC use, parity, total fruit and vegetable intake |
| Ferrucci LM et al.,16 | PLCOCST/US | Clinical Trial | 5.5 | 52,158 | 1,205 | – | 1,205 | – | 55–74 | Processed meat (bacon, cold cuts, ham, hot dogs, sausage) Total red meat (bacon, beef, cheeseburgers, cold cuts, ham, hamburgers, hot dogs, liver, pork, sausage, veal, venison, red meat from mixed dishes) (FFQ) |
Age, Ethnic group, education, study center, randomization group, family history of breast cancer, age at menarche, age at menopause, age at first birth and number of live births, history of BBD, number of mammograms during 3 years, menopausal HRT, BMI, alcohol use, total fat intake, total energy intake |
| Larsson et al.,10 | SMC/Sweden | Cohort | 17.4 | 61,433 | 2,952 | – | – | * | 39–76 | Red meat (all fresh and minced pork, beef, veal) Processed meat (ham, bacon, sausages, salami, processed meat cuts, liver pate, blood sausages) Total red meat (FFQ) |
Age, education, BMI, height, parity and age at first birth, age at menarche, age at menopause, OC use, HRT, family history of breast cancer, total energy intake, alcohol intake |
| Pala et al.,17 | EPIC/Europe | Cohort | 8.8 | 319,826 | 7,119 | 1,699 | 3,673 | – | 50.8 (25–70) | Red meat (fresh, minced and frozen beef, veal, pork, lamb) Processed meat (ham, bacon, sausages, blood sausages, liver pâté, salami, mortadella, tinned meat and others) (FFQ) |
Age, center, energy, height, weight, years of schooling, smoking, alcohol intake, menopause |
| Wirfält et al.,22 | MDC, Sweden | Cohort | 10 | 15,773 | 544 | – | – | * | 45–73 | Processed meat (sausages, all types) (mixed methods) | Energy intake, age, method version, season of data collection |
| Genkinger et al.,21 | BWHS/US | Cohort | 12 | 52,062 | 1,268 | 573 | 520 | * | 21–69 | Red meat Processed meat (FFQ) |
Energy intake, age at menarche, BMI, family history of breast cancer, education, parity and age at first live birth, OC use, menopausal status, age at menopause, HRT, physical activity, smoking status, alcohol intake |
| Pouchieu et al.,37 | SU.VI.MAX/France | Randomized clinical trial (placebo group) | 11.3 | 2,367 | 102 | – | – | – | – | Red meat (fresh, minced and frozen beef, veal, pork and lamb) Processed meat (ham, bacon, sausages, blood sausages, liver pâ té, salami, mortadella, tinned meat and others) (Dietary record) |
Age, number of dietary records, smoking status, educational level, physical activity, height, BMI, family history of breast cancer, menopausal status at baseline, HRT, number of live births, without-alcohol energy intake, alcohol intake, total lipid intake, mutual adjustment for red meat or processed meat |
| Farvid et al.,8 | NHSII/US | Cohort | 20 | 88,803 | 2,830 | 1,511 | 918 | * | 26–45 | Red meat (beef, or lamb as a sandwich, pork, beef, or lamb as a main dish, hamburger) Processed meat (sausage, salami and bologna) Total red meat (red meat and processed meat) (FFQ) |
Age, Ethnic group, family history of breast cancer, history of BBD, smoking, height, BMI, age at menarche, parity and age at firth birth, OC use, HRT, age at menopause, menopausal status, alcohol intake, energy intake |
| Inoue-Choi et al.,18 | NIH-AARP/US | Cohort | 9.4 | 193,742 | 9,305 | – | 9,305 | * | 62 ± 5.3 (50–71) | Red meat (beef, pork) Processed red meat (ham, bacon, sausages, hot dogs, cold cuts) Total red meat (red meat, processed meat) (FFQ) | Age, Ethnic group, BMI, height, education, cigarette smoking, alcohol intake, physical activity, family history of breast cancer, age at menarche, age at menopause, age at first live birth, number of live births, HRT, OC use, number of previous breast biopsy, total energy intake, total fat intake, fiber intake, intake of other types of meat. |
| Anderson, et al.,6 | UK Biobank/UK | Cohort | 7 | 262,195 | 4,819 | – | 40–69 | Red meat (beef, pork, lamb) Processed meat (FFQ) |
Age, deprivation, ethnic group, smoking, alcohol, BMI, physical activity, vegetables, type of bread | ||
| Diallo, et al.,34 | NutriNet Santé/France | Cohort | 4.1 | 45,930 | 544 | 169 | 375 | 51.7 ± 10.1 | Red meat Processed meat Total red meat (red meat and processed meat) 24 h dietary records | Age, energy intake without alcohol, number of 24-h dietary records, smoking, education, physical activity, height, BMI, alcohol, family history of cancers, lipid intake, fruits, vegetables, menopausal status, number of children, mutual adjustment for red meat or processed meat | |
BBD, Benign Breast Disease; BMI, Body Mass Index; BWHS, Black Women Health Study; CNBSS, Canadian National Breast Screening Study; CSA, California Seventh-day Adventist; EPIC, European Prospective Investigation into Cancer and Nutrition cohort; HRT, Hormone Replacement Therapy; MDC, the Malmö Diet and Cancer; MPCDRF, the Monitoring Project on Cardiovascular Disease Risk Factors; NHS, Nurses’ Health Study; NHSII, Nurses’ Health Study II; NIH-AARP, National Institute of Health- the American Association of Retired Persons; NLCS, The Netherland Cohort Study; NYS, The New York University Women’s Health Study; OC, Oral Contraceptive; PLCOCST, Prostate Lung Colorectal and Ovarian Cancer Screening Trial; RERFLSS, The Radiation Effects Research Foundation’s Life Span Study; SMC, Swedish Mammography Cohort; SU.VI.MAX, Supplemental en Vitamines et Mineraux Antioxydants; UKWCS, The UK Women’s Cohort Study.
Table 2.
Characteristics of cohort studies that evaluated red meat intake and incidence of breast cancer by NAT2 acetylator status
| Author and year | Study name and country | Design of the study | Follow-up | Total No. of participants | Number of breast cancer events | Baseline age | Red meat variables (method of assessment) | Adjustment |
|---|---|---|---|---|---|---|---|---|
| Egeberg et al.,29 | DCH/Denmark | nested case-control | 7 | Control:367 | 367 | 50–64 | Red meat (beef, veal, pork, lamb, offal) Processed meat (bacon, smoked ham, salami, frankfurter, Cumberland sausage, cold cuts, liver pâ té, processed fish that is fish prepared by pickling, salting, or smoking) (FFQ) |
Parity, age at first birth, education, duration of HRT use, intake of alcohol, BMI |
| Lee et al,30 | NHS/US | nested case-control | – | 1,560 | 579 | Total red meat (red meat and processed meat) (FFQ) | age, smoking, BMI at 18 yr, weight gain from 18 yr, age at menarche, family history of breast cancer, parity and age at first birth, postmenopausal hormone use, history of benign breast disease, total calorie and alcohol intake. |
BMI, Body Mass Index; DCH, Diet, Cancer, and Health Study; HRT, Hormone Replacement Therapy; NHS, Nurses’ Health Study.
We conducted separate analyses considering for red meat, processed meat and total red meat. The characteristics of the 18 identified cohort, nested case–control and clinical trial studies are summarized in Table 1. In each study, the number of participants ranged from 493 to 319,826, and they were followed up anywhere from 1.9 to 20 years. A total of 1,133,110 women, including 33,493 cases of breast cancer (13 studies), were included in the red meat and overall breast cancer meta-analysis; a total of 1,254,452 women, including 37,070 cases of breast cancer (15 studies) for processed meat; and a total of 531,722 women, including 21,123 cases of breast cancer (7 studies) for total red meat. Diet was generally assessed by food frequency questionnaire, except two studies (NutriNet Santé and the Supplementation en Vitamines et Mineraux Antioxydants [SU.VI.MAX]) that utilized dietary records.34,37 Red meat, processed meat and total red meat consumption was reported as gram/day or week, serving/day, or gram/1,000 kcal across studies (Supporting Information Tables S1, S2, S3). From 18 studies (including nested case–control and clinical trial studies), eight studies were from North America,8,9,12,14,16,18,21,35 nine studies were from Europe6,10,13,15,17,22,34,36,37 and one study from Japan.33 In 15 out of 18 studies (CSA, NLCS, NHS, MPCDRF, CNBSS, UKWCS, PLCOCST, SMC, EPIC, BWHS, SU.VI.MAX, NHSII, NIH-AARP, UK Biobank, NutriNet Santé), measures of associations were adjusted for known breast cancer risk factors (Table 1). Seven cohort studies (NHS, CNBSS, UKWCS, EPIC, BWHS, NHSII, NutriNet Santé) reported results for premenopausal breast cancer. Eleven out of 18 studies reported the association between red meat or processed meat intake with breast cancer in postmenopausal women.6,8,9,13–18,21,34 Three cohort studies (SMC, NHSII, NIH-AARP) reported the association between total red meat intake and breast cancer by estrogen and progesterone receptor status (Table 1). Data on red meat intake and risk of breast cancer stratified by NAT2 genotypes were available from two nested case–control studies (DCH, NHS) (Table 2).
Publication bias
For overall breast cancer, visual inspection of a funnel plot (Supporting Information Fig. 1) and the Begg’s test suggested no evidence of publication bias for red meat (p = 0.23) or processed meat (p = 0.67).
Red meat, processed meat and total red meat consumption and risk of breast cancer
Across 13 studies that examined the association between red meat and overall breast cancer, red meat consumption was associated with a nonsignificant increased risk of overall breast cancer. The random-effects summary of RRs comparing the highest vs. the lowest category of red meat was 1.06 (95% CI: 0.99, 1.14) (Fig. 2), with moderate inconsistency between studies (I2 = 56.3%).
Figure 2.
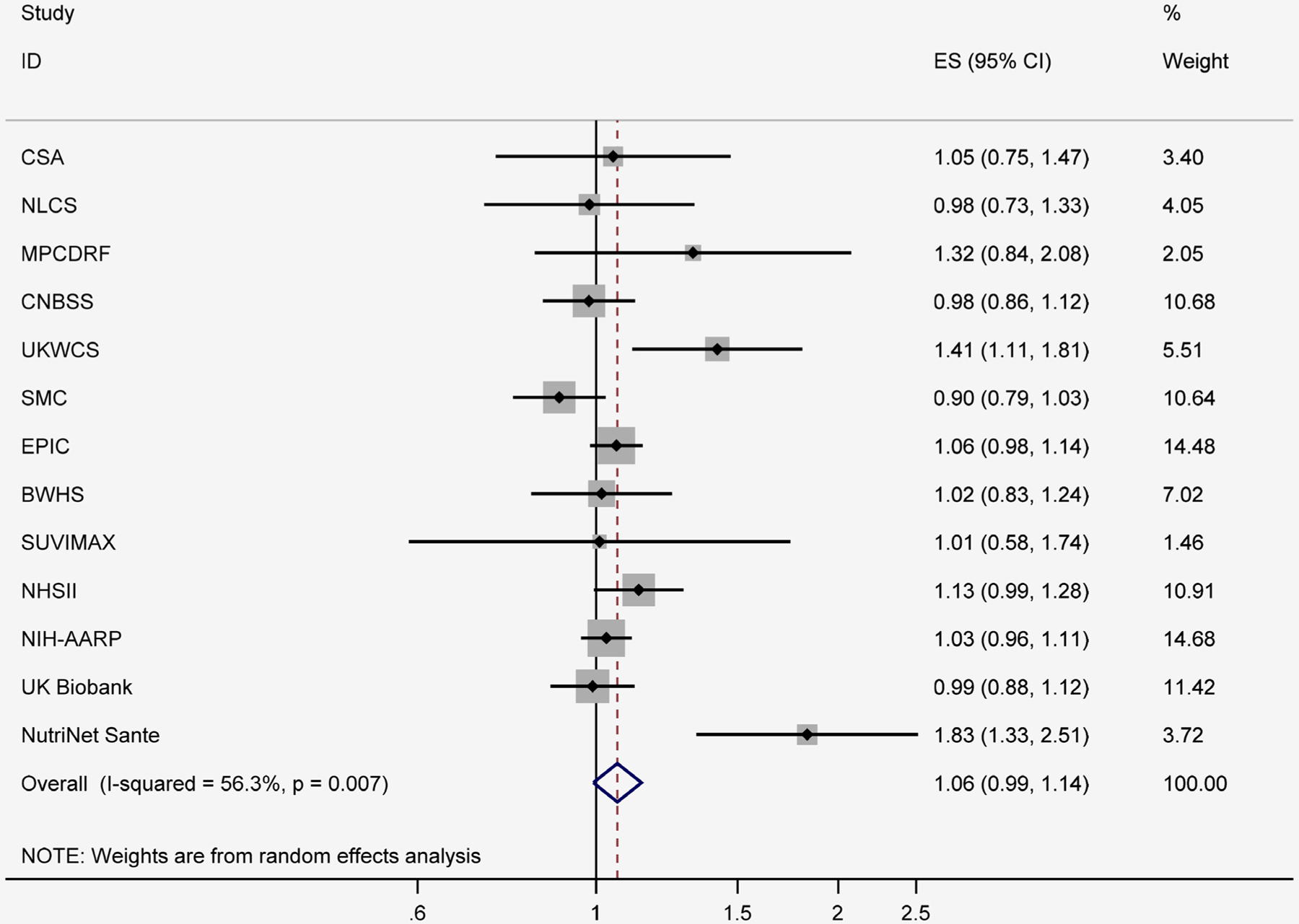
Red meat intake and relative risks of overall breast cancer (highest category vs. lowest category). BWHS, Black Women Health Study; CNBSS, Canadian National Breast Screening Study; CSA, California Seventh-day Adventist; EPIC, European Prospective Investigation into Cancer and Nutrition cohort; MPCDRF, the Monitoring Project on Cardiovascular Disease Risk Factors; NHSII, Nurses’ Health Study II; NIH-AARP, National Institute of Health- the American Association of Retired Persons; NLCS, The Netherland Cohort Study; SMC, Swedish Mammography Cohort; SU.VI.MAX, Supplemental en Vitamines et Mineraux Antioxydants; UKWCS, The UK Women’s Cohort Study.
Among 15 studies that evaluated the association between processed meat and overall breast cancer, the risk estimate comparing the highest vs. the lowest category was 1.09 (95% CI: 1.03, 1.16; I2 = 44.4%) (Fig. 3).
Figure 3.
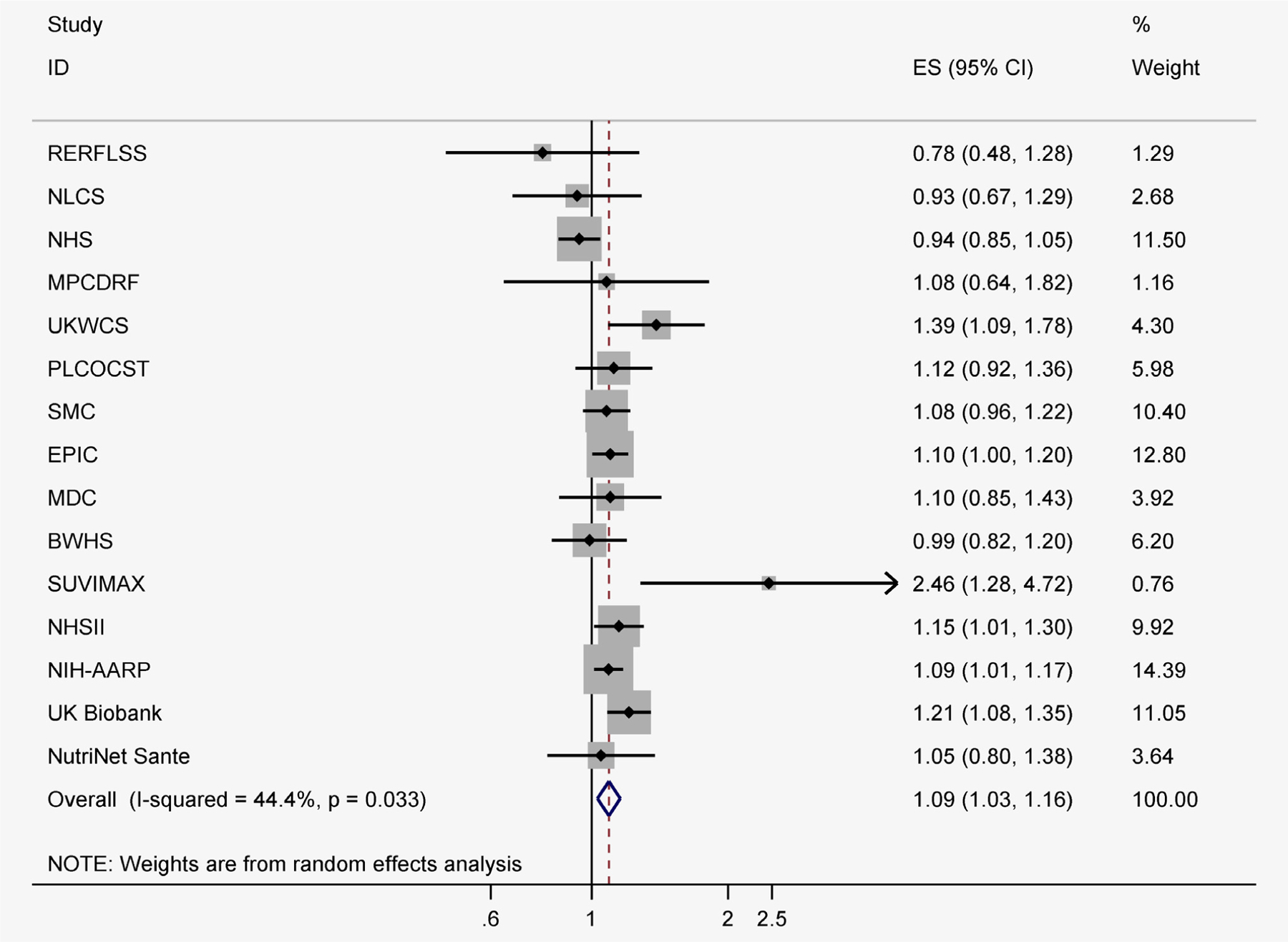
Processed meat intake and relative risks of overall breast cancer (highest category vs. lowest category). BWHS, Black Women Health Study; EPIC, European Prospective Investigation into Cancer and Nutrition cohort; MDC, the Malmö Diet and Cancer; MPCDRF, the Monitoring Project on Cardiovascular Disease Risk Factors; NHS, Nurses’ Health Study; NHSII, Nurses’ Health Study II; NIH-AARP, National Institute of Health- the American Association of Retired Persons; NLCS, The Netherland Cohort Study; PLCOCST, Prostate Lung Colorectal and Ovarian Cancer Screening Trial; RERFLSS, The Radiation Effects Research Foundation’s Life Span Study; SMC, Swedish Mammography Cohort; SU.VI.MAX, Supplemental en Vitamines et Mineraux Antioxydants; UKWCS, The UK Women’s Cohort Study.
Among seven studies that evaluated the association between total red meat and overall breast cancer, the risk estimate comparing the highest vs. the lowest category was 1.09 (95% CI: 0.99, 1.21; I2 = 65.3%) (Fig. 4).
Figure 4.
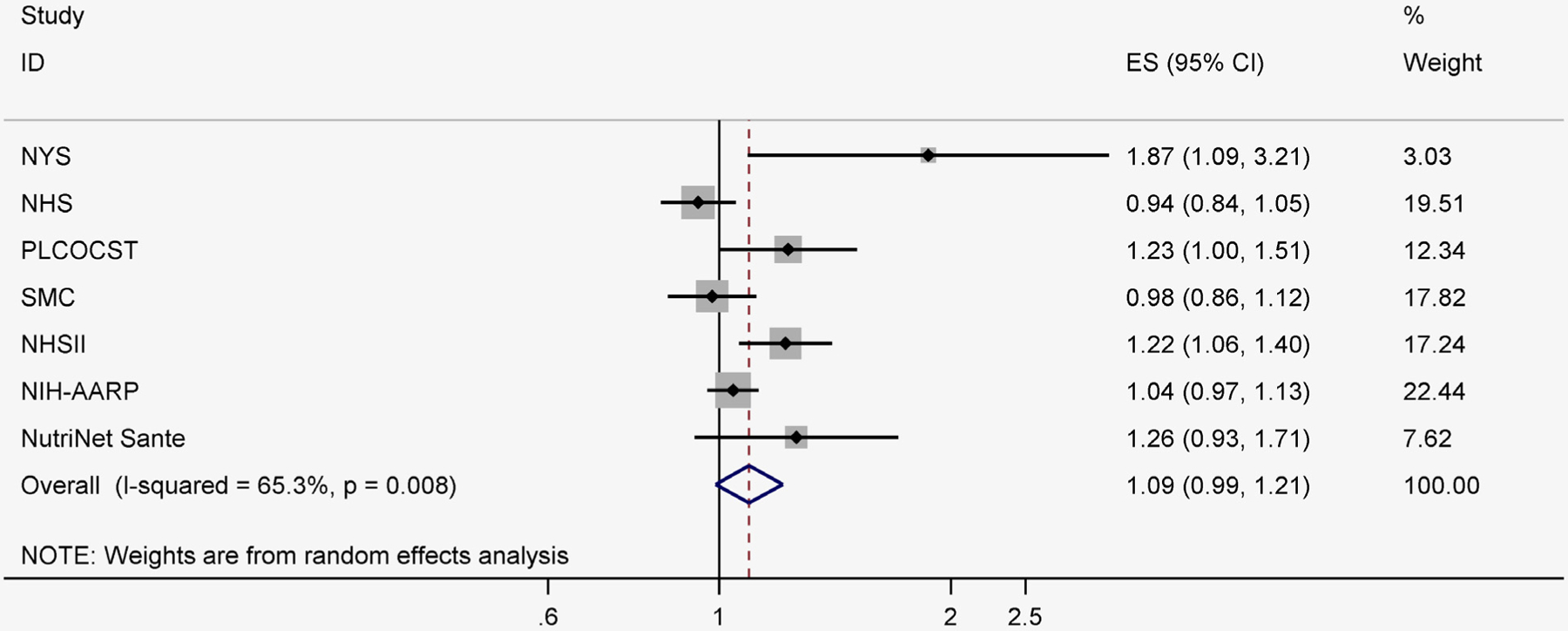
Total red meat intake and relative risks of overall breast cancer (highest category vs. lowest category). NHS, Nurses’ Health Study; NHSII, Nurses’ Health Study II; NIH-AARP, National Institute of Health- the American Association of Retired Persons; NYS, The New York University Women’s Health Study; PLCOCST, Prostate Lung Colorectal and Ovarian Cancer Screening Trial; SMC, Swedish Mammography Cohort.
No individual study had a particularly large influence on the pooled estimate of RR for red meat or processed meat and breast cancer. However, significant association was observed between red meat and breast cancer after excluding SMC (RR = 1.08, 95%CI = 1.01–1.16; I2 = 50.5%). In addition, the pooled RRs for red meat and breast cancer was changed to 1.04 (95%CI = 0.98–1.09; I2 = 27.8%) after excluding NutriNet Santé.
Red meat and processed meat consumption and risk of pre- and post-menopausal breast cancer
Among six cohort studies that examined the association between red meat intake and premenopausal breast cancer, the risk estimate comparing the highest vs. the lowest category was 1.07 (95% CI: 0.97, 1.18; I2 = 30.9%) (Supporting Information Fig. S2–A). Among nine studies that examined the association between red meat intake and postmenopausal breast cancer, the summary of RRs comparing the highest vs. the lowest category of red meat was 1.08 (95% CI: 0.99, 1.17; I2 = 53.6%) (Supporting Information Fig. S2–B). With regard to processed meat, higher intake was not associated with risk of premenopausal breast cancer (highest vs. lowest category RR = 1.09; 95%CI = 0.95, 1.25; I2 = 50.3%) (Supporting Information Fig. S3–A), whereas it was associated with a higher risk of postmenopausal breast cancer (highest vs. lowest category RR = 1.10; 95% CI: 1.03, 1.17; I2 = 30.8%) (Supporting Information Fig. S3–B).
Total red meat consumption, estrogen and progesterone receptor status and risk of breast cancer
Among the three studies with data related to hormone receptor status, total red meat intake was not significantly positively associated with risk of ER+/PR+ tumors (RR: 1.12; 95% CI: 0.92, 1.38) (Supporting Information Fig. S4–A) and ER−/PR− tumors (RR: 1.03; 95% CI: 0.85, 1.24) (Supporting Information Fig. S4–B).
Red meat consumption, NAT2 acetylator status and risk of breast cancer
Among the two studies with data on NAT2 genotype status, consumption of red meat was not associated with a higher risk of breast cancer among women with either the fast NAT2 acetylator genotype (OR: 1.18; 95% CI: 0.93, 1.50 for each 25 g/d increase of red meat) (Fig. 5-A) or the slow NAT2 acetylator genotype (OR: 0.99; 95% CI: 0.91, 1.08 for each 25 g/d increase of red meat) (Fig. 5-B).
Figure 5.
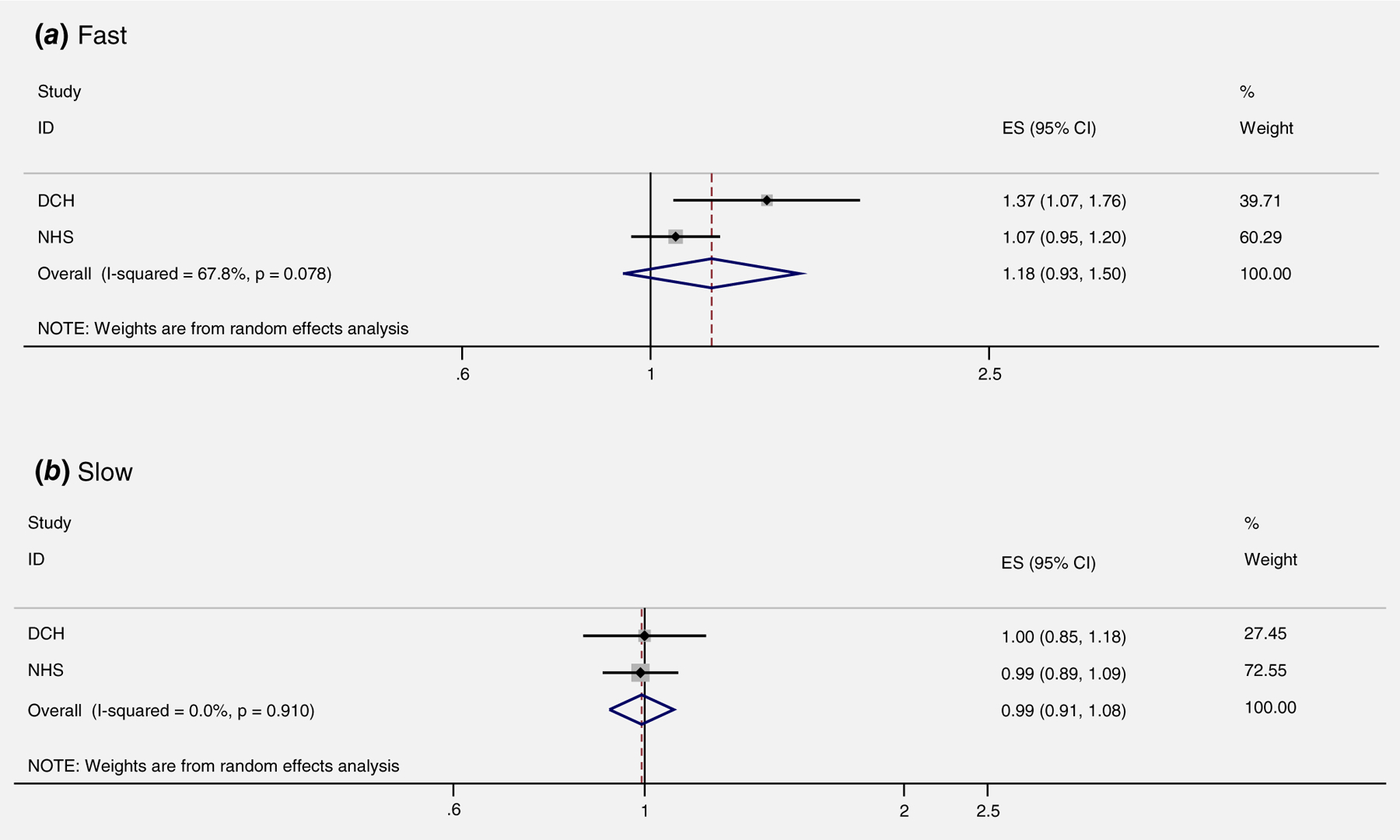
Red meat intake and risk of breast cancer based on NAT2 acetylator genotype (per 25 g/day). DCH, Diet, Cancer, and Health Study; NHS, Nurses’ Health Study.
Subgroup analyses
We did not find significant heterogeneity among studies that examined red meat or processed meat in relation to overall breast cancer incidence (Supporting Information Table S4).
Discussion
This systematic review and meta-analysis study reports significant positive associations between processed meat consumption with risk of breast cancer. When considering menopausal status, similar risk estimates were observed for association between processed meat and breast cancer risk before and after menopause, however, this association was not significant among premenopausal women. These associations were independent of traditional breast cancer risk factors. We did not observe significant association between red meat intake and risk of breast cancer among women with fast or slow NAT2 acetylator genotype.
Similar to the prior meta-analyses published in 2015 and 2018,5,6 we found positive association between processed meat intake and breast cancer risk after including several newly published data and excluding duplicate studies. In our meta-analysis, we further expanded the analyses to include the assessment by menopausal and hormone receptor status.
Although high amounts of nitrate and nitrite might link processed meat to increased risk of breast cancer,38,39 the high content of saturated fat, cholesterol and heme iron found in red meat may also underlie the association with breast cancer.40,41 A high intake of animal-derived iron was associated with an increased risk of breast cancer in Chinese women.41 Consistent with these findings, Ferrucci et al.16 reported that intake of red meat, HCA and dietary iron elevated risk of invasive breast cancer. A study of genetic variability in iron-related oxidative stress pathways suggested that women with genotypes resulting in potentially higher levels of iron-related oxidative stress might be at increased risk of breast cancer.42 However, another study was unable to demonstrate an association of iron or heme iron with breast cancer risk.14
Carcinogenic HCAs formed in red meat during high-temperature cooking may play a role in the etiology of breast cancer.43–45 The carcinogenic effect may depend upon metabolisms of HCAs and related chemicals by NAT2 genotypes. However, our findings did not support that high intake of red meat might increase risk of breast cancer for women characterized as fast acetylators of NAT2 (Fig. 5). The lack of statistical significance might be due to the meta-analysis of two studies that the RR came from red meat in the DCH and the RR came from total red meat in the NHS.
When considering menopausal status, similar risk estimates were observed for association between processed meat and breast cancer risk before and after menopause, however, this association was not significant among premenopausal women. The lack of statistical significance might be due to smaller sample size and lower statistical power among premenopausal women.
Fewer studies evaluated whether the association between total red meat intake and breast cancer varied by hormone receptor status.8,10,18,21,22 Sex steroid hormones administered to animal for growth promotion might increase risk of hormone receptor positive tumors.20 In the current analysis, however, the association between high total red meat consumption and breast cancer risk did not differ between ER+/PR+ tumors or ER−/PR− tumors.
Our analysis has several strengths. We limited our analysis to prospective cohort, nested case–control and clinical trial studies to minimize the influence of recall and selection bias that may occur in case–control studies. Also, most prospective studies adjusted for potential breast cancer risk factors. Although we observed wide variations in study population in the cohort studies, low to moderate heterogeneity across studies supported the external validity of pooling results from different studies. Additional strengths included the ability to evaluate the association of red meat intake and breast cancer events in different populations with different diets, including large variations in red meat intake and to distinguish between unprocessed and processed red meat.
A few limitations of our study should be considered. As in any meta-analysis, publication bias is possible. However, we did not observe significant publication bias for either red meat or processed meat. Although most of the studies adjusted for major breast cancer risk factors, as with most observational studies, we cannot exclude the possibility of residual confounding. In the majority of studies, because diet was assessed using an FFQ, the under- or over-reporting of the amount of food groups could cause measurement error. However, since this equally may affect cases and noncases, likely estimates will be biased toward the null, so actual effect sizes might be larger than we observed here. Moreover, we compared the highest level of intake vs. the lowest, but levels of intake do not match sometimes. In some studies, processed poultry was included in processed meat and total red meat. If data for processed red meat were reported, these data were considered in our meta-analysis. Furthermore, at this level of risk subtle biases may be present. In some analyses, especially the one by NAT2 genotype or by hormone receptor status, sample size was limited. Finally, because the majority of the studies were conducted in North America and Europe, the findings may not be directly generalizable to other racial and ethnic groups.
Conclusion
This systematic review and meta-analysis including prospective cohort studies of red meat and processed meat consumption provides evidence that higher consumption of processed meat is associated with higher risk of breast cancer. However, red meat was not a significant cause of breast cancer. Moreover, we did not find evidence for differing associations according to NAT2 genotypes. Further studies examining molecular subtypes of breast cancer are needed.
Supplementary Material
What’s new?
The International Agency for Research on Cancer concluded that consumption of red meat is a probable human carcinogen, whereas processed meat was classified as carcinogenic. However, this classification was largely based on the evidence for colorectal, pancreas and prostate cancers. This systematic review and meta-analysis including prospective cohort studies of red meat and processed meat consumption provides evidence that higher consumption of processed meat, but not red meat, is associated with higher risk of breast cancer. These associations were independent of traditional breast cancer risk factors, and no evidence was found for differing associations according to N-acetyltransferase 2 (NAT2) genotypes.
Footnotes
Disclosures
The authors of our study have no conflict of interest or any financial disclosures to make.
References
- 1.Ferlay J, Soerjomataram I, Dikshit R, et al. Cancer incidence and mortality worldwide: sources, methods and major patterns in GLOBOCAN 2012. Int J Cancer 2015;136:E359–86. [DOI] [PubMed] [Google Scholar]
- 2. http://globocan.iarc.fr/old/FactSheets/cancers/breast-new.asp .
- 3.Bouvard V, Loomis D, Guyton KZ, et al. Carcinogenicity of consumption of red and processed meat. Lancet Oncol 2015;16:1599–600. [DOI] [PubMed] [Google Scholar]
- 4.Missmer SA, Smith-Warner SA, Spiegelman D, et al. Meat and dairy food consumption and breast cancer: a pooled analysis of cohort studies. Int J Epidemiol 2002;31:78–85. [DOI] [PubMed] [Google Scholar]
- 5.Guo J, Wei W, Zhan L. Red and processed meat intake and risk of breast cancer: a meta-analysis of prospective studies. Breast Cancer Res Treat 2015;151:191–8. [DOI] [PubMed] [Google Scholar]
- 6.Anderson JJ, Darwis NDM, Mackay DF, et al. Red and processed meat consumption and breast cancer: UKbiobank cohort study and meta-analysis. Eur J Cancer 2018;90:73–82. [DOI] [PubMed] [Google Scholar]
- 7.Cho E, Chen WY, Hunter DJ, et al. Red meat intake and risk of breast cancer among premenopausal women. Arch Intern Med 2006;166:2253–9. [DOI] [PubMed] [Google Scholar]
- 8.Farvid MS, Cho E, Chen WY, et al. Dietary protein sources in early adulthood and breast cancer incidence: prospective cohort study. BMJ 2014; 348:g3437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Holmes MD, Colditz GA, Hunter DJ, et al. Meat, fish and egg intake and risk of breast cancer. Int J Cancer 2003;104:221–7. [DOI] [PubMed] [Google Scholar]
- 10.Larsson SC, Bergkvist L, Wolk A. Long-term meat intake and risk of breast cancer by oestrogen and progesterone receptor status in a cohort of Swedish women. Eur J Cancer 2009;45:3042–6. [DOI] [PubMed] [Google Scholar]
- 11.Shannon J, Ray R, Wu C, et al. Food and botanical groupings and risk of breast cancer: a case-control study in Shanghai China. Cancer Epidemiol Biomarkers Prev 2005;14:81–90. [PubMed] [Google Scholar]
- 12.Mills PK, Beeson WL, Phillips RL, et al. Dietary habits and breast cancer incidence among seventh-day Adventists. Cancer 1989;64:582–90. [DOI] [PubMed] [Google Scholar]
- 13.Voorrips LE, Brants HA, Kardinaal AF, et al. Intake of conjugated linoleic acid, fat, and other fatty acids in relation to postmenopausal breast cancer: The Netherlands cohort study on diet and cancer. Am J Clin Nutr 2002;76:873–82. [DOI] [PubMed] [Google Scholar]
- 14.Kabat GC, Miller AB, Jain M, et al. Dietary iron and heme iron intake and risk of breast cancer: a prospective cohort study. Cancer Epidemiol Biomarkers Prev 2007;16:1306–8. [DOI] [PubMed] [Google Scholar]
- 15.Taylor EF, Burley VJ, Greenwood DC, et al. Meat consumption and risk of breast cancer in the UKWomen’s cohort study. Br J Cancer 2007;96: 1139–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ferrucci LM, Cross AJ, Graubard BI, et al. Intake of meat, meat mutagens, and iron and the risk of breast cancer in the prostate, lung, colorectal, and ovarian cancer screening trial. Br J Cancer 2009;101:178–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Pala V, Krogh V, Berrino F, et al. Meat, eggs, dairy products, and risk of breast cancer in the European prospective investigation into cancer and nutrition (EPIC) cohort. Am J Clin Nutr 2009;90:602–12. [DOI] [PubMed] [Google Scholar]
- 18.Inoue-Choi M, Sinha R, Gierach GL, et al. Red and processed meat, nitrite, and heme iron intakes and postmenopausal breast cancer risk in the NIH-AARP diet and health study. Int J Cancer 2016;138:1609–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Althuis MD, Fergenbaum JH, Garcia-Closas M, et al. Etiology of hormone receptor-defined breast cancer: a systematic review of the literature. Cancer Epidemiol Biomarkers Prev 2004;13:1558–68. [PubMed] [Google Scholar]
- 20.Andersson AM, Skakkebaek NE. Exposure to exogenous estrogens in food: possible impact on human development and health. Eur J Endocrinol 1999;140:477–85. [DOI] [PubMed] [Google Scholar]
- 21.Genkinger JM, Makambi KH, Palmer JR, et al. Consumption of dairy and meat in relation to breast cancer risk in the black Women’s health study. Cancer Causes Control 2013;24:675–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wirfält E, Li C, Manjer J, et al. Food sources of fat and sex hormone receptor status of invasive breast tumors in women of the Malmö diet and cancer cohort. Nutr Cancer 2011;63:722–33. [DOI] [PubMed] [Google Scholar]
- 23.Layton DW, Bogen KT, Knize MG, et al. Cancer risk of heterocyclic amines in cooked foods: an analysis and implications for research. Carcinogenesis 1995;16:39–52. [DOI] [PubMed] [Google Scholar]
- 24.Sugimura T, Wakabayashi K, Nakagama H, et al. Heterocyclic amines: mutagens/carcinogens produced during cooking of meat and fish. Cancer Sci 2004;95:290–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Minchin RF, Reeves PT, Teitel CH, et al. N-and O-acetylation of aromatic and heterocyclic amine carcinogens by human monomorphic and polymorphic acetyltransferases expressed in COS-1 cells. Biochem Biophys Res Commun 1992;185: 839–44. [DOI] [PubMed] [Google Scholar]
- 26.Hein DW, Doll MA, Fretland AJ, et al. Molecular genetics and epidemiology of the NAT1 and NAT2 acetylation polymorphisms. Cancer Epidemiol Biomarkers Prev 2000;9:29–42. [PubMed] [Google Scholar]
- 27.Grant DM, Hughes NC, Janezic SA, et al. Human acetyltransferase polymorphisms. Mutat Res 1997; 376:61–70. [DOI] [PubMed] [Google Scholar]
- 28.Stone EM, Williams JA, Grover PL, et al. Interindividual variation in the metabolic activation of heterocyclic amines and their N-hydroxy derivatives in primary cultures of human mammary epithelial cells. Carcinogenesis 1998;19: 873–9. [DOI] [PubMed] [Google Scholar]
- 29.Egeberg R, Olsen A, Autrup H, et al. Meat consumption, N-acetyl transferase 1 and 2 polymorphism and risk of breast cancer in Danish postmenopausal women. Eur J Cancer Prev 2008; 17:39–47. [DOI] [PubMed] [Google Scholar]
- 30.Lee HJ, Wu K, Cox DG, et al. Polymorphisms in xenobiotic metabolizing genes, intakes of heterocyclic amines and red meat, and postmenopausal breast cancer. Nutr Cancer 2013;65:1122–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Deitz AC, Zheng W, Leff MA, et al. N-Acetyltransferase-2 genetic polymorphism, well-done meat intake, and breast cancer risk among postmenopausal women. Cancer Epidemiol Biomarkers Prev 2000;9:905–10. [PubMed] [Google Scholar]
- 32.Stroup DF, Berlin JA, Morton SC, et al. Meta-analysis of observational studies in epidemiology: a proposal for reporting. Meta-analysis of observational studies in epidemiology (MOOSE) group. JAMA 2000;283:2008–12. [DOI] [PubMed] [Google Scholar]
- 33.Key TJ, Sharp GB, Appleby PN, et al. Soya foods and breast cancer risk: a prospective study in Hiroshima and Nagasaki, Japan. Br J Cancer 1999;81:1248–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Diallo A, Deschasaux M, Latino-Martel P, et al. Red and processed meat intake and cancer risk: results from the prospective NutriNet-Santé cohort study. Int J Cancer 2018;142:230–7. [DOI] [PubMed] [Google Scholar]
- 35.Toniolo P, Riboli E, Shore RE, et al. Consumption of meat, animal products, protein, and fat and risk of breast cancer: a prospective cohort study in New York. Epidemiology 1994;5:391–7. [DOI] [PubMed] [Google Scholar]
- 36.van der Hel OL, Peeters PH, Hein DW, et al. GSTM1 null genotype, red meat consumption and breast cancer risk (The Netherlands). Cancer Causes Control 2004;15:295–303. [DOI] [PubMed] [Google Scholar]
- 37.Pouchieu C, Deschasaux M, Hercberg S, et al. Prospective association between red and processed meat intakes and breast cancer risk: modulation by an antioxidant supplementation in the SU.VI.MAX randomized controlled trial. Int J Epidemiol 2014;43:1583–92. [DOI] [PubMed] [Google Scholar]
- 38.Bartsch H, Montesano R. Relevance of nitrosamines to human cancer. Carcinogenesis 1984;5: 1381–93. [DOI] [PubMed] [Google Scholar]
- 39.Mirvish SS. Role of N-nitroso compounds (NOC) and N-nitrosation in etiology of gastric, esophageal, nasopharyngeal and bladder cancer and contribution to cancer of known exposures to NOC. Cancer Lett 1995;93:17–48. [DOI] [PubMed] [Google Scholar]
- 40.Farvid MS, Cho E, Chen WY, et al. Premenopausal dietary fat in relation to pre- and post-menopausal breast cancer. Breast Cancer Res Treat 2014;145:255–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kallianpur AR, Lee SA, Gao YT, et al. Dietary animal-derived iron and fat intake and breast cancer risk in the Shanghai breast cancer study. Breast Cancer Res Treat 2008;107:123–32. [DOI] [PubMed] [Google Scholar]
- 42.Hong CC, Ambrosone CB, Ahn J, et al. Genetic variability in iron-related oxidative stress pathways (Nrf2, NQ01, NOS3, and HO-1), iron intake, and risk of postmenopausal breast cancer. Cancer Epidemiol Biomarkers Prev 2007;16: 1784–94. [DOI] [PubMed] [Google Scholar]
- 43.De Stefani E, Ronco A, Mendilaharsu M, et al. Meat intake, heterocyclic amines, and risk of breast cancer: a case-control study in Uruguay. Cancer Epidemiol Biomarkers Prev 1997;6:573–81. [PubMed] [Google Scholar]
- 44.Steck SE, Gaudet MM, Eng SM, et al. Cooked meat and risk of breast cancer lifetime versus recent dietary intake. Epidemiology 2007;18:373–82. [DOI] [PubMed] [Google Scholar]
- 45.Lauber SN, Ali S, Gooderham NJ. The cooked food derived carcinogen 2-amino-1-methyl-6-phenylimidazo[4,5-b] pyridine is a potent oestrogen: a mechanistic basis for its tissue-specific carcinogenicity. Carcinogenesis 2004;25:2509–17. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


