Abstract
Background
Free radical accumulation and oxidative stress have been proposed as contributing to the progression of amyotrophic lateral sclerosis (or motor neuron disease). A range of antioxidant medications have been studied. This is an updated review.
Objectives
To examine the effects of antioxidant medication in the treatment of people with amyotrophic lateral sclerosis.
Search methods
We searched the Cochrane Neuromuscular Disease Group Specialized Register (11 May 2010), the Cochrane Central Register of Controlled Trials (CENTRAL) (The Cochrane Library 2010, Issue 2), MEDLINE (January 1966 to April 2010), EMBASE (January 1980 to May 2010).
Selection criteria
All randomized or quasi‐randomized controlled trials of antioxidant treatment for amyotrophic lateral sclerosis.
Data collection and analysis
The authors independently applied the selection criteria and assessed study quality; two authors performed independent data extraction.
Main results
The search identified 25 studies for consideration but only 10 studies met the inclusion criteria. These included a total of 1015 participants. Generally the studies were poorly designed and underpowered, with low numbers of participants and short durations. Only two studies used our predetermined primary outcome measure (survival at 12 months treatment). However, sufficient data were available from four studies to allow meta‐analysis. In the individual studies, no significant effect was observed for vitamin E 500 mg twice daily; vitamin E 1 g five times daily; acetylcysteine 50 mg/kg daily by subcutaneous infusion; a combination of L‐methionine 2 g, vitamin E 400 international units, and selenium (Alsemet) 0.03 mg three times daily; or coenzyme Q10 1800 mg/day and 2700 mg/day. No significant effect was observed on the primary outcome measure in a meta‐analysis of all antioxidants combined. No significant differences were demonstrated in any of the secondary outcome measures. The antioxidants were generally well tolerated, without serious side effects.
Authors' conclusions
There is insufficient evidence of efficacy of individual antioxidants, or antioxidants in general, in the treatment of people with amyotrophic lateral sclerosis. One study reported a mild positive effect but this was not supported in our analysis. If future trials of antioxidant medications are performed, careful attention should be given to sample size, outcome measures, and duration of the trials. The high tolerance and safety, and relatively low cost of vitamins C and E, explain the continuing use of these vitamins by physicians and people with amyotrophic lateral sclerosis. While there is no substantial clinical trial evidence to support their clinical use, there is no clear contraindication.
Keywords: Humans, Amyotrophic Lateral Sclerosis, Amyotrophic Lateral Sclerosis/drug therapy, Amyotrophic Lateral Sclerosis/mortality, Antioxidants, Antioxidants/therapeutic use, Randomized Controlled Trials as Topic
Plain language summary
Antioxidants for treating amyotrophic lateral sclerosis
There is no cure for amyotrophic lateral sclerosis, also known as motor neuron disease, which is a progressively disabling and ultimately fatal disease. Antioxidants, including vitamins C, E, selegiline, selenium, methionineacetylcysteine, and coenzyme Q10, have been suggested as possible treatments and some of these are commonly advised by physicians treating people with amyotrophic lateral sclerosis. In this updated review, we identified 10 studies involving a total of 1015 participants. We did not find any well‐designed randomized controlled trial evidence to support the use of these medications. Trials of antioxidants identified in this review were generally of poor methodological quality and lacked statistical power. However, antioxidants are generally well tolerated without serious adverse effects.
Background
Amyotrophic lateral sclerosis (ALS), also known as motor neuron disease (MND), is a progressive neurodegenerative disease that causes loss of power and function of skeletal muscles generally with difficulty walking, using the arms, speaking and swallowing. The course of the disease is variable but is usually relatively rapid, with an average length of time from the first symptoms and signs of the disease to death of around 3.5 years. Death is most frequently due to respiratory failure. For a review see Eisen 1998.
ALS occurs in individuals of all ethnic origins, worldwide. Males are affected slightly more frequently than females. Adults of all ages may be affected, with peak incidence between 50 and 70 years of age. The worldwide prevalence is 0.75 to 9 per 100,000 population, with an annual incidence of 0.3 to 2 per 100,000 population (Eisen 1998; Logroscino 2005; Sejvar 2005). The cause of ALS remains unknown, but about 5% to 10% of patients have a family history of other affected members and dominant and recessive forms of inheritance of ALS are now recognised. Mutations of the copper/zinc superoxide dismutase (SOD1) gene have been found in both familial and rarely in apparently sporadic forms (Rosen 1993). These amount to around 2% of all patients with ALS.
Until recently, treatment options have only been symptomatic and supportive. Recent studies have suggested a mild beneficial effect of riluzole in slowing progression of the disease and increasing survival (Lacomblez 1996) and are the subject of a separate Cochrane review (Miller 2007). Many other agents have been tried in ALS but generally without clear benefit.
One of the proposed mechanisms of neuronal death in ALS is free radical accumulation resulting from oxidative stress (Bergeron 1996). Free radicals are produced in normal and abnormal biological cellular metabolic processes that involve molecular oxygen. 'Oxidative stress' refers to an increase in the generation of oxygen derived species (superoxide anion, hydroxyl radical, hydrogen peroxide) beyond the ability of normal antioxidative defences to 'neutralize' them. An excess of these free radicals may lead to oxidative damage to lipids, proteins and nucleic acids, causing cell death. Free radicals are normally 'neutralized' by antioxidant enzymes (superoxide dismutase, catalase and glutathione peroxidase) and nutrient derived antioxidants such as vitamins C and E, carotenes, flavonoids, glutathione, uric acid and taurine (Sardesi 1995). Nitric oxide may interact with superoxide anion to form highly reactive peroxynitrites (Beckman 1993). Increased iron, selenium and glutathione peroxidase activity (Ince 1994) and other heavy metals and trace elements including manganese (Mitchell 1987) have been identified in the spinal cord of ALS patients. Increased oxidative stress may have a primary role in causing ALS. This increase might result from altered mitochondrial energy metabolism related to age, environmental factors that lead to increased free radical production, or glutamate mediated excitotoxicity.
Many natural biological agents have antioxidant effects. Some of these are readily available, such as vitamins C and E, and are commonly advised by physicians for patients with ALS. Patients may take individual antioxidant agents or proprietary combinations. Whilst the cost of individual agents may be low, more complex proprietary combinations can have a significant cost. It is important to determine the evidence for benefit, and possible harm, from these agents.
More commonly considered antioxidant medications in ALS include the following.
Vitamin C (ascorbic acid)
Vitamin C is a naturally occurring antioxidant. It is usually taken orally.
Vitamin E (α‐tocopherol)
Vitamin E is a naturally occurring antioxidant. It is taken orally but very little vitamin E appears to cross the blood brain barrier after oral administration (Halliwell 2001). Studies of vitamin E in the SOD1 (copper/zinc superoxide dismutase gene) mutant transgenic mouse model of ALS have suggested efficacy in delaying disease onset (Gurney 1996).
L‐deprenyl (selegiline)
Selegiline is a selective monoamine oxidase B inhibitor. Selegiline has been reported to increase SOD activity in the basal ganglia of rats, and possibly has additional antioxidant properties (Knoll 1989). It has been used in Parkinson's disease for presumed neuroprotective and antioxidant properties. Its clinical efficacy in that condition has been questioned and is currently under review. Some trials in patients with ALS have already been undertaken (Janik 1996; Jossan 1994; Lange 1998; Mazzini 1994; Mitchell 1995).
N‐acetylcysteine
N‐acetylcysteine is a precursor of glutathione, a natural intracellular antioxidant. One trial has already been undertaken (Louwerse 1995).
Dehydroepiandrosterone
Dehydroepiandrosterone is a steroid synthesized in brain glial cells. This agent has been investigated in patients with ALS and is proposed to have antioxidant properties (Eisen 1998).
Combination antioxidant therapy
Experience has been reported with an 'array' of antioxidants in patients with ALS; using N‐acetylcysteine, vitamin C, vitamin E, N‐acetylmethionine and dithiothreitol or its isomer dithioerythritol (Vyth 1996).
AEOL‐10150
AEOL‐10150 is a small molecule antioxidant analogous to the catalytic site of superoxide dismutase. This has been investigated for ALS in a phase I clinical trial (Orrell 2006).
Edavarone
Edavarone (3‐methyl‐1‐phenyl‐pyrazolin‐5‐one) is a newly developed free radical scavenging agent, which has been investigated in a range of conditions including stroke (Yoshida 2006). This has been studied in ALS (Zhang 2007).
Coenzyme Q10
Coenzyme Q10 (CoQ10, or ubiquinone) is an oil‐soluble, vitamin‐like substance present primarily in mitochondria. It is a component of the electron transport chain and a free radical scavenger. Coenyme Q10 has been suggested as a potential treatment for a wide range of conditions, because of its antioxidant effect. A phase II trial in ALS was reported (QALS Study Group 2009).
This is an update to the original review.
Objectives
We set out to examine the efficacy of antioxidant medication in the treatment of ALS. We undertook a broad approach by studying all agents with a generally accepted antioxidant mechanism, singly or in combination; and a narrower approach by studying the effects of the individual agents.
Methods
Criteria for considering studies for this review
Types of studies
We included all randomized or quasi‐randomized (for example alternate allocation) controlled trials of antioxidant treatment for ALS.
Types of participants
We included clinical trials of patients with ALS at all stages and in all subtypes. This included all sites of onset, other clinical features, and included familial and nonfamilial disease patients. Amyotrophic lateral sclerosis was defined in terms generally agreed as compatible with the clinical diagnosis, including features of upper and lower motor neuron involvement, absence of sensory signs not otherwise explicable, and a progressive course. Diagnostic criteria formulated by the World Federation of Neurology Sub‐Committee on Neuromuscular Diseases, at El Escorial (Brooks 1994) have been proposed for use in clinical trials, and were used where available. However, these criteria post date some of the trials considered and they have been further refined since their first publication.
Types of interventions
We included any form of treatment considered to have an antioxidant effect. This includes vitamin C, vitamin E, selegiline, N‐acetylcysteine, dehydroepiandrosterone, N‐acetylmethionine, dithiothreitol and coenzyme Q10.
Types of outcome measures
A summary of findings table (SOF) will be added to the next update. Outcomes to address include survival, muscle strength, respiratory function, quality of life and adverse events.
Primary outcomes
Antioxidants were analyzed initially as a group, with additional subgroup analysis of individual antioxidants. The primary outcome measure was survival (free of tracheostomy or ventilatory support) after 12 months treatment.
Secondary outcomes
Secondary measures included:
survival as a function of time (3, 6, 9, 15 and 18 months);
duration of disease as measured by time from randomization to death or mechanical ventilation with a tracheostomy;
quantitative muscle testing;
functional rating scales;
subjective rating scales;
quality of life assessments of patients and caregivers;
adverse effects ‐ severe (leading to cessation of medication), and mild (medication continued).
Search methods for identification of studies
Electronic searches
We searched the Cochrane Neuromuscular Disease Group Specialized Register (11 May 2010) using 'amyotrophic lateral sclerosis' and its synonyms 'motor neuron disease' and 'motor neuron disease' for trials of the following agents, using the search terms 'vitamin C', 'ascorbic acid', 'vitamin E', 'alpha‐tocopherol', 'selegiline', 'deprenyl', 'n‐acetyl cysteine', 'n‐acetyl‐l‐cysteine', 'n‐acetylcysteine', 'acetylcysteine', 'superoxide dismutase', 'SOD', 'dehydroepiandrosterone', the generic term 'antioxidant','AEOL‐10150', 'coenzyme Q10', 'coQ10', and 'ubiquinone'. We adapted this strategy to search the Cochrane Central Register of Controlled Trials (CENTRAL) (Issue 2, 2010 in The Cochrane Library), MEDLINE (January 1966 to April 2010) and EMBASE (January 1980 to May 2010).
For the MEDLINE, EMBASE and CENTRAL search strategies, see Appendix 1, Appendix 2 and Appendix 3.
Searching other resources
We checked the bibliographies in reports of the randomized trials and contacted their authors and other experts in the field to identify additional published or unpublished data. Manufacturers of antioxidant medication were contacted for details of any randomized trials performed.
Data collection and analysis
Three authors checked the titles and abstracts identified. We obtained the full text of all potentially relevant studies for independent assessment by all authors. The authors decided which trials fitted the inclusion criteria and assessed risk of bias. We resolved disagreements about inclusion criteria by discussion.
We assessed risk of bias using The Cochrane Collaboration 'Risk of bias' tool. The domains were: adequate sequence generation, allocation concealment, blinding (all outcomes), incomplete outcome data addressed, free of selective reporting, and free of other bias. These were scored as yes, no, or unclear, using Cochrane defined criteria (Higgins 2008).
Two authors performed data extraction independently, and all authors checked data extraction. We obtained missing data from the trial authors whenever possible. We tested for heterogeneity in the results where possible. Sensitivity analysis was performed if there was significant heterogeneity in the outcomes. Antioxidants as a group were assessed, with additional subgroup analysis of individual antioxidant agents.
We calculated relative risks for binary outcomes (such as survival) and a difference in weighted means for continuous outcomes (for example muscle strength) to determine treatment effect across trials (using a fixed‐effect model) with the Cochrane statistical package Review Manager (RevMan), where possible. Results were to be expressed as relative risks with 95% confidence intervals and risk differences with 95% confidence intervals for dichotomous outcomes and weighted mean difference and 95% confidence intervals for continuous outcomes. We analyzed all the primary and secondary outcomes under consideration whenever the data allowed.
Results
Description of studies
A search of the Cochrane Neuromuscular Disease Group (NMD) Specialized Register, MEDLINE, EMBASE and other sources as described revealed 25 possible randomized controlled trials. The number of references returned from the databases was: MEDLINE 212, EMBASE 27, CENTRAL 23, NMD register 4. Two studies in Polish were translated into English (Kwiecinski 2001; Szczudlik 1998), as was one study in Chinese (Zhang 2007).
Excluded studies (see 'Characteristics of excluded studies')
One study (Vyth 1996) was excluded as it used historical controls and was not randomized.
We excluded four study reports (Engel 1969; Janik 1996; Mitchell 1993; Stevic 1998) as they were abstracts of subsequently published studies (Dorman 1969; Kwiecinski 2001; Mitchell 1995; Stevic 2001). Mitchell 1994 described the same study as Mitchell 1995, and Professor Mitchell also provided further unpublished details of this study.
We excluded eight studies (Chio 1998; Dorman 1969; Kwiecinski 2001; Mazzini 1994; Quick 1969; Szczudlik 1998; Wechsler 1940; Zhang 2007) as there was inadequate concealment of allocation of treatment. Chio 1998 was an open unblinded cross‐over, sequentially randomized study. Dorman 1969 was an unblinded nonrandomized study without controls. Quick 1969 was an open nonrandomized study. Kwiecinski 2001 was an open randomized study. Mazzini 1994 randomized the patients sequentially and was open and unblinded. Szczudlik 1998 was an open study. Wechsler 1940 was an observational nonrandomized, uncontrolled unblinded study. Ascherio 2005 was a prospective study of prevention of occurrence of disease. Zhang 2007 was an open randomized study, not double blinded.
There remained ten trials that fulfilled our selection criteria for randomized controlled trials.
Included studies (see 'Characteristics of included studies')
A total of 1015 patients were studied in these 10 trials. Two of the studies were cross‐over studies (Jossan 1994; Mitchell 1995), comprising 66 patients. The remaining seven noncross‐over studies included a total of 383 treated patients and 367 placebo patients. One hundred and ten patients and 75 controls were involved in an adaptive, two stage, phase II design study (QALS Study Group 2009). An additional 14 patients were treated with amino acids or nimodipine (Apostolski 1998). The potential antioxidants vitamin C, vitamin E (a‐tocopherol), coenzyme Q, selenium, beta‐carotene, N‐acetylcysteine, selegiline and coenzyme Q10 were studied. Methionine was also included in combination as it may "enhance methylation of normal or abnormally hypomethylated DNA in motor neurons or possibly in other cells that influence motor neurons by deficiency or toxic mechanism" (Stevic 2001).
Apostolski 1998 This was described as a double‐blind study of Alsamin, which is a combination of vitamin E (135 international units (IU)), selenium (3 x 10‐5 g) organically bound in baker's yeast, β‐carotene (4500 IU), amino acids: L‐arginine (0.15 g), L‐methionine (2.0 g), L‐leucine (4.0 g), L‐isoleucine (3.0 g), and L‐valine (2.0 g), and the calcium channel blocker nimodipine (20 mg), taken three times daily. The study was conducted at a single site in Yugoslavia. Thirty‐five participants were studied, 60% were male and the mean age was 54 years. Patients were divided into five groups of seven. Groups took either placebo; Alsamin; a combination of selenium, vitamin E and β‐carotene; a combination of amino acids; or nimodipine 20 mg three times daily.
Diagnosis of ALS was by the World Federation of Neurology criteria.
The primary endpoint was evaluation of several antioxidative enzymes: copper zinc superoxide dismutase, glutathione peroxidase, catalase, glutathione reductase in erythrocytes and plasma glutathione transferase.
The secondary endpoint was a clinical assessment using the Norris score, made at the beginning and after nine weeks of treatment.
The study duration was 63 days.
Desnuelle 2001 This was a randomized, placebo controlled double‐blind study, of alpha‐tocopherol (vitamin E) 500 mg twice daily. The preparation Toco 500 was used. In addition all patients were taking riluzole, which has demonstrated a mild effect in prolonging survival in ALS. The study was conducted at 28 sites in France. Two hundred and eighty‐eight participants were studied, 55% were male and the mean age was 64 years.
Diagnosis of ALS was probable or definite ALS by the El Escorial criteria. To be included, all patients had to be treated with riluzole for at least three months without side effects. Exclusion criteria included dementia or other major psychiatric disorders, other serious disease or handicap, forced vital capacity less than 60%, monoclonal gammopathy, conduction blocks, abnormal liver function tests, taking vitamin E or hepatotoxic drugs.
The primary endpoint was the change in functional status of each patient using the modified Norris limb scale.
Secondary endpoints included survival, bulbar function assessed with the Norris bulbar scale, and manual muscle testing. Fatigue, limb stiffness, cramps and fasciculations were assessed using visual analogue scales. Respiratory function, quality of life using the Sickness Impact Profile (SIP) and the ALS Health State scale were measured. Biological markers of oxidative stress (plasma thiobarbituric acid, erythrocyte superoxide dismutase, and erythrocyte glutathione peroxidase) were studied in a subgroup of 122 patients.
The study duration was 12 months.
Ellis 1997 A randomized, placebo controlled, double‐blind study, of a cocktail of six antioxidants (coenzyme Q (240 mg), vitamin E (1600 IU), vitamin C (2000 mg), selenium (100 mg), beta‐carotene (10 mg) and N‐acetylcysteine (200 mg)).
The study was performed at a single centre in the USA. Ten patients were included. The study was presented in abstract form only, and age of participants, inclusion and exclusion criteria were not given. The trial included an initial six month observation period followed by randomization and six months treatment.
The primary endpoint was rate of change in the Appel Rating Scale score.
The secondary endpoints included safety and blood markers of oxidative damage.
Graf 2005 This was described as a double‐blind study of vitamin E; alpha‐tocopherol (Schwarzhaupt, Cologne, Germany) 1 g was given five times spread though the day. The study was performed at six sites in Germany. One hundred and sixty participants, 65% male, were studied. The mean age in the vitamin E group was 59 years and in the placebo group it was 57 years. Ninety‐eight per cent of patients were taking riluzole. Twenty‐three per cent were taking vitamin E before the onset of the study. Diagnosis was probable or definite ALS using the El Escorial criteria. Inclusion criteria were: less than five years disease duration, and treatment with riluzole. Exclusion criteria were not stated. The primary endpoint was survival, as measured by time to death, tracheostomy or permanent assisted ventilation during the period of the study. Secondary endpoints were rate of deterioration of function assessed by the modified Norris limb and bulbar scales, manual muscle testing (BMRC), spasticity scale, ventilatory function, and the Sickness Impact Profile (SIP ALS/19). Vitamin E levels were measured for a compliance check.
Jossan 1994 A randomized, cross‐over, double‐blind study of selegiline 10 mg daily.
The study was performed at a single centre in Sweden. Ten patients were included, with a mean age of 50 years. Patients had World Federation of Neurology (Brooks 1994) diagnosis of ALS. Inclusion and exclusion criteria were not stated.
The patients took the drug for 12 weeks, followed by 12 weeks washout and 12 weeks placebo.
The primary endpoint was not stated. Other endpoints were Norris score, bulbar and spinal scores. Monoamine oxidase B was estimated in platelets.
Lange 1998 A randomized, placebo controlled, double‐blind study of selegiline 5 mg twice daily.
The study was performed at two sites in the USA. Criteria for classical ALS were used, although El Escorial or World Federation of Neurology criteria were not stated. One hundred and thirty‐three patients were included, with a mean age of 57 years.
Inclusion criteria were: patients aged 25 to 65 years, symptom duration less than three years, mild to moderate disease with Appel ALS total score 30 to 80, and no drug therapy for at least three months before enrolment.
Exclusion criteria were: multifocal motor neuropathy with conduction block, paraproteinemia, elevated GM1 antibodies, sensorimotor peripheral neuropathy, previous infection with poliovirus, lower motor neuron disease only, primary lateral sclerosis, previous allergy to selegiline, abnormal endocrinology, serious medical problems, and poor family support.
Primary endpoint was rate of change of Appel ALS (AALS) total score.
Secondary endpoints were AALS component scores and survival analysis.
The study duration was six months. Participants with a AALS score below 115 or forced vital capacity below 39% predicted were considered treatment failures and entered an open‐label phase.
Louwerse 1995 A randomized, placebo controlled, double‐blind study, of acetylcysteine 50 mg/kg subcutaneous infusion daily. A similar placebo was used.
The study was performed at a single centre in the Netherlands. One hundred and ten patients were included, with a mean age 58 years. Diagnosis was of probable or definite ALS by the El Escorial criteria.
Inclusion criteria were not stated. Exclusion criteria were: aged younger than 20 years or older than 80 years; first or second degree family member with ALS; signs of dementia or parkinsonism; serious mental illness; life expectancy less than six months due to another disease; previously used drugs such as antioxidants, mucolytic drugs, glutamate antagonists and chelating agents.
Primary endpoint was survival, defined by death from all causes, long‐term assisted ventilation, tracheostomy, and positive‐pressure breathing.
Secondary endpoints were rates of disease progression as expressed by manual muscle strength testing, myometry, forced vital capacity, ability to perform activities of daily living, degree of independence and bulbar function.
The study duration was 12 months.
Mitchell 1995 A cross‐over, placebo controlled, double‐blind study of selegiline 10 mg daily.
The study was conducted at a single centre in England. Fifty‐six patients were included. The age was not stated. This study was published as a letter; with additional details in Mitchell 1993, Mitchell 1994, and unpublished data as provided by Professor Mitchell. Diagnosis was of motor neuron disease (MND), which is an alternative term for ALS, made by two consultant neurologists. No more formal criteria were used. It was stated that at the time of the trial these had not been formulated.
The primary outcome was not stated. Other measures included hand held myometry, hand grip strength, Barthel Disability Index, Rankin Disability Scale, Bulbar Rating Scale, quality of life scale. Whole blood and serum glutathione peroxidase, erythrocyte superoxide dismutase (SOD), serum caeruloplasmin, leucocyte and plasma ascorbic acid, and serum tocopherol were measured.
The study had a cross‐over design, both groups taking placebo for four weeks, 16 weeks on medication or placebo, four weeks placebo, 16 weeks on the alternative medication or placebo, and four weeks placebo.
An adaptive, two‐stage, bias‐adjusted, randomized, placebo controlled, double‐blind, phase II study of coenzyme Q10. Coenzyme Q10 at 1800 mg/day and 2700 mg/day
The study was conducted at 19 centres in the USA. Diagnosis was of definite, probable, or laboratory supported probable ALS, sporadic or familial by the El Escorial guidelines.
The primary outcome was decline in the ALS Functional Rating Scale‐revised (ALSFRSr) score from baseline to nine months. Secondary outcome measures were decline in forced vital capacity, fatigue severity (9‐item fatigue severity scale) and quality of life (SF‐36 with physical and mental components analyzed separately). Oxidative stress was assessed in plasma by 8‐hydroxy‐2 deoxyguanosine measurement.
The design was innovative, aiming to minimize sample size and length of follow‐up. In Stage 1, 35 patients were allocated to each of placebo, CoQ10 1800 mg/day and CoQ10 2700 mg/day, for nine months. The dose of 2700 mg/day had higher efficacy, and was chosen for Stage 2. In Stage 2, 40 patients were added to each of the placebo and CoQ10 2700 mg/day groups, and studied for a further nine months.
Stevic 2001 A double‐blind, placebo controlled trial of a combination of L‐methionine (2 g), vitamin E (400 IU) and selenium (3 x 10‐5g) (Alsemet), or placebo, taken orally three times daily.
The study was performed in Yugoslavia. Sixteen patients received medication, and 12 placebo. Randomization was not stated, but implied. The mean age of participants was 57 years. Inclusion criteria were probable or definite ALS by El Escorial criteria, age 20 to 70 years, disease duration of less than 3 years, and ambulatory. Exclusion criteria were significant compromise of bulbar or respiratory function, conduction block, M protein, significant imaging abnormality, dementia, and concurrent systemic disease.
The primary endpoints were survival and rate of disease progression as expressed by decline in limb‐function, bulbar‐function and muscle testing scores.
The secondary endpoints were the activity of antioxidative components (glutathione peroxidase, glutathione reductase, and catalase) and the level of vitamin E, in blood.
The study was initially presented in abstract form (Stevic 1998) with some difference in results. In Stevic 1998, at 12 months 6/12 patients in the placebo group were alive and 17/20 in the Alsemet group. In Stevic 2001, at 12 months 6/12 in the placebo group were alive but 13/16 were alive in the Alsemet group.
Risk of bias in included studies
See Characteristics of included studies table and Figure 1.
1.
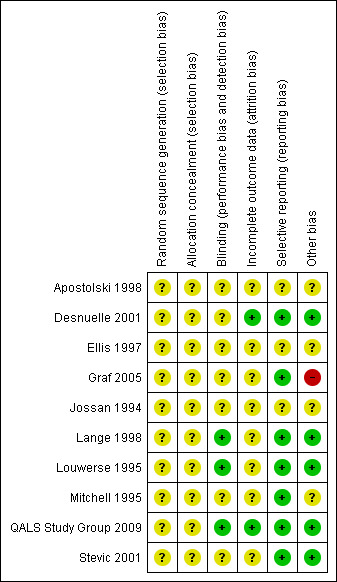
Risk of bias summary: review authors' judgements about each risk of bias item for each included study.
Adequate sequence generation and allocation concealment
Sequence generation and allocation concealment were graded unclear in all studies. Explicit details of randomization were not given. In the studies of Mitchell 1995 and Stevic 1998, randomization was not stated but was implied by being double‐blind, placebo controlled studies. Professor Mitchell has confirmed that randomization was undertaken within the pharmacy and was completely independent of the researchers.
Blinding (all outcomes)
Blinding was graded adequate in three studies (Lange 1998; Louwerse 1995; QALS Study Group 2009), and unclear in eight studies (Apostolski 1998; Desnuelle 2001; Ellis 1997; Graf 2005; Jossan 1994; Mitchell 1995; Stevic 2001). Generally the details of blinding were not clear, although the studies were stated to be blinded.
Incomplete outcome data addressed
Incomplete outcome data were adequately addressed in two studies (Desnuelle 2001; QALS Study Group 2009), but unclear in seven studies (Apostolski 1998; Ellis 1997; Graf 2005; Jossan 1994; Lange 1998; Louwerse 1995; Mitchell 1995; Stevic 2001).
Free of selective reporting
Seven studies were graded to be adequately free of selective reporting (Desnuelle 2001; Graf 2005; Lange 1998; Louwerse 1995; Mitchell 1995; QALS Study Group 2009; Stevic 2001), but unclear in three studies (Apostolski 1998; Ellis 1997; Jossan 1994).
Free of other bias
Five studies were graded to be adequately free of other bias (Desnuelle 2001; Lange 1998; Louwerse 1995; QALS Study Group 2009; Stevic 2001), and four were unclear (Apostolski 1998; Ellis 1997; Jossan 1994; Mitchell 1995). One study was graded as not being free of other bias (Graf 2005) as seven patients in the placebo group were taking open vitamin E and 23% of patients were taking vitamin E at the onset of the study.
Effects of interventions
There were generally insufficient data for subgroup analysis of individual antioxidants.
Primary outcome measures
Only two studies used the primary outcome measure we had proposed in our protocol as their primary measure (Louwerse 1995; Stevic 2001). One study used this measure as a secondary outcome (Desnuelle 2001) and in another the data were given and analyzed in a Kaplan‐Meier curve (Graf 2005).
Louwerse 1995 reported that at 12 months, 35 participants (65%) treated with acetylcysteine and 30 participants (54%) given placebo were still alive (hazard ratio (HR) 0.74 in the acetylcysteine group relative to the placebo group; 95% confidence interval (CI) 0.41 to 1.33; log‐rank test, P = 0.31). The difference in 12 month survival was 11% in favor of the treated patients (95% CI ‐30% to 7%). The reported mortality risk reduction was 26% relative to placebo. The effect remained after adjustment for baseline differences in prognostic factors by multivariate analysis, with an adjusted HR of 0.71 (95% CI 0.38 to 1.32). In a subgroup analysis confined to those with limb onset (81 patients), 74% in the treated and 51% in the placebo group survived 12 months (HR 0.50; 95% CI 0.24 to 1.04; P = 0.06); and in bulbar onset (29 patients) 44% in the treated and 62% in the placebo group survived 12 months (HR 1.66; 95% CI 0.56 to 4.99; P = 0.36). In our analysis of survival at 12 months, relative risk was 1.13 (95% CI 0.82 to 1.54) (Analysis 1.1), and risk difference was 0.07 (95% CI ‐0.11 to 0.25) (Analysis 1.2).
1.1. Analysis.

Comparison 1 All antioxidants versus placebo, Outcome 1 Survival at 12 months (relative risk).
1.2. Analysis.

Comparison 1 All antioxidants versus placebo, Outcome 2 Survival at 12 months (risk difference).
Stevic 2001 reported that at 12 months, 6/12 (50%) patients in the control group were still alive compared to 13/16 (81%) in the treatment group. Survival was analyzed using the Mantel test. A significant difference in survival (P < 0.05) was reported. In our analysis of survival at 12 months, we found no significant difference. Relative risk was 1.63 (95% CI 0.88 to 3.00) (Analysis 1.1), and risk difference was 0.31 (95% CI ‐0.03 to 0.65) (Analysis 1.2).
Desnuelle 2001 used survival as a secondary outcome measure. The log‐rank test revealed no statistically significant difference (P = 0.89) in survival at 12 months. In our analysis of survival at 12 months, the relative risk was 1.01 (95% CI 0.89 to 1.15) (Analysis 1.1), and risk difference was 0.01 (95% CI ‐0.09 to 0.11) (Analysis 1.2).
Graf 2005 used time to death as the primary endpoint, but gave data for survival at 360 days (12 months equivalent): 64/83 (77%) survived in the vitamin E group and 53/77 (69%) survived in the placebo group. (The groups appear to be incorrectly labelled in the paper, Figure 1.) Additional data were available for survival at 90, 180, 270, 450 and 540 days (Analysis 1.4; Analysis 1.5; Analysis 1.6; Analysis 1.7; Analysis 1.7; Analysis 1.8; Analysis 1.9). The effect of vitamin E just failed to reach significance at six months treatment, and otherwise was not significant.
1.4. Analysis.

Comparison 1 All antioxidants versus placebo, Outcome 4 Survival at 3 months (relative risk).
1.5. Analysis.

Comparison 1 All antioxidants versus placebo, Outcome 5 Survival at 6 months (relative risk).
1.6. Analysis.

Comparison 1 All antioxidants versus placebo, Outcome 6 Survival at 9 months (relative risk).
1.7. Analysis.

Comparison 1 All antioxidants versus placebo, Outcome 7 Survival at 15 months (relative risk).
1.8. Analysis.

Comparison 1 All antioxidants versus placebo, Outcome 8 Survival at 18 months (relative risk).
1.9. Analysis.

Comparison 1 All antioxidants versus placebo, Outcome 9 Survival at 6 months (risk difference).
Meta‐analysis of survival at 12 months in these three studies showed no significant effect of treatment. The forest plots are shown in the analysis section. The relative risk was 1.08 (95% CI 0.97 to 1.19) (Analysis 1.1). The risk difference was 0.05 (95% CI ‐0.02 to 0.13) (Analysis 1.2). Sensitivity analysis, omitting Stevic 2001, showed a relative risk of 1.06 (95% CI 0.95 to 1.17).
There were insufficient data to perform meta‐analysis of functional scales.
Secondary outcome measures
There were generally insufficient data to pool included studies for secondary outcome measures.
(1) Proportional change in survival as a function of time at 3, 6, 9, 15, 18 months
Graf 2005 gave survival numbers at 90, 180, 270, 450 and 540 days (equivalent to 3, 6, 9, 15 and 18 months). (The data appear to be incorrectly labelled in the original paper.) No significant difference was reported between placebo and treatment group with the stratified log‐rank test or stratified Wilcoxon test. The HR was calculated as 1.145 (95% CI 0.701 to 1.872).
Louwerse 1995 and Desnuelle 2001 published Kaplan‐Meier survival curves for a 12 month period, but with no data on individual time points other than at 12 months, as above. Desnuelle 2001 reported that the log‐rank test revealed no significant difference between treated and placebo groups (P = 0.89).
Lange 1998 reported that 4 of 67 participants died in the treatment group, and 3 of 66 participants died in the placebo group over six months. A 'survival function estimate' was plotted over six months, with patients censored at a progression of 22 points in AALS total score. Using this endpoint, no significant difference was reported between the two groups: Wilcoxon2 = 0.01, P = 0.98.
Stevic 2001 produced a Kaplan‐Meier survival curve over 12 months but with absolute values given only for the 12 month point.
(2) Duration of disease
Graf 2005 used duration of disease as the primary endpoint. Thirty‐two participants from each group reached the endpoint by the end of the study (18 months). In the vitamin E group the duration was 315 + 13 days and in the placebo group it was 278 + 15 days. Statistical analysis was not given. There was stated to be no significant difference using the stratified log‐rank test or the Wilcoxon test.
The other studies were not designed to measure duration of disease, which is probably not an appropriate or realistic measure in initial studies of therapy of ALS.
(3) Quantitative muscle testing (12 and 18 months)
Louwerse 1995 found no significant difference in linear rate of decline in myometric and manually tested muscle strength during 12 months. In a subgroup analysis of bulbar onset patients (16 acetylcysteine, 13 placebo) there was an increased deterioration in muscle strength measured by the MRC score (P < 0.01) and myometry (P < 0.01) in patients receiving acetylcysteine.
Desnuelle 2001 found no significant difference in muscle testing at 12 months.
Graf 2005 states that manual muscle testing was performed, but no data were given.
Mitchell 1995 in a cross‐over study with 16 weeks treatment found no significant difference in hand‐held myometry of a range of muscles; and hand grip. It was commented that selegiline may "hold" some of the indices of disease progression in ALS and that there may also be a "carry over" effect persisting several months after selegiline was stopped.
Stevic 2001 published bar charts indicating that the rate of deterioration of muscle testing scores was significantly slower in treated patients (P < 0.025) at six months, but with no difference at 12 months. Absolute values were not given.
(4) Functional rating scales (12 and 18 months)
Apostolski 1998 reported progression in Norris score in all subgroups, but an improvement in the Alsamin group. No statistical analysis was performed.
Desnuelle 2001 found no significant difference in Norris limb score at 12 months. The ALS Health State scale (AHSS), which has the four states I to IV, was used. States I and II were combined as State A, and States III and IV as State B. In the control group 53 patients (44.5%) progressed from AHSS State A to AHSS State B compared to only 39 patients (32%) in the treated group, over 12 months (P = 0.04). Ellis 1997, in a six months treatment study preceded by six months observation, found that three of five patients in the treatment group had a slower rate of change of Appel Rating Scale (ARS) during treatment versus the observation period; three of five patients in the placebo group had a faster rate of change. However, using a mixed model analysis of variance which considered treatment group, treatment period, and visits interactions there was no statistically significant effect of treatment on ARS.
Graf 2005 stated that "Functional assessments according to the scales showed a trend in favour of vitamin E without however reaching significance". Data for the Norris limb score was given as an example.
Lange 1998 found no difference in the rate of progression as measured by the Appel ALS total score over six months. The monthly rate of change was 3.4 for the treated group, and 3.5 for the control group.
Louwerse 1995 found no significant difference in pulmonary function at 12 months. There was no significant difference in bulbar function scale score. In a subgroup analysis of bulbar onset patients, there was an increased deterioration in bulbar function (P < 0.01) in patients receiving acetylcysteine.
Jossan 1994, in a cross‐over study with 12 weeks on treatment, found no statistically significant difference in Norris, spinal and bulbar scores.
Mitchell 1995, in a cross‐over study with 16 weeks on treatment, found no significant difference in bulbar rating scale.
Stevic 2001 stated that the deterioration of limb and bulbar function scores was significantly lower in treated than control groups at six months (P < 0.05) but not at 12 months. Bar charts of the results were presented but no absolute values were given. The linear rate of decline was not given.
QALS Study Group 2009 found no significant difference in mean decline of ALSFRSr over a nine month period. There was no significant difference in decline of FVC (% of predicted).
(5) Subjective rating scales (12 and 18 months)
Desnuelle 2001 found no significant difference in self assessed scoring of cramps, fatigue, stiffness, or fasciculations at 12 months.
Louwerse 1995 found no significant difference in ability to perform activities of daily living (disability) by the Barthel Index and overall degree of independence (handicap) by the modified Rankin Scale, at 12 months.
Mitchell 1995, in a cross‐over study with 16 weeks on treatment, found no significant difference in the Barthel Disability Index and Rankin Disability Scale.
QALS Study Group 2009 found no significant difference in fatigue severity measured by the nine‐item fatigue severity scale (FSS) over a nine month period.
(6) Quality of life assessments of patients and caregivers (12 and 18 months)
Desnuelle 2001 found no difference in quality of life as measured by the Sickness Impact Profile (SIP) at 12 months. This appeared to relate to patients only, and not caregivers.
Graf 2005 measured the Sickness Impact Profile, but no data were given.
Mitchell 1995, in a cross‐over study with 16 weeks on treatment, found no significant difference in the quality of life scale. This appeared to relate to patients only, and not caregivers.
QALS Study Group 2009 found no significant difference in physical or mental components of quality of life, measured by SF‐36‐PCS and SF‐36‐MCS, over a nine month period.
(7) Adverse effects
Louwerse 1995 reported three patients discontinuing the study with rashes and pain at the injection site. All three were receiving acetylcysteine. No other adverse effects were documented.
Desnuelle 2001 reported adverse events: 74.3% in the control group, and 80.5% in the treatment group. Forty‐two per cent were classified as serious. There was no statistically significant difference between the two groups. There was no common adverse event that could be unambiguously attributed to treatment with a‐tocopherol.
Lange 1998 reported one patient requiring a reduction in dose with subsequent withdrawal because of worsening depression.
Stevic 2001 stated that L‐methionine, vitamin E and selenium (Alsemet) were well tolerated and no adverse effects were observed.
QALS Study Group 2009 reported no serious adverse events related to coenzyme Q10. There was no significant difference in adverse events between the placebo and coenzyme Q10 groups.
Jossan 1994; Mitchell 1995 and Ellis 1997 did not report adverse effects.
Discussion
Of the 10 included studies only four provided sufficient data for analysis of the primary endpoint, survival at 12 months. The results of the individual studies, and the meta‐analysis, showed no benefit of the individual antioxidants vitamin E (Desnuelle 2001), acetylcysteine (Louwerse 1995) and a combination of L‐methionine, vitamin E, and selenium (Alsemet) (Stevic 2001). Significant differences in trial methodology and the selection of primary and secondary measures and endpoints caused difficulty in comparing studies, pooling results and performing meta‐analysis. QALS Study Group 2009 used an adaptive trial design aiming for a more efficient phase II study design Cudkowicz 2010.
Stevic 2001 reported significantly improved survival at 12 months using a combination of selegiline, selenium and methionine (not necessarily an antioxidant). This was a small study of 28 patients and the method of randomization was not clear. A Mantel test was used for statistical analysis. We did not find a significant improvement in survival at 12 months using our analyses of relative risk and risk difference. A further study with a larger number of patients and standardized trial methodology would be interesting. Methionine itself may deserve further study to clarify the effect. Apostolski 1998 reported an improvement in Norris score over 63 days treatment with Alsamin in seven patients. No statistical analysis was performed and the result is difficult to interpret. A more rigorous study of a larger number of patients over a longer period of time is required to prove efficacy.
The antioxidant medications used are generally well tolerated and without serious adverse effects. This probably explains their wide use by patients, with or without the advice of their physician. This is especially so when faced with a rapidly progressive, fatal condition for which there is no dramatically effective therapy. The effect of riluzole is mild, and its sole potential effect in marginally extending survival is not apparent to the individual patient. A recent meta‐analysis of high dosage vitamin E supplementation in a variety of conditions reported an increased all cause adverse mortality risk difference of 39 per 100,000, and concluded that high dosage vitamin E (> 400 IU/day) should be avoided (Greenberg 2005; Miller 2005). This may have importance for long‐term preventive intake but is unlikely to be of significance in a rapidly progressive and fatal condition such as ALS. Another recent study (Ascherio 2005) concluded that individuals participating in the American Cancer Society's Cancer Prevention Study II, who were taking regular vitamin E supplementation of an undefined dose for 10 years or more, had a reduced age and smoking adjusted relative risk of dying of ALS of 0.38. There was no effect of vitamin C or multivitamins. It was suggested that vitamin E supplementation may have a role in ALS prevention. This is an important observation on the potential benefit of antioxidants in preventing onset of ALS but the study was not a randomized controlled trial of treatment of ALS and did not fulfil the criteria for inclusion in this systematic review. Another study (Veldink 2007) assessed whether the premorbid dietary intake of fatty acids, cholesterol, glutamate or antioxidants was associated with the risk of developing ALS. This was assessed using a food‐frequency questionnaire. The authors concluded that a high intake of polyunsaturated fatty acid and vitamin E was associated with a 50% to 60% decreased risk of developing ALS, and they appeared to act synergistically. Intake of flavanols, lycopene, vitamin C, vitamin B2, glutamate, calcium and phytoestrogens was not associated with the risk of developing ALS.
Many of the antioxidants are readily available, often without prescription. We did not, however, find any evidence to support the use of these medications on the basis of well‐designed, randomized controlled trials. The cost of the individual medication is relatively low but may vary depending on the formulation used. For example, Pioro 2000 recommended the following antioxidant therapies for ALS "on the basis of available data and documented toxicities":
people with ALS should avoid activities that reduce endogenous antioxidant levels; for example smoking, which reduces serum vitamin C concentrations;
high dose vitamin E (up to 2000 IU/day);
high dose vitamin C (500 to 1000 mg/day);
consideration of catalase, trientine and lipoic acid, on the basis of animal model studies and low toxicity.
The approximate current daily cost of vitamin E 1000 IU is from GBP 0.30, and vitamin C 1000 mg is from GBP 0.05. For comparison, the approximate daily cost of riluzole 50 mg twice daily is GBP 10.00.
Although the use of vitamins C and E for the treatment of ALS is not supported by clinical trial data, the high tolerance and safety, relatively low cost, and ready availability do not contraindicate their common usage by physicians and patients. This may be an important consideration when other unproven medications may have a higher potential risk of adverse effects, and higher cost.
Authors' conclusions
Implications for practice.
We conclude that despite substantial literature on the potential scientific basis for the use of antioxidants in people with ALS and the widespread use of these medications by patients, often on the advice of their physician, there is no significant evidence that they have a beneficial effect in people with ALS.
Implications for research.
The quality of design and presentation of the earlier studies was generally poor. Methodology was often incompletely described. The studies were generally underpowered to prove any benefit which may be present, especially when compared to the studies of riluzole, which are the only studies so far to have demonstrated benefit from a therapeutic agent in people with ALS. The most recent studies show improved trial design, and novel approaches. In future, it is important that the patient entry characteristics are well defined and, if possible, comparable between trials. Inclusion and exclusion criteria should be defined. There should be some conformity in outcome measures and primary outcome measures should be defined. Adverse effects require more detailed description. In addition to survival or functional measures, quality of life measures should be included.
What's new
| Date | Event | Description |
|---|---|---|
| 13 February 2011 | New search has been performed | We have included reference to a phase II clinical study of coenzyme Q10 in ALS. Edavarone has been included in the background. Searches were updated to 22 June 2010. The Cochrane Collaboration 'Risk of bias' tool was incorporated. |
History
Protocol first published: Issue 4, 2000 Review first published: Issue 4, 2004
| Date | Event | Description |
|---|---|---|
| 1 September 2006 | New citation required and conclusions have changed | Substantive amendment |
Acknowledgements
Translator: Anna Wanstead, Wellcome Institute.
Translator: Muke Zhou.
Appendices
Appendix 1. MEDLINE OvidSP search strategy
1 randomized controlled trial.pt. 2 controlled clinical trial.pt. 3 randomized.ab. 4 placebo.ab. 5 drug therapy.fs. 6 randomly.ab. 7 trial.ab. 8 groups.ab. 9 or/1‐8 10 exp animals/ not humans.sh. 11 9 not 10 12 exp Motor Neuron Disease/ 13 (moto$1 neuron$1 disease$1 or moto?neuron$1 disease).mp. 14 amyotrophic lateral sclerosis.mp. 15 (Lou Gehrig$1 adj5 (syndrome$ or disease$)).tw. 16 or/12‐15 17 Ascorbic Acid/ 18 vitamin c.tw. 19 ascorbic acid.mp. 20 Vitamin E/ 21 vitamin E.tw. 22 alpha‐Tocopherol/ 23 alpha‐tocopherol.mp. 24 selegiline.mp. 25 Selegiline/ 26 deprenyl.mp. 27 Acetylcysteine/ 28 n‐acetyl cysteine.mp. 29 n‐acetylcysteine.mp. 30 n‐acetyl‐l‐cysteine.mp. 31 Acetylcysteine.mp. 32 Superoxide Dismutase/ 33 Superoxide Dismutase.mp. 34 SOD.mp. 35 DEHYDROEPIANDROSTERONE/ 36 DEHYDROEPIANDROSTERONE.mp. 37 ANTIOXIDANTS/ 38 antioxidant.mp. 39 anti oxidant.mp. 40 or/17‐39 41 aeol‐10150.mp. 42 (catalytic adj1 metalloporphyrin).mp. 43 (coQ10$ or coenzyme q10$ or ubiquinone).mp. 44 or/17‐43 45 11 and 16 and 44
Appendix 2. EMBASE OvidSP search strategy
1 crossover‐procedure/ 2 double‐blind procedure/ 3 randomized controlled trial/ 4 single‐blind procedure/ 5 (random$ or factorial$ or crossover$ or cross over$ or cross‐over$ or placebo$ or (doubl$ adj blind$) or (singl$ adj blind$) or assign$ or allocat$ or volunteer$).tw. 6 or/1‐5 7 human/ 8 6 and 7 9 nonhuman/ or human/ 10 6 not 9 11 8 or 10 12 exp Motor Neuron Disease/ 13 (moto$1 neuron$1 disease$ or moto?neuron$1 disease$).mp. 14 amyotrophic lateral sclerosis.mp. 15 (lou gehrig$1 adj5 (disease$ or syndrome$)).mp. 16 or/12‐15 17 Ascorbic Acid/ 18 vitamin c.tw. 19 ascorbic acid.mp. 20 vitamin E.tw. 21 alpha‐Tocopherol/ 22 alpha‐tocopherol.mp. 23 selegiline.mp. 24 Selegiline/ 25 deprenyl.mp. 26 Acetylcysteine/ 27 n‐acetyl cysteine.mp. 28 n‐acetylcysteine.mp. 29 n‐acetyl‐l‐cysteine.mp. 30 Acetylcysteine.mp. 31 Superoxide Dismutase/ 32 Superoxide Dismutase.mp. 33 SOD.mp. 34 prasterone/ 35 prasterone.mp. 36 DEHYDROEPIANDROSTERONE.mp. 37 ANTIOXIDANT/ 38 antioxidant.mp. 39 anti oxidant.mp. 40 (aeol‐10150 or aeol10150).mp. 41 (catalytic adj1 metalloporphyrin).mp. 42 (coQ10$ or coenzyme Q10$ or ubiquinone).mp. 43 or/17‐42 44 11 and 16 and 43
Appendix 3. Cochrane Central Register of Controlled Trials (CENTRAL) search strategy
#1MeSH descriptor Motor Neuron Disease explode all trees #2"motor neuron disease" OR "motor neurone disease" OR "motoneuron disease" OR "motorneuron disease" OR "amyotrophic lateral sclerosis" #3(Gehrig* NEAR syndrome*) #4(Gehrig* NEAR disease*) #5(#1 OR #2 OR #3 OR #4) #6(ascorbic acid) or (vitamin c) or (vitamin e) or (alpha tocopherol) #7selegiline or deprenyl or acetylcysteine or (acetyl NEAR/3 cysteine) #8(superoxide dismutase) or sod or dehydroepiandrosterone or antioxidants or antioxidant or (anti oxidant) #9aeol‐10150 #10catalytic NEAR metalloporphyrin #11COQ10 * or "coenzyme q10*" or ubiquinone #12(#6 OR #7 OR #8 OR #9 OR #10 OR #11) #13(#5 AND #12)
Data and analyses
Comparison 1. All antioxidants versus placebo.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Survival at 12 months (relative risk) | 4 | 586 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.08 [0.97, 1.19] |
| 2 Survival at 12 months (risk difference) | 4 | 586 | Risk Difference (M‐H, Fixed, 95% CI) | 0.05 [‐0.02, 0.13] |
| 3 Survival at 12 months (sensitivity analysis) | 3 | 558 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.06 [0.95, 1.17] |
| 4 Survival at 3 months (relative risk) | 1 | 160 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.00 [0.95, 1.05] |
| 5 Survival at 6 months (relative risk) | 1 | 160 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.09 [0.99, 1.21] |
| 6 Survival at 9 months (relative risk) | 1 | 160 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.04 [0.92, 1.19] |
| 7 Survival at 15 months (relative risk) | 1 | 160 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.04 [0.84, 1.29] |
| 8 Survival at 18 months (relative risk) | 1 | 160 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.05 [0.82, 1.34] |
| 9 Survival at 6 months (risk difference) | 1 | 160 | Risk Difference (M‐H, Fixed, 95% CI) | 0.08 [‐0.01, 0.17] |
1.3. Analysis.

Comparison 1 All antioxidants versus placebo, Outcome 3 Survival at 12 months (sensitivity analysis).
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Apostolski 1998.
| Methods | Double‐blind, placebo controlled study | |
| Participants | Country: Yugoslavia Single centre Diagnosis: ALS, World Federation of Neurology Participants: 35 (14 treated with antioxidant, 7 placebo, 7 nimodipine, 7 amino acids. 60% male) Age: mean 54 years Inclusion criteria not stated Exclusion criteria ‐ family history of neurological disease Side effects ‐ none | |
| Interventions | 1. Alsamin ‐ selenium, vitamin E, b‐carotene, L‐arginine, L‐leucine, L‐isoleucine, L‐valine, L‐methionine, nimodipine 2. selenium, vitamin E, b‐carotene 3. L‐arginine, L‐methionine, L‐leucine, L‐isoleucine, L‐valine 4. nimodipine 5. placebo | |
| Outcomes | Primary: Erythrocyte enzyme activity of Copper zinc superoxide dismutase, glutathione peroxidase, catalase, glutathione reductase, and plasma activity of glutathione transferase Secondary: Change in Norris score | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Comment: not addressed |
| Allocation concealment (selection bias) | Unclear risk | Comment: not addressed |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | "double blind" Comment: not addressed further |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Comment: not addressed |
| Selective reporting (reporting bias) | Unclear risk | Comment: insufficient information |
| Other bias | Unclear risk | Comment: insufficient information |
Desnuelle 2001.
| Methods | Randomized, placebo controlled, double‐blind study Duration: 12 months | |
| Participants | Country: France Multicentre: 28 sites Diagnosis: probable or definite ALS by El Escorial criteria Number of participants: 288 (144 treated, 144 placebo, 55% male) Age: mean 64 years Inclusion criteria: to have been treated with riluzole for at least 3 months without side effects Exclusion criteria: dementia and/or major psychiatric disorders, or other serious disease or handicap, forced vital capacity less than 60%, monoclonal gammopathy, conduction blocks, abnormal liver function tests as defined, taking vitamin E or hepatotoxic drugs | |
| Interventions | 1. a‐tocopherol (500 mg capsules twice daily, Toco 500) plus riluzole 50 mg twice daily 2. placebo twice daily (identically‐appearing capsules) plus riluzole 50 mg twice daily | |
| Outcomes | Primary: the change in functional status of each patient using the modified Norris limb scale Secondary: included survival, bulbar function assessed with the Norris bulbar scale, manual muscle testing Fatigue, limb stiffness, cramps and fasciculations were assessed using visual analogue scales. Respiratory function, quality of life using the Sickness Impact Profile, the ALS Health State scale. Biological markers of oxidative stress ‐ plasma thiobarbituric acid, erythrocyte superoxide dismutase, erythrocyte glutathione peroxidase ‐ in a subgroup of 122 patients | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "patients were randomly assigned to one of the two treatment groups according to a randomization schedule prepared by Labaratoire Rhone‐Poulenc Rorer (now trading under the name Laboratoire Aventis" Comment: probably adequate |
| Allocation concealment (selection bias) | Unclear risk | Comment: probably adequate |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | "double blind, placebo controlled". "identically appearing capsules, bid, Rhone‐Poulenc Rorer" Comment: probably adequate |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | "Intent to treat population which included all randomized patients who had received at least one treatment dose" |
| Selective reporting (reporting bias) | Low risk | Appears adequate |
| Other bias | Low risk | Appears adequate |
Ellis 1997.
| Methods | Randomized, placebo controlled, double‐blind study Duration: 6 months observation, followed by randomization and 6 months treatment | |
| Participants | Country: USA Single centre Diagnosis: ALS Number of participants: 10 (5 treated, 5 placebo, sex not stated) Age: not stated Inclusion criteria: not stated Exclusion criteria: not stated | |
| Interventions | 1. Coenzyme Q (240 mg) 2. Vitamin E (1600 IU) 3. Vitamin C (2000 mg) 4. Selenium (100 mg) 5. Beta‐carotene (10 mg) 6. N‐acetylcysteine (200 mg) | |
| Outcomes | Primary: rate of change in Appel Rating Scale score. Secondary: included safety and blood markers of oxidative damage | |
| Notes | Presented in abstract form only | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "randomized" Comment: not addressed |
| Allocation concealment (selection bias) | Unclear risk | Comment: not addressed |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | "double blind" Comment: probably adequate |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Comment: not addressed |
| Selective reporting (reporting bias) | Unclear risk | Comment: insufficient information |
| Other bias | Unclear risk | Comment: insufficient information |
Graf 2005.
| Methods | Randomized, double‐blind, placebo controlled study Duration 18 months | |
| Participants | Country: Germany Multicentre: 6 sites Diagnosis: Probable or definite ALS ‐ El Escorial criteria Number of participants: 160 (83 treated, 77 placebo) Age: mean 59 years treated, 57 years placebo Inclusion criteria: Disease duration less than 5 years. Treated with riluzole Exclusion criteria: Not stated Side effects. No difference between treated and placebo | |
| Interventions | 1. Vitamin E, a‐tocopherol, 1 g five times daily 2. Placebo | |
| Outcomes | Primary: Survival, calculated as time to death, tracheostomy, or permanent assisted ventilation Secondary: Rate of deterioration of function assessed by modified Norris limb and bulbar scales, manual muscle testing (BMRC), spasticity scale, ventilatory function, Sickness Impact Profile (SIP ALS/19) | |
| Notes | 7 patients in placebo group were taking open vitamin E 23% of patients were taking vitamin E at onset of study | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Comment: not clear |
| Allocation concealment (selection bias) | Unclear risk | Comment ‐ not clear |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | "identical capsules" Comment: probably adequate |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Comment: not addressed |
| Selective reporting (reporting bias) | Low risk | Comment: probably adequate |
| Other bias | High risk | Comment: 7 patients in placebo group were taking open vitamin E. 23% of patients were taking vitamin E at onset of study |
Jossan 1994.
| Methods | Randomized, cross‐over, double‐blind study Duration: 12 weeks drug, 12 weeks washout, 12 weeks placebo | |
| Participants | Country: Sweden Single centre Diagnosis: ALS ‐ World Federation of Neurology (1990) Number of participants: 10 (80% male) Age: mean 50 years Inclusion criteria: not stated Exclusion criteria: not stated | |
| Interventions | 1. Deprenyl (selegiline) 10 mg daily 2. Placebo | |
| Outcomes | Primary: not stated Other: Norris score, bulbar and spinal scores. MAO‐B estimation in platelets | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Comment: not addressed |
| Allocation concealment (selection bias) | Unclear risk | Comment: not addressed |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | "double blind" Comment: not addressed further |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Comment: not addressed |
| Selective reporting (reporting bias) | Unclear risk | Comment: appears adequate, but limited information |
| Other bias | Unclear risk | Comment: appears adequate, but limited information |
Lange 1998.
| Methods | Randomized, placebo controlled, double‐blind study Duration: 6 months | |
| Participants | Country: USA Multicentre: 2 sites Diagnosis: criteria for classical ALS Number of participants: 133 (67 treated, 66 placebo, 62% male) Age: mean 57 years Inclusion criteria: 25 to 65 years, symptom duration less than 3 years, mild to moderate disease with Appel ALS total score 30 to 80, no drug therapy for at least 3 months before enrolment Exclusion criteria: multifocal motor neuropathy with conduction block, paraproteinemia, elevated GM1 antibodies, sensorimotor peripheral neuropathy, previous infection with poliovirus, lower motor neuron disease only, primary lateral sclerosis, previous allergy to selegiline, abnormal endocrinology, serious medical problems, poor family support | |
| Interventions | 1. selegiline 5 mg twice daily 2. placebo | |
| Outcomes | Primary: rate of change of Appel ALS (AALS) total score Secondary: AALS component scores, survival analysis | |
| Notes | Subjects with AALS score below 115 or forced vital capacity below 39% predicted were considered treatment failures and entered an open‐label phase | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Comment: not addressed |
| Allocation concealment (selection bias) | Unclear risk | Comment: not addressed |
| Blinding (performance bias and detection bias) All outcomes | Low risk | "The research team at each site consisted of 2 investigators and a study coordinator unaware of randomization status and another investigator who reviewed laboratory data." Comment: appears adequate |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Comment: not addressed |
| Selective reporting (reporting bias) | Low risk | Comment: appears adequate |
| Other bias | Low risk | Comment: appears adequate |
Louwerse 1995.
| Methods | Randomized, placebo controlled, double‐blind study Duration: 12 months | |
| Participants | Country: Netherlands Single centre Diagnosis: Probable or definite ALS by El Escorial criteria Number of participants: 110 (54 treated, 56 placebo, 55% male) Age: mean 58 years Inclusion criteria: not stated Exclusion criteria: younger than 20 years, older than 80 years, first or second degree family member with ALS, signs of dementia or parkinsonism, serious mental illness, life expectancy less than 6 months due to other disease, previously used drugs such as antioxidants, mucolytic drugs, glutamate antagonists, chelating agents | |
| Interventions | 1. Acetylcysteine 50 mg/kg sc infusion daily 2. Placebo | |
| Outcomes | Primary: survival ‐ death from all causes, long‐term assisted ventilation, tracheostomy, positive‐pressure breathing Secondary: rates of disease progression as expressed by manual muscle strength testing, myometry, forced vital capacity, ability to perform activities of daily living, degree of independence, bulbar function | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "randomization was balanced for prognostic factors according to the minimization method described by Pocock and Simon". Comment: not clear |
| Allocation concealment (selection bias) | Unclear risk | Comment: not clear |
| Blinding (performance bias and detection bias) All outcomes | Low risk | "the drug code was known only to the hospital pharmacists until the final analysis of the trial". "double blind" Comment: probably adequate |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Comment:not addressed |
| Selective reporting (reporting bias) | Low risk | Comment: appears adequate |
| Other bias | Low risk | Comment: appears adequate |
Mitchell 1995.
| Methods | Cross‐over, placebo controlled, double‐blind study. Duration: 4 week placebo, 16 weeks medication/placebo, 4 weeks placebo, 16 weeks medication/placebo, 4 weeks placebo | |
| Participants | Country: England Single centre Diagnosis: MND diagnosed by two consultant neurologists Number of participants: 56 (66% male) Age: not stated Inclusion criteria: not stated Exclusion criteria: not stated | |
| Interventions | 1. Selegiline 10 mg daily 2. Placebo | |
| Outcomes | Primary: not stated Other: hand held myometry, hand grip strength, Barthel Disability Index, Rankin Disability Scale, Bulbar Rating Scale, Quality of Life Scale. Whole blood and serum glutathione peroxidase, erythrocyte SOD, serum caeruloplasmin, leucocyte and plasma ascorbic acid, serum tocopherol | |
| Notes | This is published as a letter with no details of clinical results. Additional details are given in Mitchell 1994, and in unpublished information provided by Professor Mitchell | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Comment: not addressed |
| Allocation concealment (selection bias) | Unclear risk | Comment: not addressed |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | "double blind" Comment: probably adequate |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Comment: not addressed |
| Selective reporting (reporting bias) | Low risk | Comment: appears adequate |
| Other bias | Unclear risk | Comment: insufficient information |
QALS Study Group 2009.
| Methods | An adaptive, two stage, bias‐adjusted, randomized, placebo‐controlled, double‐blind, Phase II study of coenzyme Q10. Coenzyme Q10 1800 mg/day and 2700 mg/day. A total of 185 patients with ALS was studied | |
| Participants | The study was conducted at 19 centres in the USA. Diagnosis was of definite, probable, or laboratory supported probable ALS, sporadic or familial, by El Escorial guidelines | |
| Interventions | The design was innovative, aiming to minimize sample size and length of follow up. In Stage 1, 35 patients were allocated to each of placebo, CoQ10 1800 mg/day and CoQ10 2700 mg/day, for 9 months. The dose of 2700 mg/day had higher efficacy, and was chosen Stage 2. In Stage 2, 40 patients were added to each of the placebo and CoQ10 2700 mg/day groups, and studied for a further 9 months | |
| Outcomes | The primary outcome was decline in the ALS Functional Rating Scale‐revised (ALSFRSr) score from baseline to 9 months. Secondary outcome measures were decline in forced vital capacity, fatigue severity (9‐item Fatigue Severity Scale), and quality of life (SF‐36 with physical and mental components analyzed separately). Oxidative stress was assessed in plasma by 8‐hydroxy‐2 deoxyguanosine measurement | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "Participants were randomized in permuted blocks, stratified by clinical site and riluzole use" Comment: probably done but sequence generation not described |
| Allocation concealment (selection bias) | Unclear risk | Comment: probably done but allocation concealment not described |
| Blinding (performance bias and detection bias) All outcomes | Low risk | "double blind" |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | "Scoring for deceased patients". Imputation for missing data". Comment: these are described in the text |
| Selective reporting (reporting bias) | Low risk | Comment: sufficient information provided |
| Other bias | Low risk | Comment: the study appears to be free of other sources of bias |
Stevic 2001.
| Methods | Double‐blind, placebo controlled study. Duration 12 months. | |
| Participants | Country: Yugoslavia. Centres: not stated (presumed single centre). Diagnosis: ALS. Number of participants: 28 (16 treated, 12 placebo; 75% male). Age: mean 57 years. Inclusion criteria: probable or definite ALS by El Escorial criteria, age 20‐70 years, disease duration <3 years, ambulatory. Exclusion criteria: significant compromise of bulbar or respiratory function, conduction block, M protein, significant imaging abnormality, dementia, concurrent systemic disease. | |
| Interventions | 1. Alsemet ‐ L‐methionine (2 g), vitamin E (400 IU), selenium (3 x 10‐5g) three times daily. 2. Placebo. | |
| Outcomes | Primary: survival and rate of disease progression as expressed by decline in limb‐function, bulbar‐function and muscle‐testing scores. Secondary: activity of antioxidative components (GSH‐Px, GR, CAT), and level of vitamin E, in blood. | |
| Notes | Published in abstract form as Stevic 1998 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "sixteen patients were randomized to Alsemet and 12 to placebo" Comment: not clear |
| Allocation concealment (selection bias) | Unclear risk | Comment: not clear |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | "double blind" Comment: not clear |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Comment: not addressed |
| Selective reporting (reporting bias) | Low risk | Appears adequate |
| Other bias | Low risk | Appears adequate |
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Ascherio 2005 | A prospective study of prevention of disease in patients taking vitamin C and E. 957,740 patients were studied, with recruitment in 1982. ALS deaths were assessed from 1989 to 1998. Regular use of vitamin E was associated with a less than half the risk of dying from ALS than in non users. No significant association was found for use of vitamin C or multivitamins. This was not a randomized controlled trial of treatment |
| Chio 1998 | An open, cross‐over, sequentially randomized trial. Thirty‐two patients were studied, and treated with reduced glutathione (GSH), 600 mg IM, daily, for 12 weeks, with 1 week washout. No significant difference in rate of progression of MRC score, bulbar score, Norris score, or forced vital capacity |
| Dorman 1969 | An unblinded nonrandomized study of 12 patients treated with pancreatic extract (Viokase) 6.3 g and DL a‐tocopherol 1500 units daily for variable periods of time. No improvement in muscle testing scores was observed |
| Janik 1996 | An abstract of preliminary results of the study Kwiecinski 2001. The study is a randomized open trial |
| Mazzini 1994 | An open randomized study. Fifty‐three patients were given selegiline 10 mg daily for six months, and 58 patients were not. There was no statistically significant difference in mortality, mean MRC and Norris disability scores, and forced vital capacity |
| Norris 1987 | Brief descriptions of uncontrolled studies of vitamin E in ALS, with no benefit |
| Quick 1969 | Nonrandomized. No concealment. No controls. Ten patients were given vitamin E (900 to 2100 IU) and pancreatic supplement Viokase (5 to 7 g) daily. The seven patients followed for six months or more showed "physical changes were we interpret as favorable, reflecting remission of the usual relentless progression of ALS. One has shown marked improvement in muscle strength and function to levels 50% greater than the initial performance. The other three have improved less dramatically but all have achieved up to 25% improvement." |
| Szczudlik 1998 | An open randomized study. Nineteen patients were given pimozide 1 mg daily, and 25 selegiline and vitamin E, for 3 to 12 months. A significant decrease of an index of progression of the disease was reported in the pimozide treated patients |
| Vyth 1996 | Nonrandomized. No concealment. Historical controls. Patients received a variety of medications including N‐acetylcysteine, vitamin C, vitamin E, N‐acetylmethionine, dithiothreitol, dithioerythritol, and meso‐2,3‐dimercaptosuccinic acid (DMSA). The median survival in the treated group was 3.4 years, and in the untreated 2.8 years |
| Wechsler 1940 | Non randomized. No concealment. No controls. Twenty patients were given a‐tocopherol acetate 30 to 200 mg oral, or 50 mg IM, plus 2 teaspoonfuls of whole wheat germ oil, 2.5 g bile salts, whole vitamin B complex, and vitamin E containing foods. Duration of treatment 3 to 42 months. Fourteen patients were reported to be improved, and 2 stabilised |
| Zhang 2007 | An open randomized study. 50 patients with ALS were randomly divided to receive edaravone 30 mg/day intravenously. All patients received vitamin E 100 mg four times a day, and polysacharidum of Ganoderma lucidum Karst 2 ml four times a day intramuscularly (an extract of Reishi mushroom). The treatment was given for 14 days, repeated every three months. There was no indication of placebo injection. The trial lasted 27 months, with follow up for 18 months. The absolute changes of ALS‐FRS score were stated to be significantly different between the two groups (P < 0.05), but the changes of ALS‐FRS score between the two groups were not significant (P = 0.08) |
Contributions of authors
Dr Orrell led the design of the review, collation and analysis of the studies, and the writing of the review. Dr Lane and Dr Ross contributed to the design of the review, analysis of the studies, and writing of the review.
Sources of support
Internal sources
None, Not specified.
External sources
None, Not specified.
Declarations of interest
None known
New search for studies and content updated (no change to conclusions)
References
References to studies included in this review
Apostolski 1998 {published data only}
- Apostolski S, Markinkovic Z, Nocolic A, Blagojevic D, Spasic MB, Michelson AM. Glutathione peroxidase in amyotrophic lateral sclerosis: the effects of selenium supplementation. Journal of Environmental Pathology, Toxicology and Oncology 1998;17(3‐4):325‐9. [PUBMED: 9726810] [PubMed] [Google Scholar]
Desnuelle 2001 {published and unpublished data}
- Desnuelle C, Dib M, Garrel C, Favier A. ALS riluzole‐tocopherol Study Group. A double‐blind, placebo‐controlled randomized clinical trial of alpha‐tocopherol (vitamin E) in the treatment of amyotrophic lateral sclerosis. Amyotrophic Lateral Sclerosis and other motor neuron disorders 2001;2(1):9‐18. [PUBMED: 11465936] [DOI] [PubMed] [Google Scholar]
Ellis 1997 {published data only}
- Ellis T, Cudkowicz ME, McKenna‐Yasek D, Eggelston PM, Brown RH, Cros D. Combination antioxidant therapy in amyotrophic lateral sclerosis. Neurology 1997;48:A127. [Google Scholar]
Graf 2005 {published data only}
- Graf M, Ecker D, Horowski R, Kramer B, Riederer P, Gerlach M, et al. on behalf of the German Vitamin E / ALS Study Group. High dose vitamin E therapy in amyotrophic lateral sclerosis as add‐on therapy to riluzole: results of a placebo‐controlled double‐blind study. Journal of Neurotransmission 2005;112(5):649‐60. [PUBMED: 15517433] [DOI] [PubMed] [Google Scholar]
Jossan 1994 {published data only}
- Jossan SS, Ekblom J, Gudjonsson O, Hagbarth KE, Aquilonius SM. Double blind cross over trial with deprenyl in amyotrophic lateral sclerosis. Journal of Neural Transmission 1994;41 Suppl:237‐41. [PUBMED: 7931231] [DOI] [PubMed] [Google Scholar]
Lange 1998 {published data only}
- Lange DJ, Murphy PL, Diamond B, Appel V, Lai EC, Younger DS, et al. Selegiline is ineffective in a collaborative double‐blind, placebo‐controlled trial for treatment of amyotrophic lateral sclerosis. Archives of Neurology 1998;55(1):93‐6. [PUBMED: 9443715] [DOI] [PubMed] [Google Scholar]
Louwerse 1995 {published data only}
- Louwerse ES, Weverling GJ, Bosuyt PM, Meyjes FE, Jong JM. Randomized, double‐blind, controlled trial of acetylcysteine in amyotrophic lateral sclerosis. Archives of Neurology 1995;52(6):559‐64. [PUBMED: 7763202] [DOI] [PubMed] [Google Scholar]
Mitchell 1995 {published data only}
- Mitchell JD, Gatt JA, Phillips TM, Houghton E, Rostron G, Wignall C. Cu/Zn superoxide dismutase free radicals, and motoneuron disease [Letter]. Lancet 1993;342(8878):1051‐2. [PUBMED: 8105281] [DOI] [PubMed] [Google Scholar]
- Mitchell JD, Houghton E, Rostron G, Wignall C, Gatt JA, Phillips TM, et al. Serial studies of free radical and antioxidant activity in motor neurone disease and the effect of selegiline [letter]. Neurodegeneration 1995;4(2):233‐5. [DOI] [PubMed] [Google Scholar]
- Mitchell JD, Houghton E, Rostron G, Wignall C, Phillips TM, Kilshaw J. Indices of free radical and antioxidant activity in amyotrophic lateral sclerosis. In: Clifford Rose F editor(s). ALS ‐ from Charcot to the present and into the future. London: Smith‐Gordon and Company Ltd, 1994:297‐307. [Google Scholar]
QALS Study Group 2009 {published data only}
- Kaufmann P, Thompson JL, Levy G, Buchsbaum R, Shefner J, Krivickas LS, et al. Phase II trial of CoQ10 for ALS finds insufficient evidence to justify phase III. Annals of Neurology 2009;66(2):235‐44. [PUBMED: 19743457] [DOI] [PMC free article] [PubMed] [Google Scholar]
Stevic 2001 {published data only}
- Stevic Z, Nicolic A, Blagjevic D, Saicia ZS, Kocev N, Apostolski S, et al. A controlled trial of combination of methionine and antioxidants in ALS patients. Jugoslavenska Medicinska Biokemija 2001;20:223‐8. [Google Scholar]
- Stevic Z, Nicolic A, Blagojevic D, Saicic ZS, Apolstolski S, Spasic M. A controlled trial of combination of methionine and antioxidants in amyotrophic lateral sclerosis. Proceedings ALS/MND International Alliance Meeting, Munich, Germany. 1998.
References to studies excluded from this review
Ascherio 2005 {published data only}
- Ascherio A, Weisskopf MG, O'Reilly EJ, Jacobs EJ, McCullough ML, Calle EE, et al. Vitamin E intake and risk of amyotrophic lateral sclerosis. Annals of Neurology 2005;57(1):104‐10. [PUBMED: 15529299] [DOI] [PubMed] [Google Scholar]
Chio 1998 {published data only}
- Chio A, Cucatto A, Terreni AA, Schiffer D. Reduced glutathione in amyotrophic lateral sclerosis: an open, crossover, randomized trial. Italian Journal of Neurological Science 1998;19(6):363‐6. [PUBMED: 10935831] [DOI] [PubMed] [Google Scholar]
Dorman 1969 {published data only}
- Dorman JD, Engel WK, Fried DM. Therapeutic trial in amyotrophic lateral sclerosis. JAMA 1969;209(2):257‐8. [PUBMED: 5819254] [PubMed] [Google Scholar]
- Engel WK, Hogenhuis LAH, Collis WJ, Schalch DS, Barlow MH, Gold GN, et al. Metabolic studies and therapeutic trials in amyotrophic lateral sclerosis. In: Norris FH, Kurland LT editor(s). Motor neuron diseases. New York: Grune and Stratton, 1969:199‐208. [Google Scholar]
Janik 1996 {published data only}
- Janik P, Kwiecinski H, Jamrozik Z, Czyzewski K, Opuchlik A. A randomised trial of antioxidative therapy in amyotrophic lateral sclerosis. Journal of Neurology 1996;243 Suppl:25. [Google Scholar]
- Kwiecinski H, Janik P, Jamrozik Z, Opuchlik A. The effect of selegiline and vitamin E in the treatment of ALS: an open randomized clinical trial [Polish]. Neurologia i Neurochirurgia Polska 2001;Suppl 1:101‐6. [PubMed] [Google Scholar]
Mazzini 1994 {published data only}
- Mazzini L, Testa D, Balzarini C, Mora G. An open‐randomized clinical trial of selegiline in amyotrophic lateral sclerosis. Journal of Neurology 1994;241(4):223‐7. [PUBMED: 8195821] [DOI] [PubMed] [Google Scholar]
Norris 1987 {published data only}
- Norris FH, Denys EH. Nutritional supplements in amyotrophic lateral sclerosis. Advances in Experimental Medicine and Biology 1987;209:183‐9. [PUBMED: 3554909] [DOI] [PubMed] [Google Scholar]
Quick 1969 {published data only}
- Quick DT. Pancreatic dysfunction in amyotrophic lateral sclerosis. In: Norris FH, Kurland LT editor(s). Motor neuron diseases. New York: Grune and Stratton, 1969:189‐98. [Google Scholar]
Szczudlik 1998 {published data only}
- Szczudilk A, Tomi B, Slowik A, Kasprzyk K. Assessment of the efficacy of treatment with pimozide in patients with amyotrophic lateral sclerosis. Neurologia i Neurochirurgia Polska 1998;T32(XLVIII):821‐9. [PubMed] [Google Scholar]
Vyth 1996 {published data only}
- Vyth A, Timmer JG, Bossuyt PMM, Louwerse ES, Jong JM. Survival in patients with amyotrophic lateral sclerosis treated with an array of antioxidants. Journal of the Neurological Sciences 1996;39 Suppl:99‐103. [PUBMED: 8899667] [DOI] [PubMed] [Google Scholar]
Wechsler 1940 {published data only}
- Wechsler IS. The treatment of amyotrophic lateral sclerosis with vitamin E (tocopherols). American Journal of Medical Science 1940;200:765‐78. [Google Scholar]
Zhang 2007 {published data only}
- Zhang YJ, Zhang J, Zhang N, Zhang HG, Wang LP, Lu M, et al. The randomized open clinical trial on a novel free radical scavenger edaravone in amyotrophic lateral sclerosis. Chinese Journal of Contemporary Neurology and Neurosurgery 2007;7:161‐4. [Google Scholar]
Additional references
Beckman 1993
- Beckman JS, Carson M, Smith CD, Koppenol WH. ALS, SOD and peroxynitrite. Nature 1993;364(6438):584. [PUBMED: 8350919] [DOI] [PubMed] [Google Scholar]
Bergeron 1996
- Bergeron C, Petrunka C, Weyer L. Oxidative stress plays a role in the pathogenesis of familial and sporadic amyotrophic lateral sclerosis. In: Fiskum G editor(s). Neurodegenerative Diseases. New York: Plenum Press, 1996:275‐80. [Google Scholar]
Brooks 1994
- Brooks BR. El Escorial World Federation of Neurology criteria for the diagnosis of amyotrophic lateral sclerosis. Subcommittee on Motor Neuron Diseases/Amyotrophic Lateral Sclerosis of the World Federation of Neurology Research Group on Neuromuscular Diseases and the El Escorial "Clinical limits of amyotrophic lateral sclerosis" workshop contributors. Journal of the Neurological Sciences 1994;124 Suppl:96‐107. [PUBMED: 7807156] [DOI] [PubMed] [Google Scholar]
Cudkowicz 2010
- Cudkowicz ME, Katz J, Moore DH, O'Neill G, Glass JD, Mitsumoto H, et al. Toward more efficient clinical trials for amyotrophic lateral sclerosis. Amyotrophic Lateral Sclerosis 2010;11(3):259‐65. [PUBMED: 19961263] [DOI] [PubMed] [Google Scholar]
Eisen 1998
- Eisen A, Krieger C. In: Eisen A, Krieger C editor(s). Amyotrophic lateral sclerosis: a synthesis of research and clinical practice. Cambridge: Cambridge University Press, 1998. [Google Scholar]
Greenberg 2005
- Greenberg ER. Vitamin E supplements: good in theory, but is the theory good?. Annals of Internal Medicine 2004;142(1):75‐6. [PUBMED: 15579660] [DOI] [PubMed] [Google Scholar]
Gurney 1996
- Gurney ME, Cutting FB, Zhai P, Doble A, Taylor CP, Andrus PK. Benefit of vitamin E, riluzole, and gabapentin in a transgenic model of familial amyotrophic lateral sclerosis. Annals of Neurology 1996;39(2):147‐57. [PUBMED: 8967745] [DOI] [PubMed] [Google Scholar]
Halliwell 2001
- Halliwell B. Role of free radicals in the neurodegenerative diseases: Therapeutic implications for antioxidant treatment. Drugs and Aging 2001;18(6):685‐716. [PUBMED: 11599633] [DOI] [PubMed] [Google Scholar]
Higgins 2008
- Higgins JPT, Green S, editors. Cochrane Handbook for Systematic Reviews of Interventions 5.0.1 [updated September 2008]. The Cochrane Collaboration, 2008. Available from www.cochrane‐handbook.org 2008.
Ince 1994
- Ince PG, Shaw PJ, Candy JM, Mantle D, Tandon L, Ehmann WD, et al. Iron, selenium and glutathione peroxidase activity are elevated in sporadic motor neuron disease. Neuroscience Letters 1994;182(1):87‐90. [PUBMED: 7891897] [DOI] [PubMed] [Google Scholar]
Knoll 1989
- Knoll J. The pharmacology of selegiline ((‐)deprenyl). New aspects. Acta Neurologica Scandinavica. Supplementum 1989;126:83‐91. [PUBMED: 2515725] [DOI] [PubMed] [Google Scholar]
Lacomblez 1996
- Lacomblez L, Bensimon G, Leigh PN, Guillet P, Menninger V, The ALS/Riluzole Study Group II. A dose‐ranging study of riluzole in amyotrophic lateral sclerosis. Lancet 1996;347(9013):1425‐31. [PUBMED: 8676624] [DOI] [PubMed] [Google Scholar]
Logroscino 2005
- Logroscino G, Beghi E, Zoccolella S, Palagano R, Fraddosio A, Simone IL, et al. the SLAP registry. Incidence of amyotrophic lateral sclerosis in southern Italy: a population based study. Journal of Neurology Neurosurgery and Psychiatry 2005;76(8):1094‐8. [PUBMED: 15956808] [DOI] [PMC free article] [PubMed] [Google Scholar]
Miller 2005
- Miller ER 3rd, Pastor‐Barriuso R, Dalal D, Riemersma RA, Appel LJ, Guallar E. Meta‐analysis: high‐dosage vitamin E supplementation may increase all‐cause mortality. Annals of Internal Medicine 2004;142:37‐46. [PUBMED: 15537632] [DOI] [PubMed] [Google Scholar]
Miller 2007
- Miller RG, Mitchell JD, Moore DH. Riluzole for amyotrophic lateral sclerosis (ALS)/motor neuron disease (MND). Cochrane Database of Systematic Reviews 2007, Issue 1. [DOI: 10.1002/14651858.CD001447.pub2] [DOI] [PubMed] [Google Scholar]
Mitchell 1987
- Mitchell JD. Heavy metals and trace elements in amyotrophic lateral sclerosis. Neurology Clinics 1987;5(1):43‐60. [PUBMED: 3550416] [PubMed] [Google Scholar]
Orrell 2006
- Orrell RW. AEOL‐10150 (Aeolus). Current Opinion in Investigational Drugs 2006;7(1):70‐80. [PUBMED: 16425674] [PubMed] [Google Scholar]
Pioro 2000
- Pioro EP. Antioxidant therapy in ALS. ALS and other motor neuron disorders 2000;1 Suppl 4:5‐12. [PUBMED: 11466960] [DOI] [PubMed] [Google Scholar]
Rosen 1993
- Rosen DR, Siddique T, Patterson D, Figlewicz DA, Sapp P, Hentati A, et al. Mutations in CuZn superoxide dismutase gene are associated with familial amyotrophic lateral sclerosis. Nature 1993;362(6415):59‐62. [PUBMED: 8446170] [DOI] [PubMed] [Google Scholar]
Sardesi 1995
- Sardesai VM. Role of antioxidants in health maintenance. Nutrition in Clinical Practice 1995;10(1):19‐25. [DOI] [PubMed] [Google Scholar]
Sejvar 2005
- Sejvar JJ, Holman RC, Bresee JS, Kochanek KD, Schonberger LB. Amyotrophic lateral sclerosis mortality in the United States, 1979‐2001. Neuroepidemiology 2005;25(3):144‐52. [PUBMED: 15990445] [DOI] [PubMed] [Google Scholar]
Veldink 2007
- Veldink JH, Kalmijn S, Groeneveld G‐J, Wunderink W, Koster A, Vries JHM, et al. Intake of polyunsaturated fatty acids and vitamin E reduces the risk of developing amyotrophic lateral sclerosis. Journal of Neurology, Neurosurgery and Psychiatry 2007;78:367‐71. [PUBMED: 16648143] [DOI] [PMC free article] [PubMed] [Google Scholar]
Yoshida 2006
- Yoshida H, Yanai H, Namiki Y, Fukatsu‐Sasaki K, Furutani N, Tada N. Neuroprotective effects of edaravone: a novel free radical scavenger in cerebrovascular injury. CNS Drug Reviews 2006;12(1):9‐20. [PUBMED: 16834755] [DOI] [PMC free article] [PubMed] [Google Scholar]


