Abstract
Increasing Helicobacter pylori resistance to antibiotics has ledthat molecular testing is appropriate as a sub to adoption of seven different bismuth quadruple therapies (BQT) in China without differentiation of first-line or second-line regimens. The objective of this study was to evaluate the efficacy of susceptibility-guided BQT for patients who had experienced previous treatment failures. A total of 133 patients was included and H. pylori was successfully cultured from 101 patients (75.9%) for subsequent antimicrobial susceptibility testing (AST). Based on the AST results, 88 patients completed one of five AST-guided 14-day BQT regimens: esomeprazole and bismuth colloidal pectin, along with either, amoxicillin and clarithromycin (EBAC), amoxicillin and levofloxacin (EBAL), amoxicillin and furazolidone (EBAF), amoxicillin and tetracycline (EBAT), or tetracycline and furazolidone (EBTF). H. pylori eradication rates were 100% for EBAC (5/5), EBAL (13/13), EBAF (14/14), and EBTF (43/43), but 76.9% for EBAT (10/13). The three patients that failed the EBAT regimen were all cured after subsequent treatment with the EBTF regimen. Our study demonstrates the excellent efficacy of the AST-guided BQT for referred H. pylori patients, and that the current EBAT regimen, used in clinics, needs to be optimized. In addition, 57 of the isolates were subjected to whole-genome sequencing. Analysis of the sequences revealed that point mutations in 23S rRNA correlated well with the phenotypic clarithromycin resistance with a concordance of 91.2%, while the concordance between phenotypic levofloxacin resistance and gyrA point mutations was 82.3%. This suggests that molecular testing is appropriate as a substitute for AST as a more rapid and cost-effective method for determining clarithromycin and levofloxacin resistance in Chinese patients.
Keywords: Helicobacter pylori, antibiotic resistance, rescue therapy, antimicrobial susceptibility testing, whole-genome sequencing
Introduction
The global increase in Helicobacter pylori antibiotic resistance is correlated with the decreased efficacy of the standard empiric triple therapy, proton pump inhibitor (PPI) plus a combination of two antibiotics, among amoxicillin (AMX), clarithromycin (CLA) or metronidazole (MTZ) for treating H. pylori infection, with eradication rates falling to an unacceptably low level (<60%).1 Consequently, for regions with a clarithromycin resistance rate >15%, the triple therapy has been abandoned as a first-line treatment option unless susceptibility testing is performed prior to treatment.2
In China, the primary rates of H. pylori resistance are very high to CLA (28.9%), MTZ (63.8%), and levofloxacin (LVX) (28.0%).3 Therefore, as bismuth is widely available, the Fifth Chinese National H. pylori Consensus recommended that empiric bismuth quadruple therapy (BQT) be used to treat H. pylori infection,4 which is consistent with recent international guidelines including the Maastricht V/Florence consensus,2 the Toronto consensus,5 and the American College of Gastroenterology (ACG) clinical guidelines on H. pylori treatment.6 Of note, apart from conventional BQT, consisting of a PPI, bismuth, MTZ, and tetracycline (TET), six other BQT regimens differing in the combination of two antibiotics: AMX plus CLA, AMX plus LVX, AMX plus TET, AMX plus MTZ, AMX plus furazolidone (FZD), and TET plus FZD, have also been tested as highly efficient regimens (with cure rates >90%).7–12 Thus, the seven different BQT regimens listed above have all been recommended for H. pylori treatment in China without differentiation between what should be considered as first-line, second-line, or third-line regimens.4 Although the choice of regimens is recommended to be based on local H. pylori resistance profiles and history of personal medications,4 such local antibiotic resistance profiles are mostly unavailable in China because of the practice of empirical H. pylori treatment, and many patients who fail therapy do not recall their previous eradication regimens. Under such circumstances, physicians are forced to ‘randomly’ choose between the non-differentiated BQT regimens, and, out of prescription habit, they choose to simply add a bismuth component to legacy triple therapies (such as PPI + AMX + CLA), which has led to a poor eradication efficacy in treatment-naïve patients (<80% by intention-to-treat analysis).8,13 Therefore, there is an increasing demand for dealing with treatment failures after an unsuccessful BQT in China.
As H. pylori infection has been defined as an infectious disease by all recent consensus guidelines,2,5,6,14,15 its treatment should be susceptibility-guided, as is done for other infectious diseases, to minimize the misuse and overuse of antibiotics, especially in the setting of an initially failed BQT. There are two types of susceptibility tests for profiling H. pylori drug resistance: 1) standard phenotypic antimicrobial susceptibility testing (AST), and 2) genotypic molecular testing. Because of the fastidious growth requirements for the culture of H. pylori, the standard AST is indeed laborious and time-consuming (~1–2 weeks).4 Nevertheless, it is currently the only way to test the susceptibility of H. pylori isolates to all clinically used antibiotics.2 Compared to the standard AST, genotypic molecular tests do not require culture and therefore are more rapid and convenient for detection of H. pylori drug resistance. However, currently these molecular-based tests can only be used for the detection of CLA and LVX resistance.2 CLA resistance mainly results from point mutations in the V domain of 23S rRNA, particularly at positions 2146 and 2147 (A2147G, A2146G, A2146C),16–22 and LVX resistance mainly results from point mutations in gyrA corresponding to the amino acid sequence positions 87 and 91.17,20–22 The presence of these point mutations has been reported to correlate well with the standard AST in clinical isolates from several countries,20–22 and therefore commercially developed kits are available.23,24 However, whether these point mutations correlate well with the corresponding standard AST in Chinese H. pylori isolates remains to be determined.
Here, we report the results of AST-guided BQT for referred patients who have mostly failed previous BQT. Furthermore, using a subset of clinical isolates subjected to whole-genome sequencing, we assessed the accuracy of the presence of point mutations in the 23S rRNA gene and gyrA in predicting phenotypic CLA and LVX resistance in Chinese H. pylori isolates.
Materials and methods
Patients
This was a study of consecutive patients who had failed at least one H. pylori empiric treatment. Persistent H. pylori infection was diagnosed using the 14C-urea breath test (UBT).2 Exclusion criteria included subjects who were naïve to H. pylori therapy; younger than 18 years old; pregnant women; mentally impaired; currently taking any medications for other medical conditions; and had a history of allergy to any of the given regimens. Informed consent was obtained from all participants and the study was conducted in accordance with the Declaration of Helsinki, and the protocol was approved by the Ethics Committee of West China Hospital of Sichuan University (2017/332). All participants were evaluated with gastric endoscopy examination with biopsy specimens taken for bacterial culture and histology.
H. pylori isolation
During gastric endoscopy, one or two biopsies were taken from the antrum and/or corpus and were transferred on ice in saline-containing tubes to the H. pylori laboratory at West China-Marshall Research Center for Infectious Diseases, for culture. The biopsies were homogenized with a FastPrep®-24 Sample Preparation System (M.P. Biomedicals, USA) and inoculated onto two agar plates (non-selective and selective). The non-selective plates used in this study were commercial Columbia Blood Agar (CBA) plates (Autobio, China), and the selective plates were CBA supplemented with 5% sheep blood and Dent antibiotics (Oxoid). Inoculated plates were incubated at 37°C for 3–5 days under microaerobic conditions generated using the Anoxomat Mark-II system (Mart Microbiology B.V., the Netherlands). After incubation, the colonies resembling H. pylori were confirmed by Gram-staining and positive oxidase, catalase, and urease tests.
Antimicrobial susceptibility testing
Minimal inhibitory concentrations (MICs) for AMX, CLA, MTZ, LVX, and TET were performed using an Epsilometer test (E-test) (Liofilchem s.r.l, Italy), whereas the MIC for FZD was determined using the 2-fold agar dilution method as previously described.25 According to the recommendations of the European Committee on Antimicrobial Susceptibility Testing (EUCAST), resistance to MTZ, CLA, LVX, AMX, and TET was defined as MIC >8 mg/l, >0.5 mg/l, >1 mg/l, >0.125 mg/l, and 1 mg/l, respectively.25 Resistance to FZD was defined as MIC > 2 mg/l.25,26 Strains with resistance to three or more of the six tested antibiotics were defined as a multidrug-resistant (MDR) phenotype.
Whole-genome sequencing and genotypic resistance
Genomic DNA isolated from 57 isolates using the QIAamp DNA Mini Kit (Qiagen), were subjected to whole-genome sequencing using an Illumina HiSeq X10 platform at Shenzhen BGI Diagnosis Technology Co., Ltd. The assembly and annotation of the generated reads were performed as previously described.27 Draft whole-genome sequences of the 57 strains were deposited at GenBank (Table S1 in the supplementary material). Of note, 32 out of the 57 genomes were used in a previous study involving bioinformatic analysis of lipopolysaccharide glycosyltransferase genes among H. pylori strains of different phylogeographic origin.27
Using the DNA sequences of 23S rRNA (HPr01) and gyrA (HP0701) from H. pylori strain 26695 (RefSeq assembly accession: GCF_000008525.1) as references, the CLA genotypes (the presence/absence of mutations at positions 2146 and 2147 in the V domain of 23S rRNA), and the LVX genotypes (the presence/absence of mutations at the HP0701 amino acid sequence positions 87 and 91) were analyzed by Ariba (v 2.13.5) with default parameters.28 Of note, strain 26695 was tested in this study to be sensitive to both CLA (MIC: 0.016 mg/l) and LVX (MIC: 0.047 mg/l).
Susceptibility-guided bismuth quadruple rescue therapies
Based on AST and the recommendations of the Fifth Chinese National Consensus report on H. pylori infection treatment,4 five different bismuth quadruple rescue regimens were used in this study (Fig. 1). These BQT regimens consisted of esomeprazole and bismuth colloidal pectin with AMX and FZD (EBAF), or with TET and FZD (EBTF), AMX and LVX (EBAL), AMX and CLA (EBAC), or AMX and TET (EBAT) (Table S2 in the supplementary material).
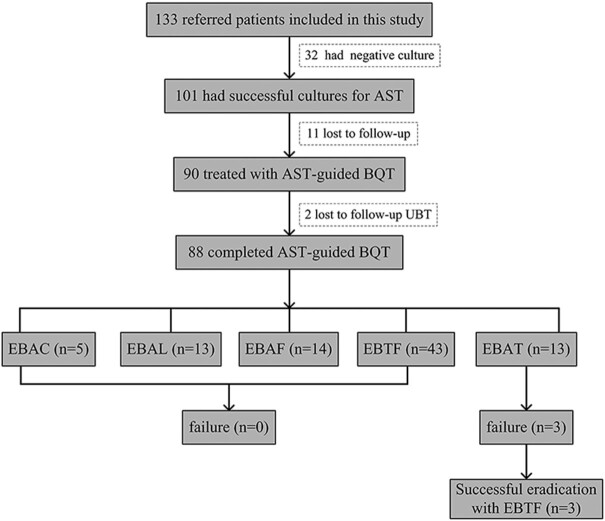
Figure 1.
Flow chart of the study. AST, antimicrobial susceptibility testing; BQT, bismuth quadruple therapy; E, esomeprazole; B, bismuth colloidal pectin; A, amoxicillin; C, clarithromycin; L, levofloxacin; F, furazolidone; T, tetracycline.
To enhance drug compliance, all patients were informed of the importance of adhering to the treatment regimen for the full course (14 days) to achieve successful eradication. Patients were instructed of the timing of doses in relation to meals (administer PPI and bismuth 30 minutes before the meals, antibiotics taken 30 minutes after meals). Systematic telephone follow-ups with each patient were taken to collect data on compliance and treatment tolerance. Eradication of the H. pylori infection was assessed using the UBT at least 8 weeks after completion of the rescue therapies.
Results
Patient demographics and clinical characteristics
From November 2017 to September 2018, a total of 133 (male/female, 67/66) consecutive patients who had failed at least one H. pylori eradication treatment were evaluated with gastric endoscopy, with biopsy specimens taken for bacterial culture and histology (Table 1). Gastritis or duodenitis was found in 106 (79.7%) patients, mucosal erosions were present in six (4.5%) patients, and peptic ulcers in 19 (14.3%) patients. The patients with complete clinical records who had one, two, and three or more previous failed treatments were 54 (40.6%), 29 (21.8%), and 23 (17.3%), respectively. However, 27 patients (20.3%) could not recall their previous treatment regimens.
Table 1.
Patients demographics and clinical characteristics.
| Variables | Patients enrolled (n = 133) |
|---|---|
| Mean age (years) | 48.3 ± 11.8 |
| Male/female | 67/66 |
| Endoscopy findings | |
| Gastritis/duodenitis | 106 (79.7%) |
| Mucosal erosions | 6 (4.5%) |
| Peptic ulcer disease | 19 (14.3%) |
| Other | 2 (1.5%) |
| Number of previous eradication therapies | |
| 1 | 54 (40.6%) |
| 2 | 29 (21.8%) |
| ≥3 | 23 (17.3%) |
| Unknown | 27 (20.3%) |
H. pylori culture rate and antibiotic resistance patterns
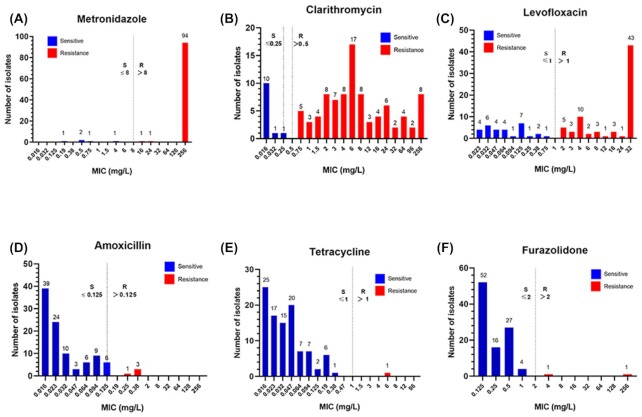
Of the 133 patients included in this study, 101 (75.9%) cases had successful bacterial cultures and subsequent susceptibility tests were completed (Fig. 1). Antibiotic resistance rates to MTZ, CLA, and LVX were high, with values of 95.0% (96/101), 88.1% (89/101), and 69.9% (71/101), respectively (Table 2). Of note, most of the MTZ-resistant strains (94/96, 97.9%) had MIC values > 250 mg/l (Fig. 2). However, a wide distribution of MICs against CLA was observed: ≤0.25 mg/l (n = 12), 0.75 (n = 5), 1–8 (n = 55), 12–96 (n = 21), and 256 (n = 8). As for the MIC values against LVX, 43 strains had an MIC ≥32 mg/l, 30 strains had an MIC ≤0.75 mg/l, 23 strains had an MIC in the range of 1–8 mg/l, with the remaining five strains having an MIC value in the range of 12–24 mg/l (Fig. 2).
Table 2.
AST results of 101 clinical H. pylori isolates.
| AST results | No. of strains | Resistance rate (%) |
|---|---|---|
| MTZ | 96 | 95.0 |
| CLA | 89 | 88.1 |
| LVX | 71 | 70.3 |
| AMX | 4 | 3.9 |
| TET | 1 | 1.0 |
| FZD | 2 | 1.9 |
| Mono resistance | 7 | 6.9 |
| MTZ | 5 | 4.9 |
| CLA | 2 | 1.9 |
| Dual resistance | 27 | 26.7 |
| MTZ + CLA | 21 | 20.8 |
| MTZ + LVX | 5 | 4.9 |
| LVX + CLA | 1 | 1.0 |
| Triple resistance | 59 | 58.4 |
| MTZ + CLA + LVX | 59 | 58.4 |
| Quadruple resistance | 5 | 4.9 |
| MTZ + CLA+ LVX + AMX | 4 | 3.9 |
| MTZ + CLA + LVX+ FZD | 1 | 1.0 |
| Quinary resistance | 1 | 1.0 |
| MTZ + CLA + LVX + TET + FZD | 1 | 1.0 |
| Pan resistance | 0 | 0 |
AMX, amoxicillin; TET, tetracycline; FZD, furazolidone; LVX, levofloxacin; CLA, clarithromycin; MTZ, metronidazole.
Figure 2.
Distribution of minimum inhibitory concentrations (MICs) found against six antibiotics in 101 clinical H. pylori isolates. Based on EUCAST, the resistance cut-off values for MTZ, CLA, LEV, AMX, and TET were defined as MIC >8 mg/l, >0.5 mg/l, >1 mg/l, >0.125 mg/l, and 1 mg/l, respectively.26 Resistance to FZD was defined as MIC >2 mg/l.26,27 The resistance cut-off value for each antibiotic was indicated by a dotted line.
Antibiotic resistance rates to AMX, TET, and FZD were found to be low, at 4.0% (4/101), 1.0% (1/101), and 2.0% (2/101), respectively (Table 2), which was in sharp contrast to the high resistance rates of MTZ, CLA, and LVX. As for the MICs of AMX, all the 101 strains (100%) had MIC ≤0.38 mg/l (Fig. 2).
Of the 101 cultured strains, seven (6.9%) were mono-antibiotic resistant (five to MTZ and two to CLA), and 27 (26.7%) were dual resistant (21 to MTZ + CLA, five to MTZ + LVX, one to LVX + CLA). Triple resistance was observed in 59 strains (58.4%), with all strains being resistant to the same three antibiotics: MTZ + CLA + LVX. Quadruple resistance was detected in five strains (5.0%), with four of them resistant to MTZ + CLA + LVX + AMX (Table 2). Quinary resistance was detected in one strain with sensitivity only to AMX. Thus, the overall MDR phenotype in the 101 isolates was 64.4% (65/101). Finally, only two of the 101 strains (patient 38# and patient 77#) were pan-sensitive to the six antibiotics tested despite previous use of AMX, CLA, MTZ, and LVX (Table S1).
As it has been reported that >15% simultaneous resistance to CLA and MTZ impairs the efficacy of all non-bismuth quadruple therapies,2,5 the strains that were resistant to both these antibiotics (including dual, triple, quadruple, and quandary resistant) were recorded as 85.1% (86/101).
Concordance between phenotypic and molecular resistance patterns
To compare molecular testing and standard AST methods, 57 clinical isolates characterized by AST were subjected to whole-genome sequencing and were analyzed for the presence of mutations at positions 2146 and 2147 (A2147G, A2146G, A2146C) in the 23S rRNA,16–22 and mutations at amino acid sequence positions 87 and 91 in gyrA,17,20–22 for the detection of CLA and LVX susceptibility, respectively.
Concordance between phenotypic and molecular resistance patterns to CLA was observed for 52 of the 57 whole-genome sequenced clinical isolates (91.2%) (Table S3 in the supplementary material). Of these strains, three strains were susceptible and the remaining 49 strains were resistant. An A2147G mutation was identified in 98.0% of the resistant isolates (48/49), and the A2146G mutation in only 2.0% (1/49). The A2146C mutation was not detected in any of the 57 sequenced genomes. There was discordance between the standard AST and genotype results in 8.8% of the isolates (5/57): two had a susceptible phenotype but contained a A2147G mutation, while three had a resistant phenotype but possessed a wild-type 23S rRNA sequence (Table S3 in the supplementary material).
For the LVX resistance profile, the concordance between phenotypic and molecular resistance patterns was observed for 82.3% of the isolates (47/57). Of these strains, 12 strains were susceptible and the remaining 35 strains were resistant. An Asn-87-Lys mutation was recorded in 51.4% of the resistant isolates (18/35), and an Asn-87-Ile mutation was recorded in 8.6% (3/35). The remaining 14 strains were found to have mutations at Asp-91-Asn (n = 6), Asp-91-Gly (n = 6), and Asp-91-Tyr (n = 2). Inconsistency between the phenotypic and genotypic results was observed for 17.5% of the sequenced isolates (10/57): four strains had a susceptible phenotype but harbored a gyrA 87 or 91 mutation, while six strains had a resistant phenotype but possessed a wild-type gyrA sequence (Table S4 in the supplementary material). Interestingly, the MIC values recorded for the resistant strains having point mutations at position 87 were higher than that of resistant strains having point mutations at position 91 (Fig. S1 in the supplementary material).
AST-guided bismuth quadruple rescue therapy
Of the 101 patients with positive H. pylori culture and subsequent AST, 11 patients were lost to follow-up. Therefore the 14-day AST-guided BQT was prescribed to 90 patients, among whom another two patients were lost to follow-up UBT. Of the remaining 88 patients who completed the 14-day AST-guided BQT (Fig. 1), 43 patients received the EBTF regimen, 14 patients received the EBAF regimen, and H. pylori eradication was achieved in all these patients. Similarly, H. pylori was successfully cured in all 13 patients receiving the EBAL regimen, and in all five patients receiving the EBAC regimen. However, in 13 patients receiving the EBAT regimen, only 10 patients achieved H. pylori eradication (Table 3). Thus, a 100% eradication rate was observed in patients receiving the EBTF, EBAC, EBAL, and EBAF regimens, whereas the AST-guided EBAT regimen only gave an eradication of 76.9% (10/13). Overall, a 96.6% (85/88) eradication rate was achieved in one-course of AST-guided bismuth quadruple rescue therapy. The three patients that failed the EBAT regimen were all cured with a second 14-day course of the EBTF regimen.
Table 3.
Eradication rate of different AST-guided BQT regimens in this study.
| Regimens | n | Cured | Eradication rate (%) |
|---|---|---|---|
| EBAC | 5 | 5 | 100.0 |
| EBAL | 13 | 13 | 100.0 |
| EBAF | 14 | 14 | 100.0 |
| EBAT | 13 | 10 | 76.9 |
| EBTF | 43 | 43 | 100.0 |
| Total | 88 | 85 | 96.6 |
E, esomeprazole; B, bismuth colloidal pectin; A, amoxicillin; C, clarithromycin; F, furazolidone; T, tetracycline; L, levofloxacin.
Discussion
H. pylori infection is an infectious disease, and thus whenever possible, treatment should be based on culture and AST to guide the selection of appropriate antibiotics, especially in the setting of referred patients with previous BQT treatment failures. With a culture rate of 75.9% (101/133) in this study, we found that the secondary resistances to MTZ, CLA, and LVX were 95.0%, 88.1% and 69.9%, respectively, with 64.4% (65/101) exhibiting triple resistance to these three antimicrobials. The high (>15%) dual MTZ and CLA resistance excludes the empiric use of non-BQT,2,5 while the high secondary LVX resistance rules out the selection of LVX-containing regimens as empiric rescue therapies.2,5 The secondary resistances to AMX, FZD, and TET were still low in this study, being 4.0%, 1.0%, and 2.0%, respectively, which is consistent with the results from other reported studies in China.9–11,29 The subsequent AST-guided bismuth quadruple rescue therapy was highly effective with a cure rate of 96.6% in this study, providing evidence to support the principle of susceptibility-guided therapy for achieving high cure rates in referred patients.
The excellent efficacy of the rescue therapies in this study can be mainly ascribed to the use of various AST-guided BQT regimens. Under the circumstances of high secondary resistance to CLA (88.1%), MTZ (95.0%), and LVX (69.9%) in this study, 64.8% of the referred patients (57/88) received the AST-guided FZD-containing BQT, achieving a cure rate of 100% (Table 3). In addition, the three patients who failed the EBAT therapy were also cured with the FZD-containing BQT regimen, EBTF. The excellent efficacy observed here, of the FZD-containing BQT in patients even with multiple previous treatment failures, is consistent with previous studies.9,12 Of note, however, as FZD is a monoamine oxidase inhibitor, is that its concurrent use with other drugs including sympathomimetic amines, as well as foods containing tyramine (cheeses, beans, and bean products such as soy sauce) should be avoided to reduce unnecessary side effects.30,31 In addition, consumption of alcohol while taking FZD should be avoided to prevent the occurrence of disulfiram-like symptoms.
The AST-guided EBAC given to five patients, and the EBAL regimen given to 13 patients were also shown to be a highly efficient rescue therapy with a cure rate of 100% (Table 3). This suggests that even in the face of high secondary resistance to CLA and LVX, these two drugs, if confirmed to be susceptible by pre-treatment AST, can still be confidently prescribed to H. pylori-infected patients. Of note, previous studies have shown that the AST-guided non-BQT or the AST-guided triple therapy were not effective in treatment-experienced patients,32,33 indicating that the bismuth may be an indispensable component of the rescue regimens.
Of the 13 patients receiving the EBAT regimen with AST-confirmed susceptibility to the AMX and TET, only 10 patients (76.9%) achieved successful eradication (Table 3). This finding is consistent with previous studies showing only 62% to 78% eradication success with the EBAT regimen.34,35 The suboptimal efficacy of the EBAT regimen may be ascribed to the antagonistic effect between AMX and TET. As AMX is a bactericidal drug that exerts antibacterial activity during the bacterial multiplying phase, whereas TET is a bacteriostatic drug, this may reduce the bactericidal effect of AMX by inhibiting cellular protein synthesis required for cell growth.36 However, Liang et al. reported an optimal efficacy of the EBAT regimen in treatment-experienced patients with an eradication rate of 94.6% by per-protocol and 83.8% by intention-to-treat analysis, possibly as a result of the use of a higher dose of AMX (1 g, three times daily).9 Thus, the current EBAT regimen with 2 g of AMX daily recommended by the Fifth Chinese National H. pylori Consensus may need to be optimized to achieve excellent eradication efficacy.
As China is a country with high primary resistance to CLA (28.9%), MTZ (63.8%), and LVX (28.0%),3 the current recommendation of the seven different BQT regimens as initial empiric therapies for H. pylori infection by the Fifth Chinese National H. pylori Consensus,4 is consistent with the recent international guidelines on H. pylori treatment.2,5,6 However, the non-differentiation of the BQT regimens as first-line, second-line, or third-line treatment in the current consensus,4 has unfortunately led to a regimen selection bias. Out of prescription habit, many physicians choose to simply add a bismuth component to triple therapies (PPI + AMX + CLA, PPI + CLA + MTZ, PPI + AMX + MTZ, or PPI + AMX + LVX). However, after analysis of the patient history collected in this study (Table S1), we found that most of the failed patients had previously been prescribed the CLA-containing or LVX-containing BQT regimens. The AST profiling of the isolates from these failed patients proved that their previous BQT eradication failure was mainly caused by drug resistance to CLA and LVX (Table S1). As China is a country with high primary and secondary resistance to these antibiotics,3,9–11,29 our study supports the conclusions from previous studies that without prior susceptibility testing, CLA- or LVX-containing BQT regimens are not suitable as the recommended initial or rescue treatment for patients in China.8,13
In contrast to the excellent efficacy using AST-guided BQT observed in this study, the culture rate was only 75.9%. Molecular-based susceptibility testing methods are culture-independent, and so may be used as an alternative for those patients without a successful culture. However, molecular-based testing for H. pylori’s antimicrobial susceptibility is currently not commercially available in China, and studies assessing the concordance between molecular and phenotypic susceptibility for CLA and LVX are lacking. By comparing the molecular testing and standard AST in 57 whole-genome sequenced isolates in this study, we demonstrated a 91.2% and 82.3% concordance between molecular and phenotypic susceptibility for CLA and LVX, respectively. Point mutations in 23S rRNA were identified in 94.2% of the 52 sequenced isolates with phenotypic CLA resistance, and gyrA mutations at amino acid positions 87 and 91 occurring in 85.4% of the 41 sequenced isolates with phenotypic LVX resistance. These data indicate that when the standard AST is not available, the molecular tests detecting the presence of point mutations in 23S rRNA and gyrA can predict CLA and LVX resistance, respectively, with reasonable accuracy.
Of note for this study, is that the MIC breakpoints for H. pylori drug resistance towards many antibiotics have not been clinically established. Thus, different studies have applied different breakpoints for the same drug, which may greatly influence not only H. pylori antimicrobial surveillance, but also affect the clinical guidance of precision H. pylori eradication. For example, when using the EUCAST standard of 0.125 mg/l as the MIC breakpoint for AMX in this study, the four strains with MICs of 0.25 and 0.38 mg/l were defined as AMX resistant (Fig. 2D). However, when applying ≥2 mg/l as the MIC breakpoint for AMX resistance used in previously published studies,25,34,37 four strains would be redefined as AMX sensitive.
Despite the excellent eradication results observed, there are limitations in this study. First, all rescue therapy regimes used in this study contained bismuth, and more than half of the regimes contained FZD and TET. These results then, although important in treatment of H. pylori infection in China, may not be applicable in other countries where bismuth, FZD, and TET are not available. Second, the high secondary resistance of our clinical H. pylori isolates to CLA, MTZ, and LVX can only be used as a reference for the prediction of drug resistance profiles in treatment-experienced populations, rather than in untreated populations.
In summary, this study demonstrated excellent efficacy of AST-guided BQT regimens (especially the FZD-containing regimens) for referred H. pylori patients with previous treatment failures. Recognizing H. pylori as a common infectious disease, we believe that a 100% cure rate is achievable with treatment based on individual susceptibility profiling by AST. If AST is not available, our study showed that the molecular-based tests could be used as an alternative for CLA and LVX resistance profiling. If neither standard AST nor molecular testing are available, BQT regimens containing the two antimicrobials chosen from AMX, FZD, and TET can be used as empiric strategies for rescue therapy.
Supplementary Material
Acknowledgements
This research was funded by the National Natural Science Foundation of China (grant No. 81701976), and 1.3.5 Project for Disciplines of Excellence, West China Hospital, Sichuan University (grant No. ZY2016201).
Contributor Information
Tiankuo Yang, West China Marshall Research Center for Infectious Diseases, Center of Infectious Diseases, Division of Infectious Diseases, State Key Laboratory of Biotherapy, West China Hospital, Sichuan University, Chengdu 610041, China.
Renwei Hu, Department of Gastroenterology, West China Hospital, Sichuan University, Chengdu 610041, China.
Xiaoqiong Tang, West China Marshall Research Center for Infectious Diseases, Center of Infectious Diseases, Division of Infectious Diseases, State Key Laboratory of Biotherapy, West China Hospital, Sichuan University, Chengdu 610041, China.
Yalin Shen, West China Marshall Research Center for Infectious Diseases, Center of Infectious Diseases, Division of Infectious Diseases, State Key Laboratory of Biotherapy, West China Hospital, Sichuan University, Chengdu 610041, China.
Alfred Tay, Helicobacter pylori Research Laboratory, School of Biomedical Sciences, Marshall Centre for Infectious Disease Research and Training, University of Western Australia, Nedlands 6009, Australia.
Xuenan Pi, Precision Medicine Key Laboratory of Sichuan Province & Precision Medicine Center, West China Hospital, Chengdu 610041, China.
Gang Wang, Precision Medicine Key Laboratory of Sichuan Province & Precision Medicine Center, West China Hospital, Chengdu 610041, China.
Aleksandra W Debowski, Helicobacter pylori Research Laboratory, School of Biomedical Sciences, Marshall Centre for Infectious Disease Research and Training, University of Western Australia, Nedlands 6009, Australia; School of Molecular Sciences, University of Western Australia, Nedlands 6009, Australia.
Keith A Stubbs, School of Molecular Sciences, University of Western Australia, Nedlands 6009, Australia.
Mohammed Benghezal, West China Marshall Research Center for Infectious Diseases, Center of Infectious Diseases, Division of Infectious Diseases, State Key Laboratory of Biotherapy, West China Hospital, Sichuan University, Chengdu 610041, China.
Barry J Marshall, Helicobacter pylori Research Laboratory, School of Biomedical Sciences, Marshall Centre for Infectious Disease Research and Training, University of Western Australia, Nedlands 6009, Australia.
Hong Li, West China Marshall Research Center for Infectious Diseases, Center of Infectious Diseases, Division of Infectious Diseases, State Key Laboratory of Biotherapy, West China Hospital, Sichuan University, Chengdu 610041, China.
Hong Tang, West China Marshall Research Center for Infectious Diseases, Center of Infectious Diseases, Division of Infectious Diseases, State Key Laboratory of Biotherapy, West China Hospital, Sichuan University, Chengdu 610041, China.
Conflict of interest statement
None declared.
References
- 1. Li H, Yang T, Tang Het al.. Helicobacter pylori infection is an infectious disease and the empiric therapy paradigm should be changed. Precis Clin Med. 2019;2:77–80. doi: 10.1093/pcmedi/pbz009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Malfertheiner P, Megraud F, O'Morain CAet al.. Management of Helicobacter pylori infection-the Maastricht V/Florence consensus report. Gut. 2017;66:6–30. doi: 10.1136/gutjnl-2016-312288. [DOI] [PubMed] [Google Scholar]
- 3. Hu Y, Zhu Y, Lu NH. Primary antibiotic resistance of Helicobacter pylori in China. Dig Dis Sci. 2017;62:1146–54. doi: 10.1007/s10620-017-4536-8. [DOI] [PubMed] [Google Scholar]
- 4. Liu WZ, Xie Y, Lu Het al.. Fifth Chinese National Consensus Report on the management of Helicobacter pylori infection. Helicobacter. 2018;23:e12475. doi: 10.1111/hel.12475. [DOI] [PubMed] [Google Scholar]
- 5. Fallone CA, Chiba N, van Zanten SVet al.. The Toronto consensus for the treatment of Helicobacter pylori infection in adults. Gastroenterology. 2016;151:51–69. doi: 10.1053/j.gastro.2016.04.006. [DOI] [PubMed] [Google Scholar]
- 6. Chey WD, Leontiadis GI, Howden CWet al.. ACG clinical guideline: treatment of Helicobacter pylori infection. Am J Gastroenterol. 2017;112:212–39. doi: 10.1038/ajg.2016.563. [DOI] [PubMed] [Google Scholar]
- 7. Chen Q, Long X, Ji Yet al.. Randomised controlled trial: susceptibility-guided therapy versus empiric bismuth quadruple therapy for first-line Helicobacter pylori treatment. Aliment Pharmacol Ther. 2019;49:1385–94. doi: 10.1111/apt.15273. [DOI] [PubMed] [Google Scholar]
- 8. Zhang W, Chen Q, Liang Xet al.. Bismuth, lansoprazole, amoxicillin and metronidazole or clarithromycin as first-line Helicobacter pylori therapy. Gut. 2015;64:1715–20. doi: 10.1136/gutjnl-2015-309900. [DOI] [PubMed] [Google Scholar]
- 9. Liang X, Xu X, Zheng Qet al.. Efficacy of bismuth-containing quadruple therapies for clarithromycin-, metronidazole-, and fluoroquinolone-resistant Helicobacter pylori infections in a prospective study. Clin Gastroenterol Hepatol. 2013;11:802–7. doi: 10.1016/j.cgh.2013.01.008. [DOI] [PubMed] [Google Scholar]
- 10. Cao Z, Chen Q, Zhang Wet al.. Fourteen-day optimized levofloxacin-based therapy versus classical quadruple therapy for Helicobacter pylori treatment failures: a randomized clinical trial. Scand J Gastroenterol. 2015;50:1185–90. doi: 10.3109/00365521.2015.1037345. [DOI] [PubMed] [Google Scholar]
- 11. Chen Q, Zhang W, Fu Qet al.. Rescue therapy for Helicobacter pylori eradication: a randomized non-inferiority trial of amoxicillin or tetracycline in bismuth quadruple therapy. Am J Gastroenterol. 2016;111:1736–42. doi: 10.1038/ajg.2016.443. [DOI] [PubMed] [Google Scholar]
- 12. Cheng H, Hu FL. Furazolidone, amoxicillin, bismuth and rabeprazole quadruple rescue therapy for the eradication of Helicobacter pylori. World J Gastroenterol. 2009;15:860–4. doi: 10.3748/wjg.15.860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Zhou L, Zhang J, Song Zet al.. Tailored versus triple plus bismuth or concomitant therapy as initial Helicobacter pylori treatment: a randomized trial. Helicobacter. 2016;21:91–9. doi: 10.1111/hel.12242. [DOI] [PubMed] [Google Scholar]
- 14. Sugano K, Tack J, Kuipers EJet al.. Kyoto global consensus report on Helicobacter pylori gastritis. Gut. 2015;64:1353–67. doi: 10.1136/gutjnl-2015-309252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. El-Serag HB, Kao JY, Kanwal Fet al.. Houston consensus conference on testing for Helicobacter pylori infection in the United States. Clin Gastroenterol Hepatol. 2018;16:992–1002. doi: 10.1016/j.cgh.2018.03.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Megraud F. H. pylori antibiotic resistance: prevalence, importance, and advances in testing. Gut. 2004;53:1374–84. doi: 10.1136/gut.2003.022111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Liou JM, Chen PY, Luo JCet al.. Efficacies of genotypic resistance-guided vs empirical therapy for refractory Helicobacter pylori infection. Gastroenterology. 2018;155:1109–19. doi: 10.1053/j.gastro.2018.06.047. [DOI] [PubMed] [Google Scholar]
- 18. Chen D, Cunningham SA, Cole NCet al.. Phenotypic and molecular antimicrobial susceptibility of Helicobacter pylori. Antimicrob Agents Chemother. 2017;61. doi: 10.1128/AAC.02530-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Wang YH, Li Z, Wang Let al.. A systematic review and meta-analysis of genotypic methods for detecting antibiotic resistance in Helicobacter pylori. Helicobacter. 2018;23:e12467. doi: 10.1111/hel.12467. [DOI] [PubMed] [Google Scholar]
- 20. Lauener FN, Imkamp F, Lehours Pet al.. Genetic determinants and prediction of antibiotic resistance phenotypes in Helicobacter pylori. J Clin Med. 2019;8. doi: 10.3390/jcm8010053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Tuan VP, Narith D, Tshibangu-Kabamba Eet al.. A next-generation sequencing-based approach to identify genetic determinants of antibiotic resistance in Cambodian Helicobacter pylori clinical isolates. J Clin Med. 2019;8. doi: 10.3390/jcm8060858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Liou JM, Chang CY, Sheng WHet al.. Genotypic resistance in Helicobacter pylori strains correlates with susceptibility test and treatment outcomes after levofloxacin- and clarithromycin-based therapies. Antimicrob Agents Chemother. 2011;55:1123–9. doi: 10.1128/AAC.01131-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Pastukh N, Binyamin D, On Aet al.. GenoType(R) HelicoDR test in comparison with histology and culture for Helicobacter pylori detection and identification of resistance mutations to clarithromycin and fluoroquinolones. Helicobacter. 2017;22. doi: 10.1111/hel.12447. [DOI] [PubMed] [Google Scholar]
- 24. Malfertheiner P, Megraud F, O'Morain CAet al.. Management of Helicobacter pylori infection--the Maastricht IV/ Florence consensus report. Gut. 2012;61:646–64. doi: 10.1136/gutjnl-2012-302084. [DOI] [PubMed] [Google Scholar]
- 25. Su P, Li Y, Li Het al.. Antibiotic resistance of Helicobacter pylori isolated in the southeast coastal region of China. Helicobacter. 2013;18:274–9. doi: 10.1111/hel.12046. [DOI] [PubMed] [Google Scholar]
- 26. Kahlmeter G, Brown DF, Goldstein FWet al.. European committee on antimicrobial susceptibility testing (EUCAST) technical notes on antimicrobial susceptibility testing. Clin Microbiol Infect. 2006;12:501–3. doi: 10.1111/j.1469-0691.2006.01454.x. [DOI] [PubMed] [Google Scholar]
- 27. Li H, Marceau M, Yang Tet al.. East-Asian Helicobacter pylori strains synthesize heptan-deficient lipopolysaccharide. PLoS Genet. 2019;15:e1008497. doi: 10.1371/journal.pgen.1008497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Hunt M, Mather AE, Sanchez-Buso Let al.. ARIBA: rapid antimicrobial resistance genotyping directly from sequencing reads. Microb Genom. 2017;3:e000131. doi: 10.1099/mgen.0.000131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Gao W, Cheng H, Hu Fet al.. The evolution of Helicobacter pylori antibiotics resistance over 10 years in Beijing. China Helicobacter. 2010;15:460–6. doi: 10.1111/j.1523-5378.2010.00788.x. [DOI] [PubMed] [Google Scholar]
- 30. Graham DY, Fischbach L. Helicobacter pylori treatment in the era of increasing antibiotic resistance. Gut. 2010;59:1143–53. doi: 10.1136/gut.2009.192757. [DOI] [PubMed] [Google Scholar]
- 31. Graham DY, Lu H. Furazolidone in Helicobacter pylori therapy: misunderstood and often unfairly maligned drug told in a story of French bread. Saudi J Gastroenterol. 2012;18:1–2. doi: 10.4103/1319-3767.91724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Tan B, Yang JC, Young CLet al.. Helicobacter pylori antimicrobial susceptibility testing-guided salvage therapy in the USA: a real life experience. Dig Dis Sci. 2018;63:437–45. doi: 10.1007/s10620-017-4880-8. [DOI] [PubMed] [Google Scholar]
- 33. Bhakta D, Graham DY, Chan Jet al.. Lessons from using culture-guided treatment after referral for multiple treatment failures for Helicobacter pylori infection. Clin Gastroenterol Hepatol. 2018;16:1531–2. doi: 10.1016/j.cgh.2017.12.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Chi CH, Lin CY, Sheu BSet al.. Quadruple therapy containing amoxicillin and tetracycline is an effective regimen to rescue failed triple therapy by overcoming the antimicrobial resistance of Helicobacter pylori. Aliment Pharmacol Ther. 2003;18:347–53. doi: 10.1046/j.1365-2036.2003.01653.x. [DOI] [PubMed] [Google Scholar]
- 35. Wu DC, Hsu PI, Tseng HHet al.. Helicobacter pylori infection: a randomized, controlled study comparing 2 rescue therapies after failure of standard triple therapies. Medicine (Baltimore). 2011;90:180–5. doi: 10.1097/MD.0b013e31821c9d1c. [DOI] [PubMed] [Google Scholar]
- 36. Lv ZF, Wang FC, Zheng HLet al.. Meta-analysis: is combination of tetracycline and amoxicillin suitable for Helicobacter pylori infection?. World J Gastroenterol. 2015;21:2522–33. doi: 10.3748/wjg.v21.i8.2522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Tay CY, Windsor HM, Thirriot Fet al.. Helicobacter pylori eradication in Western Australia using novel quadruple therapy combinations. Aliment Pharmacol Ther. 2012;36:1076–83. doi: 10.1111/apt.12089. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.