Abstract
This study investigated the efficiency of natural killer (NK) cell immunotherapy on non-small cell lung cancer with and without EGFR mutations in order to evaluate the response rate (RR) and progression-free survival (PFS). Among the 48 patients recruited, 24 were clinically confirmed to be EGFR mutation positive. The study group was treated with autologous NK cell immunotherapy. Comparisons of the lymphocyte number, serum tumour-related biomarkers, circulating tumour cells (CTC), Karnofsky Performance Status (KPS) and survival curves were carried out before and after NK cell immunotherapy. The safety and short-term effects were evaluated, followed by median PFS and RR assessments. The serum CEA and CA125 values were found lower in the NK cell therapy group than that of the non-NK treatment group (p < 0.05). The χ2 test showed a 75% RR of the study group A, significantly higher than that of the control group B (16.7%; p < 0.01). The RR of groups C (58.3%) and D (41.7%) were not statistically significant. The p values of the 4 groups were 0.012, 0.012, 0.166 and 1 from group A to group D, respectively. The median PFS was 9 months in EGFR mutation positive group undergoing NK cell infusion interference. By evaluating the changes in immune function, tumour biomarkers, CTC, KPS and PFS, we demonstrated that NK cell therapy had better clinical therapeutic effects on EGFR mutation-positive lung adenocarcinoma.
Keywords: Natural killer cells, immunotherapy, EGFR mutation, lung cancer, clinical efficacy, clinical trial
Introduction
Malignant tumours associated with lung cancer have the highest morbidity and mortality among tumours worldwide. Lung cancer represents 13.2% of all newly diagnosed cancers, and approximately 14.1 million new cancer cases and 8.2 million deaths occurred in 2012 worldwide.1 In China, there was a 1.63% increase in lung cancer incidences per year from 1988 to 2005, totaling 465% increase over the past 30 years.2 Lung cancer is clinically divided into two types: non-small cell lung cancer (NSCLC) and small cell lung cancer (SCLC). NSCLC is the major contributor in patients and represents approximately 85% of the overall lung cancer diagnoses. NSCLC consists of adenocarcinoma, squamous cell carcinoma and large cell carcinoma. Adenocarcinomas in lung cancer, in particular, are always characterized by a poor response to chemotherapy due to epithelial growth factor receptor (EGFR) mutations, which also lead to a poor prognosis.3 In East Asians, more than 40% of lung cancer adenocarcinomas have EGFR mutations.4 The practice of testing for EGFR mutations in advanced lung cancer has an important guiding significance, not only for patient prognosis but also for predicting the efficacy of EGFR tyrosine kinase inhibitors (TKIs), which are important first line treatment drugs.
The standard therapy for advanced lung cancer includes chemotherapy, radiotherapy and targeted treatment (WHO 2012, International Agency for Research on Cancer, GLOBOCAN 2012). However, our growing understanding of the immune mechanisms in lung cancer has helped to reinforce multiple disciplinary teamwork in the development of various immunotherapies. Although T-lymphocytes have received most of the attention in immunotherapy, another key immune cell in the lung cancer microenvironment worth noticing is the natural killer (NK) cell. NK cells are characterized by their large granular lymphocyte (LGL) morphologic appearance and CD3−CD56+CD16+ or CD16− phenotype. Functionally, these cells are defined by their ability to lyse target cells without prior sensitization and without restriction by major histocompatibility (MHC) antigens.5 NK cells inhibit the proliferation of tumour cells in different ways. Apart from cytotoxic cytokines, such as INF-γ, TNF-α, IL-2 and IL-4,5 NK cells are also involved in tumour-antigen targeting with monoclonal antibodies (mAbs), which can destroy tumour cells through NK cell-mediated antibody-dependent cellular cytotoxicity (ADCC).6,7 In the very early stages of cancer development, Glushkov et al and Bei et al reported the presence of anti-EGFR auto-antibodies (IgG isotype) in the serum and tumour tissue of lung cancer patients.8,9 This finding provides evidence for using the NK cell-mediated ADCC effect against EGFR mutation-positive lung cancer adenocarcinomas.
In advanced lung cancer, large number of tumour cells and their immunosuppressive microenvironments were found to largely inhibit NK cell function in vivo through the release of TGF-β1 and IL-10.7 Furthermore, many clinical studies have been published that support NK cell anti-tumour functions. The percentage of peripheral blood NK cells expressing the activating receptor, NKp46, was significantly lower in patients with NSCLC compared to healthy donors.10 A few studies have reported encouraging results on NK adoptive infusion in patients with acute myelocytic leukemia (AML). NK-donor lymphocyte infusions are well tolerated, and graft-versus-host disease (GVHD) has not been a major problem in clinical trials to date.11 Tonn et al. found that NK92 cells (a cell line isolated from a patient with lymphoma) infused into patients could result in an anti-tumour effect in 75% of patients with lung cancer.12 The survival of lung cancer patients was positively correlated with the degree of NK cell infiltration.13
In all of the above studies, the influence of NK cells on the formation of an immunosuppressive microenvironment makes these cells a potential target for adoptive immunotherapy against lung cancer. To avoid the immunosuppressive microenvironment in vivo, the proliferation of NK cells in vitro accompanied by the inhibition of NK cell suppression mechanisms and enhancement of NK cell target recognition, for example NK cell-mediated ADCC, has been considered.14,15 Recently, several preliminary clinical studies in different types of solid tumours using allogenic NK cell adoptive immunotherapy have shown clinical safety and efficiency.16–19 Based on all of the clinical data reported, we found that not all cases of NK cell immunotherapy worked well, although most of these trials showed indications of their effectiveness. In fact, the great majority of NK-based lung cancer treatment mechanisms were identified in animal models. A limited number of clinical and/or phased studies in the literature have evaluated the efficiency of autologous NK cells. To date, most clinical studies regarding NK cell-based adoptive immunotherapies have focused on haematological malignancies rather than on solid tumours, as is the case for lung cancer. Therefore, in this research we aimed to evaluate the efficiency of autologous NK cell immunotherapy in EGFR mutation-positive lung adenocarcinomas. It is of interest to explore the reason why only a portion of the previous trials demonstrated the effectiveness of NK cell therapy.
Materials and methods
This clinical trial was registered in July 2018 (registration number: NCT03662477).
Design and patients
Patients were enrolled from 2015 to 2018 in this prospective study on the effects of autologous NK cell immunotherapy against EGFR mutation-positive lung adenocarcinomas. The criteria for enrolment were as follows: (1) expected survival of > 6 months; (2) age between 35–75 years old; (3) KPS > 45; (4) platelets > 80 × 109/L, WBC > 3 × 109/L, haemoglobin > 90 g/L, prothrombin time-international normalized ratio of 0.8–1.5, adequate hepatic function (bilirubin < 20 μM, aminotransferase < 60 U/L) and renal function (serum creatinine < 130 μM, serum urea < 10 mM); (5) diagnosis confirmed by pathology and/or imaging; and (6) absence of level 3 hypertension, severe coronary artery disease, myelosuppression, respiratory disease, acute or chronic infection, and autoimmune diseases. Of the 48 patients recruited, 24 patients were clinically confirmed to be EGFR mutation-positive. These patients were divided into two groups (group A and group B, according to whether they accepted NK therapy) in accordance with the principles of randomized trials. Patients in group A and B had been treated with first-generation TKI drug gefitinib before and identified to be drug resistant. All the EGFR mutation positive patients accepted second-generation TKI drug afatinib with NK therapy in group A or without in group B. The remaining 24 patients, who were EGFR mutation-negative, were divided into two groups (group C and group D) in accordance with the principles of randomized trials and were paired with group A and group B, respectively, as shown in Fig. 1A.
Figure 1 .
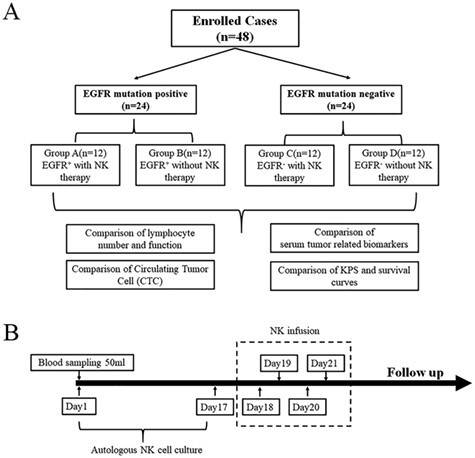
Consort diagram. Patient enrollment design of the prospective study on the effects of autologous NK cell immunotherapy against EGFR mutation-positive lung adenocarcinoma. A. Forty-eight patients were recruited and divided into 4 groups according to the EGFR mutation and NK cell treatment status. B. NK cell infusion protocol and treatment cycle design.
Cell lines
Human non-small cell lung cancer cell line H1299 (ZQ0007, Shanghai Zhong Qiao Xin Zhou Biotechnology Company, Shanghai) and human lung squamous cell carcinoma cell line NCI-H520 (ZQ0014, Shanghai Zhong Qiao Xin Zhou Biotechnology Company, Shanghai) were maintained in RPMI 1640 medium (Thermo Fisher Scientific, USA) supplemented with 10% fetal bovine serum (FBS, Thermo Fisher Scientific, Australia).
Identification of EGFR mutation genotyping
We sent the patient biopsy puncture sample containing cancer cells for Next Generation Sequencing using Illumina NextSeq CN 500 (Shanghai Da An medical Laboratory, third-party business services). Average coverage depth of the sequencing was 8917x.
Autologous NK cell expansion and treatment
NK cells were generated under good manufacturing practice (GMP) conditions. In brief, peripheral blood mononuclear cells (PBMCs) were isolated from 50 mL of patient blood with Ficoll-Hypaque (Morecell Biomedical Co. Ltd., Shenzhen, China). Then, the Human NK Cell Culture Kit (Cat. No. MCF-004, Morecell Biomedical Co. Ltd.) and Serum-free Medium for NK Cells (MCM-002, Morecell Biomedical Co. Ltd.) were used to induce NK cells according to the manufacturer’s instructions. Three days before NK cell transfusion, the NK cells were sampled and sent to the Shenzhen Cell Quality Testing and Evaluation Public Service Platform to detect the quality of the NK cells. Quality indicators were a proportion of living cells ≥95%, a proportion of CD3−CD56+ NK cells ≥80%, endotoxin content ≤ 1 EU/mL, and negative results from bacterial, fungal, and mycoplasma cultures. The cell viability was measured with trypan blue staining, and the phenotype of NK cells was verified with flow cytometry. After culturing for 17 days, NK cells were infused intravenously for four consecutive days (days 18, 19, 20, 21), with the number of NK cells injected at each time point not less than 1.2 billion (Fig. 1B).
RNA extraction and quantitative real-time polymerase chain reaction (qRT-PCR)
EGFR mRNA levels were determined by qRT-PCR. Total RNA was extracted using the Trizol reagent (Invitrogen, CA, USA) according to the manufacturer’s instructions. Then, cDNAs were synthesized using the ReverTraAce qPCR RT kit (Takara, Japan). Real-time PCR analyses were performed using Thunderbird SYBR qPCR mix (Takara, Japan) on a Quant Studio Dx Real-Time PCR Instrument (Life Technologies, USA). Glyceraldehyde-3-phosphate dehydrogenase (GAPDH) was used as an internal control, and fold changes were calculated by relative quantification (2-ΔΔCt). The nucleotide sequences of the qRT-PCR primers were as follow: The EGFR sense primer sequence was 5’-AGGCACGAGTAACAAGCTCAC-3′, and the antisense primer sequence was 5’-ATGAGGACATAACCAGC-CACC-3′. The GAPDH sense primer sequence was 5’-GGAGCGAGATCCCTCCAAAAT-3′, and the antisense primer sequence was 5’-GGCTGTTGTCATACTTCTCATGG-3′.
In vitro cytotoxicity assay
LDH release cytotoxicity assays were carried out using the LDH Cytotoxicity Assay Kit (Beyotime biotechnology, China), following the manufacturer’s protocol. NK cells and tumour cells were co-cultured at an Effector cell to Target cell (E:T) ratio of 10:1 for 4 h in 96-well plates in triplicate. The specific lysis was calculated by the following equation: percentage of specific lysis = [(experimental release–effector spontaneous release–target spontaneous release)/(target maximal release–target spontaneous release)] × 100%.
Lymphocyte subgroup detection
Numbers of CD3−CD56+ NK cells, CD3+ T cells, CD3+CD4+ T cells and CD3+CD8+ T cells in a microliter of peripheral blood from patients were obtained from the in-patient medical records. The CD4/CD8 ratio was calculated as follow: CD4/CD8 ratio equals to numbers of CD3+CD4+ T cells/numbers of CD3+CD8+ T cells.
Detection of CEA and CA125 levels in serum
Concentrations of serum carcinoembryonic antigen (CEA) and CA125 were measured with Human CEA ELISA Kit and Human CA125 ELISA Kit (R&D Systems, USA) following the manufacturer’s protocols.
Detection of CTC levels in serum
The 48 patients in our study were followed by circulating tumour cells (CTC) examination using immune fluorescence in situ hybridization (imFISH) combining FISH with chromosome 8 (orange) centromere probe (Abbott Molecular Diagnostics, Des Plaines, IL, USA). The CEP8 amplified tumor cells were quantitatively detected by using the CEP8 probe as a marker to identify the tumor cells using the principle of imFISH.20
Safety evaluation index
The main adverse events during treatment and post-treatment were closely observed and recorded.
Curative effect evaluation index
The lymphocyte numbers, concentrations of tumour-related biomarkers and circulating tumour cell numbers in patient blood were collected from clinical examination reports pre-treatment and at 3 months post-treatment.
Clinical response was based on the degree of change of the largest transverse diameter. The therapeutic effect was divided into complete response (CR), partial response (PR), stable disease (SD), or progressive disease (PD). We calculated the sum area of all tumours 3 months after NK treatment. The recent curative effect was maintained for more than 4 weeks. CR + PR denoted the effective response rate (RR). We used spiral computer tomography (CT) to assess tumor diameter. The cut-off value of the percentage change of the sum diameters was used to diagnose partial response or progressive disease after therapy, in accordance with Response Evaluation Criteria In Solid Tumours—RECIST 1.1.
The endpoints of interest for follow-up evaluations were progression-free survival (PFS). PFS was defined as the interval between NK treatment and local relapse, distant metastasis, or death, whichever occurred first. In addition, we evaluated the Karnofsky Performance Status (KPS) according to the comprehensive assessment of clinical activity, disease level, and self-care ability. The KPS scores were collected pre-treatment and at 3 months post-treatment.
Statistical analysis
Statistical analyses were performed with SPSS Statistics v22.0 f (NY: IBM Corporation). Prism v5 (GraphPad Software, San Diego, CA) was used to plot graphs. Continuous data are reported as the mean ± SD, and comparisons made on normally distributed data were performed using the paired t-test (pre-treatment and post-treatment) or two-way ANOVA (EGFR mutation * NK cell therapy). Further analyses were done using the Bonferroni test. Categorical variables were expressed as numbers and percentages, and compared by a χ2 or Fishers exact test. The Kaplan-Meier method was used to estimate the median with PFS as the endpoints of interest. A p value < 0.05 was considered to represent statistical significance.
Results
Patient demographics
A total of 48 patients were enrolled and their information was collected before the treatment began, as shown in Table 1. All together 24 male and 24 female patients were included in this study. Patients in group A and B used first-generation TKI drug gefitinib before and had been identified to be drug resistant. In this study, all these enrolled patients accepted second-generation TKI drug afatinib with NK therapy in group A or without in group B. Patients in each group were selected in accordance with the principles of randomization. Comparisons of patient’s general information were carried out between different groups.
Table 1 .
Patient characteristics.
| Group A | Group B | Group C | Group D | p value① | |
|---|---|---|---|---|---|
| (n=12) | (n=12) | (n=12) | (n=12) | ||
| Gender | p =0.862 | ||||
| Male | 7 | 6 | 5 | 6 | |
| Female | 5 | 6 | 7 | 6 | |
| Age (year) | |||||
| Median age (range) | 52 (43-75) | 65 (48-72) | 51 (42-78) | 58 (46-74) | p =0.625 |
| <50 | 7 | 1 | 5 | 2 | |
| >50 | 5 | 11 | 7 | 10 | |
| Drive gene | |||||
| EGFR+ | 12 | 12 | 0 | 0 | |
| Tumor histology | |||||
| Adenocarcinoma | 12 | 12 | 12 | 12 | |
| Squamous carcinoma | 0 | 0 | 0 | 0 | |
| Previous therapy | p =0.637 | ||||
| Surgery | 6 | 0 | 6 | 3 | |
| Chemotherapy | 8 | 3 | 8 | 10 | |
| Radiotherapy | 4 | 2 | 7 | 3 | |
| Sites of metastasis | p =0.543 | ||||
| Lymph node | 9 | 9 | 12 | 11 | |
| Brain | 2 | 5 | 2 | 6 | |
| Bone | 5 | 8 | 6 | 5 | |
| liver | 4 | 2 | 2 | 1 | |
| Clinical stage (AJCC) | p =0.807 | ||||
| III | 4 | 2 | 2 | 2 | |
| IV | 8 | 10 | 10 | 10 | |
| KPS | p =0.62 | ||||
| 50 | 0 | 3 | 1 | 1 | |
| 60 | 2 | 0 | 1 | 0 | |
| 70 | 1 | 2 | 1 | 4 | |
| 80 | 1 | 4 | 3 | 2 | |
| 90 | 3 | 2 | 2 | 2 | |
| 100 | 5 | 1 | 4 | 3 |
Group A: EGFR mutation positive with NK therapy; Group B: EGFR mutation positive without NK therapy. Patients in group A and B used first-generation TKI drug gefitinib before and had been identified to be drug resistant. In this study, all these enrolled patients accepted second-generation TKI drug afatinib with NK therapy in group A or without in group B; Group C: EGFR mutation negative with NK therapy; Group D: EGFR mutation negative without NK therapy; KPS: Karnofsky Performance Status; +: positive; ①These p values were calculated with χ2 test.
Safety evaluation
The adverse events throughout the trial were limited for all patients. Adverse events experienced by the patients were graded as I, II and III according the common Terminology Criteria of Adverse Events (CTCAE), and recorded throughout the trial. The occurrence of adverse events was compared using a χ2 test, and no significant differences were noted among the four groups (p > 0.05). All of the adverse events were below grade III, tolerable, and relievable after symptomatic treatment (Supplementary Table 1). No other side effects, including blood or bone marrow changes, were detected.
Functional analysis of autologous NK cells
Autologous NK cells originating from patient PBMCs were induced and expanded for 17 days before infusion. All cell samples were examined for purity through the detection of CD3−CD56+ NK cells by flow cytometry. The mean proportion of CD3−CD56+ NK cells was 82.54% ± 2.72% after expansion on day 17 (Fig. 2A). To examine the specific killing of autologous NK cells, we used NK cells from healthy adults against different cell lines with various EGFR expressions, in which H1299 represented a much higher level of EGFR expression than that of NCI-H520 (Fig. 2B). Although both cell lines are originally from lung cancers, our results showed that NK cells had higher cytotoxicity against H1299 than NCI-H520 (Fig. 2C).
Figure 2 .
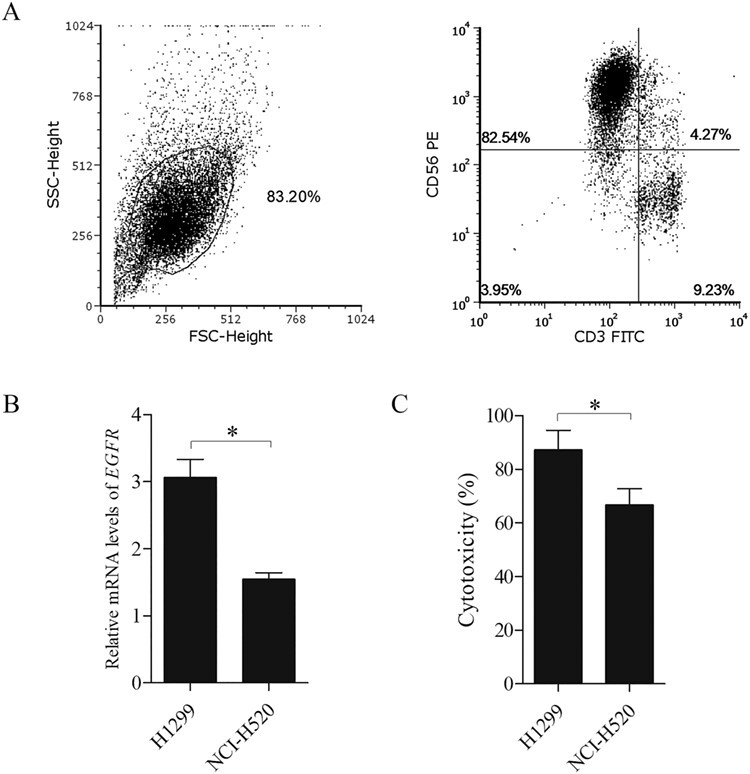
Autologous NK cell expansion and quality evaluation. A. Examination of the NK cell quality. Flow cytometry results indicate a proportion of CD3−CD56+ NK cells ≥80%. B. The level of EGFR mRNA in H1299 is much higher than that of NCI-H520 (*p < 0.05). C. Cytotoxicity of NK cells against H1299 is higher than that of NCI-H520 (*p < 0.05).
Detection of immune function
The evaluation of immunological parameters is a common method to predict the prognosis of cancer patients. Lymphocyte subgroup detection is one of the important immune function evaluations in cancer patients. We compared the NK cell number and T cell number before and after immunotherapy in different groups by paired-T test, as shown in Fig. 3A and 3B. The results indicated that NK cell immunotherapy significantly promoted both T lymphocytes and NK lymphocytes in the peripheral blood in group A (NK cell number: p < 0.001; Total T cell number: p < 0.001) and group C (NK cell number: p < 0.001; Total T cell number: p = 0.001). The CD4/CD8 ratio more accurately describes the global view of immune dysfunction and may be a better biomarker for disease progression, response to treatment, morbidity, and mortality. A greater understanding of the CD4/CD8 ratio and the impact of its manipulation should be a target, not only for HIV patients but also for cancer patients.21 Therefore, we compared in this study the CD4/CD8 ratios before and after NK cell immunotherapy among the four different groups. Our results indicated a significantly increased CD4/CD8 ratio after NK cell therapy (group A: p = 0.003; group C: p = 0.026; Fig. 3C).
Figure 3 .
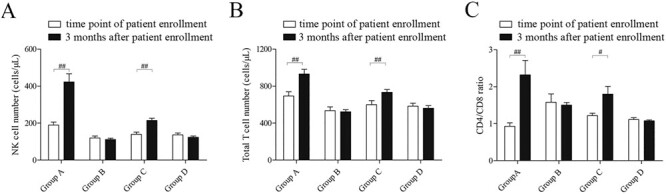
Evaluation of the immunological parameters predicts the prognosis of patients. A. Comparison of the NK cell number at the time point of patient enrollment and 3 months after in different groups (##p < 0.01). B. Comparison of the total T cell number at the time point of patient enrollment and 3 months after in different groups (##p < 0.01). C. Comparison of the CD4/CD8 ration at the time point of patient enrollment and 3 months after in different groups (#p < 0.05).
Analysis of the levels of the tumour biomarkers, CEA and CA125 in serum
Changes in serum CEA and CA125 levels are a prognostic factor to identify tumour recurrence in patients.22 CEA and CA125 levels are also able to assess the clinical efficacy in non-small cell lung cancer patients (NSCLC).23 To perform a therapeutic evaluation, we next compared the levels of CEA and CA125 in serum at the time point of patient enrolment and 3 months after. Paired-T test was performed to compare the changes of CEA pre/post treatment in each group. After NK infusion, the expression of CEA in four groups decreased significantly (n = 12; p = 0.001, 0.144, 0.347 and 0.022 for group A, B, C and D, respectively). The two-way ANOVA found in the groups significant difference of EGFR mutation F (1, 44) = 13.916, p = 0.001, but no significant difference of NK cell infusion F (1, 44) = 0.762, p = 0.388, and no significant interaction between EGFR mutation and NK cell infusion F (1, 44) = 0.216, p = 0.645. Furthermore, the result of Bonferroni multiple comparisons test revealed that EGFR mutation plays an important role in the decreasing of serum CEA level in different groups. Significant difference was showed between EGFR mutation positive groups and negative groups (p = 0.001), as shown in Fig. 4A.
Figure 4 .
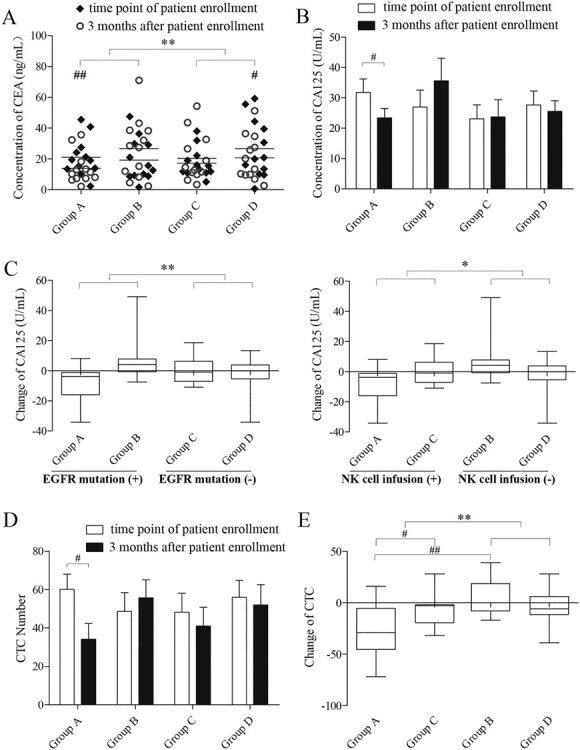
Analysis of tumour biomarkers (CEA and CA125) levels and the number of CTCs in serum at the time point of patient enrollment and 3 months after. A. Comparison of the concentration of CEA at the time point of patient enrollment and 3 months after in each group (# p < 0.05, ##p < 0.01, **p < 0.01). B. Comparison of the concentration of CA125 at the time point of patient enrollment and 3 months after within each group (# p < 0.05). C. Comparison of CA125 change between each group (*p < 0.05, **p < 0.01). The left picture displays the difference of two groups (A&B vs C&D) according to the factor of “EGFR mutation”. The right picture displays the difference of two groups (A&C vs B&D) according to the factor of “NK cell infusion” positive or negative. D, E. Comparison of the CTC number at the time point of patient enrollment and 3 months after in each group (# p < 0.05, ##p < 0.01, **p < 0.01).
We also analysis the variety of serum CA125 in different groups. Paired-T test was performed to compare the changes of CA125 at the time point of patient enrolment and 3 months after in each group. We observed significant difference in group A but not in the other three groups (n = 12; p = 0.027, 0.086, 0.826 and 0.565 for group A, B, C and D, respectively; Fig. 4B). Furthermore, ANOVA results indicated significant difference of EGFR mutation F (1, 44) = 7.822, p = 0.008 (Fig. 4C, left), and of NK cell infusion F (1, 44) = 4.187, p = 0.047 (Fig. 4C, right), but no significant interaction between EGFR mutation and NK cell infusion F (1, 44) = 0.072, p = 0.790.
Analysis of CTC levels in serum
Circulating tumour cells, which are shed from the primary tumour into the vasculature or lymphatic system, are regarded as a new prognostic factor for the metastatic process.24 We aimed to assess the NK cell therapeutic evaluation by comparing the number of CTCs in serum at the time point of patient enrolment and 3 months after. Figure 4D shows the results of paired-T test for the variety of CTC number at the time points of patient enrolment and 3 months after, indicating significant difference only in group A (n = 12; P = 0.005, 0.276, 0.146 and 0.472 for group A, B, C and D, respectively).
The presence or absence of EGFR mutation did not affect the difference in CTC assay results (F (1, 44) = 0.653, p = 0.423). On the other hand, NK cell infusion showed significant effect on the difference in CTC assay (F (1, 44) = 9.131, p = 0.004). Two way ANOVA found a significant interaction between EGFR mutation and NK cell infusion (F (1, 44) = 5.950, p = 0.019). Furthermore, we carried out the interaction effect analysis using the Bonferroni multiple comparisons test, which showed that under the condition of EGFR mutation positive, NK cell infusion had much more effect on variety of CTC number than non-NK cell infusion (p < 0.001). Under the condition of NK cell infusion, EGFR mutation positive had much more effect on variety of CTC number than EGFR mutation negative (p = 0.026) (Fig. 4E).
Clinical efficacy of NK cell therapy
The clinical response was observed at 3 months after NK cell treatment (Table 2). The response rate (RR) of group A was 75%, significantly higher than that of the control group B (16.7%) by χ2 test (p < 0.01). The RR of groups C (58.3%) and D (41.7%) were not statistically significant, however, both higher than that of group B.
Table 2 .
Clinical response at 3 months after NK treatment.
| Group | N | CR | PR | SD | PD | RR |
|---|---|---|---|---|---|---|
| A | 12 | 0 | 9 (75.0%) | 2 (16.7%) | 1 (8.3%) | 75.0% |
| B | 12 | 0 | 2 (16.7%) | 6 (50.0%) | 4 (33.3%) | 16.7%** |
| C | 12 | 0 | 7 (58.3%) | 4 (33.3%) | 1 (8.3%) | 58.3% |
| D | 12 | 0 | 5 (41.7%) | 5 (41.7%) | 2 (16.7%) | 41.7% |
Group A: EGFR mutation positive with NK therapy; Group B: EGFR mutation positive without NK therapy; Group C: EGFR mutation negative with NK therapy; Group D: EGFR mutation negative without NK therapy. N: numbers; CR: Complete response; PR: Partial response; SD: Stable disease; PD: Progressive disease; RR: Response rate. The RR was significant difference between group A and B by χ2 test. **p<0.01.
In oncology practices, a key determinant of the patient’s ability to undergo therapy is their performance status (PS). The Karnofsky Performance Status (KPS) scale has been commonly used for the general assessment of patients with cancer since its development in 1948. To perform a therapeutic evaluation, we next compared the KPS values at the time points of patient enrolment and 3 months after. The paired-sample t-tests results showed that the p values of the 4 groups were 0.012, 0.012, 0.166 and 1, from group A to group D, respectively, between samples collected at patient enrolment and 3 months after (Fig. 5A).
Figure 5 .
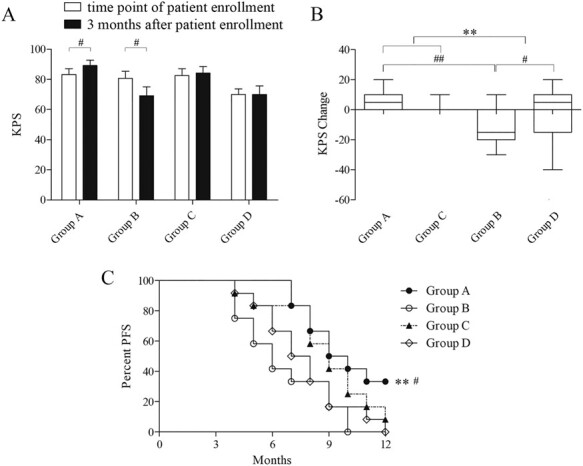
Evaluation of the clinical efficacy of NK cell therapy. A, B. Comparison of the KPS at the time point of patient enrollment and 3 months after in each group (# p < 0.05, ##p < 0.01, **p < 0.01). C. Live curve shows the splitting in different groups. The median progression-free survival (PFS) was 9 months in group A, 6 months in group B, 9 months in group C and 7 months in group D. The PFS of group A was longer than that of group B and group D (p = 0.004 and 0.02 for group A vs. group B and D, respectively. **p < 0.01, # p < 0.05).
The two-way ANOVA results about changing of KPS in different groups showed no significant difference of EGFR mutation (F (1, 44) = 1.193, p = 0.281), but significant difference of NK cell infusion (F (1, 44) = 7.790, p = 0.008), and significant interaction between EGFR mutation and NK cell infusion (F (1, 44) = 5.316, p = 0.026) (Fig. 5B). Moreover, we performed the interaction effect analysis using the Bonferroni multiple comparisons test. It indicated that under the condition of EGFR mutation positive, NK cell infusion had much more effect on KPS changing than non-NK cell infusion (p = 0.001). Additionally, under the condition of NK cell infusion, EGFR mutation positive had much more impact on KPS changing than EGFR mutation negative (p = 0.021) (Fig. 5B).
In this study, we followed up with each patient enrolled in the trial for 12 months. We found that the median PFS was 9 months in group A, 6 months in group B, 9 months in group C and 7 months in group D. As seen in Fig. 5C, the PFS of group A was longer than that of group B and group D (p = 0.004 and 0.02 for group A vs. group B and D, respectively. **p < 0.01, # p < 0.05) with χ2 test. NK cell infusion significantly prolonged PFS in different individuals with advanced lung cancer.
Discussion
Over the past ten years, cellular immunotherapy has become one of the comprehensive treatment schemes for various cancers. However, the efficacy of cellular immunotherapy is variable in clinical practice. There are few published works exploring markers for identifying which patients are suitable for cellular therapy treatment.25,26 In this preliminary clinical study, we demonstrated that NK cell therapy had better clinical therapeutic effects on EGFR mutation-positive lung adenocarcinomas than on EGFR mutation-negative tumours by evaluating the change in immune function, tumour biomarkers, and CTC, KPS and PFS values. We think that the possible reason for this phenotype can be summed up in the following aspects. First, NK cells are highly activated by anti-EGFR auto-antibodies (IgG isotype) through the ADCC effect when EGFR mutation-positive cancer cells undergo massive proliferation. Second, cancer cells with EGFR mutations grow faster than cells that are negative for this mutation, which leads to the lack of normal MHC expression on the cell surface. This breaks the self-balance between killer cell immunoglobulin-like receptor (KIR) and killer activation receptor (KAR),27 which results in a super active NK cell state. The 75% response rate in group A, which was much higher than that of group B, could result from NK cell therapy reversing the drug resistance of TKI drug and increasing sensitivity of TKI drug for these patients. It has been reported that NK cell therapy combined TKI drug can enhance cytotoxicity to lung cancer cells with EGFR resistance mutation.28 Combination of EGFR tyrokinase inhibitors and NK cells adoptive immunotherapy may achieve a higher response rate in lung cancer treatment. Recently, a study on the tumour microenvironment showed that dendritic cell (DC) accumulation in tumours often depends on NK cells that produce the DC chemo-attractants, CCL5, XCL1, and XCL2. Their transcripts closely correlate with the gene signatures of both NK cells and DCs and are associated with increased overall patient survival.29 These findings indicate that NK cells assist in breaking up tumour immune evasion and that these cells could be exploited for cancer therapy. Above all, we think that NK cell immunotherapy can help to largely improve the prognosis of lung cancers, especially those that are EGFR mutation-positive. From another aspect, our results could somehow supply key selection criteria for clinical practices in determining whether to carry out multi-cycle adoptive immunotherapy, or not to because of its expensiveness.
Although oncogenic driver mutations that activate EGFR in NSCLC predict sensitivity to specific TKIs, increasing drug resistance has appeared after first or second round therapy with the EGFR-TKIs gefitinib and erlotinib.30 For these patients, NK cell immunotherapy could be a supplemental treatment.
Supplementary Material
Acknowledgements
The authors thank all 48 participants. This study was funded by the Innovation of Science and Technology Commission of Shenzhen (Grant No: KQJSCX2017033116-0008397, JCYJ20170412155231633, JCYJ20170307171034705 and JCYJ20170816105345191). The clinical study portion was funded by the Luohu Scientific Research Project 2018-71 and the Sanming Project Medicine in Shenzhen.
Conflict of interest statement
None declared.
Data availability
All data generated in this study are available upon reasonable request to the author.
Ethics approval and consent to participate
All procedures performed in the studies involving human participants were in accordance with the ethical standards of the institutional and national research committees and with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards. This study was also approved by the Ethics Committee of Shenzhen Luohu People’s Hospital (ZLNK 03/2016, Shenzhen, China). This article does not contain any studies with animals performed by any of the authors.
Patient consent for publication
Informed consent was obtained from all individual participants included in the study.
References
- 1. Torre LA, Bray F, Siegel RL, et al. Global cancer statistics, 2012. CA Cancer J Clin 2015;65:87–108. doi: 10.3322/caac.21262. [DOI] [PubMed] [Google Scholar]
- 2. Chen W, Zhang S, Estimation ZX. Projection of lung cancer incidence and mortality in China. (in Chinese). Zhongguo Fei Ai Za Zhi 2010;13:488–93. doi: 10.3779/j.issn.1009-3419.2010.05.20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Bar J, Cyjon A, Flex D, et al. EGFR mutation testing practice in advanced non-small cell lung cancer. Lung 2014;192:759–63. doi: 10.1007/s00408-014-9604-7. [DOI] [PubMed] [Google Scholar]
- 4. Suda K, Mitsudomi T. Role of EGFR mutations in lung cancers: prognosis and tumor chemosensitivity. Arch Toxicol 2015;89:1227–40. doi: 10.1007/s00204-015-1524-7. [DOI] [PubMed] [Google Scholar]
- 5. Ljunggren HG, Malmberg KJ. Prospects for the use of NK cells in immunotherapy of human cancer. Nat Rev Immunol 2007;7:329–39. doi: 10.1038/nri2073. [DOI] [PubMed] [Google Scholar]
- 6. Wang W, Erbe AK, Hank JA, et al. NK cell-mediated antibody-dependent cellular cytotoxicity in cancer immunotherapy. Front immunol 2015;6:368. doi: 10.3389/fimmu.2015.00368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Huang AL, Liu SG, Qi WJ, et al. TGF-beta1 protein expression in non-small cell lung cancers is correlated with prognosis. Asian Pac J Cancer Prev 2014;15:8143–7. doi: 10.7314/APJCP.2014.15.19.8143. [DOI] [PubMed] [Google Scholar]
- 8. Bei R, Masuelli L, Moriconi E, et al. Immune responses to all ErbB family receptors detectable in serum of cancer patients. Oncogene 1999;18:1267–75. doi: 10.1038/sj.onc.1202442. [DOI] [PubMed] [Google Scholar]
- 9. Glushkov AN, Anosova TP, Anosov MP, et al. New approaches in evaluating the antitumor immune response in breast cancer. (in Russian). Vopr Onkol 1996;42:33–6. [PubMed] [Google Scholar]
- 10. Al Omar SY, Marshall E, Middleton D, et al. Increased killer immunoglobulin-like receptor expression and functional defects in natural killer cells in lung cancer. Immunology 2011;133:94–104. doi: 10.1111/j.1365-2567.2011.03415.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Davies JOJ, Stringaris K, Barrett AJ, et al. Opportunities and limitations of natural killer cells as adoptive therapy for malignant disease. Cytotherapy 2014;16:1453–66. doi: 10.1016/j.jcyt.2014.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Tonn T, Schwabe D, Klingemann HG, et al. Treatment of patients with advanced cancer with the natural killer cell line NK-92. Cytotherapy 2013;15:1563–70. doi: 10.1016/j.jcyt.2013.06.017. [DOI] [PubMed] [Google Scholar]
- 13. Jin S, Deng Y, Hao JW, et al. NK cell phenotypic modulation in lung cancer environment. PLoS One 2014;9:e109976. doi: 10.1371/journal.pone.0109976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Lowry LE, Zehring WA. Potentiation of natural killer cells for cancer immunotherapy: a review of literature. Front Immunol 2017;8:1061. doi: 10.3389/fimmu.2017.01061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Zhang G, Zhao H, Wu J, et al. Adoptive immunotherapy for non-small cell lung cancer by NK and cytotoxic T lymphocytes mixed effector cells: retrospective clinical observation. Int Immunopharmacol 2014;21:396–405. doi: 10.1016/j.intimp.2014.04.026. [DOI] [PubMed] [Google Scholar]
- 16. Xie S, Chen J, Zhang M, et al. Allogenic natural killer cell immunotherapy of sizeable ovarian cancer: a case report. Molecular and clinical oncology 2017;6:903–6. doi: 10.3892/mco.2017.1230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Lin M, Xu K, Liang S, et al. Prospective study of percutaneous cryoablation combined with allogenic NK cell immunotherapy for advanced renal cell cancer. Immunol Lett 2017;184:98–104. doi: 10.1016/j.imlet.2017.03.004. [DOI] [PubMed] [Google Scholar]
- 18. Lin M, Liang S, Wang X, et al. Percutaneous irreversible electroporation combined with allogeneic natural killer cell immunotherapy for patients with unresectable (stage III/IV) pancreatic cancer: a promising treatment. J Cancer Res Clin Oncol 2017;143:2607–18. doi: 10.1007/s00432-017-2513-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Lin M, Alnaggar M, Liang S, et al. An important discovery on combination of irreversible electroporation and allogeneic natural killer cell immunotherapy for unresectable pancreatic cancer. Oncotarget 2017;8:101795–807. doi: 10.18632/oncotarget.21974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Huss DJ, Saias S, Hamamah S, et al. Avian primordial germ cells contribute to and interact with the extracellular matrix during early migration. Front Cell Dev Biol 2019;7:35. doi: 10.3389/fcell.2019.00035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. McBride JA, Striker R. Imbalance in the game of T cells: What can the CD4/CD8 T-cell ratio tell us about HIV and health? PLoS Pathog 2017;13:e1006624. doi: 10.1371/journal.ppat.1006624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Li X, Asmitananda T, Gao L, et al. Biomarkers in the lung cancer diagnosis: a clinical perspective. Neoplasma 2012;59:500–7. doi: 10.4149/neo_2012_064. [DOI] [PubMed] [Google Scholar]
- 23. Ma L, Xie XW, Wang HY, et al. Clinical evaluation of tumor markers for diagnosis in patients with non-small cell lung cancer in China. Asian Pac J Cancer Prev 2015;16:4891–4. [DOI] [PubMed] [Google Scholar]
- 24. Jin XR, Zhu LY, Qian K, et al. Circulating tumor cells in early stage lung adenocarcinoma: a case series report and literature review. Oncotarget 2017;8:23130–41. doi: 10.18632/oncotarget.15506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Huang H, Young W, Chen L, et al. Clinical cell therapy guidelines for neurorestoration (IANR/CANR 2017). Cell Transplant 2018;27:310–24. doi: 10.1177/0963689717746999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Masucci GV, Cesano A, Eggermont A, et al. The need for a network to establish and validate predictive biomarkers in cancer immunotherapy. J Transl Med 2017;15:223. doi: 10.1186/s12967-017-1325-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Srivastava S, Lundqvist A, Childs RW. Natural killer cell immunotherapy for cancer: a new hope. Cytotherapy 2008;10:775–83. doi: 10.1080/14653240802648181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. He S, Yin T, Li D, et al. Enhanced interaction between natural killer cells and lung cancer cells: involvement in gefitinib-mediated immunoregulation. J Transl Med 2013;11:186. doi: 10.1186/1479-5876-11-186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Bottcher JP, Bonavita E, Chakravarty P, et al. NK cells stimulate recruitment of cDC1 into the tumor microenvironment promoting cancer immune control. Cell 2018;172:1022, e14–37. doi: 10.1016/j.cell.2018.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Faehling M, Schwenk B, Kramberg S, et al. Oncogenic driver mutations, treatment, and EGFR-TKI resistance in a Caucasian population with non-small cell lung cancer: survival in clinical practice. Oncotarget 2017;8:77897–914. doi: 10.18632/oncotarget.20857. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All data generated in this study are available upon reasonable request to the author.


