Abstract
Objective:
To describe the ultrastructure of the interface between periodontal tissues and titanium mini-implants in rat mandibles.
Materials and Methods:
A titanium mini-implant was placed between the buccal roots of the mandibular first molar of 24 adult rats. After 21, 30, 45, 60, 90, and 120 days of implantation, the mandibular portion was removed and fixed in cacodylate-buffered 2% glutaraldehyde + 2.5% formaldehyde. The material was decalcified and processed for scanning and transmission electron microscopy.
Results:
Ultrastructural analysis revealed a thin cementum-like layer at longer times after implantation at the areas in which the periodontal ligament was in contact with the implant.
Conclusions:
The alveolar bone and the periodontal ligament reorganized their constituents around the implant, and a thin cementum-like layer was formed at longer times after implantation at the areas in which the periodontal ligament was in contact with the implant.
Keywords: Dental implant, Mini-implants, Interface morphology, Orthodontic anchorage
INTRODUCTION
Titanium implants have been used largely in dentistry over past decades. The close contact between bone and titanium implants provides an ankylosis-like type of interaction, an event named osseointegration.1 Because osseointegration offers necessary conditions for load and transfer bearing, the use of dental implants as orthodontic anchorages has increased progressively over the years.2,3
Although implants provide excellent anchorage, some limitations such as the waiting time for allowing osseointegration, invasive surgery, high cost, and difficulty removing the dental implant after completion of orthodontic treatment were noted initially because of their routine use in orthodontics.4,5 Another initial difficulty was that conventional implants are placed in edentulous sites with sufficient bone for anchorage; however, most orthodontic patients are young and do not have edentulous areas. To overcome this limitation, titanium screws with smaller dimensions (miniscrews) were introduced and were referred to as orthodontic mini-implants6; these can be placed in unconventional sites such as the alveolar bone of adjacent teeth without damaging roots and without requiring time for osseointegration,7–9 while offering stable anchorage even in critical conditions.6 Although insertion of mini-implants might result in contact with or proximity to root surfaces or nerves,10,11 no evidence indicates whether they influence the periodontal tissues to be repaired around them. Although the relationship between bone and titanium implants is well known, what does occur when titanium implants are placed in contact with the periodontal ligament remains unclear.
Because few reports in the literature are based mainly on light microscopy analyses, we felt it was necessary to apply a powerful method to discern the cells and matrix of the periodontal ligament in contact with titanium mini-implants. Transmission electron microscopy offers the advantage of evaluating cells and tissues at higher magnifications while maintaining good resolution. Therefore, we carried out an ultrastructural study to examine fine details of the interface between alveolar bone and periodontal ligament, and of titanium mini-implants placed between the roots of the mandibular first rat molar.
MATERIALS AND METHODS
Mini-implants
Commercially pure titanium mini-implants 1.4 mm in length and 1.2 mm in diameter were manufactured for this study (INP System, São Paulo, Brazil). Before surgeries were performed, all instruments were sterilized in a Prosmatic T 215 autoclave (Prismatec, São Paulo, Brazil) for 30 minutes at 180°C.
Animals
Twenty-four 3-month-old male Wistar rats weighing 350 g were housed with a 12-hour light/dark cycle and were allowed a standard pellet diet and tap water ad libitum throughout the experiments. Principles of laboratory animal care (NIH publication 85-23, 1985) and national laws were observed for the present study, which was authorized by the Ethics Committee of the University of São Paulo, Brazil.
Surgery
The animals were anesthetized intramuscularly with a combination of ketamine hydrochloride (Ketalar®, Parke Davis, Brazil) and xylazine (Rompun®, Bayer SA, Brazil) at concentrations of 6 mg and 0.7 mg/100 g body weight, respectively. The surgical area was shaved with a razor blade and was cleaned with an alcoholic solution of iodine. A total flap was made by a horizontal incision in the buccal face at the level of the right first lower molar. After careful dissection of the periosteum was performed, a cortical hole was drilled between the mesial and distal buccal roots of the first molar with a slow-speed (800 rpm) dental handpiece, using a sequence of three drills (0.5, 0.9, and 1.2 mm diameter) with copious irrigation of sterile physiologic saline to avoid heating. Then, the miniscrew was installed on the right mandible (Figure 1a) in contact with the periodontal tissues, and the scrap was sutured with polyglactin 910 (Vicryl® 6.0, Ethicon, Somerville, NJ). All intraoral procedures were carried out under a surgical stereomicroscope (DF Vasconcellos, São Paulo, Brazil) at magnifications ranging from 125× to 200×.
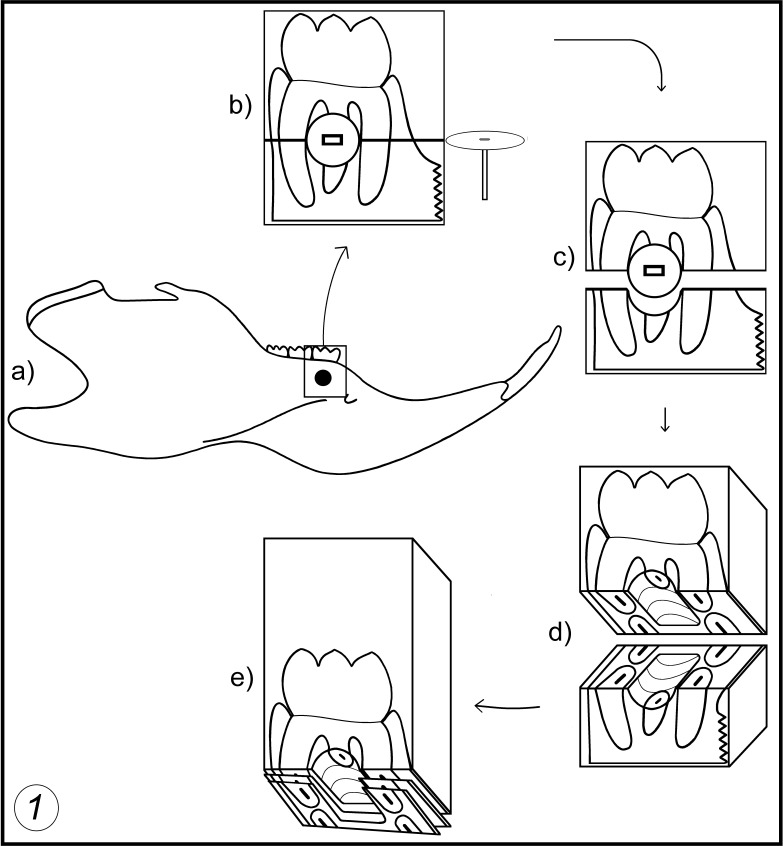
Figure 1.
Drawings show the site where the implant is placed (a), the fracture of resin block for removal of the implant (b, c, d), and how the interface between the periodontal tissues and the implant appears in the semithin sections (e).
Tissue Processing
Screws and the general health of rats were checked daily. A screw was considered successful when, at the end of the experimental period, it showed no mobility in situ, and the sound on percussion was clear; otherwise, the animal was discarded.
After 21, 30, 45, 60, 90, and 120 days, the rats were anesthetized, the miniscrews were evaluated as described previously, and the mandible portion containing the first molar and the implant were removed using a dental handpiece with a carborundum disk. The samples were dissected out and fixed in 2% glutaraldehyde + 2.5% formaldehyde (freshly prepared from paraformaldehyde) buffered at pH 7.4 with 0.1 M sodium cacodylate under microwave irradiation.12 Then, specimens were washed in the same buffer for 1 hour and were decalcified in 4.13% ethylenediaminetetraacetic acid (EDTA), pH 7.2, under microwave irradiation for 15 hours, washed in the same buffer, postfixed in 1% osmium tetroxide for 2 hours, dehydrated in graded concentrations of ethanol, and embedded in Spurr resin. The blocks were fractured into two fragments using a groove that was made with a steel disk as a guide (Figure 1b).
After the fracture occurred, the screw usually was retained on one fragment, and the other one showed the interfacial replica of resin (Figure 1c). Some specimens containing the implant and their corresponding replicas were processed for scanning electron microscopy (SEM) to correlate the grooves and ridges of the two fragments of the same block. The remaining samples had the implants removed and were re-embedded in Spurr resin (Figure 1d) and trimmed; semithin sections were obtained using a Microm HM-360 microtome (Thermo Fisher Scientific Inc, Waltham, Mass) equipped with a glass knife. Toluidine blue–stained sections 500 micrometers thick and containing alveolar bone and/or the periodontal ligament–implant interface (Figure 1e) were trimmed for cutting of 80-nanometer-thick ultrathin sections that were collected onto copper grids, stained with uranyl acetate/lead citrate, and examined in a Jeol 1010 (Jeol Ltd, Tokyo, Japan) transmission electron microscope operating to 80 kv.
The half resin blocks kept for SEM examination and the implants removed from resin-embedded tissue were mounted on aluminum stubs for sputtering with a 25-nm layer of gold in a Balzers SCD 050 apparatus (Bal-Tec AG, Principality of Liechtenstein). The samples were examined in a Jeol 6100 (Jeol Korea Ltd) scanning electron microscope operating to 10 to 15 kv.
RESULTS
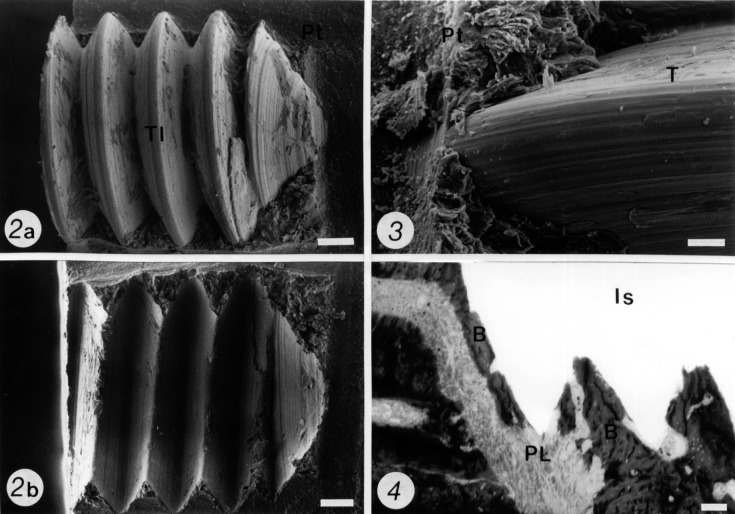
The mini-implants removed from resin blocks examined under SEM showed no residual resin. The groove/ridge surface of the resin-embedded issues (replica) exhibited shape and dimensions comparable with those of the threads from the removed implants (Figures 2a,b). The half resin blocks analyzed by SEM (Figure 3) and the semithin sections (Figure 4) showed bone in contact with the titanium surface. The periodontal tissues were in close relation to the implant space; no signs of inflammation or epithelial invasion were detected at the interfaces examined (Figure 4).
Figure 2.
Scanning electron micrographs after fracture of a resin block from a specimen at 120 days after implantation to correlate the grooves and ridges of the two fragments of the same block. In 2a, the half resin block that has retained the titanium mini-implant (TI) appears free of any residual resin. Periodontal tissues (Pt) can be observed at both sites of the implant in intimate contact with its threads. In 2b, the corresponding replica from 2a appears free from the mini-implant, exhibiting grooves and ridges on its surface, with shape and dimensions comparable with those of the threads from the implant. Bar = 100 µm.
Figure 3. Scanning electron micrograph shows a higher magnification view from a region at the interface between the periodontal tissues (Pt) and the thread (T) from a mini-implant. Observe that osseointegration has taken place. Bar = 35 µm.
Figure 4. Light micrograph shows a semithin section of periodontal tissues detached from a titanium implant in a resin-embedded specimen at 60 days after implantation. Observe the alveolar bone (B) and the periodontal ligament (PL) adjacent to the space that remains after detachment of the implant (Is). Bar = 100 µm.
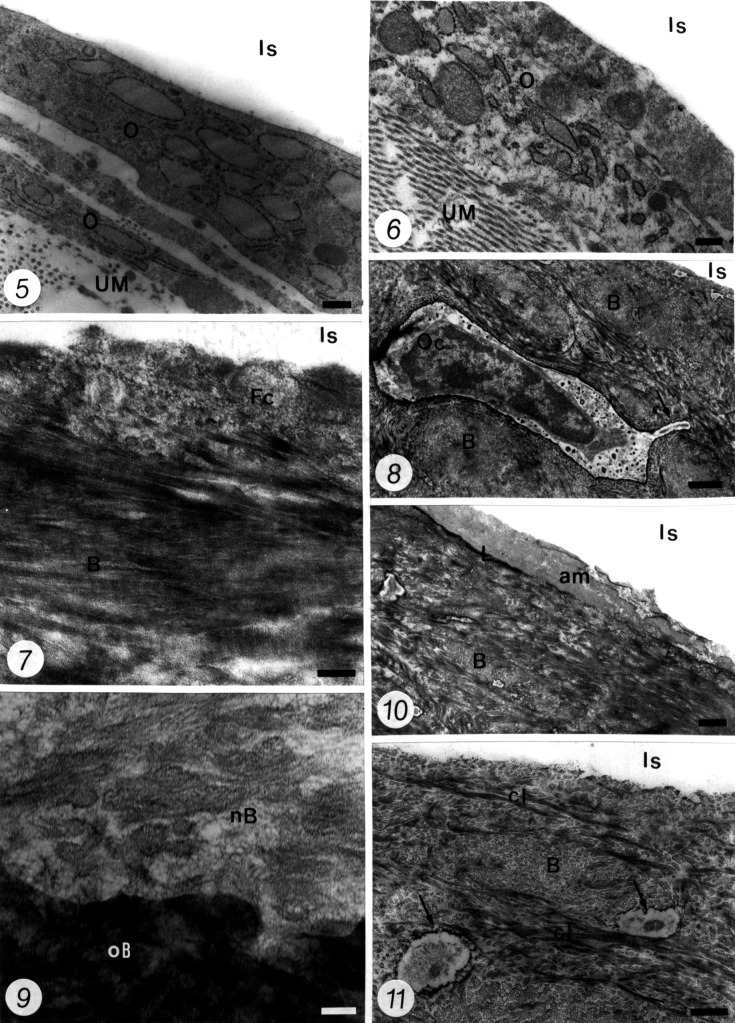
Ultrastructural analysis of the interface between the alveolar bone and the implant showed newly formed bone in all the specimens, which became mature as the time periods became longer. Twenty-one days after implantation, the bone–implant interface showed many areas in which flattened cells appeared at the implant boundary. Secretory osteoblasts showing well-developed synthetic organelles in contact with unmineralized bone matrix (osteoid) were identified over the implant (Figure 5). Active osteoclasts also were noted at this early time point. At 30 days, a layer of flattened cells, which resembled bone lining cells, was always present between the new bone and the implant. In addition, a thin electron-opaque line was seen surrounding the implant. The specimens at 45 days showed connective (osteoblast-like) cells, surrounded by a collagen-rich organic matrix at the implant boundary (Figure 6). At later times (60 and 90 days after implantation), some regions of the interface exhibited portions of flattened cells, which were entrapped against the implant (Figure 7). In other regions, the alveolar bone reached the implant surface, exhibiting some osteocytes close to the interface (Figure 8). The boundary between the newly formed bone and the original bone was observed clearly in these specimens (Figure 9). An electron-opaque line (“laminae limitans') of bone was observed in some regions. Adjacent to this region, an amorphous material or collagen fibrils were observed in contact with the implant space (Figure 10). The collagen fibrils immediately adjacent to the implant appeared to be aligned parallel to its surface; those from the interface were arranged randomly. Similar findings were observed in rats after 120 days of implantation, but the alveolar bone appeared with a typical mature aspect. Typical cement lines were frequent at these regions (Figure 11).
Figures 5–11.
Transmission electron micrographs at the interface between forming alveolar bone and the implant space.
Figure 5. The bone–implant interface at 21 days after implantation shows portions of osteoblast-like cells (O) in which well-developed synthesis organelles can be observed. These cells are present between the unmineralized bone matrix (UM) and the implant space (Is). Bar = 0.25 µm. Figure legends continued on next page.
Figure 6. A specimen at 45 days after implantation showing an osteoblast-like cell (O) adjacent to the implant space (Is) that is surrounded by a collagen-rich unmineralized bone matrix (UM). Bar = 0.25 µm.
Figure 7. Newly formed alveolar bone (B) apposed to the implant surface at 60 days after implantation is observed. A flattened cell (Fc) appears to be entrapped by the forming bone against the implant surface (Is), in which no clearly distinguished organelles are present into its cytoplasm. Bar = 0.25 µm.
Figure 8. The alveolar bone (B) is present in contact with the implant surface (Is) in a specimen at 90 days after implantation. An osteocyte (Oc) appears inside a lacuna close to the interface, and an osteocyte process arises from its cell body (arrows). Bar = 1 µm.
Figure 9. A higher magnification view at the boundary between newly formed alveolar bone (nB) and the original alveolar bone (oB) in a specimen at 60 days after implantation. Bar = 0.12 µm.
Figure 10. The interface between alveolar bone (B) and the implant at 90 days after implantation shows an electron-opaque line, similar to the “laminae limitans” (L) close of the implant space (Is). A 1.5-µm-thick layer of amorphous afibrillar material (am) with variable appearance is observed in contact with the implant space, adjacent to the “laminae limitans.” Bar = 0.5 µm.
Figure 11. Observe that the alveolar bone (B) has reached the implant surface (Is) at 120 days after implantation. Some osteocyte processes (arrows), which are observed at the interior of canaliculae, appear in the vicinity of the interface. Typical cement lines (cl) are seen in the alveolar bone. Bar = 0.5 µm.
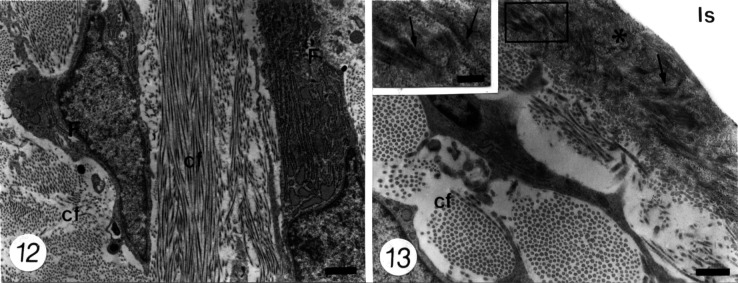
In the areas in which the implant was placed in contact with the periodontal ligament, it appeared with its typical aspect as the time periods became longer. Thus, at advanced times (60 to 120 days), fibroblasts appeared interspersed among bundles of collagen fibrils (Figure 12); however, they exhibited no special arrangement in relation to the implant surface. In some areas, a dense amorphous material that appeared to contain some collagen fibrils was detected forming a cementum-like layer, approximately 1 to 1.5 µm thick, that seemed to cover the implant; the collagen fibrils, however, were not inserted into collagen bundles or Sharpey's fibers (Figure 13).
Figures 12–13.
Transmission electron micrographs at the interface between periodontal ligament and implant space.
Figure 12. The periodontal ligament from a region that appears close to the implant surface at 60 days after implantation contains fibroblasts (F) with well-developed synthesis and secreting organelles. They are surrounded by numerous bundles of collagen fibrils (cf). Bar = 1 µm.
Figure 13. A dense amorphous material that contains some collagen fibrils (arrows) is forming a 1.35-µm-thick cementum-like layer (asterisk) over the implant surface (Is) in a specimen at 120 days after implantation. At the adjacent periodontal ligament, some bundles of cross-sectional collagen fibrils (cf) are observed. The inset shows higher magnification of the squared area at the cementum-like layer. Bar = 0.5 µm.
DISCUSSION
The present study showed that periodontal ligament around titanium mini-implants placed between the roots of rat molars formed a very thin layer of cementum-like tissue that could be observed only under transmission electron microscopy.
The present investigation evaluated unloaded mini-implants, because recent experimental animal studies reported that loading time has no statistically significant influence on osseointegration, thus yielding no differences in the percentage of bone-to-metal contact between loaded and unloaded mini-implants.13–15 Early times (less than 21 days) were not studied, so the stages in which the first reactions took place could be avoided. This was done because the study sought to determine what does occur when the periodontal ligament comes in contact with titanium.
In general, the osseointegration pattern observed in the alveolar bone resembled the one previously reported in other bones like tibia.16,17 Thus, the more staggering finding was the presence of a cementum-like layer covering the implant at longer times when titanium established contact with the periodontal ligament. Although this possibility has been stated previously by some authors,18–21 the formation of a layer of cementum in their studies was restricted to areas in which the implant touched the root, which was not necessarily the case in our study results. The earlier times exhibited areas of periodontal ligament in which collagen bundles with numerous fibroblasts appeared in close relation to the mini-implant. Later, notably after 90 days, a conspicuous cementum-like layer constituted the tissue boundary at the interface between the periodontal ligament and the mini-implant. Ultrastructural detection of a mineralized tissue in contact with the implant surface supports the idea that periodontal ligament fibroblasts are capable of forming a hard tissue.22–24 Thus, the titanium surface through its well-known biocompatibility exerts an effect on the periodontal ligament to lay down a covering hard tissue layer at later times after implant placement. The possibility of cementoblasts covering the cementum of adjacent roots, after having migrated to form the cementum-like layer, also should be considered.25 The cementum-like layer contained some collagen fibrils into its milieu; this is somewhat similar to the acellular extrinsic fiber cementum that is formed by fibroblasts during cementogenesis.20 Nevertheless, although the layer had a cementum-like appearance, it did not contain inserted collagen bundles (Sharpey's fibers), leaving it toward the adjacent periodontal ligament. Thus, it was not a true periodontal ligament if one takes into consideration that a functional periodontal ligament has its collagen bundles inserted into its neighboring hard tissues.
In summary, our findings show that repair occurred at the mini-implant surface through cementoblastic activity. In addition, the periodontal ligament space was well preserved in all specimens, and no microankylotic spots were detected.
CONCLUSIONS
A thin cementum-like layer was formed at longer times after implantation at the areas in which the periodontal ligament was in contact with the implant.
In addition, bone formation occurred in the alveolar bone in contact with the implant surface, thus showing that osseointegration actually takes place around orthodontic mini-implants when left for long times.
Acknowledgments
The authors thank Dr Tadeu T. Siqueira and INP System (Sao Paulo, Brazil) for supplying the mini-implants. Technical assistance provided by Mr Gaspar Lima and Mr Edson Oliveira is also acknowledged. This work was supported in part by FAPESP and CNPq, Brazil.
REFERENCES
- 1.Brånemark P. I, Hansson B. O, Adell R, Breine U, Lindström J, Hallén O, Ohman A. Osseointegrated implants in the treatment of the edentulous jaw: experience from a 10-year period. Scand J Plast Reconstr Surg. 1977;16:1–132. [PubMed] [Google Scholar]
- 2.Roberts W. E, Helm F. R, Marshall K. J, Gongloff R. K. Rigid endosseous implants for orthodontic and orthopedic anchorage. Angle Orthod. 1989;59:247–256. doi: 10.1043/0003-3219(1989)059<0247:REIFOA>2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- 3.Higuchi K. W, Slack J. M. The use of titanium fixtures for intraoral anchorage to facilitate orthodontic tooth movement. Int J Oral Maxillofac Implants. 1991;6:338–344. [PubMed] [Google Scholar]
- 4.Bae S. M, Park H. S, Kyung H. M, Kwon O. W, Sung J. H. Clinical application of micro-implant anchorage. J Clin Orthod. 2002;36:298–302. [PubMed] [Google Scholar]
- 5.Fritz U, Ehmer A, Diedrich P. Clinical suitability of titanium microscrews for orthodontic anchorage—preliminary experiences. J Orofac Orthop. 2004;65:410–418. doi: 10.1007/s00056-004-0408-x. [DOI] [PubMed] [Google Scholar]
- 6.Degushi T, Takano-Yamamoto T, Kanomi R, Hartsfield J. K, Roberts W. H, Garetto L. P. The use of small titanium screws for orthodontic anchorage. J Dent Res. 2003;82:377–381. doi: 10.1177/154405910308200510. [DOI] [PubMed] [Google Scholar]
- 7.Carano A, Velo S, Leone P, Siciliani G. Clinical applications of the miniscrew anchorage system. J Clin Orthod. 2005;39:9–24; quiz 29–30. [PubMed] [Google Scholar]
- 8.Arcuri C, Muzzi F, Santini F, Barlattani A, Giancotti A. Five years of experience using palatal mini-implants for orthodontic anchorage. J Oral Maxillofac Surg. 2007;65:2492–2497. doi: 10.1016/j.joms.2007.06.651. [DOI] [PubMed] [Google Scholar]
- 9.Vande-Vannet B, Sabzevar M. M, Wehrbein H, Asscherickx K. Osseointegration of miniscrews: a histomorphometric evaluation. Eur J Orthod. 2007;29:437–442. doi: 10.1093/ejo/cjm078. [DOI] [PubMed] [Google Scholar]
- 10.Coburn D. G, Kennedy D. W, Hodder S. C. Complications with intermaxillary fixation screws in the management of fractured mandibles. Br J Oral Maxillofac Surg. 2002;40:241–243. doi: 10.1054/bjom.2001.0771. [DOI] [PubMed] [Google Scholar]
- 11.Fabbroni G, Aabed S, Mizen K, Star D. G. Transalveolar screws and the incidence of dental damage: a prospective study. Int J Oral Maxillofac Surg. 2004;33:442–446. doi: 10.1016/j.ijom.2003.10.014. [DOI] [PubMed] [Google Scholar]
- 12.Massa L. F, Arana-Chavez V. E. Ultrastructural preservation of rat embryonic dental tissues after rapid fixation and dehydration under microwave irradiation. Eur J Oral Sci. 2000;108:74–77. doi: 10.1034/j.1600-0722.2000.00753.x. [DOI] [PubMed] [Google Scholar]
- 13.Zubery Y, Bichacho N, Moses O, Tal H. Immediate loading of modular transitional implants: a histologic and histomorphometric study in dogs. Int J Periodontics Restorative Dent. 1999;19:343–353. [PubMed] [Google Scholar]
- 14.Nkenke E, Lehner B, Weinzierl K, Thams U, Neugebauer J, Steveling H, Radespiel-Tröger M, Neukam F. W. Bone contact, growth and density around immediately loaded implants in the mandible of mini pigs. Clin Oral Implants Res. 2003;14:312–321. doi: 10.1034/j.1600-0501.2003.120906.x. [DOI] [PubMed] [Google Scholar]
- 15.Freire J. N, Silva N. R, Gil J. N, Magini R. S, Coelho P. G. Histomorphologic and histomorphometric evaluation of immediately and early loaded mini-implants for orthodontic anchorage. Am J Orthod Dentofacial Orthop. 2007;131:704.e1–e9. doi: 10.1016/j.ajodo.2006.09.040. [DOI] [PubMed] [Google Scholar]
- 16.Nanci A, McCarthy G. F, Zalzal S, Clokie C. M. L, Warchawski H, McKee M. D. Tissue response titanium implants in the rat tibia: ultrastructural, immunocytochemical and lectin-cytochemical characterization of the bone-titanium interface. Cells Mater. 1994;4:1–30. [Google Scholar]
- 17.Siqueira J. T, Cavalher-Machado S. C, Arana-Chavez V. E, Sannomiya P. Bone formation around titanium implants in the rat tibia: role of insulin. Implant Dent. 2003;12:242–251. doi: 10.1097/01.id.0000074440.04609.4f. [DOI] [PubMed] [Google Scholar]
- 18.Buser D, Warrer K, Karring T. Formation of a periodontal ligament around titanium implants. J Periodontol. 1990;61:597–601. doi: 10.1902/jop.1990.61.9.597. [DOI] [PubMed] [Google Scholar]
- 19.Warrer K, Karring T, Gotfredsen K. Periodontal ligament formation around different types of dental titanium implants. I. The self-tapping screw type implant system. J Periodontol. 1993;64:29–34. doi: 10.1902/jop.1993.64.1.29. [DOI] [PubMed] [Google Scholar]
- 20.Gray J. L, Vernino A. R. The interface between retained roots and dental implants: a histologic study in baboons. J Periodontol. 2004;75:1102–1106. doi: 10.1902/jop.2004.75.8.1102. [DOI] [PubMed] [Google Scholar]
- 21.Jahangiri L, Hessamfar R, Ricci J. L. Partial generation of periodontal ligament on endosseous dental implants in dogs. Clin Oral Implants Res. 2005;16:396–401. doi: 10.1111/j.1600-0501.2005.01152.x. [DOI] [PubMed] [Google Scholar]
- 22.Nojima N, Kobayashi M, Shionome M, Takahashi N, Suda T, Hasegawa K. Fibroblastic cells derived from bovine periodontal ligaments have the phenotypes of osteoblasts. J Periodontal Res. 1990;25:179–185. doi: 10.1111/j.1600-0765.1990.tb01041.x. [DOI] [PubMed] [Google Scholar]
- 23.Bosshardt D. D, Schoeder H. E. Cementogenesis reviewed: a comparison between human premolars and rodent molars. Anat Rec. 1996;245:267–292. doi: 10.1002/(SICI)1097-0185(199606)245:2<267::AID-AR12>3.0.CO;2-N. [DOI] [PubMed] [Google Scholar]
- 24.Trubiani O, Orsini G, Zini N, Di Iorio D, Piccirilli M, Piattelli A, Caputi S. Regenerative potential of human periodontal ligament derived stem cells on three-dimensional biomaterials: a morphological report. J Biomed Mater Res A. 2008;87:986–993. doi: 10.1002/jbm.a.31837. [DOI] [PubMed] [Google Scholar]
- 25.Parlar A, Bosshardt D. D, Unsal B, Cetiner D, Haytaç C, Lang N. P. New formation of periodontal tissues around titanium implants in a novel dentin chamber model. Clin Oral Implants Res. 2005;16:259–267. doi: 10.1111/j.1600-0501.2005.01123.x. [DOI] [PubMed] [Google Scholar]