Abstract
Lung cancer is the leading cause of cancer-related deaths in China, with over 690 000 lung cancer deaths estimated in 2018. The mortality has increased about five-fold from the mid-1970s to the 2000s. Lung cancer low-dose computerized tomography (LDCT) screening in smokers was shown to improve survival in the US National Lung Screening Trial, and more recently in the European NELSON trial. However, although the predominant risk factor, smoking contributes to a lower fraction of lung cancers in China than in the UK and USA. Therefore, it is necessary to establish Chinese-specific screening strategies. There have been 23 associated programmes completed or still ongoing in China since the 1980s, mainly after 2000; and one has recently been planned. Generally, their entry criteria are not smoking-stringent. Most of the Chinese programmes have reported preliminary results only, which demonstrated a different high-risk subpopulation of lung cancer in China. Evidence concerning LDCT screening implementation is based on results of randomized controlled trials outside China. LDCT screening programmes combining tobacco control would produce more benefits. Population recruitment (e.g. risk-based selection), screening protocol, nodule management and cost-effectiveness are discussed in detail. In China, the high-risk subpopulation eligible for lung cancer screening has not as yet been confirmed, as all the risk parameters have not as yet been determined. Although evidence on best practice for implementation of lung cancer screening has been accumulating in other countries, further research in China is urgently required, as China is now facing a lung cancer epidemic.
Keywords: lung cancer, China, screening, recommendation, low-dose computerized tomography, risk factor, tobacco control, pulmonary nodule management
Introduction
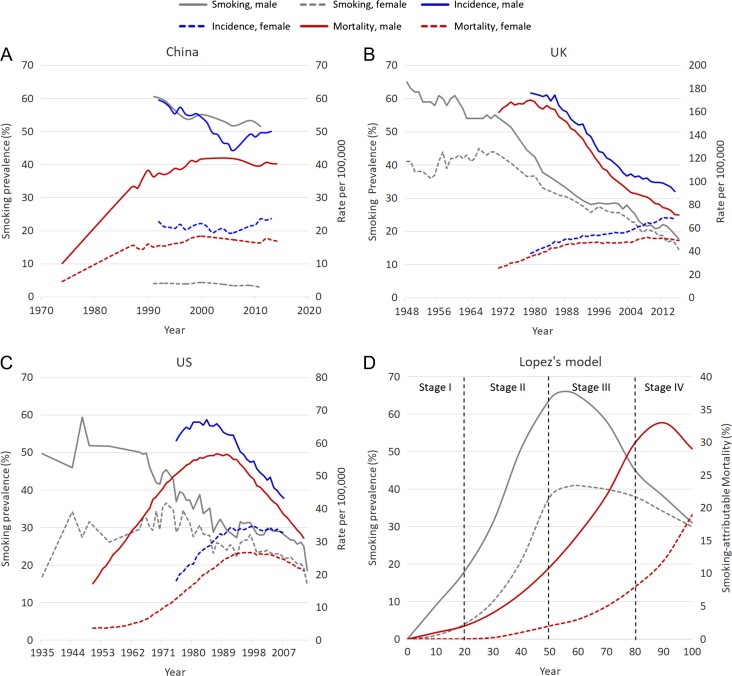
Lung cancer has an extremely high incidence and mortality rate, and is recognized as a major public health problem all over the world, increasingly so in developing economies that have not heeded the dangers associated with smoking uptake. China, the most populous country in the world, has approximately 20% of the world population but has over one-third of the newly diagnosed lung cancer cases and lung cancer deaths worldwide, which were projected at over 774 000 and 690 000 in 2018 by GLOBCAN1 (Table 1). Lung cancer is the most commonly diagnosed cancer in Chinese men and the second most commonly diagnosed in Chinese women.2 During 2000–2010, there was a slight but insignificant decrease in incidence rate in males of 0.2% per year, but an annually significant upward change of 0.9% in females.2 The male-to-female incidence ratio decreased from 1.56 to 1.35 over the period of 1989–2008.3 However, mortality has increased in recent years, from 5.47/100 000 in the mid-1970s, to 17.27/100 000 in the early 1990s, and then 30.83/100 000 in the 2000s.2,4 Since then, lung cancer has become the leading cause of cancer-related deaths for both genders2 (Fig. 1A).
Table 1.
Estimated incidence and mortality rate (world population age-standardized per 100 000) of lung cancer in China, the UK, and the US, all ages.
| Incidence | Mortality | |||||
|---|---|---|---|---|---|---|
| Total | Male | Female | Total | Male | Female | |
| China | 35.1 | 47.8 | 22.8 | 30.9 | 43.4 | 19.0 |
| UK | 32.5 | 35.5 | 30.2 | 22.2 | 25.2 | 19.7 |
| US | 35.1 | 40.1 | 30.8 | 22.1 | 25.9 | 19.0 |
Data extracted from GLOBCAN 2018.1
Figure 1.
Trends in smoking prevalence, lung cancer incidence, and mortality, by sex. (A) China,4–13 (B) the UK,14 (C) the US,6,12,46 and (D) Lopez’s model of the cigarette epidemic.15
Attributable risk factors
Internationally, smoking is considered to be the predominant risk factor for lung cancer. However, in China, the proportion of lung cancer cases attributable to smoking was 57.5% in males and 11.5% in females in 2013, respectively,16 which is much lower than that reported in the United Kingdom (UK, 85% in males and 80% in females in 2010)17 and the United States (US, 84.4% in males and 78.9% in females in 2014).18 Lung cancer incidence among male and female non-smokers estimated from the 2010 national data was over three times that of 1990 US never-smokers.19 The attributable fractions of lung cancer cases and deaths to smoking were similar.16,18 Therefore, other risk factors, including outdoor as well as indoor air pollution (i.e. second-hand smoking exposure), prior lung diseases [i.e. tuberculosis infection, chronic obstructive pulmonary disease (COPD)], and family history of cancer are considered to have a more important role in China, especially in never-smokers, than in other regions or populations.17,18,20
Second-hand smoking exposure was estimated to contribute to 3.0% of male and 22.0% of female lung cancers in never-smokers aged ≥30 years in China (2013).16 The attributable fraction of lung cancer cases in Chinese never-smoking females is much higher than their counterparts in the UK (15.4% in all ages in 2010)17 and the US (2.3% in ages ≥30 years in 2014),18 as is lung cancer deaths.16,18
Use of coal for household heating and cooking—another component of indoor air pollution—is also a significant risk factor in China.20 The lung cancer mortality in Xuanwei County, Yunnan Province, ranking among the highest in China is the best example: two to three times and four to seven times higher in local male and female residents, respectively, than in other contemporary rural areas (in the early 1990s, mid-2000s and early 2010s).21 Use of smoky coal and unimproved domestic stoves is the main reason for this.21 Outdoor air pollution [i.e. particulate matter (PM)] becomes increasingly significant in China,22 with lung cancer risk ratios of 1.03, 1.04, and 1.03 per 10 μg/m3 in relation to PM2.5, SO2, and nitrogen oxides, respectively.22,23 Occupational history (i.e. construction),24 radiation (i.e. residential radon radiation),20 and unhealthy diet (i.e. low fruit/vegetable intake)16 also have a significant influence on lung cancer risk or death in China.
Recent data have demonstrated that genetic factors modulate cancer pathogenesis. Genome-wide association analysis revealed susceptibility loci for lung cancer, e.g. the 15q2525,26 or 5p1527,28 loci, yet with different profiles of genetic variants between Chinese and Caucasians. Evidence also shows significant gene-smoking interactions in lung cancer, e.g. rs1316298 and rs4589502 in the Chinese population, which may shed light on the lung cancer aetiology.29 Investigations into familial lung cancers have indicated a number of predisposing germline mutations, e.g. EGFR T790M (mostly Caucasians), EGFR V843I, and HER2 G660D (East Asians).30 Furthermore, somatic mutation profiles differ between lung cancer subgroups in terms of smoking status, ethnicity, and histological subtypes31–33; e.g. EGFR mutations are more likely present in non-smokers [compared with smokers: 67.2% vs. 27.0% in Chinese non-small cell lung cancers (NSCLCs)],31 East Asians (compared with other ethnicities: 30% vs. 8%),32,33 and lung adenocarcinomas (compared with squamous cell cancer: 40.4% vs. 2.5% in smoking lung cancers in China).31 These results demonstrate that lung cancer is not a single disease.31,34 The nature of lung cancer in China is therefore not solely attributable to environmental factors, but is further complicated by genetic influences.
Tobacco use
Epidemiology
China is the largest tobacco producer and consumer in the world. In 2016, it manufactured over 2.9 million tons of tobacco.35,36 There were estimated to be over 300 million current smokers aged ≥15 in China including 288.1 million males and 12.6 million females in the 2010 Global Adult Tobacco Survey (GATS).37
In the China Health and Nutrition Surveys 1991–2011, the prevalence of current smoking in individuals aged ≥15 was reported to successively decline from 60.6% to 51.6% in males and 4.0% to 2.9% in females5 (Fig. 1A). However, the ever-smoking prevalence in both genders did not alter greatly during that time.5 Specifically, females’ smoking uptake rate decreased in generations who were born during the 1930s–1970s,38 but increased in the younger generations born in the 1980s and thereafter.39 The prevalence of smoking in females aged 12–17 during 1981–2010 multiplied from 2.47% to 19.72% for ever-smokers and from 0.29% to 3.26% for current smokers.39 Collapse of ‘cultural prohibitions against smoking among young women’ as a result of socio-economic and political changes39 (i.e. probably reform and opening up in China since 1978) might be responsible for the uptake increase in Chinese young women, which was similar to that witnessed in the US and UK during and after World War II40 (Fig. S1). Overall, the current smoking prevalence in both genders has slightly declined over the last 20 years5; however, a slight increase has been reported in the younger female subgroup.39 Given that China is the most populated country in the world, the number of smokers is strikingly high.
In contrast, very different trends in smoking prevalence were observed in the UK and the US (Fig. 1B and C). In the UK, the tobacco-uptake rate peaked at 82% in 1948 among males and 45% in the mid-1960s among females, respectively.41,42 This was followed by continuous decline in both men and women in the following decades42 (Fig. 1B). In 2017, overall current smoking prevalence in the UK was 15.1%,43 which is among the lowest prevalence rates in Europe44; although there are still significant gaps in smoking uptakes in specific regions within the UK (e.g. 22.0% in Manchester versus 6.4% in Chiltern located in South West England in 2017),43 which are closely related to deprivation status.45 The trend in the US46,47 is very similar to that in the UK (Fig. 1C). Caution is required when comparing these data, as differing definitions for smoking rates and statistical methods have been used in reporting smoking cessation rates in different countries.
Smoking-related mortality
It is perceived that there is a long delay between the peak of smoking prevalence and its full impact on mortality. Cigarette epidemiology was first described by Lopez et al. as a four-stage model in 199415 (Fig. 1D). The model precisely described the relationship between smoking and smoking-related deaths in males and females in economically developed countries, such as the UK and the US. It largely reflected the interaction between smoking and lung cancer mortality, as smoking was attributed to over 80% of lung cancer deaths in these countries.18,48 Both countries may be currently experiencing the fourth stage in which smoking prevalence in both genders has decreased in recent years yet with mortality converging15 (Fig. 1B and C).
In China, the situation appears more complicated. The earliest nationally representative prevalence survey on smoking in China was in 1984, only a little over 30 years ago (Figs S1–S2),49 whereas there are over 60 years of records in the UK41,42 and US.46,47 China has made great efforts to move forward in cancer surveillance, particularly following the launch of the National Central Cancer Registration (NCCR) in 2002.50,51 There has been a surge in the number of both cancer registration points in total and those included in the reports of Cancer Incidence in Five Continents (CI5), the latter taken as an indicator of data quality.50 The latest version of CI5 (CI5 Vol. XI) released in 2017, included data from 35 points collected during 2008–2012, almost three times that included in the previous version, indicating a significant improvement in data quality (Fig. S2).50,52 However, there are concerns regarding the population coverage by cancer registry, data quality control, and data representativeness, etc.50,51 Cancer registries providing data with good quality are more established in eastern, developed, and urban areas,2,7 which compromises data representativeness nationally.50,51 Most of the rural cancer registries are established in high-risk regions of cancer50,53 and have a lower level of population coverage.50 Furthermore, the overall cancer mortality estimated from rural cancer registries was 13% higher than the estimate of the third National Death Survey, indicating overestimation; the difference was even more significant in some specific cancer types.53
Substantial healthcare disparities exist across China, as indirectly evidenced by geographical variations in all cancer mortality and its 5-year survival in 2015: the estimates for rural areas were considerably worse than for urban areas [149.0 vs. 109.5 per 100 000 (age-standardized by world population) and 30.3% vs. 42.8%, respectively]; similarly Southwest China was worse than East China (170.2 vs. 115.6 per 100 000, and 24.9% vs. 40.3%, respectively).2 In contrast to the urban population, the rural population are more likely to underuse healthcare resources (e.g. less likely to choose self-care, outpatient, and inpatient care versus no care) because of inferior health insurance coverage and reimbursement procedures associated with the two-class social insurance system.54 Unbalanced health service supply54 and a lack of qualified primary healthcare providers55 impede rural individuals’ equitable access to healthcare,54 and induce a high rate of misdiagnosis and/or inappropriate treatment thus poor management of chronic diseases.55 Factors that potentially increase financial risks are also non-negligible,54 e.g. travelling distance54 and low annual household income (rural compared with urban: US$2587 vs. US$4761 on average in 2011).56 Western and central China have experienced similar healthcare inequalities, where the economy is less developed than eastern China.56 Encouragingly, the gaps between regions are shrinking as continuous efforts are made in healthcare reforms by the government.54,56,57
Thus, caution cannot be overemphasized in data interpretation because of potentially poor representation of current experience in rural and underdeveloped areas. From current data, China is most likely experiencing the ‘third stage’ of the four-stage model at this time.58 In Stage III, males’ smoking prevalence starts to decline; while females’ could rise first, because of a resurgence of uptake in the younger generation39 and peak at a later time. Both genders show a continuous increase in mortality in Stage III.15 It’s worth noting that smoking patterns are changing in younger generations, in terms of an earlier age of initiation (e.g. before 20 years old) and consumption of more cigarettes daily.38 Moreover, the attributable fraction of smoking has probably not reached its full impact to date,40 considering the lower smoking attributable fraction to lung cancer in China.16 Hence, severe health consequences are likely to occur in China in the upcoming years. However, these are only assumptions based on limited data, and the likely times at which smoking and mortality in both genders will peak or decrease are as yet undefined (this information requires data from national tobacco surveys/cancer registries in future years).
Social changes and historical events are also responsible for the different trends in smoking and mortality between China and the UK and US (Fig. S1), and these differences continue even today. Interventions have been encouraged to reduce the growth in tobacco consumption and risk of death from tobacco-related diseases.
Interventions for lung cancer
More than one-half of lung cancer cases were diagnosed at a very late stage throughout these years, as evidenced by the retrospective data from West China Hospital59 and the US national cancer registries’ statistics.60 These late-stage lung cancer patients have a minimal chance of successful therapeutic intervention, thus resulting in inferior prognosis. The 5-year survival rate in this subgroup is only 5% in the US,60 and well below 5% in the UK.14 It is now agreed that an integrated programme of tobacco control with earlier detection through low-dose computerized tomography (LDCT) screening would facilitate an improvement in lung cancer survival.
Tobacco control
The protective effect of smoking cessation increases with the quitting duration in ex-smokers who stopped smoking either by choice (while still healthy) or because of illness.38,61,62 However, the mortality risk is still somewhat higher than in never-smokers.38,61,62 Quitting before the age of 40 years would avoid over 90% of the excess deaths caused by regular smoking61,62; and adults who had quit smoking early enough would gain 10 extra years of life expectancy compared with those who continued to smoke.62
In 2005, China ratified the World Health Organization Framework Convention on Tobacco Control (WHO FCTC).63 The framework aims to reduce tobacco use among countries worldwide. It has six elemental compositions called MPOWER, including Monitoring, smoke-free Policies, Offer help to cessation, health Warnings, Enforcing advertising bans and Raising taxes.63 Enforcement of these measures in China is still weak, compared to the UK, which has adopted comprehensive MPOWER measures at a best-practice level.63 The major obstacle remains the state-owned tobacco industry.49,58 The state tobacco monopoly in China is in charge of both tobacco manufacturing and selling, and tobacco control in the WHO FCTC.49 ‘The tipping point’ was a documentary by the Party School in 2013, which discussed historical and philosophical perspectives on tobacco and tobacco control in China, including the conflicting interests of the Chinese tobacco monopoly.49 Since then, tobacco control initiatives have been conducted one after another, including the tax readjustment in 201549 (Fig. S1). Although the percentage of tax in the retail price (56%) is still lower than the WHO’s recommendation of at least 70%,64 some early positive impacts have been reported in 2018.65 Cigarette sales have dropped from 127 billion packs in 2014 to 117 billion in 2016, with a decline of 0.2–0.6% estimated in adults’ smoking prevalence during this period, i.e. 2.2–6.5 million fewer smokers, which could be related to the increased cigarette prices.65 China is moving forward in tobacco control, albeit slowly. It is crucial for China to take further action in comprehensive legislation, taxation, education, and tackling the current dual identity of the state tobacco monopoly.49
Lung cancer screening outside China
A number of lung cancer screening trials have been undertaken since the 1980s, but use of chest X-rays (CXR) with/without sputum cytology did not identify any mortality reductions.66,67 LDCT has been found to be more sensitive than CXR in detection of lung cancers in observational studies,66,68 with the potential to improve survival by detecting lung cancer at an early stage, i.e. in Early Lung Cancer Action Program (ELCAP)68 and later in International ELCAP (I-ELCAP)69. Accordingly, lung cancer screening trials, mainly randomized controlled trials (RCTs), have been undertaken in the US (National Lung Screening Trial, NLST),70 Europe (eight RCTs),71–78 and lately in Japan (Japanese randomized trial for evaluating the efficacy of low-dose thoracic CT screening for lung cancer, JECS)79 to investigate the benefits of screening by LDCT (Table S1).
Briefly, NLST was the first RCT to report mortality reduction by LDCT screening. In 2011, this trial reported a 20% reduction in lung cancer mortality and 6.7% reduction in all-cause mortality in the LDCT arm when compared with the CXR arm after a median follow-up of 6.5 years post randomization.70 Since then, multiple organizations in the US have approved annual screening for high-risk individuals based on the NLST results.80–84 Four European trials - Danish Lung Cancer Screening Trial (DLCST), Detection And screening of early lung cancer with Novel imaging TEchnology (DANTE), Italian Lung Cancer Screening Trial (ITALUNG), Multicentric Italian Lung Detection project (MILD) - reported on mortality, despite not having sufficient study power to test this, but none of them demonstrated a protective role of LDCT concerning mortality reduction.72–75 However, the Nederlands Leuvens Longkanker Screenings Onderzoek (NELSON) trial, the only fully powered trial in Europe, reported at the 2018 World Conference on Lung Cancer (WCLC 2018) a 26% decrease of lung cancer mortality in males and an even higher reduction in its smaller-sized population of females, which ranged from 39% to 61% depending on the length of follow-up of 8-10 years.71
Lung cancer screening in China
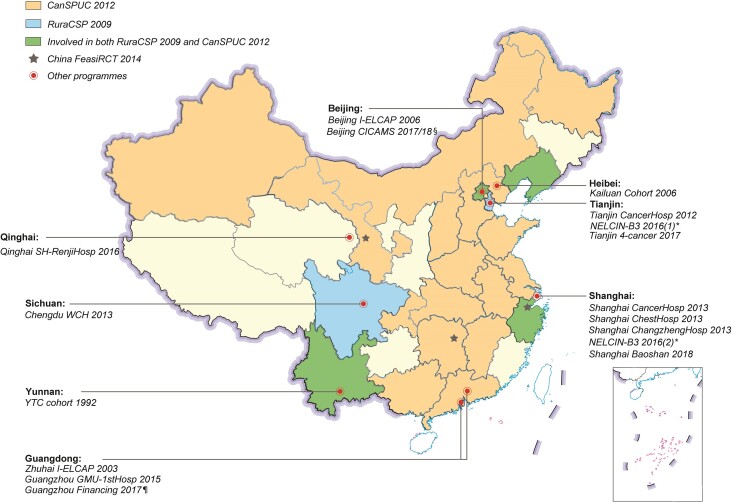
We searched four Chinese databases [China National Knowledge Infrastructure database (CNKI), Wanfang Data, Chongqing VIP database, and Chinese Clinical Trial Registry Centre Library] and four English databases (PubMed, Embase, Web of Science Core Collection Library, and Cochrane Library) as of 10 September 2018 from the earliest dates available. Other sources (e.g. references in reviews/articles, policies/news from government websites and personal communication with principal investigators) were also used (Supplementary data). Generally, most of the publications concerning LDCT and/or CXR were retrospective cohorts (e.g. in a population undergoing regular physical examinations), case-control studies (e.g. comparison in the performance of LDCT versus CXR in selected populations) or cross-sectional studies (e.g. with one-time LDCT/CXR screening). Therefore, we considered only prospective cohorts and RCTs here. Twenty-three associated programmes have been completed or are ongoing in China since the 1980s, the majority after 2000; and one has recently been planned (Fig. 2, Table 2; Supplementary data).
Figure 2.
The landscape of lung cancer screening programmes in China since the 1990s, with the coloured areas being the regions covered by the corresponding national programmes.85–88 *NELCIN-B389 has three study centres in China: two in Shanghai (Shanghai Changzheng Hospital and Shanghai General Hospital) and one in Tianjin (Tianjin Medical University Cancer Institute and Hospital). § Including three separate programmes sponsored by central government90,91: one in 2017 and another two (including a multicentre RCT) in 2018. ¶ The Guangzhou Financing project92 was proposed in 2017 and is still being discussed currently.
Table 2.
Lung cancer screening programmes in China.
| Time | Trial/study name used in the manuscript (ref.)* | Initiation year | Targeted region/population | Study design | Interventions | Entry criteria | Population (recruiting time) |
|---|---|---|---|---|---|---|---|
| Before the 1990s | Mass photofluorography in early detection of peripheral lung cancer93 | 1979 | N/A | Prospective cohort | Annual CXR for 5 years | Workers from 54 factories; no other restrictions | 211 811 person-years (1979–1983) |
| Mass screening in Hunan orpiment miners94 | 1986 | Hunan orpiment miners | Prospective cohort |
|
Orpiment miners in Hunan; aged >35 years | 601 (baseline) | |
| Screening lung cancer by Sputum Occult Blood Test (OBT) Study95,96 | 1988 | Workers in Changchun automobile industries, Tangshan and Yunnan tin mines, Xuanwei and Beijing steel factories | Cross-sectional study | Sputum OBT and cytology with/without CXR | High-risk workers from various manufacturing and mining factories, including some famers/cadres; aged ≥40 years | 14 431 (1988–1990) | |
| 2007 | Laibing County, Xuanwei (Yunnan) | Prospective cohort |
|
Residents aged 35–70 years | About 30 000 at baseline (January 2007-July 2007) | ||
| 1990s | The Yunnan Tin Corporation (YTC) cohorta97 | 1992 | Around Gejiu City, Southern Yunan | Prospective cohort | Annual sputum sampled + annual CXR | Current/retired YTC workers, aged ≥40 years, with a history of underground mining/smelting ≥10 years | 9143 (1992–1999) |
| 2000s | Zhuhai I-ELCAP cohort98 | 2003 | Zhuhai, Guangdong province | Prospective cohort | Annual LDCT | Asymptomatic participants aged ≥40 years | 3582 (2003–2009) |
| Beijing I-ELCAP cohort99 | 2006 | Beijing, China | Prospective cohort | Annual LDCT | Asymptomatic participants aged ≥40 years, no history of malignancy (except basal cell carcinoma and cervical carcinoma in situ) within 5 years | 4690 (2007–2012) | |
| Kailuan cohorta100 | 2006 | Kuailuan Group Company, Tangshan City, Heibei Province | Prospective cohort | Biennial CXR; annual follow-up in 11 hospitals affiliated to the Kailuan Company | Current or retired employees aged ≥18 years in the Kailuan Group Company (mining industry) | 133 273 (2006–2011) | |
| 2010s | Rural China Cancer Screening Programme (RuraCSP)b85,86 | 2009 | Dagang Oilfield (Tianjin), Xuanwei (Yunnan), Gejiu (Yunnan), Beijing, Chengdu (Sichuan), and Shenyang (Liaoning) | Prospective cohort | Annual LDCT and sputum cytological examination (for 3 years) | Inclusion criteria are region-dependent: 50–74 years (in Tianjin), 45–69 years (in Yunnan), staff aged 50–74 years and smoking history of ≥20 pack-years (in the Dagang Oilfield). The Xuaiwei centre included indoor air pollution as a risk factor | 19 068 (2010–2017, baseline participants) |
| Cancer Screening Program in Urban China (CanSPUC)a87,88 | 2012 | 20 provincial/municipal-level regions in China by 2018 | Prospective cohort | Annual LDCT for 5 years | Urban residents (residing >3 years) aged 40–69 (some areas defined ages at 40–74) with high risk of lung cancer; high-risk criteria are region-dependent | 210 000 (planned in the first stage during 2012–2016) | |
| The China Cancer Screening Trial Feasibility Study (China FeasiRCT) b101,102 | 2014 | Three cities (Changsha[Hunan]; Lanzhou[Gansu]; Haining[Zhejiang] | RCT |
|
Local permanent residents; aged 50–74 years; smoking >30 pack-years, quit ≤15 years if former-smokers (or second-hand smoke exposure in females: living with a regular daily smoker for >20 years); no previous history of lung cancer or colorectal cancer | 2700 (as of 31 March 2015) | |
| Beijing CICAMS programmesc,d90,91 | 2017, 2018 | Beijing | N/A | N/A | N/A | N/A | |
| Tianjin CancerHosp cohort103 | 2012 | Tianjin | Prospective cohort | LDCT at Baseline and 1 or 2 years later | Asymptomatic, aged ≥40 years-, tolerant of possible invasive procedures and not screened by CT within 1 year | 650 (2014–2016) | |
| Tianjin 4-Cancer programmeb104 | 2017 | Selected districts in Tianjin: Hexi and Jinzhou in 2017; will cover up to seven districts planned in 2018 | Prospective cohort | LDCT screening; and then follow-up for LDCT result-positive participants | Healthy residents will undergo risk assessment first and those at high risk will undergo LDCT screening | 52 092 risk assessed; 992 LDCT screened (2017) | |
| Shanghai CancerHosp cohort105 | 2013 | Seven selected communities in Minhang District, Shanghai | Prospective cohort | Annual LDCT; community-based, LDCT + CAD for screening |
|
11 332 (2013–2014) | |
| Shanghai ChestHosp RCT106 | 2013 | Six selected communities in Xuhui District, Shanghai | RCT | Biennial LDCT versus usual care arm (for three rounds) | Asymptomatic residents aged 45–70 years, with ≥1 risk factor: 1) a smoking history ≥20 pack-years, and if former-smoker, quit ≤15 years; 2) family history of cancer; 3) personal cancer history; 4) occupational exposures; 5) long-term exposure of passive smoking (>2 h/day at home/indoor workplaces for ≥10 years); 6) long-term exposure to cooking oil fumes (>50 dish-years) | 6717 (2013–2014): | |
| Shanghai-ChangzhengHosp cohort107,108 | 2013 | Physical examination centres in seven tertiary hospitals and their surrounding communities | Prospective cohort | Baseline LDCT + CAD; interval scans were not specified | Asymptomatic; any age | 14 506 (2013–2016) | |
| Netherlands-China Big-3 screening (NELCIN-B3) a,d89 | 2016 | Shanghai Changzheng Hospital, Shanghai General Hospital and Tianjin Medical University Cancer Institute & Hospital | N/A | LDCT screening | N/A | N/A | |
| Shanghai Baoshan Programmeb109,110 | 2018 | Baoshan District, Shanghai | Prospective cohort | One-time CT; referral to a hospital for further assessment if positive results; and follow-up | Ages ≥75 years, or ≥65 years yet with cough/expectoration ≥2 weeks and abnormal CXRs | 14 005 (as of September 2018) | |
| Chengdu WCH cohortd | 2013 | Chengdu, Sichuan Province | Retro-prospective cohort | Annual CXR or LDCT | Workers of specific industries/enterprises/organizations undergoing annual physical examinations (CXR or LDCT) (records back to the year 2006) | Baseline: 46 317 (by CXR); 15 996 (by LDCT) | |
| Guangzhou GMU-1stHosp Programme92,111 | 2015 | Guangzhou, Guangdong Province | Prospective cohort | Annual LDCT | Low-income residents aged ≥50 years; or residents in Yuexiu district, aged 50–74, with high risk; or volunteered residents aged ≥40 years in the whole province (the former two will get a free screening; but the latter a 1/5 discount on screening costs) | 808 (as of December 2017) | |
| Guangzhou Financing project (in planning)b92 | N/A | Guangzhou, Guangdong Province | Prospective cohort | N/A | 40–80 years; residents undergoing health checks through their employers’ health insurance or out-of-pocket payments, or occupational workers at higher risk of air pollution in working environment | 10 000 (planned) | |
| Qinghai SH-RenjiHosp programme112,113 | 2016 | Deprivation areas in Qinghai (would be expanded to Henan, Xinjiang and Shandong Province) | N/A | N/A | Aged 50–74; or aged ≥35 but with ≥1 risk factor including long-term smokers, long-term exposure to severe air pollution, radiation, coal smoke and kitchen fumes, with a family history of lung cancer, a personal history of cancer or pulmonary diseases | N/A |
*Most of the CT trial/programme (since 2010) names have been provided in the above table to identify the targeted region and the hospital in which they are undertaken otherwise stated for the purpose of this review. CAD, computer-aided diagnosis system; CICAMS, Cancer Institute & Hospital Chinese Academy of Medical Sciences; GMU-1stHosp, Guangzhou Medical University First Affiliated Hospital; LDCT, low-dose computerized tomography; N/A, not applicable or not available; RCT, randomized controlled trial; Shanghai CancerHosp, Fudan University Shanghai Cancer Centre; Shanghai ChangzhengHosp, Shanghai Changzheng Hospital; Shanghai ChestHosp, Shanghai Jiaotong University affiliated Shanghai Chest Hospital; SH-RenjiHosp, Shanghai Jiaotong University Affiliated Renji Hospital; Tianjin CancerHosp, Tianjin Medical University Cancer Institute and Hospital; WCH, West China Hospital.
aYunan Tin Corporation cohort, Kailuan cohort, CanSPUC, and NELCIN-B3 are formal names of the programmes, respectively.
bNamed after the studies’ characteristics by the author: RuraCSP, Rural China Screening Programme; China FeasiRCT, China Lung Cancer Screening Feasibility RCT; Tianjin 4-cancer programme, screening of the four common cancers (lung cancer, breast cancer, liver cancer, and stomach cancer) in Tianjin; Shanghai Baoshan programme, lung cancer screening programme in old people in Baoshan District, Shanghai; Guangzhou Financing project, a demonstration project targeting Guangzhou to expand lung cancer screening and test innovative financing models.
cIncluding three separate programmes funded by central government: one in 2017 and another two (including a multicentre RCT) in 2018.
dPersonal communication with the corresponding principal investigators Professor Wu Ning, Professor Ye Zhaoxiang, Professor Li Weimin, respectively. Please see the Supplementary data for details.
Generally, earlier studies targeted occupational populations and applied CXR and/or sputum examination for lung cancer screening.93–97,100 They mainly investigated the effectiveness of screening and lung cancer-associated risk factors [e.g. The Yunnan Tin Corporation (YTC) cohort97 and the Kailuan cohort100]. Municipal or city-level screening programmes92,103–109,111 are increasing, particularly after the central government-led programmes [Rural China Screening Programme (RuraCSP)85,86 in 2009 and Cancer Screening Program in Urban China (CanSPUC)87,88 in 2012]. Most of the programmes referred to above are pilot or feasibility studies to investigate the effectiveness of LDCT screening.
Some institutes have built collaborative relationships with international organisations [i.e. Zhuhai I-ELCAP,98 Beijing I-ELCAP,99 Netherlands-China Big-3 screening (NELCIN-B3)89], to help to clarify characteristics and to accumulate evidence on lung cancer screening in China. NELCIN-B3,89 a Netherlands-China collaborative, multicentre study, will focus on the three major diseases of the thorax—lung cancer, cardiovascular disease, and chronic obstructive pulmonary disease—using one-stop CT imaging technology in the context of LDCT screening. NELCIN-B3 is expected to provide more evidence on the management of both nodules and other thoracic diseases.89
Notably, the majority of the programmes are funded by central or local government, which is argued to be unsustainable and unaffordable for a larger-scale programme in the long run.92 The Guangzhou Financing demonstration project in planning will investigate potential financing models to cope with costs during the screening implementation.92 Charity foundations and supports of companies could also play a role in the financing [i.e. Guangzhou Medical University First Affiliated Hospital (Guangzhou GMU-1stHosp) programme92,111 and Qinghai SH-Renji programme112,113]. The reader should be aware of the limitations of the references to many of the Chinese CT screening programmes, which are based only on web pages or conference abstracts, thus caution is required in interpretation.
To date, the majority of the studies have reported only their preliminary results, suggesting possible benefits of LDCT in detecting early lung cancers. However, concerning high-risk definition, nodule management, and mortality outcomes, evidence in China is quite limited at this time. There is a different risk profile for lung cancer in China, as indicated by the baseline/preliminary results from Beijing I-ELCAP,99 Tianjin CancerHosp,103 Shanghai CancerHosp cohort105 and Shanghai ChestHosp RCT106: females and non-smokers could have a lung cancer detection rate comparable to or even higher than males and smokers in China. Therefore, risk stratification based on exotic guidelines or entry criteria could result in significant misdiagnosis in the Chinese population.
Using microsimulation modelling, Sheehan et al.114 compared eligibility criteria of Centres for Medicare & Medicaid Services in 2015 (CMS 2015: ages 55–77 and smoking ≥30 pack-years, quitting ≤15 years if former smokers)84 and the 2015 China National lung cancer screening (CNS 2015: ages 50–74 and smoking ≥20 pack-years, quitting ≤5 years if former smokers)115 in the Chinese population if annual LDCT screening was applied from 2016 to 2050. Applying CNS 2015 criteria would have a lower mortality reduction in males (6.30% vs. 6.58%), but a higher mortality reduction in females (2.79% vs. 1.97%), namely 2.9% more lung cancers prevented when compared to CMS 2015 criteria. However, more screens would be needed when using CNS 2015 criteria (1.43 billion vs. 998 billion if CMS 2015 criteria applied).114 In decision analysis, Wang et al.116 simulated a cohort of 100 000 Chinese urban smokers aged 45–80 who would receive a one-off screening. They found there would be a lung cancer mortality reduction of 17.2% and 24.2% by LDCT screening when compared to CXR screening and no screening, respectively. In the LDCT screening scenario, there would be 9387 false diagnosis and seven deaths attributed to false diagnosis; in CXR screening, the numbers would be 2497 and two, respectively. Lung cancer prevalence, LDCT sensitivity, and proportion of early stage in lung cancers detected by LDCT would influence mortality reduction the most in the LDCT screening arm when compared to no screening.116 These results demonstrate the possible benefit of mortality reduction in China and also the urgent necessity for better definition in high-risk eligible individuals.
Many hospitals have established independent programmes, but now need to collaborate to work to consensus protocols and data collection methods, to provide data which can be used throughout the whole of China. A good example of international collaboration is the European Position Statement on lung cancer screening,117 where a consensus approach throughout Europe has been agreed. Evidence specific to China is awaited as the majority of the programmes are still ongoing. It is essential to consider what other countries have done in terms of Chinese conditions; thus, we can better aim to curb lung cancer suffering in this specific population.
Integrating tobacco control into screening programmes
It is considered that ongoing lung cancer screening programmes provide a ‘teachable moment’ for the participating smokers, thereby motivating smoking cessation and maximizing overall cancer prevention benefit, as was introduced first and assessed in ELCAP in 2001.118 Subsequently, positive effects of screening programmes on quitting,119–121 and CT abnormality-dependent smoking cessation120,122,123 have been illustrated in other trials. Researchers also found that consistently negative scans were not necessarily related to a lower rate of smoking abstinence or a higher percentage of relapse.124 Quitting smoking has also been reported to benefit participants’ outcomes within the frame of lung cancer screening programmes, where the mortality reduction could be comparable to or even exceed that achieved by LDCT screening alone,125 even in late quitters who stopped smoking during follow-up after baseline scan.126 In 2018, a group of researchers formed the Smoking Cessation within the Context of Lung Cancer Screening (SCALE) collaboration to determine the optimal implementation strategy from this specific integration.127
Planning for lung cancer screening programmes
High-quality medical research is necessary for prioritizing health needs. Regarding real-world evidence, Sun et al.128 concluded that there is a desperate lack of pragmatic clinical trials in China; in total, these amount to only 16, of which nine involve traditional Chinese medicine and most featured moderate sample sizes and short follow-ups. More effort is required in terms of population-specified and highly reliable medical research in China. We reviewed current evidence on lung cancer LDCT screening both in and out of China and this is discussed below in the hope of facilitating its implementation in the Chinese population.
Population recruitment
Most of the lung screening trials (Table S2) applied combined recruitment strategies to enrol participants. Detailed information on recruitment yields was reported in a limited number of the screening trials [i.e. NELSON, ITALUNG, German Lung Cancer Screening Intervention Trial (LUSI), and United Kingdom Lung Cancer Screening Trial (UKLS)]. The overall yield of participation in those approached ranged from 1.4% to 4.5%, with all four trials approaching the population by mailing. The recruitment rate was mainly dependent on the recruitment methods (closely related to the response rate) as well as the stringency of the selection process (i.e. risk-based selection).
Recruitment methods
Current smoking stigma and deprivation are the common factors compromising uptake in a lung cancer screening trial.129,130 Younger individuals are less likely to respond to the first invitation approach.129 Conversely, after assessing lung cancer risk and when approaching the eligible high-risk cohort, older people are more likely refuse.130 Differences in risk perception can also impact participation.130,131 Practical barriers including travel and comorbidities, along with emotional barriers, were the most reported reasons for non-uptake.130
The minorities or underserved, who may be more vulnerable to morbidity and mortality,132,133 were underrepresented in the screening trials,132 which impacts the generalisability of such lung cancer screening programmes. These people are more likely to be less-educated, economically disadvantaged, uninsured,134 and also smokers.133 The barriers to their participation include lack of awareness, lack of opportunity/access, individual beliefs,134 economic obstacles, and weakness in study designs.135 Targeted strategies have been suggested for this subgroup,134,136 e.g. a more intensive face-to-face recruiting method.134,135 A second or third contact,137 or use of mobile CT scanners and one-stop lung health checks near local shopping centres138 have been demonstrated to be beneficial for uptake in deprived areas. Some tactics are probably helpful, including cooperation with community-based clinics or organisations who have built trust in local people, employment of coordinators who are proactive and knowledgeable in programmes, complimentary transportation assistance, and personalized post-screening navigation.136 Current evidence on the efficacy and effectiveness of recruitment strategies is limited, mostly because recruitment targeting the underserved was issued midway through studies134,139 and data collection on recruitment methods was incomplete.134,139,140 Considerate preparation of trial design, population approach, and cost estimation is needed. Additionally, reporting the nature and effectiveness of recruitment strategies in screening trials is an essential requirement, as it is useful for later evaluation and comparisons in different settings.
Risk-based selection
The question of how best to define the high-risk population remains unanswered. Most of the screening trials defined their entry criteria on a solo combination of age and smoking exposure (Table S2). Specifically, NELSON selected its participants based on lung cancer mortality risk estimated from two large-scale cohorts, US Cancer Prevention Study I and II (CPS I/II).141 UKLS and Pan-Canadian Early Detection of Lung Cancer Study (PanCan) were the only RCT and cohort, respectively, to apply a risk model for such a selection. However, as for Chinese screening studies, other risk factors (e.g. passive smoking, occupation, family history of cancer, kitchen fumes), parallel to smoking exposure, were also considered in entry criteria (Table 2).
Age
The age eligibility in the screening trials varies greatly, with the lower limit between 40 and 60, and the upper ranging from 69 to no limit (Table S2). The median age of the enrolled participants in all studies was normally around 60 years, ranging from 56 to 67 years old (Table S3). A lower age limit is not necessarily associated with an accordingly lower median age in enrolees of the trials. Younger individuals are less likely to participate because of a lower affective risk perception,129 or to be eligible because of a generally lower predicted risk if any prediction models were applied that included age.142,143 A lower cut-off point for age eligibility of at least 58 was suggested by the UKLS researchers because the positive response rate in the high-risk population ≥58 was much higher than in those below this age (≥4.3% vs. 1.0%).129
There is discordance in recommendations for the upper age limit80,144: 74 in American College of Chest Physicians (ACCP), American Society of Clinical Oncology, American Thoracic Society,81 American Cancer Society,83 and National Comprehensive Cancer Network (NCCN)145 (all based on the NLST results), 74 in the CNS 2015/2018,146 79 in the American Association for Thoracic Surgery guidelines82 (based on the NLST results, age-specific incidence and life expectancy in the specific nations), and 80 in the U.S. Preventive Services Task Force (USPSTF) statement80 (based on data modelling). The USPSTF modelled data from NLST, Prostate, Lung, Colorectal, and Ovarian Cancer Screening Trial (PLCO), the Surveillance, Epidemiology, and End Results program, and the U.S. Smoking History Generator. They selected the most advantageous screening scenario by maximizing lung cancer mortality reduction and reducing overdiagnosis as much as possible.80,147
However, it is widely presumed that older individuals would be not eligible for inclusion because of existing comorbidities. In this subpopulation, harm from screening might outweigh the benefits, but this can be difficult to measure because of competing causes of death. In another study using microsimulation modelling, Han et al.144 incorporated overdiagnosis into the outcome measures (including lung cancer deaths prevented and life-years gained as a result of screening). They found that stopping screening at a younger age of 75 would have higher efficiency in maximizing the benefits (mainly life-years gained per overdiagnosed case) than at 80, and there was no gender difference.
In a trend analysis of national cancer registries of lung cancers in China during 1989–2008, the average ages in male and female patients significantly increased from 65.32 to 67.87, and from 65.14 to 68.05, respectively.3 The change could be explained by the increase in the ageing population with time.3 However, in the West China Hospital, the average age at diagnosis in hospital-based lung cancer cases was 59.22 during 2008–2014.59 This difference could be attributed to data sources and geographical factors. Therefore, it is advisable to comprehensively consider age- and geographical-specific lung cancer incidence, participation rate, and also benefit-to-harm ratios before determining the age at entry.
Smoking status
Smoking is the other basic entry criteria after age. Heavy current and former smokers are the targets in most of the trials, except the Asian studies (Table 2 and S2). JECS in Japan targets only non- or light smokers. The Chinese studies [e.g. Shanghai CancerHosp cohort,105 Shanghai ChestHosp RCT,106 and China Lung Cancer Screening Feasibility RCT (China FeasiRCT)101,102] also recruited individuals exposed to other risk factors, not restricted to heavy current/former smokers only. Although PanCan and UKLS used a risk model for high-risk assessment and recruitment, the final studies included participants who were practically all ex- or current smokers (Table S3).
There are two types of smoking exposure criteria in the trials: cumulative pack-years, or smoking duration and intensity (average number of cigarettes per day), separately (Table S2). In the Liverpool Lung Project (LLP) model used by UKLS as a selection tool, smoking duration was demonstrated as the strongest predictor over other smoking-related factors, e.g. smoking status, intensity, and time since quitting.148,149 ten Haaf et al.150 concluded that there was little difference between the two criteria in the aspect of cost-effectiveness in their microsimulation modelling; the scenario with stringent smoking criteria, annual screening for persons aged 55–75 who smoked >40 pack-years and who currently or quit ≤10 years ago, was optimal.150
Most trials in the Western world, and almost all screening guidelines concentrate on the smoking subpopulation. Both Ten Haaf et al.151 and Tammemagi et al.152 demonstrated that most never-smokers would not benefit from lung cancer screening; notably, the two studies were based on a US dataset. As there are different smoking profiles in lung cancer patients in the US and China as discussed above, whether or not Chinese never-smokers could gain more significant benefits than harm, from early screening, is unknown. Given other predisposing factors, this may indicate totally distinct entry criteria for lung cancer screening in China. This is somewhat evidenced by the baseline results from the Shanghai Chest Hosp RCT106 and Shanghai Cancer Hosp cohort,105 which had less stringent smoking eligibility criteria.105,106 The former had a similar prevalence rate of lung cancer between the NLST-ineligible males (1.1%) and females (1.4%) in the LDCT arm,106 which was comparable to NLST (1.0%).153 In the latter, the incidence in never-smokers was two-fold that of smokers at baseline screening105(Table 2, Table S3). Some lessons could be learned by comparison with other Asian studies, in which never-smoker lung cancer incidence is more comparable to China. However, this is difficult because of limited data.
Thus, pre-evaluation of lung cancer risk in the local population, and pre-estimation of cost-effectiveness for different scenarios of screening criteria in the setting of the corresponding economic structure would assist in selection of optimal eligibility criteria. Establishment of a specifically optimized Chinese risk model could simplify recruitment in China and could lead to a more effective screening program on the basis of an individual’s risk.
Modelling for risk prediction in the population outside China
Many publications have implied the outperformance of risk models in improving screening effectiveness and efficiency over current eligibility criteria, used in the trials or recommended in guidelines.152,154–158 UKLS and PanCan applied risk models (the LLP model and PanCan model, respectively) in selecting high-risk individuals for eligibility entry. The high-risk cut-off threshold was defined as the risk estimation of LLPv2 risk model ≥5% in 5 years in UKLS,133,143 and PanCan model (a prototype of PLCOm2012) >2% in 6 years in PanCan.142 Generally, studies using models had a higher lung cancer detection rate133,142 and cost-effectiveness133 than their counterparts (Table S2–S3).
There are a large number of established risk models for predicting lung cancer risk.159,160 The predictors in the models vary a great deal, from the simplest combination of age and smoking to more complicated models (integrated medical conditions, medical history, ethnicity, and socioeconomic factors).159,160 Despite good discrimination (and calibration) in development datasets, the performance of most models in external validation was generally limited.159,160
A few studies155,156,161,162 assessed and compared different risk models in respect to discrimination, calibration, and clinical utility. However, there is wide variation in their performances. In a UK case-control dataset: Spitz and LLP were comparable in discrimination and positive/negative predictive values, both of which were better than Bach; LLP showed a better sensitivity but lower specificity than Spitz and Bach.161 ten Haaf, et al.155 demonstrated that PLCOm2012, Bach, and the Two-Stage Clonal Expansion (TSCE) incidence model had the best overall performance with an AUC of 0.68–0.71 in NLST and 0.74–0.79 in PLCO for 6-year lung cancer incidence, superior to the other models (including LLP, Knoke, and two versions of the TSCE model for lung cancer death). Katki et al.162 arrived at the conclusion that PLCOm2012, Bach, the Lung Cancer Risk Assessment Tool (LCRAT), and the Lung Cancer Death Risk Assessment Tool (LCDRAT) outperformed the five other models, including Spitz, LLP, the LLP incidence (LLPi) Risk model, Hoggart, and the Pittsburgh Predictor, in three US population-based datasets. However, in a German cohort, Li et al.156 demonstrated only a modest superiority of PLCOm2012 over Bach and LLP in selecting a high-risk population for screening.
On reflection, there may be a number of reasons for the varied performance. Firstly, some models, e.g. LLP and Spitz, were derived from case-control datasets, whereas others, e.g. PLCOm2012, Bach, and TSCE, were from cohorts.159,162 Risk models developed from case-control datasets may lack generalizability in the population because of selection bias in cases and controls; they may also have bias in risk estimations because recall bias exists in data collection.162 Secondly, all the models were derived from a specific ethnicity or region. This population-dependent feature could impair their performance in populations from other ethnicities and regions, e.g. PLCOm2012 under-rated lung cancer risk in Hispanic patients.162 Thirdly, some risk factors may be unavailable in another independent dataset, which may weaken the prediction. However, the impact may be limited. ten Haaf and colleagues155 found that the full versions and simplified versions (only including age, gender, and smoking) of risk models performed similarly, i.e. full PLCOm2012 and simplified PLCOm2012, full LLP and simplified LLP. Their study indicated that the three variables in simplified models contributed to lung cancer risk the most.
Evidence of long-term benefits and harms, such as trade-offs between life-years gained, mortality reduction, and overdiagnosis are limited. The optimal threshold for risk models, at which lung cancer screening programs or clinical practice should gain maximum benefits over harm, is still undetermined.155,159 Thus, no preferential risk model and risk threshold have been recommended in risk prediction for screening eligibility across different populations. The European position statement117 suggested that ‘either the PLCOm2012 or the LLPv2 would suffice if screening were to be implemented immediately’ given their high level of prediction.
There are emerging models integrating clinical factors, e.g. molecular biomarkers from blood, pulmonary function, and genetic biomarkers (e.g. single-nucleotide polymorphisms), which potentially are alternative ways to improve risk models’ overall performance. Some of them are extensions of existing models which have only epidemiological factors, but their improvement over the existing models was found to be generally moderate.159,160 Specifically, the extended LLP model has been successively integrated with different SNPs twice, with performance in discrimination increased from 0.72 to 0.75163 and from 0.73 to 0.79,164 respectively, when compared with the original epidemiological model. However, a modest enhancement in the performance of the risk models would still be significant and meaningful, as the ‘improvement space’ is limited. It is also important to note that genetic risk is already captured to some extent in the LLP risk model through inclusion of personal and family cancer history.
Risk models for participant selection in China
In mainland China, four studies explored this topic165–168 (Table S4). Among them, three models had good discrimination (AUC: 0.7037–0.885).166–168 Lin et al.166 constructed a model using the first-degree pedigrees of patients and their spouses as cases and controls (633 proband pedigrees versus 565 spouse pedigrees). The higher the risk threshold, the more accurate the prediction in clinical use (cut-off value <5, an accuracy of 68.3%; 5–10, 84.0%; ≥10, 91.9%), but no external validation was performed. Yang et al.168 developed a model from a retrospective cohort. When the risk probability was calculated at ≥0.65, that model’s sensitivity and specificity were 14.9% and 94.5% in the development dataset, and 13.0% and 98.3% in the external validation dataset, respectively. The model built by Wang et al.167 performed well in the aspects of discrimination and clinical use, but it had no external validation. All the four models were derived from hospital-based data,165–168 which potentially could introduce bias in data analysis. Further optimization is desperately needed to produce new models. A prospective cohort to observe lung cancer incidence within a specific timeframe and validate the models is also worth considering, but may cause significant delay unless performed alongside CT screening, using the best current model.
In the future, a comprehensive, systematic reporting standard in the development and validation of screening would be helpful for comparisons between models from similar or different backgrounds, enabling extensive validation of various models in a unified cross-border dataset. Undoubtedly, further research is important and should be an integral part of any screening programme.
Screen protocols and related issues
Screening interval
There were only six screening trials that applied biennial LDCT screening in their intervention arms, including PanCan, NELSON (only once), MILD, and the three Chinese trials (the China FeasiRCT,101,102 the Shanghai ChestHosp RCT106 and Tianjin CancerHosp programme103). Others, except JECS with a 5-year screening interval, used annual screens for their enrolees (Table 2, Table S1).
In NELSON,169 there were an increasing number of interval cancers (5 vs. 19 vs. 28, respectively) and higher proportions of stage IIIB/IV in screen-detected lung cancers (6.8% vs. 5.2% vs. 17.3%, respectively) after corresponding 1, 2, and 2.5-year intervals.169 These results indicated that an interval of 2.5 years is most likely too long for a population screening programme.
No significant difference between annual and biennial screening in MILD has been found in respect to interval lung cancers, specificity, sensitivity, and positive/negative predictive value.170 Note that the population in MILD was much smaller than in NELSON. In the UKLS modelling, annual screening would prevent more lung cancer deaths (956 vs. 802), but induced more overdiagnosis (457 vs. 383) and screening episodes (330 000 vs. 180 000).171 By microsimulating NLST, the biennial screening gained similar quality-adjusted life-years (QALYs) to the annual screening over 20 years (24 000 vs. 23 000), but the former was more cost-effective regarding both incremental cost-effectiveness ratios and CT scans saved.172 Therefore, a 2-year interval might be a cost-effective alternative for screening.
The risk of screen-detected lung cancer depended strongly on the results of the first scan: 1.0% with a baseline-negative scan, 5.7% with an indeterminate result, and 48.3% with a positive result over a 5.5-year follow-up.173 When compared to individuals with a nodule at the baseline scan, those without have a much lower risk in 2 years (0.2% vs. 4.6%).142 Thus, a tailored screen interval is needed. For such a low-risk probability, the subpopulation with a negative baseline result might be safely screened every 2 years or at even longer intervals; other subpopulations with distinct baseline nodule results might be managed according to their specific risk probabilities. The risk probabilities of the individuals with nodules could be implied by the cut-off value of nodule risk prediction models (discussed below). Evidence from the Chinese studies is limited because the results of interval screening rounds are not yet available.
Overdiagnosis
Overdiagnosis is often disputed in the context of screening. It is defined as the detection of a cancer that would not have been clinically apparent if there were no screening.174 Overdiagnosis can result in unnecessary treatment, psychological problems, and economic burdens.174 An upper bound of about 18%–25% of all the cancers detected in the LDCT screening were estimated to be indolent, thus probably overdiagnosed.174,175 The overdiagnosed lung cancers are more likely to be adenocarcinoma because it had a higher proportion in the LDCT arm than in the control arm,73,174 and also a longer volume doubling time (VDT) than other lung cancer subtypes.175
A contradictory indicator to overdiagnosis is stage shift. The primary aim of screening is to detect lung cancer at an earlier stage; thus we aim for a corresponding reduction in advanced lung cancers. It is therefore not expected that detected tumours are indolent. Overdiagnosis will be caused if there is no reduction in advanced lung cancers, but only an accumulation of indolent cancers categorized into early stages.72 Only NLST70 (Stage IV: 0.9% vs. 1.3%) and DLCST72 (T4N3M1: 0.4% vs. 1.0%) showed a significantly lower proportion of advanced lung cancers in the intervention arm than in the control arm. There were no evident stage shifts in DANTE73 (stage IV: 2.1% vs. 2.8%) and ITALUNG74 (stage IV: 1.7% vs. 2.2%). Reasons for this could be the larger study sizes, differing approach methods used for NLST and DLCST, or that some degree of overdiagnosis existed in these trials.
Additionally, the effects of overdiagnosis could be mixed with lead time. The latter is defined as ‘the difference between the time when diagnosis would have been made without screening and the time that the diagnosis was actually made as a result of early detection by screening’.174 A longer follow-up may be helpful to distinguish between overdiagnosis and lead time. Mean lead time were estimated of 3.6 years for non-bronchioloalveolar carcinoma (BAC) NSCLCs and 32.1 years for BACs, to when they naturally become clinically significant without screening interventions. Specifically, over 25% of the non-BAC NSCLC cases would have a lead time of >5 years, and a very low proportion of 6.3% would exceed more than one decade. However, for BACs, 73.2% would have a lead time of ≥10 years, and approximately 50% would be overdiagnosed throughout the whole life.174
In ITALUNG, the cumulative number of lung cancers in the usual care group caught up with the LDCT group after a follow-up of 6–7 years from randomization.74 However, in DANTE, after a median follow-up of 8.35 years from randomization, there was still a lung cancer excess rate of 30.76% in the LDCT arm compared with the usual care arm.73 Besides overdiagnosis, the difference could also be explained by an additional screening round in DANTE and possible different subtype distribution in the diagnosed lung cancers.
The results above indicate that certain screening rounds accompanying a specific and sufficient follow-up timeframe might minimize overdiagnosis. Moreover, overdiagnosis would be affected by the possibly different distribution of lung cancer subtypes in screening participants.
Length of screening
As discussed above, screening length is closely associated with overdiagnosis; compared with the usual care group, the LDCT group managed with three annual screens would have an overdiagnosis rate of 31% within a complete 7-year follow-up after baseline.174 Given the evidence from ITALUNG74 and DANTE73 extended follow-ups (as above), it is advisable to estimate screening length, follow-up duration, and corresponding overdiagnosis rate before a trial is started.
When compared with the unscreened Beta-Carotene and Retinol Efficacy Trial (CARET) cohort, the mortality reduction from two annual screening rounds in the New York ELCAP cohort became apparent in the fourth year and reached a maximum in the sixth to eighth year after enrolment. The overall mortality reduction would be 36% when standardized by the CARET entry criteria176; the mortality would be reduced further if the screenings continued.176 In the Continuous Observation of Smoking Subject (COSMOS) pilot cohort of 1035 individuals, a lung cancer mortality reduction of 31–61% would be expected after 7 years of annual screening when compared with the extrapolation from age- and sex-matched unscreened CPS II smokers.177 Despite a lack of statistical significance in mortality reduction after a 9-year follow-up in ITALUNG, the researchers found a significant mortality reduction in the post-screening period.74 Therefore, extensions of screening and follow-ups could enhance mortality reduction.
In summary, when planning the screening length of a trial or national programme, some factors to consider are: 1) the mortality reduction expected in screening population; 2) cost-effectiveness; 3) limiting overdiagnosis; and 4) minimizing other potential harms, e.g. radiological exposure, psychological impact.
Nodule management
The nodule management protocols of most screening trials largely follow or are modified from the ELCAP/I-ELCAP (Table S5). Henschke and colleagues published the protocols consecutively in 1999,68 2004,178 2011,179 and 2016180 when new evidence accumulated. When comparing the modified versions with the 1999 protocol, the significant changes are: 1) nodule cut-off value increased; 2) volumetric analysis and VDT introduced to define growth; 3) management differed among solid, part-solid, and non-solid nodules; 4) non-solid nodules managed less aggressively; 5) management differed in baseline nodules and new nodules detected at intervals (the latter managed more aggressively); and 6) endo-bronchial solid nodules also specified.
The NELSON protocol was derived from the 2004 I-ELCAP protocol.181 It was the first lung cancer screening trial to use volumetry as a nodule assessment method. It developed two classification systems for nodules detected at either baseline or interval scans: NODCAT (nodule categories) and GROWCAT (growth categories). Generally, the solid component, either in solid or part-solid nodules, is measured in volume (mm3), whereas the overall size of the part-solid, non-solid, and pleural-based solid nodules are measured in diameter (mm). NODCAT is applied to all nodules detected on CT scans, assisting decision-making on follow-up; GROWCAT is applied when there are follow-up scans for assessing VDT or a new solid component growth in a non-solid lesion.181
The UKLS trial nodule management largely followed that of the NELSON. The main difference between UKLS and NELSON categories is that UKLS picked 15–49 mm3 nodules as a separate category to ensure the inclusion of cancers in nodules <50mm3 to the largest degree in a single screen design.182
A variety of guidelines on pulmonary nodule management have also emerged in different countries tailored to their own circumstances.115,146,183–186 Several risk models for nodule malignancy prediction have been recommended in these guidelines: the Mayo Clinic model by ACCP185 and the Fleischner Society,187 or the Herder model and Brock model by the British Thoracic Society (BTS).184 As its guidelines are applicable to clinical practice, the Fleischner Society recommended adherence to the existing American College of Radiology Lung CT Screening Reporting and Data System (Lung-RADS) guidelines for lung cancer screening.187
Associated guidelines have also been developed in Asia or China in the clinical183 or screening settings.115,146 Evidence supporting these recommendations comes predominantly from the Western countries, so it is possible that they are inappropriate to the East with its distinct demographic, geographic, and genetic aspects. It is unclear if variation in the aetiology of lung cancer in the East is limited to the initiation of lung cancer, or extends to the biological features that influence nodule behaviour. In the absence of any large-scale LDCT trials in China and other Asian countries, slight modifications made in the Asian guidelines were taken from experts’ opinions.115,146,183 Herein, we discuss some crucial issues related to nodule management.
Measurement: diameter or volumetry?
The screening trials use several ways of evaluating nodule size: maximum axial diameter, the average of length and width, and three-dimensional (3D) volumetric computer-aid assessment.188 Specifically, NELSON, MILD, and UKLS used volumetric-based measurement for nodule assessment, with others mostly following a diameter-based protocol (e.g. NLST), with some applying a computer-aided system at follow-up scans for nodule growth and VDT assessment (e.g. DLCST, LUSI) (Table S5).
Mean axial diameter (using the average of the long-axis diameter and that taken at right angles to it) for nodule risk assessment was first adopted in ELCAP.68 In 2017, the Fleischner Society commented that, because of substantial inter- and intra-observer variability, use of the maximum dimension would lead to misclassification of nodules, especially in small nodules, thus resulting in a high false-positive rate.188 Large variance of intra-nodular diameters also exists in indeterminate nodules; this can reach up to a median value of 2.8 mm, higher than the growth threshold of 1.5 mm recommended by LUNG-RADS.189 Hence, nodule size represented by diameter is concluded to be poor. Calculation of volumes based on the diameter was also used. However, compared with volume measured semi-automatically using 3D software, a mean overestimation of volume by 85.1% and 47.2% can occur in volume calculation by the maximum and mean axial diameter, respectively.189 Therefore, the European position statement117 and BTS 2015184 recommend volumetry as the preferred assessment method.
Cut-off values
NLST defined ≥4 mm as its threshold of positive results, whereas most of the others applied a cut-off value of ≥5 mm (Table S5). With rising thresholds, the frequency of positive results and further work-ups decreases successively, thus saving medical resources. When increasing the threshold from 6 mm to 9 mm in I-ELCAP,190 the screening-positive rate dropped from 10.2% to 4.0% and the work-up would be reduced from 63% to 25%. The disadvantage was the corresponding increased rate of lung cancer diagnostics delayed up to 9 months from 0% to 6.7%. Similar results were attained in the NLST LDCT-arm dataset.191,192 The ≥6 mm threshold performed well in other aspects, including avoidance of false positivity192,193 and more positive predictive findings,194 but it impaired the sensitivity194 when compared with the cut-off of ≥4 mm. There was no statistically significant effect on survival or mortality in different nodule sizes.192 Currently, the nodule-positive threshold of 6 mm is recommended by I-ELCAP (2016),180 the Fleischner Society (2017),187 and LUNG-RADS.195 The First Brazilian Lung Cancer Screening Trial (BRELT1) also increased its threshold from the original 4 mm to 6 mm during the implementation.196
The lung cancer probabilities in different nodule sizes at baseline are also an essential factor when determining the appropriate threshold. In NELSON, the risk increased with the volumes (or diameters) of baseline non-calcified nodules: a low risk of 0.6% (or 0.4%) in nodules of <100 mm3 (or <5 mm, respectively), comparable to those without nodules (0.4%); intermediate risk of 2.4% (1.3%) in 100–300 mm3 (5–10 mm); and high risk of 16.9% (15.2%) in ≥300 mm3 (≥10 mm).197 No additional CT scans or work-up are needed for low-risk nodules, whereas the high-risk should undergo diagnostic examination immediately. Intermediate-risk nodules should be risk-stratified by VDTs and managed differently. The authors concluded that lung cancer risk increased with reduced VDTs: 0.7% for VDTs ≥600 days, 4.0% for VDTs of 400–600 days, and 9.9% for those ≤400 days.197 Therefore, the management strategies should be tailored to risk-stratification accordingly, to detect the most lung cancers while limiting the required resources.
The I-ELCAP researchers found non-solid nodules featuring slow growth and a 100% curative rate by surgery.198 In MILD, only 16.7% of the non-solid nodules progressed after a mean follow-up of over 55 months.199 Annual follow-up for non-solid nodules of all sizes (except those with a new solid component at following CT scans) is recommended in the I-ELCAP protocols.179,180 Perifissural nodules have also been reported to be of low malignancy.200,201 In PanCan, perifissural nodules have been excluded from the nodule positive definition.142
Another issue concerns de novo nodules, which are first detected at interval scans. Lung cancers derived from de novo nodules have more aggressive features and a poorer prognosis than those diagnosed from baseline-positive nodules.202 Lung cancer probabilities increased with the volumes (and diameters) of de novo nodules; in NELSON, the risk is 0.5% in nodules of <27 mm3 (3.7 mm), 3.1% in 27–206 mm3 (3.7–8.2 mm), and 16.9% in ≥206 mm3 (8.2 mm).203 A cut-off value of ≥27 mm3 would achieve a sensitivity of 95.8% and specificity of 38.3% for lung cancer.203 Therefore, new nodules at incidence rounds and those from the prevalence round should be managed separately. The 2011179 and 2016180 I-ELCAP protocols suggested a diameter threshold of 3 mm for these de novo nodules. Meanwhile, the European position statement recommended a cut-off value of >30 mm3.117
Number of nodules
The radiological features of the largest nodule detected on CT have been assessed in trials. In I-ELCAP and Mayo LDCT study, the number of nodules required for recording was up to 6; in UKLS, the number reached 20; and in NELSON, all non-calcified nodules are measured (Table S6).
It is very common to find two or more nodules in lung cancer screening participants, this was the case in about 48.5% of all NELSON baseline participants.204 Of the malignancies, 97.0% were diagnosed in the largest nodule at baseline.204 However, lung cancer probability in an individual is not necessarily associated with the nodule count at baseline: 3.6%, 4.1%, 4.8%, 6.3%, and 3.3% in those with 1, 2, 3, 4 and >4 nodules, respectively. For this reason, separate assessment of each nodule is suggested.204
In short, nodule count does not necessarily indicate a benign or malignant lesion, but the specific features of each nodule are important.
Modelling for risk prediction of nodule malignancy outside China
The aim of modelling is to reduce biopsy rate and increase malignant-to-benign ratio. BRELT1 is the only screening trial that used a risk model, namely The Mayo Clinic model, for malignancy prediction of pulmonary nodules (Table S5). The Mayo Clinic model was also the first model to be introduced for pre-test prediction by ACCP since 2007.205 It was initially developed and internally validated in a retrospective unscreened cohort of 629 patients with indeterminate solitary pulmonary nodules on CXR (malignant rate: 23%).206 However, the model did not show superior performance in the baseline biopsy rate and malignant-to-benign ratio in BRELT1 when compared to other trials196 (Table S3), indicating future efforts in optimizing.
The Brock model207 was derived from the PanCan prospective cohort (malignant rate: 5.5%) and externally validated in the British Columbia Cancer Agency chemopre-vention trials. Both datasets were in the CT screening context and included ever- and never-smokers. The model displayed great discrimination of over 0.89 in all settings and calibrated very well. It also performed well in individuals with nodules ≤10 mm. The Herder model208 was modified from the Mayo Clinic model by integrating positron emission tomography (PET) results. It was developed from a hospital-based unscreened cohort of 106 patients from the Netherlands with indeterminate solitary nodules (malignant rate: 57.5%), the same dataset that the Mayo Clinic model used for external validation. It improved the AUC by 13.6% when compared with the Mayo Clinic model. When validated in a hospital-based unscreened cohort from the UK, the Brock model (AUC 0.902) and the Mayo model (AUC 0.895) were similar in predicting nodule malignancy, but the Herder model had higher accuracy (AUC 0.916) than the other two models in patients undergoing PET-CT.209 Therefore the 2015 BTS guideline, stated that the Brock model would be used for risk assessment in nodules ≥8 mm or ≥300 mm3, and the Herder model used following PET-CT if malignancy risk was ≥10% in the Brock model.184
Additionally, the Brock model has shown excellent performance in heterogeneous populations, including LDCT screening trials, e.g. NLST (AUC 0.963),210 DLCST (AUC 0.826–0.870),211 a LDCT screen-detected sub-solid nodule cohort from Australia (AUC 0.89),212 and a multicentre unscreened cohort from the Netherlands (AUC ≥0.90).213 Nonetheless, it may be suboptimal in other aspects, such as differentiating invasive lesions from sub-solid lesions (AUC: 0.671 in non-solid, 0.746 in part-solid nodules in a Korean unscreened cohort).214 The Herder model also had good discriminatory power of 0.757 in an Italian retrospective cohort,215 albeit inferior to the value previously reported in its development and external validation datasets.208 However, the Brock and the Herder models were derived from and confirmed only in post hoc analysis (i.e. applied retrospectively in pulmonary nodule data). Whether or not they would perform well in an ongoing LDCT screening trial is unknown.
Modelling for risk prediction of nodule malignancy in China
A great many risk models for predicting malignancy in nodules have been developed in China (Table S4), with all of them constructed from hospital-based retrospective cohorts. Most do not specify calibration. The two models developed by Li et al., 2012216 and Yang et al., 2018,168 respectively, have spatially external validation. The model built by Li et al.,216 also called the Peking University People’s Hospital (PKUPH) model, discriminated quite well (AUC 0.810) when evaluated externally. At a risk threshold of 0.471, the sensitivity and specificity of the PKUPH model were 83.3% and 75.9%, respectively.217 For the model established by Yang et al., the discriminatory power was very limited in the external validation dataset (AUC 0.584).168 Additionally, three other risk models focused on sub-solid nodules218,219 or ground glass opacities.220
Notably, almost all the development datasets had a very high malignancy prevalence (except the Brock model),160 especially those in China (malignancy prevalence >50%) (Table S4). This could be because only participants undergoing surgical procedures or biopsies were eligible for the analysis. The accuracy of a model is likely to depend on the lung cancer prevalence in a target population. Hence, these derived models may not be well calibrated in other datasets with a different prevalence.160 However, because the decisions for invasive management in these datasets were often combined with the clinical experiences of doctors, models from these datasets may be more useful in real-world clinical practice. Still, it is unclear how these Chinese models would perform when applied in LDCT screen-detected nodules and ongoing screening trials.
Other screening-related issues
Significant other findings
It is believed that significant other findings on CT scans would maximize the benefits of screening programmes. Of the NLST population who were screened in LSS centres, 19.6% had potentially significant extra-pulmonary abnormalities after three screening rounds.221 Some would bear significant clinical implications and require further clinical assessment; this accounted for 1% of the NELSON baseline population.222 Extra-thoracic cancers were diagnosed in 0.39% of the screened participants during the screening period in NLST, including kidney (0.26%), thyroid (0.08%), and liver (0.05%) cancers.221 Once found, these clinically significant abnormalities could be managed immediately and systematically. In this case, the specific individual may benefit from the screening in a ‘byproduct’ way, although dangers of overdiagnosis are relevant to incidental findings.
Moreover, some conditions, e.g. idiopathic pulmonary fibrosis, are rare in the general population, but highly lethal. It is impossible to implement an independent screening trial for this kind of disease, so detection within a cancer trial is valuable. In 884 smokers from the NLST, the prevalence of interstitial lung abnormalities (ILA) was 9.7%, with fibrotic abnormalities accounting for 2.1% and non-fibrotic for 5.9%. Among them, 37% of fibrotic and 11% of non-fibrotic ILA progressed in a 2-year follow-up.223 Such epidemiological and clinical information provided through screening would facilitate optimization of current ILA management strategies.
The benefits of incidental findings are not limited to rare diseases, detection of common diseases such as cardiovascular diseases and emphysema can also be provided to assist clinical management, e.g. significant role of coronary artery calcium score in predicting all-cause mortality and cardiovascular events,224 quantification of emphysema extent225 and its potential implication on lower bone density.226
However, regarding the cost-effectiveness of management for these extra findings in screening, the evidence is very limited. Given that some abnormalities in the context of screening might be clinically non-significant or indolent in nature, such as mediastinal masses,227 it is better for us to manage these findings distinctively according to their characteristics.
Cost-effectiveness
Cost-effectiveness analysis could be used to evaluate whether one trial design is superior to another concerning value for money and also to investigate impact factors attributable to cost-effectiveness improvement. Related measures in health-economic analysis include costs, quality-adjusted life-years (QALYs), and incremental cost-effectiveness ratios (ICERs).
Compared with no screening, LDCT screenings in NLST provided an additional 0.02 QALYs per person and a corresponding ICER of $81 000 per QALY gained.228 Although, with similar QALYs gained per person, UKLS had a mean ICER of $12 106, much lower than NLST.133 By comparing UKLS with NLST, researchers identified some possible measures for improved cost-effectiveness: 1) higher lung cancer prevalence in a target population; 2) lower unit costs for management; 3) more effective selection of the high-risk population recruited; 4) fewer screens arranged in protocols; and 5) more true-positive results throughout the protocol of nodule management.133
Cressman et al.229 analysed the factors driving programme efficiency by comparing different scenarios applied to the NLST datasets. They found that mortality reductions had the greatest impact on cost-effectiveness, followed by long-term improvements to the quality of life in lung cancer-free participants. Consideration of non-lung cancer outcomes in screening participants may be necessary in the cost-effectiveness analysis.229 Using the same NLST dataset, Kumar et al.230 stratified the participants into different deciles according to their pre-screening risk of lung cancer mortality. Although lung cancer deaths prevented per 10 000 person-years increased from the lowest to the highest risk deciles (extreme decile ratio: 7.9), the gradients across deciles were attenuated in the aspects of life-years, and QALYs gained (extreme decile ratios: 3.6 and 2.4, respectively). ICERs across risk strata were similar.230 The conflicting results may be explained by comparable roles between lung cancer and other diseases in the high-risk groups as they are more likely to be older and have more comorbidities.229,230 Therefore, some scholars argued that all-cause mortality reduction should be the benchmark for cancer screening.231 However, to date, none of the CT screening trials have sufficient power to provide all-cause mortality data.
In a post hoc analysis of NLST screening participants, Young et al.232 demonstrated that smokers with higher lung cancer risk predicted by the PLCOm2012 model would have a COPD prevalence and likelihood of non-lung cancer deaths in a linearly increasing fashion. Limiting those of intermediate risk (predicted by PLCOm2012) to screening eligibility would achieve a greater reduction in lung cancer mortality compared with those of risks just over the cut-off value (28% vs. 17%). Similar conclusions could be drawn from those with normal lung function or only mild-to-moderate COPD when comparing to those with severe or very severe COPD. Regarding lung cancer mortality reduction, it is better to exclude those with high risk and severe or very severe COPD who it is presumed will not benefit from screening because of other competing causes of death and inoperability.232
Smoking cessation may be a good alternative for cost-effectiveness improvement at the population level, as indicated in a previous US health-economic analysis,233 but this did not address earlier detection of lung cancers in those currently at high risk.
In summary, cost-effectiveness varies widely in different settings. Short-term or long-term outcomes, and lung cancer per se or other health conditions, should be considered in the analysis. Overall mortality reduction may be more critical than lung cancer-specific mortality reduction in assessing the effectiveness of screening. Considerations should be taken when recruiting people who would potentially die from other causes, e.g. the effect of COPD in lung cancer screening.232 Some interventions, such as smoking cessation, managing cardiovascular risk in advance, and screening/clinical strategy optimization, may be anticipated to improve cost-effectiveness in those screened.
Psychological impact
Four trials, NELSON, NLST, DLCST, and UKLS, reported results on psychological impacts. There was temporarily increased lung cancer-specific distress in participants with a high affective risk perception234 or those with positive results133,235; but, this dropped with long-term follow-up, e.g. 6 months,234 2 years,235 or when individuals were reassured by a negative result.236
The psychological impact is presumed to be screening result-dependent. Those with false-positive scans, significant incidental findings, or negative scans in NLST had no significant increase in anxiety.237 Participants with true-positive scans who developed lung cancer within 1 year had higher anxiety and lower health-related quality of life at 1 and 6 months after screening in NLST,237 but this is to be expected (and anxiety is likely to be less than if the subjects were diagnosed later with a higher stage disease).
There was no difference in psychological impact across the LDCT and CXR screening arms in NLST.237 However in DLCST,238 compared to the LDCT arm, the usual care arm experienced more negative psychological consequences.238 This may be explained by the reassurance in those with normal screening results in the LDCT arm.236,238
In short, lung cancer screening can exert certain short-term, yet generally minimal long-term, psychological harm on participants. The impacts are usually not severe,234 or not to clinical levels.235 However, special attention should be paid to those with positive scans and help should be provided if necessary after regular psychological assessment. Those who do not receive the reassurance of an early diagnosis or a negative LDCT scan (e.g. those randomized to usual care in a trial, or unable to have a screening scan) may also need help.
Radiation exposure
New CT scanners have a much lower level of radiation than previously, e.g. in NLST, the effective dose was estimated at about 2 mSv for LDCT but 8 mSv for full-dose chest CT.239 However, extra radiation exposure associated with screening is still a concern.239 It is estimated that if a person aged 55 was followed up according to the Fleischner guidelines over 20–30 years (three full-dose CT follow-ups over 2 years if nodules >4mm), that person would experience a cumulative radiation dose of 280–420 mSv, a dose exceeding that of nuclear workers and atomic bomb survivors.239 As a result, lung cancer risk would increase.239 A male smoker and a female smoker would observe increases in lung cancer risk induced by radiation of about 0.23% and 0.85%, respectively, if he or she underwent annual LDCT screening from age 50 until 75 years.240
In ITALUNG, when assuming a lung cancer-specific mortality reduction of 20–30% in current smokers, the potential fatal cancers associated with radiation exposure were 10–100 times lower than the expected lives saved by screening in number, indicating a favourable benefit over the risk.241 However, never-smokers or former-smokers would benefit less in the same scenarios than current-smokers.241 In a secondary analysis of the COSMOS data, lung cancers and major cancers induced by 10 years of LDCT screening were 1.5 and 2.4 in number, respectively. The additional risk of induced cancer was extremely low, namely one induced major cancer for every 108 screen-detected lung cancers.242
Therefore, we could expect a very low and acceptable risk of cancers induced by LDCT screening per se,241,242 but cancers would occur if screening was conducted for long enough.239 Protocols for screening should be optimized to attenuate the possible increased cancer risk by modifying the screening frequency and age range in line with individualized lung cancer risks and emerging evidence on screening-induced cancers. A mortality reduction considerably >5%240 is required to outweigh the radiation-induced cancer risk, and this should be estimated before screening is conducted, especially for individuals aged <50 years.243
Recommendations on Chinese lung cancer screening programmes
Herein, we reproduce a figure from Field’s review244 to illustrate the current status of evidence in China (Fig. 3). The evidence for the 12 aspects given in the figure is mostly based on results of trials performed outside China, thus, moving forward with population CT screening, further research is required to put these aspects into the Chinese context. We note several issues that require caution or further investigation and give our recommendations (Panel 1).
Figure 3.
Levels of evidence for implementation of lung cancer CT screening in China in 2018, where green indicates sufficient evidence, orange is borderline evidence, and red requires further evidence (Chinese-specific).244 MDT, multidisciplinary team; CSCO, Chinese Society of Clinical Oncology.
Panel 1.
Recommendations for implementation of lung cancer screening in China.
|
|
|
|
|
|
|
|
|
|
Participation: recruitment of hard-to-reach participants
There are substantial health and healthcare disparities across different regions of China. The underserved are more likely to suffer from morbidities and mortalities, yet are less likely to participate in screening programmes. Some targeted recruitment methods can be efficient; however, in China, most programmes have targeted urban areas, which may have higher socioeconomics. The Guangzhou GMU-1stHosp programme focused on underprivileged individuals, but had low uptakes because of low awareness of preventive health care among the targeted population.111
In China, people have free access to any hospital, which leads to ‘medical migration’.245 Selection bias and higher dropout might be anticipated when recruiting participants based on hospital catchment areas as these are not fixed and people ‘migrate’. Community-based enrolment could be a favourable alternative for lung cancer screening, by which people could be organized as a whole more effectively.
A significant number of the lung cancer screening programmes in China only have references, which are based on web pages or conference abstracts, thus the detailed protocols and results are unavailable. To harmonize CT screening programmes in China, it would be beneficial to facilitate cooperation between the lung cancer screening groups, which would increase awareness and also provide consistency, governance control, and transparency of all the programmes.
Risk-based selection
Risk-based selection is presumed to focus on individuals who are most likely to be at higher risk of developing lung cancer, and to minimize unnecessary scans in the low-risk population, thus is more likely to be cost-effective. However, such high-risk populations are also more likely to be older and suffer from non-lung cancer deaths, thus bringing the net benefits into question.246 The high-risk profile for lung cancer screening is still undetermined in China. The proportion of lung cancers attributed to smoking is much lower in China than in the UK and the US. Other risk factors may play more critical roles in lung cancer incidence in China. The preliminary results of various programmes in China indicate a different risk profile from that in the US and European countries.
Risk models play a crucial role in lung cancer prediction in either general population screening or management of detected nodules, yet much work is needed on optimization. Most of the risk models developed in China have relatively poor discrimination, no calibration or no external validation.
As there are different risk profiles for lung cancer in China, we must consider to what extent these differences will influence the optimal Chinese lung cancer risk model. Whether or not risk models should be developed separately in males and females, or different thresholds should be set in different genders or those with different smoking status, are questions that remain to be answered (and might not be fully addressed until implementation based on the best model at the time and further data gathered as part of the screening effort).
It must be considered that in the Chinese context, science is advancing on an exponential scale. Current lung cancer prevalence may reflect exposure levels of risk factors many years ago, similar to the delayed impact of smoking on mortality; or the real status quo in China may be mis-represented because of potential bias in data collection, i.e. from current incomplete cancer registries. A recent publication reported a higher lung cancer incidence in young women compared to young men, noting that those in both genders were born after the mid-1960s in the US.247 Different smoking behaviours between the genders cannot fully explain this phenomenon.247 Given the changing situations, the entry criteria into lung cancer screening programmes should be reconsidered.
Screening age range
In China, lung cancer incidence is quite low in individuals aged ≤45, but it increases with ages in those over 50.115,146 Individuals in younger generations (i.e. <50 years) would suffer more harm from screening, e.g. excess cancer risk induced by radiation exposure,243 whereas an older individual might not benefit from screening because of existing comorbidities and other competing causes of deaths. After combining the evidence above and life expectancy in China, the CNS 2015/2018 recommended ages 50–74 for screening feasibility;115,146 however, the optimal screening age range is not yet specified in China.
Nodule measurement
Accumulating evidence has demonstrated that volumetrics and VDT are less variable and more sensitive in detecting nodule sizes and growth. The NELCIN-B3 study will help to further define this. It is also preferable to apply volumetry software to optimize nodule management strategies during implementation.
Identify ‘indeterminate’ nodules
Different cut-off values are associated with different lung cancer risks. Risk-stratification allows for nodules to be managed accordingly; however, it is unclear whether variations in the aetiology of lung cancer in the East extend to the biological features that influence nodule behaviour. Risk models for malignancy prediction of nodules were derived from post hoc analysis, and it is not known whether these models would perform well in an ongoing LDCT screening trial.
Mortality data
The two largest studies—NLST and NELSON—reported a benefit of mortality reduction by LDCT screening. In China, a microsimulation modelling study indicated a favourable role of LDCT screening over CXR and no screening in mortality reduction among urban smokers aged 45–80 years.116 It is uncertain to what extent LDCT screening in China would help to reduce mortality, either lung cancer-specific or all-cause, in the real world. Whether or not non-smokers in China would benefit from screening is also undetermined.
Cost-effectiveness
When it comes to real-life practice, cost-effectiveness is always a serious consideration. We should consider not just the health benefit provided by screening, but the associated financial benefits of reduced costs for cancer treatment and the improved economic output of those living longer and healthier lives.
The ageing population in China is likely to be more vulnerable to both lung cancer and other causes of death. The latest papers suggest that long-term outcomes and non-lung cancer outcomes of participants should be taken into account during assessment.229,230 There will always be compromises during the process, e.g. more screening rounds can lead to lung cancer mortality reduction but result in more overdiagnosis and radiation exposure. Management should be individualized in screened participants according to their baseline scan results and nodule risk-stratification, to reduce unnecessary scans in the low-risk and maximize the benefits. Currently, using a mathematical method to simulate different scenarios is a favourable alternative, and this may provide us with additional information that could not be obtained in real life because of limited research resources.
Screening intervals
Lung cancer risk is baseline result-dependent, but nodule size and nodule attenuation (solid, part-solid, non-solid) also affect the risk of malignancy. Similarly, cost-effectiveness analysis leads the way. Data from real practice is needed in China.
Smoking cessation
Tobacco control can provide additional benefits to those we have discussed. Apart from lung cancer, smoking is closely related to morbidities such as COPD, cardiovascular diseases, and ischemic stroke. Smoking exposure is positively associated with mortality risks of these morbidities, and cessation would help to decrease these risks.38 Thus, tobacco control could save lives not only from lung cancers but also from other highly life-disabling conditions, thus improving quality of life. By combination with tobacco control, lung cancer screening could achieve more cost-effectiveness. However, evidence on efficient and effective strategies for such a combination is still limited.
In conclusion, lung cancer and smoking prevalence in China are very different from that seen in other countries. Increasing trends for lung cancer mortality are expected following a lag from smoking exposure. Other risk factors may play a significant role alongside smoking for lung cancer risk in China; broader entry criteria to screening programmes might be more expedient in China to accommodate non-smokers. Evidence from Chinese lung cancer screening is limited, but the success of screening programmes and evidence from other countries could pave the way. Risk models should be optimized, and a prespecified analysis would be helpful for initial trials, adopting a re-iterative, adaptive approach as screening programmes develop.
Supplementary Material
Acknowledgements
We give our special appreciation to Robert Carlton for proofreading the manuscript. YIC is funded by China Scholarship Council (CSC201706240094) and West China-Liverpool Clinician-Scientist Leadership Scholarship. MPAD is funded by the Roy Castle Lung Cancer Foundation. This work is also supported by 1.3.5 Project for Disciplines of Excellence, West China Hospital. Sichuan University (ZYJC18001) the National Key Development Plan for Precision Medicine Research (2017YFC0910004), the Transformation Projects of Sci-Tech Achievements of Sichuan Province (2016CZYD0001), and the Sci-Tech Support Program of Science and Technology Department of Sichuan Province (2016SZ0073).
Conflict of interest statement
None declared.
References
- 1. Ferlay J, Ervik M, Lam F, et al. Global Cancer Observatory: Cancer Today. Lyon, France: International Agency for Research on Cancer. 2018. https://gco.iarc.fr/today.
- 2. Chen W, Zheng R, Baade PD, et al. . Cancer statistics in China, 2015. CA Cancer J Clin 2016;66:115–32. 10.3322/caac.21338. [DOI] [PubMed] [Google Scholar]
- 3. Han R, Zheng R, Zhang S, et al. . [Trend analyses on the differences of lung cancer incidence between gender, area and average age in China during 1989–2008]. Zhongguo Fei Ai Za Zhi 2013;16:445–51. 10.3779/j.issn.1009-3419.2013.09.02. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Chen W, Zheng R, Zeng H, et al. . Epidemiology of lung cancer in China. Thorac Cancer 2015;6:209–15. 10.1111/1759-7714.12169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Li S, Meng L, Chiolero A, et al. . Trends in smoking prevalence and attributable mortality in China, 1991–2011. Prev Med 2016;93:82–7. 10.1016/j.ypmed.2016.09.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Ferlay J, Colombet M, Bray F. Cancer Incidence in Five Continents Time Trends, CI5plus: IARC CancerBase No.9 [Internet]. Lyon, France: International Agency for Research on Cancer; 2018. Available from: http://ci5.iarc.fr (3 March 2019, date last accessed).
- 7. Chen W, Sun K, Zheng R, et al. . Cancer incidence and mortality in China, 2014. Chin J Cancer Res 2018;30:1–12. 10.21147/j.issn.1000-9604.2018.01.01. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Zheng R, Zeng H, Zuo T, et al. . Lung cancer incidence and mortality in China, 2011. Thorac Cancer 2016;7:94–9. 10.1111/1759-7714.12286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Chen W, Zheng R, Zuo T, et al. . National cancer incidence and mortality in China, 2012. Chin J Cancer Res 2016;28:1–11. 10.3978/j.issn.1000-9604.2016.02.08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Zheng R, Zeng H, Zhang S, et al. . Estimates of cancer incidence and mortality in China, 2013. Chin J Cancer 2017;36:66. 10.1186/s40880-017-0234-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Zhi XY, Zou XN, Hu M, et al. . Increased lung cancer mortality rates in the Chinese population from 1973–1975 to 2004–2005: An adverse health effect from exposure to smoking. Cancer 2015;121:3107–12. 10.1002/cncr.29603. [DOI] [PubMed] [Google Scholar]
- 12.World Health Organization (WHO) International Agency for Research on Cancer (IARC): Cancer Mortality Database. 2016. http://www-dep.iarc.fr/WHOdb/WHOdb.htm (3 March 2019, date last accessed).
- 13. Chen WQ, Zhang SW, Zou XN, et al. . Cancer incidence and mortality in china, 2006. Chin J Cancer Res 2011;23:3–9. 10.1007/s11670-011-0003-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cancer Research UK: Lung cancer statistics 2018. http://www.cancerresearchuk.org/health-professional/cancer-statistics/statistics-by-cancer-type/lung-cancer (3 March 2019, date last accessed).
- 15. Lopez AD, Collishaw N, Piha TA. A Descriptive Model of the Cigarette Epidemic in Developed Countries. Tob Control 1994;3:242–7. 10.1136/tc.3.3.242. [DOI] [Google Scholar]
- 16. Islami F, Chen W, Yu XQ, et al. . Cancer deaths and cases attributable to lifestyle factors and infections in China, 2013. Ann Oncol 2017;28:2567–74. 10.1093/annonc/mdx342. [DOI] [PubMed] [Google Scholar]
- 17. Parkin DM. 2. Tobacco-attributable cancer burden in the UK in 2010. Br J Cancer 2011;105:S6–S13. 10.1038/bjc.2011.475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Islami F, Goding Sauer A, Miller KD, et al. . Proportion and number of cancer cases and deaths attributable to potentially modifiable risk factors in the United States. CA Cancer J Clin 2018;68:31–54. 10.3322/caac.21440. [DOI] [PubMed] [Google Scholar]
- 19. Chen ZM, Peto R, Iona A, et al. . Emerging tobacco-related cancer risks in China: A nationwide, prospective study of 0.5 million adults. Cancer 2015;121:3097–106. 10.1002/cncr.29560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Sisti J, Boffetta P. What proportion of lung cancer in never-smokers can be attributed to known risk factors? Int J Cancer 2012;131:265–75. 10.1002/ijc.27477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Chen G, Sun X, Ren H, et al. . The mortality patterns of lung cancer between 1990 and 2013 in Xuanwei, China. Lung Cancer 2015;90:155–60. 10.1016/j.lungcan.2015.08.006. [DOI] [PubMed] [Google Scholar]
- 22. Loomis D, Huang W, Chen G. The International Agency for Research on Cancer (IARC) evaluation of the carcinogenicity of outdoor air pollution: focus on China. Chin J Cancer 2014;33:189–96. 10.5732/cjc.014.10028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Cao J, Yang C, Li J, et al. . Association between long-term exposure to outdoor air pollution and mortality in China: a cohort study. J Hazard Mater 2011;186:1594–600. 10.1016/j.jhazmat.2010.12.036. [DOI] [PubMed] [Google Scholar]
- 24. Tse LA, Yu IT, Qiu H, et al. . Occupational risks and lung cancer burden for Chinese men: a population-based case-referent study. Cancer Causes Control 2012;23:121–31. 10.1007/s10552-011-9861-1. [DOI] [PubMed] [Google Scholar]
- 25. Wu C, Hu Z, Yu D, et al. . Genetic variants on chromosome 15q25 associated with lung cancer risk in Chinese populations. Cancer Res 2009;69:5065–72. 10.1158/0008-5472.Can-09-0081. [DOI] [PubMed] [Google Scholar]
- 26. Hung RJ, McKay JD, Gaborieau V, et al. . A susceptibility locus for lung cancer maps to nicotinic acetylcholine receptor subunit genes on 15q25. Nature 2008;452:633–7. 10.1038/nature06885. [DOI] [PubMed] [Google Scholar]
- 27. Hu Z, Wu C, Shi Y, et al. . A genome-wide association study identifies two new lung cancer susceptibility loci at 13q12.12 and 22q12.2 in Han Chinese. Nat Genet 2011;43:792–6. 10.1038/ng.875. [DOI] [PubMed] [Google Scholar]
- 28. McKay JD, Hung RJ, Gaborieau V, et al. . Lung cancer susceptibility locus at 5p15.33. Nat Genet 2008;40:1404–6. 10.1038/ng.254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Zhang R, Chu M, Zhao Y, et al. . A genome-wide gene-environment interaction analysis for tobacco smoke and lung cancer susceptibility. Carcinogenesis 2014;35:1528–35. 10.1093/carcin/bgu076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Yamamoto H, Yatabe Y, Toyooka S. Inherited lung cancer syndromes targeting never smokers. Transl Lung Cancer Res 2018;7:498–504. 10.21037/tlcr.2018.06.01. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Gou LY, Niu FY, Wu YL, et al. . Differences in driver genes between smoking-related and non-smoking-related lung cancer in the Chinese population. Cancer 2015;121:3069–79. 10.1002/cncr.29531. [DOI] [PubMed] [Google Scholar]
- 32. El-Telbany A, Ma PC. Cancer genes in lung cancer: racial disparities: are there any? Genes Cancer 2012;3:467–80. 10.1177/1947601912465177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Shigematsu H, Lin L, Takahashi T, et al. . Clinical and biological features associated with epidermal growth factor receptor gene mutations in lung cancers. J Natl Cancer Inst 2005;97:339–46. 10.1093/jnci/dji055. [DOI] [PubMed] [Google Scholar]
- 34. O’Brien TD, Jia P, Aldrich MC, et al. . Lung Cancer: One Disease or Many. Hum Hered 2018;83:65–70. 10.1159/000488942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. BRICS Joint Statistical Publication 2017: English version. Beijing: China Statistics Press; 2017. http://www.gks.ru/free_doc/doc_2017/JSP-2017.pdf (3 March 2019, date last accessed). [Google Scholar]
- 36. Statista . Tobacco 2017. https://www.statista.com/study/15892/tobacco-statista-dossier/ (3 March 2019, date last accessed).
- 37. Giovino GA, Mirza SA, Samet JM, et al. . Tobacco use in 3 billion individuals from 16 countries: an analysis of nationally representative cross-sectional household surveys. Lancet 2012;380:668–79. 10.1016/s0140-6736(12)61085-x. [DOI] [PubMed] [Google Scholar]
- 38. Chen Z, Peto R, Zhou M, et al. . Contrasting male and female trends in tobacco-attributed mortality in China: evidence from successive nationwide prospective cohort studies. Lancet 2015;386:1447–56. 10.1016/S0140-6736(15)00340-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Han J, Chen X. A Meta-Analysis of Cigarette Smoking Prevalence among Adolescents in China: 1981–2010. Int J Environ Res Public Health 2015;12:4617–30. 10.3390/ijerph120504617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Thun M, Peto R, Boreham J, et al. . Stages of the cigarette epidemic on entering its second century. Tob Control 2012;21:96–101. 10.1136/tobaccocontrol-2011-050294. [DOI] [PubMed] [Google Scholar]
- 41. ASH-Action on Smoking and Health . Smoking statistics: who smokes and how much. 2016. http://ash.org.uk/wp-content/uploads/2016/06/Smoking-Statistics-Who-Smokes-and-How-Much.pdf (3 March 2019, date last accessed).
- 42. Cancer Research UK . Tobacco Statistics: Trends over time for tobacco statistics. http://www.cancerresearchuk.org/health-professional/cancer-statistics/risk/tobacco#heading-Three (3 March 2019, date last accessed).
- 43. Office for National Statistics . Adult smoking habits in the UK:2017. 2018. https://www.ons.gov.uk/peoplepopulationandcommunity/healthandsocialcare/healthandlifeexpectancies/bulletins/adultsmokinghabitsingreatbritain/2017#the-proportion-who-are-current-smokers-in-the-uk-its-constituent-countries-and-local-areas-2011-to-2017 (3 March 2019, date last accessed).
- 44. Public Health England, GOV.UK . Research and analysis: Chapter 4: European comparisons. 2017. https://www.gov.uk/government/publications/health-profile-for-england/chapter-4-european-comparisons (3 March 2019, date last accessed).
- 45. Office for National Statistics . Likelihood of smoking four times higher in England’s most deprived areas than least deprived. 2018. https://www.ons.gov.uk/peoplepopulationandcommunity/healthandsocialcare/drugusealcoholandsmoking/articles/likelihoodofsmokingfourtimeshigherinenglandsmostdeprivedareasthanleastdeprived/2018-03-14 (3 March 2019, date last accessed).
- 46. Forey B, Hamling J, Hamling J, et al. International Smoking Statistics(WEB Edition): A collection of worldwide historical data. 2016. http://www.pnlee.co.uk/iss3.htm (3 March 2019, date last accessed).
- 47. U.S. Department of Health and Human Services . The Health Consequences of Smoking—50 Years of Progress: A Report of the Surgeon General. 2014; Chapter 13 Patterns of Tobacco Use Among U.S. Youth, Young Adults, and Adults: 701–70.
- 48. Peto R, Lopez AD, Pan H, et al. Mortality from Smoking in Developed Countries 1950–2020 (updated September 2015). 2015. http://gas.ctsu.ox.ac.uk/tobacco/ (3 March 2019, date last accessed).
- 49. Mackay J. China: the tipping point in tobacco control. Br Med Bull 2016;120:15–25. 10.1093/bmb/ldw043. [DOI] [PubMed] [Google Scholar]
- 50. Shen M. Prof. Wanqing Chen: the past, present and future of cancer registry in China. Ann Transl Med 2014;2:71. 10.3978/j.issn.2305-5839.2014.04.07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Wei KR, Liang ZH, Liu J, et al. . [History of Cancer Registration in China.]. Chin J Med History 2012;42:21–5. 10.3760/cma.j.issn.0255-7053.2012.01.006. [DOI] [PubMed] [Google Scholar]
- 52. Bray F, Colombet M, Mery L, et al. . Cancer Incidence in Five Continents, Vol. XI (electronic version). Lyon: International Agency for Research on Cancer. 2017. http://ci5.iarc.fr (3 March 2019, date last accessed). [Google Scholar]
- 53. Li GL, Chen WQ. Representativeness of Population-Based Cancer Registration in China—Comparison of Urban and Rural Areas. Asian Pac J Cancer Prev 2009;10:559–64. [PubMed] [Google Scholar]
- 54. Li J, Shi L, Liang H, et al. . Urban-rural disparities in health care utilization among Chinese adults from 1993 to 2011. BMC Health Serv Res 2018;18:102. 10.1186/s12913-018-2905-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Li X, Lu JP, Hu S, et al. . The primary health-care system in China. Lancet 2017;390:2584–94. 10.1016/S0140-6736(17)33109-4. [DOI] [PubMed] [Google Scholar]
- 56. Meng Q, Xu L, Zhang Y, et al. . Trends in access to health services and financial protection in China between 2003 and 2011: a cross-sectional study. Lancet 2012;379:805–14. 10.1016/s0140-6736(12)60278-5. [DOI] [PubMed] [Google Scholar]
- 57. Jian W, Chan KY, Reidpath DD, et al. . China’s rural-urban care gap shrank for chronic disease patients, but inequities persist. Health Aff (Millwood) 2010;29:2189–96. 10.1377/hlthaff.2009.0989. [DOI] [PubMed] [Google Scholar]
- 58. Stone EC, Zhou C, International Association for the Study of Lung Cancer Tobacco Control C . Slowing the Titanic: China’s Epic Struggle with Tobacco. J Thorac Oncol 2016;11:2053–65. 10.1016/j.jtho.2016.07.020. [DOI] [PubMed] [Google Scholar]
- 59. Li L, Liu D, Zhang L, et al. . [Clinicopathological Features, Diagnoses and Treatments of 6458 Lung Cancer Patients]. Sichuan Da Xue Xue Bao Yi Xue Ban 2017;48:352–8. [PubMed] [Google Scholar]
- 60. Siegel RL, Miller KD, Jemal A. Cancer statistics, 2018. CA Cancer J Clin 2018;68:7–30. 10.3322/caac.21442. [DOI] [PubMed] [Google Scholar]
- 61. Pirie K, Peto R, Reeves GK, et al. . The 21st century hazards of smoking and benefits of stopping: a prospective study of one million women in the UK. Lancet 2013;381:133–41. 10.1016/s0140-6736(12)61720-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Jha P, Ramasundarahettige C, Landsman V, et al. . 21st-century hazards of smoking and benefits of cessation in the United States. N Engl J Med 2013;368:341–50. 10.1056/NEJMsa1211128. [DOI] [PubMed] [Google Scholar]
- 63.Geneva: World Health Organization (WHO) Report On The Global Tobacco Epidemic, 2017: Monitoring tobacco use and prevention policies 2017. http://www.who.int/tobacco/global_report/2017/en/ (3 March 2019, date last accessed).
- 64. Hu TW, Zhang X, Zheng R. China has raised the tax on cigarettes: what’s next? Tob Control 2016;25:609–11. 10.1136/tobaccocontrol-2015-052534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Goodchild M, Zheng R. Early assessment of China’s 2015 tobacco tax increase. Bull World Health Organ 2018;96:506–12. 10.2471/BLT.17.205989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Field JK, Duffy SW. Lung cancer screening: the way forward. Br J Cancer 2008;99:557–62. 10.1038/sj.bjc.6604509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Oken MM, Hocking WG, Kvale PA, et al. . Screening by chest radiograph and lung cancer mortality: the Prostate, Lung, Colorectal, and Ovarian (PLCO) randomized trial. JAMA 2011;306:1865–73. 10.1001/jama.2011.1591. [DOI] [PubMed] [Google Scholar]
- 68. Henschke CI, McCauley DI, Yankelevitz DF, et al. . Early Lung Cancer Action Project: overall design and findings from baseline screening. Lancet 1999;354:99–105. 10.1016/s0140-6736(99)06093-6. [DOI] [PubMed] [Google Scholar]
- 69. Henschke CI, Yankelevitz DF, Libby DM, et al. . Survival of patients with stage I lung cancer detected on CT screening. N Engl J Med 2006;355:1763–71. 10.1056/NEJMoa060476. [DOI] [PubMed] [Google Scholar]
- 70. Aberle DR, Adams AM, Berg CD, et al. . The National Lung Screening Trial Research Team. Reduced lung-cancer mortality with low-dose computed tomographic screening. N Engl J Med 2011;365:395–409. 10.1056/NEJMoa1102873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Konning HD. NELSON Study Shows CT Screening for Nodule Volume Management Reduces Lung Cancer Mortality by 26% in Men. IASLC 19th World Conference on Lung Cancer (WCLC 2018); Toronto, Canada. 2018.
- 72. Wille MM, Dirksen A, Ashraf H, et al. . Results of the Randomized Danish Lung Cancer Screening Trial with Focus on High-Risk Profiling. Am J Respir Crit Care Med 2016;193:542–51. 10.1164/rccm.201505-1040OC. [DOI] [PubMed] [Google Scholar]
- 73. Infante M, Cavuto S, Lutman FR, et al. . Long-Term Follow-up Results of the DANTE Trial, a Randomized Study of Lung Cancer Screening with Spiral Computed Tomography. Am J Respir Crit Care Med 2015;191:1166–75. 10.1164/rccm.201408-1475OC. [DOI] [PubMed] [Google Scholar]
- 74. Paci E, Puliti D, Lopes Pegna A, et al. . Mortality, survival and incidence rates in the ITALUNG randomised lung cancer screening trial. Thorax 2017;72:825–31. 10.1136/thoraxjnl-2016-209825. [DOI] [PubMed] [Google Scholar]
- 75. Pastorino U, Rossi M, Rosato V, et al. . Annual or biennial CT screening versus observation in heavy smokers: 5-year results of the MILD trial. Eur J Cancer Prev 2012;21:308–15. 10.1097/CEJ.0b013e328351e1b6. [DOI] [PubMed] [Google Scholar]
- 76. Becker N, Motsch E, Gross ML, et al. . Randomized Study on Early Detection of Lung Cancer with MSCT in Germany: Results of the First 3 Years of Follow-up After Randomization. J Thorac Oncol 2015;10:890–6. 10.1097/JTO.0000000000000530. [DOI] [PubMed] [Google Scholar]
- 77. Blanchon T, Brechot JM, Grenier PA, et al. . Baseline results of the Depiscan study: a French randomized pilot trial of lung cancer screening comparing low dose CT scan (LDCT) and chest X-ray (CXR). Lung Cancer 2007;58:50–8. 10.1016/j.lungcan.2007.05.009. [DOI] [PubMed] [Google Scholar]
- 78. Coureau G, Salmi LR, Etard C, et al. . Low-dose computed tomography screening for lung cancer in populations highly exposed to tobacco: A systematic methodological appraisal of published randomised controlled trials. Eur J Cancer 2016;61:146–56. 10.1016/j.ejca.2016.04.006. [DOI] [PubMed] [Google Scholar]
- 79. Sagawa M, Nakayama T, Tanaka M, et al. . A randomized controlled trial on the efficacy of thoracic CT screening for lung cancer in non-smokers and smokers of <30 pack-years aged 50–64 years (JECS study): research design. Jpn J Clin Oncol 2012;42:1219–21. 10.1093/jjco/hys157. [DOI] [PubMed] [Google Scholar]
- 80. Moyer VA. Screening for lung cancer: U.S. Preventive Services Task Force recommendation statement. Ann Intern Med 2014;160:330–8. 10.7326/m13-2771. [DOI] [PubMed] [Google Scholar]
- 81. Bach PB, Mirkin JN, Oliver TK, et al. . Benefits and harms of CT screening for lung cancer: a systematic review. JAMA 2012;307:2418–29. 10.1001/jama.2012.5521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Jaklitsch MT, Jacobson FL, Austin JH, et al. . The American Association for Thoracic Surgery guidelines for lung cancer screening using low-dose computed tomography scans for lung cancer survivors and other high-risk groups. J Thorac Cardiovasc Surg 2012;144:33–8. 10.1016/j.jtcvs.2012.05.060. [DOI] [PubMed] [Google Scholar]
- 83. Wender R, Fontham ET, Barrera E Jr, et al. . American Cancer Society lung cancer screening guidelines. CA Cancer J Clin 2013;63:107–17. 10.3322/caac.21172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Centres for Medicare & Medicaid Services (CMS) . Decision Memo for Screening for Lung Cancer with Low Dose Computed Tomography (LDCT) (CAG-00439N). 2015. https://www.cms.gov/medicare-coverage-database/details/nca-decision-memo.aspx?NCAId=274 (3 March 2019, date last accessed).
- 85. Zhou Q, Fan Y, Wu N, et al. . Demonstration program of population-based lung cancer screening in China: Rationale and study design. Thorac Cancer 2014;5:197–203. 10.1111/1759-7714.12078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Zhou Q. MS 16.02 NELCIN-B3 SCREENING PROGRAM IN CHINA. The IASLC 19th World Conference on Lung Cancer (WCLC 2018). 23–25 September. Toronto, Canada, 2018.
- 87. Dai M, Shi J, Li N. [The design and expectation of the Cancer Screening Program in Urban China]. Chin J Prev Med 2013;47:179–82. 10.3760/cma.j.issn.0253-9624.2013.02.018. [DOI] [Google Scholar]
- 88. Jincheng General Hospital . Implementation Protocol of Cancer Screening Program in Urban China. 2016. http://www.jmjtzyy.com/index.php/Index/newsContent/id/1237 (3 March 2019, date last accessed).
- 89.Netherlands-China Big-3 screening: NELCIN-B3. https://www.rug.nl/news/2016/11/grote-subsidie-voor-samenwerking-in-onderzoek-vroege-opsporing-longkanker_-copd-en-hart--en-vaatziekten?lang=en (3 March 2019, date last accessed).
- 90. Cancer Hospital Chinese Academy of Medical Sciences . Results of project funding from the National Key Reseach and Development Program of China in 2017. http://www.cicams.ac.cn/Html/News/Articles/2631.html (3 March 2019, date last accessed).
- 91. National Science and Technology Information System, Public Service Platform . Results of project funding from the National Key Reseach and Development Program of China in 2018. http://service.most.gov.cn/2015tztg_all/20180727/2659.html (3 March 2019, date last accessed).
- 92. Chng B, Maclean C. Milken Institute. Financing Lung cancer screening in China: financial innovations lab report. 2017.
- 93. Sun XK, Tu DH, Li JF, et al. . [Mass Photofluorography in early detection of peripheral lung cancer.]. Chin J Oncol 1986;8:370–2. [PubMed] [Google Scholar]
- 94. Shi ZT, Zhang YL. [Mass screening of lung cancer: reports in orpiment miners in Hunan.]. Chin J Tubercul Respirat Dis 1989;12:230–1. [PubMed] [Google Scholar]
- 95. Qin DX, Li YY, Zuo JH, et al. . [Stage II test report for screening lung cancer by sputum of occult blood test.]. Cancer 1991;10. [Google Scholar]
- 96. Qin DX, Xu ZJ, Sun Y, et al. . [Comparison of lung cancer screening methods sputum occult blood detection versus chest X-ray.]. Chin J Clin Oncol Rehabil 2009;16. 10.13455/j.cnki. [DOI] [Google Scholar]
- 97. Gao G, Yao S, Sun X, et al. . [Establishment of cohort to study lung cancer in Yunnan tin miners]. Zhongguo Fei Ai Za Zhi 2002;5:87–91. 10.3779/j.issn.1009-3419.2002.02.03. [DOI] [PubMed] [Google Scholar]
- 98. Liu X, Liang M, Wang Y, et al. . The outcome differences of CT screening for lung cancer pre and post following an algorithm in Zhuhai, China. Lung Cancer 2011;73:230–6. 10.1016/j.lungcan.2010.11.012. [DOI] [PubMed] [Google Scholar]
- 99. Tang W, Wu N, Huang Y, et al. . [Results of low-dose computed tomography (LDCT) screening for early lung cancer: prevalence in 4690 asymptomatic participants]. Zhonghua Zhong Liu Za Zhi 2014;36:549–54. [PubMed] [Google Scholar]
- 100. Guo L, Li N, Wang G, et al. . [Body mass index and cancer incidence:a prospective cohort study in northern China]. Zhonghua Liu Xing Bing Xue Za Zhi 2014;35:231–6. [PubMed] [Google Scholar]
- 101. Dai M, Hu P, Shi J-F, et al. . The China Cancer Screening Trial Feasibility Study. Lancet 2015;386. 10.1016/s0140-6736(15)00616-9. [DOI] [Google Scholar]
- 102. Chinese Clinical Trial Registry . The Feasibility Study of the Randomized Cancer Screening Trial in China 2015. http://www.chictr.org.cn/showproj.aspx?proj=11888 (3 March 2019, date last accessed).
- 103. Gao ZS, Ye ZX, Zhang P, et al. . [Low-dose computed tomography screening for lung cancer in Tianjin: a preliminary clinical analysis of baseline screening and follow-up results]. Chin J Clin Oncol 2017;44:1034–9. [Google Scholar]
- 104. Tianjin Medical University Cancer Institute and Hospital . News Centre: Initiation of Tianjin Cancer Screening Programme in 2018. http://www.tjmuch.com/system/2018/03/28/011271257.shtml (3 March 2019, date last accessed).
- 105. Luo X, Zheng S, Liu Q, et al. . Should Nonsmokers Be Excluded from Early Lung Cancer Screening with Low-Dose Spiral Computed Tomography? Community-Based Practice in Shanghai. Transl Oncol 2017;10:485–90. 10.1016/j.tranon.2017.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106. Yang W, Qian F, Teng J, et al. . Community-based lung cancer screening with low-dose CT in China: Results of the baseline screening. Lung Cancer 2018;117:20–6. 10.1016/j.lungcan.2018.01.003. [DOI] [PubMed] [Google Scholar]
- 107. Wang Y, Fan L, Zhou Y, et al. . [Preliminary results of baseline low-dose computed tomography screening for lung cancer in Shanghai]. Chin J Health Manage 2018;12:51–4. [Google Scholar]
- 108. Shanghai Changzheng Hospital . Programme declaration of Liu Shiyuan: Study on early detection and diagnosis of peripheral lung cancer by multi-modality imaging. 2018. http://www.shcz.com/front/newOne.aspx?id=1637. (3 March 2019, date last accessed).
- 109. Baoshan District Commission of Health and Family Planning, Shanghai Municipality . Government policy. Implementation of lung cancer screening programme in old people in Baoshan District. 2018. http://bsxx.baoshan.sh.cn/xxgk_website/HTML/bsxxgk/bsxxgk_zfjg_qwsjsw_gkml/2018-04-02/Detail_298737.htm (3 March 2019, date last accessed).
- 110. Shanghai Municipal Government . Progress Report of governmental programmes in September, 2018. http://bsxx.baoshan.sh.cn/xxgk_website/html/bsxxgk_m/bsxxgk_xbzfssxm_zfssxmjz_m/2018-10-08/Detail_301895.htm (3 March 2019, date last accessed).
- 111. The Guangzhou Medical University First Affiliated Hospital . News report: initiation of lung cancer screening in Guangdong Province. 2017. http://www.gyfyy.com/cn/list-133-7854.html (3 March 2019, date last accessed).
- 112. Department of Housing and Urban-Rural Development of Qinghai Province . Government news: lung cancer screening programme in Qinghai initiated by Shanghai Cijing Charity Fund with support of Qinghai Red Cross Hospital. 2017. http://www.qhcin.gov.cn/info/1640/15629.htm (3 March 2019, date last accessed).
- 113. More Love Foundation . News report: cancer screening in Minhe County, Qinghai Province by Cijing Special Charity Fund. 2017. http://www.morelove.org.cn/html/20170112256.html (3 March 2019, date last accessed).
- 114. Sheehan DF, Criss SD, Gazelle GS, et al. . Evaluating lung cancer screening in China: Implications for eligibility criteria design from a microsimulation modeling approach. PLoS One 2017;12. 10.1371/journal.pone.0173119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115. Zhou QH, Fan YG, Bu H, et al. . China national lung cancer screening guideline with low-dose computed tomography (2015 version). Thorac Cancer 2015;6:812–8. 10.1111/1759-7714.12287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116. Wang Z, Han W, Zhang W, et al. . Mortality outcomes of low-dose computed tomography screening for lung cancer in urban China: a decision analysis and implications for practice. Chin J Cancer 2017;8:367–79. 10.1186/s40880-017-0221-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117. Oudkerk M, Devaraj A, Vliegenthart R, et al. . European position statement on lung cancer screening. Lancet Oncol 2017;18:e754–e66. 10.1016/s1470-2045(17)30861-6. [DOI] [PubMed] [Google Scholar]
- 118. Ostroff JS, Buckshee N, Mancuso CA, et al. . Smoking cessation following CT screening for early detection of lung cancer. Prev Med 2001;33:613–21. 10.1006/pmed.2001.0935. [DOI] [PubMed] [Google Scholar]
- 119. van der Aalst CM, van den Bergh KA, Willemsen MC, et al. . Lung cancer screening and smoking abstinence: 2 year follow-up data from the Dutch-Belgian randomised controlled lung cancer screening trial. Thorax 2010;65:600–5. 10.1136/thx.2009.133751. [DOI] [PubMed] [Google Scholar]
- 120. Brain K, Carter B, Lifford KJ, et al. . Impact of low-dose CT screening on smoking cessation among high-risk participants in the UK Lung Cancer Screening Trial. Thorax 2017;72:912–8. 10.1136/thoraxjnl-2016-209690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121. Ashraf H, Tonnesen P, Holst Pedersen J, et al. . Effect of CT screening on smoking habits at 1-year follow-up in the Danish Lung Cancer Screening Trial (DLCST). Thorax 2009;64:388–92. 10.1136/thx.2008.102475. [DOI] [PubMed] [Google Scholar]
- 122. Tammemagi MC, Berg CD, Riley TL, et al. . Impact of lung cancer screening results on smoking cessation. J Natl Cancer Inst 2014;106:dju084. 10.1093/jnci/dju084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123. Taylor KL, Cox LS, Zincke N, et al. . Lung cancer screening as a teachable moment for smoking cessation. Lung Cancer 2007;56:125–34. 10.1016/j.lungcan.2006.11.015. [DOI] [PubMed] [Google Scholar]
- 124. Anderson CM, Yip R, Henschke CI, et al. . Smoking cessation and relapse during a lung cancer screening program. Cancer Epidemiol Biomarkers Prev 2009;18:3476–83. 10.1158/1055-9965.Epi-09-0176. [DOI] [PubMed] [Google Scholar]
- 125. Tanner NT, Kanodra NM, Gebregziabher M, et al. . The Association between Smoking Abstinence and Mortality in the National Lung Screening Trial. Am J Respir Crit Care Med 2016;193:534–41. 10.1164/rccm.201507-1420OC. [DOI] [PubMed] [Google Scholar]
- 126. Pastorino U, Boffi R, Marchiano A, et al. . Stopping Smoking Reduces Mortality in Low-Dose Computed Tomography Screening Participants. J Thorac Oncol 2016;11:693–9. 10.1016/j.jtho.2016.02.011. [DOI] [PubMed] [Google Scholar]
- 127. Joseph AM, Rothman AJ, Almirall D, et al. . Lung Cancer Screening and Smoking Cessation Clinical Trials. SCALE (Smoking Cessation within the Context of Lung Cancer Screening) Collaboration. Am J Respir Crit Care Med 2018;197:172–82. 10.1164/rccm.201705-0909CI. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128. Sun X, Tan J, Tang L, et al. . Real world evidence: experience and lessons from China. BMJ 2018;360:j5262. 10.1136/bmj.j5262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129. McRonald FE, Yadegarfar G, Baldwin DR, et al. . The UK Lung Screen (UKLS): demographic profile of first 88,897 approaches provides recommendations for population screening. Cancer Prev Res (Phila) 2014;7:362–71. 10.1158/1940-6207.CAPR-13-0206. [DOI] [PubMed] [Google Scholar]
- 130. Ali N, Lifford KJ, Carter B, et al. . Barriers to uptake among high-risk individuals declining participation in lung cancer screening: a mixed methods analysis of the UK Lung Cancer Screening (UKLS) trial. BMJ Open 2015;5:e008254. 10.1136/bmjopen-2015-008254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131. van den Bergh KA, Essink-Bot ML, van Klaveren RJ, et al. . Informed participation in a randomised controlled trial of computed tomography screening for lung cancer. Eur Respir J 2009;34:711–20. 10.1183/09031936.00098908. [DOI] [PubMed] [Google Scholar]
- 132. Tanner NT, Gebregziabher M, Hughes Halbert C, et al. . Racial Differences in Outcomes within the National Lung Screening Trial. Implications for Widespread Implementation. Am J Respir Crit Care Med 2015;192:200–8. 10.1164/rccm.201502-0259OC. [DOI] [PubMed] [Google Scholar]
- 133. Field JK, Duffy SW, Baldwin DR, et al. . The UK Lung Cancer Screening Trial: a pilot randomised controlled trial of low-dose computed tomography screening for the early detection of lung cancer. Health Technol Assess 2016;20:1–146. 10.3310/hta20400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134. Duda C, Mahon I, Chen MH, et al. . Impact and costs of targeted recruitment of minorities to the National Lung Screening Trial. Clin Trials 2011;8:214–23. 10.1177/1740774510396742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135. Ford ME, Havstad SL, Davis SD. A randomized trial of recruitment methods for older African American men in the Prostate, Lung, Colorectal and Ovarian (PLCO) Cancer Screening Trial. Clin Trials 2004;1:343–51. 10.1191/1740774504cn029oa. [DOI] [PubMed] [Google Scholar]
- 136. Lee C. Screening for Lung Cancer: Effective Recruitment Methods. AJR Am J Roentgenol 2018;210:514–7. 10.2214/AJR.17.18755. [DOI] [PubMed] [Google Scholar]
- 137. Field JK, Marcus M, Duffy SW, et al. Liverpool Healthy Lung Programme: Preliminary report for the first three neighbourhoods: Everton, Picton and Speke. 2017. https://www.liverpoolccg.nhs.uk/media/2665/liverpool-healthy-lung-project-report_final.pdf (3 March 2019, date last accessed).
- 138. Crosbie PA, Balata H, Evison M, et al. . Implementing lung cancer screening: baseline results from a community-based ‘Lung Health Check’ pilot in deprived areas of Manchester. Thorax 2018. Nov 12. pii: thoraxjnl-2018-212547. [Epub ahead of print] 10.1136/thoraxjnl-2017-211377. [DOI] [PubMed] [Google Scholar]
- 139. Marcus PM, Lenz S, Sammons D, et al. . Recruitment methods employed in the National Lung Screening Trial. J Med Screen 2012;19:94–102. 10.1258/jms.2012.012016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140. Gren L, Broski K, Childs J, et al. . Recruitment methods employed in the Prostate, Lung, Colorectal, and Ovarian Cancer Screening Trial. Clin Trials 2009;6:52–9. 10.1177/1740774508100974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141. van Iersel CA, de Koning HJ, Draisma G, et al. . Risk-based selection from the general population in a screening trial: selection criteria, recruitment and power for the Dutch-Belgian randomised lung cancer multi-slice CT screening trial (NELSON). Int J Cancer 2007;120:868–74. 10.1002/ijc.22134. [DOI] [PubMed] [Google Scholar]
- 142. Tammemagi MC, Schmidt H, Martel S, et al. . Participant selection for lung cancer screening by risk modelling (the Pan-Canadian Early Detection of Lung Cancer [PanCan] study): a single-arm, prospective study. Lancet Oncol 2017;18:1523–31. 10.1016/s1470-2045(17)30597-1. [DOI] [PubMed] [Google Scholar]
- 143. Field JK, Duffy SW, Baldwin DR, et al. . UK Lung Cancer RCT Pilot Screening Trial: baseline findings from the screening arm provide evidence for the potential implementation of lung cancer screening. Thorax 2016;71:161–70. 10.1136/thoraxjnl-2015-207140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144. Han SS, Ten Haaf K, Hazelton WD, et al. . The impact of overdiagnosis on the selection of efficient lung cancer screening strategies. Int J Cancer 2017;140:2436–43. 10.1002/ijc.30602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145. National Comprehensive Cancer Network . National Comprehensive Cancer Network Clinical Practice Guidelines in Oncology: Lung Cancer Screening. Fort Washington, PA: National Comprehensive Cancer Network; version 1.2019—11 June 2018. https://www.nccn.org/ (3 March 2019, date last accessed). [DOI] [PubMed]
- 146. Zhou Q, Fan Y, Wang Y, et al. . [China National Lung Cancer Screening Guideline with Low-dose Computed Tomography (2018 version)]. Zhongguo Fei Ai Za Zhi 2018;21:67–75. 10.3779/j.issn.1009-3419.2018.02.01. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147. de Koning HJ, Meza R, Plevritis SK, et al. . Benefits and harms of computed tomography lung cancer screening strategies: a comparative modeling study for the U.S. Preventive Services Task Force. Ann Intern Med 2014;160:311–20. 10.7326/m13-2316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148. Cassidy A, Myles JP, van Tongeren M, et al. . The LLP risk model: an individual risk prediction model for lung cancer. Br J Cancer 2008;98:270–6. 10.1038/sj.bjc.6604158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149. Raji OY, Duffy SW, Agbaje OF, et al. . Predictive accuracy of the Liverpool Lung Project risk model for stratifying patients for computed tomography screening for lung cancer: a case-control and cohort validation study. Ann Intern Med 2012;157:242–50. 10.7326/0003-4819-157-4-201208210-00004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150. Ten Haaf K, Tammemagi MC, Bondy SJ, et al. . Performance and Cost-Effectiveness of Computed Tomography Lung Cancer Screening Scenarios in a Population-Based Setting: A Microsimulation Modeling Analysis in Ontario, Canada. PLoS Med 2017;14:e1002225. 10.1371/journal.pmed.1002225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151. Ten Haaf K, de Koning HJ. Should Never-Smokers at Increased Risk for Lung Cancer Be Screened? J Thorac Oncol 2015;10:1285–91. 10.1097/JTO.0000000000000593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152. Tammemagi MC, Church TR, Hocking WG, et al. . Evaluation of the lung cancer risks at which to screen ever- and never-smokers: screening rules applied to the PLCO and NLST cohorts. PLoS Med 2014;11:e1001764. 10.1371/journal.pmed.1001764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153. Church TR, Black WC, Aberle DR, et al. . The National Lung Screening Trial Research Team. Results of initial low-dose computed tomographic screening for lung cancer. N Engl J Med 2013;368:1980–91. 10.1056/NEJMoa1209120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154. Katki HA, Kovalchik SA, Berg CD, et al. . Development and Validation of Risk Models to Select Ever-Smokers for CT Lung Cancer Screening. JAMA 2016;315:2300–11. 10.1001/jama.2016.6255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155. ten Haaf K, Jeon J, Tammemagi MC, et al. . Risk prediction models for selection of lung cancer screening candidates: A retrospective validation study. PLoS Med 2017;14:e1002277. 10.1371/journal.pmed.1002277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156. Li K, Husing A, Sookthai D, et al. . Selecting High-Risk Individuals for Lung Cancer Screening: A Prospective Evaluation of Existing Risk Models and Eligibility Criteria in the German EPIC Cohort. Cancer Prev Res (Phila) 2015;8:777–85. 10.1158/1940-6207.CAPR-14-0424. [DOI] [PubMed] [Google Scholar]
- 157. Tammemagi MC, Katki HA, Hocking WG, et al. . Selection criteria for lung-cancer screening. N Engl J Med 2013;368:728–36. 10.1056/NEJMoa1211776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158. Weber M, Yap S, Goldsbury D, et al. . Identifying high risk individuals for targeted lung cancer screening: Independent validation of the PLCOm2012 risk prediction tool. Int J Cancer 2017;141:242–53. 10.1002/ijc.30673. [DOI] [PubMed] [Google Scholar]
- 159. Gray EP, Teare MD, Stevens J, et al. . Risk Prediction Models for Lung Cancer: A Systematic Review. Clin Lung Cancer 2016;17:95–106. 10.1016/j.cllc.2015.11.007. [DOI] [PubMed] [Google Scholar]
- 160. Sakoda LC, Henderson LM, Caverly TJ, et al. . Applying Risk Prediction Models to Optimize Lung Cancer Screening: Current Knowledge, Challenges, and Future Directions. Curr Epidemiol Rep 2017;4:307–20. 10.1007/s40471-017-0126-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161. D’Amelio AM Jr, Cassidy A, Asomaning K, et al. . Comparison of discriminatory power and accuracy of three lung cancer risk models. Br J Cancer 2010;103:423–9. 10.1038/sj.bjc.6605759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162. Katki HA, Kovalchik SA, Petito LC, et al. . Implications of Nine Risk Prediction Models for Selecting Ever-Smokers for Computed Tomography Lung Cancer Screening. Ann Intern Med 2018;169:10–9. 10.7326/M17-2701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163. Raji OY, Agbaje OF, Duffy SW, et al. . Incorporation of a genetic factor into an epidemiologic model for prediction of individual risk of lung cancer: the Liverpool Lung Project. Cancer Prev Res (Phila) 2010;3:664–9. 10.1158/1940-6207.CAPR-09-0141. [DOI] [PubMed] [Google Scholar]
- 164. Marcus MW, Raji OY, Duffy SW, et al. . Incorporating epistasis interaction of genetic susceptibility single nucleotide polymorphisms in a lung cancer risk prediction model. Int J Oncol 2016;49:361–70. 10.3892/ijo.2016.3499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165. Li H, Yang L, Zhao X, et al. . Prediction of lung cancer risk in a Chinese population using a multifactorial genetic model. BMC Med Genet 2012;13:118. 10.1186/1471-2350-13-118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166. Lin H, Zhong WZ, Yang XN, et al. . A clinical model to estimate the pretest probability of lung cancer, based on 1198 pedigrees in China. J Thorac Oncol 2012;7:1534–40. 10.1097/JTO.0b013e3182641b82. [DOI] [PubMed] [Google Scholar]
- 167. Wang X, Ma K, Cui J, et al. . An individual risk prediction model for lung cancer based on a study in a Chinese population. Tumori 2015;101:16–23. 10.5301/tj.5000205. [DOI] [PubMed] [Google Scholar]
- 168. Yang D, Zhang X, Powell CA, et al. . Probability of cancer in high-risk patients predicted by the protein-based lung cancer biomarker panel in China: LCBP study. Cancer 2018;124:262–70. 10.1002/cncr.31020. [DOI] [PubMed] [Google Scholar]
- 169. Yousaf-Khan U, van der Aalst C, de Jong PA, et al. . Final screening round of the NELSON lung cancer screening trial: the effect of a 2.5-year screening interval. Thorax 2017;72:48–56. 10.1136/thoraxjnl-2016-208655. [DOI] [PubMed] [Google Scholar]
- 170. Sverzellati N, Silva M, Calareso G, et al. . Low-dose computed tomography for lung cancer screening: comparison of performance between annual and biennial screen. Eur Radiol 2016;26:3821–9. 10.1007/s00330-016-4228-3. [DOI] [PubMed] [Google Scholar]
- 171. Duffy SW, Field JK, Allgood PC, et al. . Translation of research results to simple estimates of the likely effect of a lung cancer screening programme in the United Kingdom. Br J Cancer 2014;110:1834–40. 10.1038/bjc.2014.63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172. Goffin JR, Flanagan WM, Miller AB, et al. . Biennial lung cancer screening in Canada with smoking cessation-outcomes and cost-effectiveness. Lung Cancer 2016;101:98–103. 10.1016/j.lungcan.2016.09.013. [DOI] [PubMed] [Google Scholar]
- 173. Horeweg N, van der Aalst CM, Vliegenthart R, et al. . Volumetric computed tomography screening for lung cancer: three rounds of the NELSON trial. Eur Respir J 2013;42:1659–67. 10.1183/09031936.00197712. [DOI] [PubMed] [Google Scholar]
- 174. Patz EF Jr, Pinsky P, Gatsonis C, et al. . Overdiagnosis in low-dose computed tomography screening for lung cancer. JAMA Intern Med 2014;174:269–74. 10.1001/jamainternmed.2013.12738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175. Veronesi G, Maisonneuve P, Bellomi M, et al. . Estimating overdiagnosis in low-dose computed tomography screening for lung cancer: a cohort study. Ann Intern Med 2012;157:776–84. 10.7326/0003-4819-157-11-201212040-00005. [DOI] [PubMed] [Google Scholar]
- 176. Henschke CI, Boffetta P, Gorlova O, et al. . Assessment of lung-cancer mortality reduction from CT Screening. Lung Cancer 2011;71:328–32. 10.1016/j.lungcan.2010.10.025. [DOI] [PubMed] [Google Scholar]
- 177. Veronesi G, Maisonneuve P, Spaggiari L, et al. . Long-term outcomes of a pilot CT screening for lung cancer. Ecancermedicalscience 2010;4:186. 10.3332/ecancer.2010.186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178. Libby DM, Smith JP, Altorki NK, et al. . Managing the small pulmonary nodule discovered by CT. Chest 2004;125:1522–9. [DOI] [PubMed] [Google Scholar]
- 179. Henschke CI. International Early Lung Cancer Action Program: Enrollment and Screening Protocol, 2011. http://www.ielcap.org/sites/default/files/ielcap.pdf (3 March 2019, date last accessed).
- 180. Henschke CI. International Early Lung Cancer Action Program: Screening Protocol, 2016. http://www.ielcap.org/sites/default/files/I-ELCAP-protocol.pdf (3 March 2019, date last accessed).
- 181. Xu DM, Gietema H, de Koning H, et al. . Nodule management protocol of the NELSON randomised lung cancer screening trial. Lung Cancer 2006;54:177–84. 10.1016/j.lungcan.2006.08.006. [DOI] [PubMed] [Google Scholar]
- 182. Baldwin DR, Duffy SW, Wald NJ, et al. . UK Lung Screen (UKLS) nodule management protocol: modelling of a single screen randomised controlled trial of low-dose CT screening for lung cancer. Thorax 2011;66:308–13. 10.1136/thx.2010.152066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183. Bai C, Choi CM, Chu CM, et al. . Evaluation of Pulmonary Nodules: Clinical Practice Consensus Guidelines for Asia. Chest 2016;150:877–93. 10.1016/j.chest.2016.02.650. [DOI] [PubMed] [Google Scholar]
- 184. Callister ME, Baldwin DR, Akram AR, et al. . British Thoracic Society guidelines for the investigation and management of pulmonary nodules. Thorax 2015;70:ii1–ii54. 10.1136/thoraxjnl-2015-207168. [DOI] [PubMed] [Google Scholar]
- 185. Gould MK, Donington J, Lynch WR, et al. . Evaluation of individuals with pulmonary nodules: when is it lung cancer? Diagnosis and management of lung cancer, 3rd ed: American College of Chest Physicians evidence-based clinical practice guidelines. Chest 2013;143:e93S–e120S. 10.1378/chest.12-2351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186. Ruparel M, Quaife SL, Navani N, et al. . Pulmonary nodules and CT screening: the past, present and future. Thorax 2016;71:367–75. 10.1136/thoraxjnl-2015-208107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187. MacMahon H, Naidich DP, Goo JM, et al. . Guidelines for Management of Incidental Pulmonary Nodules Detected on CT Images: From the Fleischner Society 2017. Radiology 2017;284:228–43. 10.1148/radiol.2017161659. [DOI] [PubMed] [Google Scholar]
- 188. Bankier AA, MacMahon H, Goo JM, et al. . Recommendations for Measuring Pulmonary Nodules at CT: A Statement from the Fleischner Society. Radiology 2017;285:584–600. 10.1148/radiol.2017162894. [DOI] [PubMed] [Google Scholar]
- 189. Heuvelmans MA, Walter JE, Vliegenthart R, et al. . Disagreement of diameter and volume measurements for pulmonary nodule size estimation in CT lung cancer screening. Thorax 2018;73:779–781. 10.1136/thoraxjnl-2017-210770. [DOI] [PubMed] [Google Scholar]
- 190. Henschke CI, Yip R, Yankelevitz DF, et al. . Definition of a positive test result in computed tomography screening for lung cancer: a cohort study. Ann Intern Med 2013;158:246–52. 10.7326/0003-4819-158-4-201302190-00004. [DOI] [PubMed] [Google Scholar]
- 191. Yip R, Henschke CI, Yankelevitz DF, et al. . CT screening for lung cancer: alternative definitions of positive test result based on the national lung screening trial and international early lung cancer action program databases. Radiology 2014;273:591–6. 10.1148/radiol.14132950. [DOI] [PubMed] [Google Scholar]
- 192. Gierada DS, Pinsky P, Nath H, et al. . Projected outcomes using different nodule sizes to define a positive CT lung cancer screening examination. J Natl Cancer Inst 2014;106. 10.1093/jnci/dju284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193. McKee BJ, Regis SM, McKee AB, et al. . Performance of ACR Lung-RADS in a clinical CT lung screening program. J Am Coll Radiol 2015;12:273–6. 10.1016/j.jacr.2014.08.004. [DOI] [PubMed] [Google Scholar]
- 194. Pinsky PF, Gierada DS, Black W, et al. . Performance of Lung-RADS in the National Lung Screening Trial: a retrospective assessment. Ann Intern Med 2015;162:485–91. 10.7326/m14-2086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195. American College of Radiology . Lung CT Screening Reporting & Data System: Lung-RADS™ Version 1.0. 2014. https://www.acr.org/Clinical-Resources/Reporting-and-Data-Systems/Lung-Rads (3 March 2019, date last accessed).
- 196. dos Santos RS, Franceschini JP, Chate RC, et al. . Do Current Lung Cancer Screening Guidelines Apply for Populations With High Prevalence of Granulomatous Disease? Results From the First Brazilian Lung Cancer Screening Trial (BRELT1). Ann Thorac Surg 2016;101:481–6; discussion 7–8. 10.1016/j.athoracsur.2015.07.013. [DOI] [PubMed] [Google Scholar]
- 197. Horeweg N, van Rosmalen J, Heuvelmans MA, et al. . Lung cancer probability in patients with CT-detected pulmonary nodules: a prespecified analysis of data from the NELSON trial of low-dose CT screening. Lancet Oncol 2014;15:1332–41. 10.1016/s1470-2045(14)70389-4. [DOI] [PubMed] [Google Scholar]
- 198. Yankelevitz DF, Yip R, Smith JP, et al. . CT Screening for Lung Cancer: Nonsolid Nodules in Baseline and Annual Repeat Rounds. Radiology 2015;277:555–64. 10.1148/radiol.2015142554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199. Silva M, Sverzellati N, Manna C, et al. . Long-term surveillance of ground-glass nodules: evidence from the MILD trial. J Thorac Oncol 2012;7:1541–6. 10.1097/JTO.0b013e3182641bba. [DOI] [PubMed] [Google Scholar]
- 200. de Hoop B, van Ginneken B, Gietema H, et al. . Pulmonary perifissural nodules on CT scans: rapid growth is not a predictor of malignancy. Radiology 2012;265:611–6. 10.1148/radiol.12112351. [DOI] [PubMed] [Google Scholar]
- 201. Ahn MI, Gleeson TG, Chan IH, et al. . Perifissural nodules seen at CT screening for lung cancer. Radiology 2010;254:949–56. 10.1148/radiol.09090031. [DOI] [PubMed] [Google Scholar]
- 202. Schabath MB, Massion PP, Thompson ZJ, et al. . Differences in Patient Outcomes of Prevalence, Interval, and Screen-Detected Lung Cancers in the CT Arm of the National Lung Screening Trial. PLoS One 2016;11:e0159880. 10.1371/journal.pone.0159880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203. Walter JE, Heuvelmans MA, de Jong PA, et al. . Occurrence and lung cancer probability of new solid nodules at incidence screening with low-dose CT: analysis of data from the randomised, controlled NELSON trial. Lancet Oncol 2016;17:907–16. 10.1016/s1470-2045(16)30069-9. [DOI] [PubMed] [Google Scholar]
- 204. Heuvelmans MA, Walter JE, Peters RB, et al. . Relationship between nodule count and lung cancer probability in baseline CT lung cancer screening: The NELSON study. Lung Cancer 2017;113:45–50. 10.1016/j.lungcan.2017.08.023. [DOI] [PubMed] [Google Scholar]
- 205. Gould MK, Fletcher J, Iannettoni MD, et al. . Evaluation of patients with pulmonary nodules: when is it lung cancer?: ACCP evidence-based clinical practice guidelines (2nd edition). Chest 2007;132:108S–30S. 10.1378/chest.07-1353. [DOI] [PubMed] [Google Scholar]
- 206. Swensen SJ, Silverstein MD, Ilstrup DM, et al. . The probability of malignancy in solitary pulmonary nodules. Application to small radiologically indeterminate nodules. Arch Intern Med 1997;157:849–55. [PubMed] [Google Scholar]
- 207. McWilliams A, Tammemagi MC, Mayo JR, et al. . Probability of cancer in pulmonary nodules detected on first screening CT. N Engl J Med 2013;369:910–9. 10.1056/NEJMoa1214726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208. Herder GJ, van Tinteren H, Golding RP, et al. . Clinical prediction model to characterize pulmonary nodules: validation and added value of 18F-fluorodeoxyglucose positron emission tomography. Chest 2005;128:2490–6. 10.1378/chest.128.4.2490. [DOI] [PubMed] [Google Scholar]
- 209. Al-Ameri A, Malhotra P, Thygesen H, et al. . Risk of malignancy in pulmonary nodules: A validation study of four prediction models. Lung Cancer 2015;89:27–30. 10.1016/j.lungcan.2015.03.018. [DOI] [PubMed] [Google Scholar]
- 210. White CS, Dharaiya E, Campbell E, et al. . The Vancouver Lung Cancer Risk Prediction Model: Assessment by Using a Subset of the National Lung Screening Trial Cohort. Radiology 2017;283:264–72. 10.1148/radiol.2016152627. [DOI] [PubMed] [Google Scholar]
- 211. Winkler Wille MM, van Riel SJ, Saghir Z, et al. . Predictive Accuracy of the PanCan Lung Cancer Risk Prediction Model -External Validation based on CT from the Danish Lung Cancer Screening Trial. Eur Radiol 2015;25:3093–9. 10.1007/s00330-015-3689-0. [DOI] [PubMed] [Google Scholar]
- 212. Zhao H, Marshall HM, Yang IA, et al. . Screen-detected subsolid pulmonary nodules: long-term follow-up and application of the PanCan lung cancer risk prediction model. Br J Radiol 2016;89:20160016. 10.1259/bjr.20160016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213. Chung K, Mets OM, Gerke PK, et al. . Brock malignancy risk calculator for pulmonary nodules: validation outside a lung cancer screening population. Thorax 2018;73:857–63. 10.1136/thoraxjnl-2017-211372. [DOI] [PubMed] [Google Scholar]
- 214. Kim H, Park CM, Jeon S, et al. . Validation of prediction models for risk stratification of incidentally detected pulmonary subsolid nodules: a retrospective cohort study in a Korean tertiary medical centre. BMJ Open 2018;8:e019996. 10.1136/bmjopen-2017-019996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215. Perandini S, Soardi GA, Larici AR, et al. . Multicenter external validation of two malignancy risk prediction models in patients undergoing 18F-FDG-PET for solitary pulmonary nodule evaluation. Eur Radiol 2017;27:2042–6. 10.1007/s00330-016-4580-3. [DOI] [PubMed] [Google Scholar]
- 216. Li Y, Wang J. A mathematical model for predicting malignancy of solitary pulmonary nodules. World J Surg 2012;36:830–5. 10.1007/s00268-012-1449-8. [DOI] [PubMed] [Google Scholar]
- 217. Xiao F, Liu D, Guo Y, et al. . Novel and convenient method to evaluate the character of solitary pulmonary nodule-comparison of three mathematical prediction models and further stratification of risk factors. PLoS One 2013;8(10):e78271. 10.1371/journal.pone.0078271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218. Jin C, Cao J, Cai Y, et al. . A nomogram for predicting the risk of invasive pulmonary adenocarcinoma for patients with solitary peripheral subsolid nodules. J Thorac Cardiovasc Surg 2017;153:462–9 e1. 10.1016/j.jtcvs.2016.10.019. [DOI] [PubMed] [Google Scholar]
- 219. Zheng B, Zhou X, Chen J, et al. . A Modified Model for Preoperatively Predicting Malignancy of Solitary Pulmonary Nodules: An Asia Cohort Study. Ann Thorac Surg 2015;100:288–94. 10.1016/j.athoracsur.2015.03.071. [DOI] [PubMed] [Google Scholar]
- 220. Hu H, Wang Q, Tang H, et al. . Multi-slice computed tomography characteristics of solitary pulmonary ground-glass nodules: Differences between malignant and benign. Thorac Cancer 2016;7:80–7. 10.1111/1759-7714.12280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 221. Nguyen XV, Davies L, Eastwood JD, et al. . Extrapulmonary Findings and Malignancies in Participants Screened With Chest CT in the National Lung Screening Trial. J Am Coll Radiol 2017;14:324–30. 10.1016/j.jacr.2016.09.044. [DOI] [PubMed] [Google Scholar]
- 222. van de Wiel JC, Wang Y, Xu DM, et al. . Neglectable benefit of searching for incidental findings in the Dutch-Belgian lung cancer screening trial (NELSON) using low-dose multidetector CT. Eur Radiol 2007;17:1474–82. 10.1007/s00330-006-0532-7. [DOI] [PubMed] [Google Scholar]
- 223. Jin GY, Lynch D, Chawla A, et al. . Interstitial lung abnormalities in a CT lung cancer screening population: prevalence and progression rate. Radiology 2013;268:563–71. 10.1148/radiol.13120816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 224. Sverzellati N, Cademartiri F, Bravi F, et al. . Relationship and prognostic value of modified coronary artery calcium score, FEV1, and emphysema in lung cancer screening population: the MILD trial. Radiology 2012;262:460–7. 10.1148/radiol.11110364. [DOI] [PubMed] [Google Scholar]
- 225. Gietema HA, Schilham AM, van Ginneken B, et al. . Monitoring of smoking-induced emphysema with CT in a lung cancer screening setting: detection of real increase in extent of emphysema. Radiology 2007;244:890–7. 10.1148/radiol.2443061330. [DOI] [PubMed] [Google Scholar]
- 226. Pompe E, de Jong PA, van Rikxoort EM, et al. . Smokers with emphysema and small airway disease on computed tomography have lower bone density. Int J Chron Obstruct Pulmon Dis 2016;11:1207–16. 10.2147/copd.S103680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 227. Henschke CI, Lee IJ, Wu N, et al. . CT screening for lung cancer: prevalence and incidence of mediastinal masses. Radiology 2006;239:586–90. 10.1148/radiol.2392050261. [DOI] [PubMed] [Google Scholar]
- 228. Black WC, Gareen IF, Soneji SS, et al. . Cost-effectiveness of CT screening in the National Lung Screening Trial. N Engl J Med 2014;371:1793–802. 10.1056/NEJMoa1312547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 229. Cressman S, Peacock SJ, Tammemagi MC, et al. . The Cost-Effectiveness of High-Risk Lung Cancer Screening and Drivers of Program Efficiency. J Thorac Oncol 2017;12:1210–22. 10.1016/j.jtho.2017.04.021. [DOI] [PubMed] [Google Scholar]
- 230. Kumar V, Cohen JT, van Klaveren D, et al. . Risk-Targeted Lung Cancer Screening: A Cost-Effectiveness Analysis. Ann Intern Med 2018;168:161–9. 10.7326/M17-1401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 231. Prasad V, Lenzer J, Newman DH. Why cancer screening has never been shown to ‘save lives’—and what we can do about it. BMJ 2016;352:h6080. 10.1136/bmj.h6080. [DOI] [PubMed] [Google Scholar]
- 232. Young RP, Hopkins RJ. Chronic obstructive pulmonary disease (COPD) and lung cancer screening. Transl Lung Cancer Res 2018;7:347–60. 10.21037/tlcr.2018.05.04. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 233. McMahon PM, Kong CY, Bouzan C, et al. . Cost-effectiveness of computed tomography screening for lung cancer in the United States. J Thorac Oncol 2011;6:1841–8. 10.1097/JTO.0b013e31822e59b3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 234. Bunge EM, van den Bergh KA, Essink-Bot ML, et al. . High affective risk perception is associated with more lung cancer-specific distress in CT screening for lung cancer. Lung Cancer 2008;62:385–90. 10.1016/j.lungcan.2008.03.029. [DOI] [PubMed] [Google Scholar]
- 235. Brain K, Lifford KJ, Carter B, et al. . Long-term psychosocial outcomes of low-dose CT screening: results of the UK Lung Cancer Screening randomised controlled trial. Thorax 2016;71:996–1005. 10.1136/thoraxjnl-2016-208283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 236. van den Bergh KA, Essink-Bot ML, Borsboom GJ, et al. . Short-term health-related quality of life consequences in a lung cancer CT screening trial (NELSON). Br J Cancer 2010;102:27–34. 10.1038/sj.bjc.6605459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 237. Gareen IF, Duan F, Greco EM, et al. . Impact of lung cancer screening results on participant health-related quality of life and state anxiety in the National Lung Screening Trial. Cancer 2014;120:3401–9. 10.1002/cncr.28833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 238. Rasmussen JF, Siersma V, Pedersen JH, et al. . Psychosocial consequences in the Danish randomised controlled lung cancer screening trial (DLCST). Lung Cancer 2015;87:65–72. 10.1016/j.lungcan.2014.11.003. [DOI] [PubMed] [Google Scholar]
- 239. McCunney RJ, Li J. Radiation risks in lung cancer screening programs: a comparison with nuclear industry workers and atomic bomb survivors. Chest 2014;145:618–24. 10.1378/chest.13-1420. [DOI] [PubMed] [Google Scholar]
- 240. Brenner DJ. Radiation risks potentially associated with low-dose CT screening of adult smokers for lung cancer. Radiology 2004;231:440–5. 10.1148/radiol.2312030880. [DOI] [PubMed] [Google Scholar]
- 241. Mascalchi M, Belli G, Zappa M, et al. . Risk-benefit analysis of X-ray exposure associated with lung cancer screening in the Italung-CT trial. AJR Am J Roentgenol 2006;187:421–9. 10.2214/ajr.05.0088. [DOI] [PubMed] [Google Scholar]
- 242. Rampinelli C, De Marco P, Origgi D, et al. . Exposure to low dose computed tomography for lung cancer screening and risk of cancer: secondary analysis of trial data and risk-benefit analysis. BMJ 2017;356:j347. 10.1136/bmj.j347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 243. Berrington de Gonzalez A, Kim KP, Berg CD. Low-dose lung computed tomography screening before age 55: estimates of the mortality reduction required to outweigh the radiation-induced cancer risk. J Med Screen 2008;15:153–8. 10.1258/jms.2008.008052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 244. Field JK, Devaraj A, Duffy SW, et al. . CT screening for lung cancer: Is the evidence strong enough? Lung Cancer 2016;91:29–35. 10.1016/j.lungcan.2015.11.003. [DOI] [PubMed] [Google Scholar]
- 245. Zhang L, Wang H, Li Q, et al. . Big data and medical research in China. BMJ 2018;360:j5910. 10.1136/bmj.j5910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 246. Tammemagi MC. Selecting lung cancer screenees using risk prediction models-where do we go from here. Transl Lung Cancer Res 2018;7:243–53. 10.21037/tlcr.2018.06.03. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 247. Jemal A, Miller KD, Ma J, et al. . Higher Lung Cancer Incidence in Young Women Than Young Men in the United States. N Engl J Med 2018;378:1999–2009. 10.1056/NEJMoa1715907. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.