Abstract
A 51-year-old, male, non-smoker with a 3.4 cm mass in the right middle lobe was diagnosed with large cell neuroendocrine carcinoma (LCNEC). Fluorescence in situ hybridization revealed anaplastic lymphoma kinase (ALK) gene translocation, in agreement with the immunohistochemistry result obtained with use of ALK-Ventana. Radiographic examinations showed both bone and brain metastasis. After two cycles of chemotherapy consisting of etoposide and cisplatin, the patient achieved stable disease, and was subsequently switched to crizotinib. Both computed tomography and magnetic resonance imaging revealed partial response after 4 months of crizotinib, but progressed after treatment for 10 months, when several hard lymph nodes were palpable in the left supraclavicular fossa. Lymph node biopsy showed similar histology of tumor cells and targeted next-generation sequencing revealed ALK F1174L on exon 23 with two rare forms of ALK rearrangements. This case provides evidence of responsiveness of ALK inhibitors for a rare pattern of ALK-rearranged LCNEC, and suggests that F1174L, a common resistant mutation found in non-small-cell lung cancer, also causes crizotinib resistance in LCNEC.
Keywords: anaplastic lymphoma kinase, large cell neuroendocrine carcinoma, next-generation sequencing
Introduction
Anaplastic lymphoma kinase (ALK) rearrangements account for 2–7% of mutations in non-small-cell lung cancer (NSCLC), especially in adenocarcinomas. Crizotinib, an ALK inhibitor, has been shown to improve survival rate and quality of life in patients with ALK-rearranged NSCLC.1,2 There are also other histologic subtypes harboring ALK rearrangement, such as squamous cell carcinoma and neuroendocrinal carcinoma (NEC). NECs harboring epidermal growth factor receptor (EGFR) mutations were reported to be resistant to EGFR-tyrosine kinase inhibitors, probably because of the absence of EGFR expression on the membranes of NEC cells.3 However, NECs such as small-cell lung cancer (SCLC) are sometimes locally positive for ALK-Ventana but negative for ALK fusion by fluorescence in situ hybridization (FISH). A previous case was reported with large cell neuroendocrine carcinoma (LCNEC) that tested positive for ALK by immunohistochemistry, but negative by FISH or reverse transcription polymerase chain reaction, suggesting that the presence of ALK fusion genes diagnosed by immunohistochemistry should be confirmed by FISH or reverse transcription polymerase chain reaction.4 LCNECs with ALK rearrangements are extremely rare, and there are only a few case reports. One case with LCNEC harboring ALK rearrangement reportedly showed a lack of response to crizotinib,5 and two cases achieved partial response to crizotinib and alectinib.6,7 Here, we report a case of LCNEC harboring rare ALK fusion that initially responded to crizotinib and later acquired F1174L mutation causing resistance.
Case report
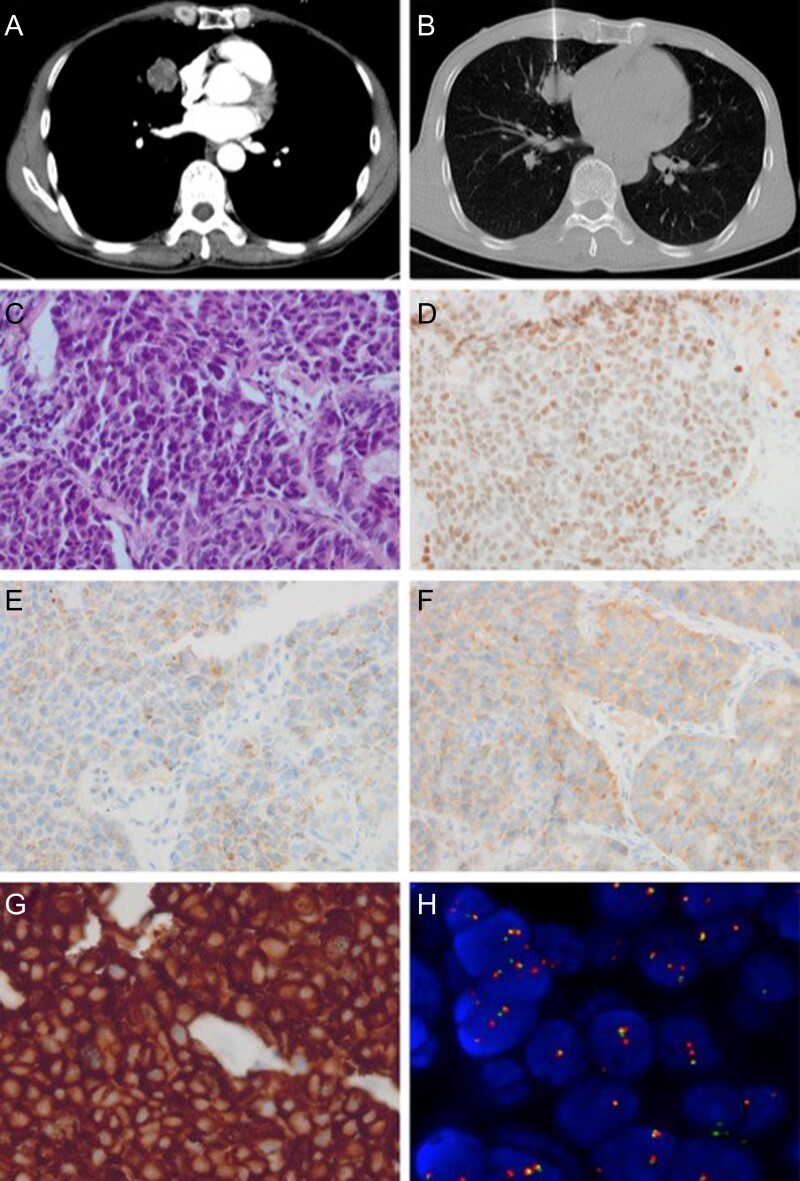
A 51-year-old, male, non-smoker presented with mild hemoptysis and chest pain. Computed tomography (CT) revealed a 3.4-cm mass in the right middle lobe (Fig. 1A). Neuron-specific enolase was 20.55 ng/ml. Percutaneous transthoracic needle biopsy revealed organoid nesting and peripheral palisading patterns, with moderate to abundant cytoplasm and prominent nucleoli. Immunohistochemistry showed positive staining for thyroid transcription factor (TTF)-1 on the nucleus, CgA and Syn in the cytoplasma (Fig. 1B–F). The patient was diagnosed with LCNEC. FISH revealed ALK gene translocation, in agreement with the immunohistochemistry result by ALK-Ventana (Fig. 1G,H). Radiographic examinations showed both bone and brain metastasis. After two cycles of chemotherapy consisting of etoposide and cisplatin, the patient achieved stable disease and was subsequently switched to crizotinib.
Figure 1.
Histopathology and immunochemistry of primary tumor. A, B. CT showed a 3.1 cm tumor in middle lobe of lung. A coaxial cutting needle was placed into the tumor for biopsy. C. In the percutaneous puncture biopsy tissue of the lung, the tumor cells showed organoid nesting and peripheral palisading patterns, with moderate to abundant cytoplasm and prominent nucleoli. (hematoxylin and eosin stain, original magnification × 300). D–F. The tumor cells showed positive staining of TTF-1 on nucleus, CgA and Syn in the cytoplasma, respectively (immunohistochemistry stain, original magnification × 300). G. The tumor cells showed positive staining of ALK-Ventana immunohistochemistry (original magnification × 400). H. ALK gene translocation was demonstrated by FISH, with an isolated red signal pattern. (original magnification × 1000)
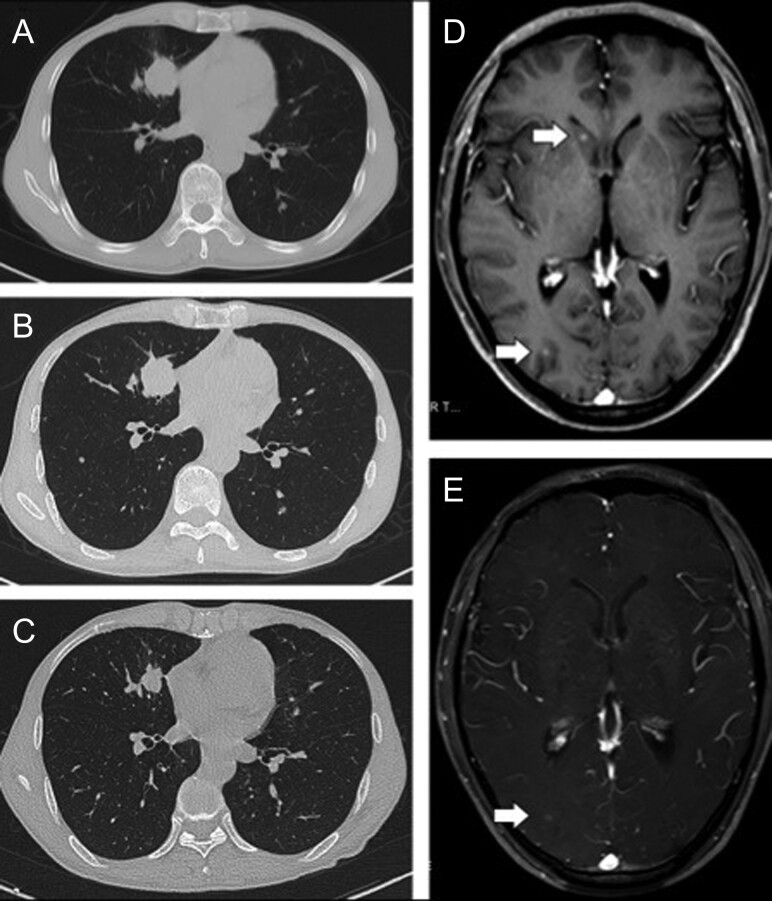
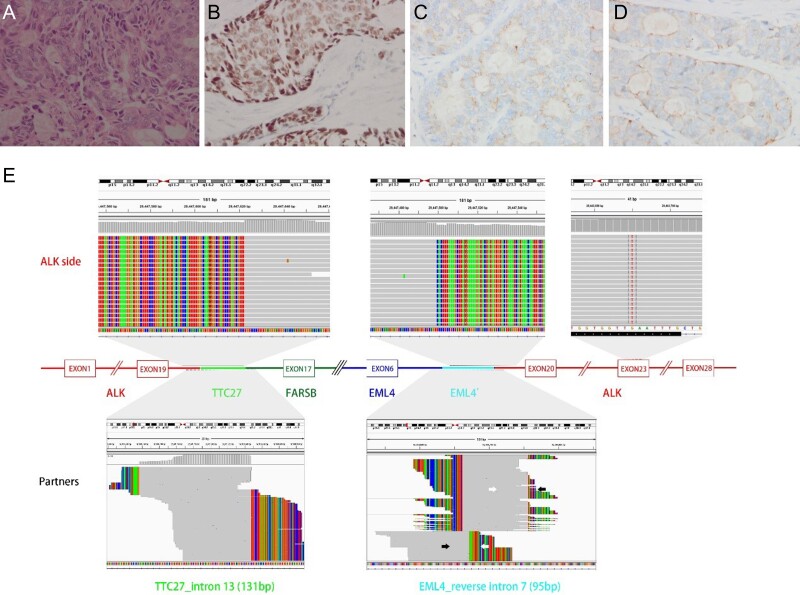
After 4 months of crizotinib, both CT and magnetic resonance imaging revealed that the patient achieved partial response, with the best response accompanied by tumor shrinkage in the lung as well as the disappearance of several nodules in the brain (Fig. 2). After treatment with crizotinib for 10 months, several hard lymph nodes were palpable in the left supraclavicular fossa. Lymph node biopsy showed similar histology of tumor cells found in the original lung biopsy: positive staining for TTF-1 on nucleus, CgA and Syn in the cytoplasma (Fig. 3 A–D). Targeted next-generation sequencing revealed ALK F1174L on exon 23, and two rare forms of ALK rearrangements. One was ALK containing exons encoding kinase region fused to exon 6 of echinoderm microtubule-associated protein-like 4 (EML4) (Allele Frequency (AF): 37.9%), but a reverse intronic sequence of EML4 (96 bp) inserted into the break point. On the other ALK side, a sequence of Gallus gallus tetratricopeptide repeat domain 27 with 131 bp linked to the break point of ALK on 3′ side, and penylalanyl-tRNA synthetase beta on 5′ side (AF: 37.6%) (Fig. 3E). According to the genetic profile, this is an adenocarcinoma such as LCENC. The tumor in the lungs and the brain remained stable. Crizotinib was continued after removal of the lymph node.
Figure 2.
Tumor response to chemotherapy and targeted therapy (crizotinib). A–C. The patient received etoposide and cisplatin for two cycles, but the primary tumor remained stable in size. After switching to crizotinib for 4 months, CT revealed shrinkage of primary tumor. D, E. Enhanced MRI of brain showed several nodules, and some of them disappeared after treatment with crizotinib for 4 months.
Figure 3.
Rebiopy of lymphnode and NGS for resistant mechanism. A. In the lymph node resection specimen, the tumor cells showed similar histology to that of lung biopsy. B–D. The tumor cells still showed positive staining of TTF-1 on nucleus, CgA and Syn in the cytoplasma, respectively (immunohistochemistry stain, original magnification × 300). E. Targeted next-generation sequencing revealed ALK F1174L on exon 23, and two rare forms of ALK rearrangements.
Discussion
In the present case, an ALK-rearranged LCENC responded to crizotinib as second line therapy, with a progression free survival of 10 months. In the first reported case, an ALK-rearranged LCENC was resistant to crizotinib.5 The authors guessed that neuroendocrine tumors with ALK rearrangement might be less responsive to ALK inhibitors. It is known that EGFR-mutated SCLCs are resistant to EGFR-tyrosine kinase inhibitors because EGFR is not expressed in SCLC.3 However, ALK is expressed in neuroendocrine tumors and other histologic tumors. Therefore, ALK rearrangement could act as a driver gene in neuroendocrine tumors, resulting in a potential favorable response to ALK inhibitors. Two previous cases reported by Zheng et al.6 and Hayashi et al.,7 and now this case have achieved partial responses, providing evidence of responsiveness of ALK-rearranged LCNEC to ALK inhibitors.
After progression, repeat biopsy from left cervical lymph nodes verified the nature of the tumor, LCENC. Targeted next-generation sequencing revealed a previously reported resistant mutation ALK F1174L, and a rare rearrangement pattern. Based on similar allelic frequencies of the two ALK rearrangements, we assumed that they occurred on one DNA strand. EML4-6/ALK-20 is a driver mutation, resulting in the responsiveness to ALK inhibitors. This case provides evidence of responsiveness of ALK inhibitors to a rare pattern of ALK-rearranged LCNEC, and suggests that F1174L, a common resistant mutation found in NSCLC, also causes crizotinib resistance in LCNEC.
Acknowledgements
The authors would like to thank Dr. Zhou Zhang for next-generation sequencing data analysis. This work was supported by the National Key Development Plan for Precision Medicine Research (2017YFC0910004), the Transformation Projects of Sci-Tech Achievements of Sichuan Province (2016CZYD0001), and the Sci-Tech Support Program of Science and Technology Department of Sichuan Province (2016SZ0073).
Conflict of interest statement
None declared.
References
- 1. Shaw AT, Kim DW, Nakagawa K, et al. . Crizotinib versus chemotherapy in advanced ALK-positive lung cancer. N Engl J Med 2013;368:2385–94. [DOI] [PubMed] [Google Scholar]
- 2. Solomon BJ, Mok T, Kim DW, et al. . First-line crizotinib versus chemotherapy in ALK-positive lung cancer. N Engl J Med 2014;371:2167–77. [DOI] [PubMed] [Google Scholar]
- 3. Le X, Desai NV, Majid A, et al. . De novo pulmonary small cell carcinomas and large cell neuroendocrine carcinomas harboring EGFR mutations: Lack of response to EGFR inhibitors. Lung Cancer 2015;88:70–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Miyoshi K, Adachi Y, Nakaji H, et al. . Neuroendocrine carcinoma of the lung expressing anaplastic lymphoma kinase on high-sensitivity immunohistochemistry: A case report. Mol Clin Oncol 2017;7:188–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Omachi N, Shimizu S, Kawaguchi T, et al. . A case of large-cell neuroendocrine carcinoma harboring an EML4-ALK rearrangement with resistance to the ALK inhibitor crizotinib. J Thorac Oncol 2014;9:e40–2. [DOI] [PubMed] [Google Scholar]
- 6. Zheng Q, Zheng MJ, Jin Y, et al. . ALK-rearrangement neuroendocrine carcinoma of the lung: a comprehensive study of a rare case series and review of literature. Onco Targets Ther 2018;11:4991–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Hayashi N, Fujita A, Saikai T, et al. . Large Cell Neuroendocrine Carcinoma Harboring an Anaplastic Lymphoma Kinase (ALK) Rearrangement with Response to Alectinib. Intern Med 2018;57:713–6. [DOI] [PMC free article] [PubMed] [Google Scholar]