Introduction
Hydrocephalus is characterized by abnormal accumulation, and impaired circulation and clearance, of cerebrospinal fluid (CSF). CSF accumulation results in distention of the ventricular system, leading to accelerated head growth and increased intracranial pressure, and often requires surgical intervention1,2. Syndromic hydrocephalus encompasses a diverse group of disorders and genetic variants in which hydrocephalus is a symptom, due to congenital structural malformations, or a range of emerging pathology associated with recently described genetic variants3. In this review we discuss several of the major syndromic causes of hydrocephalus, as well as emerging research on the genetic basis for congenital hydrocephalus as part of recently described genetic mutations (Table 1).
Table 1.
Genetic Basis of Syndromes Associated with Hydrocephalus
| Syndrome | Type of Disorder | Mode of Inheritance | Genetic Locus |
|---|---|---|---|
| L1 Syndrome, and X-Linked Hydrocephalus | Neuronal Adhesion | X-linked | L1CAM |
| Syndromic Craniosynostoses (Pfeiffer, Crouzon, Apert, Muenke) | Primary cerebral maldevelopment | Heterogeneous | FGFR1 (Pfeiffer), FGFR2 (Crouzon; Apert; Pfeiffer), FGFR3 (Muenke) |
| Achondroplasia | Growth Factor | Autosomal Dominant | FGFR3 |
| NF 1 | RASopathy | Autosomal Dominant | NF1 (17q11.2) |
| NF 2 | RASopathy | Autosomal Dominant | NF2 (22q12) |
| Down’s Syndrome | Trisomy | Non-disjunction | Chromosome 21 |
| Tuberous Sclerosis | mTOR related | Autosomal Dominant | DNAH5, DAIC1, CCDC151, MCIDAS, FOXJ1 |
| Walker-Warburg Syndrome/ Brain-muscle-eye disease | Dystroglycanopathies | Autosomal Recessive | POMT1, POMT2, POMGNT1, FKTN, FKRP, LARGE, ISPD |
| Primary Ciliary Dyskinesia | Ciliopathy | Heterogeneous | DNAH5, MCIDAS, FOXJ1 |
| Osteogenesis Imperfecta | Connective tissue | Autosomal Dominant | COL1A1 and COL1A2 |
| Pettigrew Syndrome | Vesicle trafficking | X-linked | AP1S2 |
| Costello Syndrome | RASopathy | Autosomal Dominant | HRAS |
| Noonon Syndrome | RASopathy | Autosomal Dominant | CBL, KRAS, NRAS, PTPN11, SOS1, SHOC2 and RAF1 |
| Cardio-facio-cutaneous (CFC) syndrome | RASopathy | Autosomal Dominant | BRAF, MEK1, KRAS and MEK2 |
| Megalencephaly-polymicrogyria-polydactyly-hydrocephalus (MPPH) | PI3K-AKT-mTOR pathway | Autosomal Dominant | AKT3, CCND2 and PIK3R2 |
| Megalencephaly-capillary-malformation (MCAP) | PI3K-AKT-mTOR pathway | N/A | PIK3CA |
L1 Syndrome, and X-Linked Hydrocephalus
X-linked hydrocephalus comprises 5% of all cases of congenital hydrocephalus (Table 1)1.X-linked hydrocephalus, associated with stenosis of the aqueduct of Sylvius (cerebral aqueduct), is the most severe phenotype associated with L1 syndrome, an X-linked recessive disorder (Fig. 1)4. Other phenotypes of L1 syndrome include MASA (mental retardation, aphasia, spastic paraplegia, adducted thumbs) and X-linked complicated corpus callosum and/or pyramidal tract agenesis3,5. Many of these phenotypic features commonly co-occur with X-linked hydrocephalus, especially intellectual disability6.
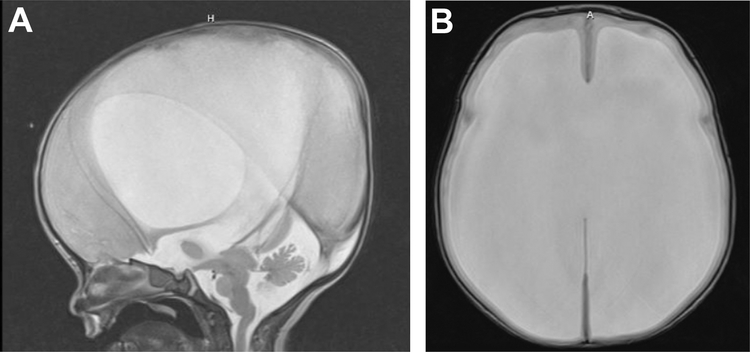
Figure 1.
1-day-old with L1 syndrome resulting in hydrocephalus and aqueductal stenosis treated on day-of-life 1 with a VP shunt. T2-weighted MRI (A) sagittal and (B) axial views.
This syndrome results from mutations in L1CAM on chromosome region Xq28, affecting the locus of a gene coding for the neural cell adhesion molecule L1. Mutations in this gene are associated with disordered neuronal migration which is considered a key mechanism in the pathogenesis of this syndrome7. Knockout rat models of the disease have revealed early pathologic changes of periventricular white matter tracts following the development of hydrocephalus, evidenced by reductions in fractional anisotropy and axial diffusivity on DTI in the corpus callosum, external capsule, and internal capsule. Histology also revealed hypomyelination and increased extracellular fluid in the corpus callosum, yielding some insight into how mutations of L1CAM contribute not only to hydrocephalus, but also to the developmental delays and intellectual deficits observed in this condition8.
Point mutations at branch points in introns of L1CAM, causing abnormal splicing, were among the first mutations implicated in the disease. Recently, duplication affecting the intracellular domain, frameshift mutations affecting translation of fibronectin type-III of L1CAM, and novel nonsense mutations affecting ependymal cilia have also been implicated in this syndrome9,10,11,12,13.
Beyond L1CAM, other X-linked mutations have recently been associated with syndromic hydrocephalus including missense mutations in OTUD5 resulting in severe neurodevelopmental delay, hydrocephalus, and early lethality14. Duplications of Xp22.33 also lead to an L1-like phenotype of hydrocephalus associated with stenosis of the cerebral aqueduct, and dysgenesis of the corpus callosum15. Modern sequencing techniques may reveal other X linked mutations associated with L1-like syndromic hydrocephalus, though L1CAM remains the most common and thoroughly explored locus causing this condition.
Once an L1CAM pathogenic variant has been identified in a family, carrier testing, prenatal testing, and preimplantation genetic testing are available to patients and families. Treatment of individuals with X-linked hydrocephalus may vary depending on the timing of presentation; however, because many of these patients present with symptomatic hydrocephalus at birth, treatment often involves ventriculoperitoneal shunting (VPS), as the efficacy of endoscopic third ventriculostomy (ETV) with choroid plexus catheterization (CPC) in young infants is variable4.
Syndromic Craniosynostosis
Syndromic craniosynostosis is associated with an increased incidence of hydrocephalus16. Hydrocephalus is more common in syndromic compared to non-syndromic or isolated craniosynostosis, and is seen in Crouzon’s and Pfeiffer’s syndromes17. Mechanisms underlying hydrocephalus in this group of syndromes relate to primary cerebral maldevelopment and residual structural outflow obstruction, not significantly ameliorated by posterior vault distraction strip craniectomy or cranial vault reconstruction commonly utilized in the treatment of craniosynostosis16,18. Conversely, early shunting (or over-shunting) can cause iatrogenic premature fusion of the cranial sutures in patients without craniosynostosis19. Treatment of hydrocephalus in patients with syndromic craniosynostosis includes VP shunt and ETV +/− CPC and is usually influenced by future need for cranial vault reconstruction; therefore, location of shunt placement may vary to accommodate future planned surgeries.
Pfeiffer Syndrome
Of the syndromes associated with craniosynostosis, Pfeiffer syndrome is most frequently associated with hydrocephalus, with up to 60–80% of patients requiring ventriculoperitoneal shunt insertion or other treatment for hydrocephalus20,21. This syndrome is also associated with speech, language, hearing and feeding issues related to mutations in FGFR221,22.
Crouzon Syndrome
Crouzon syndrome, related to mutations in FGFR223, can be associated with a small foramen magnum and outlet obstruction associated with hydrocephalus.[24] Because of the structural predisposition to developing hydrocephalus in this form of craniosynostosis, up to 40% of patients with Crouzon’s can present with or develop ventricular dilation25. While ventriculomegaly is common, a smaller number will ultimately require surgical treatment of hydrocephalus26.
Apert Syndrome
Apert syndrome, also related to mutations in FGFR2, is characterized by multi-suture craniosynostosis, midface retrusion, syndactyly of the hands, fusion of the second through fourth nails, and nonprogressive ventriculomegaly27,28. Fewer patients with Apert syndrome have true or progressive hydrocephalus29. Rates of ventriculoperitoneal shunt placement in one study of a cohort of patients with Apert syndrome was 24.3%, lower that that seen in Crouzon or Pfeiffer syndromes30.
Muenke Syndrome
Muenke syndrome is an autosomal dominant syndrome associated with mutations in FGFR3 characterized by coronal craniosynostosis and variable extracranial anomalies31. Though this syndrome is not regularly associated with hydrocephalus, there has been a rare familial variant of this syndrome (p.Pro250Arg) with hydrocephalus, without craniosynostosis32.
Achondroplasia
Achondroplasia is an autosomal dominant skeletal dysplasia caused by a gain of function G380R mutation in FGFR3 on chromosome 433,34. This mutation alters bone growth resulting in obstruction or stenosis of the cranial skull base foramina. Increases in venous pressure secondary to stenosis of the jugular foramen can result in macrocephaly, ventriculomegaly and hydrocephalus33,34,35. The etiology of hydrocephalus in achondroplasia is likely multifactorial, with contributions from alterations in CSF flow at the foramen magnum, venous outflow alterations, and potentially decreased CSF egress along cranial nerve sheaths due to narrowing of their respective foramina 34,36,37.
Many patients with achondroplasia have some degree of ventriculomegaly which stabilizes overtime 37,38. As the natural history of this ventriculomegaly and the contributions from stenosis at the foramen magnum and cervical medullary junction is better understood, there are far fewer patients who receive treatment with ventriculoperitoneal shunting, which in the past was associated with significant complications39. Some patients are successfully treated with cervicomedullary decompression, which is associated with decreased need for shunting and stabilization of both ventriculomegaly and intracranial pressure40,37.
NF 1 and NF 2
Neurofibromatosis 1 (NF1), also known as von Recklinghausen’s disease, is an autosomal dominant disorder caused by mutations on chromosome 17q11.2 that affect neurofibromin production41,42. NF 1 has variable expression and predisposes individuals to several tumors and hamartomas in the central and peripheral nervous system, including optic pathway gliomas (OPG) and gliomas outside the optic pathway, which in some cases can lead to obstructive hydrocephalus (Fig. 2) 43,44,45,46. Hydrocephalus in NF-1 can occur from alterations in CSF circulation that results from OPGs, as well as periaqueductal gliosis, aqueductal webs, nontumor hamartomatous changes, and tectal and midbrain tumors causing obstruction or narrowing of the ventricular system, particularly at the level of the cerebral aqueduct.45,47. Treatment may involve the tumor directly, or in some cases, surgical treatment for symptomatic hydrocephalus. 42,47. Direct treatment of a tumor may involve surgical removal or treatment with chemotherapy including BRAF/MEK inhibitors 47. Finally, treatment of NF-1 associated hydrocephalus with ETV can be successful regardless of the type or level of obstruction48.
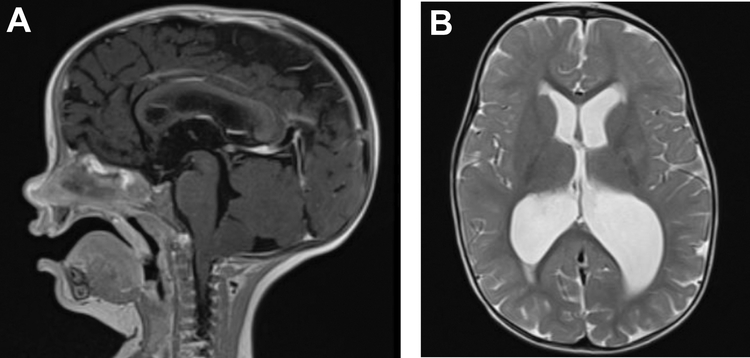
Figure 2.
14-month-old with NF-1 presented with enlarged ventricles, transependymal CSF, and low tonsil position. Patient underwent Chiari decompression and subsequent treatment with ETV. T2-weighted MRI (A) sagittal and (B) axial views
Neurofibromatosis 2 (NF2), another autosomal dominant disorder, is caused by deletions or loss of function of the tumor suppressor gene on chromosome 22q12 that encodes the protein Merlin49,50. Compared to NF1 with a prevalence of 1 in 4,000–5,000 births, NF2 is more rare with an incidence of 1 in 40,000 births43,51. Patients with NF2 can develop bilateral vestibular schwannomas or other intracranial tumors that can rarely cause hydrocephalus through obstruction of CSF circulation50,52.
Down Syndrome
There have been several case reports of hydrocephalus in patients with Down syndrome. Two early reports from the 1970s describe children with Down syndrome and hydrocephalus, aqueductal stenosis, and partial agenesis of the corpus callosum53,54. A more recent case report describes a patient with hydrocephalus detected during pregnancy who required treatment with ventriculoperitoneal shunt55. Two additional case reports describe two patients with Down syndrome and normal pressure hydrocephalus, both of whom were treated with ventriculoperitoneal shunts56. The second report describes a patient with tetraventricular hydrocephalus caused by obstruction of the foramina of Luschka and Magendie treated using ETV57.
Although Down syndrome is not associated with hydrocephalus, mouse models have demonstrated ventriculomegaly related to the genetic abnormalities in Down syndrome. The Ts1Rhr mouse model, with a shorter trisomic segment than previous models, exhibits PcP4-dependent ciliopathy sufficient to trigger ventricular enlargement58. Thus, it is possible that despite their varying presentations and treatment, some of the reported human hydrocephalus cases may be related to trisomy 21 itself rather than non-syndromic findings. Finally, there is an increased prevalence of ventriculomegaly in very low birthweight (VLBW) infants with Down syndrome compared to other VLBW infants, suggesting that some Down Syndrome-related pathologic process may contribute to the development of hydrocephalus or ventriculomegaly in a subset of patients with this condition59.
Tuberous Sclerosis
Tuberous sclerosis complex (TSC) is an autosomal dominant genetic syndrome with dysfunction of the mTOR pathway resulting in cortical and subcortical tubers, subependymal nodules along the lateral ventricles, and subependymal giant cell astrocytomas (SEGAs) 60,61. SEGAs are associated with obstructive hydrocephalus in the first two decades of life, and are monitored for growth using frequent, serial imaging in patients with TSC60,61. Though they grow slowly, SEGAs can cause obstructive hydrocephalus secondary to blockage of CSF circulation at the Foramen of Monro.62.
Management of hydrocephalus in these cases depends on management of the SEGAs themselves. Treatment strategies include surgical resection or mTOR inhibition. Early surgical resection was once the standard for management of SEGAs that showed growth on serial imaging, and surgery was curative for small lesions, with low complication rates63. However for larger or bilateral lesions, complication rates were higher, and in many patients with both SEGA and hydrocephalus, VPS was still necessary63,64. More recently, medical management using mTOR inhibitors has been used alone or in combination with surgical resection, though some concerns remain about its long-term safety considering effects on linear growth, puberty, and immunosuppression65,66,67,68. In patients with SEGA and asymptomatic obstructive hydrocephalus, mTOR inhibition is effective in treating hydrocephalus even in the setting of acute, symptomatic increases in intracranial pressure, though surgery is often still considered in these cases66,69. Endoscopic tumor removal, laser interstitial thermal therapy (LITT), and biologics targeting of the MAPK/ERK pathway, may also be utilized in the treatment of SEGA and associated hydrocephalus in TSC patients70,71.
Walker-Warburg Syndrome/ Brain-muscle-eye disease
Walker-Warburg Syndrome (WWS), the most severe congenital muscular disorder, is caused by an autosomal recessive mutation that leads to type II Lissencephaly and severe hydrocephalus (Fig. 3)72,73. WWS is characterized by defective O-glycosylation of α-dextroglycan but the disease is highly heterogenous, as hypoglycosylation can occur in the Protein O-Mannosyltransferase 1 (POMT1) gene, present in 10–20% of patients, as well as in the POMT2, POMGNT1, FKTN, FKRP, LARGE, ISPD or other genes associated with dystroglycanopathy phenotypes73,74,75.
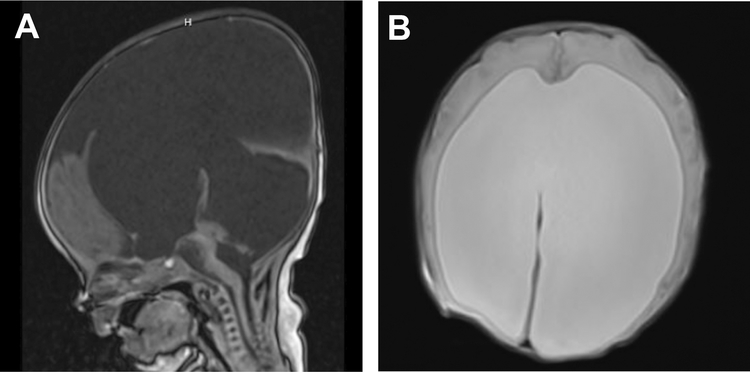
Figure 3.
1 day old with POMT-1 P273L Walker-Warburg syndrome, born at 35 weeks gestation. MRI A) sagittal (T1) and B) axial (T2) views showing hydrocephalus with tectal dysplasia contributing to aqueductal stenosis. This patient was treated with a VP shunt on day of life 4
While the incidence of WWS is rare, estimated at 1.2 per 100,000 births, many cases of WWS are complicated by hydrocephalus76,77,78. Similar to the clinical and genetic variability of WWS, the nature of the concurrent hydrocephalus is also variable. Hydrocephalus can be associated with tectal enlargement resulting in aqueductal stenosis as well as cobblestone cortex79. One patient with WWS presented with ventriculomegaly and a bulging third ventricle along with progressive hydrocephalus, while another WWS patient presented with macrocephaly, triventricular enlargement, hypoplasia of the corpus collosum, and obstructive hydrocephalus; both were treated with VP shunts77. Other case studies reported prenatal findings of posterior fossa anomalies and associated hydrocephalus with a POMT2 mutation confirming WWS, and three affected siblings with varying levels of fetal and congenital hydrocephalus, all with fatal prognosis 76,78. ETV-CPC was used to treat a patient with cobblestone lissencephaly, increased bulging of the anterior fontanelle and sutural separation, with a POMT1 mutation80.
It is important to note that the etiology of hydrocephalus in WWS is likely due to stenosis of the aqueduct secondary to altered brain development and an enlarged tectum. This differs from an aqueductal web, in which the brain stem is otherwise normal. Given the rates of hydrocephalus and abnormal brain development in WWS, close monitoring for hydrocephalus is warranted, although many patients present at birth with symptomatic hydrocephalus requiring treatment76. Given the early presentation, although many patients do have aqueductal stenosis, VP shunting may be more efficacious than ETV or ETV-CPC due to the young age at presentation.
Primary Ciliary Dyskinesia/Kartagener’s
Primary ciliary dyskinesia (PCD) is a rare, genetically heterogeneous syndrome associated with defects in cilia and flagella motility81,82. Many of the mutations associated with PCD affect the dynein axonemal heavy chain 5 or dynein axonemal intermediate chain 1 genes which encode the outer dynein arms of cilia83,84,85. PCD has a worldwide prevalence of 1 in 16,000 children86. Common manifestations include Kartagener’s syndrome (bronchiectasis, situs inversus, and chronic sinusitis), as well as neonatal respiratory distress, and male infertility86. While hydrocephalus is infrequently seen in humans with PCD, mouse models of this disease frequently develop severe symptomatic hydrocephalus82. Insertional mutations in the mouse axonemal dynein heavy chain gene, Mdnah5, cause loss of axonemal outer arms, and reproduce most of the classic features of PCD such as recurrent respiratory infections, situs inversus, and ciliary immobility87. In these mice, the mutation also causes hydrocephalus and death in the perinatal period and this mouse model suggest that the degree of ciliary dysfunction caused by the mutation is causally related to the severity of PCD and the development of hydrocephalus87. In both human and mouse models, functional loss of the Ccdc151 gene has also been associated with PCD, as this gene is expressed by ependymal cells and affects the ciliary axoneme. This mutation, too, is associated with hydrocephalus and perinatal death in mouse models88. The exact role of ciliary motion in the development of hydrocephalus has yet to be determined, including whether cilia are capable of producing or maintaining bulk flow of CSF throughout the ventricular system.
While there are case reports of hydrocephalus in Kartagener’s syndrome or PCD, in humans hydrocephalus is relatively infrequent 82,89,90,91,92. One case of PCD and hydrocephalus was associated with aqueductal stenosis and abnormal ultrastructure of the respiratory epithelium cilia93. Another report of autosomal recessively inherited PCD and intellectual disability in 3 generations of a Jordanian family notes that 4 of 9 affected individuals also had hydrocephalus94. Mutations in the multicilin gene (MCIDAS) are associated with choroid plexus hyperplasia and hydrocephalus in humans, possibly related to reduced generation of motile cilia95. Finally, mutations in FOXJ1, encoding a transcription factor involved in ciliogenesis, are associated with an autosomal dominant motile ciliopathy like PCD, with obstructive hydrocephalus in all reported cases. Hydrocephalus in these cases was associated with the inability to maintain patency, and resulting stenosis, of the aqueduct and/or foramina of Luschka/Magendie due to insufficient motile ciliary function96. In all affected individuals, pathological specimens showed mis-localized basal bodies and incorrect localization of adhesion proteins, leading to deficits in ciliary function and inadequate fluid flow96. Overall, while PCD is not consistently linked with hydrocephalus in humans, findings from mouse models and several human reports suggest that severe dysfunction of ciliary motility may contribute to hydrocephalus in some cases of PCD and related ciliopathies. Possible mechanisms for symptomatic hydrocephalus include stenosis of the aqueduct due to decreased flow of fluid across this channel or from collapse of the ependymal walls around the aqueduct due to changes in the integrity of the ependyma. Finally, as in other syndromes, the concurrent altered brain development may play a role in the development of hydrocephalus.
Osteogenesis Imperfecta
Osteogenesis Imperfecta (OI) is a group of disorders of connective tissue that affects bone growth and fragility, caused by defective COL1A1 and COL1A2 genes and is grouped into four types— Type II, type III, type IV and type I — ordered from most to least severe97,98. OI can be associated with macrocephaly, hydrocephalus, basilar invagination and cerebral atrophy99. Concurrent basilar invagination can lead to obstructive hydrocephalus. While the true incidence of hydrocephalus in OI is not well known, several studies and case reports have documented OI with associated hydrocephalus99,100.
In one study, OI was associated with sulcal prominence and hydrocephalus with no clear intraventricular obstruction in 22% of patients99. In another study of 5 neonates with OI, 4 had hydrocephalus associated with foramen magnum stenosis, cerebral parenchymal and intraventricular hemorrhage, and occipital-bone fractures101. All 4 infants died soon after birth101. One other case reported a patient that presented with a novel mutation of the COL1A2 gene and was diagnosed with type II OI with obstructive hydrocephalus; in this case the patient was treated with ETV98.
Emerging Genetic Syndromes
Through the use of next generation sequencing, novel genetic mutations associated with hydrocephalus have been identified, accounting for many cases previously classified as sporadic or as “congenital hydrocephalus”3,102,103. It is unclear in all cases how these genetic alterations directly lead to hydrocephalus, however mechanisms beyond disruptions to CSF dynamics or structural barriers to CSF flow such as abnormal neuronal proliferation, differentiation, and maintenance may be involved in hydrocephalus102.
Pettigrew Syndrome
Pettigrew syndrome is an X-linked disorder, characterized by mutations in the AP1S2 gene that encodes a subunit of the AP1 adaptin protein essential in regulating lysosomal protein sorting104. Mutations lead to intellectual disabilities, iron and calcium deposition, and hydrocephalus3. The related Lethal giant larva (Lgl) protein is involved in maintaining cell polarity in mice. Loss of this protein in Lgl1−/− mice pups leads to severe hydrocephalus and neonatal death105.
RASopathies
Various RASopathies, through mutations in the RAS-MAPK pathway, have been associated with hydrocephalus; among these disorders are NF1, discussed previously, Costello syndrome, Noonan syndrome and cardio-facio-cutaneous (CFC) syndrome, all with autosomal dominant inheritance3. Noonan syndrome is associated with mutations in CBL (regulators of activated TRKs), KRAS, NRAS (RAS proteins), PTPN11, SOS1, SHOC2 (modulators of RAS function) and RAF1 (downstream signal transducers); it is also associated with hydrocephalus in addition to hindbrain herniation and syringomyelia106,107. Costello syndrome is caused by mutations in the HRAS protooncogene that lead to failure-to-thrive, along with macrocephaly, posterior fossa crowding, low cerebellar tonsil position and hydrocephalus108. CFC syndrome is most frequently caused by mutations in BRAF, but can occur due to mutations in MEK1, KRAS and MEK2 as well. This can lead to cervical stenosis, torticollis, Chiari malformation, and hydrocephalus109.
RAS has a multifaceted role in CNS development. Through its involvement in the RAS-ERK pathway, it is involved in the regulation and maintenance of neural stem cells, as well as in the regulation of oligodendrocyte differentiation110,111. Through its regulation of the PI3K-AKT pathway, RAS is also involved in synaptic plasticity112,113. Despite these interactions with CNS cell growth, differentiation, and maintenance, the role of RAS in CSF clearance and flow is not well characterized, and it is possible that structural deficits due to defective neurogenesis and differentiation contribute to hydrocephalus in these cases rather than direct effects on CSF flow.
PI3K-AKT-mTOR pathway
Four different mutations of genes in the PI3K-AKT-mTOR pathway, involved in cell proliferation, growth and function, have been shown to cause megalencephaly-associated symptoms, leading to hydrocephalus3. Mutations in the AKT3, CCND2 and PIK3R2 genes cause different types of Megalencephaly-polymicrogyria-polydactyly-hydrocephalus (MPPH), while mutations in the PIK3CA gene causes Megalencephaly-capillary-malformations (MCAP). Both MPPH and MCAP cause megalencephaly, polymicrogyria and ventriculomegaly that can lead to hydrocephalus114. PI3K pathway genes, including PIK3CA, PTEN, and MTOR contribute to neural stem cell growth, proliferation, and differentiation, especially in the developing ventricular zone, and mutations in these genes predispose patients to tumorigenesis and overgrowth syndromes115,103. As these syndromes have only recently been characterized, it remains to be seen whether therapeutics targeting affected molecular pathways will eventually aid in the treatment of hydrocephalus in these cases.
Discussion
Hydrocephalus occurs in the setting of many well-characterized syndromes of childhood. Treatment involves a combination of treating the primary pathology (e.g. tumors that cause outflow obstruction) and treatment for hydrocephalus with VPS and/or ETV +/− CPC. Next-generation sequencing has allowed for the characterization of novel genetic syndromes associated with hydrocephalus and the identification of mutations in sporadic cases of hydrocephalus. These mutations shed light on how alterations to development and proliferation of neurons and neural stem cells contribute to sporadic hydrocephalus, and may eventually contribute to our understanding of novel syndromic causes of hydrocephalus.
Four genes regulating neural stem cell fate have recently been described in congenital hydrocephalus102. TRIM71 loss is associated with defective neural tube closure, and decreased proliferation of neural progenitor cells (NPCs) in mouse models116. SMARCC1 encodes a subunit of a chromatin remodeling complex important to the survival of NPCs and transcriptional control of telencephalon development, and knockouts are associated with hydrocephalus and aqueductal stenosis117. PTCH1 is involved in the mechanism by which primary cilia in neuroepithelial cells sense and respond to SHH gradients, which is important in NPC differentiation and fate118. SHH encodes the ligand responsible for NPC migration along the dorsal-ventral axis of the neural tube119. TRIM71 mutations are associated with communicating hydrocephalus, while SMARCC1 and PTCH1 mutations are more likely to be associated with aqueductal stenosis. These mutations highlight that abnormal neurogenesis or brain development likely play a role in the development of hydrocephalus, beyond deficits in CSF accumulation or clearance102. In a follow-up study, mutations in the PI3K pathway genes previously discussed, as well as FOXJ1, FMN2, and FXYD2 were present in up to 22% of sporadic congenital hydrocephalus cases. Their involvement is likely related to their role in supporting embryonic neurogenesis103. These findings highlight that the dysregulation of neurogenesis, proliferation, or migration, especially in the ventricular zone, are emerging areas of interest that may contribute to CSF accumulation or disordered circulation in some cases of hydrocephalus.
Summary
Hydrocephalus is a common phenotype in various syndromes of childhood with diverse genetic etiologies. The mechanisms behind these disorders include structural deficits, mutations affecting neuronal adhesion, vesicle trafficking, growth factors, PI3K-AKT-mTOR pathway and Dystroglycanopathies, Ciliopathies and RASopathies3. The pathophysiology of syndromic hydrocephalus is multifactorial, and treatment is often multimodal, addressing both the underlying condition and the associated hydrocephalus. While the incidence or the underlying pathogenesis of hydrocephalus in certain conditions is not fully known, next generation genetic sequencing has begun to shed light on the complex underlying pathways affecting development of the brain which result in hydrocephalus.
KEY POINTS.
Hydrocephalus is a phenotypic feature associated with a diverse set of genetic syndromes in childhood
Pathogenesis and accompanying phenotypic features,as well as inheritance patterns,vary between and within syndromes
Next-generation sequencing studies now identify underlying genetic causes of hydrocephalus, previously categorized as “congenital hydrocephalus”.
SYNOPSIS.
Hydrocephalus, the abnormal accumulation and impaired circulation/clearance of cerebrospinal fluid, occurs as a common phenotypic feature of a diverse group of genetic syndromes. In this review we outline the genetic mutations, pathogenesis, and accompanying symptoms underlying syndromic hydrocephalus in the context of: L1 syndrome, syndromic craniosynostoses, achondroplasia, NF 1/2, Down’s syndrome, tuberous sclerosis, Walker-Warburg syndrome, primary ciliary dyskinesia, and osteogenesis imperfecta. Further, we discuss emerging genetic variants associated with syndromic hydrocephalus.
CLINICS CARE POINTS.
Management of syndromic hydrocephalus may require direct surgical treatment with VPS or ETV (±CPC) and/or treatment of associated pathology resulting in hydrocephalus such as tumors obstructing CSF flow.
Treatment options vary depending on a variety of patient- and syndrome-specific factors.
In sporadic cases of congenital hydrocephalus, genetic screening for recently described variants may eventually influence treatment decisions, though at this stage few pathway-specific therapeutics are available
Footnotes
DISCLOSURE STATEMENT
The Authors have nothing to disclose.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Schrander-Stumpel C, Fryns J-P Congenital hydrocephalus: Nosology and guidelines for clinical approach and genetic counselling. Eur J Pediatr. 1998;157(5):355–362. doi: 10.1007/s004310050830 [DOI] [PubMed] [Google Scholar]
- 2.Rekate HL. The definition and classification of hydrocephalus: a personal recommendation to stimulate debate. Cerebrospinal Fluid Res. 2008;5:2. doi: 10.1186/1743-8454-5-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kousi M, Katsanis N. TheGenetic Basis of Hydrocephalus. Annu Rev Neurosci. 2016;39(1):409–435. doi: 10.1146/annurev-neuro-070815-014023 [DOI] [PubMed] [Google Scholar]
- 4.Stumpel C, Vos YJ. Syndrome L1.In: Adam MP, Ardinger HH, Pagon RA, et al. ,eds.GeneReviews®. University of Washington, Seattle; 1993. Accessed January 10, 2021. http://www.ncbi.nlm.nih.gov/books/NBK1484/ [Google Scholar]
- 5.Adle-Biassette H, Saugier-Veber P, Fallet-Bianco C,et al. Neuropathological review of 138 cases genetically tested for X-linked hydrocephalus: evidence for closely related clinical entities of unknown molecular bases. Acta Neuropathol (Berl). 2013;126(3):427–442. doi: 10.1007/s00401-013-1146-1 [DOI] [PubMed] [Google Scholar]
- 6.Willems PJ, Brouwer OF, Dijkstra I, Wilmink J, Opitz JM, Reynolds JF. X-linkedhydrocephalus. Am J Med Genet. 1987;27(4):921–928. doi: 10.1002/ajmg.1320270419 [DOI] [PubMed] [Google Scholar]
- 7.Rosenthal A, Jouet M, Kenwrick S. Aberrant splicing of neural cell adhesion molecule L1mRNA in a family with X–linked hydrocephalus. Nat Genet. 1992;2(2):107–112.doi: 10.1038/ng1092-107 [DOI] [PubMed] [Google Scholar]
- 8.Emmert AS, Vuong SM, Shula C,et al. Characterization of a novel rat model of X-linked hydrocephalus by CRISPR-mediated mutation in L1cam. J Neurosurg. Published online February 8, 2019:1–14. doi: 10.3171/2018.10.JNS181015 [DOI] [PubMed] [Google Scholar]
- 9.Van Camp G, Vits L, Coucke P,et al. A duplication in the L1CAM gene associated with X–linked hydrocephalus. Nat Genet. 1993;4(4):421–425.doi: 10.1038/ng0893-421 [DOI] [PubMed] [Google Scholar]
- 10.Kong W, Wang X, Zhao J, Kang M, Xi N, Li S. A new frameshift mutation in L1CAM producing X-linked hydrocephalus. Mol Genet Genomic Med. 2020;8(1):e1031. doi: 10.1002/mgg3.1031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Guo D, Shi Y, Jian W,et al. A novel nonsense mutation in the L1CAM generesponsible for X-linked congenital hydrocephalus. J Gene Med. 2020;22(7):e3180. doi: 10.1002/jgm.3180 [DOI] [PubMed] [Google Scholar]
- 12.Wu Q, Sun L, Xu Y, Yang X, Sun S, Wang W. [Diagnosis of a fetus with X-linked hydrocephalus due to mutation of L1CAM gene]. Zhonghua Yi Xue Yi Chuan Xue Za Zhi Zhonghua Yixue Yichuanxue Zazhi Chin J Med Genet. 2019;36(9):897–900. doi: 10.3760/cma.j.issn.1003-9406.2019.09.011 [DOI] [PubMed] [Google Scholar]
- 13.Ferese R, Zampatti S, Griguoli AMP,et al. A New Splicing Mutation in the L1CAM Gene Responsible for X-Linked Hydrocephalus (HSAS). J Mol Neurosci MN. 2016;59(3):376–381. doi: 10.1007/s12031-016-0754-3 [DOI] [PubMed] [Google Scholar]
- 14.Tripolszki K, Sasaki E, Hotakainen R,et al. An X-linked syndrome with severe neurodevelopmental delay, hydrocephalus, and early lethality caused by a missense variation in the OTUD5 gene. Clin Genet. Published online November 1, 2020. doi: 10.1111/cge.13873 [DOI] [PubMed] [Google Scholar]
- 15.Alhousseini A, Zeineddine S, Husseini A,et al. Familial Hydrocephalus and Dysgenesis of the Corpus Callosum Associated with Xp22.33 Duplication and Stenosis of the Aqueduct of Sylvius with X-Linked Recessive Inheritance Pattern. Gynecol Obstet Invest. 2019;84(4):412–416. doi: 10.1159/000499505 [DOI] [PubMed] [Google Scholar]
- 16.Lin LO, Zhang RS, Hoppe IC,et al. Onset and Resolution of Chiari Malformations and Hydrocephalus in Syndromic Craniosynostosis following Posterior Vault Distraction. Plast Reconstr Surg. 2019;144(4):932–940. doi: 10.1097/PRS.0000000000006041 [DOI] [PubMed] [Google Scholar]
- 17.Cinalli G, Sainte-Rose C, Kollar EM,et al. Hydrocephalus and craniosynostosis. J Neurosurg. 1998;88(2):209–214. doi: 10.3171/jns.1998.88.2.0209 [DOI] [PubMed] [Google Scholar]
- 18.Collmann H, Sörensen N, Krauss J, Mühling J. Hydrocephalus in craniosynostosis. Childs Nerv Syst ChNS Off J Int Soc Pediatr Neurosurg. 1988;4(5):279–285. doi: 10.1007/BF00271924 [DOI] [PubMed] [Google Scholar]
- 19.Wang JC, Nagy L, Demke JC. Syndromic Craniosynostosis. Facial Plast Surg Clin N Am. 2016;24(4):531–543. doi: 10.1016/j.fsc.2016.06.008 [DOI] [PubMed] [Google Scholar]
- 20.Fearon JA, Rhodes J. Pfeiffer syndrome: a treatment evaluation. Plast Reconstr Surg. 2009;123(5):1560–1569. doi: 10.1097/PRS.0b013e3181a2057e [DOI] [PubMed] [Google Scholar]
- 21.Kilcoyne S, Potter KR, Gordon Z,et al. Feeding,Communication,Hydrocephalus,and Intracranial Hypertension in Patients With Severe FGFR2-Associated Pfeiffer Syndrome. J Craniofac Surg. 2021;32(1):134–140. doi: 10.1097/SCS.0000000000007153 [DOI] [PubMed] [Google Scholar]
- 22.Moore MH, Hanieh A. Hydrocephalus in pfeiffer syndrome. J Clin Neurosci Off J Neurosurg Soc Australas. 1994;1(3):202–204. doi: 10.1016/0967-5868(94)90030-2 [DOI] [PubMed] [Google Scholar]
- 23.Al-Namnam NM, Hariri F, Thong MK, Rahman ZA. Crouzon syndrome:Genetic and intervention review. J Oral Biol Craniofacial Res. 2019;9(1):37–39. doi: 10.1016/j.jobcr.2018.08.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Coll G, Arnaud E, Selek L,et al. The growth of the foramen magnum in Crouzon syndrome. Childs Nerv Syst ChNS Off J Int Soc Pediatr Neurosurg. 2012;28(9):1525–1535. doi: 10.1007/s00381-012-1805-x [DOI] [PubMed] [Google Scholar]
- 25.Hanieh A, Sheen R, David DJ. Hydrocephalus in Crouzon’s syndrome. Childs Nerv Syst ChNS Off J Int Soc Pediatr Neurosurg. 1989;5(3):188–189. doi: 10.1007/BF00272125 [DOI] [PubMed] [Google Scholar]
- 26.Abu-Sittah GS, Jeelani O, Dunaway D, Hayward R. Raised intracranial pressure in Crouzon syndrome: incidence, causes, and management. J Neurosurg Pediatr. 2016;17(4):469–475. doi: 10.3171/2015.6.PEDS15177 [DOI] [PubMed] [Google Scholar]
- 27.Wenger TL, Hing AV, Evans KN. Apert Syndrome. In: Adam MP, Ardinger HH, Pagon RA, et al. , eds. GeneReviews®. University of Washington, Seattle; 1993. Accessed January 10, 2021. http://www.ncbi.nlm.nih.gov/books/NBK541728/ [Google Scholar]
- 28.Ibrahimi OA, Chiu ES, McCarthy JG, Mohammadi M. Understanding the molecular basis of Apert syndrome. Plast Reconstr Surg. 2005;115(1):264–270. [PubMed] [Google Scholar]
- 29.Breik O, Mahindu A, Moore MH, Molloy CJ, Santoreneos S, David DJ. Apert syndrome:Surgical outcomes and perspectives. J Cranio-Maxillo-fac Surg Off Publ Eur Assoc Cranio-Maxillo-fac Surg. 2016;44(9):1238–1245. doi: 10.1016/j.jcms.2016.06.001 [DOI] [PubMed] [Google Scholar]
- 30.Munarriz PM, Pascual B, Castaño-Leon AM,et al. Apert syndrome: Cranial procedures and brain malformations in a series of patients. Surg Neurol Int. 2020;11:361. doi: 10.25259/SNI_413_2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kruszka P, Addissie YA, Agochukwu NB, Doherty ES, Muenke M.Muenke Syndrome. In: Adam MP, Ardinger HH, Pagon RA, et al. , eds. GeneReviews®. University of Washington, Seattle; 1993. Accessed February 10, 2021. http://www.ncbi.nlm.nih.gov/books/NBK1415/ [Google Scholar]
- 32.González-DelAngel A, Estandía-Ortega B, Alcántara-Ortigoza MA,et al. Expansion of the variable expression of Muenke syndrome: Hydrocephalus without craniosynostosis. Am J Med Genet A. 2016;170(12):3189–3196. doi: 10.1002/ajmg.a.37951 [DOI] [PubMed] [Google Scholar]
- 33.Baujat G, Legeai-Mallet L, Finidori G, Cormier-Daire V, LeMerrer M Achondroplasia. Best Pract Res Clin Rheumatol. 2008;22(1):3–18. doi: 10.1016/j.berh.2007.12.008 [DOI] [PubMed] [Google Scholar]
- 34.Bodensteiner JB. Neurological Manifestations of Achondroplasia. Curr Neurol Neurosci Rep. 2019;19(12):105. doi: 10.1007/s11910-019-1008-x [DOI] [PubMed] [Google Scholar]
- 35.Steinbok P, Hall J, Flodmark O. Hydrocephalus in achondroplasia: the possible role of intracranial venous hypertension. J Neurosurg. 1989;71(1):42–48. doi: 10.3171/jns.1989.71.1.0042 [DOI] [PubMed] [Google Scholar]
- 36.Cohen ME, Rosenthal AD, Matson DD. Neurological abnormalities in achondroplastic children. J Pediatr. 1967;71(3):367–376. doi: 10.1016/S0022-3476(67)80296-8 [DOI] [PubMed] [Google Scholar]
- 37.White KK, Bompadre V, Goldberg MJ,et al. Best practices in the evaluation and treatment of foramen magnum stenosis in achondroplasia during infancy. Am J Med Genet A. 2016;170(1):42–51. doi: 10.1002/ajmg.a.37394 [DOI] [PubMed] [Google Scholar]
- 38.Pierre-Kahn A, Hirsch JF, Renier D, Metzger J, Maroteaux P. Hydrocephalus and Achondroplasia. Pediatr Neurosurg. 1980;7(4):205–219. doi: 10.1159/000119948 [DOI] [PubMed] [Google Scholar]
- 39.King JAJ, Vachhrajani S, Drake JM, Rutka JT. Neurosurgical implications of achondroplasia. J Neurosurg Pediatr. 2009;4(4):297–306. doi: 10.3171/2009.3.PEDS08344 [DOI] [PubMed] [Google Scholar]
- 40.Kashanian A, Chan J, Mukherjee D, Pressman BD, Krakow D, Danielpour M. Improvement in ventriculomegaly following cervicomedullary decompressive surgery in children with achondroplasia and foramen magnum stenosis. Am J Med Genet A. 2020;182(8):1896–1905. doi: 10.1002/ajmg.a.61640 [DOI] [PubMed] [Google Scholar]
- 41.Jett K, Friedman JM. Clinical and genetic aspects of neurofibromatosis 1. Genet Med. 2010;12(1):1–11. doi: 10.1097/GIM.0b013e3181bf15e3 [DOI] [PubMed] [Google Scholar]
- 42.Roth J, Ber R, Constantini S. Neurofibromatosis Type 1-Related Hydrocephalus: Treatment Options and Considerations. World Neurosurg. 2019;128:e664–e668. doi: 10.1016/j.wneu.2019.04.231 [DOI] [PubMed] [Google Scholar]
- 43.Ferner RE. Neurofibromatosis 1. EurJ Hum Genet. 2007;15(2):131–138. doi: 10.1038/sj.ejhg.5201676 [DOI] [PubMed] [Google Scholar]
- 44.Tonsgard JH. Clinical Manifestations and Management of Neurofibromatosis Type 1. Semin Pediatr Neurol. 2006;13(1):2–7. doi: 10.1016/j.spen.2006.01.005 [DOI] [PubMed] [Google Scholar]
- 45.Glombova M, Petrak B, Lisy J, Zamecnik J, Sumerauer D, Liby P. Brain gliomas, hydrocephalus and idiopathic aqueduct stenosis in children with neurofibromatosis type 1. Brain Dev. 2019;41(8):678–690. doi: 10.1016/j.braindev.2019.04.003 [DOI] [PubMed] [Google Scholar]
- 46.Tanrikulu B, Özek MM. Neurofibromatosis and Hydrocephalus. In: Cinalli G, Özek MM, Sainte-Rose C, eds. Pediatric Hydrocephalus. Springer International Publishing; 2019:1107–1118. doi: 10.1007/978-3-319-27250-4_65 [DOI] [Google Scholar]
- 47.Roth J, Constantini S, Cinalli G. Neurofibromatosis type 1-related hydrocephalus: causes and treatment considerations. Childs Nerv Syst ChNS Off J Int Soc Pediatr Neurosurg. 2020;36(10):2385–2390. doi: 10.1007/s00381-020-04719-y [DOI] [PubMed] [Google Scholar]
- 48.Dinçer A, Yener U, Özek MM. Hydrocephalus in Patients with Neurofibromatosis Type 1:MR Imaging Findings and the Outcome of Endoscopic Third Ventriculostomy. Am J Neuroradiol. 2011;32(4):643–646. doi: 10.3174/ajnr.A2357 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Gutmann DH, Aylsworth A, Carey JC,et al. The Diagnostic Evaluation and Multidisciplinary Management of Neurofibromatosis 1 and Neurofibromatosis 2. JAMA. 1997;278(1):51–57. doi: 10.1001/jama.1997.03550010065042 [DOI] [PubMed] [Google Scholar]
- 50.Petrilli AM, Fernández-Valle C. Role of Merlin/NF2 inactivation in tumor biology. Oncogene. 2016;35(5):537–548. doi: 10.1038/onc.2015.125 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Cinalli G, Maixner WJ, Sainte-Rose C.Pediatric Hydrocephalus. Springer Science & Business Media;2012. [Google Scholar]
- 52.Dirks PB. Genetics of Hydrocephalus. In: Cinalli G, Sainte-Rose C, Maixner WJ,eds. Pediatric Hydrocephalus. Springer; Milan; 2005:1–17. doi: 10.1007/978-88-470-2121-1_1 [DOI] [Google Scholar]
- 53.Jayaraman A, Ballweg GP, Donnenfeld H, Chusid JG. Hydrocephalus in Down’ssyndrome. Childs Brain. 1976;2(3):202–207. [PubMed] [Google Scholar]
- 54.Zadikoff C Down’s syndrome with hydrocephalus treated by compressive head binding. South Afr Med J Suid-Afr Tydskr Vir Geneeskd. 1977;51(11):353–355. [PubMed] [Google Scholar]
- 55.Forcelini CM, Mallmann AB, Crusius PS,et al. Down syndrome with congenital hydrocephalus: case report. Arq Neuropsiquiatr. 2006;64(3B):869–871. doi: 10.1590/s0004-282x2006000500031 [DOI] [PubMed] [Google Scholar]
- 56.Marano M, Pompucci A, Motolese F,et al. Normal Pressure Hydrocephalus in Down Syndrome: The Report of Two Cases. J Alzheimers Dis JAD. 2020;77(3):979–984. doi: 10.3233/JAD-200409 [DOI] [PubMed] [Google Scholar]
- 57.Orlando V, Spennato P, De Liso M, Trischitta V, Imperato A, Cinalli G. Fourth Ventricle Outlet Obstruction and Diverticular Enlargement of Luschka Foramina in a Child with Down Syndrome. Pediatr Neurosurg. Published online December 28, 2020:1–4. doi: 10.1159/000511088 [DOI] [PubMed] [Google Scholar]
- 58.Raveau M, Nakahari T, Asada S,et al. Brain ventriculomegaly in Down syndrome mice is caused by Pcp4 dose-dependent cilia dysfunction. Hum Mol Genet. 2017;26(5):923–931. doi: 10.1093/hmg/ddx007 [DOI] [PubMed] [Google Scholar]
- 59.Movsas TZ, Spitzer AR, Gewolb IH. Ventriculomegaly in very-low-birthweight infants with Down syndrome. Dev Med Child Neurol. 2016;58(11):1167–1171. doi: 10.1111/dmcn.13191 [DOI] [PubMed] [Google Scholar]
- 60.Lu DS, Karas PJ, Krueger DA, Weiner HL. Central nervous system manifestations of tuberous sclerosis complex. Am J Med Genet C Semin Med Genet. 2018;178(3):291–298. doi: 10.1002/ajmg.c.31647 [DOI] [PubMed] [Google Scholar]
- 61.Hsieh DT, Whiteway SL, Rohena LO, Thiele EA. Tuberous sclerosis complex. NeurolClin Pract. 2016;6(4):339–347. doi: 10.1212/CPJ.0000000000000260 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Roth J, Roach ES, Bartels U,et al. Subependymal giant cell astrocytoma: diagnosis, screening, and treatment. Recommendations from the International Tuberous Sclerosis Complex Consensus Conference 2012. Pediatr Neurol. 2013;49(6):439–444. doi: 10.1016/j.pediatrneurol.2013.08.017 [DOI] [PubMed] [Google Scholar]
- 63.Kotulska K, Borkowska J, Roszkowski M,et al. Surgical treatment of subependymal giant cell astrocytoma in tuberous sclerosis complex patients. Pediatr Neurol. 2014;50(4):307–312. doi: 10.1016/j.pediatrneurol.2013.12.004 [DOI] [PubMed] [Google Scholar]
- 64.Fohlen M, Ferrand-Sorbets S, Delalande O, Dorfmüller G. Surgery for subependymal giant cell astrocytomas in children with tuberous sclerosis complex. Childs Nerv Syst ChNS Off J Int Soc Pediatr Neurosurg. 2018;34(8):1511–1519. doi: 10.1007/s00381-018-3826-6 [DOI] [PubMed] [Google Scholar]
- 65.Józwiak S, Nabbout R, Curatolo P, participants of the TSC Consensus Meeting for SEGA and Epilepsy Management. Management of subependymal giant cell astrocytoma (SEGA) associated with tuberous sclerosis complex (TSC): Clinical recommendations. Eur J Paediatr Neurol EJPN Off J Eur Paediatr Neurol Soc. 2013;17(4):348–352. doi: 10.1016/j.ejpn.2012.12.008 [DOI] [PubMed] [Google Scholar]
- 66.Ebrahimi-Fakhari D, Franz DN. Pharmacological treatment strategies for subependymal giant cell astrocytoma (SEGA). Expert Opin Pharmacother. 2020;21(11):1329–1336. doi: 10.1080/14656566.2020.1751124 [DOI] [PubMed] [Google Scholar]
- 67.Giordano F, Moscheo C, Lenge M,et al. Neurosurgical treatment of subependymal giant cell astrocytomas in tuberous sclerosis complex: a series of 44 surgical procedures in 31 patients. Childs Nerv Syst ChNS Off J Int Soc Pediatr Neurosurg. 2020;36(5):951–960.doi: 10.1007/s00381-019-04449-w [DOI] [PubMed] [Google Scholar]
- 68.Somers MJG, Paul E. Safety considerations of mammalian target of rapamycin inhibitors in tuberous sclerosis complex and renal transplantation. J Clin Pharmacol. 2015;55(4):368–376. doi: 10.1002/jcph.428 [DOI] [PubMed] [Google Scholar]
- 69.Weidman DR, Palasamudram S, Zak M,et al. The effect of mTOR inhibition on obstructive hydrocephalus in patients with tuberous sclerosis complex (TSC) related subependymal giant cell astrocytoma (SEGA). J Neurooncol. 2020;147(3):731–736. doi: 10.1007/s11060-020-03487-8 [DOI] [PubMed] [Google Scholar]
- 70.Frassanito P, Noya C, Tamburrini G. Current trends in the management of subependymal giant cell astrocytomas in tuberous sclerosis. Childs Nerv Syst ChNS Off J Int Soc Pediatr Neurosurg. 2020;36(10):2527–2536. doi: 10.1007/s00381-020-04889-9 [DOI] [PubMed] [Google Scholar]
- 71.Bongaarts A, van Scheppingen J, Korotkov A,et al. The coding and non-coding transcriptional landscape of subependymal giant cell astrocytomas. Brain J Neurol. 2020;143(1):131–149. doi: 10.1093/brain/awz370 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Dobyns WB, Pagon RA, Armstrong D,et al. Diagnostic criteria for Walker-Warburg syndrome. Am J Med Genet. 1989;32(2):195–210. doi: 10.1002/ajmg.1320320213 [DOI] [PubMed] [Google Scholar]
- 73.Vajsar J, Schachter H. Walker-Warburg syndrome. Orphanet J Rare Dis. 2006;1(1):29. doi: 10.1186/1750-1172-1-29 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Reeuwijk J van, Janssen M, Elzen C van den, et al. POMT2 mutations cause α-dystroglycan hypoglycosylation and Walker-Warburg syndrome. J Med Genet. 2005;42(12):907–912. doi: 10.1136/jmg.2005.031963 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Tully HM, Dobyns WB. Infantile hydrocephalus: A review of epidemiology, classification and causes. Eur J Med Genet. 2014;57(8):359–368. doi: 10.1016/j.ejmg.2014.06.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Rodgers BL, Vanner LV, Pai GS, Sens MA. Walker-Warburg syndrome: Report of three affected sibs. Am J Med Genet. 1994;49(2):198–201. doi: 10.1002/ajmg.1320490207 [DOI] [PubMed] [Google Scholar]
- 77.Preuss M, Heckmann M, Stein M, Nestler U. Two Cases of Walker-Warburg Syndrome Complicated by Hydrocephalus. Pediatr Neurosurg. 2010;46(1):34–38. doi: 10.1159/000314999 [DOI] [PubMed] [Google Scholar]
- 78.Brasseur-Daudruy M, Vivier PH, Ickowicz V, Eurin D, Verspyck E. Walker-Warburg syndrome diagnosed by findings of typical ocular abnormalities on prenatal ultrasound. Pediatr Radiol. 2012;42(4):488–490. doi: 10.1007/s00247-011-2242-9 [DOI] [PubMed] [Google Scholar]
- 79.Alharbi S, Alhashem A, Alkuraya F, Kashlan F, Tlili-Graiess K. Neuroimaging manifestations and genetic heterogeneity of Walker-Warburg syndrome in Saudi patients. Brain Dev. 2021;43(3):380–388. doi: 10.1016/j.braindev.2020.10.012 [DOI] [PubMed] [Google Scholar]
- 80.Tanaka T, Harris CJ, Barnett SS, Litofsky NS. A Successful Treatment of Endoscopic Third Ventriculostomy with Choroid Plexus Cauterization for Hydrocephalus in Walker-Warburg Syndrome. Case Rep Neurol Med. 2016;2016:7627289. doi: 10.1155/2016/7627289 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Leigh MW, Pittman JE, Carson JL,et al. Clinical and genetic aspects of primary ciliary dyskinesia/Kartagener syndrome. Genet Med. 2009;11(7):473–487. doi: 10.1097/GIM.0b013e3181a53562 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Lee L Riding the wave of ependymal cilia: genetic susceptibility to hydrocephalus in primary ciliary dyskinesia. J Neurosci Res. 2013;91(9):1117–1132.doi: 10.1002/jnr.23238 [DOI] [PubMed] [Google Scholar]
- 83.Guichard C, Harricane M-C, Lafitte J-J,et al. Axonemal Dynein Intermediate-Chain Gene(DNAI1) Mutations Result in Situs Inversus and Primary Ciliary Dyskinesia (Kartagener Syndrome). Am J Hum Genet. 2001;68(4):1030–1035. doi: 10.1086/319511 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Zariwala M, Noone PG, Sannuti A, et al. Germline Mutations in an Intermediate Chain Dynein Cause Primary Ciliary Dyskinesia. Am J Respir Cell Mol Biol. 2001;25(5):577–583. doi: 10.1165/ajrcmb.25.5.4619 [DOI] [PubMed] [Google Scholar]
- 85.Omran H, Häffner K, Völkel A,et al. Homozygosity Mapping of a Gene Locus for Primary Ciliary Dyskinesia on Chromosome 5p and Identification of the Heavy Dynein Chain DNAH5 as a Candidate Gene. Am J Respir Cell Mol Biol. 2000;23(5):696–702. doi: 10.1165/ajrcmb.23.5.4257 [DOI] [PubMed] [Google Scholar]
- 86.Lee L Mechanisms of mammalian ciliary motility: Insights from primary ciliary dyskinesia genetics. Gene. 2011;473(2):57–66. doi: 10.1016/j.gene.2010.11.006 [DOI] [PubMed] [Google Scholar]
- 87.Ibañez-Tallon I, Gorokhova S, Heintz N. Loss of function of axonemal dynein Mdnah5 causes primary ciliary dyskinesia and hydrocephalus. Hum Mol Genet. 2002;11(6):715–721. doi: 10.1093/hmg/11.6.715 [DOI] [PubMed] [Google Scholar]
- 88.Chiani F, Orsini T, Gambadoro A,et al. Functional loss of Ccdc151 leads to hydrocephalus in a mouse model of primary ciliary dyskinesia. Dis Model Mech. 2019;12(8). doi: 10.1242/dmm.038489 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Greenstone MA, Jones RW, Dewar A, Neville BG, Cole PJ. Hydrocephalus and primary ciliary dyskinesia. Arch Dis Child. 1984;59(5):481–482. doi: 10.1136/adc.59.5.481 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Jabourian Z, Lublin FD, Adler A, Gonzales C, Northrup B, Zwillenberg D. Hydrocephalus in Kartagener’s syndrome. Ear Nose Throat J. 1986;65(10):468–472. [PubMed] [Google Scholar]
- 91.Santi MMD, Magni A, Valletta EA, Gardi C, Lungarella G. Hydrocephalus, bronchiectasis, and ciliary aplasia. Arch Dis Child. 1990;65(5):543–544. doi: 10.1136/adc.65.5.543 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Picco P, Leveratto L, Cama A,et al. Immotile cilia syndrome associated with hydrocephalus and precocious puberty: a case report. Eur J Pediatr Surg Off J Austrian Assoc Pediatr Surg Al Z Kinderchir. 1993;3 Suppl 1:20–21. [PubMed] [Google Scholar]
- 93.Vieira JP, Lopes P, Silva R. Primary ciliary dyskinesia and hydrocephalus with aqueductal stenosis. J Child Neurol. 2012;27(7):938–941. doi: 10.1177/0883073811429856 [DOI] [PubMed] [Google Scholar]
- 94.al-Shroof M, Karnik AM, Karnik AA, Longshore J, Sliman NA, Khan FA. Ciliary dyskinesia associated with hydrocephalus and mental retardation in a Jordanian family. Mayo Clin Proc. 2001;76(12):1219–1224. doi: 10.4065/76.12.1219 [DOI] [PubMed] [Google Scholar]
- 95.Robson EA, Dixon L, Causon L,et al. Hydrocephalus and diffuse choroid plexus hyperplasia in primary ciliary dyskinesia-related MCIDAS mutation. Neurol Genet. 2020;6(4):e482. doi: 10.1212/NXG.0000000000000482 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Wallmeier J, Frank D, Shoemark A,et al. De Novo Mutations in FOXJ1 Result in a Motile Ciliopathy with Hydrocephalus and Randomization of Left/Right Body Asymmetry. Am J Hum Genet. 2019;105(5):1030–1039. doi: 10.1016/j.ajhg.2019.09.022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Cole DE, Carpenter TO. Bone fragility, craniosynostosis, ocular proptosis, hydrocephalus, and distinctive facial features: a newly recognized type of osteogenesis imperfecta. J Pediatr. 1987;110(1):76–80. doi: 10.1016/s0022-3476(87)80292-5 [DOI] [PubMed] [Google Scholar]
- 98.Hachiya Y, Hayashi M, Negishi T, Atsumi S, Kubota M, Nishihara T. A case of osteogenesis imperfecta type II caused by a novel COL1A2 gene mutation: endoscopic third ventriculostomy to prevent hydrocephalus. Neuropediatrics. 2012;43(4):225–228. doi: 10.1055/s-0032-1324405 [DOI] [PubMed] [Google Scholar]
- 99.Charnas LR, Marini JC. Communicating hydrocephalus, basilar invagination, and other neurologic features in osteogenesis imperfecta. Neurology. 1993;43(12):2603–2608. doi: 10.1212/wnl.43.12.2603 [DOI] [PubMed] [Google Scholar]
- 100.Sasaki-Adams D, Kulkarni A, Rutka J, Dirks P, Taylor M, Drake JM. Neurosurgical implications of osteogenesis imperfecta in children: Report of 4 cases. J Neurosurg Pediatr. 2008;1(3):229–236. doi: 10.3171/PED/2008/1/3/229 [DOI] [PubMed] [Google Scholar]
- 101.Knisely AS, Frates RE, Ambler MW, Singer DB. Hydrocephalus of intrauterine onset in perinatally lethal osteogenesis imperfecta: clinical, sonographic, and pathologic correlations. Pediatr Pathol. 1988;8(4):367–376. doi: 10.3109/15513818809041570 [DOI] [PubMed] [Google Scholar]
- 102.Furey CG, Choi J, Jin SC, et al. De Novo Mutation in Genes Regulating Neural Stem Cell Fate in Human Congenital Hydrocephalus. Neuron. 2018;99(2):302–314.e4. doi: 10.1016/j.neuron.2018.06.019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Jin SC, Dong W, Kundishora AJ, et al. Exome sequencing implicates genetic disruption of prenatal neuro-gliogenesis in sporadic congenital hydrocephalus. Nat Med. 2020;26(11):1754–1765. doi: 10.1038/s41591-020-1090-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Reusch U, Bernhard O, Koszinowski U, Schu P. AP-1A and AP-3A Lysosomal Sorting Functions. Traffic. 2002;3(10):752–761. doi: 10.1034/j.1600-0854.2002.31007.x [DOI] [PubMed] [Google Scholar]
- 105.Klezovitch O, Fernandez TE, Tapscott SJ, Vasioukhin V. Loss of cell polarity causes severe brain dysplasia in Lgl1 knockout mice. Genes Dev. 2004;18(5):559–571. doi: 10.1101/gad.1178004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Heye N, Dunne JW. Noonan’s syndrome with hydrocephalus, hindbrain herniation, and upper cervical intracord cyst. J Neurol Neurosurg Psychiatry. 1995;59(3):338–339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Roberts AE, Allanson JE, Tartaglia M, Gelb BD. Noonan syndrome. The Lancet. 2013;381(9863):333–342. doi: 10.1016/S0140-6736(12)61023-X [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Gripp KW, Hopkins E, Doyle D, Dobyns WB. High incidence of progressive postnatal cerebellar enlargement in Costello syndrome: Brain overgrowth associated with HRAS mutations as the likely cause of structural brain and spinal cord abnormalities. Am J Med Genet A. 2010;152A(5):1161–1168. doi: 10.1002/ajmg.a.33391 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Reinker KA, Stevenson DA, Tsung A. Orthopaedic Conditions in Ras/MAPK Related Disorders. J Pediatr Orthop. 2011;31(5):599–605. doi: 10.1097/BPO.0b013e318220396e [DOI] [PubMed] [Google Scholar]
- 110.Campos LS, Leone DP, Relvas JB, et al. Beta1 integrins activate a MAPK signalling pathway in neural stem cells that contributes to their maintenance. Dev Camb Engl. 2004;131(14):3433–3444. doi: 10.1242/dev.01199 [DOI] [PubMed] [Google Scholar]
- 111.Fressinaud C, Vallat JM, Labourdette G. Basic fibroblast growth factor down-regulates myelin basic protein gene expression and alters myelin compaction of mature oligodendrocytes in vitro. J Neurosci Res. 1995;40(3):285–293. doi: 10.1002/jnr.490400302 [DOI] [PubMed] [Google Scholar]
- 112.Kim J-I, Lee H-R, Sim S, et al. PI3Kγ is required for NMDA receptor-dependent long-term depression and behavioral flexibility. Nat Neurosci. 2011;14(11):1447–1454. doi: 10.1038/nn.2937 [DOI] [PubMed] [Google Scholar]
- 113.Choi J-H, Park P, Baek G-C, et al. Effects of PI3Kγ overexpression in the hippocampus on synaptic plasticity and spatial learning. Mol Brain. 2014;7:78. doi: 10.1186/s13041-014-0078-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Mirzaa GM, Conway RL, Gripp KW, et al. Megalencephaly-capillary malformation (MCAP) and megalencephaly-polydactyly-polymicrogyria-hydrocephalus (MPPH) syndromes: Two closely related disorders of brain overgrowth and abnormal brain and body morphogenesis. Am J Med Genet A. 2012;158A(2):269–291. doi: 10.1002/ajmg.a.34402 [DOI] [PubMed] [Google Scholar]
- 115.Li L, Liu F, Ross AH. PTEN regulation of neural development and CNS stem cells. J Cell Biochem. 2003;88(1):24–28. doi: 10.1002/jcb.10312 [DOI] [PubMed] [Google Scholar]
- 116.Chen J, Lai F, Niswander L. The ubiquitin ligase mLin41 temporally promotes neural progenitor cell maintenance through FGF signaling. Genes Dev. 2012;26(8):803–815. doi: 10.1101/gad.187641.112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Narayanan R, Pirouz M, Kerimoglu C, et al. Loss of BAF (mSWI/SNF) Complexes Causes Global Transcriptional and Chromatin State Changes in Forebrain Development. Cell Rep. 2015;13(9):1842–1854. doi: 10.1016/j.celrep.2015.10.046 [DOI] [PubMed] [Google Scholar]
- 118.Palma V, Ruiz i Altaba A. Hedgehog-GLI signaling regulates the behavior of cells with stem cell properties in the developing neocortex. Dev Camb Engl. 2004;131(2):337–345. doi: 10.1242/dev.00930 [DOI] [PubMed] [Google Scholar]
- 119.Lupo G, Harris WA, Lewis KE. Mechanisms of ventral patterning in the vertebrate nervous system. Nat Rev Neurosci. 2006;7(2):103–114. doi: 10.1038/nrn1843 [DOI] [PubMed] [Google Scholar]