Abstract
Inflammatory myofibroblastic tumor (IMT) is a rare mesenchymal tumor that can develop in numerous organs, most commonly in the lungs and rarely in the brain. Here, we reported a 55-year-old patient with nasopharyngeal IMT and the recurrence in the skull base, slope and pterygoid sinus who underwent cranial base and slope tumor resection. Postoperative magnetic resonance imaging (MRI) and multiplex immunohistochemistry (mIHC) showed tumor recurrence and metastasis to the intracalvarium. While genetic testing revealed no significant related gene mutations, tertiary mutations in NSD1 and SOX9 genes were identified in the tumor tissues. The patient achieved partial remission after receiving 7 cycles of immunotherapy (toripalimab 240 mg for 1 cycle followed by 6 cycles of sintilimab 200 mg), and MRI examination indicated an almost complete remission of intracranial IMT after 16 cycles of immunotherapy. In summary, the novel class of immune-targeted agents may be effective in clinical management of rare intracranial IMT.
Keywords: inflammatory myofibroblastic tumor, sintilimab, multiplex immunohistochemistry, magnetic resonance imaging, immunotherapy
Introduction
Inflammatory myofibroblastic tumor (IMT), also known as inflammatory pseudotumor and plasmacytoid granuloma, is a tumor commonly occurring in the lungs, abdomen, skin, soft tissues, genital system, and mediastinum.1,2 Its origin, etiology and behavior remain a matter of debate. While metastases have been reported in up to 5% of IMT cases, potential kinase mutations, most commonly involving anaplastic lymphoma kinase (ALK), have been identified in these tumors.3,4 Recurrent metastasis of IMT may be associated with incomplete resection of the lesion, involvement of infiltrating adjacent vital organs, and TP53 expression in tumor cells.
Herein, we reported a rare case with intracranial IMT who achieved complete remission (CR) after receiving immunotherapy. This report provided important guiding significance to clinical treatment of the disease.
Case Presentation
A 55-year-old female was admitted to our hospital with recurrent IMT and invasion of skull base, slope and sphenoid sinus after tumor resection. On January 13th, 2019, the patient had a sudden onset of slurred speech without obvious incentive and her condition improved spontaneously after 30 seconds. She was treated with mannitol and vasodilator in Weifang Yidu Central Hospital, but the symptoms were not significantly alleviated. The patient was then referred to Shandong Provincial Hospital and underwent cranial base and slope tumor resection through nasal endoscopy on March 12th, 2019. Postoperative pathological examination confirmed the diagnosis of IMT. She was discharged with a good postoperative recovery and did not receive any further treatment after surgery.
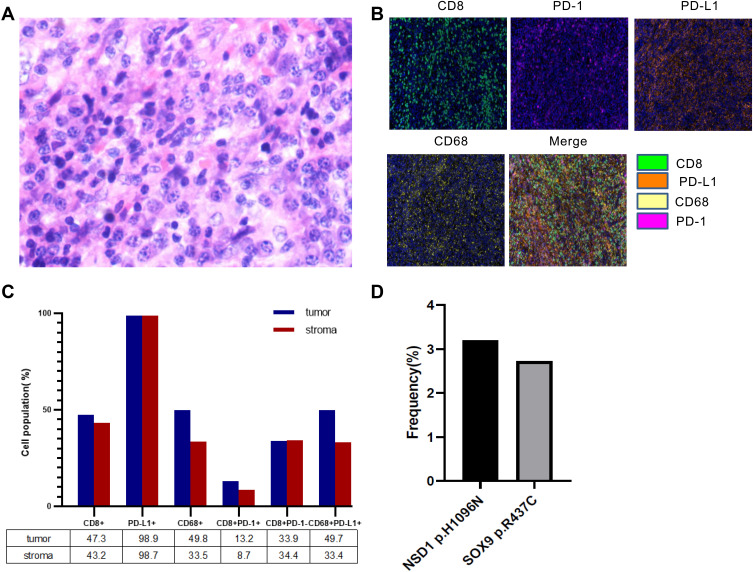
On April 9th, 2019, the patient was admitted to Shandong Institute of Medical Imaging. Craniocerebral magnetic resonance imaging (MRI) (April 15th) showed abnormal thickening of the middle cranial fossa region, skull base and right temporal meninges (Figure 1), suggestive of tumor recurrence and metastasis following surgery. Hematoxylin and eosin staining suggested inflammatory myofibroblastoma, with abundant cells and active growth (Figure 2A). Meanwhile, multiplex immunohistochemistry (mIHC) revealed a relatively high infiltration of CD8+/CD68+ lymphocytes as well as a high expression of PD-L1 (SP142) in the tumor tissues (Figure 2B). Moreover, quantitative analysis of tumor cells, macrophages and other subsets of immune cells found that the proportion of CD8+, pD-L1+, CD68+, CD8+PD-1+, CD8+PD-1-, and CD68+PD-L1+ cells in the tumor region was 47.3%, 98.9%, 49.8%, 13.2%, 33.9% and 49.7%, respectively, while the stromal region harbored the different percentage of CD8+ (43.2%), pD-L1+ (98.7%), CD68+ (33.5%), CD8+PD-1+ (8.7%), CD8+PD-1- (34.4%), and CD68+PD-L1+ cells (33.4%) (Figure 2C).
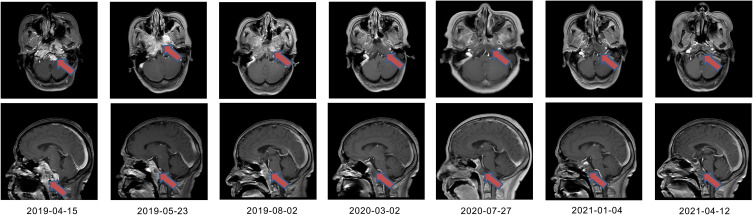
Figure 1.
Representative magnetic resonance imaging (MRI) scans. MRI images taken during radiotherapy, at the end of radiotherapy, after 2 cycles of immunotherapy, after 7 cycles of immunotherapy, after 12 cycles of immunotherapy, after 16 cycles of immunotherapy, and during follow-up were shown respectively.
Figure 2.
(A) Hematoxylin and eosin staining of the recurrent lesions. (B) The expression of CD8, PD-1, PD-L1, and CD68 in resected tumor tissues was detected by multiple immunohistochemistry (mIHC). Nuclei (blue) were counter-stained by DAPI. Magnification ×200. (C) Quantification analysis of data in B. (D) The frequencies of two shared pathogenic mutations in the recurrent lesions.
After consultation in the radiotherapy department of our hospital, the patient received radiotherapy with a total dose of 54 Gy in 27 fractions between April 17th and May 23rd, 2019. This trial was approved by the Ethics Committee of Shandong Cancer Hospital, and written informed consent was obtained from the patient. During radiotherapy, next generation sequencing (NGS) of her blood cells and paraffin-embedded tissues was carried out using a 543 cancer-related gene panel (Genecast, Wuxi, China) to identify the possible gene mutations suitable for immunotherapy. As shown in Figure 2D, the frequency of NSD1 c.3286C>A p.H1096N and SOX9 c.1309C>T p.R437C in the tissues was found to be 3.2% and 2.73%, respectively. MRI (May 23rd, 2019) revealed that at the end of radiotherapy, IMT invaded the skull base and brain with right dural metastasis after surgery (Figure 1).
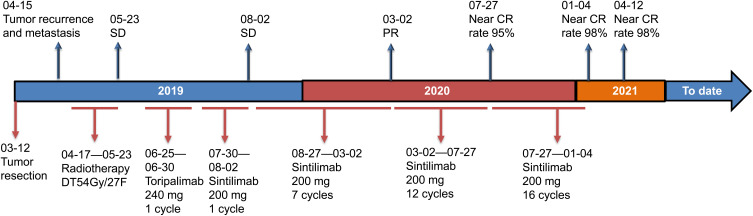
The patient was then treated with immunotherapy for further suppressing IMT. After excluding the immune contraindications, she was first given intravenous drip of Toripalimab (240 mg) for 1 cycle, and no obvious side effects were observed. Thereafter, she was administered with Sintilimab (200 mg) for another cycle, and a subsequent craniocerebral MRI examination (August 2nd, 2019) showed that the tumor was shrinking. Following another 7 cycles of immunotherapy (Sintilimab, 200 mg), most of the lesions were in remission (March 2nd, 2020). On July 27th, 2020, craniocerebral MRI demonstrated that the lesions had a near-CR rate of 98% after 12 cycles of Sintilimab 200 mg (Figure 1). Since then, the patient visited our hospital monthly for receiving Sintilimab 200 mg. After 16 cycles of Sintilimab 200 mg, the curative effects of the patient almost reached CR with no significant changes in the tumor (January 4th, 2021). In this case, the immunotherapy was well tolerated with no significant toxic and side effects. And no other therapy was given after the end of the treatment with Sintilimab. On April 12th, 2021, craniocerebral MRI demonstrated an improvement in postoperative nasopharyngeal IMT with invasion of the skull base, brain and right dural metastasis (Figure 1). The clinical and disease course of the patient is illustrated in Figure 3.
Figure 3.
Timeline of clinical events of the patient.
Abbreviations: SD, stable disease; PR, partial response; CR, complete response.
Discussion
IMT is a relatively rare tumor of mesenchymal origin that is common in children and adolescents. It can develop in various organs, with the lung and liver being commonly affected and the skull being less involved.5,6 Surgical resection is the most common treatment for IMT.4 In this case, while the patient was first diagnosed with nasopharyngeal IMT, the tumor recurred and metastasized to the brain after tumor resection. Genetic testing failed to identify representative gene mutations in IMT. However, the patient was found to harbor tertiary mutations in NSD1 and SOX9 genes as well as a high expression of PD-L1 in the tumor tissue. SOX9 acts as a key determinant of cancer cell plasticity. NSD1 is a histone methyltransferase containing the catalytic domain of SET, and its abnormal expression could be closely associated with Sotos syndrome. It has been reported that NSD1 affects chondrocyte differentiation by regulating the expression of Sox9.7 To date, the significance of mutations in NSD1 and SOX9 genes in IMT has yet to be defined.
Identification of underlying kinase mutations, including those in ALK, has provided a potential targeted therapy option for patients with unresectable and/or advanced IMT. It has been shown that not all IMT patients harbor actionable mutations. In the past 10 years, a total of 18 cases with metastatic IMT have been reported;8–25 most of them received surgical resection for primary IMT, while undergoing radiotherapy, chemotherapy and/or targeted therapy for metastatic tumors (Table 1). In one study, Carcamo et al showed that PD-L1 was expressed in 50% of the tumor cells in a 16-year-old male patient who failed to respond to PD-L1 inhibitor Nivolumab.9 In this case, we found that treatment with Sintilimab can block the binding of PD-1 with PD-L1 and alleviate tumor cell suppression via immune T cells. Sixteen cycles of immunotherapy led to a significant inhibition in the tumor cells of the patient. Sintilimab is PD-1 monoclonal antibody that has recently been approved for cancer treatment.26 In China, Phase I/II/III clinical trials of Sintilimab for the treatment of various solid tumors are being conducted.27–29 Wang et al have reported that Sintilimab possesses stronger anti-tumor activity with an acceptable safety profile in vivo as compared to certain monoclonal antibodies,30 while it obviously has some inevitable side effects and causes potential damage to patients, including pneumonia, diarrhea, colitis, hepatitis, and nephritis. At present, there is no report on research of the effects of Sintilimab and Toripalimab on IMT. Surgery remains predominant in clinical management of intracranial IMT due to the lack of definitive treatment and its unknown pathogenesis. In addition to radiotherapy, long-term treatment with clarithromycin can be administered when ALK1 and immunoglobulin deficiency are diagnosed, or when chronic inflammation worsens the patient’s condition.31 This study provides the first demonstration that Sintilimab exerts a good therapeutic effect on a patient with recurred IMT and intracranial metastasis.
Table 1.
A Summary of 18 Cases Reported Metastatic Inflammatory Myofibroblastic Tumors in Recent 10 Years
| Age (Years)/Sex | Presentation | Tumor Primary Site | IHC | Gene Mutation | Recurrence | Tumor Metastasis Site | Treatment | Follow-up and Prognosis | Reference |
|---|---|---|---|---|---|---|---|---|---|
| 55/F | A sudden slurred speech | Nasopharynx | Highly expressed PD-L1 | Tertiary mutations in NSD1 and SOX9 genes | 1 month | Skull base, slope and pterygoid sinus | Surgery, radiotherapy and immunotherapy (PD-L1 inhibitor Sintilimab) | Better than before to date | This case |
| 19/M | Macroscopic hematuria and progressive anemia | Bladder | ALK pos | NA | 7 months | Lung and left iliac bone and | Surgery and targeted therapy | Complete remission | Bonvini et al, 20218 |
| 16/M | NR | Right chest wall with pleural involvement | ALK neg, highly expressed PD-L1 | TFG-ROS1 fusion, an acquired G2032R mutation in the TKD of ROS1 and MAPK1 amplification | Continuing progression | Brain, right triceps | Antiinflammatory therapy, chemotherapy, targeted therapy and immunotherapy (PD-L1 inhibitor Nivolumab) | Died of respiratory complications | Carcamo et al, 20219 |
| 40/M | Dyspnoea and productive cough | Upper lobe of right lung | ALK pos | TPM4-ALK fusion | Transient improvement and continuing progression | Hilar lymph nodes, right trapezius muscle, left frontal lobe, left adrenal, left gluteal | Chemotherapy, radiotherapy and targeted therapy | Dead | Wong et al, 202010 |
| 57/F | Tightening sensation around the retrosternal region | Anterior mediastinum with left pleural invasion | ALK neg | 6 germline mutations (PARP1 p.V69I, ATR p.S1007N, GRM8 p.T97A, MLLT10 p.G409R, TCF7L2 p.N185S, SMARCA4 p.A321T) and 1 somatic mutation (TSHR p.Q720H) | Recurring at the left anterior mediastinum after nine months post first surgery, and at the left anterior mediastinum and right anterior pleural space at the age of 65 | Left anterior mediastinum and right anterior pleural space | Surgery and radiotherapy | NR | Hou et al, 202011 |
| 59/M | Consistent fatigue | Medium lobe of right lung | ALK neg | NA | Continuing progression | Bone and abdominal cavity | Targeted therapy | Stable condition on follow-up 9 months | Liu et al, 201912 |
| 81/M | Anal pain | The posterior rectal wall | ALK neg | NA | 2 months after surgery | Liver | Surgery | Dead | Shimodaira et al, 202013 |
| 76/M | Upper back pain and motor weakness | Upper lobe of right lung | ALK neg | NA | NA | Right renal hilum | Hormonotherapy and radiotherapy | Improvement in symptoms on follow-up 1 month | Na et al, 201814 |
| 55/M | Left pneumonia | Lower lobe of left lung | NA | NA | 3 months after first surgery | Lingula, lung and liver | Surgery and radiotherapy | The patient was referred to another oncological center. | Rodrigues et al, 201715 |
| 37/F | Cough and stridor | Upper lobe of left lung | ALK pos | NA | 1 year after first surgery | Uterine | Surgery | Dead 1 year after second surgery | Zhang et al, 201816 |
| 18/F | Headaches | Lung | ALK pos | ALK-1 gene rearranged | Continuing progression | Brain | Targeted therapy | Alive and well on follow-up 2.5 years since primary diagnosis | Yuan et al, 201717 |
| 43/F | Heart failure symptoms | Small intestinal | ALK neg | NA | 1 year | Left ventricle, stomach, liver, vertebra, and pelvic bones | Surgery and chemotherapy | Dead 9 months after surgery | Zorinas et al, 201718 |
| 16/F | A localized left shoulder mass around the subacromial region | Left shoulder | ALK pos | EML4-ALK translocation | 45 months | Left clavicle, the arm, and the anterior chest wall soft tissues | Surgery, chemotherapy, radiotherapy and targeted therapy | Remains in complete remission on follow-up 3 years | Gaudichon et al, 201619 |
| 49/F | Cough | Lower lobe of left lung | ALK pos | ALK-gene rearrangement | 4 months | Right anterior-end of third-rib and right adrenal gland | Surgery and targeted therapy | NR | Sethi et al, 201520 |
| 28/F | Post-prandial abdominal pain | Abdominal extensive solid masses involving multiple viscera | ALK neg | NA | Continuing progression | Vertebral body, liver and peritoneum | Surgery and chemotherapy | Dead | Kim et al, 201521 |
| 27/F | A painless palpable mass in the upper outer quadrant of the right breast | Right breast | ALK neg | NA | 2 years | The upper inner quadrant of the right breast and right cervical area | Surgery | NR | Choi et al, 201522 |
| 36/M | Hematochezia, tenesmus, and constipation | Rectum | ALK pos | NA | 18 months | The pelvic floor muscles, sacrococcyx, pre-sacral fascia | Surgery and chemotherapy | Follow-up every 6 months and disease-free | Sun et al, 201423 |
| 26/M | Chronic nonproductive cough | Mediastinum | ALK neg | NA | Continuing progression | Lymph nodes and the thoracic vertebra | Hormonotherapy and chemotherapy | No radiological evidence of tumor progression or recurrence for 7 months | Kubo et al, 201224 |
| 52/M | Dyspnea and cough | Upper lobes of bilateral lung | NA | NA | No | Left adrenal gland | Surgery | Alive and well, without recurrence on follow-up 1 year | Carillo et al, 201125 |
Abbreviations: ALK, anaplastic lymphoma kinase; F, female; M, male; NA, not available; neg, negative; NR, not reported; pos, positive.
Conclusion
The presence of tertiary mutations in NSD1 and SOX9 genes could potentially serve as an indicator for the diagnosis of IMT. Meanwhile, Sintilimab may be a good choice for immunotherapy against the recurrence and metastasis of IMT.
Ethics and Informed Consent Statements
This study was approved by the Ethics Committee of Shandong Cancer Hospital. Written informed consent was obtained from the patient for publication of this case report and any accompanying images.
Author Contributions
All authors contributed to data analysis, drafting or revising the article, have agreed on the journal to which the article will be submitted, gave final approval of the version to be published, and agree to be accountable for all aspects of the work.
Disclosure
The authors declare that they have no conflict of interest.
References
- 1.Silva W, Zavarez LB, Zanferrari FL, et al. Inflammatory myofibroblastic tumor: rare manifestation in face. J Craniofac Surg. 2017;28(8):e751–e2. doi: 10.1097/SCS.0000000000003954 [DOI] [PubMed] [Google Scholar]
- 2.Barata F, Marques D, Figueiredo A. Rare and unpredictable inflammatory myofibroblastic tumor. Rev Port Cir Cardiotorac Vasc. 2020;27(2):79–80. [PubMed] [Google Scholar]
- 3.Cottrell TR, Duong AT, Gocke CD, et al. Pd-l1 expression in inflammatory myofibroblastic tumors. Mod Pathol. 2018;31(7):1155–1163. doi: 10.1038/s41379-018-0034-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lovly CM, Gupta A, Lipson D, et al. Inflammatory myofibroblastic tumors harbor multiple potentially actionable kinase fusions. Cancer Discov. 2014;4(8):889–895. doi: 10.1158/2159-8290.cd-14-0377 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ding Y, Yang HY, Zhang D, et al. Diagnosis and treatment of inflammatory myofibroblastoma in children and adolescents. Chin Med J. 2019;132(9):1110–1112. doi: 10.1097/CM9.0000000000000176 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chuah YY, Tashi T, Shy CG, Shyu JS, Dong MJ, Hsueh EJ. Intracranial inflammatory myofibroblastic tumor with sarcomatous local recurrence. World Neurosurg. 2016;93(484):e1–e4. doi: 10.1016/j.wneu.2016.07.060 [DOI] [PubMed] [Google Scholar]
- 7.Shao R, Zhang Z, Xu Z, et al. H3k36 methyltransferase nsd1 regulates chondrocyte differentiation for skeletal development and fracture repair. Bone Res. 2021;9(1):30. doi: 10.1038/s41413-021-00148-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bonvini P, Rossi E, Zin A, et al. Case report: circulating tumor cells as a response biomarker in alk-positive metastatic inflammatory myofibroblastic tumor. Front Pediatr. 2021;9:652583. doi: 10.3389/fped.2021.652583 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Carcamo B, Bista R, Wilson H, Reddy P, Pacheco J. Rapid response to lorlatinib in a patient with tfg-ros1 fusion positive inflammatory myofibroblastic tumor of the chest wall metastatic to the brain and refractory to First and second generation ros1 inhibitors. J Pediatr Hematol Oncol. 2021;43(5):e718–e22. doi: 10.1097/mph.0000000000002185 [DOI] [PubMed] [Google Scholar]
- 10.Wong HH, Bentley H, Bulusu VR, et al. Lorlatinib for the treatment of inflammatory myofibroblastic tumour with tpm4-alk fusion following failure of entrectinib. Anticancer Drugs. 2020;31(10):1106–1110. doi: 10.1097/cad.0000000000000994 [DOI] [PubMed] [Google Scholar]
- 11.Hou TC, Wu PS, Huang WY, et al. Over expression of cdk4 and mdm2 in a patient with recurrent alk-negative mediastinal inflammatory myofibroblastic tumor: a case report. Medicine. 2020;99(12):e19577. doi: 10.1097/md.0000000000019577 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Liu Q, Wei J, Liu X, Wang J. Anaplastic lymphoma kinase-negative pulmonary inflammatory myofibroblastic tumor with multiple metastases and its treatment by apatinib: a case report. Medicine. 2019;98(52):e18414. doi: 10.1097/md.0000000000018414 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Shimodaira Y, Sugawara K, Fukuda S, et al. Aggressive inflammatory myofibroblastic tumor without anaplastic lymphoma kinase gene rearrangement in the rectum with liver metastasis. Intern Med. 2020;59(4):495–499. doi: 10.2169/internalmedicine.3686-19 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Na YS, Park SG. Inflammatory myofibroblastic tumor of the pleura with adjacent chest wall invasion and metastasis to the kidney: a case report. J Med Case Rep. 2018;12(1):253. doi: 10.1186/s13256-018-1796-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rodrigues C, Cabral D, Almodovar T, et al. Unusual behavior of a lung inflammatory myofibroblastic tumor: case report. Rev Port Cir Cardiotorac Vasc. 2017;24(3–4):140. [PubMed] [Google Scholar]
- 16.Zhang J, Li Y, Lou L. Uterine metastases originating from a pulmonary inflammatory myofibroblastic tumor. J Cancer Res Ther. 2018;14(Supplement):S257–s9. doi: 10.4103/0973-1482.172129 [DOI] [PubMed] [Google Scholar]
- 17.Yuan C, Ma MJ, Parker JV, Mekhail TM. Metastatic anaplastic lymphoma kinase-1 (alk-1)-rearranged inflammatory myofibroblastic sarcoma to the brain with leptomeningeal involvement: favorable response to serial alk inhibitors: a case report. Am J Case Rep. 2017;18:799–804. doi: 10.12659/ajcr.903698 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zorinas A, Austys D, Janusauskas V, et al. Small intestinal inflammatory myofibroblastic metastasis in the left ventricle. Ann Thorac Surg. 2017;103(1):e31–e3. doi: 10.1016/j.athoracsur.2016.05.099 [DOI] [PubMed] [Google Scholar]
- 19.Gaudichon J, Jeanne-Pasquier C, Deparis M, et al. Complete and repeated response of a metastatic alk-rearranged inflammatory myofibroblastic tumor to crizotinib in a teenage girl. J Pediatr Hematol Oncol. 2016;38(4):308–311. doi: 10.1097/mph.0000000000000498 [DOI] [PubMed] [Google Scholar]
- 20.Sethi B, Pai T, Allam A, Epari S. Anaplastic lymphoma kinase-positive pulmonary inflammatory myofibroblastic tumor with sarcomatous morphology and distant metastases: an unusual histomorphology and behavior. Indian J Pathol Microbiol. 2015;58(4):509–512. doi: 10.4103/0377-4929.168866 [DOI] [PubMed] [Google Scholar]
- 21.Kim S, Bakkum-Gamez JN, Okuno S, Kerr S, Dowdy SC. Abdominopelvic inflammatory myofibroblastic tumor that metastasized to the vertebrae and liver: a case report and review of the literature. Gynecol Oncol Rep. 2015;12:9–12. doi: 10.1016/j.gore.2015.01.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Choi EJ, Jin GY, Chung MJ, Moon WS, Youn HJ. Primary inflammatory myofibroblastic tumors of the breast with metastasis: radiographic and histopathologic predictive factors. J Breast Cancer. 2015;18(2):200–205. doi: 10.4048/jbc.2015.18.2.200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sun L, Tu L, Wang X, et al. Management of rectal inflammatory myofibroblastic tumor recurrence. J Cancer Res Ther. 2014;10(2):425–427. doi: 10.4103/0973-1482.136679 [DOI] [PubMed] [Google Scholar]
- 24.Kubo N, Harada T, Anai S, et al. Carboplatin plus paclitaxel in the successful treatment of advanced inflammatory myofibroblastic tumor. Intern Med. 2012;51(17):2399–2401. doi: 10.2169/internalmedicine.51.7599 [DOI] [PubMed] [Google Scholar]
- 25.Carillo C, Anile M, De Giacomo T, Venuta F. Bilateral simultaneous inflammatory myofibroblastic tumor of the lung with distant metastatic spread. Interact Cardiovasc Thorac Surg. 2011;13(2):246–247. doi: 10.1510/icvts.2011.271932 [DOI] [PubMed] [Google Scholar]
- 26.Gao S, Li N, Gao S, et al. Neoadjuvant pd-1 inhibitor (sintilimab) in NSCLC. J Thorac Oncol. 2020;15(5):816–826. doi: 10.1016/j.jtho.2020.01.017 [DOI] [PubMed] [Google Scholar]
- 27.Shi Y, Su H, Song Y, et al. Safety and activity of sintilimab in patients with relapsed or refractory classical Hodgkin lymphoma (orient-1): a multicentre, single-arm, Phase 2 trial. Lancet Haematol. 2019;6(1):e12–e9. doi: 10.1016/s2352-3026(18)30192-3 [DOI] [PubMed] [Google Scholar]
- 28.Yang Y, Wang Z, Fang J, et al. Efficacy and safety of sintilimab plus pemetrexed and platinum as first-line treatment for locally advanced or metastatic nonsquamous NSCLC: a randomized, double-blind, Phase 3 study (Oncology program by innovent anti-pd-1-11). J Thorac Oncol. 2020;15(10):1636–1646. doi: 10.1016/j.jtho.2020.07.014 [DOI] [PubMed] [Google Scholar]
- 29.Jiang H, Li N, Wang H, et al. Assessment of tmb, pd-l1, and lymphocyte to monocyte ratio as predictive potential in a phase ib study of sintilimab in patients with advanced solid tumors. Am J Cancer Res. 2021;11(9):4259–4276. [PMC free article] [PubMed] [Google Scholar]
- 30.Wang J, Fei K, Jing H, et al. Durable blockade of pd-1 signaling links preclinical efficacy of sintilimab to its clinical benefit. MAbs. 2019;11(8):1443–1451. doi: 10.1080/19420862.2019.1654303 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Watanabe H, Uruma T, Tazaki G, et al. Remission of alk-negative primary pulmonary inflammatory myofibroblastic tumor on treatment with clarithromycin: a case report and review of the literature. Oncol Lett. 2016;11(3):1757–1761. doi: 10.3892/ol.2016.4119 [DOI] [PMC free article] [PubMed] [Google Scholar]