Abstract
Paternal environmental perturbations can influence the physiology and behavior of offspring. For example, our previous work showed reduced cocaine reinforcement in male, but not female, progeny of rat sires that self-administered cocaine. The information transfer from sire to progeny may occur through epigenetic marks in sperm, encompassing alterations in small noncoding RNAs, including microRNAs (miRNAs) and/or DNA methylation. Here, no reliable changes in miRNAs in the sperm of cocaine- relative to saline-experienced sires were identified. In contrast, 272 differentially methylated regions were observed in sperm between these groups. Two hypomethylated promoter regions in the sperm of cocaine-experienced rats were upstream of cyclin-dependent kinase inhibitor 1a (Cdkn1a). Cdkn1a mRNA also was selectively increased in the NAc of cocaine-sired male (but not female) offspring. Cocaine self-administration also enhanced Cdkn1a expression in the accumbens of cocaine-sired rats. These results suggest that changes in Cdkn1a may play a role in the reduced cocaine reinforcing efficacy observed in cocaine-sired male rats. Introducing a 90 d delay between sire self-administration and breeding reversed both cocaine resistance and the increase in accumbens Cdkn1a mRNA in male offspring, indicating that cocaine-induced epigenetic modifications are eliminated with sperm turnover. Collectively, our results indicate that cocaine self-administration produces hypomethylation of Cdkn1a in sperm and a selective increase in the expression of this gene in the NAc of male offspring, which is associated with blunted cocaine reinforcement.
SIGNIFICANCE STATEMENT The relatively new field of transgenerational epigenetics explores the effects of environmental perturbations on offspring behavior and physiology. Our prior work in rats indicated that male, but not female, progeny of sires that self-administered cocaine displayed reduced cocaine reinforcement. The information transfer from sire to progeny may occur through heritable epigenetic marks in sperm, including DNA methylation. The present findings revealed two hypomethylated promoter regions upstream of the Cdkn1a gene in sire sperm. Remarkably, Cdkn1a expression was selectively decreased in offspring NAc, a brain region that regulates cocaine reinforcement.
Keywords: addiction, epigenetics, intergenerational, self-administration, transgenerational
Introduction
It is now clear that environmental changes experienced by males, including chronic stress or a high-fat diet as well as prolonged exposure to toxins or drugs of abuse, can result in physiological and behavioral changes in offspring (Bale, 2015; Pierce et al., 2018; Cunningham et al., 2021). For example, the offspring of male rats subjected to chronic stress displayed dysregulation in the hypothalamic-pituitary-adrenal axis response to stress both physiologically and behaviorally (Dietz et al., 2011; Rodgers et al., 2013; Gapp et al., 2014). These stress-associated changes in offspring were associated with increases in sire sperm miRNAs (Rodgers et al., 2013, 2015; Gapp et al., 2014). Moreover, injection of these miRNAs into fertilized WT embryos recapitulated the behavioral and metabolic changes in offspring (Gapp et al., 2014; Rodgers et al., 2015). In terms of drugs of abuse, several studies have assessed the effects of rodent sire cocaine exposure on various offspring behaviors. Examining addiction-related behaviors in cocaine-sired offspring generally revealed reductions in cocaine self-administration (Vassoler et al., 2013; Le et al., 2017), cocaine preference (Yaw et al., 2019), and conditioned place preference (Fischer et al., 2017). Interestingly, in studies where sires voluntarily self-administered cocaine (Vassoler et al., 2013; Le et al., 2017) or consumed oral cocaine (Yaw et al., 2019), the effects were specific to male offspring. It also has been shown that paternal cocaine self-administration resulted in spatial object learning deficits (Wimmer et al., 2017) and inhibited cocaine-evoked locomotor sensitization (Wimmer et al., 2019) selectively in male offspring. The effects of paternal cocaine on other behaviors produced equivocal results with reports of increases (White et al., 2016; Fischer et al., 2017) or no effect (Killinger et al., 2012; Le et al., 2017) on anxiety-like behavior in offspring. Likewise, some but not all studies have indicated cognitive deficits in progeny of cocaine-exposed sires (He et al., 2006; Killinger et al., 2012; Fischer et al., 2017; Wimmer et al., 2017). There are a number of experimental discrepancies (species, mode of drug administration, etc.) that may explain the lack of agreement among studies in some cases. Overall, however, this emerging literature has identified several reliable examples of cross-generational effects of paternal cocaine exposure.
In experiments of this sort, the sires are removed immediately after breeding and therefore have no impact on offspring during gestation or rearing. Previous work indicated that dam exposure to a cocaine-experienced sire also had no influence on maternal behaviors (Vassoler et al., 2013). Therefore, the mechanism whereby information is inherited in as little as one generation is almost certainly reprogramming of germline epigenetic marks, such as DNA methylation and histone post-translational modifications as well as changes in miRNA or small RNA expression. The vast majority of DNA methyl marks are erased following fertilization, and nearly all histones and their associated post-translational modifications are replaced in sperm by protamines, which facilitate even greater chromatin compaction. Moreover, following fertilization, paternal protamines are replaced by histones of maternal origin (Puri et al., 2010). However, some parental DNA methylation is conserved in the germline (Hackett et al., 2013), and a small but significant proportion of paternal histones and their epigenetic marks are retained following fertilization (Hammoud et al., 2009; Brunner et al., 2014; Carone et al., 2014), both of which can produce profound effects on development. Paternal cocaine self-administration produced changes in sperm DNA methylation (Le et al., 2017) as well as the acetylation of sperm histone 3 (H3) associated with Bdnf (Vassoler et al., 2013). Paternal cocaine self-administration also increased BDNF expression in the mPFC (Vassoler et al., 2013; Le et al., 2017) of male offspring and blocking BDNF signaling in this nucleus reversed the cocaine resistance phenotype (Vassoler et al., 2013). These findings, together, are consistent with conserved paternal germline epigenetic marks representing the primary mechanism for rapid cocaine-induced cross-generational inheritance.
Mature sperm cells are epigenetically and transcriptionally inert, suggesting that potential reprogramming of epigenetic marks must occur during spermatogenesis, which occurs over 6-8 weeks in rodents (Bale, 2015). In contrast, paternal small RNAs, including miRNAs and tsRNAs, can interact with mature sperm in the epididymis, resulting in developmental impacts following fertilization (Rodgers and Bale, 2015; Rodgers et al., 2015; Chen et al., 2016; Sharma et al., 2016; Conine et al., 2018, 2019; Sharma et al., 2018; Chan et al., 2020; Trigg et al., 2021; Wang et al., 2021; Zhang et al., 2021). For example, paternal ethanol exposure altered levels of several noncoding RNAs similarly in both sperm and epididymosomes (Finegersh et al., 2015; Rompala et al., 2018), results that are consistent with transfer of miRNAs via exosomes as sperm travels through the epididymis. The current work focused on the effects of paternal cocaine on DNA methylation and miRNA abundance in sperm, the potential persistence of these germline effects as well as potential functional effects of these germline epigenetic modifications on offspring behavior. Results indicated that two hypomethylated promoter regions in the sperm of cocaine-experienced rats were upstream of the cyclin-dependent kinase inhibitor 1a (Cdkn1a) gene, a prominent regulator of cell cycle progression (Liu and Lozano, 2005; Dutto et al., 2015). Cdkn1a also is expressed in adult brain neurons (Renthal et al., 2009; Scholpa et al., 2016; Riessland et al., 2019), and its mRNA was shown to be selectively increased in the NAc of cocaine-sired male offspring, suggesting that Cdkn1a (which encodes for the p21 protein) may play a previously unappreciated role in NAc behavioral plasticity associated with cocaine exposure.
Materials and Methods
Animals and housing
Sires and dams (F0 generation) or naive rats were Sprague Dawley rats weighing 250-300 g obtained from Taconic Laboratories. Sires were housed individually, except for 1 week of pair housing during the mating period. Food and water were available ad libitum for all experiments. Rats were kept on a 12 h-12 h light-dark cycle with experiments performed during the light phase. All animal care and experiments were approved by the Institutional Animal Care and Use Committee and conducted in accordance with the National Institute of Health guidelines.
Surgery
Rats were anesthetized using a ketamine/xylazine cocktail (80 and 12 mg/kg, respectively). Silastic catheters were implanted into the right jugular vein, sutured in place, and mounted on the shoulder blade using a mesh backmount. Catheters (SAI) were flushed daily with an antibiotic (Timentin, 0.93 mg/ml) dissolved in heparinized saline and sealed using plastic obturators when not in use. For virally mediated gene delivery experiments, immediately following the implantation of indwelling catheters, the rats were mounted in a stereotaxic apparatus (Kopf Instruments) and 1 µl of pLenti-C-mGFP (TR30021V; Origene), pLenti-Cdkn1a shRNA-mGFP (TL712469V; Origene), pLenti-C-mGFP (PS100071; Origene), or pLenti-Cdkn1a ORF-mGFP (RR209648L2V; Origene) was infused bilaterally over 5 min into the NAc shell according to the following coordinates, relative to bregma (Paxinos and Watson, 1997): 1.0 mm AP, ±1.0 mm ML, −7.0 mm DV. Rats recovered for at least 7 d.
Drugs
Cocaine and cocaine methiodide, both gifts from the U.S. National Institute on Drug Abuse, were dissolved in bacteriostatic saline.
Self-administration
Sire and offspring self-administration experiments were performed as previously described (Vassoler et al., 2013). Self-administration was conducted in Med Associates operant chambers comprised of two response levers (active and inactive), house light, and injection pumps. The self-administration equipment was enclosed in ventilated, sound-attenuating chambers. Cocaine sires were placed in the operant chambers and allowed to lever press for cocaine infusions (0.25 mg cocaine per 56 μl saline per infusion over 5 s) on a fixed ratio 1 (FR1) schedule. Saline sires were yoked to cocaine sires and received an infusion of saline for each cocaine infusion earned by a conspecific. Yoked-cocaine sires received a passive infusion of cocaine each time an infusion was earned by a designated cocaine self-administering sire. Yoked-cocaine methiodide sires received a passive infusion of cocaine methiodide (0.33 mg/infusion; equimolar dose of cocaine) each time an infusion was earned by a designated cocaine self-administering sire. Each infusion was followed by a 20 s timeout period. Sires were limited to a maximum of 75 infusions for each daily 2 h self-administration session for 60 d.
F1 offspring rats were trained to self-administer cocaine (1.0 mg/kg/inf) as described, except that there were 10 daily self-administration sessions. A separate group of F1 offspring were trained to self-administer sucrose under identical parameters, except that responding on the active lever on an FR1 schedule resulted in delivery of a sucrose pellet (45 mg/pellet) in 10 daily 1-2 h sessions. For experiments with cocaine methiodide-sired rats, following 10 d of cocaine self-administration, rats were switched to an FR5 schedule for 2 d and then to a progressive ratio (PR) schedule for 1 training day and 1 test day. Under the PR schedule, the response requirement for subsequent drug delivery increased until the session was terminated 30 min after the last completed schedule. The response requirement for the “ith” reinforcement was R(1) = [5e°.2i-5] (Richardson and Roberts, 1996). The breakpoint was operationally defined as the total number of responses for the last completed schedule. Upon completion of behavioral experiments, catheter patency of cocaine self-administering rats was confirmed by intravenous infusion of ketamine (0.1 ml).
Virally infused rats were given 2.5-3.5 weeks after surgery to ensure knockdown or overexpression of Cdkn1a and expression of GFP control before initiation of cocaine (1.0 mg/kg/inf) self-administration as described above. Rats responded for cocaine on an FR1 schedule for 10 d. The following day, rats were switched to an FR5 schedule for 2 d and then to a PR schedule for 2 d. Under the PR schedule, the response requirement for subsequent drug delivery increased until the session was terminated 30 min after the last completed schedule. The response requirement for the “ith” reinforcement was R(1) = [5e°.2i-5] (Richardson and Roberts, 1996). The breakpoint was operationally defined as the total number of responses for the last completed schedule. Upon completion of behavioral experiments, catheter patency was confirmed by intravenous infusion of ketamine (0.1 ml). Twenty-four hours after the last self-administration session, rats were killed, viral placement was visualized using NIGHTSEA DFP Dual Fluorescent Protein Excitation Flashlight with NIGHTSEA Barrier Filter Glasses (Electron Microscopy Services), and the fluorescent region of the NAc was harvested.
Breeding
Sires were pair-housed with drug-naive females 24 h after the last session. Animals remained co-housed until vaginal plugs were found or for a maximum of 7 d, then sires were killed and sperm was harvested. The resulting F1 offspring were weaned at 21 d and remained group-housed (2 or 3 per cage) until the onset of experiments (beginning at ∼60 d).
Delayed mating experiments were identical, except that sires were returned to individual housing and again paired for up to 7 d with unique drug-naive females at 45 and 90 d following cessation of self-administration. Blank mating at 45 d was intended to eliminate remaining cocaine-exposed sperm; females were killed and no litters were generated. At 90 d after cocaine self-administration, sires were mated with new drug-naive females. F1 offspring were weaned as above. Only 1 or 2 males/litter/experiment were used, so no litter was overrepresented.
Reduced representation bisulfite sequencing (RRBS)
RRBS libraries were generated using previously published protocols and the gel-free technique (Gu et al., 2011; Boyle et al., 2012). Briefly, 500 ng of sperm DNA was digested using the methylation-sensitive restriction enzyme, MspI, followed by end repair and A-tailing. Small fragments of DNA were then removed using AMPure XP beads (Beckman Coulter). After ligation to methylated adapters (Illumina), DNA samples underwent two rounds of bisulfite conversion followed by bead clean-up. RRBS libraries were prepared by large-scale PCR and assessed for quality before sequencing. Sperm DNA libraries (n = 6 for each condition) were then multiplexed for paired end balanced sequencing in two lane of a HiSeq 2000 Sequencer (Illumina) followed by initial data processing and alignment of reads by the software pipeline bsmap version 2.6 (Xi and Li, 2009). MethylKit software (version 0.5.3) was used for determination of differentially methylated regions (DMRs) between cocaine-exposed and saline-exposed sperm. This software implements the Benjamini-Hochberg false discovery rate-based method for p-value correction, and only DMRs passing the q-value threshold (q = 0.01) were considered. Analysis was based on 100 bp stepwise tiling windows, ≥2 CpG per tile as well as ≥10 × CpG coverage of each tile per sample. The methylation level of a 100 bp tile was the average of all CpGs within the tile. If significant changes of DNA methylation between cocaine- and saline-exposed sperm exceeded 20%, the tile was designated as a DMR; further annotation of DMRs was performed by the HOMER software version 3.51. All data and protocols will be made available in a freely accessible public repository.
Small RNA sequencing
For each sperm sample (n = 6/group, cocaine- and saline-exposed), small RNAs were extracted using the mirVana miRNA Isolation Kit (Thermo Fisher Scientific) according to the manufacturer's instructions. RNA quality was assessed on a BioAnalyzer 2100 (Agilent), and sequencing libraries were prepared with 10-50 ng of RNA as template using the TruSeq Small RNA Library Prep Kit (Illumina) according to the manufacturer's instructions. Libraries were size-selected using electrophoresis to enrich for mature miRNAs. Libraries were sequenced on a NextSeq500. Raw .fastq sequencing files were then quality-filtered, trimmed, and aligned on an index of all mature rat miRNA sequences obtained from miRBase (http://mirbase.org) using the STAR aligner (https://github.com/alexdobin/STAR) (Dobin et al., 2013). Principal component analysis was performed in R. Differential expression analysis was performed using the DESeq2 package (Love et al., 2014).
qRT-PCR
We extracted total RNA using the RNeasy kit (QIAGEN) according to the manufacturer's instructions. RNA concentration and purity were quantified by NanoDrop spectrophotometry (Thermo Fisher Scientific). Generation of cDNA was performed using the SuperScriptIII Reverse Transcriptase kit (Thermo Fisher Scientific) with 50 ng of RNA as template. For miRNA qPCRs, cDNA content was first preamplified using the TaqMan PreAmp Master Mix (Thermo Fisher Scientific). Reactions were prepared in 96-well optical reaction plates (ABI) with optical adhesive covers (ABI) using Taqman Fast Universal PCR Master Mix (Invitrogen). Reactions were conducted in the Step One Plus with an initial incubation at 95°C for 10 min, and 40 subsequent cycles of 95°C for 15 s, 60°C for 30 s. The Taqman Assay IDs for the probes used in these experiments are as follows: Cdkn1a, Rn00589996_m1; SGCE, Rn01456231_m1; 18S RNA, 4333760F; GAPDH, Rn01775763_g1; rno-miR-1b, 002222; rno-miR-1-3p, 002064; rno-miR-206-3p, 000510; rno-miR-362-3p, 002616; and rno-miR-101b-3p, 002531. ΔCt values were corrected using housekeeping gene expression levels for each sample, and fold change was calculated as 2(–ΔΔCt). Presented is the mean of three technical replicates per biological replicates with n = rats/group.
Statistical analyses
Data were analyzed in GraphPad Prism 7, and p < 0.05 was the criteria for significance, unless otherwise stated above. Offspring cocaine and sucrose self-administration were analyzed with separate two-way mixed model ANOVA with a between-subject factor of sire exposure (saline-sired, yoked-cocaine-sired, and cocaine-sired) and repeated measures over time (days). Dunnett's was used for post hoc comparisons with saline-sired rats as the control group. PR responding was analyzed by one-way ANOVA with the factor of sire (cocaine, yoked-saline, yoked-cocaine methiodide) and Bonferroni post hoc comparison. Cocaine and sucrose self-administration in delayed mating offspring were analyzed with separate two-way mixed model ANOVA with a between-subject factor of sire exposure (saline-sired vs cocaine-sired) and repeated measures over time (days). Unpaired two-tailed t tests were used to analyze RNA and mi-RNA levels in cocaine-sired versus saline sired rats.
Cocaine self-administration in Cdkn1a shRNA rats relative to GFP controls was analyzed with two-way mixed model ANOVA with a between-subject factor of viral vector infusion (Cdkn1a shRNA vs GFP) and repeated measures over time (days). Cocaine self-administration in Cdkn1a-overexpressing rats relative to GFP controls was analyzed with two-way mixed model ANOVA with a between-subject factor of viral infusion (Cdkn1a overexpression vs GFP) and repeated measures over time (days). Linear regression was used to assess the relationship between cocaine self-administration and Cdkn1a expression in virally mediated Cdkn1a-overexpressing or GFP control rats. Unpaired two-tailed t test was used to analyze breakpoint on a PR test in Cdkn1a shRNA rats or Cdkn1a-overexpressing rats versus GFP controls. Unpaired two-tailed t test was used to analyze RNA levels in virally infused rats versus GFP controls.
Results
Cocaine reprograms sperm DNA methylation
Male rats were trained to self-administer cocaine, whereas yoked conspecifics received passive infusions of saline (Fig. 1A). We examined gene expression of the primary physiological targets of cocaine (i.e., the dopamine transporter, the norepinephrine transporter, and the serotonin transporter) in the testes of cocaine and saline sires. Liver tissue was included as a negative control for the dopamine and serotonin transporters (Vairetti et al., 2012). Each biogenic amine transporter was expressed in the testes, and levels were similar between cocaine and saline sires (Fig. 1B), indicating that there are substrates for direct action of cocaine in testes, as previously reported (Li et al., 1997).
Figure 1.
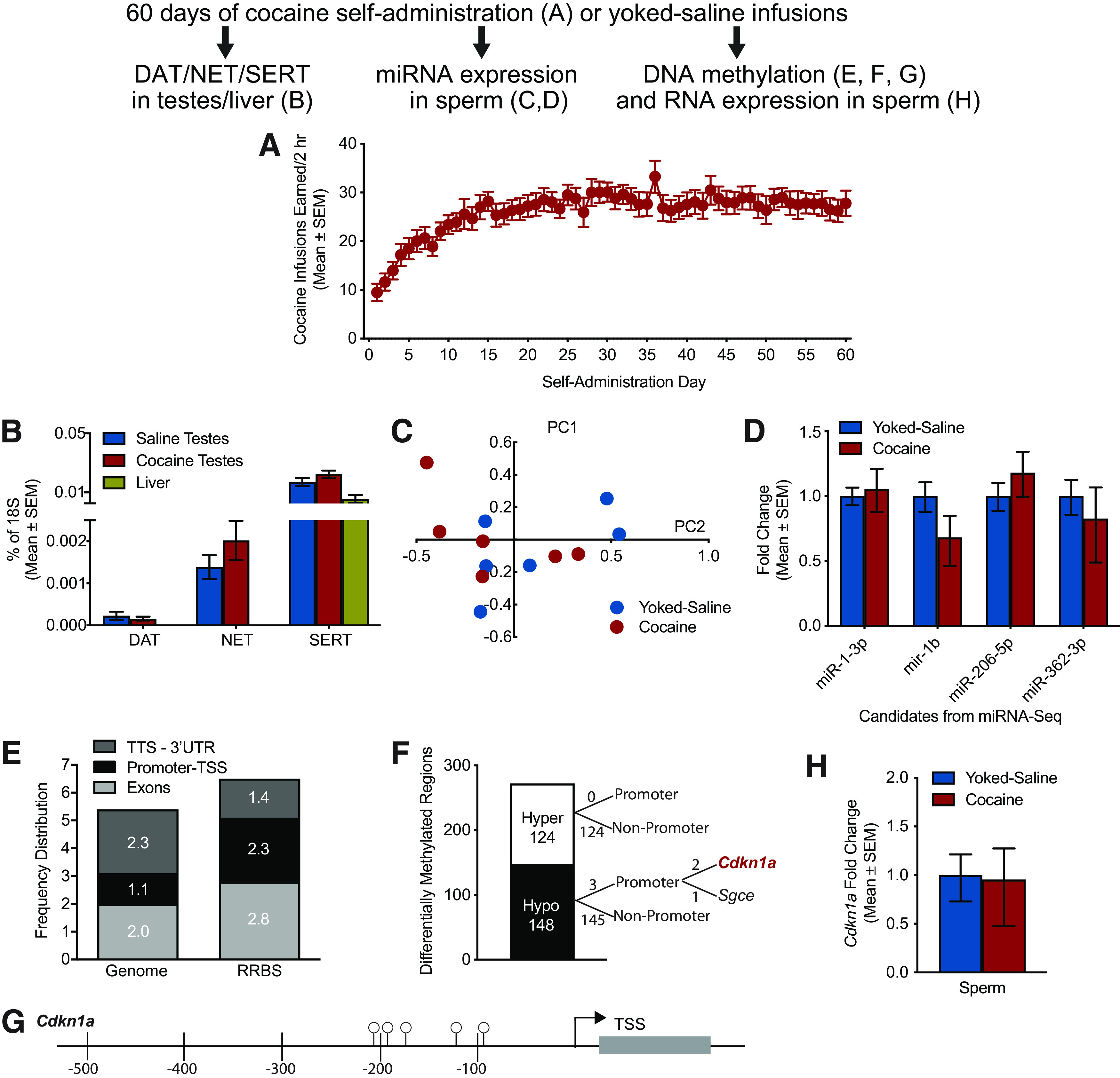
Cocaine self-administration elicits changes in DNA methylation but not miRNA content of sperm. Heading indicates the timeline of experiments. A, Mean daily (±SEM) infusions earned for cocaine self-administering sires across 60 d (n = 18). B, The dopamine transporter (DAT), norepinephrine transporter (NET), and serotonin transporter (SERT) are expressed in the testes and at similar levels in saline sires versus cocaine sires (n = 3-5/group). C, Principal component analysis of miRNA-seq data revealed important overlap between the cocaine-exposed and saline-exposed sperm samples (n = 6/group). D, qPCR analysis on a second cohort of sperm samples (n = 5/group) failed to validate the candidate gene expression changes identified by miRNA sequencing. E, The frequency distribution of methylation in distinct regions of the genome is plotted for a representative whole genome (left) and for the RRBS results (right; n = 6/group). Not depicted are intergenic regions (55.3% Genome vs 65.9% RRBS) and introns (39.3% Genome vs 27.6% RRBS). The RRBS approach enriched for promoter regions (2.3% vs 1.1% of the genome). F, Of 272 differentially methylated regions identified in cocaine sire versus saline sire sperm, 124 were hypermethylated and 148 were hypomethylated. There were three hypomethylated sites in promoter regions: two for Cdkn1a and one for Sgce. G, Five hypomethylated CpG islands within two hypomethylated regions of the Cdkn1a promoter are depicted. The full list of differentially methylated regions in the sperm of cocaine sires versus saline sires is presented in Extended Data Table 1-1. H, Cdkn1a RNA expression is identical in the sperm of cocaine and yoked-saline sires (n = 7/group).
Supplementary material. Download Table 1-1, XLS file (120.5KB, xls) .
Small RNA-seq was performed on sperm samples from cocaine-exposed rats and their saline-yoked conspecifics to investigate potential changes in sperm miRNA content. Six to 9 million reads were obtained per sample, of which 35%-45% mapped to the annotated miRNA database. Principal component analysis revealed significant overlap between the cocaine-exposed samples and the saline-yoked samples (Fig. 1C); importantly, within-cohort variability was on the same scale as the variability between cohort. Although typical for sperm samples, such variability hinders statistical analyses and can lead to potential false negatives or false positives. Nonetheless, analysis of small RNA-seq results revealed differential expression of 4 miRNAs (false discovery rate < 0.1): rno-miR-1-3p, rno-miR-1b, rno-miR-206-3p, and rno-miR-362-3p. However, qPCR validation experiments on a second sperm sample cohort revealed no significant differences in expression for those 4 candidates (rno-miR-1-3p: t(1,8) = 0.3241, p = 0.7542; rno-miR-1b: t(1,8) = 1.036, p = 0.3306; rno-miR-206-3p: t(1,8) = 0.9188, p = 0.3851; rno-miR-362-3p: t(1,8) = 0.5712, p = 0.6190; Fig. 1D). Together, these results suggest that sperm miRNA content and composition are not consistently and reliably affected by cocaine exposure.
Figure 1E shows the frequency distribution of methylated sites in distinct regions of the genome for a representative whole genome and for the current RRBS results. Eleven to 20 million reads were sequenced per sample, and the average coverage ranged from 16× to 30×. As expected, methylation detected by the RRBS approach was enriched in promoter regions (2.3%) versus expected genomic distribution (1.1%). Of 272 DMRs identified in cocaine sire versus saline sire sperm, 124 were hypermethylated and 148 were hypomethylated (Fig. 1F). There were three hypomethylated regions localized to gene promoters: two upstream of cyclin dependent kinase inhibitor 1a (Cdkn1a) and one upstream of sarcoglycan e (Sgce). Promoters were conservatively defined according to the rat genome annotation as the 500 bp regions upstream of an annotated gene. This region usually encompasses the minimal, TATA-box containing promoter (which it does in the case of Cdkn1a). In addition, this proximal region has been shown to effectively control Cdkn1a expression in human cells (Scibetta et al., 2010), and its methylation has been shown to affect gene expression in rat cells (Allan et al., 2000). The only two other promoter-related DMRs were located upstream of a pseudogene (Hmg1l1) and an automatically annotated miRNA (miR-678) and were therefore not considered. The two 100 bp hypomethylated regions of Cdkn1a, beginning at −287 and −187 bp upstream of the TSS, containing 5 hypomethylated CpG islands, are depicted in Figure 1G. The full list of differentially methylated regions in the sperm of cocaine sires versus saline sires is presented in Extended Data Table 1-1. Cdkn1a RNA expression was identical in the sperm of cocaine sires and yoked-saline controls (t(1,12) = 0.0979, p = 0.9236; Fig. 1H), supporting a role for epigenetic inheritance rather than a local effect of altered Cdkn1a RNA levels within sperm. These data suggest that cocaine-evoked hypomethylation of Cdkn1a and Sgce in sperm may be a putative mechanism for altered cocaine self-administration phenotypes in male offspring.
Cocaine-sired male offspring display cocaine resistance concomitant with Cdkn1a overexpression in the NAc
Saline-sired, cocaine-sired, and yoked-cocaine-sired male rat offspring were trained to self-administer cocaine (Fig. 2A) or sucrose (Fig. 2B) on an FR1 schedule of reinforcement. Cocaine-sired and yoked-cocaine-sired rats self-administered less cocaine than saline-sired rats. For cocaine self-administration, there was no main effect of sire (F(2,21) = 1.575, p = 0.2305), a main effect of day (F(9,189) = 6.356, p < 0.0001), and a sire by day interaction (F(18,189) = 1.848, p = 0.0226). Dunnett's post hoc comparisons revealed that, relative to saline-sired rats, there was a significant difference in yoked-cocaine-sired rats on day 10 (#p < 0.05) and a significant difference in cocaine-sired and yoked-cocaine-sired rats on day 9 (*p < 0.05). For sucrose self-administration, there was no main effect of sire (F(2,29) = 0.2426, p = 0.7862), a main effect of day (F(9,261) = 39.27, p < 0.0001), and no sire by day interaction (F(18,261) = 0.5521, p = 0.9303). These data suggest motivated responding for cocaine in sires is not necessary to evoke reduced cocaine self-administration in male offspring.
Figure 2.
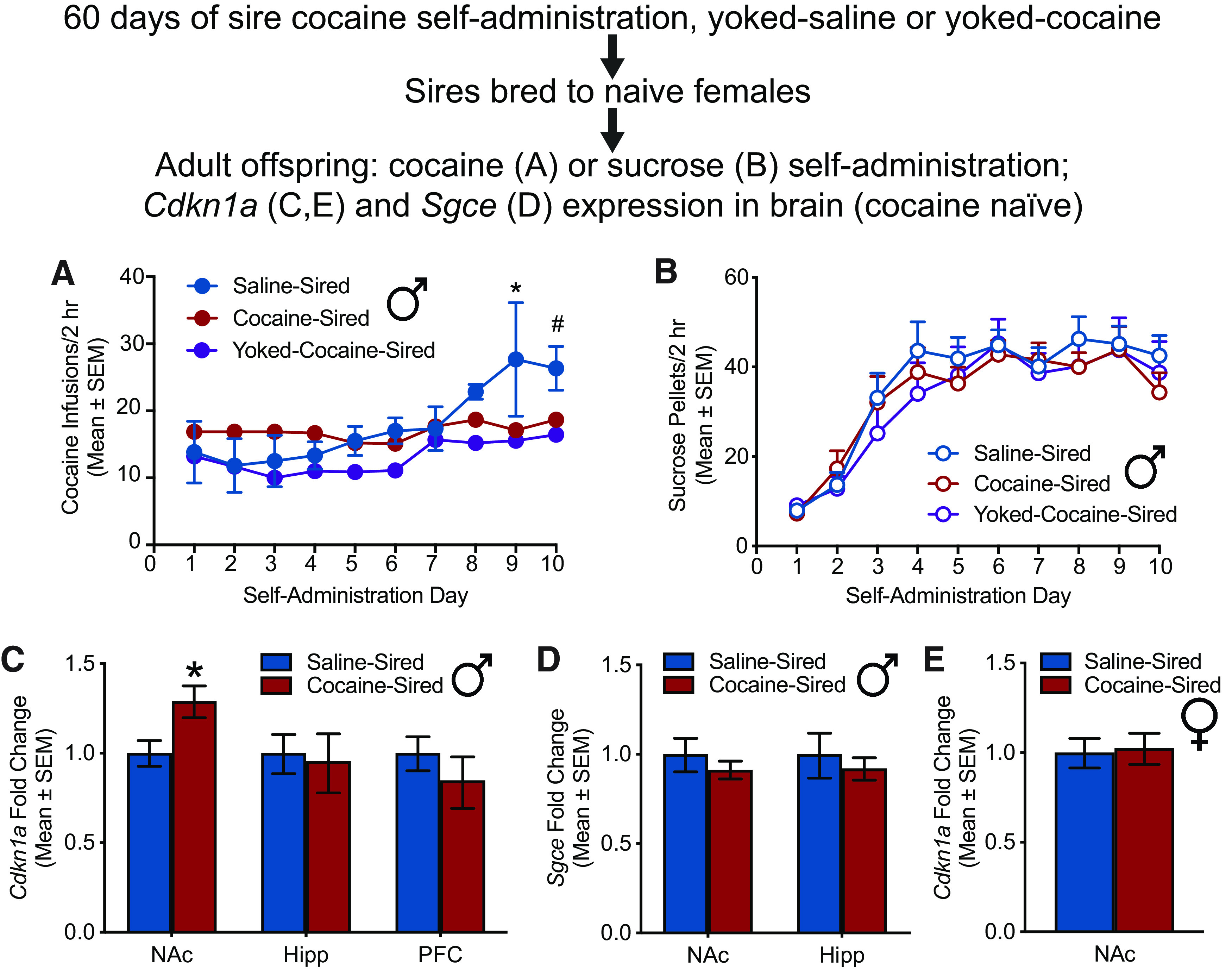
Reduced cocaine self-administration in cocaine-sired male offspring is associated with higher gene expression of Cdkn1a in NAc. A, Cocaine self-administration was attenuated in cocaine-sired (*p < 0.05 vs saline-sired on day 9; n = 9 from 7 litters) and yoked-cocaine-sired (*p < 0.05 vs saline-sired on day 9; #p < 0.05 vs saline-sired on day 10; n = 9 from 5 litters) relative to saline-sired male F1 offspring (n = 6 from 3 litters). B, Sucrose self-administration did not differ among cocaine-sired (n = 12 from 6 litters), saline-sired (n = 10 from 6 litters), and yoked-cocaine-sired male F1 offspring (n = 10 from 5 litters). C, Cdkn1a gene expression was higher in the NAc of cocaine-sired (n = 5 from 5 litters) versus saline-sired male F1 offspring (*p < 0.05 vs saline-sired, n = 6 from 6 litters). There was no difference in Cdkn1a expression in the hippocampus or mPFC of saline-sired (n = 6 from 6 litters) and cocaine-sired offspring (n = 6 from 6 litters). D, Sgce gene expression in the NAc or hippocampus was not altered in cocaine-sired (n = 6 from 6 litters) versus saline-sired male F1 offspring (n = 6 from 6 litters). E, Cdkn1a gene expression in the NAc did not differ in cocaine-sired (n = 6 from 6 litters) versus saline-sired female F1 offspring (n = 6 from 6 litters).
NAc, hippocampus, and mPFC were harvested from drug-naive F1 saline-sired and cocaine-sired rats, and gene expression of Cdkn1a and Sgce was evaluated by qPCR (Fig. 2C–E). In the NAc, Cdkn1a mRNA levels were higher in cocaine-sired versus saline-sired male rats (t(1,9) = 2.508, p = 0.0334; Fig. 2C). There was no difference in Cdkn1a expression in the hippocampus (t(1,10) = 0.2143, p = 0.8346) or the mPFC (t(1,10) = 0.8536, p = 0.4133), which suggests that paternal cocaine exposure results in selective Cdkn1a overexpressed in particular regions of the brain. There was no difference in Sgce expression in the NAc (t(1,10) = 1.445, p = 0.1791) or hippocampus (t(1,10) = 0.5796, p = 0.5750; Fig. 2D). We have previously demonstrated that, in contrast to male offspring, cocaine-sired female offspring show no reduction in cocaine self-administration (Vassoler et al., 2013). Consistent with these findings, there was no difference in NAc Cdkn1a expression in cocaine-sired versus saline-sired female offspring (t(1,10) = 0.141, p = 0.8907; Fig. 2E). Together, these results suggest that overexpression of accumbens Cdkn1a in the male offspring of cocaine sires may contribute to the cocaine resistance phenotype.
Motivation for cocaine is similarly blunted in male offspring of sires exposed to cocaine or the blood–brain barrier impermeable analog cocaine methiodide
The data described above illustrate a potential role for the direct action of cocaine on sperm to produce epigenetic modifications and behavioral effects in offspring. Cocaine methiodide, an analog of cocaine that does not penetrate the blood–brain barrier, was used to determine whether direct effects of cocaine on the brains of sires are required to suppress the motivation for cocaine in male offspring. Saline-sired, cocaine-sired, and yoked-cocaine methiodide-sired male offspring were trained to self-administer cocaine, and motivation for cocaine was assessed in a PR session (Fig. 3). Two rats were removed from the study because of loss of catheter patency. There was no main effect of sire (F(2,32) = 0.093, p = 0.9115), a main effect of day (F(9,285) = 43.70, p < 0.0001), and no sire by day interaction (F(18,285) = 0.731, p = 0.7788) for cocaine self-administration on an FR1 schedule in this group of rats. Rats were then trained to self-administer cocaine on an FR5 schedule for 2 d before consecutive PR sessions training and test sessions to assess motivation for cocaine. There was no difference in infusions earned on an FR5 schedule. There was no main effect of sire (F(2,32) = 0.5071, p = 0.6070), no main effect of day (F(1,32) = 1.440, p = 0.2390), and no vector by day interaction (F(2,32) = 0.5699, p = 0.5712). Breakpoint, operationally defined as the last schedule completed in the PR test session, was used at the index of motivation. A one-way ANOVA revealed a significant main effect (F(2,32) = 4.393, p = 0.0206). Bonferroni post hoc comparisons showed that, relative to saline-sired offspring, motivation for cocaine was significantly and similarly suppressed in cocaine-sired and cocaine methiodide-sired male offspring (p < 0.05). These data indicate that a direct effect of cocaine in the brains of sires is not necessary to elicit behavioral consequences in offspring.
Figure 3.
Motivation for cocaine is similarly diminished in male offspring of sires exposed to cocaine or the analog cocaine methiodide, which does not penetrate the blood–brain barrier. Heading indicates the timeline of experiments. Sire self-administration was truncated at 55 d because of limited availability of cocaine methiodide. Cocaine-sired (n = 12 from 11 litters) and cocaine methiodide-sired (n = 12 from 8 litters) male F1 offspring display reduced motivation for cocaine, indicated by reduced breakpoint in a PR session relative to saline-sired offspring (*p < 0.05; n = 11 from 10 litters).
Protracted abstinence from cocaine in sires reverses altered cocaine-taking and Cdkn1a gene expression in offspring
A delayed mating strategy was used to determine whether cocaine resistance and associated epigenetic changes in the sperm were embedded in the germline or are constrained to the developing sperm and are thus eliminated once the population of sperm is refreshed. Saline and cocaine sires were mated 90 d after cocaine self-administration with blank mating occurring after 45 d (timeline depicted in Fig. 4A). Adult male offspring from sires mated 90 d following saline or cocaine self-administration were evaluated for cocaine (Fig. 4B) and sucrose self-administration (Fig. 4C). Two rats were removed from the cocaine self-administration study because of loss of catheter patency. For cocaine self-administration, there was no main effect of sire (F(1,17) = 0.002, p = 0.9647), a main effect of day (F(9,153) = 22.47, p < 0.0001), and no sire by day interaction (F(9,153) = 1.235, p = 0.2776). For sucrose self-administration, there was no main effect of sire (F(1,10) = 2.054, p = 0.1823), a main effect of day (F(9,90) = 8.351, p < 0.0001), and no sire by day interaction (F(9,90) = 0.6237, p = 0.7740). Cdkn1a expression was measured by qPCR of the NAc of delayed mating saline-sired and cocaine-sired male offspring. There was no difference in mRNA levels of Cdkn1a in the NAc of cocaine-sired versus saline-sired male offspring (t(1,10) = 0.1469, p = 0.8862; Fig. 4D). These results suggest that reduced cocaine self-administration and overexpression of accumbens Cdkn1a expression in the male offspring of cocaine sires are normalized to saline-sired levels when the mating of the sires is delayed, suggesting that, if these phenotypes are indeed caused by cocaine-evoked hypomethylation at the Cdkn1a promoter, epigenetic marks may be washed out when sperm are replenished, rather than permanently encoded in the germline.
Figure 4.
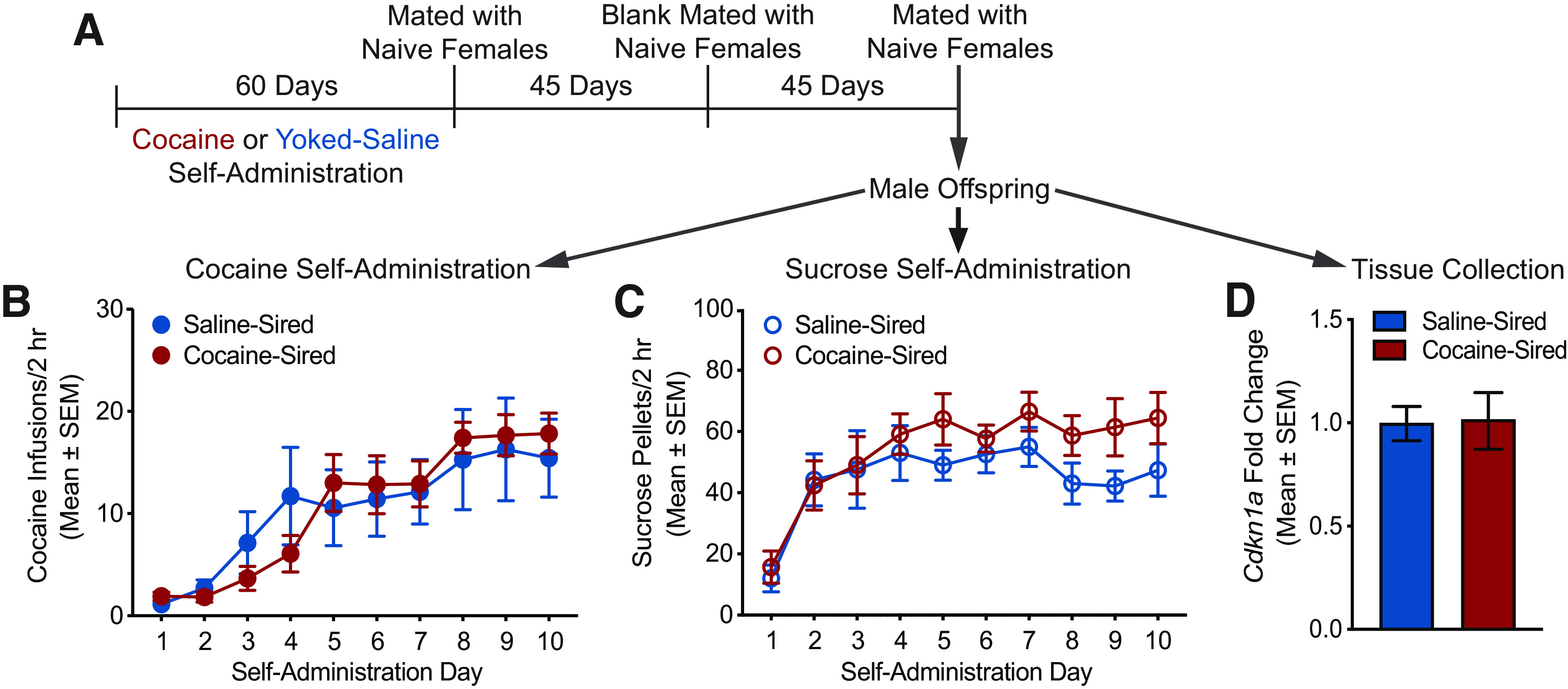
Delayed mating of cocaine sires does not alter cocaine self-administration or Cdkn1a expression in male offspring. A, Timeline showing F1 male offspring were produced from cocaine and saline sires mated 90 d after cessation of cocaine self-administration. Cocaine-sired and saline-sired F1 offspring do not differ in (B) cocaine self-administration (saline-sired n = 7 from 4 litters; cocaine-sired n = 12 from 7 litters) or (C) sucrose self-administration (saline sired n = 5 from 5 litters; cocaine-sired n = 7 from 7 litters). D, Cdkn1a mRNA levels in the NAc are identical in cocaine-sired (n = 6 from 6 litters) and saline-sired offspring (n = 6 from 5 litters) generated by delayed mating.
Cocaine self-administration reverses virally mediated knockdown of accumbens Cdkn1a in adult cocaine-sired male offspring resulting in no changes in cocaine self-administration or motivation for cocaine
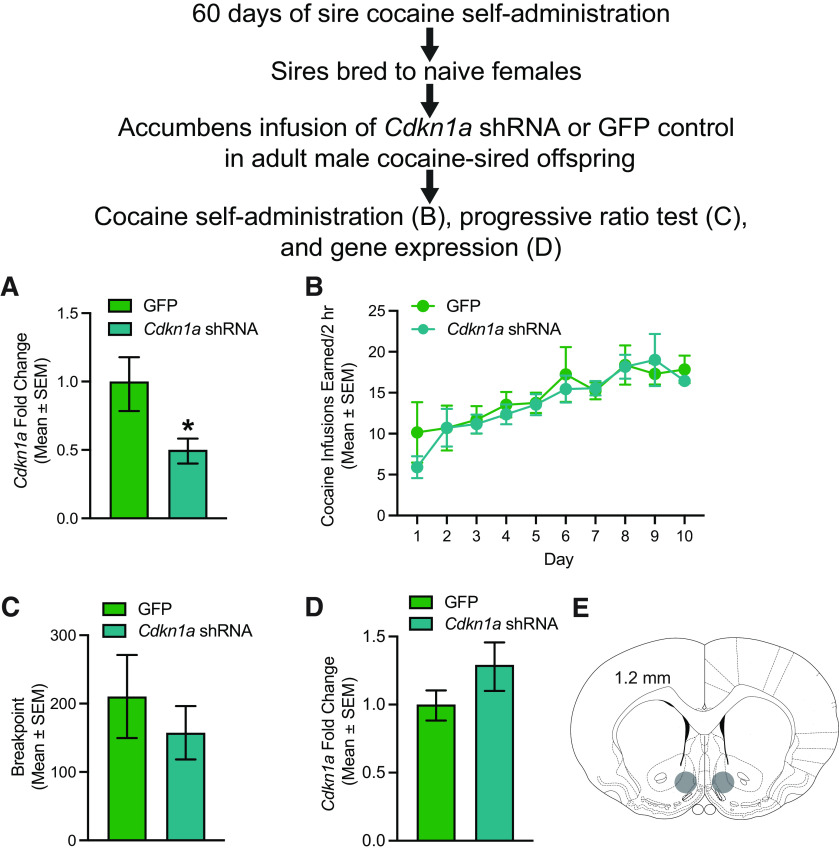
Cdkn1a was knocked down in the NAc shell of adult cocaine-sired male rats that were allowed to self-administer cocaine to determine whether “normalizing” Cdkn1a expression in cocaine-sired males reversed the resistance to cocaine self-administration. Cocaine-sired male offspring that received intra-accumbens GFP infusions were used as controls. Knockdown of Cdkn1a expression by the viral vector was first confirmed, and Cdkn1a expression was reduced by 50% in a separate group of procured naive rats (t(1,8) = 2.370, p = 0.0452; Fig. 5A). Cocaine-sired rats expressing GFP or Cdkn1a shRNA were trained to self-administer cocaine on an FR1 schedule of reinforcement (Fig. 5B). Three rats were removed from the cocaine self-administration study because of loss of catheter patency. There was no main effect of vector (F(1,22) = 0.1511, p = 0.7012), a main effect of day (F(9,198) = 9.262, p = 0.0003), and no vector by day interaction (F(9,198) = 0.4818, p = 0.8858). Rats were then allowed to self-administer cocaine on an FR5 schedule for 2 d before two consecutive PR sessions to assess motivation for cocaine. There was no difference between groups in terms of infusions earned on an FR5 schedule. There was no main effect of vector (F(1,25) = 0.1759, p = 0.6785), no main effect of day (F(1,25) = 0.0159, p = 0.9006), and no vector by day interaction (F(1,25) = 0.2519, p = 0.6201). There was no difference in motivation for cocaine as measured by breakpoint in a PR test (t(1,22) = 0.7063, p = 0.4874; Fig. 5C). To confirm that Cdkn1a shRNA knocked down Cdkn1a expression in experimental rats following cocaine exposure, rats were killed 1 day following the last session and the fluorescent region of the accumbens shell was harvested for qPCR (Fig. 5D). Surprisingly, there was no difference in Cdkn1a expression between GFP controls and Cdkn1a shRNA cocaine-sired offspring (t(1,22) = 1.381, p = 0.1813), indicating that cocaine self-administration reversed the virally mediated decrease in Cdkn1a expression, thereby obviating any potential effects of accumbens Cdkn1a knockdown on behavior. The cocaine-induced increase in Cdkn1a expression is consistent with previous findings indicating increased acetylation of Cdkn1a in the accumbens following cocaine (Renthal et al., 2009).
Figure 5.
Cocaine self-administration reversed virally mediated knockdown of Cdkn1a shRNA in the NAc of adult cocaine-sired male offspring resulting in no changes in cocaine self-administration or motivation for cocaine. A, In procured, experimentally naive rats, Cdkn1a shRNA significantly knocked down Cdkn1a expression in the NAc relative to GFP controls (*p < 0.05; n = 5/group). Cocaine-sired male offspring expressing GFP (n = 13 from 9 litters) and cocaine-sired male offspring expressing Cdkn1a shRNA (n = 11 from 8 litters) do not differ in (B) cocaine self-administration on an FR1 schedule of reinforcement or (C) motivation for cocaine measured by breakpoint in a PR test. D, Cdkn1a expression from cocaine-experienced cocaine-sired male offspring expressing GFP or Cdkn1a shRNA was not significantly different. E, Representative diagram showing region of appropriate GFP fluorescence in Cdnk1a shRNA and GFP control rats.
Virally mediated Cdkn1a overexpression dampens the relationship between Cdkn1a expression and cocaine self-administration
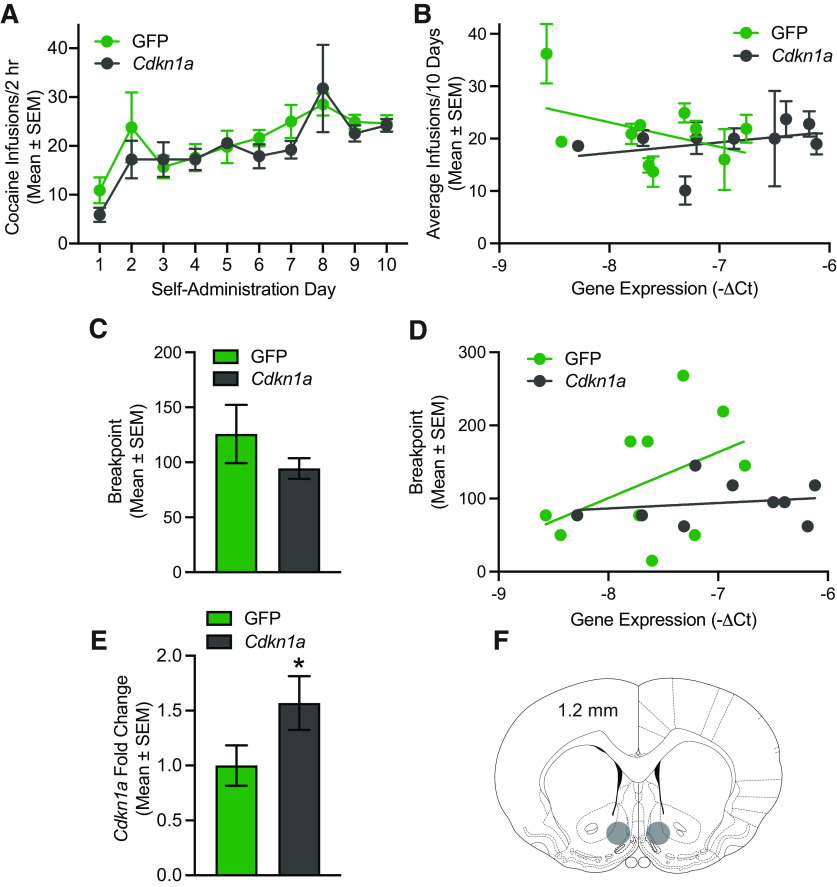
A Cdkn1a overexpression experiment was performed in an effort to demonstrate the behavioral significance of alterations in the expression of this gene in the NAc. Given the potential for a ceiling effect in the ability of a viral vector to overexpress this gene in cocaine-sired rats, Cdkn1a was overexpressed in the NAc shell of naive male rats. The acquisition of cocaine self-administration was assessed 2.5 weeks after surgery. Two rats were removed from the analyses because of loss of catheter patency. There was no difference in cocaine self-administration on an FR1 schedule of reinforcement between rats that overexpressed Cdkn1a or expressed a GFP control (Fig. 6A). There was no main effect of vector (F(1,17) = 0.5735, p = 0.4593), a main effect of day (F(9,153) = 6.741, p = 0.0007), and no vector by day interaction (F(9,153) = 0.5749, p = 0.8162). However, there was substantial variability in Cdkn1a expression in both GFP controls and Cdkn1a-overexpressing rats, so the relationship between average cocaine infusions and Cdkn1a expression was interrogated by linear regression (Fig. 6B). The relation between Cdkn1a expression and cocaine infusions earned was stronger in GFP controls (R2 = 0.05540) than Cdkn1a-overexpressing rats (R2 = 0.01371). There was a significant difference in the slopes of the regression lines for Cdkn1a overexpression (−4.692 ± 1.957) relative to GFP controls (2.011 ± 1.818; F(1,186) = 6.219; p = 0.0135). The slope of the regression line for GFP controls was significantly non-zero (F(1,98) = 5.748; p = 0.0184), whereas the slope for Cdkn1a-overexpressing rats did not significantly deviate from zero (F(1,88) = 1.223; p = 0.2718). Together, these data suggest that there is an inherent inverse relationship between endogenous Cdkn1a levels and cocaine self-administration, which was blunted by virally mediated overexpression of Cdkn1a.
Figure 6.
Overexpression of Cdkn1a in the NAc of naive rats blunts the relation between Cdkn1a expression and cocaine self-administration. A, Cocaine self-administration was not altered in Cdkn1a-overexpressing rats relative to GFP controls (n = 9 or 10/group). B, Linear regressions show the relation between Cdkn1a mRNA and cocaine infusions in Cdkn1a-overexpressing relative to GFP control rats. There was a significant difference in slope of the regression lines (p < 0.05). C, Motivation for cocaine was not altered in Cdkn1a-overexpressing rats relative to GFP controls (n = 9 or 10/group). D, Linear regressions show the relation between Cdkn1a mRNA and breakpoint in PR responding for cocaine in Cdkn1a-overexpressing relative to GFP control rats. There was no significant difference in the slope or intercept of the regression lines. E, Cdkn1a was overexpressed in the NAc of rats that received infusion of a Cdkn1a overexpression vector relative to GFP controls (*p < 0.05 vs GFP; n = 9 or 10/group). F, Representative diagram showing region of appropriate GFP fluorescence in Cdnk1a-overexpressing and GFP control rats.
Rats were then trained to self-administer cocaine on an FR5 schedule for 2 d before two consecutive PR sessions to assess motivation for cocaine. There was no difference in infusions earned on an FR5 schedule. There was no main effect of vector (F(1,17) = 0.3881, p = 0.5415), no main effect of day (F(1,17) = 0.4985, p = 0.4897), and no vector by day interaction (F(1,17) = 2.635, p = 0.1230). There was no difference in motivation for cocaine as measured by breakpoint in a PR test between rats that overexpressed Cdkn1a or expressed a GFP control (t(1,17) = 1.067, p = 0.3010; Fig. 6C). Linear regression was used to examine the relation between motivation for cocaine and Cdkn1a expression (Fig. 6D). The relationship between Cdkn1a expression and breakpoint was slightly stronger in Cdkn1a-overexpressing rats (R2 = 0.1896) than GFP controls (R2 = 0.0375). However, there was no significant difference in the regression slopes for Cdkn1a overexpression relative to GFP controls (F(1,15) = 1.427; p = 0.2508). There was no significant difference in intercept in Cdkn1a overexpression relative to GFP controls (F(1,16) = 2.471; p = 0.1355). Cdkn1a overexpression was confirmed in the NAc of rats receiving the Cdkn1a overexpression vector relative to GFP controls (t(1,17) = 2.146, p = 0.0466; Fig. 6E) among rats that displayed appropriate GFP signal in the NAc (Fig. 6F).
Discussion
Our working hypothesis is that the intergenerational effects of paternal cocaine exposure are because of epigenetic changes in sperm that occur only during spermatogenesis. To that end, we used a genome-wide approach to assess sperm DNA methylation. The results of this experiment revealed surprisingly selective methylation changes, highlighted by hypomethylation of promoter regions upstream of Cdkn1a in the sperm of cocaine-experienced sires. In offspring, Cdkn1a expression was selectively increased in the NAc of drug-naive cocaine-sired male, but not female, offspring. Physiologic changes in the accumbens play a critical role in the development and maintenance of cocaine self-administration, and prior studies showed decreased cocaine self-administration in male, but not female, offspring of cocaine-exposed sires (Vassoler et al., 2013; Le et al., 2017). Yoked-cocaine and yoked-cocaine methiodide experiments support a direct effect of cocaine on sperm to mediate epigenetic changes and behavioral effects in offspring. In order to determine whether cocaine-induced epigenetic changes in sperm are confined to the period of active spermatogenesis, which constitutes just less than 2 months in rat, a 3 month delay was imposed between sire cocaine self-administration and breeding. With this 3 month delay, the decrease in cocaine self-administration and increase in Cdkn1a expression in the NAc of male cocaine-sired offspring were no longer observed, suggesting that these influences of cocaine occur only during sperm development. Virally mediated knockdown of Cdkn1a in the accumbens of cocaine-sired male offspring failed to reverse the cocaine resistance phenotype, as measured by cocaine self-administration and PR responding for cocaine. This is likely because, following 14 d of cocaine self-administration, Cdkn1a expression was normalized in cocaine-sired rats infused with Cdkn1a shRNA relative to GFP controls. Virally mediated overexpression of Cdkn1a in procured rats similarly did not decrease cocaine self-administration or motivation for cocaine relative to GFP controls. Surprisingly, however, GFP-infused control animals with endogenously higher accumbens Cdkn1a took less cocaine. Virally mediated overexpression of accumbens Cdkn1a in previously naive rats disrupted the inverse relationship between endogenous accumbens Cdkn1a levels and cocaine self-administration. These results, collectively, suggest that cocaine's effects during spermatogenesis can lead to selective changes in gene expression in a specific progeny brain nucleus known to regulate cocaine self-administration behavior.
Previous work on the effects of cocaine on epigenetic marks in sperm is limited. Although the vast majority of sperm histones are replaced by protamines, physiologically relevant epigenetic changes in sperm histones persist and influence DNA methylation as well as offspring development (Gatewood et al., 1987; Wykes and Krawetz, 2003). Increased association of acetylated H3 with brain-derived neurotrophic factor (Bdnf) was observed in the sperm of cocaine-exposed sires, which corresponded with behaviorally relevant increases in BDNF expression the mPFC of male offspring (Vassoler et al., 2013). The current study assessed, for the first time, the potential influence of cocaine on sperm miRNAs. Despite the fact that transgenerational paternal stress studies have identified increased sperm miRNAs and diminished stress-induced responsivity of the hypothalamic-pituitary axis in offspring (Rodgers et al., 2013, 2015; Gapp et al., 2014), we failed to identify reliable changes in miRNA expression in the sperm of cocaine-experienced sires. In terms of DNA methylation, a recent study showed that paternal cocaine self-administration resulted in changes in sperm DNA methylation, with hypomethylation predominating (Le et al., 2017). Here, 242 differentially methylated DNA sites were identified in sperm of cocaine-exposed sires, 148 of which were hypomethylated. Somewhat surprisingly, there were only three differentially hypomethylated DNA sites in promoter regions, and two of these were associated with the same gene: Cdkn1a. Cdkn1a is a marker of cell cycle arrest in proliferating cells, and expression of Cdkn1a is altered in ∼1% of all cancers (Abbas and Dutta, 2009). In the brain, Cdkn1a restrains neuronal proliferation associated with hippocampal neurogenesis, a process that is influenced by antidepressant administration (Pechnick et al., 2011; Epp et al., 2013).
We assessed the expression of Cdkn1a mRNA in several brain regions of cocaine-sired male rats, with results indicating increased Cdkn1a mRNA in the NAc but not the dorsal hippocampus or mPFC. There is no simple or specific mechanism linking DNA hypomethylation in sperm and gene expression in the offspring brain, and the regional specificity of Cdkn1a overexpression in the NAc of male offspring is particularly puzzling. It is likely that the mechanism of transmission between sperm and region-specific changes in offspring brains are not as simple or direct as conserved epigenetic marks, and may involve upstream and/or downstream regulators of Cdkn1a. Elucidation of the sequence of molecular changes by which epigenetic marks in sperm alter epigenetic or transcriptional profiles in offspring brains is a challenging but important avenue for exploration, for this study but also more broadly for studies of transgenerational epigenetics as a whole.
Although the mechanisms governing regional specificity are unclear, increased Cdkn1a expression in the NAc, but not the hippocampus, suggests it plays a role in cocaine reinforcement, but likely not in the spatial memory deficits also observed in cocaine-sired offspring (Wimmer et al., 2017). To determine whether this change in Cdkn1a mRNA expression was behaviorally relevant, we used virally mediated gene transfer to knock down Cdkn1a in the NAc of adult cocaine-sired male rats. Surprisingly, there was no difference in cocaine self-administration on a fixed ratio schedule of reinforcement or motivation for cocaine on a PR schedule of reinforcement between cocaine-sired male offspring that received Cdkn1a shRNA or GFP controls. The viral vector-expressing Cdkn1a shRNA produced a significant knockdown in naive rats; however, when we examined Cdkn1a expression in experimental rats following cocaine self-administration, there was no significant difference in Cdkn1a levels. We hypothesize that cocaine exposure increased Cdkn1a expression in the NAc, consistent with a previous study in mice (Renthal et al., 2009), which obfuscated the effect of Cdkn1a shRNA and may explain why we were unable to functionally validate the role of Cdkn1a in cocaine self-administration. Alternatively, the inability of virally mediated Cdkn1a knockdown in cocaine-sired rats to alter cocaine self-administration could suggest a critical role for elevated Cdkn1a expression during development, and intervention during that sensitive period may be necessary to influence offspring behavior. This may similarly explain why virally mediated overexpression of Cdkn1a in adult naive procured rats was insufficient to attenuate mean cocaine self-administration relative to GFP controls. Among controls that only expressed GFP, there was a surprising degree of variability in Cdkn1a expression in the NAc. Interestingly, the range of natural variation of accumbens Cdkn1a levels appeared to influence cocaine self-administration in that animals with higher expression of Cdkn1a in the NAc had lower cocaine intake. Virally mediated overexpression of Cdkn1a in the NAc blunted the inverse relationship between Cdkn1a expression and cocaine self-administration. Our knowledge of differentially expressed genes in the NAc of cocaine-sired versus saline-sired offspring is currently limited to Cdkn1a. RNA sequencing experiments may provide insight to additional differentially expressed genes, potentially including regulators of Cdkn1a.
The current results suggest that accumbens Cdkn1a may play a role in regulating cocaine intake, findings that are similar to a recent study demonstrating that global Cdkn1a KO in mice enhanced cocaine conditioned place preference (Scholpa et al., 2016). The relationship between Cdkn1a and cocaine conditioned reward led to a focus on the ventral hippocampus, which processes drug-associated context learning through glutamatergic projections to the NAc (Vorel et al., 2001; Marchant et al., 2015). Cocaine self-administration led to a persistent elevation of ventral hippocampal Cdkn1a and an increased association of acetyl H3 with Cdkn1a (Scholpa et al., 2016); no cocaine-induced changes in Cdkn1a expression in the striatal complex were observed (Scholpa et al., 2016). The present results showed increased Cdkn1a in the NAc but not in the dorsal hippocampus of cocaine-sired male rats. Beyond differences in the region of the hippocampus examined between the current study and that of Scholpa et al. (2016), it is important to emphasize that cocaine-sired rats in the current experiments were completely drug-naive with the cocaine exposure restricted to their sires. As noted above, Cdkn1a in the brain has been studied primarily in the context of proliferating cells. A genome-wide study which examined the effect of cocaine on chromatin remodeling found increased histone acetylation at Cdkn1a (among other genes), and Cdkn1a gene expression was elevated in the NAc (Renthal et al., 2009). This cocaine-induced upregulation of Cdkn1a expression may explain why Cdkn1a shRNA successfully knocked down Cdkn1a expression in naive rats but failed to significantly reduce Cdkn1a expression relative to cocaine-sired controls following 14 d of cocaine self-administration. Acute injection of morphine similarly increased Cdkn1a expression in the NAc, specifically in oligodendrocytes (Avey et al., 2018), which adds to a growing literature focusing on roles for glial cells in the action of drugs of abuse (Spencer et al., 2016; Stellwagen et al., 2019). However, Cdkn1a expression is not restrained to proliferative cells in the brain; it is also expressed in dopaminergic neurons, where it promotes cellular senescence, and cortical neurons, where its function is yet unknown (Riessland et al., 2019). This study did not explicitly examine the cellular distribution and function of Cdkn1a in the brain, but it is feasible that Cdkn1a may be expressed on neuronal and non-neuronal populations within the NAc. The downstream consequences of Cdkn1a expression in nonproliferating cells, such as neurons in the NAc, has not been established but is clearly a research avenue worthy of exploration.
Delayed mating experiments demonstrated that the effects of paternal cocaine intake on accumbens Cdkn1a expression and on offspring cocaine self-administration were not permanent. That is, when a delay (here, 90 d) was imposed between cocaine self-administration and breeding that exceeded the time frame of spermatogenesis, the increase in Cdkn1a expression in the NAc as well as the intergenerational cocaine resistance phenotype was not observed in male progeny. Similarly, the blunted cocaine preference observed in male offspring of cocaine-exposed mice was normalized when sires were subjected to 4 months of drug abstinence before mating (Yaw et al., 2019). Together, these data indicate that cocaine acts on developing sperm during spermatogenesis rather than permanently encoding changes in the germline by, for example, stably modifying epigenetic marks in spermatogonial stem cells. Our protocol used a 60 d self-administration protocol to ensure that sperm was exposed to cocaine throughout the entirety of spermatogenesis. Limiting the duration of cocaine exposure to specific phases of spermatogenesis may further narrow the vulnerable period during which cocaine-evoked epigenetic modifications in sperm can occur.
In conclusion, the present work assessed DNA methylation in the sperm of sires who self-administered cocaine, revealing two hypomethylated promoter regions upstream of the Cdkn1a gene. Male offspring of cocaine-experienced sires show blunted cocaine reinforcement, which the current findings indicate is due at least in part to selective increases in Cdkn1a expression in the NAc. These results support accumbens Cdkn1a as a novel regulator of cocaine self-administration in rats. These findings, collectively, raise the intriguing possibility that identification of epigenetic changes in the sperm of cocaine-exposed sires can reveal novel targets for cocaine addiction therapeutics.
Footnotes
This work was supported by National Institutes of Health Grants R01 DA33641 to R.C.P., T32 DA28874 to S.E.S.-J., K01 DA39308 to M.E.W., and DA46537 to M.E.W. We thank Tracy Bale, Jacquetta Trasler, and Allison Rodgers for technical and conceptual assistance.
The authors declare no competing financial interests.
References
- Abbas T, Dutta A (2009) p21 in cancer: intricate networks and multiple activities. Nat Rev Cancer 9:400–414. 10.1038/nrc2657 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allan LA, Duhig T, Read M, Fried M (2000) The p21(WAF1/CIP1) promoter is methylated in Rat-1 cells: stable restoration of p53-dependent p21(WAF1/CIP1) expression after transfection of a genomic clone containing the p21(WAF1/CIP1) gene. Mol Cell Biol 20:1291–1298. 10.1128/MCB.20.4.1291-1298.2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Avey D, Sankararaman S, Yim AK, Barve R, Milbrandt J, Mitra RD (2018) Single-cell RNA-Seq uncovers a robust transcriptional response to morphine by glia. Cell Rep 24:3619–3629.e3614. 10.1016/j.celrep.2018.08.080 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bale TL (2015) Epigenetic and transgenerational reprogramming of brain development. Nat Rev Neurosci 16:332–344. 10.1038/nrn3818 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boyle P, Clement K, Gu H, Smith ZD, Ziller M, Fostel JL, Holmes L, Meldrim J, Kelley F, Gnirke A, Meissner A (2012) Gel-free multiplexed reduced representation bisulfite sequencing for large-scale DNA methylation profiling. Genome Biol 13:R92. 10.1186/gb-2012-13-10-r92 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brunner AM, Nanni P, Mansuy IM (2014) Epigenetic marking of sperm by post-translational modification of histones and protamines. Epigenetics Chromatin 7:2. 10.1186/1756-8935-7-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carone BR, Hung JH, Hainer SJ, Chou MT, Carone DM, Weng Z, Fazzio TG, Rando OJ (2014) High-resolution mapping of chromatin packaging in mouse embryonic stem cells and sperm. Dev Cell 30:11–22. 10.1016/j.devcel.2014.05.024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan JC, Morgan CP, Adrian Leu N, Shetty A, Cisse YM, Nugent BM, Morrison KE, Jasarevic E, Huang W, Kanyuch N, Rodgers AB, Bhanu NV, Berger DS, Garcia BA, Ament S, Kane M, Neill Epperson C, Bale TL (2020) Reproductive tract extracellular vesicles are sufficient to transmit intergenerational stress and program neurodevelopment. Nat Commun 11:1499. 10.1038/s41467-020-15305-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Q, Yan M, Cao Z, Li X, Zhang Y, Shi J, Feng GH, Peng H, Zhang X, Zhang Y, Qian J, Duan E, Zhai Q, Zhou Q (2016) Sperm tsRNAs contribute to intergenerational inheritance of an acquired metabolic disorder. Science 351:397–400. 10.1126/science.aad7977 [DOI] [PubMed] [Google Scholar]
- Conine CC, Sun F, Song L, Rivera-Perez JA, Rando OJ (2018) Small RNAs gained during epididymal transit of sperm are essential for embryonic development in mice. Dev Cell 46:470–480.e473. 10.1016/j.devcel.2018.06.024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conine CC, Sun F, Song L, Rivera-Perez JA, Rando OJ (2019) MicroRNAs absent in caput sperm are required for normal embryonic development. Dev Cell 50:7–8. 10.1016/j.devcel.2019.06.007 [DOI] [PubMed] [Google Scholar]
- Cunningham AM, Walker DM, Nestler EJ (2021) Paternal transgenerational epigenetic mechanisms mediating stress phenotypes of offspring. Eur J Neurosci 53:271–280. 10.1111/ejn.14582 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dietz DM, Laplant Q, Watts EL, Hodes GE, Russo SJ, Feng J, Oosting RS, Vialou V, Nestler EJ (2011) Paternal transmission of stress-induced pathologies. Biol Psychiatry 70:408–414. 10.1016/j.biopsych.2011.05.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dobin A, Davis CA, Schlesinger F, Drenkow J, Zaleski C, Jha S, Batut P, Chaisson M, Gingeras TR (2013) STAR: ultrafast universal RNA-seq aligner. Bioinformatics 29:15–21. 10.1093/bioinformatics/bts635 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dutto I, Tillhon M, Cazzalini O, Stivala LA, Prosperi E (2015) Biology of the cell cycle inhibitor p21(CDKN1A): molecular mechanisms and relevance in chemical toxicology. Arch Toxicol 89:155–178. 10.1007/s00204-014-1430-4 [DOI] [PubMed] [Google Scholar]
- Epp JR, Beasley CL, Galea LA (2013) Increased hippocampal neurogenesis and p21 expression in depression: dependent on antidepressants, sex, age, and antipsychotic exposure. Neuropsychopharmacology 38:2297–2306. 10.1038/npp.2013.132 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finegersh A, Rompala GR, Martin DI, Homanics GE (2015) Drinking beyond a lifetime: new and emerging insights into paternal alcohol exposure on subsequent generations. Alcohol 49:461–470. 10.1016/j.alcohol.2015.02.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischer DK, Rice RC, Martinez Rivera A, Donohoe M, Rajadhyaksha AM (2017) Altered reward sensitivity in female offspring of cocaine-exposed fathers. Behav Brain Res 332:23–31. 10.1016/j.bbr.2017.05.054 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gapp K, Jawaid A, Sarkies P, Bohacek J, Pelczar P, Prados J, Farinelli L, Miska E, Mansuy IM (2014) Implication of sperm RNAs in transgenerational inheritance of the effects of early trauma in mice. Nat Neurosci 17:667–669. 10.1038/nn.3695 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gatewood JM, Cook GR, Balhorn R, Bradbury EM, Schmid CW (1987) Sequence-specific packaging of DNA in human sperm chromatin. Science 236:962–964. 10.1126/science.3576213 [DOI] [PubMed] [Google Scholar]
- Gu H, Smith ZD, Bock C, Boyle P, Gnirke A, Meissner A (2011) Preparation of reduced representation bisulfite sequencing libraries for genome-scale DNA methylation profiling. Nat Protoc 6:468–481. 10.1038/nprot.2010.190 [DOI] [PubMed] [Google Scholar]
- Hackett JA, Sengupta R, Zylicz JJ, Murakami K, Lee C, Down TA, Surani MA (2013) Germline DNA demethylation dynamics and imprint erasure through 5-hydroxymethylcytosine. Science 339:448–452. 10.1126/science.1229277 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hammoud SS, Nix DA, Zhang H, Purwar J, Carrell DT, Cairns BR (2009) Distinctive chromatin in human sperm packages genes for embryo development. Nature 460:473–478. 10.1038/nature08162 [DOI] [PMC free article] [PubMed] [Google Scholar]
- He F, Lidow IA, Lidow MS (2006) Consequences of paternal cocaine exposure in mice. Neurotoxicol Teratol 28:198–209. 10.1016/j.ntt.2005.12.003 [DOI] [PubMed] [Google Scholar]
- Killinger CE, Robinson S, Stanwood GD (2012) Subtle biobehavioral effects produced by paternal cocaine exposure. Synapse 66:902–908. 10.1002/syn.21582 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le Q, Yan B, Yu X, Li Y, Song H, Zhu H, Hou W, Ma D, Wu F, Zhou Y, Ma L (2017) Drug-seeking motivation level in male rats determines offspring susceptibility or resistance to cocaine-seeking behaviour. Nat Commun 8:15527. 10.1038/ncomms15527 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li H, George VK, Crossland WJ, Anderson GF, Dhabuwala CB (1997) Characterization of cocaine binding sites in the rat testes. J Urol 158:962–965. 10.1016/S0022-5347(01)64371-4 [DOI] [PubMed] [Google Scholar]
- Liu G, Lozano G (2005) p21 stability: linking chaperones to a cell cycle checkpoint. Cancer Cell 7:113–114. 10.1016/j.ccr.2005.01.019 [DOI] [PubMed] [Google Scholar]
- Love MI, Huber W, Anders S (2014) Moderated estimation of fold change and dispersion for RNA-seq data with DESeq2. Genome Biol 15:550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marchant NJ, Kaganovsky K, Shaham Y, Bossert JM (2015) Role of corticostriatal circuits in context-induced reinstatement of drug seeking. Brain Res 1628:219–232. 10.1016/j.brainres.2014.09.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paxinos G, Watson C (1997) The Rat Brain in Stereotaxic Coordinates. Academic Press: New York. [Google Scholar]
- Pechnick RN, Zonis S, Wawrowsky K, Cosgayon R, Farrokhi C, Lacayo L, Chesnokova V (2011) Antidepressants stimulate hippocampal neurogenesis by inhibiting p21 expression in the subgranular zone of the hipppocampus. PLoS One 6:e27290. 10.1371/journal.pone.0027290 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pierce RC, Fant B, Swinford-Jackson SE, Heller EA, Berrettini WH, Wimmer ME (2018) Environmental, genetic and epigenetic contributions to cocaine addiction. Neuropsychopharmacology 43:1471–1480. 10.1038/s41386-018-0008-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Puri D, Dhawan J, Mishra RK (2010) The paternal hidden agenda: epigenetic inheritance through sperm chromatin. Epigenetics 5:386–391. 10.4161/epi.5.5.12005 [DOI] [PubMed] [Google Scholar]
- Renthal W, Kumar A, Xiao G, Wilkinson M, Covington HE 3rd, Maze I, Sikder D, Robison AJ, LaPlant Q, Dietz DM, Russo SJ, Vialou V, Chakravarty S, Kodadek TJ, Stack A, Kabbaj M, Nestler EJ (2009) Genome-wide analysis of chromatin regulation by cocaine reveals a role for sirtuins. Neuron 62:335–348. 10.1016/j.neuron.2009.03.026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richardson NR, Roberts DC (1996) Progressive ratio schedules in drug self-administration studies in rats: a method to evaluate reinforcing efficacy. J Neurosci Methods 66:1–11. 10.1016/0165-0270(95)00153-0 [DOI] [PubMed] [Google Scholar]
- Riessland M, Kolisnyk B, Kim TW, Cheng J, Ni J, Pearson JA, Park EJ, Dam K, Acehan D, Ramos-Espiritu LS, Wang W, Zhang J, Shim JW, Ciceri G, Brichta L, Studer L, Greengard P (2019) Loss of SATB1 induces p21-dependent cellular senescence in post-mitotic dopaminergic neurons. Cell Stem Cell 25:514–530.e518. 10.1016/j.stem.2019.08.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodgers AB, Bale TL (2015) Germ cell origins of posttraumatic stress disorder risk: the transgenerational impact of parental stress experience. Biol Psychiatry 78:307–314. 10.1016/j.biopsych.2015.03.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodgers AB, Morgan CP, Bronson SL, Revello S, Bale TL (2013) Paternal stress exposure alters sperm microRNA content and reprograms offspring HPA stress axis regulation. J Neurosci 33:9003–9012. 10.1523/JNEUROSCI.0914-13.2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodgers AB, Morgan CP, Leu NA, Bale TL (2015) Transgenerational epigenetic programming via sperm microRNA recapitulates effects of paternal stress. Proc Natl Acad Sci USA 112:13699–13704. 10.1073/pnas.1508347112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rompala GR, Mounier A, Wolfe CM, Lin Q, Lefterov I, Homanics GE (2018) Heavy chronic intermittent ethanol exposure alters small noncoding RNAs in mouse sperm and epididymosomes. Front Genet 9:32. 10.3389/fgene.2018.00032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scholpa NE, Briggs SB, Wagner JJ, Cummings BS (2016) Cyclin-dependent kinase inhibitor 1a (p21) modulates response to cocaine and motivated behaviors. J Pharmacol Exp Ther 357:56–65. 10.1124/jpet.115.230888 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scibetta AG, Wong PP, Chan KV, Canosa M, Hurst HC (2010) Dual association by TFAP2A during activation of the p21cip/CDKN1A promoter. Cell Cycle 9:4525–4532. 10.4161/cc.9.22.13746 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharma U, Conine CC, Shea JM, Boskovic A, Derr AG, Bing XY, Belleannee C, Kucukural A, Serra RW, Sun F, Song L, Carone BR, Ricci EP, Li XZ, Fauquier L, Moore MJ, Sullivan R, Mello CC, Garber M, Rando OJ (2016) Biogenesis and function of tRNA fragments during sperm maturation and fertilization in mammals. Science 351:391–396. 10.1126/science.aad6780 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharma U, Sun F, Conine CC, Reichholf B, Kukreja S, Herzog VA, Ameres SL, Rando OJ (2018) Small RNAs are trafficked from the epididymis to developing mammalian sperm. Dev Cell 46:481–494.e486. 10.1016/j.devcel.2018.06.023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spencer S, Scofield M, Kalivas PW (2016) The good and bad news about glutamate in drug addiction. J Psychopharmacol 30:1095–1098. 10.1177/0269881116655248 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stellwagen D, Kemp GM, Valade S, Chambon J (2019) Glial regulation of synaptic function in models of addiction. Curr Opin Neurobiol 57:179–185. 10.1016/j.conb.2019.02.010 [DOI] [PubMed] [Google Scholar]
- Trigg NA, Skerrett-Byrne DA, Xavier MJ, Zhou W, Anderson AL, Stanger SJ, Katen AL, De Iuliis GN, Dun MD, Roman SD, Eamens AL, Nixon B (2021) Acrylamide modulates the mouse epididymal proteome to drive alterations in the sperm small non-coding RNA profile and dysregulate embryo development. Cell Rep 37:109787. 10.1016/j.celrep.2021.109787 [DOI] [PubMed] [Google Scholar]
- Vairetti M, Ferrigno A, Rizzo V, Ambrosi G, Bianchi A, Richelmi P, Blandini F, Armentero MT (2012) Impaired hepatic function and central dopaminergic denervation in a rodent model of Parkinson's disease: a self-perpetuating crosstalk? Biochim Biophys Acta 1822:176–184. 10.1016/j.bbadis.2011.11.008 [DOI] [PubMed] [Google Scholar]
- Vassoler FM, White SL, Schmidt HD, Sadri-Vakili G, Pierce RC (2013) Epigenetic inheritance of a cocaine-resistance phenotype. Nat Neurosci 16:42–47. 10.1038/nn.3280 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vorel SR, Liu X, Hayes RJ, Spector JA, Gardner EL (2001) Relapse to cocaine-seeking after hippocampal theta burst stimulation. Science 292:1175–1178. 10.1126/science.1058043 [DOI] [PubMed] [Google Scholar]
- Wang Y, Chen ZP, Hu H, Lei J, Zhou Z, Yao B, Chen L, Liang G, Zhan S, Zhu X, Jin F, Ma R, Zhang J, Liang H, Xing M, Chen XR, Zhang CY, Zhu JN, Chen X (2021) Sperm microRNAs confer depression susceptibility to offspring. Sci Adv 7:eabd7605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White SL, Vassoler FM, Schmidt HD, Pierce RC, Wimmer ME (2016) Enhanced anxiety in the male offspring of sires that self-administered cocaine. Addict Biol 21:802–810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wimmer ME, Briand LA, Fant B, Guercio LA, Arreola AC, Schmidt HD, Sidoli S, Han Y, Garcia BA, Pierce RC (2017) Paternal cocaine taking elicits epigenetic remodeling and memory deficits in male progeny. Mol Psychiatry 22:1641–1650. 10.1038/mp.2017.8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wimmer ME, Vassoler FM, White SL, Schmidt HD, Sidoli S, Han Y, Garcia BA, Pierce RC (2019) Impaired cocaine-induced behavioral plasticity in the male offspring of cocaine-experienced sires. Eur J Neurosci 49:1115–1126. 10.1111/ejn.14310 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wykes SM, Krawetz SA (2003) The structural organization of sperm chromatin. J Biol Chem 278:29471–29477. 10.1074/jbc.M304545200 [DOI] [PubMed] [Google Scholar]
- Xi Y, Li W (2009) BSMAP: whole genome bisulfite sequence MAPping program. BMC Bioinformatics 10:232. 10.1186/1471-2105-10-232 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yaw AM, Prosser RA, Jones PC, Garcia BJ, Jacobson DA, Glass JD (2019) Epigenetic effects of paternal cocaine on reward stimulus behavior and accumbens gene expression in mice. Behav Brain Res 367:68–81. 10.1016/j.bbr.2019.02.043 [DOI] [PubMed] [Google Scholar]
- Zhang Y, Ren L, Sun X, Zhang Z, Liu J, Xin Y, Yu J, Jia Y, Sheng J, Hu GF, Zhao R, He B (2021) Angiogenin mediates paternal inflammation-induced metabolic disorders in offspring through sperm tsRNAs. Nat Commun 12:6673. 10.1038/s41467-021-26909-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary material. Download Table 1-1, XLS file (120.5KB, xls) .