Abstract
Oxidative stress has a major role in disease pathogenesis. However, limited studies have investigated the effect of various sample collection tubes on oxidative biomarkers. The present study aimed to evaluate the effect of different collection tubes on the variation of malondialdehyde (MDA), nitric oxide (NO), total thiol (t-SH), and ferric reducing ability of plasma (FRAP) levels. A total of 35 individuals participated in this study and each collected sample was separated into three different tubes: glass tubes (GTs), plain plastic tubes (PTs), and gel separator tubes (GSTs). The results of PTs and GSTs were compared to those of GTs as the reference tube. The comparison between the means of biomarkers in various tubes indicated that there was no significant difference in MDA results between tubes. In contrast, t-SH and NO content were significantly decreased in GSTs and PTs compared to GTs. However, the Bland-Altman analysis showed an acceptable concordance for the mentioned analytes and the statistically significant differences were not clinically significant for NO, MDA, and t-SH antioxidant parameters. Moreover, the FRAP level was considerably lower in GSTs compared to GTs. Nevertheless, the Bland-Altman analysis showed a high bias percentage for the FRAP assay when using PTs and GSTs. According to the present results, it can be concluded that switching to plastic blood collection tubes or serum separation tubes could influence the FRAP results. However, there was no interference for the interpretation of other antioxidant assays in different types of collection tubes. Hence, it is suggested to use GTs for total antioxidant capacity evaluations, especially the FRAP assay.
Introduction
Blood collection is a critical step in pre-analytical laboratory testing and plays an integral role in the accuracy of the results. Previously, the most prevalent tubes used for blood collection were glass ones. However, they were replaced by plastic tubes which are usually made up of materials such as polyesters, polyolefins, polysiloxane, polyacrylonitrile, polyacrylic, polyvinyl chloride, polytetrafluoroethylene, and polystyrene [1, 2]. Since these substances are hydrophobic, the activation of the coagulation process is postponed in these tubes, and additives, e.g., polymer gels, clot activators, and surfactants are employed to resolve this issue [1]. Clot activators are composed of inorganic silicate or substances such as thromboplastin, thrombin, and ellagic acid. However, it is likely that these substances may not sediment with the clot after centrifugation, stay in serum, and cause interference with various assays [2–5]. Moreover, surfactants are usually fabricated from polymers that are soluble in water and it has been demonstrated that some biochemical factors such as thyroid hormones are altered in the presence of surfactants [2, 6–9]. Nevertheless, plastic tubes are still widely employed in laboratories owing to their ease of use, ability to increase serum volume, and reduced risk of contamination [10–12]. Despite these advantages, there are controversies around the precision of laboratory tests on samples gathered in different kinds of tubes, and several studies have proposed alternations in the concentration of biochemical analytes in plastic tubes versus glass ones. It has been established that tubes containing separator gels are not only able to absorb hydrophobic compounds such as specific drugs but also release an oily film to serum under extreme temperature because of their instability, which clogs the instrument probe in the analytical step [13]. Consequently, it has been suggested that serum/plasma separator tubes may have a slight analytical effect on several assays including hormonal, drug monitoring, and immunoassay tests because total triiodothyronine (TT3) and total thyroxine (TT4) concentrations are increased in plain red tubes, serum separator tubes, and rapid serum tubes compared to glass tubes (GTs) [8, 10, 14]. Moreover, the concentration of some drugs such as carbamazepine is significantly elevated in plastic tubes after 24 h [14]. Despite considering a wide spectrum of laboratory assays, we found that the effect of various collection tubes on stability and the accuracy of measured metabolites produced during oxidative stress has never been studied.
Oxidative stress status caused by the impairment of oxidant/antioxidant equilibrium is shown to have a major role in disease pathogenesis and has been studied in a wide spectrum of disciplines such as chemistry, biology, biochemistry, and physiology owing to its importance in cell survival and maintaining the crucial functions of the cell [15]. During oxidative stress, reactive species (RS) trigger modifications in biomolecules including DNA, lipids, and proteins [16]. Nitric oxide (NO) is one of these products, which is generated by intact endothelium and plays a role in oxidative stress alongside other reactive oxygen species (ROS) [17]. In addition, lipid peroxidation of polyunsaturated fatty acids (PUFAs) in membranes is usually evaluated by the formation of malondialdehyde (MDA), which is one of the principal breakdown products of the endoperoxidase activity in various diseases [18]. In contrast, a number of assays have been developed to assess the total antioxidant power of plasma. For instance, the measurement of the ferric reducing ability of plasma (FRAP) and total thiol (t-SH) is introduced to evaluate the antioxidant status [19, 20]. Several studies have indicated that investigating the profile of protein modifications (thiol groups) caused by oxidative stress would be a favorable approach for facilitating the diagnosis of pathological conditions and several disorders including inflammatory responses, atherosclerosis, and neurodegenerative disorders. As a result, studying oxidative stress biomarkers is crucial in determining the oxidative stress status in individuals to help the identification of pathological conditions [21–24]. Therefore, the collection of blood in an appropriate tube has great importance in the precision and reliability of the test results. However, many laboratories have made little effort to evaluate the quality of blood collection tubes and monitor their performance.
To the best of the authors’ knowledge, the impact of using different blood collection tubes on the variations of oxidative stress metabolites is not well-known and only a few authors have sporadically addressed this issue. A study investigating the alternations of myeloperoxidase collected in various tubes revealed that heparin plasma tubes show higher myeloperoxidase concentrations than EDTA or citrate tubes [25]. However, this study focused on the effects of two different anticoagulants not the type of tube itself. Hence, the present study aimed to investigate the impact of GTs, plain plastic tubes (PTs), and gel separator tubes (GSTs) on the oxidative (MDA and NO) and antioxidative markers including t-SH levels as well as total antioxidant capacity, which is measured by the FRAP assay, in order to suggest an appropriate blood collection tube to minimize the spontaneous effects on antioxidant markers.
Materials and methods
Sample collection
Three types of blood collection tubes were examined in this study, including GT (non-silicone coated glass), PT (plastic with clot activator), and GST (gel separator with clot activator). All tubes were 14 x 100 mm blood collection tubes with a sterile interior. In the present study, GTs were considered the control tubes since they have been the conventional serum collecting tubes over the past few decades and contain neither surfactants for the coverage of internal tube surface nor clot activators and separator gels [8]. The project was approved by the ethics committee of Kerman University of Medical Sciences (Ethical Approval Code: IR.KMU.REC.1398.177). Written informed consent, as a crucial requirement for providing the participants with the details of the proposed trial, was obtained from all the contributors according to the principles of the Declaration of Helsinki. Afterward, 10 mL of venous blood was drawn from 35 healthy volunteers aged between 18 and 50 years after 10 h of fasting. The blood samples were separated into the previously mentioned tubes and inverted eight times to ensure the proper mixing of blood and additives. The samples were incubated for 30 min at room temperature and serum was separated by centrifugation at 800g for 8 min. All the serum samples were stored at -70°C until analysis.
Oxidative stress markers
Determination of MDA
Serum MDA level was determined according to the method introduced by Buege and Aust (1978) as described in detail in our previous study [26]. The color produced as a result of thiobarbituric acid (TBA) and MDA reaction was assessed at 532 nm. Consequently, a molar absorption coefficient of 1.56 × 105 M−1 cm−1 was employed to estimate the MDA level and the results are expressed as μmol/L.
Determination of the NO metabolite
The Griess method was applied to measure the NO content. For this purpose, the serum samples were deproteinized using acetonitrile followed by 30 min incubation at 37°C after the addition of 0.1 mL of the Griess reagent. Subsequently, the samples’ absorbance was determined at 546 nm and the NO level was evaluated using a standard curve confirmed by 0–50 μmol/L sodium nitrite. The results are expressed as μmol/L [27].
Determination of t-SH
To calculate the t-SH content in the cell lysate, the spectrophotometric procedure was performed. In this assay, the 5,5′-dithiobis (2-nitrobenzoic acid) (DTNB) reagent was used, which produces a yellow complex when it reacts with thiol groups and has the maximum absorption at 412 nm [28]. The t-SH amount was determined using the molar absorption coefficient of 13,600 M−1 cm−1.
Determination of FRAP levels
The FRAP assay was carried out based on the Benzie and Strain method in which the reduction ability of ferric (III) to ferrous (II) ion at a low pH is evaluated [20]. Concisely, the addition of cellular supernatant to the FRAP reagent creates a blue color, which is spectrophotometrically assessed at 593 nm followed by 5 min incubation at 37°C. The FRAP level of unknown samples was evaluated using the standard curve of FeSO4.7H2O solution (0–1000 μmol/L [29]).
Statistical analysis
The results of antioxidant assays on samples collected in PTs and GSTs were compared with those of GTs as the reference tube. For this purpose, the normality test was carried out, which showed a non-normal distribution of data, and then the Friedman test was performed. Moreover, the means and medians of MDA, NO, t-SH, and FRAP results in different groups were calculated and reported as mean ± standard error of the mean (SEM) and median (first quartile to the third quartile). The Wilcoxon matched-pairs test was used to compare mean differences between tubes. Furthermore, the Bland-Altman and Passing-Bablok regression analyses were employed to visualize the scatter of differences between glass vials and the other two collection tubes. Both the mean bias and the percentage bias were determined using the Bland-Altman analysis for medical decision points in different tubes. The evaluation of clinical significance was based on desirable bias calculation [30]. Bias was determined for each analyte in each tube as follows: Bias% = (Average absolute deviation from the target value/Target) × 100. Subsequently, the Pearson correlation coefficient (r) was used to determine the analyte concentrations correlation between tubes. The statistical analysis was carried out using GraphPad Prism software version 8 for Windows (GraphPad Software Inc., San Diego, CA, USA), and P-values less than 0.05 were considered statistically significant.
Results and discussion
Preventing pre-analytical errors remains an ongoing problem in clinical and research laboratories and may affect the validity and precision of outcomes. Therefore, proper handling and usage of suitable collection tubes seem crucial. The aim of the present study was to investigate the effects of three different types of collection tubes on the measurement of MDA, FRAP, NO, and t-SH levels in order to recommend the most appropriate blood collection tube for the assessment of antioxidants. To the best of the authors’ knowledge, several studies have evaluated the effects of using various collection tubes on the alternation of chemical analytes, hormones, and drugs; however, their effect on oxidative stress factors has not been studied and it is recommended to design further studies with larger sample sizes to reevaluate these findings [8, 10, 14].
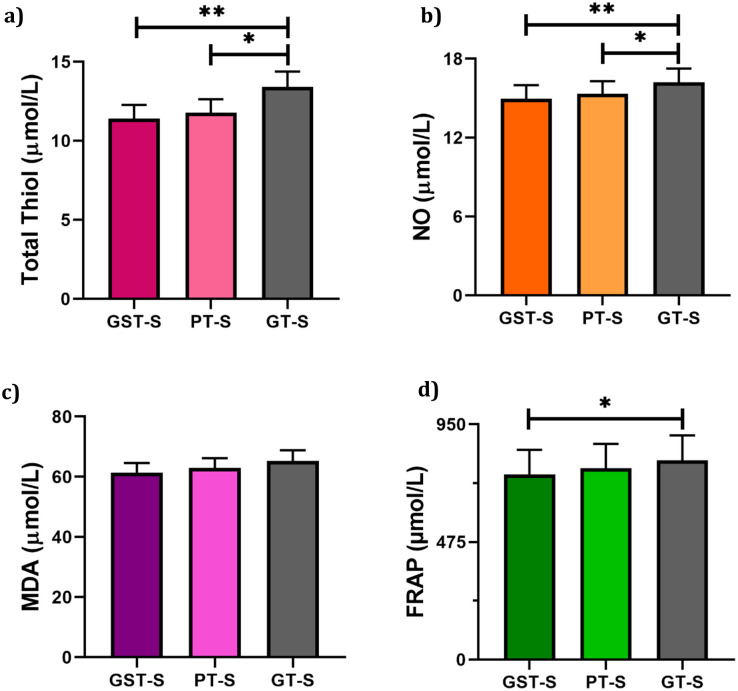
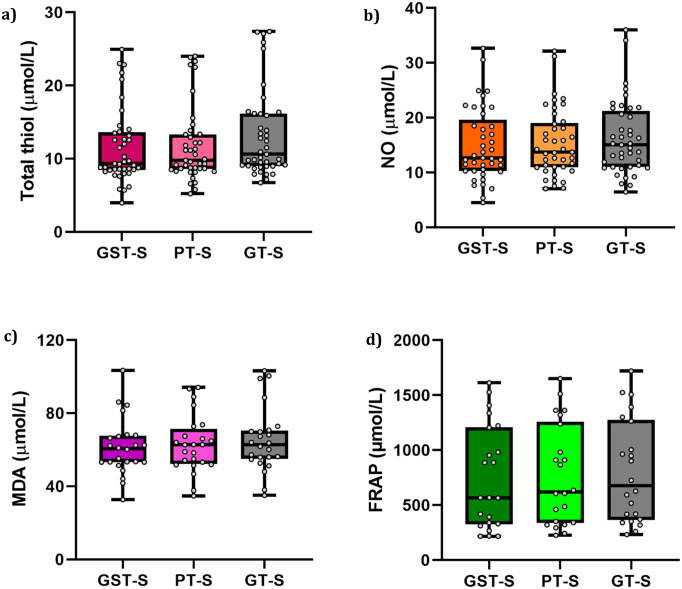
The blood sample collected from the participants was separated into three different types of collection tubes to determine the possible impact of blood collection tube materials on serum MDA, NO, FRAP, and t-SH levels. The comparison between the means of various tubes and their significance is depicted in Fig 1. From a statistical standpoint, the findings of this study revealed that NO, FRAP, and t-SH levels were significantly lower in GSTs and/or PTs compared to GTs. We found that the t-SH content was markedly decreased in GSTs and PTs (11.42 ± 0.82, and 11.79 ± 0.83, respectively) compared to GTs (13.41 ± 0.96), and its levels were the lowest in GSTs compared to the other tubes. These data also indicated that the t-SH content in GTs showed a statistically significant difference compared to PTs and GSTs (P-value < 0.05). In addition, it was shown that the NO content was significantly lower in GSTs and PTs (14.96 ± 1.03 and 15.34 ± 0.96, respectively) than in GTs (16.20 ± 1.04). Moreover, the FRAP level was considerably lower in GSTs compared to GTs (P-value < 0.05). Based on the data obtained in this study, the MDA concentration in PTs, GTs, or GSTs did not differ significantly (P-value > 0.05). Current data suggests that PTs and GSTs may absorb or interact with these metabolites and subsequently lower their levels. It has been proposed that some tube additives such as clot activators and surfactants may absorb certain cellular materials from the whole blood specimens [8, 31]. Although we did not directly evaluate the effects of the additives in the present study, since all of the metabolites were measured in blood, this phenomenon may also apply to oxidative factors. Several studies have investigated alternations in clinical chemistry, endocrinology, serology, and molecular testing, as well as coagulation assays in PTs and GTs and have reported a statistically significant difference in some analytes [5, 32, 33]. Detailed information on the median as well as first and third quartiles of the measured factors is presented in Fig 2.
Fig 1. The comparison of mean concentration of (a) total Thiol, (b) NO, (c) MDA, and (d) FRAP levels in GST, GT, and PT.
Wilcoxon matched paired test was used, * < 0.05, ** < 0.01.
Fig 2. The comparison of median, mean, first, and third quartile concentration of (a) total Thiol, (b) NO, (c) MDA, and (d) FRAP in GST, GT, and PT.
The lower concentration of factors in PTs versus GTs might be because of two reasons. The first reason is the hydrophobic surface of plastic tubes, which not only interacts with the non-polar groups of compounds but also leads to the cohesion of clotted blood on the tube wall as a result of non-smooth blood flow on the plastic surface [34, 35]. The second reason is that the clots created on the internal wall of PTs are gelatinous, which disrupts the tidy separation of clot and serum by centrifugation and correspondingly may cause hemolysis; thus, it could interfere with spectrophotometric methods employed for the assessment of antioxidants. It is also reported that clot activators fabricate a gelatinous clot and cause cell lysis as explained above [36]. To obviate this issue, it is suggested to coat the tube wall with surfactants, which are used to reduce the non-specific adsorption of red blood cells (RBCs), platelets, and proteins to the tube wall while also improving the blood flow on the tube surface [36]. These surfactants change the permeability of the cellular membrane as well as lipophilic structures due to their detergent characteristics [5]. Therefore, an increased probability of cell lysis is proposed in the case of using surfactants as an additive, which can lead to disruption in the photometric process as previously explained.
Additionally, the presence of the separator gel, which is made of viscous liquid, fillers, and/or tackifiers and can interfere with laboratory tests via several mechanisms, may explain the reduced level of factors in GSTs [1, 37]. Separator gels absorb some drugs such as phenytoin, phenobarbital, and carbamazepine through hydrophobic interactions in addition to causing a time-dependent reduction in several hormones including progesterone and testosterone [38–41]. Moreover, separator gels may also release substances such as silicone oil and gel pieces into the specimen, which would interfere with the proper function of solid-phase immunoassay systems, electrode surfaces, sample probes, and absorbance reading in the cuvettes [40, 42]. It should be noted that their rate of degradation and release may rise in the case of extreme temperatures and improper storage, which reveals the importance of suitable sample collection [40].
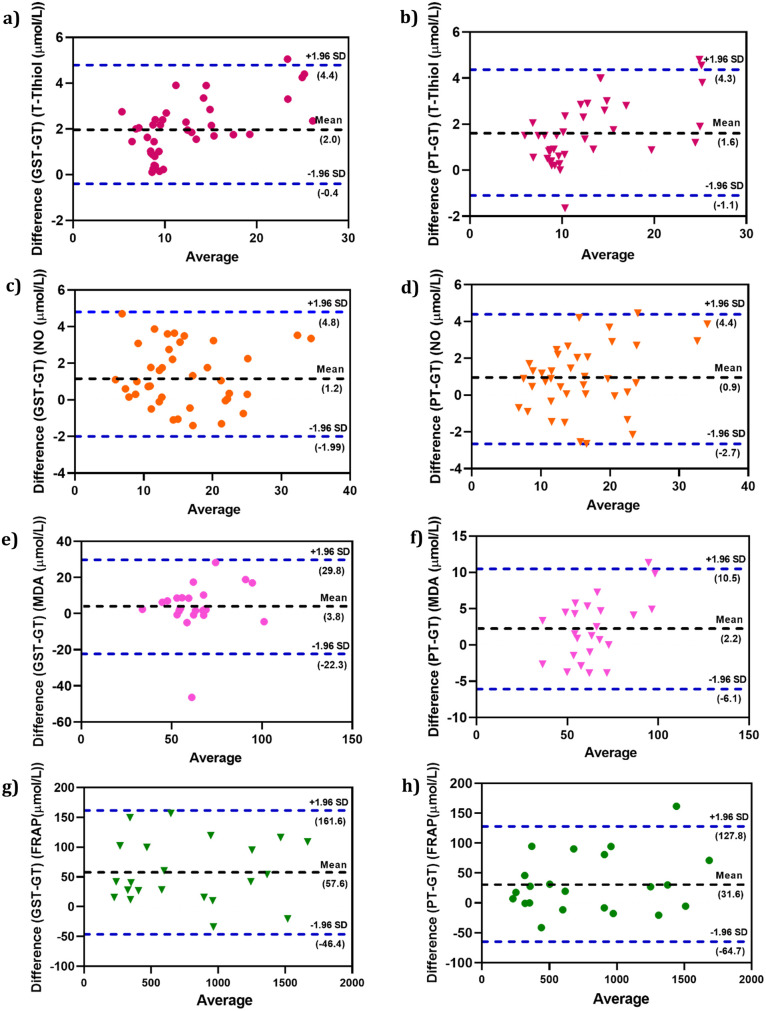
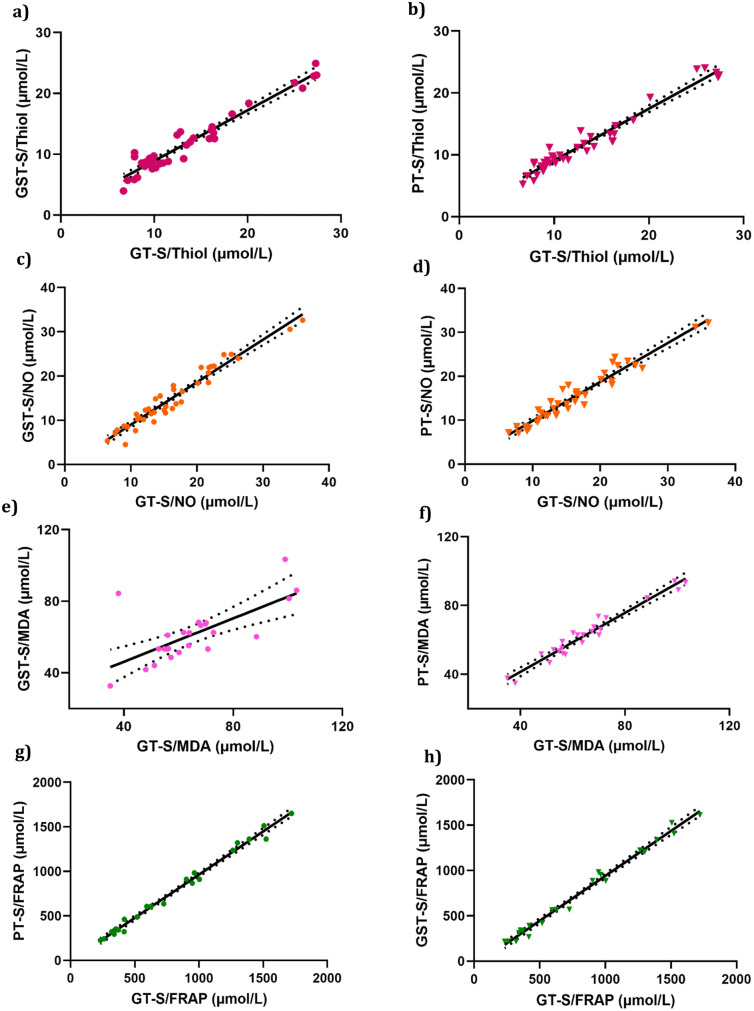
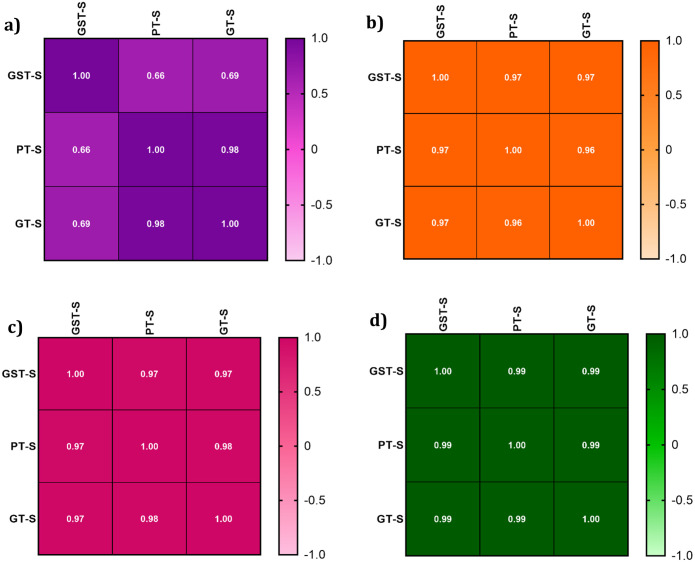
The Bland–Altman plot denoted that the different tubes had no influence on the mean NO, MDA, and t-SH values in our results (Fig 3). The mean differences (bias percentage) of NO, MDA, and t-SH ranged from 0.8% to 5.5% and were lower than the desirable calculated error or total allowable error (TAE) percentage. Variations of the obtained bias percentage are not of clinical significance. However, we found an upper 10% bias for FRAP that should be considered when using PTs and GSTs. The Passing-Bablok regression analysis (Fig 4) was performed for all the parameters. The values of the parameters in different types of tubes were strongly correlated according to the Passing-Bablok regression. In addition, Pearson correlation coefficients (r) for all tests with P < 0.05 showed a good correlation between the results in PTs vs. GTs and GSTs vs. GTs (Fig 5).
Fig 3. Bland-Altman difference plots for the antioxidant parameters obtained with PT, GST and GT tubes.
(a, b) total Thiol, (c, d) NO, (e, f) MDA, (g, h) FRAP values. The dashed lines are the limit of agreements (LOA), which correspond to the mean ± 1.96 SD of the difference between the tubes.
Fig 4. Comparison of assay results after collection of blood in PT, GT, and GST tubes, using passing and bablok regression analysis.
Regression line (full line) with its 95% confidence interval (broken lines). Comparison of results for PT, GST and GT tubes for the antioxidants parameters.
Fig 5. Pearson correlation coefficient (r) values of correlation analysis for the antioxidant parameters obtained with PT, GST and GT tubes.
(a) total Thiol, (b) NO, (c) MDA, and (d) FRAP.
Conclusion
Biomarkers of oxidative stress can be used in the evaluation of disease status such as cardiovascular diseases and cancer and etc. However, the use of oxidative biomarkers in prognosis of the diseases require further management. A vast number of methods have been developed and used in almost all diseases to measure the extent of oxidative stress. Nevertheless, the type of blood collection tubes has been overlooked. Based on higher Bias % in FRAP assay than TAE, it could be concluded that some of the materials used in blood collection tubes interfere with the FRAP measurement. These materials are in the tube wall and in some additives including surfactants, separating gels, and clot activators. Despite statistical differences in NO and t-SH, there was no clinically significant change between the samples. It is suggested to use GTs rather than PTs or GSTs as they show less interference in the assessment of oxidative stress biomarkers. Moreover, it is recommended to investigate the influence of different anticoagulants on the variation of these factors in prospective studies.
Supporting information
(DOCX)
Acknowledgments
The authors would like to express their deep gratitude towards participants who provided us with their precious assistance in performing this study.
Data Availability
All relevant data are within the paper and the Supporting information files.
Funding Statement
The work was supported by the Kerman University of Medical Sciences (project no. 98000218) and the funder had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Bowen RA, Remaley AT. Interferences from blood collection tube components on clinical chemistry assays. Biochemia medica: Biochemia medica. 2014;24(1):31–44. doi: 10.11613/BM.2014.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Anraku H, Shoji Y, inventors; Sekisui Chemical Co Ltd, assignee. Vacuum blood collection tubes. United States patent US4856533A. 1989 Aug.
- 3.Kessler SB. Apparatus for the separation of blood components. U.S. Patent No. 4,257,886; 1981.
- 4.Bennett MC, inventor; Becton Dickinson and Co, assignee. Novel assembly for separating blood. United States patent US3976579A. 1976.
- 5.Bowen RA, Vu C, Remaley AT, Hortin GL, Csako G. Differential effect of blood collection tubes on total free fatty acids (FFA) and total triiodothyronine (TT3) concentration: a model for studying interference from tube constituents. Clinica chimica acta. 2007;378(1–2):181–93. [DOI] [PubMed] [Google Scholar]
- 6.Vogler EA, Harper GR, inventors; Becton Dickinson and Co, assignee. Surface modified blood collection tubes. Canada patent CA2099097C. 1998.
- 7.Vogler EA, Harper GR, Graper JC, inventors; Becton Dickinson and Co, assignee. Vacuum actuated blood collection assembly including tube of clot-accelerating plastic. Europe patent EP0629445A2. 1997.
- 8.Bowen RA, Sattayapiwat A, Gounden V, Remaley AT. Blood collection tube-related alterations in analyte concentrations in quality control material and serum specimens. Clinical biochemistry. 2014;47(3):150–7. doi: 10.1016/j.clinbiochem.2013.11.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bowen RA, Chan Y, Ruddel ME, Hortin GL, Csako G, Demosky SJ Jr, et al. Immunoassay interference by a commonly used blood collection tube additive, the organosilicone surfactant silwet L-720. Clinical chemistry. 2005;51(10):1874–82. doi: 10.1373/clinchem.2005.055400 [DOI] [PubMed] [Google Scholar]
- 10.Smets EM, Dijkstra-Lagemaat JE, Blankenstein MA. Influence of blood collection in plastic vs. glass evacuated serum-separator tubes on hormone and tumour marker levels. Clinical Chemistry and Laboratory Medicine (CCLM). 2004;42(4):435–9. doi: 10.1515/CCLM.2004.076 [DOI] [PubMed] [Google Scholar]
- 11.Hill B, Laessig R, Koch D, Hassemer D. Comparison of plastic vs. glass evacuated serum-separator (SSTTM) blood-drawing tubes for common clinical chemistry determinations. Clinical chemistry. 1992;38(8):1474–8. [PubMed] [Google Scholar]
- 12.Dasgupta A, Blackwell W, Bard D. Stability of therapeutic drug measurement in specimens collected in VACUTAINER plastic blood-collection tubes. Therapeutic drug monitoring. 1996;18(3):306–9. doi: 10.1097/00007691-199606000-00016 [DOI] [PubMed] [Google Scholar]
- 13.Dasgupta A, Dean R, Saldana S, Kinnaman G, McLawhon RW. Absorption of Therapeutic Drugs by Barrier Gels in Serum Separator Blood Collection Tubes: Volume-and Time-dependent Reduction in Total and Free Drug Concentrations a. American journal of clinical pathology. 1994;101(4):456–61. doi: 10.1093/ajcp/101.4.456 [DOI] [PubMed] [Google Scholar]
- 14.Boeynaems J-M, De Leener A, Dessars B, Villa-Lobos HR, Aubry J-C, Cotton F, et al. Evaluation of a new generation of plastic evacuated blood-collection tubes in clinical chemistry, therapeutic drug monitoring, hormone and trace metal analysis. Clinical Chemistry and Laboratory Medicine (CCLM). 2004;42(1):67–71. doi: 10.1515/CCLM.2004.013 [DOI] [PubMed] [Google Scholar]
- 15.Valko M, Leibfritz D, Moncol J, Cronin MT, Mazur M, Telser J. Free radicals and antioxidants in normal physiological functions and human disease. The international journal of biochemistry & cell biology. 2007;39(1):44–84. doi: 10.1016/j.biocel.2006.07.001 [DOI] [PubMed] [Google Scholar]
- 16.Knasmüller S, Nersesyan A, Mišík M, Gerner C, Mikulits W, Ehrlich V, et al. Use of conventional and-omics based methods for health claims of dietary antioxidants: a critical overview. British Journal of Nutrition. 2008;99(E-S1):ES3–ES52. [DOI] [PubMed] [Google Scholar]
- 17.Lubos E, Handy DE, Loscalzo J. Role of oxidative stress and nitric oxide in atherothrombosis. Front Biosci. 2008;13:5323–44. doi: 10.2741/3084 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Paydar P, Asadikaram G, Nejad HZ, Moazed V, Poursayedi B, Nematollahi MH, et al. The Role of Acetylcholinesterase, Paraoxonase, and Oxidative Stress in Breast Tumors. International journal of cancer management. 2018;11(11). [Google Scholar]
- 19.Mao C, Yuan J-Q, Lv Y-B, Gao X, Yin Z-X, Kraus VB, et al. Associations between superoxide dismutase, malondialdehyde and all-cause mortality in older adults: a community-based cohort study. BMC Geriatr. 2019;19(1):1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Benzie IF, Strain JJ. The ferric reducing ability of plasma (FRAP) as a measure of “antioxidant power”: the FRAP assay. Anal Biochem. 1996;239(1):70–6. doi: 10.1006/abio.1996.0292 [DOI] [PubMed] [Google Scholar]
- 21.Firuzi O, Mladěnka P, Riccieri V, Spadaro A, Petrucci R, Marrosu G, et al. Parameters of oxidative stress status in healthy subjects: their correlations and stability after sample collection. Journal of clinical laboratory analysis. 2006;20(4):139–48. doi: 10.1002/jcla.20122 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Butterfield DA, Gu L, Domenico FD, Robinson RA. Mass spectrometry and redox proteomics: applications in disease. Mass spectrometry reviews. 2014;33(4):277–301. doi: 10.1002/mas.21374 [DOI] [PubMed] [Google Scholar]
- 23.Baraibar MA, Ladouce R, Friguet B. Proteomic quantification and identification of carbonylated proteins upon oxidative stress and during cellular aging. Journal of proteomics. 2013;92:63–70. doi: 10.1016/j.jprot.2013.05.008 [DOI] [PubMed] [Google Scholar]
- 24.Marrocco I, Altieri F, Peluso I. Measurement and clinical significance of biomarkers of oxidative stress in humans. Oxidative medicine and cellular longevity. 2017. doi: 10.1155/2017/6501046 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Shih J, Datwyler SA, Hsu SC, Matias MS, Pacenti DP, Lueders C, et al. Effect of collection tube type and preanalytical handling on myeloperoxidase concentrations. Clinical chemistry. 2008;54(6):1076–9. doi: 10.1373/clinchem.2007.101568 [DOI] [PubMed] [Google Scholar]
- 26.Juybari KB, Ebrahimi G, Moghaddam MAM, Asadikaram G, Torkzadeh-Mahani M, Akbari M, et al. Evaluation of serum arsenic and its effects on antioxidant alterations in relapsing-remitting multiple sclerosis patients. Multiple sclerosis and related disorders. 2018;19:79–84. doi: 10.1016/j.msard.2017.11.010 [DOI] [PubMed] [Google Scholar]
- 27.Arya A, Azarmehr N, Mansourian M, Doustimotlagh AH. Inactivation of the superoxide dismutase by malondialdehyde in the nonalcoholic fatty liver disease: a combined molecular docking approach to clinical studies. Archives of Physiology and Biochemistry. 2019:1–8. doi: 10.1080/13813455.2019.1659827 [DOI] [PubMed] [Google Scholar]
- 28.Bastin A, Sadeghi A, Nematollahi MH, Abolhassani M, Mohammadi A, Akbari H. The effects of malvidin on oxidative stress parameters and inflammatory cytokines in LPS-induced human THP-1 cells. Journal of Cellular Physiology. 2021;236(4):2790–9. doi: 10.1002/jcp.30049 [DOI] [PubMed] [Google Scholar]
- 29.Ahmadi Z, Moradabadi A, Abdollahdokht D, Mehrabani M, Nematollahi MH. Association of environmental exposure with hematological and oxidative stress alteration in gasoline station attendants. Environmental Science and Pollution Research. 2019;26(20):20411–7. doi: 10.1007/s11356-019-05412-7 [DOI] [PubMed] [Google Scholar]
- 30.Biswas S, Bindra M, Jain V, Gokhale P. Evaluation of imprecision, bias and total error of clinical chemistry analysers. Indian Journal of Clinical Biochemistry. 2015;30(1):104–8. doi: 10.1007/s12291-014-0448-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Miller W, Erek A, Cunningham TD, Oladipo O, Scott MG, Johnson RE. Commutability limitations influence quality control results with different reagent lots. Clinical chemistry. 2011;57(1):76–83. doi: 10.1373/clinchem.2010.148106 [DOI] [PubMed] [Google Scholar]
- 32.Bowen RA, Hortin GL, Csako G, Otañez OH, Remaley AT. Impact of blood collection devices on clinical chemistry assays. Clinical biochemistry. 2010;43(1–2):4–25. doi: 10.1016/j.clinbiochem.2009.10.001 [DOI] [PubMed] [Google Scholar]
- 33.Bowen RA, Chan Y, Cohen J, Rehak NN, Hortin GL, Csako G, et al. Effect of blood collection tubes on total triiodothyronine and other laboratory assays. Clinical Chemistry. 2005;51(2):424–33. doi: 10.1373/clinchem.2004.043349 [DOI] [PubMed] [Google Scholar]
- 34.Vogler EA, Harper GR, Graper JC. Vacuum actuated blood collection assembly including tube of clot-accelerating plastic. U.S. Patent No. 5,344,611; 1994.
- 35.Anraku H, Shoji Y. Vacuum blood-collection tube. US Patent; 1989.
- 36.Dubrowny NE, Harrop AJ. Collection device. U.S. Patent No. 6,686,204; 2004.
- 37.Anraku H SY. “Vacuum blood collection tubes”, U.S. Patent No. 8,565,334. August 1989.
- 38.Bergqvist Y, Eckerbom S, Funding L. Effect of use of gel-barrier sampling tubes on determination of some antiepileptic drugs in serum. Clinical chemistry. 1984;30(3):465–6. [PubMed] [Google Scholar]
- 39.Koch TR, Platoff G. Suitability of collection tubes with separator gels for therapeutic drug monitoring. Therapeutic drug monitoring. 1990;12(3):277–80. doi: 10.1097/00007691-199005000-00011 [DOI] [PubMed] [Google Scholar]
- 40.Wilde C. Subject preparation, sample collection, and handling. In: Wild D, editor. The immunoassay handbook. NY: Elsevier. 2005. 13. [Google Scholar]
- 41.Shi RZ, van Rossum HH, Bowen RA. Serum testosterone quantitation by liquid chromatography-tandem mass spectrometry: Interference from blood collection tubes. Clinical biochemistry. 2012;45(18):1706–9. doi: 10.1016/j.clinbiochem.2012.08.008 [DOI] [PubMed] [Google Scholar]
- 42.Ji S, Evenson MA. Effects of contaminants in blood-collection devices on measurements of therapeutic drugs. Clinical chemistry. 1983;29(3):456–61. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(DOCX)
Data Availability Statement
All relevant data are within the paper and the Supporting information files.