Abstract
Objective:
To examine changes in gravity fluctuation caused by experimentally altering the area of occlusal contact.
Materials and Methods:
Subjects consisted of 15 adult Japanese males with normal stomatognathic function, no missing teeth except for the third molars, and equivalent occlusal contact in the anterior and bilateral posterior regions. Silicon biteplates fabricated for each subject to evaluate gravity fluctuation in relation to changes in occlusal contact area were as follows: RP(−)–OC(+) (entire occlusal surface covered in centric occlusion); RP(+)–OC(+) (entire occlusal surface covered with bite slightly raised); Ant or Pos/RP(+)–OC(+) (anterior or posterior region selectively covered); and RP(+)–OC(−) (only retromolar pads covered, no occlusal coverage).
Results:
No significant differences in gravity fluctuation were noted between subjects wearing biteplates covering the entire occlusal surface. Subjects wearing biteplates with no occlusal contact showed greater gravity fluctuation than those with occlusal contact. In addition, gravity fluctuation for the Ant/RP(+)–OC(+) group (no occlusal contact in the posterior region) was greater than for RP(+)–OC(+) and Pos/RP(+)–OC(+). However, groups with unilateral occlusal contact in the posterior region exhibited large right and left sway amplitude.
Conclusions:
These results suggest that occlusal contact, especially posterior occlusal contact, affects gravity fluctuation, and that appropriate occlusion attained by maintaining even occlusal contact in the posterior region is crucial for gravity fluctuation.
Keywords: Occlusion, Gravity fluctuation, Silicon biteplate, Occlusal area, Posture
INTRODUCTION
Humans exist in a physically unstable state in the standing position because the supporting plantar area is narrow. And the center of gravity is positioned high because the heavy skull is located at the highest point of the vertebral column.1 Maintenance of such a posture is related to gravity fluctuation, a phenomenon that is said to be controlled by information from the ocular region, the three semicircular canals, and the antigravity muscles.2,3
Previous studies have shown a relationship between occlusion and systemic conditions, and that the position and functional factors of the mandible affect the center of gravity.4–8 Other reports have found that as malocclusion improved, stiff neck, muscular neck pain, migraine, and lower back pain were alleviated,9–14 and that proper teeth clenching played an effective role in the enhancement of sports performance.15
Previous studies reported that stimulation of the periodontal membrane affects antigravity muscles.16 However, no studies have evaluated the detailed effects of stimulation of the periodontal membrane on gravity fluctuation.
Although it is thought that tooth loss is a risk factor for postural instability, and that incisors and molars play different roles,17,18 no studies have evaluated the effects of occlusal contact area on posture or gravity fluctuation. Therefore, in this study, we evaluated the effects of experimental changes in the occlusal contact area on gravity fluctuation.
MATERIALS AND METHODS
Subjects
Subjects were selected using the following selection criteria: (1) adult males; (2) no abnormality in the oral cavity, temporomandibular joint, or head and neck muscles; (3) no missing teeth except for the third molars; and (4) no history of ophthalmologic or otolaryngologic disease.
Among candidates who fitted the above selection criteria, 15 adult Japanese males (26 to 33 years old; average age, 29.5 years) with occlusal contact in the anterior and bilateral posterior regions with 50±10% equilibration of left and right occlusal contact were selected as subjects after occlusal contact was measured using occlusal force meters (Dental Prescale; Fuji Film, Tokyo, Japan/Occluzer; GC, Tokyo, Japan). The subjects' average occlusal contact area was 22 mm2 (SD±11 mm2), and the average occlusal force was 1200 N (SD±520 N).
Assessment took place after the meaning, purpose, and methods of the study were explained to subjects and informed consent was obtained in accordance with the principles of the Declaration of Helsinki.
Fabrication of Silicon Biteplates and Occlusal Conditions
Conventional alginate impressions of the maxillary and mandibular dentitions were taken to fabricate working casts using dental stone. Working casts were mounted on a semiadjustable articulator.
Using silicone rubber impression material (Exafine putty type; GC), two types of silicon biteplates were fabricated: RP(−), in which the teeth were in centric occlusion; and RP(+), in which the occlusal dimension was greater than the centric position. The change in occlusal height was within 2 mm of the “rest position” of the mandible in the maxillary and mandibular central incisor region,19,20 to eliminate the effects of muscles that can be caused by increased occlusal dimension. The biteplates were prepared in the following seven configurations of mandibular position and occlusal contact [OC(+), in which there was occlusal contact; and OC(−), in which no occlusal contact occurred (Figure 1)]: (1) RP(−)–OC(+): coverage of entire occlusal surface in RP(−); (2) RP(+)–OC(+): coverage of entire occlusal surface in RP(+)—the same mandibular position as those following in 3, 4, 5, 6, and 7; (3) Ant/RP(+)–OC(+): coverage of anterior region (canine to canine) only; (4) R,L Ant/RP(+)–OC(+): coverage of right or left anterior region; (5) Pos/RP(+)–OC(+): coverage of posterior region (premolar to molar) only; (6) R,L Pos/RP(+)–OC(+): coverage of right or left posterior region; and (7) RP(+)–OC(−): coverage of retromolar pads only without occlusal contact: OC(−). The connection between right and left retromolar pads was checked to ensure that there was no contact with teeth or mucous membranes.
Figure 1.
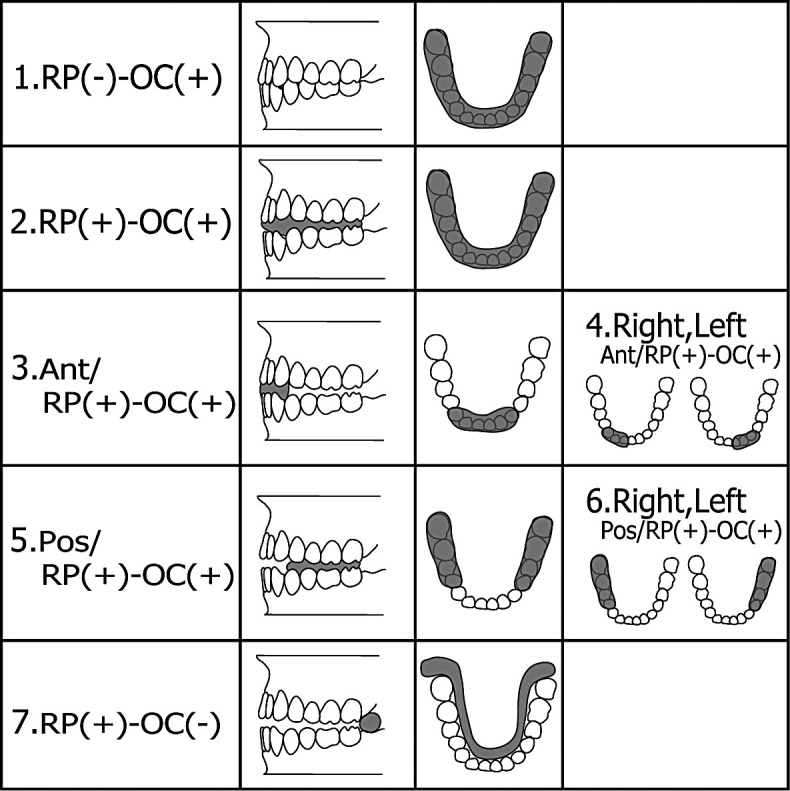
Silicon biteplate configurations. (1) RP(−)–OC(+): bite not raised, coverage of the entire occlusal surface; (2) RP(+)–OC(+): occlusal dimension greater than the centric position within 2 mm extraocclusal height; the same mandibular position as those following in 3–7, with coverage of the entire occlusal surface; (3) Ant/RP(+)–OC(+): anterior biteplates; (4) R,L Ant/RP(+)–OC(+): right or left anterior biteplates; (5) Pos/RP(+)–OC(+): posterior biteplates; (6) R,L Pos/RP(+)–OC(+): right or left posterior biteplates; and (7) RP(+)–OC(−): no occlusal contact.
The silicon biteplates were trimmed on the buccal and lingual sides where necessary to relieve discomfort following insertion.
Conditions for Biting Force
Before measurements were taken, subjects were asked to use maximum biting force in centric occlusion three times for approximately 3 seconds each time. The maximum root mean square (RMS value) of the masseter, as measured by a surface electromyogram, was taken to be the maximum voluntary contraction (MVC). The index of biting force was taken as 50% of the RMS value of the masseter during maximum biting, as measured during electromyography (50% MVC). Subjects were asked to monitor the RMS value of the masseter muscles on the screen, so that they could practice obtaining 50% MVC by visual feedback.
Measurement and Analysis of Gravity Fluctuation
A gravity fluctuation analyzing system (Kyowa, Tokyo, Japan) was used in this study. As a sensor, a gravicorder (ECG-1010DSA1B; Kyowa) was used. Through a sensor interface board (PCD-100A; Kyowa), data were sent to a personal computer (EpsonPC486; Seiko Epson, Nagano, Japan) for recording and analysis.
Measurement was performed in accordance with the equilibrium test21 developed by Equilibrium Research (Bristol, UK), where possible. Therefore, we used a quiet testing room with a uniform amount of light, without disruption by additional light and noise. Subjects were asked to take a natural standing position, holding both arms close to the sides of the body, with the feet in a closed position on the foot position guiding board on the sensor. In addition, subjects were asked to gaze at a target (about 1 cm in diameter) placed at eye level approximately 1 m in front of them. Measurement was started 20 seconds after eye closure. Data sampling was recorded at 20 Hz of frequency for 60 seconds. Measurements for each biteplate were taken once a day for 3 days, and the mean scores were used for analysis.
The analysis items are set out below (Figure 2):
Figure 2.
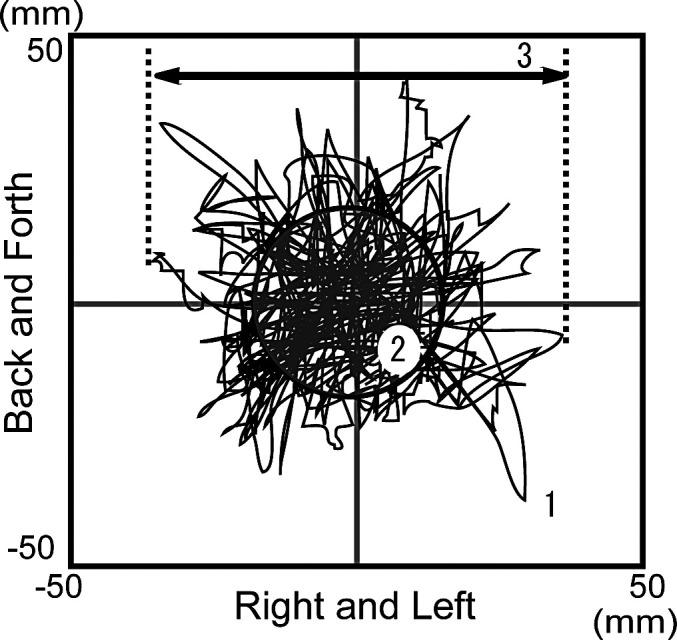
Measurement of gravity fluctuation. (1) Total length of trace; (2) RMS value; (3) maximum right and left sway amplitude.
Total length of trace (mm): Distance moved by the center of gravity on the X-Y coordinate during the measurement time, reflecting the velocity of gravity fluctuation.
RMS value area (mm2): Area of a circle with the standard deviation of the X-Y directions as a radius; an area obtained by subtracting the trace deviates from the circle with the standard deviation as a radius from the outer area.
Maximum right and left sway amplitude (mm): Distance between the maximum and minimum values in the X direction, reflecting left and right gravity fluctuation.
The items for comparison are set out below:
The effect of bite raising: RP(−)–OC(+) and RP(+)–OC(+)
The presence of occlusal contact: RP(+)–OC(−) and RP(+)–OC(+)
The presence of anteroposterior occlusal contact: RP(+)–OC(+), Ant/RP(+)–OC(+), and Pos/RP(+)–OC(+)
The presence of right-left occlusal contact: Ant/RP(+)–OC(+) and R,L Ant/RP(+)–OC(+), Pos/RP(+)–OC(+) and R,L Pos/RP(+)–OC(+)
For statistical analysis, a Wilcoxon matched-pairs signed-rank test was performed on the parameters of the gravity fluctuations obtained by wearing each silicon biteplate. All data were expressed as mean±standard error of the mean (* P < .05; ** P < .01).
RESULTS
I. The Effect of Raising the Bite on Gravity Fluctuation
No significant difference was noted among the RP(−)–OC(+) and RP(+)–OC(+) groups for each analysis item (Figure 3a,b). This finding suggests that bite raising had no effect on gravity fluctuation.
Figure 3.
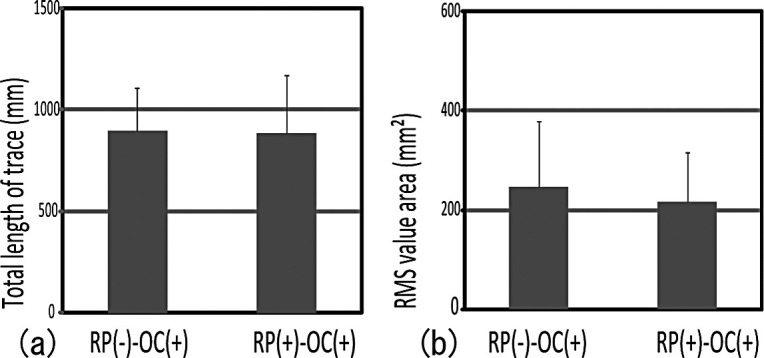
Gravity fluctuations both with and without bite raising had no effect on the masseter muscle spindle. No significant difference was noted between RP(−)–OC(+) and RP(+)–OC(+).
II. The Presence of Occlusal Contact and Gravity Fluctuation
Significant differences were observed between RP(+)–OC(−) and RP(+)–OC(+) in the total length of trace and RMS value area (Figure 4a,b). RP(+)–OC(+) showed significantly lower gravity fluctuation. These results suggest that occlusal contact made gravity fluctuation smaller.
Figure 4.
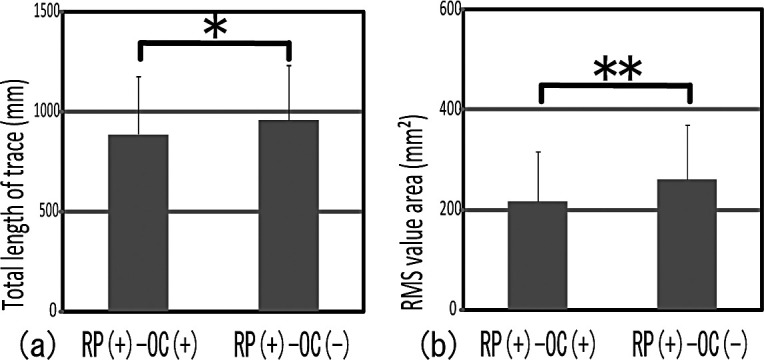
Gravity fluctuations both with and without occlusal contact had an effect on the periodontal membrane. RP(+)–OC(+) showed significantly lower gravity fluctuation than did RP(+)–OC(−). * P < .05; ** P < .01.
III. The Presence of Anteroposterior Occlusal Contact and Gravity Fluctuation
No difference was seen between RP(+)–OC(+) and Pos/RP(+)–OC(+) for each analysis item (Figure 5a,b). However, significant differences were observed in RMS value area not only between RP(+)–OC(+) and Ant/RP(+)–OC(+), but also between Pos/RP(+)–OC(+) and Ant/RP(+)–OC(+) (Figure 5a,b). RP(+)–OC(+) and Pos/RP(+)–OC(+) showed significantly lower gravity fluctuation than Ant/RP(+)–OC(+). These findings suggest that posterior occlusal contact made gravity fluctuation smaller.
Figure 5.
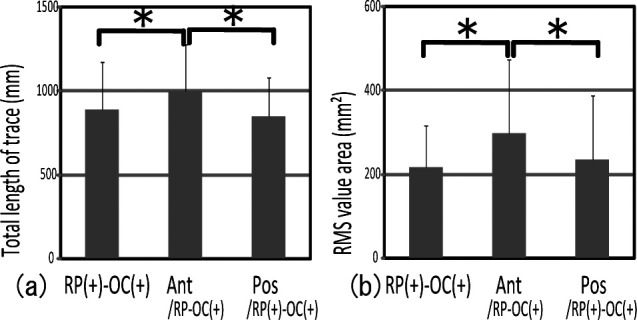
Comparison of gravity fluctuation between biteplates with anterior and posterior occlusal contact showed significantly greater gravity fluctuation in Ant/RP(+)–OC(+) than in RP(+)–OC(+) and Pos/RP(+)–OC(+). * P < .05.
IV. The Presence of Right-Left Occlusal Contact and Gravity Fluctuation
No significant difference was noted between Ant/RP(+)–OC(+) and R,L Ant/RP(+)–OC(+) for each analysis item (Figure 6a,b,c). On the other hand, significant differences between Pos/RP(+)–OC(+) and R,L Pos/RP(+)–OC(+) were noted in the total length of trace, the RMS value area, and the maximum right and left sway amplitude (Figure 7a,b,c). Pos/RP(+)–OC(+) presented significantly lower gravity fluctuation than did R,L Pos/RP(+)–OC(+). These results suggest that unequal posterior occlusion made gravity fluctuation greater.
Figure 6.
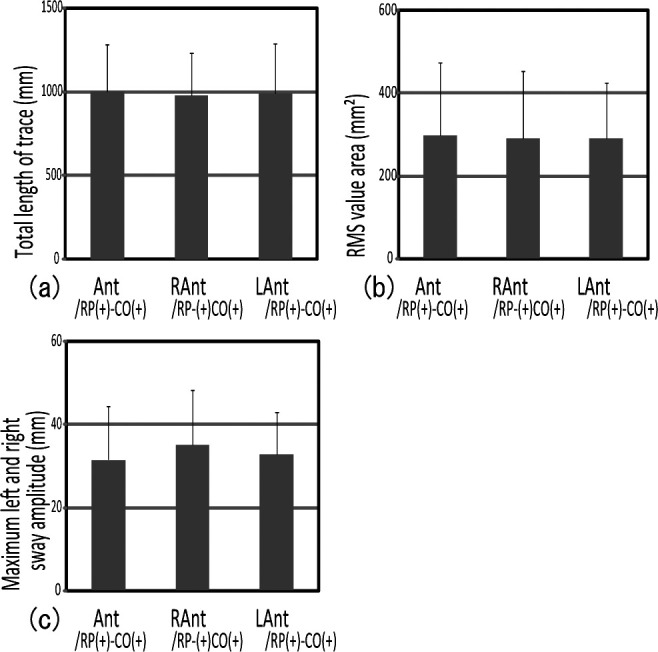
Comparison of gravity fluctuation between biteplates with bilateral and unilateral anterior occlusal contact showed no significant difference between Ant/RP(+)–OC(+) and R,L Ant/RP(+)–OC(+).
Figure 7.
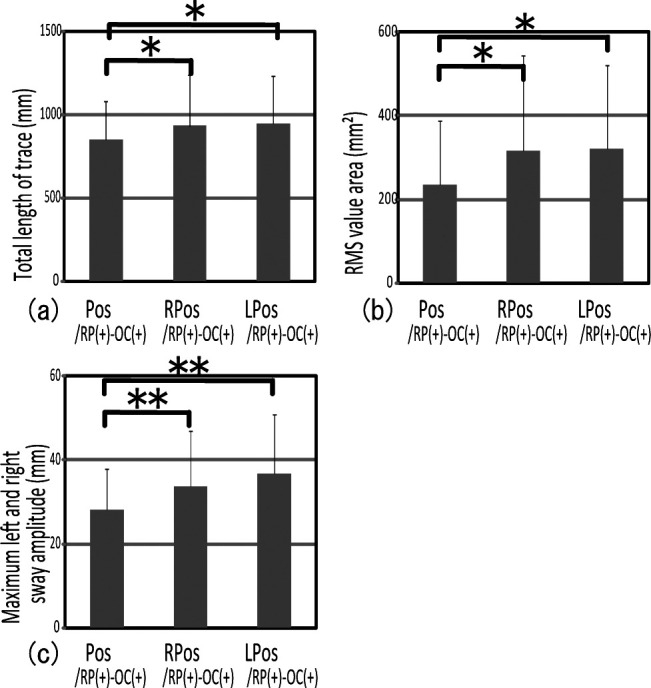
Comparison of gravity fluctuation between biteplates with bilateral and unilateral posterior occlusal contact showed that R,L Pos/RP(+)–OC(+) presented a significantly higher gravity fluctuation than Pos/RP(+)–OC(+). * P < .05; ** P < .01.
DISCUSSION
Subjects and Measurement Conditions
Previous studies have reported that in the normal occlusion of Japanese male individuals, the occlusal contact area is 18.9 ± 0.9 mm2 and the occlusal force is 1001.26 ± 411.00 N.22,23 Subjects in this study were selected on the basis of these values. In addition, a biting force of 50% was considered to exhibit relatively stable gravity fluctuation and therefore was used as an indicator.
Analysis Items
The total length of trace indicates the moving distance of the center of gravity. The greater the value, the faster the center of gravity moves continuously. In this study, significant differences were noted between RP(+)–OC(+) and RP(+)–OC(−) (Figure 4a), between RP(+)–OC(+) and Ant/RP(+)–OC(+), Pos/RP(+)–OC(+) and Ant/RP(+)–OC(+) (Figure 5a), and between Pos/RP(+)–OC(+) and R,L Pos/RP–OC(+) (Figure 7a). In this study, we found that in subjects for whom the occlusion was unequal on the right and left sides, the total length of the trace tended to be greater. These findings confirm that the condition of the posterior occlusion affects the velocity of gravity fluctuation.
The RMS value area that indicates the area of a circle with the standard deviation of the outer area as a radius is effective for evaluating the size of the standard range of the constantly moving gravity center position. In this study, significant differences were seen between RP(+)–OC(+) and RP(+)–OC(−) (Figure 4b), between RP(+)–OC(+) and Ant/RP(+)–OC(+), between Pos/RP(+)–OC(+) and Ant/RP(+)–OC(+) (Figure 5b), and between Pos/RP(+)–OC(+) and R,L Pos/RP(+)–OC(+) (Figure 7b). These results confirm that gravity fluctuation is greater in cases where there is no or unequal posterior occlusal contact, and that posture is more stable where there are posterior equal occlusal contacts.
The maximum right and left sway amplitude is effective for evaluating right and left gravity fluctuation of subjects. In this study, a significant difference was seen between Pos/RP(+)–OC(+) and R,L Pos/RP(+)–OC(+) (Figure 7c). This difference became greater when right and left posterior occlusion was unequal. Therefore, well balanced right and left posterior occlusion seemed to minimize and stabilize the sway of the center of gravity. The maximum anteroposterior sway amplitude (indicating front and back sway) of the center of gravity was measured; however, we observed no significant differences among all of silicon biteplate groups.
Silicon Biteplates
In this study, we used silicone biteplates to ensure stability in RP(−) samples, in which the bite was not raised. In the RP(+) samples, occlusal dimension was increased up to 2 mm for setting the occlusal contact area selectively. No significant difference was observed between RP(−)–OC(+) and RP(+)–OC(+), despite different mandibular positions (Figure 3a,b). We considered no significant differences between the two mandibular positions could influence masticatory muscle spindles, etc.
In addition, RP(+)–OC(−) had no occlusal contact but a coverage of retromolar pads. For the purpose of examining the effects of coverage of the retromolar pads, we fabricated one more silicon biteplate: RP(+)–OC(−)/RP(+)–OC(+), covering the entire occlusal surface and the retromolar pads (Figure 8). We noted no significant differences in gravity fluctuation with and without coverage of the retromolar pads (Figure 9). We suggest that contact in the retromolar pad region has no effect on gravity fluctuation.
Figure 8.
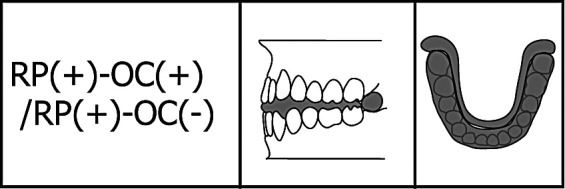
Silicon biteplate RP(+)–OC(+)/RP(+)–OC(−): coverage of entire occlusal surface and retromolar pads.
Figure 9.
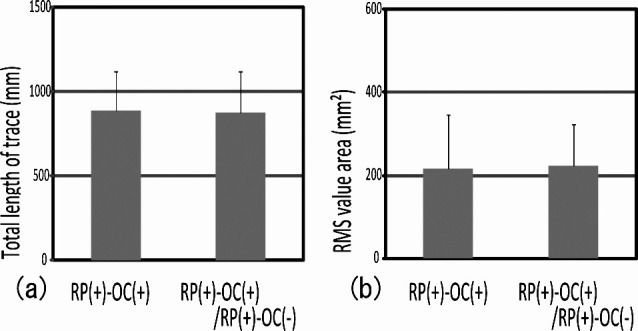
Comparison of gravity fluctuation between biteplates with and without coverage of the retromolar pads showed no significant difference between RP(+)–OC(+) and RP(+)–OC(+)/RP(+)–OC(−).
Occlusal Contact and Gravity Fluctuation
Previous studies reported that gravity fluctuation is controlled by information from the ocular region, the three semicircular canals, and antigravity muscles. In this study, we focused attention on the periodontal mechanoreceptors affecting the antigravity muscles.16 Changes in gravity fluctuation were observed both with occlusal contact and without occlusal contact. Physiologically, mechanical receptors in the periodontal membrane control mandibular movements and various responses.24,25 Some studies have reported that muscle activity in the head and neck region occurred following stimulation of the periodontal membrane.26–29 Our results suggest that the presence of occlusal contact affects gravity fluctuation. These facts suggest that stimulation of the periodontal membrane in occlusal contact affects antigravity muscles, thus playing an important role in posture control.
Molar-Anterior and Right-Left Occlusal Contact and Gravity Fluctuation
Differences were observed in periodontal mechanoreceptor responses in incisor and molar receptors in rabbits.30 It is possible that molar and incisal periodontal mechanoreceptors play different roles. Our results suggest that posterior occlusion has a significant impact on gravity fluctuation.
Previous reports have shown that the effects of stimulation to the periodontal membrane in the posterior region on antigravity muscles occur with physiologic right and left specificity.26,27 Gravity fluctuation increases when disharmony occurs in muscles of mastication.31 Therefore, our results suggest that bilaterally unequal posterior occlusion may cause disharmony in various antigravity muscles that control posture, with a consequent increase in gravity fluctuation.
CONCLUSIONS
Occlusal contact is one of the factors that affect gravity fluctuation; the posterior region in particular plays an important role in posture control.
Bilaterally equivalent occlusal contacts stabilize maximum right and left body sway amplitude.
The results of this study emphasize the importance of appropriate occlusion to the general condition.
REFERENCES
- 1.Shephard R. J. Physiology and Biochemistry of Exercise. New York: Praeger Publishers; 1982. Control mechanisms: neuromuscular system; pp. 245–289. [Google Scholar]
- 2.Mints V. W. Disease of the temporomandibular apparatus. In: Morgan D. H, editor. The Orthopedic Influence. St Louis, Mo: CV Mosby; 1977. pp. 197–201. [Google Scholar]
- 3.Brodie A. G. Anatomy and physiology of head and neck musculature. Am J Orthod. 1950;36:831–844. doi: 10.1016/0002-9416(50)90038-8. [DOI] [PubMed] [Google Scholar]
- 4.Kibana Y, Ishijima Y, Hirai T. Occlusal support and head posture. J Oral Rehabil. 2002;29:58–63. doi: 10.1046/j.1365-2842.2002.00794.x. [DOI] [PubMed] [Google Scholar]
- 5.Miles T. S. Postural control of the human mandible. Arch Oral Biol. 2007;52:347–352. doi: 10.1016/j.archoralbio.2006.12.017. [DOI] [PubMed] [Google Scholar]
- 6.Moulton R. E. Psychiatric consideration in maxillofacial pain. J Am Dent Assoc. 1955;51:408–414. doi: 10.14219/jada.archive.1955.0208. [DOI] [PubMed] [Google Scholar]
- 7.Lagaida M, White G. E. Unilateral mastication and facial formation. J Pedod. 1983;7:127–134. [PubMed] [Google Scholar]
- 8.von Treuenfels H. Dysgnathism, posture problems and deformities of the spine: their significance in functional orthodontics 2. Zahnarzt. 1984;28:591–604. [PubMed] [Google Scholar]
- 9.Kelly H. T, Goodfriend D. J. Vertigo attributable to dental and temporomandibular joint causes. J Prosthet Dent. 1964;14:159–173. [Google Scholar]
- 10.Gelb H, Calderone J. P, Gross S. M, Kantor M. E. The role of the dentist and the otolaryngologist in evaluating temporomandibular joint syndromes. J Prosthet Dent. 1967;18:497–503. doi: 10.1016/0022-3913(67)90173-4. [DOI] [PubMed] [Google Scholar]
- 11.Kibana Y, Ishijima T, Hirai T. Occlusal support and head posture. J Oral Rehabil. 2002;29:58–63. doi: 10.1046/j.1365-2842.2002.00794.x. [DOI] [PubMed] [Google Scholar]
- 12.Forgione A. G, Mehta N. R, McQuade C. F. Strength and bite, Part II, Testing isometric strength using a MORA set to a functional criterion. J Craniomandib Pract. 1992;10:13–20. doi: 10.1080/08869634.1992.11677886. [DOI] [PubMed] [Google Scholar]
- 13.Nakamura Y, Takatori M, Nozaki S, Kikuchi M. Monosynaptic reciprocal control of trigeminal motoneurons from the medical bulbar reticula formation. Brain Res. 1975;89:144–148. doi: 10.1016/0006-8993(75)90142-0. [DOI] [PubMed] [Google Scholar]
- 14.Nakamura Y, Hiraba K, Enomoto S. Bulbar reticular unit activity during food ingestion in the cat. Brain Res. 1982;253:312–316. doi: 10.1016/0006-8993(82)90699-0. [DOI] [PubMed] [Google Scholar]
- 15.Tanaka S, Hirai T, Koshino H, Yokoyama Y, Ishijima T. Influence of teeth clenching on the bodily equilibrium against striking weight impact. Prosthodont Res Pract. 2006;5:143–149. [Google Scholar]
- 16.Yasunaga K. Human muscle activities of the erector spinalis during clenching. J Jpn Dent Soc Oriental Med. 2009;28:9–13. [Google Scholar]
- 17.Yoshida M, Kikutani T, Okada G, Kawamura T, Kimura M, Akagawa Y. The effect of tooth loss on body balance control among community-dwelling elderly persons. Int J Prosthodont. 2009;22:137–139. [PubMed] [Google Scholar]
- 18.Noribumi I, Kunimichi S, Kazuo T. Response properties of periodontal mechanoreceptors in rats, in vitro. Brain Res. 2002;58:357–361. doi: 10.1016/s0361-9230(02)00771-2. [DOI] [PubMed] [Google Scholar]
- 19.Ramfjord S. P, Ash M. M., Jr . Occlusion 2nd ed. Philadelphia/London/Toronto: WB Saunders; 1971. p. 14. [Google Scholar]
- 20.Shohet H. Mouth rehabilitation and bite raising. J Am Dent Assoc. 1946;33:963–976. doi: 10.14219/jada.archive.1946.0126. [DOI] [PubMed] [Google Scholar]
- 21.Tokumasu K, Okubo H, Kato I. Data for standardization of equilibrium test. Equilibrium Res. 1988;47:221–224. [Google Scholar]
- 22.Yokota Y, Ogata Y. Measurement of occlusal force using a dental prescale system—relation to masticatory efficiency. Jpn J Conserv Dent. 2002;45:967–971. [Google Scholar]
- 23.Inoue M, Nagata Y, Yotsui Y, Shimizutani K, Kawamoto T. Relationship between bite force and maxillofacial morphology in normal occlusion. Jpn J Jaw Deform. 2006;16:176–183. [Google Scholar]
- 24.Linden R. W. A. Periodontal mechanoreceptors and their functions. In: Taylor A, editor. Neurophysiology of the Jaw and Teeth. London: Macmillan; 1990. pp. 52–88. [Google Scholar]
- 25.Hannam A. G. The innervation of the periodontal ligament. In: Berkovitz B. K. B, editor. Disease. Oxford: Pergamon Press; 1982. pp. 173–196. [Google Scholar]
- 26.Kirimoto H, Seki Y, Soma K. Differential roles of periodontal mechanoreceptors of working-side posterior teeth in triggering non working-side temporalis activities. J Med Dent Sci. 2003;40:47–52. [PubMed] [Google Scholar]
- 27.Zeredo J. L, Toda K, Soma K. Neck motor unit activities induced by inputs from periodontal mechanoreceptors in rats. J Dent Res. 2002;81:39–42. doi: 10.1177/002203450208100109. [DOI] [PubMed] [Google Scholar]
- 28.Shimazaki K, Matsubara N, Hisano M, Soma K. Functional relationships between the masseter and sternocleidomastoid muscle activities during gum chewing. Angle Orthod. 2005;76:452–458. doi: 10.1043/0003-3219(2006)076[0452:FRBTMA]2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- 29.Kamata S, Fujita Y, Soma K. Reflex response of temporal muscle induced by mechanical stimulation of human canine-comparison of normal overjet and cross bite responses. J Jpn Soc Stomatognath Funct. 1995;1:281–287. [Google Scholar]
- 30.Appenteng K, Lund J. P, Seguin J. J. Intraoral mechanoreceptor activity during jaw movement in the anesthetized rabbit. J Neurophysiol. 1982;48:27–37. doi: 10.1152/jn.1982.48.1.27. [DOI] [PubMed] [Google Scholar]
- 31.Ferrario V. F, Sforza C, Schmitz J. H, Taroni A. Occlusion and center of foot pressure variation: is there a relationship? J Prosthet Dent. 1996;76:302–308. doi: 10.1016/s0022-3913(96)90176-6. [DOI] [PubMed] [Google Scholar]


