Abstract
Background:
Genetic variability in LRRK2 has been unequivocally established as a major risk factor for familial and sporadic forms of PD in ethnically diverse populations.
Objectives:
To resolve the role of LRRK2 in the Indian population.
Methods:
We performed targeted resequencing of the LRRK2 locus in 288 cases and 298 controls and resolved the haplotypic structure of LRRK2 in a combined cohort of 800 cases and 402 controls in the Indian population. We assessed the frequency of novel missense variants in the white and East Asian population by leveraging exome sequencing and densely genotype data, respectively. We did computational modeling and biochemical approach to infer the potential role of novel variants impacting the LRRK2 protein function. Finally, we assessed the phosphorylation activity of identified novel coding variants in the LRRK2 gene.
Results:
We identified four novel missense variants with frequency ranging from 0.0008% to 0.002% specific for the Indian population, encompassing armadillo and kinase domains of the LRRK2 protein. A common genetic variability within LRRK2 may contribute to increased risk, but it was nonsignificant after correcting for multiple testing, because of small cohort size. The computational modeling showed destabilizing effect on the LRRK2 function. In comparison to the wild-type, the kinase domain variant showed 4-fold increase in the kinase activity.
Conclusions:
Our study, for the first time, identified novel missense variants for LRRK2, specific for the Indian population, and showed that a novel missense variant in the kinase domain modifies kinase activity in vitro.
Keywords: neurodegeneration, Parkinson’s disease, LRRK2
Mutations in the leucine-rich repeat kinase 2 (LRRK2) gene are the most commonly identified genetic variations in familial Parkinson’s disease (PD), with seven definite pathogenic mutations cosegregating with the disease in well-defined pedigrees.1,2 Genetic variants in the LRRK2 gene arc also increasingly recognized as risk factors associated with apparently sporadic PD.3,4 The LRRK2 gene has elicited much interest both as a novel disease marker (because LRRK2-related PD closely mimics sporadic PD phenotypically) and as a potential therapeutic target in PD, owing to the putative role for kinase inhibitors.5,6 The distribution of LRRK2 variants differs significantly among genetically diverse populations.4,7-11 The most commonly reported LRRK2 mutation, G2019S, accounts for ~41% of PD in North African Arab-Berbers, ~31% in Ashkenazi Jews with familial PD2, and is under-represented in the Asian PD population.7,12 Haplotype linkage disequilibrium studies suggest a common founder effect in these populations.13,14 Another LRRK2 variant, the R1441C/G/H, has been primarily reported in Basque cohorts with familial PD, whereas the I2020T substitution was identified in a Japanese pedigree.9,15 The low-penetrance PD risk variants in LRRK2, identified by genetic association studies, are also ethnogeographically restricted. For instance, the G2385R variant is a risk factor in Asian subjects with PD, particularly ethnic Chinese with a population-attributable risk of around 4%.16 Similarly, the R1628P, A419V variants were associated with PD in Asian cohorts, whereas the M1646 T conferred risk in whites.4,8,17,18 In a large East Asian cohort assessing nonsynonymous coding variations in the known PD genes, only variations in the LRRK2 were found to be enriched in PD patients.19,20 Population-specific differences in the minor allele frequencies of the LRRK2 variants may contribute toward the relative significance of a particular variant in genetically diverse populations.21 In this context, studying genetically diverse populations is important to identify hitherto uncharacterized variations in the LRRK2 gene. The Indian population is under-represented in studies of human genetic variation in general and specifically in PD, and the variations in the Indian genome are poorly represented by ethnically different populations.22 In addition to the characterization of novel disease-associated variants in a global context, there may be population-specific benefits in the identification of population-specific genetic risk variants, particularly in the context of LRRK2 and its role as a putative therapeutic target. Specifically, in India, the G2019S substitution was found to be an uncommon cause of familial or sporadic PD.23,24 The G2019S, R1441G/C/H, 12012T, and 12020T were also uncommon in a large cohort of Indian PD patients, including probands from autosomal-dominant pedigrees.25 The common mutations, as well as the Asian risk-associated variant, G2385R, were not identified in an East Indian cohort and a haplotype-tagging single-nucleotide polymorphism (SNP) approach failed to identify a contributory role for variations in LRRK2 in this cohort.26 Taken together, these data suggest that as-yet-unidentified common and rare genetic variations in the LRRK2 may be contributing to PD risk in the Indian population. Indeed, linkage disequilibrium evidence suggests that PD-associated LRRK2 variations in the Indian genome are likely to parallel those reported from the white, rather than the Japanese, cohorts.27 We conducted a clinicogenetic association study to identify such PD-associated genomic variations in the LRRK2 gene in the Indian population.
Materials and Methods
We formed a trilateral consortium, Lux-GIANT (Luxembourg German Indian Alliance on Neurodegenerative diseases and therapeutics), to understand the genetic causes of neurodegenerative diseases, in particular PD and atypical parkinsonian syndromes such as MSA, PSP, dementia with Lewy bodies, and cortical basal syndrome, in the Indian population, which has so far been under-represented in genomic research. The PD cohort consisted of 800 cases and 402 controls. The diagnosis of PD was made by experienced movement disorder specialists and based on the UK Parkinson’s Disease Society Brain Bank criteria.28 Mean age at onset was 49.9 years (range, 24–80). All the clinical data were prospectively collected (age of onset, duration of symptoms, neurological examination findings, non-neurological examination findings, treatment details, and response to treatment) both for sporadic and familial cases. All apparently sporadic cases were interviewed in detail to identify a positive family history. Data related to both sporadic and familial PD were compiled for research purposes in the data bank of the Movement Disorder Clinic. Ethnically matched healthy controls, unrelated to patients, were also regularly recruited to build a comprehensive control group for the study. Before inclusion in the study, the controls were examined for any neurological disorders and queried for any family history of neurodegenerative disorders. The institutional ethics committee approved the study. All participants signed an informed consent.
Resequencing of LRRK2 Locus
Resequencing was performed at the Core facility of Applied Transcriptomics and Genomics at the Institute of Medical Genetics and Applied Genomics, University Hospital of Tübingen, (Tübingen, Germany). A total of 288 cases and 298 controls (post-QC [quality control]) were selected for resequencing. A Nextera rapid capture custom enrichment was designed (Illumina, San Diego, CA) targeting 216 kilobase pair (kbp) of the LRRK2 locus, including introns and the 5’ and 3’ untranslated regions. Enrichment was performed with two amplification cycles according to the manufacturer’s protocol. Sequencing was performed on a NextSeq500 producing 75 base pairs paired-end reads (NextSeq MidOutput Kit 150 cycles; Illumina). Raw read data were analyzed using an in-house analysis pipeline consisting of the following tools in the given order: SeqPurge for adapter trimming, BWA mem for read mapping, samblaster for duplicate removal, ABRA for indel realignment, freebayes for variant calling, and SnpEff/SnpSift29-32 for variant annotation. In order to find new risk factor variants for PD, we filtered the raw variant using two criteria: (1) The variant must be a missense variant, and (2) the variant should not be present in known public databases such as 1000 genomes, Exome variant server, ExAC, and GnomAD, including subpopulations. For all analyses, the hg19 genome was used as a reference.
International Parkinson Disease Genomics Consortium and COURAGE-PD Cohort
To compare the frequency of novel variants in the white and East Asian population, we leveraged the genetic data resource from the International Parkinson disease Genomics Consortium (IPDGC) and COURAGE-PD (Comprehensive Unbiased Risk Factor Assessment for Genetics and Environment in Parkinson’s Disease; http://www.neurodegenerationresearch.eu/tr/publication/couragc-pd/). In brief, we first used the whole-exome sequencing (WES) data set from the IPDGC, which includes 1,167 PD cases and 1,685 controls (post-QC) to determine ancestry. Additionally, we also leveraged NeuroX data from the IPDGC.33 In brief, Standard GATK filter steps were applied, together with a minimum genotype quality Phred-score of 20 and depth of 8, to only select high-quality variants. The NeuroX data set encompasses 6,801 PD cases and 5,970 controls (post-QC) or European ancestry. Over-lapping samples with the WES data set were excluded. For individual QC in both the WES and the NeuroX data sets, samples were removed when showing sex ambiguity, dubious heterozygosity/genotype calls, evidence of relatedness, or being a population outlier. Further details of the cohort were described elsewhere.34,35 For COURAGE-PD data, we leveraged the results from targeted resequencing of European (800 PD cases and 800 controls) and Korean (690 PD patients and 690 controls) cohorts.36,37 In brief, DNA pools were generated, each representing 10 individuals, and target enrichment was performed using a 5 Mb HaloPlex (Agilent Technologies, Santa Clara, CA) kit designed to capture 776 targets, including genome-wide association study (GWAS) regions, Mendelian genes, and candidate loci in PD. Libraries were sequenced on an Illumina HiSeq instrument, followed by bioinformatic processing including alignment with bwa 0.5.9 variant calling, filtering and recalibration with GATK 3.4, and annotation with ANNOVAR version 2015 Jun 17.
Common Variants and LRRK2 Variability
A two-stage strategy was used to assess the role of genetic variability of LRRK2. In the first stage, a total of 288 cases and 298 controls were used to define common genetic variability. A standard QC pipeline, as implemented in PLINK software (v.1.9), was applied.38 For the screening stage, resequencing cohort samples with a call rate less than 90% (n = 48 cases and 14 control subjects), SNPs with a minor allele frequency less than 0.05 (n = 1841), SNPs with significant departure from Hardy–Weinberg equilibrium (P < 0.001; n = 31), SNPs with a missingness rate greater than 5% (n = 0), or SNPs with inaccurate clustering (n = 0) were excluded from analysis. A total of 523 SNPs were selected. For stage 1 analysis, a trend model was used to test for the association for each SNP. Using Haploview, we determined the linkage disequilibrium (LD) structure of LRRK2 in the Indian population. A multimarker strategy, as implemented in Haploview, was used to define tagSNPs. A cutoff of r2 > 0.8 was used to select tagSNPs. For the replication stage, a threshold of 1 × 10−5 (0.05/523 = 1 × 10−5) was used to select SNPs for replication stage. For the replication stage, a total of 550 cases and 125 controls were selected for genotyping. The genotyping core was blinded to case–control status. Genotyping was performed using a matrix-assisted laser desorption/ionization time-of-flight mass spectrometry on a MassArray System (Agena Bioscience, San Diego, CA). Cleaned extension products were analyzed by a mass spectrometer (Bruker Daltonics Inc., Billerica, MA), and peaks were identified using MassArray Typer software (version 4.0.2.5; Agena Bioscience). Assays were designed by the AssayDesigner software (version 4.0; Agena Bioscience) with the default parameters for the iPLEX Gold chemistry and the Human GenoTyping Tools, ProxSNP and PreXTEND (Agena Bioscience). All variants were genotyped in one multiplex assay. An experienced investigator blinded to case or control status of the samples visually checked genotype clustering. The average call rate of the variants was 97%. For combined analysis, a logistic regression model adjusted for age, sex was used to test for association. A Bonferroni correction was used to control for multiple testing. Power calculations showed that with 800 cases and 400 controls, our study would have at least 44% power to detect an allele-based odds ratio of 1.15 for minor allele frequencies of 15% or higher for alpha = 0.05. Power would be only 5% for a minor allele frequency of 2% and odds ratio of 1.15, but it would be 37% for the same minor allele frequency of 2% and odds ratio of 3.0.
Computational Modeling of Novel Missense Variants
We utilized a preminimized structural model of the Armadillo domain that we previously generated to predict the full-length, quaternary structure of LRRK2.39 For the three mutants, we performed energy calculations by using the ddG_monomer protocol from Rosetta software using standard settings.40 A total of 50 cycles of energy calculations, for both the wild-type (WT) and mutant forms, were carried out both in low-resolution (i.e., with a fixed backbone) and high-resolution (i.e., allowing backbone degrees of freedom) modes. ΔΔG values were finally obtained by comparing the total energies of the top models generated for the mutant and WT forms and are expressed in Rosetta energy units. Finally, the pathogenicity of the variants was calculated using the Combined Annotation Dependent Depletion (CADD) score. A threshold greater than 13 was used to define the pathogenicity of variants.
Biochemical Analysis
LRRK2 kinase activity has been determined by cell-based Rab-assays, which have been adapted based on previous work.41,42 Briefly, for this purpose, Strep/Flag-tagged LRRK2 variants, generated by site-directed mutagenesis, have been coexpressed with Elag/HA-tagged Rab10 in HEK293T cells. For this purpose, the human Rab10 CDS was subcloned in an in-house FLAG-HA pcDNA3.0-based Gateway destination vector. The generation of LRRK2 expression construct has been described elsewhere.43 Two days after transfection with a polyethylenimine-based reagent, cells were lysed in lysis buffer (30 mM of Tris–HCl [pH 7.4], 150 mM of NaCl, 0.5% Nonidet-P40, protease inhibitor cocktail [Roche, Indianapolis, IN], and phosphatase inhibitor cocktails I II and III [Sigma-Aldrich, St. Louis, MO]). Tagged proteins were purified by Anti-FLAG M2 Affinity Gel (Sigma-Aldrich) and eluted from the affinity resin by the FLAG peptide (Sigma-Aldrich), as previously described.44 Samples were analyzed by sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS-PAGE). The amount of the FLAG/HA-Rab10 protein was determined by densitometric comparison of the corresponding protein bands with a bovine serum albumin (BSA) standard visible after staining of the polyacrylamide gel with colloidal Coomassie using ImageJ (v1.51 rv1.46r; National Institutes of Health, Bethesda, MD). For western blot analysis, samples were adjusted to equal amounts of Rab10. Polyvinylidene difluoride membranes were blocked in 1% polyvinylpyrrolidone (Sigma-Aldrich) and 0.5% BSA in Tris-buffered saline with Tween 20. Rab-10 phosphorylation was determined by probing with a monoclonal motif-specific antibody (RxxS/T, clone 110B7E, 1:10,000; Cell Signaling Technology, Peabody, MA) and a secondary horseradish peroxidase–conjugated antirabbit antibody (1:15,000; Jackson ImmunoResearch, West Grove, PA) in blocking buffer. Detection of signals was performed with ECL+ (GE Healthcare, Waukesha, WI).
Statistical Analysis
Densitometric analysis of signals was performed with ImageJ (National Institutes of Health). Statistical analysis (analysis of variance and Tukey’s post-hoc test) has been performed by in-house R-scripts.
Results
Resequencing of LRRK2 Locus
Overall, for all samples, an average sequencing depth of 244× was achieved. On average, 97.85% of the 366-kbp target region was covered at least 20×. Of the 599 samples (298 cases and 301 controls), nine were found to be of low quality (average depth on the target region less than 50× or less than 93% of the target region covered at least 20×). These 10 samples, and three more samples that were found to he duplicated, were excluded from further analysis. Thus, a total of 288 PD cases and 298 controls were available for the variant analysis. On average, each sample yielded 800 variants, resulting in approximately 472,000 variants overall. Using the above filtering criteria, we were able to identify four novel variants (Table 1). These variants observed on exons 11, 12, 14, and 39 encompassed the armadillo and kinase domains of LRRK2. Frequency of novel variants ranged from 0.0008% to 0.002%, suggesting that these variants are a rare cause of PD in the Indian population (Table 1). Using exome and an array-based cohort from the IPDGC and targeted resequencing cohorts from COURAGE-PD respectively, we observed that these variants are not present in the white and South Korean populations, further suggesting that these variants are population specific (Table 1).
TABLE 1.
Novel rare variants in the Indian population
| Gene | Chromosomal Position |
Variant Type |
Amino Acid Change |
CDNA Change |
REFSEQ ID | EXDN | Public Databases |
CADD Score |
IPDGC |
COURAGE-PD |
Rare Variants in Overall Cohorts (1202) |
||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| dbSNP | 1000 g | ExAC | GME | ExAC_ SAS |
1000 g_ SAS |
Cases (N = 6,801) |
Controls (N = 5,970) |
Cases (N = 1,490) |
Controls (N = 1,490) |
Cases (freq) |
Controls | ||||||||
| LRRK2 | chr12: 40646719 | MISSENSE | Ala397Thr | C.1189G > A | NM_198578.3 | 11 | 0 | 0 | 0 | 0 | 0 | 0 | 23.40 | 0 | 0 | 0 | 0 | 2 (0.001) | 0 |
| LRRK2 | chr12: 40651175 | MISSENSE | Gly472Arg | C.1414G > A | NM_198578.3 | 12 | 0 | 0 | 0 | 0 | 0 | 0 | 31.00 | 0 | 0 | 0 | 0 | 1 (0.0008) | 0 |
| LRRK2 | Chr12: 40657696 | MISSENSE | Leu550Trp | C.1649 T > G | NM_198578.3 | 14 | 0 | 0 | 0 | 0 | 0 | 0 | 27.50 | 0 | 0 | 0 | 0 | 3 (0.002) | 0 |
| LRRK2 | chr12: 40722165 | MISSENSE | Asp1887Gly | c.5660A > G | NM_198578.3 | 39 | 0 | 0 | 0 | 0 | 0 | 0 | 25.30 | 0 | 0 | 0 | 0 | 2 (0.001) | 0 |
Abbreviations: ExAC, Exome Aggregation Consortium; ExAC SAS, Exome Aggregation Consortium South Asian; 1000 g, 1000 genomes; 1000 g SAS, 1000 genomes South Asian; GME, Greater Middle East
Common Genetic Variability and LRRK2 Risk in Indian Population
A total of 523 SNPs were selected for the first-stage analysis. A trend model was used to test for association. Four large LD blocks with large D’ values were defined. In addition, few small LD blocks covering the upstream and downstream regions of the LRRK2 were identified (Supporting Information Figure S1). In the stage 1 analysis, a number of markers showed a significant association with disease status, but were found to be not significant after adjustment for multiple testing (Table 2). We selected, on average, one SNP per haplotype blocks for validation. For the replication stage, SNPs with the lowest P value could not be replicated, though we observed a trend for the association. Lack of confirmation could be attributed to the small cohort size. Previously published studies identified a protective haplotype (N551 K-R1398H-K1423 K) in the white population.4 We observed all three markers in our resequencing data. The haplotypic association showed a minor haplotype frequency of 0.0041% in cases and 0.0027% in controls. In contrast to the protective effect of the haplotype, our study observed an increased risk, though the haplotype association was not significant in our data set (odds ratio = 1.52; P = 0.711).4,21 The smaller sample size of our cohort is a plausible reason for not obtaining a significant association. Interestingly, we observed R1628P only in 1 case and did not identify any G2385R carrier, indicating that East Asian–specific variants are rare in the Indian population.
TABLE 2.
Common genetic variability and LRRK2 Risk in the Indian population
| Gene | Common SNPS | Chromosomal Position | Stage 1 (Cases = 250) (Controls = 277) |
Stage 2 (Cases = 550) (Controls = 125) |
Combined Analysis (Cases = 800) (Controls = 402) |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Odds Ratio | Confidence Interval |
p Values | Odds Ratio | Confidence Interval |
p Values | Odds Ratio | Confidence Interval |
P Values (UNADJ) | ||||||
| L95 | U95 | L95 | U95 | L95 | U95 | |||||||||
| LRRK2 | rs2708404 | chr12:40575630 | 1.484 | 1.108 | 1.988 | 0.007909 | 0.9135 | 0.6517 | 1.28 | 0.5992 | 1.281 | 0.9612 | 1.708 | 0.09104 |
| LRRK2 | rs79410089 | chr12:40592064 | 1.543 | 1.126 | 2.115 | 0.00675 | 0.8677 | 0.605 | 1.245 | 0.4404 | 1.216 | 0.891 | 1.659 | 0.2181 |
| LRRK2 | rs4768221 | chr12:40597545 | 0.7298 | 0.5753 | 0.9257 | 0.009333 | 0.931 | 0.7054 | 1.229 | 0.6135 | 0.928 | 0.7348 | 1.172 | 0.5302 |
| LRRK2 | rs4767967 | Chr12:40597869 | 0.7349 | 0.5793 | 0.9322 | 0.01105 | 0.931 | 0.7054 | 1.229 | 0.6135 | 0.9278 | 0.7347 | 1.172 | 0.5294 |
| LRRK2 | rs10878220 | chr12:40608880 | 0.7146 | 0.5634 | 0.9063 | 0.005529 | 0.9114 | 0.6909 | 1.202 | 0.5112 | 0.9255 | 0.7335 | 1.168 | 0.5138 |
| LRRK2 | rs12827541 | chr12:40609979 | 0.7236 | 0.5703 | 0.9181 | 0.007667 | 0.8844 | 0.6704 | 1.167 | 0.3849 | 0.9003 | 0.7117 | 1.139 | 0.3812 |
| LRRK2 | rs10878224 | chr12:40614601 | 0.7287 | 0.5743 | 0.9246 | 0.009107 | 0.9137 | 0.6922 | 1.206 | 0.5238 | 0.9172 | 0.7246 | 1.161 | 0.4725 |
| LRRK2 | rs10748014 | chr12:40615069 | 0.7333 | 0.5778 | 0.9306 | 0.01065 | 0.9287 | 0.7034 | 1.226 | 0.6019 | 0.9158 | 0.7232 | 1.16 | 0.4657 |
| LRRK2 | rs7973058 | Chr12:40708845 | 0.7066 | 0.5558 | 0.8985 | 0.004542 | 1.071 | 0.8029 | 1.428 | 0.6421 | 0.8526 | 0.6763 | 1.075 | 0.1773 |
| LRRK2 | rs7302841 | chr12:40729655 | 0.7073 | 0.5564 | 0.8991 | 0.004611 | 1.04 | 0.78 | 1.386 | 0.791 | 0.8432 | 0.6696 | 1.062 | 0.1472 |
| LRRK2 | rs11609433 | Chr12:40764769 | 0.716 | 0.5608 | 0.9141 | 0.007254 | 1.016 | 0.7606 | 1.358 | 0.9129 | 0.8752 | 0.6911 | 1.108 | 0.2686 |
| LRRK2 | rs17444605 | chr12:40787912 | 0.7235 | 0.5718 | 0.9155 | 0.006973 | 1.075 | 0.8152 | 1.416 | 0.6098 | 0.8845 | 0.7018 | 1.115 | 0.2988 |
| LRRK2 | rs10784589 | chr12:40791214 | 0.7054 | 0.5545 | 0.8972 | 0.004405 | 0.9384 | 0.706 | 1.247 | 0.6615 | 0.799 | 0.6323 | 1.01 | 0.06024 |
Clincial Features
All PD patients who carried potential pathogenic variants (Ala397Thr, Gly472Arg, Lcu550Trp, and Asp1887G) were clinically diagnosed with PD. None of the affected individuals had a positive family history. Affected individuals exhibit classical symptoms of PD (resting tremor, bradykinesia, rigidity) (Table 3). Non-motor symptoms were present in all the PD patients carrying a pathogenic variant (Table 3).
TABLE 3.
Clinical description of carriers of novel variants in the Indian population
| Serial No. | Sex |
LRRK2 Carriers |
Age of Onset in Years |
Duration of Illness in Years |
Motor Phenotype | Time to l-dopa in Years |
Time to Motor Fluctuations in years |
Time to Dyskinesias In Years |
Additional Features | CT Brain |
MRI Brain |
Surgical Treatment | Response to Surgery |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | F | Ala397Thr | 56 | 9 | Rigidity, bradykinesia | 1 | 4 | 8 | Cognitive dysfunction | — | Normal | Right pallidotomy | Excellent |
| 2 | F | Ala397Thr | 54 | 16 | Rigidity, bradykinesia | 2 | 4 | 5 | Urinary urgency, constipation, anxiety, depression | — | Normal | Bilateral STN DBS | Excellent |
| 3 | F | Gly472Arg | 56 | 15 | Tremor, rigidity, bradykinesia | 1 | 5 | 9 | Constipation, RBD, urinary urgency, depression, anxiety | Normal | — | Bilateral STN DBS | Excellent |
| 4 | F | Leu550Trp | 47 | 17 | Rigidity, bradykinesia | 3 | 1 | 1.5 | Nil | — | Normal | — | — |
| 5 | M | Leu550Trp | 45 | 27 | Tremor, rigidity, bradykinesia | 9 | 1 | 3 | Constipation, urinary urgency, excessive day time somnolence | — | — | — | — |
| 6 | F | Leu550Trp | 58 | 9 | Rigidity, bradykinesia | 2 | 2 | Nil | insomnia | — | Normal | — | — |
| 7 | F | Asp1887Gly | 48 | 26 | Rigidity, bradykinesia | 1 | 4 | 4 | Insomnia, dysarthria, blepharospasm | — | Normal | Left pallidotomy, Right subthalamotomy, right pallidotomy | Excellent |
| 8 | M | Asp1887Gly | 50 | 9 | Rigidity, bradykinesia | 1 | 0.5 | 1 | Erectile dysfunction, mania, hypersexuality, insomnia | normal | — | — | — |
Abbreviation: RBD, REM sleep behavior disorder.
Computational Modeling of Rare Variants and LRRK2 Function
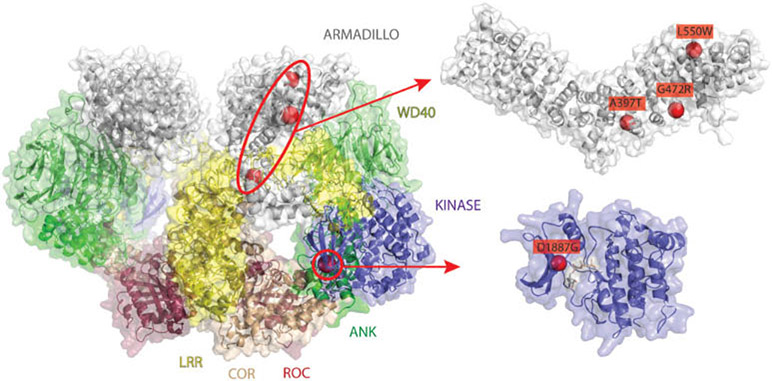
Mutations Ala397Thr, Gly472Arg, and Leu550Trp are found within the C-terminal half of LRRK2 Armadillo domain and in close contact with the leucine-rich repeat (LRR) domain interaction interface (Fig. 1). To better understand the functional consequences of these rare variants, we performed energy calculations using the structure of the armadillo domain alone (see Materials and Methods). This approach predicts the stabilizing or destabilizing effect of a mutation as respectively negative or positive differences in free energy (or ΔΔG) between the mutant and the WT protein. The three mutations are similarly predicted to have a destabilizing effect on the armadillo domain structure (Supporting Information Table S1 with prediction energies).
FIG. 1.
Novel PD variants represented on LRRK2 quaternary structure: (a) overview of variants, represented as red spheres, on the structural model of dimeric LRRK2 (ref 27357661), represented as cartoon and surface. The following domain-specific color scheme is used: Armadillo in white, Ankyrin (ANK) in green, LRR in yellow, Roc in raspberry, COR in wheat, Kinase in slate, and WD40 in lime; (B) zoom of mutations affecting the Armadillo domain; (C) zoom of the mutation affecting the kinase domain. The ATP analogue, AppCp, fitted from the Roco4 Kinase domain (PDB ID: 4F0F), is shown as gray sticks; (D) sequence conservation logo of the G-loop region obtained from Pfam. Kinase family seed alignments and visualized with Jalview.
Given that mutations Gly472Arg, Leu550Trp are spatially proximal to the interaction interface with the LRR domain in the LRRK2 quaternary structure model (Fig. 1), it is possible that their downstream effect is a perturbation of intramolecular regulatory interactions mediated by LRRK2’s N-terminal. Interestingly, the mutation which is predicted to cause the most detrimental effect, that is, Leu550Trp (ΔΔG = 6.579 REU), is adjacent to a motif region (i.e., amino acids [a.a.] 538–547) that has been recently described to mediate the interaction with Fas-associated protein with death domain (FADD), thus linking LRRK2 signaling to apoptotic pathways.45 Hence, by similarly altering the armadillo domain structure, these mutations might affect, either directly or indirectly, LRRK2 intramolecular regulatory mechanisms or mediated interactions and downstream signaling pathways.
Pathogenic LRRK2 Variants Have Been Shown to Increase Kinase Activity
In vitro assays demonstrated a robust increase in autophosphorylation for pathogenic variants in the kinase domain, at least for the most prominent Gly2019Ser variant, which also shows a consistent increase in LRRKtide assays over various studies.45-47 In addition, the recent identification of a subset of Rab proteins as physiological substrates by a mass-spectrometry–based screen allowed the development of cell-based LRRK2 activity assays.41,42,48
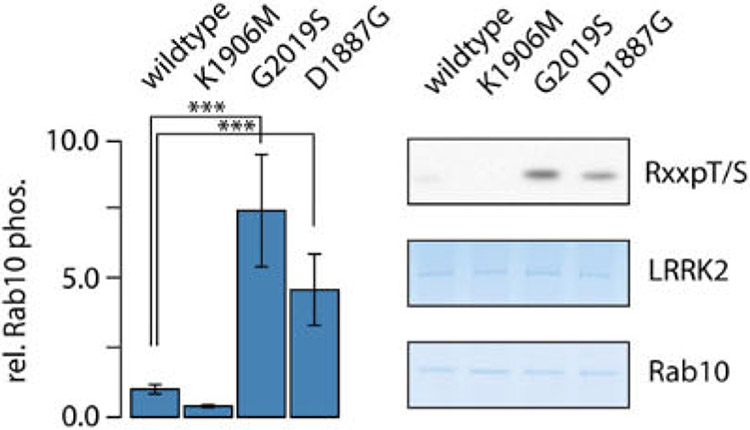
Like Gly2019Ser, one of the variants, Asp1887Gly, falls in the LRRK2 kinase domain. Whereas Gly2019ser alters the DYG motif preceding the activation loop, the novel variant is localized in the Glycine-rich loop (G-loop), a conserved motif which is involved in adenosine triphosphate (ATP) binding and therefore critical for the phospho-transfer activity of a kinase.49 It consists of three conserved glycines (GxGxxG motif), corresponding to the LRRK2 residues Gly1886, Gly1888, and Gly1891. The new PD variant, Asp1887Gly, was found just downstream of the first conserved Glycine residue. Given the importance of the G-loop in the catalytic process of a kinase, we wondered whether this variant has an impact on LRRK2 kinase activity. In a cell-based assay, the novel Asp1887Gly LRRK2 G-loop variant showed a significant increase in Rab10 phosphorylation by approximately 4-fold of the WT level. Furthermore, the levels observed for the G-loop variant were comparable to LRRK2 Gly2019Ser, which increased Rab10 phosphorylation by approximately 7-fold (Fig. 2). Taken together, the functional assay supports the genetic data, demonstrating that the novel variant in the LRRK2 kinase domain indeed shares biochemical features of confirmed pathogenic LRRK2 variants.
FIG. 2.
Cell-based Rab10 phospho-assay comparing the Asp1887Gly (D1887G) variant with WT LRRK2, the most common PD variant Gly2019Ser (G2019S) and a kinase-dead control Lys1906Met (K1906 M). Strep/FLAG-LRRK2 variants have been coexpressed with Flag/HA-Rab10 (N = 3 biological +3 technical replicates). Statistical significance has been determined by ANOVA and Tukey’s post-hoc test. Left panel: relative Rab10 phosphorylation levels observed for different LRRK2 variants. Right panel: SDS-PAGE and western blot analysis after FLAG-IP. Rab10 phosphorylation has been detected by a phospho-pattem–specific antibody. Equal loading has been determined by colloidal Coomassie stain. For the western blot analysis and for the colloidal Coomassie stain, approximately 8 and 37 pmole of FLAG/HA-Rab10 were loaded, respectively.
Discussion
To date, LRRK2 has been consistently shown to be a major risk factor both for familial and sporadic forms of PD in the majority of ethnically diverse populations. A majority of studies from the Indian subcontinent assessed the role of known variants (e.g., G2019S) primarily identified in the white population—and excluded the role of these LRRK2 variants in the Indian PD population.23,25,50,51 Here, we performed the first comprehensive study using targeted sequencing in Indian PD patients, which led to the identification of population-specific novel variants and underscores the relevance of allelic heterogeneity in PD.
The identification of population-specific variants in the armadillo and kinase domain, if confirmed, will help to understand the molecular mechanism of LRRK2 related PD. For example, LRRK2 has been shown to induce apoptotic neuronal cell death by the FADD pathway.52 Interestingly, the Leu550Trp variant is localized right downstream of a recently identified FADD binding motif within the armadillo domain.45 Another PD variant within the LRRK2 armadillo domain, Glu193Lys, has recently been functionally characterized. This work showed that this variant alters LRRK2 binding to dynamin-related protein 1, thereby preventing mitochondrial fission upon MPP* treatment.48 Taken together, these reports suggest that pathogenic variants within the N-terminus might alter the scaffolding properties of LRRK2. The variant Asp1887Gly is part of the G-loop motif. Beside its importance in the ATP binding/hydrolysis has been shown to be involved in the binding of the cdc37 cochaperone of heat shock protein 90 (Hsp90) client kinases.49 LRRK2 has been previously shown to bind Hsp90 and cdc37.53 Further functional studies are warranted to understand their role in PD pathogenesis. The carriers identified have a negative family history, indicating the reduced penetrance of these variants. Of note, the Asp1887Gly variant is observed in the kinase domain, and most of the studies have excluded the role of the G2019S variant in the Indian population. It is important to note that the populations which have been under-represented in the genomic research, such as the Indian population, will invariably identify novel variants which have not been documented in well-characterized publically available resources. We have taken every precaution to define our variants using the guidelines published by the American College of Medical Genetics and Genomics and the Association for Molecular Biology.54 Based on the genetic, computational, and functional assays, the kinase variant identified in our cohort should be considered “likely pathogenic,” and the three remaining variants should be categorized as “variants of uncertain significance.” Therefore, further genetic screening of the LRRK2 gene in an expanded cohort from the Indian population is highly warranted before any conclusive claims related to their pathogenicity can be confirmed.
Replicating association signals, either from GWAS or candidate gene studies, relies on the existence of similar patterns of LD between the unknown causal variants and the genotyped SNPs; the likelihood of replicating the association signal is greater, where patterns of LD are more similar across populations.22 A previous study comparing reference populations using HapMap and the Singapore Genome Variation Project concluded that the likelihood of replicating association signal obtained from the white population is greater in the Indian population as compared to other ethnically diverse populations.27 Indeed, the frequency of the common variants used in our study is comparable to that of the white population.54,55 For common variants, the consistency of directionality of effect estimates observed in both stages showed that observed finding is not an artifact. The lack of association observed in our cohort reflects the small sample size.
There are few drawbacks of our study. Our study has 44% power to detect the allele-based odds ratio of 1.15 or higher for alpha = 0.05. Given the effect estimates observed in our study, we would need more than ~10,000 subjects to confirm the observed association, as has been shown in previously published large-scale GWAS.55,56 Here, given the currently limited number of samples available from India, we are confident that our network will provide fresh impetus in developing a comprehensive biobank and database for PD and other neurodegenerative diseases in India to conduct large-scale, population-based studies.
In conclusion, our study, for the first time, performed a comprehensive genetic screen of LRRK2 in the Indian population and showed that allelic heterogeneity is important in PD pathogenesis. Thus, a large-scale screening of known PD loci identified in the white population should be performed to catalogue population-specific variants. The cataloging of such variants will help to define population-specific diagnostic screen panels, which will eventually help to define enriched cohorts for clinical trials. ■
Supplementary Material
Acknowledgments:
We thank all subjects who have contributed in this study.
Funding agencies:
The project underlying this publication was funded by the Michael J Fox Foundation, USA (M S. and A.K.). This work was further supported by the grants from the German Research Council (DFG/SH 599/6-1 to M.S.), the EU Joint Program-Neurodegenerative diseases (JPND; COURAGE-PD to M.S., R.K., and T.G.), and Multiple System Atrophy Coalition, USA (to M.S.). R.K. gratefully acknowledges the support from the Luxembourg National Research Fund (FNR) within the National Centre of Excellence in Research on Parkinson’s disease (NCER-PD), PEARL programme (FNR; FNR/P13/6682797 to R.K.), the German Research Council (DFG; KR2119/8-1 to R.K. and T.G.), and by the European Union’s Horizon2020 research and innovation program under grant agreement no. 692320 (WIDESPREAD; CENTRE-PD).
Footnotes
Supporting Data
Additional Supporting Information may be found in the online version of this article at the publisher’s web-site.
Relevant conflicts of interest/financial disclosures: Manu Sharma and Asha Kishore received funding for this study from the Michael J Fox Foundation. Christian Johannes Gloeckner, the work which was done for the article, was financed by iMed and the Michael J Fox Foundation.
Full financial disclosures and author roles may be found in the online version of this article.
A full list of the authors can be found in the supplementary document.
References
- 1.Paisán-Ruiz C LRRK2 gene variation and its contribution to Parkinson disease. Hum Mutat 2009;30:1153–1160. [DOI] [PubMed] [Google Scholar]
- 2.Healy DG, Falchi M, O’Sullivan SS, et al. Phenotype, genotype, and worldwide genetic penetrance of LRRK2-associated Parkinson’s disease: a case-control study. Lancet Neurol 2008;7:583–590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mata IF, Checkoway H, Hutter CM, et al. Common variation in the LRRK2 gene is a risk factor for Parkinson’s disease. Mov Disord 2012;27:1822–1825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ross OA, Soto-Ortolaza AI, Heckman MG, et al. Association of LRRK2 exonic variants with susceptibility to Parkinson’s disease: a case-control study. Lancet Neurol 2011;10:898–908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Fraser KB, Moehle MS, Alcalay RN, West AB. Urinary LRRK2 phosphorylation predicts parkinsonian phenotypes in G2019S LRRK2 carriers. Neurology 2016;86:994–999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gilligan PJ. Inhibitors of leucine-rich repeat kinase 2 (LRRK2): progress and promise for the treatment of Parkinson’s disease. Curr Topics Med Chem 2015;15:927–938. [DOI] [PubMed] [Google Scholar]
- 7.Tan EK, Shen H, Tan LC, et al. The G2019S LRRK2 mutation is uncommon in an Asian cohort of Parkinson’s disease patients. Neurosci Lett 2005;384:327–329. [DOI] [PubMed] [Google Scholar]
- 8.Gopalai AA, Lim SY, Chua JY, et al. LRRK2 G2385R and R1628P mutations are associated with an increased risk of Parkinson’s disease in the Malaysian population. BioMed Res Int 2014;2014:867321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Di Fonzo A, Tassorelli C, De Mari M, et al. Comprehensive analysis of the LRRK2 gene in sixty families with Parkinson’s disease. Eur J Hum Cienet 2006;14:322–331. [DOI] [PubMed] [Google Scholar]
- 10.Xie CL, Pan JL, Wang WW, et al. The association between the LRRK2 G2385R variant and the risk of Parkinson’s disease: a meta-analysis based on 23 case-control studies. Neurol Sci 2014;35:1495–1504. [DOI] [PubMed] [Google Scholar]
- 11.Zabetian CP, Yamamoto M, Lopez AN, et al. LRRK2 mutations and risk variants in Japanese patients with Parkinson’s disease. Mov Disord 2009;24:1034–1041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Vilas D, Ispierto L, Alvarez R, et al. Clinical and imaging markers in premotor LRRK2 G2019S mutation carriers. Parkinsonism Relat Disord 2015;21:1170–1176. [DOI] [PubMed] [Google Scholar]
- 13.Lesage S, Ibanez P, Lohmann E, et al. G2019S LRRK2 mutation in French and North African families with Parkinson’s disease. Ann Neurol 2005;58:784–787. [DOI] [PubMed] [Google Scholar]
- 14.Warren L, Gibson R, Ishihara L, et al. A founding LRRK2 haplotype shared by Tunisian, US, European and Middle Eastern families with Parkinson’s disease. Parkinsonism Relat Disord 2008;14:77–80. [DOI] [PubMed] [Google Scholar]
- 15.Ohta E, Hasegawa K, Gasser T, Obata F. Independent occurrence of I2020T mutation in the kinase domain of the leucine rich repeat kinase 2 gene in Japanese and German Parkinson’s disease families. Neurosci Lett 2007;417:21–23. [DOI] [PubMed] [Google Scholar]
- 16.Tan EK, Zhao Y, Skipper L, et al. The LRRK2 Gly2385Arg variant is associated with Parkinson’s disease: genetic and functional evidence. Hum Genet 2007;120:857–863. [DOI] [PubMed] [Google Scholar]
- 17.Tan EK, Tan LC, Lim HQ, et al. LRRK2 R1628P increases risk of Parkinson’s disease: replication evidence. Hum Genet 2008;124:287–288. [DOI] [PubMed] [Google Scholar]
- 18.Pulkes T, Papsing C, Thakkinstian A, et al. Confirmation of the association between LRRK2 R1628P variant and susceptibility to Parkinson’s disease in the Thai population. Parkinsonism Relat Disord 2014;20:1018–1021. [DOI] [PubMed] [Google Scholar]
- 19.Foo JN, Tan LG, Liany H, et al. Analysis of non-synonymous-coding variants of Parkinson’s disease-related pathogenic and susceptibility genes in East Asian populations. Hum Mol Genet 2014;23:3891–3897. [DOI] [PubMed] [Google Scholar]
- 20.Skipper L, Li Y, Bonnard C, et al. Comprehensive evaluation of common genetic variation within LRRK2 reveals evidence for association with sporadic Parkinson’s disease. Hum Mol Genet 2005;14:3549–3556. [DOI] [PubMed] [Google Scholar]
- 21.Heckman MG, Soto-Ortolaza AI, Aasly JO, et al. Population-specific frequencies for LRRK2 susceptibility variants in the Genetic Epidemiology of Parkinson’s Disease (GEO-PD) Consortium. Mov Disord 2013;28:1740–1744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Altshuler DM, Gibbs RA, Peltonen L, et al. Integrating common and rare genetic variation in diverse human populations. Nature 2010;467:52–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Vijayan B, Gopala S, Kishore A. LRRK2 G2019S mutation does not contribute to Parkinson’s disease in South India. Neurol India 2011;59:157–160. [DOI] [PubMed] [Google Scholar]
- 24.Vishwanathan Padmaja M, Jayaraman M, Srinivasan AV, Srikumari Srisailapathy CR, Ramesh A. The SNCA (A53T, A30P, E46K) and LRRK2 (G2019S) mutations are rare cause of Parkinson’s disease in South Indian patients. Parkinsonism Relat Disord 2012;18:801–802. [DOI] [PubMed] [Google Scholar]
- 25.Punia S, Behari M, Govindappa ST, et al. Absence/rarity of commonly reported LRRK2 mutations in Indian Parkinson’s disease patients. Neurosci Lett 2006;409:83–88. [DOI] [PubMed] [Google Scholar]
- 26.Sanyal J, Sarkar B, Ojha S, Banerjee TK, Ray BC, Rao VR. Absence of commonly reported leucine-rich repeat kinase 2 mutations in Eastern Indian Parkinson’s disease patients, Genet Test Mol Biomarkers 2010;14:691–694. [DOI] [PubMed] [Google Scholar]
- 27.Li H, Teo YY, Tan EK. Patterns of linkage disequilibrium of LRRK2 across different races: implications for genetic association studies. PLoS One 2013;8:e75041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hughes AJ, Daniel SE, Kilford L, Lees AJ. Accuracy of clinical diagnosis of idiopathic Parkinson’s disease: a clinico-pathological study of 100 cases. J Neurol Neurosurg Psychiatry 1992;55:181–184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Li H, Durbin R. Fast and accurate short read alignment with Burrows-Wheeler transform. Bioinformatics 2009;25:1754–1760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mose LE, Wilkerson MD, Hayes DN, Perou CM, Parker JS. ABRA: improved coding indel detection via assembly-based realignment. Bioinformatics 2014;30:2813–2815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Cingolani P, Patel VM, Coon M, et al. Using Drosophila melanogaster as a model for genotoxic chemical mutational studies with a new program, SnpSift. Front Genet 2012;3:35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Faust GG, Hall IM. SAMBLASTER: fast duplicate marking and structural variant read extraction. Bioinformatics 2014;30:2503–2505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Nalls MA, Bras J, Hernandez DG, et al. ; International Parkinson’s Disease Genomics Consortium (IPDGC); Parkinson’s Disease meta-analysis consortium. NeuroX, a fast and efficient genotyping platform for investigation of neurodegenerative diseases. Neurobiol Aging 2015;36(3):1605.e7–e12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Jansen IE, Bras JM, Lesage S, et al. CHCHD2 and Parkinson’s disease. Lancet Neurol 2015;14:678–679. [DOI] [PubMed] [Google Scholar]
- 35.Jansen IE, Ye H, Heetveld S, et al. Discovery and functional prioritization of Parkinson’s disease candidate genes from large-scale whole exome sequencing. Genome Biol 2017;18:22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Blauwendraat C, Faghri F, Pihlstrom L, et al. NeuroChip, an updated version of the NeuroX genotyping platform to rapidly screen for variants associated with neurological diseases. Neurohiol Aging 2017;57:247.e9–247.e13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Blauwendraat C, Kia DA, Pihlstrom L, et al. Insufficient evidence for pathogenicity of SNCA His50Gln (H50Q) in Parkinson’s disease. Neurobiol Aging 2018;64:159.e5–159.e8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Purcell S, Neale B, Todd-Brown K, et al. PLINK: a tool set for whole-genome association and population-based linkage analyses. Am J Hum Genet 2007;81:559–575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Guaitoli G, Raimondi F, Gilsbach BK, et al. Structural model of the dimeric Parkinson’s protein LRRK2 reveals a compact architecture involving distant interdomain contacts. Proc Natl Acad Sci U S A 2016;113:E4357–E4366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kellogg EH, Leaver-Fay A, Baker D. Role of conformational sampling in computing mutation-induced changes in protein structure and stability. Proteins 2011;79:830–838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ito G, Katsemonova K, Tonelli F, et al. Phos-tag analysis of Rab10 phosphorylation by LRRK2: a powerful assay for assessing kinase function and inhibitors. Biochem J 2016;473:2671–2685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Gloeckner CJ, Boldt K, von Zweydorf F, et al. Phosphopeptide analysis reveals two discrete clusters of phosphorylation in the N-terminus and the Roc domain of the Parkinson-disease associated protein kinase LRRK2. J Proteome Res 2010;9:1738–1745. [DOI] [PubMed] [Google Scholar]
- 43.Gloeckner CJ, Boldt K, Schumacher A, Roepman R, Ueffing M. A novel tandem affinity purification strategy for the efficient isolation and characterisation of native protein complexes. Proteomics 2007;7:4228–4234. [DOI] [PubMed] [Google Scholar]
- 44.Anand VS, Braithwaite SP. LRRK2 in Parkinson’s disease: biochemical functions. FEBS J 2009;276:6428–6435. [DOI] [PubMed] [Google Scholar]
- 45.Antoniou N, Vlachakis D, Memou A, et al. A motif within the armadillo repeat of Parkinson’s-linked LRRK2 interacts with FADD to hijack the extrinsic death pathway. Sci Rep 2018;8:3455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Steger M, Tonelli F, Ito G, et al. Phosphoproteomics reveals that Parkinson’s disease kinase LRRK2 regulates a subset of Rab GTPases. Elife 2016;5:e12813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Taylor SS, Kornev AP. Protein kinases: evolution of dynamic regulatory proteins. Trends Biochem Sci 2011;36:65–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Perez Carrion MD, Pischedda F, Biosa A, et al. The LRRK2 variant E193K prevents mitochondrial fission upon MPP+ treatment by altering LRRK2 binding to DRP1. Front Mol Neurosci 2018;11:64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Terasawa K, Yoshimatsu K, Iemura S, Natsume T, Tanaka K, Minami Y. Cdc37 interacts with the glycine-rich loop of Hsp90 client kinases. Mol Cell Biol 2006;26:3378–3389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Madegowda RH, Kishore A, Anand A. Mutational screening of the parkin gene among South Indians with early onset Parkinson’s disease. J Neurol Neurosurg Psychiatry 2005;76:1588–1590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Das SK, Ghosh B, Das G, Biswas A, Ray J. Movement disorders: Indian scenario: a clinico-genetic review. Neurol India 2013;61:457–466. [DOI] [PubMed] [Google Scholar]
- 52.Iaccarino C, Crosio C, Vitale C, Sanna G, Carri MT, Barone P. Apoptotic mechanisms in mutant LRRK2-mediated cell death. Hum Mol Genet 2007;16:1319–1326. [DOI] [PubMed] [Google Scholar]
- 53.Gloeckner CJ, Kinkl N, Schumacher A, et al. The Parkinson disease causing LRRK2 mutation I2020T is associated with increased kinase activity. Hum Mol Genet 2006;15:223–232. [DOI] [PubMed] [Google Scholar]
- 54.Nalls MA, Plagnol V, Hernandez DG, et al. Imputation of sequence variants for identification of genetic risks for Parkinson’s disease: a meta-analysis of genome-wide association studies. Lancet 2011;377:641–649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Nalls MA, Pankratz N, Lill CM, et al. Large-scale meta-analysis of genome-wide association data identifies six new risk loci for Parkinson’s disease. Nat Genet 2014;46:989–993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Chang D, Nalls MA, Hallgrimsdottir IB, et al. A meta-analysis of genome-wide association studies identifies 17 new Parkinson’s disease risk loci. Nat Genet 2017;49:1511–1516. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.