Abstract
Cefprozil, an oral semisynthetic cephalosporin, is commonly utilized in the treatment of respiratory-tract infections in children. While this agent has provided acceptable clinical success over a number of years, this study was undertaken to better define its pharmacodynamic profile against Streptococcus pneumoniae. Nineteen clinical isolates of S. pneumoniae were utilized in the neutropenic murine thigh infection model. To simulate the pharmacokinetic profile of cefprozil in children, the renal function of mice was impaired with uranyl nitrate, and a commercially available cefprozil suspension (6 mg/kg of body weight) was administered orally every 12 h. Mice were infected with 106 to 107 CFU per thigh, and therapy was initiated 2 h later. At 0 and 24 h postinfection, thighs were harvested to determine bacterial density. Survival was assessed during 96 h of therapy. The magnitude of bacterial kill ranged from 0.5 to 4.4 log10 CFU per thigh over 24 h, and the extent of microbial eradication was dependent on the MIC. Killing of more than 2.6 log10 CFU per thigh was observed with MICs of ≤3 μg/ml, while either minimal killing or growth was detected with MICs of ≥4 μg/ml. Mortality in untreated control animals was 100%. Animals infected with strains for which the MICs were ≤2 μg/ml survived the infection, whereas MICs exceeding 2 μg/ml resulted in substantial mortality. These studies demonstrate the effectiveness of cefprozil against isolates of the pneumococcus for which the MICs are ≤2 μg/ml using a drug exposure typically observed in children. These data support a susceptibility breakpoint of ≤2 μg/ml for cefprozil.
Cefprozil is a semisynthetic broad-spectrum cephalosporin antibiotic which is currently available in an oral dosage form (i.e., tablet and suspension) for the treatment of respiratory-tract and skin or skin structure infections in both adults and children. While this agent has provided acceptable clinical success rates for its approved indications when the infecting pathogen is Streptococcus pneumoniae (2, 3, 11, 12), the pharmacodynamic profile of cefprozil against this important pathogen has not been fully described. The availability of these data not only will assist with optimizing the effectiveness of the prescribed antimicrobial regimen in clinical practice but also has assisted with the assessment of appropriate National Committee for Clinical Laboratory Standards (NCCLS) breakpoints for this antimicrobial agent.
Therefore, the present study was undertaken to better define the in vivo activity of cefprozil against S. pneumoniae using a neutropenic murine thigh infection model.
MATERIALS AND METHODS
Antimicrobial test agents.
Penicillin and cefprozil analytical-grade standards were obtained for in vitro testing from Sigma Chemicals, St. Louis, Mo., and Bristol-Myers Squibb, Princeton, N.J., respectively. For all in vivo studies, a commercially available cefprozil (Cefzil; lot no. J8E18B; expiration date, Oct. 2001; Bristol-Myers Squibb) suspension was obtained from the manufacturer and administered via the oral route as outlined.
Bacterial isolates and susceptibilities.
Nineteen clinical isolates of S. pneumoniae were included in this study. The MICs of penicillin and cefprozil were determined using the microdilution method according to NCCLS guidelines (9). The MICs were determined in cation-adjusted Mueller-Hinton broth (20 to 25 mg of calcium/liter and 10 to 12.5 mg of magnesium/liter) with 5% lysed horse blood in ambient air. Trypticase soy agar with 5% sheep blood was used as the growth medium for S. pneumoniae.
Thigh infection model.
Specific-pathogen-free female ICR mice weighing approximately 25 g were obtained from Harlan Sprague Dawley, Inc. (Indianapolis, Ind.) and used throughout the experiment. Mice were rendered transiently neutropenic by injecting cyclophosphamide intraperitoneally (i.p.) at a dose of 150 mg/kg of body weight at 4 days and a second time, at a dose of 100 mg/kg, 1 day before bacterial inoculation. This regimen has been shown to induce neutropenia in the model for 5 days (1, 8, 10). In addition, renal impairment was produced by a single i.p. injection of uranyl nitrate (Mallinckrodt, Inc., Paris, Ky.) 3 days prior to the initiation of antimicrobial therapy (1, 10). Broth cultures of the test organism were grown overnight and subsequently diluted to an inoculum range of 106 to 107 CFU/ml. Final inoculum concentrations were confirmed by serial dilution and plating techniques. Thigh infection with each of the test isolates was produced by injecting 0.1 ml of the inoculum into each thigh of each mouse 2 h prior to the initiation of antimicrobial therapy.
Pharmacokinetic studies and dosing regimen determination.
The purpose of these studies was to find a cefprozil regimen in the murine model that simulated the pharmacokinetic profile observed in children receiving 15 mg/kg every 12 h (14). Since drug accumulation over the 12-h dosing interval is not observed with cefprozil, single-dose pharmacokinetic studies were undertaken. In an attempt to optimally design the pharmacokinetic profile of cefprozil, the dosages of both uranyl nitrate and cefprozil were varied to determine the most suitable concentration-versus-time profile of the β-lactam in the neutropenic infected murine model. Two hours after pneumococcal thigh inoculation as described above, mice were administered the commercially available cefprozil suspension orally. Animals were euthanized by CO2 exposure followed by cervical dislocation prior to the intracardiac puncture. Blood was obtained from three to five mice at 0.08, 0.16, 0.25, 0.5, 1, 2, 4, and 6 h postdosing. The blood was centrifuged at 4,000 × g for 10 min; the serum was transferred into a polypropylene tube and stored at −80°C until analysis.
Concentrations of cefprozil in murine serum were determined using a previously validated high-performance liquid chromatography (HPLC) procedure (17). The assay was linear over a range of 0.2 to 25 μg/ml. Intraday coefficients of variation for the low (5-μg/ml) and high (20-μg/ml) check samples were 1.4 and 2.9%, respectively. Interday coefficients of variation for the low and high check samples were 2.8 and 0.9%, respectively.
The pharmacokinetic parameters for each of the administered doses, including the terminal-phase elimination rate constant (β), elimination half-life (t1/2β), apparent volume of the central compartment (Vc), apparent steady-state volume of distribution (VSS), area under the serum drug concentration-time curve (AUC), and total-body clearance (CLT), were calculated with first-order elimination, by nonlinear least-squares techniques (PCNONLIN, version 4.2; Statistical Consultants, Lexington, Ky.). Compartment model selection was based on visual inspection of the fit and use of the correlation between the observed and the calculated concentrations.
Efficacy as assessed by bacterial density.
Once the animals had been prepared as described above and inoculated, treatment was initiated on mice (six per isolate) at 2 h after inoculation with an orally administered cefprozil regimen (6 mg/kg every 12 h [q12h]) which simulated the concentration-time-curve observed in children (14). Control animals received water orally in the same volume (0.2 ml) and on the same schedule as cefprozil. Untreated control mice (four per group) were sacrificed just prior to antibiotic initiation and after 24 h. Cefprozil was administered for two doses (for a total of 24 h of therapy), and animals were euthanized by CO2 exposure followed by cervical dislocation.
After sacrifice, both thighs were removed and individually homogenized in normal saline. Serial dilutions were plated on Trypticase soy agar with 5% sheep blood for CFU determinations. Efficacy (change in bacterial density) was calculated by subtracting the mean log CFU per thigh of the control mice obtained just prior to antibiotic administration from the log CFU per thigh of cefprozil-treated or untreated control mice at the end of therapy (24 h).
Efficacy as assessed by survival.
Groups of 35 mice were similarly infected with each test strain for evaluation of survival after 96 h of therapy. Cefprozil therapy (6 mg/kg orally q12h) was initiated 2 h after inoculation in 30 animals. The remaining five animals received the same oral volume of water q12h and served as the control population for each isolate. Cumulative mortality was calculated during 96 h of therapy. Although death has historically been used as an end point for studies of this type, this end point is no longer suitable in the current era of animal research. Therefore, our study methodology has been modified to contemporary standards, which have been used recently in studies conducted at our institution (7). Animals were monitored not less than three times daily by individuals experienced in recognizing the signs of illness and abnormal behavior. Animals that appeared to have substantial alterations in posture (e.g., abnormal posture or head tucked into abdomen), coat, exudate around the eyes and/or nose, and breathing or movement were removed from the group housing and were euthanized. The term “mortality” has been used as an end point for this study; however, it should be clearly understood that when possible, every attempt was made to minimize the pain and suffering of the animals. Animals were euthanized prior to naturally succumbing to infection if the above symptoms of impending death were observed. For the purposes of this study, whether an animal died due to the natural infection process or was euthanized, both were considered the same end point for experimental and statistical purposes.
Data analysis.
Spearman's rank correlation coefficient was used to evaluate the relationship between mortality and the duration of the time that the serum drug concentration remained above the MIC (T>MIC) for cefprozil after 96 h of therapy. This test was also used to evaluate the relationship between change in CFU and T>MIC for cefprozil after 24 h of therapy. In addition, the relation between the pharmacodynamic parameter of T>MIC and mortality or change in CFU was fitted to a sigmoid Emax model using the computer program WinNonlin, version 3.0 (Pharsight Corp., Mountain View, Calif.).
RESULTS
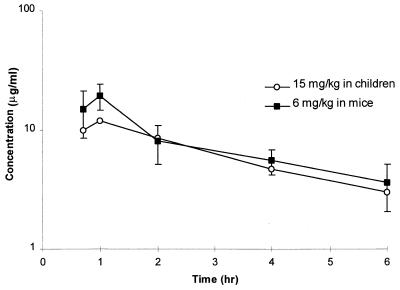
The pharmacokinetic parameter estimates for several cefprozil and uranyl nitrate dosages are presented in Table 1. These data reveal the dose proportionality related to the maximum concentration and the influence of the uranyl nitrate in substantially altering the clearance of cefprozil in this infection model. As a result of these data, a 12-h dosing regimen was simulated to meet the anticipated pharmacokinetic goals. This simulated regimen (cefprozil at 6 mg/kg and uranyl nitrate at 4.4 mg/kg) was administered to another group of infected neutropenic mice to confirm the PCNONLIN-predicted values of the modeling scheme (Table 1). As shown in Fig. 1, the selected regimen produced a concentration-versus-time profile in this murine model comparable to that observed in children receiving 15 mg of cefprozil/kg q12h (14).
TABLE 1.
Single-dose pharmacokinetic parameters for cefprozil in S. pneumoniae-infected micea with uranyl nitrate-induced renal impairment
| Doseb (mg/kg)
|
Parameterc (SE)
|
||||||
|---|---|---|---|---|---|---|---|
| Uranyl nitrate | Cefprozil | Cmax (μg/ml) | Tmax (h) | t1/2 β (h) | AUC0–T (μg · h/ml) | CLT/F (litersc/h/kg) | Vc (liters/kg) |
| 10 | 15 | 23.44 (3.0) | 1.58 (0.26) | 5.82 (3.63) | 237.6 (100.0) | 0.06 | 0.53 (0.13) |
| 10 | 30 | 52.32 (4.0) | 0.96 (0.1) | 3.71 (0.79) | 335.3 (43.2) | 0.09 | 0.48 (0.06) |
| 5 | 30 | 53.31 (6.5) | 0.86 (0.14) | 2.77 (0.62) | 266.0 (33.2) | 0.11 | 0.45 (0.08) |
| 4.4 | 13 | 23.66 (4.9) | 0.68 (0.22) | 1.77 (0.80) | 79.0 (21.7) | 0.16 | 0.42 (0.14) |
| 4.4b | 6b | 17.59 (3.9) | 0.96 (0.63) | 1.89 (0.73) | 45.5 (14.2) | 0.13 | 0.36 (0.07) |
Bacteria were injected into each thigh.
The regimen of 4.4 mg of uranyl nitrate/kg and 6 mg of cefprozil/kg was used during in vivo studies and was used to produce Fig. 1.
Cmax, maximum concentration of drug in serum; Tmax, time to maximum concentration of drug in serum; AUC0–T, AUC from time zero to the time of the last quantifiable concentration; CLT/F, apparent CLT.
FIG. 1.
Mean serum concentration-versus-time profile of an oral suspension of cefprozil in children (14) and the murine thigh infection model.
The MICs of cefprozil and penicillin for the S. pneumoniae isolates incorporated into this study are given in Table 2. The selected test organisms represent a wide range of sensitivities to cefprozil and allow the opportunity for pharmacodynamic modeling over a representative range of anticipated values in clinical practice.
TABLE 2.
Relationship between the MIC for S. pneumoniae, cumulative mortality, and T>MIC after 4 days of therapy
| Isolate no. | MIC (μg/ml) of:
|
% Mortalitya (no. of animals dying/ no. in group) | T>MICb | |
|---|---|---|---|---|
| Penicillin | Cefprozil | |||
| 39 | 0.25 | 0.09 | 3 (1/30) | 100 |
| 53 | 0.03–0.06 | 0.125 | 0 (0/30) | 100 |
| 32 | 0.25–0.5 | 0.25 | 0 (0/27) | 97 |
| 10 | 2.0 | 0.5 | 0 (0/30) | 82 |
| 77 | 0.5 | 0.5 | 7 (2/30) | 82 |
| 74 | NDc | 1.0 | 0 (0/30) | 66 |
| 80 | 0.5 | 1.0 | 0 (0/31) | 66 |
| 81 | 0.13 | 1.0 | 3 (1/32) | 66 |
| 73 | ND | 2.0 | 0 (0/30) | 50 |
| 85 | 2.0 | 2.0 | 0 (0/32) | 50 |
| 84 | 2.0 | 3.0d | 17 (5/29) | 42 |
| 51 | 2.0 | 4.0 | 13 (4/31) | 35 |
| 63 | 0.5 | 4.0 | 23 (7/30) | 35 |
| 70 | ND | 4.0 | 58 (11/19) | 35 |
| 54 | 2.0 | 6.0d | ND | ND |
| 34 | 4.0 | 8.0 | 23 (7/30) | 19 |
| 75 | ND | 8.0 | 50 (15/30) | 19 |
| 59 | 2.0–4.0 | 8.0 | 70 (21/30) | 19 |
| 71 | ND | 16.0 | 23 (7/31) | 5 |
See Materials and Methods for the definition of mortality in this study.
Expressed as a percentage of the total dosing interval.
ND, not determined.
Multiple duplicate analysis yielded MICs of 2 and 4 μg/ml for isolate 84 and of 4 and 8 μg/ml for isolate 54. For the purposes of the pharmacodynamic analysis, these data were presented as averaged MICs.
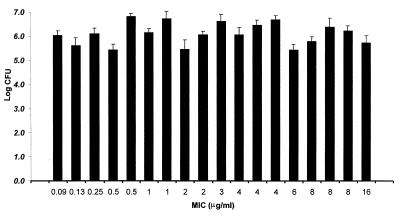
As presented in Fig. 2, excellent bacterial recovery from infected thighs was observed for all isolates. These data support the accuracy of inoculum preparation and in vitro quantitative assessment prior to the initiation of therapy. In addition, these results support the reproducibility of infection via the thigh injection technique over the 4-week study period, as 5 of the 19 isolates were randomly selected for study each week.
FIG. 2.
Bacterial density of S. pneumoniae in the thighs of infected animals at the start of therapy. Values represents means ± standard deviations for eight thighs.
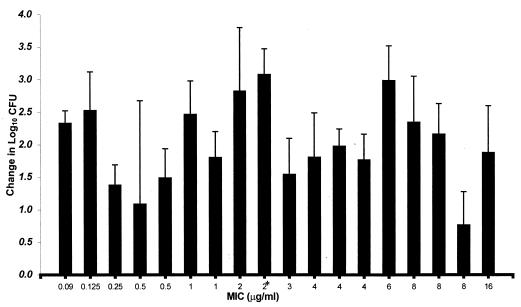
Figure 3 displays the growth of each organism in control mice over the 24-h postinfection period. Organisms grew at 0.8 to 3.1 log10 CFU per thigh over 24 h in untreated control animals. Organism growth (positive values) or killing (negative values) at the conclusion of 24 h of cefprozil therapy (6 mg/kg q12h) are presented in Fig. 4. The magnitude of killing ranged from 0.5 to 4.4 log10 CFU per thigh over 24 h, and the extent of microbial eradication was dependent on the MIC for the pneumococcal isolate. Killing of more than 2.6 log10 CFU per thigh was observed with pneumococci for which the MIC was ≤3 μg/ml, while minimal killing or growth was detected with MICs of ≥4 μg/ml.
FIG. 3.
Growth of S. pneumoniae in the thighs of infected mice not receiving cefprozil (controls) after 24 h. Values are means ± standard deviations for six to eight thighs (∗, mean for 4 thighs).
FIG. 4.
Change in CFU of S. pneumoniae in the thighs of infected mice treated with cefprozil for 24 h. Values are means ± standard deviations for 10 to 12 thighs.
The cumulative mortality over 96 h of cefprozil treatment is reported in Table 2. Observed mortality in untreated control animals was 100% over this observation period for all isolates. In agreement with CFU-per-thigh data after 24 h of therapy, the MICs for the isolates were predictive of cumulative mortality. Animals infected with organisms for which the MICs were ≤2 μg/ml survived the infection, whereas MICs exceeding 2 μg/ml resulted in substantial mortality.
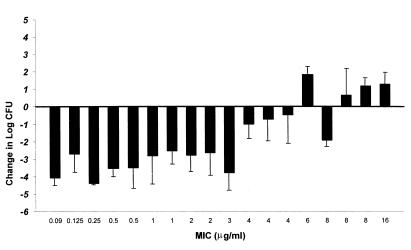
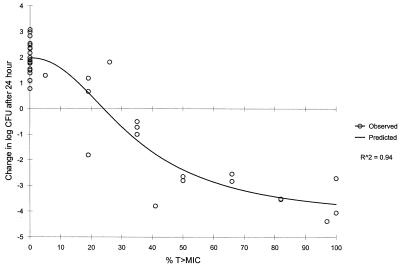
A significant correlation (Spearman's rank correlation coefficient; P ≤ 0.001; R2 = 0.96) was observed for the relationship between mortality and T>MIC for cefprozil (Table 2). Maximum survival was observed when T>MIC was ≥50% of the 12-h dosing interval. Similarly, a significant correlation (P ≤ 0.001) was noted for the relationship between the change in CFU and T>MIC for cefprozil after 24 h of therapy (Fig. 5). A static effect (no net growth) was observed when T>MIC was 20 to 30%. Maximal bactericidal effects were detected when T>MIC was 40 to 50% of the dosing interval.
FIG. 5.
Relationship between change in CFU and T>MIC) for cefprozil after 24 h of therapy. Values are mean data derived from Fig. 4.
DISCUSSION
S. pneumoniae is the most common cause of otitis media and community-acquired pneumonia. As a result of the prevalence of this infecting organism and its evolving antimicrobial-resistance patterns, a pharmacodynamic evaluation of commonly used therapies is warranted for the optimization of clinical outcomes. In the present study, we evaluated the pharmacodynamic profile of cefprozil against 19 clinical isolates of S. pneumoniae with a wide range of sensitivities using the neutropenic murine thigh infection model. This model was selected because it is an accepted pharmacodynamic tool in the assessment of antimicrobial effectiveness. Also, the results obtained by using this technique appear to provide pharmacodynamic end points similar to those observed in the clinical setting with otitis media (1, 4). As a result, both the in vivo animal data and data obtained in the clinical setting suggest that the pharmacodynamic parameter for the β-lactams which is most closely related to outcome is T>MIC (1, 4, 5). Additionally, in otitis media the pharmacodynamic indices of T>MIC and the middle-ear fluid/MIC ratio appear to predict bacteriologic efficacy with similar accuracy (4). Using the same data, it has been shown that adequate bacterial killing is present when T>MIC is maintained for 40 to 50% of the dosing interval for this class of antimicrobials. Therefore, in an attempt to more closely assimilate the data obtained in this animal model to that which is observed in the clinical arena, the pharmacokinetic profile of cefprozil in children was reproduced by altering both the renal function of mice and the dosage administered. Using these techniques, we were able to simulate in the model the pharmacokinetic and pharmacodynamic profile observed in children after the commonly used cefprozil regimen of 15 mg/kg every 12 hours (Fig. 1). While the apparent serum pharmacokinetic profile obtained in our model was quite similar to the targeted values in children, an assessment of the interspecies difference in protein binding had not been made prior to this study. For that reason, cefprozil protein binding determinations were conducted by the sponsor in the mouse species utilized in our study. Results from these studies revealed that the percentages of cefprozil bound at concentrations of 10 and 100 μg/ml in mouse serum were 26.3 and 31.6%, respectively. These values are consistent with the low protein binding (36%) of cefprozil in humans (Cefzil oral suspension prescribing information, document 53-004156-03, July 1997, Bristol-Myers Squibb, Princeton, N.J.).
Over the course of the study, excellent bacterial recovery from infected thighs was noted for all isolates prior to the initiation of therapy. While the growth of organisms in the untreated animals was generally consistent over 24 h (2 to 3 log10 CFU per thigh), these data did reveal the inherent variability of in vivo growth of the S. pneumoniae isolates utilized. Using this simulated pediatric exposure with a number of infecting pathogens for which the MICs varied yielded a wide range of bacterial killing over the first 24 h of therapy. Furthermore, the extent of microbial eradication was dependent on the MIC of cefprozil for the pneumococcal isolate. In this study, killing of more than 2.6 log10 CFU per thigh was demonstrated for pneumococci for which the MIC was ≤3 μg/ml, while minimal killing or growth was detected with MICs of ≥4 μg/ml.
Additionally, the MICs for the isolates were predictive of the cumulative mortality rate over the course of the study. Animals infected with organisms for which the MICs were ≤2 μg/ml survived the infection, whereas MICs exceeding 2 μg/ml resulted in substantial mortality. These observations of an apparent breakpoint value for both the reduction in CFU and survival have been reported by other investigators utilizing a variety of β-lactams and drug exposure profiles (1, 5, 6, 13, 15, 18). As expected from our reported data with cefprozil, the pharmacodynamic evaluation revealed a significant correlation both for the relationship between mortality and T>MIC and for that between the change in CFU and T>MIC with cefprozil. Maximum survival was observed when the T>MIC was ≥50% of the dosing interval. Also, the maximal bactericidal effect in tissue was detected when the T>MIC was 40 to 50% of the dosing interval, values which closely approximate those reported by other investigators studying the pharmacodynamics of the β-lactams (1, 5, 6, 13, 15, 18).
Moreover, clinical trial data obtained from one new drug application (NDA) and two post-NDA trials with children (66% of whom were less than 3 years old) undergoing cefprozil therapy (15 mg/kg q12h) for pneumococcal otitis media apparently correspond with our observations. Patients infected with strains for which the MICs were 0.5 to 2 μg/ml had a clinical response rate of 85% (11 of 13), whereas those infected with strains for which the MICs were 4 or 8 μg/ml (n = 18) had a clinical response rate of 67% (data on file, Bristol-Myers Squibb, Princeton, N.J.). These data corroborate the utility of this in vivo model in predicting outcomes in the infected patient and provide additional data concerning the appropriateness of breakpoint values to ensure good clinical outcomes for those infected with this pathogen.
Although we chose to simulate the pediatric pharmacokinetic profile of cefprozil observed after the 15-mg/kg q12h schedule due to the high use of this agent in this population, it should be recognized that a regimen of 500 mg q12h in adults produces a similar pharmacokinetic profile (16). Therefore, for the purposes of our pharmacodynamic analysis and the utilization of these data for breakpoint determinations, similar therapeutic outcomes should be observed for either population providing the appropriate dosage is given.
In conclusion, the effectiveness (as assessed by both mortality and the reduction in CFU per thigh) of cefprozil in the murine thigh model at a drug exposure which is typically used for children reveals that cefprozil maintains maximal efficacy against S. pneumoniae when the MIC is 2 μg/ml or lower. Our data and the clinical data obtained for patients with pneumococcal otitis media support the susceptibility breakpoint of ≤2 μg/ml for cefprozil.
ACKNOWLEDGMENTS
We thank Jeff Mather for statistical consultation and Dennis O. Taylor and Junius M. Clark of Bristol-Myers Squibb, Wallingford, Conn., for assessing the protein binding of cefprozil in mice. We also thank Ron Jones of the University of Iowa and Joan Fung-Tomc of Bristol-Myers Squibb, Wallingford, Conn., for providing clinical isolates which were used in these experiments.
This study was supported by a grant from Bristol-Myers Squibb, Princeton, N.J.
REFERENCES
- 1.Andes D, Craig W A. In vivo activities of amoxicillin and amoxicillin-clavulanate against Streptococcus pneumoniae: application to breakpoint determinations. Antimicrob Agents Chemother. 1998;42:2375–2379. doi: 10.1128/aac.42.9.2375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Areguedas A G, Zaleska M, Stutman H R, Blummer J L, Hains C S. Comparative trial of cefprozil vs. amoxicillin clavulanate potassium in the treatment of children with acute otitis media with effusion. Pediatr Infect Dis J. 1991;10:375–380. doi: 10.1097/00006454-199105000-00006. [DOI] [PubMed] [Google Scholar]
- 3.Aronovitz G H, Doyle C A, Durham S J, Wilber R B, Materman E, Simonson C. Cefprozil Multicenter Study Group. Cefprozil vs. amoxicillin/clavulanate in the treatment of acute otitis media. Infect Med. 1992;9(Suppl. C):19–31. [Google Scholar]
- 4.Craig W A, Andes D. Pharmacokinetics and pharmacodynamics of antibiotics in otitis media. Pediatr Infect Dis J. 1996;15:255–259. doi: 10.1097/00006454-199603000-00015. [DOI] [PubMed] [Google Scholar]
- 5.Craig W A. Interrelationship between pharmacokinetics and pharmacodynamics in determining dosage regimens for the cephalosporins. Diagn Microbiol Infect Dis. 1995;22:89–96. doi: 10.1016/0732-8893(95)00053-d. [DOI] [PubMed] [Google Scholar]
- 6.Gavalda J, Capdevila J A, Almirante B, Otero J, Ruiz I, Laguarda M, Allende H, Crespo E, Pigrau C, Pahissa A. Treatment of experimental pneumonia due to penicillin-resistant Streptococcus pneumoniae in immunocompetent rats. Antimicrob Agents Chemother. 1997;41:795–801. doi: 10.1128/aac.41.4.795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hamm T E. Proposed institutional animal care and use committee guidelines for death as an end point in rodent studies. AALAS Contemp Top. 1995;34:69–71. [Google Scholar]
- 8.Joly-Guillou M-L, Wolff M, Pocidalo J-J, Walker F, Carbon C. Use of a new mouse model of Acinetobacter baumannii pneumonia to evaluate the postantibiotic effect of imipenem. Antimicrob Agents Chemother. 1997;41:345–351. doi: 10.1128/aac.41.2.345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.National Committee for Clinical Laboratory Standards. Methods for dilution antimicrobial susceptibility tests for bacteria that grow aerobically. 4th ed. 1997. Approved standard M7-A4. National Committee for Clinical Laboratory Standards, Wayne, Pa. [Google Scholar]
- 10.Onyeji C O, Bui K Q, Owens R C, Jr, Nicolau D P, Quintiliani R, Nightingale C H. Comparative efficacies of levofloxacin and ciprofloxacin against Streptococcus pneumoniae in a mouse model of experimental septicemia. Int J Antimicrob Agents. 1999;12:107–114. doi: 10.1016/s0924-8579(98)00087-9. [DOI] [PubMed] [Google Scholar]
- 11.Pelletier L L., Jr Treatment of respiratory tract infections: overview of cefprozil clinical trials. Infect Dis Clin Pract. 1998;7(Suppl. 5):S316–S323. [Google Scholar]
- 12.Pichichero M E, McLinn S, Aronovitz G, Fiddes R, Blumer J, Nelson K, Dashefsky B. Cefprozil treatment of persistent and recurrent acute otitis media. Pediatr Infect Dis J. 1997;16:471–478. doi: 10.1097/00006454-199705000-00003. [DOI] [PubMed] [Google Scholar]
- 13.Ponte C, Parra A, Nieto E, Soriano F. Development of experimental pneumonia by infection with penicillin-sensitive Streptococcus pneumoniae in guinea pigs and their treatment with amoxicillin, cefotaxime, and meropenem. Antimicrob Agents Chemother. 1996;40:2698–2702. doi: 10.1128/aac.40.12.2698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Saez-Llorens X, Shyu W C, Shelton S, Kumiesz H, Nelson J. Pharmacokinetics of cefprozil in infants and children. Antimicrob Agents Chemother. 1990;34:2152–2155. doi: 10.1128/aac.34.11.2152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sauve C, Azoulay-Dupris E, Moine P, Darras-Joly C, Rieux V, Carbon C, Bedos J P. Efficacies of cefotaxime and ceftriaxone in a mouse model of pneumonia induced by two penicillin- and cephalosporin-resistant strains of Streptococcus pneumoniae. Antimicrob Agents Chemother. 1996;40:2829–2834. doi: 10.1128/aac.40.12.2829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Shyu W C, Shah V R, Campbell D A, Wilber R B, Pittman K A, Barbhaiya R H. Oral absolute bioavailability and intravenous dose-proportionality of cefprozil in humans. J Clin Pharmacol. 1992;32:798–803. doi: 10.1002/j.1552-4604.1992.tb03885.x. [DOI] [PubMed] [Google Scholar]
- 17.Shyu W C, Shukla U A, Shah V R, Papp E A, Barbhaiya R H. Simultaneous high-performance liquid chromatographic analysis of cefprozil diastereomers in a pharmacokinetic study. Pharm Res. 1991;8:992–996. doi: 10.1023/a:1015896722170. [DOI] [PubMed] [Google Scholar]
- 18.Woodnutt G, Berry V. Two pharmacodynamic models for assessing the efficacy of amoxicillin-clavulanate against experimental respiratory tract infections caused by strains of Streptococcus pneumoniae. Antimicrob Agents Chemother. 1999;43:29–34. doi: 10.1128/aac.43.1.29. [DOI] [PMC free article] [PubMed] [Google Scholar]