Abstract
Left sided valvular heart disease poses major impact on life and lifestyle. Medical therapy merely palliates chronic severe valve disease and once symptoms or haemodynamic sequelae appear, life expectancy is markedly truncated. In this article, we review the mechanisms of valve pathology, latest evidence in the quest for pharmacological options, means by which to predict deterioration, and standard and novel treatment options.
Global variation in the epidemiology of valve disease
Valve disease is a formidable phenomenon, particularly in the elderly, limiting life and lifestyle. Structural abnormalities of a native valve can only be treated by valve intervention due to lack of proven pharmacotherapy altering progression or prognosis. This, as with many other pathologies in recent decades, has evolved from a communicable to a degenerative disease in aging populations—the late cardiovascular sequelae of rheumatic fever have vastly declined due to control of preceding infection. There are still, however, 32 million people currently living with rheumatic heart disease and this accounts for 2% of all deaths from cardiovascular disease worldwide (http://www.who.int). Over 5 million migrants now live in Europe, many of whom originate from countries where rheumatic heart disease is endemic, and require ongoing health care (http://www.unhcr.org/uk/statistics/country/5a8ee0387/unhcr-statistical-yearbook-2016-16th-edition.html).
According to Global Health Observatory data, 31% of deaths in the United Kingdom are caused by cardiovascular disease (declining each year), compared to 45% in China (trending upwards). In China, the prevalence of any mitral or aortic stenosis, or moderate or severe regurgitation is 9.65%1 and significantly correlated with age and hypertension. The prevalence of moderate to severe left sided valvular heart disease in patients over 65 years of age in the United Kingdom is 11.3% with over 50% having at least mild disease.2 There is a striking difference in bicuspid aortic valve disease however, with nearly 50% of patients undergoing transcatheter aortic valve implantation (TAVI) in China having bicuspid valve morphology3 in comparison to a UK prevalence of 1.6–6.7%. This is perhaps because patients undergo TAVI at a younger age in China, since the background incidence of bicuspid aortic valve disease in the general population as detected by echocardiography, is similar,4 although computed tomography has greater sensitivity and specificity. When considering transcatheter treatment, bicuspidity can pose challenges with valve sizing, device selection, and the increased likelihood of paravalvular leak.
The financial cost of aortic and mitral valve disease in the US has been calculated as nearly $15billion.5 Whilst valve disease is less common than coronary and cerebrovascular disease, it poses a health economic burden.6 To manage this population requires adequate infrastructure to provide long-term clinical monitoring and follow-up, frequent investigations and costly valve intervention.
Aetiology, physiological and clinical implications
Degenerative valve disease has an insidious progression and patients are often unaware of symptoms, compensating by refraining from activity and ascribing perceived changes to advancing age. The onset of arrhythmia, sepsis or anaemia can herald rapid decompensation. Symptoms are therefore subjective and confounded by co-morbidities. Echocardiographic valve parameters may not fit neatly into predetermined categories (‘mild’, ‘moderate’ or ‘severe’) and are often discordant or misleading in the context of multiple valve pathologies (see Table 17). Valve disease causes impaired left ventricular (LV) strain parameters, subclinical myocardial damage and eventual overt heart failure, with or without LV impairment. The mortality for untreated severe symptomatic disease is high, and patients should rarely be denied treatment in an era where simple treatments are available for most cases.
Table 1.
The interplay of multiple valve pathology.
| Impacts the diagnosis of: | ||||||
|---|---|---|---|---|---|---|
| Aortic Stenosis | Aortic Regurgitation | Mitral Stenosis | Mitral Regurgitation | |||
| The presence of | AS |
|
|
|
||
| AR |
|
|
|
|||
| MS |
|
|
|
|||
| MR |
|
|
|
|||
Table Key: PHT—pressure half time, LFLG—low flow-low gradient, MS—mitral stenosis, MR—mitral regurgitation, ROA—regurgitant orifice area, LVOT—left ventricular outflow tract, Vmax—maximum velocity, AR—aortic regurgitation, AS—aortic stenosis, MVA—mitral valve area, CW—continuous wave
Acute valve regurgitation, which can be caused by infective endocarditis, trauma, aortic dissection or myocardial infarction, is poorly tolerated and leads to rapid onset pulmonary oedema and cardiogenic shock. Initial treatment should focus on urgent echocardiography, delineation of the underlying aetiology and reason for decompensation, instigation of circulatory support and timely intervention. Surgery should not be delayed since mortality is at least 75% within the first 48 hours.8 Mechanical circulatory support accompanied by vasodilator therapy and inotropic support may provide temporary stability in patients with cardiogenic shock secondary to acute mitral regurgitation prior to early surgery. Acute aortic regurgitation launches rapid volume and pressure overload of the normally-sized LV, escalating end diastolic pressure (LVEDP) and reducing forward flow. Intravenous vasodilators (nitrates, nitroprusside) may improve LVEDP and enhance forward flow but inotropes, although often necessary, are best avoided since increasing systemic pressures may worsen AR. This article will focus on chronic left sided valve pathology.
Volume Loading Conditions: Aortic Regurgitation
Chronic aortic regurgitation (AR) can result from an aortopathy or leaflet abnormality. Aortic root dilatation caused by hypertension or connective tissue disease (such as Marfan syndrome) leads to poor coaptation of the valve cusps resulting in a central jet of regurgitation. Leaflet pathology can be congenital (bicuspid, quadricuspid or unicuspid aortic valve disease) or acquired, such as in rheumatic heart disease, collagen vascular disease, infective endocarditis or atherosclerotic degeneration. Diagnosis and follow-up by echocardiography is ample although this can be supplemented by cardiac magnetic resonance imaging where there are poor echocardiographic windows or ambiguity about valve disease severity or LV function.
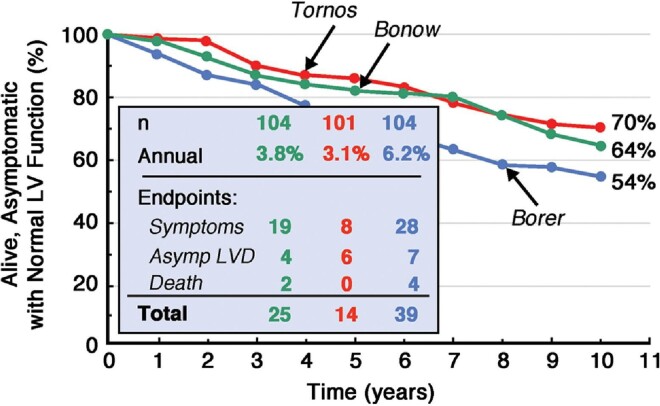
Chronic AR leads to increased preload (regurgitant volume overload) and afterload (increased wall stress), leading to ventricular dilatation and eventual systolic impairment, often preceding the onset of symptoms. Careful surveillance is necessary—whilst progression is slow at an event rate of around 4% per year9–11 (Fig. 1), surgery is clearly mandated once symptoms manifest since event rates and mortality rise sharply.
Figure 1.
Natural history of asymptomatic aortic regurgitation.12
Volume Loading Conditions: Mitral Regurgitation
Mitral valve anatomy is intricate and there are numerous ways in which valve function can be impacted. Primary mitral regurgitation (MR) relates to disease of the valve leaflets, such as myxomatous degeneration and damage from infective endocarditis, and predominantly causes eccentric regurgitant jets that can be challenging to quantify by echocardiography. In contrast, secondary MR arises as a consequence of cardiomyopathy, often in the context of ischaemic heart disease. This so called ‘ischaemic MR’ results from disruption of the sub-valvar apparatus (the chordae or papillary muscles) by regional wall motion abnormalities (RWMA) which disrupt the normal chordal tethering of the valve leaflets, or papillary muscle dysfunction or rupture following acute myocardial infarction (the latter leading to acute, torrential MR). Similarly, the mitral annulus is subject to stretch in ischaemic heart disease, dilated cardiomyopathy and volume overloaded states, resulting in central, functional MR.
During ventricular contraction, blood is ejected from the LV both into the aorta and through the regurgitant mitral valve into the left atrium (LA), thereby increasing LA pressure. Chronic MR causes compensatory LV dilatation and eccentric hypertrophy due to increased preload and afterload, increased total stroke volume and left atrial dilatation (with higher likelihood of atrial fibrillation [AF]). As a consequence of the bidirectional ejection of blood into the aorta and LA, LV ejection fraction is falsely elevated (and should in fact be supra-normal in this state). Functional recovery is possible if mitral valve intervention is undertaken at an early stage. However, if intervention is performed late in the disease process, MR results in irreversible structural and functional deterioration and surgical outcomes are poor.
Pressure Loading Conditions: Aortic Stenosis
Aortic stenosis (AS) usually results from degenerative calcification of a trileaflet valve, thickening and calcification of a congenital bicuspid valve or, less frequently, rheumatic heart disease. Progressive thickening of the aortic valve over years leads to breathlessness (raised diastolic filling pressures), angina (impaired coronary flow reserve), decompensated heart failure (left ventricular dysfunction) and exertional syncope (low cardiac output due to fixed LV outflow tract obstruction). Once symptomatic, life-expectancy is shorter than most cancers, and decompensation is often swift and accelerated.
AS is characterized by progressive valve narrowing, and the adaptive compensatory LV hypertrophy which increases contractile force and reduces systolic wall stress eventually becomes maladaptive, resulting in cardiac decompensation. Myocardial hypertrophy is detrimental to overall survival and correlates with myocardial fibrosis, impaired longitudinal shortening and worsening diastolic function.13 Myocardial fibrosis is a crucial determinant of cardiac dysfunction and prognosis, and has long been associated with AS, even in the absence of significant epicardial coronary artery disease.14 Replacement fibrosis may be the result of myocyte apoptosis owing to extensive hypertrophy and eventually accounts for progression to heart failure.15
Pressure Loading Conditions: Mitral Stenosis
The declining incidence of mitral stenosis (MS) parallels that of acute rheumatic fever, which induces an exaggerated immune response to group A β-haemolytic streptococcal infection and an associated pancarditis. Proteins produced by streptococci display molecular mimicry triggering an immune response, particularly to M-proteins and human cardiac antigens such as myosin and valvular endothelium. T-cells infiltrate the valve, activated by the binding of antistreptococcal carbohydrates, with release of tumour necrosis factor and interleukins. The resulting multiple inflammatory foci (Aschoff bodies, perivascular mononuclear infiltrate) become progressively thickened and calcified over subsequent decades, with commissural fusion leading to a small effective orifice area and increased pressure gradient across the valve during ventricular diastole. Occasionally, mitral annular calcification (MAC) can result in severe MS—a degree of MAC is found in 8.5–10% of the population above the age of 50 but rarely has significant haemodynamic impact.
MS manifests as gradual obstruction to left ventricular inflow and decreased preload. Progressive valve stenosis curtails the free flow of blood from the LA to the LV during diastole, eventually causing LA dilatation and impaired LV filling with reduced stroke volume and cardiac output. As stenosis severity progresses, pulmonary congestion and reduced cardiac output manifest as congestive cardiac failure despite preserved LV ejection fraction. Back pressure and congestion through the lung capillary bed and into the right ventricle (RV) cause pressure overload, pulmonary hypertension and right heart failure. Symptoms can include dyspnoea, chest pain due to pulmonary hypertension, and haemoptysis secondary to alveolar, capillary or bronchial vein rupture. Progressive LA dilatation can compress surrounding structures: the left recurrent laryngeal nerve causing hoarseness; the oesophagus causing dysphagia; and the left main bronchus causing left lung collapse. The onset of AF can precipitate decompensation and increases the risk of systemic embolism. All attempts should be made to maintain sinus rhythm or optimize rate control in AF where restoration of sinus rhythm is not possible. Beta blockers prolong LV diastolic filling and are of particular value. Anticoagulation with warfarin (but not direct thrombin inhibitors) is recommended in patients with AF, LA thrombus or a previous embolic event.16 Diuretic and nitrate therapy provide symptomatic relief in the setting of pulmonary congestion.
Paucity of Evidence for Medical Therapy
Symptoms of valve disease can merely be palliated until definitive intervention. Cautious diuretics (which relieve preload but may cause hypovolaemia and syncope) and beta-blockade (which reduce myocardial oxygen demand) are used whilst awaiting intervention in the setting of severe AS. Attempts should be made to restore sinus rhythm since AF can lead to rapid decompensation in the setting of a hypertrophied ventricle with impaired diastolic filling. Agents which reduce afterload (e.g. nitrates) should be avoided. Angiotensin-converting enzyme inhibitors have been traditionally contraindicated although a study of asymptomatic patients with moderate to severe AS demonstrated a modest but progressive reduction in LV mass associated with Ramipril, coupled with improved myocardial physiology and slower progression of valve stenosis.17 Despite promising retrospective studies suggesting that statin therapy may slow the progression of AS, large prospective randomised controlled trials (ASTRONOMER,18 SEAS19 and SALTIRE20) found no change with aggressive lipid-lowering therapy.
Once AR is established, medical therapy offers minimal respite. In patients with LV dilatation, beta-blockers and angiotensin-converting enzyme inhibitors (or angiotensin receptor blockers) can be beneficial, and vasodilator therapy may be considered in patients with LV dilatation and preserved systolic function.21 Betablockers should be used with caution in tachycardic patients with cardiac decompensation since the tachycardia to reduce regurgitant time may be a last-remaining physiological holding measure. While historical data suggest that vasodilator therapy can slow the natural history of AR and delay the need for valve intervention,22,23 evidence is relatively inconsistent.
MR more commonly results in pulmonary hypertension due to the regurgitation of blood into the LA. A common mistake in clinical practice is to overuse medical therapy in chronic primary MR (despite the absence of evidence),24 and underuse evidence-based medical therapy in chronic secondary MR. In the latter, treatment should be steered by guideline algorithms for LV dysfunction, including angiotensin-converting enzyme inhibitors or angiotensin-receptor antagonists, beta-blockers and aldosterone antagonists. Cardiac resynchronization should be considered in patients with QRS widening whose symptoms persist despite medical treatment.16
MS also results in significant pulmonary hypertension. No evidence exists for improved survival with medical therapy, although prolongation of diastole with rate controlling medication (e.g. beta blockers) can maximise the ejection of blood through the mitral valve orifice and improve cardiac output.
Predicting Deterioration—the Role of Imaging and Biomarkers
Patients (particularly the elderly) can be poor diagnosticians, potentially ignoring or confusing symptoms with those related to co-morbidities. Clinicians therefore face a dilemma in objectively teasing out key information and selecting the most appropriate treatment strategy. The role of exercise stress electrocardiography and echocardiography are of particular use in risk stratification in asymptomatic patients with severe MR or AS25—exercise can unmask symptoms and determine haemodynamic consequences. In AS, stress testing can demonstrate high-risk features of exercise-induced haemodynamic instability—an indication for intervention (class IIa, level C). In asymptomatic severe MR, significant elevation of pulmonary artery pressure with exercise (>56 mmHg) is prognostically significant.26 In both AS and MR, failure to enhance systolic function (or pathological reduction in LV function) with exercise signals the need for intervention. Once symptoms develop, there is a clear mandate for intervention.
The role of cardiac biomarkers in stratifying the risk and timing of intervention is key, especially when symptoms are confounded by comorbidities. Neurohormonal activation, stimulating the release of enzymes such as troponin and b-natriuretic peptide (BNP) correlates well with symptom-free survival27 and allows monitoring using a simple blood test.
Calcium scoring using multi-slice computed tomography (MSCT) can be extremely helpful in discordant cases of AS with an aortic valve area of <1 cm2 but preserved LV systolic function and mean pressure gradient <40 mmHg. Severe AS is very likely with an Agatston score ≥3000 and ≥1600 in men and women, respectively, and likely with scores ≥2000 and ≥1200. Not only is the distribution and burden of calcium helpful, but CT also provides additional information concerning the geometry of the annulus and aorta. 18F-FDG positron emission tomography is being used as a research tool to quantify metabolic activity and prove an inflammatory basis for the progression of AS.
Several studies are underway to address whether early intervention is indicated in severe, asymptomatic AS. These investigations either take all-comers with asymptomatic AS (e.g. Evaluation of Transcatheter Aortic Valve Replacement Compared to SurveilLance for Patients with AsYmptomatic Severe Aortic Stenosis, EARLY-TAVR, ClinicalTrials.gov Identifier NCT03042104) or use markers of left ventricular decompensation to guide intervention28 (e.g. Early valve surgery for severe asymptomatic aortic stenosis, EVoLVeD trial, ClinicalTrials.gov Identifier NCT03094143). The latter study randomises asymptomatic patients with mid-wall late gadolinium enhancement on cardiac magnetic resonance imaging to early intervention or conventional management. Another area of intense discussion is moderate AS with coexistent left ventricular dysfunction. The Transcatheter Aortic Valve Replacement to UNload the Left Ventricle in Patients with ADvanced Heart Failure (TAVR-UNLOAD ClinicalTrials.gov Identifier NCT02661451) study’s objective is to determine if there is potential benefit of TAVI over optimal heart failure treatment in the setting of LV dysfunction (ejection fraction 20–50%). Several imaging tools, such as global longitudinal strain, are increasingly being used to determine the timing of intervention by identifying early LV decompensation.29–31
Timing and Mechanism of Intervention
The optimal timing of intervention for patients with valve disease is the point at which risks associated with disease progression (i.e. sudden death, irreversible LV dysfunction and heart failure) outweigh those of the procedure (and, where appropriate, living with a prosthetic heart valve). The onset of symptoms generally portends rapid deterioration and poor prognosis and is therefore a strong indicator for intervention. However, irreversible myocardial damage can become established before symptom onset, and asymptomatic patients may derive more benefits from earlier intervention.
Periodic clinical and echocardiographic follow-up, the frequency tailored according to disease severity, is critical in asymptomatic patients with significant valve disease to identify a trigger for intervention. While conventional open-heart surgery remains the standard of care for most patients, the advent of transcatheter valve therapy during the past decade has heralded a changing landscape. Where multiple valves are diseased, the heart team must carefully assess all available imaging in order to delineate the underlying mechanism of pathology. A meta-analysis has demonstrated that in patients with moderate–severe MR undergoing TAVI, 50.5% of cases improved at a median follow-up of 180 (30–360) days, with a more marked improvement following balloon-expandable in comparison to self-expanding prostheses.32
Aortic Stenosis—Timing of Intervention
Patients with severe AS may remain asymptomatic for years until eventual left ventricular decompensation as a result of excess preload and afterload. The prognosis of symptomatic AS is dismal - 2-year mortality can be as high as 50%.33 Therefore, the presence of symptoms is a class I indication for surgical aortic valve replacement (SAVR) or TAVI according to both European [European Society of Cardiology (ESC)/European Association for Cardio-Thoracic Surgery (EACTS)]16 and American [American Heart Association (AHA)/American College of Cardiology (ACC)] guidelines for the management of valve disease.34,35
The assessment and management of asymptomatic patients with severe AS remains challenging and controversial. Exercise stress testing uncovers symptoms in about 30% (pseudo-asymptomatic)36 while the remainder are truly asymptomatic. Routine intervention in asymptomatic patients with severe AS is not recommended, though certain adverse features justify early intervention rather than watchful waiting: (1) left ventricular ejection fraction (LVEF) <50% (class I); (2) very severe AS defined as peak jet velocity >5.5 m/s in European guidelines or >5.0 m/s in US guidelines (class IIa); (3) severe aortic valve calcification and peak velocity progression ≥0.3 m/s per year (class IIa in European guidelines, IIb in US guidelines); (4) blood pressure fall below baseline during exercise testing (class IIa); (5) resting systolic pulmonary artery pressure >60 mmHg by invasive measurement (class IIa in European guidelines); (6) elevated B-type natriuretic peptide >3x normal (class IIa in European guidelines).16,34,35
Aortic Stenosis—Mode of Intervention
To date, there has been no robust evidence supporting the prognostic benefits of conservative medical therapy (including balloon aortic valvuloplasty) in patients with AS. Traditionally, SAVR has been the gold standard and the only effective treatment for AS. Since its introduction in 2002, minimally invasive TAVI has evolved as the standard of care for patients with prohibitive surgical risk and a preferable alternative to SAVR in those at intermediate or high surgical risk.37–39 Currently, large head-to-head randomised trials comparing TAVI and SAVR in low-risk patients are ongoing and may justify the extension of TAVI into this patient cohort.
The choice between SAVR and TAVI should be individualized, depending on patient’s age, surgical risk, procedure-related anatomical features, and the presence or absence of coexisting cardiac conditions.16 SAVR is preferred in younger patients at low surgical risk, or in those with less favourable anatomy for TAVI (e.g. poor vascular access, bicuspid valve morphology, non-calcified or extremely calcified aortic valve, annular size beyond the range of available prostheses), or coexisting cardiac conditions necessitating concomitant intervention (e.g. coronary artery bypass grafting [CABG], severe mitral or tricuspid valve disease, or aortic dilatation).16 TAVI is favoured in anatomically suitable patients who are older, and at intermediate or high surgical risk (calculated using the Society of Thoracic Surgeons’ Online Risk Calculator, the STS score). Patients with frailty, severe comorbidities not captured or inadequately represented by surgical risk algorithms, or unfavourable features for SAVR (e.g. sequelae of chest radiation, porcelain aorta, severe chest deformation or scoliosis, or small aortic root with high likelihood of patient-prosthesis mismatch) are more suited to transcatheter intervention.16 Where transfemoral access is feasible, TAVI is preferred over SAVR in elderly patients16 (class I, level of evidence B), since all-cause mortality is similar in high-risk patients, and transfemoral access confers a survival benefit in patients of intermediate risk.37,40
Aortic Regurgitation—timing of intervention
Optimal timing of intervention is more challenging in chronic AR when compared to AS, due to frequent abnormalities of ventricular function even before the onset of symptoms. Therefore, although the presence of symptoms (either spontaneous or induced by exercise testing) is a strong guideline-recommended indication (class I, level B) for surgical intervention,16,35 close observation of LV dimensions and function is paramount in asymptomatic patients. Delayed intervention and irreversible LV impairment place the patient at increased perioperative risk and impair subsequent recovery.41 Truly asymptomatic severe AR has a favourable long-term outcome with conservative management (annual mortality rate ~0.2%) and intervention should therefore only be performed if there is evidence of decompensation. Subnormal LVEF and enlarged LV end-systolic/end-diastolic diameters (LVESD and LVEDD) are well established predictors of adverse outcomes.9,10 Current guidelines recommend aortic valve surgery in asymptomatic patients with LVEF ≤50% (class I, level B), LVESD > 50 mm or indexed LVESD >25 mm/m2 (class IIa, level B), or LVEDD > 65 mm (US guideline)/LVEDD >70 mm (European guideline, class IIa, level B).16,35
A long-term follow up study showed that asymptomatic patients with AR and modest LV dysfunction (LVEF 45–50% and/or LVESD 50–55 mm) and mildly symptomatic patients had significantly better outcomes after aortic valve surgery than patients with severe symptoms (New York Heart Association [NYHA] functional class III-IV) or more significant LV dysfunction (LVEF < 45% or LVESD > 55 mm),42 supporting the pursuit of earlier surgery according to guideline recommendations. Another study demonstrated that patients with preserved systolic function without LV dilatation (LVESD < 50 mm, LVESD < 25 mm/m2, LVEDD < 65 mm) had significantly improved long-term outcomes following surgery compared to those with abnormal LV function or size.43 Notably, in-hospital mortality after isolated SAVR in this contemporary cohort was very low at 0.6%. Based on these findings, the authors proposed additional benefits of early intervention before the onset of triggers recommended by current guidelines. Future large-scale prospective studies are required to validate or modify the guideline-recommended thresholds for intervention, and explore novel imaging and biomarker surrogates for early detection and accurate quantification of LV dysfunction.
Aortic Regurgitation—Mode of Intervention
Surgery remains the mainstay of treatment for pure AR due to accompanying variations in aortic root anatomy and the lack of dedicated TAVI prostheses. A wide range of surgical procedures may be required to treat AR, including conventional SAVR, aortic valve repair, full aortic root replacement, and valve sparing root replacement, depending on the mechanism of AR and the presence or absence of associated ascending aortic lesions. While the majority of patients are treated with conventional SAVR, aortic valve repair can produce satisfactory long-term outcomes in carefully selected cases when undertaken by experienced surgeons and is an attractive treatment choice, especially for young patients.44
Anatomical features associated with AR, particularly non-calcified leaflets, large annular dimensions, associated aortopathy, and LV and outflow tract dilatation, pose significant challenges for TAVI.45 Whilst TAVI is feasible in selected patients, device success rate is relatively low even when using newer-generation devices, primarily due to the frequent need for a second valve.46 TAVI using devices with specific designs, such as a leaflet ‘clip’, ‘clasper’ or ‘crown’, may help to anchor the prosthesis and prevent embolization, and may be a valuable alternative to surgery for selected patients at high or prohibitive surgical risk.47–49
Mitral Stenosis
With few exceptions, MS is the consequence of rheumatic involvement of the mitral valve. Historically, surgical commissurotomy was the only effective treatment until the introduction of percutaneous mitral balloon commissurotomy (PMC) in the 1980s,50,51 which dramatically changed treatment strategy. PMC is less invasive and the treatment of choice for rheumatic MS when feasible, with randomized trials demonstrating excellent safety and efficacy comparable to surgery, and at lower cost. The procedure also avoids the need for thoracotomy and cardiopulmonary bypass, facilitating faster recovery.52,53
Current guidelines recommend PMC for symptomatic patients with severe MS (mitral valve area ≤1.5 cm2) and favourable anatomical characteristics, and in those at high or prohibitive surgical risk (class I).16,34,35 Unfavourable clinical characteristics include old age, previous commissurotomy, NYHA class IV, permanent AF, or severe pulmonary hypertension. Anatomical suitability for PMC can be assessed by the Wilkins score based on the extent and severity of leaflet restriction, leaflet thickening, leaflet calcification and subvalvular thickening (1 to 4 points for each, total score 4–16).54 Unfavourable characteristics include: Wilkins score > 8, fluoroscopic leaflet calcification (Cormier score 3), very small mitral valve area, or severe tricuspid regurgitation.16 Left atrial thrombus and concomitant ≥ moderate MR are primary contraindications. PMC is also reasonable in certain groups of asymptomatic patients on the premise that valve morphology is favourable and there are no contraindications: (1) high thromboembolic risk (history of system embolism, new-onset or paroxysmal AF)16,35; (2) significant pulmonary hypertension (systolic pressure > 50 mmHg at rest); (3) requiring major non-cardiac surgery; or (4) planning pregnancy.16
A prospective study involving 244 asymptomatic patients with moderate MS and favourable anatomy showed that early PMC was associated with significant reduction in the composite event rate of cardiovascular mortality, cerebral infarction, systemic embolism, and PMC-related complications over median follow-up of 8.3 years.55 These results suggest that anatomically suitable patients with MS may derive greater benefits from PMC performed earlier than recommended by current guidelines. Isolated mitral valve surgery (open commissurotomy or mitral valve replacement ([MVR]) is now mainly reserved for severely symptomatic patients with MS (NYHA class III or IV) in whom the estimated surgical risk is acceptable and anatomical characteristics suboptimal for PMC (or when attempted PMC has failed).16,35 Surgery should be deferred in patients with mild or moderate symptoms (NYHA class I or II) with unsuitable anatomy for PMC or failed PMC, given the limited impact of MS on LV function, and the risks of open-heart surgery.
In the Western world, degenerative MS resulting from severe MAC and extended calcification of the mitral leaflets has become more common, mainly affecting the very elderly population. Surgery carries substantial technical challenges (inability to fully decalcify the annulus, place sutures and attach the prosthesis) and very high mortality. Since there is no commissural fusion, PMC has no role to play. Transcatheter placement of a balloon-expandable TAVI valve in severe MAC has recently proved feasible and emerged as a viable therapeutic option for selected patients with suitable anatomy who are poor candidates for surgery. In the largest case series reported to date, thirty-day and 1-year mortality were high (25.0% and 53.7%, respectively), although it should be noted that these patients were at extreme surgical risk (mean STS score 15.3 ± 11.6%).56 The majority (63.6%) of patients who survived 30 days were alive at 1 year with sustained functional improvement and satisfactory valve hemodynamics.
Mitral Regurgitation
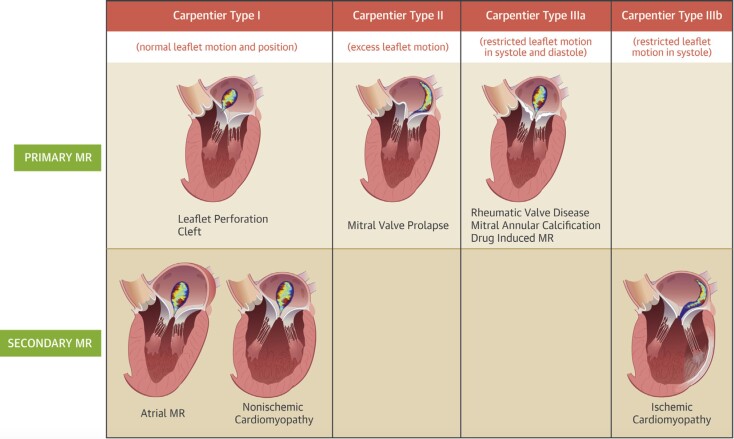
Primary and secondary MR have distinct aetiologies and differing responses to mechanical intervention (Fig. 2).
Figure 2.
Central illustration: Classification of the etiology of MR.57
Primary Mitral Regurgitation
Mitral valve surgery is the standard of care for patients with primary MR, with a strong preference for valve repair over replacement when there is high probability of a successful and durable repair (class I, level B in European guidelines).16 Surgical expertise and valve anatomy play an important part in this outcome. The guidelines even recommend that MVR is contraindicated when the primary MR lesion is confined to less than one half of the posterior leaflet, unless repair has already been attempted (class III level B).34,35 MVR is usually reserved for patients in whom repair is not feasible.
Symptoms are a clear stimulus for surgery in patients with severe primary MR (class I, level B).16,34,35 However, symptom onset is frequently preceded by LV decompensation which adversely affects postoperative outcomes. Therefore, surgery is also indicated in asymptomatic patients with LV dysfunction, defined as an LVESD ≥45 mm (European guidelines) or ≥40 mm (US guidelines) and/or LVEF ≤60% (class I, level B).16,34,35 Surgery is also indicated when new-onset AF or pulmonary hypertension (systolic pulmonary arterial pressure >50 mmHg at rest) develop in asymptomatic patients with severe MR and normal LV size and function, particularly if the likelihood of durable repair is high and surgical risk low (class IIa).16,34,35 Data from the Mitral Regurgitation International Database (MIDA) registry showed that among 1021 patients with primary MR and no class I triggers (only 20% had a class II trigger), early surgery compared with watchful waiting was associated with significantly better long-term survival (86% vs 69% at 10 years, P < 0.001).58 A further study suggested that mitral valve repair should be performed when LVEF ≥ 64% and LVESD < 37 mm for the best preservation and post-operative recovery of LV function in patients with primary MR.59 The latest US guidelines therefore recommend early mitral valve repair in advance of conventional thresholds (LVEF < 60% or LVESD ≥ 40 mm) if there is a trend towards these thresholds on serial imaging studies (class IIa, level C).34
The timing of surgery for asymptomatic patients with primary MR will continue to be a subject of debate. Trends toward earlier intervention can be expected in view of the excellent outcomes following successful valve repair and as emerging imaging surrogates or biomarkers help earlier identification of potential LV dysfunction or other complications.
Secondary Mitral Regurgitation
The prognosis of secondary MR is largely determined by the underlying cardiomyopathy and functional status. Guideline-directed optimal medical therapy (OMT) is of fundamental importance16,35 while the benefits of correcting secondary MR by surgery or transcatheter intervention remain uncertain.
Valve surgery is indicated in patients with severe secondary MR undergoing CABG (class I, level C, European guidelines; class IIa, US guidelines) or SAVR (class IIa, US guidelines).16,34,35 Valve repair for moderate ischaemic MR at the time of CABG has been placed under scrutiny following important data from a randomized trial showing no benefit in terms of survival or LV remodeling (but increased rates of neurologic events and supraventricular arrhythmias) at 2-year follow-up.60
In the absence of an indication for revascularization or SAVR, mitral valve surgery may be considered in patients with severe secondary MR who remain severely symptomatic despite OMT (including cardiac resynchronization therapy; class IIb),16,34,35 although there is little evidence to demonstrate survival benefit or even sustained symptom improvement. The latest US guidelines favour chordal-sparing mitral valve replacement over downsized annuloplasty repair in ischaemic MR (class IIa),34 noting the more frequent recurrence of moderate or severe MR after 2 years in the latter cohort (58.8% vs 3.8%, p < 0.001), despite similar survival and LV remodeling.61
The Role of Transcatheter Mitral Valve Intervention
At least 50% of patients with severe MR do not undergo surgery,62 often due to high operative risk and the lack of supportive evidence. Transcatheter mitral valve repair is safe and less invasive and has emerged as an attractive alternative. Guidelines suggest transcatheter mitral valve repair may be considered in severely symptomatic patients (NYHA class III–IV) with severe primary MR who satisfy echocardiographic criteria for eligibility, have reasonable life expectancy and are deemed at high or prohibitive surgical risk (class IIb).16,34,35 At present, the Abbott MitraClip® edge-to-edge repair system is the most widely used device worldwide and the only clinically available device in the US (where the device is only indicated for primary MR). In Europe, however, about 70% of MitraClip procedures are performed as an adjunct to OMT for symptom relief in patients with secondary MR (European guidelines class IIb).16
The recently announced 2-year outcomes of the Cardiovascular Outcomes Assessment of the MitraClip Percutaneous Therapy for Heart Failure Patients with Functional Mitral Regurgitation (COAPT, ClinicalTrials.gov Identifier: NCT01626079) trial showed that transcatheter mitral valve repair using the MitraClip in addition to maximally tolerated OMT was superior to OMT alone in reducing hospitalisation for heart failure (35.8% vs 67.9% per patient-year, p < 0.001) and all-cause mortality (29.1% vs 46.1%; p < 0.001) (alongside improvements in quality of life and functional capacity) in symptomatic heart failure patients with moderate-to-severe or severe secondary MR.63 These findings suggest that secondary MR contributes to adverse outcomes and that MR reduction in properly selected patients with heart failure could improve prognosis. The promising outcomes shown in this landmark trial may lead to a paradigm shift in the management of patients with heart failure and secondary MR.
Transcatheter mitral valve replacement represents the new frontier in cardiac structural intervention and remains at a preliminary stage of development, with numerous devices under investigation in pre-clinical or early feasibility studies. Device design can be grouped into edge-to-edge repair (e.g. MitraClip), direct and indirect annuloplasty, mitral valve replacement, and other techniques including chordal repair. Initial results using the transapically delivered self-expanding Intrepid System (Medtronic, Inc., Redwood City, California) are very promising. Pilot data demonstrated low 30-day mortality (14%), a reduction in MR to mild or none in all patients, and a short procedure time with minimal learning curve in a high-risk patient cohort.64 This field will grow much more slowly than TAVI in view of the complexities of mitral valve anatomy and pathology. However, transcatheter mitral valve replacement might serve as an important therapeutic option for patients with severe MR who are unsuitable for valve repair or surgical valve replacement in the near future.
Conclusion
Valve disease is a highly prevalent disorder, particularly in the elderly, and carries substantial morbidity, mortality and economic burden in both developing and developed countries. Despite rapid advances in diagnostic and therapeutic techniques, it remains challenging to determine the best timing and mode of intervention. Incorporation of advanced imaging-derived parameters and cardiac biomarkers can address important limitations of conventional parameters, better stratify patient risk, and help determine the optimal timing of intervention in asymptomatic patients. The advent of transcatheter valve therapy has revolutionized the management of valve disease. Indications are expected to expand with further device iteration, accumulated experience, and confirmation of durable long-term outcomes. As more robust data becomes available, determination of the best timing and mode of intervention will become firmly evidenced-based and individualized according to disease, procedure and patient-related variables within a shared decision-making process.
Disclosure
The authors have no disclosures or conflicts of interest to declare
References
- 1. Wang Y-T, Tao J, Maimaiti A, et al. Prevalence of valvular heart diseases and associated risk factors in Han, Uygur and Kazak population in Xinjiang, China. Taniyama Y, ed. PLoS One 2017;12:e0174490. 10.1371/journal.pone.0174490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. d’Arcy JL, Coffey S, Loudon MA, et al. Large-scale community echocardiographic screening reveals a major burden of undiagnosed valvular heart disease in older people: the OxVALVE Population Cohort Study. Eur Heart J 2016;37:3515–22. 10.1093/eurheartj/ehw229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Jilaihawi H, Wu Y, Yang Y, et al. Morphological characteristics of severe aortic stenosis in China: imaging corelab observations from the first Chinese transcatheter aortic valve trial. Catheter Cardiovasc Interv 2015;85:752–61. 10.1002/ccd.25863. [DOI] [PubMed] [Google Scholar]
- 4. Li Y, Wei X, Zhao Z, et al. Prevalence and Complications of Bicuspid Aortic Valve in Chinese According to Echocardiographic Database. Am J Cardiol 2017;120:287–91. 10.1016/j.amjcard.2017.04.025. [DOI] [PubMed] [Google Scholar]
- 5. Rizzo JA, Chen J, Mallow PJ, et al. The Total Direct Healthcare Cost of Aortic And Mitral Valvular Disease: Evidence From Us National Survey Data. Value Health 2015;18:A144. 10.1016/j.jval.2015.03.837. [DOI] [Google Scholar]
- 6. Coffey S, Cairns BJ, Iung B. The modern epidemiology of heart valve disease. Heart 2016;102:75–85. 10.1136/heartjnl-2014-307020. [DOI] [PubMed] [Google Scholar]
- 7. Unger P, Rosenhek R, Dedobbeleer C, et al. Management of multiple valve disease. Heart 2011;97:272–7. 10.1136/hrt.2010.212282. [DOI] [PubMed] [Google Scholar]
- 8. Thompson CR, Buller CE, Sleeper LA, et al. Cardiogenic shock due to acute severe mitral regurgitation complicating acute myocardial infarction: a report from the SHOCK Trial Registry. SHould we use emergently revascularize Occluded Coronaries in cardiogenic shocK? J Am Coll Cardiol 2000;36:1104–9. [DOI] [PubMed] [Google Scholar]
- 9. Bonow RO, Lakatos E, Maron BJ, et al. Serial long-term assessment of the natural history of asymptomatic patients with chronic aortic regurgitation and normal left ventricular systolic function. Circulation 1991;84:1625–35. 10.1161/01.CIR.84.4.1625. [DOI] [PubMed] [Google Scholar]
- 10. Tornos MP, Olona M, Permanyer-Miralda G, et al. Clinical outcome of severe asymptomatic chronic aortic regurgitation: a long-term prospective follow-up study. Am Heart J 1995;130:333–9. 10.1016/0002-8703(95)90450-6. [DOI] [PubMed] [Google Scholar]
- 11. Borer JS, Hochreiter C, Herrold EM, et al. Prediction of indications for valve replacement among asymptomatic or minimally symptomatic patients with chronic aortic regurgitation and normal left ventricular performance. Circulation 1998;97:525–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Bonow RO. Chronic Mitral Regurgitation and Aortic Regurgitation. J Am Coll Cardiol 2013;61:693–701. 10.1016/j.jacc.2012.08.1025. [DOI] [PubMed] [Google Scholar]
- 13. Shah ASV, Chin CWL, Vassiliou V, et al. Left ventricular hypertrophy with strain and aortic stenosis. Circulation 2014;130:1607–16. 10.1161/CIRCULATIONAHA.114.011085. [DOI] [PubMed] [Google Scholar]
- 14. Treibel TA, López B, González A, et al. Reappraising myocardial fibrosis in severe aortic stenosis: an invasive and non-invasive study in 133 patients. Eur Heart J 2018;39:699–709. 10.1093/eurheartj/ehx353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Hein S. Progression From Compensated Hypertrophy to Failure in the Pressure-Overloaded Human Heart: Structural Deterioration and Compensatory Mechanisms. Circulation 2003;107:984–91. 10.1161/01.CIR.0000051865.66123.B7. [DOI] [PubMed] [Google Scholar]
- 16. Baumgartner H, Falk V, Bax JJ, et al. 2017 ESC/EACTS Guidelines for the management of valvular heart disease: The Task Force for the Management of Valvular Heart Disease of the European Society of Cardiology (ESC) and the European Association for Cardio-Thoracic Surgery (EACTS). Eur Heart J August2017. 10.1093/eurheartj/ehx391. [DOI] [Google Scholar]
- 17. Bull S, Loudon M, Francis JM, et al. A prospective, double-blind, randomized controlled trial of the angiotensin-converting enzyme inhibitor Ramipril In Aortic Stenosis (RIAS trial). Eur Heart J Cardiovasc Imaging 2015;16:834–41. 10.1093/ehjci/jev043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Chan KL, Teo K, Dumesnil JG, et al. ASTRONOMER Investigators . Effect of Lipid lowering with rosuvastatin on progression of aortic stenosis: results of the aortic stenosis progression observation: measuring effects of rosuvastatin (ASTRONOMER) trial. Circulation 2010;121:306–14. 10.1161/CIRCULATIONAHA.109.900027. [DOI] [PubMed] [Google Scholar]
- 19. Rossebø AB, Pedersen TR, Boman K, et al. Intensive lipid lowering with simvastatin and ezetimibe in aortic stenosis. N Engl J Med 2008;359:1343–56. 10.1056/NEJMoa0804602. [DOI] [PubMed] [Google Scholar]
- 20. Cowell SJ, Newby DE, Prescott RJ, et al. A Randomized Trial of Intensive Lipid-Lowering Therapy in Calcific Aortic Stenosis. N Engl J Med 2005;352:2389–97. 10.1056/NEJMoa043876. [DOI] [PubMed] [Google Scholar]
- 21. Evangelista A, Tornos P, Sambola A, et al. Long-term vasodilator therapy in patients with severe aortic regurgitation. N Engl J Med 2005;353:1342–9. 10.1056/NEJMoa050666. [DOI] [PubMed] [Google Scholar]
- 22. Greenberg B, Massie B, Bristow JD, et al. Long-term vasodilator therapy of chronic aortic insufficiency. A randomized double-blinded, placebo-controlled clinical trial. Circulation 1988;78:92–103. [DOI] [PubMed] [Google Scholar]
- 23. Scognamiglio R, Rahimtoola SH, Fasoli G, et al. Nifedipine in asymptomatic patients with severe aortic regurgitation and normal left ventricular function. N Engl J Med 1994;331:689–94. 10.1056/NEJM199409153311101. [DOI] [PubMed] [Google Scholar]
- 24. Borer JS, Sharma A. Drug Therapy for Heart Valve Diseases. Circulation 2015;132:1038–45. 10.1161/CIRCULATIONAHA.115.016006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Picano E, Pibarot P, Lancellotti P, et al. The emerging role of exercise testing and stress echocardiography in valvular heart disease. J Am Coll Cardiol 2009;54:2251–60. 10.1016/j.jacc.2009.07.046. [DOI] [PubMed] [Google Scholar]
- 26. Magne J, Lancellotti P, Pierard LA. Exercise pulmonary hypertension in asymptomatic degenerative mitral regurgitation. Circulation 2010;122:33–41. 10.1161/CIRCULATIONAHA.110.938241. [DOI] [PubMed] [Google Scholar]
- 27. Pizarro R, Bazzino OO, Oberti PF, et al. Prospective validation of the prognostic usefulness of B-type natriuretic peptide in asymptomatic patients with chronic severe aortic regurgitation. J Am Coll Cardiol 2011;58:1705–14. 10.1016/j.jacc.2011.07.016. [DOI] [PubMed] [Google Scholar]
- 28. Everett RJ, Clavel M-A, Pibarot P, et al. Timing of intervention in aortic stenosis: a review of current and future strategies. Heart July2018:heartjnl–2017–312304. 10.1136/heartjnl-2017-312304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Kim HM, Cho G-Y, Hwang I-C, et al. Myocardial Strain in Prediction of Outcomes After Surgery for Severe Mitral Regurgitation. JACC Cardiovasc Imaging 2018;11:1235–44. 10.1016/j.jcmg.2018.03.016. [DOI] [PubMed] [Google Scholar]
- 30. Alashi A, Mentias A, Abdallah A, et al. Incremental Prognostic Utility of Left Ventricular Global Longitudinal Strain in Asymptomatic Patients With Significant Chronic Aortic Regurgitation and Preserved Left Ventricular Ejection Fraction. JACC Cardiovasc Imaging 2018;11:673–82. 10.1016/j.jcmg.2017.02.016. [DOI] [PubMed] [Google Scholar]
- 31. Vollema EM, Sugimoto T, Shen M, et al. Association of Left Ventricular Global Longitudinal Strain With Asymptomatic Severe Aortic Stenosis: Natural Course and Prognostic Value. JAMA Cardiol 2018;3:839–47. 10.1001/jamacardio.2018.2288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Nombela-Franco L, Eltchaninoff H, Zahn R, et al. Clinical impact and evolution of mitral regurgitation following transcatheter aortic valve replacement: a meta-analysis. Heart 2015;101:1395–1405. 10.1136/heartjnl-2014-307120. [DOI] [PubMed] [Google Scholar]
- 33. Carabello BA, Paulus WJ. Aortic stenosis. Lancet 2009;373:956–66. 10.1016/S0140-6736(09)60211-7. [DOI] [PubMed] [Google Scholar]
- 34. Nishimura RA, Otto CM, Bonow RO, et al. 2017 AHA/ACC Focused Update of the 2014 AHA/ACC Guideline for the Management of Patients With Valvular Heart Disease: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. J Am Coll Cardiol 2017;70:252–89. 10.1016/j.jacc.2017.03.011. [DOI] [PubMed] [Google Scholar]
- 35. Nishimura RA, Otto CM, Bonow RO, et al. 2014 AHA/ACC Guideline for the Management of Patients With Valvular Heart Disease: executive summary: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. Circulation 2014;129:2440–92. 10.1161/CIR.0000000000000029. [DOI] [PubMed] [Google Scholar]
- 36. Maréchaux S, Hachicha Z, Bellouin A, et al. Usefulness of exercise-stress echocardiography for risk stratification of true asymptomatic patients with aortic valve stenosis. Eur Heart J 2010;31:1390–7. 10.1093/eurheartj/ehq076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Leon MB, Smith CR, Mack MJ, et al. Transcatheter or Surgical Aortic-Valve Replacement in Intermediate-Risk Patients. N Engl J Med 2016;374:1609–20. 10.1056/NEJMoa1514616. [DOI] [PubMed] [Google Scholar]
- 38. Smith CR, Leon MB, Mack MJ, et al. Transcatheter versus surgical aortic-valve replacement in high-risk patients. N Engl J Med 2011;364:2187–98. 10.1056/NEJMoa1103510. [DOI] [PubMed] [Google Scholar]
- 39. Leon MB, Smith CR, Mack M, et al. Transcatheter Aortic-Valve Implantation for Aortic Stenosis in Patients Who Cannot Undergo Surgery. N Engl J Med 2010;363:1597–1607. 10.1056/NEJMoa1008232. [DOI] [PubMed] [Google Scholar]
- 40. Mack MJ, Leon MB, Smith CR, et al. 5-year outcomes of transcatheter aortic valve replacement or surgical aortic valve replacement for high surgical risk patients with aortic stenosis (PARTNER 1): a randomised controlled trial. Lancet 2015;385:2477–84. 10.1016/S0140-6736(15)60308-7. [DOI] [PubMed] [Google Scholar]
- 41. Dujardin KS, Enriquez-Sarano M, Schaff HV, et al. Mortality and morbidity of aortic regurgitation in clinical practice. A long-term follow-up study. Circulation 1999;99:1851–7. 10.1161/01.CIR.99.14.1851. [DOI] [PubMed] [Google Scholar]
- 42. Tornos P, Sambola A, Permanyer-Miralda G, et al. Long-term outcome of surgically treated aortic regurgitation: influence of guideline adherence toward early surgery. J Am Coll Cardiol 2006;47:1012–7. 10.1016/j.jacc.2005.10.049. [DOI] [PubMed] [Google Scholar]
- 43. Mentias A, Feng K, Alashi A, et al. Long-Term Outcomes in Patients With Aortic Regurgitation and Preserved Left Ventricular Ejection Fraction. J Am Coll Cardiol 2016;68:2144–53. 10.1016/j.jacc.2016.08.045. [DOI] [PubMed] [Google Scholar]
- 44. Pettersson GB, Crucean AC, Savage R, et al. Toward predictable repair of regurgitant aortic valves: a systematic morphology-directed approach to bicommissural repair. J Am Coll Cardiol 2008;52:40–9. 10.1016/j.jacc.2008.01.073. [DOI] [PubMed] [Google Scholar]
- 45. Roy DA, Schaefer U, Guetta V, et al. Transcatheter aortic valve implantation for pure severe native aortic valve regurgitation. J Am Coll Cardiol 2013;61:1577–84. 10.1016/j.jacc.2013.01.018. [DOI] [PubMed] [Google Scholar]
- 46. Yoon SH, Schmidt T, Bleiziffer S, et al. Transcatheter Aortic Valve Replacement in Pure Native Aortic Valve Regurgitation. J Am Coll Cardiol 2017;70:2752–63. 10.1016/j.jacc.2017.10.006. [DOI] [PubMed] [Google Scholar]
- 47. Seiffert M, Bader R, Kappert U, et al. Initial German experience with transapical implantation of a second-generation transcatheter heart valve for the treatment of aortic regurgitation. JACC Cardiovasc Interv 2014;7:1168–74. 10.1016/j.jcin.2014.05.014. [DOI] [PubMed] [Google Scholar]
- 48. Wei L, Liu H, Zhu L, et al. A New Transcatheter Aortic Valve Replacement System for Predominant Aortic Regurgitation Implantation of the J-Valve and Early Outcome. JACC Cardiovasc Interv 2015;8:1831–41. 10.1016/j.jcin.2015.08.021. [DOI] [PubMed] [Google Scholar]
- 49. Toggweiler S, Cerillo AG, Kim WK, et al. Transfemoral Implantation of the Acurate neo for the Treatment of Aortic Regurgitation. J Invasive Cardiol 2018;30:329–33. [PubMed] [Google Scholar]
- 50. Lock JE, Khalilullah M, Shrivastava S, et al. Percutaneous catheter commissurotomy in rheumatic mitral stenosis. N Engl J Med 1985;313:1515–8. 10.1056/NEJM198512123132405. [DOI] [PubMed] [Google Scholar]
- 51. Inoue K, Owaki T, Nakamura T, et al. Clinical application of transvenous mitral commissurotomy by a new balloon catheter. J Thorac Cardiovasc Surg 1984;87:394–402. [PubMed] [Google Scholar]
- 52. Arora R, Nair M, Kalra GS, et al. Immediate and long-term results of balloon and surgical closed mitral valvotomy: a randomized comparative study. Am Heart J 1993;125:1091–4. [DOI] [PubMed] [Google Scholar]
- 53. Reyes VP, Raju BS, Wynne J, et al. Percutaneous balloon valvuloplasty compared with open surgical commissurotomy for mitral stenosis. N Engl J Med 1994;331:961–7. 10.1056/NEJM199410133311501. [DOI] [PubMed] [Google Scholar]
- 54. Wilkins GT, Weyman AE, Abascal VM, et al. Percutaneous balloon dilatation of the mitral valve: an analysis of echocardiographic variables related to outcome and the mechanism of dilatation. Br Heart J 1988;60:299–308. 10.1136/hrt.60.4.299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Kang DH, Lee CH, Kim DH, et al. Early percutaneous mitral commissurotomy vs. conventional management in asymptomatic moderate mitral stenosis. Eur Heart J 2012;33:1511–7. 10.1093/eurheartj/ehr495. [DOI] [PubMed] [Google Scholar]
- 56. Guerrero M, Urena M, Himbert D, et al. 1-Year Outcomes of Transcatheter Mitral Valve Replacement in Patients With Severe Mitral Annular Calcification. J Am Coll Cardiol 2018;71:1841–53. 10.1016/j.jacc.2018.02.054. [DOI] [PubMed] [Google Scholar]
- 57. El Sabbagh A, Reddy YNV, Nishimura RA, et al. Mitral Valve Regurgitation in the Contemporary Era: Insights Into Diagnosis, Management, and Future Directions. JACC Cardiovasc Imaging 2018;11(4):628–643. 10.1016/j.jcmg.2018.01.009. [DOI] [PubMed] [Google Scholar]
- 58. Suri RM, Vanoverschelde JL, Grigioni F, et al. Association between early surgical intervention vs watchful waiting and outcomes for mitral regurgitation due to flail mitral valve leaflets. JAMA 2013;310:609–16. 10.1001/jama.2013.8643. [DOI] [PubMed] [Google Scholar]
- 59. Tribouilloy C, Rusinaru D, Szymanski C, et al. Predicting left ventricular dysfunction after valve repair for mitral regurgitation due to leaflet prolapse: additive value of left ventricular end-systolic dimension to ejection fraction. Eur J Echocardiogr 2011;12:702–10. 10.1093/ejechocard/jer128. [DOI] [PubMed] [Google Scholar]
- 60. Michler RE, Smith PK, Parides MK, et al. Two-Year Outcomes of Surgical Treatment of Moderate Ischemic Mitral Regurgitation. N Engl J Med 2016;374:1932–41. 10.1056/NEJMoa1602003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Goldstein D, Moskowitz AJ, Gelijns AC, et al. Two-Year Outcomes of Surgical Treatment of Severe Ischemic Mitral Regurgitation. N Engl J Med 2016;374:344–53. 10.1056/NEJMoa1512913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Mirabel M, Iung B, Baron G, et al. What are the characteristics of patients with severe, symptomatic, mitral regurgitation who are denied surgery? Eur Heart J 2007;28:1358–65. 10.1093/eurheartj/ehm001. [DOI] [PubMed] [Google Scholar]
- 63. Stone GW, Lindenfeld J, Abraham WT, et al. Transcatheter Mitral-Valve Repair in Patients with Heart Failure. N Engl J Med September2018. 10.1056/NEJMoa1806640. [DOI] [PubMed] [Google Scholar]
- 64. Bapat V, Rajagopal V, Meduri C, et al. Early Experience With New Transcatheter Mitral Valve Replacement. J Am Coll Cardiol 2018;71:12–21. 10.1016/j.jacc.2017.10.061. [DOI] [PubMed] [Google Scholar]