Abstract
Objective:
This study aimed to determine acaricide resistance in Rhipicephalus decoloratus ticks collected from grazing cattle between November 2018 and May 2019 in Elundini, Senqu, and Walter Sisulu Local Municipalities in the northeastern region of the Eastern Cape Province.
Materials and Methods:
A sample of 20–30 adult engorged female R. decoloratus ticks were collected from at least 10 randomly selected cattle (highly tick-infested) at each dip tank and placed into the labelled plastic collection bottles containing absorbent paper and with a perforated lid at a constant room temperature of ±28°C and >70% relative humidity until resistance testing commenced. The Shaw larval immersion test method was used to determine R. decoloratus larvae resistance to various acaricide concentration levels [amidines, organophosphate (OP), and synthetic pyrethroids (SPs)].
Results:
This study found that most ticks were susceptible to exposure to different acaricide field concentrations of amidines (49% at 250 ppm), OPs (33% and 47% at 300 ppm and 500 ppm, respectively), and SPs (44% and 23% at 150 ppm and 300 ppm, respectively). The resistance testing results showed no resistance to amidines at any localities and no resistance to OP in the Senqu region. However, resistance development of the larvae to amines, OPs, and SPs was extensively observed in Senqu (18%, 6%, and 7%), Elundini (15%, 15%, and 17%), and Walter Sisulu (13%, 19%, and 9%) regions, respectively.
Conclusions:
The larvae’s resistance is a cause for worry. Hence, the continuous monitoring of tick resistance to commonly used acaricides will help mitigate widespread acaricidal resistance and sustain livestock productivity.
Keywords: Acaricide resistance, cattle, Rhipicephalus decoloratus larvae, Shaw larval immersion test
Introduction
Among other tick species globally, Rhipicephalus decoloratus is the most widely distributed tick species and considered the most crucial external parasite to livestock, particularly in cattle [1]. The tickis known as the indigenous tick to the African continent and is widely distributed in tropical and subtropical regions. Rhipicephalus decoloratus is regarded as the major vector for transmission of tick-borne diseases, such as Babesia bigemina, Anaplasma marginale, and Anaplasma central, to cattle, whereas its counteracting species, Rhipicephalus microplus, can also transmit Babesia bovis, amongst other pathogens [2–4]. Heavy infestation of ticks results in the production and economic losses by reducing milk yield in cows, meat, and damage to the skin. However, over time, R. decoloratus tickshave become resistant to almost every application of registered acaricide, thus increasing its rapidity and spreading into nonendemic areas [5,6]. The tick’s lifecycle, worldwide distribution, and indiscriminate acaricide use by cattle farmers have proven to be the most significant contributing factors to the rapid establishment of tick-resistant acaricide compounds [7].
In South Africa, three synthetic acaricide chemical groups are known to be used to control ticks, including organophosphates (OPs), amidines, and pyrethroids [8,9]. Of these acaricide groups, pyrethroids and OPs are the most commonly used insecticides by most resource-limited farmers [10,11]. Sodium arsenate was the first successful acaricide used in South Africa until the first report of resistance detection on R. decoloratus. After that, benzene hexachloride (BHC) took over from arsenic as an alternative in the form of dichlorodiphenyltrichloroethane [12]. Since then, the incidence of arsenic–BHC-resistant blue ticks has increased along the country’s coastal regions [13]. In addition to this, Perez-Cogollo et al. [19] reported the first record of R. microplus being resistant to OP in the eastern regions of the Eastern Cape Province (ECP), and no reports have yet been made on the northern part of the province.
Several bioassays used in evaluating tick susceptibility to acaricide chemicals include the adult immersion test, larval packed test (LPT) [14], and larval immersion test [15]. Recently, a new bioassay method, the Larval Tarsal Test (LTT), was developed and compared to the LPT. Both tests successfully detected resistance to OP, amitraz, and coumaphos [16]. The LTT has proven to be more advantageous than the LPT since it allows a large volume of doses and compounds in a short time and fewer engorged females [17]. Tick resistance is typically established by exposing ticks to a unique type of dosage guided by the information of a susceptible reference strain; after that, a discriminating dose survival indicates acaricide resistance [17]. The objective of the current study was to determine the acaricide resistance of R. decoloratus larvae collected from cattle herds in the northeastern region of the ECP.
Materials and Method
Ethical approval
Before data collection, ethical approval was obtained from the University of Fort Hare’s Research Ethics Committee (Reference number: MUC021SYAW01). All experimental procedures adhered to the moral standards for experimentation established by the Society for the Prevention of Cruelty to Animals’ ethics committee on animal use.
Study site description
This study was conducted in 33 communities within the municipalities of Elundini, Senqu, and Walter Sisulu in the ECP’s Joe Gqabi District. Elundini municipality is located at an elevation of 1,600 m above sea level. In the Southern Drakensberg Grassland, the average annual rainfall is between 800 and 1,200 mm; the average annual minimum temperature is 13°C; and the maximum temperature is 22°C. [18]. Senqu municipality is located between 1,000 and 1,500 m above sea level, with an annual average temperature of −16°C in the winter and 42°C in the summer. Montana Shrubland receives an annual rainfall between 1,000 and 1,400 mm [18]. The municipality of Walter Sisulu is located between 1,000 and 1,500 m above sea level. Under the Mixed Nama Karoo vegetation type, the annual average temperature ranges between 15°C in winter and 30°C in summer, and the annual rainfall ranges between 1,000 and 1,400 mm [18].
Experimental animals
Ten cattle herds were randomly selected for tick sampling at each dip tank from November 2018 to September 2019. At all times, all animals selected were over the age of 12 months and included both sexes. Cattle in the study areas were of various breeds. However, the study did not focus on the breed effect. Each locality conducted cattle dipping twice a month during the summer and once a month during the winter season. Ticks were sampled from cattle prior to dipping to ensure that the tick population was not skewed. Water supply to the dip tank has been a major issue in these areas, limiting farmers’ use of the plunge dipping system during the dry season. With a continuous grazing system, cattle rely heavily on natural pastures for feeding.
Ticks collection and transportation for acaricide testing
Between 0800 and 0900 h on dipping days, engorged female ticks were collected from grazing cattle; this was done because the majority of engorged ticks drop off the host in the early morning. At each dip tank, a sample of 20–30 adult female R. decoloratus ticks was collected from at least 10 cattle and placed in plastic collection bottles containing absorbent paper and perforated lids at a constant room temperature of 28°C and a relative humidity of >70%. Each collection bottle was labeled with the collection date, the name of the farm, and the number of cattle sampled. Ticks were immediately transported to the Acaricide Resistance Testing Laboratory at the University of the Free State’s Department of Zoology and Entomology for acaricide resistance testing. Engorged female ticks were washed on a sieve with clean tap water upon arrival at the laboratory, and all damaged and undersized ticks (weighing less than 150–350 mg) were discarded. After air-drying ticks on an absorbent paper, they were placed in a glass flask and incubated [19]. Ticks were checked daily until oviposition began. After the ticks produced their first egg at approximately +35 days, they were monitored daily for hatch date establishment, which was determined to be the day when about 70% of the larvae hatched. Then acaricide resistance testing was carried out on larvae between 15 and 21 days [19].
Acaricides used in the study
The study tested for acaricide resistance using three dip formulations: (i) OP, (ii) synthetic pyrethroids (SPs), and (iii) amidines. These compounds were chosen because they are commonly used in South Africa, are commercially available, and have all been registered for tick control under Act 36 of 1947. For tick larvae resistance testing, the acaricide compound concentrations used are the standard recommended field concentrations for each acaricide, prepared from a 1% stock solution diluted from each acaricide group (Table 1). The concentrations used were cypermethrin 0.015 and 0.03 ppm, chlorfenvinnphos 0.03 and 0.05 ppm, and amitraz 0.025 ppm. These concentrations were prepared using double-distilled water, with one serving as a control. Ten milliliters of each concentration test and distilled water were placed in the labeled test tubes for the subsequent acaricide testing step. During the preparation process, the concentration and water were thoroughly mixed to ensure the uniformity of the acaricide solution.
Table 1. Acaricide resistance test dilutions.
| Dilutions made from a 30% (m/v) chlorfenvinphos solution | ||||
|---|---|---|---|---|
| Dilution number | Concentration (% m/v) | Dip | 2× Distilled water (ml) | Total (ml) |
| Stock 1 | 1% | 1.67 ml chlorofenvinphos solution | 48.33 | 50 |
| 2 | 0.03 | 3 ml of stock 1 | 97 | 100 |
| 3 | 0.05 | 5 ml of stock 1 | 95 | 100 |
| 4 | Control | - | 10 | 10 |
| Dilutions made from a 12.5% (m/v) amitraz solution | ||||
| Stock 1 | 1% | 4 ml amitraz solution | 46 | 50 |
| 2 | 0.03 | 2.5 ml of stock 1 | 97.5 | 100 |
| 3 | Control | - | 10 | 10 |
| Dilutions made from a 20% (m/v) cypermethrin solution | ||||
| Stock 1 | 1% | 1.67 ml chlorofenvinphos solution | 48.33 | 50 |
| 2 | 0.03 | 3 ml of stock 1 | 97 | 100 |
| 3 | 0.05 | 5 ml of stock 1 | 95 | 100 |
| 4 | Control | - | 10 | 10 |
Shaw larval immersion test (SLIT)
Engorged female ticks were handled in accordance with the laboratory’s standard operating procedure (SOP) upon arrival [20]. In summary, the SOP requires that engorged females be washed on a sieve with clean tap water and that all damaged, non-engorged, and pre-laying females be discarded. Tick larvae were exposed to the field concentration of SLIT for acaricide resistance testing, and the percentage of larvae killed was used to determine efficacy. A mortality rate of at least 80% was considered adequate. In comparison, a mortality rate of less than 80% but greater than 50% was considered an indicator of resistance development, while a mortality rate of less than 50% was deemed resistant [20].
Larvae exposure to the acaricide
The conical flask containing the larvae samples was submerged in water on a petri dish. After that, a round of filter paper with a diameter of 24 cm was placed in the stainless steel tray to absorb any water droplets that may have spilled during the start of the actual test. A foil plate comprising two circular filter papers with a diameter of approximately 11 cm was inserted into the 24 cm filter paper. The resistant test was initiated by removing the cotton stopper from the flask and inserting it into the side of the 11 cm filter paper in the pie plate using forceps. The remaining larvae in the flask’s neck were then removed with a demarcated control brush and brushed onto one sheet of filter paper, which was then covered. The flask was sealed with a cotton stopper, and the control test tubes were vortexed for 10 sec before being poured over the filter paper sandwich. After pouring the concentrations into the filter paper, the timer was started, and the procedure was repeated for 60 sec. Tick larvae were transferred to the filter paper using the uncontaminated brush. After determining the concentrations, masking tape was used to remove any larvae that had escaped into the cotton stopper. The flask was returned to the incubator box and relocated to the incubator room.
Larvae postexposure to acaricide
Using forceps, the sandwich filter paper was removed from the plate precisely 10 min after the larvae were exposed to the acaricide. A small amount of water was drained into one of the 24 cm filter paper’s corners, and the foil plate was then discarded. The sandwich filter paper was then separated and placed in the dry sections of the 24 cm filter paper to extract excess liquid. Again, the designated brush was used to transfer larvae into the pre-labeled filter paper envelope, and masking tape was used to prevent larvae from escaping through the filter paper. Two envelopes containing chemicals were then clipped together and placed in the incubator for 72 h at a temperature of ±28°C and a relative humidity greater than 70%. This procedure was repeated for each field concentration, and a new foil plate and 24 cm of paper were used for testing. Between each chemical concentration used, acetone was used to clean the trays, and separate incubator boxes were used to separate the chemical and control envelopes in the incubator room.
Larvae mortality counting
After 72 h, the filter paper envelopes were removed from the incubator, and all the larvae, alive and dead, were counted. Live larvae were counted on a 24 cm filter paper, taking care to avoid larvae that might flee during the counting process. The total number of live larvae was recorded in the envelope’s corner. All dead larvae were poured into the 24 cm filter paper below and counted. As a result, the mortality percentage was calculated by counting live and dead larvae. Corrected mortalities were determined using the formula described elsewhere [21]:
Where;
CM% = corrected mortality;
% i = % mortality in concentration; and
% c = % mortality in water control.
Results
The SLIT was only carried out on R. decoloratus larvae as engorged R. microplus ticks could not meet the required sample size for resistance testing. Each resistance range was presented by its specific color, from red = indications of resistant, yellow = developing resistance, blue = effective reservation, and pink = susceptible/effective, as shown in Table 2.
Table 2. The range of resistance percentages used to display the larvae resistance.
| Mortality count range (%) | Color | Result interpretation |
|---|---|---|
| 0%<50% | Red | Indications of resistant |
| 50%<80% | Yellow | Developing resistance |
| 80%<90% | Blue | Effective reservation |
| 90%–100% | Pink | Susceptible |
Resistance profile of amidine, OP, and SPs
Table 3 shows the defined resistance development of R. decoloratus larvae exposed at different field concentration levels of amidine, OP, and SPs. A total of 49% of the larvae were susceptible to amidine, with 30% developing resistance and 21% ineffective reservation at a concentration of 250 ppm. At a concentration of 300 ppm, OP displayed the greatest proportion of effective reservations (45%), with 33% being susceptible to the chemical, 18% developing resistance, and only 4% being considered resistant. On the other hand, 47% susceptibility was observed when larvae were exposed to OP at a concentration of 500 ppm, 32% showed effective reservation, and 21% indicated developing resistance. The SP results at a concentration of 150 ppm showed 44% susceptibility, 37% effective reservation, 12% developing resistance, and 7% resistance. However, on the other hand, SPs at a concentration of 300 ppm recorded the most significant proportion of effective reservations (34%), followed by developing resistance (30%), with 23% of the samples susceptible and 13% resistant.
Table 3. Resistance status of amidine, OP, and SPs used at 150 ppm, 250 ppm, 300 ppm and 500 ppm field concentration levels.
| Field concentration levels | |||||
|---|---|---|---|---|---|
| Resistant test | 150 ppm | 250 ppm | 300 ppm | 500 ppm | |
| Developing resistance | - | 21% | - | - | |
| Amidine | Effective reservation | - | 30% | - | - |
| Susceptible | - | 49% | - | - | |
| Resistant | - | - | 4% | - | |
| OP | Developing resistance | - | - | 18% | 21% |
| Effective reservation | - | - | 45% | 32% | |
| Susceptible | - | - | 33% | 47% | |
| Resistant | 7% | - | 13% | - | |
| Pyrethroids | Developing resistance | 12% | - | 30% | - |
| Effective reservation | 37% | - | 34% | - | |
| Susceptible | 44% | - | 23% | - | |
Rhiphicephalus decoloratus larvae resistance profiles exposed to amidine
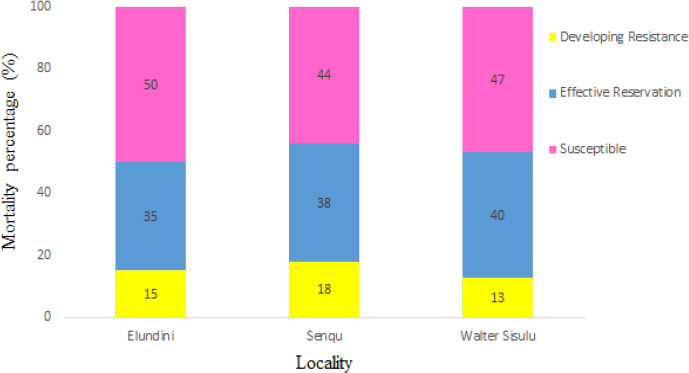
Figure 1 shows the mortality counts of R. decoloratus tick’s larvae collected from Elundini, Senqu, and Walter Sisulu exposed to amidine field concentration. Ticks collected from Elundini, Walter Sisulu, and Senqu were susceptible to amidine and showed 50%, 47%, and 44% mortality counts, respectively. More so, mortality counts for effective reservation were observed mainly in Walter Sisulu (40%), 38% at Senqu, and the lowest mortality counts at the Elundini region (35%). Resistance development of the larvae to the chemical was extensively observed in Senqu (18%), Elundini (15%), and Walter Sisulu (13%) regions. Ticks did not show resistance when exposed to this chemical across the localities.
Figure 1. Resista~nce profiles of R. decoloratus larvae exposed to amidine.
Rhiphicephalus decoloratus larvae resistance profiles exposed to OP
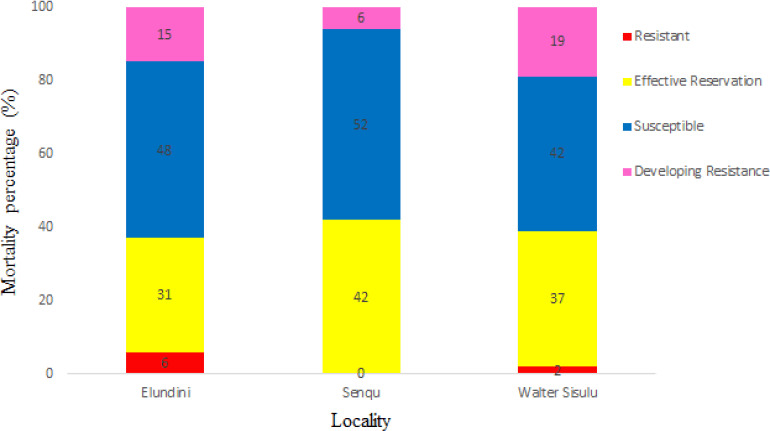
Figure 2 shows the mortality counts of R. decoloratus tick’s larvae collected from Elundini, Senqu, and Walter Sisulu exposed to OP field concentration. Susceptible mortality counts were primarily observed in Senqu (52%), Elundini (48%), and Walter Sisulu (42%). The highest mortality counts for effective reservations were recorded in Senqu (42%), Walter Sisulu (37%), and Elundini (31%). The highest larvae within the resistance development range to the chemical were found in Walter Sisulu (19%), followed by Elundini (15%), and the lowest counts (6%) in the Senqu region. 6% and 2% mortality counts were observed in the Elundini and Walter Sisulu regions, respectively. There was no tick resistance to OP in the Senqu region.
Figure 2. Resistance profiles of R. decoloratus larvae exposed to OP.
Rhiphicephalus decoloratus larvae resistance profiles exposed to SPs
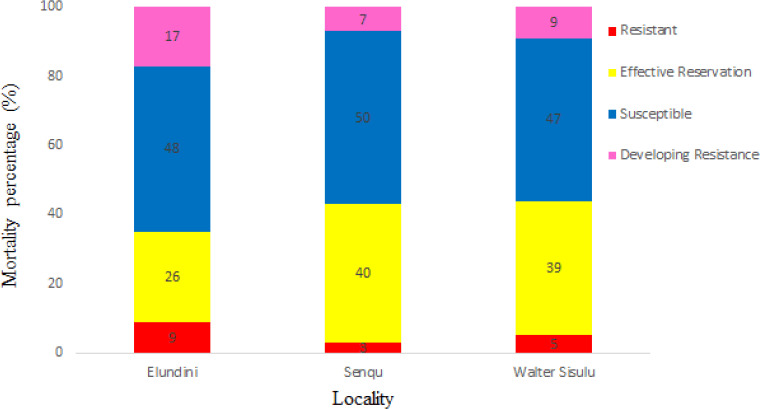
Figure 3 shows the mortality counts of R. decoloratus tick’s larvae collected from Elundini, Senqu, and Walter Sisulu exposed to SP concentrations. Larvae mortality counts were susceptible in Senqu (50%), Elundini (48%), and Walter Sisulu (47%), respectively. Similarly, larvae mortality counts in the effective reservation category were 40% in Senqu, 39% in Walter Sisulu, and the lowest counts in Elundini (26%). The highest larvae within the resistance development range to the chemical were observed in Elundini (17%), Walter Sisulu (9%), and Senqu (7%). On the other hand, the greatest mortality counts of larvae described as resistant to the applied chemical were observed mainly in Elundini (9%), Walter Sisulu (5%), and Senqu had the lowest counts (3%) compared to the other localities.
Figure 3. Resistance profiles of R. decoloratus larvae exposed to SPs.
Discussion
Tick infestation is the most economically important ectoparasite of livestock and has been reported globally [22]. Many South African studies [23–26,17,27–30] have documented studies of R. microplus resistance to the most commonly used commercial acaricides for tick control. The common acaricides used for the management of ticks include formamidines, phenylpyrazoles, macrocyclic lactones, SPs, and OPs. The indiscriminate use and misapplication of acaricides has escalated selection pressure for tick resistance to acaricides [31]. Studies have further reported the displacement of the African blue tick (R. decoloratus) by the Asian cattle blue tick (R. microplus). However, no such displacement was observed in the current study. The present study found patchy engorged R. microplus tick specimens in each study site. Similar findings were also reported by Pottinger [32], who reported fewer R. microplus counts in the study conducted in the coastal regions of the ECP. The collected R. microplus samples did not meet the sample size for resistance testing. These findings were attributed to the susceptibility of R. microplus to currently used acaricides in the study localities. Also, the resistance information of R. decoloratus in South Africa, particularly in the ECP, is mostly outdated as more focus has been shifted toward the invasive tick species, R. microplus [33,8,34,9].
Over the past 10 years, the three acaricide compounds (amidine, OP, and SPs)have been used to control ticks as they are known for their low toxicity to cattle and other animal species on which ticks feed. This chemical acts as an octopamine receptor when applied to the tick, leading to reduced numbers of active neurons, resulting in tick paralysis and death [3]. The mechanism of tick resistance to acaricide is described by an increase in the tick’s metabolic activity that produces metabolic enzymes that detoxify any toxic substance as soon as possible before it gets to the target sites [35]. The current study found that the majority of the ticks were susceptible to exposure to different field-level concentrations of the acaricide amidines (49% at 250 ppm), OPs (33% and 47% at 300 ppm and 500 ppm, respectively), and SPs (44% and 23% at 150 ppm and 300 ppm, respectively). Tick susceptibility may be responsible for the low tick counts in the study areas, particularly the blue tick. The low tick count may also be linked to the prolonged drought in the ECP over the past 5 years, which has limited adequate tick habitat for an increasing population [36].
The current study reported R. decoloratus larvae resistance when exposed to OP and SPs, with no resistance reported to amidines. These findings concur with the report conducted elsewhere [34,30]. They found similar results where all the tick larvae did not show resistance during exposure to the amidines. It was further argued that even though the three acaricide groups have been used over the years, amidines have not been commonly used in high tick areas, which lowers the chances for tick selection pressure on amidines compared with the OPs and SPs [37,30]. Hence, it is further anticipated that amidines effectively control single and multi-host ticks [38].
Of the three localities, except for Senqu, the tested larvae did not show resistance when exposed to all the acaricides. This was attributed to the fact that there was a well-developed tick control program at Senqu. In these regions, it was compulsory for every cattle farmer to bring the cattle for dipping. This action suppressed the tick population in the region and subsequently made it difficult for ticks to develop resistance. The majority of the cattle farmers indicated that they increase acaricide concentration during the high tick season, resulting in complete mortalities of ticks after acaricide application. The only worry seemed to be the presence of acaricidal residues [11]. Among other tick resistance factors, acaricide use over a long period has been the major contributing factor toward the larvae’s resistance to OPs and SPs [39,8]. However, even though OPs and SPs showed resistance, findings from the studies conducted earlier [9] and [7] suggested that the two acaricides effectively decrease tick populations when applied at an effective therapeutic concentration. Thus, the emergence of acaricide resistance in R. decoloratus ticks should motivate tick control programs in the regions to mitigate the chances of complete resistance to the commonly used chemicals [40].
Conclusion
This study documented R. decoloratus larvae’s resistance to OP and SPs except for amidines. This is an important finding given the regular use of OP and SPs in the management of ticks in South Africa. We further observed that amidine acaricides were the most effective for controlling R. decoloratus larvae. Thus, this study recommends that future acaricide application strategies incorporate knowledge of tick dynamics, such as available tick species, as the tick population differs from region to region based on host availability and vegetation. Moreover, our study recommends that acaricides be diluted by trained personnel and guided by the manufacturers’ recommendations for effectiveness. Also, the rotation of the acaricides should be practiced using different modes of action.
Acknowledgments
The authors express their thanks to the Joe Gqabi District, Department of Rural Development and Agrarian Reform (DRDAR), for their assistance during this study. They are also grateful to the farmers who brought their animals for participating in this study during data collection. They acknowledge the National Research Foundation, South Africa (NRF) (Grant number: 102941), for funding this study.
List of Abbreviations
BHC, Benzene hexachloride; cm, Centimeter; ECP, Eastern Cape Province; h, hour; LPT, Larval packed test; LTT, Larval tarsal test; mm, Millimeter; ppm, Parts per million; SLIT, Shaw larval immersion test; SOP, Standard operating procedure.
Conflict of interest
The authors declare that they have no conflict of interest.
Authors’ contributions
Mandla Yawa designed, organized the research experiments, collected data and materials, and wrote the first draft of the manuscript. Nkululeko Nyangiwe, Ishmael Festus Jaja, Munyaradzi Christopher Marufu, and Charles T. Kadzere supervised the student, analyzed the data, monitored the project, and provided research funds for research.
REFERENCES
- [1].de Castro JJ. Sustainable tick and tick-borne disease control in livestock improvement in developing countries. Vet Parasitol. 1997;71:77–97. doi: 10.1016/s0304-4017(97)00033-2. https://doi.org/10.1016/S0304-4017(97)00033-2. [DOI] [PubMed] [Google Scholar]
- [2].Abdul Alim M, Roy K, Sikder S, Das S, Masuduzzaman M, Mahmudul Hassan M, et al. Prevalence of hemoprotozoan diseases in cattle population of Chittagong division, Bangladesh. Pak Vet J. 2016;32:221–4. [Google Scholar]
- [3].Janadaree Bandara KMU, Parakrama Karunaratne SHP. Mechanisms of acaricide resistance in the cattle tick Rhipicephalus (Boophilus) microplus in Sri Lanka. Pestic Biochem Physiol. 2017;139:68–72. doi: 10.1016/j.pestbp.2017.05.002. https://doi.org/10.1016/j.pestbp.2017.05.002. [DOI] [PubMed] [Google Scholar]
- [4].Valente PP, Moreira GHFA, Serafini MF, Facury-Filho EJ, Carvalho AÚ, Faraco AAG, et al. In vivo efficacy of a biotherapic and eugenol formulation against Rhipicephalus microplus. Parasitol Res. 2017;116:929–38. doi: 10.1007/s00436-016-5366-x. https://doi.org/10.1007/s00436-016-5366-x. [DOI] [PubMed] [Google Scholar]
- [5].Castro-Janer E, Rifran L, Piaggio J, Gil A, Miller RJ, Schumaker TTS. In vitro tests to establish LC50 and discriminating concentrations for fipronil against Rhipicephalus (Boophilus) microplus (Acari: Ixodidae) and their standardization. Vet Parasitol. 2009;162:120–8. doi: 10.1016/j.vetpar.2009.02.013. https://doi.org/10.1016/j.vetpar.2009.02.013. [DOI] [PubMed] [Google Scholar]
- [6].Klafke GM, Castro-Janer E, Mendes MC, Namindome A, Schumaker TTS. Applicability of in vitro bioassays for the diagnosis of ivermectin resistance in Rhipicephalus microplus (Acari: Ixodidae) Vet Parasitol. 2012;184:212–20. doi: 10.1016/j.vetpar.2011.09.018. https://doi.org/10.1016/j.vetpar.2011.09.018. [DOI] [PubMed] [Google Scholar]
- [7].Guerrero FD, Lovis L, Martins JR. Acaricide resistance mechanisms in Rhipicephalus (Boophilus) microplus. Rev Bras Parasitol Vet. 2012;21:1–6. doi: 10.1590/s1984-29612012000100002. https://doi.org/10.1590/S1984-29612012000100002. [DOI] [PubMed] [Google Scholar]
- [8].Mekonnen S, Bryson NR, Fourie LJ, Peter RJ, Spickett AM, Taylor RJ, et al. Acaricide resistance profiles of single- and multi-host ticks from communal and commercial farming areas in the Eastern Cape and North-West Provinces of South Africa. Onderstepoort J Vet Res. 2002;69:99–105. [PubMed] [Google Scholar]
- [9].Ntondinia Z, van Dalenb EMSP, Horak IG. The extent of acaricide resistance in 1-, 2- and 3-host tickson communally grazed cattle in the eastern region of theEastern Cape Province, South Africa. J S Afr Vet Assoc. 2008;79:130–5. doi: 10.4102/jsava.v79i3.259. https://doi.org/10.4102/jsava.v79i3.259. [DOI] [PubMed] [Google Scholar]
- [10].Baffi MA, de Souza GRL, Vieira CU, de Sousa CS, Gourlart LR, Bonetti AM. Identification of point mutations in a putative carboxylesterase and their association with acaricide resistance in Rhipicephalus (Boophilus) microplus (Acari: Ixodidae) Vet Parasitol. 2007;148:301–9. doi: 10.1016/j.vetpar.2007.06.016. https://doi.org/10.1016/j.vetpar.2007.06.016. [DOI] [PubMed] [Google Scholar]
- [11].Sungirai M, Moyo DZ, De Clercq P, Madder M. Communal farmers’ perceptions of tick-borne diseases affecting cattle and investigation of tick control methods practiced in Zimbabwe. Ticks Tick Borne Dis. 2016;7:1–9. doi: 10.1016/j.ttbdis.2015.07.015. https://doi.org/10.1016/j.ttbdis.2015.07.015. [DOI] [PubMed] [Google Scholar]
- [12].Whitehead GB. Resistance to acaricides in ticks in the Eastern Cape Province. South African Med J. 1973;47:342–4. [PubMed] [Google Scholar]
- [13].Rajput ZI, Hu S, Chen W, Arijo AG, Xiao C. Importance of ticks and their chemical and immunological control in livestock. J Zhejiang Univ Sci B. 2006;7:912–21. doi: 10.1631/jzus.2006.B0912. https://doi.org/10.1631/jzus.2006.B0912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Sabatini G, Kemp D, Hughes S, Nari A, Hansen J. Tests to determine LC50 and discriminating doses for macrocyclic lactones against the cattle tick, Boophilus microplus. Vet Parasitol. 2001;95:53–62. doi: 10.1016/s0304-4017(00)00406-4. https://doi.org/10.1016/S0304-4017(00)00406-4. [DOI] [PubMed] [Google Scholar]
- [15].Shaw RD. Culture of an organophosphorus-resistant strain of Boophilus microplus (Can.) and an assessment of its resistance spectrum. Bull Entomol Res. 1966;56:389–405. doi: 10.1017/s0007485300056480. https://doi.org/10.1017/S0007485300056480. [DOI] [PubMed] [Google Scholar]
- [16].Lovis L, Perret JL, Bouvier J, Fellay JM, Kaminsky R, Betschart B, et al. A new in vitro test to evaluate the resistance level against acaricides of the cattle tick, Rhipicephalus (Boophilus ) microplus. Vet Parasitol. 2011;182:269–80. doi: 10.1016/j.vetpar.2011.06.004. https://doi.org/10.1016/j.vetpar.2011.06.004. [DOI] [PubMed] [Google Scholar]
- [17].Lovis L, Mendes MC, Perret JL, Martins JR, Bouvier J, Betschart B, et al. Use of the Larval Tarsal Test to determine acaricide resistance in Rhipicephalus (Boophilus) microplus Brazilian field populations. Vet Parasitol. 2013;191:323–31. doi: 10.1016/j.vetpar.2012.09.011. https://doi.org/10.1016/j.vetpar.2012.09.011. [DOI] [PubMed] [Google Scholar]
- [18].Jaja IF, Mushonga B, Green E, Muchenje V. A quantitative assessment of causes of bovine liver condemnation and its implication for Food Security in the Eastern Cape Province South Africa. Sustainability. 2017;9:736. https://doi.org/10.3390/su9050736. [Google Scholar]
- [19].Perez-Cogollo LC, Rodriguez-Vivas RI, Ramirez-Cruz GT, Miller RJ. First report of the cattle tick Rhipicephalus microplus resistant to ivermectin in Mexico. Vet Parasitol. 2010;168:165–9. doi: 10.1016/j.vetpar.2009.10.021. https://doi.org/10.1016/j.vetpar.2009.10.021. [DOI] [PubMed] [Google Scholar]
- [20].Vudriko P, Okwee-Acai J, Tayebwa DS, Byaruhanga J, Kakooza S, Wampande E, et al. Emergence of multi-acaricide resistant Rhipicephalus ticks and its implication on chemical tick control in Uganda. Parasit Vectors. 2016;9:1–13. doi: 10.1186/s13071-015-1278-3. https://doi.org/10.1186/s13071-015-1278-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Abbott WS. A method of computing the effectiveness of an insecticide. J Economic Entomology. J Econ Entomol. 1925;18:265–7. https://doi.org/10.1093/jee/18.2.265a. [Google Scholar]
- [22].Kumar R. Molecular markers and their application in the monitoring of acaricide resistance in Rhipicephalus microplus. Exp Appl Acarol. 2019;78:149–72. doi: 10.1007/s10493-019-00394-0. https://doi.org/10.1007/s10493-019-00394-0. [DOI] [PubMed] [Google Scholar]
- [23].Adehan SB, Biguezoton A, Adakal H, Assogba MN, Zoungrana S, Gbaguidi AM, et al. Acaricide resistance of Rhipicephalus microplus ticks in Benin. Afr J Agric Res. 2016;11:1199–208. https://doi.org/10.5897/AJAR2015.10619. [Google Scholar]
- [24].Baron S, Barrero RA, Black M, Bellgard MI, van Dalen E, Maritz-Olivier C. Differentially expressed genes in response to amitraz treatment suggests a proposed model of resistance to amitraz in R. decoloratus ticks. Int J Parasitol Drugs Drug Resist. 2018;8:361–71. doi: 10.1016/j.ijpddr.2018.06.005. https://doi.org/10.1016/j.ijpddr.2018.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Baron S, van der Merwe NA, Madder M, Maritz-Olivier C. SNP analysis infers that recombination is involved in the evolution of amitraz resistance in Rhipicephalus microplus. PLoS One. 2015;10:e0131341. doi: 10.1371/journal.pone.0131341. https://doi.org/10.1371/journal.pone.0131341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Horak IG, Nyangiwe N, De Matos C, Neves L. The onderstepoort journal of veterinary research. Onderstepoort J Vet Res. 2009;76:263–76. doi: 10.4102/ojvr.v76i3.28. https://doi.org/10.4102/ojvr.v76i3.28. [DOI] [PubMed] [Google Scholar]
- [27].Nyangiwe N. 2017. [20/10/2020]. Distribution and ecology of economically important ticks on cattle, with special reference to the Eastern Cape Province, South Africa and Namibia. Theses and Dissertations (University of Stellenbosch). https://scholar.sun.ac.za/handle/10019.1/102576. [Google Scholar]
- [28].Nyangiwe N, Harrison A, Horak IG, Harrison A. Displacement of Rhipicephalus decoloratus by Rhipicephalus microplus (Acari: Ixodidae) in the Eastern Cape Province, South Africa. Exp Appl Acarol. 2013;61:371–82. doi: 10.1007/s10493-013-9705-7. https://doi.org/10.1007/s10493-013-9705-7. [DOI] [PubMed] [Google Scholar]
- [29].Tønnesen MH, Penzhorn BL, Bryson NR, Stoltsz WH, Masibigiri T. Displacement of Boophilus decoloratus by Boophilus microplus in the Soutpansberg region, Limpopo Province, South Africa. Exp Appl Acarol. 2004;32:199–208. doi: 10.1023/b:appa.0000021789.44411.b5. https://doi.org/10.1023/B:APPA.0000021789.44411.b5. [DOI] [PubMed] [Google Scholar]
- [30].Wyk RDJ, Baron S, Maritz-Olivier C. An integrative approach to understanding pyrethroid resistance in Rhipicephalus microplus and R. decoloratus ticks. Ticks Tick Borne Dis. 2016;7:586–94. doi: 10.1016/j.ttbdis.2016.01.007. https://doi.org/10.1016/j.ttbdis.2016.01.007. [DOI] [PubMed] [Google Scholar]
- [31].Sagar SV, Saini K, Sharma AK, Kumar S, Kumar R, Fular A, et al. Acaricide resistance in Rhipicephalus microplus collected from selected districts of Madhya Pradesh, Uttar Pradesh and Punjab states of India. Trop Anim Heal Prod. 2019;52:611–8. doi: 10.1007/s11250-019-02048-0. https://doi.org/10.1007/s11250-019-02048-0. [DOI] [PubMed] [Google Scholar]
- [32].Pottinger M. [20/10/2020]. The distribution of Rhipicephalus (Boophilus) microplus and Rhipicephalus (Boophilus) decoloratus on a farm in the Eastern Cape Province, South Africa Ethical Statement Acknowledgement’s. Bloemfontein: University of the Free State, 2019 p. Theses and Dissertations (University of Free State) https://scholar.ufs.ac.za/handle/11660/9960. [Google Scholar]
- [33].Chen AC, He H, Davey RB. Mutations in a putative octopamine receptor gene in amitraz-resistant cattle ticks. Vet Parasitol. 2007;148:379–83. doi: 10.1016/j.vetpar.2007.06.026. https://doi.org/10.1016/j.vetpar.2007.06.026. [DOI] [PubMed] [Google Scholar]
- [34].Mekonnen S, Bryson NR, Fourie LJ, Peter RJ, Spickett AM, Taylor RJ, et al. Comparison of 3 tests to detect acaricide resistance in Boophilus decoloratus on dairy farms in the Eastern Cape Province, South Africa. J S Afr Vet Assoc. 2003;74:41–4. doi: 10.4102/jsava.v74i2.502. https://doi.org/10.4102/jsava.v74i2.502. [DOI] [PubMed] [Google Scholar]
- [35].Aguilar G, Olvera AM, Carvajal BI, Mosqueda J. SNPs and other polymorhisms associated with acaricide resistance in Rhipicephalus microplus. Front Biosci. 2018;23:65–82. doi: 10.2741/4582. https://doi.org/10.2741/4582. [DOI] [PubMed] [Google Scholar]
- [36].Léger E, Vourc’h G, Vial L, Chevillon C, McCoy KD. Changing distributions of ticks: causes and consequences. Exp Appl Acarol. 2013;59:219–44. doi: 10.1007/s10493-012-9615-0. https://doi.org/10.1007/s10493-012-9615-0. [DOI] [PubMed] [Google Scholar]
- [37].Fourie JJ, Ollagnier C, Beugnet F, Luus HG, Jongejan F. Prevention of transmission of Ehrlichia canis by Rhipicephalus sanguineus ticks to dogs treated with a combination of fipronil, amitraz and (S)-methoprene (CERTIFECT®) Vet Parasitol. 2013;193:223–8. doi: 10.1016/j.vetpar.2012.12.009. https://doi.org/10.1016/j.vetpar.2012.12.009. [DOI] [PubMed] [Google Scholar]
- [38].Peter R, De Bruin C, Odendaal D, Thompson PN. The use of a pour-on and spray dip containing Amitraz to control ticks (Acari: Ixodidae) on cattle. J South Africa Vet Assoc. 2006;77:66–9. doi: 10.4102/jsava.v77i2.346. https://doi.org/10.4102/jsava.v77i2.346. [DOI] [PubMed] [Google Scholar]
- [39].Malan RC. 2016. [20/10/2020]. Acaricide resistance in Rhipicephalus (Boophilus) species at a communal dipping system in the Mnisi community Mpumalanga Province. Theses and Dissertations (University of Pretoria). https://repository.up.ac.za/handle/2263/53302. [Google Scholar]
- [40].Sangster NC, Cowling A, Woodgate RG. Ten events that defined anthelmintic resistance research. Trends Parasitol. 2018;34:553–63. doi: 10.1016/j.pt.2018.05.001. https://doi.org/10.1016/j.pt.2018.05.001. [DOI] [PubMed] [Google Scholar]


