Abstract
Combinations of artemisinin and quinine for uncomplicated falciparum malaria were studied. A total of 268 patients were randomized to 7 days of quinine at 10 mg/kg of body weight three times a day (Q) or to artemisinin at 20 mg/kg of body weight followed by 3 (AQ3) or 5 (AQ5) days of quinine. Recrudescence rates were 16, 38, and 15% for the Q, AQ3, and AQ5 groups, respectively (P < 0.001). Recrudescence was associated with shorter parasite clearance time (PCT) and longer treatment after the blood smear had become negative (eradication time). However, classification of patients to outcome—recrudescence or radical cure—was correct in only 77% of patients. The population kinetics of the parasitemia was estimated with nonlinear mixed-effect models. Several models were tested, but the best model was a monoexponential decline of the parasitemia in which the mean parasite elimination half-life was shorter after artemisinin (5.1 h; 95% confidence interval [CI], 4.9 to 5.2 h) than after quinine (8.0 h [95% CI, 7.5 to 8.3 h]). Attempts to simulate the initial increase of the parasitemia did not result in better models with a biologically plausible interpretation. Recrudescence was associated with slower parasite clearance and a higher simulated terminal parasitemia (Pterm). The classification of patients to outcome groups based on Pterm was correct in 78% of patients. The data suggest that parasite strains with reduced sensitivity to quinine are prevalent in Vietnam, with slower parasite clearance and consequent recrudescence. A single dose of artemisinin induces rapid parasite reduction and lowers the value of Pterm, but to prevent recrudescence, this should be followed by quinine for at least 3 days after parasite clearance, or 5 days in total.
Combination of drugs is an important contribution to the rational design of anti-infective treatment regimens. There are several reasons for this, among which are drug resistance, pharmacological arguments, and arguments concerning the therapeutic index. Combination of antimalarial drugs has been studied and applied for more than 30 years. To date it has been felt that such combinations should contain an artemisinin drug (16). The theoretical risk of selection of resistant strains is probably less when the biomass of parasites is eliminated early, and thus combination with an artemisinin drug may retard the development of resistance (15). However, there are other arguments in favor of combination with artemisinin drugs. They induce rapid clinical recovery and parasite clearance, and this feature might allow for shortening of treatment courses of drugs with a moderately short half-life, such as quinine and tetracyclines. This would enhance compliance with therapy, and, in the case of quinine, lead to a shorter duration of symptoms of cinchonism.
There are also arguments for why artemisinin drugs should not be used alone. Since the introduction of the artemisinin drugs, it is known that recrudescence is frequent when monotherapy is applied (5). Initially this was explained by the short residence time of artemisinin, but recently another argument was added. It was shown that after repeated dosing, the concentrations of artemisinin in plasma decline, which may limit the efficacy of monotherapy (2, 6).
Artemisinin and its derivatives are often used in combination with mefloquine, especially in Thailand, where multidrug resistance has become a problem (9, 10, 18). Due to its slow elimination, mefloquine can be administered as a single dose or a split dose. Nowadays in Vietnam, mefloquine is used as the first choice in combination with artemisinin or artesunate for treatment of confirmed falciparum malaria (8, 13). However, other regimens, some adequate and some inadequate, are also being applied (4). Quinine is also used for the treatment of uncomplicated malaria (1, 7). It is cheap, widely available, and generally considered to be effective, but nowadays is less popular than the artemisinin drugs. The combination of quinine and artemisinin has been studied to a limited extent only, although it offers a relatively cheap and effective treatment. Quinine doesn't have as long a residence time as mefloquine, and therefore repeated dosages are required. The question is how many dosages are required, or, in other words, what is the minimum duration of quinine therapy after a starting dose of artemisinin?
Previously we reported on the interim analysis of a study of treatment regimens with a single dose of artemisinin combined with quinine or doxycycline in comparison with quinine monotherapy (3). Three days of doxycycline in combination with a single dose of artemisinin appeared unsatisfactory. To investigate whether this could be improved, doxycycline was replaced by quinine and treatment was extended to 5 days after the initial dose of artemisinin. This restructured the study into an open-label comparison of 7 days of quinine monotherapy (Q) and a single dose of artemisinin followed by either 3 (AQ3) or 5 days (AQ5) of quinine.
The reasons for comparing these three regimens were as follows. Quinine monotherapy for 7 days was the standard recommended treatment in Vietnam, and in many areas where malaria is endemic, it still is. As mentioned above, quinine monotherapy has several drawbacks: drug administration longer than 3 days suffers from poor compliance, because cinchonism starts after a few days of treatment, when the patient is already recovering, and because of declining efficacy of quinine in Southeast Asia. It was hypothesized that a combination with artemisinin would retain the benefits and overcome the drawbacks. Regimen AQ3 was kept in the study because at the interim analysis, as reported previously, this regimen was comparable to quinine monotherapy, and we wanted to increase the sample size as originally planned (3). AQ5 was added to the study to establish its clinical efficacy, but also because in this way the three regimens were principally different with respect to the kinetics of the parasite clearance. Mathematical models that describe the parasitemia over time are difficult to design (17), but some characteristics of the parasitemia during treatment may have a clinical meaning and can be used for prediction of recrudescence (15). These characteristics include the initial parasitemia, the elimination rate of parasites, and the duration of treatment. The three regimens in the study differed from each other in two important aspects, namely, in antiparasitic activity—artemisinin versus quinine—and in duration of therapy; initial parasitemia was expected to vary equally in each treatment group.
(Part of this information was presented at the 2nd European Congress on Tropical Medicine, September 1998, Liverpool, United Kingdom.)
MATERIALS AND METHODS
All patients were admitted to the hospital and discharged only after full clinical recovery, parasite clearance, and completion of drug treatment. Intake of drugs was supervised. The methods of this study have been described in detail elsewhere (3). Informed consent was obtained from all patients who participated in the study before randomization. The study protocol was approved by the medical ethics committee of the Academic Medical Center Amsterdam and the boards of Cho Ray Hospital, Ho Chi Minh City, and Bao Loc Provincial Hospital. In aggregate, patients admitted to Lam Dong Provincial Hospital II, Bao Loc, Vietnam, for uncomplicated falciparum malaria, with a parasite density of between 1,000 and 100,000/μl, and between 8 and 65 years of age were included. Among others, exclusion criteria included inability to take oral medication; allergy to one of the study drugs; and verbal confirmation of the intake of quinine in the previous 12 h; artemisinin or derivatives in the previous 24 h; or mefloquine, tetracycline, or doxycycline during the previous 7 days. As reported previously, traces of quinine could be detected in many patients in this area, but concentrations were low. In this study, we did not assess prereferral use of effective antimalarial agents, but this was probably not a significant bias, because antimalarials such as artemisinin and mefloquine were not available outside the official health sector. Examination of the original sample size of 360 patients, aimed at detecting a difference in cure rate in three regimens with statistical significance at α = 0.05 and β = 0.2 (power, 0.8), was completed, and after deduction of the artemisinin- and doxycycline-treated patients, 268 patients were available for the analysis presented here.
Treatment.
The patients were treated with one of the following regimens: quinine at 10 mg/kg of body weight three times a day (t.i.d.) orally (250-mg quinine sulfate tablets; Pharmaceutical Factory no. 24, Hanoi, Vietnam) for 7 days (Q), or a single dose of artemisinin at 20 mg/kg of body weight orally (250-mg capsules; ACE Chemie, Maarssen, The Netherlands) followed after 6 h by quinine at 10 mg/kg of body weight t.i.d. orally for either 3 (AQ3) or 5 (AQ5) days.
Closed envelopes, containing the computer-generated randomization codes, were consecutively drawn after inclusion. A total of 120 randomized numbers were originally allocated to each of the three treatment regimens. The numbers of the original artemisinin-doxycycline group were used for the AQ5 group. Toward the end of the study, the regimens Q and AQ3 were discontinued, and the study continued with AQ5 as the only treatment regimen for the last 36 consecutive patients. In this way, the three groups would be approximately the same size at the end of the study.
The patients of groups Q and AQ3 who had been analyzed in the previous report were included again in this analysis. The reasons for this are that the numbers of patients in these groups had increased since then and the interpretation of the kinetic data of parasite clearance had not been performed yet. Besides, the change of treatment regimens was regarded not to introduce bias because the procedures for inclusion had not been changed.
Clinical assessments.
All patients were admitted to the hospital. Vital signs were recorded every 8 h, and a complete physical examination was performed every day. A full blood count and liver tests were performed prior to patient inclusion and on the third day thereafter. Giemsa-stained thick and thin blood smears were obtained for identification and counting of asexual parasites by light microscopy prior to patient inclusion. The parasitemia was then counted every 8 h until three negative smears had been obtained. Hereafter, blood smears were taken 7, 14, 21, and 28 days after the start of treatment on an outpatient basis. The parasite density was expressed as the number of parasites per microliter of blood, calculated as the ratio with the leukocyte count in 100 microscopic oil immersion fields in the thick smear or with the erythrocyte count in the thin smear.
Fever clearance time and parasite clearance time (PCT) were defined as the time from initiation of treatment to the first of three consecutive normal temperature readings (<37°C axillary) or negative blood smears, respectively. Clinical and parasitological outcome were assessed separately. Clinical failure was defined as no improvement, necessitating additional treatment within the first 48 h of treatment (early failure) or after 48 h of therapy (late failure). Additional therapy consisted of artesunate with quinine or mefloquine.
Parasitological response was defined, independently from clinical outcome, as follows. Radical cure means parasite clearance by day 7 without recrudescence up to day 28. R1 represents initial disappearance of parasites with recrudescence before day 14 (early R1) or from day 14 to day 28 (late R1). This is a conventional subdivision with the notification that late recrudescence cannot be discriminated from reinfection in our situation. R2 represents an initial decrease of parasite count to <25% of the initial value, followed by resurgence, without clearance by day 7. R3 represents no response or a small decrease in parasitemia to not less than 25% of the initial value, assessed at 48 h after initiation of therapy.
Population kinetics of the parasitemia.
The population kinetics of the time course of the parasitemia was estimated by using several nonlinear mixed-effect models (see Appendix). These models estimated the kinetic parameters of the time course of the parasite density.
The initial parasitemia [P(0)], the duration of effective drug treatment, and the elimination rate converge in a single value: the parasitemia at the end of the treatment (Pterm). The end of therapy was defined as the time of the last dose of quinine plus 8 h, which represents the duration of one dosing interval. Pterm can be derived from the kinetic models of the parasitemia.
At the end of treatment, the replication of parasites leading to recrudescence can be simulated as an exponential increase, the replication rate being the slope of the line connecting Pterm and the parasite density of the recrudescence on a semilogarithmic plot. This value is a rather crude estimate, connecting a simulated value and a single data point. However, it may serve as a reference to other literature.
Another parameter, the eradication time (ET), was defined as the duration of treatment after parasite clearance. Thus, PCT plus ET equals the duration of effective therapy. ET was introduced to evaluate if this could be a better predictor for recrudescence than PCT and if this would be useful in individualizing the duration of therapy in future studies.
Statistical analysis.
The individual data were analyzed with the aid of the statistical package SPSS (version 8.0; SPSS Inc., Chicago, Ill.). All statistics concerning parasitemia were calculated according to its log transformation. The clinical outcome was analyzed with contingency tables and χ2 tests with continuity correction for categorical parameters and with analysis of variance (ANOVA) or nonparametric tests for numerical parameters. The effects of treatment and kinetic parameters on the occurrence of parasite clearance or recrudescence were analyzed by logistic regression and in a Cox proportional hazard model. Statistical significance was accepted at P < 0.05.
RESULTS
Clinical assessment.
Some baseline patient characteristics are shown in Table 1. There were no significant differences between the groups. Some patients with hyperparasitemia had been introduced into the study. They were not excluded from the analysis, because there were no complications of malaria. The outcome is shown in Table 2. In two patients, hemoglobinuria was observed after 24 h of treatment. This was regarded as an adverse effect of quinine, and the patients were withdrawn from the study and treated with artesunate. These patients could not be evaluated for clinical and parasitological outcome. Other side effects were not considered to be a clinically significant problem in this study. Cinchonism and malaria-related complaints were reported in all three groups.
TABLE 1.
Characteristics of patients treated with three different regimens for uncomplicated falciparum malaria
| Characteristic | Result with regimena
|
||
|---|---|---|---|
| Quinine (Q) | Artemisinin + 3 days of quinine (AQ3) | Artemisinin + 5 days of quinine (AQ5) | |
| Sex (no. male/no. female) | 70/14 | 76/20 | 70/18 |
| Median age (yr) [range] | 26.0 [7–60] | 26.0 [7–64] | 26.0 [7–60] |
| Mean wt (kg) | 47.9 (46.1–49.7) | 46.3 (44.3–48.3) | 48.3 (45.9–50.7) |
| Mean temp (°C) | 38.6 (38.5–38.7) | 38.6 (38.5–38.7) | 38.7 (38.6–38.9) |
| Geometric mean P(0) (per μl) | 16,157 (12,642–20,646) | 16,123 (12,611–20,611) | 23,202 (17,888–30,091) |
Values in parentheses represent the 95% CI of the geometric mean.
TABLE 2.
Outcome of treatment for the malaria patients in this study
| Patient outcome | No. (%) of patients with result
|
|||
|---|---|---|---|---|
| Quinine (Q) | Artemisinin + 3 days of quinine (AQ3) | Artemisinin + 5 days of quinine (AQ5) | Total | |
| Total | 84 | 96 | 88 | 268 |
| Early dropout, not evaluable | 3 | 1 | 1 | 5 |
| Lost before day 7 | 2 | 3 | 5 | |
| Failure | ||||
| Early | 1 | |||
| Late | 1a | |||
| Radical cure | 56 | 46 | 66 | 168 |
| Recrudescenceb | 11 (16) | 28 (38) | 12 (15) | 51 (30) |
| Early | 3 | 11 | 14 | |
| Late | 8 | 17 | 12 | 37 |
| Lost between days 7 and 28 | 10 | 18 | 9 | 37 |
The parasitological response was R3.
P < 0.001 (χ2 test). The 95% CIs of the differences are as follows: Q versus AQ3, 8 to 36%; Q versus AQ5, −11 to 13%; and AQ3 versus AQ5, 9 to 37%.
Four patients left the hospital before any endpoint was reached. There was one early clinical failure, as well as one late clinical failure with a parasitological R3 response, both in regimen Q. These two patients needed additional intravenous therapy.
The recrudescence (R1 response) rate in regimen AQ3 was significantly higher than in the other two regimens. When the recrudescence rate was calculated in a best-case or worst-case scenario (i.e., all patients who were lost to follow-up classified as radically cured or as recrudescent, respectively), the difference between regimen AQ3 and the other two regimens was still significant (P = 0.007 and P = <0.001, respectively). The median days of diagnosing recrudescence were day 21 (earliest, day 11; latest, day 28) in group Q7, day 18 (earliest, day 7; latest, day 27) in group AQ3, and day 23 (earliest, day 17; latest, day 28) in group AQ5. The proportional cumulative recrudescence of patients, including those who were lost to follow-up between days 7 and 28, is shown in Fig. 1. Recrudescence was less frequent in groups Q and AQ5 than in regimen AQ3 (relative risk for Q and AQ5 versus AQ3, 0.37; 95% CI, 0.19 to 0.72).
FIG. 1.
Kaplan Meier curve of the cumulative recrudescence of P. falciparum after treatment with quinine monotherapy for 7 days (Q [○]) or a single dose of artemisinin followed by either 3 days of quinine (AQ3 [●]) or 5 days of quinine (AQ5 [□]) (P < 0.001; log-rank test).
The mean fever clearance times were not different among the three treatment groups: 47 h (95% CI, 41 to 53 h) in regimen Q, 41 h (95% CI, 37 to 46 h) in regimen AQ3, and 43 h (95% CI, 38 to 47 h) in regimen AQ5. The mean observed PCTs were 62 h (95% CI, 57 to 67 h), 41 h (95% CI, 38 to 44 h), and 42 h (95% CI, 39 to 46 h) for regimens Q, AQ3, and AQ5, respectively. The observed P(0), PCT, and ET for treatment groups and outcome are shown in Table 3.
TABLE 3.
Kinetic parameters in an exponential elimination model of the parasitemia per treatment group and parasitological outcome
| Parameter | Result with regimena:
|
P (ANOVA) | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Quinine for 7 days (Q)
|
Artemisinin + 3 days of quinine (AQ3)
|
Artemisinin + 5 days of quinine (AQ5)
|
|||||||
| Radical cure (n = 56) | Recrudescence
|
Radical cure (n = 44) | Recrudescence
|
Radical cure (n = 66) | Recrudescence (late; n = 11) | ||||
| Late (n = 8) | Early (n = 3) | Late (n = 17) | Early (n = 11) | ||||||
| Observed values | |||||||||
| Geometric mean P(0) (per μl) | 16,282 (11,722–22,617) | 20,317 (8,646–47,740) | 13,111 (5,651–30,420) | 17,541 (12,123–25,379) | 26,733 (14,344–49,823) | 13,461 (6,645–27,267) | 24,917 (18,619–33,345) | 16,987 (5,520–52,279) | 0.429 |
| Mean PCT (h) | 58 (53–64) | 72 (51–93) | 107 (76–137) | 40 (35–45) | 46 (40–52) | 52 (41–63) | 42 (41–46) | 48 (34–62) | 0.004 |
| Mean ET (h) | 110 (104–115) | 96 (75–117) | 61 (31–92) | 39 (34–44) | 32 (26–38) | 26 (15–37) | 84 (80–88) | 75 (60–89) | 0.000 |
| Model-estimated values | |||||||||
| Mean lag time (h) | 5 (3–8) | 5 (0–12) | 14 (12–16) | 3 (2–5) | 2 (1–4) | 5 (1–10) | 3 (2–4) | 7 (2–13) | 0.003 |
| Mean t1/2el (h) | 7.8 (7.3–8.3) | 8.6 (7.4–9.8) | 11.4 (6.7–16.2) | 4.7 (4.5–4.9) | 5.0 (4.7–5.4) | 5.5 (4.8–6.2) | 5.3 (5.1–5.5) | 5.8 (5.0–6.5) | 0.000 |
| Mean PCTcalc (h) | 92 (85–99) | 102 (86–119) | 142 (76–207) | 55 (51–58) | 60 (55–65) | 66 (56–77) | 63 (59–66) | 69 (58–81) | 0.000 |
| Geometric mean Pterm (per μl) | 0.003 (0.001–0.008) | 0.021 (0.003–0.156) | 0.969 (0.005–183) | 0.138 (0.074–0.255) | 0.396 (0.160–0.982) | 0.959 (0.238–3.862) | 0.001 (0.000–0.002) | 0.003 (0.000–0.038) | 0.000 |
| Mean replication t1/2 (h) | 21 (13–29) | 22 (−20–63) | 39 (34–44) | 32 (14–50) | 23 (18–28) | 0.029 | |||
Values in parentheses represent 95% CIs.
Kinetic models.
Population kinetic models of the parasitemia were fitted with the mixed nonlinear regression program of S-Plus (version 4.5; Math Soft, Inc., Seattle, Wash.). The buildup of the models is shown in the Appendix. In two patients of regimen Q with radical cure, the time series was incomplete and could not be used for the kinetic analysis.
As shown in the Appendix, model I with a monoexponential decline of the parasitemia in which the clearance rate depends on the regimen, yielded the best fits. The mean estimates of the elimination half-life (t1/2el) in groups Q7, AQ3, and AQ5 were 8.0 h (95% CI, 7.5 to 8.3 h), 4.8 h (95% CI, 4.6 to 5.0 h), and 5.3 h (95% CI, 5.2 to 5.5 h), respectively. The model estimates and derivatives, specified for treatment and outcome, are shown in Table 3. Instead of the estimated initial parasitemia (expressed as A in the formulas), lag time (tlag) is presented. This was calculated according to formula 4 in the Appendix. The elimination rate has been recalculated in response to t1/2el. As a reference value for the observed data, the PCT was calculated from the kinetic estimates (PCTcalc), setting the detection limit at 6 parasites/μl, the lowest parasitemia observed in this study. The kinetic models yielded the estimates of Pterm.
The parasite elimination half-life was significantly shorter in the groups that started with artemisinin than the quinine monotherapy group and was also significantly shorter for the radical cure response than for early and late recrudescence (P < 0.01 for both effects; two-factor ANOVA). However, there was a strong interaction effect between treatment group and outcome. The CIs of the estimates in Table 3 give an indication of the respective differences. There were also significant differences between the treatment groups with respect to PCT, ET, and the estimated value of Pterm.
The outcome, i.e., the occurrence of recrudescence, was fitted in a Cox proportional hazards model. Since PCT and ET are not independent of each other, it is not possible to construct a Cox model which adequately describes the hazard function based on both parameters. However, the effects of PCT and ET are both reflected in the value of Pterm. The same was the case for P(0) or the estimated intercept and the estimates of k (and thus t1/2el) and Pterm. These were all entered separately into the Cox model. Pterm was shown to be a significant predictor of recrudescence (P < 0.001; relative risk for 1 log increase of Pterm, 1.7 [95% CI, 1.4 to 2.1]), but PCT and ET were also associated with a greater hazard function of recrudescence (data not shown).
To evaluate if the duration of therapy could be individualized, the chance of recrudescence was estimated in a logistic regression model. P(0), PCT, ET, and Pterm were entered separately in the model. No more than 80% of cases were classified correctly as recrudescence or radical cure, with no important differences between the respective parameters.
Figure 2 illustrates the simulated time course of the parasitemia in model C. The figure shows that in regimen Q, as well as in regimen AQ3, Pterm is lower for the patients with radical cure than for the patients with recrudescence. The model did not discriminate between early and late recrudescence. In group AQ5, there was no difference between radical cure and late recrudescence with respect to Pterm.
FIG. 2.
P. falciparum parasitemia simulated by an exponential elimination model (see text) during treatment with quinine monotherapy for 7 days (Q) or a single dose of artemisinin followed by either 3 days of quinine (AQ3) or 5 days of quinine (AQ5). The arrow indicates the single dose of 1 g of artemisinin, and the shaded bar indicates the duration of treatment with quinine. L.d., limit of detection. ——, patients with radical cure; ............, patients with late recrudescence; ······, patients with early recrudescence.
The mean replication rate by which Pterm evolves into recrudescence was 0.03 h−1 on a natural logarithmic scale, which corresponds to a 0.013-log increase per h, or a 0.6-log increase per 48-h cycle. There was a slight difference in replication half-life between the subgroups of recrudescence.
DISCUSSION
This study shows that for uncomplicated falciparum malaria in southern Vietnam the combination of a single dose of artemisinin with 5 days of quinine (AQ5) is as effective as 7 days of quinine monotherapy (Q) and superior to artemisinin with 3 days of quinine (AQ3). The standard 7-day quinine treatment is still rather effective, with initial parasite clearance in most patients, but with a rate of recrudescence of 17%. Recrudescence (by convention an indicator of resistance) was associated with a slower parasite clearance.
The study was the continuation of a study in which one of the study arms had been replaced (3). Although this is an unusual procedure, it is unlikely that this introduced bias. The differences between the three regimens added interesting information to the study. First, regimen AQ3 was confirmed to be less effective than regimen Q7 in terms of preventing recrudescence. The number of patients included was larger than at the interim analysis, and this increased the power of the comparisons enough to detect a significant difference. Second, the analysis of the population kinetics of the parasitemia yielded new information—namely that Pterm is an important determinant of the chance of recrudescence.
In the parasite kinetic model I, three parameters determine the value of Pterm: the estimate of the initial parasitemia, k, and the duration of therapy. P(0) belonged to the inclusion criteria, and therefore it was within a relatively narrow range. The decline of the parasitemia started earlier after the artemisinin regimens than after quinine. This illustrates the fast antiparasitic activity of artemisinin and the great range of the parasite development cycle on which it exerts its action, which confirms in vitro findings (14). The impact of the lag phase on Pterm is small though. The parasite elimination rate is more important, and when this is slow, the eradication time, and thus duration of therapy, becomes critical. A slower parasite clearance, and thus a higher value of Pterm, was associated with recrudescence in groups Q and AQ3. It was also shown that the longer eradication time in regimen AQ5 lowers Pterm and improves efficacy, in comparison to those of regimen AQ3. In regimen AQ5 itself, there was no difference between radical cure and recrudescence with respect to Pterm. The lack of this difference is not clear, but it should be noted that a late recrudescence could not be discriminated from a reinfection. In the low-transmission study area, reinfection is probably infrequent, and an extra argument is that the replication rate in the cases of recrudescence attained a realistic value. A more plausible explanation for the lack of difference in Pterm between radical cure and recrudescence in regimen AQ5 is at the same time the Achilles heel of the kinetic model. During the first 24 to 48 h of therapy, the elimination rate constant is dominated by the effects of artemisinin. In this period, the exponential decline is an adequate description of the time course of the parasitemia. However, later, when the clearance rate slows down to that of quinine, a second elimination constant should be incorporated into the model. The effect of a second elimination constant on the parasitemia was not detected by the model, probably because by that time the parasitemia has decreased to or is under the detection level in most cases. However, there was a significant difference in elimination rates after artemisinin or quinine. The low precision of the low parasite counts and the relatively high limit of detection are important limitations for more refined kinetic modeling.
In regimen Q, the slow parasite clearance in patients with recrudescence suggests reduced sensitivity, and not therapy that is too short. Reduced sensitivity to quinine expressed as slower parasite clearance has been observed in Thailand (10). In Vietnam, this phenomenon has been mentioned, but not confirmed (1, 4). This is the first report which shows that parasite strains that are less sensitive to quinine circulate in Vietnam. In regimen AQ3, there was a difference in clearance rates between radical cure and recrudescence. Whether this should be explained by a difference in sensitivities to artemisinin could not be ascertained. This could be studied further in a study with artemisinin monotherapy.
The mean replication rate corresponded to a 0.6-log increase per 48-h cycle. This value is comparable to what has been reported in the literature (15). However, that the replication rates may differ for certain parasites and/or their hosts cannot be excluded.
The predictive accuracy of our results was limited. Outcome could be classified correctly in approximately four of every five patients, based on either PCT, ET, or Pterm. This does not provide a satisfactory algorithm with which to predict recrudescence or to individualize the duration of therapy. With our current knowledge, the best advice for an individual patient is to aim at an eradication time of at least 3 days with quinine after a single dose of artemisinin. A design in which patients would be randomized to eradication time could give a better idea of the possibilities of individualization of treatment.
The concept that eradication of a pathogen requires a certain minimum duration of treatment is not new. In the first half of this century, when dosing schedules of quinine were not yet uniform, the duration of quinine treatment was guided by the duration of fever in several recommended treatment regimens for (mainly tertian) malaria (11). At present, in the era of antibiotic and antiviral treatment, the required eradication of the pathogen is still a major determinant of the duration of therapy. Since eradication occurs beyond our level of detection, we can only make assumptions based on extrapolation. The process of extrapolation requires a model that describes the amount of parasites until below the detection level. Such models are not easily available. Malaria parasites in blood can be visualized and counted by simple techniques, but the kinetics of Plasmodium falciparum are not yet fully understood. However, White recently presented arguments, based on pharmacodynamic concepts, explaining how mefloquine resistance developed so quickly in Thailand (15).
The model of parasite elimination applied in this study confirmed these concepts and showed that they are also valid for artemisinin and quinine. The model shows that Pterm is an important determinant for outcome: radical cure or recrudescence. It suggests that there is a point of no return the actual value of which may depend on treatment regimen.
The population kinetics model confirmed and quantified several pharmacodynamic concepts of antimalarial treatment, which are not yet common practice. These concepts of duration of treatment, eradication time, and Pterm could only be studied because of the short residence time of artemisinin and, to a lesser extent, of quinine. With chloroquine and mefloquine, the extremely long residence times preclude accurate estimation of the eradication time. Nevertheless, we feel that these concepts of parasite kinetics can be generalized to other drugs also and that they may provide tools for a rational design of new antimalarial treatment regimens.
We conclude that a 7-day treatment course of quinine is still effective in the initial treatment of uncomplicated falciparum malaria in southern Vietnam. However, the rate of recrudescence is rather high, and the results suggest that this is caused by reduced sensitivity to quinine. The addition of a single dose of artemisinin increases the parasite elimination rate, and this benefits the cure rate. It is prudent to aim at an eradication time of at least 3 days for single-dose artemisinin-plus-quinine combinations. This rule of thumb allows individualizing of the duration of treatment of patients, provided that the parasitemia is determined regularly and that the parasite clearance time is known. Otherwise after a single dose of artemisinin, a minimum of 5 days of quinine treatment should be advised.
ACKNOWLEDGMENT
This study was part of a research and development program supported by the Ministry of Development Cooperation of The Netherlands.
Appendix
Population kinetics of parasitemia.
The buildup of the kinetic models started with inspection of the natural logarithms of the parasite count, P(t), excluding the negative blood smears. It appeared that for most individuals the decline of ln P(t) was more or less constant over all 8-h intervals, with the exception of the first interval. In a plot of the geometric mean values (Fig. A1), this is less clear, because the tail of the mean curve is distorted by blood smears becoming negative. So it seemed rational to start with a simple log-linear (exponential) decline of the parasitemia as the basic model and build from this. In the formula
 |
1 |
or
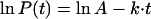 |
2 |
A is the estimated initial parasitemia and k is the elimination rate constant. k can be recalculated into a more conventional elimination half-life, t1/2el, according to the formula
 |
In a mixed-effects population kinetics model, parameters can be entered as fixed, which means that they have a certain value, or be nonfixed, which means that they can vary at random with a mean value of zero. Parameters can also vary depending on another factor, for example, the treatment regimen. The formula of such an exponential decline of the parasitemia looks like
 |
3 |
In this way, several models can be applied to the data from all patients. Models were compared by using the Bayesian information criterion (BIC) (12). The method of maximum likelihoods was used when different models were to be compared. After choosing the best model, restricted maximum likelihood was used to estimate the parameters.
FIG. A1.
Geometric mean parasitemia of P. falciparum during treatment with quinine monotherapy for 7 days (Q [○]) or a single dose of artemisinin followed by either 3 days of quinine (AQ3 [●]) or 5 days of quinine (AQ5 [□]). The bars indicate the 95% CIs. A parasitemia measurement below the detection level (negative blood smear) was entered as 3 parasites per μl.
A model with random intercepts and slopes and with independent residuals was tested as well as a comparable model in which the residuals, ɛ, are normal with autocorrelation, ρ. Autocorrelation in this context means that the repeated measurements over time are correlated; in other words, the value of parasitemia at a certain time predicts the following value. A third model, model I, in which k was allowed to change per treatment regimen, was also constructed, and this model gave the best fit (BIC = 4,720.6). Negative blood smears, with a value of zero, were not included in the models. The three models were also fitted to ln [P(t) + 0.5], with zero values included, but this did not have a significant effect on the BIC and estimates. In further modeling, zero values were excluded from the data set.
Although the initial parasite counts were not different among the three treatment groups, a modification of model I was made in which the intercept, ln A, was allowed to change per regimen. As expected (treatment groups were similar with respect to baseline parasite count), this did not improve the fit, so that the variation of this term could be interpreted as a random effect.
In model I, the estimate of A was greater than the observed initial parasitemia, P(0), or put simply, after drug intake, it takes some time before the parasitemia starts to decline. This time can conceptually be simplified to a lag phase, tlag. Although the lag phase is not readily explained in biologically plausible terms, in clinical experience, a lag phase is usually interpreted as the time until P(t) has decreased to values lower than P(0). Moreover, the definitions of the in vivo response to drug treatment are based on decrease of the parasite count relative to the initial parasitemia, thereby ignoring that, in many patients, the parasitemia increases initially and that the lag times may be different for individuals. When the lag time is incorporated into the kinetic models, these have the form
 |
4 |
From this formula it can easily be seen that in log-linear models, A and tlag are interdependent, which means that a difference in tlag also affects the value of A. Nevertheless, the models were fitted, again incorporating tlag, and this did not yield better fits than model I.
To investigate further if a mathematical function could give an better description of the initial part of the curve, irrespective of biological interpretation, a quadratic term was added to the basic model:
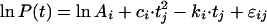 |
5 |
This quadratic model was worked out analogously to the simple (log)linear model with normal residuals ɛ with autocorrelation, with c and k changing per regimen, or with only k changing per regimen. The latter two models were also investigated with k as a fixed factor. Model II, the model with a fixed quadratic term, c, for every patient and a linear term, k, variable per patient, but depending on regimen, yielded the best fit (BIC = 4,719.4). In both models I and II, the estimates of k were comparable for regimens AQ3 and AQ5 and different from those for regimen Q. The difference in BIC for models I and II was small, and model II had 1 df more than model I. Another approach to describe the initial rise in the parasite count was to build models in which the logarithm of t was factored in. The basic model of this approach looks like
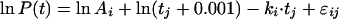 |
6 |
This model did not improve the fits.
Alternatively, a model with an exponential term added to this logarithm of t is described by
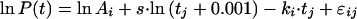 |
7 |
or written as
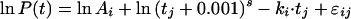 |
8 |
Model III, with s fixed for all patients, independent of treatment regimen, and k, with random variation, but with a value depending on the regimen, yielded the best fits (BIC = 4,640) of this group of models.
The problem with this model was that there was no plausible biological explanation behind it. Since antimalarial drugs are parasite stage specific, and since replication may continue in the lag phase, a term for parasite stage was incorporated in the model. For this purpose, we adopted a model constructed by White, who assumed a normal distribution in four patients who failed to respond to treatment was applied (17). It was assumed that the distribution of the parasites' age has a mean value, μ, with a standard deviation (SD). For the convenience of calculations, a logistic distribution of the parasites' age was assumed. This is a small difference from White's model. This model, model IV, which is basically an extension of model III, but with a biologically plausible interpretation of the lag phase, can be described by
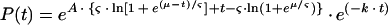 |
9 |
simplified to
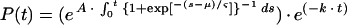 |
10 |
where ς = (SD · π)/√3 and s is the stage in the parasites' cycle.
However, the fits of model IV were not better than those of model III, but also were not better than those of model I or II. In addition, the lag phase is probably determined not only by parasite factors, but also by pharmacokinetic and other factors. Because model IV did not improve the fits, and because models II and III lacked any plausible biological explanation of the parameters c and s, respectively, it was decided that the simplest model, model I, would be taken as the best description for the data. In this model, the concept of Pterm, fitted by this model, gave a plausible explanation of the mechanism of recrudescence.
REFERENCES
- 1.Arnold K, Hien T T, Chinh N T, Phu N H, Mai P P. A randomized comparative study of artemisinin (qinghaosu) suppositories and oral quinine in acute falciparum malaria. Trans R Soc Trop Med Hyg. 1990;84:499–502. doi: 10.1016/0035-9203(90)90012-4. [DOI] [PubMed] [Google Scholar]
- 2.Ashton M, Sy N D, Gordi T, Hai T N, Thach D C, Huong N V, Farah M H, Johansson M, Cong L D. Evidence for time-dependent artemisinin kinetics in adults with uncomplicated malaria. Pharm Pharmacol Lett. 1996;3:127–130. [Google Scholar]
- 3.Bich N N, De Vries P J, Thien H V, Phong T H, Hung L N, Eggelte T A, Anh T K, Kager P A. Efficacy and tolerance of artemisinin in short combination regimens for the treatment of uncomplicated falciparum malaria. Am J Trop Med Hyg. 1996;55:438–443. doi: 10.4269/ajtmh.1996.55.438. [DOI] [PubMed] [Google Scholar]
- 4.Cong L D, Yen P T, Nhu T V, Binh L N. Use and quality of antimalarial drugs in the private sector in Viet Nam. WHO Bull. 1998;76(Suppl.1):51–58. [PMC free article] [PubMed] [Google Scholar]
- 5.De Vries P J, Dien T K. Clinical pharmacology and therapeutic potential of artemisinin and its derivatives in the treatment of malaria. Drugs. 1996;52:818–836. doi: 10.2165/00003495-199652060-00004. [DOI] [PubMed] [Google Scholar]
- 6.Hassan Alin M, Ashton M, Kihamia C M, Mtey G J B, Bjorkman A. Multiple dose pharmacokinetics of oral artemisinin and comparison of its efficacy with that of oral artesunate in falciparum malaria patients. Trans R Soc Trop Med Hyg. 1996;90:61–65. doi: 10.1016/s0035-9203(96)90480-0. [DOI] [PubMed] [Google Scholar]
- 7.Hien T T, Tam D T H, Cuc N T, Arnold K. Comparative effectiveness of artemisinin suppositories and oral quinine with acute falciparum malaria. Trans R Soc Trop Med Hyg. 1991;85:210–211. doi: 10.1016/0035-9203(91)90024-s. [DOI] [PubMed] [Google Scholar]
- 8.Hung L N, De Vries P J, Thuy L T D, Lien B, Long H P, Hung T N, Nam N V, Anh T K, Kager P A. Single dose artemisinin-mefloquine versus mefloquine alone for uncomplicated falciparum malaria. Trans R Soc Trop Med Hyg. 1997;91:191–194. doi: 10.1016/s0035-9203(97)90221-2. [DOI] [PubMed] [Google Scholar]
- 9.Nosten F, ter Kuile F, Chongsuphajaisiddhi T, Luxemburger C, Webster H K, Edstein M, Phaipun L, Thew K L, White N J. Mefloquine-resistant falciparum malaria on the Thai Burmese border. Lancet. 1991;337:1140–1143. doi: 10.1016/0140-6736(91)92798-7. [DOI] [PubMed] [Google Scholar]
- 10.Pukrittayakamee S, Supanaranond W, Looareesuwan S, Vanijanonta S, White N J. Quinine in severe malaria: evidence of declining efficacy in Thailand. Trans R Soc Trop Med Hyg. 1994;88:324–327. doi: 10.1016/0035-9203(94)90102-3. [DOI] [PubMed] [Google Scholar]
- 11.Rogers L. Recent advances in tropical medicine. London, United Kingdom: J.&-A. Churchill; 1928. pp. 79–80. [Google Scholar]
- 12.Schwarz G. Estimating the dimension of a model. Ann Stat. 1978;6:461–464. [Google Scholar]
- 13.Sy N D, Hoan D B, Dung N P, Huong N V, Binh L N, Son M V, Meshnick S R. Treatment of malaria in Vietnam with oral artemisinin. Am J Trop Med Hyg. 1993;48:398–402. [PubMed] [Google Scholar]
- 14.Ter Kuile F, White N J, Holloway P, Pasvol G, Krishna S. Plasmodium falciparum: in vitro studies of the pharmacodynamic properties of drugs used for the treatment of severe malaria. Exp Parasitol. 1993;76:85–95. doi: 10.1006/expr.1993.1010. [DOI] [PubMed] [Google Scholar]
- 15.White N J. Assessment of the pharmacodynamic properties of antimalarial drugs in vivo. Antimicrob Agents Chemother. 1997;41:1413–1422. doi: 10.1128/aac.41.7.1413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.White N J. Antimalarial drug resistance and combination chemotherapy. Phil Trans R Soc Lond B. 1999;354:739–749. doi: 10.1098/rstb.1999.0426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.White N J, Chapman D, Watt G. The effects of multiplication and synchronicity on the vascular distribution of parasites in falciparum malaria. Trans R Soc Trop Med Hyg. 1992;86:590–597. doi: 10.1016/0035-9203(92)90141-x. [DOI] [PubMed] [Google Scholar]
- 18.Wongsrichanalai C, Webster H K, Wimonwattrawatee T, Sookto P, Chuanak N, Thimasarn K, Wernsdorfer W H. Emergence of multidrug-resistant Plasmodium falciparum in Thailand: in vitro tracking. Am J Trop Med Hyg. 1992;47:112–116. doi: 10.4269/ajtmh.1992.47.112. [DOI] [PubMed] [Google Scholar]





