Abstract
Objective
We investigated whether comorbidities differentially impacted health-related quality of life (HRQOL) for rheumatoid arthritis (RA) and osteoarthritis (OA) patients.
Methods
Adult patients with self-reported doctor-diagnosed RA (n=159) or OA (n=149) were recruited from multiple sources and completed an online cross-sectional survey. Patients self-reported sociodemographic variables, arthritis severity and comorbid conditions. HRQOL was assessed using the SF-12v2 and comorbidity counts were assigned using an expanded Functional Comorbidities Index. HRQOL (8 domain and 2 composite (physical and mental health) scores) was compared with norm-based general US population scores and between RA and OA patients to determine if they significantly differed from one another. Linear regression was used to test whether comorbidity count was associated with the physical and mental health of RA and OA patients.
Results
OA and RA patients experienced significantly worse HRQOL across all dimensions compared with that of the general US population. There were no significant differences between RA and OA patients on any HRQOL dimension. A higher comorbidity count was associated with worse physical (p=0.0007) and mental (p=0.0295) health scores when controlling for patient gender, age, education, and arthritis severity.
Conclusion
Arthritis negatively impacted patients’ HRQOL. OA patients in our sample perceived their condition as similarly disabling in terms of physical and mental health as RA patients. Arthritis patients with more chronic comorbid conditions may be at particular risk for poor physical and mental health. Providers should discuss management of comorbid conditions with arthritis patients.
Keywords: osteoarthritis, rheumatoid arthritis, comorbidity, quality of life
Introduction
Affecting 1 in 5 adults (1), arthritis is particularly common among individuals with multiple chronic conditions and is the most frequent cause of disability in the US (2). Rheumatoid arthritis (RA) and osteoarthritis (OA) differ in etiology, course, and medical management, yet share similar features (3, 4). RA is a systemic, autoimmune disease with no known cause (5). OA, by contrast, can occur in any joint and results from factors including mechanical stress and biological processes over time (6, 7). While RA is generally considered more disabling and its management more intensive compared to OA, which tends to be slowly progressive and generally occurs later in life (7, 8), both diseases cause pain, decreased motion, and compromised quality of life resulting from cartilage destruction and joint dysfunction (3, 9).
Individuals with arthritis score significantly lower on health-related quality of life (HRQOL) measures (an individual’s perceived physical and mental health functioning) as compared to the general US population and other disease populations (e.g. hypertension and diabetes) (10–12). Specifically, RA and OA patients report that their physical health is negatively impacted to a greater extent than their mental health (11–13). Findings from studies considering differences in specific HRQOL domains (14–17) suggest a general trend where RA patients report worse HRQOL (14) for a larger number of domains (15–17) when compared to OA patients.
Comorbidity has been implicated as an important factor differentiating degrees of functioning related to quality of life (18–20). Comorbidity is particularly important to consider with arthritis patients, since individuals with arthritis have a greater number of comorbidities when compared with individuals without arthritis (21) and comorbidities have been associated with reduced HRQOL (1, 13). Six studies have examined the impact of comorbidity on HRQOL among individuals with RA (22–27), four studies among OA patients (13, 28–30), and one study among a combined sample of OA and RA patients (considered “those with arthritis” for comorbidity analysis) (17). Collectively, these findings suggest comorbid conditions are associated with poorer physical HRQOL among individuals with RA and OA and having a greater number of concurrent chronic conditions negatively affects HRQOL scores for arthritis patients. To our knowledge, no study has examined whether comorbidity counts differentially impact HRQOL for OA and RA patients.
Table I provides a summary of research on comorbidities and HRQOL for arthritis patients. Three general methods have been used to consider or measure comorbidity among the aforementioned studies (i.e. dichotomisation, count, and weighting). Only one study used a comorbidity measure designed to include diagnoses impacting physical function (19). Counts, defined as adding the number of diseases in one person (31), are the most commonly used comorbidity measure (32). Comorbidity counts provide an understanding of the potentially additive impact of multiple comorbid diseases on RA or OA patients, and provide richer information when compared to simply considering whether a patient has any comorbid condition (dichotomous method). While there may be advantages to using weighted measures (combining the number and severity of each disease (31)), there are no weighting standards, weights are not necessarily more effective at predicting outcomes than simple counts, and counts are easier to score and use (19, 32).
Table I.
RA and OA studies considering comorbidity and HRQOL
| Reference / Year | *Method | Location | Source of data | Study type | Sample size | HRQOL measures | Comorbidity treatment | Main comorbidity findings/results |
|---|---|---|---|---|---|---|---|---|
| Wolfe F, Michaud K, Li T, et al. (22) /2010 | 3 | US | Rheumatology practices | Longitudinal | 13,722 with RA | EQ-5D | Self-reported comorbidities, asked about 22 “health problems”; created summary variables and composite index | EQ-5D quality of life score decreased by about 0.04 units for RA. Regardless of comorbid condition, current comorbidity always resulted in a lower EQ-5D score than lifetime comorbidity. |
| Lee T-J, Park BH, Son HK, et al. (23) /2012 | 1 | Seoul, South Korea | Rheumatology clinic | Cross-sectional; Face-to-face interview survey | 196 RA patients | KEQ-5D | Dichotomised comorbidity; assessment of comorbidities not outlined | Patients with comorbidity had lower HRQOL scores than did those without. |
| Radner H, Smolen JS, Aletaha D. (24) /2011 | 3 | Vienna, Austria | Outpatient clinic | Longitudinal; chart review | 380 RA patients with established RA | SF-36 | Age-adjusted Charlson Comorbidity Index (CCIA) | Significant (p<0.03) increase of disability within each domain of HAQ with increasing level of comorbidity. Similar results were observed using the physical component score (p=0.003) of the SF-36 and its domains, whereas mental component score (p=0.31) and its domains were unaffected by comorbidities. |
| Rupp I, Boshuizen HC, Jacobi CE, et al.. (25) /2004 | 1 | Amsterdam, The Netherlands | Outpatient center for rheumatology and rehabilitation | Follow-up study | 679 patients with RA | RAND-36 | Self-administered questionnaire including 17 chronic diseases; asked whether they had had any of the conditions of the list in the previous 12 months. | The effect of comorbidity on HRQOL depended on both the type of comorbid condition and the dimension of HRQOL. Gastrointestinal (GI) diseases, cancer, dizziness with falling (and less severe chronic pulmonary disease and heart complaints) resulted in significant adverse changes in HRQOL. For the other conditions under study no influence could be detected. |
| Crilly MA, Johnston MC, Black C. (26) /2013 | 2 | Scotland, United Kingdom | Clinic patients | Cross-sectional; Interviews and medical records checks | 114 ambulatory RA patients | EQ-5D | Medical records were reviewed by a rheumatologist for co-existing conditions. | EQ-5D scores were inversely correlated with the overall number of co-existing conditions (Spearman’s q −0.31, p=0.001), number of comorbidities (q −0.22, p=0.02). There was a linear trend of lower EQ-5D with increasing number of co-existing conditions (p=0.003). EQ-5D was −0.18 (95% Cl −0.33 to −0.02) lower in the presence of more than two coexisting conditions compared to none. |
| Krishnan E, Hakkinen A, Sokka T, et al. (27) /2005 | 2 | Central Finland District, Finland | Finnish population registry | Retrospective; record review | 1530 adults with RA | Global assessment of general hedth | Self- reported comorbidities. | Outcomes worsened steadily with increasing number of comorbidities. Number of comorbidities was a statistically significant correlate of general health. |
| Hopman W, Harrison M, Coo H, et al. (13) /2009 | 1 | Ottawa, Canada | Examined data collected in 10 studies | Retrospective; Interviews and chart reviews | 366 with OA | SF-36 or SF-12 | Only two comorbidities (cardiovascular disease, diabetes) plus a category of “additional comorbidities” (i.e. defined as yes/no) could be drawn from databases | Comorbid conditions were associated with poorer HRQOL. |
| Hosseini K, Gaujoux-Viala C, Coste J, et al. (28) /2012 | 2 | Paris, France | Random sample of households; clinical confirmation of diagnosis | Cross-sectional; Population-based survey | 878 OA patients | SF-6D from the MOS36-item SF-36 | Functional Comorbidity Index; Used comorbidity count and categories (grouped the 18 conditions of this index into nine categories) | For each additional co-morbidity (range 0–9), the mean utility score decreased 0.03 point (beta = - 0.03, p<0.0001) |
| Tuominen U, Blom M, Hirvonen J, et al. (29) /2007 | 1 | Helsinki, Finland | Orthopaedic patients from 4 hospitals | Cross-sectional | 893 OA patients | Generic 15D and VAS | Comorbidities considered dichotomously and collected from the patients’ reported co-morbidities as diagnosed by a medical doctor | The mean number of co-morbidities among the patients was two. Comorbidity was significantly associated with a reduced HRQOL. |
| Salaffi F, Carotti M, Stancati A,et al. (30) /2005 | 2,3 | Italy | Department of Rheumatology | Cross-sectional; survey | 264 OA patients | SF-36 | Number of comorbidities and study generated comorbidity index score | There was a significant inverse association with measures of comorbidity (number of comorbidities and comorbidity index score) and both physical and mental SF-36 summary scores. |
| Dominick K, Kelli L, Ahern F, et al. (17) /2004 | 3 | Pennsylvania, United States | State-wide sample enrolled in Pharm Assistance Contract for the Elderly | Cross-sectional; mailed surveys | 41,467 older adults with RA and OA | CDC’s Core and Optional HRQOL Modules | Outpatient visit (Medicare Part B) records Charlson Comorbidity Score | Analysis of relationship of comorbidity to HRQOL used combined OA and RA arthritis sample. Among individuals with arthritis those with greater numbers of comorbid illnesses were significantly more likely to report fair or poor general health and to have poorer scores on one or more of the “days” HRQOL items. |
Method used to measure comorbidity: 1) considered individuals as either having or not having a comorbid condition; 2) used simple counts of diseases in each individual (e.g. patient self-report or chart review); and, 3) used indices including count, weighting conditions and/or assigning severity to assess comorbidity burden.
To address the lack of existing data considering differences between RA and OA patient groups, this study investigated the association of comorbidity and HRQOL among a sample of 308 arthritis patients. We hypothesised that HRQOL domains would, on average, be lower for arthritis patients compared to national norms and that HRQOL scores would be lower for RA patients when compared with OA patients, with physical composite scores being lower than mental health composite scores. We further hypothesised that increasing comorbidity count would be associated with worse HRQOL among RA patients compared to OA patients.
Subjects and methods
Subjects
All data were derived from Information Networks for Osteoarthritis Resources and Medications (INFORM), a cross-sectional on-line survey assessing self-reported health information seeking behaviours and health status of arthritis patients. Eligible participants had a self-reported doctor-diagnosis of osteoarthritis or rheumatoid arthritis, were at least 18 years of age, could read and write in English, had Internet access, and were currently taking at least one medication to treat their arthritis on a routine basis. All participants indicated agreement to participate after reading a study fact sheet. The INFORM study was approved by the University of North Carolina’s Institutional Review Board.
Recruitment methods have been described in detail elsewhere (33). Briefly, recruitment mailings were sent to persons having a diagnosis of osteoarthritis or rheumatoid arthritis according to University of North Carolina Hospital System records and general recruitment announcements were distributed via patient websites, local clinics, arthritis support groups, and in local media publications and advertising outlets. A total of 424 patients accessed the study survey between May 2010 and January 2011. Among those, 71 individuals were ineligible (34 did not meet eligibility criteria; 7 were missing screeners; 30 surveys were incomplete or duplicate); 25 declined to participate after reading the fact sheet. Three hundred and twenty-eight patients completed the study survey; 124 were recruited from the hospital mailing and 204 from general announcements. For these analyses, twenty patients were excluded because they were either unsure what type of arthritis they had (n=18) or reported having a pain disorder (n=2). Thus, the current analysis is limited to 308 patients.
Measures
Sociodemographic and clinical characteristics
Patients reported their gender, race/ethnicity, and education level (i.e. 8th grade or less, some high school but no diploma, high school graduate or GED, some college but no degree, associate’s degree, bachelor’s degree, post-graduate school or degree). Patients reported year of arthritis diagnosis and doctor-diagnosed arthritis type. Arthritis severity during the past 4 weeks was assessed using one item (“Based on how you have been feeling during the past 4 weeks, please select the one number that best represents how severe you consider your arthritis to be.”); responses ranged from 1–10 (1=not at all severe; 5=moderately severe; and 10=extremely severe).
Health-related quality of life
HRQOL was assessed using the 12-item Medical Outcomes Study Short-Form Health Survey (SF-12v2) (34). The SF-12v2 is a widely-used, self-administered questionnaire measuring perceived health status over the last 4 weeks with respect to 8 dimensions: physical functioning, social functioning, role limitations caused by physical health problems, role limitations due to emotional health problems, body pain, mental health, vitality, and general health. The 8 subscale scores can be scored independently and also combined into two composite scores: the Physical Component Summary (PCS) and the Mental Component Summary (MCS). The RAND-12 scoring method (35) was used to generate summary scores (36).
Scores in the range of 0–100 were computed for each of the 8 subscales and 2 summary scales, with lower scores indicating poorer health status (e.g. lower scores on the bodily pain dimension correspond to more pain). The SF-12v2 was scored using norm-based methods and transformed into t-scores for comparison to the general population (mean of 50 and standard deviation of 10; scores greater than 50 are considered above average, a score of 50 is considered average, and scores less than 50 are considered below average) (37). The SF-12v2, has demonstrated validity when used with RA and OA patients (38). In the current study, patient SF-12v2 scores were compared to 2005–2006 US population means (39).
Co-morbidities
Patients reported the presence and type of major medical conditions (other than arthritis) when asked, “Do you have any major medical conditions other than arthritis? (yes/no)” followed by an open-ended item “Please list the other major medical conditions that you have.” Patients’ responses to the open-ended item were then classified using the Functional Comorbidity Index (FCI) (includes 18 conditions found to be significantly associated with the Short-Form Health Survey (SF-36) physical functioning scale (40)) and seven additional comorbid conditions (i.e. hypertension, anaemia, bowel disease, cancer, liver disease, kidney disease, and migraine), identified in the first stage of the FCI index development (19). We expanded the FCI list to include those 7 conditions because, in addition to physical functioning, we are interested in mental HRQOL domains (i.e. vitality, social functioning, role-emotional, and mental health) and those conditions may exert influence on the outcomes of interest. Using the expanded FCI, we calculated a comorbidity count for each patient, which ranged from 0, indicating no comorbidities, to 24, indicating the highest number of comorbidities. Table II lists the comorbid conditions. Self-reported conditions (e.g. high cholesterol and osteopenia), that were not included in the expanded FCI, were excluded from the comorbidity count.
Table II.
The Expanded Functional Comorbidity Index items.
|
*1 Arthritis (rheumatoid and osteoarthritis 2 Osteoporosis 3 Asthma 4 Chronic obstructive pulmonary disease, acquired respiratory distress syndrome, or emphysema 5 Angina 6 Congestive heart failure (or heart disease) 7 Heart attack (myocardial infarct) 8 Neurological disease (such as multiple sclerosis or Parkinson’s) 9 Stroke or transient ischaemic attack 10 Peripheral vascular disease 11 Diabetes types I and II 12 Upper gastrointestinal diseases (ulcer, hernia, reflux). 13 Depression 14 Anxiety or panic disorders 15 Visual impairment (such as cataracts, glaucoma, macular degeneration) 16 Hearing Impairment (very hard of hearing, even with hearing aids) 17 Degenerative disc disease (back disease, spinal stenosis, or severe chronic back pain) 18 Obesity and/or body mass index >30 (weight in kg/height in meters2) 19 Hypertension 20 Anaemia 21 Bowel disease 22 Cancer 23 Liver disease 24 Kidney disease 25 Migraine |
arthritis not retained for this study.
Statistical analysis
Analyses were conducted using SAS software, version 9.3 (SAS Institute, Cary, NC). Simple descriptive statistics (frequencies and percentages for categorical variables, mean and standard deviations for continuous variables) were used to summarise sociodemographic and clinical characteristics and SF-12v2 scores of study participants. Any differences in characteristics between OA patients and RA patients, as well as the percentage of RA and OA patients with average or above average HRQOL scores for the 8 subscales and the two composite scores (PCS and MCS),were assessed using the Chi-square statistic (for categorical variables) or t-tests (for continuous variables). The Cochran-Mantel-Haenszel test with adjustment for age and gender was used to test the association between arthritis type (RA vs. OA) and HRQOL scores.
In order to compare the HRQOL of RA and OA patients with the general 2005–2006 US population, HRQOL subscale scores were converted to norm-based scores using procedures previously described by Hays (1998). Maglinte and Hays’s estimates for the 2005–2006 non-institutionalised US population were derived from the Medical Expenditure Panel Survey (a random-digit dial telephone survey) and represent 3,844 households that were weighted to the 2000 US census data on age (35–44, 45–64, 65–89 years), race (African American, white, other), and gender (male, female) (39). The weighted mean age for the 2005–2006 sample was 54 years; 53% were female, and the majority (82%) were white. Most respondents (60%) had at least some college education or had a college degree. Regarding interpretation, a norm-based score of 50 indicates the population of interest scored exactly the same as the general 2005–2006 US general population (all ages). Similarly, norm-based scores >50 indicate the population of interest scored higher than the general 2005–2006 US population (all ages), whereas scores <50 indicate the population scored lower than the 2005–2006 general US population. For this study, norm-based HRQOL scores for patients were presented along with mean standard norm-based scale scores for the general US population. Independent sample t-tests were then used to determine whether OA and RA patients differed significantly (α=0.05) from the general US population (ages 35–89 years) on the 8 HRQOL subscales and the overall physical and mental composite scales. Adjustments for multiple comparisons were made with the Bonferonni method with the level of significance set at p<0.005. Norm-based scores were used in all subsequent analyses.
Logistic regression was used to model HRQOL physical and mental function by arthritis group, controlling for age and gender. Two linear regression models were used to test whether comorbidity count was associated with the physical and mental HRQOL of RA and OA patients, controlling for the potential effects of gender, age, education, and arthritis severity. ORs with 95% CIs were determined by logistic regression in unadjusted models and in adjusted models controlling for potential confounding.
Results
The sociodemographic and clinical characteristics of the 308 patients are shown in Table III; presented by arthritis type. In the overall sample, the mean age of patients was 56 years (±13; range 19–85). Most patients were female (80%) and white (80%) and the median arthritis duration was 11 years. A little over half (51%) of the arthritis patients in this sample did not have a comorbid condition and among the 49% who did have one or more comorbidity, 58% had one, 25% two, 11% three, 5% four, and 1% five. The maximum number of comorbidities in this sample was 5. Hypertension, diabetes, and cancer were the most frequently reported comorbid conditions. RA patients were younger (53±14; range 19–84 years vs. 60±11; range 22–85 years and had lower mean comorbidity counts (.72±1.0; range 1–4 vs. .92±1.1; range 1–5) compared to OA patients.
Table III.
Socio-demographic and clinical characteristics of OA and RA patients.
| OA (n=149) | RA (n=159) | |||
|---|---|---|---|---|
| n | (%) | n | (%) | |
| Sex | ||||
| Female | 118 | 47.8 | 129 | 52.2 |
| Race/Ethnicity | ||||
| White | 129 | 52.2 | 118 | 47.8 |
| African American | 10 | 24.4 | 31 | 75.6 |
| Other | 10 | 50.0 | 10 | 50.0 |
| Age group (yrs) | ||||
| 18–44 | 9 | 18.4 | 40 | 81.6 |
| 45–64 | 95 | 53.4 | 83 | 46.6 |
| ≥65 | 45 | 55.6 | 36 | 44.4 |
| Education level | ||||
| <High school diploma | 1 | 20.0 | 4 | 80.0 |
| High school diploma | 22 | 53.7 | 19 | 46.3 |
| At least some college | 47 | 43.9 | 60 | 56.1 |
| Completed college or greater | 79 | 51.0 | 76 | 49.0 |
| Disease duration (yrs) | ||||
| <5 yrs | 43 | 46.7 | 49 | 53.3 |
| 6–20 yrs | 71 | 48.0 | 77 | 52.0 |
| over 20 | 26 | 50.0 | 26 | 50.0 |
| Self-reported severity | ||||
| Not at all severe to moderately severe | 46 | 45.5 | 55 | 54.5 |
| Moderately severe | 41 | 47.7 | 45 | 52.3 |
| Moderately severe to extremely severe | 61 | 50.8 | 59 | 49.2 |
| Comorbidity | ||||
| Yes | 81 | 53.6 | 70 | 46.4 |
HRQOL data by arthritis type are shown in Figure 1. SF-12v2 mean composite scores for arthritis patients in this sample were 39 (±8; range 18–58) for the PCS and 42 (±6; range 23–57) for the MCS. Arthritis patients in this sample had domain and composite scores that were all significantly lower than those of the 2005–2006 general US population means (all p<0.005 with Bonferonni correction). No statistically significant mean differences were found between RA and OA patients for any of the HRQOL subscale or summary scores when adjusting for patient age and gender.
Fig. 1.
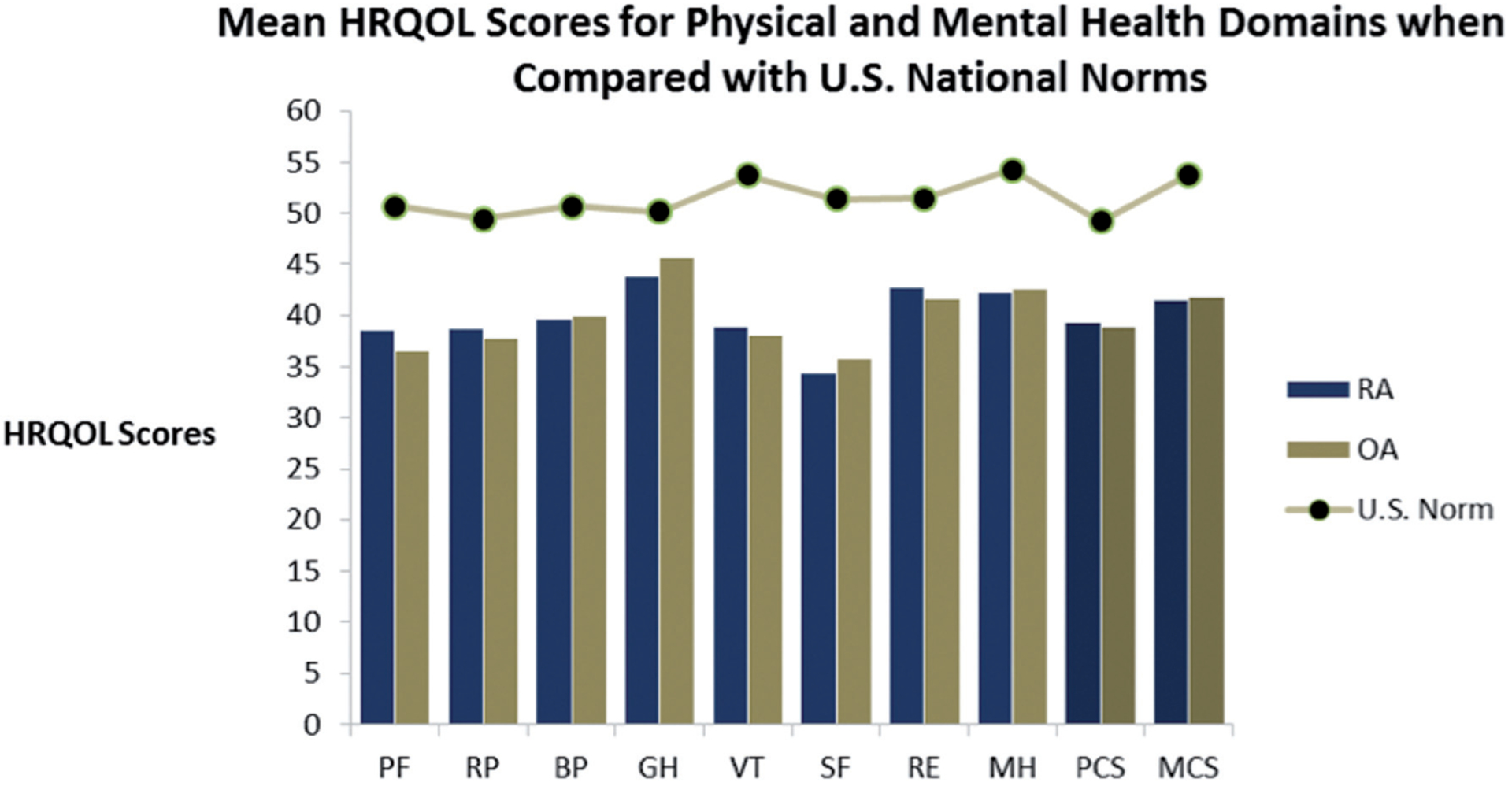
Norm-based health-related quality of life subscale and composite scores for RA patients (n=159), OA patients (n=149), and the 2005–2006 general US population (ages 35–89 years; n=3,844) (ref. Maglinte, 2012). PF physical functioning; RP physical role limitations; BP bodily pain; GH general health; VT vitality/energy; SF social functioning; RE emotional role limitations; MH mental health/ emotional well-being, PCS physicaal composite score; MCS mental composite
The percentage of RA and OA patients with average or above average HRQOL scores for the 8 subscales and the two composite scores (PCS and MCS) are shown in Figure 2. Logistic regression modeling physical function by arthritis type indicated that the odds of having a below average physical HRQOL are 2.4 times higher in OA versus RA patients, even after controlling for age and gender (p=0.009).
Fig. 2.
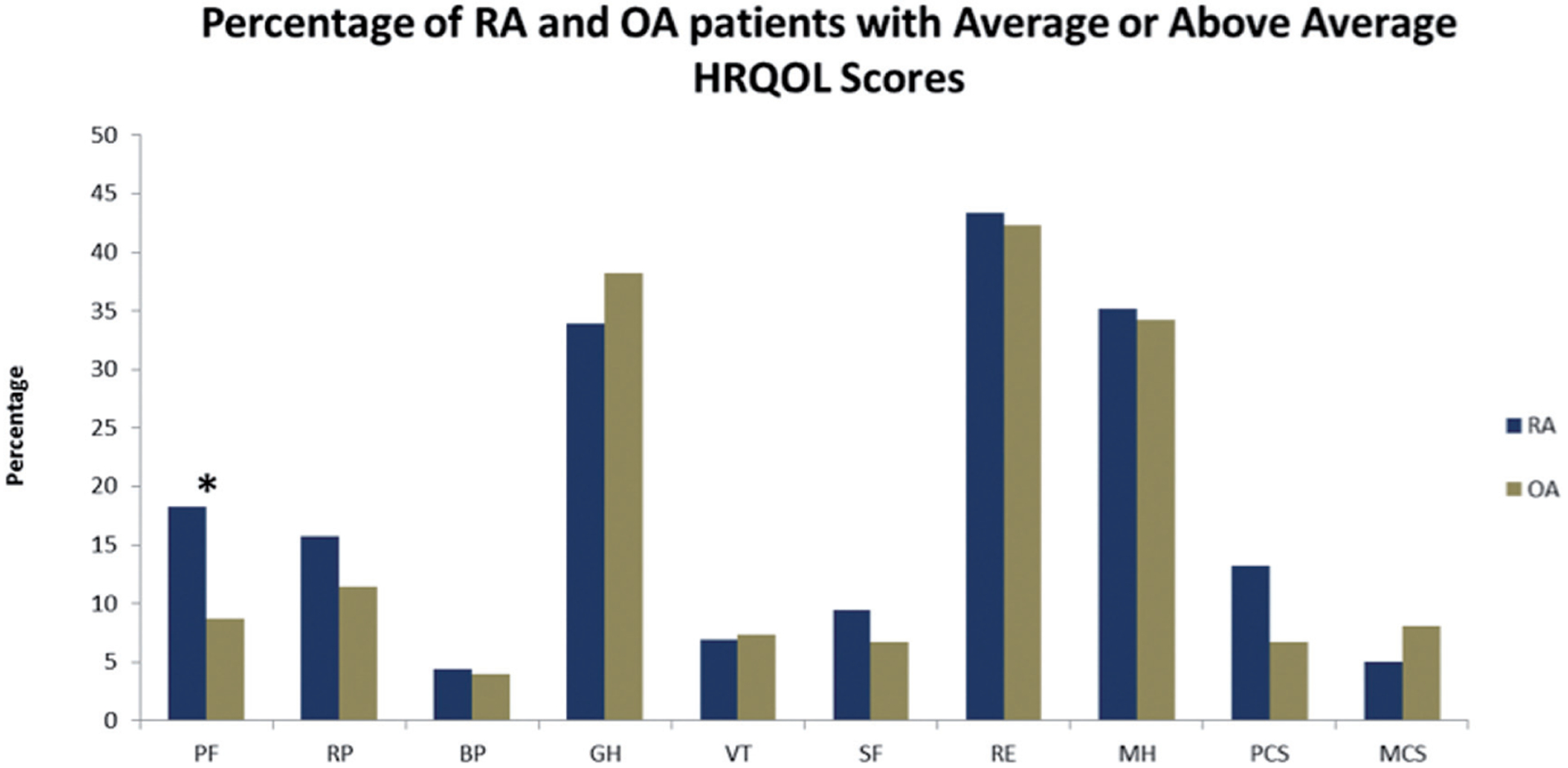
HRQOL subscales: PF physical functioning; RP physical role limitations; BP bodily pain; GH general health; VT vitality/energy; SF social functioning; RE emotional role limitations; MH mental health/ emotional well-being, PCS physical composite score; MCS mental composite.
*Significant by t-test (0.05).
The summary relationship between comorbidity count and HRQOL using the physical (PCS) and mental (MCS) composite scores are shown in Figure 3. As comorbidities increased, the odds of patients with either RA or OA having an average or above average HRQOL PCS score decreased significantly (OR 0.51; (p=0.02). Patients’ physical composite scores were negatively and significantly associated with comorbidity count. Each additional comorbid condition was associated with a mean decrease in the PCS score of −2.22 (p<0.0001). Although not statistically significant, similar to PCS scores, there was a negative linear trend between increasing comorbidity count and decreasing MCS score. Patients’ MCS were negatively and significantly predicted by comorbidity count (mean decrease for every additional comorbidity = −0.68, p=0.0378). After adjusting for gender, age, education, and arthritis severity, the effect of comorbidity count on MCS remained significant (p=0.0295). Additionally, in the adjusted model, age was positively and significantly associated with MCS (p=0.0007). The effect of comorbidity count on PCS also remained significant (p=<0.0001) in the adjusted model and severity was also significantly associated with PCS (p=<0.0001).
Fig. 3.
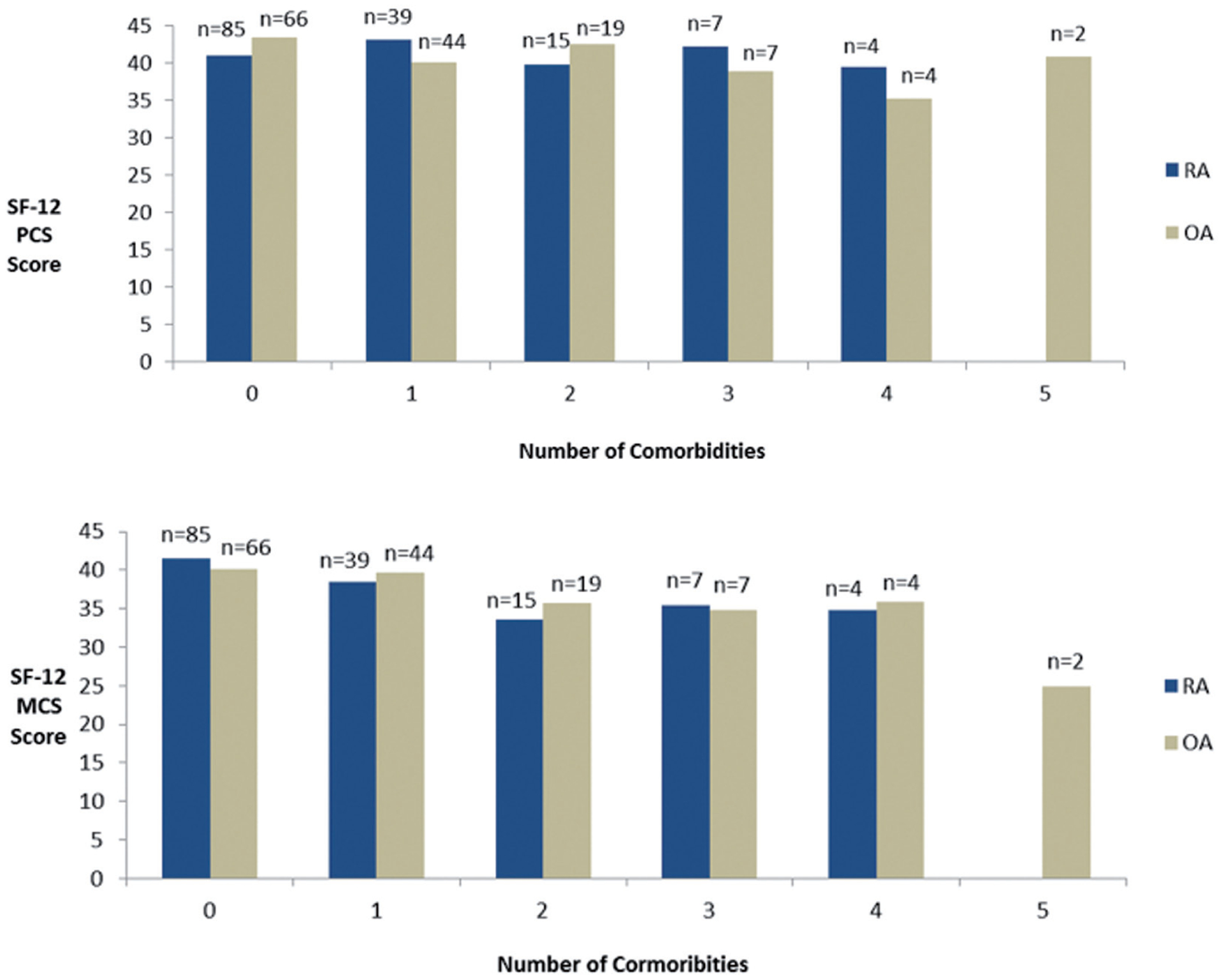
Summary relationship between comorbidity count and HRQOL using the SF-12v2 physical (PCS) and mental (MCS) composite scores.
Discussion
Independent of arthritis type, patients in our sample showed substantially diminished HRQOL compared to the general US population, which supports previous study findings (10–13). The present study also demonstrates the multidimensional nature of HRQOL and the impact of RA and OA is not restricted to physical aspects only, but also influences other domains of HRQOL including social, emotional, and mental health, supporting other research findings (11, 17, 41).
We had expected to find systematic differences related to HRQOL when comparing the two patient groups, due to the fact that RA is generally considered more disabling and management intensive than OA. Contrary to our hypothesis, no statistically significant differences in HRQOL were found between RA and OA patients, even after controlling for age and gender. A previous study found OA only impacted physical functioning, physical role limitations and pain (17), yet findings from our convenience sample show that similar to RA, OA negatively impacted all domains of HRQOL. Further, contrary to previous research, we did not find evidence that RA patients scored lower on quality of life domains than OA patients in our sample (14, 16). Our findings support evidence that physical health is the most negatively impacted domain for both RA and OA patients; however, unlike Picavet et al. (2004), who found bodily pain scores to be second lowest, we found social functioning scores were the second most negatively impacted domain for arthritis patients. Our eligibility criterion requiring patients to take medications regularly may have limited the number of patients with mild OA who participated and partially explain the lack of significant differences between OA and RA patients in our sample. However, it is also possible that because arthritis disease progression is patient specific (4) and because both diseases cause significant pain and decreased joint motion, patient perceptions of the impact of arthritis on HRQOL domains may not differ substantially.
This study is the first to examine whether higher comorbidity counts differentially impact HRQOL for RA and OA patients. The comorbidity burden of patients with RA and OA was far-reaching in this sample. Our examination of the burden of chronic comorbidities on HRQOL revealed a significant linear relationship between increasing comorbidity count and lower physical health scores. Although not significant, a similar negative linear trend between increasing comorbidity count and decreasing RA and OA mental HRQOL scores was identified. These results support the few existing study findings examining the relationship between increasing comorbidity count and HRQOL for RA (26, 27) and OA (28, 30) patients. Only one (28) of the aforementioned studies used the FCI, a measure designed to include diagnoses that impact physical function (19). Typically, comorbidity indices are designed for mortality or administrative outcomes, such as length of hospital stay. They often include asymptomatic diagnoses (19) and may not be the best choice for studies concerned with arthritis and outcomes associated with HRQOL. The expanded FCI allowed us to assess the additive impact that comorbid diseases had on the physical and mental HRQOL of OA and RA patients.
The cross-sectional nature of the data do not allow for determination of causal relationship between arthritis and comorbidity and functional status. However, determination of causation was not the goal of this study. It is also possible that patients with severely disabling RA or OA, those with mild OA, those without internet access or computer literacy skills were underrepresented in our online survey sample, which impedes our ability to generalise the results to the greater population of OA and RA patients. We controlled for patient age, gender, education and perceived arthritis severity in our analyses since these variables have been associated with HRQOL in previous studies and likely vary between RA and OA patients. Further, results were based on self-reported data (e.g. diagnosis and comorbidities) not verified through clinical records or other assessment. Additionally, participants are more likely to under-report rather than over-report diagnoses (42) and patients may be less likely to recall a comorbid condition when asked an open-ended question rather than when prompted with a list. However, self-reported medical information for chronic diseases has been shown to have good sensitivity and specificity compared to patient medical record information (42). Additionally, several self-reported comorbidities were too vague or abbreviated to be assigned a comorbidity type in our sample.
Certain chronic conditions may have more impact on HRQOL than others and another possible limitation is the use of comorbidity counts rather than differential weighting of severity as some comorbidity indexes use (32). However, Groll et al. did not find that simple counts outperformed weighted counts (19). Further, count indexes are much easier for providers to use than weighted counts (19). The Functional Comorbidity Index is the first measure developed for use in the general population with physical function, not mortality, as the outcome of interest (19) and the expanded FCI allowed us to assess additional conditions, such as cancer, which have been shown to negatively and significantly impact multiple domains of HRQOL, including mental health (43).
Despite its limitations, this study has important clinical implications. In this sample, OA patients reported similar HRQOL as RA patients. Given the high prevalence of OA and that 10% of the population over 60 years of age have clinical problems attributable to OA (8), more focused research and clinical attention should be paid to this finding and future research would do well to consider other rheumatologic conditions. The SF-12 can be valuable in helping clinicians distinguish differences in patients’ physical and mental well-being and also help to better inform focus of treatment.
Several factors, including an aging population, an increasing prevalence of arthritis with age, and evidence that older people are at risk for comorbid conditions, point to an increasing likelihood that individuals seeking care for arthritis will commonly present with comorbid conditions (31). Comorbidity and poor HRQOL are factors that have been shown to affect arthritis patient outcomes, treatment adherence and reliance on health services (31). The important finding of the impact that increasing comorbidity has on functioning for both RA and OA patients in this study underscores the clinical importance of comprehensively considering and monitoring comorbidity over time because they are tied to worsening HRQOL status. Taking cognisance of comorbidity counts is a more feasible target for clinical consideration than weighting specific diseases (19). HRQOL and comorbidity assessment should be included as part of education interventions and arthritis self-management programmes and compliment other measures designed to assess patients’ experience with arthritis, such as the Patient Reported Experience Measures (44).
Conclusion
Arthritis negatively impacts patient HRQOL. In our sample, it seems that there are more similarities than differences in the shared experiences on the impact of OA and RA on HRQOL. Findings also highlight the importance of the cumulative impact of chronic comorbidities on HRQOL measures in patients with RA and OA.
Our results illustrate the complementary roles of the SF-12v2 and the expanded Functional Comorbidity Index in understanding how RA and OA and associated comorbidities affect HRQOL. Since the management of arthritis is an iterative process, generally focusing on controlling rather than curing the waxing and waning course of disease (5), RA and OA patients should be periodically and simultaneously evaluated for evidence of existing or emerging comorbid conditions and HRQOL status.
Acknowledgment
We would like to acknowledge the TraCS Institute at the University of North Carolina at Chapel Hill for assistance with recruitment, and Kristen Morella for her analytical support.
Funding:
this project was supported by the Thurston Arthritis Research Center Postdoctoral Fellowship (5T32-AR007416), Novartis Pharmaceuticals, and the ACR REF/Abbott Health Professional Graduate Student Research Preceptorship.
This project was also supported by UL1RR025747 from the National Center for Research Resources.
The content is solely the responsibilityof the authors and does not necessarily represent the official views of the National Center for Research Resources or the National Institutes of Health.
Footnotes
Competing interests: none declared.
References
- 1.Centers for Disease Conrol and Prevention: Prevalence of doctor-diagnosed arthritis and arthritis-attributable activity limitation -United States, 2010–2012. 2013: 869–73. [PMC free article] [PubMed] [Google Scholar]
- 2.Centers for Disease Control and Prevention: Prevalence and most common causes of disability among adults—United States. MMWR 2009; 58: 421–6.19407734 [Google Scholar]
- 3.BROOKS PM: The burden of musculoskeletal disease—a global perspective. Clin Rheumatol 2006; 25: 778–81. [DOI] [PubMed] [Google Scholar]
- 4.BREEDVELD FC, COMBE B: Understanding emerging treatment paradigms in rheumatoid arthritis. Arthritis Res Ther 2011; 13 (Suppl. 1): S3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.YOOD RA Guidelines ACORSORA: Guidelines for the management of rheumatoid arthritis: 2002. update. 2002.
- 6.MARTEL-PELLETIER J, BOILEAU C, PELLETIER J-P, ROUGHLEY PJ: Cartilage in normal and osteoarthritis conditions. Best Pract Res Clin Rheumatol 2008; 22: 351–84. [DOI] [PubMed] [Google Scholar]
- 7.WOOLF AD, PFLEGER B: Burden of major musculoskeletal conditions. Bull World Health Organ 2003; 81: 646–56. [PMC free article] [PubMed] [Google Scholar]
- 8.COOPER C, DENNISON E, EDWARDS M, LITWIC A: Epidemiology of osteoarthritis. Medicographia 2013; 35: 145–51. [Google Scholar]
- 9.MURPHY G, NAGASE H: Reappraising metalloproteinases in rheumatoid arthritis and osteoarthritis: destruction or repair? Nat Clin Pract Rheum 2008; 4: 128–35. [DOI] [PubMed] [Google Scholar]
- 10.MILI F, HELMICK CG, MORIARTY DG: Health related quality of life among adults reporting arthritis: analysis of data from the Behavioral Risk Factor Surveillance System, US, 1996–99. J Rheumatol 2003; 30: 160–6. [PubMed] [Google Scholar]
- 11.FURNER SE, HOOTMAN JM, HELMICK CG, BOLEN J, ZACK MM: Health-related quality of life of US adults with arthritis: analysis of data from the behavioral risk factor surveillance system, 2003, 2005, and 2007. Arthritis Care Res 2011; 63: 788–99. [DOI] [PubMed] [Google Scholar]
- 12.ALONSO J, FERRER M, GANDEK B et al. : Health-related quality of life associated with chronic conditions in eight countries: results from the International Quality of Life Assessment (IQOLA) Project. Qual Life Res 2004; 13: 283–98. [DOI] [PubMed] [Google Scholar]
- 13.HOPMAN W, HARRISON M, COO H, FRIEDBERG E, BUCHANAN M, VanDenKERKHOF E: Associations between chronic disease, age and physical and mental health status. Chronic Dis Can 2009; 29: 108–16. [PubMed] [Google Scholar]
- 14.PICAVET H, HOEYMANS N: Health related quality of life in multiple musculoskeletal diseases: SF-36 and EQ-5D in the DMC3 study. Ann Rheum Dis 2004; 63: 723–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.STRAND V, CRAWFORD B, SINGH J, CHOY E, SMOLEN J, KHANNA D: Use of “spydergrams” to present and interpret SF-36 health-related quality of life data across rheumatic diseases. Ann Rheum Dis 2009; 68: 1800–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.SLATKOWSKY-CHRISTENSEN B, MOWINCKEL P, LOGE JH, KVIEN TK: Health-related quality of life in women with symptomatic hand osteoarthritis: a comparison with rheumatoid arthritis patients, healthy controls, and normative data. Arthritis Care Res 2007; 57: 1404–9. [DOI] [PubMed] [Google Scholar]
- 17.DOMINICK KL, AHERN FM, GOLD CH, HELLER DA: Health-related quality of life among older adults with arthritis. Health Qual Life Outcomes 2004; 2: 5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.CHARLSON ME, CHARLSON RE, PETERSON JC, MARINOPOULOS SS, BRIGGS WM, HOLLENBERG JP: The Charlson comorbidity index is adapted to predict costs of chronic disease in primary care patients. J Clin Epidemiol 2008; 61: 1234–40. [DOI] [PubMed] [Google Scholar]
- 19.GROLL DL, TO T, BOMBARDIER C, WRIGHT JG: The development of a comorbidity index with physical function as the outcome. J Clin Epidemiol 2005; 58: 595–602. [DOI] [PubMed] [Google Scholar]
- 20.HUNGER M, THORAND B, SCHUNK M et al. : Multimorbidity and health-related quality of life in the older population: results from the German KORA-Age study. Health Qual Life Outcomes 2011; 9: 53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.GABRIEL S, CROWSON C, O’FALLON W: Comorbidity in arthritis. J Rheumatol 1999; 26: 2475–9. [PubMed] [Google Scholar]
- 22.WOLFE F, MICHAUD K, LI T, KATZ RS: Chronic conditions and health problems in rheumatic diseases: comparisons with rheumatoid arthritis, noninflammatory rheumatic disorders, systemic lupus erythematosus, and fibromyalgia. J Rheumatol 2010; 37: 305–15. [DOI] [PubMed] [Google Scholar]
- 23.LEE T-J, PARK BH, SON HK et al. : Cost of illness and quality of life of patients with rheumatoid arthritis in South Korea. Value Health 2012; 15: S43–S9. [DOI] [PubMed] [Google Scholar]
- 24.RADNER H, SMOLEN JS, ALETAHA D: Comorbidity affects all domains of physical function and quality of life in patients with rheumatoid arthritis. Rheumatology 2011; 50: 381–8. [DOI] [PubMed] [Google Scholar]
- 25.RUPP I, BOSHUIZEN HC, JACOBI CE, DINANT HJ, Van den BOS G: Comorbidity in patients with rheumatoid arthritis: effect on health-related quality of life. J Rheumatol 2004; 31: 58–65. [PubMed] [Google Scholar]
- 26.CRILLY MA, JOHNSTON MC, BLACK C: Relationship of EQ-5D quality of life with the presence of co-morbidity and extra-articular features in patients with rheumatoid arthritis. Qual Life Res 2013: 1–9. [DOI] [PubMed] [Google Scholar]
- 27.KRISHNAN E, HÄKKINEN A, SOKKA T, HANNONEN P: Impact of age and comorbidities on the criteria for remission and response in rheumatoid arthritis. Ann Rheum Dis 2005; 64: 1350–2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.HOSSEINI K, GAUJOUX-VIALA C, COSTE J et al. : Impact of co-morbidities on measuring indirect utility by the Medical Outcomes Study Short Form 6D in lower-limb osteoarthritis. Best Pract Res Clin Rheumatol 2012; 26: 627–35. [DOI] [PubMed] [Google Scholar]
- 29.TUOMINEN U, BLOM M, HIRVONEN J et al. : The effect of co-morbidities on health-related quality of life in patients placed on the waiting list for total joint replacement. Health Qual Life Outcomes 2007; 5: 16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.SALAFFI F, CAROTTI M, STANCATI A, GRASSI W: Health-related quality of life in older adults with symptomatic hip and knee osteoarthritis: a comparison with matched healthy controls. Aging Clin Exp Res 2005; 17: 255–63. [DOI] [PubMed] [Google Scholar]
- 31.GIJSEN R, HOEYMANS N, SCHELLEVIS FG, RUWAARD D, SATARIANO WA, van den BOS GA: Causes and consequences of comorbidity: a review. J Clin Epidemiol 2001; 54: 661–74. [DOI] [PubMed] [Google Scholar]
- 32.HUNTLEY AL JOHNSON R, PURDY S, VALDERAS JM, SALISBURY C: Measures of multimorbidity and morbidity burden for use in primary care and community settings: a systematic review and guide. Ann Fam Med 2012; 10: 134–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.CARPENTER DM, ELSTAD EA, BLALOCK SJ, DEVELLIS RF: Conflicting medication information: Prevalence, sources, and relationship to medication adherence. J Health Commun 2014; 1: 67–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.WARE JE, KOSINSKI M, TURNER-BOWKER DM, GANDEK B: How to score version 2 of the SF-12 health survey (with a supplement documenting version 1). QualityMetric Incorporated; 2002. [Google Scholar]
- 35.HAYS RD: Scoring the SF-12 version 2.0. 2003. [updated 2003; cited 2014 2/26/2014]; Available from: http://gim.med.ucla.edu/FacultyPages/Hays/utils/.
- 36.WARE JE Jr, KOSINSKI M, KELLER SD: A 12-item Short-Form Health Survey: construction of scales and preliminary tests of reliability and validity. Med Care 1996; 34: 220–33. [DOI] [PubMed] [Google Scholar]
- 37.HAYS RD, PRINCE-EMBURY S, CHEN H-Y: RAND-36 health status inventory. Psychological Corporation San Antonio, TX; 1998. [Google Scholar]
- 38.GANDHI SK, WARREN SALMON J, ZHAO SZ, LAMBERT BL, GORE PR, CONRAD K: Psychometric evaluation of the 12-item short-form health survey (SF-12) in osteoarthritis and rheumatoid arthritis clinical trials. Clin Ther 2001; 23: 1080–98. [DOI] [PubMed] [Google Scholar]
- 39.MAGLINTE GA, HAYS RD, KAPLAN RM: US general population norms for telephone administration of the SF-36v2. J Clin Epidemiol 2012; 65: 497–502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.WARE JE, KOSINSKI M: SF-36 physical & mental health summary scales: a manual for users of version 1. Quality Metric Inc; 2001. [Google Scholar]
- 41.CHIU Y, LAI M, LIN H et al. : Disease activity affects all domains of quality of life in patients with rheumatoid arthritis and is modified by disease duration. Clin Exp Rheumatol 2014; 32: 898–903. [PubMed] [Google Scholar]
- 42.FOWLES JB, FOWLER EJ, CRAFT C: Validation of claims diagnoses and self-reported conditions compared with medical records for selected chronic diseases. J Ambul Care Manage 1998; 21: 24–34. [DOI] [PubMed] [Google Scholar]
- 43.WEAVER KE, FORSYTHE LP, REEVE BB et al. : Mental and Physical Health–Related Quality of Life among US Cancer Survivors: Population Estimates from the 2010 National Health Interview Survey. Cancer Epidem Biomar 2012; 21: 2108–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.EL MIEDANY Y, EL GAAFARY M, YOUSSEF S, AHMED I, PALMER D: The arthritic patients’ perspective of measuring treatment efficacy: Patient Reported Experience Measures (PREMs) as a quality tool. Clin Exp Rheumatol 2014; 32: 547–52. [PubMed] [Google Scholar]


