Abstract
The noradrenergic locus coeruleus (LC) mediates key aspects of arousal, memory, and cognition in structured tasks, but its contribution to naturalistic behavior remains unclear. LC activity is thought to multiplex distinct signals by superimposing sustained (“tonic”) firing patterns reflecting global brain states, such as arousal and anxiety, and rapidly fluctuating (“phasic”) bursts signaling discrete behaviorally significant events. Manipulations of the LC noradrenergic system broadly impair social behavior, but the temporal structure of LC firing and its relationship to social interaction is unknown. One possibility is that tonic firing may increase in the presence of social partners; it is also possible that phasic bursts may accompany specific social events. We used chronic in vivo electrophysiology and fiber photometry to measure single-unit and population neural activity in LC of freely behaving mice during their interactions with pups. We find that pup retrieval elicits remarkably precise phasic activity in LC that cannot be attributed merely to sensory stimuli, motor activity, or reward. Correlation of LC activity with retrieval events shows that phasic events are most closely related to specific subsequent behaviors. The reliability and magnitude of phasic responses strongly suggest that these events are coordinated across LC and broadcast noradrenaline (NA) release throughout the brain. We also observed slow changes in tonic firing when females performed distinct maternal behaviors such as nest building and pup grooming. We therefore propose that LC signals state changes during sustained interactions and contributes to goal-directed action selection during social behavior with globally broadcast NA release.
SIGNIFICANCE STATEMENT Locus coeruleus (LC) releases noradrenaline (NA) brain wide, influencing many cognitive, emotional, and physiological processes. Multifunctionality of LC is maintained by multiplexing NA signaling via brief “phasic” patterns of bursting and slowly changing “tonic” firing. Manipulations of NA impair social behavior, yet the structure of LC activity with respect to specific social events is unknown. We measured LC activity in mice freely interacting with pups. We find that pup retrieval elicits precisely timed and pervasive phasic activation of LC that anticipates specific behaviors. We also found that LC neurons exhibited slow fluctuations in firing during sustained behaviors. We propose that LC simultaneously contributes to goal-directed social action selection with globally broadcast NA release and signals social state changes with increased tonic firing.
Keywords: electrophysiology, fiber photometry, locus coeruleus, maternal behavior, noradrenaline
Introduction
Neurons in the pontine nucleus locus coeruleus (LC) release noradrenaline (NA) broadly throughout the CNS, constituting a key regulator of emotion, arousal, stress, and memory (Berridge and Waterhouse, 2003). Classically, temporal patterns of firing in LC are understood to be composed of the following two distinct processes: slowly evolving, sustained (“tonic”) firing, which is thought to reflect global brain states such as arousal; and rapidly fluctuating, bursty (“phasic”) firing (Aston-Jones and Cohen, 2005). Phasic bursts are typically associated with highly salient stimuli and events (Aston-Jones and Bloom, 1981; Aston-Jones et al., 1994; Vankov et al., 1995) or shifts in behavioral strategy and task contingencies (Sara and Segal, 1991; Bouret and Sara, 2004, 2005). While early work interpreted phasic bursts in LC as responses to behaviorally significant sensory events, over the past 2 decades it has become clear that LC neural activity is more precisely aligned with subsequent execution of conditioned behavioral responses (Clayton et al., 2004; Rajkowski et al., 2004; Bouret and Richmond, 2009; Bouret et al., 2012; Kalwani et al., 2014). Phasic bursts are therefore perhaps better thought of as linking salient sensory events with learned actions (Aston-Jones and Cohen, 2005). The spatial extent and temporal precision of coordinated phasic firing among individual LC neurons remains a subject of investigation (Chandler et al., 2014; Schwarz et al., 2015; Totah et al., 2018), but they may be highly dependent on behavioral context (Poe et al., 2020). Nevertheless, based on their relationship to significant events in structured tasks, LC phasic bursts are an established participant in shaping goal-directed behavior.
Seemingly independent from this central role in navigating experimenter-orchestrated tasks, LC and NA have long been linked to a number of natural social behaviors. For example, microdialysis measurements reveal that NA undergoes elevated and sustained release during conspecific encounters, such as mating (Brennan et al., 1995). In the main and accessory olfactory systems, NA is essential for establishing memories of mating partners (Keverne and de la Riva, 1982; Rosser and Keverne, 1985; Brennan et al., 1995; Shea et al., 2008), for maternal bonding and subsequent recognition of offspring in rats and sheep (Kendrick et al., 1992; Moffat et al., 1993), and for imprinting offspring to odors associated with maternal care (Sullivan et al., 1989). Female mice lacking the enzyme dopamine-β-hydroxylase (Dbh), which is essential for synthesizing NA from dopamine, showed profound disruption of maternal behaviors, often resulting in pup death because of neglect (Thomas and Palmiter, 1997). Importantly, in that study, maternal care was restored in Dbh−/− mutant mothers when NA synthesis was reactivated shortly before birth. Neuroanatomical evidence suggests that adrenergic projections from LC and surrounding areas as well as from the nucleus of the solitary tract (NTS) terminate in the medial preoptic area (MPOA; Numan et al., 1990; Numan and Numan, 1996), a key regulator of maternal behavior.
Collectively, these observations compellingly inculpate NA and LC in a range of natural social behaviors, including maternal care and motivation in particular. However, the technical approaches used lacked sufficient spatial and temporal resolution to ascertain the timing and structure of the underlying neuronal activity in LC (but see Rasmussen et al., 1986). Therefore, the relative contributions of tonic and phasic firing patterns to social interactions are unknown, and the timing of these patterns relative to salient social events is undetermined. Specifically, it is unclear whether fluctuations in NA release occur in response to specific social sensory stimuli, or whether they anticipate specific goal-directed social behaviors.
Here we used chronic in vivo electrophysiology and fiber photometry to measure single-unit and population neural activity in LC of freely behaving surrogate mice during their interactions with pups. The term “surrogate” here refers to a virgin female who is cohoused with a dam and her pups beginning before birth, and who begins to perform maternal care over the first few postnatal days (Rosenblatt, 1967; Stolzenberg and Champagne, 2016). We found that several aspects of maternal care were reliably associated with fluctuations in LC activity. Contact with pups during retrieval events precisely coincided with phasic bursts in individual LC neurons and rapid, transient increases in optically detected bulk fluorescence that continued until the pup was dropped in the nest. The ubiquity of this response among LC neurons, and its reliability and magnitude in fiber photometry recordings, strongly suggest that these events are coordinated across LC and broadcast NA release throughout the brain. We also observed slow changes in tonic firing rate when females performed distinct maternal behaviors such as nest building and pup grooming. Retrieval-related LC bursts could not be explained merely by responses to sensory stimuli, general motor activity, or reward, and changes in tonic firing were not seen during highly similar, but nonsocial motor activities. Analysis of the relationship between phasic events and retrieval behavior indicates that LC activity specifically correlates with impending behavior. We conclude that LC likely modulates social behavior by promoting specific context-dependent actions with rapid fluctuations in activity while simultaneously signaling socially relevant states with slow firing rate changes.
Materials and Methods
Animals.
All procedures were conducted in accordance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals and approved by the Cold Spring Harbor Laboratory Institutional Animal Care and Use Committee. Adult (age, 8–12 weeks) female mice were used in all experiments. All single-unit electrophysiology experiments were conducted in C57BL/6 mice (The Jackson Laboratory), and all fiber photometry experiments were conducted in Dbh-Cre mice [Tg(Dbh-cre)KH212Gsat/Mmucd, unfrozen stock; Mutant Mouse Resource & Research Centers MMRRC]. Animals were maintained on a reversed 12 h light/dark cycle (lights off at 9:00 A.M.), and all experiments were performed during the dark cycle of the animals. Food and water were available ad libitum.
Genotyping.
Hemizygous male Dbh-Cre were crossed with wild-type (C57BL/6) females. The resulting offspring were genotyped according to the vendor protocol (MMRRC). After weaning on postnatal day 21 (P21), tail samples were collected under brief isoflurane anesthesia. Samples were dissolved in lysis buffer (10 mm NaOH and 0.1 mm EDTA) and proteinase K at 37°C for 4 h, and the proteinase K was deactivated in a 95°C water bath. The PCR solution included 1 µl of the DNA solution, 10 µl of PCR master mix (GoTaq Green Master Mix M7123, Promega), 7 µl of nuclease-free water, and 1 µl of each primer (10 µm; 5′, FAATGGCAGAGTGGGGTTGGG; 3′, CGGCAAACGGACAGAAGCATT; Sigma-Aldrich).
Viruses.
Cre-dependent adeno-associated virus (AAV; serotypes 5 or 9) was used to express GCaMP6s (AAV5-Syn-Flex-GCaMP6s, Addgene), or GCaMP7f (AAV9-Syn-Flex-jGCaMP7f-WPRE, Addgene) in the LC of Dbh-Cre mice (Chen et al., 2013; Dana et al., 2019). GCaMP7f has faster response kinetics and improved signal-to-noise ratio (SNR) compared with GCaMP6s.
Surgery.
All surgeries were performed under isoflurane anesthesia (induction, 2−3%; maintenance, 0.7−1.2%) in a stereotaxic frame. To reduce pain and inflammation, mice were injected with meloxicam (5 mg/kg) before all surgeries, and meloxicam gel (ClearH2O) was provided to singly housed mice after the surgery.
For electrophysiology implants, a ∼1 × 1 mm craniotomy was made above the estimated LC location [distance from bregma: anteroposterior (AP), −5.1 to −5.4 mm; mediolateral (ML), 0.8–1.0 mm]. LC was first located using a single tungsten electrode (1 MΩ; Microprobes) by well established neurophysiological criteria (slow tonic firing, wide spike shape, phasic response to tail pinch; Shea et al., 2008). After successful location of LC, an 8- or 16-channel movable microwire bundle (Innovative Electrophysiology) was attached to an 18-pin connector (Omnetics Connector) and was implanted with the tip of the bundle ∼800 µm above LC. The implant was secured to the skull with adhesive luting cement (Parkell). For additional support, two machine screws (Amazon Supply) were secured to the skull and the ground wire was looped around both. Additional luting cement was then applied to cover and secure the implant. Mice were allowed to recover for 7 d before the bundle was advanced.
For fiber photometry implants, a similar craniotomy was made at the same position. Injection of a Cre-dependent AAV driving expression of the Ca2+ sensor GCaMP (either AAV5-Syn-Flex-GCaMP6s or AAV9-Syn-Flex-jGCaMP7f-WPRE) was slowly (∼100 nl/min) injected local to LC [distance from bregma: AP, −5.2 mm; ML, 0.85 mm; distance from brain surface: dorsoventral (DV), 2.9 mm]. After completion of the injection, the injection pipette was left in place for an additional 5 min before withdrawal. Subsequently, a 200-µm-diameter optic fiber (numerical aperture, 0.37; Doric Lenses) was implanted slightly above the injection site and LC (DV, 2.7 mm from brain surface) and was cemented in place. Mice were allowed to recover and express GCaMP for at least 3 weeks before experiments began.
Behavior.
In most cases, surrogates were nulliparous female mice that were cohoused with primiparous CBA/CaJ females (The Jackson Laboratory) beginning 1–5 d before delivery. We never observed aggression between the mother and the surrogate. Nor did we ever observe signs of defeat or injuries on either mouse. A subset of surrogates was exposed to the same litter of pups for only 30 min/d through P5 (see Fig. 5). The same subset was injected with GCaMP7f. All behavior was conducted in the dark, in a Plexiglas arena (42 × 28 cm) inside a custom-built, double-walled anechoic chamber (IAC Acoustics). To reduce anxiety, mice were introduced to handling and the experimental arena for at least 1 h/d for a week before commencing experiments. All experimental subjects were habituated to our experimental arena for prolonged periods of time before the initiation of experiments (3–5 d, 2–3 h/d) and supplied with nesting material (shredded paper) from which the mice built nests during this period. The pups were retrieved to this established nest during the actual experiments. Neither the nest nor the corncob bedding was replaced until the end of the experiment.
Figure 5.
LC activation on contact with pups predicts subsequent retrieval and is not experience dependent. a, Heatmap and mean trace showing an example of the lack of response to pup contact when not followed by retrieval. Data were taken from P1, before the emergence of retrieval behavior in a mouse with controlled exposure to pups. We refer to this as investigation. b, Heatmap and mean trace showing an example of the response to pup contact when it was followed by retrieval. Data were taken from P3 to P5, after the emergence of retrieval behavior in a mouse with controlled exposure to pups. c, Heatmap and mean traces showing an example of the response to retrieval as it emerged in a mouse with controlled exposure to pups. Data are aligned to pup lift. All retrievals performed by this mouse are represented in this panel. The mean traces below compare the mean of data from the first 10 retrieval trials exhibited by this mouse to the mean of all trials in the experiment. d, Scatterplot comparing the baseline activity to the behavior activity for investigation, all retrievals, and the 10 earliest retrievals. Responses during investigation were not significantly different from baseline (n = 6 mice; baseline, 0.15 ± 0.11 z score; retrieval, 0.28 ± 0.19 z score; paired t test, p = 0.24). Significant responses relative to baseline were observed for all retrievals (n = 6 mice; baseline, 0.10 ± 0.11 z score; retrieval, 1.51 ± 0.22 z score; paired t test, ***p < 0.001) as well as for early retrievals only (n = 6 mice; baseline, 0.25 ± 0.18 z score; retrieval, 1.89 ± 0.22 z score; paired t test, *p < 0.05). The amplitude of early retrievals did not significantly differ from the mean amplitude of all retrievals (n = 6 mice; paired t test, p = 0.08). e, Heatmap depicting the time course and amplitude of mean retrieval responses for all subjects.
Pup retrieval behavior was elicited as follows. Each electrophysiology recording session had a duration of 40–90 min. The sessions began with the surrogate alone in the arena. After a short habituation period, the entire litter of pups (5–10) was scattered around the arena and allowed to be retrieved back to the nest by the surrogate. This procedure was repeated several times during each session (approximately every 10 min). Between each retrieval, surrogates were allowed to freely interact with the pups. Fiber photometry sessions were similar, but shorter (∼10 min), and pups were only scattered once. Post hoc scoring of various behaviors was conducted manually using BORIS (Friard and Gamba, 2016) from recorded videos (30 frames/s). Pup contact was defined as the first frame in which the snout of the mouse was on top of a pup. Retrieval onset was defined as the first frame in which the pup was lifted from the bedding and will henceforth be referred to as “pup lift.” In addition to its conventional use to refer to the general behavior, for the purposes of quantification, we use the term “retrieval” to refer to the time between pup lift and pup drop.
Histology and immunohistochemistry.
Mice were perfused with 4% paraformaldehyde/PBS, and brains were extracted and postfixed overnight at 4°C. Brains were then treated with 30% sucrose/PBS overnight at room temperature and sectioned on a freezing microtome at 50 µm. For fiber photometry subjects, free-floating sections were immunostained using standard protocols. Briefly, sections were blocked in 5% normal goat serum plus 2% BSA and 2% Triton X-100 for 1 h and incubated with the following primary antibodies overnight at 4°C: rabbit anti-GFP (1:1000; Thermo Fisher Scientific) and chicken anti- tyrosine hydroxylase (TH; 1:1000; Alves Lab). The next day, the sections were washed in PBS and incubated with secondary antibodies (1:500, Alexa Fluor 488 nm goat anti-rabbit; and 1:500, Alexa Fluor 594 nm goat anti-chicken; Thermo Fisher Scientific) for an additional 1 h and mounted in Fluoromount-G (Southern Biotech). Images were acquired using a microscope (X4 or X10 objective, UPlanFL N; model BX43, Olympus).
For mice implanted with wire bundles, sections were Nissl stained with cresyl violet to identify the deepest location of the bundle. For mice injected with GCaMP, we verified that the expression of GCaMP was restricted to LC and that the visible fiber tip was just above or in LC. In those cases, we observed ongoing fluctuations in the signal, and when the mouse retrieved pups there was always an associated fluorescent transient. In mice where the fiber was mistargeted, we observed neither baseline nor retrieval-associated fluctuations. We never saw ongoing fluctuations in a mouse that did not exhibit phasic bursts during pup retrieval. Mice that either had a misplaced optical fiber or wire bundle or inadequate viral expression (7 of 11 bundles and 10 of 23 fibers) were excluded from the study.
In vivo electrophysiology.
The electrode bundle was advanced daily by 50–100 µm until putative LC neurons were reached (defined by depth and known electrophysiological properties, as described previously; Shea et al., 2008). Once the bundle reached the location of LC, cohousing of the implanted mouse with a pregnant CBA/CaJ female began, and the surrogate was introduced to daily behavioral tests as described above. Electrodes were connected by a tether to a headstage and amplified (Tucker-Davis Technologies). This allowed the mice to move freely while performing natural behaviors. Recordings were digitized at 24 kHz, bandpass filtered from 300 Hz to 3 kHz, and thresholded online by the user and saved for further analysis using Synapse software (Tucker-Davis Technologies). Post hoc spike sorting was first automatically performed using OpenSorter software (Tucker-Davis Technologies) and was further refined manually. Standard criteria were used to ensure single-unit isolation (e.g., manual inspection of cluster separation, SNR, and autocorrelation histograms).
Fiber photometry.
GCaMP signals were detected and measured as follows. A 200 µm optical fiber cable (numerical aperture, 0.39) was mated to the fiber implant at the beginning of each optical recording session, and it was used to deliver 470 and 565 nm excitation light to the brain. The intensity of the light for excitation was adjusted to ∼30 µW at the tip of the patch cord. The two wavelengths were sinusoidally modulated 180° out of phase at 211 Hz. Green and red emitted light signals were filtered and split to separate photodetectors and digitally sampled at 6100 Hz via a data acquisition board (model #NI USB-6211, National Instruments). Peaks were extracted by custom MATLAB software to achieve an effective sampling rate of 211 Hz. Each signal was separately corrected for photobleaching by fitting the decay with a double exponential. A robust regression algorithm was used to compute the coefficients of a linear transformation between the red and green signal, which was then applied to the red signal to generate a prediction of the green trace (Gp). This predicted trace was subtracted from the measured green trace and the residual (Gr) was used to calculate ΔF/F according to the following equation:
The resulting traces from each recording session were collectively converted to a z score to compare data between subjects.
Experimental design and statistical analysis.
Unless specified otherwise all data analyses were performed in MATLAB (MathWorks) using custom-written code. Recordings from single units were sorted into the following three groups according to defined electrophysiological characteristics: (1) putative LC units exhibited a wide spike shape (1.5–2 ms), slow (0.5–5 Hz) regular tonic firing rates, no jaw-related firing events (e.g., while gnawing on corncob bedding), and phasic responses to salient stimuli (e.g., a sudden sound or the experimenter's hand); (2) inhibited units exhibited narrow spike shapes (0.7–0.9 ms), fast (>5 Hz) irregular (coefficient of variation = 2.07 ± 0.76) tonic firing rates, and strong firing rate suppression during pup retrieval; and (3) unidentified units exhibited heterogenous characteristics and could not be clearly separated into one of the other groups. We cannot rule out the possibility that some unidentified cells are LC neurons. Peristimulus time histograms (PSTHs) were generated for 20 s periods (±10 s from pup lift) by binning spike rates into 0.2 s bins. Individual cell PSTHs were z scored, and heatmaps of averaged z scores were calculated. Maximum z scores for baseline (−2.8 to −0.8 s from behavior onset) and behavior (pup retrieval, toy mouse retrieval, snack; −0.8 to 1.6 s from behavior onset) were extracted.
Instantaneous firing rate (IFR) was calculated as the inverse of each interspike interval. To perform correlations between simultaneously recorded neurons, we binned the spike train of each neuron into 1 s bins. The correlation value at each time point was computed as a Pearson correlation between the bins of each neuron for a 30 s window centered around that time point. Retrieval trials were shuffled by randomly permuting trial identities for one of the pair of neurons. Nonretrieval activity was shuffled by taking the mean correlation value for all time steps as one set of bins was circularly permuted in time with respect to the other.
DeepLabCut (Mathis et al., 2018) was used to track the position of the mouse throughout each recording session. We achieved the most reliable tracking by training DLC to follow the connection of the tip of the patch cord to the fiber. The x–y coordinates returned by automated tracking were used to compute a directionless velocity signal (speed), which was then smoothed by convolution with a 7-point boxcar kernel. ΔF/F values were resampled at 30 Hz to match the video frame rate. The optimal lag for speed relative to ΔF/F was found by calculating the maximum of the cross-correlogram of speed versus the ΔF/F signal from 0.5 s before pup lift through the end of retrieval, defined as when the pup was dropped in the nest and henceforth referred to as “pup drop.” Speed was shifted accordingly, and Pearson correlations were calculated for the baseline period (−2.5 to −0.5 s before pup lift) and retrieval (−0.5 s to pup drop).
Statistical analyses were performed using parametric or nonparametric tests as noted in the text, with corrections for multiple comparisons where appropriate. Paired tests were used when comparing two quantities computed using data from the same neurons or mice.
Results
Individual LC neurons emit brief phasic bursts locked to pup retrieval
To directly observe the firing of noradrenergic LC (LC-NA) neurons during maternal interaction, we chronically implanted miniature movable microwire drives in nulliparous female mice (n = 4). Drives were lowered daily until putative LC-NA neurons were located based on well established electrophysiological criteria (see Materials and Methods; Aston-Jones and Bloom, 1981; Berridge and Waterhouse, 2003; Shea et al., 2008). Immediately following the initial identification of putative LC-NA neurons, each subject was cohoused with a female in late-stage pregnancy. After several days in these housing conditions, nulliparous females begin to exhibit characteristic maternal behaviors, including retrieval of pups who become separated from the nest and emit ultrasonic distress vocalizations (USVs; Galindo-Leon et al., 2009; Cohen et al., 2011; Lin et al., 2013; Marlin et al., 2015; Krishnan et al., 2017). We used surrogates for most of our experiments because it allowed us to control exposure to pups and to thereby observe the activity patterns in LC during the early emergence of maternal care. Following birth, the now maternally experienced females (surrogates) were placed with the familiar pups in the experimental arena for extended sessions of free interaction (40–90 min).
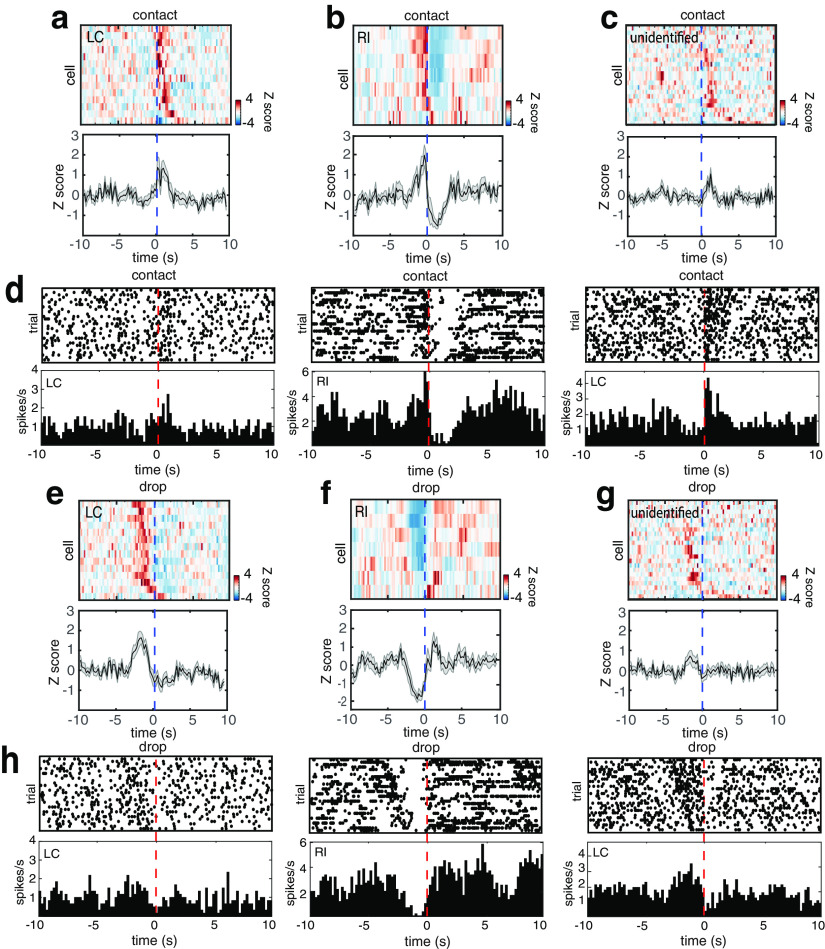
Pup retrieval was elicited several times during each session when the experimenter scattered the pups across the arena and waited for the surrogate to return them to the nest (Fig. 1a). By doing this, we were able to collect LC neural data associated with numerous individual retrieval events from each session (mean, 21.2 retrieval events; range, 5–30 retrieval events). Fourteen putative LC-NA neurons (from four mice) reliably exhibited phasic bursts close to the time of each retrieval event (Fig. 1b, Movie 1). These bursts strongly resembled the typical burst–pause response seen in LC-NA neurons in response to significant or surprising events such as a tail pinch (Aston-Jones and Bloom, 1981). To determine the timing of these bursts for each neuron relative to each retrieval, we compared the alignment of the spike train for each trial from a neuron to three different components of retrieval (pup contact, pup lift, pup drop; Figs. 1c–e, 2a–c, 3). Once each neuron was aligned, we compared the PSTH width at half-maximum (Fig. 1f) and measured the mean lead or lag of the neuronal activity relative to the specific behavior (Fig. 1g). When aligned to contact, the half-maximum width of the PSTH was 2.12 ± 0.22 s and the peak of firing lagged by 1.05 ± 0.20 s. (Unless otherwise stated, all values are mean ± SEM.) When aligned to pup lift, the half-maximum width of the PSTH was 2.18 ± 0.18 s and the peak of firing lagged by 0.44 ± 0.18 s. When aligned to drop, the half-maximum width of the PSTH was 2.51 ± 0.12 s and the peak of firing led by 1.12 ± 0.16 s. These values for pup drop were significantly different from those for contact and pup lift (paired t test comparing the values for each alignment within cells; p < 0.05). Based on the narrow peak seen in the PSTH, and the fact that the firing was centered around the mouse lifting the pup, for the remainder of this study, we will use the term pup lift to refer to this point. When we aligned to this point, we found that individual bursts were narrow but exhibited different phases relative to retrieval; some neurons increased firing immediately following contact and others at later times during the return trip of the surrogate to the nest. All neurons but one exhibited a characteristic inhibition (pause) just after pup drop (Fig. 3e).
Figure 1.

LC-NA neurons emit brief phasic bursts timed to pup retrieval. a, Schematic of the behavior. The pup emits USVs, summoning the female to pick it up and return it to the nest. b, Example single-unit data. Left, A trace of 30 s of a recording from an LC-NA neuron that includes three retrieval events. Three key components in the behavioral sequence are marked with colored dots: yellow, pup contact; green, lifting the pup (pup lift); and red, the time the pup was dropped in the nest. Right, The mean of 100 spikes from the neuron depicted on the left. The gray-shaded band around the mean is the SEM. c, Raster plot and PSTH of neural data recorded from the neuron in b during 19 retrieval events from one session. Data in these plots are aligned to the contact of the female with the pup. d, Same as c, but the data are aligned to the time that the female lifts the pup. e, Same as c, but the data are aligned to the time that the female drops the pup in the nest. f, Scatterplot comparing the width of the peak in the PSTH at half-maximum height above baseline between data aligned to contact, pup lift, and pup drop (contact, 2.12 ± 0.22 s; lift, 2.18 ± 0.18 s; drop, 2.51 ± 0.12 s). The width of the peak was significantly greater when spikes were aligned to drop compared with contact or pup lift (paired t test; *p < 0.05). g, Scatterplot comparing the lag (+) or lead (–) of the peak in the PSTH relative to contact, lift, and drop (contact, 1.05 ± 0.20 s; lift, 0.44 ± 0.18 s; drop, −1.12 ± 0.26 s). The lag of the peak was significantly different for drop compared with contact or lift (paired t test; *p < 0.05).
Figure 2.

Physiologically distinct local neurons show activity that is inversely correlated to LC-NA neurons during retrieval. a, A 2D PSTH of the mean activity of all LC-NA neurons (n = 12) during retrieval (top) and a plot of the mean z-score firing rate over all cells (bottom). In the top panel, each row is the mean firing rate for one neuron, and the data are aligned to pup lift. b, Data from all RI neurons (n = 7) organized as in a. c, Data from all unidentified neurons (n = 18) organized as in a. d, Examples of typical spike shapes from LC-NA neurons (top) and RI neurons (bottom). Each plot is the mean of 100 spikes from the corresponding neuron. The gray-shaded band around the mean is the SEM. e, Scatterplot comparing the mean activity during retrieval to the immediately preceding baseline activity for all neurons of all three types. LC-NA neurons showed significantly higher firing rates during retrieval than at baseline (n = 14; baseline, −0.098 ± 0.07 z score; retrieval, 1.03 ± 0.19 z score; paired t test, ***p < 0.001). RI neurons showed significantly lower firing rates during retrieval than at baseline (n = 7; baseline, 0.28 ± 0.15 z score; retrieval, −0.59 ± 0.20 z score; paired t test, *p < 0.05). The unidentified cells as a group showed a small but significant increase in firing rate relative to baseline (n = 22; baseline, −0.086 ± 0.07 z score; retrieval, 0.31 ± 0.13 z score; paired t test, *p < 0.05). f, Raster plots and PSTHs of neural data recorded simultaneously from three neurons (two LC-NA neurons and one RI neuron) during 28 retrieval events from one session. Data are aligned to pup lift. g, A plot of the fluctuating correlation (see Materials and Methods) of one simultaneously recorded LC-NA/RI pair. The red tick marks denote the times of retrieval. The strongest negative correlations were seen during clusters of retrieval events. h, Scatterplots comparing the mean correlation values for all three neuron types paired with simultaneously recorded LC-NA neurons. Correlation values are plotted separately for firing during retrieval episodes and nonretrieval activity, and all values are also computed for shuffled data. Correlation values were significantly more negative in LC-NA/RI pairs during retrieval than in nonretrieval periods or shuffled data (n = 7; retrieval, −0.21 ± 0.5; nonretrieval, 0.00 ± 0.05; shuffled retrieval, −0.05 ± 0.02; one-way ANOVA, **p < 0.01).
Figure 3.
RI neurons show an increase in firing rate before pup contact and strong activity rebound following pup drop. a–h, Same data as in Figure 2a–f aligned to pup contact (a–d) or pup drop (e–h).
In this clip, the audible clicks correspond to the spikes of a well isolated LC-NA single unit. Bursts of accelerated spiking can be heard time locked to each retrieval event. The inset in the bottom right corner is a streaming visual representation of the neuronal spike train.
Faster spiking neurons in or near LC are sharply inhibited during pup retrieval
In the course of performing these experiments, we incidentally recorded other single units in the immediate vicinity of LC that did not match the characteristics of LC-NA neurons (n = 29 neurons; recorded from four mice). For example, post hoc analysis revealed a group of bursty, faster-spiking (8.55 ± 1.2 spikes/s) neurons with a narrow shape (Fig. 2d), which were consistently and strongly inhibited around the time of pup retrievals [retrieval-inhibited (RI) neurons]. These neurons consequently exhibited a reciprocal response to LC-NA neurons including a strong rebound in firing rate just after the time the pup was dropped in the nest (n = 7 neurons; recorded from two mice; Figs. 2b,d, 3b,f). Alignment to contact revealed a sharp increase in the firing rate of these neurons beginning a few seconds before and peaking at contact (Figs. 2b, 3b,d). Closer examination of our retrieval videos revealed that this corresponded to the mouse initiating motion toward the pups.
In addition, we collected another 22 neurons that did not match the profiles of LC-NA or RI neurons; these neurons were designated “unidentified” and rarely exhibited activity related to retrieval (Figs. 2c, 3c,d,h). We quantified the sign and magnitude of retrieval activity in each of these three classes by converting the firing rates of all neurons to z scores and comparing the mean baseline activity just before pup lift to the mean activity during the rest of retrieval (Fig. 2e). Putative LC-NA neurons showed a significant increase in firing rate during retrieval (n = 14; paired t test comparing baseline firing rate to retrieval firing rate for each neuron, p < 0.001), while RI neurons showed a significant decrease in firing rate during retrieval (n = 7; paired t test comparing baseline firing rate to retrieval firing rate for each neuron, p < 0.05). As a group, the unidentified neurons also showed a significant change in firing (n = 22; paired t test comparing baseline firing rate to retrieval firing rate for each neuron, p < 0.05).
We were not able to definitively identify the location of the cell bodies of the RI neurons; however, the apparently reciprocal firing pattern during pup retrieval raises the possibility that RI neurons correspond to GABAergic neurons in and around LC that have been proposed to exert inhibitory control of LC-NA neurons (Aston-Jones et al., 2004; Jin et al., 2016; Breton-Provencher and Sur, 2019; Kuo et al., 2020). Many of our recording sessions yielded multiple simultaneously recorded neurons (Fig. 2e). We therefore examined correlated activity between LC-NA neurons and all three types of neurons (other LC-NA, RI, and unidentified; 30 neuron pairs recorded in three mice; Fig. 2f,g). We binned firing rate for both neurons in each pair at 1 s/bin and then performed a Pearson correlation on the bin values of the two histograms.
Correlations between LC-NA and RI neurons fluctuated in time, typically reaching maximum inverse correlation during episodes of retrieval (Fig. 2f). We therefore separately computed the correlations for bins falling in the 8 s following a retrieval event from those falling elsewhere in the record (Fig. 2g). The mean correlation value for LC-NA/RI neuron pairs during retrieval episodes was significantly more negative than that for all other time points (n = 7 pairs recorded in two mice; paired t test comparison of data during retrieval to all other data for each pair, p < 0.05). The inverse correlation of these two cell types during retrieval was not because of a nonspecific synchronizing effect of retrieval because it disappeared when retrieval trials were shuffled between the paired neurons (n = 7 pairs; paired t test comparison of data to shuffled data for each pair, p < 0.01). Mean correlation value for LC-NA/RI neuron pairs during nonretrieval periods did not significantly differ from that obtained when the temporal shift between the spike trains of the paired neurons was circularly permuted (n = 7 pairs; paired t test comparison of data to circularly permuted data for each pair, p = 0.95).
No significant differences in correlation values were seen between retrieval and nonretrieval data, or between shuffled and nonshuffled data, for LC-NA/LC-NA neuron pairs or for LC-NA/unidentified neuron pairs.
Optical recordings reveal that retrieval activity in LC is pervasive and synchronous
Our electrophysiology data have two limitations. First, mounting evidence argues that LC may have a more modular organization anatomically and functionally than was previously appreciated (Schwarz et al., 2015; Kebschull et al., 2016; Uematsu et al., 2017; Totah et al., 2018). Hence, despite the fact that all of our putative LC-NA neurons exhibited sharply elevated phasic activity during pup retrievals, it is possible that LC neurons projecting to different parts of the brain fire differently during pup interaction. Second, because of the challenging nature of electrical recordings in freely behaving mice, data are sporadic and low yield, making longitudinal experiments nearly impossible. To overcome these limitations, we used fiber photometry to measure bulk Ca2+ signals from LC-NA neurons. This method captures the neuronal population activity, so if the timing of LC-NA neurons' participation in pup retrieval is heterogenous, the signal fluctuations will presumably be of relatively low amplitude. Fiber photometry also allows us to monitor the same neural population day by day, so we can monitor any changes in the responses as the surrogate begins to perform maternal behavior.
We injected a Cre-dependent AAV carrying the genetically encoded Ca2+ sensor GCaMP6s into the LC of Dbh-Cre mice, thereby restricting GCaMP expression to noradrenergic neurons (Fig. 4a–c). Surrogates were cohoused with a pregnant female. Following birth, retrieval experiments were conducted over several consecutive days in a familiar experimental arena. Consistent with what we observed in single-unit recordings from putative LC-NA neurons, pup retrievals were accompanied by a steep, strong rise in the population Ca2+ signal (Fig. 4d, Movie 2) beginning around contact with the pup and reaching its peak just after pup lift (Fig. 4f; n = 7 mice; paired t test comparison of baseline fluorescence to retrieval fluorescence for each mouse with Holm–Bonferroni correction, p < 0.001). The signal remained elevated through the retrieval until the pup was dropped in the nest. Signals typically subsequently exhibited a dip below baseline (n = 7 mice; paired t test comparison of baseline fluorescence to postretrieval dip fluorescence for each mouse with Holm–Bonferroni correction, p < 0.05) that resembled the burst-pause response typical of phasic activity in LC-NA neurons (Aston-Jones and Bloom, 1981; Fig. 4g,h). Comparing the signal across 5 consecutive days, we found no significant changes in the relative amplitudes and timing of the Ca2+ peaks (Fig. 4i,j; one-way ANOVA, p > 0.05).
Figure 4.

Fiber photometry reveals that LC-NA neurons emit pervasive and synchronous population bursts during retrieval in surrogates and dams. a, Experimental strategy. Dbh-Cre mice were injected with a Cre-dependent AAV driving expression of the genetically encoded Ca2+ indicator GCaMP6s and were implanted ipsilaterally with an optical fiber. b, Photomicrograph showing GCaMP expression and the location of the fiber. Scale bar, 200 µm. c, Photomicrographs showing the overlap in expression of GCaMP (green) and TH (red), a marker of noradrenergic neurons. Scale bar, 100 µm. d, Plot of 110 s of raw ΔF/F data from one example subject performing eight retrievals (red arrowheads). Each retrieval is accompanied by a large transient in fluorescence. e, Heatmap of 99 individual retrieval trials taken from P0 through P6. f, g, Plots of mean responses during pup retrieval for each mouse (n = 7; top) and the mean ± SEM across all mice, aligned to pup lift (f) and pup drop (g). h, Scatterplot comparing the mean z-score values from baseline, retrieval, and the postretrieval dip for all mice. All values were significantly different from the other values (n = 7; baseline, 0.025 ± 0.07 z score; retrieval, 1.36 ± 0.13 z score; postdrop dip, −0.50 ± 0.13 z score; paired t test with Holm–Bonferroni correction: ***p < 0.001; *p < 0.05). i, j, Plots of the stability of signals over 5 d for all mice. Mean amplitude relative to day 1 (normalized to 1; i) and mean timing of peak activity relative to day 1 (defined as 0; j) are shown for all mice (n = 7). Fiber photometry in LC of dams during pup retrieval shows a pervasive burst similar to surrogates. k, Example of bulk Ca2+ signal fluctuations measured during pup retrieval in the same dam over 6 d (n = 40 individual trials; P0–P5) aligned to pup lift (dashed line). The same female was previously used as surrogate (Fig. 4E). The lower SNR is probably because of degradation of the signal after close to 3 months postinjection. l, Mean z-scored ΔF/F responses across all retrievals aligned to pup lift [n = 3 dams (1 GCaMP6s, 2 GCaMP7f); top] and the mean ± SEM responses across all dams. m, Scatterplot comparing the baseline activity (bl; gray circles) to the retrieval activity (ret; black circles). Red lines represented the mean of each condition (baseline, −0.18 ± 0.07; retrieval, 1.03 ± 0.1; paired t test, ***p < 0.001).
In this clip, the audio track consists of a carrier tone that is frequency modulated by the fluorescent fiber photometry signal. Sharp peaks in the signal that are time locked to retrieval can easily be heard as brief, steep increases in pitch. The inset at the bottom also depicts a streaming trace of ΔF/F synced to the video.
Pregnancy is associated with hormonal fluctuations that trigger comprehensive changes in the physiology of the dam, including neural circuitry and brain function. Although surrogates learn to perform pup retrieval and other maternal behaviors, it is possible that pregnancy leads to a different LC-NA activation profile compared with surrogates. We tested this by mating former surrogates expressing GCaMP (n = 3 mice; one female was injected with GCaMP6s, two females were injected with GCaMP7f) and performing retrievals with their pups beginning after delivery (P0–P5; Fig. 4k–m). Pup retrieval in dams elicited strong phasic activation of LC beginning around pup contact and persisting until pup drop (paired t test comparison of baseline fluorescence to retrieval fluorescence for each mouse, p < 0.001). This activity pattern was qualitatively indistinguishable from the corresponding events in surrogates. Thus, we conclude that LC activity in surrogates is representative of that in dams.
The strength and precision of the phasic activity in the LC-NA population during pup retrieval leads us to conclude that these events are likely pervasive and reflect synchronous activity among a large proportion of the population of LC-NA neurons. If that is correct, then this specific behavior may evoke coordinated “broadcast” release of NA at many downstream targets.
LC activation at pup contact predicts subsequent retrieval and is not experience dependent
We next asked how phasic LC activity during retrieval emerges as the female acquires maternal experience. Naive virgin female Dbh-Cre mice were injected in LC with a Cre-dependent AAV carrying the Ca2+ sensor GCaMP7f. Following recovery, the mice were exposed to pups daily (postnatal days 1–5) for 30–45 min in a familiar experimental arena. Similar protocols have been used to induce maternal behavior in naive rats and mice without the need for continuous cohousing, a process termed “sensitization” (Rosenblatt, 1967; Martin-Sanchez et al., 2015). At the end of each exposure, pups were scattered across the arena several times, as above, to elicit maternal retrieval. Initially (P1–P2), inexperienced females only investigated pups (transiently touching them with their snout) without initiating retrieval to the nest with a pup lift. These brief contacts did not elicit any detectable increase in population Ca2+ signals in LC (Fig. 5a,d; n = 6 mice; paired t test comparison of baseline fluorescence to retrieval fluorescence for each mouse, p = 0.24). By P3, all mice (n = 6) were reliably retrieving the scattered pups. Investigatory contact with pups, only when followed by pup lift, was accompanied by a rise in fluorescence that persisted until the pup was dropped in the nest (Fig. 5b; n = 6 mice; paired t test comparison of baseline fluorescence to retrieval fluorescence for each mouse, p < 0.001). Therefore, LC activation in surrogates at the time of initial contact with a pup predicted subsequent retrieval to the nest. This set of observations is consistent with evidence from structured tasks that phasic LC activity is not driven by sensory stimulation by itself. Rather, bursts in LC are elicited in a context-dependent manner, reliably preceding goal-directed actions made in response to sensory cues (Clayton et al., 2004; Rajkowski et al., 2004; Bouret and Richmond, 2009; Bouret et al., 2012; Kalwani et al., 2014).
We speculated that the magnitude of phasic activity associated with pup retrieval may gradually increase with improving retrieval performance on early trials, but this was not the case. The magnitude of fluorescence on early trials was significantly higher than baseline fluorescence (n = 6 mice; paired t test comparison of baseline fluorescence to retrieval fluorescence for each mouse on the first 10 trials, p = 0.001). We compared the mean amplitude of Ca2+ signals on the first 10 retrievals performed by each mouse with the mean amplitude of all retrievals from that mouse (Fig. 5c,d). The magnitude of early trials did not significantly differ from all trials, although there was a strong trend (n = 6 mice; paired t test comparison of mean early trial amplitude to mean amplitude over all trials for each mouse, p = 0.08). This result shows that retrieval-related activation of LC emerges at nearly full amplitude on the very first retrievals performed by the mouse and is not experience dependent. The change in behavior may reflect in part a removal of neophobic aversion to pups (Stolzenberg and Champagne, 2016).
Pup retrieval activity in LC is distinct from nonsocial motor or reward responses
The above results seem to indicate that sensory stimuli from the pups (e.g., pup odors) alone are not sufficient to account for LC activity during pup retrieval. However, it remains possible that the responses are evoked by other, nonsocial motor or reward aspects of the task. For example, one recent study suggested that LC-NA neurons mediate the effort/reward trade-off (Borderies et al., 2020). To compare LC responses to nonsocial motor, effort-exerting, and rewarding activities, we measured LC activation patterns during digging, appetitive reward (snack), and the retrieval of a toy (fake mouse) using both fiber photometry and single-unit electrophysiology.
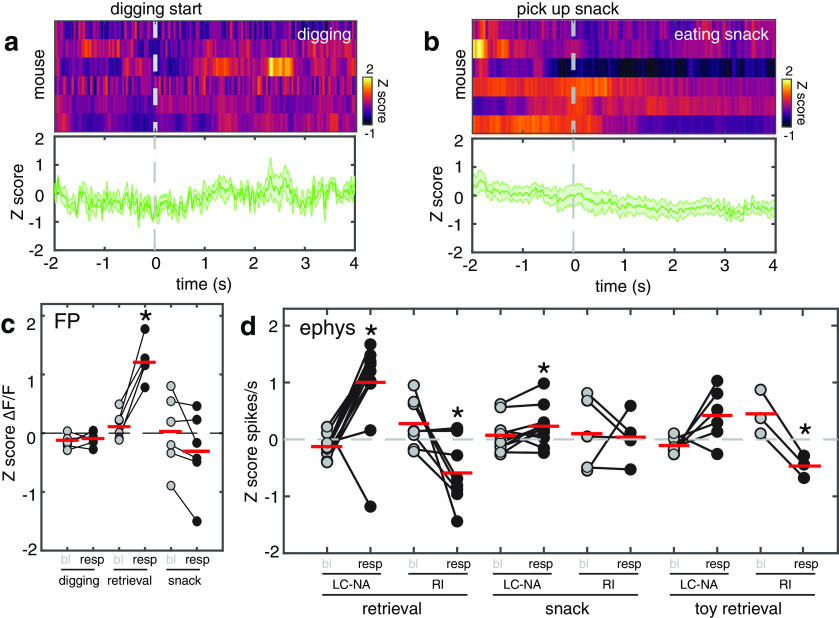
Laboratory mice tend to dig in their corncob bedding, looking for pieces to gnaw on. While not directly comparable to pup retrievals, this activity both expends effort and is potentially rewarding. Nevertheless, when we measured LC activity aligned to the onset of digging, there were no significant changes in either Ca2+ signal (Fig. 6a,c; n = 6 mice; paired t test comparison of baseline fluorescence to digging fluorescence for each mouse, p = 0.32) or in the firing rate of single units (Fig. 7a,d; n = 11 cells; paired t test comparison of baseline firing to retrieval firing rate for each neuron, p = 0.30).
Figure 6.
LC maternal retrieval responses are not replicated by motor activity, unexpected reward, or retrieval of an inanimate object. a, Plots of the mean fluorescence signal in LC aligned to bouts of vigorous digging in the cage bedding. The mean response of LC to the onset of digging in six mice is shown in the heat map (top). Each row is the mean response of one mouse. Bottom, Mean response to digging across all mice. b, Plots of the mean fluorescence signal in LC aligned to locating a hidden snack buried in the bedding. The mean response of LC to the snack in three mice is shown in the heat map (top). Bottom, Mean response to snack discovery across all mice. c, Scatterplot comparing the magnitude of GCaMP fluorescence responses to digging and snack discovery with responses to pup retrieval. In contrast with the robust retrieval responses, neither digging (n = 6 mice; baseline, −0.15 ± 0.05 z score; retrieval, −0.09 ± 0.05 z score; paired t test, p = 0.33) nor snack discovery (n = 6 mice; baseline, 0.02 ± 0.25 z score; retrieval, −0.32 ± 0.28 z score; paired t test, p = 0.08) resulted in a significant increase above baseline activity. d, Scatterplot comparing the magnitude of neuronal spiking responses to snack discovery and retrieval of a stuffed mouse toy with responses to pup retrieval for LC-NA and RI neurons. In contrast with the reliable and robust responses in both neuron types to pup retrieval, firing rate changes to the other events were weak and inconsistent. Snack discovery did evoke a significant increase in firing above baseline in LC-NA neurons, but it was far smaller (n = 11 neurons; baseline, 0.07 ± 0.13 z score; snack, 0.23 ± 0.15 z score; paired t test, *p < 0.05). Retrieval of the toy mouse resulted in a significant drop in firing rate only in RI neurons (n = 3 neurons; baseline, 0.45 ± 0.19 z score; toy retrieval, −0.47 ± 0.22 z score; paired t test, *p < 0.05).
Figure 7.
Distinct maternal behaviors are associated with tonic increases in LC neural activity. a, Plot of log10(instantaneous firing rate) from one LC-NA neuron over 50 min of recording in a freely behaving pup-experienced female. Some of the points are color coded to reflect that those data were collected during the specific behavior indicated by the color legend. Nesting with pups refers to active nest building while one or more pups were in the nest. Nesting without pups refers to the same behavior performed without any pups in the nest. The black points found at the start of the trace while the subject was alone in the arena were used as control data to compare with other behaviors. b–g, Comparison of log firing rates associated with various behaviors, including the following: nesting with pup versus control (b), nesting with pups versus nesting without pups (c), nesting with pups versus digging (d), retrieval versus control (e), licking pup versus control (f), and chewing the toy mouse versus control (g). Nesting with pups had significantly higher firing rates than control (nesting with pups, 2.53 ± 1.4 spikes/s; control, 1.79 ± 1.5 spikes/s; paired t test, *p < 0.05), nesting without pups (nesting with pups, 1.37 ± 1.3 spikes/s; nesting without pups, 0.96 ± 1.3 spikes/s; paired t test, *p < 0.05), and digging (nesting with pups, 2.06 ± 1.3 spikes/s; digging, 1.31 ± 1.4 spikes/s; paired t test, **p < 0.01). Firing during retrieval was also higher than during control (retrieval, 4.46 ± 1.2 spikes/s; control, 2.07 ± 1.5 spikes/s; paired t test, *p < 0.05). h, Plot of 150 s of GCaMP fluorescence data containing two examples of episodes of the mouse licking and grooming pups; denoted by the blue bars. i, Comparison of fluorescence signals during licking and grooming of pup and control periods alone in the arena. Activity was significantly higher during licking and grooming (n = 6 mice; licking, 0.32 ± 0.15 z score; control, −0.29 ± 0.04 z score; paired test, *p < 0.05).
Introducing a toy mouse to the arena elicited subsequent investigation and, in a few cases, retrieval of the toy to the nest. Retrieval of the toy was accompanied by a trending, but not statistically significant increase in firing rate of LC-NA single units compared with baseline (Fig. 6d; n = 6 cells; paired t test, p = 0.08) and a significant decrease in the firing rate of RI units (Fig. 6d; n = 3 cells; paired t test, p < 0.05). This partial response could reflect effort associated with retrieval of the toy; however, it seems unlikely that the steeper rise in LC-NA neurons firing during pup retrieval is entirely related to predicted effort as the toy was substantially larger than a pup and demanded no less effort to retrieve (Fig. 6d). Moreover, retrieval responses did not increase as pups gained in weight (Figs. 4, 5).
To test for nonsocial reward response, mice were given a snack (chocolate pellet). As recently reported (Sciolino et al., 2019), snack consumption by satiated mice (food and water was provided ad libitum to all experimental animals; see Materials and Methods) was accompanied by a drop in Ca2+ signal that was not significant, but in any case trended away from the sharp excitation during retrieval (Fig. 6b,c; n = 6 mice; paired t test comparison of baseline fluorescence to fluorescence during snack consumption for each mouse, p = 0.08). There was a mixed response in the two single-unit types (LC-NA and RI) with a minor statistically significant increase in the firing rate of LC-NA units while mice ate the snack compared with baseline (Fig. 6d; n = 11 cells; paired t test, p < 0.05). These results demonstrate that strong phasic activation of LC during pup retrieval cannot simply be attributed to motor response, effort exertion, or nonsocial appetitive reward.
Tonic firing of LC-NA neurons is associated with distinct maternal behaviors
Pup retrieval is one of a suite of maternal behaviors exhibited by dams and surrogate mice including, for example, nest building and maintenance, licking and grooming, and crouching (Stolzenberg and Mayer, 2019). Disruption of NA signaling in pregnant female rodents with neurotoxins, lesions, or genetic manipulation causes severe deficiencies in these behaviors postpartum, leading to pup negligence and elevated mortality (Rosenberg et al., 1977; Steele et al., 1979; Thomas and Palmiter, 1997). The relatively extended time frame for our single-unit recordings (40–90 min) afforded us the opportunity to monitor LC-NA neuron firing patterns during a broader set of maternal behaviors beyond pup retrieval. By measuring tonic spiking as the mean log of IFR, we identified several maternal behaviors that were associated with sustained elevations in LC-NA activity relative to control conditions when the surrogate is alone in the arena (Fig. 7a, black dots).
Nest building and maintenance are important components of maternal behavior in mice that become more intensive and elaborate in peripartum females (Bond et al., 2002). Mean IFR significantly increased while surrogates were organizing disturbed nesting material and covering pups with it (Fig. 7a,b). Importantly, mean IFR was not only higher than control periods, but it was also significantly higher than the IFR during episodes of nest maintenance when pups were not present in the nest and during digging, another active behavior (Fig. 7c,d). Licking and grooming pups is another fundamental maternal behavior that is critical for infant–dam bonding (Sullivan et al., 1989; Landers and Sullivan, 2012). Although when surrogates licked pups, LC-NA single units did show a trend toward elevated FR, it never reached statistical significance compared with the control period (before pup introduction to the experimental arena; Fig. 7f). Importantly, this trend was not observed when surrogates gnawed on a toy mouse (Fig. 7g). Pup licking in Dbh-Cre surrogates injected with GCaMP6s, however, led to significant an increase in calcium signal that persisted as long as the licking continued (Fig. 7h,i; n = 6 mice; paired t test comparison of baseline fluorescence to licking fluorescence for each mouse; paired test, p < 0.05). This discrepancy could be because of the spatiotemporally integrative nature of calcium sensors, collecting fluorescence from throughout the neural population. These results are consistent with LC-NA neurons influencing distinct maternal behavior patterns at short timescales and over longer intervals.
Ca2+ signals in LC correlate with subsequent retrieval but not other locomotion
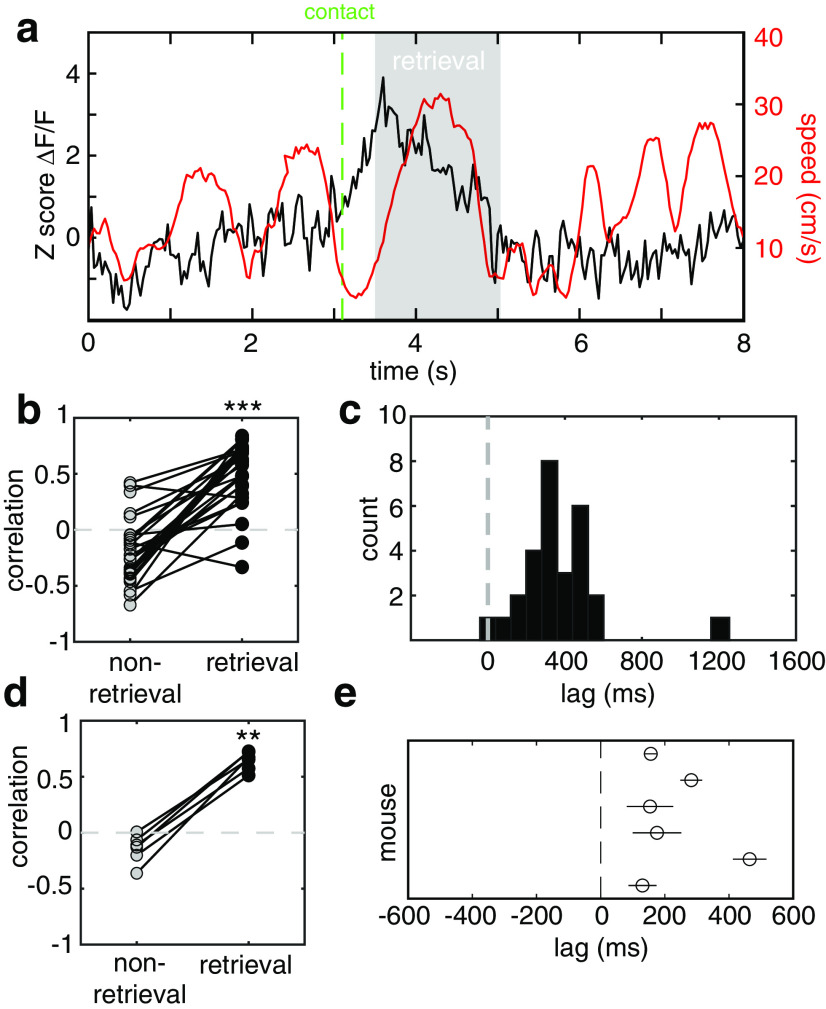
The onsets of phasic bursts in LC reliably preceded pup retrieval. To examine the temporal relationship between these two events in greater detail, we used automatic position-tracking software (DeepLabCut; Mathis et al., 2018) to calculate a directionless instantaneous velocity signal (speed). Inspection of plots of the speed trace aligned to the concurrently recorded GCaMP signal revealed a peak in Ca2+ signals leading the peak in speed corresponding to the return trip of the female to the nest with the pup in its mouth by several hundred milliseconds (Fig. 8a). No such Ca2+ transient was evident before other movements with similar temporal profiles.
Figure 8.
Ca2+ signals in LC correlate with subsequent retrieval but not other locomotion. a, Plot of z score ΔF/F signal (black) and the concurrent speed signal computed from automated tracking (red) for a single retrieval trial. The dashed green line marks the point of contact with the pup, and the gray-shaded region marks the time of retrieval, beginning at pup lift and ending in pup drop. This is a typical example showing that the fluorescence signal is unrelated to the speed of the animal at all times other than retrieval, including approaching the pup. b, Plot comparing speed–ΔF/F correlation values between retrieval and preretrieval times for 28 different retrieval trials during one session from the mouse depicted in a. Correlation values were significantly greater during retrieval than during nonretrieval (n = 28 trials; nonretrieval, −0.20 ± 0.05; retrieval, 0.73 ± 0.04; paired t test, ***p < 0.001). c, Histogram of the latency between the ΔF/F signal and the speed signal for each trial in the session depicted in a and b, computed as the maximum of the cross-correlogram. The mean and SEM of the latency were 464 ± 52 ms. d, Plot comparing the mean speed−ΔF/F correlation between retrieval and preretrieval times over all trials for six mice. The correlation values were significantly higher during retrieval (n = 6; baseline correlation, −0.14 ± 0.05; retrieval correlation, 0.61 ± 0.03; paired t test, **p < 0.01). e, Scatterplot of the mean ± SEM of the lag between the ΔF/F signal and the speed signal for each of the mice in d.
We quantitatively verified this impression by performing for each retrieval trial a sliding cross-correlation between the GCaMP signal and the mouse speed trace spanning a lag of −0.5 to +n seconds for speed with respect to GCaMP (where the n is the duration of retrieval in seconds). We measured the lag and the Pearson correlation value at the peak of the cross-correlation function, which we designated as the optimal lag. We compared this to the correlation values at the same lag for the prior 2.5 s of data. Figure 8, b and c, shows the results of this analysis of all retrieval trials from the mouse depicted in Figure 8a. Comparison of the correlation values during retrieval to those before retrieval shows that mean correlation during retrieval was significantly higher (Fig. 8b; baseline correlation, −0.20 ± 0.05; retrieval correlation, 0.51 ± 0.06; paired t test, p < 0.001), and the histogram in Figure 8c shows that the optimal lag for all trials was short, consistent, and always GCaMP leading (lag = 460 ± 52 ms). This pattern was consistent across animals (Fig. 8d,e). Mean correlation values during retrieval were significantly higher than those at other times when the mouse was still making similar movements, including pup approach (n = 6; baseline correlation, −0.14 ± 0.05; retrieval correlation, 0.61 ± 0.03; paired t test, p < 0.01). Based on these data, we propose that the role of LC in action selection during maternal behavior is context dependent and goal directed.
Discussion
For decades, the LC-NA system has been understood to be a critical participant in arousal and attention in structured cognitive tasks. Specifically, LC neurons respond to novelty (Vankov et al., 1995), behavioral significance (Aston-Jones and Bloom, 1981; Aston-Jones et al., 1994), effort (Borderies et al., 2020), and changes in strategy (Sara and Segal, 1991; Bouret and Sara, 2004, 2005), and they also promote goal-directed action selection (Clayton et al., 2004; Rajkowski et al., 2004; Bouret and Richmond, 2009; Bouret et al., 2012; Kalwani et al., 2014). Largely independently, LC is also closely tied to social behaviors, such as individual recognition (Rosser and Keverne, 1985; Kendrick et al., 1992; Shea et al., 2008), mating choices (Keverne and de la Riva, 1982; Brennan et al., 1995), and, importantly for our work, maternal behavior (Sullivan et al., 1989; Thomas and Palmiter, 1997). Until now, it was unclear how these diverse functions are compartmentalized or multiplexed within the output of this very small population of neurons. It was also virtually unknown at what timescale LC regulates natural behavioral interactions with conspecifics. A prior study recorded LC units in cats during unrestricted behavior, including interactions with other cats, but reported little change in firing rate in the presence of conspecifics (Rasmussen et al., 1986). Our results begin to provide the first answers to these questions, revealing an unexpectedly high level of precision in the control of maternal behavior of LC, and also highlighting some common principles that govern the role of phasic NA activity in both behavioral contexts.
Here we used chronic in vivo electrophysiology and fiber photometry to measure single-unit firing and population activity in LC of female mice while they freely interacted with pups. Individual neurons in maternally experienced surrogates consistently showed a brief phasic burst near the initiation of pup retrievals. Optical recordings using genetically encoded calcium sensors restricted to expression only in LC-NA neurons confirmed this phasic response during pup retrievals, and further revealed it to be a pervasive event, reflecting relative synchrony among LC-NA neurons. Importantly, we observed very similar activity in dams that delivered pups themselves, which adds to a growing body of evidence that overlapping neural processes govern the emergence of maternal care in surrogates (hormone-independent sensitization) and dams (hormone-dependent; Stolzenberg and Champagne, 2016).
Phasic bursts in LC were not primarily driven by sensory responses to pups, because they were only seen on trials where the female actually lifted the pup, and they appeared at full magnitude on the first trials where the mouse began to retrieve. LC retrieval responses also did not simply reflect vigorous motor activity, general effort, or unexpected discovery of a desirable treat. For all these reasons, we conclude that phasic LC bursts are not signaling reward. Instead, they were most closely correlated with the specific, impending goal-directed action of returning the pup to the nest. At the same time, other sustained maternal behavioral states were reflected in the ongoing tonic of LC activity (e.g., nest building in the presence of pups as opposed to the same behavior without pups). Therefore, we propose that LC regulation of maternal behavior shares a common blueprint with activity underlying structured tasks: tonic firing levels encode slowly fluctuating state variables such as arousal and are punctuated by phasic events that promote goal-directed behavioral choices (Clayton et al., 2004; Rajkowski et al., 2004; Bouret and Richmond, 2009; Bouret et al., 2012; Kalwani et al., 2014).
In our electrophysiology experiments, we observed two distinct types of cells that responded during pup retrieval. First, we observed putative LC-NA neurons that bore the characteristics of TH+/Dbh+ neurons and all increased firing during retrieval. Second, we saw another type of cell that responded with a sharp decrease in firing rate during retrieval. Because these cells were consistently anticorrelated with LC-NA neurons during retrieval, we speculate that they may represent some of the inhibitory neurons that are intermingled with LC or adjacent to it. Based on ultrastructural neuroanatomy, in vitro optogenetic activation, and in vivo manipulation of pupillary dilation, several groups have reported evidence of local inhibitory control of LC from different cell clusters. Since we cannot be sure about the precise location of the neurons we recorded, further work will be needed to clarify the specific involvement of any of these sources of inhibition.
Although LC was once viewed as a syncytium, firing largely in a highly coordinated fashion across the nucleus, recent evidence has cast doubt on this model. Closer examination of the anatomic distribution of inputs and outputs of LC suggests that there is greater specificity to them than previously appreciated, potentially forming separate parallel circuits that link specific afferent populations to specific targets (Schwarz et al., 2015; Kebschull et al., 2016). Functional analysis of neuronal activity in multichannel recordings is consistent with this revised view, showing relatively little correlation among neurons (Totah et al., 2018). In contrast, our data seem to suggest that, at least under certain conditions, the population of LC-NA neurons may act in close coordination, constituting a broadcast signal that simultaneously mobilizes a wide swath of downstream circuits. This apparent synchrony is of course relative because individual LC-NA neurons exhibited a diversity of activation latencies ranging from time of contact with the pup through the end of retrieval several seconds later. Indeed, the averaged firing from all LC-NA neurons closely resembled the population response as measured by bulk Ca2+ signal. This phase diversity could arise from distinct sets of afferents driven by various behavioral components (e.g., sensory, motivational, motoric). Further work dissecting distinct LC-NA neurons ensembles by, for example, retrogradely labeling them from different efferent structures may clarify this. Nevertheless, we submit that the timescale of phasic events during maternal retrieval reveals a surprising level of precision and coordination among LC-NA neurons.
Further work will inevitably reveal more about the maternal brain networks that are accessed by LC and how they are influenced by NA. However, based on existing work, we can speculate on some likely key targets. The MPOA, which is an important nexus of circuits that mediate maternal behavior, likely receives adrenergic inputs from LC and the NTS in rats (Numan et al., 1990; Numan and Numan, 1996). LC also sends a robust projection to the anterior cingulate and other regions that are sometimes referred to as the prefrontal cortex in rodents (Koga et al., 2020). Finally, another major downstream target of LC is the amygdala. The basolateral amygdala (BLA) is interconnected with several areas that regulate maternal behavior (Numan and Stolzenberg, 2009), and lesions of the BLA selectively impair pup retrieval (Numan et al., 2010).
We closely examined the temporal relationship between Ca2+ signals and locomotion during retrieval. We found that LC activity consistently preceded the change in speed associated with initiating retrieval by several hundred milliseconds. LC fluorescence was highly correlated with speed during retrieval periods, yet these two measures were either not correlated or negatively correlated during pup approach. Several recent studies have reported significant correlations between phasic activation of LC and motion on approach to a learned reward location (Xiang et al., 2019; Kaufman et al., 2020). One interpretation of our results that is concordant with these previous findings is that an important feature of the regulation of LC of maternal behavior is the promotion of actions that achieve the motivational goal of delivering the pup to a specific reward-associated location (the nest).
In conclusion, we propose that our results begin to integrate and align the participation of LC-NA neurons in both unstructured natural social interactions and experimenter-designed and -instructed cognitive tasks into a common framework. Moving forward, we are optimistic that our results delineate a novel approach for deconstructing the ethological building blocks that have been adapted to solve closely controlled problems devised for the laboratory setting.
Footnotes
This work was supported by a grant to S.D.S. from the National Institute of Mental Health (R01-MH-119250) and a National Alliance for Research on Schizophrenia and Depression Young Investigator Grant to R.D. from the Brain and Behavior Research Foundation. We thank C. Kelahan, J. Sturgill, and L. Huang for technical assistance; and A. Kepecs, M. Smear, and Shea Laboratory members for helpful comments and discussion.
The authors declare no competing financial interests.
References
- Aston-Jones G, Bloom FE (1981) Nonrepinephrine-containing locus coeruleus neurons in behaving rats exhibit pronounced responses to non-noxious environmental stimuli. J Neurosci 1:887–900. 10.1523/JNEUROSCI.01-08-00887.1981 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aston-Jones G, Cohen JD (2005) An integrative theory of locus coeruleus-norepinephrine function: adaptive gain and optimal performance. Annu Rev Neurosci 28:403–450. 10.1146/annurev.neuro.28.061604.135709 [DOI] [PubMed] [Google Scholar]
- Aston-Jones G, Rajkowski J, Kubiak P, Alexinsky T (1994) Locus coeruleus neurons in monkey are selectively activated by attended cues in a vigilance task. J Neurosci 14:4467–4480. 10.1523/JNEUROSCI.14-07-04467.1994 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aston-Jones G, Zhu Y, Card JP (2004) Numerous GABAergic afferents to locus ceruleus in the pericerulear dendritic zone: possible interneuronal pool. J Neurosci 24:2313–2321. 10.1523/JNEUROSCI.5339-03.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berridge CW, Waterhouse BD (2003) The locus coeruleus-noradrenergic system: modulation of behavioral state and state-dependent cognitive processes. Brain Res Brain Res Rev 42:33–84. 10.1016/s0165-0173(03)00143-7 [DOI] [PubMed] [Google Scholar]
- Bond TL, Neumann PE, Mathieson WB, Brown RE (2002) Nest building in nulligravid, primigravid and primiparous C57BL/6J and DBA/2J mice (Mus musculus). Physiol Behav 75:551–555. 10.1016/s0031-9384(02)00659-5 [DOI] [PubMed] [Google Scholar]
- Borderies N, Bornert P, Gilardeau S, Bouret S (2020) Pharmacological evidence for the implication of noradrenaline in effort. PLoS Biol 18:e3000793. 10.1371/journal.pbio.3000793 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bouret S, Richmond BJ (2009) Relation of locus coeruleus neurons in monkeys to Pavlovian and operant behaviors. J Neurophysiol 101:898–911. 10.1152/jn.91048.2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bouret S, Sara SJ (2004) Reward expectation, orientation of attention and locus coeruleus-medial frontal cortex interplay during learning. Eur J Neurosci 20:791–802. 10.1111/j.1460-9568.2004.03526.x [DOI] [PubMed] [Google Scholar]
- Bouret S, Sara SJ (2005) Network reset: a simplified overarching theory of locus coeruleus noradrenaline function. Trends Neurosci 28:574–582. 10.1016/j.tins.2005.09.002 [DOI] [PubMed] [Google Scholar]
- Bouret S, Ravel S, Richmond BJ (2012) Complementary neural correlates of motivation in dopaminergic and noradrenergic neurons of monkeys. Front Behav Neurosci 6:40. 10.3389/fnbeh.2012.00040 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brennan PA, Kendrick KM, Keverne EB (1995) Neurotransmitter release in the accessory olfactory bulb during and after the formation of an olfactory memory in mice. Neuroscience 69:1075–1086. 10.1016/0306-4522(95)00309-7 [DOI] [PubMed] [Google Scholar]
- Breton-Provencher V, Sur M (2019) Active control of arousal by a locus coeruleus GABAergic circuit. Nat Neurosci 22:218–228. 10.1038/s41593-018-0305-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chandler DJ, Gao WJ, Waterhouse BD (2014) Heterogeneous organization of the locus coeruleus projections to prefrontal and motor cortices. Proc Natl Acad Sci U S A 111:6816–6821. 10.1073/pnas.1320827111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen TW, Wardill TJ, Sun Y, Pulver SR, Renninger SL, Baohan A, Schreiter ER, Kerr RA, Orger MB, Jayaraman V, Looger LL, Svoboda K, Kim DS (2013) Ultrasensitive fluorescent proteins for imaging neuronal activity. Nature 499:295–300. 10.1038/nature12354 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clayton EC, Rajkowski J, Cohen JD, Aston-Jones G (2004) Phasic activation of monkey locus ceruleus neurons by simple decisions in a forced-choice task. J Neurosci 24:9914–9920. 10.1523/JNEUROSCI.2446-04.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen L, Rothschild G, Mizrahi A (2011) Multisensory integration of natural odors and sounds in the auditory cortex. Neuron 72:357–369. 10.1016/j.neuron.2011.08.019 [DOI] [PubMed] [Google Scholar]
- Dana H, Sun Y, Mohar B, Hulse BK, Kerlin AM, Hasseman JP, Tsegaye G, Tsang A, Wong A, Patel R, Macklin JJ, Chen Y, Konnerth A, Jayaraman V, Looger LL, Schreiter ER, Svoboda K, Kim DS (2019) High-performance calcium sensors for imaging activity in neuronal populations and microcompartments. Nat Methods 16:649–657. 10.1038/s41592-019-0435-6 [DOI] [PubMed] [Google Scholar]
- Friard O, Gamba M (2016) BORIS: a free, versatile open-source event-logging software for video/audio coding and live observation. Methods Ecol Evol 7:1325–1330. 10.1111/2041-210X.12584 [DOI] [Google Scholar]
- Galindo-Leon EE, Lin FG, Liu RC (2009) Inhibitory plasticity in a lateral band improves cortical detection of natural vocalizations. Neuron 62:705–716. 10.1016/j.neuron.2009.05.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin X, Li S, Bondy B, Zhong W, Oginsky MF, Wu Y, Johnson CM, Zhang S, Cui N, Jiang C (2016) Identification of a group of GABAergic neurons in the dorsomedial area of the locus coeruleus. PLoS One 11:e0146470. 10.1371/journal.pone.0146470 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalwani RM, Joshi S, Gold JI (2014) Phasic activation of individual neurons in the locus ceruleus/subceruleus complex of monkeys reflects rewarded decisions to go but not stop. J Neurosci 34:13656–13669. 10.1523/JNEUROSCI.2566-14.2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaufman AM, Geiller T, Losonczy A (2020) A role for the locus coeruleus in hippocampal CA1 place cell reorganization during spatial reward learning. Neuron 105:1018–1026. 10.1016/j.neuron.2019.12.029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kebschull JM, Garcia da Silva P, Reid AP, Peikon ID, Albeanu DF, Zador AM (2016) High-throughput mapping of single-neuron projections by sequencing of barcoded RNA. Neuron 91:975–987. 10.1016/j.neuron.2016.07.036 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kendrick KM, Lévy F, Keverne EB (1992) Changes in the sensory processing of olfactory signals induced by birth in sheep. Science 256:833–836. 10.1126/science.1589766 [DOI] [PubMed] [Google Scholar]
- Keverne EB, de la Riva C (1982) Pheromones in mice: reciprocal interaction between the nose and brain. Nature 296:148–150. 10.1038/296148a0 [DOI] [PubMed] [Google Scholar]
- Koga K, Yamada A, Song Q, Li XH, Chen QY, Liu RH, Ge J, Zhan C, Furue H, Zhuo M, Chen T (2020) Ascending noradrenergic excitation from the locus coeruleus to the anterior cingulate cortex. Mol Brain 13:49. 10.1186/s13041-020-00586-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krishnan K, Lau BY, Ewall G, Huang ZJ, Shea SD (2017) MECP2 regulates cortical plasticity underlying a learned behaviour in adult female mice. Nat Commun 8:14077. 10.1038/ncomms14077 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuo CC, Hsieh JC, Tsai HC, Kuo YS, Yau HJ, Chen CC, Chen RF, Yang HW, Min MY (2020) Inhibitory interneurons regulate phasic activity of noradrenergic neurons in the mouse locus coeruleus and functional implications. J Physiol 598:4003–4029. 10.1113/JP279557 [DOI] [PubMed] [Google Scholar]
- Landers MS, Sullivan RM (2012) The development and neurobiology of infant attachment and fear. Dev Neurosci 34:101–114. 10.1159/000336732 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin FG, Galindo-Leon EE, Ivanova TN, Mappus RC, Liu RC (2013) A role for maternal physiological state in preserving auditory cortical plasticity for salient infant calls. Neuroscience 247:102–116. 10.1016/j.neuroscience.2013.05.020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marlin BJ, Mitre M, D'Amour JA, Chao MV, Froemke RC (2015) Oxytocin enables maternal behaviour by balancing cortical inhibition. Nature 520:499–504. 10.1038/nature14402 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin-Sanchez A, Valera-Marin G, Hernandez-Martinez A, Lanuza E, Martinez-Garcia F, Agustin-Pavon C (2015) Wired for motherhood: induction of maternal care but not maternal aggression in virgin female CD1 mice. Front Behav Neurosci 9:197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mathis A, Mamidanna P, Cury KM, Abe T, Murthy VN, Mathis MW, Bethge M (2018) DeepLabCut: markerless pose estimation of user-defined body parts with deep learning. Nat Neurosci 21:1281–1289. 10.1038/s41593-018-0209-y [DOI] [PubMed] [Google Scholar]
- Moffat SD, Suh EJ, Fleming AS (1993) Noradrenergic involvement in the consolidation of maternal experience in postpartum rats. Physiol Behav 53:805–811. 10.1016/0031-9384(93)90192-i [DOI] [PubMed] [Google Scholar]
- Numan M, Numan M (1996) A lesion and neuroanatomical tract-tracing analysis of the role of the bed nucleus of the stria terminalis in retrieval behavior and other aspects of maternal responsiveness in rats. Dev Psychobiol 29:23–51. [DOI] [PubMed] [Google Scholar]
- Numan M, Stolzenberg DS (2009) Medial preoptic area interactions with dopamine neural systems in the control of the onset and maintenance of maternal behavior in rats. Front Neuroendocrinol 30:46–64. 10.1016/j.yfrne.2008.10.002 [DOI] [PubMed] [Google Scholar]
- Numan M, McSparren J, Numan MJ (1990) Dorsolateral connections of the medial preoptic area and maternal behavior in rats. Behav Neurosci 104:964–979. 10.1037/0735-7044.104.6.964 [DOI] [PubMed] [Google Scholar]
- Numan M, Bress JA, Ranker LR, Gary AJ, Denicola AL, Bettis JK, Knapp SE (2010) The importance of the basolateral/basomedial amygdala for goal-directed maternal responses in postpartum rats. Behav Brain Res 214:368–376. 10.1016/j.bbr.2010.06.006 [DOI] [PubMed] [Google Scholar]
- Poe GR, Foote S, Eschenko O, Johansen JP, Bouret S, Aston-Jones G, Harley CW, Manahan-Vaughan D, Weinshenker D, Valentino R, Berridge C, Chandler DJ, Waterhouse B, Sara SJ (2020) Locus coeruleus: a new look at the blue spot. Nat Rev Neurosci 21:644–659. 10.1038/s41583-020-0360-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rajkowski J, Majczynski H, Clayton E, Aston-Jones G (2004) Activation of monkey locus coeruleus neurons varies with difficulty and performance in a target detection task. J Neurophysiol 92:361–371. 10.1152/jn.00673.2003 [DOI] [PubMed] [Google Scholar]
- Rasmussen K, Morilak DA, Jacobs BL (1986) Single unit activity of locus coeruleus neurons in the freely moving cat. I. During naturalistic behaviors and in response to simple and complex stimuli. Brain Res 371:324–334. 10.1016/0006-8993(86)90370-7 [DOI] [PubMed] [Google Scholar]
- Rosenberg P, Halaris A, Moltz H (1977) Effects of central norepinephrine depletion on the initiation and maintenance of maternal behavior in the rat. Pharmacol Biochem Behav 6:21–24. 10.1016/0091-3057(77)90155-1 [DOI] [PubMed] [Google Scholar]
- Rosenblatt JS (1967) Nonhormonal basis of maternal behavior in the rat. Science 156:1512–1514. 10.1126/science.156.3781.1512 [DOI] [PubMed] [Google Scholar]
- Rosser AE, Keverne EB (1985) The importance of central noradrenergic neurones in the formation of an olfactory memory in the prevention of pregnancy block. Neuroscience 15:1141–1147. 10.1016/0306-4522(85)90258-1 [DOI] [PubMed] [Google Scholar]
- Sara SJ, Segal M (1991) Plasticity of sensory responses of locus coeruleus neurons in the behaving rat: implications for cognition. Prog Brain Res 88:571–585. 10.1016/s0079-6123(08)63835-2 [DOI] [PubMed] [Google Scholar]
- Schwarz LA, Miyamichi K, Gao XJ, Beier KT, Weissbourd B, DeLoach KE, Ren J, Ibanes S, Malenka RC, Kremer EJ, Luo L (2015) Viral-genetic tracing of the input-output organization of a central noradrenaline circuit. Nature 524:88–92. 10.1038/nature14600 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sciolino NR, Mazzone CM, Plummer NW, Evsyukova I, Amin J, Smith KG, McGee CA, Fry SA, Yang CX, Powell JM, Bruchas MR, Kravitz AV, Cushman JD, Krashes MJ, Cui G, Jensen P (2019) A role for locus coeruleus in the modulation of feeding. bioRxiv. doi: 10.1101/2019.12.18.881599. [DOI] [Google Scholar]
- Shea SD, Katz LC, Mooney R (2008) Noradrenergic induction of odor-specific neural habituation and olfactory memories. J Neurosci 28:10711–10719. 10.1523/JNEUROSCI.3853-08.2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steele MK, Rowland D, Moltz H (1979) Initiation of maternal behavior in the rat: possible involvement of limbic norepinephrine. Pharmacol Biochem Behav 11:123–130. 10.1016/0091-3057(79)90001-7 [DOI] [PubMed] [Google Scholar]
- Stolzenberg DS, Champagne FA (2016) Hormonal and non-hormonal bases of maternal behavior: the role of experience and epigenetic mechanisms. Horm Behav 77:204–210. 10.1016/j.yhbeh.2015.07.005 [DOI] [PubMed] [Google Scholar]
- Stolzenberg DS, Mayer HS (2019) Experience-dependent mechanisms in the regulation of parental care. Front Neuroendocrinol 54:100745. 10.1016/j.yfrne.2019.04.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sullivan RM, Wilson DA, Leon M (1989) Norepinephrine and learning-induced plasticity in infant rat olfactory system. J Neurosci 9:3998–4006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas SA, Palmiter RD (1997) Impaired maternal behavior in mice lacking norepinephrine and epinephrine. Cell 91:583–592. 10.1016/s0092-8674(00)80446-8 [DOI] [PubMed] [Google Scholar]
- Totah NK, Neves RM, Panzeri S, Logothetis NK, Eschenko O (2018) The locus coeruleus is a complex and differentiated neuromodulatory system. Neuron 99:1055–1068.e6. 10.1016/j.neuron.2018.07.037 [DOI] [PubMed] [Google Scholar]
- Uematsu A, Tan BZ, Ycu EA, Cuevas JS, Koivumaa J, Junyent F, Kremer EJ, Witten IB, Deisseroth K, Johansen JP (2017) Modular organization of the brainstem noradrenaline system coordinates opposing learning states. Nat Neurosci 20:1602–1611. 10.1038/nn.4642 [DOI] [PubMed] [Google Scholar]
- Vankov A, Hervé-Minvielle A, Sara SJ (1995) Response to novelty and its rapid habituation in locus coeruleus neurons of the freely exploring rat. Eur J Neurosci 7:1180–1187. 10.1111/j.1460-9568.1995.tb01108.x [DOI] [PubMed] [Google Scholar]
- Xiang L, Harel A, Gao H, Pickering AE, Sara SJ, Wiener SI (2019) Behavioral correlates of activity of optogenetically identified locus coeruleus noradrenergic neurons in rats performing T-maze tasks. Sci Rep 9:1361. 10.1038/s41598-018-37227-w [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
In this clip, the audible clicks correspond to the spikes of a well isolated LC-NA single unit. Bursts of accelerated spiking can be heard time locked to each retrieval event. The inset in the bottom right corner is a streaming visual representation of the neuronal spike train.
In this clip, the audio track consists of a carrier tone that is frequency modulated by the fluorescent fiber photometry signal. Sharp peaks in the signal that are time locked to retrieval can easily be heard as brief, steep increases in pitch. The inset at the bottom also depicts a streaming trace of ΔF/F synced to the video.