Abstract
Radiotherapy as one of the four pillars of cancer therapy plays a critical role in the multimodal treatment of thoracic cancers. Due to significant improvements in overall cancer survival, radiotherapy-induced heart disease (RIHD) has become an increasingly recognized adverse reaction which contributes to major radiation-associated toxicities including non-malignant death. This is especially relevant for patients suffering from diseases with excellent prognosis such as breast cancer or Hodgkin’s lymphoma, since RIHD may occur decades after radiotherapy. Preclinical studies have enriched our knowledge of many potential mechanisms by which thoracic radiotherapy induces heart injury. Epidemiological findings in humans reveal that irradiation might increase the risk of cardiac disease at even lower doses than previously assumed. Recent preclinical studies have identified non-invasive methods for evaluation of RIHD. Furthermore, potential options preventing or at least attenuating RIHD have been developed. Ongoing research may enrich our limited knowledge about biological mechanisms of RIHD, identify non-invasive early detection biomarkers and investigate potential treatment options that might attenuate or prevent these unwanted side effects. Here, we present a comprehensive review about the published literature regarding clinical manifestation and pathological alterations in RIHD. Biological mechanisms and treatment options are outlined, and challenges in RIHD treatment are summarized.
Keywords: Radiotherapy, heart injury, thoracic cancer, radiation-induced heart injury, cancer treatment
Background
Thoracic irradiation is a fundamental part of the standard therapy for lung, breast, esophageal and thymic carcinoma, and one of the most common uses of radiotherapy. Although modern planning and irradiation techniques have greatly improved since the introduction of intensity-modulated radiotherapy (IMRT), image-guided radiotherapy (IGRT) and stereotactic radiotherapy, adjacent organs at risk limit the application of high radiotherapy doses. Simultaneous chemoradiotherapy and combination of radiotherapy with novel agents including monoclonal antibodies, tyrosine kinase in hibitors and checkpoint inhibitors can increase radiation-induced toxicities.1 Especially patients suffering from diseases with excellent prognosis such as breast cancer or Hodgkin’s lymphoma may suffer from delayed side effects2–6 including radiation-induced heart disease (RIHD) in a dose-dependent manner.7 The number of long term survivors after radiotherapy is increasing even for lung cancer patients due to new targeted therapies and checkpoint inhibitors.8,9 Therefore, a profound understanding of RIHD is becoming more important in the future. While numbers do vary, an increased risk with up to 62% of cardiac deaths was reported among breast cancer patients after radiotherapy,10,11 and 4% (50/1261) of Hodgkin’s lymphoma patients receiving radiotherapy died from cardiovascular diseases including ischemic heart disease and myocardial infarction.12,13 Patients who received thoracic radiotherapy in their childhood have a 5.0 to 18.4-fold increase in the risk to develop RIHD when average cardiac radiation dose exceeded 5 Gy.14–16
The pathological spectrum of RIHD includes conduction abnormalities, valvular disease, coronary artery disease, pericarditis and pericardial constriction or effusion, cardiomyopathy and myocardial fibrosis.17–20 Although physical progress allowing for more conformal radiation techniques have decreased radiation doses to normal tissues, significant heart doses still can not completely be avoided.21,22 Until now, no effective treatment has been established for RIHD, partially because the underlying mechanisms of the RIHD remain largely unknown.23
Here, we present the clinical manifestations and possible radiobiological mechanisms of RIHD. Furthermore, we discuss how a deeper understanding of RIHD might help to discover strategies in order to reduce the risk of cardiovascular diseases in thoracically irradiated cancer patients.
Clinical manifestation
Coronary artery disease
Radiation-induced coronary artery changes have become a serious reason for morbidity in breast cancer and other mediastinal malignancy patients treated with radiotherapy.24 For example, ischemic heart disease has become the most common reason for cardiac death in cancer patients after thoracic radiotherapy.11 A retrospective analysis involving 2168 breast cancer patients who received radiotherapy showed a linear correlation between radiation dose and coronary artery changes, showing the risk of coronary events increasing by 7.4% per Gy without obvious ceiling.11,25 Symptoms of radiation-induced coronary artery disease are the same as for regular coronary artery disease including angina and myocardial infarction, thereby complicating the differential diagnosis between radiation-induced coronary artery disease and conventional coronary heart disease. The diagnosis of radiation-induced coronary artery disease depends mainly on the history of thoracic radiotherapy.
Valvular disease
Thoracic radiotherapy may directly affect heart valves, leading to both stenotic and regurgitant valve diseases. Pathologic changes involve not only leaflet retraction but also fibrotic thickening and finally calcification.26 Aortic and mitral valves are affected more frequently compared to tricuspid and pulmonic valves. Although valvular heart changes are found in up to 81% of RIHD patients, over 70% of affected patients show no symptoms.27,28 Mean development time of asymptomatic valvular lesions is estimated to be 11.5 years, while the average time to symptomatic dysfunctional valvular disease is about 16.5 years.27 Until now, no specific radiation-induced valvular damage model has been established in vivo, and further animal data about the radiation-induced valvular disease are needed.
Conduction system disease
For radiation-induced conduction system injuries, it is difficult to confirm the causal link to radiotherapy and to determine the incidence, because conduction abnormalities typically are not detected until many years after radiation. However, reported conduction system diseases after thoracic irradiation include all degrees of atrioventricular block (AV block), atrioventricular nodal bradycardia and sick sinus syndrome.29 Other conduction anomalies reported to be connected with radiotherapy include right bundle branch block,30 prolongation of the corrected QT interval,31 T-wave changes and ST depression.32 So far, the biological mechanisms underlying radiation-induced conduction system disease remain unclear as it is challenging to establish the disease model in animals.
Pericardial disease
In necropsy studies, 70% of RIHD patients were found to have pericardial abnormalities.33 Hereby, radiation-induced pericarditis is marked by both protein-rich exudates in the pericardial sac and fibrin accumulation in the mesothelial lining pericardial cavity.17,34 Clinical spectrum of pericardial disease ranges from acute pericarditis to delayed chronic pericardial effusion, tamponade and constrictive pericarditis, according to the severity and the development of the disease.
Myocardial injury
Microvascular impairment by chest radiotherapy may lead to chronic ischemia, which eventually can result in myocardial fibrosis.35 Clinically, most patients suffer from restrictive cardiomyopathy leading to diastolic dysfunction which is partially accompanied by a slight reduction of systolic function in the left ventricle.36 Less than 5% of patients develop a dilated cardiomyopathy accompanied by reduction of left ventricular ejection fraction.37 Although the majority of studies associated with RIHD focus on the myocardial damage, the underlying mechanisms of the myocardial injury itself remain largely unknown. Elucidation of biological mechanisms underlying RIHD may give the opportunity to attenuate RIHD both in the early and delayed stage after thoracic irradiation.
Mechanisms of RIHD
Finding the biological mechanisms of RIHD is challenging due to many confounding factors, including the difficulty of sampling and the the long observation time of over 10 years for clinical development of RIHD. However, preclinical studies may yield information on pathologic changes and potential treatments.
The response of tissues to radiotherapy can be estimated using a dose-response model, the so-called linear quadratic model,38 where the α/β-ratio indicates the fractionation sensitivity of irradiated cells.19 Cardiomyocytes exhibit a low α/β-ratio (about 2) which is typical for late-responding normal tissues. Most preclinical research about mechanisms of RIHD has been performed using a single high dose (20-30 Gy) or a low number of fractions, which is an increasingly used fractionation method for many tumors. However, many clinical irradiation protocols still use multiple fractions (~30) with a relatively low dose (~2 Gy) per fraction.39–42 High radiation doses show different biological effects compared to doses used in normal fractionated treatments; thus, available data from animal models in which high doses were used are difficult to compare with the majority of clinical situations. Moreover, although pathological findings in animals appear to be similar to those in cardiac tissue samples of human beings, it remains uncertain how well preclinical in vivo findings correspond to RIHD in humans.19
Coronary artery disease and vessel injury
Radiation-induced atherosclerosis plays an essential role in the development of RIHD. While myocardial changes in response to a high dose of radiotherapy are alike to those detected in human beings, atherosclerosis does not occur in regular laboratory animals. Therefore, additional vascular risk factors are included in these models to accelerate atherosclerosis formation such as apolipoprotein E (ApoE) knock-out, high lipid diet, or high levels of troponin in plasma.43–46 Heart irradiation in ApoE knock-out mice was found to induce both elevated microvascular detriment and atherosclerotic plaque formation in coronary arteries.47 The transcription factor peroxisome proliferator-activated receptor alpha (PPARα), an important regulator of lipid metabolism in heart tissue, was activated in wild-type mice after exposure to a single radiation dose of 16 Gy40,48; and reduced activation of PPARα resulted in the sudden death of 40 weeks old mice.49 Analogously, the increment of PPARα activity by administrating simvastatin partly prevented the progress of cardiac fibrosis and hypertrophy in hypercholesterolemic and atherosclerosis-prone apolipoprotein E knock-out (ApoE-/-) mice, which exhibited elevated cholesterol levels and developed age-related atherosclerosis and fibrosis.50 These in vivo findings are consistent with observations in both tissue specimens and ultrasound examination from patients showing vessel wall lesions after radiation.51,52
Both perivascular and interstitial collagen deposition in rat myocardium was found increased for several months after a single radiation dose or a limited number of fractions.53,54 Irradiation-related myocardial fibrosis was accompanied by altered microvascular density, leading to impaired myocardium microvasculature function.55 Mice with an endothelial cell-specific deletion of p53 showed a significant increase of cardiac dysfunction and myocardial necrosis in response to local heart radiation with 12 Gy, indicating the importance of myocardial vessels in sustaining cardiac structure and function after heart irradiation.56
Eventually, atherosclerotic lesions in coronary vessels caused by irradiation are morphologically identical to those in patients with regular atherosclerosis and are marked by intimal proliferation, lipid-rich macrophages accumulation, and finally plaque formation.57 Risk of radiation-caused coronary artery alteration is known to be correlated with radiation dose and duration of radiotherapy.
Compared to thoracic radiation alone, 10 Gy whole body irradiation was found to result in pronounced cardiac vascular density reduction,58 suggesting that RIHD may be enhanced by irradiation of non-cardiac structures in the body. This might reflect an “abscopal” irradiation effect against normal tissue, which is then not in favour of patients as is the “abscopal” effect against tumors in the standard literature about radiation immune effects. However, this hypothetical negative systemic “abscopal” phenomenon against cardiac tissue has not yet been systematically investigated.
Conduction system disease and pericardial disease
Few researchers have focused on the mechanisms of the conduction system and pericardial disease in RIHD. Cardiac arrhythmia induced by exposure to ionizing radiation is a long-term consequence. The beat rate of differentiated cardiomyocytes derived from human induced pluripotent stem cells (iPS) decreased at 48 hours after irradiation with 5 Gy or 10 Gy. With higher irradiation doses, alterations in electrophysiological function were observed.59 In contrast, the beat rate of chicken cardiac cells raised in a dose-dependent manner for more than one week after irradiation with 0.5 Gy to 7 Gy. Duration of a single action potential was mildly shortened, and the number of mitotic and S-phase cells decreased after radiation. Though the number of γH2AX foci was found increased after irradiation, no obvious changes in the quantity of reactive oxygen species (ROS) were observed.60 Furthermore, the irradiation reactions intracellular and intranuclear in the sinus node P cells and Purkinje cells remain absent.
Pericardial fibrosis is caused by collagen deposition in the interstitium and parietal region of the thickened pericardium. These findings in animals are similar to those observed in patients after radiotherapy.17,49 Radiation-caused microvascular impairment is believed to result in increased capillary permeability and quick development of protein-rich exudates.29
Myocardial fibrosis
Myocardial fibrosis is characterized by collagen deposition throughout the cardiac tissue and finally the replacement of cardiomyocytes.17,61 Furthermore, myocardial fibrosis may develop under the condition of chronic myocarditis when functional tissue is substituted by connective tissue including collagen, fibronectin and tenascin C as part of an adaptive process.62,63 Animal models have been established in mice, rats, rabbits and dogs in which loss of cardiac function and myocardial fibrosis usually develop from 4 to 12 months after irradiation.17,64
Irradiation induces both morphological and functional changes in hearts that can be measured by histology and echocardiography. In one study, heart weight and heart-to-body weight ratio were found decreased, while diastolic pressure of the left ventricle increased after exposure to ionizing radiation.65 After irradiation, thickening of the left ventricular anterior wall was observed reducing both the inner diameters and the volumes of the left and right ventricle.66,67 Both fractional shortening and ejection fraction were increased in the irradiated hearts, whereas stroke volume was not changed.65,67
In animal models, a single dose of up to 8 Gy led to a significant increase in cardiac fibrosis, although higher total doses with lower dose per fraction seemed to be less harmful to cardiomyocytes than a lower single dose.67–69 Rat cardiac fibroblasts isolated from irradiated hearts displayed cytoskeletal remodellings such as actin filaments changes and stress fibres formation.70 Formation of cytoplasmatic actin stress fibres was associated with an increased number of myofibroblasts producing collagen71 leading to impaired myocardial contractility.65,67,72 Additionally, cardiomyocytes responded to stress signals by launching an inflammatory reaction activating macrophages, which resulted in reduced myocyte contractility both in vitro and in vivo, eventually leading to impaired diastolic and systolic function.73,74 This inflammatory response also reduced the capillary density of irradiated hearts, which may contribute to myocardial injury.47,75
Oxidative stress induced by various cytokines and growth factors including TGF-β,76,77 TNF-α,78–80 IL-1,81 IL-11,82–84 CTGF,85 PDGFs,86,87 VEGF and FGF88,89 has been demonstrated to contribute to the induction of fibrosis (Fig. 1). Many of these factors as well as CK-MB and BNP were considered to qualify as potential markers in predicting and evaluating RIHD.90 Overexpression of TGF-β1 was associated with radiation-induced cardiac fibrosis, suggesting that increased growth factor levels may deteriorate RIHD.91–93 Another important mediator of heart fibrogenesis are factors belonging to the PDGF family. Some studies have shown that overexpression of cardiac PDGF-C and PDGF-D by transgenic technology resulted in extensive cardiac fibrosis,86,87,94 whereas the PDGF-receptor blocker imatinib (which blocks also other kinases) significantly attenuated fibrosis in mice.95 Antifibrotic effects of PDGF signalling inhibitors such as imatinib have also been published for other organs including the lung and the kidney.96–99 PDGF signalling generally seems to plays an important role in fibrogenesis. The small molecule PDGF tyrosine kinase inhibitor BIBF 11200 (Ofev) has been approved by the Food and Drug Administration (FDA) in the USA for the treatment of idiopathic lung fibrosis. Furthermore, in vivo experiments revealed beneficial effects regarding RIHD when the pro-fibrotic protein CTGF was blocked.100 Recently, an interesting study identified circulating microRNAs (pre-treatment c-miRNA) as biomarkers of radiation-induced cardiac toxicity in non-small-cell lung cancer, highlighting the important role of microRNA in RIHD.101
Figure 1 .
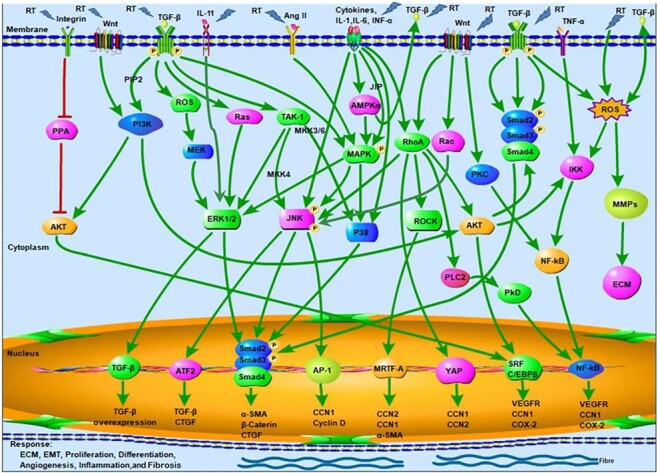
Putative pathway overview how radiation therapy is involved in the development of cardiac fibrosis upon thoracic radiotherapy for cancer. RT, radiation therapy; PPA, protein phosphatase; AKT, protein kinase B; TGF-β, transforming growth factor beta; PIP2, phosphatidylinositol Biphosphate; PI3K, phosphoinositide 3-kinases; ROS, reactive oxygen species; ERK, extracellular signal-regulated kinase; TAK, TGF-β-activated kinase; MKK, mitogen-activated protein kinase kinase; JNK, the c-Jun NH2-terminal protein kinase; ATF, activating transcriptional factor; CTGF, connective tissue growth factor; α-SMA, α smooth muscle actin; Ang II, angiotensin II; AMPKα, AMP-activated protein kinase α; MAPK, mitogen-activated protein kinase; P38, P38 mitogen-activated protein kinase; JIP, JNK-interacting protein; AP-1, activating protein-1; CCN, the first number of CYR61, CTGF & NOV family; IL, interleukin; INF-α, interferon-α; RhoA, Ras homolog gene family, member A; ROCK, Rho-associated protein kinase; MRTF, myocardin-related transcription factor; YAP, yes-associated protein; PLC-2, Phospholipase C 2; PkD, Protein kinase D; PKC, Protein kinase C; SRF, serum response factor; C/EBPβ, CCAAT-enhancer-binding protein β; VEGFR, vascular endothelial growth factor receptors; NF-κB, nuclear factor-κB; COX-2, cyclooxygenase-2; TNF-α, tumour necrosis factor alpha; IKK, NF-κB kinase; MMPs, matrix metallopeptidases; ECM, extracellular matrix; Activate.
In rodent hearts receiving single high dose irradiation, long term pathological changes are associated with altered protein expression and impaired cardiac mitochondria function.102–104 The mitochondrial transcription factor, nuclear factor erythroid 2 [NF-E2]-related factor 2 (Nrf2), regulates the expression of various anti-oxidant enzymes.105 Although the exact mechanisms of the interaction between the Nrf2 pathway and mitochondrial alterations in RIHD are unclear, deficiency of Nrf2 was observed to reduce the life span of mice receiving thoracic radiation, suggesting that increment of Nrf2 levels may reduce radiation damages including RIHD.42,106–108
Some researchers proposed that cardiac irradiation can increase the number of mast cells which could be associated with progression of RIHD53; however, in other studies, mast cell-deficient rats showed more severe alterations than controls.109 Furthermore, high throughput transcriptomic and genetic studies have identified new molecular signals and pathways related to the whole fibrotic process after irradiation. Pathogenesis of cardiac fibrosis includes several molecular pathways, which can be activated by ionizing radiation110 (Table 1). However, the exact roles for the various cytokines and transcription factors in RIHD still need to be clarified, and it is unlikely to find a unique driver biological mechanism of RIHD.
Table 1.
Cytokines, signalling pathways and transcription factors involved in cardiac fibrosis.
| Cytokines | Pathway | Transcription factors | ||||
| TNF-α78–80 | CTGF100 | MCP-1111 | Smad-independent pathway112 | Smad113 | PPAR-γ114 | Nrf2.42,105–108 |
| IL-1β81 | ET-1110,115,116 | IL-1182–84 | AMPKα signaling pathway117 | MRTF118,119 | AP-1120 | ERK121 |
| IL-6122 | Ang II111 | FGF88 | Wnt signaling pathway123 | YAP124 | NF-κB120 | JNK125,126 |
| VEGF88 | TGF-β76,77,91,92 | PDGF86,87,94 | Smad-dependent pathway113 | SRF119 | ATF2127 | C/EBPβ128 |
IL, interleukin; TNF-α, tumor necrosis factor alpha; CTGF, connective tissue growth factor; ET-1, endothelin-1; Ang II, angiotensin II; MCP-1, anti-monocyte chemotactic protein-1; TGF-β, transforming growth factor beta; PDGF, platelet-derived growth factor; VEGF, vascular endothelial growth factor; FGF, fibroblast growth factor; AMPKα, AMP-activated protein kinase α; MRTF, myocardin-related transcription factor; YAP, yes-associated protein; JNK,c-Jun N-terminal kinase; SRF, serum response factor; PPAR-γ, peroxisome proliferators-activated receptor gamma; AP-1, activating protein-1; NF-κB, nuclear factor-κB; ATF2, activating transcriptional factor 2; Nrf2, Nuclear factor erythroid 2 [NF-E2]-related factor 2; ERK, extracellular signal-regulated kinase; C/EBPβ, CCAAT-enhancer-binding protein β.
Potential countermeasures
Since early RIHD is mostly asymptomatic, pathologic changes are often diagnosed in late stages, generally over 10 years after irradiation. Currently, the only eligible approach for prevention of RIHD is by reducing cardiac irradiation doses. Since some exposure will remain inevitable, pharmacological treatments have been studied in order to attenuate RIHD.
Previous studies have shown that oxidative stress plays a significant role in the progression of RIHD.41,42,129 Antioxidants like pentoxifylline (PTX) and α-tocopherol applied 24 hours to one week prior to local heart irradiation significantly attenuated radiation-induced increments of left ventricular diastolic pressure in vivo. Furthermore, mast cell number in the left ventricle, collagen deposition and myocardial degeneration were found to be reduced after application of antioxidants.65,130 Regardless of clinical suitability, these studies suggested that antioxidants in principal may be able to reduce radiotherapy-caused collagen deposition and cardiac fibrosis.65 In another study, colchicine was prophylactically used against radiation-induced coronary artery impairment by reducing inflammation and hindering platelet aggregation.131 Additionally, the cytoprotective agent amifostine was reported to protect against myocardial fibrosis and loss of function by scavenging free radicals when applied before single high dose heart irradiation.132,133 Amifostine is one of few clinically approved drugs for radiation protection but is rarely used due to significant adverse effects such as hypotension, Stevens-Johnson syndrome, erythema multiforme and epidermal necrolysis.19
Oral administration of molecular hydrogen saturated water or black grape juice prior to local heart irradiation reduced RIHD in animal models, probably by free radical scavenging.134,135 Similarly, melatonin application 15 minutes before radiation decreased both necrosis and fibrosis by free radical scavenging in a rat model.136 L-carnitine has also been found to attenuate radiation-induced cardiac function loss in mice by activating the p38MAPK/Nrf2 pathway, triggering the expression of NQO1 and HO1. Additionally, L-carnitine exhibited anti-apoptotic and anti-oxidative effects in irradiated mice hearts.137
Cardioprotective drugs, which are used in ischemic heart disease and chronic heart failure, have been investigated for RIHD, too. In some studies, statins have shown promising effects for RIHD,138,139 but in other studies, atorvastatin (and the anti-platelet drug clopidogrel) did not ameliorate atherosclerosis induction after 14 Gy irradiation in ApoE-/- mice.140 Nitric oxide-releasing aspirin (NCX 4016) and aspirin are known to attenuate age-related atherosclerosis; however, both NCX 4016 and aspirin did not significantly reduce number of atherosclerosis lesions when a single irradiation dose of 14 Gy was delivered.141 Moreover, the angiotensin-converting enzyme inhibitor (ACEI) captopril has demonstrated beneficial effects regarding RIHD in vivo.142,143 With rheological agent PTX in combination with α-tocopherol, cardiac fibrosis was mitigated, and cardiac function was preserved. The authors reported about inhibition of pathways in which TGFβ and CTGF were involved. However, induction of arrhythmia and bradycardia neutralized these beneficial effects.65,130 Thalidomide was used to reduce infiltration of inflammatory cells by inactivating macrophages but did not change long-term radiation damage in mice receiving a single radiation dose of 16 Gy.144 The tyrosine kinase inhibitor sunitinib reduced systolic left ventricular inner diameter and volume, when administered once a day for 14 days after irradiation.129 Rabender and colleagues investigated the effects of IPW-5371, a TGF-β receptor 1 inhibitor. Administration of 30 mg/kg IPW-5371 for 20 weeks preserved cardiac contractile reserve and resulted in significantly decreased cardiac fibrosis. Furthermore, IPW-5371 treatment at either 10 mg/kg or 30 mg/kg for 6 weeks extended the survival time of irradiated mice.145 Mesenchymal stem cells (MSC) are known for their regenerative abilities in radiation-induced tissue injuries. Tail vein injection of bone marrow MSC improved RIHD and may be a new therapeutic option for myocardial injured patients after chemo- or radiotherapy.146–150 Whether bone marrow MSCs or adipose tissue MSCs are superior regarding their regenerative effects for RIHD is unknown; at least, radioresistance was shown to be independent of their tissue of origin.151 In a rat model, palladium alpha-lipoic acid complex (POLY-MVA) was administrated after irradiation with 45 Gy delivered in 5 fractions of 9 Gy. POLY-MVA reduced inflammatory infiltration markers such as CD2 and CD68 in irradiated hearts and attenuated radiation effects on mitochondria. However, the reversal of cardiac remodelling was not observed.152 Moreover, chronic intermittent hypobaric hypoxia promoted cardiac function in RIHD and decreased interstitial and perivascular cardiac fibrosis by reducing oxidative stress.153 Some other agents such as tetrahydrobiopterin might protect cardiomyocytes from radiation-induced injury by decreasing oxidative stress in vitro.154 Potential countermeasures to mitigate RIHD are summarized in Table 2. Currently, it seems far away to find an effective therapy for attenuation of RIHD and simulatenous tumor sensitisation.
Table 2.
The potential countermeasures to attenuate the RIHD.
| Antioxidants | Results | Non-antioxidant agents | Mechanism | Results |
| Amifostine132,133 | + | Statins138,139 | Cholesterol-lowering drugs | + |
| Black grape juice134,135 | + | Captopril142,143 | ACE inhibitor | + |
| Water saturated with molecular hydrogen134,135 | + | Nitric oxide-releasing aspirin141 | Anti-platelet agent | - |
| Tetrahydrobiopterin (in vitro)154 | + | Thalidomide144 | Inactivate macrophages | - |
| Melatonin136,155 | + | Pentoxifylline plus α-tocopherol65,130 | Inhibits intracellular signals in response to TGFβ and CTGF | + |
| L-carnitine137 | + | IPW-5371145 | TGF-β receptor 1 inhibitor | + |
| Chronic intermittent hypobaric hypoxia153 | -- | MSC146–148 | DNA repair | + |
| Palladium lipoic acid complex152 | Targets mitochondrial complex I | -- | ||
| -- | -- | Sunitinib129 | Tyrosine kinase receptor inhibitor | + |
| -- | -- | L-carnitine137 | Inhibiting reactive oxygen species production and apoptosis | + |
| -- | -- | Colchicine131 | Inhibiting the inflammation and anti-platelet-aggregation | + |
| -- | -- | Huangqi Shengmai Yin156 | Regulating the TGF-β1/Smads and MMPs | + |
Clinical management
Countermeasures for radiation-induced coronary artery disease in patients are challenging. Potential therapeutic concepts are similar to those used in patients with regular coronary artery disease which include lifestyle modifications, medical therapy, percutaneous coronary intervention157 and coronary artery bypass grafting.158 Regarding RIHD, percutaneous coronary intervention is generally preferable to coronary artery bypass grafting since radiation-induced lung injuries, valvular diseases, internal thoracic artery stenosis and fibrosis of surrounding structures may increase the risk of surgical procedures.29,159,160 For patients with irradiation-caused valvular disease, surgical valve replacement is generally recommended. Due to perioperative risk factors, increased long-term morbidity and death associated with open heart surgery, transcatheter aortic valve implantation (TAVI) might also be an appropriate alternative.161
Furthermore, patients with acute pericarditis are often treated with diuretics and non-steroidal anti-inflammatory drugs for symptom control, whereas chronic pericarditis can be treated surgically.17,29
Management of radiation-caused cardiomyopathy is similar to treatment of other types of cardiomyopathy and is typically based on symptomatic treatment. Heart transplantation may be a choice for highly selected patients in the terminal heart failure stage.162–164 Most mechanical interventions for fibrosis-related diseases are helpful to a certain degree, but a more biological approach that can interfere with fibrogenesis on a cellular level seems mandatory. Clearly, further preclinical and clinical research is needed to develop new compounds for RIHD.159,165,166
Conclusion
Despite significant technical and physical improvements of thoracic radiotherapy such as IMRT, IGRT, stereotactic radiotherapy, proton and heavy ion irradiation, RIHD remains a relevant risk. Preclinical and clinical studies have widely investigated various manifestations of RIHD including coronary vessel, heart valve, conduction system, pericardium and myocardium injuries. However, present knowledge of the underlying biological mechanisms is insufficient, and reported data from clinical studies are scarce, hampering a personalized and effective treatment approach for RIHD.
A deeper understanding of RIHD mechanisms is essential to initiate appropriate non-invasive screening methods for diagnosis and monitoring. Potential diagnostic modalities are specific biomarkers and radiological techniques such as echocardiography, high resolution computed tomography (HRCT), magnetic resonance imaging (MRI), positron emission magnetic resonance imaging (PET-MRI) and positron emission computed tomography (PET-CT). Beneficial effects of several compounds have been demonstrated in preclinical studies but data regarding these drugs in RIHD patients are limited. Some pharmacological drugs may provide new approaches to treat or prevent RIHD; however, randomized trials are essential to evaluate the role of these biological approaches.
Acknowledgements
This study was supported in part by the China Scholarship Council, and Science and Technology Department of Sichuan Province (Grant No. 2017SZ0057).
Conflict of interest
The authors declare that they have no competing interests.
References
- 1. Yang H, Jin T, Li M, et al. Synergistic effect of immunotherapy and radiotherapy in non-small cell lung cancer: current clinical trials and prospective challenges. Prec Clin Med 2019;2:57-70. doi: 10.1093/pcmedi/pbz004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Siegel RL, Miller KD, Jemal A. Cancer statistics, 2016. CA: Cancer J Clin 2016;66:7–30. doi: 10.3322/caac.21332. [DOI] [PubMed] [Google Scholar]
- 3. Yu AF, Ho AY, Braunstein LZ, et al. Assessment of early radiation-induced changes in left ventricular function by myocardial strain imaging after breast radiation therapy. J Am Soc Echocardiogr 2019;32:521–528. doi: 10.1016/j.echo.2018.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Recht A. Radiation-induced heart disease after breast cancer treatment: how big a problem, and how much can-and should-we try to reduce it? J Clin Oncol 2017;35:1146–8. doi: 10.1200/JCO.2016.71.4113. [DOI] [PubMed] [Google Scholar]
- 5. Tuohinen SS, Skyttä T, Huhtala H, et al. Detection of early radiotherapy-induced changes in intrinsic myocardial contractility by ultrasound tissue characterization in patients with early-stage breast cancer. Echocardiography 2017;34:191–8. doi: 10.1111/echo.13433. [DOI] [PubMed] [Google Scholar]
- 6. Rühle A, Huber PE. Normal tissue: radiosensitivity, toxicity, consequences for planning. Radiologe 2018;58:746–53. doi: 10.1007/s00117-018-0430-4. [DOI] [PubMed] [Google Scholar]
- 7. Luo L, Yan C, Urata Yet al. Dose-dependency and reversibility of radiation-induced injury in cardiac explant-derived cells of mice. Sci Rep 2017;7:40959. doi: 10.1038/srep40959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Antonia SJ, Villegas A, Daniel D, et al. Overall survival with durvalumab after chemoradiotherapy in stage III NSCLC. N Engl J Med 2018;379:2342–50. doi: 10.1056/NEJMoa1809697. [DOI] [PubMed] [Google Scholar]
- 9. Soria JC, Ohe Y, Vansteenkiste J, et al. Osimertinib in untreated EGFR-mutated advanced non-small-cell lung cancer. N Engl J Med 2018;378:113–25. doi: 10.1056/NEJMoa1713137. [DOI] [PubMed] [Google Scholar]
- 10. Yusuf SW, Sami S, Daher IN. Radiation-induced heart disease: a clinical update. Cardiol Res Pract 2011;2011:317659. doi: 10.4061/2011/317659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Darby SC, Ewertz M, McGale P, et al. Risk of ischemic heart disease in women after radiotherapy for breast cancer. N Engl J Med 2013;368:987–98. doi: 10.1056/NEJMoa1209825. [DOI] [PubMed] [Google Scholar]
- 12. Aleman BM, Belt-Dusebout AW, Klokman WJ, et al. Long-term cause-specific mortality of patients treated for Hodgkin's disease. J Clin Oncol 2003;21:3431–9. doi: 10.1200/JCO.2003.07.131 [DOI] [PubMed] [Google Scholar]
- 13. Nimwegen FA, Schaapveld M, Janus CP, et al. Cardiovascular disease after Hodgkin lymphoma treatment: 40-year disease risk. JAMA Intern Med 2015;175:1007–17. doi: 10.1001/jamainternmed.2015.1180. [DOI] [PubMed] [Google Scholar]
- 14. Tukenova M, Guibout C, Oberlin O, et al. Role of cancer treatment in long-term overall and cardiovascular mortality after childhood cancer. J Clin Oncol 2010;28:1308–15. doi: 10.1200/JCO.2008.20.2267. [DOI] [PubMed] [Google Scholar]
- 15. Haddy N, Diallo S, El-Fayech C, et al. Cardiac diseases following childhood cancer treatment: cohort study. Circulation 2016;133:31–8. doi: 10.1161/CIRCULATIONAHA.115.016686. [DOI] [PubMed] [Google Scholar]
- 16. Ruiz CR, Mesa-Pabón M, Soto K, et al. Radiation-induced coronary artery disease in young patients. Heart Views 2018;19:23–6. doi: 10.4103/HEARTVIEWS.HEARTVIEWS_64_17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Darby SC, Cutter DJ, Boerma M, et al. Radiation-related heart disease: current knowledge and future prospects. Int J Radiat Oncol Biol Phys 2010;76:656–65. doi: 10.1016/j.ijrobp.2009.09.064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Clarke M, Collins R, Darby S, et al. Effects of radiotherapy and of differences in the extent of surgery for early breast cancer on local recurrence and 15-year survival: an overview of the randomised trials. Lancet 2005;366:2087–106. doi: 10.1016/S0140-6736(05)67887-7. [DOI] [PubMed] [Google Scholar]
- 19. Boerma M, Sridharan V, Mao XW, et al. Effects of ionizing radiation on the heart. Mutat Res 2016;770:319–27. doi: 10.1016/j.mrrev.2016.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Tadic M, Cuspidi C, Hering D, et al. Radiotherapy-induced right ventricular remodelling: The missing piece of the puzzle. Arch Cardiovasc Dis 2017;110:116–23. doi: 10.1016/j.acvd.2016.10.003. [DOI] [PubMed] [Google Scholar]
- 21. Lipshultz SE, Adams MJ, Colan SD, et al. Long-term cardiovascular toxicity in children, adolescents, and young adults who receive cancer therapy: pathophysiology, course, monitoring, management, prevention, and research directions: a scientific statement from the American Heart Association. Circulation 2013;128:1927–95. doi: 10.1161/CIR.0b013e3182a88099. [DOI] [PubMed] [Google Scholar]
- 22. Jaworski C, Mariani JA, Wheeler G, et al. Cardiac complications of thoracic irradiation. J Am Coll Cardiol 2013;61:2319–28. doi: 10.1016/j.jacc.2013.01.090. [DOI] [PubMed] [Google Scholar]
- 23. Gujral DM, Lloyd G, Bhattacharyya S. Radiation-induced valvular heart disease. Heart 2016;102:269–76. doi: 10.1136/heartjnl-2015-308765. [DOI] [PubMed] [Google Scholar]
- 24. Cuomo JR, Javaheri SP, Sharma GK, et al. How to prevent and manage radiation-induced coronary artery disease. Heart 2018;104:1647–53. doi: 10.1136/heartjnl-2017-312123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Báez-Ferrer N, Izquierdo-Gómez MM, Beyello-Belkasem Cet al. Long-term radiotherapy-induced cardiac complications: A case report. Am J Case Rep 2019;20:1182–8. doi: 10.12659/AJCR.917224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Brand MD, Abadi CA, Aurigemma GP, et al. Radiation-associated valvular heart disease in Hodgkin's disease is associated with characteristic thickening and fibrosis of the aortic-mitral curtain. J Heart Valve Dis 2001;10:681–5. [PubMed] [Google Scholar]
- 27. Carlson RG, Mayfield WR, Normann S, et al. Radiation-associated valvular disease. Chest 1991;99:538–45. doi: 10.1378/chest.99.3.538. [DOI] [PubMed] [Google Scholar]
- 28. Tamura A, Takahara Y, Mogi K, et al. Radiation-induced valvular disease is the logical consequence of irradiation. Gen Thorac Cardiovasc Surg 2007;55:53–6. doi: 10.1007/s11748-006-0070-x. [DOI] [PubMed] [Google Scholar]
- 29. Donnellan E, Phelan D, McCarthy CP, et al. Radiation-induced heart disease: A practical guide to diagnosis and management. Cleve Clin J Med 2016;83:914–22. doi: 10.3949/ccjm.83a.15104. [DOI] [PubMed] [Google Scholar]
- 30. Adams MJ, Lipshultz SE, Schwartz C, et al. Radiation-associated cardiovascular disease: manifestations and management. Semin Radiat Oncol 2003;13:346–56. doi: 10.1016/S1053-4296(03)00026-2. [DOI] [PubMed] [Google Scholar]
- 31. Orzan F, Brusca A, Gaita F, et al. Associated cardiac lesions in patients with radiation-induced complete heart block. Int J Cardiol 1993;39:151–6. doi: 10.1016/0167-5273(93)90027-e. [DOI] [PubMed] [Google Scholar]
- 32. Larsen RL, Jakacki RI, Vetter VL, et al. Electrocardiographic changes and arrhythmias after cancer therapy in children and young adults. Am J Cardiol 1992;70:73–7. doi: 10.1016/0002-9149(92)91393-i. [DOI] [PubMed] [Google Scholar]
- 33. Veinot JP, Edwards WD. Pathology of radiation-induced heart disease: a surgical and autopsy study of 27 cases. Hum Pathol 1996;27:766–73. doi: 10.1016/s0046-8177(96)90447-5. [DOI] [PubMed] [Google Scholar]
- 34. Monceau V, Llach A, Azria D, et al. Epac contributes to cardiac hypertrophy and amyloidosis induced by radiotherapy but not fibrosis. Radiother Oncol 2014;111:63–71. doi: 10.1016/j.radonc.2014.01.025. [DOI] [PubMed] [Google Scholar]
- 35. Heidenreich PA, Hancock SL, Vagelos RH, et al. Diastolic dysfunction after mediastinal irradiation. Am Heart J 2005;150:977–82. doi: 10.1016/j.ahj.2004.12.026. [DOI] [PubMed] [Google Scholar]
- 36. Lipshultz SE, Adams MJ. Cardiotoxicity after childhood cancer: beginning with the end in mind. J Clin Oncol 2010;28:1276–81. doi: 10.1200/JCO.2009.26.5751. [DOI] [PubMed] [Google Scholar]
- 37. Cella L, Liuzzi R, Conson M, et al. Multivariate normal tissue complication probability modeling of heart valve dysfunction in Hodgkin lymphoma survivors. Int J Radiat Oncol Biol Phys 2013;87:304–10. doi: 10.1016/j.ijrobp.2013.05.049. [DOI] [PubMed] [Google Scholar]
- 38. Brenner DJ. The linear-quadratic model is an appropriate methodology for determining isoeffective doses at large doses per fraction. Semin Radiat Oncol 2008;18:234–9. doi: 10.1016/j.semradonc.2008.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Ferreira-Machado SC, Salata C, Rocha NN, et al. Caspase-3 activation and increased procollagen type I in irradiated hearts. An Acad Bras Ciênc 2013;85:215–22. doi: 10.1590/S0001-37652013005000009. [DOI] [PubMed] [Google Scholar]
- 40. Gao S, Wu R, Zeng YC. Up-regulation of peroxisome proliferator-activated receptor gamma in radiation-induced heart injury in rats. Radiat Environ Biophys 2012;51:53–9. doi: 10.1007/s00411-011-0390-9. [DOI] [PubMed] [Google Scholar]
- 41. Sridharan V, Tripathi P, Aykin-Burns N, et al. A tocotrienol-enriched formulation protects against radiation-induced changes in cardiac mitochondria without modifying late cardiac function or structure. Radiat Res 2015;183:357–66. doi: 10.1667/RR13915.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Boerma M, Singh P, Sridharan V, et al. Effects of local heart irradiation in a glutathione S-transferase alpha 4-null mouse model. Radiat Res 2015;183:610–9. doi: 10.1667/RR13979.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Hoving S, Heeneman S, Gijbels MJJ, et al. Single-dose and fractionated irradiation promote initiation and progression of atherosclerosis and induce an inflammatory plaque phenotype in ApoE–/– mice. Int J Radiat Oncol 2008;71:848–57. [DOI] [PubMed] [Google Scholar]
- 44. Stewart FA, Heeneman S, Te Poele J, et al. Ionizing radiation accelerates the development of atherosclerotic lesions in ApoE-/- mice and predisposes to an inflammatory plaque phenotype prone to hemorrhage. Am J Pathol 2006;168:649–58. doi: 10.2353/ajpath.2006.050409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Hoving S, Heeneman S, Gijbels MJ, et al. Irradiation induces different inflammatory and thrombotic responses in carotid arteries of wildtype C57BL/6J and atherosclerosis-prone ApoE(-/-) mice. Radiother Oncol 2012;105:365–70. doi: 10.1016/j.radonc.2012.11.001. [DOI] [PubMed] [Google Scholar]
- 46. Skyttä T, Tuohinen S, Boman Eet al. Troponin T-release associates with cardiac radiation doses during adjuvant left-sided breast cancer radiotherapy. Radiat Oncol 2015;10:141. doi: 10.1186/s13014-015-0436-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Gabriels K, Hoving S, Seemann I, et al. Local heart irradiation of ApoE(-/-) mice induces microvascular and endocardial damage and accelerates coronary atherosclerosis. Radiother Oncol 2012;105:358–64. doi: 10.1016/j.radonc.2012.08.002. [DOI] [PubMed] [Google Scholar]
- 48. Azimzadeh O, Sievert W, Sarioglu H, et al. PPAR alpha: a novel radiation target in locally exposed Mus musculus heart revealed by quantitative proteomics. J Proteome Res 2013;12:2700–14. doi: 10.1021/pr400071g. [DOI] [PubMed] [Google Scholar]
- 49. Tapio S. Pathology and biology of radiation-induced cardiac disease. J Radiat Res 2016;57:439–48. doi: 10.1093/jrr/rrw064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Qin YW, Ye P, He JQ, et al. Simvastatin inhibited cardiac hypertrophy and fibrosis in apolipoprotein E-deficient mice fed a “Western-style diet” by increasing PPAR α and γ expression and reducing TC, MMP-9, and Cat S levels. Acta Pharmacol Sin 2010;31:1350–8. doi: 10.1038/aps.2010.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Gianicolo ME, Gianicolo EA, Tramacere Fet al. Effects of external irradiation of the neck region on intima media thickness of the common carotid artery. Cardiovasc Ultrasound 2010;8:8. doi: 10.1186/1476-7120-8-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Russell NS, Hoving S, Heeneman S, et al. Novel insights into pathological changes in muscular arteries of radiotherapy patients. Radiother Oncol 2009;92:477–83. doi: 10.1016/j.radonc.2009.05.021. [DOI] [PubMed] [Google Scholar]
- 53. Boerma M, Zurcher C, Esveldt I, et al. Histopathology of ventricles, coronary arteries and mast cell accumulation in transverse and longitudinal sections of the rat heart after irradiation. Oncol Rep 2004;12:213–9. [DOI] [PubMed] [Google Scholar]
- 54. Sridharan V, Tripathi P, Sharma S, et al. Roles of sensory nerves in the regulation of radiation-induced structural and functional changes in the heart. Int J Radiat Oncol Biol Phys 2014;88:167–74. doi: 10.1016/j.ijrobp.2013.10.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Boerma M, Kruse JJ, Loenen M, et al. Increased deposition of von Willebrand factor in the rat heart after local ionizing irradiation. Et Al 2004;180:109–16. doi: 10.1007/s00066-004-1138-0. [DOI] [PubMed] [Google Scholar]
- 56. Lee CL, Moding EJ, Cuneo KC, et al. P53 functions in endothelial cells to prevent radiation-induced myocardial injury in mice. Sci Signal 2012;5:ra52. doi: 10.1126/scisignal.2002918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Cheng RK, Lee MS, Seki A, et al. Radiation coronary arteritis refractory to surgical and percutaneous revascularization culminating in orthotopic heart transplantation. Cardiovasc Pathol 2013;22:303–8. doi: 10.1016/j.carpath.2012.12.005. [DOI] [PubMed] [Google Scholar]
- 58. Baker JE, Fish BL, Su JD, et al. 10 Gy total body irradiation increases risk of coronary sclerosis, degeneration of heart structure and function in a rat model. Int J Radiat Biol 2009;85:1089–100. doi: 10.3109/09553000903264473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Becker BV, Seeger T, Beiert T, et al. Impact of ionizing radiation on electrophysiological behavior of human-induced ipsc-derived cardiomyocytes on multielectrode arrays. Health Phys 2018;115:21–8. [DOI] [PubMed] [Google Scholar]
- 60. Frieß JL, Heselich A, Ritter Set al. Electrophysiologic and cellular characteristics of cardiomyocytes after X-ray irradiation. Mutat Res 2015;777:1–10. doi: 10.1016/j.mrfmmm.2015.03.012. [DOI] [PubMed] [Google Scholar]
- 61. Fajardo LF, Stewart JR. Experimental radiation-induced heart disease. I. Light microscopic studies. Am J Pathol 1970;59:299–316. [PMC free article] [PubMed] [Google Scholar]
- 62. Tamura A, Kusachi S, Nogami K, et al. Tenascin expression in endomyocardial biopsy specimens in patients with dilated cardiomyopathy: distribution along margin of fibrotic lesions. Heart 1996;75:291–4. doi: 10.1136/hrt.75.3.291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Maisch B, Rupp H. Myocardial fibrosis: a cardiopathophysiologic Janus head. Herz 2006;31:260–8. doi: 10.1007/s00059-006-2823-9 [DOI] [PubMed] [Google Scholar]
- 64. Unthank JL, Ortiz M, Trivedi H, et al. Cardiac and renal delayed effects of acute radiation exposure: organ differences in vasculopathy, inflammation, senescence and oxidative balance. Radiat Res 2019;191:383–97. doi: 10.1667/RR15130.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Boerma M, Roberto KA, Hauer-Jensen M. Prevention and treatment of functional and structural radiation injury in the rat heart by pentoxifylline and alpha-tocopherol. Int J Radiat Oncol Biol Phys 2008;72:170–7. doi: 10.1016/j.ijrobp.2008.04.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Boerma M, Wees CG, Vrieling Het al. Microarray analysis of gene expression profiles of cardiac myocytes and fibroblasts after mechanical stress, ionising or ultraviolet radiation. BMC Genomics 2005;6:6. doi: 10.1186/1471-2164-6-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Seemann I, Gabriels K, Visser NL, et al. Irradiation induced modest changes in murine cardiac function despite progressive structural damage to the myocardium and microvasculature. Radiother Oncol 2012;103:143–50. doi: 10.1016/j.radonc.2011.10.011. [DOI] [PubMed] [Google Scholar]
- 68. Puukila S, Lemon JA, Lees SJ, et al. Impact of ionizing radiation on the cardiovascular system: A review. Radiat Res 2017;188:539–46. doi: 10.1667/RR14864.1. [DOI] [PubMed] [Google Scholar]
- 69. Herskind C, Rodemann HP. Spontaneous and radiation-induced differentiationof fibroblasts. Exp Gerontol 2000;35:747–55. doi: 10.1016/s0531-5565(00)00168-6 [DOI] [PubMed] [Google Scholar]
- 70. Hinz B, Mastrangelo D, Iselin CE, et al. Mechanical tension controls granulation tissue contractile activity and myofibroblast differentiation. Am J Pathol 2001;159:1009–20. doi: 10.1016/S0002-9440(10)61776-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Hinz B, Gabbiani G. Mechanisms of force generation and transmission by myofibroblasts. Curr Opin Biotechnol 2003;14:538–46. doi: 10.1016/j.copbio.2003.08.006. [DOI] [PubMed] [Google Scholar]
- 72. Krüse JJ, Zurcher C, Strootman EG, et al. Structural changes in the auricles of the rat heart after local ionizing irradiation. Radiother Oncol 2001;58:303–11. doi: 10.1016/S0167-8140(00)00327-3. [DOI] [PubMed] [Google Scholar]
- 73. Boyd JH, Kan B, Roberts H, et al. S100A8 and S100A9 mediate endotoxin-induced cardiomyocyte dysfunction via the receptor for advanced glycation end products. Circ Res 2008;102:1239–46. doi: 10.1161/CIRCRESAHA.107.167544. [DOI] [PubMed] [Google Scholar]
- 74. Simms MG, Walley KR. Activated macrophages decrease rat cardiac myocyte contractility: importance of ICAM-1-dependent adhesion. Am J Physiol 1999;277:H253–60. doi: 10.1152/ajpheart.1999.277.1.H253. [DOI] [PubMed] [Google Scholar]
- 75. Patties I, Haagen J, Dörr W, et al. Late inflammatory and thrombotic changes in irradiated hearts of C57BL/6 wild-type and atherosclerosis-prone ApoE-deficient mice. Et Al 2015;191:172–9. doi: 10.1007/s00066-014-0745-7. [DOI] [PubMed] [Google Scholar]
- 76. Rosenkranz S, Flesch M, Amann K, et al. Alterations of beta-adrenergic signaling and cardiac hypertrophy in transgenic mice overexpressing TGF-beta(1). Am J Physiol Heart Circ Physiol 2002;283:H1253–62. doi: 10.1152/ajpheart.00578.2001 [DOI] [PubMed] [Google Scholar]
- 77. Krüse JJ, Bart CI, Visser A, et al. Changes in transforming growth factor-beta (TGF-beta 1), procollagen types I and II mRNA in the rat heart after irradiation. Int J Radiat Biol 1999;75:1429–36. doi: 10.1080/095530099139296. [DOI] [PubMed] [Google Scholar]
- 78. Li X, Moody MR, Engel D, et al. Cardiac-specific overexpression of tumor necrosis factor-alpha causes oxidative stress and contractile dysfunction in mouse diaphragm. Circulation 2000;102:1690–6. doi: 10.1161/01.cir.102.14.1690. [DOI] [PubMed] [Google Scholar]
- 79. Kubota T, McTiernan CF, Frye CS, et al. Cardiac-specific overexpression of tumor necrosis factor-alpha causes lethal myocarditis in transgenic mice. J Card Fail 1997;3:117–24. doi: 10.1016/s1071-9164(97)90045-2. [DOI] [PubMed] [Google Scholar]
- 80. Bryant D, Becker L, Richardson J, et al. Cardiac failure in transgenic mice with myocardial expression of tumor necrosis factor-alpha. Circulation 1998;97:1375–81. doi: 10.1161/01.cir.97.14.1375 [DOI] [PubMed] [Google Scholar]
- 81. Isoda K, Kamezawa Y, Tada N, et al. Myocardial hypertrophy in transgenic mice overexpressing human interleukin 1alpha. J Card Fail 2001;7:355–64. doi: 10.1054/jcaf.2001.28221 [DOI] [PubMed] [Google Scholar]
- 82. Schafer S, Viswanathan S, Widjaja AA, et al. IL-11 is a crucial determinant of cardiovascular fibrosis. Nature 2017;552:110–15. doi: 10.1038/nature24676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Widjaja AA, Singh BK, Adami E, et al. Inhibiting interleukin 11 signaling reduces hepatocyte death and liver fibrosis, inflammation, and steatosis in mouse models of nonalcoholic steatohepatitis. Gastroenterology 2019;157:777–792. doi: 10.1053/j.gastro.2019.05.002. [DOI] [PubMed] [Google Scholar]
- 84. Obana M, Maeda M, Takeda K, et al. Therapeutic activation of signal transducer and activator of transcription 3 by interleukin-11 ameliorates cardiac fibrosis after myocardial infarction. Circulation 2010;121:684–91. doi: 10.1161/CIRCULATIONAHA.109.893677. [DOI] [PubMed] [Google Scholar]
- 85. Matsui Y, Sadoshima J. Rapid upregulation of CTGF in cardiac myocytes by hypertrophic stimuli: implication for cardiac fibrosis and hypertrophy. J Mol Cell Cardiol 2004;37:477–81. doi: 10.1016/j.yjmcc.2004.05.012. [DOI] [PubMed] [Google Scholar]
- 86. Pontén A, Folestad EB, Pietras K, et al. Platelet-derived growth factor D induces cardiac fibrosis and proliferation of vascular smooth muscle cells in heart-specific transgenic mice. Circ Res 2005;97:1036–45. doi: 10.1161/01.RES.0000190590.31545.d4. [DOI] [PubMed] [Google Scholar]
- 87. Pontén A, Li XR, Thorén P, et al. Transgenic overexpression of platelet-derived growth factor-C in the mouse heart induces cardiac fibrosis, hypertrophy, and dilated cardiomyopathy. Am J Pathol 2003;163:673–82. doi: 10.1016/S0002-9440(10)63694-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Gao M, Shirato H, Miyasaka K, et al. Induction of growth factors in rat cardiac tissue by X irradiation. Radiat Res 2000;153:540–7. doi: 10.1667/0033-7587(2000)153[0540:iogfir]2.0.co;2 [DOI] [PubMed] [Google Scholar]
- 89. Frangogiannis NG. Cardiac fibrosis: Cell biological mechanisms, molecular pathways and therapeutic opportunities. Mol Aspects Med 2019;65:70–99. doi: 10.1016/j.mam.2018.07.001. [DOI] [PubMed] [Google Scholar]
- 90. Slezak J, Kura B, Babal P, et al. Potential markers and metabolic processes involved in the mechanism of radiation-induced heart injury. Can J Physiol Pharmacol 2017;95:1190–203. doi: 10.1139/cjpp-2017-0121. [DOI] [PubMed] [Google Scholar]
- 91. Khan R, Sheppard R. Fibrosis in heart disease: understanding the role of transforming growth factor-beta in cardiomyopathy, valvular disease and arrhythmia. Immunology 2006;118:10–24. doi: 10.1111/j.1365-2567.2006.02336.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Seeland U, Haeuseler C, Hinrichs R, et al. Myocardial fibrosis in transforming growth factor-beta(1) (TGF-beta(1)) transgenic mice is associated with inhibition of interstitial collagenase. Eur J Clin Invest 2002;32:295–303. doi: 10.1046/j.1365-2362.2002.00985.x. [DOI] [PubMed] [Google Scholar]
- 93. Sakata Y, Chancey AL, Divakaran VG, et al. Transforming growth factor-beta receptor antagonism attenuates myocardial fibrosis in mice with cardiac-restricted overexpression of tumor necrosis factor. Basic Res Cardiol 2008;103:60–8. doi: 10.1007/s00395-007-0689-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Heldin CH, Eriksson U, Ostman A. New members of the platelet-derived growth factor family of mitogens. Arch Biochem Biophys 2002;398:284–90. doi: 10.1006/abbi.2001.2707. [DOI] [PubMed] [Google Scholar]
- 95. Leipner C, Grün K, Müller A, et al. Imatinib mesylate attenuates fibrosis in coxsackievirus b3-induced chronic myocarditis. Cardiovasc Res 2008;79:118–26. doi: 10.1093/cvr/cvn063. [DOI] [PubMed] [Google Scholar]
- 96. Wang SN, Wilkes MC, Leof EB, et al. Imatinib mesylate blocks a non-Smad TGF-beta pathway and reduces renal fibrogenesis in vivo. FASEB J 2005;19:1–11. doi: 10.1096/fj.04-2370com. [DOI] [PubMed] [Google Scholar]
- 97. Dadrich M, Nicolay NH, Flechsig P, et al. Combined inhibition of TGFβ and PDGF signaling attenuates radiation-induced pulmonary fibrosis. Oncoimmunology 2016;5:e1123366. doi: 10.1080/2162402X.2015.1123366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98. Flechsig P, Dadrich M, Bickelhaupt S, et al. LY2109761 attenuates radiation-induced pulmonary murine fibrosis via reversal of TGF-β and BMP-associated proinflammatory and proangiogenic signals. Clin Cancer Res 2012;18:3616–27. doi: 10.1158/1078-0432.CCR-11-2855. [DOI] [PubMed] [Google Scholar]
- 99. Li ML, Abdollahi A, Gröne HJet al. Late treatment with imatinib mesylate ameliorates radiation-induced lung fibrosis in a mouse model. Radiat Oncol 2009;4:66. doi: 10.1186/1748-717X-4-66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100. Bickelhaupt S, Erbel C, Timke C, et al. Effects of CTGF blockade on attenuation and reversal of radiation-induced pulmonary fibrosis. J Natl Cancer Inst 2017;109. doi: 10.1093/jnci/djw339. [DOI] [PubMed] [Google Scholar]
- 101. Hawkins PG, Sun YL, Dess RT, et al. Circulating microRNAs as biomarkers of radiation-induced cardiac toxicity in non-small-cell lung cancer. J Cancer Res Clin Oncol 2019;145:1635–43. doi: 10.1007/s00432-019-02903-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102. Barjaktarovic Z, Shyla A, Azimzadeh O, et al. Ionising radiation induces persistent alterations in the cardiac mitochondrial function of C57BL/6 mice 40 weeks after local heart exposure. Radiother Oncol 2013;106:404–10. doi: 10.1016/j.radonc.2013.01.017. [DOI] [PubMed] [Google Scholar]
- 103. Sridharan V, Aykin-Burns N, Tripathi P, et al. Radiation-induced alterations in mitochondria of the rat heart. Radiat Res 2014;181:324–34. doi: 10.1667/RR13452.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104. Barjaktarovic Z, Schmaltz D, Shyla A, et al. Radiation-induced signaling results in mitochondrial impairment in mouse heart at 4 weeks after exposure to X-rays. PLoS One 2011;6:e27811. doi: 10.1371/journal.pone.0027811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105. Lo SC, Hannink M. PGAM5 tethers a ternary complex containing Keap1 and Nrf2 to mitochondria. Exp Cell Res 2008;314:1789–803. doi: 10.1016/j.yexcr.2008.02.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106. Mathew B, Jacobson JR, Siegler JH, et al. Role of migratory inhibition factor in age-related susceptibility to radiation lung injury via NF-E2-related factor-2 and antioxidant regulation. Am J Respir Cell Mol Biol 2013;49:269–78. doi: 10.1165/rcmb.2012-0291OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107. Travis EL, Rachakonda G, Zhou XH, et al. NRF2 deficiency reduces life span of mice administered thoracic irradiation. Free Radic Biol Med 2011;51:1175–83. doi: 10.1016/j.freeradbiomed.2011.05.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108. Chute JP. NRF2 mitigates radiation-induced hematopoietic death. J Clin Invest 2014;124:960–1. doi: 10.1172/JCI74143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109. Boerma M, Wang JR, Wondergem J, et al. Influence of mast cells on structural and functional manifestations of radiation-induced heart disease. Cancer Res 2005;65:3100–7. doi: 10.1158/0008-5472.CAN-04-4333. [DOI] [PubMed] [Google Scholar]
- 110. Kong P, Christia P, Frangogiannis NG. The pathogenesis of cardiac fibrosis. Cell Mol Life Sci 2014;71:549–74. doi: 10.1007/s00018-013-1349-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111. Haudek SB, Cheng JZ, Du J, et al. Monocytic fibroblast precursors mediate fibrosis in angiotensin-II-induced cardiac hypertrophy. J Mol Cell Cardiol 2010;49:499–507. doi: 10.1016/j.yjmcc.2010.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112. Sullivan KE, Black LD. The role of cardiac fibroblasts in extracellular matrix-mediated signaling during normal and pathological cardiac development. J Biomech Eng 2013;135:71001. doi: 10.1115/1.4024349 [DOI] [PubMed] [Google Scholar]
- 113. Wang L, Jiang P, He Y, et al. A novel mechanism of Smads/miR-675/TGFβR1 axis modulating the proliferation and remodeling of mouse cardiac fibroblasts. J Cell Physiol 2019;234:20275–85. doi: 10.1002/jcp.28628. [DOI] [PubMed] [Google Scholar]
- 114. Subramanian V, Borchard S, Azimzadeh O, et al. PPARα is necessary for radiation-induced activation of noncanonical TGFβ signaling in the heart. J Proteome Res 2018;17:1677–89. doi: 10.1021/acs.jproteome.8b00001. [DOI] [PubMed] [Google Scholar]
- 115. Yamamoto K, Masuyama T, Sakata Y, et al. Roles of renin-angiotensin and endothelin systems in development of diastolic heart failure in hypertensive hearts. Cardiovasc Res 2000;47:274–83. doi: 10.1016/s0008-6363(00)00101-2. [DOI] [PubMed] [Google Scholar]
- 116. Tsutamoto T, Wada A, Maeda K, et al. Transcardiac extraction of circulating endothelin-1 across the failing heart. Am J Cardiol 2000;86:524–8. doi: 10.1016/s0002-9149(00)01006-7. [DOI] [PubMed] [Google Scholar]
- 117. Feng YN, Zhang YY, Xiao H. AMPK and cardiac remodelling. Sci China Life Sci 2018;61:14–23. doi: 10.1007/s11427-017-9197-5. [DOI] [PubMed] [Google Scholar]
- 118. Sharma V, Dogra N, Saikia UN, et al. Transcriptional regulation of endothelial-to-mesenchymal transition in cardiac fibrosis: role of myocardin-related transcription factor A and activating transcription factor 3. Can J Physiol Pharmacol 2017;95:1263–70. doi: 10.1139/cjpp-2016-0634. [DOI] [PubMed] [Google Scholar]
- 119. Quijada P, Misra A, Velasquez LSet al. Pre-existing fibroblasts of epicardial origin are the primary source of pathological fibrosis in cardiac ischemia and aging. J Mol Cell Cardiol 2019;129:92–104. doi: 10.1016/j.yjmcc.2019.01.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120. Siwik DA, Colucci WS. Regulation of matrix metalloproteinases by cytokines and reactive oxygen/nitrogen species in the myocardium. Heart Fail Rev 2004;9:43–51. doi: 10.1023/B:HREV.0000011393.40674.13. [DOI] [PubMed] [Google Scholar]
- 121. Cheng M, Wu G, Song Y, et al. Celastrol-induced suppression of the MiR-21/ERK signalling pathway attenuates cardiac fibrosis and dysfunction. Cell Physiol Biochem 2016;38:1928–38. doi: 10.1159/000445554. [DOI] [PubMed] [Google Scholar]
- 122. Plenz G, Song ZF, Reichenberg S, et al. Left-ventricular expression of interleukin-6 messenger-RNA higher in idiopathic dilated than in ischemic cardiomyopathy. Thorac Cardiovasc Surg 1998;46:213–6. doi: 10.1055/s-2007-1010227. [DOI] [PubMed] [Google Scholar]
- 123. Tao H, Yang JJ, Shi KH, et al. Wnt signaling pathway in cardiac fibrosis: New insights and directions. Metab Clin Exp 2016;65:30–40. doi: 10.1016/j.metabol.2015.10.013. [DOI] [PubMed] [Google Scholar]
- 124. Byun J, Del Re DP, Zhai PY, et al. Yes-associated protein (YAP) mediates adaptive cardiac hypertrophy in response to pressure overload. J Biol Chem 2019;294:3603–17. doi: 10.1074/jbc.RA118.006123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125. Vainio LE, Szabó Z, Lin RZ, et al. Connective tissue growth factor inhibition enhances cardiac repair and limits fibrosis after myocardial infarction. JACC Basic Transl Sci 2019;4:83–94. doi: 10.1016/j.jacbts.2018.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126. Li C, Li J, Xue Ket al. MicroRNA-143-3p promotes human cardiac fibrosis via targeting sprouty3 after myocardial infarction. J Mol Cell Cardiol 2019;129:281–92. doi: 10.1016/j.yjmcc.2019.03.005. [DOI] [PubMed] [Google Scholar]
- 127. Akiyama-Uchida Y, Ashizawa N, Ohtsuru A, et al. Norepinephrine enhances fibrosis mediated by TGF-beta in cardiac fibroblasts. Hypertension 2002;40:148–54. doi: 10.1161/01.HYP.0000025443.61926.12. [DOI] [PubMed] [Google Scholar]
- 128. Li X, Sun MH, Men SZ, et al. The inflammatory transcription factor C/EBPβ plays a critical role in cardiac fibroblast differentiation and a rat model of cardiac fibrosis induced by autoimmune myocarditis. Int Heart J 2018;59:1389–97. doi: 10.1536/ihj.17-446. [DOI] [PubMed] [Google Scholar]
- 129. Sridharan V, Thomas CJ, Cao MH, et al. Effects of local irradiation combined with sunitinib on early remodeling, mitochondria, and oxidative stress in the rat heart. Radiother Oncol 2016;119:259–64. doi: 10.1016/j.radonc.2016.03.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130. Sridharan V, Tripathi P, Sharma S, et al. Effects of late administration of pentoxifylline and tocotrienols in an image-guided rat model of localized heart irradiation. PLoS One 2013;8:e68762. doi: 10.1371/journal.pone.0068762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131. O'Herron T, Lafferty J. Prophylactic use of colchicine in preventing radiation induced coronary artery disease. Med Hypotheses 2018;111:58–60. doi: 10.1016/j.mehy.2017.12.021. [DOI] [PubMed] [Google Scholar]
- 132. Kruse JJ, Strootman EG, Wondergem J. Effects of amifostine on radiation-induced cardiac damage. Acta Oncol 2003;42:4–9. doi: 10.1080/0891060310002168. [DOI] [PubMed] [Google Scholar]
- 133. Tokatli F, Uzal C, Doganay L, et al. The potential cardioprotective effects of amifostine in irradiated rats. Int J Radiat Oncol Biol Phys 2004;58:1228–34. doi: 10.1016/j.ijrobp.2003.09.071. [DOI] [PubMed] [Google Scholar]
- 134. Freitas RB, Boligon AA, Rovani BT, et al. Effect of black grape juice against heart damage from acute gamma TBI in rats. Molecules 2013;18:12154–67. doi: 10.3390/molecules181012154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135. Qian LR, Cao F, Cui JG, et al. The potential cardioprotective effects of hydrogen in irradiated mice. J Radiat Res 2010;51:741–7. doi: 10.1269/jrr.10093. [DOI] [PubMed] [Google Scholar]
- 136. Gürses I, Özeren M, Serin M, et al. Histopathological evaluation of melatonin as a protective agent in heart injury induced by radiation in a rat model. Pathol Res Pract 2014;210:863–71. doi: 10.1016/j.prp.2014.08.006. [DOI] [PubMed] [Google Scholar]
- 137. Fan ZG, Han Y, Ye YPet al. L-carnitine preserves cardiac function by activating p38 MAPK/Nrf2 signalling in hearts exposed to irradiation. Eur J Pharmacol 2017;804:7–12. doi: 10.1016/j.ejphar.2017.04.003. [DOI] [PubMed] [Google Scholar]
- 138. Zhang KY, He XY, Zhou YL, et al. Atorvastatin ameliorates radiation-induced cardiac fibrosis in rats. Radiat Res 2015;184:611–20. doi: 10.1667/RR14075.1. [DOI] [PubMed] [Google Scholar]
- 139. Lenarczyk M, Su JD, Haworth ST, et al. Simvastatin mitigates increases in risk factors for and the occurrence of cardiac disease following 10 Gy total body irradiation. Pharmacol Res Perspect 2015;3:e00145. doi: 10.1002/prp2.145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140. Hoving S, Heeneman S, Gijbels MJ, et al. Anti-inflammatory and anti-thrombotic intervention strategies using atorvastatin, clopidogrel and knock-down of CD40L do not modify radiation-induced atherosclerosis in ApoE null mice. Radiother Oncol 2011;101:100–8. doi: 10.1016/j.radonc.2011.09.019. [DOI] [PubMed] [Google Scholar]
- 141. Hoving S, Heeneman S, Gijbels MJ, et al. NO-donating aspirin and aspirin partially inhibit age-related atherosclerosis but not radiation-induced atherosclerosis in ApoE null mice. PLoS One 2010;5:e12874. doi: 10.1371/journal.pone.0012874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142. Veen SJ, Ghobadi G, Boer RA, et al. ACE inhibition attenuates radiation-induced cardiopulmonary damage. Radiother Oncol 2015;114:96–103. doi: 10.1016/j.radonc.2014.11.017. [DOI] [PubMed] [Google Scholar]
- 143. Yarom R, Harper IS, Wynchank S, et al. Effect of captopril on changes in rats' hearts induced by long-term irradiation. Radiat Res 1993;133:187–97. [PubMed] [Google Scholar]
- 144. Hoving S, Seemann I, Visser NL, et al. Thalidomide is not able to inhibit radiation-induced heart disease. Int J Radiat Biol 2013;89:685–91. doi: 10.3109/09553002.2013.788797. [DOI] [PubMed] [Google Scholar]
- 145. Rabender C, Mezzaroma E, Mauro AG, et al. IPW-5371 proves effective as a radiation countermeasure by mitigating radiation-induced late effects. Radiat Res 2016;186:478–88. doi: 10.1667/RR14403.2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146. Gao S, Zhao ZY, Wu R, et al. Bone marrow mesenchymal stem cell transplantation improves radiation-induced heart injury through DNA damage repair in rat model. Radiat Environ Biophys 2017;56:63–77. doi: 10.1007/s00411-016-0675-0. [DOI] [PubMed] [Google Scholar]
- 147. Nicolay NH, Lopez Perez R, Saffrich R, et al. Radio-resistant mesenchymal stem cells: mechanisms of resistance and potential implications for the clinic. Oncotarget 2015;6:19366–80. doi: 10.18632/oncotarget.4358 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148. Nicolay NH, Lopez Perez R, Debus J, et al. Mesenchymal stem cells—A new hope for radiotherapy-induced tissue damage? Cancer Lett 2015;366:133–40. doi: 10.1016/j.canlet.2015.06.012. [DOI] [PubMed] [Google Scholar]
- 149. Rühle A, Huber PE, Saffrich R, et al. The current understanding of mesenchymal stem cells as potential attenuators of chemotherapy-induced toxicity. Int J Cancer 2018;143:2628–39. doi: 10.1002/ijc.31619. [DOI] [PubMed] [Google Scholar]
- 150. Rühle A, Lopez Perez R, Zou BW, et al. The therapeutic potential of mesenchymal stromal cells in the treatment of chemotherapy-induced tissue damage. Stem Cell Rev Rep 2019;15:356–73. doi: 10.1007/s12015-019-09886-3 [DOI] [PubMed] [Google Scholar]
- 151. Rühle A, Xia O, Perez RL, et al. The radiation resistance of human multipotent mesenchymal stromal cells is independent of their tissue of origin. Int J Radiat Oncol Biol Phys 2018;100:1259–69. doi: 10.1016/j.ijrobp.2018.01.015. [DOI] [PubMed] [Google Scholar]
- 152. Sridharan V, Seawright JW, Antonawich FJ, et al. Late administration of a palladium lipoic acid complex (POLY-MVA) modifies cardiac mitochondria but not functional or structural manifestations of radiation-induced heart disease in a rat model. Radiat Res 2017;187:361–6. doi: 10.1667/RR14643.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153. Wang J, Wu YJ, Yuan F, et al. Chronic intermittent hypobaric hypoxia attenuates radiation induced heart damage in rats. Life Sci 2016;160:57–63. doi: 10.1016/j.lfs.2016.07.002. [DOI] [PubMed] [Google Scholar]
- 154. Zhang ZY, Li Y, Li R, et al. Tetrahydrobiopterin protects against radiation-induced growth inhibition in H9c2 cardiomyocytes. Chin Med J 2016;129:2733–40. doi: 10.4103/0366-6999.193455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155. Farhood B, Aliasgharzadeh A, Amini P, et al. Radiation-induced dual oxidase upregulation in rat heart tissues: protective effect of melatonin. Medicina (Kaunas) 2019;55:E317. doi: 10.3390/medicina55070317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156. Gu J, Liu YQ, Wu HY, et al. Huangqi shengmai yin protects against radiation-induced cardiac fibrosis injury by regulating the TGF-β1/smads and MMPs. Evid Based Complement Alternat Med 2019;2019:1358469. doi: 10.1155/2019/1358469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157. Balanescu DV, Donisan T, Dayah T, et al. Refractory radiation-induced coronary artery disease: mapping the path and Guiding treatment with optical coherence tomography. Int J Cardiovasc Imaging 2019;35:759–60. doi: 10.1007/s10554-019-01533-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158. Mousavi N, Nohria A. Radiation-induced cardiovascular disease. Curr Treat Options Cardiovasc Med 2013;15:507–17. doi: 10.1007/s11936-013-0259-0. [DOI] [PubMed] [Google Scholar]
- 159. Moat NE, Ludman P, Belder MA, et al. Long-term outcomes after transcatheter aortic valve implantation in high-risk patients with severe aortic stenosis: the U.K. TAVI (United Kingdom Transcatheter Aortic Valve Implantation) Registry. J Am Coll Cardiol 2011;58:2130–8. doi: 10.1016/j.jacc.2011.08.050. [DOI] [PubMed] [Google Scholar]
- 160. Gharagozloo F, Clements IP, Mullany CJ. Use of the internal mammary artery for myocardial revascularization in a patient with radiation-induced coronary artery disease. Mayo Clin Proc 1992;67:1081–4. doi: 10.1016/s0025-6196(12)61124-0. [DOI] [PubMed] [Google Scholar]
- 161. McConkey H, Zhao ZG, Redwood S, et al. Timing and mode of intervention for patients with left sided valvular heart disease: an individualized approach. Precis Clin Med 2018;1:118–28. doi: 10.1093/pcmedi/pby017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162. DePasquale EC, Nasir K, Jacoby DL. Outcomes of adults with restrictive cardiomyopathy after heart transplantation. J Heart Lung Transplant 2012;31:1269–75. doi: 10.1016/j.healun.2012.09.018. [DOI] [PubMed] [Google Scholar]
- 163. Al-Kindi SG, Oliveira GH. Heart transplantation outcomes in radiation-induced restrictive cardiomyopathy. J Card Fail 2016;22:475–8. doi: 10.1016/j.cardfail.2016.03.014. [DOI] [PubMed] [Google Scholar]
- 164. Saxena P, Joyce LD, Daly RC, et al. Cardiac transplantation for radiation-induced cardiomyopathy: the mayo clinic experience. Ann Thorac Surg 2014;98:2115–21. doi: 10.1016/j.athoracsur.2014.06.056. [DOI] [PubMed] [Google Scholar]
- 165. Nieto DC. Advanced interventional therapy for radiation-induced cardiovascular disease. Tex Heart Inst J 2016;43:315-7. doi: 10.14503/THIJ-16-5806 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166. Latib A, Montorfano M, Figini F, et al. Percutaneous valve replacement in a young adult for radiation-induced aortic stenosis. J Cardiovasc Med (Hagerstown) 2012;13:397–8. doi: 10.2459/JCM.0b013e3283517c4a. [DOI] [PubMed] [Google Scholar]


