BACKGROUND:
Darvadstrocel is an expanded allogeneic adipose-derived mesenchymal stem cell therapy for the treatment of complex perianal fistulas in patients with Crohn’s disease. Safety and efficacy outcomes from the clinical trial known as “Adipose derived mesenchymal stem cells for induction of remission in perianal fistulizing Crohn’s disease,” or ADMIRE-CD (NCT01541579), from up to 52 weeks posttreatment were previously reported. Here, the outcomes from an extended 104-week follow-up are reported.
OBJECTIVE:
The goal of this study was to assess the long-term safety and efficacy of darvadstrocel at 2 years post-treatment in patients with Crohn’s disease and complex perianal fistulas.
DESIGN:
This was a phase 3 double-blind randomized controlled study (ADMIRE-CD) in patients with perianal fistulizing Crohn’s disease.
SETTINGS:
This study extension was conducted in multiple hospitals across 7 European countries and Israel.
PATIENTS:
Forty patients entered the extended follow-up period: 25 patients in the darvadstrocel treatment group and 15 in the control group.
INTERVENTIONS:
Darvadstrocel or saline solution (control group) was administered once, locally, after fistula tract curettage and internal opening closure (with previous seton placement). All patients were permitted to continue ongoing medical treatments for fistulas.
MAIN OUTCOME MEASURES:
Treatment-emergent serious adverse events were recorded through week 104. Clinical remission, defined as closure of all treated external openings that were draining at baseline despite gentle finger compression, was assessed at week 104.
RESULTS:
Of 40 patients, 37 completed the extended follow-up. Through week 104, 7 treatment-emergent serious adverse events were reported, of which 4 occurred between weeks 52 and 104. At week 104, clinical remission was reported in 14/25 (56%) patients in the darvadstrocel group and 6/15 (40%) patients in the control group.
LIMITATIONS:
Limitations include the small number of patients who entered the extended follow-up period, and no imaging examinations were performed at the 104-week time point.
CONCLUSIONS:
Darvadstrocel was well tolerated and clinical remission after treatment with darvadstrocel may be sustained for up to 104 weeks in patients with perianal fistulizing Crohn’s disease. See Video Abstract at http://links.lww.com/DCR/B812.
ESTUDIO DE SEGUIMIENTO PARA EVALUAR LA SEGURIDAD Y EFICACIA A LARGO PLAZO DE DARVADSTROCEL (TRATAMIENTO CON CÉLULAS MADRE MESENQUIMALES) EN PACIENTES CON ENFERMEDAD DE CROHN PERIANAL FISTULIZANTE: ENSAYO CONTROLADO ALEATORIZADO DE FASE 3 ADMIRE-CD
ANTECEDENTES:
Darvadstrocel es una terapia con células madre mesenquimales alogénicas expandidas derivadas de tejido adiposo para el tratamiento de fístulas perianales complejas en pacientes con enfermedad de Crohn. Los resultados del ensayo clínico conocido como “Células madre mesenquimales derivadas de tejido adiposo para la inducción de la remisión en la enfermedad de Crohn fistulizante perianal” o ADMIRE-CD (NCT01541579), en cuanto a la seguridad y eficacia hasta 52 semanas después del tratamiento, fueron previamente informados. Seguidamente, se presentan los resultados de un seguimiento extendido de 104 semanas.
OBJETIVO:
Evaluar la seguridad y eficacia a largo plazo de darvadstrocel a dos años del tratamiento en pacientes con enfermedad de Crohn y fístulas perianales complejas.
DISEÑO:
Este fue un estudio de fase 3, aleatorizado, a doble ciego, controlado (ADMIRE-CD) en pacientes con enfermedad de Crohn perianal fistulizante.
DESARROLLO:
Esta extensión del estudio se realizó en varios hospitales de siete países europeos e Israel.
PACIENTES:
Cuarenta pacientes participaron en la extensión de seguimiento: tratamiento con darvadstrocel (n = 25); grupo control (n = 15).
INTERVENCIONES:
Se administró Darvadstrocel o solución salina (grupo control) una vez, localmente, tras el legrado del trayecto fístuloso y cierre del orificio interno (con la colocación previa de setón). A todos los pacientes se les permitió continuar con los tratamientos médicos en curso para las fístulas.
PRINCIPALES MEDIDAS DE RESULTADO:
Los eventos de efectos adversos graves derivados del tratamiento se registraron hasta la semana 104. La remisión clínica, definida como el cierre de todas las aberturas externas tratadas que drenaban al inicio espontáneamente o por compresión suave de los dedos, fue evaluado en la semana 104.
RESULTADOS:
Del total de 40 pacientes, 37 completaron la extensión de seguimiento. Hasta la semana 104, se reportaron 7 eventos de efectos adversos graves resultantes del tratamiento, de los cuales 4 ocurrieron entre las semanas 52 y 104. En la semana 104, se reportó remisión clínica en 14/25 (56%) pacientes en el grupo de darvadstrocel y 6/15 (40%) pacientes en el grupo de control.
LIMITACIONES:
Solo una pequeña cantidad de pacientes participaron en el período de seguimiento extendido y no se realizaron exámenes por técnicas de imagen en la visita a 104 semanas.
CONCLUSIONES:
Darvadstrocel fue bien tolerado y la remisión clínica después del tratamiento con darvadstrocel puede mantenerse hasta 104 semanas en pacientes con enfermedad de Crohn perianal fistulizante. Consulte Video Resumen en http://links.lww.com/DCR/B812. (Traducción—Dr Osvaldo Gauto and Dr Julian Panés.)
Keywords: Crohn’s disease, Expanded allogeneic adipose-derived mesenchymal stem cells, Perianal fistula remission
Crohn’s disease (CD) is a chronic inflammatory condition of the gastrointestinal tract.1 The development of anal fistulas in CD is common, and approximately half are reported to be complex.2,3 Complex perianal fistulas are difficult to manage because they have a high rate of recurrence and may cause sphincter damage and fecal incontinence.2 Furthermore, proctitis and rectal ulceration can lead to anorectal stricture, which reduces therapeutic options. Common symptoms of complex perianal fistulas include chronic anal pain, perianal itching, and drainage of pus, stool, or blood from fistula openings, which can lead to a substantial impairment in quality of life (QOL).4–6 Complex perianal fistulas are estimated to occur in 12% to 28% of patients with CD, although social stigma around the symptoms could lead to under-reporting.3,6–9
Treatment options for complex perianal fistulas in CD include medical and surgical therapies, yet studies have shown that most interventions are ineffective in providing long-term healing, and a high proportion of patients experience an inadequate response to treatment or a relapse/recurrence of fistulas.5,10 Seton placement for drainage in combination with antibiotics is a common initial treatment for complex perianal fistulas.5 Anti-tumor necrosis factor (anti-TNF) agents, such as infliximab, have been recommended in the 2019 European Crohn’s and Colitis Organisation (ECCO) Guidelines on Therapeutics in Crohn’s Disease as a first-line medical therapy following adequate drainage, and efficacy has been demonstrated in several clinical trials.5,11–14 However, there is a high rate of fistula recurrence once treatment is stopped after 1 year.15 A combination of anti-TNF therapy and thiopurine immunomodulators have also been used, although the evidence for improvement in outcomes is weak.13 Surgical procedures to close fistulas include advancement flaps and ligation of the intersphincteric fistula tract. Surgery can be performed as a complement to, or after failure of, medical therapy. However, surgical intervention carries an increased risk of fecal incontinence.16
Consequently, there is a clear need for an effective therapy that provides long-term healing of complex perianal fistulas in patients with CD, without the risk of fecal incontinence. The key long-term therapeutic goals for the treatment of complex perianal fistulas are to 1) resolve fistula discharge, 2) achieve fistula healing, 3) prevent fistula recurrence, 4) maintain fecal continence, 5) avoid long-term diversion (protectomy with stoma), and hence, 6) improve and maintain QOL for patients.17
Owing to their regenerative properties, mesenchymal stem cells have been investigated for the treatment of complex perianal fistulas in patients with CD and have received increasing attention in recent years, with beneficial effects on fistula healing reported in clinical trials.5,18,19 Complex perianal fistulas are thought to arise from an epithelial defect (eg, ulcer), which may be caused by ongoing inflammation in patients with CD.20 Adipose-derived mesenchymal stem cells have anti-inflammatory and immunomodulatory potential and are therefore an attractive option for the treatment of complex perianal fistulas in CD.19,21
Darvadstrocel (Cx601) is a suspension of expanded allogeneic adipose-derived mesenchymal stem cells (eASCs) developed as a one-time local injection for the treatment of complex perianal fistulas in patients with CD. In the ADMIRE-CD phase 3 randomized controlled trial, administration of darvadstrocel after fistula tract curettage and internal opening closure demonstrated efficacy in patients with CD and treatment-refractory complex perianal fistulas, and it was well tolerated.22,23 The primary endpoint of combined remission at 24 weeks posttreatment (defined as a clinical assessment of closure of all treated external openings that were draining at baseline and the absence of collections >2cm diameter in the treated perianal fistulas) was met, with 51.5% (53/103) versus 35.6% (36/101) of patients observed to be in combined remission in the darvadstrocel-treated group versus the control group, respectively.22 The secondary endpoint of combined remission at 52 weeks posttreatment was also met with 56.3% (58/103) and 38.6% (39/101) remission rates in the darvadstrocel and control groups, respectively.23
The ADMIRE-CD study protocol was amended to extend the follow-up period by an additional year to bring the total follow-up period to 104 weeks, to assess the long-term safety of this treatment, and to determine whether the responses to darvadstrocel treatment observed up to 52 weeks after treatment were maintained through 104 weeks after treatment. The 2019 ECCO Guidelines note that there is a lack of long-term follow-up data (>1 year) available for complex perianal fistulas in patients with CD treated with eASCs; this study therefore contributes toward addressing this evidence gap.24 This article describes the results for patients included in the prospective follow-up extension of the ADMIRE-CD study (weeks 52 to 104). It was hypothesized that the efficacy of darvadstrocel could be sustained for 2 years after treatment.
METHODS
Study Design and Patients
ADMIRE-CD (NCT01541579) was a phase 3 double-blind randomized controlled study conducted in 49 hospitals in 7 European countries and Israel to assess the efficacy and safety of darvadstrocel for the treatment of complex perianal fistulas in patients with CD (study start date: December 13, 2011). Patients were enrolled in the 104-week extended follow-up at 24/49 study sites (study sites and Principal Investigators provided in Supplementary Table 1 at http://links.lww.com/DCR/B813). The study protocol was approved by the central or local Independent Ethics Committee or Institutional Review Board prior to initiation in each study center. The study was conducted in accordance with the 2008 World Medical Association Declaration of Helsinki: Ethical Principles for Medical Research Involving Human Subjects, as well as all relevant international, national, and local rules and regulations. Written informed consent to take part in the study was obtained from all patients.
Full details of the study design and patient eligibility criteria have been published previously.22 In brief, eligible patients were aged ≥18 years with non-active or mildly active luminal CD (Crohn’s Disease Activity Index score <220) diagnosed ≥6 months before enrollment. Patients had complex perianal fistulas with ≤2 internal openings and ≤3 external openings, which had been draining for ≥6 weeks and were refractory to ≥1 medical treatment (including antibiotics, immunosuppressants, or anti-TNF therapies). The study allowed continuation of previous treatment with immunosuppressants and anti-TNF therapies, and these treatments were maintained at stable doses throughout the study. To balance concomitant treatments between groups, a stratified randomization was used based on concomitant anti-TNF treatment (yes/no), and concomitant immunosuppressant treatment (yes/no). Patients were excluded if they were naïve to any treatment for complex perianal fistulas, were treated with oral steroids in the 4 weeks before study entry, or had abscesses or collections >2 cm diameter (unless resolved in the preparation procedure), concomitant rectovaginal fistulas or diverting stomas, and rectal and/or anal stenosis and/or active proctitis or surgical treatment of fistulas other than drainage or seton placement. Study treatment was administered by a non-blinded surgeon and the therapeutic effect was assessed by a blinded gastroenterologist.
Study Treatment
Vigorous curettage of all fistula tracts and placement of setons was performed in accordance with minimally invasive surgical procedures 2–3 weeks before the administration of darvadstrocel or placebo. Where abscesses were present, incision and drainage were also conducted. Before the administration of darvadstrocel, surgeons were required to exclude the presence of an abscess. Immediately before the administration of darvadstrocel, fistula tracts were conditioned: setons (if in place) were removed and a second vigorous curettage of all fistula tracts was performed, followed by the closing of internal openings with sutures. Patients then received a single administration of darvadstrocel around the internal opening and the fistula tract (suspension of 5 × 106 cells/mL; 120 × 106 cells in total, in sterile buffered solution) or placebo (24 mL saline solution) at a ratio of 1:1, stratified by co-treatment.
Extended Follow-up
A protocol amendment was approved on December 8, 2014 to extend the follow-up period to 104 weeks from the initial treatment date. Patients who were still participating in the ADMIRE-CD study at the time of the protocol amendment and subsequently completed the 52-week follow-up period had the option to participate in the extended follow-up.
Safety Endpoints
Treatment-emergent serious adverse events (TESAEs) related and not related to study treatment were recorded through week 104.
Efficacy Endpoint
Clinical remission was defined as the closure of all treated external openings that were draining at baseline despite gentle finger compression, as clinically assessed at weeks 24, 52, and 104.
Statistical Analysis
Safety and efficacy data were summarized only for those patients who entered the extended follow-up. Clinical remission rates at weeks 24, 52, and 104 were summarized by treatment arm (darvadstrocel and control groups); 95% CIs were calculated using Wald’s asymptomatic method. For patients who discontinued the study before week 104 or who had missing data at week 104, “no remission” was imputed. TESAE related and not related to study treatment were compared with TESAE data at week 52 for the 40 patients who entered the follow-up extension. Summary statistics were used to describe TESAEs starting between the day of study treatment administration and fistula assessment at week 104 visit (or early withdrawal visit, whichever came first).
RESULTS
Patient Disposition
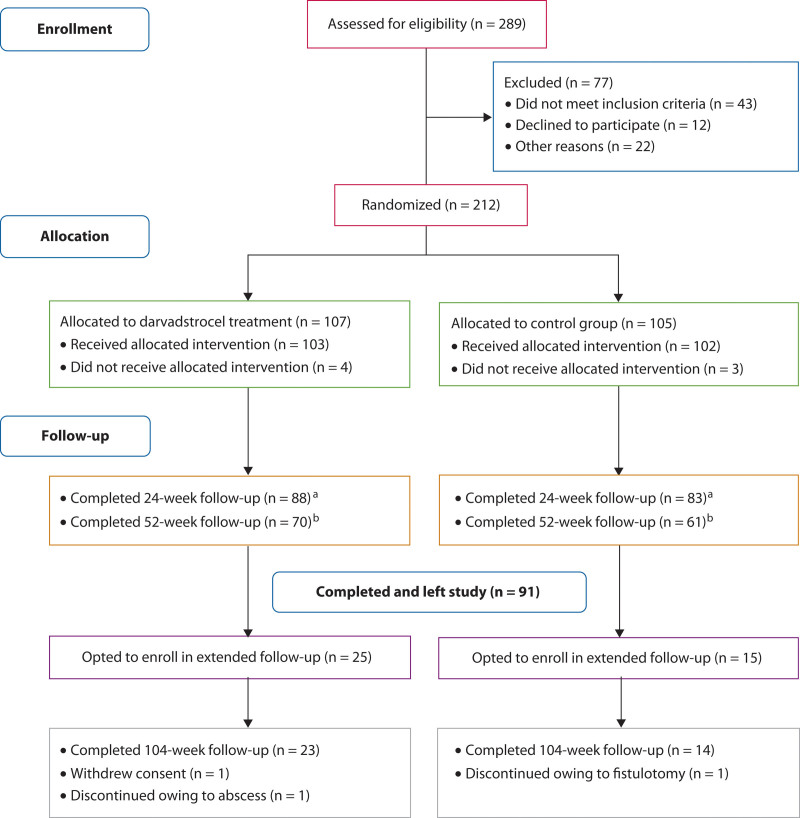
A total of 131 patients completed the 52-week follow-up in the ADMIRE-CD study. Of the 77 patients who were still enrolled at the time of the protocol amendment, 40 opted to continue in the extended follow-up; of these, 25 had received darvadstrocel treatment and 15 had received saline solution (control group). A total of 37 patients completed the 104-week follow-up (23 in the darvadstrocel group; 14 in the control group; Fig. 1).
FIGURE 1.
CONSORT flow diagram for ADMIRE-CD study with extended follow-up.
Patient Demographics and Disease Characteristics
Demographics and disease characteristics for patients who completed 52 weeks in ADMIRE-CD and entered the extended follow-up (n = 40; Table 1) were similar to those in the full ADMIRE-CD study population (n = 212).22 The mean (standard deviation) age of patients in the darvadstrocel and control groups in the extension was 38.6 (14.4) and 42.7 (14.8) years, respectively, and just over 50% of patients in each group were male. In the darvadstrocel group, the severity of fistula prior to treatment was greater than in the control group: 60.0% of patients in the darvadstrocel group had more than 1 internal or more than 1 external fistula opening, compared with 33.3% in the control group (Table 1). The remaining patients in each group had only 1 internal and 1 external fistula opening. Concomitant treatment (anti-TNF only, immunosuppressants only, or both) was received by 17/25 (68%) patients and 12/15 (80%) patients in the darvadstrocel and control groups, respectively.
TABLE 1.
Patient demographics and disease characteristics for patients enrolled in the extended follow-up (n = 40)
| Characteristic | Darvadstrocel (n = 25) |
Control (n = 15) |
|---|---|---|
| Sex, n (%) | ||
| Male | 14 (56.0) | 8 (53.3) |
| Female | 11 (44.0) | 7 (46.7) |
| Age, y, mean (SD) | 38.6 (14.4) | 42.7 (14.8) |
| Age category, n (%) | ||
| 0 to ≤65 y | 25 (100.0) | 14 (93.3) |
| >65 to ≤75 y | 0 (0.0) | 1 (6.7) |
| Weight, kg, mean (SD) | 73.4 (14.8) | 70.2 (11.0) |
| Smoking status, n (%) | ||
| Current smoker | 11 (44.0) | 4 (26.7) |
| Ex-smoker | 3 (12.0) | 2 (13.3) |
| Never smoked | 7 (28.0) | 5 (33.3) |
| Unknown | 4 (16.0) | 4 (26.7) |
| CD duration, y, mean (SD) | 9.9 (7.9) | 10.7 (7.5) |
| Prior CD treatment in past 6 mo before study entry, n (%) | ||
| Anti-TNF only | 4 (16.0) | 1 (6.7) |
| Immunosuppressants only | 2 (8.0) | 0 (0.0) |
| Anti-TNF and immunosuppressants | 15 (60.0) | 12 (80.0) |
| Neither | 4 (16.0) | 2 (13.3) |
| Concomitant CD treatment, n (%) | ||
| Anti-TNF only | 10 (40.0) | 4 (26.7) |
| Immunosuppressants only | 0 (0.0) | 2 (13.3) |
| Anti-TNF and immunosuppressants | 7 (28.0) | 6 (40.0) |
| Neither | 8 (32.0) | 3 (20.0) |
| Fistula severity, n (%) | ||
| Patients with 1 IO and 1 EO | 10 (40.0) | 10 (66.7) |
| Patients with 1 IO and ≥2 EOs | 10 (40.0) | 3 (20.0) |
| Patients with 2 IOs and ≥1 EO | 5 (20.0) | 2 (13.3) |
Control: curettage with placebo administration.
CD = Crohn’s disease; EO = external opening; IO = internal opening; TNF = tumor necrosis factor.
Safety Outcomes Through Week 104
For the 40 patients enrolled in the extended follow-up, 7 TESAEs were reported between study treatment and week 104. Of these TESAEs, 4 occurred between week 52 and week 104: 3 patients in the darvadstrocel group had a moderate TESAE and 1 patient in the control group had a severe TESAE (Table 2). None of the TESAEs were considered to be related to study treatment and no new safety signals were identified from week 52 through week 104. Safety data through week 52 have previously been reported.23
TABLE 2.
TESAEs reported between weeks 52 and 104 for patients in the extended follow-up (n = 40)
| TESAE details | Darvadstrocel (n = 25) | Control (n = 15) | ||
|---|---|---|---|---|
| Patients, n (%) | Events, n | Patients, n (%) | Events, n | |
| Any TESAEs | 3 (12.0) | 3 | 1 (6.7) | 1 |
| TESAE intensity | ||||
| Mild | 0 | 0 | 0 | 0 |
| Moderate | 3 (12.0) | 3 | 0 | 0 |
| Severe | 0 | 0 | 1 (6.7) | 1 |
| TESAE relationship to study treatment | ||||
| Related | 0 | 0 | 0 | 0 |
| Not related | 3 (12.0) | 3 | 1 (6.7) | 1 |
| TESAEs by system organ class and preferred term, start–end date | ||||
| Gastrointestinal disorders | ||||
| Anal fistula | 1 (4.0) day 377–378 |
1 | 0 | 0 |
| Infections and infestations | ||||
| Anal abscess | 1 (4.0) day 538–542 |
1 | 0 | 0 |
| Musculoskeletal and connective tissue disorders | ||||
| Fistula discharge | 1 (4.0) day 399–402 |
1 | 1 (6.7) day 627–629 |
1 |
Control: curettage with placebo administration.
TESAE = treatment-emergent serious adverse event.
Efficacy Outcomes Through Week 104
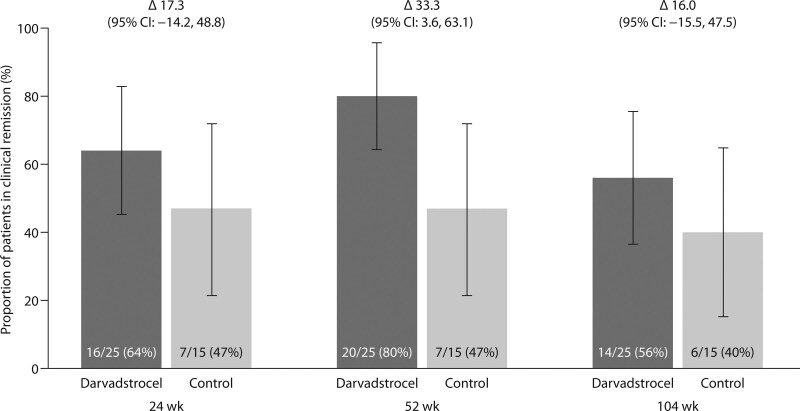
At weeks 24, 52 and 104, clinical remission for the sub-group of patients in the extended follow-up (n = 40) was reported in 16/25 (64%), 20/25 (80%), and 14/25 (56%) patients in the darvadstrocel treatment group versus 7/15 (47%), 7/15 (47%), and 6/15 (40%) patients in the control group (Fig. 2). The difference in proportion of patients in clinical remission between the two groups was 17.3% (95% CI, −14.2 to 48.8), 33.3% (95% CI, 3.6 to 63.1) and 16.0% (95% CI, −15.5 to 47.5) at weeks 24, 52, and 104, respectively.
FIGURE 2.
Proportion of patients with clinical remission at weeks 24, 52, and 104 after fistula tract curettage and administration of darvadstrocel or placebo (control).
At week 104, clinical remission after darvadstrocel treatment was reported in 10/17 (59%) patients receiving concomitant anti-TNF therapy and in 4/8 (50%) patients not receiving anti-TNF therapy, irrespective of immunosuppressant therapy. In the control group, clinical remission was reported in 3/10 (30%) patients receiving anti-TNF therapy and 3/5 (60%) patients not receiving anti-TNF therapy.
DISCUSSION
This study reports the outcomes from an extension to the 52-week ADMIRE-CD trial in which, following a protocol amendment, patients were permitted to enter an extended follow-up period of 104 weeks posttreatment. The aim of this extension was to obtain longer-term data on the safety and efficacy of darvadstrocel administration after fistula tract curettage and internal opening closure of complex perianal fistulas in patients with CD. The outcome for patients during this extended follow-up period after darvadstrocel treatment is important because 1) there is a lack of long-term (>1 year after administration) safety data of stem cell therapeutic approaches for complex perianal fistulas in patients with CD, and 2) the ultimate goal of treatment is to provide long-term fistula healing and improve patient QOL. This study is the first to report outcomes for patients up to 104 weeks after darvadstrocel treatment. Patient demographics and disease characteristics of the 40 patients who entered the extended follow-up were broadly similar to those of the full 52-week ADMIRE-CD study population; however, for patients in the extended follow-up, the overall baseline characteristics of the fistulas were more severe in the darvadstrocel group than in the control group (Table 1).22
In the 40 patients who participated in the extended follow-up, the safety profile of darvadstrocel through 104 weeks was comparable to that through 52 weeks in the ADMIRE-CD trial, with only 4 new TESAEs reported during the extended follow-up period; none of these were considered to be related to study treatment.23 No new safety signals were reported during the extended follow-up, and all 4 TESAEs reported during the extension (3 in the darvadstrocel group and 1 in the control group) were related to anal fistula or abscess, which were among the most frequent TESAEs throughout the trial.23 Furthermore, the proportion of patients who experienced TESAEs in the darvadstrocel group during the extended follow-up was lower than that reported through 52 weeks (12% and 24%).23
The clinical remission rate in the sub-group of patients followed up through 104 weeks after darvadstrocel administration was greater than that of the control group, irrespective of anti-TNF concomitant treatment, and was similar to that previously reported by Panés et al for the modified intention-to-treat (ITT) population, in which 57/103 (55%) and 61/103 (59%) patients in the darvadstrocel treatment group and 43/101 (43%) and 42/101 (42%) patients in the control group were in clinical remission at 24 and 52 weeks after treatment.23 Clinical remission was, however, also observed in the control group, and this may be attributed to the minimally invasive surgical techniques employed before administration of placebo, including vigorous fistula tract curettage and closure of internal openings, as well as the permitted use of ongoing medical therapies. Combined remission (clinical remission plus absence of collections >2 cm diameter confirmed by MRI) was not evaluated in this study. However, in the previous report for the modified ITT population, clinical remission at weeks 24 and 52 was also greater in the darvadstrocel treated group compared with the control group, respectively: 53/103 (51.5%) versus 36/101 (35.6%) at week 24, and 58/103 (56.3%) versus 39/101 (38.6%) at week 52.23
Although darvadstrocel has been commercially available since 2019, eASC therapy has been systematically explored over a longer period of time, including real-world studies of the treatment of complex perianal fistulas in patients with CD.24,25 Other therapies for complex perianal fistulas in CD have failed to demonstrate long-term remission. For example, retrospective studies of infliximab have shown that discontinuation of infliximab treatment after successful fistula healing is associated with an increased likelihood of relapse (approximately half of patients who initially responded to treatment with infliximab had a relapse within 1 year of treatment).26 The cell-mediated closure of fistula tracts with darvadstrocel, coupled with minimally invasive surgery, offers a treatment option for complex perianal fistulas with the potential for achieving long-term remission. Darvadstrocel may also represent a valuable option for the treatment of patients who should avoid immunosuppression and for whom agents such as infliximab may not be suitable.27
There are several limitations to this study. At the time of the ADMIRE-CD protocol amendment, many patients had already completed the 52-week follow-up and had left the study; hence, they could not take part in this extended follow-up period to 104 weeks, and the relatively small sample size (n = 40) is a limitation of this study. Furthermore, there is an imbalance in the number of patients in this extension study who were treated with darvadstrocel versus those in the control group (n = 25 versus n = 15, respectively), which could lead to potential bias in the analyses. Another limitation is the absence of MRI examination at the end of the 104-week follow-up, hence combined remission rates (which include both clinical remission and absence of collections >2 cm diameter, confirmed by MRI) could not be determined (as previously reported for modified ITT population at weeks 24 and 52).23 It is anticipated that some of these limitations will be addressed by the ongoing INSPIRE global registry (EU PAS Register number: EUPAS24267), which was set up to capture real-world effectiveness and safety data on a large number of patients with complex perianal fistulas in CD treated with darvadstrocel and followed up for over 36 months.28
CONCLUSION
Darvadstrocel administration with minimally invasive surgery was well tolerated and had a safety profile comparable to the control arm. No apparent new safety signals were identified during this extended follow-up period of the ADMIRE-CD study. These data also suggest that clinical remission of complex perianal fistulas, after minimally invasive surgery and administration of darvadstrocel in patients with CD, may be sustained in the long term (up to 104 weeks).
Supplementary Material
Footnotes
Supplemental digital content is available for this article. Direct URL citations appear in the printed text, and links to the digital files are provided in the HTML and PDF versions of this article on the journal’s website (www.dcrjournal.com).
Funding/Support: This study was sponsored by Takeda Pharmaceuticals International Co. Medical writing support was provided by Sally McTaggart, PhD, of Oxford PharmaGenesis, Oxford, UK, and was funded by Takeda Pharmaceuticals International Co.
Financial Disclosures: Julian Panés has received consultant or speaker fees from Takeda Pharmaceuticals Int. Co., AbbVie, Boehringer Ingelheim, Celgene, Celltrion, Genentech, GSK, Immunic Therapeutics, Janssen, Nestlé, Novartis, Origo Pharmaceuticals, Pandion Therapeutics, Pfizer, Progenity, Roche, Takeda Pharmaceuticals Inc., Theravance Biopharma, and Wasserman. Antonino Spinelli has received consultant fees from Takeda Pharmaceuticals Int. Co. and consultant/speaker fees from Johnson & Johnson, Janssen, and Oasis. Francesco Selvaggi has received consultant fees from Takeda Pharmaceuticals Int. Co. Dirk Lindner is an employee of Takeda Pharmaceuticals Int. Co. and has received stock/stock options. André D´Hoore has received consultant fees from Takeda Pharmaceuticals Int. Co. and Johnson & Johnson. Matthias Binek is an employee of Takeda Pharmaceuticals Int. Co. and has received stock/stock options. Inmaculada Gilaberte is an employee of Takeda Pharmaceuticals Int. Co. and has received stock/stock options. Damián Garcia-Olmo has received consultant fees from Takeda Pharmaceuticals Int. Co. and is a named inventor on patents related to this study.
REFERENCES
- 1.Baumgart DC, Sandborn WJ. Crohn’s disease. Lancet. 2012;380:1590–1605. [DOI] [PubMed] [Google Scholar]
- 2.Sandborn WJ, Fazio VW, Feagan BG, Hanauer SB; American Gastroenterological Association Clinical Practice Committee. AGA technical review on perianal Crohn’s disease. Gastroenterology. 2003;125:1508–1530. [DOI] [PubMed] [Google Scholar]
- 3.Chaparro M, Burgueno P, Vera I, et al. Epidemiological study of perianal fistulas in patients with Crohn’s disease. Gastroenterology. 2011;140:S736. [Google Scholar]
- 4.Kasparek MS, Glatzle J, Temeltcheva T, Mueller MH, Koenigsrainer A, Kreis ME. Long-term quality of life in patients with Crohn’s disease and perianal fistulas: influence of fecal diversion. Dis Colon Rectum. 2007;50:2067–2074. [DOI] [PubMed] [Google Scholar]
- 5.Aguilera-Castro L, Ferre-Aracil C, Garcia-Garcia-de-Paredes A, Rodriguez-de-Santiago E, Lopez-Sanroman A. Management of complex perianal Crohn’s disease. Ann Gastroenterol.2017;30:33–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Adegbola SO, Dibley L, Sahnan K, et al. Burden of disease and adaptation to life in patients with Crohn’s perianal fistula: a qualitative exploration. Health Qual Life Outcomes. 2020;18:370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Schwartz DA, Loftus EV, Jr, Tremaine WJ, et al. The natural history of fistulizing Crohn’s disease in Olmsted County, Minnesota. Gastroenterology. 2002;122:875–880. [DOI] [PubMed] [Google Scholar]
- 8.Schwartz DA, Tagarro I, Carmen Díez M, Sandborn WJ. Prevalence of fistulizing Crohn’s disease in the United States: estimate from a systematic literature review attempt and population-based database analysis. Inflamm Bowel Dis. 2019;25:1773–1779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Eglinton TW, Barclay ML, Gearry RB, Frizelle FA. The spectrum of perianal Crohn’s disease in a population-based cohort. Dis Colon Rectum. 2012;55:773–777. [DOI] [PubMed] [Google Scholar]
- 10.Panes J, Reinisch W, Rupniewska E, et al. Burden and outcomes for complex perianal fistulas in Crohn’s disease: Systematic review. World J Gastroenterol. 2018;24:4821–4834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Present DH, Rutgeerts P, Targan S, et al. Infliximab for the treatment of fistulas in patients with Crohn’s disease. N Engl J Med. 1999;340:1398–1405. [DOI] [PubMed] [Google Scholar]
- 12.Sands BE, Anderson FH, Bernstein CN, et al. Infliximab maintenance therapy for fistulizing Crohn’s disease. N Engl J Med. 2004;350:876–885. [DOI] [PubMed] [Google Scholar]
- 13.Torres J, Bonovas S, Doherty G, et al. ECCO Guidelines on Therapeutics in Crohn’s Disease: Medical Treatment. J Crohns Colitis. 2020;14:4–22. [DOI] [PubMed] [Google Scholar]
- 14.Gionchetti P, Dignass A, Danese S, et al.; ECCO. 3rd European Evidence-based Consensus on the Diagnosis and Management of Crohn’s Disease 2016: Part 2: Surgical Management and Special Situations. J Crohns Colitis. 2017;11:135–149. [DOI] [PubMed] [Google Scholar]
- 15.Ardizzone S, Maconi G, Colombo E, Manzionna G, Bollani S, Bianchi Porro G. Perianal fistulae following infliximab treatment: clinical and endosonographic outcome. Inflamm Bowel Dis. 2004;10:91–96. [DOI] [PubMed] [Google Scholar]
- 16.Norton C, Dibley LB, Bassett P. Faecal incontinence in inflammatory bowel disease: associations and effect on quality of life. J Crohns Colitis. 2013;7:e302–e311. [DOI] [PubMed] [Google Scholar]
- 17.Gecse KB, Bemelman W, Kamm MA, et al.; World Gastroenterology Organization, International Organisation for Inflammatory Bowel Diseases IOIBD, European Society of Coloproctology and Robarts Clinical Trials; World Gastroenterology Organization International Organisation for Inflammatory Bowel Diseases IOIBD European Society of Coloproctology and Robarts Clinical Trials. A global consensus on the classification, diagnosis and multidisciplinary treatment of perianal fistulising Crohn’s disease. Gut. 2014;63:1381–1392. [DOI] [PubMed] [Google Scholar]
- 18.García-Olmo D, García-Arranz M, Herreros D, Pascual I, Peiro C, Rodríguez-Montes JA. A phase I clinical trial of the treatment of Crohn’s fistula by adipose mesenchymal stem cell transplantation. Dis Colon Rectum. 2005;48:1416–1423. [DOI] [PubMed] [Google Scholar]
- 19.de la Portilla F, Alba F, García-Olmo D, Herrerías JM, González FX, Galindo A. Expanded allogeneic adipose-derived stem cells (eASCs) for the treatment of complex perianal fistula in Crohn’s disease: results from a multicenter phase I/IIa clinical trial. Int J Colorectal Dis. 2013;28:313–323. [DOI] [PubMed] [Google Scholar]
- 20.Scharl M, Rogler G. Pathophysiology of fistula formation in Crohn’s disease. World J Gastrointest Pathophysiol. 2014;5:205–212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.DelaRosa O, Dalemans W, Lombardo E. Mesenchymal stem cells as therapeutic agents of inflammatory and autoimmune diseases. Curr Opin Biotechnol. 2012;23:978–983. [DOI] [PubMed] [Google Scholar]
- 22.Panés J, García-Olmo D, Van Assche G, et al.; ADMIRE CD Study Group Collaborators. Expanded allogeneic adipose-derived mesenchymal stem cells (Cx601) for complex perianal fistulas in Crohn’s disease: a phase 3 randomised, double-blind controlled trial. Lancet. 2016;388:1281–1290. [DOI] [PubMed] [Google Scholar]
- 23.Panés J, García-Olmo D, Van Assche G, et al.; ADMIRE CD Study Group Collaborators. Long-term efficacy and safety of stem cell therapy (Cx601) for complex perianal fistulas in patients with Crohn’s disease. Gastroenterology. 2018;154:1334–1342.e4. [DOI] [PubMed] [Google Scholar]
- 24.Adamina M, Bonovas S, Raine T, et al. ECCO Guidelines on Therapeutics in Crohn’s Disease: Surgical Treatment. J Crohns Colitis. 2020;14:155–168. [DOI] [PubMed] [Google Scholar]
- 25.Cabalzar-Wondberg D, Turina M, Biedermann L, Rogler G, Schreiner P. Allogeneic expanded adipose-derived mesenchymal stem cell therapy for perianal fistulas in Crohn’s disease: a case series. Colorectal Dis. 2021;23:1444–1450. [DOI] [PubMed] [Google Scholar]
- 26.Lopez N, Ramamoorthy S, Sandborn WJ. Recent advances in the management of perianal fistulizing Crohn’s disease: lessons for the clinic. Expert Rev Gastroenterol Hepatol. 2019;13:563–577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lichtenstein GR, Feagan BG, Cohen RD, et al. Infliximab for Crohn’s disease: more than 13 years of real-world experience. Inflamm Bowel Dis. 2018;24:490–501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zmora O, Panés J, Drohan C, et al. P491 inspire: design and implementation aspects of a registry of complex perianal fistulas in crohn’s disease patients treated with darvadstrocel. J Crohn’s Colitis. 2019;13(suppl 1):S357–S358. [Google Scholar]